Abstract
Nondioxin-like polychlorinated biphenyls (NDL PCBs) activate ryanodine-sensitive Ca2+ channels (RyRs) and this activation has been associated with neurotoxicity in exposed animals. RyR-active congeners follow a distinct structure–activity relationship and a quantitative structure–activity relationship (QSAR) predicts that a large number of PCBs likely activate the receptor, which requires validation. Additionally, previous structural based conclusions have been established using receptor ligand binding assays but the impact of varying PCB structures on ion channel gating behavior is not understood. We used [3H]Ryanodine ([3H]Ry) binding to assess the RyR-activity of 14 previously untested PCB congeners evaluating the predictability of the QSAR. Congeners determined to display widely varying potency were then assayed with single channel voltage clamp analysis to assess direct influences on channel gating kinetics. The RyR-activity of individual PCBs assessed in in vitro assays followed the general pattern predicted by the QSAR but binding and lipid bilayer experiments demonstrated higher potency than predicted. Of the 49 congeners tested to date, tetra-ortho PCB 202 was found to be the most potent RyR-active congener increasing channel open probability at 200 pM. Shifting meta-substitutions to the para-position resulted in a > 100-fold reduction in potency as seen with PCB 197. Non-ortho PCB 11 was found to lack activity at the receptor supporting a minimum mono-ortho substitution for PCB RyR activity. These findings expand and support previous SAR assessments; where out of the 49 congeners tested to date 42 activate the receptor demonstrating that the RyR is a sensitive and common target of PCBs.
Keywords: nondioxin-like PCBs, ryanodine receptor, single-channel gating, neurotoxicity.
Polychlorinated biphenyls (PCBs) represent one of the leading groups of chemicals detected in environmental media and human samples (Domingo and Bocio, 2007; Faroon and Ruiz, 2015; Stahl et al., 2009). Of the 209 PCB congeners, varying by the position and number of chlorine substitutions, the nondioxin-like PCBs (NDL PCB) have been identified as potential neurological toxicants due to observed cognitive deficits in PCB exposed organisms (Boix et al., 2011; Lilienthal et al., 2015; Schantz et al., 1997, 2001; Yang et al., 2009). Numerous modes of action have been described that may contribute to neurotoxicity. This includes altered neurotransmitter signaling (Fonnum and Mariussen, 2009; Mariussen and Fonnum, 2001; Wigestrand et al., 2013), altered GABAA receptor activity (Fernandes et al., 2010), altered Ca2+ homeostasis through several mechanisms (Choi et al., 2016; Gafni et al., 2004; Kodavanti and Tilson, 1997; Pessah et al., 2010; Westerink, 2014), induced reactive oxygen species (Stenberg et al., 2011), altered cell viability (Dickerson et al., 2009), and alterations of neuro/endocrine processes (Bell, 2014; Fonnum and Mariussen, 2009; Kodavanti and Curras-Collazo, 2010).
Of the modes identified, a particularly sensitive endpoint is the ability of NDL PCBs to enhance the activity of ryanodine sensitive Ca2+ channels embedded in the sarco/endoplasmic reticulum (SR/ER), namely, the ryanodine receptor (RyR), in both mammals (Pessah et al., 2006) and fish (Fritsch and Pessah, 2013). There are 3 RyR-isoforms (Berridge, 2012) with varying expression by developmental stage and brain region and the channels are essential to both peripheral and central nervous systems (for a comprehensive review see Pessah et al., 2010). Briefly, RyR-isoform 1 (RyR1) and RyR-isoform 2 (RyR2) are both found to predominate in the brain, where the third isoform, RyR3, represents only about 2%. RyR Ca2+ release from intercellular stores contributes to neurotransmission and vesicle mobilization and cellular transcription, translation and secretion processes that are directly tied to synaptic plasticity. The RyR has also been implicated as a primary mechanism by which dendritic growth is attenuated; a measure of neuronal connectivity. In primary neuronal cultures, NDL PCB exposure increases intracellular Ca2+ levels, increases spontaneous Ca2+ oscillations (Wayman et al., 2012a,b) and interferes with normal neuronal dendritic growth and plasticity in a RyR dependent manner. The expression, role and toxicity data support the RyR as a contributor to NDL PCB induced neurotoxicity and suggest that RyR-activation by NDL PCBs could disrupt several aspects of neuronal signaling.
For RyR-active PCB congeners a distinct structure–activity relationship (SAR) has been suggested (Pessah et al., 2006) and the SAR is consistent for NDL PCB activity at RyR1 and RyR2 channels (Pessah et al., 2010; Wong et al., 1997). Congeners with 2 or more ortho-substitutions display high potency at the RyR whereas those lacking ortho substitution lack receptor activity. The most detailed SAR conducted to date has been done with RyR1, assessing RyR-activity for a subset of PCBs (35 of the 209 congeners). The SAR suggests that those congeners containing ortho substitution would likely contribute to neurotoxic outcomes and this prediction is supported by a recent quantitative structure–activity relationship (QSAR), which predicted the RyR activity of all 209 PCBs. The QSAR was built using the RyR1 data from previous studies (Pessah et al., 2010) and predicted that approximately 190 PCBs would display RyR-activity (Rayne and Forest, 2010). Further evaluating the predictions of the in silico QSAR would help describe the extent to which individual congeners may contribute to mixture induced neurotoxicity.
Whereas studies have evaluated the SAR of congeners towards causing RyR activation the consequent changes in the Ca2+ channel’s gating behavior is poorly understood. In single channel voltage clamp analysis PCB 95, thought to be the most potent RyR-active PCB, causes the RyR1 to display an increased open probability due to prolonged time in the open state together with short lived closed state transitions (Samsó et al., 2009). Only 2 potent RyR active PCBs, namely, PCB 95 and enantiomers of PCB 136 (Pessah et al., 2009), have been tested in single channel gating and it is unclear whether less potent RyR-active PCBs may display similar or distinct impacts on channel behavior. Studies addressing this knowledge gap would provide valuable information regarding functional changes in the RyR in the presence of various PCB congeners, especially in light of structural differences. Single channel gating is also the most direct approach for measuring the inherent potency and efficacy of compounds and mutations that modify the RyR channel (Pessah et al., 2010) and could be monitored at lower concentrations.
The goals of the current study were to (1) evaluate the predictions of the previously developed QSAR by testing whether previously untested PCB congeners could alter RyR activity measured in [3H]ryanodine ([3H]Ry) binding assays and to (2) compare toxicity observed in [3H]Ry-binding assays with changes in the RyR’s functional behavior as measured through single channel voltage clamp analysis. We selected 14 congeners with 2–9 chlorine substitutions (Table 1A) that have not been previously assessed for RyR activity. Congeners were chosen based on their chlorine substitution patterns and predicted, or lack of, RyR activity (Rayne and Forest, 2010). Special focus was placed on tetra-ortho PCB 202, lacking para-substitution, which the QSAR suggested would be the most potent RyR-active congener. Additional focus was placed on PCB 11 and PCB 197, which were lacking or limited in original Aroclor mixtures (Frame, 2001; Koh et al., 2015) but are detected in human serum (Koh et al., 2015). As a non-ortho congener, PCB 11 is predicted to lack RyR-activity. PCB-197, like PCB 202, has 8 chlorines, but is predicted to have relatively lower RyR-activity due to its para-chloro substitutions. All the congeners selected have been detected in human samples (Koh et al., 2015; Marek et al., 2013; http://www.ewg.org/sites/humantoxome/chemicals/index), with several congeners, including, PCB 11, 20, 28, 95, 99, and 118, being frequently detected in both humans and the environment (Grimm et al., 2015; Stahl et al., 2009).
TABLE 1.
Potency and Efficacy of PCB Congeners Toward the Ryanodine Receptor, Isoform 1, in Skeletal Muscle
| A. Ryanodine receptor activity of previously untested congeners | |||||||
|---|---|---|---|---|---|---|---|
| PCB Congener | Substitution | Maximum Response (%) | EC50 (µM) | EC50 95%CI (µM) | EC2X (µM) | EC2X 95% CI (µM) | Predicted EC2X (µM)b |
| 11 | 3,3' | – | – | – | – | – | 10.00 |
| 20 | 2,3,3' | 570 ± 41 | 1.08 | 0.58–2.1 | 0.25 | 0.13–0.43 | 2.00 |
| 28 | 2,4,4' | 219 ± 12 | 0.86 | 0.40–1.85 | 1.37 | 0.7– 50.00 | 5.00 |
| 40 | 2,2',3,3' | 502 ± 19 | 1.83 | 1.43–2.35 | 0.75 | 0.62–0.90 | 1.00 |
| 44 | 2,2',3,5' | 1374 ± 142 | 1.78 | 1.21–2.61 | 0.15 | 0.07–0.27 | 0.80 |
| 56 | 2,3,3',4' | 178 ± 4 | 0.87 | 0.62–1.22 | – | – | 3.00 |
| 77 | 3,3',4,4' | 134 ± 9 | 3.07 | 0.6–15.0 | – | – | 5.00 |
| 99 | 2,2',4,4',5 | 426 ± 25 | 0.74 | 0.44–1.23 | 0.28 | 0.20–0.37 | 2.00 |
| 118 | 2,3',4,4',5 | 203 ± 5 | 0.82 | 0.62–1.08 | 2.35 | 1.21–50.00 | 3.00 |
| 179 | 2,2',3,3',5,6,6' | 442 ± 14 | 1.45 | 1.17–1.80 | 0.77 | 0.66–0.89 | 0.17 |
| 194 | 2,2',3,3',4,4',5,5' | 618 ± 46 | 0.47 | 0.24–0.93 | 0.07 | 0.04–0.13 | 0.80 |
| 197a | 2,2',3,3',4,4',6,6' | 261 ± 6 | 1.38 | 1.14–1.68 | 1.76 | 1.51–2.10 | 0.60 |
| 202 | 2,2',3,3',5,5',6,6' | 457 ± 45 | 0.31 | 0.08–1.10 | 0.05 | 0.02–0.10 | 0.12 |
| 208 | 2,2'3,3',4,5,5',6,6' | 243 ± 21 | 0.03 | 0.003–0.11 | 0.10 | 0.03–0.42 | 0.18 |
|
B. Re-evaluation of previously confirmed ryanodine receptor active congeners | |||||||
| PCB Congener | Substitution | Maximum Response (%) | EC50 (µM) | EC50 95% CI (µM) | EC2X (µM) | EC2X 95% CI (µM) | Previous EC2X (µM)c |
| 18 | 2,2',5 | 610 ± 50 | 2.46 | 1.44–4.18 | 0.66 | 0.46–0.89 | 1.05 |
| 95a | 2,2',3,5',6 | 1130 ± 30 | 0.46 | 0.39–0.55 | 0.14 | 0.07–0.21 | 0.22 |
| 183a | 2,2',3,4,4',5',6 | 445 ± 21 | 0.82 | 0.51–1.32 | 0.35 | 0.16–0.61 | 0.55 |
Congener displays chirality (Lehmler et al., 2010).
Estimated EC2x as predicted by a recent QSAR (Rayne and Forest, 2010).
Previously published EC2X (Pessah et al., 2006).
CI—confidence interval.
MATERIALS AND METHODS
Chemicals
Polychlorinated biphenyls (PCB) congeners were purchased neat from AccuStandard (99–100% pure). The 14 PCB congeners previously untested for RyR activity can be found in Table 1A and are displayed in comparison to 3 PCB congeners (PCB 18, 95, and 183), with published RyR-activity (Table 1B; Pessah et al. 2006), that were re-evaluated to provide a measure of assay reproducibility and a comparison of the current findings to that from previously published work. A stock of PCB 11 was custom synthesized (Supplementary Figs. 1 and 2) and characterized as described in the Supplementary Data. According to gas chromatography-mass spectrometric analysis and relative peak area the PCB 11 preparation was 99.99% pure (Supplementary Figs. 3 and 4).
Membrane preparation
Microsomal fractions enriched with RyR1 were created from white skeletal muscle of male New Zealand White rabbits (Pel-Freez Biologicals) as previously described (Pessah et al., 1985, 1987). Briefly, muscle was ground and homogenized in buffer containing 300 mM sucrose, 10 mM 4-(2-hydroxyethyl)-1-piperazineethane sulfonic acid (HEPES), 1 pheylmethylsulfonyl fluoride and 5 µg/ml leupeptin (pH 7.4). The junctional sarcoplasmic reticulum (JSR) fraction was then isolated using differential centrifugation followed by purification through a discontinuous sucrose gradient. The JSR preparation was resuspended in 300 mM sucrose and 10 mM HEPES, pH 7.4 and protein concentrations were determined in triplicate using a BCA assay (Fisher).
[3H]Ryanodine binding assays
The ligand ryanodine binds preferentially to the open state of the RyR, where increased ligand binding signifies increased open state, or active state, of the receptor. We used [3H]Ryanodine ([3H]Ry; 61.8 Ci mmol−1; Perkin Elmer) to assess the open state of the RyR in the presence of PCB congeners. Assays followed the exact experimental conditions utilized previously (Pessah et al., 2006). Specifically, assays utilized 12 µg protein and 1 nM [3H]Ryanodine in the presence of 0.5% DMSO or 0.01–50 µM PCB in 0.5% DMSO. Assays were conducted in 500 µL of buffer containing 140 mM KCl, 15 mM NaCl, 20 mM HEPES, and 50 µM CaCl2 (pH 7.4) and incubated for 3 h at 37°C. Nonspecific binding was determined with the addition of 1 µM ryanodine. Tests were run in triplicate and concentration-responses were conducted at least twice using different JSR preparations.
RyR1 single channel recordings in planar lipid bilayer
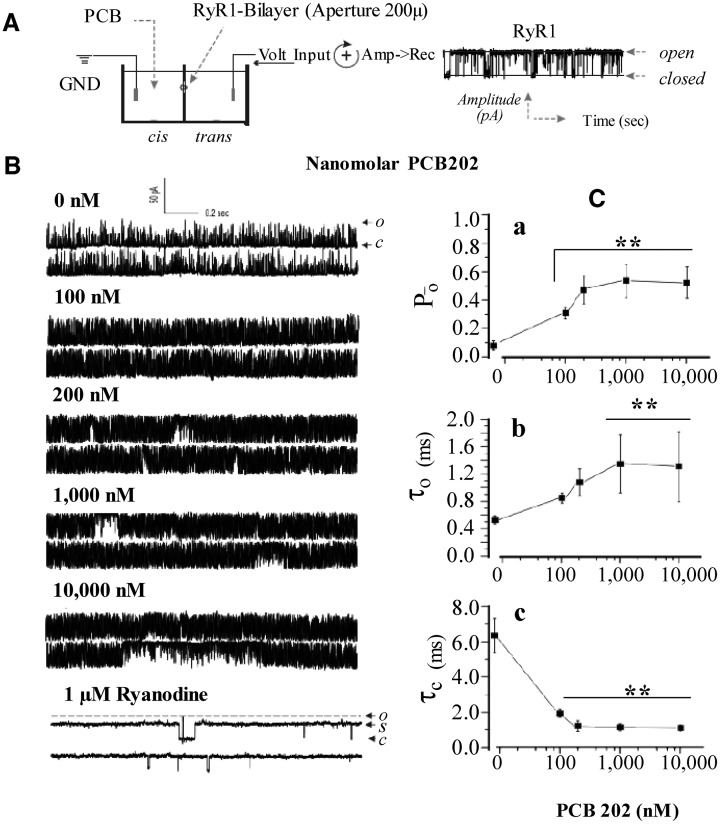
Single channel recordings were completed following previously described methods (Pessah et al., 2009). Briefly, measurements of single RyR1 channels were conducted by inducing JSR vesicle fusion with BLM; composed of 30 mg/ml phosphatidylethanolamine, phosphatidylserine, and phosphatidylcholine in n-decane (Avanti). A 10:1 cytoplasmic (cis) to lumen (trans) side chemical gradient of Cs+ was built by 500–50 mM of CsCl, buffered with 20 mM HEPES, pH 7.4. Channel currents were recorded at a holding potential of −40 mV applied to the trans lumen side that had 100 μM Ca2+. JSR vesicles (1–4 µg/ml), test PCBs, and defined free Ca2+ (2 μM) were introduced to the cis-cytosolic channel face, which served as virtual ground. Prior to the addition of a given treatment, baseline recordings (control) were acquired for approximately 60 s. After baseline recordings, chemical treatments were added to the channel sequentially and channels monitored extended periods of time that lasted up to 5 min after the addition of varying PCBs or PCB concentrations. Thus, chemical exposure could be compared directly to a given channels’ baseline gating behavior. Recordings in the presence of DMSO tested up to 1% (v/v) showed negligible changes in channel gating (data not shown and see O’Neill et al., 2003) and in the current study never exceeded 0.5% (v/v). Data acquisition was made through Digidata 1440A (Axon-Molecular Devices), digitized at 10 kHz after 1 kHz low-pass filter (Low-Pass Bessel Filter 8 Pole). A schematic graph briefly depicting the process is shown in the Figure 3A.
FIG. 3.
PCB 202 influences RyR1 channel gating kinetics in a concentration-dependent manner. (A) Schematic diagram of the RyR1 channel reconstitution across the BLM chamber. (B) Representative traces of single RyR1 channel gating activity before and after stepwise increase in PCB 202 concentrations. Recordings of channel gating activity were measured for several minutes before the sequential addition of PCB 202 on the cis (cytoplasmic) side of the RyR1 channel. Traces shown are representative sections of the RyR1 channel. (C) Concentration-dependent change in open probability (Po, a) and corresponding changes in the mean open dwell time (τo, b) and the mean closed time (τc, c). Channel kinetic data in (C) are the Mean ± SD from 4 different recording time periods (eg, parameter during the initial 20 s exposure to given treatment). Abbreviations: Ground (GND), polychlorinated biphenyl (PCB), cytoplasmic side (cis), luminal side (trans), voltage input (volt-input), amplifier to recorded (Amp -> Rec), open channel state (O); closed channel state (C); sub-conductance state (S). Differences indicated in panel (C) were assessed using repeated measures ANOVA with a Tukey post-hoc analysis. **P ≤ .01 relative to the control; †P ≤ .05 relative to PCB 202 at 100 nM. n = 4 time periods.
Statistics and data analysis
For [3H]Ry binding assays, specific binding was calculated by subtracting the nonspecific binding from the observed total binding as measured in disintegrations per minute (DPM). Concentration responses were tested in at least 2 different JSR preparations (n ≥ 2) and each concentration was run in triplicate per experiment (3 technical replicates). Under these analyses, assuming 80% power and an α ≤ 0.05, n = 2 would detect a difference of 200 DPM (Minitab; SD = 36; geometric mean of the SD observed in DMSO controls; where DMSO controls were run with every congener each experiment). This change in DPM would be approximately 136% change in [3H]Ry binding compared with the DMSO control (assuming mean = 560 DPM; geometric mean of the average DPM for the DMSO controls).
Concentration response curves were created using specific binding normalized to the DMSO control from the same experiment and displayed as a percent. Percent responses were fit to sigmoidal concentration-response curves (PRISM 6.0) that were used to estimate maximal percent activation, the effective concentration causing 50% of maximal response (EC50), and the concentration that would cause a 200% over activation of the receptor (EC2X). Nonlinear regression models had ≥ 32 degrees of freedom for goodness of fit assessments. To evaluate the predictions of previously developed QSAR (Rayne and Forest, 2010), the predicted versus experimental log-EC2x were compared via Pearson’s correlation. Inactive or low efficacy congeners, as determined in [3H]Ry binding assays, were ascribed an EC2x of 30 µM to remain consistent with the concentration range utilized in the developed QSAR (Rayne and Forest, 2010), which predicted that only 14 congeners would completely lack RyR activity.
Single channel recording data was analyzed using pClamp 10.4 (Molecular Devices); where recorded traces were analyzed for channel open probability (PΟ) and current amplitude distribution. Open probability represents the ratio of time that the channel spends in the open state (τO ) relative to the dwell time in the closed state (τC). The baseline gating activity of each reconstituted channel was recorded for approximately 1 min followed by the sequential addition of PCB 202, PCB 197, or PCB 11 from low to high concentration. Channel gating activity for each PCB at a given PCB concentration was recorded for an extended period of time that was up to 5 min after chemical addition. Thus, chemical affects were determined relative to the respective baseline recording. For PCB 202, affects between 100 nM to 10 µM were conducted on a single channel recorded for prolonged periods of time under baseline or a given PCB 202 concentration. Time periods of the channel recording under the baseline or chemical treatments were then compared using a repeated measures analysis of variance (ANOVA; Origin 9.0) and differences between treatments compared using a Tukey’s post-hoc analysis. This method was used to determine the lowest concentration of PCB 202 that would cause the maximum change in receptor gating kinetics. Once determined, the lowest maximal effect concentration (see results; 200 nM PCB 202; Figure 3) was assessed in 6 individual RyR1 channels and compared with kinetics in the presence of picomolar concentrations of PCB 202 (n = 6). Similarly, the effect of PCB 197 were assessed in multiple individual channels at 200 pM (n = 3) and 200 nM (n = 4). Here, the Po in the presence of PCB 202 or PCB 197 in the pM–nM range were normalized to their respective baseline and significance determined using a paired sample t-test. The pairwise SD was 39.5% for PCB treatment relative to the baseline control, where assuming 80% power and an α ≤ 0.05, a n = 6 would detect a difference of 56% from the baseline (eg, 100% compared with 156% increase in PΟ). An n = 3 would detect a difference of 128.9% compared with baseline (eg, 100–228.9%).
RESULTS
Predicted Versus Experimental Activity of PCBs Towards RyR1
We assess the RyR-based toxicity of 14 PCB congeners that have not previously been assessed for channel activity (Table 1A), expanding our previous SAR (Pessah et al., 2006). We also re-assessed the binding activity of previously published congeners (Pessah et al., 2006) and demonstrated high reproducibility of the [3H]Ry binding assay (Table 1B). Of the 14 congeners tested in [3H]Ry binding assays, 11 PCBs were found to cause a 200%, or greater, overactivation (EC2X) of the RyR. Together with previous work this brings the total number of active RyR congeners to 42, out of the 49 congeners that have been tested to date (current work and Pessah et al., 2006). Maximum effects and effective concentrations for all congeners are summarized in Table 1 and are shown next to their corresponding predicted (Table 1A) or previously published EC2X.
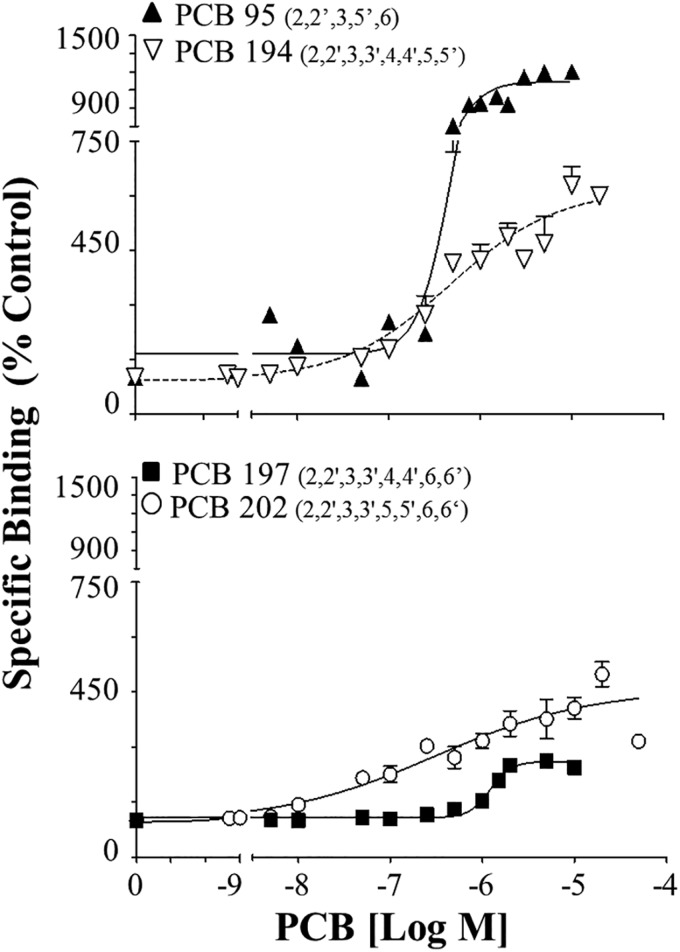
Tetra-ortho PCB 202, lacking para-substitution, displayed the highest RyR1 potency of the congeners assessed with an EC2x of 0.05 µM in [3H]Ry binding assays (Figure 1 and Table 1A). The finding that PCB 202 is the most potent RyR PCB congener agrees with the predictions of the QSAR by Rayne and Forest (2010) but we demonstrate a higher potency than the QSAR predicted (Table 1A). The concentration response curves for PCB 202 compared with PCB 95, 194, and 197 can be found in Figure 1 and are displayed in different panels to help demonstrate the differences in RyR-activation curves by these structurally distinct congeners. When compared with PCB 95, a tri-ortho PCB previously suggested in experimental assessments as the most potent RyR congener, PCB 202 displayed an almost 2-fold higher apparent potency, but lower efficacy (maximum response; Figure 1 and Table 1A and B). When PCB 202 is compared with PCB 194 and PCB 197, all containing 8 chlorine substitutions, the importance of meta-substitution adjacent to the ortho-chlorines is clearly demonstrated. PCB 194, lacking 6,6′ortho-chlorines but containing both 5,5′ meta-substitutions, is highly efficacious and potent displaying an EC2X of 0.07 µM and causing a maximum 618% overactivation of the channel. Conversely, tetra-ortho PCB 197, which lacks the 5,5′ meta-substitution pattern and contains para-substitution, shows ∼35-fold lower EC2X of 1.76 µM and nearly 2-fold lower maximum response at just 261% overactivation of the channel.
FIG. 1.
Binding of [3H]Ry to the RyR1 in the presence of PCB 202 in comparison to PCB 95, 194, and PCB 197 to demonstrate the importance of ortho, meta, and para-chlorine substitutions. Specific binding is shown relative to DMSO control (100%). Congeners were placed in different panel to aid visualization. Concentration response curves were assessed using nonlinear regression. Numbers represent Mean ± SEM; from 2 protein preparations (n ≥ 2) where each treatment was run in triplicate for each protein preparation.
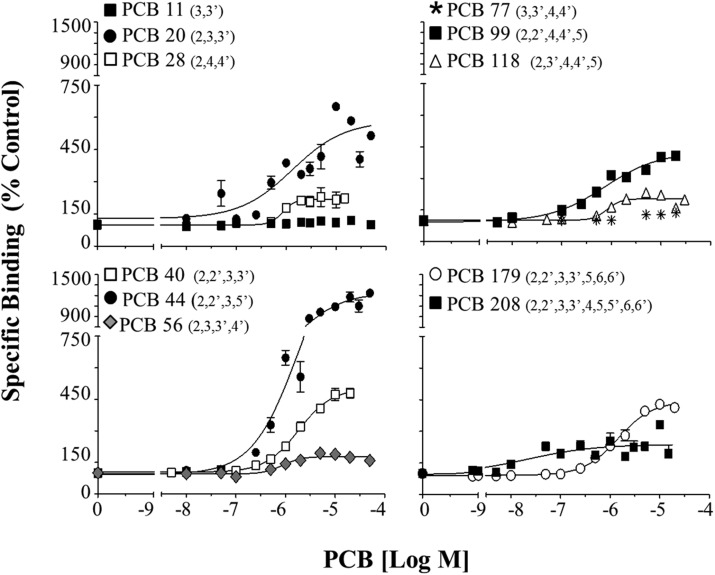
Additional concentration response curves for the remaining 11 PCBs that were tested for the first time in the current study can be found in Figure 2 and are separated into separate panels based on chlorine number to aid visualization. One pattern that can be seen in Figure 2 is the impact of para-substitution on the RyR-activity of different PCBs; for example when comparing the tri-ortho congeners PCB 44 and PCB 99. Both congeners activate the channel but PCB 44, without para substitution, displays a 3-fold great maximum efficacy and an approximate 2-fold higher potency compared with PCB 99, which has both para positions occupied (Table 1A and Figure 2). It should be noted however that the 95% confidence intervals for the PCB 44 and PCB 99 EC2X’s overlap. The non- and mono-ortho-substituted congeners tested in the current study displayed little to no RyR activity (Table 1A and Figure 2). This included non-ortho congeners PCB 11, which caused no activation, and PCB 77 that unexpectedly caused a slight activation of the RyR (maximum 130%) at concentrations higher than 10 µM. Mono-ortho congeners tested included PCB 20, 28, 56, and 118. Of the mono-ortho congener PCB 20 (chlorines in the 2, 3, 3′ position) displayed a high potency and efficacy (EC2X = 0.25; 570% maximum overactivation) and was more RyR-active than the mono-ortho congeners containing para substitution (ie, PCB 28, 56, and 118). These findings support a minimal mono-orthro substitution for RyR-activity and that congeners with ortho-substitution in the presence of para-substitution will likely display lower RyR activity than similar chlorine substituted congeners lacking para-substitution.
FIG. 2.
Concentration response curves for the remaining 11 PCB congeners tested for the first time in the current study for RyR binding activity. Specific binding is shown relative to DMSO control (100%). RyR1 activity of the different PCB congeners is shown in different panels based on chlorine number to aid visualization. Concentration response curves were assessed using nonlinear regression. Numbers represent Mean ± SEM; from 2 protein preparations (n ≥ 2) where each treatment was run in triplicate for each protein preparation.
The RyR1 based NDL PCB QSAR (Rayne and Forest, 2010) displayed a moderate predictive capability, such that, the predictive and experimentally derived EC2X values were significantly correlated (r = 0.71; P = 0.004, Supplementary Fig. 5). When the experimental versus predictive EC2X values of single congeners are compared (Table 1A) the experimental measurements describe receptor activation occurring at lower concentrations. Several exceptions included PCB 179 and 197, both displaying effective concentrations at higher concentrations than predicted by the QSAR. The QSAR model suggested that the ortho-substitution number was, in large part, driving potency at the RyR1 (Rayne and Forest, 2010) but both PCB 179 and PCB 197 have 4 ortho-substitutions but lack one or both chlorines in the meta position, respectively. These findings support the importance of chlorines in the meta position, in addition to the ortho position, for PCB congener activity at the RyR.
NDL PCBs Alter RyR1 Single Channel Gating Behavior
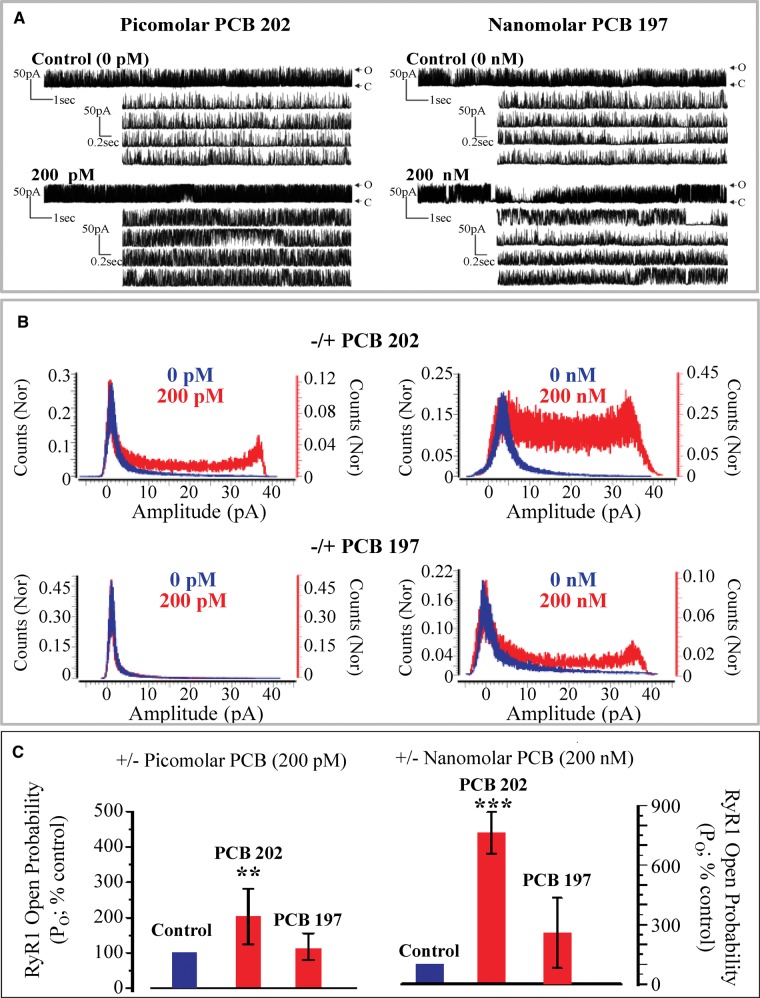
[3H]Ry binding assay revealed drastic differences in the RyR-activity of PCB 202 and PCB 197 (Figure 1 and Table 1A, EC2X 50 nM and 1.76 µM, respectively). Both PCB 202 and PCB 197 are tetra-ortho congeners with 8 total chlorines but PCB 202 lacks para-substitution and PCB 197 lacks meta-substitution but contains para-substitutions. Due to these distinct potency differences, especially in light of structural similarities and differences, these congeners were chosen for further evaluation in single channel gating analysis in comparison to non-ortho PCB 11, which was completely inactive in [3H]Ry binding assays.
Figure 3A shows a schematic graph depicting the recording process of an incorporated RyR1 channel in BLM and representative traces of RyR1 at baseline or in the presence of increasing concentrations of PCB 202 can be found in Figure 3B. Overall, Figure 3 shows that PCB 202 concentrations between 100 nM and 10 µM caused significant changes in the open probability (Po) of RyR1 compared with the baseline (control) conditions (Figure 3C; repeated measures ANOVA; P ≤ 0.0001). Specifically, starting at 100 nM, addition to the cis-side of the channel, PCB 202 caused a significant increase in the Po of the RyR channel (traces Figure 3B) that was approximately 300% greater than baseline conditions. The increase in Po was due to an increased trend in the mean open dwell time (τO ) and a significant decrease in the mean closed dwell time (τC) (see Figure 3C: (a) Po, (b) τO, and (c) τC). Subsequent sequential additions of PCB 202 to a final concentration 200 nM caused a greater increase in the PΟ of the RyR1 channel due to further increased τO and decreased τC (Figure 3C). Further escalation of PCB 202 concentrations in the cis chamber (1–10 µM) did not cause additional changes to the gating kinetics of the RyR1-channel. Final addition of the ligand ryanodine (1 μM) to the PCB 202 modified channel was able to lock the channel into the characteristic long-lasting sub-conductance state as anticipated from previous studies. This demonstrates saturability with the maximum increase in PCB 202-induced channel Po observed at just 200 nM and that PCB 202 induced changes in PΟ are specifically due to increased time in the open state in combination with shorter-lived transitions in the closed state.
We then aimed to determine the threshold concentration of PCB 202 that could cause a statistically significant change in RyR1 gating. PCB 202 as low as 200 pM induced changes in RyR1 channel gating kinetics (Figure 4A; representative traces) characterized by a significant right-shift in the current frequency histogram (Figure 4B). The change in current amplitude distribution was consistent with a significantly increased Po as compared with the channel’s baseline behavior before exposure to PCB 202 (Figure 4C). Here, PCB 202 caused an approximate 200% increase in Po compared with baseline recordings and these findings were consistent in 6 independently recorded channels (Mean Po = 199.0% compared with baseline; pairwise SD = 39.5%; paired sample t-test, P ≤ .001). The magnitude of channel activation by PCB 202 as seen in both the current amplitude distribution and the channel’s Po was concentration dependent (compare 200 pM vs 200 nM).
FIG. 4.
Comparison of RyR1 channel gating behavior in the presence of PCB 202 or PCB 197 at 200 pM or 200 nM. (A) Representative traces of (PCB 202 and PCB 197 at 200 pM or 200 nM, respectively). Note PCB 202 at 200 nM and PCB 197 at 200 pM traces are not shown for space purposes. Abbreviations: open channel state (O); closed channel state (C). (B) Frequency histograms of the RyR1 channel current amplitudes before (Blue) and after (Red) addition of PCBs. Bin width 0.1 pA, Y-axis are the normalized (Nor) counts relative to number of events. (C) Percent changes in the open probability (Po) of the PCB exposed channel relative to the Po under baseline (control) conditions. Mean ± SD; PCB 202 n = 6; PCB 197 at 200 pM n = 3 and PCB 197 at 200 nM n = 4,***P ≤ .001 relative to baseline (100%) as determined with a paired sample t-test. (Full color version available online).
Consistent with [3H]Ry binding results, PCB 197 displayed a much lower potency in single-channel recordings as compared with PCB 202. PCB 197 at 200 pM did not have detectable influence (representative traces not shown) on channel gating activity, where neither channel current amplitude distributions (Figure 4B) nor Po (Figure 4C) were significantly different from the baseline recording period. Overall, 200 nM of PCB 197 was required to appreciably shift channel current amplitude distributions and channel current amplitudes to a level similar to that produced by 200 pM PCB 202 (Figure 4C). Here, PCB 197 caused a mean change in Po of 350% as compared with the baseline Po. There was a large amount of variability in the change induced by PCB 197 at 200 nM (Mean Po= 353.6%; pairwise SD = 259.3%; paired sample t-test; P = .15).
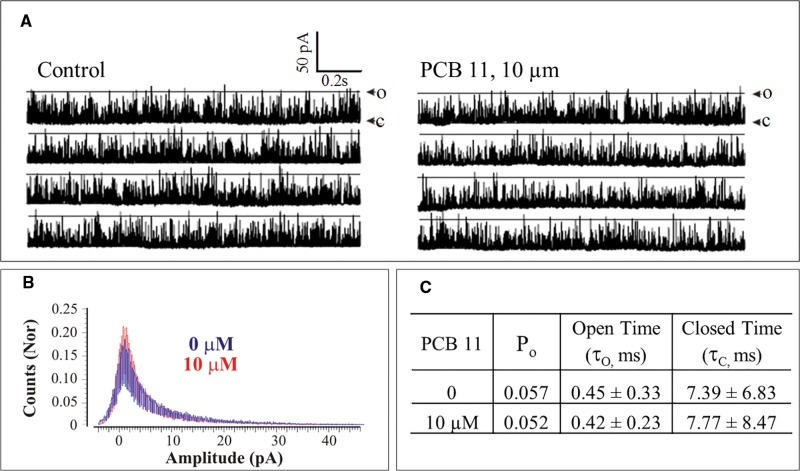
As demonstrated in [3H]Ry binding assays, PCB 11 at 10 µM did not affect channel Po (103% of baseline activity; n = 2; Figure 5). To further confirm lack of RyR activity, we measured the influence of PCB 11 on macroscopic Ca2+ fluxes across microsomes enriched in SERCA pumps and RyR1 channels, which act dynamically to accumulate and release Ca2+ across the microsomal vesicles. As shown in Supplementary Figure 6, after the Ca2+ loading phase of 3 sequential additions of 45 nmol Ca2+ to each cuvette (Supplemental Methods), the addition of DMSO or PCB 11 did not trigger release of accumulated Ca2+ by the vesicles in the presence of SERCA activity. PCB 95, a RyR1 activator, and ruthenium red, a RyR1 blocker, were then added to the DMSO cuvette as a positive and negative control for RyR1 function, respectively. The QSAR predicted that PCB 11 would have minimal RyR-activity (predicted EC2X of ∼10 µM) but with the current findings PCB 11 would be considered inactive supporting a minimal mono-ortho substitution for RyR activity.
FIG. 5.
Analysis of single RyR1 channel gating behavior before and after exposure to PCB 11 on the cytoplasmic side of the channel. (A) Representative traces at baseline and in the presence of PCB 11. Abbreviations: open channel state (O); closed channel state (C). (B) Frequency histograms of the RyR1 channel current amplitudes before (Blue) and after (Red) addition of PCB 11. Bin width 0.1 pA, Y-axis are the normalized (Nor) counts relative to number of events. (C) Summary analysis of kinetic parameters for the channel shown in panel A in the absence or presence of PCB 11: the open probability (Po), mean open dwell time (τo), and mean closed dwell time (τC). This experiment was replicated twice with similar results. (Full color version available online).
DISCUSSION
The current study expanded and supported the SAR for NDL PCBs towards the RyR, an important target linked to neurotoxicity. We demonstrate that in vitro [3H]Ry-binding assays are highly reproducible and likely act as conservative measures of RyR-activation. Specifically, we found that pM levels of PCBs can alter receptor channel gating behavior. So far, 49 of the 209 PCB congeners have been tested for RyR activity. Of the 49 tested, 42 alter RyR activity (current work and Pessah et al., 2006); however; the total number of PCBs, and other noncoplanar compounds, including PBDEs and hydroxylated-PCBs (Fritsch and Pessah, 2013; Kim et al., 2010; Niknam et al., 2013), that could contribute to RyR mediated toxicity in environmental mixtures, is probably higher (Rayne and Forest, 2010). Work suggests that RyR-active PCBs and PBDEs display additivity at the receptor (Fritsch and Pessah, 2013; Kostyniak et al., 2005; Pessah et al., 2006) demonstrating that the Ca2+ channel is likely a common target of contaminants mixtures currently found in the environment.
The prediction of the QSAR developed by Rayne and Forest (2010) were supported in the current study. Tetra-ortho PCB 202 was found to be the most potent congener identified to date instead of the previously recognized tri-ortho PCB 95. However, RyR1 mediated toxicity is differentially affected by PCB enantiomers (Pessah et al., 2009). There are 19 chiral PCBs, including PCB 95, and thus 1 enantiomer of PCB 95 may display a greater potency toward the RyR then that of the nonchiral PCB 202. The current work also demonstrated higher potency than that outlined by QSAR predictions suggesting that the QSAR predictions may act as a conservative measure of PCB mixture toxicity. Several congener’s experimental potencies did not match QSAR predictions, including PCB 179 and 197 which both displayed lower potency than predicted. It is suggested that too much emphasis may have been placed on ortho substitution because the QSAR predicted that potency was directly related to chlorine number whereby higher chlorinated congeners automatically have more ortho substitution (Rayne and Forest, 2010). Experimental data demonstrate the importance of ortho with adjacent meta-substitution (eg, PCB 194 vs PCB 197, Figure 1) and a new QSAR with emphasis on chlorines in both positions may provide even more predictive potency values.
The SAR for RyR active PCBs developed using [3H]Ry binding assays is consistent with the SAR observed in single channel voltage clamp analysis, demonstrating the predictive power of the SAR found in [3H]Ry binding assays. However, whereas the SAR was consistent our findings suggest that the [3H]Ry binding assay significantly underestimates the true potency of NDL PCBs towards RyR because the 2 assays demonstrated different sensitivities to NDL PCBs. The difference in potency; 200 pM or 50 nM of PCB 202 altered receptor gating or receptor ligand binding, respectively, may be due to the different endpoints being measured. The [3H]Ry binding analysis represents the activity of the receptors in a tissue extract after reaching saturation but BLM recording represents activation and monitoring of a single channel in real-time. [3H]Ry binding assessments also contain more microsomal lipid in the aqueous assay, which would appreciably reduce the free PCBs. In single channel voltage clamp the total lipid composition would be at least 6- to 24-fold lower (JSR concentrations; 24 µg/ml in [3H]Ry assays vs 1–4 µg/ml in BLM) presenting an insignificant effect on NDL PCB concentration when introduced to the cytoplasmic side of the channel.
The findings that NDL PCBs increase the open probability of the RyR are consistent with previous studies with NDL PCBs and other RyR-active compounds, namely Bastadin 10 (Chen et al., 1999; Pessah et al., 2009; Samsó et al., 2009). But in the current study we show that much lower, environmentally relevant, concentrations of PCBs could lead to distinct changes in the gating behavior and functional regulation of RyR channels (Niknam et al., 2013; Pessah et al., 2009). Currently the direct site(s) of interaction between the RyR and NDL PCBs is unknown but it is thought to be within the FKBP12–RyR1 interaction domain because NDL PCB-induced RyR activity can be eliminated by pretreatment with the immunophilin drugs FK506 and rapamycin (Wong and Pessah, 1997). The FK506 binding protein 12 kDa (ie, FKBP12) is an accessory protein that stabilizes the conformational state of the RyR. Disruption of the FKBP12–RyR complex, through genetic or pharmacological manipulation, causes an increase in channel open probability and causes the channel to exhibit a sub-conductance state, or conductance below the channel’s maximum conductance, that leaks Ca2+ (Ahern et al., 1997; Bellinger et al., 2008; Zalk et al., 2007). Thus, it is suggested that the increased open probability caused by NDL PCB toxicity at the RyR would lead to channels that leak Ca2+ from the SR/ER stores due to the disrupted FKBP12–RyR complex and the changes in intracellular Ca2+ would contribute to altered neuromuscular function.
The current work focused on NDL PCB-activation of RyR1 using skeletal muscle preparations. Skeletal muscle may be a target of NDL PCB toxicity at the RyR (Niknam et al., 2013) but developmental neurotoxicity has arisen as the prime toxic outcome of concern for NDL PCB exposure. Here, we use the skeletal muscle as a model for NDL PCBs on neuronal systems because (1) it represents a large source of primarily 1 RyR channel, namely, RyR1, eliminating potential differences in concentration responses due to the presence of multiple channel types as seen in neuronal tissue, (2) RyR1 is highly expressed in the brain, (3) NDL PCB toxicity at the so-called cardiac isoform, RyR2, also highly expressed in the brain, follows the same SAR (Pessah et al., 2010), and (4) the impact of NDL PCBs on dendritic growth in cultured hippocampal neurons can be eliminated by siRNA knockdown of either RyR1 or RyR2 (Wayman et al., 2012b). NDL PCB activity at the RyR also follows the same SAR in different regions of the brain (Wong et al., 1997) but potency values were reported in the micromolar range. The difference in potency, as compared with the pM–nM range reported here, may be due to drastic differences in assay conditions. Specifically, Wong et al. (1997) used crude microsomal preparations, that were not purified through a differential sucrose gradient as in the current study, and [3H]Ry binding assays contained ∼700 µg/ml of protein. The [3H]Ry binding assays in the 1997 publication also utilized 10 µM CaCl2, versus 50 µM in the current study, where the RyR channel’s open state is highly sensitive to buffer Ca2+ levels (Pessah et al., 1987). Further assessments addressing NDL PCB activity at the RyR in different regions of the brain, but under the current [3H]Ry binding conditions, would strengthen the connection between the current SAR and predictions of neurotoxic risk. However, research has demonstrated that pM to low nM PCB 95 can alter dendritic growth in cultured hippocampal neurons (Wayman et al., 2012b; Yang et al., 2009) supporting the pM to nM toxicity range demonstrated in the current study.
Additional studies provide evidence that RyR in vitro toxicity could contribute to in vivo neurotoxic outcomes. Organisms exposed during development to PCB 95 and Aroclor 1254, a mixture with increased ortho-substituted PCBs, have altered dendritic arborization in the hippocampus (Yang et al., 2009) and altered RyR1 and RyR, isoform 2 (RyR2) expression in their cerebellum. In these studies, the exposed animals had altered learning and had PCB congeners in their brains that consisted of numerous RyR active congeners including PCB 99, 153, 170, 180, 183, and 187 but did not contain PCB 77, 126, and 169 (Yang et al., 2009). Another study with weanling mice exposed to a mixture of PCB 28, 52, 101, 138, 153, and 180 through lactation displayed age specific disruption in motor function and persistent anxiety like behavior (Elnar et al., 2012). These PCBs all display RyR activity and the weanlings displayed anxiety likely behavior that was correlated with the expression of RyR, isoform 3.
Polychlorinated biphenyls (PCB) concentrations found in human samples have also been correlated with cognitive or behavioral deficits in adults (Fitzgerald et al., 2008; Schantz et al., 2001) and children (Jacobson and Jacobson, 1996; Lonky et al., 1996; Stewart et al., 2008). Deficits in children have included lowered IQ scores, impaired learning and memory, and attention deficit (Jacobson and Jacobson, 1996; Lonky et al., 1996; Stewart et al., 2008). It is well recognized that PCB residues in these samples are complex mixtures but neurotoxic outcomes due to PCB mixture exposure have been largely ascribed to NDL congeners because they are found in relatively high concentrations in the brains of human and experimental animals that display neuropsychological deficits (Mitchell et al., 2012; Pessah et al., 2010) NDL PCBs also constitute the majority of the total PCB burden in both human and environmental samples (Hwang et al., 2001, 2006; Marek et al., 2013; Stahl et al., 2009) and further understanding the contribution of the RyR, alone or in combination with other modes of NDL PCB neurotoxicity (see “Introduction” section), would help further elucidate risks in human populations.
In addition to NDL PCB induced neurotoxicity, enhanced RyR activity has implications on the health and performance of nonneuronal systems (Niknam et al., 2013; Pessah et al., 2010). The RyR was first identified and characterized for its role in excitation-contraction coupling (EC) (Pessah et al., 1985), which is essential to the normal function of both cardiac and skeletal muscle. NDL PCBs disrupt important aspects of EC coupling in exposed skeletal myotubes demonstrating a direct impact on muscle performance (Niknam et al., 2013). The cardiac RyR-isoform, RyR2, is also sensitive to NDL PCBs (Pessah et al., 2009) but whether NDL PCB activity at RyR2 causes cardiac EC-coupling dysfunction requires further elucidation. Such studies may help explain the observed effects of PCB exposure that has been associated with an increase in cardiac physiological dysfunction (Bergkvist et al., 2015). Finally, the RyR and related Ca2+ signaling proteins have been demonstrated to have roles in diseases ranging from cardiac arrhythmias (Lacampagne et al., 2008) and malignant hypothermia (Robinson et al., 2006) to Alzheimer’s disease (Bruno et al., 2012; Stutzmann et al., 2006) and autism spectrum disorder (Pessah et al., 2010; Shelton et al., 2012; Stamou et al., 2013). Currently, it is unclear whether NDL PCB toxicity at the RyR may contribute to these disease states but the potential gene by environment interaction warrants further investigation.
CONCLUSION
As evidence regarding the impact of NDL PCBs mounts, numerous research groups have started developing and applying new risk assessment tools to define NDL PCB neurotoxic risk. This includes the development of a relative potency scheme called the neurotoxic equivalency (NEQ) scheme. The NEQ is similar to and would complement the well-known TCDD equivalency scheme (TEQ), commonly used to assess PCB mixture toxicity. Whereas still in its infancy, the NEQ faces several challenges before establishment in risk assessment. First, numerous modes of neurotoxicity have been documented (see “Introduction” section), often displaying different SAR requirements (eg, current work and Stenberg et al., 2011), and the question arises regarding what modes and how many modes should be included to establish an NEQ. To date the RyR is the most sensitive target of NDL PCBs and has been included in the development of NEQ schemes (Rayne and Forest, 2010; Simon et al., 2007). Additionally, few congeners have been tested in the various neurotoxic assays; making QSAR studies essential. Here, we evaluated and supported the predictions of a RyR-based neurotoxic QSAR that has been used to define NEQs for NDL PCBs (Rayne and Forest, 2010). As predicted, PCB 202 is a highly RyR potent PCB congener, and whereas it is detected in human and environmental samples, it likely does not represent a large percentage of total PCB burdens (Marek et al., 2013; Stahl et al., 2009). However, similar to TCDD in the TEQ scheme, PCB 202 could act as a reference compound for which PCB mixture toxicity toward the RyR is based. Therefore, additional in vitro and in vivo assessments, focused specifically on PCB 202, could provide valuable information for understanding or further modeling NDL PCB mixture induced neurotoxicity and these efforts would further establish the NEQ for application in risk assessment.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
ACKNOWLEDGMENT
The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH or USEPA. The authors declare no competing financial interests.
FUNDING
National Institute of Environmental Health Sciences (NIEHS) Superfund Research Program at UC Davis (INP and EBH; P42-ES004699); Iowa Superfund Research Program (HJL; P42- ES013661); National Heart Lung and Blood Institute (EBH; UC Davis T32-HL086350); NIEHS (1R01-ES014901, 1R01-ES017425); the UC Davis Center for Children’s Environmental Health (1P01-ES011269); U.S. Environmental Protection Agency through the Science to Achieve Results Program Grant (Nos. R829388 and R833292); IDDRC Core Center U54 HD079125.
REFERENCES
- Ahern G. P., Junankar P. R., Dulhunty A. F. (1997). Subconductance states in single-channel activity of skeletal muscle ryanodine receptors after removal of FKBP12. Biophys. J. 72, 146.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M. R. (2014). Endocrine-disrupting actions of PCBs on brain development and social and reproductive behaviors. Curr. Opin. Pharmacol. 19, 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger A. M., Mongillo M., Marks A. R. (2008). Stressed out: The skeletal muscle ryanodine receptor as a target of stress. J. Clin. Investig. 118, 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergkvist C., Berglund M., Glynn A., Wolk A., Åkesson A. (2015). Dietary exposure to polychlorinated biphenyls and risk of myocardial infarction—A population-based prospective cohort study. Int. J. Cardiol. 183, 242–248. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. 2012. Cell signaling pathways—Module 2. Cell Signal. Biol. 2, 1–130. [Google Scholar]
- Boix J., Cauli O., Leslie H., Felipo V. (2011). Differential long-term effects of developmental exposure to polychlorinated biphenyls 52, 138 or 180 on motor activity and neurotransmission. Gender dependence and mechanisms involved. Neurochem. Int. 58, 69–77. [DOI] [PubMed] [Google Scholar]
- Bruno A. M., Huang J. Y., Bennett D. A., Marr R. A., Hastings M. L., Stutzmann G. E. (2012). Altered ryanodine receptor expression in mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging 33, 1001.e1001– 1001.e1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Molinski T. F., Pessah I. N. (1999). Bastadin 10 stabilizes the open conformation of the ryanodine-sensitive Ca2+ channel in an FKBP12-dependent Manner. J. Biol. Chem. 274, 32603–32612. [DOI] [PubMed] [Google Scholar]
- Choi S. Y., Lee K., Park Y., Lee S. H., Jo S. H., Chung S., Kim K. T. (2016). Non-dioxin-like polychlorinated biphenyls inhibit G-protein coupled receptor-mediated Ca2+ signaling by blocking store-operated Ca2+ entry. PloS One 11, e0150921.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson S. M., Guevara E., Woller M. J., Gore A. C. (2009). Cell death mechanisms in GT1-7 GnRH cells exposed to polychlorinated biphenyls PCB74, PCB118, and PCB153. Toxicol. Appl. Pharmacol. 237, 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo J. L., Bocio A. (2007). Levels of PCDD/PCDFs and PCBs in edible marine species and human intake: A literature review. Environ. Int. 33, 397–405. [DOI] [PubMed] [Google Scholar]
- Elnar A. A., Diesel B., Desor F., Feidt C., Bouayed J., Kiemer A. K., Soulimani R. (2012). Neurodevelopmental and behavioral toxicity via lactational exposure to the sum of six indicator non-dioxin-like-polychlorinated biphenyls (∑6 NDL-PCBs) in mice. Toxicology 299, 44–54. [DOI] [PubMed] [Google Scholar]
- Faroon O., Ruiz P. 2015. Polychlorinated biphenyls: New evidence from the last decade. Toxicol. Ind. Health. doi: 10.1177/0748233715587849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes E. C. A., Hendriks H. S., van Kleef R. G., Reniers A., Andersson P. L., van den Berg M., Westerink R. H. (2010). Activation and Potentiation of Human GABAA Receptors by Non-Dioxin–Like PCBs Depends on Chlorination Pattern. Toxicol. Sci 118, 183–190. [DOI] [PubMed] [Google Scholar]
- Fitzgerald E. F., Belanger E. E., Gomez M. I., Cayo M., McCaffrey R. J., Seegal R. F., Jansing R. L., Hwang S., et al. (2008). Polychlorinated biphenyl exposure and neuropsychological status among older residents of upper Hudson River communities. Environ. Health Perspect 116, 209.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F., Mariussen E. (2009). Mechanisms involved in the neurotoxic effects of environmental toxicants such as polychlorinated biphenyls and brominated flame retardants. J. Neurochem. 111, 1327–1347. [DOI] [PubMed] [Google Scholar]
- Frame G. E. 2001. The curent state-of-the-art of comprehensive, quantitative, congener-specific PCB analysis, and what we now know about the distribution of individual congeners in commercial aroclor mixtures In PCBs, Recent Advances in Environmental Toxicology and Health Effects ( Hansen L. G., L. W. Robertson, Eds.). University Press of Kentuky, Lexington. [Google Scholar]
- Fritsch E. B., Pessah I. N. (2013). Structure–activity relationship of non-coplanar polychlorinated biphenyls toward skeletal muscle ryanodine receptors in rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 140–141, 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni J., Wong P. W., Pessah I. N. (2004). Non-coplanar 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95) amplifies ionotropic glutamate receptor signaling in embryonic cerebellar granule neurons by a mechanism involving ryanodine receptors. Toxicol. Sci. 77, 72–82. [DOI] [PubMed] [Google Scholar]
- Grimm F. A., Hu D., Kania-Korwel I., Lehmler H. J., Ludewig G., Hornbuckle K. C., Duffel M. W., Bergman Å., et al. (2015). Metabolism and metabolites of polychlorinated biphenyls. Crit. Rev. Toxicol. 45, 245–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang H. M., Green P. G., Young T. M. (2006). Tidal salt marsh sediment in California, USA. Part 1: Occurrence and sources of organic contaminants. Chemosphere 64, 1383–1392. [DOI] [PubMed] [Google Scholar]
- Hwang S. A., Yang B. Z., Fitzgerald E. F., Bush B., Cook K. (2001). Fingerprinting PCB patterns among Mohawk women. J. Exposure Anal. Environ. Epidemiol. 11, 184–192. [DOI] [PubMed] [Google Scholar]
- Jacobson J. L., Jacobson S. W. (1996). Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N. Engl. J. Med. 335, 783–789. [DOI] [PubMed] [Google Scholar]
- Kim K. H., Bose D. D., Ghogha A., Riehl J., Zhang R., Barnhart C. D., Lein P. J., Pessah I. N. (2010). Para- and ortho-substitutions are key determinants of polybrominated diphenyl ether activity toward ryanodine receptors and neurotoxicity. Environ. Health Perspect. 119, 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti P. R. S., Curras-Collazo M. C. (2010). Neuroendocrine actions of organohalogens: Thyroid hormones, arginine vasopressin, and neuroplasticity. Front. Neuroendocrinol. 31, 479–496. [DOI] [PubMed] [Google Scholar]
- Kodavanti P. R. S., Tilson H. A. (1997). Structure-activity relationships of potentially neurotoxic PCB congeners in the rat. Neurotoxicology 18, 425–442. [PubMed] [Google Scholar]
- Koh W. X., Hornbuckle K. C., Thorne P. S. (2015). Human serum from urban and rural adolescents and their mothers shows exposure to polychlorinated biphenyls not found in commercial mixtures. Environ. Sci. Technol. 49, 8105–8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyniak P. J., Hansen L. G., Widholm J. J., Fitzpatrick R. D., Olson J. R., Helferich J. L., Kim K. H., Sable H. J. K., et al. (2005). Formulation and characterization of an experimental PCB mixture designed to mimic human exposure from contaminated fish. Toxicol. Sci. 88, 400–411. [DOI] [PubMed] [Google Scholar]
- Lacampagne A., Fauconnier J., Richard S. (2008). Ryanodine receptor and heart disease. Méd. Sci. 24, 399–405.. [DOI] [PubMed] [Google Scholar]
- Lehmler H. J., Harrad S. J., Hühnerfuss H., Kania-Korwel I., Lee C. M., Lu Z., Wong C. S. (2010). Chiral polychlorinated biphenyl transport, metabolism, and distribution: A review. Environ. Sci. Technol. 44, 2757–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienthal H., Korkalainen M., Andersson P. L., Viluksela M. (2015). Developmental exposure to purity-controlled polychlorinated biphenyl congeners (PCB74 and PCB95) in rats: Effects on brainstem auditory evoked potentials and catalepsy. Toxicology 327, 22–31. [DOI] [PubMed] [Google Scholar]
- Lonky E., Reihman J., Darvill T., Mather J., Daly H. (1996). Neonatal behavioral assessment scale performance in humans influenced by maternal consumption of environmentally contaminated Lake Ontario fish. J. Great Lakes Res. 22, 198–212. [Google Scholar]
- Marek R. F., Thorne P. S., Wang K., DeWall J., Hornbuckle K. C. (2013). PCBs and OH-PCBs in serum from children and mothers in urban and rural US communities. Environ. Sci. Technol. 47, 3353–3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariussen E., Fonnum F. (2001). The effect of polychlorinated biphenyls on the high affinity uptake of the neurotransmitters, dopamine, serotonin, glutamate and GABA, into rat brain synaptosomes. Toxicology 159, 11–21. [DOI] [PubMed] [Google Scholar]
- Mitchell M. M., Woods R., Chi L. H., Schmidt R. J., Pessah I. N., Kostyniak P. J., LaSalle J. M. (2012). Levels of select PCB and PBDE congeners in human postmortem brain reveal possible environmental involvement in 15q11-q13 duplication autism spectrum disorder. Environ. Mol. Mutag. 53, 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niknam Y., Feng W., Cherednichenko G., Dong Y., Joshi S. N., Vyas S. M., Lehmler H. J., Pessah I. N. (2013). Structure-activity relationship of selected meta- and para-hydroxylated non-dioxin like polychlorinated biphenyls: From single RyR1 channels to muscle dysfunction. Toxicol. Sci. 136, 500–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill E. R., Sakowska M. M., Laver D. R. (2003). Regulation of the calcium release channel from skeletal muscle by suramin and the disulfonated stilbene derivatives DIDS, DBDS, and DNDS. Biophys. J. 84, 1674–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah, Cherednichenko G., Lein P. J. (2010). Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol. Ther. 125, 260–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah I. N., Hansen L. G., Albertson T. E., Garner C. E., Ta T. A., Do Z., Kim K. H., Wong P. W. (2006). Structure−activity relationship for noncoplanar polychlorinated biphenyl congeners toward the ryanodine receptor-Ca2+ channel complex type 1 (RyR1). Chem. Res. Toxicol. 19, 92–101. [DOI] [PubMed] [Google Scholar]
- Pessah I. N., Lehmler H. J., Robertson L. W., Perez C. F., Cabrales E., Bose D. D., Feng W. (2009). Enantiomeric specificity of (−)-2,2′,3,3′,6,6′-hexachlorobiphenyl toward ryanodine receptor types 1 and 2. Chem. Res. Toxicol. 22, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah I. N., Stambuk R. A., Casida J. E. (1987). Ca2+-activated ryanodine binding: Mechanisms of sensitivity and intensity modulation by Mg2+, caffeine, and adenine nucleotides. Mol. Pharmacol. 31, 232–238. [PubMed] [Google Scholar]
- Pessah I. N., Waterhouse A. L., Casida J. E. (1985). The calcium-ryanodine receptor complex of skeletal and cardiac muscle. Biochem. Biophys. Res. Commun. 128, 449–456. [DOI] [PubMed] [Google Scholar]
- Rayne S., Forest K. (2010). Quantitative structure-activity relationship (QSAR) studies for predicting activation of the ryanodine receptor type 1 channel complex (RyR1) by polychlorinated biphenyl (PCB) congeners. J. Environ. Sci. Health A 45, 355–362. [DOI] [PubMed] [Google Scholar]
- Robinson R., Carpenter D., Shaw M. A., Halsall J., Hopkins P. (2006). Mutations in RYR1 in malignant hyperthermia and central core disease. Hum. Mutat. 27, 977–989. [DOI] [PubMed] [Google Scholar]
- Samsó M., Feng W., Pessah I. N., Allen P. (2009). Coordinated movement of cytoplasmic and transmembrane domains of RyR1 upon gating. PLoS Biol. 7, e1000085.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz S., Seo B., Wong P., Pessah I. (1997). Long-term effects of developmental exposure to 2, 2', 3, 5', 6-pentachlorobiphenyl (PCB 95) on locomotor activity, spatial learning and memory and brain ryanodine binding. Neurotoxicology 18, 457–467.. [PubMed] [Google Scholar]
- Schantz S. L., Gasior D. M., Polverejan E., McCaffrey R. J., Sweeney A. M., Humphrey H., Gardiner J. C. (2001). Impairments of memory and learning in older adults exposed to polychlorinated biphenyls via consumption of Great Lakes fish. Environ. Health Perspect. 109, 605–611.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton J. F., Hertz-Picciotto I., Pessah I. N. (2012). Tipping the balance of autism risk: Potential mechanisms linking pesticides and autism. Environ. Health Perspect. 120, 944–951.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon T., Britt J. K., James R. C. (2007). Development of a neurotoxic equivalence scheme of relative potency for assessing the risk of PCB mixtures. Regul. Toxicol. Pharmacol. 48, 148–170. [DOI] [PubMed] [Google Scholar]
- Stahl L., Snyder B., Olsen A., Pitt J. (2009). Contaminants in fish tissue from US lakes and reservoirs: A National Probabilistic Study. Environ. Monit. Assess. 150, 3–19. [DOI] [PubMed] [Google Scholar]
- Stamou M., Streifel K. M., Goines P. E., Lein P. J. (2013). Neuronal connectivity as a convergent target of gene × environment interactions that confer risk for Autism Spectrum Disorders. Neurotoxicol. Teratol. 36, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg M., Hamers T., Machala M., Fonnum F., Stenius U., Lauy A. A., van Duursen M. B., Westerink R. H., et al. (2011). Multivariate toxicity profiles and QSAR modeling of non-dioxin-like PCBs—An investigation of in vitro screening data from ultra-pure congeners. Chemosphere 85, 1423–1429. [DOI] [PubMed] [Google Scholar]
- Stewart P. W., Lonky E., Reihman J., Pagano J., Gump B. B., Darvill T. (2008). The relationship between prenatal PCB exposure and intelligence (IQ) in 9-year-old children. Environ. Health Perspect. 116, 1416–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann G. E., Smith I., Caccamo A., Oddo S., LaFerla F. M., Parker I. (2006). Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer's disease mice. J. Neurosci. 26, 5180–5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman G. A., Bose D. D., Yang D., Lesiak A., Bruun D., Impey S., Ledoux V., Pessah I. N., et al. (2012a). PCB-95 modulates the calcium-dependent signaling pathway responsible for activity-dependent dendritic growth. Environ. Health Perspect. 120, 1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman G. A., Yang D., Bose D. D., Lesiak A., Ledoux V., Bruun D., Pessah I. N., Lein P. J. (2012b). PCB-95 promotes dendritic growth via ryanodine receptor-dependent mechanisms. Environ. Health Perspect. 120, 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerink R. H. (2014). Modulation of cell viability, oxidative stress, calcium homeostasis, and voltage-and ligand-gated ion channels as common mechanisms of action of (mixtures of) non-dioxin-like polychlorinated biphenyls and polybrominated diphenyl ethers. Environ. Sci. Pollut. Res. 21, 6373–6383. [DOI] [PubMed] [Google Scholar]
- Wigestrand M. B., Stenberg M., Walaas S. I., Fonnum F., Andersson P. L. (2013). Non-dioxin-like PCBs inhibit [3H]WIN-35,428 binding to the dopamine transporter: A structure–activity relationship study. Neurotoxicology 39, 18–24. [DOI] [PubMed] [Google Scholar]
- Wong P. W., Brackney W. R., Pessah I. N. (1997). Ortho-substituted polychlorinated biphenyls alter microsomal calcium transport by direct interaction with ryanodine receptors of mammalian brain. J. Biol. Chem. 272, 15145–15153. [DOI] [PubMed] [Google Scholar]
- Wong P. W., Pessah I. N. (1997). Noncoplanar PCB 95 alters microsomal calcium transport by an immunophilin FKBP12-dependent mechanism. Mol. Pharmacol. 51, 693–702. [DOI] [PubMed] [Google Scholar]
- Yang D., Kim K. H., Phimister A., Bachstetter A. D., Ward T. R., Stackman R. W., Mervis R. F., Wisniewski A. B., et al. (2009). Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ. Health Perspect. 117, 426–435.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalk R., Lehnart S. E., Marks A. R. (2007). Modulation of the ryanodine receptor and intracellular calcium. Annu. Rev. Biochem. 76, 367–385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.