Abstract
Background:
Although widespread reduced white matter (WM) integrity is a consistent finding in cross-sectional diffusion tensor imaging (DTI) studies of schizophrenia, little is known about the course of these alterations. This study examined to what degree microstructural WM alterations display differential trajectories over time as a function of level of psychosis liability.
Methods:
Two DTI scans with a 3-year time interval were acquired from 159 participants (55 patients with a psychotic disorder, 55 nonpsychotic siblings and 49 healthy controls) and processed with tract-based spatial statistics. The mean fractional anisotropy (FA) change over time was calculated. Main effects of group, as well as group × region interactions in the model of FA change were examined with multilevel (mixed-effects) models.
Results:
Siblings revealed a significant mean FA decrease over time compared to controls (B = −0.004, P = .04), resulting in a significant sibling-control difference at follow-up (B = −0.007, P = .03). Patients did not show a significant change over time, but their mean FA was lower than controls both at baseline and at follow-up. A significant group × region interaction (χ2 = 105.4, P = .01) revealed group differences in FA change in the right cingulum, left posterior thalamic radiation, right retrolenticular part of the internal capsule, and the right posterior corona radiata.
Conclusion:
Whole brain mean FA remained stable over a 3-year period in patients with psychotic disorder and declined over time in nonaffected siblings, so that at follow-up both groups had lower FA with respect to controls. The results suggest that liability for psychosis may involve a process of WM alterations.
Key words: psychotic disorder, diffusion tensor imaging, disease progression, siblings
Introduction
The functional dysconnectivity hypothesis of schizophrenia suggests that symptoms originate as a result of miscommunication between different brain areas,1,2 associated with microstructural white matter (WM) alterations. Diffusion tensor imaging (DTI) is used to identify potential WM microstructural correlates. Fractional anisotropy (FA) combines information on myelination, fiber density, and number of axons in 1 measure, whereas additional diffusion parameters (axial-, radial-, and mean diffusivity) may provide more specific information on WM integrity.3 A large number of cross-sectional DTI studies of patients in early and later stages of schizophrenia have shown decreased FA with respect to controls in several WM tracts throughout the brain.4,5 However, it is not clear when these WM alterations occur and how they develop over time.6 Both a neurodevelopmental model,7,8 implying that WM alterations are present before disease onset and a postonset progression model, indicating that structural brain abnormalities progress over time after disease onset, have been proposed.9,10 In support of the neurodevelopmental model, several cross-sectional DTI studies have revealed microstructural WM alterations in frontotemporal and -parietal connections in first-degree relatives without symptoms,11,12 in clinical high-risk populations before onset,13,14 and in patients with early onset psychosis.15 Indeed, the lifetime trajectory of WM alterations in schizophrenia suggests higher percentages of WM loss in the first years of the illness, implicating altered neurodevelopment.6,16 The literature on longitudinal WM changes, however, is too limited to draw firm conclusions on neurodevelopmental or progressive changes, as well as the role of medication.16 A study by Carletti and colleagues (2012) showed a significant progressive reduction in FA in the left frontal WM in individuals at “Ultra-High Risk” (UHR) who developed psychosis compared to UHR subjects who did not make a “transition”.17 Reis Marques and colleagues (2014) found an increase in FA in first episode patients (both responders and nonresponders to antipsychotic [AP] medication) over a 12-week period,18 and Garver and colleagues (2008) reported reduced mean diffusivity after 28 days of treatment in drug-responding patients with schizophrenia, which was not found in nonresponders.19 A fourth study compared WM FA changes between individuals during a more chronic course of schizophrenia (n = 49) and healthy controls (n = 16), reporting a stronger FA decline over 4 years in frontal, temporal, and parietal WM in the controls. A subgroup of patients with poor outcome could be differentiated from a group with good outcome by regional progression of WM alterations in an adjacent precentral/postcentral area.20
Not only DTI, but also longitudinal volumetric studies are scarce. Decreases over time have been described in recent onset and first-episode patients in frontal lobe WM volume21 and temporal lobe WM volume, respectively.22 However, in patients aged up to 51 years, van Haren and colleagues (2008) described an abnormal curved trajectory of volume change, with normalization of age-related volume change later in life.23
In our recent cross-sectional diffusion analyses of patients with a psychotic disorder, siblings and controls (n = 258), patient-specific microstructural WM alterations were found in the corpus callosum and other WM tracts.24 This sample was re-scanned approximately 3 years later to examine whether the evolution of FA over time varies as a function of familial risk for psychotic disorder.
Methods
Participants
Participants were recruited in the context of a multicenter longitudinal study (Genetic Risk and Outcome of Psychosis, G.R.O.U.P.) in the Netherlands.25 The magnetic resonance imaging (MRI) add-on study was conducted in Maastricht, the Netherlands (see Domen and colleagues24 or the Supplemental Method section for full information on in- and exclusion criteria of the participants and diagnostic assessments). For the baseline MRI study, 300 participants were included of which 258 provided a valid DTI scan: 85 patients with a psychotic disorder, 93 siblings without a psychotic disorder, and 80 healthy controls. At follow-up, approximately 3 years later (mean: 3.3 year), a second DTI scan was acquired from 180 participants (loss to follow-up of 40%). The final sample comprised 159 participants (55 patients with a psychotic disorder, 55 siblings without a psychotic disorder, and 49 healthy controls), for which a pair of DTI scans was available for longitudinal analysis (table 1).
Table 1.
Demographic Characteristics of the Participants (n = 159)
| Controls (n = 49) | Siblings (n = 55) | Patients (n = 55) | ||||
|---|---|---|---|---|---|---|
| Time Point | 0 | 1 | 0 | 1 | 0 | 1 |
| Scan interval (d) | 1222±198 | 1177±101 | 1196±121 | |||
| Age at scan (y) | 31.0±11.0 | 34.4±10.9 | 30.9±8.5 | 34.1±8.5 | 28.7±6.3 | 32.0±6.2 |
| Sex, male (%) | 19 (39%) | 29 (53%) | 40 (73%) | |||
| Handedness | 83.2 | 78.0 | 72.2 | |||
| Level of education | 5.4±2.0 | 5.4±2.1 | 4.5±1.9 | |||
| Age of onset (y) | — | — | 21.8±6.3 | |||
| Illness duration (y) | — | — | 5.7±3.5 | 10.3±4.0 | ||
| AP medication | — | — | 6693±6254a | 5335±5715b | ||
| Diagnosis | ||||||
| Schizophrenia | — | — | 33 | |||
| Schizoaffective disorder | — | — | 16 | |||
| Psychotic disorder NOS | — | — | 6 | |||
| Major depressive disorderc | 11 | 15 | — | |||
| Substance use | ||||||
| Cannabis | 4.8±32.0d | 7.1±49.1d | 36.5±105.2d | |||
| Other drugs | 0.0 | 0.0 | 15.2±57.0d | |||
| Alcohol | 6.3±10.8e | 6.0±5.4e | 4.9±7.6e | |||
| PANSS scores | ||||||
| Positive symptoms | 7.3±1.2 | 7.4±0.8 | 7.4±0.9 | 7.4±0.7 | 9.6±3.7 | 11.6±5.7 |
| Negative symptoms | 8.2±1.2 | 8.0±0.2 | 8.2±1.3 | 8.0±0.3 | 11.4±5.2 | 11.1±4.2 |
| Disorganization | 10.3±1.5 | 10.1±0.3 | 10.3±0.6 | 10.1±0.4 | 11.7±2.6 | 11.8±2.5 |
| Excitement | 8.4±1.3 | 8.3±0.5 | 8.5±1.2 | 8.3±0.6 | 9.4±1.9 | 9.7±2.5 |
| Emotional distress | 9.3±2.1 | 9.4±1.7 | 9.7±2.2 | 9.7±2.2 | 12.9±5.2 | 14.1±5.0 |
| Remission (percentage) | — | — | — | — | 61% | 62% |
Note: Means ± SDs are reported. AP, antipsychotic; NOS, not otherwise specified; PANSS, Positive and Negative Syndrome Scale.
aCumulative exposure (in haloperidol equivalents), lifetime until baseline assessment.
bExposure (in haloperidol equivalents) over last 3 y.
cHistory of major depressive disorder, no current episodes at baseline or in last 3 y.
dMean number of times; last 12 mo.
eWeekly consumptions last 12 mo.
The sample included 129 families of which 16 families contributed 1 patient and 1 healthy sibling and 1 family contributed 1 patient and 2 healthy siblings. Six families contributed 2 healthy siblings, 1 family contributed 3 healthy siblings, and 4 families contributed 2 healthy controls. In addition, 38 families contributed 1 patient, 22 families contributed 1 sibling, and 41 families contributed 1 control.
The standing ethics committee approved the study protocol, and all participants gave written informed consent in accordance with the committee’s guidelines.
Measures
Symptoms.
At both time points, symptoms were assessed with the Positive and Negative Syndrome Scale (PANSS).26 The 5-factor model by van der Gaag and colleagues (2006) was used, dividing the PANSS in positive symptoms, negative symptoms, disorganization symptoms, excitement, and emotional distress.27 The scores of the individual items of the 5 symptom dimensions were summed. To assess clinical remission, the operationalized criteria described by Andreasen and colleagues (2005)28 were applied.
Educational level (at baseline) was defined as the highest accomplished level of education. Handedness was assessed using the Annett Handedness Scale.29
Medication.
In the patient group, AP medication use was determined by patient report and verified with the treating consultant psychiatrist. Best estimate lifetime (cumulative) AP use at baseline was determined by multiplying the number of days of AP use with the corresponding haloperidol equivalents and summing these scores for all periods of AP use (including the exposure period between baseline assessment for the G.R.O.U.P. study and the moment of baseline MRI scanning), using the published converting formulas for AP dose equivalents described by Andreasen and colleagues.30 The same procedure was used for calculating cumulative AP exposure during the 3-year follow-up period.
Substance Use.
Substance use was measured at both time points with the Composite International Diagnostic Interview (CIDI) sections B-J-L.31 Alcohol use was defined as the reported number of weekly consumptions during the last 12 months. As data on drug use of the last 3 years were not available, cannabis and other drugs were assessed as reported frequency of use during the last 12 months, as well as lifetime use. CIDI frequency data on alcohol, lifetime cannabis, and other drug use were available at baseline for respectively 158 participants (1% missing data), 155 participants (3% missing data), and 157 participants (1% missing data).
Image Acquisition
MRI scans were obtained at Maastricht University, the Netherlands, using an Allegra Magnetom MR (Siemens) operating at 3.0 Tesla. At both measurement points, microstructural anatomy was examined using DTI with an echo-planar-imaging sequence (field of view 230×230mm2, TR 10800ms, TE 84ms, voxel size 1.8×1.8×1.8mm3, b-value 1000s/mm2, 85 slices, no overlap). As a result of a scanner update at the baseline measurement, 2 DTI sequences were used: one with 76 directions (of which 4 diffusion-unweighted [B0] and 72 diffusion-weighted [B1000]) and one with 81 directions (8xB0 and 73xB1000). A potential association between the proportion of baseline scans and group was investigated using a Pearson chi-square test. At follow-up, the DTI sequence comprised 81 directions (8xB0 and 73xB1000). Total acquisition time of the DTI sequence was 15 minutes.
DTI Analysis
DTI data were processed using tract-based spatial statistics (TBSS) v1.2 in FSL 4.1.6 (FMRIB Analysis Group, http://www.fmrib.ox.ac.uk/analysis/research/tbss). First, standard Siemens DICOM files were transformed into compressed NIFTI format using a custom built in-house software named GIANT (General Image ANalysis Tools developed by E.G.). Raw data were corrected for head movement and eddy currents invoked during scanning. The B0 volume was skull-stripped using FSL’s Brain Extraction Tool32 and this served as a brain mask for all B volumes.
The next step was fitting a diffusion tensor model at each voxel using data output from the brain extraction, diffusion weighted data, and gradient directions following a general linear model (FreeSurfer v4.5.0, http://www.freesurfer.net). After tensor fitting (using the DT-Recon script) the process continued working on FA volumes, eroding them slightly. Nonlinear registration aligned each FA volume to 1×1 × 1mm standard FMRIB58_FA space. The standard FMRIB58_FA contains a template derived from high-resolution images of 58 participants in a well-aligned population (both males and females ranging between 20 and 50 years of age).33
After nonlinear transformation of the FA volumes into standard space, 2 mean FA skeletons were created; (1) one based on 3 groups (n = 159: controls, siblings, patients) for the cross-sectional analysis at follow-up and (2) one based on 6 groups (3 groups × 2 time-points) for the longitudinal analysis. The mean FA skeleton follows the major WM tracts in each individual participant (normalized in MNI152 space) and provides a way to compare between (groups of) participants. The FA threshold was set, using visual inspection of the FA skeleton, at a level of 0.25, to include major WM tracts whilst removing small peripheral tracts that would cause excess inter-participant variability. In addition, this threshold setting avoided inclusion of regions that are likely to be composed of multiple tissue types or fiber orientations. In the final step, a binary skeleton mask was created and used to extract FA values of the individual participants. The Johns Hopkins University International Consortium for Brain Mapping (JHU ICBM)-DTI-81 WM atlas labels34 were used to label all voxels and assign a specific tract name. If the voxels did not match with the JHU ICBM labels, they were identified using the JHU WM tractography atlas.35
Statistical Analyses
From the 38 JHU labeled WM tracts, skeleton mean FA values per participant per time point were extracted and exported to R (version 3.2.0), a free software environment for statistical computing and graphics.36
Longitudinal Analyses.
Within-group paired t-tests were done to examine the difference in regional mean FA between baseline and follow-up. Subsequently, a mean FA “change” (delta, Δ per participant per region) was calculated by subtracting mean FA (baseline) from mean FA (follow-up). The data set was transformed from a wide to a hierarchically structured data set, with 38 regional ΔFA measures (Level 1) nested in subjects (Level 2) who were part of the same families (Level 3). A mixed- effects model was used to examine the model with ΔFA measures as the dependent variable and scan-interval as additional covariate. In addition, since the outcome represents means based on varying number of voxels (depending on the region), we used a model in which the error variance for a particular observation was inversely weighted by the number of voxels within the corresponding region (ie, since the variance of a mean is equal to the variance of a single observation—in this case voxel—divided by the number of values used for the averaging). Main effects of group, corrected for age, sex, handedness, level of education and scan-interval, as well as group × sex and group × region interactions in the model of ΔFA were examined. In each of the 38 regions, between-group factor significance was tested. Regions with a significant group effect were examined with pairwise comparisons (ie, it was tested whether ΔFA differed between patients and controls, between siblings and controls, and between patients and siblings).
Due to the large number of regions (and hence tests), Simes’ procedure37,38 was used to control the false discovery rate when testing the regional within-group mean FA differences between baseline and follow-up, the between-group effects within the 38 regions, and the pairwise comparisons.
Sensitivity analyses were performed with last year cannabis use, lifetime cannabis use, and scan type (76 or 81 directions) as additional covariates in 3 separate models. Since a subgroup of participants in the control (n = 11) and sibling group (n = 15) had a history of a depressive disorder (and a number of DTI studies with patients with a major depressive disorder have shown decreased FA in several cortical and subcortical WM tracts39), additional sensitivity analyses were conducted controlling for history of depression.
AP Medication.
In patients only, the association between ΔFA and respectively last 3-year and lifetime AP use was examined. These variables were entered both as linear and as factored variables (ie, representing the distribution of scores divided by its tertiles: low, moderate, or high AP exposure), allowing visualization of dose-response. Sensitivity analyses were done using patient subgroups (with low, moderate, and high AP exposure), ie, the association between group and ΔFA was examined per AP subgroup to examine whether patients with differential AP exposure would have differential FA change over time in comparison to controls and siblings.
Cross-sectional Analyses at Follow-up.
To examine group differences at follow-up (n = 159), a whole-brain mean FA was computed with the 38 WM regions conform the above-described procedure. Whole-brain FA was the dependent variable and random effects (intercepts) were added for each subject and family, including a priori hypothesized confounding variables age, sex, handedness, and level of education as fixed effects.
Results
Participant Characteristics
The characteristics of the 159 individuals with a baseline and follow-up scan are displayed in table 1. The majority of the patients were not in need of inpatient care or intensive treatment, as reflected by the low PANSS scores at both time points and the proportion of patients in remission. At baseline, 54 patients were receiving AP medication with a mean dosage in terms of standard haloperidol equivalents of 5.4 milligrams (mg) (SD = 3.4). At follow-up, 45 patients used AP medication (second generation: n = 42; first generation: n = 3), with a mean dosage of 4.7mg (SD = 5.1). The lifetime cumulative AP exposure was 13079.7mg (SD = 10977.2).
The proportion of baseline scans with 76 directions did not differ between the groups (84% in controls, 82% in siblings, and 71% in patients: χ2 = 3.02, df = 2, P = .22).
Whole Brain Group Differences in FA Change Over Time (ΔFA)
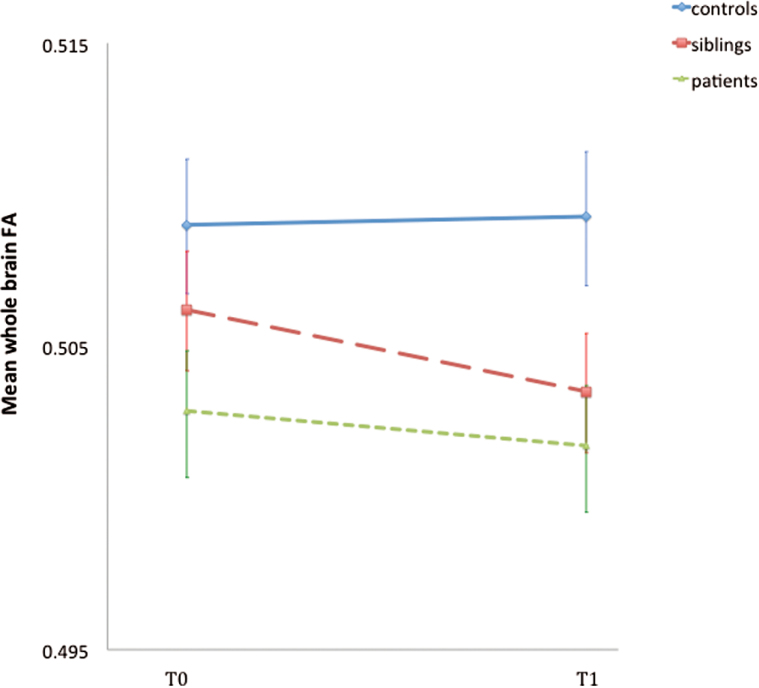
The mean ΔFA revealed a slight increase over time in the controls (0.001) and a decrease over time in the siblings (−0.003) and patients (−0.0002), with the mean FA (0.504) of the siblings at follow-up in between that of the controls (0.509) and the patients (0.502) (figure 1). At follow-up, there were more WM tracts showing a decrease than an increase in patients and siblings (27 and 23 of the 38 tracts, respectively), whereas in controls the proportion of decreases and increases was balanced (table 2).
Fig. 1.
Group differences in whole brain FA at follow-up. Error bars represent the SE of the mean ΔFA at baseline and at follow-up. FA, fractional anisotropy.
Table 2.
Mean FA per Group at Baseline and at Follow-up
| Brain Region | Mean FA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Controls | Siblings | Patients | |||||||
| T0 | T1 | Δ | T0 | T1 | Δ | T0 | T1 | Δ | |
| Genu of corpus callosum | .7570 | .7557 | −.0012 | .7551 | .7515 | −.0036 | .7466 | .7446 | −.0020 |
| Body of corpus callosum | .7214 | .7216 | .0003 | .7175 | .7155 | −.0020 | .7035 | .7007 | −.0028 |
| Splenium of corpus callosum | .7877 | .7915 | .0037 | .7891 | .7873 | −.0018 | .7880 | .7897 | .0017 |
| Forceps major | .7004 | .7042 | .0038* | .6974 | .6956 | −.0018 | .6904 | .6896 | −.0009 |
| Forceps minor | .5850 | .5848 | −.0002 | .5827 | .5800 | −.0026 | .5785 | .5779 | −.0006 |
| Fornix (column and body) | .5278 | .5199 | −.0078 | .5305 | .5208 | −.0097 | .4812 | .4855 | .0043 |
| Anterior limb of internal capsule, right | .6311 | .6213 | −.0098* | .6242 | .6154 | −.0088* | .6215 | .6129 | −.0086* |
| Anterior limb of internal capsule, left | .6083 | .6194 | .0111* | .6024 | .6102 | .0079* | .6003 | .6116 | .0113* |
| Posterior limb of internal capsule, right | .6949 | .6903 | −.0046 | .6924 | .6859 | −.0065* | .6954 | .6906 | −.0048 |
| Posterior limb of internal capsule, left | .6934 | .6942 | .0008 | .6917 | .6903 | −.0014 | .6977 | .6953 | −.0024 |
| Retrolenticular part of internal capsule, right | .5807 | .5993 | .0186* | .5819 | .5901 | .0082 | .5834 | .5901 | .0066 |
| Retrolenticular part of internal capsule, left | .5985 | .5883 | −.0102* | .5951 | .5859 | −.0092* | .5935 | .5902 | −.0033 |
| Anterior corona radiata, right | .5192 | .5119 | −.0073 | .5150 | .5043 | −.0108* | .5056 | .4947 | −.0110* |
| Anterior corona radiata, left | .5037 | .5084 | .0047 | .4979 | .4980 | .0002 | .4885 | .4942 | .0057 |
| Superior corona radiata, right | .5267 | .5297 | .0030 | .5239 | .5240 | .0001 | .5186 | .5191 | .0006 |
| Superior corona radiata, left | .5319 | .5207 | −.0111* | .5261 | .5169 | −.0091* | .5235 | .5121 | −.0114* |
| Posterior corona radiata, right | .5172 | .5115 | −.0057 | .5094 | .4945 | −.0150* | .5062 | .4873 | −.0189* |
| Posterior corona radiata, left | .5010 | .5134 | .0125* | .4892 | .5018 | .0125* | .4835 | .4999 | .0163* |
| Posterior thalamic radiation, right | .6269 | .6260 | −.0009 | .6207 | .6162 | −.0045 | .6180 | .6081 | −.0099 |
| Posterior thalamic radiation, left | .6179 | .6255 | .0076 | .6105 | .6094 | −.0011 | .6011 | .6123 | .0111 |
| Sagittal stratuma, right | .5732 | .5744 | .0012 | .5687 | .5637 | −.0050 | .5656 | .5635 | −.0020 |
| Sagittal stratuma, left | .5706 | .5746 | .0040 | .5609 | .5625 | .0016 | .5617 | .5641 | .0024 |
| External capsule, right | .4769 | .4770 | .0001 | .4741 | .4690 | −.0050 | .4711 | .4679 | −.0032 |
| External capsule, left | .4731 | .4755 | .0024 | .4679 | .4698 | .0020 | .4652 | .4715 | .0063 |
| Cingulum (cingulate gyrus), right | .6104 | .6403 | .0299* | .6099 | .6333 | .0233* | .6072 | .6378 | .0305* |
| Cingulum (cingulate gyrus), left | .6391 | .6048 | −.0343* | .6344 | .6016 | −.0328* | .6344 | .6023 | −.0320* |
| Cingulum (hippocampus), right | .5556 | .5585 | .0029 | .5602 | .5393 | −.0210* | .5535 | .5445 | −.0090 |
| Cingulum (hippocampus), left | .5486 | .5702 | .0216* | .5421 | .5552 | .0131 | .5417 | .5599 | .0182* |
| Fornix, stria terminalis, right | .5581 | .5806 | .0225* | .5547 | .5701 | .0153* | .5454 | .5671 | .0217* |
| Fornix, stria terminalis, left | .5625 | .5562 | −.0063 | .5590 | .5433 | −.0156* | .5509 | .5426 | −.0084 |
| Superior longitudinal fasciculus, right | .5298 | .5313 | .0015 | .5325 | .5297 | −.0028 | .5320 | .5241 | −.0079 |
| Superior longitudinal fasciculus, left | .5303 | .5243 | −.0060 | .5305 | .5258 | −.0047 | .5271 | .5233 | −.0038 |
| Superior fronto-occipital fasciculus, right | .5380 | .5308 | −.0072 | .5329 | .5281 | −.0048 | .5214 | .5155 | −.0060 |
| Superior fronto-occipital fasciculus, left | .5276 | .5252 | −.0023 | .5189 | .5188 | −.0001 | .5097 | .5123 | .0026 |
| Uncinate fasciculus, right | .5523 | .5516 | −.0006 | .5393 | .5413 | .0019 | .5395 | .5387 | −.0008 |
| Uncinate fasciculus, left | .5263 | .5179 | −.0084 | .5109 | .5074 | −.0035 | .5071 | .5108 | .0037 |
| Tapetum, right | .6164 | .6121 | −.0042 | .5907 | .5824 | −.0083 | .5910 | .5714 | −.0196* |
| Tapetum, left | .6345 | .6343 | −.0002 | .6106 | .5983 | −.0124 | .5942 | .5812 | −.0130 |
Note: Δ = mean FA difference (mean FA follow-up − mean FA baseline), with values in italics representing FA decreases and bold representing FA increases. FA, fractional anisotropy.
aIncl. inferior longitudinal fasciculus and inferior fronto-occipital fasciculus.
*Results of a within-group paired t-test examining significant within group ΔFA differences per brain region (P < .05), accounting for a false discovery rate with the Simes’ procedure (38 regions × 3 groups) (*P Simes < .003).
There was no significant association between group and ΔFA (χ2 = 4.9, df = 2, P = .09). Although the direction of effect for patients compared to controls indicated a decrease in FA over time, the difference was not significant (B = −0.002, P = .19); additional correction for last year cannabis use, lifetime cannabis use, and scan type did not change the results of the patient-control comparisons (B = −0.002, P = .22; B = −0.002, P = .30 and B = −0.002, P = .17, respectively). In siblings compared to controls, there was a significant decrease in FA (B = −0.004, P = .04). This effect remained significant when controlled for scan type (B = −0.004, P = .04) and last year cannabis use (B = −0.004, P = .04), and close to significant when controlled for lifetime cannabis use (B = −0.003, P = .07) and history of depression (B = −0.003, P = .06). No significant group × sex interaction in the model of ΔFA (χ2 = 0.9, df = 2, P = .62) was found.
Regional Group Differences in ΔFA
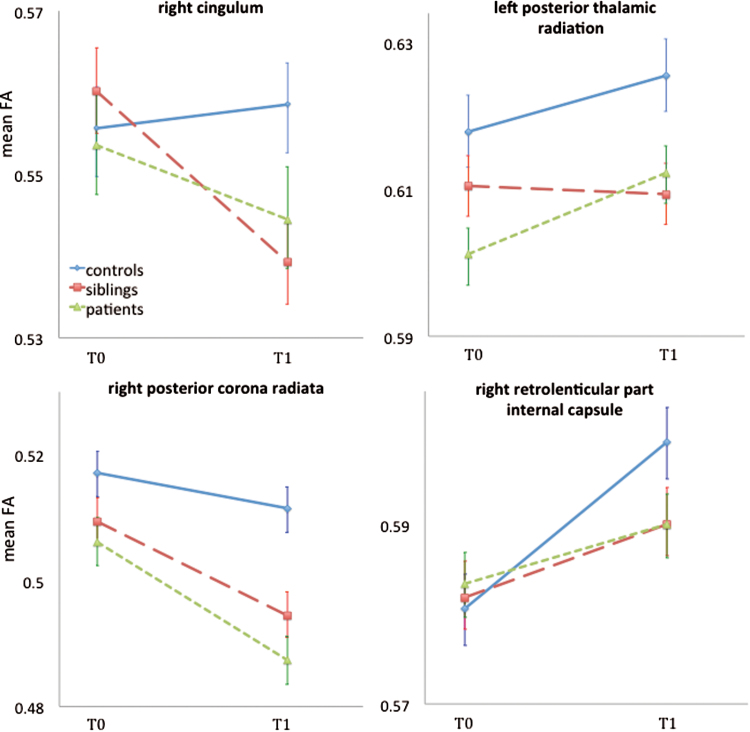
There was a significant group × region interaction in the model of ΔFA (χ2 = 105.4, df = 74, P = .01), indicating that group differences varied as a function of region. The group factor was significant in 6 of the 38 regions, of which 4 regions survived after Simes’ correction. Patients had a significant smaller FA increase in the right retrolenticular part of the internal capsule (RPIC), and a decrease in the right cingulum and the right posterior corona radiate (PCR) compared to controls. Siblings had a significant smaller FA increase in the right RPIC and a decrease in the right cingulum compared to controls. In comparison to patients and controls, siblings had a decrease in the left posterior thalamic radiation (PTR) (table 3, figure 2).
Table 3.
Group Comparisons in Regions With a Significant Group Effect
| Brain Region | Between-Group Effect | Patient-Control | Sibling-Control | Patient-Sibling |
|---|---|---|---|---|
| (χ2, df = 2, P) | (B, P) | (B, P) | (B, P) | |
| Retrolenticular part of internal capsule, right | 11.8, .003† | −0.01, .001* | −0.01, .008* | −0.003, .49 |
| Posterior corona radiata, right | 10.5, .005† | −0.01, .001* | −0.009, .03 | −0.004, .29 |
| Posterior thalamic radiation, right | 9.0, .01 | −0.01, .003 | −0.004, .27 | −0.007, .05 |
| Posterior thalamic radiation, left | 14.2, .0008† | 0.004, .31 | −0.009, .01* | 0.01, .0003* |
| Cingulum, right | 19.6, .0001† | −0.01, .01* | −0.02, 9.7×10−6* | 0.01, .05 |
| Superior longitudinal fasciculus, right | 9.4, .009 | −0.01, .002 | −0.004, 0.15 | −0.005, .08 |
Note: The χ2 and the P-values represent the significant results of the between-group factor analyses per region in multilevel modeling († P Simes < .005). B and P-values of the individual group comparisons are shown (*P Simes < .02). Analyses are controlled for age, sex, handedness, level of education, and scan interval.
Fig. 2.
Group × region interactions in the model of ΔFA. Group differences in mean ΔFA in 4 WM tracts are displayed (P Simes < .02), corresponding with table 3. Error bars represent the SE of the mean ΔFA at baseline and at follow-up. FA, fractional anisotropy.
AP Medication
Within-patients analyses showed that there was a close to significant association between lifetime AP use (linear) and whole brain mean ΔFA (B = −2.5×10−7, P = .08). Compared to low lifetime AP exposure, patients with high AP exposure showed significantly more FA decrease over time (B = −0.008, P = .04), which was not the case for patients with moderate exposure (B = −0.005, P = .15). With regard to AP exposure over the last 3 years, a significant negative association was found between cumulative AP exposure (linear) and whole brain mean ΔFA over the last 3 years (B = −5.6×10−7, P = .01). Higher levels of AP medication over the last 3 years predicted a stronger decrease in FA: moderate vs low exposure: B = −0.006, P = .04; high vs low exposure (B = −0.009, P = .004).
The group analyses based on AP medication subgroups (low, moderate, and high cumulative AP medication exposure) showed that there was a significant decrease in FA over time in patients with the highest level of AP exposure (lifetime and over the last 3 years) compared to controls, but not in patients with moderate or low AP exposure. Siblings showed a significant stronger decrease in FA than patients with the lowest AP exposure (see supplementary table 1).
Whole Brain Group Differences in Mean FA at Follow-up
There was a significant association between group and FA at follow-up (χ2 = 10.0, df = 2, P = .007): siblings (B = −0.007, P = .03) and patients (B = −0.010, P = .005) showed a significantly lower mean FA compared to the controls. The sibling-patient comparison was neither large nor significant (B = −0.001, P = .46, figure 1).
Discussion
This longitudinal DTI study showed a relatively stable whole brain mean FA course in patients with schizophrenia after the critical phase (ie, >5 years of illness duration), whereas a significant decline in mean FA was observed in siblings. The effect of group varied as a function of region, as indicated by a significant group by region interaction. Overall, there were more tracts showing a decrease than an increase over time in both patients and siblings, with a significant group difference in 4 WM tracts.
Findings in Patients
The study showed that, over a 3-year period, the overall mean FA remained stable in patients, being continuously lower than the mean FA of controls and siblings. This data is partly in line with the results of the study by Mitelman and colleagues (2009), examining an older (than the present) sample (of ±41 years) of patients with a diagnosis of schizophrenia and healthy controls, also showing a rather stable FA in patients, and a greater, probably normal age-dependent, FA decline over time in frontal, temporal, and parietal WM in healthy controls.20
The results suggest that major WM alterations in patients may have occurred in the early stages of the illness, as described previously,15 without ongoing progression. Alternatively, WM may have been modulated by AP medication, which was supported in the current study by the small but significant negative within-group effect of AP medication on ΔFA, with a dose-response effect for AP exposure over the last 3 years. Moreover, patients with the highest AP exposure levels (lifetime, as well as over the last 3 years) had a significant decrease in FA over time compared to controls, whereas this was not the case for patients with low or moderate AP exposure compared to controls.
Together with the widespread microstructural WM alterations presented in cross-sectional studies of at-risk populations and first episode psychotic patients,5 the data add to the evidence for an early developmental origin of WM alterations in schizophrenia.8 Indeed, a significant progressive reduction in FA of the left frontal WM has been found in UHR subjects who later developed psychosis compared to UHR subjects who did not make the transition.17
Despite the overall mean FA being constant, most WM tracts in the 3 groups showed minor (nonsignificant) changes in individual FA trajectories (table 2). WM tracts develop at different ages in different curvilinear patterns (inverted U-shape), with FA increases from the newborn period to adolescence, shows decelerated maturation until mid-adulthood and subsequently displays more rapid decline during old age.40,41 The WM tracts of the controls in this study may be at the top of the normal age trajectory curve because of the proportionally equal number of WM tracts with an FA increase and decrease. In contrast, patients showed a higher number of WM tracts with FA decreases, suggesting an altered developmental pattern, ie, an earlier than expected age-related decrease of WM. Specifically, a smaller FA increase in the RPIC and decrease in the PCR and the cingulum (all right-sided) was found in patients. These tracts have face-validity, as they are frequently described in relation to fronto-temporal disconnection in schizophrenia.4,42
Findings in Siblings
This is the first longitudinal study showing whole brain WM alterations over time in a sample of healthy participants at higher than average risk for psychotic disorder (siblings of patients with psychotic disorder). At baseline, there was no significant difference in whole brain mean FA between siblings and controls, whereas such a difference was apparent between siblings and patients.24 At follow-up, siblings showed a significant lower whole brain mean FA with respect to the controls and the significant difference with patients was no longer apparent. The difference at follow-up was confirmed by a significant FA decrease over time in siblings compared to controls, which was not observed in patients. At the level of individual tracts, almost two-third of the WM tracts in siblings revealed a decrease in FA over time, compared to the more balanced ratio of FA increases and decreases in the controls. Specifically, the left PTR and the right cingulum revealed a significant decrease of mean FA over time in siblings, whereas controls showed an increase. The PTR has previously been associated with schizophrenia,15,43 eg, with impaired emotional self-awareness.44 To our knowledge subtle FA decreases in the PTR have only been associated with bipolar45 but not psychosis liability. As mentioned before, all WM tracts have different maturational trajectories.46 The cingulum has one of the most prolonged maturation periods and reaches its peak FA only after 40 years of age.47 Given the mean age of 34 years in the siblings and of 32 years in the patients, the earlier than expected FA decline may reflect disturbed WM maturation from an early age, suggesting a neurodevelopmental origin. Alternatively, it may suggest progression associated with illness vulnerability, though the findings in the patients did not support this.
Decreased anisotropy in the cingulum has also been described in several cross-sectional studies in patients with schizophrenia48,49 and in the longitudinal study of Mitelman and colleagues (2009), where the left anterior cingulate gyrus was 1 of the 2 areas that showed a greater decline in FA in patients with schizophrenia compared to healthy participants.20 Regarding the present finding of a patient-control difference in this region, current and previous cross-sectional DTI findings50 in siblings may be suggestive of a WM intermediate phenotype.
Clinically, WM alterations in (sub-)regions of the cingulum have been related to impairments in impulsivity51 and executive functioning52 as well as to positive and negative symptoms53 in patients with schizophrenia. As mild cognitive alterations are present in non-affected relatives,54,55 it may be hypothesized that subclinical expression of symptoms are associated with this WM intermediate phenotype, which will be the topic of further investigation.
Methodological Considerations
Although the present study has several strengths, such as the rather large sample size, the longitudinal design covering a 3-year period, and the inclusion of both patients and their healthy siblings, there are some limitations that need to be taken into account when interpreting the results.
AP medication may have an effect on WM.15 Until now, only a handful of longitudinal diffusion studies have been published, examining (short-term) effects of AP medication on microstructural WM (pre-post treatment measurements).56–59 The results of the present study were supportive of an effect of (especially the highest) cumulative medication exposure levels on FA change over time, both in within-patients analyses and in between-group analyses based on AP exposure subgroups (with one-third of the patients in each subgroup). However, as the FA change in siblings, who were not using AP medication, was also significantly different from controls, AP exposure may be one of the contributing factors of microstructural WM alteration in patients with psychotic disorder.
The same applies to drug use. The present study sample was not drug-free which may have influenced our results. Study results differ with respect to the potential influence of cannabis use on WM alterations in patients with schizophrenia.60 Although the significant results in the control-sibling comparison remained stable after controlling for cannabis use (last year), lifetime cannabis use appeared to exert some influence, although the effect size was not affected much.
Given the absence of (1) differences in alcohol consumption across groups, (2) other drug use in the controls and siblings, and (3) effects of other drug use on microstructural WM in the baseline study,24 sensitivity analyses for these substances were not considered additional informative.
Inconsistencies in the results of the limited longitudinal studies conducted to date may be due to varying patient samples,17,20 as well as varying acquisition and analysing techniques. Although Reis Marques and colleagues (2012) used TBSS, their procedures and analyses differed from the present study.18 Furthermore, limited knowledge is available about the margins of across-session reproducibility errors.61,62 As this was the first longitudinal DTI study including healthy siblings, we used a whole brain, voxel-based analyses, given the fact that evidence for differential WM regional time-trajectories is missing to date. The results of the group by region interactions are thus hypothesis-generating and may be of use in future longitudinal studies that examine individual WM tracts within distinct development trajectories.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was supported by the Dutch organization for scientific research NWO (Genetic Risk and Outcome of Psychosis [G.R.O.U.P]) and the European Community’s Seventh Framework Programme under Grant Agreement No. HEALTH-F2-2009-241909 (European Network of National Schizophrenia Networks Studying Gene-Environment Interactions Consortium).
Both funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Supplementary Material
Acknowledgments
We thank Truda Driesen and Inge Crolla for their coordinating roles in the data collection, as well as the G.R.O.U.P. investigators: Richard Bruggeman, Wiepke Cahn, Lieuwe de Haan, René S. Kahn, Carin Meijer, Inez Myin-Germeys, Jim van Os, and Durk Wiersma. J.v.O. is or has been an unrestricted research grant holder with or has received financial compensation as an independent symposium speaker from, Lilly, BMS, Lundbeck, Organon, Janssen, GlaxoSmithKline, AstraZeneca, Pfizer, and Servier. M.M. has received financial compensation as an independent symposium speaker from Lilly and Janssen. P.D. has received financial compensation as an independent symposium speaker from AstraZeneca. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1. Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- 2. Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neuro Biobehav Rev. 2011;35:1110–1124. [DOI] [PubMed] [Google Scholar]
- 3. Pierpaoli C, Barnett A, Pajevic S, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. NeuroImage. 2001;13(6 Pt 1):1174–1185. [DOI] [PubMed] [Google Scholar]
- 4. Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108:3–10. [DOI] [PubMed] [Google Scholar]
- 5. Samartzis L, Dima D, Fusar-Poli P, Kyriakopoulos M. White matter alterations in early stages of schizophrenia: a systematic review of diffusion tensor imaging studies. J Neuroimaging. 2014;24:101–10. [DOI] [PubMed] [Google Scholar]
- 6. Kochunov P, Hong LE. Neurodevelopmental and neurodegenerative models of schizophrenia: white matter at the center stage. Schizophr Bull. 2014;40:721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pantelis C, Yücel M, Wood SJ, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–696. [DOI] [PubMed] [Google Scholar]
- 8. Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Molecular Psychiatr. 2012;17(12):1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andreasen NC. The lifetime trajectory of schizophrenia and the concept of neurodevelopment. Dialogues Clin Neurosci. 2010;12:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meyer-Lindenberg A. Neuroimaging and the question of neurodegeneration in schizophrenia. Prog Neurobiol. 2011;95:514–516. [DOI] [PubMed] [Google Scholar]
- 11. Clark KA, Nuechterlein KH, Asarnow RF, et al. Mean diffusivity and fractional anisotropy as indicators of disease and genetic liability to schizophrenia. J Psychiatr Res. 2011;45:980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Knöchel C, Oertel-Knöchel V, Schönmeyer R, et al. Interhemispheric hypoconnectivity in schizophrenia: fiber integrity and volume differences of the corpus callosum in patients and unaffected relatives. Neuroimage. 2012;59:926–934. [DOI] [PubMed] [Google Scholar]
- 13. von Hohenberg CC, Pasternak O, Kubicki M, et al. White matter microstructure in individuals at clinical high risk of psychosis: a whole-brain diffusion tensor imaging study. Schizophr Bull. 2014;40:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol Psychiatry. 2009;66:562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peters BD, Blaas J, de Haan L. Diffusion tensor imaging in the early phase of schizophrenia: what have we learned? J Psychiatr Res. 2010;44:993–1004. [DOI] [PubMed] [Google Scholar]
- 16. Canu E, Agosta F, Filippi M. A selective review of structural connectivity abnormalities of schizophrenic patients at different stages of the disease. Schizophr Res. 2015;161:19–28. [DOI] [PubMed] [Google Scholar]
- 17. Carletti F, Woolley JB, Bhattacharyya S, et al. Alterations in white matter evident before the onset of psychosis. Schizophr Bull. 2012;38:1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reis Marques T, Taylor H, Chaddock C, et al. White matter integrity as a predictor of response to treatment in first episode psychosis. Brain: J Neurol. 2014;137(Pt 1):172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garver DL, Holcomb JA, Christensen JD. Compromised myelin integrity during psychosis with repair during remission in drug-responding schizophrenia. Int J Neuropsychopharmacol. 2008;11:49–61. [DOI] [PubMed] [Google Scholar]
- 20. Mitelman SA, Canfield EL, Newmark RE, et al. Longitudinal assessment of gray and white matter in chronic schizophrenia: a combined diffusion-tensor and structural magnetic resonance imaging study. Open Neuroimaging J. 2009;3:31–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60:585–594. [DOI] [PubMed] [Google Scholar]
- 22. Whitford TJ, Grieve SM, Farrow TF, et al. Volumetric white matter abnormalities in first-episode schizophrenia: a longitudinal, tensor-based morphometry study. Am J Psychiatry. 2007;164:1082–1089. [DOI] [PubMed] [Google Scholar]
- 23. van Haren NE, Hulshoff Pol HE, Schnack HG, et al. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol Psychiatry. 2008;63:106–113. [DOI] [PubMed] [Google Scholar]
- 24. Domen PA, Michielse S, Gronenschild E, et al. Microstructural white matter alterations in psychotic disorder: a family-based diffusion tensor imaging study. Schizophr Res. 2013;146:291–300. [DOI] [PubMed] [Google Scholar]
- 25. Korver N, Quee PJ, Boos HB, Simons CJ, de Haan L. Genetic Risk and Outcome of Psychosis (GROUP), a multi-site longitudinal cohort study focused on gene-environment interaction: objectives, sample characteristics, recruitment and assessment methods. Int J Methods Psychiatr Res. 2012;21:205–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 27. van der Gaag M, Hoffman T, Remijsen M, et al. The five-factor model of the Positive and Negative Syndrome Scale II: a ten-fold cross-validation of a revised model. Schizophr Res. 2006;85:280–287. [DOI] [PubMed] [Google Scholar]
- 28. Andreasen NC, Carpenter WT, Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162:441–449. [DOI] [PubMed] [Google Scholar]
- 29. Annett M. A classification of hand preference by association analysis. Br J Psychol. 1970;61:303–321. [DOI] [PubMed] [Google Scholar]
- 30. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. WHO. Composite International Diagnostic Interview (CIDI). Geneva, Switzerland: World Health Organization; 1990. [Google Scholar]
- 32. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. [DOI] [PubMed] [Google Scholar]
- 34. Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. R Core Team. R: A Language and Environment for Statistical Computing [Computer Program]. Version 3.2.0. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 37. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B: Methodol. 1995;57:289–300. [Google Scholar]
- 38. Simes R. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73:751–754. [Google Scholar]
- 39. Liao Y, Huang X, Wu Q, et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. 2013;38:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Imperati D, Colcombe S, Kelly C, et al. Differential development of human brain white matter tracts. PLoS ONE. 2011;6:e23437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Michielse S, Coupland N, Camicioli R, et al. Selective effects of aging on brain white matter microstructure: a diffusion tensor imaging tractography study. Neuroimage. 2010;52:1190–1201. [DOI] [PubMed] [Google Scholar]
- 42. Voineskos AN, Lobaugh NJ, Bouix S, et al. Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain: J Neurol. 2010;133(Pt 5):1494–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Melicher T, Horacek J, Hlinka J, et al. White matter changes in first episode psychosis and their relation to the size of sample studied: a DTI study. Schizophr Res. 2015;162:22–28. [DOI] [PubMed] [Google Scholar]
- 44. Kubota M, Miyata J, Sasamoto A, et al. Alexithymia and reduced white matter integrity in schizophrenia: a diffusion tensor imaging study on impaired emotional self-awareness. Schizophr Res. 2012;141:137–143. [DOI] [PubMed] [Google Scholar]
- 45. Sprooten E, Brumbaugh MS, Knowles EE, et al. Reduced white matter integrity in sibling pairs discordant for bipolar disorder. Am J Psychiatry. 2013;170:1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yap QJ, Teh I, Fusar-Poli P, Sum MY, Kuswanto C, Sim K. Tracking cerebral white matter changes across the lifespan: insights from diffusion tensor imaging studies. J Neural Transm. 2013;120:1369–1395. [DOI] [PubMed] [Google Scholar]
- 47. Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60:340–352. [DOI] [PubMed] [Google Scholar]
- 48. Fujiwara H, Namiki C, Hirao K, et al. Anterior and posterior cingulum abnormalities and their association with psychopathology in schizophrenia: a diffusion tensor imaging study. Schizophr Res. 2007;95:215–222. [DOI] [PubMed] [Google Scholar]
- 49. Wang L, Hosakere M, Trein JC, et al. Abnormalities of cingulate gyrus neuroanatomy in schizophrenia. Schizophr Res. 2007;93:66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Arat HE, Chouinard VA, Cohen BM, Lewandowski KE, Öngür D. Diffusion tensor imaging in first degree relatives of schizophrenia and bipolar disorder patients. Schizophr Res. 2015;161:329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hoptman MJ, Ardekani BA, Butler PD, Nierenberg J, Javitt DC, Lim KO. DTI and impulsivity in schizophrenia: a first voxelwise correlational analysis. Neuroreport. 2004;15:2467–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kubicki M, Westin CF, Nestor PG, et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry. 2003;54:1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Whitford TJ, Lee SW, Oh JS, et al. Localized abnormalities in the cingulum bundle in patients with schizophrenia: a diffusion tensor tractography study. Neuroimage Clin. 2014;5:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Meijer J, Simons CJ, Quee PJ, Verweij K, Investigators G. Cognitive alterations in patients with non-affective psychotic disorder and their unaffected siblings and parents. Acta Psychiatr Scand. 2012;125:66–76. [DOI] [PubMed] [Google Scholar]
- 55. Sitskoorn MM, Aleman A, Ebisch SJ, Appels MC, Kahn RS. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophr Res. 2004;71:285–295. [DOI] [PubMed] [Google Scholar]
- 56. Ebdrup BH, Raghava JM, Nielsen MO, Rostrup E, Glenthoj B. Frontal fasciculi and psychotic symptoms in antipsychotic-naive patients with schizophrenia before and after 6 weeks of selective dopamine D2/3 receptor blockade. J Psychiatry Neurosci. 2016;41:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ozcelik-Eroglu E, Ertugrul A, Oguz KK, Has AC, Karahan S, Yazici MK. Effect of clozapine on white matter integrity in patients with schizophrenia: a diffusion tensor imaging study. Psychiatry Res. 2014;223:226–235. [DOI] [PubMed] [Google Scholar]
- 58. Szeszko PR, Robinson DG, Ikuta T, et al. White matter changes associated with antipsychotic treatment in first-episode psychosis. Neuropsychopharmacol. 2014;39:1324–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang Q, Cheung C, Deng W, et al. White-matter microstructure in previously drug-naive patients with schizophrenia after 6 weeks of treatment. Psychol Med. 2013;43:2301–2309. [DOI] [PubMed] [Google Scholar]
- 60. DeLisi LE. The effect of cannabis on the brain: can it cause brain anomalies that lead to increased risk for schizophrenia? Curr Opin Psychiatry. 2008;21:140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jovicich J, Marizzoni M, Bosch B, et al. Multisite longitudinal reliability of tract-based spatial statistics in diffusion tensor imaging of healthy elderly subjects. Neuroimage. 2014;101:390–403. [DOI] [PubMed] [Google Scholar]
- 62. Vollmar C, O’Muircheartaigh J, Barker GJ, et al. Identical, but not the same: intra-site and inter-site reproducibility of fractional anisotropy measures on two 3.0T scanners. Neuroimage. 2010;51:1384–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.