Abstract
DNA primases are responsible for the synthesis of the short RNA primers that are used by the replicative DNA polymerases to initiate DNA synthesis on the leading- and lagging-strand at the replication fork. In this study, we report the purification and biochemical characterization of a DNA primase (Sso DNA primase) from the thermoacidophilic crenarchaeon Sulfolobus solfataricus. The Sso DNA primase is a heterodimer composed of two subunits of 36 kDa (small subunit) and 38 kDa (large subunit), which show sequence similarity to the eukaryotic DNA primase p60 and p50 subunits, respectively. The two polypeptides were co-expressed in Escherichia coli and purified as a heterodimeric complex, with a Stokes radius of about 39.2 Å and a 1:1 stoichiometric ratio among its subunits. The Sso DNA primase utilizes poly-pyrimidine single-stranded DNA templates with low efficiency for de novo synthesis of RNA primers, whereas its synthetic function is specifically activated by thymine-containing synthetic bubble structures that mimic early replication intermediates. Interestingly, the Sso DNA primase complex is endowed with a terminal nucleotidyl-tranferase activity, being able to incorporate nucleotides at the 3′ end of synthetic oligonucleotides in a non-templated manner.
INTRODUCTION
DNA primases are essential components of all characterized systems of DNA replication. These enzymes catalyze the synthesis of short RNA primers on single-stranded (ss) DNA templates, which are used by DNA polymerases to initiate DNA synthesis at the replication fork (1). In addition, DNA primase activity is required for the checkpoint pathway, coupling DNA replication to repair (2). Saccharomyces cerevisiae cells that contain mutations in the DNA primase small subunit gene fail to delay entry into S phase and do not stop replication in response to DNA-damaging agents (3).
DNA primases from bacteria are usually associated with replicative DNA helicases (4). In Escherichia coli, the synthetic function of DNA primase (DnaG) is modulated by the association with the replicative hexameric DNA helicase (DnaB; 5). In the T7 (6), P4 (7) and SP6 (8) bacteriophages the DNA primase and helicase activities are combined in a single polypeptide. On the other hand, in eukaryotes, the DNA primase activity co-purifies with DNA polymerase α as a complex composed of four subunits with approximate molecular masses of 180, 70, 60 and 50 kDa. Within this complex, the 180 kDa polypeptide (DNA polymerase α A subunit) is responsible for the polymerase activity; the 60 and 50 kDa polypeptides form the DNA primase; and the 70 kDa protein (B subunit) was proposed to have a regulatory role, being phosphorylated in a cell-cycle dependent manner, and to tether the entire complex to the replication fork via interactions with other protein factors [for a review see (9)]. The genes coding for all the subunits of the DNA polymerase α-primase complex were identified in humans, rats, mice, Drosophila and Saccharomyces cerevisiae, and the protein complexes were purified and biochemically characterized from these sources (4). It was demonstrated that the primase 50 kDa subunit is sufficient for RNA primer synthesis, although it is highly unstable and far less efficient when compared with the heterodimeric complex (10–12). On the other hand, the 60 kDa subunit was shown to bind ssDNA and the RNA–DNA product formed after primer synthesis, to stabilize the 50 kDa subunit and to physically interact with the 180 kDa DNA polymerase α polypeptide. Nevertheless, the precise function of the 60 kDa subunit is not yet well understood and it was hypothesized to mediate the transfer of the newly generated RNA primer to the DNA polymerase α active site (13–14).
An important feature of the bacterial and viral DNA primases is their ability to recognize specific DNA sequences, termed ‘primase recognition sites’, and to start RNA synthesis at these sites. These sequences differ for each of the DNA primases examined and may play an important role in coordinating lagging-strand synthesis (4). On the other hand, eukaryotic DNA primases did not display a stringent requirement for a specific recognition site, although a number of them were reported to possess a certain sequence specificity (15). An additional important feature of the DNA primases is their ability to synthesize in vitro RNA primers of defined length (usually 7–14 nt). These RNA primers can be elongated further into longer products that are primer multimers. Presently, the molecular mechanism by which DNA primases discriminate the length of their RNA products has not yet been elucidated. In the E.coli system, the interaction of the DnaG protein with the DNA polymerase III holoenzyme limits the size of the nascent primers to the range of 9–14 nt (16), whereas in the case of the eukaryotic DNA primases, the 60 kDa subunit was proposed to play a role in the specification of the product length (17).
Whereas no evident sequence similarity can be detected among DNA primases from Bacteria and Eukarya, Archaea were reported to possess heterodimeric DNA primases that share a significant sequence similarity with the subunits of eukaryotic primases (18–20). An unique feature of the archaeal DNA primases is their ability to preferentially utilize deoxyribonucleotides (dNTPs) in vitro for the de novo synthesis. The incorporation of dNTPs is ∼10-fold more efficient than the RNA primers synthesis (21). However, similar to eukaryotic systems, the small subunit of archaeal DNA primase contains the catalytic core, whereas the large non-enzymatic subunit stabilizes the heterodimer and modulates the synthetic activity of the small subunit (19–20).
In this study, we report the biochemical characterization of the eukaryotic-like heterodimeric DNA primase (Sso DNA primase) of the hyperthermophilic crenarchaeon Sulfolobus solfataricus. The ability of this enzyme to synthesize RNA primers is specifically activated by thymine-containing synthetic bubble structures that mimic early replication intermediates. Interestingly, we found that Sso DNA primase is also endowed with a terminal nucleotidyl-tranferase activity, being able to incorporate nucleotides at the 3′ end of synthetic oligonucleotides in a non-templated manner.
MATERIALS AND METHODS
Materials
All the chemicals were of reagent grade. The restriction and modification enzymes were obtained from New England Biolabs. Radioactive nucleotides were purchased from Amersham Biosciences. The oligonucleotides were synthesized by Proligo (Paris, France). The Methanococcus jannaschii DNA primase was purified as described previously (18).
Cloning of the Sso DNA primase complex
The open reading frame (ORF) that encodes the large subunit of the S.solfataricus DNA primase (Sso Pri1) was amplified from the P2 strain genomic DNA by PCR using the High Fidelity PCR system (Roche Diagnostics) with the oligonucleotide Pri1-for (5′-GGTTCATATGGGGACTTTTACATTGCACCAAGGG-3′) as the 5′-primer (the engineered NdeI site is underlined) and the oligonucleotide Pri1-rev (5′-GGTTGGATCCTCATCTAACATAAGCCTTTACCTCACCC-3′) as the 3′-primer (the engineered BamHI site is underlined). The PCR product was cloned into NdeI/BamHI-linearized E.coli expression vector pET29a (Novagen) to create the pET29–Pri1 construct. The ORF that encodes the small subunit (SsoPri2) of the S.solfataricus DNA primase was amplified from the P2 strain genomic DNA by PCR using the High Fidelity PCR system (Roche Diagnostics) with the oligonucleotide Pri2-RBS (5′-GGTTGAATTCTTTAAGAAGGAGATATACCATGGCATTAGACGTTAAAAAGTATCCTTTT-3′) as the 5′-primer (the engineered EcoRI site and an additional RBS are underlined) and the oligonucleotide Pri2-rev (5′GGGTTTGGGCTCGAGCTATTCGTTACTTAGGAAGTAGAGCTGTAAGGGATTTTTAACG-3′) as the 3′-primer (the engineered XhoI site is underlined). The PCR product was cloned into EcoRI/XhoI-linearized pET29a–Pri1 vector. This construct was then digested with BamHI, filled in and self-ligated to eliminate the unique BamHI site. This plasmid was named pET29a–PriComplex. All the cloned PCR products were sequenced at the DNA Sequencing Core [Telethon Institute of Genetics and Medicine (TIGEM)/Institute of Genetics and Biophysics (IGB)-CNR, Naples, Italy].
Expression and purification of the Sso DNA primase complex
The E.coli BL21-CodonPlus(DE3)-RIL cells (Novagen), transformed with the plasmid pET29a–PriC, were grown at 37°C in 1 litre of LB (Luria–Bertani) medium containing 30 μg/ml chloramphenicol and 30 μg/ml kanamycin. When the culture reached an A600 nm of 1 OD, protein expression was induced by addition of IPTG to 0.2 mM. The bacterial culture was then incubated overnight at 37°C. The cells were harvested by centrifugation and the pellet was resuspended in 30 ml of Buffer A (25 mM HEPES–NaOH, pH 7.5, 25 mM NaCl, 1 mM MgCl2, 1 mM MnCl2, 10% glycerol and 0.1% Triton X-100), supplemented with Protease Inhibitor Cocktail (Sigma). The cells were broken by three consecutive passages through a French pressure cell apparatus (Aminco Co., Silver Spring, MD) at 1500 p.s.i. The resulting lysate was centrifuged for 20 min at 30 000 r.p.m. (Beckman rotor 50.2 Ti) at 10°C. The supernatant was filtered through a 0.22 μm filter (Millipore) and loaded onto a Heparine Sepharose™ 6 Fast Flow column (10 ml) pre-equilibrated in Buffer A, connected to an AKTA system (Amersham Biosciences). The elution of proteins was performed with a 90 ml linear gradient of NaCl (0.025–1.0 M, flow rate: 1.0 ml/min). The Sso DNA primase complex eluted at 0.5 M NaCl concentration. The fractions containing the protein were pooled and the pool was dialyzed against Buffer B (50 mM HEPES–NaOH, pH 7.5, 100 mM NaCl, 15% glycerol, 0.1% Triton X-100). This sample was then loaded onto a Mono S column (HR 10/10, Amersham Biosciences). A 90 ml linear gradient of NaCl (0.1–1.0 M, flow rate: 1.0 ml/min) was applied to the column. The SsoDNA primase complex eluted at a NaCl concentration of 0.15 M. The pooled fractions were concentrated on a YM10 ultrafiltration membrane (Amicon) up to 2.0 ml. This sample was loaded onto a Superdex 200 gel filtration column (HiLoad 26/60 prep grade, Amersham Biosciences) pre-equilibrated in Buffer B. The chromatographic run was carried out at a flow rate of 0.2 ml/min at room temperature. The column was calibrated by running a set of gel filtration markers that included tyroglobulin, ferritin, BSA and ovalbumin. The fractions containing the Sso DNA primase complex were pooled, concentrated on a Millipore YM10 ultra-filtration membrane (Amicon) and stored at −20°C in small aliquots. The final yield of the recombinant protein after this purification procedure was ∼1.3 mg.
DNA substrates
The poly(dT) (average length: 229 nt) or poly(dC) (average length: 369 nt) were obtained from Amersham Biosciences. For the preparation of other DNA substrates, complementary oligonucleotides were annealed by incubation of their mixtures at 95°C for 5 min followed by slow cooling at room temperature. The oligo(rA)15/poly(dT) was prepared by annealing oligo(rA)15 (250 pmol) to poly(dT) (84 pmol) in a mixture of volume 20 μl, as described above.
DNA substrates containing a bubble made of duplex surrounding poly(dT) (T-Bubble), or poly(dC) (C-Bubble), were prepared by annealing oligonucleotides having the following sequences: 5′-TCTACCTGGACGACCGGG(N)20GGGCCAGCAGGTCCATCA-3′ (as the top strand), 5′-TGATGGACCTGCTGGCCC(N)20CCCGGTCGTCCAGGTAGA-3′ (as the bottom strand), where N = T, or C.
The oligonucleotides used to prepare the T-Bubble structure with arms of 28 nt (T-Bubble-la) had the following sequence: 5′-GCTCGCTACCTCTACCTGGACGACCGGG(N)20GGGCCAGCAGGTCCATCACCATCGCTCG-3′ (as the top strand) and 5′-CGAGCGATGGTGATGGACCTGCTGGCCC(N)20CCCGGTCGTCCAGGTAGAGGTAGCGAGC-3′ (as the bottom strand).
Blunt DNA duplexes of 25 bp were prepared by annealing the following fully complementary oligonucleotides: 25merTop (5′-GCTCGGTACCCGGGGATCCTCTAGA-3′), as the top strand, and 25merBottom (5′-TCTAGAGGATCCCCGGGTACCGAGC-3′), as the bottom strand.
When labelled substrates were needed, the top strand oligonucleotides were 5′-terminally labelled using T4 polynucleotide kinase and [γ-32P]ATP. Unincorporated nucleotide was removed using Quantum Prep PCR Kleen Spin columns (Bio-Rad Laboratories). The probed strand was then annealed to a 3-fold molar excess of a cold complementary oligonucleotide.
DNA primase activity assays
The reaction mixtures (20 μl) for DNA primase assays contained: 50 mM MES–NaOH, pH 6.0 (at 25°C; pH 5.5 at 60°C), 10 mM MnCl2, poly(dT) (250 fmol/μl) or poly(dC) (155 fmol/μl) or the bubble-containing substrate (10 fmol/μl), 50 μM [α-32P]ATP or 50 μM [α-32P]GTP (specific activity: 1–5 μCi/nmol), 0.5–1 μM enzyme. Samples were incubated at 60°C for 20 min. The reactions were stopped by adding 10 μl of Stop solution (97.5% formamide, 10 mM EDTA, pH 8.0, 0.3% bromophenol blue). The samples were incubated at 95°C for 5 min and 6 μl aliquots were loaded onto polyacrylamide/urea gels. The radioactive signals were analyzed using a Molecular Dynamics Storm PhosphorImager (ImageQuant software). Reactions to assay the M.jannaschii DNA primase were carried out in the conditions described above, but the buffer used was 50 mM glycine–NaOH, pH 9.0 (at 25°C; pH 8.06 at 60°C), 20 mM KCl, 10 mM MnCl2.
pH optimum
The dependence of the Sso DNA primase activity on pH was assayed at 60°C in buffer solutions whose pH was adjusted to the desired value at 60°C. The buffers systems used (50 mM final concentration) were as follows: MES–NaOH (pH 5.0 and 5.5); MOPS–NaOH (pH 5.8); HEPES–NaOH (pH 6.4 and 7.5) and Glycine–NaOH (pH 8.2). The reactions were carried out using the T-Bubble DNA substrate as the template, as described above. The amount of incorporated radioactivity was quantified using a phosphorimager.
Thermostability test
The thermal stability of the Sso DNA primase activity was measured by pre-incubating enzyme solutions (protein concentration was 0.9 mg/ml in Buffer B) at 50, 60, 70 and 80°C. After 15 min, aliquots of the incubated samples were centrifuged in a microcentrifuge at maximum speed to eliminate any precipitated material. The supernatant was assayed at 60°C using the T-Bubble DNA substrate as the template, as described above.
Terminal nucleotidyl-transferase activity assays
The 3′-terminal nucleotidyl-transferase activity was assayed in the same conditions used for the DNA primase activity. The reaction mixtures (20 μl) contained 5′-terminally labelled oligonucleotides (the C-Bubble and T-Bubble substrates, blunt DNA duplexes of 25 bp as a double-stranded DNA, and the oligonucleotide 25merTop as a ssDNA) and rNTPs (or dNTPs). The samples were incubated at 60°C for 1 h and the aliquots (6 μl) were analyzed by electrophoresis using polyacrylamide/urea gels. The radioactive signals were detected by using a phosphorimager.
RNA primer elongation assays
The reaction mixtures (20 μl) contained: 50 mM MES–NaOH, pH 6.0 (at 25°C; pH 5.5 at 60°C), 10 mM MnCl2 (or 10 mM MgCl2), oligo(rA)15/poly(dT) [210 fmol/μl, with respect to poly(dT)], 50 μM [α-32P]ATP (specific activity: 1–5 μCi/nmol), 0.5–1 μM enzyme. The samples were incubated at 60°C for 20 min. The reactions were stopped by the addition of 10 μl of Stop solution and incubated at 95°C for 5 min. Aliquots of 6 μl were loaded onto polyacrylamide/urea gels, which were analyzed by using a phosphorimager.
RESULTS
Expression and purification of Sso DNA primase complex
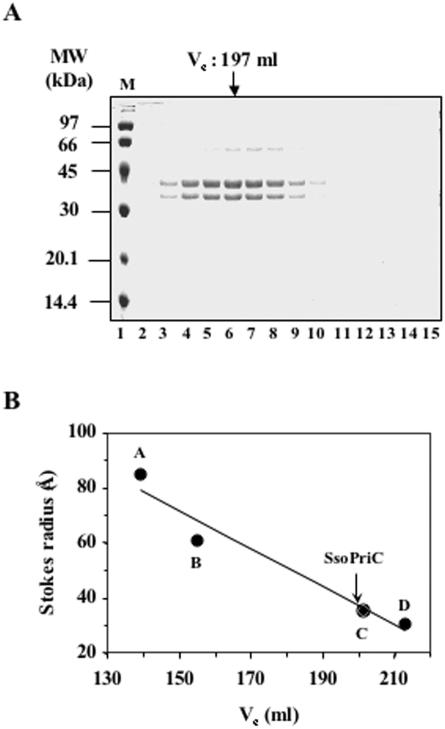
The analysis of the complete genomic sequence of the P2 strain of S.solfataricus (www.archbac.u-psud.fr/projects/sulfolobus) revealed the presence of genes coding for putative homologues of the eukaryotic DNA primase subunits (ORF SSO1048, Sso DNA primase large subunit; ORF SSO0557, Sso DNA primase small subunit). These genes were individually expressed in E.coli as hexa-Histidine tagged proteins using a pET vector. The recombinant proteins, purified by Ni2+-chelate chromatography, were both found to be poorly soluble (data not shown). In order to overcome these solubility problems, we decided to co-express the genes that code for the two Sso DNA primase subunits using a pET vector that contained the two ORFs in tandem, each preceded by an E.coli ribosome-binding site, as described previously (22). The E.coli cells harbouring this construct were found to produce both polypeptides in a soluble form upon IPTG induction. They showed apparent Mr of ∼36 and 38 kDa and their identity was confirmed by N-terminal sequence analyses (data not shown). The two polypeptides co-purified through three chromatographic steps, as described in Materials and Methods, suggest that they truly associated to form a complex. Densitometric analysis of a Coomassie-stained SDS gel of the purified Sso DNA primase revealed that the two protein bands were in a 1:1 stoichiometric ratio. In addition, a Stokes radius of about 39.2 Å was estimated by gel filtration chromatography. This value corresponds to a molecular mass of about 80 kDa, if we assume that the protein complex possesses a globular shape. These findings suggest that the Sso DNA primase complex has a heterodimeric structure (see Figure 1).
Figure 1.
Gel filtration chromatography on the recombinant Sso DNA primase complex. (A) SDS–PAGE analysis of the fractions (2 ml) from the gel filtration chromatography carried out on a Superdex 200 column, as described in Materials and Methods. The elution volume of the Sso DNA primase complex is indicated. The gel was stained with Coomassie blue. Lanes 2–15 were loaded with 20 μl aliquots of fractions 94–107; lane 1 contains marker proteins. (B) The Sso DNA primase complex Stokes radius was estimated to be about 39.2 Å from the reported plot. The gel filtration column was calibrated with the following standard proteins: A, tyroglobulin (Stokes radius: 85.0 Å, Ve: 139 ml); B, ferritin (Stokes radius: 61.0 Å, Ve: 155 ml); C, BSA (Stokes radius: 35.5 Å, Ve: 201 ml); D, ovalbumin (Stokes radius: 30.5 Å, Ve: 213 ml).
Sso DNA primase is activated by T-Bubble containing DNA substrates
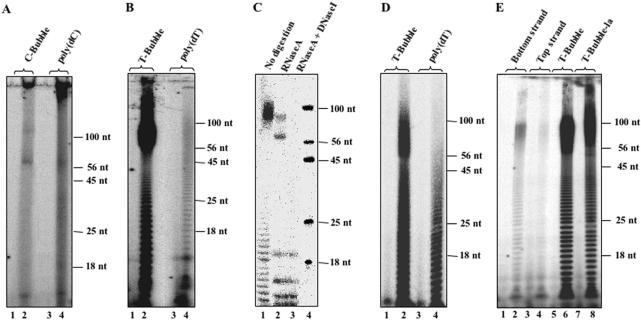
Since DNA primases are usually more active on poly-pyrimidine templates, we initially tested the ability of the Sso DNA primase to synthesize RNA primers on poly(dT) and poly(dC). We found that the enzyme was poorly active on these DNA templates, even when the reactions were carried out in the presence of radiolabelled nucleotides at high specific activity (10–50 μCi/nmol; see Figure 2A and B). In contrast, the DNA primase from the euryarchaeon M.jannaschii efficiently initiated oligoribonucleotide synthesis on either poly(dT) (Figure 2D) or poly(dC), as already reported (18). In addition, we found that the Sso DNA primase was also unable to utilize poly(dA) as a template for the synthesis of oligoribonucleotides (data not shown).
Figure 2.
Activity of the Sso DNA primase on homo-polymeric and bubble-containing DNA templates. Sso DNA primase assays (reaction volume: 20 μl) were carried out using radioactive nucleotides at 5 μM with a specific activity of 10–50 μCi/nmol, as described in Materials and Methods. Aliquots of the reaction products (6 μl) were run on 20% polyacrylamide/urea gels. Assays were carried out using the following templates: the C-Bubble substrate and poly(dC) (A); the T-Bubble substrate and poly(dT) (B); the oligonucleotides used to prepare the T-Bubble substrate (bottom and top strands), the T-Bubble substrate and the T-Bubble substrate with arms of 28 nt (T-Bubble-la; E). (C) Reaction products synthesized by Sso DNA primase on the T-Bubble substrate in standard conditions were subjected to the following treatments: no digestion (lane 1) or extensive digestion with Dnase-free Rnase A (lane 2) or with DNAse I plus Rnase A (lane 3). 5′-radiolabelled synthetic DNA oligonucleotides were run as reference (lane 4) and their length is indicated on the right side of the gel. (D) M.jannaschii DNA primase was assayed on the T-Bubble substrate and poly(dT), as described in Materials and Methods. Lanes 1 and 3 of the gels shown in A, B and D and lanes 1, 3, 5 and 7 of the gel shown in E were loaded with control reaction with no DNA template.
Since most chromosomal DNA replication origins contain AT-rich stretches and DNA primases are believed to be recruited at these sites when they are partially melted, we decided to test the ability of the Sso DNA primase to synthesize oligoribonucleotides on T-rich bubble structures that mimic an activated replication origin. The bubble substrate was assembled from two partially complementary 56 nt long oligonucleotides which formed a 20T-Bubble structure between arms of 18 bp (referred to as T-Bubble substrate). Interestingly, we found that the Sso DNA primase was able to synthesize oligoribonucleotides on this kind of DNA template in the presence of Mn2+ ions (Figure 2B), whereas Mg2+ or Zn2+ ions were not used by the enzyme as co-factors (data not shown). On the other hand, we found that synthetic DNA substrates that contain bubble structures made of cytosine residues (C-Bubble substrate) were not used by the Sso DNA primase (see Figure 2A). These results indicate that the enzyme is specifically activated by bubble structures made of thymine residues. We also found that the M.jannaschii DNA primase synthetic activity was enhanced on the T-Bubble substrate with respect to poly(dT), as shown in Figure 2D.
We also tested the ability of the Sso DNA primase to utilise the single-stranded form of the T-Bubble substrate as the template. Activity assays were carried out separately on these synthetic oligonucleotides. They were present in the assay mixtures at the same concentration that was used to prepare the T-Bubble substrate (10 fmol/μl for the bottom strand and 30 fmol/μl for the top strand). The activity of the enzyme was found to be very low on this template, confirming that the effect we observed on the T-Bubble substrate is truly due to the presence of the bubble structure (see Figure 3E). To test whether the observed activation was dependent on the distance between the bubble and the end of the duplex, assays were carried out on a T-Bubble with longer arms (T-Bubble-la, lanes 7 and 8 of Figure 2E), as described in Materials and Methods. We found that the activity of the enzyme on this template is comparable with the one observed on the T-Bubble substrate, which suggests that the activation does not depend on the length of the arms.
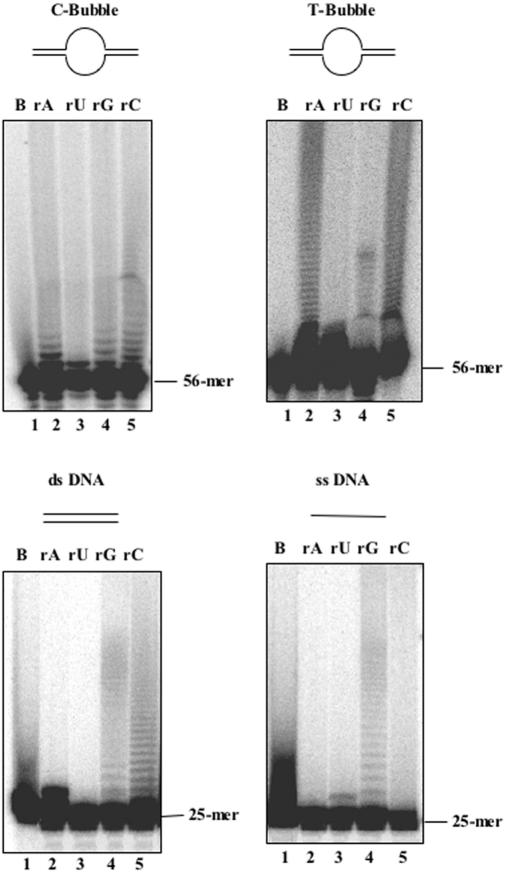
Figure 3.
Sso DNA primase has 3′-terminal nucleotidyl-transferase activity. The 3′-terminal nucleotidyl-transferase activity of Sso DNA primase was assayed on the indicated 5′-terminally labelled synthetic substrates (reaction volume: 20 μl), as described in Materials and Methods. Reaction products (6 μl of each reaction mixture) were analyzed by electrophoresis using 20% or 6% polyacrylamide/urea gels for the 25 or 56mer oligonucleotides, respectively. The radioactivity was detected by using a phosphorimager. Lanes 2–5 of each gel were loaded with reaction mixtures containing only the indicated rNTP in addition to the DNA template and the enzyme, whereas lane 1 contains a control reaction without enzyme.
As shown in Figure 2B and 2D, when the T-Bubble substrate was used, the reaction products displayed a bimodal distribution, with the bulk of synthesis centred around 10–40 nt and from 56 to >100 nt. Since each bubble arm was 20 nt long, we hypothesized that the products ranging from 20 to 40 nt could be due to the ability of DNA primases to switch from one strand of the bubble to the other one, whereas the products longer than 56 nt could be derived from a 3′-terminal ribonucleotidyl-transferase activity of the DNA primases. If these hypotheses were correct, the products shorter than 40 nt should consist entirely of RNA, whereas the products longer than 56 nt should correspond to the 56mer oligonucleotide that is used to prepare the bubble-substrate, bearing a RNA 3′-tail synthesized by the DNA primases, in a non-templated fashion. To test these hypotheses, we treated the reaction mixtures with Dnase-free Rnase A or with Rnase A plus Dnase I. As shown in Figure 2C for the Sso DNA primase, digestion with Dnase-free Rnase A caused an almost complete disappearance of the reaction products shorter than 40 nt, whereas molecules longer than 56 nt were found to be resistant to this treatment, but they disappeared after digestion with both Rnase A and Dnase I. Based on these findings, we postulated that the long products corresponded to the 56mer DNA oligonucleotide, which contained labelled AMP residues at its 3′-end as the result of 3′-terminal ribonucleotidyl-transferase activity of Sso DNA primase.
Sso DNA primase has 3′-terminal nucleotidyl-transferase activity
To demonstrate that the Sso DNA primase was truly endowed with a 3′-terminal nucleotidyl-transferase activity, we tested whether 5′-end labelled oligonucleotides could be elongated by the enzyme in the presence of unlabelled rNTPs (or dNTPs). Synthetic oligonucleotides in single- and double-stranded form were used in these assays, as well as the C- and T-Bubble substrates. The reaction products were analyzed by electrophoresis on polyacrylamide/urea gels. As shown in Figure 3, Sso DNA primase was able to incorporate individually all four tested rNTPs at the 3′-end of these DNA molecules, although, with different efficiency. The efficiency of incorporation seems to be dependent on the kind of DNA molecules used in the assay. The transferase activity seems to prefer the T-Bubble over the C-Bubble, the double- and the single-stranded substrates. When the T-Bubble substrate was used, rATP and rCTP were incorporated more efficiently than rUTP and rGTP (compare lanes 2 and 5 with lanes 3 and 4 of the top right panel in Figure 3). In contrast, rATP was incorporated very inefficiently on the single- and double-stranded DNA substrates (see lanes 2 of the bottom panels in Figure 3). Similarly, the above synthetic oligonucleotides were also elongated when dNTPs, instead of rNTPs, were present in the reaction mixtures. The M.jannaschii DNA primase was also found to possess the same synthetic capability (data not shown). Since the enzymatic assays were carried out at 60°C and since the DNA hairpin-like secondary structures are quite likely unstable at this temperature, we could rule out that the observed elongation products were produced in a template-directed fashion. In addition, all four tested rNTPs (and dNTPs) were incorporated (although with different efficiency), when incubated individually with the above DNA molecules, which indicates that nucleotide addition was not dictated by the sequence of the substrates used in these assays.
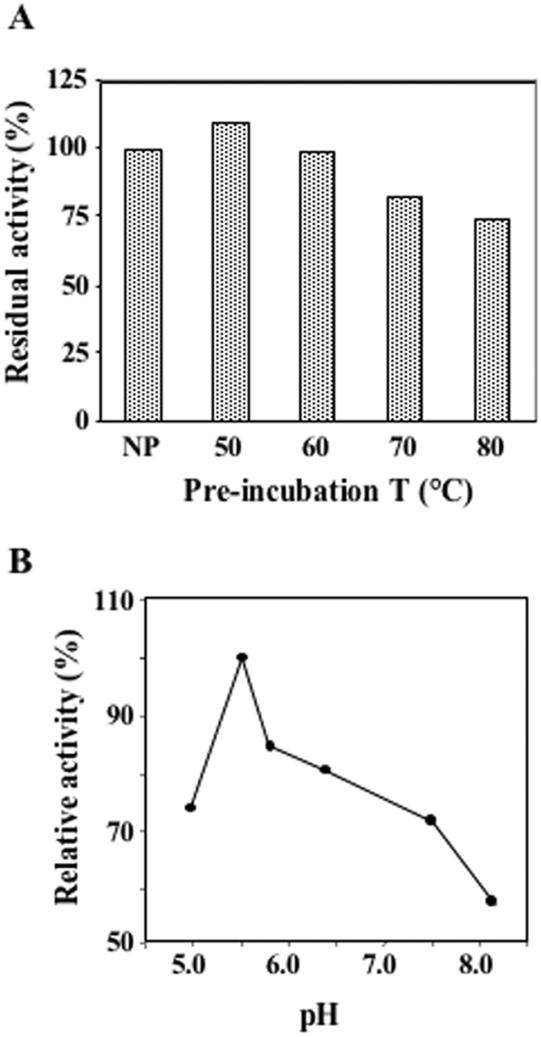
Thermal stability and pH optimum
The thermal stability of the Sso DNA primase was tested by assaying the residual activity of the enzyme after incubation for 15 min at temperatures ranging from 50 to 80°C. The T-Bubble substrate was used in these assays. Analysis of the polyacrylmide/urea gels that was used to separate the reaction products indicated that both primase (reaction products shorter than 40 nt) and terminal ribonucleotidyl-transferase (reaction products ranging from 56 to >100 nt) activities were resistant to heat treatment, and only a moderate reduction of ATP incorporation was observed after incubation for 15 min at 80°C with respect to the non-preincubated sample (see Figure 4A). These results allowed us to rule out that the observed synthetic activities could be due to any E.coli contaminant protein present in the enzyme preparations. Next, we investigated the optimal pH conditions to assay the synthetic functions of the Sso DNA primase on the T-Bubble substrate. As shown in Figure 4B, the enzyme was found to be maximally active in the incorporation of ATP when the MES–NaOH buffer system at pH 5.5 was used.
Figure 4.
Reaction requirements for the Sso DNA primase activity. (A) The thermal stability of Sso DNA primase was tested by pre-incubating the enzyme for 15 min at the indicated temperatures (NP stands for Not Pre-incubated); then, the enzyme residual activity was assayed on the T-Bubble DNA substrate, as described in Materials and Methods. (B) The influence of pH on the Sso DNA primase activity was studied in the following buffer systems (50 mM final concentration and pH measured at 60°C): MES–NaOH (pH: 5.0 and 5.5); MOPS–NaOH (pH: 5.8); HEPES–NaOH (pH: 6.4 and 7.5); Glycine–NaOH (pH: 8.2), as described in Materials and Methods.
Sso DNA primase is able to incorporate rATP (and dATP) on oligo(rA)15/poly(dT)
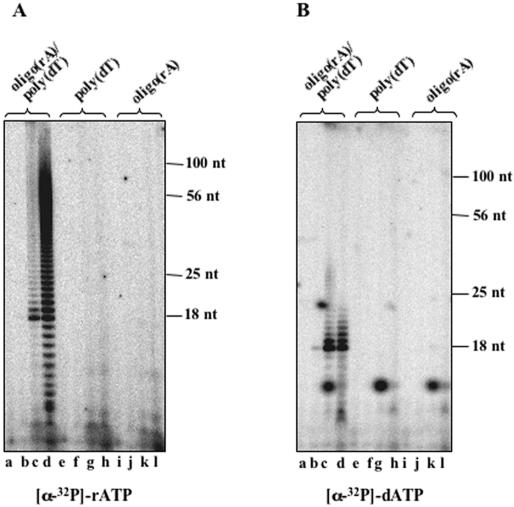
Archaeal DNA primases were reported to be able to incorporate dATP (in addition to rATP) on DNA templates. Therefore, we tested the ability of Sso DNA primase to elongate an oligo(rA)15 annealed to poly(dT) in the presence of either rATP or dATP. As shown in Figure 5A, the enzyme incorporated rATP using the above primer/template with higher efficiency in the presence of Mn2+ than with respect to Mg2+ ions. Since we found that the synthetic 32P-labelled oligo(rA)15 used in these assays contained molecules shorter than 15 bases, the reaction products that had <15 bases are probably due to the enzyme utilization of these non-full-sized primers. The enzyme was also able to incorporate dATP on oligo(rA)15/poly(dT) but with lower efficiency with respect to rATP (see Figure 5B). In addition, the Sso DNA primase was not able to elongate oligo(rA)15 when it was not annealed to poly(dT) or to initiate de novo synthesis on poly(dT) with dATP, whereas rATP was incorporated with very low efficiency on this template in the described experimental conditions.
Figure 5.
Sso DNA primase is able to elongate oligo(rA)15 primers annealed to poly(dT). Reaction mixtures (reaction volume: 20 μl) contained the indicated substrates and radioactive (A) rATP or (B) dATP. Samples (6 μl of each reaction mixture) were subjected to electrophoresis using 20% polyacrylamide/urea gels. For each gel, lanes a, e, i were loaded with control reactions without enzyme; lanes b, f, j; lanes c, g, k and lanes d, h, l were loaded with reactions carried out without any metal ion, in the presence of Mg2+ or Mn2+ ions, respectively. The migration of 32P-labelled synthetic oligonuclotides used as reference is indicated on the right side of each gel.
DISCUSSION
In this paper we analyzed the biochemical properties of the eukaryotic-like DNA primase of S.solfataricus. This enzyme is composed of two subunits that associate to form an hetero-dimeric complex, that was also demonstrated for the DNA primase of Pyrococcus furiosus (19) and P.horikoshii (20). Quite interestingly, de novo synthesis of oligoribonucleotides by Sso DNA primase is specifically activated by a thymine-rich synthetic bubble structure (T-Bubble substrate), whereas poly(dT) was found to be a poor template for this enzyme. Similarly, we also found that the DNA primase from M.jannaschii utilizes the T-Bubble substrate with higher efficiency, with respect to poly(dT). The presence of thymine residues in the bubble structure seems to be crucial for Sso DNA primase activation since we detected almost no RNA primer synthesis by the enzyme, on a template containing a bubble made of cytosine residues. Clusters of AT-rich sequences are usually found near the replication origins and they are believed to play an important role in initial unwinding of DNA at these sites (23,24). It was recently demonstrated that the chromosome of S.solfataricus possesses three active DNA replication origins (25,26); two of them were finely mapped and found to contain AT-rich sequences close to the sites where the Cdc6/Orc1-like initiator factors are specifically bound (25). Activation of these origins is likely to require unwinding of the AT-rich stretches as the initial step in the establishment of active replication bubbles. The ability of DNA primase to specifically recognize the structure, as well as the sequence of these early replication intermediates, may be critical for its affinity to the replication origins and subsequent activation. In line with this, we have recently found that the MCM-like DNA helicase of S.solfataricus does bind to DNA molecules that contain bubble structures, with high affinity (27). Similarly, it was recently reported that thymine-rich bubble-like substrates activate human MCM4/6/7 DNA helicase and this property may be the determinant for selecting initiation sites on mammalian genomes (28). All that considered, it is tempting to speculate that specific recognition of these peculiar DNA structures could be a feature shared by initiation factors of DNA replication from various organisms.
Furthermore, our biochemical analysis revealed that the Sso DNA primase is endowed with 3′-terminal nucleotidyl-transferase activity. This is the first evidence that a DNA primase is able to elongate the 3′-end of DNA molecules in a non-templated manner. On the other hand, this property is shared by DNA polymerases from eukaryotic, viral and prokaryotic organisms. DNA polymerase I from E.coli, DNA polymerase from Thermus aquaticus, DNA polymerase I from S.cerevisiae, DNA polymerase β from rat, reverse transcriptase from avian myeloblastosis virus were all reported to be able to add one deoxy-ribonucleotide (preferentially dATP) to the 3′-end of a blunt-ended duplex DNA substrate (29,30). In addition, DNA polymerases λ (31) and μ (32) from mammalian cells, as well as various RNA- and DNA-dependent RNA polymerases (such as the replicases of hepatitis C virus and bovine viral diarrhea virus, 33), and the T7 RNA polymerase (34) were reported to possess terminal nucleotidyl-transferase activity. Our analysis indicates that the DNA primases from the crenarchaeon S.solfataricus and the euryarchaeon M.jannaschii possess a non-templated synthetic function, but its biological role is not clear at present. In the eukaryotic organisms, nucleotide addition events without template instruction take place during the rearrangement of immunoglobulin genes, and are also involved in the replication of the telomeric ends of chromosomes. It would be interesting to test whether eukaryotic DNA primases are also endowed with any terminal nucleotidyl-transferase activity, considering that these enzymes consists of subunits similar in primary structure to the archaeal counterparts.
Acknowledgments
ACKNOWLEDGEMENTS
This work was supported by grants from the European Union (Contract N° QLK3-CT-2002-0207); from MIUR/CNR (Progetto Legge 449/97-DM 30/10/2000 and MIUR-DM prot. n. 1105/2002). Dr Giovanni Maga (IGM–CNR, Pavia, Italy) is gratefully acknowledged for helpful discussions and critical reading of the manuscript.
REFERENCES
- 1.Kornberg A. and Baker,T.A. (1992) Primases, primosomes, and priming. In DNA Replication. W.H. Freeman and Co., New York, NY, pp. 275–306. [Google Scholar]
- 2.Michael W.M., Ott,R., Fanning,E. and Newport,J. (2000) Activation of the DNA replication checkpoint through RNA synthesis by primase. Science, 289, 2133–2137. [DOI] [PubMed] [Google Scholar]
- 3.Marini F., Pellicioli,A., Paciotti,V., Lucchini,G., Plevani,P., Stern,D.F. and Foiani,M. (1997) A role for DNA primase in coupling DNA replication to DNA damage response. EMBO J., 16, 639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frick D.N. and Richardson,C.C. (2001) DNA primases. Annu. Rev. Biochem., 70, 39–80. [DOI] [PubMed] [Google Scholar]
- 5.Arai K. and Kornberg,A. (1979) A general priming system employing only DnaB protein and primase for DNA replication. Proc. Natl Acad. Sci. USA, 76, 4308–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein J.A. and Richardson,C.C. (1988) A 7 kDa region of the bacteriophage T7 gene 4 protein is required for primase but not for helicase activity. Proc. Natl Acad. Sci. USA, 85, 396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziegelin G., Scherzinger,E., Lurz,R. and Lanka,E. (1993) Phage P4 alpha protein is multifunctional with origin recognition, helicase and primase activities. EMBO J., 12, 3703–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng Y., Frick,D.N. and Richardson,C.C. (2000) Characterization of a novel DNA primase from the Salmonella typhimurium bacteriophage SP6. Biochemistry, 39, 1643–1654. [DOI] [PubMed] [Google Scholar]
- 9.Arezi B. and Kuchta,R.D. (2000) Eukaryotic DNA primase. Trends Biochem. Sci., 25, 572–576. [DOI] [PubMed] [Google Scholar]
- 10.Santocanale C., Foiani,M., Lucchini,G. and Plevani,P. (1993) The isolated 48 000-dalton subunit of yeast DNA primase is sufficient for RNA primer synthesis. J. Biol. Chem., 268, 1343–1348. [PubMed] [Google Scholar]
- 11.Bakkenist C.J. and Cotterill,S. (1994) The 50 kDa primase subunit of Drosophila melanogaster DNA polymerase α. Molecular characterization of the gene and functional analysis of the over-expressed protein. J. Biol. Chem., 269, 26759–26766. [PubMed] [Google Scholar]
- 12.Schneider A., Smith,R.W.P., Kautz,A.R., Weisshart,K., Grosse,F. and Nasheuer,H.-P. (1998) Primase activity of human DNA polymerase α-primase, divalent cations stabilize the enzyme activity of the p48 subunit. J. Biol. Chem., 273, 21608–21615. [DOI] [PubMed] [Google Scholar]
- 13.Arezi B., Kirk,B.W., Copeland,W.C. and Kuchta,R.D. (1999) Interactions of DNA with human DNA primase monitored with photoactivatable cross-linking agents: implications for the role of the p58 subunit. Biochemistry, 38, 12899–12907. [DOI] [PubMed] [Google Scholar]
- 14.Copeland W.C. and Wang,T.S.F. (1993) Enzymatic characterization of the individual mammalian primase subunits reveals a biphasic mechanism for initiation of DNA replication. J. Biol. Chem., 268, 26179–26189. [PubMed] [Google Scholar]
- 15.Davey S.K. and Faust,E.A. (1990) Murine DNA polymerase alpha-primase initiates RNA-primed DNA synthesis preferentially upstream of a 3′-CC(C/A)-5′ motif. J. Biol. Chem., 265, 3611–3614. [PubMed] [Google Scholar]
- 16.Zechner E.L., Wu,C.A. and Marians,K.J. (1992) Coordinated leading- and lagging-strand synthesis at the Escherichia coli DNA replication fork III. A polymerase-primase interaction governs primer size. J. Biol. Chem., 267, 4054–4063. [PubMed] [Google Scholar]
- 17.Zerbe L.K. and Kuchta,R.D. (2002) The p58 subunit of human DNA primase is important for primer initiation, elongation, and counting. Biochemistry, 41, 4891–4900. [DOI] [PubMed] [Google Scholar]
- 18.Desogus G., Onesti,S., Brick,P., Rossi,M. and Pisani,F.M. (1999) Identification and characterization of a DNA primase from the hyperthermophilic archaeon Methanococcus jannaschii. Nucleic Acids Res., 27, 4444–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L., Komori,K., Ishino,S., Bocquier,A.A., Cann,I.K.O., Kohda,D. and Ishino,Y. (2001) The archaeal DNA primase: biochemical characterization of the p41-p46 complex from Pyrococcus furiosus. J. Biol. Chem., 276, 45484–45490. [DOI] [PubMed] [Google Scholar]
- 20.Matsui E., Nishio,M., Yokohama,H., Harata,K., Darnis,S. and Matsui,I. (2003) Distinct domain functions regulating de novo DNA synthesis of thermostable DNA primase from the hyperthermophile Pyrococcus horikoshii. Biochemistry, 42, 14968–14976. [DOI] [PubMed] [Google Scholar]
- 21.Bocquier A., Liu,L., Cann,I., Komori,K., Kohda,D. and Ishino,Y. (2001) Archaeal primase: bridging the gap between RNA and DNA polymerases. Curr. Biol., 11, 452–456. [DOI] [PubMed] [Google Scholar]
- 22.Pisani F.M., De Felice,M., Carpentieri,F. and Rossi,M. (2000) Biochemical characterization of a clamp-loader complex homologous to eukaryotic Replication Factor C from the hyperthermophilic archaeon Sulfolobus solfataricus. J. Mol. Biol., 301, 61–73. [DOI] [PubMed] [Google Scholar]
- 23.DePamphilis M.L. (1996) Origin of DNA replication. In DePamphilis, M.L. (ed.), DNA Replication in Eukaryotic Cells. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 45–86. [Google Scholar]
- 24.Kelman Z. (2000) The replication origin of archaea is finally revealed. Trends Biochem. Sci., 25, 521–523. [DOI] [PubMed] [Google Scholar]
- 25.Robinson N.P., Dionne,I., Lundgren. M., Marsh,V.L., Bernander,R. and Bell,S.D. (2004) Identification of two origins of replication in the single chromosome of the archaeon Sulfolobus solfataricus. Cell, 116, 25–38. [DOI] [PubMed] [Google Scholar]
- 26.Lundgren M., Andersson,A., Lanming,C., Nilsson,P. and Bernander,R. (2004) Three replication origins in Sulfolobus species: synchronous initiation of chromosome replication and asynchronous termination. Proc. Natl Acad. Sci. USA, 101, 7046–7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pucci B., De Felice,M., Onesti,S., Rossi,M. and Pisani,F.M. (2004) Amino acids of the Sulfolobus solfataricus MCM DNA helicase involved in DNA binding/remodeling. J. Biol. Chem., in press. [DOI] [PubMed] [Google Scholar]
- 28.You Z., Ishimi,Y., Mizuno,T., Sugasawa,K., Hanaoka,F. and Masai,H. (2003) Thymine-rich single-stranded DNA activates Mcm 4/6/7 helicase on Y-fork and bubble-like substrates. EMBO J., 22, 6148–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark J.M., Joyce,C.M. and Beardsley,G.P. (1987) Novel blunt-end addition reactions catalyzed by DNA polymerase I of Escherichia coli. J. Mol. Biol., 198, 123–127. [DOI] [PubMed] [Google Scholar]
- 30.Clark J.M. (1988) Novel non-templated nucleotide addition reactions catalyzed by prokaryotic and eukaryotic DNA polymerases. Nucleic Acids Res., 16, 9677–9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramadan K., Maga,G., Shevelev,I.V., Villani,G., Blanco,L. and Hubscher,U. (2003) Human DNA polymerase λ possesses terminal deoxyribonucleotidyl transferase activity and can elongate RNA primers: implications for novel functions. J. Mol. Biol., 328, 63–72. [DOI] [PubMed] [Google Scholar]
- 32.Covo S., Blanco,L. and Livneh,Z. (2004) Lesion bypass by human DNA polymerasae mu reveals a template-dependent, sequence-independent nucleotidyl transferase activity. J. Biol. Chem., 279, 859–865. [DOI] [PubMed] [Google Scholar]
- 33.Ranjith-Kumar C.T., Gajewski,J., Gutshall,L., Maley,D., Sarisky,R.T. and Kao,C.C. (2001) Terminal nucleotidyl transferase activity of recombinant Flaviviridae RNA-dependent RNA polymerases: implications for viral RNA synthesis. J. Virol., 75, 8615–8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milligan J.F., Groebe,D.R., Witherell,G.W. and Uhlenbeck,O.C. (1987) Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res., 15, 8783–8798. [DOI] [PMC free article] [PubMed] [Google Scholar]