Abstract
The multiple galactosemia disease states manifest long-term neurological symptoms. Galactosemia I results from loss of galactose-1-phosphate uridyltransferase (GALT), which converts galactose-1-phosphate + UDP-glucose to glucose-1-phosphate + UDP-galactose. Galactosemia II results from loss of galactokinase (GALK), phosphorylating galactose to galactose-1-phosphate. Galactosemia III results from the loss of UDP-galactose 4’-epimerase (GALE), which interconverts UDP-galactose and UDP-glucose, as well as UDP-N-acetylgalactosamine and UDP-N-acetylglucosamine. UDP-glucose pyrophosphorylase (UGP) alternatively makes UDP-galactose from uridine triphosphate and galactose-1-phosphate. All four UDP-sugars are essential donors for glycoprotein biosynthesis with critical roles at the developing neuromuscular synapse. Drosophila galactosemia I (dGALT) and II (dGALK) disease models genetically interact; manifesting deficits in coordinated movement, neuromuscular junction (NMJ) development, synaptic glycosylation, and Wnt trans-synaptic signalling. Similarly, dGALE and dUGP mutants display striking locomotor and NMJ formation defects, including expanded synaptic arbours, glycosylation losses, and differential changes in Wnt trans-synaptic signalling. In combination with dGALT loss, both dGALE and dUGP mutants compromise the synaptomatrix glycan environment that regulates Wnt trans-synaptic signalling that drives 1) presynaptic Futsch/MAP1b microtubule dynamics and 2) postsynaptic Frizzled nuclear import (FNI). Taken together, these findings indicate UDP-sugar balance is a key modifier of neurological outcomes in all three interacting galactosemia disease models, suggest that Futsch homolog MAP1B and the Wnt Frizzled receptor may be disease-relevant targets in epimerase and transferase galactosemias, and identify UGP as promising new potential therapeutic target for galactosemia neuropathology.
Introduction
Galactose is metabolized in a series of reactions catalyzed by the three consecutive enzymes that comprise the Leloir pathway: galactokinase (GALK1), galactose-1-phosphate uridyltransferase (GALT) and UDP-galactose 4’ epimerase (GALE). Human deficits in the activity of any of these enzymes result in galactosemia disease states. Classic galactosemia (CG, OMIM 230400) results from loss of GALT activity (1) the second pathway enzyme (Figure 1A) . After GALK1 phosphorylation, GALT converts galactose-1-phosphate and uridine diphosphate-glucose (UDP-glucose) into glucose-1-phosphate and UDP-galactose. GALE catalyzes the final step converting UDP-galactose to UDP-glucose, as well as their N-acetylated forms: UDP N-acetylglucosamine (UDP-GlcNAc) to UDP N-acetylgalactosamine (UDP-GalNAc; Figure 1A) (2). These four UDP sugars are critical for the biosynthesis of glycoproteins and proteoglycans (3,4), which heavily populate the cell surface and secreted surroundings, including the extracellular synaptomatrix of the synaptic cleft and perisynaptic space (5). UDP-glucose pyrophosphorylase (UGP2) synthesizes UDP-glucose from glucose-1-phosphate and UTP, and alternatively forms UDP-galactose from galactose-1-phosphate and UTP (6), independent of GALT activity (Figure 1A). UGP2 is present in a wide array of organisms (7,8) since UDP-glucose, the active form of glucose, is a central player in almost all living systems. The dual roles of UGP2 in both glucose and galactose metabolic pathways underscore its central importance as an alternative enzymatic route in galactosemia patients (9).
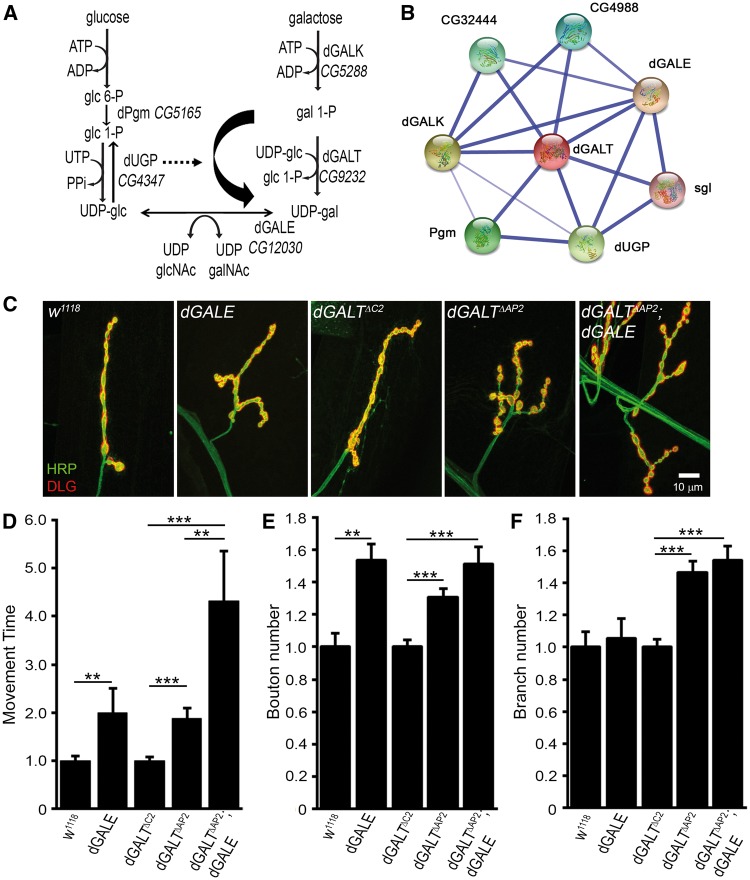
Figure 1.
dGALE phenocopies dGALT and co-removal worsens coordinated movement. (A) Schematic diagram of glucose/galactose pathways showing targeted dGALT, dGALE and dUGP gene products. (B) The dGALT interaction network generated with the Search Tool for Retrieval of Interacting Genes (STRING). Line thickness represents the strength of predicted interactions. (C) Representative NMJs co-labelled with anti-horseradish peroxidase (HRP; green) and anti-Discs Large (DLG; red) in background control (w1118) and homozygous dGALE mutant (dGALEh); precise excision dGALT control (dGALTΔC2), homozygous dGALT null mutant (dGALTΔAP2) and double mutant (dGALTΔAP2; dGALE) combinations. Movement time for coordinated roll-over behaviour. (D) NMJ bouton. (E) and branch. (F) number in the same genotypes, normalized to respective genetic controls. Sample size: ≥8 animals per genotype. Error bars show SEM with significance indicated as *P≤0.05, **P≤0.01 and ***P≤0.001.
Classic galactosemia (aka galactosemia I) is the second most common inherited metabolic disorder among US children (10), and the most frequent and clinically severe galactosemia disease state (11). Galactosemia II (OMIM 230200) results from loss of GALK1, which we have previously established as a genetically interacting condition (12). Galactosemia III (OMIM 230250) results from loss of GALE, and is the rarest and least well understood form of galactosemia (13). Galactosemia I is often detected pre-symptomatically in newborn screening, and lifelong dietary galactose withdrawal, the current standard of care, resolves at least the acute life-threatening symptoms. Dietary intervention also resolves early onset cataracts in galactosemia II, the main disease symptom (14,15). However, long-term neurological symptoms arise in maturing galactosemia patients, including movement defects, speech delay and cognitive disability (11), which are not prevented by dietary galactose control. Moreover, galactosemia III is a continuum disorder that can present life-threatening symptoms and long-term neurodevelopmental sequelae (11,16), and galactose restriction is particularly problematic as GALE plays a pivotal role maintaining UDP sugar balance during the synthesis of glycoproteins. Thus, the current treatment for GALE-associated galactosemia requires a balance between restricting galactose and providing enough for UDP sugar synthesis (17).
A large body of research documents glycosylation defects in galactosemia patients (18–20). Previous studies reveal conserved glycosylation defects in the Drosophila galactosemia I model (dGALT null mutant), and show the correction of glycosylation defects with co-removal of dGALK, in the Drosophila galactosemia II model (12). dGALT nulls exhibit severe coordinated locomotor movement deficits, as well as underlying strikingly overelaborated neuromuscular junction (NMJ) synaptic architecture. Importantly, the disease state is restricted to morphological defects without changes in neurotransmission strength. Assaying the heavily-glycosylated NMJ synaptomatrix with a series of lectin probes shows profound differences in synaptic glycan landscape dependent on dGALT activity (12). In particular, the heparan sulphate proteoglycan (HSPG) Wnt co-receptor Dally-like protein (Dlp) is lost from the NMJ synaptic interface, together with extracellular accumulation of the Wingless (Wg) Wnt glycoprotein signalling ligand. Wg signalling via its Frizzled 2 (Fz2) receptor modulated by the Dlp co-receptor is a key trans-synaptic signalling pathway regulating NMJ synaptogenesis (21–23). Importantly, coordinated movement, NMJ development, synaptomatrix glycan composition and Wnt pathway defects in the Drosophila galactosemia I disease model are all significantly corrected by co-removal of dGALK (12). This work strongly suggests NMJ glycan-dependent Wnt signalling defects as a basis for movement deficits in galactosemia I.
In the current study, we set forth to characterize a Drosophila galactosemia III model (dGALE mutant), as well as test the by-pass pathway (dUGP mutant) for galactose metabolism. We find both dGALE and dUGP phenocopy coordinated movement deficits and impaired NMJ synaptogenesis characterizing dGALT. Loss of dGALE elevates neurotransmission strength, which is further heightened by co-removal of dGALT. Defects in all three mutants include synaptomatrix glycosylation losses, elevated synaptic bouton formation and differences in the core Wg/Fz2/Dlp trans-synaptic signalling pathway components. Both dGALT and dGALE display increased Futsch/MAP1b microtubule remodeling in the presynaptic neuron, whereas dUGP exhibits elevated Frizzled nuclear import (FNI) in the postsynaptic muscle. These new findings reveal 1) key glycosylation pathway modifiers of Wnt trans-synaptic signalling, and 2) identify two new diseases-relevant targets, Futsch/MAP1b and Wnt Frizzled receptor, for intervention in galactosemia-associated neuropathology. Both dGALE and dUGP interact with dGALT, modifying behavioural deficits and changes in synaptic architecture in the Drosophila galactosemia I model. Synaptic glycosylation losses, particularly of N-acetylgalactosamine residues, are exacerbated. These findings indicate that differential glycosylation at the NMJ synaptomatrix modulates neurological movement outcomes in interacting galactosemia disease states, and further suggest UGP as a promising new therapeutic target for galactosemia treatment.
Results
Neuronal dGALE loss worsens behavioural outcomes in dGALT null mutants
Movement defects are common among GALT-deficient galactosemia patients (24–28). Similarly, dGALT is necessary for proper coordinated movement in the Drosophila disease model, and dGALK co-removal corrects this behavioural deficit (12). In the galactose pathway, dGALE intersects with dGALT downstream (Figure 1A), and the Search Tool for Retrieval of Interacting Genes (STRING) (29) identified dGALE as a promising candidate interactor (Figure 1B), consistent with previous evidence of an interactive relationship (30). GALE-deficient galactosemia patients also exhibit deficits in coordinated motor skills that appear early in development (31,32). However, most of the severe patient cases arise from consanguineous parents, which raises the possibility that homozygosity of autosomal recessive alleles other than GALE may underlie some of the severe movement symptoms reported in galactosemia III (31). Animal models have shown GALE activity is essential for viability (33) and normal development (34,35), but movement phenotypes have not been investigated despite evidence of GALE expression in neurons and muscle in C. elegans (35). We therefore set out to assay coordinated movement in our Drosophila model. Null dGALE mutants manifest early lethality, confirming that dGALE is essential for viability, and therefore viable dGALEhhypomorphic mutants with residual ∼8% dGALE activity (34) were tested.
A well-established test for Drosophila coordinated movement is the larval rollover assay, which requires a complex set of integrated behaviours to be properly executed, as previously described (12). In this assay, wandering L3 larvae (wL3) are placed in an inverted position and the time to righting is measured. Compared to genetic background controls (w1118) showing rapid and well-coordinated movement (8.2 ± 1.09 s, n = 24 animals), dGALEh mutants exhibit clearly uncoordinated and >2-fold slower responses (17.7 ± 5.64 s, n = 23, P = 0.01; Figure 1D). Mutant larvae make several attempts to twist and roll before they successfully right themselves. Similarly, as previously reported (12), dGALT null (dGALTΔAP2) animals are significantly movement impaired, also manifesting ∼2-fold slower righting time compared to precise-excision genetic background (dGALTΔC2) matched controls (n = 36,38; P < 0.001; Figure 1D). We hypothesized dGALE would exacerbate dGALT movement impairments since dGALE synthesizes UDP-sugar precursors when limited by dGALT deficiency (Figure 1A). Consistently, the double mutants (homozygous dGALTΔAP2; dGALEh) manifest >2- and >4-fold longer period to complete the coordinated movement (56.3 ± 17.77 s, n = 15) compared to dGALT single mutants (P < 0.01) and genetic controls (P < 0.001, Figure 1D), respectively. Our findings suggest that dGALT and dGALE contribute equally to the control of coordinated movement, and that loss of the two genes has an additive effect on coordinated movement deficits.
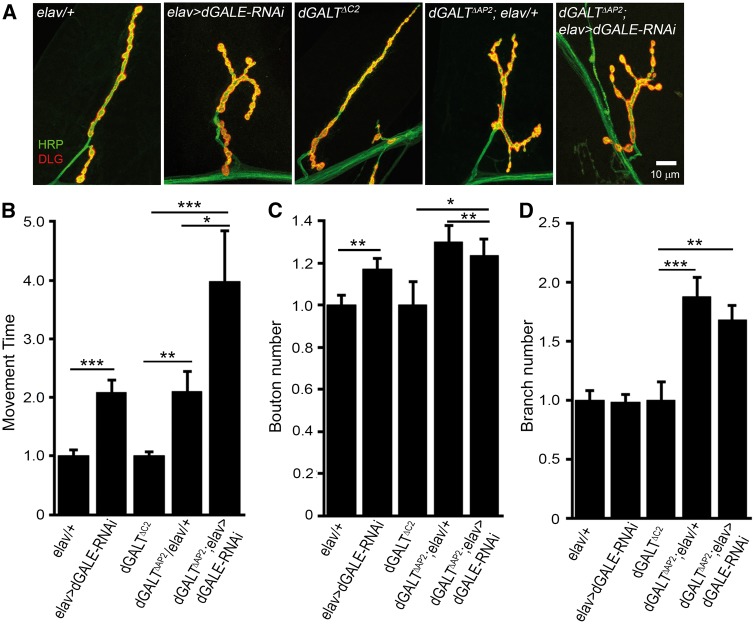
To test the cellular requirements for dGALE in bilateral coordinated movement control, dGALE was knocked-down with transgenic RNAi (Figure 2). Like dGALE null mutants, ubiquitous (UH1-Gal4) dGALE knockdown results in 100% early lethality, and therefore could not be assayed. Like the dGALEh mutants, targeted neuronal dGALE RNAi (elav-Gal4 > dGALE-RNAi) also results in overtly uncoordinated animals, which likewise manifest >2-fold slower roll-over behaviour (18.7 ± 1.96 s, n = 16) compared to their driver alone (elav-Gal4/+) genetic controls (9.0 ± 0.94 s, n = 14, P = 0.0002; Figure 2B). Similarly, targeted muscle dGALE RNAi (24B-Gal4 > dGALE-RNAi) causes >2-fold slower rollover (10.4 ± 1.64 s, n = 24) compared to genetic controls (24B-Gal4/+, 4.7 ± 0.46 s, n = 28, P = 0.002). These findings identify a neuronal and muscle dGALE requirement for properly controlled coordinated movement. Similar to the above dGALT; dGALE double mutant condition, targeted dGALE neuronal knockdown in the dGALT null background (homozygous dGALTΔAP2; elav-Gal4 > dGALE-RNAi) causes further slowing of roll-over time (34.6 ± 11.46 s, n = 25) compared to dGALT with driver alone (dGALTΔAP2; elav-Gal4/+) animals (15.7 ± 1.61 s, n = 29), as well as precise excision control (dGALTΔC2) animals (8.8 ± 0.77 s, n = 51, P < 0.001; Figure 2B). Conversely, postsynaptic targeted dGALE knockdown in the dGALT null background (homozygous dGALTΔAP2; 24B-Gal4 > dGALE-RNAi) does not further compromise locomotor coordination (20.8 ± 2.14 s, n = 22) compared to dGALT alone (dGALTΔAP2; elav-Gal4/+: 18.4 ± 2.67 s, n = 9). Taken together, these results demonstrate that neuronal and muscle dGALE both contribute to properly controlled coordinated movement, and that presynaptic, but not postsynaptic, dGALE is a strong genetic modifier of dGALT disease model behavioural deficits in an additive manner. As these movement defects have been closely associated with changes in NMJ morphological development (12), we next examined the synaptic architecture in dGALE single mutants and in dGALE; dGALT double mutant animals.
Figure 2.
Targeted neuronal dGALE knockdown also phenocopies dGALT mutants. (A) Representative NMJs co-labelled with presynaptic anti-HRP (green) and postsynaptic anti-DLG (red) in driver alone controls (elav-Gal4/+), neuronal-targeted dGALE RNAi (elav-Gal4>dGALE-RNAi); precise excision dGALT controls (dGALTC2), dGALT nulls with driver alone (dGALTΔAP2; elav-Gal4/+), and the double mutant animals (dGALTΔAP2; elav-Gal4>dGALE-RNAi). Movement time for coordinated roll-over behaviour. (B) NMJ bouton. (C) and branch. (D) number in the same genotypes, normalized to respective genetic controls. Sample size: ≥10 animals for each genotype. Error bars show SEM with significance; *P≤0.05, **P≤0.01 and ***P≤0.001.
Neuronal dGALE regulates neuromuscular synaptogenesis and transmission strength
We conducted a Drosophila glycogene screen using transgenic RNAi knockdown of a wide range of N/O-linked glycans, glycosaminoglycans, glycosyltransferases and glycan-binding lectins to test effects on NMJ structure and function (21). This screen identified dGALT as a strong negative regulator of NMJ structural synaptogenesis, but not functional differentiation, and we subsequently confirmed that dGALT acts to restrict terminal arbour branching and synaptic bouton formation (12). Since defects in NMJ morphogenesis have been shown to underlie locomotor deficits (12,36,37), we hypothesized that similar defects could account for the movement limitations occurring in the absence of neuronal dGALE, as well as for the worsening of movement deficits in dGALE; dGALT double mutant conditions. To assay synapse architecture, muscle 4 NMJs from wL3 animals were co-labelled with presynaptic anti-horseradish-peroxidase (HRP, green) and postsynaptic anti-Discs Large (DLG, red) in single and double mutant animals. Synaptic boutons (>1µm in minimum diameter) and branches (axonal processes with >2 boutons) were counted. Representative images and quantification for mutant and control genotypes are shown in Figures 1 and 2.
The synaptic bouton number is increased by >50% in dGALEh mutants (30.3 ± 2.0, n = 8 animals) compared to w1118 genetic background controls (19.7 ± 1.7, n = 10, P = 0.002; Figure 1E). Targeted neuronal dGALE knockdown (elav-Gal4 > dGALE-RNAi) animals similarly show a significant, although smaller (∼17%), increase in bouton number compared to driver alone (elav-Gal4/+) genetic controls (n = 13,16; P = 0.01; Figure 2C). While both dGALE mutants and neuron-targeted dGALE-RNAi animals display obvious NMJ overelaboration (Figures 1C, 2A), muscle-targeted dGALE-RNAi does not cause a detectable change in synaptic architecture or a significant increase in synaptic bouton number compared to genetic controls (25.1 ± 1.1 boutons, n = 35 vs. 26.9 ± 1.2 boutons, n = 35, respectively). These findings show a specific requirement for dGALE in the neuron, in this case to restrict NMJ synaptogenesis. In double mutant conditions, the NMJ architectural complexity characterizing dGALT nulls is exacerbated by simultaneously reducing dGALE activity (Figure 1C). Compared with the significantly greater number of boutons in dGALT single mutants (34.5 ± 1.6, n = 33), double mutant synapses (homozygous dGALTΔAP2; dGALEh) develop ∼50% and ∼20% more boutons (39.8 ± 2; n = 40), compared to genetic control (dGALTΔC2) (26.4 ± 1.3; n = 26, P < 0.001; Figure 1E) and dGALT single mutants (Figure 1E), respectively. Neuronal dGALE knockdown in the dGALT null background did not further increase NMJ structural complexity (Figure 2C,D). Single dGALEh and dGALE-RNAi also did not increase synaptic branching (Figures 1F,2D), in contrast to dGALT mutants (3.8 ± 0.2, n = 33) which increased synaptic branching compared to controls (2.6 ± 0.1, n = 24, P < 0.001; Figure 1F). There is a tendency for branching to increase further in dGALE; dGALT double mutants (4 ± 0.2, n = 40), but the effect is not significant (Figure 1F).
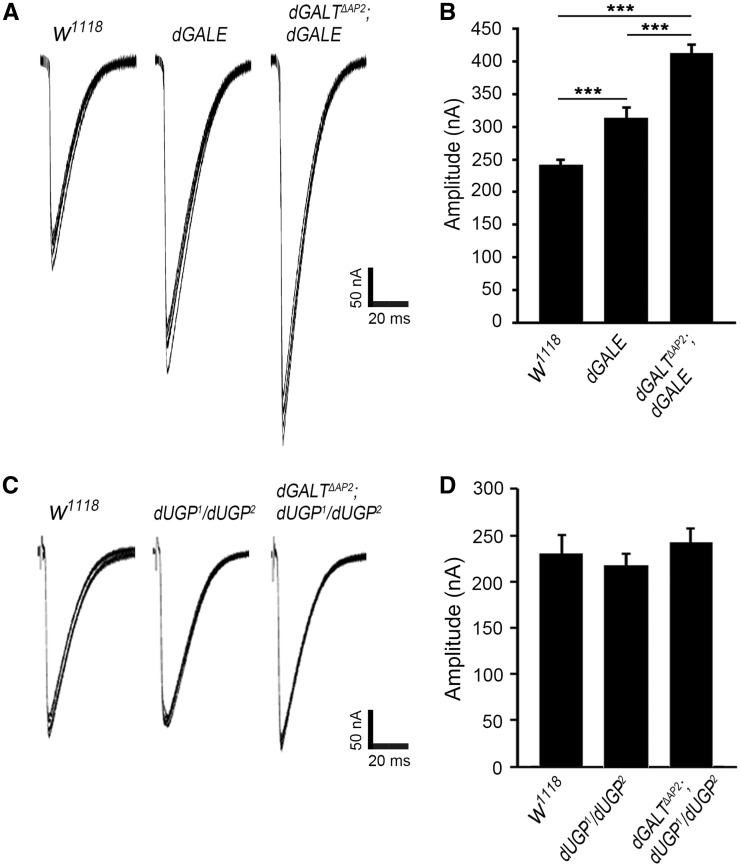
To test NMJ functional differentiation, we performed two-electrode voltage clamp (TEVC) electrophysiological recordings (12). The innervating motor nerve was stimulated with a glass suction electrode while recording from the voltage-clamped muscle 6. Excitatory junctional current (EJC) records were made at 0.2 Hz frequency with 0.5 ms duration stimuli at a suprathreshold voltage. ≥25 NMJs from ≥10 different wL3 animals were recorded from each of three genotypes: genetic background control (w1118), dGALE alone (dGALEh) and a double mutant in combination with dGALT (dGALTΔAP2; dGALEh). Neurotransmission strength is very obviously elevated with loss of dGALE activity (Figure 3A). Mean EJC amplitudes significantly increased in mutants compared to control (313.3 ± 15.6 nA vs. 241.4 ± 8.6 nA; P ≤ 0.001; Figure 3B). Strikingly, the double mutant combination exhibits further heightened neurotransmission (Figure 3A). Mean EJC amplitudes significantly increased in double mutants (dGALTΔAP2; dGALEh: 413.2 ± 12.4 nA) compared to both w1118 control (P ≤ 0.001, Figure 3B) and single dGALE mutants (P ≤ 0.001, Figure 3B). In summary, these results show that dGALE is a strong genetic modulator of both neuromuscular structural synaptogenesis and functional differentiation alone and in combination with dGALT loss at the NMJ synapse.
Figure 3.
dGALE loss elevates transmission and dGALT co-removal worsens the defect. (A) Representative EJC traces from genetic background control (w1118), dGALE single mutant (dGALEh) and double mutant animals (dGALTΔAP2; dGALEh). (B) EJC peak amplitude quantification for all three genotypes. Sample size: ≥25 NMJs for each genotype. Error bars show SEM with significance indicated: ***P<0.001. (C) Representative EJC traces from control (w1118), single dUGP mutant (dUGP1/dUGP2) and double mutant animals (dGALTΔAP2; dUGP1/dUGP2). D) EJC peak amplitude quantification for all three genotypes. Sample size: ≥12 NMJs for each genotype. There is no significant differences.
dGALT genetic modifier dUGP regulates coordinated movement and NMJ architecture
STRING analyses (29) further identified dUGP as a second highly-associated dGALT interactor (Figure 1A,B). In Drosophila, CG4347 encodes UDP-glucose pyrophosphorylase (EC 2.7.7.9) as the only enzyme capable of producing UDP-glucose from glucose-1-P and UTP (38–41) (Figure 1A). Like dGALE, dUGP is essential for viability and, consistently, no human patients with UGP loss-of-function mutations have been reported. We therefore characterized two dUGP hypomorphic mutations (dUGP1, dUGP2) generated by transposable element insertion (42). Neither mutant is homozygous viable, but viability is restored with significantly reduced survival in heteroallelic combination (dUGP1/dUGP2). We first tested dUGP mutants for coordinated movement behaviour using the same larval roll-over assay as above. Compared to w1118 genetic background controls (7.6 ± 0.71 s, n = 29), UGP1/+ (11.6 ± 1.33 s, n = 15, P < 0.05) and UGP2/+ (16.0 ± 1.82 s, n = 15, P < 0.001) animals are both significantly slower and obviously less coordinated (Figure 4B). The coordinated movement time for UGP1/+ is not significantly different from UGP2/+, although UGP2/+ appears qualitatively more behaviourally compromised. Combined dUGP1/dUGP2 mutants display a significant further reduction in coordinated movement (18.6 ± 1.73 s, n = 15) compared to single dUGP heterozygotes (P < 0.05) and w1118 controls (P < 0.001; Figure 4B). Ubiquitous dUGP knockdown (UH1-Gal4 > dUGP-RNAi) caused the strongest effect, with >2.5-fold longer time to upright position (25.2 ± 2.78 s, n = 10) compared to control (UH1-Gal4/+: 9.3 ± 1.47 s, n = 9, P = 0.004). Tissue-targeted neural (elav-Gal4 > dUGP-RNAi: 13.6 ± 1.82 s, n = 29, P < 0.05) and muscle (24B-Gal4 > dUGP-RNAi: 19.7 ± 4.40 s, n = 12, P < 0.05) knockdown both significantly impaired coordinated movement compared to the driver alone controls (elav-Gal4/+: 8.8 ± 0.70 s, n = 23; 24B-Gal4/+: 8.2 ± 1.0 s, n = 14).
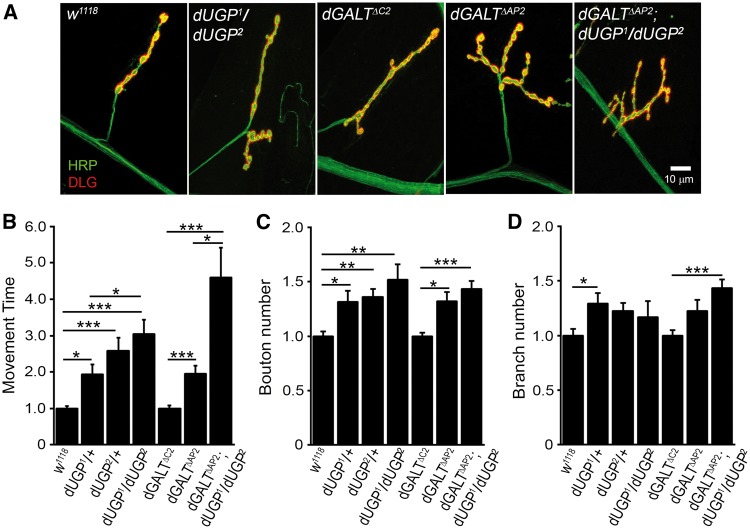
Figure 4.
dUGP phenocopies dGALT and co-removal worsens coordinated movement. (A) Representative NMJs imaged with anti-HRP (green) and anti-DLG (red) in genetic control (w1118), dUGP mutant (dUGP1/dUGP2); precise excision control (dGALTΔC2), dGALT null (dGALTΔAP2), and double mutant combinations (dGALTΔAP2; dUGP1/dUGP2). Movement time for coordinated roll-over behaviour (B), NMJ bouton (C) and branch (D) number in the same genotypes, normalized to respective genetic controls. Sample size: ≥15 animals per genotype. Error bars show SEM with significance indicated: *P≤0.05, **P≤0.01 and ***P≤0.001.
UGP2 loss causes a dramatic reduction in UDP-glucose, an essential precursor for the biosynthesis of proteoglycans (43), which are key components of the NMJ synaptomatrix and established regulators of NMJ development (5). Although UGP2 has a higher affinity for glucose-1-P, it also catalyzes UDP-galactose from galactose-1-P and UTP (6,9), particularly at the high galactose-1-P levels in GALT-deficient galactosemia patients (11). Thus, UGP2 represents an alternate GALT-independent pathway for galactose metabolism (9) (Figure 1A). We therefore hypothesized that dUGP loss would enhance behavioural impairments in dGALT mutants and produce an exacerbated phenotype. Since dUGP1/dUGP2 is the most severely impaired conditioned, coordinated movement was next assayed in the homozygous dGALTΔAP2; dUGP1/dUGP2 combination (Figure 4B). As predicted, these double mutant animals are obviously less coordinated than either of the single mutant conditions, with a 2-fold slower roll-over time (44.1 ± 10.94 s, n = 27) compared to dGALT nulls alone (22.4 ± 2.53 s, n = 23, P < 0.05) and >4-fold change compared to genetic background controls (10.3 ± 0.90 s, n = 50, P < 0.001; Figure 4B). These results show that dUGP plays a particularly important role enabling coordinated movement, and that co-removal of dUGP and dGALT combinatorially further impairs the ability to move in a coordinated manner.
We next assayed NMJ synaptogenesis in dUGP mutants (Figure 4A). Like both dGALT and dGALE mutants, loss of dUGP causes NMJ overgrowth and structural overelaboration in UGP1/+, UGP2/+ and UGP1/UGP2 mutants. Compared to the w1118 genetic background control (21.2 ± 1.0 boutons, n = 22), both UGP1/+ (28.3 ± 2.1, n = 12, P < 0.05) and UGP2/+ (30.7 ± 1.4, n = 17, P < 0.01), as well as dUGP1/dUGP2 (28.8 ± 2.7, n = 6, P < 0.001), develop significantly more synaptic boutons (Figure 4C). Synaptic branching tends to increase in all dUGP mutants, but is only significantly greater in UGP1/+ (2.8 ± 0.2, n = 12) compared to w1118 genetic controls (2.3 ± 0.2, n = 22, P < 0.05; Figure 4D). Single dGALT nulls (dGALTΔAP2/dGALTΔAP2: 32.9 ± 1.9; P < 0.05, n = 19) and particularly, the double mutant combination (dGALTΔAP2/dGALTΔAP2; dUGP1/dUGP2: 35.7 ± 1.6; P < 0.001, n = 29) develop significantly more synaptic boutons compared to the dGALTΔC2 precise excision controls (24.9 ± 1, n = 22; Figure 4C). Synaptic branching increases in dGALTΔAP2 mutants (3.3 ± 0.3, n = 19) compared to dGALTΔC2 controls (2.7 ± 0.1, n = 22), but there is a further significant increase in branching in double mutants (3.9 ± 0.2, n = 29, P < 0.001; Figure 4D).Consistently, ubiquitous dUGP knockdown (UH1-Gal4 > dUGP-RNAi) significantly increases synaptic bouton (35.5 ± 1.56, n = 11, P < 0.0003) and branch (3.36 ± 0.20, n = 11, P < 0.0017) numbers compared to driver alone controls (UH1-Gal4/+: 25.2 ± 1.56, n = 10 and 2.25 ± 0.23, n = 10, respectively). However, structure is overelaborated in pan-neuronal dUGP-knockdown animals (26.3 ± 1.0 boutons, n = 10, P = 0.007), although unaffected in muscle-targeted dUGP-RNAi larvae (25.4 ± 2.4 boutons, n = 11) compared to their appropriate controls (22 ± 1.1, n = 12 and 20.2 ± 1.4, n = 15, respectively).
As above, we next tested NMJ functional differentiation with TEVC electrophysiological recordings (Figure 3). ≥12 NMJs from ≥6 different wL3 animals were recorded from each of three genotypes: genetic background control (w1118), dUGP trans-heterozygotes (dUGP1/dUGP2) and double mutant animals (dGALTΔAP2; dUGP1/dUGP2). Unlike dGALE, neurotransmission strength is closely comparable between all three genotypes (Figure 3C). Mean EJC amplitudes from single mutants (219.35 ± 12.60 nA) are not significantly different from w1118 control (230.36 ±19.81 nA) or the double mutant combination (242.58 ± 15.09 nA, P > 0.05, Figure 3D). Taken together, these results show that dUGP is important for coordinated movement and neuromuscular structural synaptogenesis, but is not detectably required for synapse function. These findings show that co-removal of dUGP and dGALT interact to modify the severity of behavioural and structural mutant phenotypes. Since there is extensive evidence showing synaptic glycosylation restricts NMJ morphogenesis (12,21,22), we next proceeded to examine the synaptic glycan environment in single and double mutant combinations.
dGALE and dUGP both shape NMJ synaptomatrix glycan composition
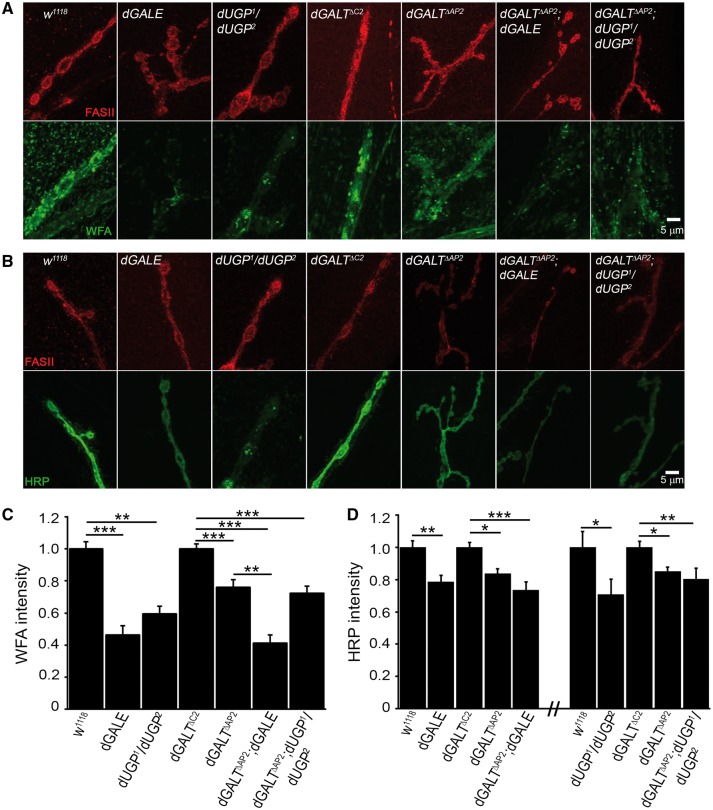
GALE plays a crucial role maintaining UDP-sugar balance for glycosylation (2), with a primarily role in UDP-galNAc biosynthesis. Unlike UDP-glc and UDP-glcNAc, UDP-galNAc cannot be synthesized by a GALE-independent pathway (Figure 1A). Indeed, recent studies reveal that GALE loss-of-function in C. elegans causes a strong reduction of UDP-galNAc levels accompanied by developmental defects unique to this UDP-sugar (35). We previously found similar glycan losses at the NMJ synaptomatrix in the absence of dGALT (12). Since this is the only glycan deficit corrected by transgenic hGALT expression in the dGALT null background (12), it provides a potential mechanistic basis for the coordinated movement and morphological synaptogenesis defects in this disease model. We hypothesized that dGALE deficiency, like loss of dGALT, would compromise galNAc abundance in the NMJ synaptomatrix in the new Drosophila epimerase galactosemia model, driving the synaptogenesis and movement deficits in dGALE mutants. To test this hypothesis, we first probed with Wisteria floribunda lectin (WFA, green) to label terminal galNAc residues in NMJs marked with Fasciclin-II (FasII, red). Representative images and data summary are shown in Figure 5.
Figure 5.
dGALE and dUGP mutants manifest glycan losses in the NMJ synaptomatrix. Representative NMJs co-labelled with anti-Fasciclin II (FASII, red). (A) Wisteria floribunda lectin (WFA, green) or (B) anti-horseradish peroxidase (HRP; green) in genetic control (w1118), dGALE (dGALEh) and dUGP (dUGP1/dUGP2) mutants; precise excision control (dGALTC2), dGALT null (dGALTΔAP2) and double mutant combinations (dGALTΔAP2; dGALE and dGALTΔAP2; dUGP1/dUGP2). Quantification of WFA. (C) and HRP. (D) intensities in the same genotypes, normalized to respective genetic controls. Sample size: ≥10 NMJs/animals per genotype. Error bars show SEM with significance indicated as *P≤0.05, **P≤0.01, ***P≤0.001 and ****P≤0.0001.
The synaptic marker FasII does not vary significantly between tested genotypes, comparing w1118 genetic background control and dGALEh mutant NMJs (Figure 5A). In sharp contrast, while w1118 control NMJs (n = 38) are very highly enriched with WFA-labelled galNAc residues, dGALEh synapses (n = 10) show a dramatic ∼60% loss of WFA label (P < 0.001; Figure 5A,C). To test whether dGALT co-removal would worsen the phenotype, dGALT; dGALE double mutants were next examined. The dGALTΔAP2 single mutant decreases WFA labelling by ∼25% compared to precise controls (n = 42,46; P < 0.001; Figure 5A,C), and the double mutants (dGALEh/dGALEh; dGALTΔAP2/dGALTΔAP2) exhibit a further ∼35% reduction, significantly different from the single mutant condition (dGALTΔAP2/dGALTΔAP2, P < 0.05; Figure 5C). Fucosylation defects are also reported in galactosemia (44), and the Drosophila dGALT disease model manifests a loss of anti-HRP labelling at the NMJ (12), a commonly employed synaptic marker recognizing α1,3-fucosylation. We therefore hypothesized dGALE mutants would manifest a similar glycosylation defect that would be exacerbated by co-removal of dGALT. Consistently, dGALEh NMJs show significantly reduced HRP glycan levels compared to controls (n = 6,6; P = 0.006), similar to dGALTΔAP2 NMJs compared to controls (n = 16,17; P < 0.05), with the dGALE; dGALT double mutants exhibiting a stronger ∼30% reduction in HRP glycan levels (n = 16, P < 0.001 compared to dGALTΔC2; Figure 5D). These results show that dGALE and dGALT both shape the glycan composition of the developing NMJ synaptomatrix.
We next tested dUGP single and double mutants with the same WFA and HRP probes (Figure 5A,B). While WFA-labelling in single dUGP mutants (UGP1/+, n = 8; UGP2/+, n = 9) is not significantly different from matched controls (n = 26), the stronger UGP1/UGP2 combination shows a ∼40% reduction in WFA-labelled galNAc residues (n = 9, P < 0.01; Figure 5C). The dGALTΔAP2 single mutant shows a ∼25% decrease (n = 42) compared to controls (n = 46, P < 0.001; Figure 5C), and the double mutants (dGALTΔAP2/dGALTΔAP2; dUGP1/dUGP2) exhibit a further decrease in WFA labelling (∼30% reduction, n = 26) compared to matched controls (n = 46, P < 0.001; Figure 5C). Anti-HRP labelling for fucosylation defects shows dUGP1/dUGP2 exhibits a ∼30% reduction (n = 10) compared to w1118 genetic controls (n = 11, P < 0.05; Figure 5D). Both single dGALTΔAP2 (n = 29) and double dGALTΔAP2/dGALTΔAP2; dUGP1/dUGP2 (n = 18) combinations show a similar ∼20% decrease in fucosylated residues at the NMJ, a significant decrease compared to controls (n = 30; P < 0.05 and P < 0.01, respectively; Figure 5D). Taken together, these results show that dGALE and dUGP are strong genetic modifiers of NMJ synaptomatrix glycan composition, partially overlapping with dGALT requirements in synaptic glycosylation. Since we have previously found that these synaptomatrix defects alter NMJ synaptogenesis via modulation of the Wg trans-synaptic signalling pathway (12), we next test whether this pathway is compromised in dGALE and dUGP single and double mutant combinations.
dGALE and dUGP both regulate wnt trans-synaptic signalling at the NMJ
The heavily-glycosylated cell surface and extracellular space modulates ligand-mediated signalling in normal and disease states (45), and the Drosophila NMJ glycosylated synaptomatrix has been repeatedly shown to fine-tune trans-synaptic signalling driving synaptogenesis (5,12,22). In the core Wnt pathway, UDP-sugar availability could also impact the glycosylation status of the secreted Wingless (Wg) glycoprotein ligand (46) and biosynthesis of Wg co-receptor HSPG Dlp (21), required for the optimal processing, availability and presentation of Wg within the synaptic cleft. We have previously shown that dGALT mutants increase Wg and reduce Dlp levels at the Drosophila NMJ (12). We therefore hypothesized that dGALE and dUGP mutants would likewise impact the Wg trans-synaptic signalling pathway to similarly alter NMJ synaptogenesis and impair coordinated movement. We tested this hypothesis by assaying the dual outputs of the Wg pathway; 1) presynaptic Wg activation of Futsch/MAP1b remodeling of the synaptic bouton microtubule cytoskeleton (47) and 2) postsynaptic Wg activation of the Frizzled nuclear import (FNI) pathway involving cleavage of the Frizzled-2 (Fz2) receptor and trafficking of the carboxyl-terminal signalling domain (Fz2-C) to the muscle nuclei (48–50). Representative images and data summaries of these analyses are shown in Figures 6 and 7.
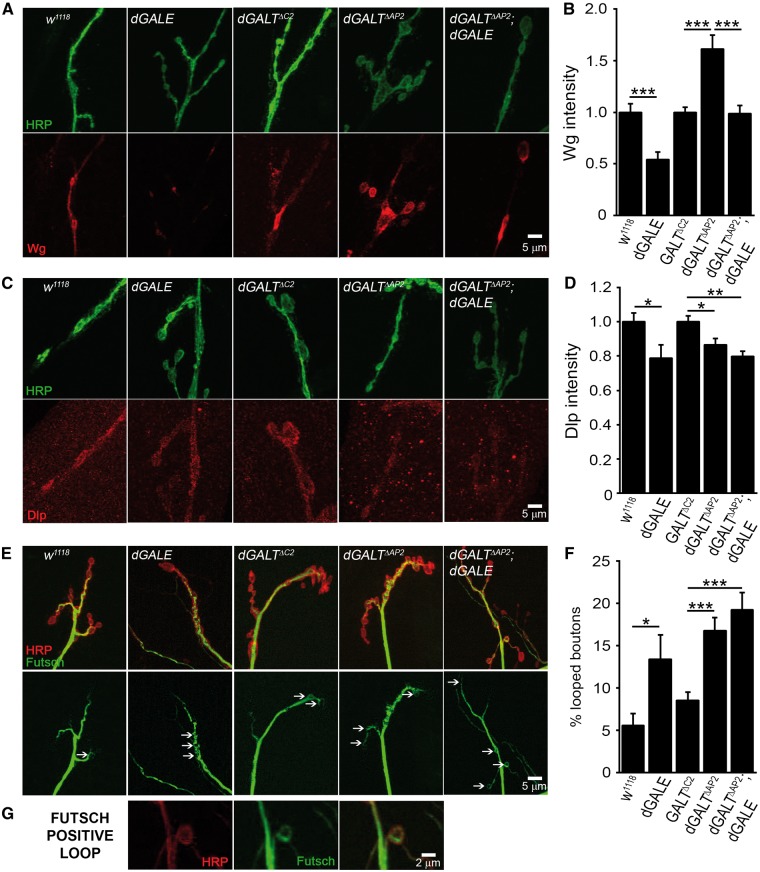
Figure 6.
Presynaptic Wnt signalling upregulated in dGALE and dGALT double mutant. (A) Representative NMJ boutons co-labelled with anti-HRP (green) and anti-Wingless (Wg, red) in genetic control (w1118) and dGALE mutant (dGALEh); precise excision controls (dGALTΔC2), dGALT mutant (dGALTΔAP2), and double mutant (dGALTΔAP2; dGALEh). (B) Wg intensity in single and double mutants normalized to appropriate genetic controls. (C) NMJ boutons imaged with anti-HRP (green) and anti-Dally-like Protein (Dlp, red) in the same 5 genotypes. (D) Dlp intensity in single and double mutants normalized to genetic controls. (E) Representative NMJs probed with anti-HRP (red) and anti-Futsch (green) in the same 5 genotypes. (F) Percentage of Futsch-positive loop boutons in single and double mutants. (G) Higher magnification image of a microtubule loop within a single synaptic bouton probed with membrane anti-HRP (red), anti-Futsch (green) and the merged channels. Sample size: ≥10 NMJs/animals per genotype. Error bars show SEM with significance indicated as *P≤0.05, **P≤0.01 and ***P≤0.001.
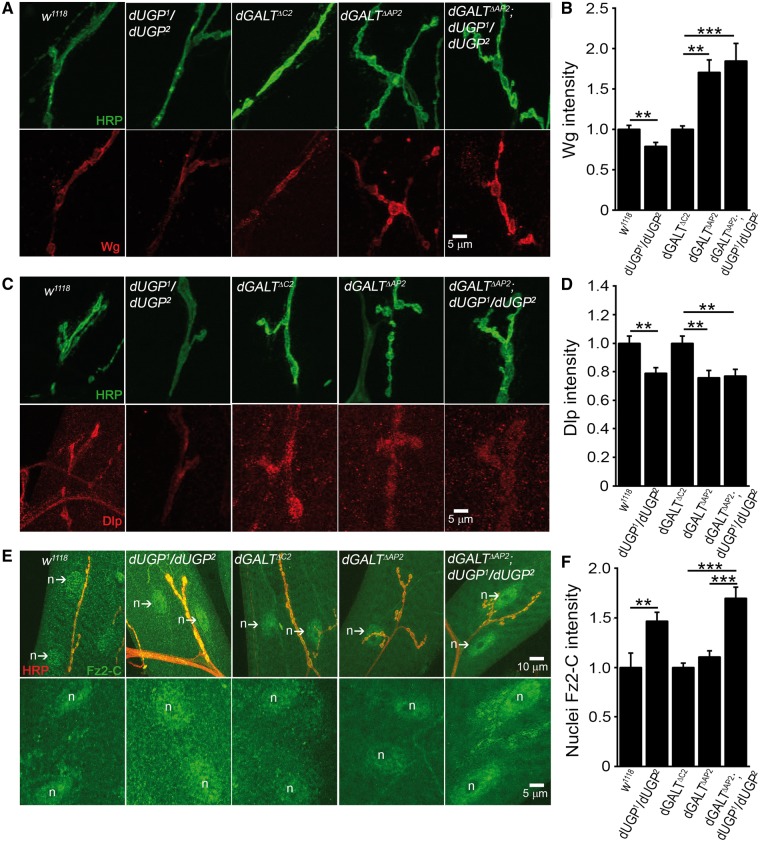
Figure 7.
Postsynaptic Wnt signalling upregulated in dUGP and dGALT double mutant. (A) Representative NMJ boutons co-labelled with anti-HRP (green) and anti-Wg (red) in genetic control (w1118), dUGP mutant (dUGP1/dUGP2); precise excision control (dGALTΔC2), dGALT single (dGALTΔAP2) and double (dGALTΔAP2; dUGP1/dUGP2) mutants. (B) Quantification of Wg intensity normalized to genetic controls. (C) NMJ boutons imaged with anti-HRP (green) and anti-Dlp (red) in the same 5 genotypes. (D) Quantification of Dlp intensity in single and double mutants normalized to genetic controls. (E) NMJs probed with anti-HRP (red) and anti-Fz2-C (Fz2-C, green) in the same 5 genotypes. The bottom row shows nuclear Fz2-C labelling only. The “n” labels postsynaptic muscle nuclei. (F) Quantification of Fz2-C intensity within muscle nuclei. Sample size: ≥7 NMJs/animals per genotype. Error bars show SEM with significance indicated as *P≤0.05, **P≤0.01 and ***P≤0.001.
Compared to w1118 genetic background controls, dGALE mutant NMJs display a striking and immediately obvious loss of the Wg signalling ligand (Figure 6A). In quantifying Wg label intensity, dGALEh exhibits a highly significant ∼50% decrease in Wg compared to matched controls (n = 10,10; P = 0.0007; Figure 6B). This contrasts sharply with dGALT mutants, which show the opposite ∼50% increase in Wg levels compared to matched controls (n = 31,36; P < 0.001; Figure 6A,B). Perhaps additively, double mutants (dGALEh/dGALEh; dGALTΔAP2/dGALTΔAP2) show a restoration of Wg ligand levels to the wildtype condition (n = 23; P < 0.001 compared to dGALTΔAP2; Figure 6A,B). In contrast, compared to genetic controls both dGALE and dGALT mutant NMJs display lower levels of the Wg co-receptor Dlp (Figure 6C). Quantification of Dlp labelling intensity shows a similar ∼20% loss in dGALEh compared to controls (n = 15,16; P = 0.03) and dGALTΔAP2 compared to controls (n = 56,59; P < 0.05; Figure 6D). Double mutants were not significantly worse than the single mutants alone, but display a very significant loss of Dlp compared to matched controls (n = 26,59; P < 0.01; Figure 6D). These results show that dGALE strongly impacts Wg pathway components, but that dGALE effects on Wg ligand are different compared to dGALT, with a similar effect on the Wg co-receptor.
In presynaptic Fz2 receptor activation, Wg binds the receptor to drive phosphorylation of Futsch/MAP1b, which mediates NMJ growth and bouton formation via regulation of the synaptic microtubule cytoskeleton (48). To assay this pathway, dGALE and dGALT single and double mutants were co-labelled with anti-Futsch (green) compared to anti-HRP (red), with Futsch labelling quantified in synaptic boutons as absent, bundled, diffuse or looped (48) (Figure 6E). In quantifying these different categories, dGALEh single mutants display a significantly greater percentage of looped boutons (0.13 ± 0.03, n = 14) compared to w1118 controls (0.05 ± 0.01, n = 15, P = 0.023; Figure 6F). Similarly, dGALTΔAP2mutants show a higher level of Futsch loops (0.17 ± 0.02, n = 28, Figure 6G) compared to dGALTΔC2 genetic background controls (0.09 ± 0.01, n = 36, P < 0.001; Figure 6F). The double mutants (dGALEh/dGALEh; dGALTΔAP2/dGALTΔAP2) exhibit greatly elevated Futsch loops in synaptic boutons (0.19 ± 0.02, n = 24), a highly significant increase compared to matched controls (P < 0.001; Figure 6F). These results show dGALE and dGALT display Futsch-driven microtubule changes predictive of the above NMJ overelaboration defects (47). In postsynaptic receptor activation, Wg binding causes Fz2-C cleavage and trafficking to muscle nuclei (49,50), which can be quantified by measuring anti-Fz2-C nuclear fluorescence intensity. For all dGALE and dGALT single and double mutants, Fz2-C accumulation in the nuclei is not significantly different from matched controls (control: 1 ± 0.05; dGALT single mutants: 0.96 ± 0.08; and double mutant animals: 0.90 ± 0.09). These results indicate that both dGALE and dGALT selectively impact presynaptic Wg signalling.
We next turned to testing dUGP roles in Wg signalling, either in the presence and absence of dGALT (Figure 7). Like dGALE, dUGP1/dUGP2 mutants exhibit significantly lower Wg ligand levels at the NMJ compared to w1118 controls (n = 28,36; P = 0.0038; Figure 7A,B). However, unlike dGALE, the double mutants (dGALTΔAP2/dGALTΔAP2; dUGP1/dUGP2) did not revert the elevated Wg ligand levels characterizing dGALTΔAP2 alone (n = 34,46; P < 0.01 compared to dGALTΔC2 control), but rather display a modest further increase (∼15%) in Wg ligand abundance (n = 33, P < 0.001; Figure 7A,B). Like dGALE, dUGP mutants (dUGP1/dUGP2) show a ∼20% decrease in the Wg co-receptor Dlp (n = 18) compared to w1118 controls (n = 19, P = 0.002; Figure 7C,D). Similar to dGALT nulls (n = 28), double mutants also display a significant Dlp depletion at the NMJ (n = 20), which is very significantly lower than matched controls (n = 31, P < 0.01; Figure 7D). Pre- and postsynaptic Wg signalling was next explored. In the presynaptic pathway, the number of Futsch-positive bouton loops in dUGP single mutants (Futsch-positive bouton loops/total number of boutons: 0.09 ± 0.02, n = 6) is indistinguishable from appropriate controls (Futsch-positive bouton loops/total number of boutons: 0.05 ± 0.01, n = 15). Our findings confirm the elevated Futsch loops that characterized the single dGALT mutant condition (Futsch-positive bouton loops/total number of boutons: 0.20 ± 0.02, n = 11, P = 0.002), compared to the precise excision control group (Futsch-positive bouton loops/total number of boutons: 0.08 ± 0.02, n = 10). This trait remains unaffected by the co-removal of dUGP (Futsch-positive bouton loops/total number of boutons: 0.20 ± 0.03, n = 10) In the postsynaptic pathway, however, nuclear Fz2-C accumulation is very significantly increased by ∼50% in dUGP1/dUGP2 mutants compared to w1118 genetic controls (n = 16, 7; P < 0.01; Figure 7E, F). As reported above, dGALT nulls again show no significant difference in nuclear Fz2-C levels compared to controls (n = 48,40; Figure 7E,F), however the double mutants (dGALTΔAP2/dGALTΔAP2; dUGP1/dUGP2, n = 25) exhibit a very striking increase in Fz2-C nuclear localization compared to both controls (P < 0.001) and dGALT alone (P < 0.001; Figure 7E,F). These findings suggest that specifically increased activation of the postsynaptic FNI pathway underlies dUGP NMJ synaptogenesis and coordinated movement deficits.
Discussion
Galactosemias result from deficits in any of the three enzymes of the Leloir pathway: GALK, GALT and GALE. Through this highly conserved cascade, galactose is converted into precursors for the galactosylation of proteins and lipids (2). This mechanism is thought to be the key to disease state chronic neurological symptoms (51,52), particularly in the transferase- and epimerase-associated galactosemias. We previously discovered severe galactose glycan losses, synaptogenesis defects and changes in Wnt trans-synaptic signalling components at the neuromuscular synapse correlated with coordinated movement impairments in the Drosophila GALT-deficient galactosemia disease model (12). Since the long-term neurodevelopmental and movement symptoms reported for GALE-deficient galactosemia are similar (11), we set forth here to characterize a new Drosophila GALE-deficient neurological disease model. Unlike GALT and GALK, no human patient completely lacking GALE activity has been reported (13), consistent with the essential requirement for GALE in Drosophila and C. elegans (34,35). Thus, while most severe GALT-deficient patients have no detectable enzymatic activity (53), the most severe GALE-deficient patients reported exhibit ≥5% residual activity (54). Consistently, we report here on dGALE mutants with ∼8% enzymatic activity, as well as targeted neuronal dGALE knockdown, and show both display compromised coordinated movement and NMJ synaptogenesis defects as severe as dGALT nulls completely lacking enzymatic activity (55).
Both dGALT and dGALE mutants show striking impairments in coordinated movement. These mutant classes both move in an overtly uncoordinated manner, and roll over slowly after being placed in an inverted position (56). This twist-and-roll response is a complex behaviour requiring the animal to contract multiple muscles on one side in a close sequence, while simultaneously relaxing contralateral muscles. Such bilateral motor control requires tight regulation of neuromuscular connectivity (12,57). Consistently, aberrant NMJ architecture is associated with the striking coordination deficits in both dGALT and dGALE mutant animals. Double dGALE; dGALT mutants show an additive exacerbation of the behavioural phenotypes. Since dGALE is downstream of dGALT in the pathway, one might predict that double mutant animals would behave like dGALT single mutants. The fact that this is not the case indicates that these enzymes must have non-overlapping functions outside of the linear Leloir pathway (58). What might be the basis of this genetic interaction? Unlike dGALT, dGALE mediates an energetically-reversible enzymatic reaction (Figure 1A). In the absence of dGALT, there is little UDP-galactose present, which has been suggested to shift the reversible dGALE reaction towards the formation of UDP-galactose from UDP-glucose (30). Loss of dGALE activity would not allow for such a responsive shift and would account for the worsening of the dGALT coordinated movement phenotypes reported here.
UDP-glucose pyrophosphorylase represents an alternate GALT-independent route for galactose metabolism (9). UGP catalyzes the formation of UDP-glucose from glucose-1-phosphate and UTP, but can also catalyze the formation of UDP-galactose from galactose-1-P and UTP (6,9). This second function is proposed to occur particularly with high levels of galactose-1-P, such as occurs in GALT-deficient galactosemia (11). Importantly, overexpression of human UGP2 (hUGP2), the ortholog for dUGP, in the yeast system has shown the ability to rescue galactose-dependent survival of GALT-deficient strains (59). It has therefore been hypothesized that UGP2 upregulation could also protect against long-term neurological complications in GALT-deficient galactosemia. Consistently, our studies reveal that dUGP deficiency is indeed a strong negative modulator of neurobehavioural outcomes. Null dGALT coordinated movement deficits are exacerbated in dGALT; dUGP double mutant combinations. Similar to dGALE, dUGP mediates a reversible enzymatic reaction (Figure 1A), and can alternately use galactose as the enzymatic substrate (6,9). Under dGALT null conditions where there are increasing amounts of galactose-1-P, the dUGP reaction would shift towards the formation of UDP-galactose from galactose-1-P, in order to restore the UDP-sugar balance for proper glycosylation. Based on this interaction, co-removal of dUGP would be predicted to aggravate coordinated movement deficits in the double mutants, as reported here.
NMJ synaptogenesis is regulated by distinct mechanisms controlling axonal branching and synaptic bouton maturation (60,61). Indeed, while new bouton formation is plastic, activity-dependent and occurs at variable rates throughout postembryonic development, the number of synaptic branches is established early and remains relatively stable thereafter (60,61). Like dGALT, dGALE mutants strongly restrict synaptic bouton development but, unlike dGALT, do not affect synaptic branching. Loss of dGALE augments only the number of synaptic boutons, whereas double dGALE; dGALT mutant animals display an increase in both branching and supernumerary bouton formation. Likewise, loss of dUGP significantly increases new synaptic bouton formation in both heterozygous and transheterozygous conditions, demonstrating the need for >50% dUGP activity for proper NMJ synaptic morphogenesis. Like dGALE, dUGP mutants have only a minimal impact on NMJ branching, although co-removal of dGALT augments the axonal branching defect in double mutant conditions. These findings show a clear need for dGALT, dGALE and dUGP in limiting synaptic bouton development, whereas dGALT and to a lesser extent dUGP are also required earlier to establish a proper synaptic branching architecture. This dUGP involvement is not surprising, since it is the only enzyme in Drosophila capable of synthesizing UDP-glucose, which is well-known to have a central role in anabolic and catabolic pathways regulating cell growth and development (38–40).
We have previously shown that dGALT activity did not significantly alter functional differentiation driving neurotransmission strength at the NMJ, but rather appears to specifically modulate synaptic architecture (12). This is possible since NMJ structure and function are well established to be independently regulated, and coordinated movement defects can occur with NMJ structural defects in the absence of overt synaptic transmission defects (56). The result is surprising, however, since N-glycans have been causally implicated in both Drosophila NMJ structural and functional synaptogenesis (21,22). Consistent with the essential role of dGALE in the biosynthesis of glycoconjugates (62–64), we find here that dGALE is a strong determinant of neurotransmission strength, with significantly elevated synaptic function under conditions of reduced dGALE activity. Moreover, neurotransmission is further elevated with co-removal of dGALT, showing a significant genetic interaction upstream of synaptic function. However, like dGALT, viable levels of dUGP loss do not have a similar impact on neurotransmission strength. There is no significant change in NMJ function either in the single dUGP mutant condition, or with co-removal of dGALT. Unlike dGALE, dUGP is not at the crossroads of UDP sugar balance and this perhaps explains the differential requirement of the two enzymes in synaptic functional differentiation.
NMJ synaptogenesis is highly dependent on extracellular glycan mechanisms (5). Indeed, we previously demonstrated dGALT null NMJ overelaboration is driven by a striking depletion of galactosyl, N-acetylgalactosamine and fucosylated HRP moieties within the synaptomatrix (12). However, only loss of N-acetylgalactosamine is rescued by hGALT expression, and is reduced in wildtype animals after galactose overfeeding, suggesting this is the primary causative synaptic glycan change (12). Similar to dGALT, C. elegans GALE loss-of-function causes strong reduction of UDP-galNAc levels, accompanied by developmental defects associated with UDP-galNAc deficits (35). Importantly, GALE is primarily responsible for UDP-galNAc biosynthesis, since UDP-galNAc cannot be synthesized by GALE-independent pathways (Figure 1A). Consistently, our Drosophila GALE-deficient galactosemia model exhibits a dramatic loss of GalNAc in the NMJ synaptomatrix. Furthermore, concomitant removal of dGALE and dGALT strikingly exacerbates GalNAc losses compared to the single dGALT null condition. dUGP- deficient animals also manifest loss of GalNAc in the NMJ synaptomatrix. Moreover, both dGALE and dUGP mutants similarly display synaptic loss of HRP epitope, revealing bifucosylated N-glycans. This apparent cross-talk between Leloir and GDP-Fucose production pathways is intriguing, especially in the absence of a Fucose salvage route in Drosophila. It would be interesting to determine how altered nucleotide sugar metabolism affects protein glycosylation in addition to processing. In eukaryotes, fucosylation occurs primarily in the Golgi, and recent evidence suggests a close connection between UDP-sugar balance and Golgi N-glycan branching (65), which may explain these findings. Furthermore, recent studies from our lab (12,22) provide evidence that HRP glycan loss is accompanied by increased synaptic growth, similar to what we report here for both dGALE and dUGP mutants.
NMJ synaptogenesis is modulated by trans-synaptic signalling that occurs via secreted glycoprotein ligands traversing the highly-glycosylated synaptomatrix (5,66,67). In particular, the founding Wnt Wingless (Wg) signalling ligand is a potent driver of synaptic development (50,68). Wg is secreted from presynaptic neuron and glia to bind Fz2 receptors on both neuron and postsynaptic muscle via interaction with the Wg co-receptor HSPG Dlp, a well-known regulator of Wg extracellular distribution and signalling (21,68,69). Postsynaptic Wg activates the Frizzled nuclear import (FNI) pathway, in which Fz2 is endocytosed followed by the cleavage and nuclear trafficking of the C-terminus (Fz2-C) to regulate synaptogenesis (50). Presynaptic Wg activates Futsch/MAP1B phosphorylation and binding to the synaptic microtubule cytoskeleton, driving the budding of new synaptic boutons and NMJ expansion (47,48) Null dGALT NMJs exhibit elevated Wg and reduced Dlp12, but the impact on Wg signal transduction was not previously investigated. We discover here that postsynaptic FNI signalling is not affected in the dGALT-deficient galactosemia model, but rather there is a strong upregulation of presynaptic Futsch signalling, consistent with the observed elevated synaptic bouton formation (47). Likewise, the new dGALE-deficient galactosemia model shows specific hyper-activation of presynaptic Futsch-mediated microtubule rearrangements modulating NMJ morphogenesis. Thus, both galactosemia disease models similarly activate the presynaptic Wnt pathway.
In contrast to dGALT and dGALE requirements, dUGP mutants specifically activate the postsynaptic FNI pathway. Presynaptic Wg activation requires the inhibition of the Glycogen Synthase Kinase 3β (GSK3β) homolog Shaggy (Sgg) (70), a protein kinase with the ability to phosphorylate glycogen synthase. Previous experiments have demonstrated that UGP loss is associated with increased phosphorylation of glycogen synthase and subsequent reduction of its catalytic activity (38). This predicts that GSK3β is hyper-activated in dUGP mutants, which would prevent Wg activation of the presynaptic pathway. As we have shown many times previously (21–23), predicting the directionality of Wg signalling changes based on Wg ligand, Fz2 receptor levels and Dlp co-receptor is profoundly difficult owing to the interplay between the 3 players, as described in the ‘Exchange Factor Mechanism’(21,23,71). Wg may be actively signalling or sequestered extracellularly in a non-signalling state, and Dlp plays both positive and negative roles in signalling, dependent on the relative abundance of Wg and Fz2 (71), as well as other factors. In the galactosemia disease models, extracellular Wg abundance is oppositely misregulated in dGALT vs. dGALE mutants, although the presynaptic signalling activation is similar. Likewise, dGALT and dUGP mutants exhibit an opposite effect on Wg ligand levels, but only dUGP mutants show a significant activation of the postsynaptic FNI pathway. We hypothesize that the fundamental defect in Leloir pathway mutants is the altered levels of the Wg co-receptor HSPG Dlp, which are low in both single and double mutant combinations. Previous studies have associated reduced Dlp levels to locally increased extracellular Wg levels (69,72), as is the case for dGALT mutants, and activation of Wg signalling, similar to what we report here. The direction of Wg activation, pre- or postsynaptic, appears different in dGALT, dGALE and dUGP mutants due to additional factors that we have yet to resolve. The identity and role of Wnt pathway ligand/receptor/coreceptor changes, and directional signalling defects at the NMJ, will be the focus of our future studies.
In conclusion, the results presented here are the first to reveal coordinated movement deficits, NMJ structure and functional defects, NMJ glycosylation losses and differential Wnt trans-synaptic signalling dysfunction under conditions of GALE-deficient galactosemia and UGP2 deficiency. Unlike GALT-deficient galactosemia, both GALE and UGP2 are indispensable for viability, and substantial residual enzymatic activities are required for survival, but nevertheless partial loss-of-function manifests severe neurobehavioural defects due to a neuronal requirement. GALE-deficient galactosemia shares with classic galactosemia numerous pathogenic factors underlying the long-term neurological impairments characterized by coordinated movement disabilities. Our results suggest that changes in Wnt signalling and Futsch/MAP1B microtubule cytoskeletal organization underlie neuromuscular synapse development defects and impaired coordinated movement in both disease states. Our results also reveal UGP2 activity and GalNAc glycosylation roles in neuromuscular synaptogenesis, raising the intriguing possibilities that targeted UGP2 strategies and/or UDP-galNAc supplementation might relieve neurological complications in the galactosemia disease states, as previously suggested (59,62).
Materials and Methods
Drosophila genetics
Figure 1A shows in parallel the enzymatic steps of the glucose and galactose metabolic pathways, listing the names and Drosophila CG numbers of all the genes targeted in this study, as well as the biochemical functions of the encoded proteins. All stocks were obtained from the Bloomington Drosophila Stock Center and Vienna Drosophila Resource Center, and reared at 25ºC under low-density rearing conditions on standard molasses-based food. We used two dGALT excision alleles generated by mobilizing a P-element insertion in the 5'-untranslated region of CG9232 (KG00049); dGALTΔC2 (precise excision control) and dGALTΔAP2 (null deficiency), with measured normal and undetectable enzymatic activity, respectively (55). A dGALE (CG12030) allele, dGALEh, was generated by mobilizing P-element insertion P{EPgy2}CG12030EY22205with H{PDelta2-3}HoP8,y1w*;Dr/TM3,Sb (34). Activity assays show dGALEhis a strong hypomorphic allele (measured ∼8% enzymatic activity), with unaltered dGALK and dGALT activities (34). The UAS-dGALE-RNAi line w1118; P{GD7464}v47408 was compared to Gal4 driver alone controls. UH1-Gal4 driven UAS-dGALE-RNAi results in 100% lethality, consistent with the essential requirement for the gene (33). We used two dUGP stocks; w1118; PBac{WH}UGPf03515/TM6B, Tb1 (dUGP1) and w1118; P{XP}UGPd07256/TM6B, Tb1 (dUGP2) generated by P-element insertion (42); and UAS-dUGP-RNAi line P{GD11288}v21832 for tissue-targeted experiments. Pan-neuronal elav-Gal4 and muscle 24B-Gal4 driver were used for tissue-specific studies, compared to driver alone controls. dGALE and dUGP hypomorphic alleles were used to create double mutant stocks with dGALTΔAP2. w1118 was used as the genetic background control for dGALE and dUGP, and precise excision controls dGALTΔC2 were used in double mutant combinations of these alleles.
Behavioural assays
Movement assays were performed as previously described in isolated wandering third-instar (wL3) male larvae (56). Larvae were placed individually on a fresh, room temperature (RT) 1% agar plate and allowed to move freely for ∼2 min before the assay. Using forceps, the larva was rolled to an inverted position as defined by the upright ventral midline. Once released, a timer recorded the amount of time the larva took to completely right itself as defined by the upright dorsal midline (56). Three consecutive time measurements were recorded for each larva and then averaged to produce one data point. The maximum amount of time allowed for a given animal to rollover was 5 minutes. Behavioural experiments were done on ≥15 individual animals for each genotype. Data were analyzed by student’s t-test for pairwise comparisons, and ANOVA tests for all data sets of ≥3 comparisons.
Immunocytochemical imaging
Wandering L3 larvae were used for all immunocytochemistry imaging as previously described (21,73). Reagents were purchased from Sigma-Aldrich unless otherwise specified. Larvae were dissected in physiological saline (128mM NaCl, 2mM KCl, 4mM MgCl2, 0.2mM CaCl2, 70mM sucrose, 5mM trehalose, 5mM HEPES), fixed in 4% paraformaldehyde for 10 minutes at RT, and then either processed with PBTX (PBS + 1% BSA + 0.2% Triton X-100) detergent, for cell permeabilized studies, or detergent-free (PBS with 1% BSA or without BSA when using lectins) conditions, for non-permeabilized studies. Primary antibodies included: Alexa Fluor-488 goat anti-horseradish peroxidase (HRP, 1:200, Jackson Labs); Cy3-conjugated goat anti-HRP (1:200, Jackson Labs); mouse anti-Fasciclin II (FasII, 1:10, Developmental Studies Hybridoma Bank (DSHB), University of Iowa); mouse anti-Discs Large (DLG, 1:250; DSHB); mouse anti-Wingless (Wg, 1:2; DSHB); mouse anti-Dally-like Protein (Dlp, 1:4; DSHB); rabbit anti-dFz-C (1:500) (75); mouse anti-Futsch (1:100; DSHB). Secondary antibodies included: Alexa Fluor-488-conjugated goat anti-mouse IgG (1:200, Invitrogen-Molecular Probes) and Alexa Fluor-488-conjugated goat anti-rabbit IgG (1:250, Invitrogen-Molecular Probes). Wisteria floribunda (WFA-Fitc, 1:250, Vector Labs) was used as fluorophore-conjugated lectin to label N-acetyl galactosamine residues (12). Primary antibodies and lectins were incubated at 4 °C overnight; secondary antibodies were incubated at RT for 2 hrs. Dissections were mounted on slides in Fluoromount G (Electron Microscopy Sciences).
All mutant and control larvae were dissected, labelled and imaged completely in parallel. Z-stacks were taken with a Zeiss LSM 510 META laser-scanning confocal, using either 40x or 63x Plan Apo oil-immersion objectives. Optical sections were taken starting immediately above and ending immediately below the NMJ. The stacks were projected on the Z-axis, with NMJ signals highlighted and the average intensity for each NMJ recorded. Intensities were quantified using ImageJ software. For structural analyses, preparations were double-labelled with anti-HRP and anti-DLG, with counts made at muscle 4 in segments A2/3 on both right and left sides. Structural data from hemisegments were averaged for each animal to produce one data point. For quantification, a bouton was defined as an axon varicosity >1µm in minimum diameter, and >2 boutons on an axon defined a NMJ branch. For quantification of Wg trans-synaptic signalling components (i.e. Wg ligand, HSPG Dlp, Fz2-C and Futsch), each NMJ was treated as an independent replicate. For Futsch labelling, NIH ImageJ was used to count bouton numbers, with high magnification imaging used to classify all boutons as empty (no Futsch labelling), diffuse (Futsch-positive, unorganized), bundled (Futsch-positive bundle) and looped (Futsch-positive loop) (48).
Electrophysiology
Two-electrode voltage-clamp (TEVC) electrophysiology was performed as previously described (74). Briefly, wL3 were secured with 3M Vetbond tissue adhesive (World Precision Instruments) to sylgard-coated glass coverslips, cut longitudinally along the dorsal midline, internal organs removed, and the larval cuticle glued down laterally to allow access to the neuromusculature. Peripheral nerves were then cut at the base of the ventral nerve cord (VNC). Dissections and recordings were performed at 18 °C in saline solution consisting of 128mM NaCl, 2mM KCl, 4mM MgCl2, 1.0mM CaCl2, 70mM sucrose, 5mM trehalose and 5mM HEPES, with pH adjusted to 7.1 using NaOH. Preparations were imaged using a Zeiss Axioskop microscope with 40X water immersion objective. Muscle 6 in abdominal segments 2/3 was impaled with two microelectrodes of ∼15 MΩ resistance filled with 3M KCl. The muscle was clamped at -60 mV using an Axoclamp-2B amplifier. A fire-polished glass suction electrode containing saline was used for evoked nerve stimulation of the severed motor nerve with a 0.5 ms suprathreshold stimuli at 0.2 Hz from a Grass S88 stimulator (74). Excitatory junctional current (EJC) records were filtered at 2 kHz. To quantify EJC amplitudes, 10 consecutive traces were averaged and the peak of the averaged trace recorded. Clampex software was used for all data acquisition and Clampfit software was used for all data analyses.
Statistical Analyses
All behavioural and NMJ structural data were averaged per genotype and each replicate value was calculated as the fold-change relative to the average value of the appropriate control. For signal intensity and structural data, control and mutant animals were always dissected, labelled and imaged in parallel. Intensity was calculated as the fold-change relative to the average control value recorded in the same experiment. The proportion of boutons displaying Futsch-positive loops was calculated relative to the total number of boutons at each NMJ. Unpaired t-tests (pairwise comparisons) or Mann-Whitney tests (≥3 comparisons) were used to compare differences between mutants and controls as indicated in figure legends. The criterion for statistical significance was P ≤ 0.05, with higher levels of significance classified as P ≤ 0.01, P ≤ 0.001 and P ≤ 0.0001. All statistical analyses were performed using GraphPad InStat version 3.0 (GraphPad Software, San Diego California, USA).
Acknowledgements
This work was solely supported by National Institutes of Health grant MH096832 to K.B. We are grateful to Vivian Budnik (University of Massachusetts Medical School, Worchester MA) for antibody reagents. We also particularly thank the Developmental Studies Hybridoma Bank at the University of Iowa for essential antibodies, and the Drosophila Bloomington Stock Center at Indiana University and Vienna Drosophila Resource Center for essential genetic lines.
Conflict of Interest statement. None declared.
Funding
This work was fully funded by NIH grant R01 MH096832 to K.B.
References
- 1.Bosch A.M. (2006) Classical galactosaemia revisited. J. Inherit. Metab. Dis., 29, 516–525. [DOI] [PubMed] [Google Scholar]
- 2.Holden H.M., Rayment I., Thoden J.B. (2003) Structure and function of enzymes of the Leloir pathway for galactose metabolism. J. Biol. Chem., 278, 43885–43888. [DOI] [PubMed] [Google Scholar]
- 3.Freeze H.H., Elbein A.D. (2009). Glycosylation Precursors Essentials of Glycobiology, 2nd edition, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 4.Prydz K., Dalen K.T. (2000) Synthesis and sorting of proteoglycans. J. Cell Sci., 113, 193–205. [DOI] [PubMed] [Google Scholar]
- 5.Dani N., Broadie K. (2012) Glycosylated synaptomatrix regulation of trans-synaptic signaling. Dev. Neurobiol., 72, 2–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnquist R.L., Gillett T.A., Hansen R.G. (1974) Uridine diphosphate glucose pyrophosphorylase. Crystallization and properties of the enzyme from rabbit liver and species comparisons. J. Biol. Chem., 249, 7695–7700. [PubMed] [Google Scholar]
- 7.Geisler M., Wilczynska M., Karpinski S., Kleczkowski L.A. (2004) Toward a blueprint for UDP-glucose pyrophosphrylase structure/function properties: homology-modeling analyses. Plant Mol./Biol., 56, 783–794. [DOI] [PubMed] [Google Scholar]
- 8.Yu Q., Zheng X. (2012) The crystal structure of human UDP-glucose pyrophosphorylase reveals a latch effect that influences enzymatic activity. Biochem. J., 442, 283–2913. [DOI] [PubMed] [Google Scholar]
- 9.Isselbacher K.J. (1958) A mammalian uridinediphosphate galactose pyrophosphorylase. J. Biol. Chem., 232, 429–444. [PubMed] [Google Scholar]
- 10.CDC Grand Rounds (2012): Newborn Screening and Improved Outcomes Morbidity and Mortality Weekly Report (MMWR). Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6121a2.htm. [PubMed] [Google Scholar]
- 11.Fridovich-Keil J.L. (2006) Galactosemia: the good, the bad, and the unknown. J. Cell Physiol., 209, 701–705. [DOI] [PubMed] [Google Scholar]
- 12.Jumbo-Lucioni P., Parkinson W., Broadie K. (2014) Overelaborated synaptic architecture and reduced synaptomatrix glycosylation in a Drosophila classic galactosemia disease model. Dis. Model Mech., 7, 1365–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fridovich-Keil J., Bean L., He M., Schroer R. (2011). Epimerase Deficiency Galactosemia. GeneReviews® [Internet]. Seattle (WA: ): University of Washington, Seattle; 1993-2015. Available from: http://www.ncbi.nlm.nih.gov/books/NBK51671/. [PubMed] [Google Scholar]
- 14.Hennermann J.B., Schadewaldt P., Vetter B., Shin Y.S., Monch E., Klein J. (2011) Features and outcome of galactokinase deficiency in children diagnosed by newborn screening. J. Inherit. Metab. Dis., 34, 399–407. [DOI] [PubMed] [Google Scholar]
- 15.Janzen N., Illsinger S., Meyer U., Shin Y.S., Sander J., Lucke T., Das A.M. (2011) Early cataract formation due to galactokinase deficiency: impact of newborn screening. Arch. Med. Res., 42, 608–612. [DOI] [PubMed] [Google Scholar]
- 16.Timson D.J. (2015) The molecular basis of galactosemia - Past, present and future. Gene, doi: 10.106/j.gene.2015.06.077. [DOI] [PubMed] [Google Scholar]
- 17.Holton J.B., Gillett M.G., MacFaul R., Young R. (1981) Galactosaemia: a new severe variant due to uridine diphosphate galactose-4-epimerase deficiency. Arch. Dis. Child., 56, 885–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haberland C., Perou M., Brunngraber E.G., Hof H. (1971) The neuropathology of galactosemia. A histopathological and biochemical study. J. Neuropathol. Exp. Neurol., 30, 431–447. [DOI] [PubMed] [Google Scholar]
- 19.Petry K., Greinix H.T., Nudelman E., Eisen H., Hakomori S., Levy H.L., Reichardt J.K.V. (1991) Characterization of a novel biochemical abnormality in galactosemia: deficiency of glycolipids containing galactose or N-acetylgalactosamine and accumulation of precursors in brain and lymphocytes. Biochem. Med. Metab. Biol., 46, 93–104. [DOI] [PubMed] [Google Scholar]
- 20.Charlwood J., Clayton P., Keir G., Mian N., Winchester B. (1998) Defective galactosylation of serum transferrin in galactosemia. Glycobiology, 8, 351–357. [DOI] [PubMed] [Google Scholar]
- 21.Dani N., Nahm M., Lee S., Broadie K. (2012) A targeted glycan-related gene screen reveals heparan sulfate proteoglycan sulfation regulates WNT and BMP trans-synaptic signaling. PLoS Genet., 8, e1003031.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parkinson W., Dear M.L., Rushton E., Broadie K. (2013) N-glycosylation requirements in neuromuscular synaptogenesis. Development, 140, 4970–4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman S.H., Dani N., Rushton E., Broadie K. (2013) Fragile X mental retardation protein regulates trans-synaptic signaling in Drosophila. Dis. Model Mech., 6, 1400–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waggoner D.D., Buist N.R., Donnell G.N. (1990) Long-term prognosis in galactosaemia: results of a survey of 350 cases. J. Inherit. Metab. Dis., 13, 802–818. [DOI] [PubMed] [Google Scholar]
- 25.Schweitzer S., Shin Y., Jakobs C., Brodehl J. (1993) Long-term outcome in 134 patients with galactosaemia. Eur. J. Pediatr., 152, 36–43. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman F.R., McBride-Chang C., Manis F.R., Wolff J.A., Nelson M.D. (1995) Cognitive functioning, neurologic status and brain imaging in classical galactosemia. Eur. J. Pediatr., 154, S2–S5. [DOI] [PubMed] [Google Scholar]
- 27.Hughes J., Ryan S., Lambert D., Geoghegan O., Clark A., Rogers Y., Hendroff U., Monaravi A., Twomey E., Treacy E.P. (2009) Outcomes of siblings with classical galactosemia. J. Pediatr., 154, 721–726. [DOI] [PubMed] [Google Scholar]
- 28.Rubio-Agusti I., Carecchio M., Bhatia K.P., Kojovic M., Parees I., Chandrashekar H.S., Footitt E.J., Burke D., Edwards M.J., Lachmann R.H., Murphy E. (2013) Movement disorders in adult patients with classical galactosemia. Mov. Disord., 28, 804–810. [DOI] [PubMed] [Google Scholar]
- 29.Szklarczyk D., Franceschini A., Wyder S., Forslund K., Heller D., Huerta-Cepas J., Simonovic M., Roth A., Santos A., TsafouK P., et al. (2015) STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res., 43, D447–D452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prodan-Zitnik I., Karas-Kuzelicki N., Lukac-Bajalo J. (2009) Positive correlation between galactose-1-phosphate uridyltransferase (GALT) and UDP-galactose-4'-epimerase (GALE) activities. Clin. Biochem., 42, 1561–1564. [DOI] [PubMed] [Google Scholar]
- 31.Walter J.H., Roberts R.E., Besley G.T., Wraith J.E., Cleary M.A., Holton J.B., MacFaul R. (1999) Generalised uridine diphosphate galactose-4-epimerase deficiency. Arch. Dis. Child., 80, 374–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alano A., Almashanu S., Chinsky J.M., Costeas P., Blitzer M.G., Wulfsberg E.A., Cowan T.M. (1998) Molecular characterization of a unique patient with epimerase-deficiency galactosaemia. J. Inherit. Metab. Dis., 21, 341–350. [DOI] [PubMed] [Google Scholar]
- 33.Daenzer J.M., Sanders R.D., Hang D., Fridovich-Keil J.L. (2012) UDP-galactose 4'-epimerase activities toward UDP-Gal and UDP-GalNAc play different roles in the development of Drosophila melanogaster. PLoS Genet., 8, e1002721.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanders R.D., Sefton J.M., Moberg K.H., Fridovich-Keil J.L. (2010) UDP-galactose 4' epimerase (GALE) is essential for development of Drosophila melanogaster. Dis. Model Mech., 3, 628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brokate-Llanos A.M., Monje J.M., Murdoch Pdel S., Munoz M.J. (2014) Developmental defects in a Caenorhabditis elegans model for type III galactosemia. Genetics., 198, 1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia R., Liu Y., Yang L., Gal J., Zhu H., Jia J. (2012) Motor neuron apoptosis and neuromuscular junction perturbation are prominent features in a Drosophila model of Fus-mediated ALS. Mol. Neurodegener., 7, 10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Courchesne S.L., Pazyra-Murphy M.F., Lee D.J., Segal R.A. (2011) Neuromuscular junction defects in mice with mutation of dynein heavy chain 1. PLoS One, 6, e16753.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higuita J.C., Alape-Giron A., Thelestam M., Katz A. (2003) A point mutation in the UDP-glucose pyrophosphorylase gene results in decreases of UDP-glucose and inactivation of glycogen synthase. Biochem. J., 370, 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flores-Diaz M., Alape-Giron A., Persson B., Pollesello P., Moos M., von Eichel-Streiber C., Thelestam M., Florin I. (1997) Cellular UDP-glucose deficiency caused by a single point mutation in the UDP-glucose pyrophosphorylase gene. J. Biol. Chem., 272, 23784–23791. [DOI] [PubMed] [Google Scholar]
- 40.Flores-Diaz M., Alape-Giron A., Titball R.W., Moos M., Guillouard I., Cole S., Howells A.M., von Eichel-Streiber C., Florin I., Thelestam M. (1998) UDP-glucose deficiency causes hypersensitivity to the cytotoxic effect of Clostridium perfringens phospholipase C. J. Biol. Chem., 273, 24433–24438. [DOI] [PubMed] [Google Scholar]
- 41.Daran J.M., Bell W., Francois J. (1997) Physiological and morphological effects of genetic alterations leading to a reduced synthesis of UDP-glucose in Saccharomyces cerevisiae. FEMS Microbiol. Lett., 153, 89–96. [DOI] [PubMed] [Google Scholar]
- 42.Bellen H.J., Levis R.W., Liao G., He Y., Carlson J.W., Tsang G., Evans-Holm M., Hiesinger P.R., Schulze K.L., Rubin G.M., et al. (2004) The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics, 167, 761–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hacker U., Lin X., Perrimon N. (1997) The Drosophila sugarless gene modulates Wingless signaling and encodes an enzyme involved in polysaccharide biosynthesis. Development, 124, 3565–3573. [DOI] [PubMed] [Google Scholar]
- 44.Sturiale L., Barone R., Fiumara A., Perez M., Zaffanello M., Sorge G., Pavone L., Tortorelli S., O’Brien J.F., Jaeken J., Garozzo D. (2005) Hypoglycosylation with increased fucosylation and branching of serum transferrin N-glycans in untreated galactosemia. Glycobiology, 15, 1268–1276. [DOI] [PubMed] [Google Scholar]
- 45.Dennis J.W., Nabi I.R., Demetriou M. (2009) Metabolism, cell surface organization, and disease. Cell, 139, 1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka K., Kitagawa Y., Kadowaki T. (2002) Drosophila segment polarity gene product porcupine stimulates the posttranslational N-glycosylation of wingless in the endoplasmic reticulum. J. Biol. Chem., 277, 12816–12823. [DOI] [PubMed] [Google Scholar]
- 47.Roos J., Hummel T., Ng N., Klambt C., Davis G.W. (2000) Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron, 26, 371–382. [DOI] [PubMed] [Google Scholar]
- 48.Packard M., Koo E.S., Gorczyca M., Sharpe J., Cumberledge S., Budnik V. (2002) The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell, 111, 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stamatakou E., Salinas P.C. (2014) Postsynaptic assembly: a role for Wnt signaling. Dev. Neurobiol., 74, 818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koles K., Budnik V. (2012) Wnt signaling in neuromuscular junction development. Cold Spring Harb. Perspect Biol., 4, pii: a008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coss K.P., Hawkes C.P., Adamczyk B., Stockmann H., Crushell E., Saldova R., Knerr I., Rubio-Gozalbo M.E., Monaravi A.A., Rudd P.M., Treacy E.P. (2014) N-glycan abnormalities in children with galactosemia. J. Proteome Res., 13, 385–394. [DOI] [PubMed] [Google Scholar]
- 52.Freeze H.H., Eklund E.A., Ng B.G., Patterson M.C. Neurology of inherited glycosylation disorders. Lancet Neurol., 11, 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holton J.B., Walter J.H., Tyfield L.A. (2000). Metabolic and Molecular Bases of Inherited Disease, Eighth ed., McGraw Hill, 1553–1587. [Google Scholar]
- 54.Wohlers T.M., Fridovich-Keil J.L. (2000) Studies of the V94M-substituted human UDPgalactose-4-epimerase enzyme associated with generalized epimerase-deficiency galactosaemia. J. Inherit. Metab. Dis., 23, 713–729. [DOI] [PubMed] [Google Scholar]
- 55.Kushner R.F., Ryan E.L., Sefton J.M., Sanders R.D., Lucioni P.J., Moberg K.H., Fridovich-Keil J.L. (2010) A Drosophila melanogaster model of classic galactosemia. Dis. Model Mech., 3, 618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bodily K.D., Morrison C.M., Renden R.B., Broadie K. (2001) A novel member of the Ig superfamily, turtle, is a CNS-specific protein required for coordinated motor control. J. Neurosci., 21, 3113–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Landmesser L.T. (2001) The acquisition of motoneuron subtype identity and motor circuit formation. Int. J. Dev. Neurosci., 19, 175–182. [DOI] [PubMed] [Google Scholar]
- 58.Frey P.A. (1996) The Leloir pathway: a mechanistic imperative for three enzymes to change the stereochemical configuration of a single carbon in galactose. FASEB J., 10, 461–470. [PubMed] [Google Scholar]
- 59.Lai K., Elsas L.J. (2000) Overexpression of human UDP-glucose pyrophosphorylase rescues galactose-1-phosphate uridyltransferase-deficient yeast. Biochem. Biophys. Res. Commun., 271, 392–400. [DOI] [PubMed] [Google Scholar]
- 60.Zito K., Parnas D., Fetter R.D., Isacoff E.Y., Goodman C.S. (1999) Watching a synapse grow: noninvasive confocal imaging of synaptic growth in Drosophila. Neuron, 22, 719–729. [DOI] [PubMed] [Google Scholar]
- 61.Miller D.L., Ballard S.L., Ganetzky B. (2012) Analysis of synaptic growth and function in Drosophila with an extended larval stage. J. Neurosci., 32, 13776–13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kingsley D.M., Kozarsky K.F., Hobbie L., Krieger M. (1986) Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-Gal/UDP-GalNAc 4-epimerase deficient mutant. Cell., 44, 749–759. [DOI] [PubMed] [Google Scholar]
- 63.Schulz J., Ross K., Malmstrom K., Krieger M., Fridovich-Keil J. (2005) Mediators of galactose sensitivity in UDP-galactose 4′-epimerase-impaired mammalian cells. J. Biol. Chem., 280, 13493–13502. [DOI] [PubMed] [Google Scholar]
- 64.Kalckar H.M. (1965) Galactose metabolism and cell “sociology”. Science, 150, 305–313. [DOI] [PubMed] [Google Scholar]
- 65.Ryczko M., Pawling J., Chen R., Abdel Rahman A.M., Yau K., Copeland J.K., Zhang C., Surendra A., Guttman D.S., Figeys D., Dennis J.W. (2016) Metabolic Reprogramming by Hexosamine Biosynthetic and Golgi N-Glycan Branching Pathways. Scientific Reports, 6, 23043.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Collins C.A., DiAntonio A. (2007) Synaptic development: insights from Drosophila. Curr. Opin. Neurobiol., 17, 35–42. [DOI] [PubMed] [Google Scholar]
- 67.Miech C., Pauer H.U., He X., Schwarz T.L. (2008) Presynaptic local signaling by a canonical wingless pathway regulates development of the Drosophila neuromuscular junction. J. Neurosci., 28, 10875–10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han C., Yan D., Belenkaya T.Y., Lin X. (2005) Drosophila glypicans Dally and Dally-like shape the extracellular Wingless morphogen gradient in the wing disc. Development., 132, 667–679. [DOI] [PubMed] [Google Scholar]
- 69.Kirkpatrick C.A., Dimitroff B.D., Rawson J.M., Selleck S.B. (2004) Spatial regulation of Wingless morphogen distribution and signaling by Dally-like protein. Dev. Cell., 7, 513–523. [DOI] [PubMed] [Google Scholar]
- 70.Franco B., Bogdanik L., Bobinnec Y., Debec A., Bockaert J., Parmentier M.L., Grau Y. (2004) Shaggy, the homolog of glycogen synthase kinase 3, controls neuromuscular junction growth in Drosophila. J. Neurosci., 24, 6573–6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan D., Wu Y., Feng Y., Lin S.C., Lin X. (2009) The core protein of glypican Dally-like determines its biphasic activity in wingless morphogen signaling. Dev. Cell., 17, 470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kreuger J., Perez L., Giraldez A.J., Cohen S.M. (2004) Opposing activities of Dally-like glypican at high and low levels of Wingless morphogen activity. Dev. Cell, 7, 503–512. [DOI] [PubMed] [Google Scholar]
- 73.Rushton E., Rohrbough J., Deutsch K., Broadie K. (2012) Structure-function analysis of endogenous lectin mind-the-gap in synaptogenesis. Dev. Neurobiol., 72, 1161–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rohrbough J., Broadie K. (2002) Electrophysiological analysis of synaptic transmission in central neurons of Drosophila larvae. J. Neurophysiol., 88, 847–860. [DOI] [PubMed] [Google Scholar]
- 75.Mathew D., Ataman B., Chen J., Zhang Y., Cumberledge S., Budnik V. (2005) Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science, 310, 1344–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]