Abstract
Expansion of a CGG-repeat tract in the 5’-untranslated region of the FMR1 gene to >200 repeats results in epigenetic silencing of the gene by a mechanism that is still unknown. FMR1 gene silencing results in fragile X syndrome (FXS), the most common heritable cause of intellectual disability. We have previously shown that reactivation of the FMR1 gene in FXS cells with 5-azadeoxycytidine (AZA) leads to the transient recruitment of EZH2, the polycomb repressive complex 2 (PRC2) component responsible for H3K27 trimethylation, and that this recruitment depends on the presence of the FMR1 transcript. However, whether H3K27 trimethylation was essential for FMR1 re-silencing was not known. We show here that EZH2 inhibitors increased FMR1 expression and significantly delayed re-silencing of the FMR1 gene in AZA-treated FXS cells. This delay occurred despite the fact that EZH2 inhibition did not prevent the return of DNA methylation. Treatment with compound 1a, a small molecule that targets CGG-repeats in the FMR1 mRNA, also resulted in sustained expression of the FMR1 gene in AZA-treated cells. This effect of 1a was also associated with a decrease in the levels of H3K27 trimethylation but not DNA methylation. Thus, our data show that EZH2 plays a critical role in the FMR1 gene silencing process and that its inhibition can prolong expression of the FMR1 gene even in the presence of its transcript.
Introduction
Fragile X syndrome (FXS) is the most frequent form of inherited cognitive impairment and the most common monogenic cause of autism (reviewed in (1)). It results from the loss of functional fragile X mental retardation protein (FMRP), an RNA binding protein that is ubiquitously expressed with particularly high levels in brain and gonads. Loss of FMRP results in translational dysregulation that ultimately results in the loss of synaptic plasticity and thus problems with learning and memory (reviewed in (2)). FMRP is also involved in the DNA damage response (3) and cancer progression (4).
Most patients with FXS have >200 CGG-repeats in the 5’-untranslated region of fragile X mental retardation-1 (FMR1), the gene that encodes FMRP. Such FMR1 alleles, known as full mutation (FM) alleles, undergo transcriptional gene silencing by a mechanism that is still not well understood (5–7). The chromatin signature of the silenced FMR1 alleles is unusual in that it has the features of both the facultative and constitutive heterochromatin (8). The facultative marks, H3K9me2 and H3K27me3, are generally associated with developmentally silenced genes whereas the constitutive heterochromatin is enriched for H3K9me3 and H4K20me3 similar to many repeated DNA elements in the human genome. We have previously shown that the constitutive heterochromatin marks are maximal in the vicinity of the expanded CGG-repeats at the FMR1 locus suggesting that the signal for their recruitment is inherent to the CGG-repeats (8). The facultative heterochromatin marks are more broadly distributed at the FMR1 locus consistent with their ability to spread in cis. The FMR1 transcript has been implicated in the silencing process in two recent studies (9,10). We showed that the FMR1 mRNA is responsible for recruitment of EZH2, the H3K27 trimethylase, to FM alleles that have been reactivated by treatment with 5-azadeoxycytidine (AZA) (10). It has also been suggested that the formation of an RNA:DNA hybrid is responsible for the silencing seen during neuronal differentiation of human embryonic stem cells (hESCs) (9). However, since the FMR1 gene in the cell lines used in that study were already substantially methylated before differentiation (Supplementary Material, Fig. S1 in (9)), as indeed are the majority of FXS ESCs (11), how this silencing recapitulates silencing in vivo remains unclear.
In an effort to better understand the contribution of H3K27me3 to FMR1 gene silencing and potentially to find ways to prevent the re-silencing that typically occurs when AZA is withdrawn, we tested a number of known EZH2 inhibitors along with compound 1a (9-hydroxy-5,11-dimethyl-2-(2-(piperidin-1-yl)ethyl)-6H-pyrido[4,3-b]carbazol-2-ium). This compound has been suggested to prevent silencing in neurons derived from FXS embryonic stem cells by preventing the formation of an RNA:DNA hybrid between the FM transcript and the 5’ end of the FMR1 gene (9). We show that both 1a and known inhibitors of EZH2 decreased H3K27 trimethylation and increased the levels of FMR1 transcript in AZA treated cells. Furthermore, both known EZH2 inhibitors and compound 1a significantly extended the time that the gene stays transcriptionally active after AZA withdrawal. However, our data suggest that 1a is not acting to prevent the formation of an RNA:DNA hybrid. Nor is it acting by disrupting some other association of the transcript with the FMR1 gene, such as a triplex. Nevertheless, our data support the idea that the use of epigenetic modifiers may have therapeutic potential in FXS and has implications for our understanding of the sequence of events involved in gene silencing in FXS.
Results
EZH2 inhibitors delay re-silencing of reactivated FM alleles
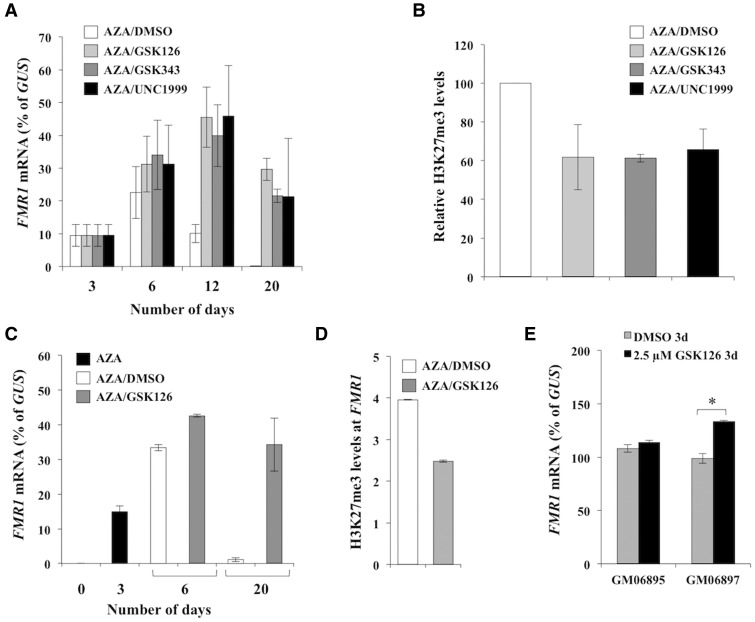
Treatment of FXS cells with 10 µM AZA for 3 days results in reactivation of the FMR1 gene (10,12). However, this reactivation is transient, reaching a maximum 3–7 days after drug withdrawal (at day 6–10 of treatment), with the FMR1 gene being completely re-silenced at ∼20 days after AZA removal (10). We previously showed that FMR1 re-silencing is preceded by an increase in the levels of H3K27me3 on the reactivated allele that is dependent on the presence of the FMR1 transcript (10). The increase in trimethylation of H3K27 is also transient, dropping to pre-reactivation levels as re-silencing occurs. We hypothesized that if the increase in H3K27me3 on the FMR1 gene was required for subsequent DNA methylation and gene silencing, then treatment with small molecules that reduce H3K27me3 accumulation on the reactivated allele would prevent or slow the re-silencing process. We therefore tested three specific EZH2 inhibitors, GSK126 (13), GSK343 (14) and UNC1999 (15). Treatment of FXS cells (GM04025) with 10 µM AZA for 3 days, followed by treatment with 5 µM of these inhibitors resulted in slightly higher levels of FMR1 mRNA at day 6 than were seen with AZA alone (Figure 1A). Treatment with EZH2 inhibitors was also associated with a ∼40% reduction in H3K27me3 levels at FMR1 exon1 at day 6 (Figure 1B). Furthermore, while the levels of FMR1 transcript were still rising in all of the EZH2 inhibitor treated cells at day 12, the levels of FMR1 mRNA were already declining in cells treated with vehicle (DMSO) (Figure 1A). Remarkably, FMR1 mRNA levels in inhibitor-treated cells were as high at day 20 as at day 6 (Figure 1A). In contrast, in vehicle-treated cells, the FMR1 mRNA was barely detectable. Thus, all three EZH2 inhibitors delayed the gene silencing that typically occurs after AZA is withdrawn. Since all three drugs had a similar effect on FMR1 expression, subsequent experiments were limited to trials of GSK126.
Figure 1.
EZH2 inhibitors delay re-silencing of the FMR1 gene in AZA treated FXS patient cells. GM04025 cells were treated with 10 µM AZA for 3 days. The medium was then replaced with fresh medium containing either 5 µM of GSK126, GSK343 or UNC1999 or an equivalent volume of the vehicle alone (DMSO) (A and B) or 2.5 µM of GSK126 or the equivalent volume of DMSO (C and D). In both sets of experiments medium was replaced with fresh drug containing medium every 3 days. The FMR1 mRNA levels were determined at the indicated time points and were normalized to GUS mRNA levels. The data shown are an average of three independent treatments and error bars represent the standard deviation. The levels of H3K27me3 present on the FMR1 exon 1 in GM04025 cells were analyzed at day 6. The data shown are an average of two independent treatments. The ChIP values were first normalized to the input DNA and are expressed relative to the levels observed in cells treated with AZA alone (B) and relative to GAPDH (D). The error bars represent the standard deviation. E) GM06895 (control) and GM06897 (UFM) cells were treated with either DMSO or 2.5 µM of GSK126 for 3 days. Data shown are an average of 2 independent experiments and error bars represent the standard deviation. * p < 0.05 by unpaired t test.
As is the case with AZA, treatment with 5 µM of the EZH2 inhibitors reduced cell growth. This could potentially confound data interpretation in a variety of ways. To attempt to reduce this as far as possible, we tested the effect of reducing the concentration of the drug as well as reducing the frequency of its addition. Treatment with multiple doses of 2.5 µM GSK126 had a similar effect on the levels of FMR1 mRNA in GM04025 cells as was seen with 5 µM GSK126 (Figure 1C). Adding a single dose of 2.5 µM/week was also effective (Supplementary Material, Fig. S1), perhaps reflecting the long residence time of GSK126 on EZH2 (16). While there was variability in the levels of FMR1 mRNA in different FXS cell lines treated with AZA and 2.5 µM GSK126, all of the treated cells showed higher FMR1 mRNA levels than cells treated with AZA/DMSO at day 6 and day 10 (Supplementary Material, Fig. S2). Moreover, in all cell lines re-silencing was delayed, with GSK126 delaying silencing most effectively on those cell lines that were the slowest to return to the silenced state in the absence of the inhibitor (Supplementary Material, Fig. S2). Why some cells are more rapidly silenced than others is unclear since all of these lines showed similar levels of methylation at the start of treatment (∼100%) (Supplementary Material, Fig. S3). As with 5 µM GSK126 treatment, a decrease in the levels of H3K27me3 was seen with 2.5 µM GSK126 in GM04025 cells (Figure 1D). This decrease was not associated with any additional decline in DNA methylation levels on the FMR1 gene above and beyond what was seen with AZA/DMSO as measured by a quantitative methylation-sensitive PCR (qMS-PCR) assay that measures the methylation of a single HpaII site (Supplementary Material, Fig. S3). This assay also showed that methylation was fully restored at day 20 in AZA/GSK126 treated cells, when substantial amounts of FMR1 transcript could still be seen (Supplementary Material, Fig. S3). To confirm that this assay reflected the methylation status of the region as a whole, we also used a previously described high resolution melting curve analysis (MS-MCA) that measures the methylation status of a 105 bp region of FMR1 promoter 5’ of the CGG-repeats (17). The results of this assay were also consistent with the return of DNA methylation by day 20 in AZA/GSK126 treated cells (Supplementary Material, Fig. S4). Thus GSK126 delays re-silencing without preventing the return of DNA methylation.
Although treatment with 2.5 µM GSK126 alone had minimal effect on FMR1 gene reactivation in FXS patient cells (Supplementary Material, Fig. S2), it did increase FMR1 expression in GM06897 cells that carry an unmethylated full mutation (UFM) and that makes normal levels of FMR1 mRNA (Figure 1E). However, it had no effect on FMR1 expression in control cells (Figure 1E). This suggests that in addition to delaying the re-silencing of reactivated alleles, GSK126 has a repeat-dependent effect on the transcription of alleles that have never been silenced. Whether this effect contributes to the elevated levels of transcript produced from reactivated alleles remains to be seen.
Treatment with compound 1a also decreases the levels of H3K27me3 on the reactivated alleles and extends the time that the gene remains active
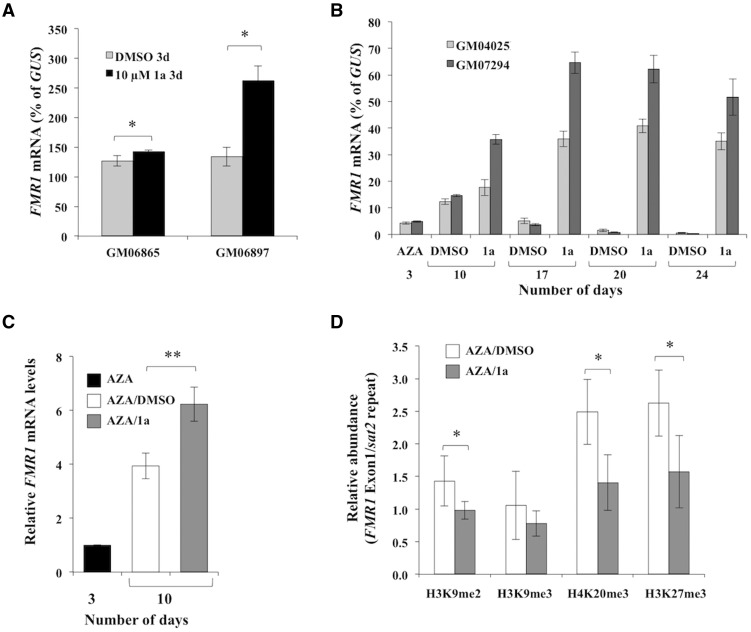
Compound 1a is a small molecule that was identified from a screen for compounds that target the expanded CGG-repeats in the mRNA (18). It has been reported to prevent silencing of the FMR1 gene in FXS ESC-derived neurons with its proposed mode of action being to prevent the formation of an RNA:DNA hybrid between the FMR1 gene and its transcript (9). In the previous study, no effect of 1a was seen on alleles that were already silenced (9). We observed a very low level reactivation of the FMR1 gene in two FXS cell lines after treatment with 10 µM compound 1a for 6 days (0.02% and 0.07% of GUS mRNA in GM04025 and GM07294 cells respectively), suggesting that 1a was also not effective at reactivating silenced alleles in FXS lymphoblastoid cells. A very small increase in FMR1 mRNA levels was also seen in control cells (GM06865) after treatment with 10 µM 1a, however, a much larger effect was observed in GM06897 cells that carry an UFM allele (Figure 2A). This differential effect of 1a on FMR1 mRNA levels in control cells and cells with UFM allele suggests that, as with GSK126, 1a may also have a repeat-specific effect on transcription of active alleles.
Figure 2.
Treatment with compound 1a increases FMR1 transcript levels and also delays re-silencing. (A) GM06865 (control) and GM06897 (UFM) cells were treated with either DMSO or 10 µM of compound 1a for 3 days. The data shown is an average of 3 independent experiments for GM06865 cells and 2 independent experiments for GM06897 cells. The error bars represent the standard deviation. * p < 0.05 by unpaired t test. (B) FXS cells (GM04025 and GM07294) were treated with 10 µM AZA for 3 days followed by addition of either DMSO or 10 µM compound 1a in DMSO once a week. The FMR1 mRNA levels were determined at indicated times and normalized to GUS mRNA. Data shown are an average from three independent experiments and the error bars represent the standard deviation. (C–D) GM04025 cells were treated with 10 µM for 3 days followed by addition of either DMSO or 10 µM compound 1a in DMSO. (C) The FMR1 mRNA levels are shown relative to the levels seen at day 3. The data shown are an average of 5 independent experiments and error bars represent the standard deviation. ** p = 0.0053 by paired t test. (D) The abundance of H3K9me2, H3K9me3, H4K20me3 and H3K27me3 at FMR1 exon1 was analyzed at day 10 and is shown relative to Sat2 repeats. The data shown are an average of 5 independent experiments and the error bars represent the standard deviation. *p < 0.05 by paired t test.
To test if treatment with compound 1a would prevent re-silencing of the FMR1 gene after reactivation with AZA treatment, we treated cells from two different FXS patients, GM04025 and GM07294, with 10 µM AZA for 3 days followed by treatment with compound 1a once a week and followed the expression of the FMR1 gene for 24 days. As seen before, FMR1 mRNA dropped to background levels by day 24 in cells treated with AZA alone, whereas in cells treated with compound 1a following AZA treatment, the FMR1 mRNA levels remained high (Figure 2B). Similar results were obtained for an additional FXS cell line tested, GM09145 (Supplementary Material, Fig. S5). Compound 1f, a structurally related compound that does not interact with the FX repeats (18) and does not delay silencing in ESC derived neurons (9), had a much more modest effect (Supplementary Material, Fig. S5).
We then analyzed the abundance of repressive histone marks associated with the FMR1 gene at day 10 after treatment with AZA alone or in cells given a single dose of 1a at day 3. At this time point, the level of FMR1 mRNA in the 1a treated GM04025 cells was ∼1.5-fold higher than it was in the cells treated with AZA alone (Figure 2C). Even at this time point it is apparent that treatment with compound 1a significantly decreased the levels of not only H3K27me3, but H3K9me2 and H4K20me3 as well (Figure 2D). This was particularly notable since we have previously shown that the levels of the H3K9me2 and H4K20me3 on the FMR1 gene do not change appreciably with AZA treatment alone (10). Treatment of AZA treated cells with 1a did not further reduce DNA methylation on the FMR1 gene (Supplementary Material, Fig. S6). Thus the increase in FMR1 transcript with 1a treatment was not the result of an effect on DNA methylation per se.
Treatment with compound 1a does not reduce the amount of RNA:DNA hybrid formed on the reactivated allele
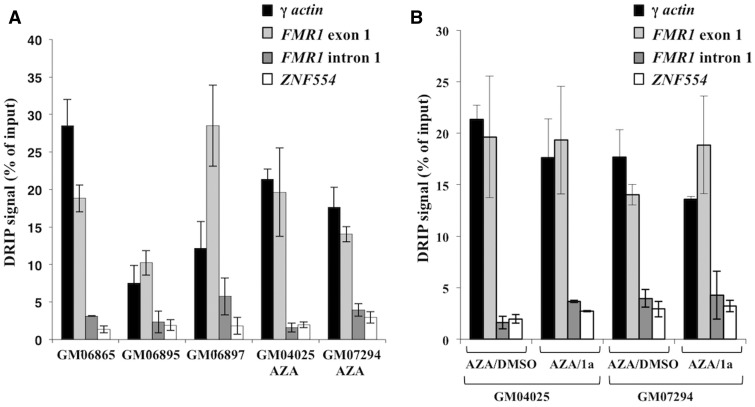
A previous study suggested that an RNA:DNA hybrid forms only on FM alleles in a specific window of time during neuronal differentiation with the duplex acting to initiate FMR1 gene silencing (9). However, work by others has suggested that such hybrids form even on normal alleles in differentiated cells (19). To address the question of which FMR1 alleles form RNA:DNA hybrids we tested cells containing normal and FM alleles using a DNA:RNA immunoprecipitation (DRIP) assay with the S9.6 antibody that is widely used for this purpose (20–24). Evidence of an RNA:DNA hybrid at the FMR1 exon 1 region was seen in control cells (GM06865 and GM06895) (Figure 3A and Supplementary Material, Fig. S7). Thus, our data are consistent with the report of Loomis et al. (19) and confirms the idea that hybrid formation is not confined to FM alleles. We also observed a hybrid in cells with a UFM allele that makes normal levels of FMR1 mRNA (GM06897) and FXS patient cells where the FMR1 gene was reactivated by AZA treatment (GM04025 and GM07294) (Figure 3A and Supplementary Material, Fig. S7). In normal cells the levels of DRIP signal at the FMR1 exon 1 region were comparable to the DRIP signal at the γ-actin locus, a region known to form an RNA:DNA hybrid (25). In the UFM line, the amount of hybrid at FMR1 was more than twice that at γ-actin. In the AZA reactivated FM cells, the levels of hybrid at FMR1 were again more similar to that at γ-actin. The higher levels of RNA:DNA hybrid formed by UFM alleles would be consistent with the idea that the extent of hybrid formation is directly related to repeat length. The drop in the RNA:DNA hybrid relative to γ-actin in cells carrying reactivated FM alleles would be consistent with the fact that while the CGG-repeat number in these alleles is high, the total amount of FMR1 mRNA is relatively low since the alleles were only partially reactivated. While our data support the idea that an RNA:DNA hybrid forms at reactivated FXS alleles, treatment with 1a did not reduce the amount of this hybrid (Figure 3B and Supplementary Material, Fig. S7), suggesting that 1a is not affecting re-silencing by preventing the formation of the RNA:DNA hybrid.
Figure 3.
Compound 1a does not reduce RNA:DNA hybrid formation. The RNA:DNA hybrid was immunoprecipitated with S9.6 antibody from untreated control (GM06865 and GM06895), UFM (GM06897) and AZA treated FXS patient cells at day 6 (GM04025 and GM07294) (A) and AZA treated FXS patient cells (GM04025 and GM07294) with and without 1a treatment at day 6 (B). DNA:RNA immunoprecipitation (DRIP) signal shown is an average of two independent experiments. The error bars represent the standard deviation. The γ-actin locus is a positive control region and ZNF554 is a negative control region for RNA:DNA hybrid.
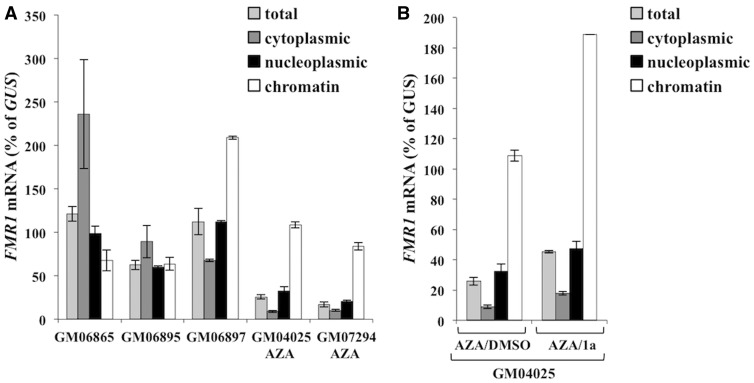
To address the possibility that 1a affects the association of the transcript with the FMR1 gene in some other way, for example, by disrupting triplex formation, we analyzed the distribution of FMR1 mRNA in the subcellular fractions prepared from normal and AZA treated FXS patient cells where the FMR1 gene was reactivated. NEAT1 RNA, a non-coding RNA enriched in the chromatin fraction, was used to monitor the efficiency of subcellular fractionation (data not shown). A greater proportion of the FMR1 mRNA was retained in the chromatin fraction in UFM (GM06897) and AZA treated FM cells (GM04025 and GM07294) than in control cells (GM06865 and GM06895) (Figure 4A). However, while treatment of FXS cells with AZA followed by treatment with 1a resulted in an increase in the levels of FMR1 mRNA in all fractions, it did not alter its enrichment in the chromatin fraction (Figure 4B). This suggests that 1a may not be affecting FMR1 silencing via affecting the association of FMR1 mRNA with the FMR1 promoter as previously suggested (9).
Figure 4.
Compound 1a does not affect the enrichment of the FMR1 mRNA in the chromatin fraction. Total RNA and RNA from the indicated fractions was isolated from untreated control (GM06865 and GM06895), UFM (GM06897) and AZA treated FXS cells at day 7 (GM04025 and GM07294) (A) and AZA treated GM04025 cells with and without 1a treatment at day 7 (B). FMR1 mRNA levels are expressed relative to GUS mRNA. Data shown are an average of at least two independent experiments and error bars represent the standard deviation.
Discussion
We have previously shown that FMR1 mRNA is responsible for the transient enrichment of the repressive histone modification, H3K27me3, on FM alleles that have been reactivated with AZA (10). We show here that preventing trimethylation of H3K27 with different EZH2 inhibitors results in the sustained expression of FMR1 long after AZA withdrawal (Figure 1). This firmly establishes H3K27 trimethylation as a key event in the FXS gene silencing process, despite the transient nature of its increase. Furthermore, it suggests that despite the fact that the FMR1 transcript is the trigger for re-silencing as we previously demonstrated (10), it is possible to circumvent this Catch-22 situation to maintain the gene in the active state in the presence of ongoing FMR1 transcription. Our data also show that while EZH2 inhibitors prevent or delay re-silencing of FXS alleles, they do so despite the fact that they do not delay DNA re-methylation (Supplementary Materials, Fig. S3 and S4). This would be consistent with DNA methylation either preceding or being independent of H3K27 trimethylation.
We also show that a greater proportion of FMR1 mRNA from reactivated FM alleles is present in the chromatin fraction than in cells with normal alleles (Figure 4A). The abnormal nuclear localization of FM transcripts is reminiscent of what has been reported for the DMPK transcripts responsible for a related repeat expansion disorder, myotonic dystrophy type 1 (26). This abnormal nuclear retention of the FMR1 transcript may contribute to the reduced FMRP expression seen in reactivated cells with larger FM alleles. Some of the retained transcript is involved in the formation of an RNA:DNA hybrid in these cells as illustrated by pull-down of the 5’ end of the FMR1 gene with an antibody specific for RNA:DNA hybrids (Figure 3A). Our data also confirm previous observations that even normal alleles form RNA:DNA hybrids (19), as do cells carrying UFM alleles. It is possible that silencing does not occur on normal alleles because the RNA:DNA hybrid is only long and/or stable enough to trigger silencing when the repeat length exceeds the FM threshold. UFM alleles may not become silenced because they are missing a key factor downstream of hybrid formation that is required to complete the silencing process (27).
Compound 1a, like the EZH2 inhibitors, also prevented re-silencing of AZA reactivated alleles (Figure 2B). However, treatment with 1a did not change the amount of RNA:DNA hybrid produced on the FMR1 5’ end (Figure 3B). Nor did it prevent the association of the FMR1 transcript with the chromatin fraction (Figure 4B). Thus, 1a is not acting to prevent the formation of the RNA:DNA hybrid or some other association of the transcript with the FMR1 gene like a triplex. Since 1a has also been shown to block binding of a number of CGG-repeat binding proteins (18), it is possible that this compound delays the silencing of reactivated alleles by preventing the recruitment of repressive histone modifiers that bind the CGG-repeats. It is also possible that 1a inhibits the expression of these or other related proteins that are involved in the re-silencing process. These possibilities would be consistent with the fact that in addition to reducing H3K27me3, treatment with 1a reduced the levels of repressive histone marks H3K9me2 and H4K20me3 (Figure 2D). This may also explain why 1a treatment resulted in higher levels of FMR1 expression than the EZH2 inhibitors in AZA-treated cells.
GSK126 did not affect FMR1 expression in control cells in the absence of AZA treatment and 1a showed only a modest effect. However, they both significantly increased the yield of full-length transcript in cells with a UFM (Figures 1E and 2A) suggesting that these compounds also have a repeat-mediated effect on promoting FMR1 expression from active alleles. It is possible that these compounds affect the expression of a repeat-binding protein that promotes FMR1 transcription or that improves transcription elongation through the repeat in some way.
We have previously shown that the FMR1 transcript is responsible, either directly or indirectly, for the recruitment of the PRC2 complex to the FMR1 locus (10). The fact that the FMR1 mRNA from FM alleles is required for recruiting PRC2 suggests analogies to what has been proposed for some long non-coding RNAs (lncRNAs) involved in gene silencing (28–32). However, the idea that PRC2 is recruited to its target genes by direct binding to lncRNAs has recently been challenged (33,34). In particular, it has been shown that in general it is a promoter silencing that leads to the recruitment of PRC2 rather than the other way around (35). This has led to the suggestion that a high density of unmethylated CpG residues, nucleosome depletion or transcription factor eviction is responsible for genome-wide PRC2 recruitment rather than any specific transcript (36–38). However, since knockdown of the FMR1 transcript reduced the levels of EZH2 on the FM allele (10), it is unlikely that PRC2 recruitment is simply due to any of these events. Thus, it may be that PRC2-recruitment to FM alleles differs from how it is recruited to normal PRC2 target genes. In any event, the fact that H3K27me3 only increases transiently after AZA withdrawal would be consistent with a model in which proteins in the PRC2 pathway recruit other downstream epigenetic marks that result in a decrease in FMR1 transcription initiation as illustrated in Supplementary Material, Fig. S8. This in turn would lead to a drop in PRC2 recruitment to the FM allele and thus a decrease in H3K27me3 levels.
Whether re-silencing of FX alleles after AZA treatment resembles de novo silencing in the embryo is uncertain. For one thing, AZA treatment does not remove all of the repressive histone marks from reactivated FMR1 alleles. Nonetheless, the fact that inhibition of H3K27me3 deposition, either by using EZH2 inhibitors or compounds like 1a, causes FMR1 gene re-silencing to be significantly delayed, might be useful therapeutically. Restoring FMRP expression for therapeutic purposes poses a number of challenges that still need to be overcome, including the fact that long CGG-repeats reduce the efficacy of translation of any transcript generated (39,40) and that too much RNA with long CGG-repeat tracts can be deleterious (41–44) perhaps via its ability to sequester important cellular proteins (45,46) or to generate a toxic protein via Repeat-Associated Non-ATG (RAN) translation (47,48). However, since FMRP levels are thought to vary widely in human population, and women with the FM who make no FMRP in half their cells often do not meet the criteria for a FXS diagnosis, it may be that only partially restoring FMR1 expression may still have some therapeutic value. The use of AZA followed by EZH2 inhibitors, perhaps in combination with factors that improve the export of FMR1 mRNA to the cytoplasm and thus FMRP translation, may allow prolonged gene reactivation while reducing any potential negative effect of continuous AZA use and minimizing any impact of the CGG-containing transcript.
Materials and Methods
Cell lines and reagents
All the lymphoblastoid cell lines used in this study were male cell lines obtained from Coriell Cell Repositories (Camden, NJ). These include the control cell lines (GM06865 and GM06895), FXS patient cell lines carrying the methylated full mutation allele (GM04025, GM03200B, GM09145 and GM07294), and a FXS patient cell line carrying the unmethylated full mutation (UFM) allele (GM06897). The cells were grown in RPMI 1640 supplemented with 10% FBS and 1X antibiotic-antimycotic liquid consisting of penicillin, streptomycin and fungisone (ThermoFisher Scientific, Waltham, MA). The DNA methyl transferase inhibitor, 5-azadeoxycytidine (AZA), and EZH2 inhibitors, GSK126, GSK343 and UNC1999 were obtained from Cayman Chemical (Ann Arbor, MI) and used at the indicated concentrations. Compound 1a was a kind gift of Dr. Matthew Disney (The Scripps Research Institute, Jupiter, FL). Chromatin immunoprecipitation assay kits, anti-histone H3K27me3 antibody (07–449), normal mouse IgG (12–371) and normal rabbit IgG (12–370) were purchased from EMD Millipore (Billerica, MA). Antibodies against histone H3K9me2 (ab1220), and histone H3K9me3 (ab8898) were from Abcam (Cambridge, MA). Antibodies against histone H4K20me3 (39180) were from Active Motif (Carlsbad, CA).
Chromatin immunoprecipitation (ChIP) assays
ChIP assays were performed as described before (10) using a ChIP assay kit from EMD-Millipore. To prepare chromatin for immunoprecipitation, cells were fixed with 1% formaldehyde for 10 minutes at room temperature and lysed as per the kit manufacturer’s instructions. The chromatin was sonicated into <500 bp fragments using Bioruptor® (Diagenode, Denville, NJ). Real-time PCRs on the immunoprecipitated DNAs were carried out in triplicate in 20 µl final volume using the Power SYBR™ Green PCR master mix (ThermoFisher Scientific) and 200 nM of each primer and 2 µl of DNA. For amplification of FMR1 exon1, the primer pair Exon1-F and Exon1-R was used. This primer pair amplifies the region +236 to +312 relative to the transcription start site. GAPDH was amplified with primers hsGAPDH exon1F1 and hsGAPDH intron1R1. Sat2 repeat region was amplified with primers hsSat2 repeat-F1 and hsSat2 repeat-R1. The primer sequences are listed in Supplementary Material, Table S1. For quantitation the comparative threshold (Ct) method was used. Enrichment over 5% of input was calculated and where indicated was normalized to GAPDH or Sat2 repeat region. Students’s t test was used to calculate p values (GraphPad Software, Inc., La Jolla, CA).
RNA methods
Isolation of RNA from cytoplasmic, nucleoplasmic and chromatin fractions was done exactly as described previously (49). Total RNA was isolated from untreated or cells treated with various drugs using Trizol® reagent (ThermoFisher Scientific) as per manufacturer’s recommendation. Quantitative reverse transcription-PCR (qRT-PCR) was done as previously described (50). For the subcellular fractionation experiments, the RNAs were treated with TURBOTM DNase (ThermoFisher Scientific) for 30 minutes at 37 °C and cleaned by phenol-chloroform and ethanol precipitation before using it for qRT-PCR. Real-time PCRs were done in triplicate in a final volume of 20 µl using TaqMan® Fast Universal PCR Mastermix (ThermoFisher Scientific), 2 µl cDNA and 1X Taqman® probe-primer pair. The Taqman® probe-primer pair (FAMTM for FMR1 and NEAT1, and VIC® for β-glucuronidase (GUS)) were obtained from ThermoFisher Scientific.
DNA methylation analysis
DNA was isolated from cells using the salting out method (51). DNA methylation at the FMR1 locus was analyzed using a quantitative methylation sensitive PCR (qMS-PCR) strategy as previously described (52). Briefly, exon 1 region 5’ of the CGG-repeats in the FMR1 gene was amplified by qPCR using primers FMR1 ex1 (F) and FMR1 ex1 (R) on samples that had been either mock digested or digested with HpaII. The region amplified by these primers contains a recognition site for HpaII and thus digestion prevents the amplification of unmethylated alleles. The extent of methylation can then be determined by comparison of the yields of PCR product with and without HpaII predigestion. The GAPDH region was used as a control for HpaII digestion and was amplified with primers hsGAPDH exon1F1 and hsGAPDH intron1R1. A high resolution methylation-specific melting curve analysis (MS-MCA) assay was also used to analyze DNA methylation at the FMR1 promoter region, where indicated, as previously described (17). Briefly, 500 ng of genomic DNA was bisulfite modified using the EZ DNA Methylation-LightningTM kit (Zymo Research Corp, Irvine, CA) as per the manufacturer’s instructions by incubating the samples at 98 °C for 10 minutes and 64 °C for 90 minutes. The bisulfite converted DNA was purified using the kit reagents and eluted in 10 µl of elution buffer. Realtime PCR was done using 3 µl of purified DNA, 200 nM each of primers 2541 and 2645R and Power SYBR™ Green PCR master mix on ViiATM7 Real-Time PCR system (ThermoFisher Scientific). Melting curve analysis was performed immediately after amplification using ViiATM7 RUO software v1.2. The primer sequences are listed in Supplementary Material, Table S1.
DNA-RNA immunoprecipitation (DRIP) assay
DRIP assay was performed as described earlier (25) with slight modifications. DNA was isolated from cells using the salting out method (51). The anti RNA:DNA hybrid antibody (S9.6) was a gift of Dr. Stephen Leppla (National Institute of Allergy and Infectious Diseases). For each DNA sample, three DRIPs were performed: No antibody control, S9.6 antibody and RNAse H treatment followed by S9.6 antibody. A total of 25 µg DNA was either mock digested or digested with 30 units of RNAse H (New England Biolabs, Ipswich, MA) in 100 µl final volume at 37 °C for 6 hours. The volume was made up to 400 µl with 300 µl of ChIP dilution buffer (from the ChIP assay Kit from EMD Millipore) and sonicated to fragments <500 bp using Bioruptor® (6 minutes at medium setting, 30 seconds ON/30 seconds OFF). To 350 µl of the sonicated DNA, 650 µl of the ChIP dilution buffer and 10 µl of protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) was added and mixed. An aliquot (1%) was saved as input sample. The sonicated DNA was then precleared with 50 µl of Protein A agarose beads/Salmon sperm DNA slurry (EMD Millipore, Catalog # 16–157) for one hour on a rotator in cold. The precleared supernatant was incubated with or without ∼4 µg S9.6 antibody overnight on a rotator in cold. The sample was then incubated with 60 µl of the Protein A agarose beads/Salmon sperm DNA slurry to collect the immune complexes. The material was washed as in the ChIP assay (1 X low salt wash buffer, 1 X high salt wash buffer, 1 X LiCl wash buffer, 2 X TE pH 8.0). The immunoprecipitated material was then eluted from the beads using elution buffer as in the ChIP assay. The input and DRIP samples were treated with phenol chloroform and precipitated overnight at −20 °C with 0.3 M sodium acetate and ethanol. After washing with 70% ethanol, the samples were resuspended in 50 µl 0.1X TE pH 8.0. Real-time PCR was carried out in triplicate in 20 µl final volume using the Power SYBR™ Green PCR master mix, 2 µl of DNA and 200 nM of each primer. The following primer pairs were used for amplification of the FMR1 gene, Exon1-F and Exon1-R and Intron1-F and Intron1-R. The γ-actin-F and γ-actin-R primers (25) were used to amplify a positive control region and ZNF554-F and ZNF554-R primers (19) were used to amplify a negative control region for the presence of an RNA:DNA hybrid. The primer sequences are listed in Supplementary Material, Table S1.
Supplementary Material
Supplementary Material is available at HMG online.
Acknowledgements
We thank Dr. Matthew Disney (The Scripps Research Institute, Jupiter, FL) for providing compound 1a and Dr. Stephen Leppla (National Institute of Allergy and Infectious Diseases) for providing the anti RNA:DNA hybrid antibody (S9.6).
Conflict of Interest statement. None declared.
Funding
This work was supported by the intramural funds of National Institute of Diabetes, Digestive and Kidney Diseases, National Institutes of Health (DK05760218 to K.U.)
References
- 1.Loesch D.Z., Huggins R.M., Hagerman R.J. (2004) Phenotypic variation and FMRP levels in fragile X. Ment. Retard. Dev. Disabil. Res. Rev., 10, 31–41. [DOI] [PubMed] [Google Scholar]
- 2.Darnell J.C., Klann E. (2013) The translation of translational control by FMRP: therapeutic targets for FXS. Nat. Neurosci., 16, 1530–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alpatov R., Lesch B.J., Nakamoto-Kinoshita M., Blanco A., Chen S., Stutzer A., Armache K.J., Simon M.D., Xu C., Ali M., et al. (2014) A chromatin-dependent role of the fragile X mental retardation protein FMRP in the DNA damage response. Cell, 157, 869–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luca R., Averna M., Zalfa F., Vecchi M., Bianchi F., La Fata G., Del Nonno F., Nardacci R., Bianchi M., Nuciforo P., et al. (2013) The fragile X protein binds mRNAs involved in cancer progression and modulates metastasis formation. EMBO Mol. Med., 5, 1523–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu Y.H., Kuhl D.P., Pizzuti A., Pieretti M., Sutcliffe J.S., Richards S., Verkerk A.J., Holden J.J., Fenwick R.G., Jr., Warren S.T., et al. (1991) Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell, 67, 1047–1058. [DOI] [PubMed] [Google Scholar]
- 6.Yu S., Pritchard M., Kremer E., Lynch M., Nancarrow J., Baker E., Holman K., Mulley J., Warren S., Schlessinger D., et al. (1991) Fragile X genotype characterized by an unstable region of DNA. Science, 252, 1179–1181. [DOI] [PubMed] [Google Scholar]
- 7.Verkerk A.J., Pieretti M., Sutcliffe J.S., Fu Y.H., Kuhl D.P., Pizzuti A., Reiner O., Richards S., Victoria M.F., Zhang F.P., et al. (1991) Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell, 65, 905–914. [DOI] [PubMed] [Google Scholar]
- 8.Kumari D., Usdin K. (2010) The distribution of repressive histone modifications on silenced FMR1 alleles provides clues to the mechanism of gene silencing in fragile X syndrome. Hum. Mol. Genet., 19, 4634–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colak D., Zaninovic N., Cohen M.S., Rosenwaks Z., Yang W.Y., Gerhardt J., Disney M.D., Jaffrey S.R. (2014) Promoter-bound trinucleotide repeat mRNA drives epigenetic silencing in fragile X syndrome. Science, 343, 1002–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumari D., Usdin K. (2014) Polycomb group complexes are recruited to reactivated FMR1 alleles in Fragile X syndrome in response to FMR1 transcription. Hum. Mol. Genet., 23, 6575–6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avitzour M., Mor-Shaked H., Yanovsky-Dagan S., Aharoni S., Altarescu G., Renbaum P., Eldar-Geva T., Schonberger O., Levy-Lahad E., Epsztejn-Litman S., et al. (2014) FMR1 epigenetic silencing commonly occurs in undifferentiated fragile X-affected embryonic stem cells. Stem Cell Reports, 3, 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biacsi R., Kumari D., Usdin K. (2008) SIRT1 inhibition alleviates gene silencing in Fragile X mental retardation syndrome. PLoS Genet., 4, e1000017.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCabe M.T., Ott H.M., Ganji G., Korenchuk S., Thompson C., Van Aller G.S., Liu Y., Graves A.P., Della Pietra A., 3rd, Diaz E., et al. (2012) EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature, 492, 108–112. [DOI] [PubMed] [Google Scholar]
- 14.Verma S.K., Tian X., LaFrance L.V., Duquenne C., Suarez D.P., Newlander K.A., Romeril S.P., Burgess J.L., Grant S.W., Brackley J.A., et al. (2012) Identification of Potent, Selective, Cell-Active Inhibitors of the Histone Lysine Methyltransferase EZH2. ACS Med. Chem. Lett., 3, 1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konze K.D., Ma A., Li F., Barsyte-Lovejoy D., Parton T., Macnevin C.J., Liu F., Gao C., Huang X.P., Kuznetsova E., et al. (2013) An orally bioavailable chemical probe of the Lysine Methyltransferases EZH2 and EZH1. ACS Chem. Biol., 8, 1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Aller G.S., Pappalardi M.B., Ott H.M., Diaz E., Brandt M., Schwartz B.J., Miller W.H., Dhanak D., McCabe M.T., Verma S.K., et al. (2014) Long residence time inhibition of EZH2 in activated polycomb repressive complex 2. ACS Chem. Biol., 9, 622–629. [DOI] [PubMed] [Google Scholar]
- 17.Dahl C., Gronskov K., Larsen L.A., Guldberg P., Brondum-Nielsen K. (2007) A homogeneous assay for analysis of FMR1 promoter methylation in patients with fragile X syndrome. Clin. Chem., 53, 790–793. [DOI] [PubMed] [Google Scholar]
- 18.Disney M.D., Liu B., Yang W.Y., Sellier C., Tran T., Charlet-Berguerand N., Childs-Disney J.L. (2012) A small molecule that targets r(CGG)(exp) and improves defects in fragile X-associated tremor ataxia syndrome. ACS Chem. Biol., 7, 1711–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loomis E.W., Sanz L.A., Chedin F., Hagerman P.J. (2014) Transcription-associated R-loop formation across the human FMR1 CGG-repeat region. PLoS Genet., 10, e1004294.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boguslawski S.J., Smith D.E., Michalak M.A., Mickelson K.E., Yehle C.O., Patterson W.L., Carrico R.J. (1986) Characterization of monoclonal antibody to DNA.RNA and its application to immunodetection of hybrids. J. Immunol. Methods, 123–130. [DOI] [PubMed] [Google Scholar]
- 21.Hu Z., Zhang A., Storz G., Gottesman S., Leppla S.H. (2006) An antibody-based microarray assay for small RNA detection. Nucleic Acids Res., 34, e52.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ginno P.A., Lim Y.W., Lott P.L., Korf I., Chedin F. (2013) GC skew at the 5' and 3' ends of human genes links R-loop formation to epigenetic regulation and transcription termination. Genome Res., 23, 1590–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pohjoismaki J.L., Holmes J.B., Wood S.R., Yang M.Y., Yasukawa T., Reyes A., Bailey L.J., Cluett T.J., Goffart S., Willcox S., et al. (2010) Mammalian mitochondrial DNA replication intermediates are essentially duplex but contain extensive tracts of RNA/DNA hybrid. J. Mol. Biol., 397, 1144–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skourti-Stathaki K., Proudfoot N.J., Gromak N. (2011) Human senataxin resolves RNA/DNA hybrids formed at transcriptional pause sites to promote Xrn2-dependent termination. Mol. Cell, 42, 794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groh M., Lufino M.M., Wade-Martins R., Gromak N. (2014) R-loops associated with triplet repeat expansions promote gene silencing in Friedreich ataxia and fragile X syndrome. PLoS Genet., 10, e1004318.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis B.M., McCurrach M.E., Taneja K.L., Singer R.H., Housman D.E. (1997) Expansion of a CUG trinucleotide repeat in the 3' untranslated region of myotonic dystrophy protein kinase transcripts results in nuclear retention of transcripts. Proc. Natl. Acad. Sci. U. S. A., 94, 7388–7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Esch C.E., Ghazvini M., Loos F., Schelling-Kazaryan N., Widagdo W., Munshi S.T., van der Wal E., Douben H., Gunhanlar N., Kushner S.A., et al. (2014) Epigenetic characterization of the FMR1 promoter in induced pluripotent stem cells from human fibroblasts carrying an unmethylated full mutation. Stem Cell Reports, 3, 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao J., Sun B.K., Erwin J.A., Song J.J., Lee J.T. (2008) Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science, 322, 750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cifuentes-Rojas C., Hernandez A.J., Sarma K., Lee J.T. (2014) Regulatory interactions between RNA and polycomb repressive complex 2. Mol. Cell, 55, 171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanhere A., Viiri K., Araujo C.C., Rasaiyaah J., Bouwman R.D., Whyte W.A., Pereira C.F., Brookes E., Walker K., Bell G.W., et al. (2010) Short RNAs are transcribed from repressed polycomb target genes and interact with polycomb repressive complex-2. Mol. Cell, 38, 675–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rinn J.L., Kertesz M., Wang J.K., Squazzo S.L., Xu X., Brugmann S.A., Goodnough L.H., Helms J.A., Farnham P.J., Segal E., et al. (2007) Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell, 129, 1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandey R.R., Mondal T., Mohammad F., Enroth S., Redrup L., Komorowski J., Nagano T., Mancini-Dinardo D., Kanduri C. (2008) Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol. Cell, 32, 232–246. [DOI] [PubMed] [Google Scholar]
- 33.Davidovich C., Cech T.R. (2015) The recruitment of chromatin modifiers by long noncoding RNAs: lessons from PRC2. rna, 21, 2007–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aranda S., Mas G., Di Croce L. (2015) Regulation of gene transcription by Polycomb proteins. Sci Adv, 1, e1500737.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riising E.M., Comet I., Leblanc B., Wu X., Johansen J.V., Helin K. (2014) Gene silencing triggers polycomb repressive complex 2 recruitment to CpG islands genome wide. Mol. Cell, 55, 347–360. [DOI] [PubMed] [Google Scholar]
- 36.Wu H., Coskun V., Tao J., Xie W., Ge W., Yoshikawa K., Li E., Zhang Y., Sun Y.E. (2010) Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science, 329, 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu X., Johansen J.V., Helin K. (2013) Fbxl10/Kdm2b recruits polycomb repressive complex 1 to CpG islands and regulates H2A ubiquitylation. Mol. Cell, 49, 1134–1146. [DOI] [PubMed] [Google Scholar]
- 38.Farcas A.M., Blackledge N.P., Sudbery I., Long H.K., McGouran J.F., Rose N.R., Lee S., Sims D., Cerase A., Sheahan T.W., et al. (2012) KDM2B links the Polycomb Repressive Complex 1 (PRC1) to recognition of CpG islands. Elife, 1, e00205.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kenneson A., Zhang F., Hagedorn C.H., Warren S.T. (2001) Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum. Mol. Genet., 10, 1449–1454. [DOI] [PubMed] [Google Scholar]
- 40.Feng Y., Zhang F., Lokey L.K., Chastain J.L., Lakkis L., Eberhart D., Warren S.T. (1995) Translational suppression by trinucleotide repeat expansion at FMR1. Science, 268, 731–734. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Arocena D., Yang J.E., Brouwer J.R., Tassone F., Iwahashi C., Berry-Kravis E.M., Goetz C.G., Sumis A.M., Zhou L., Nguyen D.V., et al. (2010) Fibroblast phenotype in male carriers of FMR1 premutation alleles. Hum. Mol. Genet., 19, 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Handa V., Goldwater D., Stiles D., Cam M., Poy G., Kumari D., Usdin K. (2005) Long CGG-repeat tracts are toxic to human cells: implications for carriers of Fragile X premutation alleles. FEBS Lett., 579, 2702–2708. [DOI] [PubMed] [Google Scholar]
- 43.Hoem G., Raske C.R., Garcia-Arocena D., Tassone F., Sanchez E., Ludwig A.L., Iwahashi C.K., Kumar M., Yang J.E., Hagerman P.J. (2011) CGG-repeat length threshold for FMR1 RNA pathogenesis in a cellular model for FXTAS. Hum. Mol. Genet., 20, 2161–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hukema R.K., Buijsen R.A., Raske C., Severijnen L.A., Nieuwenhuizen-Bakker I., Minneboo M., Maas A., de Crom R., Kros J.M., Hagerman P.J., et al. (2014) Induced expression of expanded CGG RNA causes mitochondrial dysfunction in vivo. Cell Cycle, 13, 2600–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sellier C., Rau F., Liu Y., Tassone F., Hukema R.K., Gattoni R., Schneider A., Richard S., Willemsen R., Elliott D.J., et al. (2010) Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. EMBO J., 29, 1248–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sellier C., Freyermuth F., Tabet R., Tran T., He F., Ruffenach F., Alunni V., Moine H., Thibault C., Page A., et al. (2013) Sequestration of DROSHA and DGCR8 by expanded CGG RNA repeats alters microRNA processing in fragile X-associated tremor/ataxia syndrome. Cell Rep., 3, 869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Todd P.K., Oh S.Y., Krans A., He F., Sellier C., Frazer M., Renoux A.J., Chen K.C., Scaglione K.M., Basrur V., et al. (2013) CGG repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron, 78, 440–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh S.Y., He F., Krans A., Frazer M., Taylor J.P., Paulson H.L., Todd P.K. (2015) RAN translation at CGG repeats induces ubiquitin proteasome system impairment in models of fragile X-associated tremor ataxia syndrome. Hum. Mol. Genet, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gagnon K.T., Li L., Janowski B.A., Corey D.R. (2014) Analysis of nuclear RNA interference in human cells by subcellular fractionation and Argonaute loading. Nat. Protoc., 9, 2045–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumari D., Bhattacharya A., Nadel J., Moulton K., Zeak N.M., Glicksman A., Dobkin C., Brick D.J., Schwartz P.H., Smith C.B., et al. (2014) Identification of fragile X syndrome specific molecular markers in human fibroblasts: a useful model to test the efficacy of therapeutic drugs. Hum. Mutat., 35, 1485–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller S.A., Dykes D.D., Polesky H.F. (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res., 16, 1215.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayward B.E., Zhou Y., Kumari D., Usdin K. (2016) A simple set of assays for the comprehensive analysis of FMR1 alleles in the Fragile X-related disorders. J. Mol. Diagn, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.