Abstract
Long non-coding RNAs (lncRNAs) are over 200 nucleotides in length and are transcribed from the mammalian genome in a tissue-specific and developmentally regulated pattern. There is growing recognition that lncRNAs are novel biomarkers and/or key regulators of toxicological responses in humans and animal models. Lacking protein-coding capacity, the numerous types of lncRNAs possess a myriad of transcriptional regulatory functions that include cis and trans gene expression, transcription factor activity, chromatin remodeling, imprinting, and enhancer up-regulation. LncRNAs also influence mRNA processing, post-transcriptional regulation, and protein trafficking. Dysregulation of lncRNAs has been implicated in various human health outcomes such as various cancers, Alzheimer’s disease, cardiovascular disease, autoimmune diseases, as well as intermediary metabolism such as glucose, lipid, and bile acid homeostasis. Interestingly, emerging evidence in the literature over the past five years has shown that lncRNA regulation is impacted by exposures to various chemicals such as polycyclic aromatic hydrocarbons, benzene, cadmium, chlorpyrifos-methyl, bisphenol A, phthalates, phenols, and bile acids. Recent technological advancements, including next-generation sequencing technologies and novel computational algorithms, have enabled the profiling and functional characterizations of lncRNAs on a genomic scale. In this review, we summarize the biogenesis and general biological functions of lncRNAs, highlight the important roles of lncRNAs in human diseases and especially during the toxicological responses to various xenobiotics, evaluate current methods for identifying aberrant lncRNA expression and molecular target interactions, and discuss the potential to implement these tools to address fundamental questions in toxicology.
Keywords: gene regulation, toxicoepigenomics, long non-coding RNAs.
The Encyclopedia of DNA Elements (ENCODE) Project revealed that less than 2% of the human genome is coded into proteins (Djebali et al., 2012; ENCODE Project Consortium, 2012). The functional transcripts longer than 200 nucleotides that do not translate into proteins are referred to as long non-coding RNAs (lncRNAs). Previously thought to be transcriptional noise, lncRNAs have been recently found to be integral to human health and disease. The NONCODE database has identified over 90 000 human lncRNA genes and over 140 000 human lncRNA transcripts (Zhao et al., 2016a). LncRNAs have diverse regulatory functions in cell physiology, including chromatin remodeling, gene transcription and translation, protein trafficking, and cellular signaling (Geisler and Coller, 2013; Karlsson and Baccarelli, 2016). Dysregulation of lncRNAs due to environmental exposures, genetic mutations, or other causes are associated with various human diseases, such as cancer, neurological disorders, and metabolic syndrome (Geisler and Coller, 2013). Here, we summarize the current literature on the biological functions of lncRNAs, examine their roles in human diseases and toxicological responses to xenobiotics, and evaluate the current methods to determine lncRNA expression and functions.
LNCRNA BIOGENESIS AND BIOLOGICAL FUNCTIONS
Biogenesis
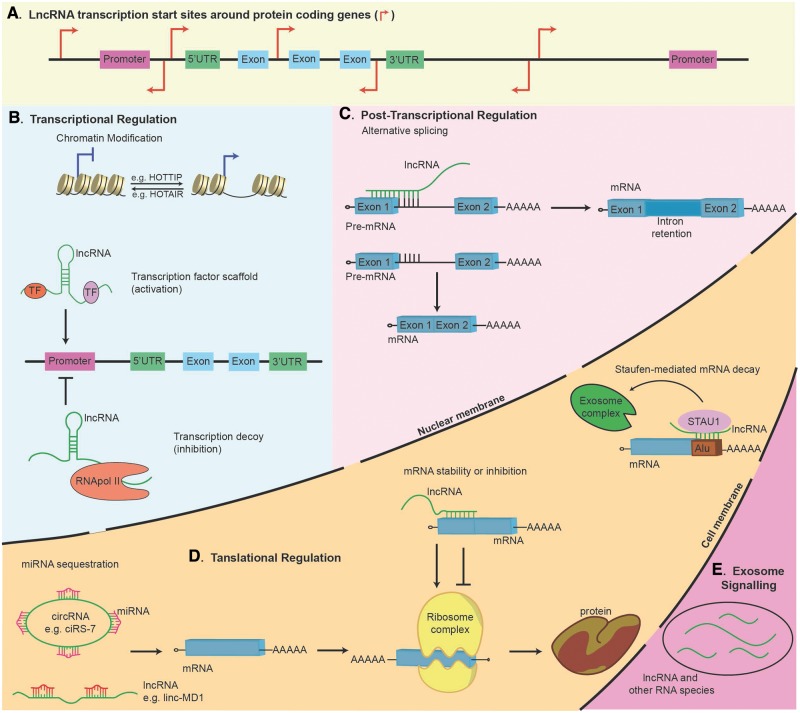
LncRNAs can be transcribed from either the Watson or the Crick DNA strand and can be intergenic, exonic, in enhancer regions, or in regions distal to protein-coding genes (Figure 1A) (Geisler and Coller, 2013; Wang and Chang, 2011). Similar to mRNAs, lncRNAs are transcribed by RNA polymerase II (RNApol II), can undergo alternative splicing, may carry single nucleotide polymorphisms (SNPs), have 5′ caps, and are usually polyadenylated (Derrien et al., 2012; Karlsson and Baccarelli, 2016). It has been estimated that the majority of lncRNAs have more than two exons, and can have secondary and tertiary structures (Derrien et al., 2012; Novikova et al., 2012).
FIG. 1.
LncRNA transcriptional start sites and examples of lncRNA functions. (A) LncRNAs are transcribed from the Watson or Crick strand and the arrows represent lncRNA transcriptional start sites that are intergenic, exonic, in enhancer regions, or in regions distal to protein-coding genes. (B) LncRNAs, such as HOTAIR and HOTTIP, modify nuclear architecture through organization of histone modification proteins (top), whereas other lncRNAs (bottom) influence transcription by localizing transcription factors or inhibiting RNA polymerase II. (C) LncRNAs regulate alternative splicing of nascent protein-coding transcripts by intron retention. (D) LncRNAs modulate translation through miRNA sequestration (left), interacting with mRNA to increase transcript stability or inhibit translation (middle), and mRNA turnover (right). (E) LncRNAs are also emerging as possible extracellular signals in exosomal shuttle RNA to regulate the biological functions of neighboring or distal cells.
LncRNAs have their own promoters, unique DNA-binding motifs, and preferred transcription factors (TFs) (Alam et al., 2014; Derrien et al., 2012; Popadin et al., 2013). Computational analysis of RNA-Seq data has suggested that lncRNA transcription may be independent from and influence the transcription of neighboring protein-coding genes, and may have more cis expression quantitative trait loci than protein-coding genes (Derrien et al., 2012; Popadin et al., 2013). LncRNAs are also regulated by epigenetic mechanisms including DNA methylation (Popadin et al., 2013). Only 11–29% of lncRNAs are ubiquitously expressed in all tissues, as compared with 65% of protein-coding genes, and lncRNAs are generally expressed at much lower levels than protein-coding mRNAs (Derrien et al., 2012; Djebali et al., 2012).
Enhancer RNAs (eRNAs) are a category of ncRNAs (of which a subset are lncRNAs) derived from enhancer regions of genes, and they interact with DNA to up-regulate gene transcription through two possible mechanisms: enhancer–promoter looping and tracking of transcriptional machinery (Li et al., 2016a). For example, the AS1eRNA mediates the spatial interactions with the lncRNA gene DHRS4-AS1 promoter by chromosomal looping to enhance transcription (Yang et al., 2016). This suggests that eRNAs contribute to enhancer function and influence the transcription of genes, including lncRNAs.
There is no unified nomenclature for lncRNAs; therefore, we refer to lncRNAs by the abbreviated names given by the originating authors with the full names and NONCODE gene IDs (if available) in Table 1.
TABLE 1.
Selected lncRNA Names and NONCODE Gene ID
| Abbreviation | Name | NONCODE Gene ID |
|---|---|---|
| AGAP2-AS1 | ADP-ribosylation factor GTPase activating protein 2-antisense RNA 1 | NONHSAG011446.2 |
| AT102202 | NONHSAG040743.2 | |
| BACE1-AS | β-secretase-1 antisense | NONHSAG009728.2 |
| CASC2 | Cancer susceptibility candidate 2 | NONHSAG006966.2 |
| CCAT1 | Colon cancer associated transcript 1 | |
| CDKN2B-AS1 | Cyclin-dependent kinase inhibitor 2B | NONHSAG051899.2 |
| ciRS-7 | Cerebellar degeneration-related protein 1 antisense circRNA | NONHSAG055442.2 |
| DHRS4-AS1 | Dehydrogenase/reductase (SDR Family) member 4 antisense 1 | NONHSAG014515.2 |
| DILC | Down-regulated in liver cancer stem cells | |
| DYNLRB2-2 | Dynein light chain roadblock-type 2-2 | NONHSAG020076.2 |
| E330013P06 (CARMN) | Cardia mesoderm enhancer-associated non-coding RNA | NONHSAG041903.2 |
| FIRRE | Functional intergenic repeating RNA element | |
| FLJ33630 (LINC01184) | Long intergenic non-protein coding RNA 1184 | |
| GABPB1-AS1 | GA binding protein transcription factor beta 1 antisense RNA 1 | NONHSAG016861.2 |
| GAS5 | Growth arrest specific 5 | NONHSAG003504.2 |
| H19 | Imprinted maternally expressed transcript | NONHSAG007409.2 |
| HAR1 | Human accelerated region 1 | NONHSAG032267.2 |
| HI-LNC25 | Human islet long non-coding RNA 25 | |
| HOTAIR | HOX transcript antisense RNA | NONHSAG011264.2 |
| HOTTIP | HOXA distal transcript antisense RNA | NONHSAG047197.2 |
| HULC | Highly up-regulated in liver cancer | NONHSAG042970.2 |
| LINC00152 | Loong intergenic non-protein coding RNA 152 | NONHSAG028438.2 |
| LINC01133 | Long intergenic non-protein coding RNA 1133 | NONHSAG001133.2 |
| linc-MD1 | Long intergenic non-protein coding RNA muscle differentiation 1 | NONMMUG000202.2 |
| LINK-A | Long intergenic non-coding RNA for kinase activation | |
| lnc-HC | LncRNA up-regulated by high cholesterol | |
| lncLGR | Long non-coding RNA liver glucokinase repressor | |
| lncLSTR | Liver-specific triglyceride regulator | |
| lncPHx2 | Long non-coding RNA induced by partial hepatectomy 2 | |
| lncRNA-422 | NONHSAG032910.2 | |
| LOC728228 | Long intergenic non-protein coding RNA 1433 | |
| MALAT1 | Metastasis-associated lung adenocarcinoma transcript 1 | NONHSAG008675.2 |
| MDC1-AS1 | Mediator of DNA damage checkpoint 1 anti-sense | NONHSAG043427.2 |
| MEG3 | Maternally expressed gene 3 | NONHSAG015923.2 |
| MIAT | Myocardial infarction associated transcript | NONHSAG033587.2 |
| MIR31HG | MicroRNA31 host gene | NONHSAG051889.2 |
| NBR2 | Neighbor of BRCA1 gene 2 | NONHSAG021874 |
| NEAT1 | Neuro-protective nuclear enriched abundant transcript 1 | NONHSAG008670.2 |
| NR_045623 | Nucleic acid bind protein variant 4 | |
| NRG1 | Nickel-related gene 1 | |
| PANDA | Promoter of CDKN1A antisense DNA damage activated RNA | NONHSAG094129.1 |
| PCGEM1 | Prostate-specific transcript 1 | NONHSAG030128.2 |
| PRAL | p53 regulation associated lncRNA | |
| PRINS | Psoriasis associated non-protein coding RNA induced by stress | NONHSAG005407.2 |
| PVT1 | Plasmacytoma Variant Translocation 1 | NONHSAG051258.2 |
| RIAN | RNA imprinted and accumulated in nucleus | NONHSAG015927.2 |
| RNCR3 | Retinal non-coding RNA 3 | NONMMUG013211.2 |
| RP5-833A20.1 | Nuclear factor I A antisense RNA 1 | NONHSAG001661.2 |
| SAL-RNA1 | Senescence-associated lncRNA 1 | |
| SBF2-AS1 | SET binding factor 2-antisense RNA 1 | NONHSAG007631.2 |
| SCAL1 | Smoke and cancer associated lncRNA | NONHSAG041002.2 |
| Scarna3a | Small cajal body-specific RNA 3a | |
| Sra | Steroid receptor RNA Activator | |
| TRIBAL | Tribbles pseudokinase 1-associated locus | |
| Tsix | X-inactive specific transcript antisense | NONHSAG054779.2 |
| TUG1 | Taurine up-regulated 1 | NONHSAG033691.2 |
| TUNA | Tcl1 upstream neuron-associated | NONHSAG015808.2 |
| UCA1 | Urothelial carcinoma 1 | NONHSAG025011.2 |
| XIST | X-inactive specific transcript | NONHSAG54780.2 |
Transcriptional Regulation
As shown in Figure 1B, lncRNAs are epigenetic modifiers of transcription through histone methylation and the subsequent modulation of chromatin structure. Among various types of histone methylation patterns, histone H3 lysine-4 (H3K4) methylation is an active mark for transcriptional activation, whereas H3K27 tri-methylation (H3K27Me3) is a suppressive mark for gene silencing. The lncRNA HOTAIR provides an epigenetic-protein scaffold with multiple binding domains for distinct proteins. At the 3′ end, HOTAIR contributes to the demethylation of H3K4 by interacting with lysine-specific histone demethylase 1A (LSD1), restrictive element 1-silencing transcription factor (REST), and REST corepressor 1. At the 5′ end, evidence from the homeobox D (HOXD) gene cluster shows that HOTAIR leads to transcriptional gene silencing by recruiting the polycomb repressive complex 2 (PRC2) and reinforcing H3K27Me3 (Wang and Chang, 2011). In contrast, evidence from the HOXA gene cluster shows that the lncRNA HOTTIP promotes gene transcriptional activation by methylating H3K4 (Figure 1B) (Wang et al., 2011). Other lncRNAs, such as XIST and FIRRE, alter 3D nuclear architecture (Mele and Rinn, 2016). Transcription of XIST from one X-chromosome spreads in cis to initiate X-chromosome inactivation, and FIRRE modulates nuclear architecture across chromosomes by localizing five trans-chromosomal loci in proximity to the X-chromosome (Augui et al., 2011; Hacisuleyman et al., 2014). Knowing that transcription and nuclear architecture are linked, it was proposed that lncRNA transcription contributes to nuclear organization in the 3D genome through a molecular “cat’s cradle” model, which suggests that during cell fate transitions, transcription of cell-type-specific lncRNAs produces new binding sites for proteins to reshape the 3D organization of the genome (Mele and Rinn, 2016).
Transcriptional inhibition can occur through lncRNA decoys that bind to RNApol II, such as the short interspersed response element Alu transcripts during heat shock (Figure 1B), or a lncRNA-DNA triplex obstructing the assembly of the pre-initiation complex (Mariner et al., 2008; Martianov et al., 2007). However, in the Saccharomyces cerevisae galactose (GAL) gene cluster, GAL lncRNAs form lncRNA-DNA hybrids (R-loops) on the GAL gene promoter, activating transcription (Cloutier et al., 2016). LncRNAs also bind and localize transcription factors for gene activation or repression (Martens et al., 2004; Willingham et al., 2005). After the formation of the nascent transcripts from the protein-coding genes, lncRNAs can regulate mRNA processing through alternative splicing (Figure 1C) and other RNA-editing processes (Faghihi and Wahlestedt, 2009).
Translational Regulation and Signaling
Competitive endogenous RNAs (ceRNAs), which are lncRNAs that sequester miRNAs and inhibit miRNA functions, have two structurally distinct forms: non-circular and circular (Figure 1D). Non-circular lncRNAs are single-stranded molecules that bind miRNAs. One known example is linc-MD1 that binds miR-133 and miR-135 to promote muscle differentiation (Cesana et al., 2011). Circular RNAs (circRNAs) are a type of ring-forming lncRNA that form by linking the 3′ and 5′ ends with a back-splicing covalent bond (Ashwal-Fluss et al., 2014; Ebbesen et al., 2016). For example, the circRNA ciRS-7 found in the brain of both humans and mice contains 73 conserved miR-7 binding domains for sequestration (Hansen et al., 2013). Because miR-7 is a regulator of many pathways and is involved in cancer and Parkinson’s disease pathology, ciRS-7 may be crucial to neurological disorders and brain tumor formation. Other lncRNAs bind to mRNA to increase translation, inhibit translation, or promote mRNA decay (Carrieri et al., 2012; Faghihi et al., 2010; Gong and Maquat, 2011).
Some transcripts that were previously thought to be lncRNAs may have translation potential. For example, a recent study found that a muscle-specific transcript, previously identified as a lncRNA in the NONCODE database as NONMMUG026737 in mice and lncRNA LOC100507527 in humans, is translated into a peptide of 34 amino acids called dwarf open reading frame. The peptide, shown in murine models, binds to the Ca2+ re-uptake transporter sarco-endoplasmic reticulum Ca2+ adenosine triphosphate by displacing inhibitors (Nelson et al., 2016). A new computational algorithm (RibORF) has been developed to predict the translation potential of ncRNAs into small proteins (Ji et al., 2015; Zeisel and Baumert, 2016). Therefore, more research utilizing proteomics is needed to further clarify which transcripts that are currently thought to be lncRNAs are actually translated, and what biological functions they may carry.
Exosome Signaling
LncRNAs can be transferred between cells in exosomes (Figure 1E) (Huang et al., 2013). Referred to as exosomal shuttle RNAs, these exosomes promote cell-to-cell communication both in the local microenvironment and remotely (Valadi et al., 2007). Although it has been suggested that fragmented lncRNAs in exosomes merely represent a possible disposal mechanism for degraded RNAs (Huang et al., 2013), recent studies have suggested that exosomal lncRNAs are mechanistically involved in diseases and may be used as non-invasive biomarkers or therapeutic targets (Enderle et al., 2015).
LNCRNAS AND HUMAN DISEASES
Understanding how lncRNAs are involved in disease pathology reveals novel disease mechanisms and possible targets for various treatments. Cancers caused by multiple gene mutations can dysregulate lncRNAs. LncRNAs associated with neurological diseases such as Alzheimer’s disease (AD) may be directly associated with disease progression, whereas the collective dysregulation of lncRNAs in metabolic syndrome may promote and progress diseases. Below, we describe the short list of lncRNAs in Table 2 associated with human diseases.
TABLE 2.
LncRNAs and Human Diseases
| Disease | lncRNA | Role in Disease | References |
|---|---|---|---|
| Breast cancer | UCA1 (↑) | Prevents inhibition of AKT phosphorylation allowing invasiveness of tumor cells induced | (Chen et al., 2015) |
| UCA1 (↑) | Competes with tumor suppressor p27 for hnRNP limiting cell cycle arrest | (Huang et al., 2014) | |
| LINK-A (↑) | Facilitates activation of BRK and HIF1α stabilization under normoxic conditions | (Lin et al., 2016) | |
| Colorectal cancer | HOTAIR (↑) | Association between select HOTAIR SNPs and cancer risk; involved in tumor formation mechanism | (Xue et al., 2015a, b) |
| lncRNA-422 (↓) | Involved in tumor formation mechanism | (Xue et al., 2015b) | |
| CASC2 (↓) | miRNA-18a sequestration which upregulates PIAS3; promotes uncontrolled cell proliferation | (Huang et al., 2016) | |
| UCA1 (↑) | miR-204-5p sequestration which upregulates CREB1; promotes proliferation and drug-resistance | (Bian et al., 2016) | |
| Hepatocellular cancer | HULC (↑) | miR-107 sequestration promotes tumor angiogenesis | (Lu et al., 2016b) |
| Deregulates lipid metabolism through positive feedback-loop of cholesterol | (Cui et al., 2015) | ||
| Perturbs circadian rhythm by CLOCK-accelerated proliferation of hepatoma cells | (Cui et al., 2015) | ||
| DILC | Prevents HCC stem cell expansion | (Wang et al., 2016; Zhou et al., 2016a) | |
| MEG3 (↑) | Induces cell cycle arrest and apoptosis | (Liu et al., 2016b) | |
| GAS5 (↓) | Tumor suppressor through negative regulation of miR-21 preventing cell migration and invasion | (Hu et al., 2016) | |
| Non-small cell lung cancer | SBF2-AS1 (↑) | Promotes proliferation by negatively regulating p21 | (Lv et al., 2016) |
| AGAP2-AS1 (↑) | Promotes tumor growth through suppression of tumor suppressors KLF2 and LATS2 | (Li et al., 2016b) | |
| LINC01133 (↑) | EZH2 and LSD1 scaffold for promoter suppression of KLF2, P21, or E-cadherin | (Zang et al., 2016) | |
| PVT1 (↑) | EZH2 interaction suppresses LATS2 dysregulating cell proliferation and apoptosis | (Wan et al., 2016) | |
| Bladder cancer | MDC1-AS (↓) | Failure of cell cycle control and tumor suppression | (Xue et al., 2015c) |
| Gastric cancer | HOTAIR (↑) | Genotype-associated risk; promotion of HOXC11 | (Du et al., 2015) |
| Multiple cancer cell lines | NBR2 (↓) | Prevents energy stress activation of AMPK leading to tumor development | (Liu et al., 2016c) |
| Prostate cancer | PCGEM1 (↑) | Genotype-associated risk | (Xue et al., 2013) |
| Alzheimer's disease | BACE1-AS (↑) | BACE1 mRNA stability increasing Aβ 1-42 expression; silencing reduces Aβ oligomers | (Faghihi et al., 2008; Liu et al., 2014b) |
| Huntington's disease | TUNA (↓) | Role in pluripotency, neural differentiation of embryonic stem cells, and neurological function | (Lin et al., 2014) |
| MEG3 (↓) | Suggestive evidence epigenetic regulation may alter gene expression in the brain | (Johnson, 2012) | |
| NEAT1 (↑) | Neuroprotective against oxidative stress; no part of disease pathology | (Sunwoo et al., 2016) | |
| Diabetes | HI-LNC25 (↓) | Regulates GLIS family zinc finger 3 mRNA | (Zhou et al., 2016a) |
| H19 (↓) | Insulin resistance | ||
| Cholesterol Metabolism | lnc-HC (↑) | Negatively regulates hepatic cholesterol metabolism | (Lan et al., 2016) |
| Lipid and bile acid homeostasis | LncLSTR (↓) | Regulates apoC2 and Cyp8b1 expression; reduces plasma triglycerides | (Li et al., 2015d) |
| Atherosclerosis | CDKN2B-AS1 (↑ and SNPs) | Lipid homeostasis | (Uchida and Dimmeler, 2015; Zhou et al., 2016b) |
| TRIBAL (SNPs), lncLSTR | Lipid and glucose homeostasis | ||
| AT102202, E330013P06 | Cholesterol accumulation in macrophages | ||
| DYNLRB2-2, RP5-833A20.1 (↑) | Reverse cholesterol transport | ||
| RNCR3 (↑) | Atheroprotective; regulates endothelial cell function | (Shan et al., 2016) | |
| Autoimmune diseases | GAS5 (↑ and ↓) | Loss of promoter or suppression inhibits cell cycle and apoptosis | (Mayama et al., 2016) |
| Celiac disease | lnc13 (↓) | Associated with subset of inflammatory genes in small intestine and risk-associated haplotype | (Castellanos-Rubio et al., 2016) |
| Psoriasis | PRINS (↑) | Cellular stress response; deregulation inhibits keratinocyte apoptosis via G1P3 | (Szegedi et al., 2010) |
Cancer
Breast Cancer
LncRNAs are mediators of breast cancer development through the inhibition of cell cycle regulation and tumor growth signaling. In particular, the lncRNA UCA1 is up-regulated in breast cancer cells treated with media isolated from cultured macrophages, which contains factors mimicking macrophage-induced invasiveness of breast cancer cells (Huang et al., 2014). UCA1 competitively binds to heterogeneous ribonucleoprotein I (hnRNP I) required for the translation of the tumor suppressor gene p27 (Kip1) and acts as a post-transcriptional inhibitor of cell cycle regulation (Chen et al., 2015; Huang et al., 2014). Another breast cancer associated lncRNA, LINK-A, promotes angiogenesis under normoxic conditions. Upon interaction with LINK-A, breast tumor kinase undergoes a conformation change that activates kinase activities of hypoxia inducible factor 1α (HIF1α), promoting angiogenesis and glycolytic reprogramming in tumors (Lin et al., 2016).
Colorectal Cancer (CRC)
In addition to breast cancer, UCA1 has been implicated in the development of CRC by a unique mechanism. UCA1 is associated with CRC cell proliferation and it decreases the effectiveness of 5-fluorouricil treatment by inhibiting apoptosis. UCA1 sequesters the miRNA miR-204-5p, allowing for increased protein expression of the following miR-204-5p target genes: cAMP responsive element binding protein 1 (CREB1), B-cell lymphoma 2 (BCL2), and Ras-related protein RAB22A (Bian et al., 2016). The overexpression of these genes inhibits apoptosis, allowing for uncontrolled cell proliferation. From microarray studies, two lncRNAs were found to be associated with cancer-related genes: down-regulated lncRNA-422 and up-regulated HOTAIR (Ma et al., 2015a; Xue et al., 2015b). SNPs in the HOTAIR gene are also correlated with decreased risks of CRC and gastric cancer (Du et al., 2015; Xue et al., 2015a, b). Decreased expression of the anti-cancer lncRNA CASC2 and increased expression of its target miR-18a are characteristics of CRC. Specifically, CASC2 suppresses the tumorigenic signal transducer and activator of transcription 3 (STAT3) by sequestering its translational repressor miR-18a (Huang et al., 2016).
Hepatocellular Carcinoma
Many lncRNAs, such as HULC, HOTAIR, MALAT1, and H19, have been implicated in the dysregulation of oncogenes and the promotion of hepatocellular carcinoma (HCC) (Li et al., 2015b). HULC, which is activated by cholesterol, increases DNA methylation of miR-9 CpG promoter sites. This results in suppression of miR-9 expression, and reactivation of the protein synthesis of its target peroxisome proliferator activated receptor α (PPARα), which is a liver-enriched lipid-sensing nuclear receptor. Subsequently, the PPARα-target gene acyl-CoA synthetase long-chain family member 1 (ASCL1) is up-regulated, leading to more cholesterol production and feed-forward regulation promoting hepatocellular proliferation (Cui et al., 2015). HULC also up-regulates the sphingolipid activator sphingosine kinase 1, promoting tumor angiogenesis, as well as disturbing the circadian rhythm of HCC cells (Lu et al., 2016b). Other lncRNAs have antitumor effects in hepatoma cells. For example, the lncRNA PRAL inhibits p53 ubiquitination and increases p53 stability, whereas the lncRNA DILC binds to the interleukin-6 promoter, preventing STAT3 signaling and liver cancer stem cell expansion (Wang et al., 2016; Zhou et al., 2016a). The lncRNA MEG3 increases the expression of p53 to promote anticancer effects (Liu et al., 2016b). The lncRNA GAS5, which is decreased in HCC cells, negatively regulates oncogene miR-21 to prevent migration and invasion of cancer cells (Hu et al., 2016).
Lung Cancer
Many lncRNAs are associated with non-small cell lung cancer (NSCLC) with identifiable genetic pathways (Khandelwal et al., 2015). Increased expression of the lncRNAs LINC01133 and AGAP2-AS1 promote cell proliferation and migration in NSCLC. LINC01133, which interacts with the methylation enzyme enhancer of zeste homolog 2 (EZH2) and the demethylase LSD1, suppresses regulatory genes kruppel like factor 2 (KLF2) and P21 as well as the cell adhesion gene E-cadherin (Zang et al., 2016). Similar inhibitory functions are observed with AGAP2-AS1, which also represses KLF2 in addition to large tumor suppressor kinase 2 (LATS2) (Li et al., 2016b). The lncRNA SBF2-AS1 is correlated with lymph node metastasis from NSCLC and binds to SUZ12 polycomb repressive complex 2 subunit, negatively regulating histone methylation of the P21 promoter region (Lv et al., 2016). Another metastatic-associated lncRNA, PVT1, recruits EZH2 to repress LATS2 transcription (Wan et al., 2016). Collectively, the lncRNA mediated down-regulation of EZH2 and LSD1 promotes NSCLC, making these lncRNAs potential prognostic biomarkers and therapeutic targets for NSCLC treatment.
Other Types of Cancer
Several lncRNAs are dysregulated in other types of cancer as described in Table 2. SNPs in the lncRNA PCGEM1 may contribute to prostate cancer risk (Xue et al., 2013). The lncRNA MDC1-AS, which regulates the tumor suppressor gene mediator of DNA damage checkpoint 1, is down-regulated in bladder cancer (Xue et al., 2015c). The lncRNA NBR2 is down-regulated and depletion of NBR2 is shown to attenuate energy-stress-induced AMP-activated protein kinase activation, which causes increased tumor development (Liu et al., 2016c).
Neurological Disorders
Alzheimer’s disease is a chronic, neurodegenerative disease associated with a decline in cognitive function. Microarray analyses have found many dysregulated lncRNAs in AD patients with unknown mechanisms, including a down-regulated lncRNA that is expressed in the brain, which is associated with protein ubiquitination (Magistri et al., 2015; Zhou and Xu, 2015). One characteristic and hypothesized mechanism of AD is the increased deposition of amyloid-β (Aβ) 1–42 and 1–40. These deposits are produced by β-secretase-1 (BACE1), which is elevated in AD patients and stabilized by a duplex-forming antisense lncRNA called BACE1-AS (Faghihi et al., 2008). Stabilization of BACE1 mRNA increases BACE1 protein expression and Aβ levels, and concentrations are attenuated by BACE1-AS siRNA in vitro (Liu et al., 2014b).
Huntington’s disease (HD) is an inherited neurodegenerative disorder caused by a mutation in the huntingtin gene (Htt) resulting in the loss of motor neurons in the brain. Associated with neuro-development, the lncRNA HAR1 is down-regulated in the brains of HD patients (Johnson et al., 2010). Mutated Htt increases the nuclear accumulation of the transcriptional repressor REST that down-regulates its targets including HAR1. A microarray study revealed other target genes repressed by REST, including the suppression of the lncRNA MEG3. MEG3 is a brain-specific tumor suppressor, is an epigenetic regulator, and is associated with PRC2 and nervous system development (Johnson, 2012). The lncRNA NEAT1 is up-regulated in the brain of HD patients, whereas NEAT1 under non-mutated Htt acts as a transcriptional regulator of immune response and is a required component for nuclear paraspeckle formation (Bond and Fox, 2009; Mao et al., 2011; Sunwoo et al., 2016). In the brains of HD patients, increased paraspeckle formation can alter the transcriptome. In addition, the up-regulation of lncRNA TUNA, which is specifically expressed in the central nervous system and acts as a protein scaffold for transcriptional promoters, is associated with severity of HD (Lin et al., 2014).
Metabolic Syndrome and Diseases
Metabolic syndrome is a set of conditions characterized by glucose metabolism impairment, hypertension, obesity, and dyslipidemia, and it is a major risk factor for many interrelated diseases, such as Type 2 Diabetes (T2D) and cardiovascular diseases (CVDs). Emerging research shows that lncRNAs have crucial roles in glucose and lipid homeostasis, adipogenesis, obesity, atherosclerosis, hypertension, CVDs, and aging (Abdelmohsen et al., 2015; Chen, 2016; Kim et al., 2016; Kour and Rath, 2016; Ruan, 2016; Uchida and Dimmeler, 2015; Wei et al., 2016; Yang et al., 2015a; Zhou et al., 2016b). For example, variation in the sequence of the lncRNA MIAT was first shown to increase the risk of myocardial infarction, and it is also associated with other types of CVDs and diabetic retinopathy (Liao et al., 2016).
Dysregulation of glucose, cholesterol, and bile acid metabolism are major risk factors for T2D. Insulin-secreting human pancreatic β cells, responsible for glucose homeostasis, express cell-specific lncRNAs such as TUG1 (Moran et al., 2012). Some T2D patients have decreased levels of TUG1, leading to decreases in insulin levels (Yin et al., 2015). Depletion of the β cell-specific lncRNA HI-LNC25 showed decreased mRNA expression of the GLIS family zinc finger 3, which is associated with T2D (Moran et al., 2012). People with T2D or insulin resistance have decreased lncRNA H19, and this may alter glucose homeostasis in muscle tissue (Zhou et al., 2016b).
Several lncRNAs affect CVD by regulating key processes in atherosclerosis. Plasma triglyceride regulation is associated with the lncRNAs TRIBAL and lncLSTR, whereas other lncRNAs directly mediate the development of foam cells from macrophages in atherosclerotic lesions (Zhou et al., 2016b). Expressed in the macrophages of diet-induced T2D mice, the lncRNA E330013P06 up-regulates inflammatory response genes and increases foam cell formation (Reddy et al., 2014). The lncRNA DYNLRB2-2, which is induced by oxidized lipids and expressed in foam cells, stimulates inflammation and cholesterol efflux (Hu et al., 2014). Additionally, the lncRNA RP5-833A20.1 is up-regulated in foam cells and down-regulates the atheroprotective nuclear factor IA (Hu et al., 2015b). Expression of and SNPs in the lncRNA CDKN2-AS1 are associated with coronary artery disease, atherosclerosis, and other cardiovascular diseases (Zhou et al., 2016b). Although the lncRNAs listed above exacerbated the development of atherosclerosis, the lncRNA RNCR3 is atheroprotective. Expressed in mouse and human endothelial cells and vascular smooth muscle cells, RNCR3 positively regulates cell function under atherosclerotic conditions by preventing cell proliferation and migration near plaque inflamed regions (Shan et al., 2016). New treatment strategies may target these lncRNAs to improve health outcomes for CVD patients by limiting the development of atherosclerosis.
Autoimmune Disease
Several lncRNAs are dysregulated in autoimmune diseases. The lncRNA GAS5 is up-regulated in multiple sclerosis, sarcoidosis, and tuberculosis and down-regulated in rheumatoid arthritis and systemic lupus erythematosus (Mayama et al., 2016). In the small intestine of patients with celiac disease, the lncRNA lnc13, which represses expression of inflammation related genes under homeostatic conditions, is down-regulated, contains a disease-associated SNP, and may contribute to the observed inflammation (Castellanos-Rubio et al., 2016). The lncRNA PRINS is up-regulated in psoriasis and regulates interferon alpha inducible protein 6, which is an anti-apoptotic protein in keratinocytes, suggesting that PRINS may contribute to psoriasis by inhibiting keratinocyte apoptosis (Szegedi et al., 2010).
LNCRNAS AND TOXICOLOGICAL RESPONSE TO CHEMICALS
The current understanding of the mechanistic involvement of lncRNAs in various toxicological responses is still in its infancy. Many descriptive and correlative studies have linked the differential expression profiles of lncRNAs and toxic exposure to chemicals. It is necessary to establish such correlations, so as to identify candidate molecular targets and guide further mechanistic research on the potential roles of lncRNAs in the toxication or detoxification processes in response to chemicals. A few studies utilizing lncRNA knockdown or overexpression approaches have provided a mechanistic link between lncRNAs and disease phenotypes. In this section, a few leading topics regarding the roles of lncRNAs during toxic exposure to chemicals are summarized, including lncRNAs and chemical carcinogens, heavy metals, bile acids, as well as alterations in caloric intake; in addition, we discuss the roles of lncRNAs in vulnerable populations, including during embryonic development and the elderly, as well as patients undergoing surgical interventions (Table 3).
TABLE 3.
LncRNAs and Toxic Exposures
| Xenobiotic Exposure | Toxicity Outcome | lncRNAs and Regulation | System Evaluated | Functional Assessment | References |
|---|---|---|---|---|---|
| Benzene | Hematotoxicity | NR_045623 (↑) | Humans | Unknown | (Bai et al., 2014) |
| NR_028291 (↑) | |||||
| Cigarette smoke | Lung cancer | CCAT1 (↑) | HBE cells | Alters cell cycle; negative regulation of CCAT1 | (Lu et al., 2016b) |
| HOTAIR (↑) | HBE cells | Decreases p21 and alters cell cycle progression | (Liu et al., 2016d) | ||
| SCAL1 (↑) | Cancer cells, human primary bronchial epithelial cells | Regulation of NRF2 and oxidative stress protection | (Thai et al., 2013) | ||
| Benzo(a)pyrene | Lung cancer | DQ786227 (↑) | Immortalized HBE cells (BEAS-2B) cells | Oncogene-like | (Gao et al., 2013) |
| Anti-Benzo(a) pyrene-7,8-diol-9,10-epoxide | Lung cancer | LOC728228 (↑) | 16HBE-T cells | Oncogene-like | (Hu et al., 2015a) |
| Polycyclic aromatic hydrocarbons | Lung cancer | HOTAIR, MALAT1, and TUG1(↑) | Coke oven workers | Mediation of stress-induced adverse health effects | (Gao et al., 2016) |
| Furan and benzo(a)pyrene | Hepatocellular carcinogenicity | lincRNA-p21 (↑) | Mice | Repress p53-dependent apoptosis | (Recio et al., 2013) |
| Phenobarbital | Liver cancer | Meg3 and Rian (↑) | Mice | Chromatin modification and reprogramming to cellular stemness | (Lempiainen et al., 2013) |
| p-Dichlorobenzene | Carcinogenicity | Snora41, Gm19947, Scarna3a (↑) | Mouse embryonic stem cells | Unknown, biomarkers of chemical stress response | (Tani et al., 2016) |
| Cadmium | Lung cancer | ENST00000414355 (↑) | 16HBE cells, rats, and humans | Correlation to DNA damage and repair genes | (Zhou et al., 2015a) |
| Cadmium and arsenic | Metal toxicity | GABPB1-AS1, LINC00152, LINC054147_v2, MIR22HG, CDKN2B-AS1, FLJ33630 (↑) | Human-induced pluripotent stem cells | Dose-dependent cellular stress response | (Tani et al., 2014) |
| Nickel | Metal toxicity | NRG1 | Rats | Involvement in nickel-induced lung cancer | (Zhang et al., 2012) |
| Phthalates | Endocrine disruption | H19 (↓) | Human placentas | Correlative decreased methylation | (LaRocca et al., 2014) |
| Chlorpyrifos-methyl | Genomic methylation | H19 (↑) | Pregnant mice | Unknown | (Shin et al., 2014) |
| Bisphenol A | Neurodevelopmental disorders | Xist (↓) | Mice | Insufficient X-chromosome inactivation | (Kumamoto and Oshio, 2013) |
| Tsix (↑) | |||||
| Endocrine disruption and male fertility | H19 (↓) | Rats | Hypomethylation in spermatozoa of neonatally exposed rats and offspring | (Doshi et al., 2013) | |
| 2,3,7,8-Tetrachlorodibenzo-p-dioxin | DNA hypermethylation | H19 (↓) | Mice | Decreased fetal weight and gene expression | (Wu et al., 2004) |
| High fat diet | Metabolic syndrome | Lnc-HC (↑) | Rats, rat hepatoma cells | Negative regulation of cholesterol metabolism | (Lan et al., 2016) |
LncRNAs and Toxic Exposure to Chemicals
LncRNAs and Chemical Carcinogens
The involvement of lncRNAs in chemical-induced carcinogenesis is among the most extensively studied areas in lncRNA research. A classic example is the organic solvent benzene, which is a potent human hematotoxicant and leukemogen. In blood samples from humans exposed to benzene, the lncRNAs NR_045623 and NR_028291 are up-regulated with increasing levels of benzene exposure. LncRNA-mRNA co-expression network and functional analysis have identified that these lncRNAs are involved in immune responses, hematopoiesis, B cell receptor signaling, and chronic myeloid leukemia, and may be the key molecular targets for benzene hematotoxicity (Bai et al., 2014).
Cigarette smoking is a strong risk factor for the development of lung cancer. Human bronchial epithelial (HBE) cells have been widely used as a model to recapitulate chemical-induced lung carcinogenesis. In HBE cells exposed to cigarette smoke extract (CSE), the microRNA miR-218 is down-regulated and the lncRNA CCAT1 is up-regulated; silencing of CCAT1 attenuates the CSE-induced decrease of miR-218 to basal levels, suggesting that miR-218 is suppressed by CCAT1. The CCAT1-mediated epigenetic silencing of miR-218 induces an altered cell cycle transition through the BMI1 proto-oncogene, and provides a new mechanism for CSE-induced lung carcinogenesis (Lu et al., 2016a). The lncRNA HOTAIR is also up-regulated by CSE in HBE cells, which is associated with increased histone epigenetic modifier EZH2 and H3K27Me3. HOTAIR regulates the epigenetic silencing of p21 via EZH2-mediated H3K27Me3, and this contributes to changes in the cell cycle induced by CSE (Liu et al., 2016d). Conversely, in multiple lung cancer cell lines, the lncRNA SCAL1 was transcriptionally up-regulated by the anti-oxidative stress sensor NRF2 and it protects against CSE-induced cytotoxicity (Thai et al., 2013). In benzo(a)pyrene-transformed HBE cells, the up-regulated lncRNA DQ786227 may serve as an oncogene for human lung cancer (Gao et al., 2013). In a HBE cell line transformed by the active benzo(a)pyrene metabolite anti-benzo(a)pyrene-7,8-diol-9,10-epoxide, the lncRNA LOC728228 is up-regulated, whereas its knockdown inhibits cell proliferation and inhibits tumor growth in a nude mouse xenograft model in vivo (Hu et al., 2015a). These results suggest that LOC728228 is also an oncogene involved in human lung cancer.
Coke production is another risk factor for lung cancer among workers in coke oven plants. Coke is a solid carbonaceous material derived from destructive distillation of low-ash, low-sulfur bituminous coal during high temperature, driving off volatile and semi-volatile chemicals that include known or suspected carcinogens such as polycyclic aromatic hydrocarbons (PAHs). In peripheral blood lymphocytes of male coke oven workers exposed to PAHs, the lncRNAs HOTAIR, MALAT1, and TUG1 are positively correlated with the levels of external PAH exposure, whereas HOTAIR and MALAT1 are also positively associated with the levels of internal PAH exposure (urinary 1-hydroxypyrene) and the degree of DNA damage (Gao et al., 2016).
LncRNAs and chemical carcinogens have also been investigated in animal models. In mice exposed to furan, which is an industrially manufactured heterocyclic organic carcinogen, the lncRNA lincRNA-p21 appears to be co-transcribed with the protein-coding gene cyclin dependent kinase n1a (Cdkn1a) and promotes p53-dependent apoptosis, suggesting that lincRNA-p21 is an anti-cancer regulator (Huarte et al., 2010; Recio et al., 2013).
The anticonvulsant phenobarbital is a carcinogen in mice, but not in humans. Phenobarbital progressively increases the hepatic expression of lncRNAs originating from the delta-like homolog 1 (Dlk1)-iodothyronine deiodinase 3 (Dio3) imprinted gene cluster, which is a locus associated with various neoplasms in humans. Phenobarbital-mediated up-regulation of the lncRNAs Meg3 and Rian within the Dlk1-Dio3 gene cluster involves β-catenin and constitutive androstane receptor signaling (Lempiainen et al., 2013). In mouse embryonic stem cells, p-dichlorobenzene, which is a pesticide and a potential carcinogen, up-regulates the lncRNAs Snora41, GM19947, and Scarna3a and these lncRNAs may serve as indicators of p-dichlorobenzene exposure (Tani et al., 2016).
LncRNAs and Heavy Metals
Cadmium is a heavy metal that has been widely used in industrial processes such as during the fabrication of nickel-cadmium batteries (Godt et al., 2006). Human exposure to cadmium primarily occurs through eating wheat and rice from cadmium-contaminated soil, as well as inhalation and cigarette smoking. Cadmium has a wide spectrum of toxic effects including nephrotoxicity, neurotoxicity, carcinogenicity, teratogenicity, endocrine and reproductive disorders, interference with DNA repair mechanisms, generation of oxidative stress, and induction of apoptosis (Rani et al., 2014). Cadmium up-regulates the lncRNA ENST00000414355 in 16HBE cells and in the lungs of rats in a dose-dependent manner, whereas blood lncRNA ENST00000414355 positively correlates with urinary cadmium of exposed workers and is associated with DNA damage in blood cells. Silencing of ENST00000414355 in 16HBE cells inhibits the growth of DNA-damaged cells and decreases the expression of genes involved in DNA damage, but increases the expression of genes involved in DNA repair (Zhou et al., 2015a). These data demonstrate that the lncRNA ENST00000414355 is an effect biomarker, abrogating DNA damage associated with cadmium exposure in the lung. Another study using human-induced pluripotent stem cells has shown that cadmium rapidly increased the lncRNAs GABPB1-AS1 and LINC00152, whereas the pluripotency-related protein-coding genes were only minimally regulated. These data suggest the potential of utilizing lncRNAs as early biomarkers for toxic cadmium exposure (Tani et al., 2014).
Inorganic arsenic is a human carcinogen producing tumors in various organs including skin, lung, urinary bladder, and liver (IARC, 2004; Liu and Waalkes, 2008). The lncRNAs LINC00152, LINC0541471_v1, and LINC0541471_v2 are increased in undifferentiated human-induced pluripotent stem cells following treatment with arsenic (As2O3) and are suggested to be early indicators of arsenic toxicity (Tani et al., 2014).
Nickel is widely used in modern industry and human exposure occurs primarily through inhalation and ingestion. Nickel toxicities include skin allergies, lung fibrosis, and lung cancer (Zhao et al., 2009). A recent study has identified a novel 1280 bp non-coding RNA NRG1 associated with nickel-induced lung cancer and has suggested the potential role of lncRNAs in nickel carcinogenesis (Zhang et al., 2012).
Bile Acids
Bile acids are mainly synthesized from cholesterol in the liver and are secreted into bile to regulate the digestion and absorption of lipophilic nutrients from the diet. In addition, bile acids are also signaling molecules that regulate intermediary and xenobiotic metabolism pathways in liver, intestine, and other metabolic organs such as brown fat and skeletal muscle (Li and Chiang, 2015; Watanabe et al., 2006). At exceedingly high concentrations, such as during cholestasis, bile acids injure the hepatocyte membrane and produce a wide spectrum of toxicities such as necrosis, inflammation, and cancer. Recent studies in mice have demonstrated that the lncRNAs H19 and lncLSTR play important roles in bile acid homeostasis.
H19 is induced in mouse liver by overexpression of the anti-apoptotic protein Bcl2, accompanied by a marked increase in serum bile acids and bilirubin levels, as well as decreased expression of bile acid synthesizing enzymes and transporters (Zhang et al., 2016b). The up-regulation of H19 in Bcl2-overexpressed mice is due to loss of the transcriptional repression mediated by the nuclear receptor small heterodimer partner (Shp/Nr0b2), whereas H19 knockdown or Shp re-expression can rescue the Bcl2-induced liver injury. Notably, Shp is critically involved in the suppression of bile acid synthesis in liver (Kong et al., 2012). In healthy adult liver, H19 and Bcl2 are minimally expressed, but they are markedly increased during liver fibrosis and cirrhosis in both humans and mice. Therefore, the lncRNA H19 is critical in the pathogenesis of hepatic cholestasis and fibrosis at least partially through modulating the bile acid homeostasis (Zhang et al., 2016b).
LncLSTR is a liver-enriched lncRNA that forms a molecular complex with the RNA/DNA binding protein TDP-43. Together they maintain the constitutive expression of Cyp8b1, which is a bile acid synthesizing enzyme that increases the ratio of cholic acid (CA) over muricholic acid (MCA). Lack of lncLSTR results in a decrease in both Cyp8b1 expression in liver and conjugated CA/MCA ratio in gallbladder of mice (Li et al., 2015d). LncLSTR depletion also leads to increased apoC2 expression, robust lipoprotein lipase activation, and increased plasma triglyceride clearance, through the bile acid sensor farnesoid X receptor-mediated pathway, and depletion of lncLSTR at least partially corrects hyperlipidemia in the ApoE-null mouse model (Li et al., 2015d). Together, these data demonstrate that lncLSTR regulates hepatic bile acid synthesis and composition to maintain systemic lipid homeostasis.
LncRNAs and the Toxicity of Calories
There is growing recognition that aberrant caloric intake and the subsequent malnutrition, including both starvation and obesity, can produce multi-organ toxicities (Klaassen et al., 2013). Hepatic lncRNA lncLSTR is down-regulated during starvation in mice, whereas depletion of lncLSTR lowers plasma hepatic triglyceride levels during both fasting and re-feeding (Li et al., 2015d). Conversely, the lncRNA lncLGR is up-regulated by starvation. Overexpression of lncLGR using an adenoviral system suppresses hepatic expression of glucokinase (GCK) and reduces hepatic glycogen content in mice, whereas lncLGR knockdown does the opposite (Ruan et al., 2016). Mechanistic investigations have found that lncLGR binds to hnRNP L, which is a transcriptional repressor of GCK, and facilitates its recruitment to the GCK promoter to down-regulate GCK transcription (Ruan et al., 2016).
Other lncRNAs are involved in obesity caused by a high-fat diet or a genetic modification (ob/ob) in mice. As highlighted in a recent review, the lncRNA lnc-HC, which is located at the 3′ end of the mitochondrial carnitine transporter gene Slc25a14 open reading frame, is up-regulated in rat liver by a high-fat diet (Ananthanarayanan, 2016; Lan et al., 2016). Lnc-HC down-regulates the rate-limiting bile acid synthetic enzyme Cyp7a1 as well as the cholesterol efflux transporter ATP-binding cassette a1, through association with hnRNP A2/B1. In vivo silencing of lnc-HC in rats on a high-fat diet ameliorates insulin resistance and improves serum biomarkers including triglyceride, cholesterol, and high-density lipoprotein cholesterol, suggesting that lnc-HC can be harnessed therapeutically for treating obesity and the metabolic syndrome (Lan et al., 2016).
Mice lacking the adipose-enriched lncRNA Sra are resistant to high-fat diet-induced obesity, are more sensitive to insulin, and have decreased fat mass and increased lean content, fewer lipid droplets, decreased expression of a subset of adipocyte marker and lipogenesis associated genes, and reduced plasma levels of tumor necrosis factor α (Liu et al., 2014a). The Sra-mediated metabolic changes are attributed at least partially to its role as a transcriptional coactivator of PPARγ. It is now recognized that Sra coordinates with the functions of various transcription factors to enhance steroid receptor-dependent gene expression and also serve as a distinct protein scaffold (Liu et al., 2016a).
The lncRNA Meg3 is up-regulated in hepatocytes of mice fed a high-fat diet and ob/ob mice, and Meg3 expression is also increased by palmitate, oleate, and linoleate (Zhu et al., 2016). Meg3 overexpression increases hepatic gluconeogenesis and suppresses insulin-stimulated glycogen synthesis in primary hepatocytes correlated with increased expression of forkhead box protein O1, glucose-6-phosphatase, and phosphoenolpyruvate carboxykinase, whereas Meg3 interference reverses the palmitate-induced increase of these genes. Meg3 interference also reverses the up-regulation of triglycerides, impairs glucose tolerance, and down-regulates glycogen content in high-fat diet treated mice and ob/ob mice (Zhu et al., 2016). Similar to Meg3, the lncRNA Malat1 is also up-regulated in livers of ob/ob mice and in hepatocytes exposed to palmitate. Knockdown of Malat1 suppresses palmitate-induced lipid accumulation, increases nuclear SREBP-1c protein in HepG2 cells, and prevents hepatic lipid accumulation and insulin resistance in ob/ob mice (Yan et al., 2016). Malat1 interacts with SREBP-1c to stabilize the nuclear SREBP-1c protein, and this has provided a mechanistic explanation of Malat1-mediated hepatic steatosis and insulin resistance (Yan et al., 2016).
LncRNAs in Vulnerable Populations
LncRNAs and Developmental Exposure to Xenobiotics
The maternally expressed lncRNA gene H19 is located on human chromosome 11 and is approximately 100 kb away from the paternally expressed gene insulin-like growth factor 2 (IGF2). The H19/IGF2 locus has been studied extensively during development in response to xenobiotics including endocrine disrupting chemicals. Phthalates are used as plasticizers and solvents, and exposure to these compounds has raised safety concerns especially during pregnancy and development (Skinner, 2016). In human placenta, a decrease in H19 methylation is associated with high levels of the sum of phthalate metabolites, as well as metabolites of low molecular weight phthalates (LaRocca et al., 2014). These metabolites are also inversely associated with IGF2 DNA methylation region 0. Variation in methylation is not associated with changes in allele-specific expression, but increased deviation of allele-specific expression of H19 is associated with the sum of di(2-ethylhexyl) phthalate metabolites and high molecular weight phthalates. However, the methylation and expression of these imprinted regions do not appear to impact newborn length or weight significantly (LaRocca et al., 2014).
Chlorpyrifos-methyl (CPM) is a broad-spectrum organophosphate insecticide that is used widely in agriculture, and it has also been shown to have endocrine disruption effects, especially anti-androgenic effects. Exposure to CPM during pregnancy in mice alters the methylation status of the H19 gene in primordial germ cells and embryonic liver and intestine (Shin et al., 2014). Exposure of mouse pre-implantation embryos to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), which is a highly toxic and persistent environmental contaminant and an activator of the xenobiotic-sensing transcription factor aryl hydrocarbon receptor, leads to decreased fetal weight as well as decreased expression of H19 and Igf2. The methylation of the 430 bp H19/Igf2 imprint control region and methyltransferase activity are higher in TCDD-exposed embryos than in controls (Wu et al., 2004). Taken together, xenobiotic exposure during development may lead to epigenetic modifications (eg, DNA methylation) of the lncRNA H19 gene and influence fetal development and health outcomes.
Bisphenol A (BPA) is an estrogenic compound that is commonly used as a plasticizer in many consumer products. The pups (F1) of male rats neonatally exposed to BPA (F0) have decreased expression of Igf2 and H19 in the F0 spermatozoa and the F1 embryo, and this correlates with DNA hypomethylation at the H19 imprinting control regions. This provides a possible mechanism for post-implantation loss and male fertility status following BPA exposure (Doshi et al., 2013).
The lncRNAs Xist and Tsix are involved in the inactivation of the second X chromosome in mammals (Chow et al., 2005). In pregnant mice, exposure to bisphenol A decreased expression of Xist as well as neurodevelopmental genes, but increased Tsix in the cerebellum of female pups, and this likely contributes to the bisphenol A induce neurodevelopmental disorders (Kumamoto and Oshio, 2013).
LncRNAs and Aging
Aging is a complex physiological process with a progressive decline of adaptation and functional capacities in multiple organs of the body. The expression of many lncRNAs is affected during different types of senescence, and many lncRNAs govern the major senescent pathways such as p53/p21, pRB/p16, as well as the senescence-associated secretory phenotype (Abdelmohsen and Gorospe, 2015; Costa et al., 2016; Grammatikakis et al., 2014; Kim et al., 2016; Montes and Lund, 2016). This subsequently alters the status of aging in essential metabolic organs such as the heart and nervous system (Devaux et al., 2015; Greco et al., 2015; Szafranski et al., 2015). In human fibroblasts, the senescence-associated lncRNA SAL-RNA1 delays senescence because reducing its levels enhances the appearance of phenotypic traits of senescence including enlarged morphology, positive β-galactosidase activity, and increased p53 levels (Abdelmohsen et al., 2013). The lncRNA MIR31HG is up-regulated in oncogene-induced senescence, whereas its knockdown promotes a strong tumor-suppressor p16-dependent senescence phenotype (Montes et al., 2015). In epithelial cells, the mitochondrial lncRNA ASncmtRNA-2 is induced by replicative senescence caused by endogenous telomere erosion, resulting in cell cycle arrest in G1 and G2 phases. Increased ASncmtRNA-2 is detected in mouse aorta during aging and is co-regulated with p16 (Bianchessi et al., 2015). In proliferating cells, the lncRNA PANDA interacts with the scaffold-attachment factor A to recruit polycomb repressive complexes to repress the transcription of senescence-promoting genes; conversely, the loss of such interactions allows for the senescence program (Puvvula et al., 2014). Together these observations indicate that lncRNAs may serve as novel targets for aging and aging-related human diseases.
LncRNAs and Side Effects of Therapeutic Interventions
A recent review has concluded that various pharmaceutical drugs can impact the expression of lncRNAs in various cell types (Marrone et al., 2014), thus this topic will not be emphasized in the present review. Various surgical interventions can also regulate lncRNA expression as well as the subsequent cellular responses as described below.
During liver transplantation, cold-induced liver injury is a major problem affecting the health outcome of patients. A recent study has shown that TUG1 is down-regulated by cold storage, whereas overexpression of TUG1 attenuates cold-induced apoptosis in mouse hepatocytes and hepatic sinusoidal endothelial cells, at least in part by blocking mitochondrial apoptosis and endoplasmic reticulum stress pathways. TUG1 also attenuates apoptosis, inflammation, and oxidative stress in vivo in livers subjected to cold storage and improves hepatocyte function and prolonged hepatic graft survival rate (Su et al., 2016).
After partial hepatectomy, which is a commonly used treatment option for liver cancer, a mouse microarray study demonstrated that 400 lncRNAs are altered during liver regeneration, especially lncPHx2 that is markedly up-regulated. LncPHx2 depletion leads to a transient increase in hepatocyte proliferation and more rapid liver regeneration, and this correlates with an up-regulation of genes involved in cell proliferation (Huang et al., 2015). Therefore, lncPHx2 appears to prevent excessive hepatocyte proliferation following partial hepatectomy.
Following hematopoietic stem cell transplantation (HSCT), which is often performed in patients with multiple myeloma or leukemia (Park et al., 2015), a serious complication is hepatic veno-occlusive disease, resulting from the damage to sinusoidal endothelial cells and hepatocytes. Using a mouse HSCT model, differentially expressed lncRNAs in hepatocytes have been identified using microarray and are correlated with dysregulation in the expression of many adjacent protein-coding genes including up-regulation of the T-cell receptor and down-regulation of the vascular endothelial growth factor precursor pathway (Qiao et al., 2016).
CURRENT TECHNOLOGIES FOR LNCRNA PROFILING AND FUNCTIONAL CHARACTERIZATIONS
Several lncRNA annotation databases are available online, including lncRNAdb, NONCODE, ALDB, and PLNlncrBASE, as described in detail in Table 4. The reference general transfer format (GTF) file for lncRNAs can be downloaded from the Gencode website. Vespucci is a recently developed algorithm for de novo transcript identification from global run-on sequencing (Allison et al., 2014).
TABLE 4.
LncRNA Annotation Resources
| Name | Purpose | Website | Sources |
|---|---|---|---|
| lncRNAdb | Includes 287 eukaryotic lncRNAs; new features include Illumina Body Atlas expression profiles, nucleotide sequence information, BLAST search tool, and easy export of content | http://lncrnadb.org | (Amaral et al., 2011; Quek et al., 2015) |
| NONCODE | ncRNA annotations in 16 species; new features include conservation annotation, lncRNA-disease relationships, literature support, and long-read sequencing method support | http://www.bioinfo.org/noncode/ | (Bu et al., 2012; Zhao et al., 2016a,b) |
| ALDB | lncRNAs in domestic animals | http://res.xaut.edu.cn/aldb/index.jsp | (Li et al., 2015a) |
| PLNlncRbase | lncRNAs in plants | http://bioinformatics.ahau.edu.cn/PLNlncRbase | (Xuan et al., 2015) |
| Gencode | Annotation of evidence-based gene features for humans and mice for protein-coding genes with alternative splicing variants, non-coding loci, and pseudogenes; has GTF files for lncRNAs | https://www.gencodegenes.org/ | |
| Vespucci | Algorithm for de novo transcript identification from global run-on sequencing that enables new insights into transcriptional dynamics within a cell; expands annotations of mRNAs and lncRNAs beyond primary transcript (eg, polyadenylation sites and un-annotated ncRNAs in enhancer regions) | http://www.ncbi.nlm.nih.gov/pubmed/?term=24304890 | (Allison et al., 2014) |
Table 5 summarizes current methods for lncRNA profiling and regulation of transcription, including Northern blotting and RT-qPCR for individual lncRNA quantification, FISH for lncRNA visualization, and PtPd nanodendrite/nano-flower-like@GO signal amplification for improved sensitivity of lncRNA detection. Transcriptome-wide profiling tools for lncRNA detection include microarrays, RNA-Seq, as well as Capture-Seq. DNase Hi-C is a genome-wide method to assess the 3D interactions between lncRNA promoters and super-enhancers as well as polycomb repressive complexes.
TABLE 5.
LncRNA Profiling Tools
| Name | Description | Source |
|---|---|---|
| Northern blotting | Individual lncRNA quantification | (Zhu et al., 2013) |
| RT-qPCR | Individual lncRNA quantification | (Zhu et al., 2013) |
| Fluorescent in situ hybridization | lncRNA visualization | (Dunagin et al., 2015) |
| PtPd BND/BNF@GO/Au/HRP | PtPd bimetallic nanodendrite/nano-flower-like cluster on grapheme oxide/Au/horseradish peroxidase signal amplification system for ultrasensitive detection of lncRNAs | (Liu et al., 2015) |
| lncRNA arrays | Arrays in humans, mice, and rats from Arraystar (http://www.arraystar.com/store/products/lncrna-microarrays/) and Gencode custom lncRNA expression microarrays in humans (http://www.gencodegenes.org/lncrna_microarray.html) | |
| RNA-Seq | Quantification of mRNAs and lncRNAs | (Zhu et al., 2013) |
| CaptureSeq | Method that first enriches transcripts of interest by hybridizing them to magnetic bead-linked oligonucleotides that are tiled across the region of interest, allowing for targeted purification, multiplexed library preparation, and RNA-Seq at a higher depth | (Clark et al., 2015) |
| DNase Hi-C | Comprehensively maps global chromatin contacts and has used to determine the 3D organization between lncRNA promoters and super-enhancers as well as the polycomb repressive complex | (Ma et al., 2015b) |
Table 6 summarizes lncRNA functional characterization tools, including CHART and CHIRP for lncRNA-DNA interactions (CHART and CHIRP), as well as RNA–protein interactions (RIP, CLIP methods, HiTS-RAP, and lncRNA pulldown with mass spectrometry). Table 7 describes the current bioinformatics tools for lncRNA functional prediction (LncReg, lncRNAtor, LncTar, CLIPdb, LncPro, DIANA-LncBase, and starBase v2.0).
TABLE 6.
LncRNA Functional Characterization Tools
| Name | Description | Sources |
|---|---|---|
| CHART | Capture Hybridization Analysis of RNA Targets enriches endogenous RNAs using complementary, biotinylated capture oligonucleotides along with their DNA targets from reversibly cross-linked chromatin extracts; can be extended by deep sequencing to obtain the DNA targets of lncRNAs within the genome | (Davis and West, 2015) |
| CHIRP | Chromatin Isolation by RNA Purification using tiling oligonucleotides to retrieve specific lncRNAs with bound protein and DNA and can be coupled with deep sequencing to reveal lncRNA–chromatin interactions | (Chu et al., 2012) |
| RIP and RIP-Seq | RNA Immunoprecipitation to identify lncRNA interactions with a protein of interest; can be coupled with deep sequencing to reveal all RNA species that interact with a protein of interest | (Gonzalez-Buendia et al., 2015; Zhang et al., 2016a) |
| CLIP and CLIP-Seq (HiTS-CLIP) | Crosslinking-Immunopurification reveals all the RNA species that are associated with a particular protein of interest; ultraviolet light-mediated, irreversible crosslinking improves specificity; can be coupled with deep sequencing to reveal all RNA species that interact with a protein of interest | (Darnell, 2010; Sugimoto et al., 2012; Ule et al., 2003; Zhu et al., 2013) |
| iCLIP | Individual-nucleotide resolution CLIP is a refinement that allows single nucleotide resolution of binding sites for RNA binding proteins (RBPs) | (Sugimoto et al., 2012) |
| miCLIP | Methylation Individual-nucleotides-resolution Crosslinking and Immunoprecipitation is a specialized version of iCLIP designed to determine which RNA nucleotides are methylated by the RNA methyltransferase Nsun2 | (Hussain et al., 2013) |
| PAR-CLIP and PAR-CLIP-Seq | Photoactivatable-Ribonucleoside-Enhanced CLIP incorporates photoreactive ribonucleoside analogs into nascent RNA transcripts by living cells, followed by UV-crosslinking between RNA and RBPs, and immunoprecipitation of the RBP of interest; identifies RBP binding sites on target RNAs with nucleotide-level resolution; can be couple with sequencing to probe on a transcriptomic scale | (Danan et al., 2016; Jalali et al., 2013) |
| HiTS-RAP | High-Throughput Sequencing-RNA Affinity Profiling quantifies the binding of fluorescently labeled protein to millions of RNAs anchored to sequenced cDNA templates | (Tome et al., 2014) |
| lncRNA pulldown with mass spectrometry | RNA probe to detect its binding with a protein/protein complex in a cell lysate; proteins are then eluted from the RNA and detected using western blot or mass spectrometry | (Xing et al., 2016) |
TABLE 7.
LncRNAs and Bioinformatics Tools for Functional Prediction
| Name | Purpose | Sources | |
|---|---|---|---|
| LncReg | Determines lncRNA-associated regulatory networks and overall functional roles of lncRNAs | http://bioinformatics.ustc.edu.cn/lncreg/ | (Zhou et al., 2015b) |
| lncRNAtor | Functional investigations of lncRNA-mRNA and lncRNA–protein interactions | http://lncrnator.ewha.ac.kr/ | (Park et al., 2014) |
| LncTar | Prediction tool for RNA targets of lncRNAs with experimentally based 80% accuracy | http://www.cuilab.cn/lnctar | (Li et al., 2015c) |
| CLIPdb | Database that describes RNA–protein interactions and from 395 CLIP-Seq datasets in human, mouse, worm, and yeast; method to identify transcriptome-wide binding sites | http://lulab.life.tsinghua.edu.cn/clipdb/ | (Yang et al., 2015b) |
| LncPro | Predicts the interactions between lncRNAs and proteins by converting RNA and protein sequences into vectors and scoring each RNA-protein pair using matrix multiplication, and applying this method on human proteins has shown that lncRNAs tend to interact with nuclear proteins and RBPs | http://bioinfo.bjmu.edu.cn/lncpro/ | (Lu et al., 2013) |
| DIANA-LncBase | Experimentally verified (>5000) and computationally predicted (>10 million) miRNA targets on human and mouse lncRNAs | http://www.microrna.gr/LncBase | (Paraskevopoulou et al., 2013) |
| starBase v2.0 | Identifies RNA–RNA and RNA–protein interaction networks; RBP–lncRNA interactions have been deposited in starBase v2.0 under the “protein-RNA” section, and an improved deepView Genome Browser has been developed in starBase v2.0 | http://starbase.sysu.edu.cn | (Li et al., 2014a, b) |
| http://starbase.sysu.edu.cn/rbpLncRNA.php | |||
| http://starbase.sysu.edu.cn/browser.php |
Due to page limitations, the detailed methodologies for lncRNA profiles and functional characterizations are not described; readers are encouraged to review the relevant information in the online resources and original publications listed in Tables 4– 7.
Future Directions
LncRNAs are not mere transcriptional noise as has been previously speculated because they have multifaceted roles in modulating the transcriptional output, mRNA stability, as well as protein functions in the cell. In the field of toxicology, there are three important aspects to consider: (1) implementing sensitive and high-throughput quantification methods to identify lncRNAs as specific and early biomarkers to predict toxic effects accurately and human diseases; (2) identifying important lncRNA targets, including DNA, RNA, and proteins that may contribute to the toxication or detoxification following xenobiotic exposure; and (3) establishing a comprehensive toxicology-oriented database on lncRNA signatures and functions that will allow toxicologists to share data and use as a reference for risk assessment and policy-making. Much more exploratory work on lncRNA profiling is still needed in this field, and more mechanistic investigations on the lncRNA–target interactions are imperative as next steps.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
ACKNOWLEDGMENTS
The authors would like to acknowledge Michelle Gray for her technical assistance with Figure 1, as well as Drs. Lucio Costa, Terry Kavanagh, Curtis Klaassen, and members of Dr. Cui’s laboratory for proofreading the manuscript.
FUNDING
This was supported by National Institutes of Health grants ES025708, GM111381, ES019487, and the University of Washington Center for Exposures, Diseases, Genomics, and Environment (P30 ES007033), as well as the Murphy Endowment.
REFERENCES
- Abdelmohsen K., Gorospe M. (2015). Noncoding RNA control of cellular senescence. Wiley Interdiscip Rev RNA 6, 615–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmohsen K., Panda A., Kang M. J., Xu J., Selimyan R., Yoon J. H., Martindale J. L., De S., Wood W. H., III, Becker K. G., et al. (2013). Senescence-associated lncRNAs: senescence-associated long noncoding RNAs. Aging Cell 12, 890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmohsen K., Panda A. C., De S., Grammatikakis I., Kim J., Ding J., Noh J. H., Kim K. M., Mattison J. A., de Cabo R., et al. (2015). Circular RNAs in monkey muscle: age-dependent changes. Aging (Albany NY) 7, 903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam T., Medvedeva Y. A., Jia H., Brown J. B., Lipovich L., Bajic V. B. (2014). Promoter analysis reveals globally differential regulation of human long non-coding RNA and protein-coding genes. PLoS One 9, e109443.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison K. A., Kaikkonen M. U., Gaasterland T., Glass C. K. (2014). Vespucci: A system for building annotated databases of nascent transcripts. Nucleic Acids Res 42, 2433–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral P. P., Clark M. B., Gascoigne D. K., Dinger M. E., Mattick J. S. (2011). lncRNAdb: A reference database for long noncoding RNAs. Nucleic Acids Res 39, D146–D151. 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthanarayanan M. (2016). A novel long non-coding RNA (lncRNA) regulating Cholesterol and Bile acid homeostasis: A new kid on the block and a potential Therapeutic Target? Hepatology 64, 16–18. [DOI] [PubMed] [Google Scholar]
- Ashwal-Fluss R., Meyer M., Pamudurti N. R., Ivanov A., Bartok O., Hanan M., Evantal N., Memczak S., Rajewsky N., Kadener S. (2014). circRNA biogenesis competes with pre-mRNA splicing. Mol Cell 56, 55–66. [DOI] [PubMed] [Google Scholar]
- Augui S., Nora E. P., Heard E. (2011). Regulation of X-chromosome inactivation by the X-inactivation centre. Nat Rev Genet 12, 429–442. [DOI] [PubMed] [Google Scholar]
- Bai W., Yang J., Yang G., Niu P., Tian L., Gao A. (2014). Long non-coding RNA NR_045623 and NR_028291 involved in benzene hematotoxicity in occupationally benzene-exposed workers. Exp Mol Pathol 96, 354–360. [DOI] [PubMed] [Google Scholar]
- Bian Z., Jin L., Zhang J., Yin Y., Quan C., Hu Y., Feng Y., Liu H., Fei B., Mao Y., et al. (2016). LncRNA-UCA1 enhances cell proliferation and 5-fluorouracil resistance in colorectal cancer by inhibiting miR-204-5p. Sci Rep 6, 23892.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchessi V., Badi I., Bertolotti M., Nigro P., D'Alessandra Y., Capogrossi M. C., Zanobini M., Pompilio G., Raucci A., Lauri A. (2015). The mitochondrial lncRNA ASncmtRNA-2 is induced in aging and replicative senescence in endothelial cells. J Mol Cell Cardiol 81, 62–70. [DOI] [PubMed] [Google Scholar]
- Bond C. S., Fox A. H. (2009). Paraspeckles: nuclear bodies built on long noncoding RNA. J Cell Biol 186, 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu D., Yu K., Sun S., Xie C., Skogerbo G., Miao R., Xiao H., Liao Q., Luo H., Zhao G., et al. (2012). NONCODE v3.0: integrative annotation of long noncoding RNAs. Nucleic Acids Res 40, D210–D215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrieri C., Cimatti L., Biagioli M., Beugnet A., Zucchelli S., Fedele S., Pesce E., Ferrer I., Collavin L., Santoro C., et al. (2012). Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature 491, 454–457. [DOI] [PubMed] [Google Scholar]
- Castellanos-Rubio A., Fernandez-Jimenez N., Kratchmarov R., Luo X., Bhagat G., Green P. H., Schneider R., Kiledjian M., Bilbao J. R., Ghosh S. (2016). A long noncoding RNA associated with susceptibility to celiac disease. Science 352, 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesana M., Cacchiarelli D., Legnini I., Santini T., Sthandier O., Chinappi M., Tramontano A., Bozzoni I. (2011). A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 147, 358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Shao C., Xu M., Ji J., Xie Y., Lei Y., Wang X. (2015). Macrophage infiltration promotes invasiveness of breast cancer cells via activating long non-coding RNA UCA1. Int J Clin Exp Pathol 8, 9052–9061. [PMC free article] [PubMed] [Google Scholar]
- Chen Z. (2016). Progress and prospects of long noncoding RNAs in lipid homeostasis. Mol Metab 5, 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J. C., Yen Z., Ziesche S. M., Brown C. J. (2005). Silencing of the mammalian X chromosome. Annu Rev Genomics Hum Genet 6, 69–92. [DOI] [PubMed] [Google Scholar]
- Chu C., Quinn J., Chang H. Y. (2012). Chromatin isolation by RNA purification (ChIRP). J Vis Exp e3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. B., Mercer T. R., Bussotti G., Leonardi T., Haynes K. R., Crawford J., Brunck M. E., Cao K. A., Thomas G. P., Chen W. Y., et al. (2015). Quantitative gene profiling of long noncoding RNAs with targeted RNA sequencing. Nat Methods 12, 339–342. [DOI] [PubMed] [Google Scholar]
- Cloutier S. C., Wang S., Ma W. K., Al Husini N., Dhoondia Z., Ansari A., Pascuzzi P. E., Tran E. J. (2016). Regulated formation of lncRNA-DNA hybrids enables faster transcriptional induction and environmental adaptation. Mol Cell 61, 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE Project Consortium. (2012). An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M. C., Leitao A. L., Enguita F. J. (2016). Noncoding transcriptional landscape in human aging. Curr Top Microbiol Immunol 394, 177–202. [DOI] [PubMed] [Google Scholar]
- Cui M., Xiao Z., Wang Y., Zheng M., Song T., Cai X., Sun B., Ye L., Zhang X. (2015). Long noncoding RNA HULC modulates abnormal lipid metabolism in hepatoma cells through an miR-9-mediated RXRA signaling pathway. Cancer Res 75, 846–857. [DOI] [PubMed] [Google Scholar]
- Danan C., Manickavel S., Hafner M. (2016). PAR-CLIP: A method for transcriptome-wide identification of RNA binding protein interaction sites. Methods Mol Biol 1358, 153–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell R. B. (2010). HITS-CLIP: Panoramic views of protein-RNA regulation in living cells. Wiley Interdiscip Rev RNA 1, 266–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P., West J. A. (2015). Purification of specific chromatin regions using oligonucleotides: Capture hybridization analysis of RNA targets (CHART). Methods Mol Biol 1262, 167–182. [DOI] [PubMed] [Google Scholar]
- Derrien T., Johnson R., Bussotti G., Tanzer A., Djebali S., Tilgner H., Guernec G., Martin D., Merkel A., Knowles D. G., et al. (2012). The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res 22, 1775–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux Y., Zangrando J., Schroen B., Creemers E. E., Pedrazzini T., Chang C. P., Dorn G. W., 2nd, Thum T., Heymans S., et al. (2015). Long noncoding RNAs in cardiac development and ageing. Nat Rev Cardiol 12, 415–425. [DOI] [PubMed] [Google Scholar]
- Djebali S., Davis C. A., Merkel A., Dobin A., Lassmann T., Mortazavi A., Tanzer A., Lagarde J., Lin W., Schlesinger F., et al. (2012). Landscape of transcription in human cells. Nature 489, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi T., D'Souza C., Vanage G. (2013). Aberrant DNA methylation at Igf2-H19 imprinting control region in spermatozoa upon neonatal exposure to bisphenol A and its association with post implantation loss. Mol Biol Rep 40, 4747–4757. [DOI] [PubMed] [Google Scholar]
- Du M., Wang W., Jin H., Wang Q., Ge Y., Lu J., Ma G., Chu H., Tong N., Zhu H., et al. (2015). The association analysis of lncRNA HOTAIR genetic variants and gastric cancer risk in a Chinese population. Oncotarget 6, 31255–31262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunagin M., Cabili M. N., Rinn J., Raj A. (2015). Visualization of lncRNA by single-molecule fluorescence in situ hybridization. Methods Mol Biol 1262, 3–19. [DOI] [PubMed] [Google Scholar]
- Ebbesen K. K., Kjems J., Hansen T. B. (2016). Circular RNAs: Identification, biogenesis and function. Biochim Biophys Acta 1859, 163–168. [DOI] [PubMed] [Google Scholar]
- Enderle D., Spiel A., Coticchia C. M., Berghoff E., Mueller R., Schlumpberger M., Sprenger-Haussels M., Shaffer J. M., Lader E., Skog J., et al. (2015). Characterization of RNA from exosomes and other extracellular vesicles isolated by a novel spin column-based method. PLoS One 10, e0136133.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghihi M. A., Modarresi F., Khalil A. M., Wood D. E., Sahagan B. G., Morgan T. E., Finch C. E., St Laurent G., 3rd, Kenny P. J., Wahlestedt C. (2008). Expression of a noncoding RNA is elevated in Alzheimer's disease and drives rapid feed-forward regulation of beta-secretase. Nat Med 14, 723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghihi M. A., Wahlestedt C. (2009). Regulatory roles of natural antisense transcripts. Nat Rev Mol Cell Biol 10, 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghihi M. A., Zhang M., Huang J., Modarresi F., Van der Brug M. P., Nalls M. A., Cookson M. R., St-Laurent G., 3rd, Wahlestedt C. (2010). Evidence for natural antisense transcript-mediated inhibition of microRNA function. Genome Biol 11, R56.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C., He Z., Li J., Li X., Bai Q., Zhang Z., Zhang X., Wang S., Xiao X., Wang F., et al. (2016). Specific long non-coding RNAs response to occupational PAHs exposure in coke oven workers. Toxicol Rep 3, 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Mai A., Li X., Lai Y., Zheng J., Yang Q., Wu J., Nan A., Ye S., Jiang Y. (2013). LncRNA-DQ786227-mediated cell malignant transformation induced by benzo(a)pyrene. Toxicol Lett 223, 205–210. [DOI] [PubMed] [Google Scholar]
- Geisler S., Coller J. (2013). RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol 14, 699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt J., Scheidig F., Grosse-Siestrup C., Esche V., Brandenburg P., Reich A., Groneberg D. A. (2006). The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol 1, 22.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong C., Maquat L. E. (2011). lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3' UTRs via Alu elements. Nature 470, 284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Buendia E., Saldana-Meyer R., Meier K., Recillas-Targa F. (2015). Transcriptome-wide identification of in vivo interactions between RNAs and RNA-binding proteins by RIP and PAR-CLIP assays. Methods Mol Biol 1288, 413–428. [DOI] [PubMed] [Google Scholar]
- Grammatikakis I., Panda A. C., Abdelmohsen K., Gorospe M. (2014). Long noncoding RNAs(lncRNAs) and the molecular hallmarks of aging. Aging (Albany NY) 6, 992–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco S., Gorospe M., Martelli F. (2015). Noncoding RNA in age-related cardiovascular diseases. J Mol Cell Cardiol 83, 142–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacisuleyman E., Goff L. A., Trapnell C., Williams A., Henao-Mejia J., Sun L., McClanahan P., Hendrickson D. G., Sauvageau M., Kelley D. R., et al. (2014). Topological organization of multichromosomal regions by the long intergenic noncoding RNA Firre. Nat Struct Mol Biol 21, 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T. B., Jensen T. I., Clausen B. H., Bramsen J. B., Finsen B., Damgaard C. K., Kjems J. (2013). Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388. [DOI] [PubMed] [Google Scholar]
- Hu G., Yang T., Zheng J., Dai J., Nan A., Lai Y., Zhang Y., Yang C., Jiang Y. (2015a). Functional role and mechanism of lncRNA LOC728228 in malignant 16HBE cells transformed by anti-benzopyrene-trans-7,8-dihydrodiol-9,10-epoxide. Mol Carcinog 54 Suppl 1, E192–E204. [DOI] [PubMed] [Google Scholar]
- Hu L., Ye H., Huang G., Luo F., Liu Y., Liu Y., Yang X., Shen J., Liu Q., Zhang J. (2016). Long noncoding RNA GAS5 suppresses the migration and invasion of hepatocellular carcinoma cells via miR-21. Tumour Biol 37, 2691–2702. [DOI] [PubMed] [Google Scholar]
- Hu Y. W., Yang J. Y., Ma X., Chen Z. P., Hu Y. R., Zhao J. Y., Li S. F., Qiu Y. R., Lu J. B., Wang Y. C., et al. (2014). A lincRNA-DYNLRB2-2/GPR119/GLP-1R/ABCA1-dependent signal transduction pathway is essential for the regulation of cholesterol homeostasis. J Lipid Res 55, 681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y. W., Zhao J. Y., Li S. F., Huang J. L., Qiu Y. R., Ma X., Wu S. G., Chen Z. P., Hu Y. R., Yang J. Y., et al. (2015b). RP5-833A20.1/miR-382-5p/NFIA-dependent signal transduction pathway contributes to the regulation of cholesterol homeostasis and inflammatory reaction. Arterioscler Thromb Vasc Biol 35, 87–101. [DOI] [PubMed] [Google Scholar]
- Huang G., Wu X., Li S., Xu X., Zhu H., Chen X. (2016). The long noncoding RNA CASC2 functions as a competing endogenous RNA by sponging miR-18a in colorectal cancer. Sci Rep 6, 26524.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zhou N., Watabe K., Lu Z., Wu F., Xu M., Mo Y. Y. (2014). Long non-coding RNA UCA1 promotes breast tumor growth by suppression of p27 (Kip1). Cell Death Dis 5, e1008.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Damle S. S., Booten S., Singh P., Sabripour M., Hsu J., Jo M., Katz M., Watt A., Hart C. E., et al. (2015). Partial hepatectomy induced long noncoding RNA inhibits hepatocyte proliferation during liver regeneration. PLoS One 10, e0132798.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Yuan T., Tschannen M., Sun Z., Jacob H., Du M., Liang M., Dittmar R. L., Liu Y., Liang M., et al. (2013). Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics 14, 319.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarte M., Guttman M., Feldser D., Garber M., Koziol M. J., Kenzelmann-Broz D., Khalil A. M., Zuk O., Amit I., Rabani M., et al. (2010). A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 142, 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S., Sajini A. A., Blanco S., Dietmann S., Lombard P., Sugimoto Y., Paramor M., Gleeson J. G., Odom D. T., Ule J., et al. (2013). NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep 4, 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (2004). Some drinking-water disinfectants and contaminants, including arsenic. IARC Monogr Eval Carcinog Risks Hum 84, 1–477. [PMC free article] [PubMed] [Google Scholar]
- Jalali S., Bhartiya D., Lalwani M. K., Sivasubbu S., Scaria V. (2013). Systematic transcriptome wide analysis of lncRNA-miRNA interactions. PLoS One 8, e53823.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Z., Song R., Regev A., Struhl K. (2015). Many lncRNAs, 5'UTRs, and pseudogenes are translated and some are likely to express functional proteins. Elife 4, e08890.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. (2012). Long non-coding RNAs in Huntington's disease neurodegeneration. Neurobiol Dis 46, 245–254. [DOI] [PubMed] [Google Scholar]
- Johnson R., Richter N., Jauch R., Gaughwin P. M., Zuccato C., Cattaneo E., Stanton L. W. (2010). Human accelerated region 1 noncoding RNA is repressed by REST in Huntington's disease. Physiol Genomics 41, 269–274. [DOI] [PubMed] [Google Scholar]
- Karlsson O., Baccarelli A. A. (2016). Environmental health and long non-coding RNAs. Curr Environ Health Rep 3, 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal A., Bacolla A., Vasquez K. M., Jain A. (2015). Long non-coding RNA: A new paradigm for lung cancer. Mol Carcinog 54, 1235–1251. [DOI] [PubMed] [Google Scholar]
- Kim J., Kim K. M., Noh J. H., Yoon J. H., Abdelmohsen K., Gorospe M. (2016). Long noncoding RNAs in diseases of aging. Biochim Biophys Acta 1859, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen C. D., Casarett L. J., Doull J. (2013) Casarett and Doull's toxicology: the basic science of poisons. McGraw-Hill Education, New York. [Google Scholar]
- Kong B., Wang L., Chiang J. Y., Zhang Y., Klaassen C. D., Guo G. L. (2012). Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology 56, 1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kour S., Rath P. C. (2016). Long noncoding RNAs in aging and age-related diseases. Ageing Res Rev 26, 1–21. [DOI] [PubMed] [Google Scholar]
- Kumamoto T., Oshio S. (2013). Effect of fetal exposure to bisphenol A on brain mediated by X-chromosome inactivation. J Toxicol Sci 38, 485–494. [DOI] [PubMed] [Google Scholar]
- Lan X., Yan J., Ren J., Zhong B., Li J., Li Y., Liu L., Yi J., Sun Q., Yang X., et al. (2016). A novel long noncoding RNA Lnc-HC binds hnRNPA2B1 to regulate expressions of Cyp7a1 and Abca1 in hepatocytic cholesterol metabolism. Hepatology 64, 58–72. [DOI] [PubMed] [Google Scholar]
- LaRocca J., Binder A. M., McElrath T. F., Michels K. B. (2014). The impact of first trimester phthalate and phenol exposure on IGF2/H19 genomic imprinting and birth outcomes. Environ Res 133, 396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempiainen H., Couttet P., Bolognani F., Muller A., Dubost V., Luisier R., Del Rio Espinola A., Vitry V., Unterberger E. B., Thomson J. P., et al. (2013). Identification of Dlk1-Dio3 imprinted gene cluster noncoding RNAs as novel candidate biomarkers for liver tumor promotion. Toxicol Sci 131, 375–386. [DOI] [PubMed] [Google Scholar]
- Li A., Zhang J., Zhou Z., Wang L., Liu Y., Liu Y. (2015a). ALDB: A domestic-animal long noncoding RNA database. PLoS One 10, e0124003.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Chen J., Zhang K., Feng B., Wang R., Chen L. (2015b). Progress and prospects of long noncoding RNAs (lncRNAs) in hepatocellular carcinoma. Cell Physiol Biochem 36, 423–434. [DOI] [PubMed] [Google Scholar]
- Li J., Ma W., Zeng P., Wang J., Geng B., Yang J., Cui Q. (2015c). LncTar: A tool for predicting the RNA targets of long noncoding RNAs. Brief Bioinform 16, 806–812. [DOI] [PubMed] [Google Scholar]
- Li J. H., Liu S., Zheng L. L., Wu J., Sun W. J., Wang Z. L., Zhou H., Qu L. H., Yang J. H. (2014a). Discovery of protein-lncRNA interactions by integrating large-scale CLIP-seq and RNA-seq datasets. Front Bioeng Biotechnol 2, 88.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. H., Liu S., Zhou H., Qu L. H., Yang J. H. (2014b). starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res 42, D92–D97. 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Ruan X., Yang L., Kiesewetter K., Zhao Y., Luo H., Chen Y., Gucek M., Zhu J., Cao H. (2015d). A liver-enriched long non-coding RNA, lncLSTR, regulates systemic lipid metabolism in mice. Cell Metab 21, 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Chiang J. Y. (2015). Bile acids as metabolic regulators. Curr Opin Gastroenterol 31, 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Notani D., Rosenfeld M. G. (2016a). Enhancers as non-coding RNA transcription units: recent insights and future perspectives. Nat Rev Genet 17, 207–223. [DOI] [PubMed] [Google Scholar]
- Li W., Sun M., Zang C., Ma P., He J., Zhang M., Huang Z., Ding Y., Shu Y. (2016b). Upregulated long non-coding RNA AGAP2-AS1 represses LATS2 and KLF2 expression through interacting with EZH2 and LSD1 in non-small-cell lung cancer cells. Cell Death Dis 7, e2225.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J., He Q., Li M., Chen Y., Liu Y., Wang J. (2016). LncRNA MIAT: Myocardial infarction associated and more. Gene 578, 158–161. [DOI] [PubMed] [Google Scholar]
- Lin A., Li C., Xing Z., Hu Q., Liang K., Han L., Wang C., Hawke D. H., Wang S., Zhang Y., et al. (2016). The LINK-A lncRNA activates normoxic HIF1alpha signalling in triple-negative breast cancer. Nat Cell Biol 18, 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N., Chang K. Y., Li Z., Gates K., Rana Z. A., Dang J., Zhang D., Han T., Yang C. S., Cunningham T. J., et al. (2014). An evolutionarily conserved long noncoding RNA TUNA controls pluripotency and neural lineage commitment. Mol Cell 53, 1005–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Wu H. T., Zhu N., Shi Y. N., Liu Z., Ao B. X., Liao D. F., Zheng X. L., Qin L. (2016a). Steroid receptor RNA activator: Biologic function and role in disease. Clin Chim Acta 459, 137–146. [DOI] [PubMed] [Google Scholar]
- Liu F., Xiang G., Jiang D., Zhang L., Chen X., Liu L., Luo F., Li Y., Liu C., Pu X. (2015). Ultrasensitive strategy based on PtPd nanodendrite/nano-flower-like@GO signal amplification for the detection of long non-coding RNA. Biosens Bioelectron 74, 214–221. [DOI] [PubMed] [Google Scholar]
- Liu J., Waalkes M. P. (2008). Liver is a target of arsenic carcinogenesis. Toxicol Sci 105, 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. X., Deng W., Zhou X. T., Chen R. P., Xiang M. Q., Guo Y. T., Pu Z. J., Li R., Wang G. F., Wu L. F. (2016b). The mechanism of adenosine-mediated activation of lncRNA MEG3 and its antitumor effects in human hepatoma cells. Int J Oncol 48, 421–429. [DOI] [PubMed] [Google Scholar]
- Liu S., Sheng L., Miao H., Saunders T. L., MacDougald O. A., Koenig R. J., Xu B. (2014a). SRA gene knockout protects against diet-induced obesity and improves glucose tolerance. J Biol Chem 289, 13000–13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Huang Y., Chen J., Chi H., Yu Z., Wang J., Chen C. (2014b). Attenuated ability of BACE1 to cleave the amyloid precursor protein via silencing long noncoding RNA BACE1AS expression. Mol Med Rep 10, 1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Xiao Z. D., Han L., Zhang J., Lee S. W., Wang W., Lee H., Zhuang L., Chen J., Lin H. K., et al. (2016c). LncRNA NBR2 engages a metabolic checkpoint by regulating AMPK under energy stress. Nat Cell Biol 18, 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wang B., Liu X., Lu L., Luo F., Lu X., Shi L., Xu W., Liu Q. (2016d). Epigenetic silencing of p21 by long non-coding RNA HOTAIR is involved in the cell cycle disorder induced by cigarette smoke extract. Toxicol Lett 240, 60–67. [DOI] [PubMed] [Google Scholar]
- Lu L., Xu H., Luo F., Liu X., Lu X., Yang Q., Xue J., Chen C., Shi L., Liu Q. (2016a). Epigenetic silencing of miR-218 by the lncRNA CCAT1, acting via BMI1, promotes an altered cell cycle transition in the malignant transformation of HBE cells induced by cigarette smoke extract. Toxicol Appl Pharmacol 304, 30–41. [DOI] [PubMed] [Google Scholar]
- Lu Q., Ren S., Lu M., Zhang Y., Zhu D., Zhang X., Li T. (2013). Computational prediction of associations between long non-coding RNAs and proteins. BMC Genomics 14, 651.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Xiao Z., Liu F., Cui M., Li W., Yang Z., Li J., Ye L., Zhang X. (2016b). Long non-coding RNA HULC promotes tumor angiogenesis in liver cancer by up-regulating sphingosine kinase 1 (SPHK1). Oncotarget 7, 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J., Qiu M., Xia W., Liu C., Xu Y., Wang J., Leng X., Huang S., Zhu R., Zhao M., et al. (2016). High expression of long non-coding RNA SBF2-AS1 promotes proliferation in non-small cell lung cancer. J Exp Clin Cancer Res 35, 75.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G., Wang Q., Lv C., Qiang F., Hua Q., Chu H., Du M., Tong N., Jiang Y., Wang M., et al. (2015a). The prognostic significance of HOTAIR for predicting clinical outcome in patients with digestive system tumors. J Cancer Res Clin Oncol 141, 2139–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Ay F., Lee C., Gulsoy G., Deng X., Cook S., Hesson J., Cavanaugh C., Ware C. B., Krumm A., et al. (2015b). Fine-scale chromatin interaction maps reveal the cis-regulatory landscape of human lincRNA genes. Nat Methods 12, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistri M., Velmeshev D., Makhmutova M., Faghihi M. A. (2015). Transcriptomics profiling of alzheimer's disease reveal neurovascular defects, altered amyloid-beta homeostasis, and deregulated expression of long noncoding RNAs. J Alzheimers Dis 48, 647–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y. S., Sunwoo H., Zhang B., Spector D. L. (2011). Direct visualization of the co-transcriptional assembly of a nuclear body by noncoding RNAs. Nat Cell Biol 13, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariner P. D., Walters R. D., Espinoza C. A., Drullinger L. F., Wagner S. D., Kugel J. F., Goodrich J. A. (2008). Human Alu RNA is a modular transacting repressor of mRNA transcription during heat shock. Mol Cell 29, 499–509. [DOI] [PubMed] [Google Scholar]
- Marrone A. K., Beland F. A., Pogribny I. P. (2014). Noncoding RNA response to xenobiotic exposure: An indicator of toxicity and carcinogenicity. Expert Opin Drug Metab Toxicol 10, 1409–1422. [DOI] [PubMed] [Google Scholar]
- Martens J. A., Laprade L., Winston F. (2004). Intergenic transcription is required to repress the Saccharomyces cerevisiae SER3 gene. Nature 429, 571–574. [DOI] [PubMed] [Google Scholar]
- Martianov I., Ramadass A., Serra Barros A., Chow N., Akoulitchev A. (2007). Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature 445, 666–670. [DOI] [PubMed] [Google Scholar]
- Mayama T., Marr A. K., Kino T. (2016). Differential expression of glucocorticoid receptor noncoding RNA repressor Gas5 in autoimmune and inflammatory diseases. Horm Metab Res 48, 550–557. [DOI] [PubMed] [Google Scholar]
- Mele M., Rinn J. L. (2016). “Cat's Cradling” the 3D genome by the act of lncRNA transcription. Mol Cell 62, 657–664. [DOI] [PubMed] [Google Scholar]
- Montes M., Lund A. H. (2016). Emerging roles of lncRNAs in senescence. FEBS J 283, 2414–2426. [DOI] [PubMed] [Google Scholar]
- Montes M., Nielsen M. M., Maglieri G., Jacobsen A., Hojfeldt J., Agrawal-Singh S., Hansen K., Helin K., van de Werken H. J., Pedersen J. S., et al. (2015). The lncRNA MIR31HG regulates p16(INK4A) expression to modulate senescence. Nat Commun 6, 6967.. [DOI] [PubMed] [Google Scholar]
- Moran I., Akerman I., van de Bunt M., Xie R., Benazra M., Nammo T., Arnes L., Nakic N., Garcia-Hurtado J., Rodriguez-Segui S., et al. (2012). Human beta cell transcriptome analysis uncovers lncRNAs that are tissue-specific, dynamically regulated, and abnormally expressed in type 2 diabetes. Cell Metab 16, 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B. R., Makarewich C. A., Anderson D. M., Winders B. R., Troupes C. D., Wu F., Reese A. L., McAnally J. R., Chen X., Kavalali E. T., et al. (2016). A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science 351, 271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikova I. V., Hennelly S. P., Sanbonmatsu K. Y. (2012). Sizing up long non-coding RNAs: Do lncRNAs have secondary and tertiary structure? Bioarchitecture 2, 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevopoulou M. D., Georgakilas G., Kostoulas N., Reczko M., Maragkakis M., Dalamagas T. M., Hatzigeorgiou A. G. (2013). DIANA-LncBase: experimentally verified and computationally predicted microRNA targets on long non-coding RNAs. Nucleic Acids Res 41, D239–D245. 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B., Yoo K. H., Kim C. (2015). Hematopoietic stem cell expansion and generation: The ways to make a breakthrough. Blood Res 50, 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C., Yu N., Choi I., Kim W., Lee S. (2014). lncRNAtor: A comprehensive resource for functional investigation of long non-coding RNAs. Bioinformatics 30, 2480–2485. [DOI] [PubMed] [Google Scholar]
- Popadin K., Gutierrez-Arcelus M., Dermitzakis E. T., Antonarakis S. E. (2013). Genetic and epigenetic regulation of human lincRNA gene expression. Am J Hum Genet 93, 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvvula P. K., Desetty R. D., Pineau P., Marchio A., Moon A., Dejean A., Bischof O. (2014). Long noncoding RNA PANDA and scaffold-attachment-factor SAFA control senescence entry and exit. Nat Commun 5, 5323.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J., Yao H., Xia Y., Chu P., Li M., Wu Y., Li W., Ding L., Qi K., Li D., et al. (2016). Long non-coding RNAs expression profiles in hepatocytes of mice after hematopoietic stem cell transplantation. IUBMB Life 68, 232–241. [DOI] [PubMed] [Google Scholar]
- Quek X. C., Thomson D. W., Maag J. L., Bartonicek N., Signal B., Clark M. B., Gloss B. S., Dinger M. E. (2015). lncRNAdb v2.0: Expanding the reference database for functional long noncoding RNAs. Nucleic Acids Res 43, D168–D173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani A., Kumar A., Lal A., Pant M. (2014). Cellular mechanisms of cadmium-induced toxicity: A review. Int J Environ Health Res 24, 378–399. [DOI] [PubMed] [Google Scholar]
- Recio L., Phillips S. L., Maynor T., Waters M., Jackson A. F., Yauk C. L. (2013). Differential expression of long noncoding RNAs in the livers of female B6C3F1 mice exposed to the carcinogen furan. Toxicol Sci 135, 369–379. [DOI] [PubMed] [Google Scholar]
- Reddy M. A., Chen Z., Park J. T., Wang M., Lanting L., Zhang Q., Bhatt K., Leung A., Wu X., Putta S., et al. (2014). Regulation of inflammatory phenotype in macrophages by a diabetes-induced long noncoding RNA. Diabetes 63, 4249–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan X. (2016). Long non-coding RNA central of glucose homeostasis. J Cell Biochem 117, 1061–1065. [DOI] [PubMed] [Google Scholar]
- Ruan X., Li P., Cangelosi A., Yang L., Cao H. (2016). A long non-coding RNA, lncLGR, regulates hepatic glucokinase expression and glycogen storage during fasting. Cell Rep 14, 1867–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan K., Jiang Q., Wang X. Q., Wang Y. N., Yang H., Yao M. D., Liu C., Li X. M., Yao J., Liu B., et al. (2016). Role of long non-coding RNA-RNCR3 in atherosclerosis-related vascular dysfunction. Cell Death Dis 7, e2248.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H. S., Seo J. H., Jeong S. H., Park S. W., Park Y., Son S. W., Kim J. S., Kang H. G. (2014). Exposure of pregnant mice to chlorpyrifos-methyl alters embryonic H19 gene methylation patterns. Environ Toxicol 29, 926–935. [DOI] [PubMed] [Google Scholar]
- Skinner M. K. (2016). Endocrine disruptors in 2015: Epigenetic transgenerational inheritance. Nat Rev Endocrinol 12, 68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Liu J., He K., Zhang M., Feng C., Peng F., Li B., Xia X. (2016). Overexpression of the long noncoding RNA TUG1 protects against cold-induced injury of mouse livers by inhibiting apoptosis and inflammation. FEBS J 283, 1261–1274. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y., Konig J., Hussain S., Zupan B., Curk T., Frye M., Ule J. (2012). Analysis of CLIP and iCLIP methods for nucleotide-resolution studies of protein-RNA interactions. Genome Biol 13, R67.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunwoo J. S., Lee S. T., Im W., Lee M., Byun J. I., Jung K. H., Park K. I., Jung K. Y., Lee S. K., Chu K., et al. (2016). Altered expression of the long noncoding RNA NEAT1 in Huntington's disease. Mol Neurobiol. doi:10.1007/s12035-016-9928-9. [DOI] [PubMed] [Google Scholar]
- Szafranski K., Abraham K. J., Mekhail K. (2015). Non-coding RNA in neural function, disease, and aging. Front Genet 6, 87.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegedi K., Sonkoly E., Nagy N., Nemeth I. B., Bata-Csorgo Z., Kemeny L., Dobozy A., Szell M. (2010). The anti-apoptotic protein G1P3 is overexpressed in psoriasis and regulated by the non-coding RNA, PRINS. Exp Dermatol 19, 269–278. [DOI] [PubMed] [Google Scholar]
- Tani H., Onuma Y., Ito Y., Torimura M. (2014). Long non-coding RNAs as surrogate indicators for chemical stress responses in human-induced pluripotent stem cells. PLoS One 9, e106282.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H., Takeshita J. I., Aoki H., Abe R., Toyoda A., Endo Y., Miyamoto S., Gamo M., Torimura M. (2016). Genome-wide gene expression analysis of mouse embryonic stem cells exposed to p-dichlorobenzene. J Biosci Bioeng 122, 329–333. [DOI] [PubMed] [Google Scholar]
- Thai P., Statt S., Chen C. H., Liang E., Campbell C., Wu R. (2013). Characterization of a novel long noncoding RNA, SCAL1, induced by cigarette smoke and elevated in lung cancer cell lines. Am J Respir Cell Mol Biol 49, 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tome J. M., Ozer A., Pagano J. M., Gheba D., Schroth G. P., Lis J. T. (2014). Comprehensive analysis of RNA-protein interactions by high-throughput sequencing-RNA affinity profiling. Nat Methods 11, 683–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Dimmeler S. (2015). Long noncoding RNAs in cardiovascular diseases. Circ Res 116, 737–750. [DOI] [PubMed] [Google Scholar]
- Ule J., Jensen K. B., Ruggiu M., Mele A., Ule A., Darnell R. B. (2003). CLIP identifies Nova-regulated RNA networks in the brain. Science 302, 1212–1215. [DOI] [PubMed] [Google Scholar]
- Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J. J., Lotvall J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9, 654–659. [DOI] [PubMed] [Google Scholar]
- Wan L., Sun M., Liu G. J., Wei C. C., Zhang E. B., Kong R., Xu T. P., Huang M. D., Wang Z. X. (2016). Long noncoding RNA PVT1 promotes non-small cell lung cancer cell proliferation through epigenetically regulating LATS2 expression. Mol Cancer Ther 15, 1082–1094. [DOI] [PubMed] [Google Scholar]
- Wang K. C., Chang H. Y. (2011). Molecular mechanisms of long noncoding RNAs. Mol Cell 43, 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. C., Yang Y. W., Liu B., Sanyal A., Corces-Zimmerman R., Chen Y., Lajoie B. R., Protacio A., Flynn R. A., Gupta R. A., et al. (2011). A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472, 120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Sun W., Shen W., Xia M., Chen C., Xiang D., Ning B., Cui X., Li H., Li X., et al. (2016). Long non-coding RNA DILC regulates liver cancer stem cells via IL-6/STAT3 axis. J Hepatol 64, 1283–1294. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Houten S. M., Mataki C., Christoffolete M. A., Kim B. W., Sato H., Messaddeq N., Harney J. W., Ezaki O., Kodama T., et al. (2006). Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439, 484–489. [DOI] [PubMed] [Google Scholar]
- Wei S., Du M., Jiang Z., Hausman G. J., Zhang L., Dodson M. V. (2016). Long noncoding RNAs in regulating adipogenesis: New RNAs shed lights on obesity. Cell Mol Life Sci 73, 2079–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham A. T., Orth A. P., Batalov S., Peters E. C., Wen B. G., Aza-Blanc P., Hogenesch J. B., Schultz P. G. (2005). A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science 309, 1570–1573. [DOI] [PubMed] [Google Scholar]
- Wu Q., Ohsako S., Ishimura R., Suzuki J. S., Tohyama C. (2004). Exposure of mouse preimplantation embryos to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters the methylation status of imprinted genes H19 and Igf2. Biol Reprod 70, 1790–1797. [DOI] [PubMed] [Google Scholar]
- Xing Z., Lin C., Yang L. (2016). LncRNA pulldown combined with mass spectrometry to identify the novel lncRNA-associated proteins. Methods Mol Biol 1402, 1–9. [DOI] [PubMed] [Google Scholar]
- Xuan H., Zhang L., Liu X., Han G., Li J., Li X., Liu A., Liao M., Zhang S. (2015). PLNlncRbase: A resource for experimentally identified lncRNAs in plants. Gene 573, 328–332. [DOI] [PubMed] [Google Scholar]
- Xue Y., Gu D., Ma G., Zhu L., Hua Q., Chu H., Tong N., Chen J., Zhang Z., Wang M. (2015a). Genetic variants in lncRNA HOTAIR are associated with risk of colorectal cancer. Mutagenesis 30, 303–310. [DOI] [PubMed] [Google Scholar]
- Xue Y., Ma G., Gu D., Zhu L., Hua Q., Du M., Chu H., Tong N., Chen J., Zhang Z., et al. (2015b). Genome-wide analysis of long noncoding RNA signature in human colorectal cancer. Gene 556, 227–234. [DOI] [PubMed] [Google Scholar]
- Xue Y., Ma G., Zhang Z., Hua Q., Chu H., Tong N., Yuan L., Qin C., Yin C., Zhang Z., et al. (2015c). A novel antisense long noncoding RNA regulates the expression of MDC1 in bladder cancer. Oncotarget 6, 484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y., Wang M., Kang M., Wang Q., Wu B., Chu H., Zhong D., Qin C., Yin C., Zhang Z., et al. (2013). Association between lncrna PCGEM1 polymorphisms and prostate cancer risk. Prostate Cancer Prostatic Dis 16, 139–144. S131. [DOI] [PubMed] [Google Scholar]
- Yan C., Chen J., Chen N. (2016). Long noncoding RNA MALAT1 promotes hepatic steatosis and insulin resistance by increasing nuclear SREBP-1c protein stability. Sci Rep 6, 22640.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Su Z., Song X., Liang B., Zeng F., Chang X., Huang D. (2016). Enhancer RNA-driven looping enhances the transcription of the long noncoding RNA DHRS4-AS1, a controller of the DHRS4 gene cluster. Sci Rep 6, 20961.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Xi P., Xie Y., Zhao C., Xu J., Jiang J. (2015a). Notoginsenoside R1 reduces blood pressure in spontaneously hypertensive rats through a long non-coding RNA AK094457. Int J Clin Exp Pathol 8, 2700–2709. [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Di C., Hu B., Zhou M., Liu Y., Song N., Li Y., Umetsu J., Lu Z. J. (2015b). CLIPdb: A CLIP-seq database for protein-RNA interactions. BMC Genomics 16, 51.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D. D., Zhang E. B., You L. H., Wang N., Wang L. T., Jin F. Y., Zhu Y. N., Cao L. H., Yuan Q. X., De W., et al. (2015). Downregulation of lncRNA TUG1 affects apoptosis and insulin secretion in mouse pancreatic beta cells. Cell Physiol Biochem 35, 1892–1904. [DOI] [PubMed] [Google Scholar]
- Zang C., Nie F. Q., Wang Q., Sun M., Li W., He J., Zhang M., Lu K. H. (2016). Long non-coding RNA LINC01133 represses KLF2, P21 and E-cadherin transcription through binding with EZH2, LSD1 in non-small cell lung cancer. Oncotarget 7, 11696–11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel M. B., Baumert T. F. (2016). Translation and protein expression of lncRNAs: Impact for liver disease and hepatocellular carcinoma. Hepatology 64, 671–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zhou Y., Wu Y., Ma L., Fan Y., Kang X., Shi H. (2012). Isolation and characterization of a novel noncoding RNA from nickel-induced lung cancer. Biol Trace Elem Res 150, 258–263. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Feng Y., Hu Z., Hu X., Yuan C. -X., Fan Y., Zhang L. (2016a) Characterization of long noncoding RNA-associated proteins by RNA-immunoprecipitation. In Long Non-Coding RNAs: Methods and Protocols (Y. Feng and L. Zhang, Eds), pp. 19–26. Springer, New York. [DOI] [PMC free article] [PubMed]
- Zhang Y., Liu C., Barbier O., Smalling R., Tsuchiya H., Lee S., Delker D., Zou A., Hagedorn C. H., Wang L. (2016b). Bcl2 is a critical regulator of bile acid homeostasis by dictating Shp and lncRNA H19 function. Sci Rep 6, 20559.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Shi X., Castranova V., Ding M. (2009). Occupational toxicology of nickel and nickel compounds. J Environ Pathol Toxicol Oncol 28, 177–208. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Li H., Fang S., Kang Y., Wu W., Hao Y., Li Z., Bu D., Sun N., Zhang M. Q., et al. (2016a). NONCODE 2016: An informative and valuable data source of long non-coding RNAs. Nucleic Acids Res 44, D203–D208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Yuan J., Chen R. (2016b). NONCODEv4: Annotation of noncoding RNAs with emphasis on long noncoding RNAs. Methods Mol Biol 1402, 243–254. [DOI] [PubMed] [Google Scholar]
- Zhou C. C., Yang F., Yuan S. X., Ma J. Z., Liu F., Yuan J. H., Bi F. R., Lin K. Y., Yin J. H., Cao G. W., et al. (2016a). Systemic genome screening identifies the outcome associated focal loss of long noncoding RNA PRAL in hepatocellular carcinoma. Hepatology 63, 850–863. [DOI] [PubMed] [Google Scholar]
- Zhou T., Ding J. W., Wang X. A., Zheng X. X. (2016b). Long noncoding RNAs and atherosclerosis. Atherosclerosis 248, 51–61. [DOI] [PubMed] [Google Scholar]
- Zhou X., Xu J. (2015). Identification of Alzheimer's disease-associated long noncoding RNAs. Neurobiol Aging 36, 2925–2931. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Liu H., Wang C., Lu Q., Huang Q., Zheng C., Lei Y. (2015a). Long non-coding RNAs as novel expression signatures modulate DNA damage and repair in cadmium toxicology. Sci Rep 5, 15293.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Shen Y., Khan M. R., Li A. (2015b). LncReg: A reference resource for lncRNA-associated regulatory networks. Database (Oxford). doi: 10.1093/database/bav083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Fu H., Wu Y., Zheng X. (2013). Function of lncRNAs and approaches to lncRNA-protein interactions. Sci China Life Sci 56, 876–885. [DOI] [PubMed] [Google Scholar]
- Zhu X., Wu Y. B., Zhou J., Kang D. M. (2016). Upregulation of lncRNA MEG3 promotes hepatic insulin resistance via increasing FoxO1 expression. Biochem Biophys Res Commun 469, 319–325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.