Abstract
Psychotic symptoms are prevalent in schizophrenia, bipolar disorder, and other psychiatric and neurological disorders, yet the neurobiological underpinnings of psychosis remain obscure. In the last decade, a large number of magnetic resonance imaging studies have shown differences in local gray matter volume between patients with different psychiatric syndromes and healthy controls. Few studies have focused on the symptoms, which these syndromes are constituted of. Here, we test the association between psychosis and gray matter volume by using a sample of 167 subjects with bipolar disorder, with and without a history of psychosis, and 102 healthy controls. Magnetic resonance images were analyzed on group level using a voxel-wise mass univariate analysis (Voxel-Based Morphometry). We found that patients with a history of psychosis had smaller gray matter volume in left fusiform gyrus, the right rostral dorsolateral prefrontal cortex, and the left inferior frontal gyrus compared with patients without psychosis and with healthy controls. There was no volume difference in these areas between the no-psychosis group and healthy controls. These areas have previously been structurally and functionally coupled to delusions and hallucinations. Our finding adds further evidence to the probability of these regions as key areas in the development of psychotic symptoms.
Key words: psychosis, magnetic resonance imaging, bipolar disorder, voxel-based morphometry, prefrontal cortex
Introduction
Psychosis entails a reduced ability to interpret delusive thoughts or hallucinations as false, and this has been suggested to be associated with structural and neurochemical alterations in the brain. There are several Magnetic Resonance Imaging-studies (MRI) comparing brain morphology between patients with schizophrenia or bipolar disorder (BD) with healthy controls.1,2 As psychosis occurs in both schizophrenia and BD, studies have focused on shared brain morphology changes. Even though gray matter volume loss is more wide-spread and severe in schizophrenia than in BD,3 there are also shared structural changes including enlarged ventricles,4 as well as gray matter volume reduction in the anterior cingulate cortex and bilateral insula.3 However, these shared morphological alterations are more likely to reflect nonspecific pathological processes related to other common symptoms in schizophrenia and BD, as recent research has not been able to link these exclusively to psychosis.5 Enlarged ventricles are also found in major depression6 and post-traumatic stress disorder7 and the anterior cingulate cortex and insula are rather believed to be involved in interoception and emotional regulation. Hence, this diagnosis-based strategy reveals important leads on the general pathophysiology of the respective disorder, but does not inform on specific structural changes related to the symptoms of psychosis.
A better strategy to reveal brain changes specifically related to psychosis is to compare patients with the same psychiatric illness, presenting with and without psychotic symptoms. Such a case-case design would control for general structural changes related to the disorder and enable the study of specific traits associated with the psychosis. This design is not possible in schizophrenia since psychotic symptoms are part of the definition of the disorder. By contrast, not all bipolar patients have a history of psychosis: psychotic symptoms have been reported in up to 68% of patients with manic episodes,8 in 11% of patients with bipolar depression, and in 25% of patients with mixed states.9 Most other symptoms and clinical characteristics are, however, shared between the psychosis and no-psychosis bipolar patients, ie, recurrent episodes of mania, hypomania, and depression, and euthymic periods when the patient at best is asymptomatic and regains function.
Two previous structural MRI studies have directly compared psychosis (PBD) and no-psychosis (NPBD) bipolar subjects with respect to volumes of local brain areas.10,11 These studies were, however, hampered by small sample sizes (PBD n = 23, NPBD n = 15, and PBD n = 15, NPBD n = 12, respectively). The one finding reported was a decreased gray matter volume in the left prefrontal cortex in the PBD group.11 One larger study compared bipolar patients who had experienced delusions during depressive episodes with those who had no delusions. This analysis revealed smaller gray matter volume in the insula and the dorsolateral prefrontal cortex (DLPFC) in the group with delusions.12 One diffusion tensor imaging study revealed reduced white matter integrity along the corpus callosum in patients with a history of psychosis,13 and one study of ventricular volume showed larger lateral ventricles in the PBD group.14
The aim of this study was to test if psychosis per se is related to local gray matter volume alterations. To this end, we compared a comparatively large cohort of bipolar patients with and without a history of psychotic affective episodes, by means of voxel-based morphometry (VBM) analysis of magnetic resonance images of the brain in a case-case design. Any significant clusters of volumetric difference were compared to healthy controls, to determine if they are caused by an increase in one group or a decrease in the other.
Methods
The study was approved by the Regional Ethical Review Board in Stockholm, Sweden.
Subjects
Subjects were recruited from a long-term follow-up program, the St Göran bipolar project, at the Northern Stockholm psychiatric clinic, Stockholm, Sweden (for details, see Ekman et al15). Briefly, the catchment area for the recruiting clinic consists of urban communities with diverse socioeconomic status. The population is approximately 320 000 over the age of 18. During the time of inclusion for this study, all patients in this area who presented with signs of BD were referred to this tertiary unit for evaluation and treatment. Consecutive new outpatients and continuing patients at the unit were invited to participate, provided that they were diagnosed with BD.
Assessment of Diagnosis
The clinical diagnosis of BD was established according to the structured interview instrument Affective Disorder Evaluation (ADE), which has previously been employed in the STEP-BD project.16 The Swedish version of this instrument is described in supplementary material. Also, the structured psychiatric interview Mini International Neuropsychiatry Interview (M.I.N.I.) was completed at baseline in order to screen for comorbidities. The ADE and M.I.N.I. interviews were conducted by board-certified psychiatrists, working at the tertiary bipolar outpatient unit, or by residents in psychiatry completing their training at this unit. The assessments were based on the patients’ self-reports and the clinicians’ evaluation, and, when needed, supplemented with information from medical records and interviews with next of kin. To assess alcohol and drug misuse, the patients completed 2 self-report questionnaires: the Alcohol Use Disorders Identification Test (AUDIT) and the Drug Use Disorders Identification Test (DUDIT). Illness severity was rated using the Clinical Global Impression scale (CGI).17 The final diagnoses were set at a diagnostic case-conference where all information was presented. A consensus panel of experienced board-certified psychiatrists, specialized in BD (n = 2–5), made a best-estimate diagnostic decision. Using this procedure, the risk of inter-rater bias in the inclusion process was reduced. If the patient was at least 18 years old and met the DSM-IV-TR criteria for BD type I, II, or Not Otherwise Specified (NOS), they were provided detailed written and oral information about the study. Patients were excluded if they were unable to complete the standard clinical assessment or were incapable of providing informed consent. All enrolled patients consented orally and in writing in order to participate in the follow-up program. The subjects were not remunerated for participation.
Subjects were euthymic and did not meet criteria for depression or mania/hypomania when MRI scans were performed. Euthymia was defined by Montgomery Åsberg Depression Rating Scale (MADRS)18 and Young Ziegler Mania Rating Scale (YMRS)19 scores of <14.
Assessment of Psychosis
We used the ICD-10 definitions of psychosis. The diagnosis of psychotic depression requires “the presence of hallucinations, delusions, psychomotor retardation, or stupor so severe that ordinary social activities are impossible”.20 Psychotic symptoms in mania are diagnosed when “delusions or hallucinations are present, or the excitement, excessive motor activity, and flight of ideas are so extreme that the subject is incomprehensible or inaccessible to ordinary communication”.20 The presence of psychosis is in the ADE determined under the heading “Determine Psychotic Disorder Diagnosis.” The rater scores a binary “yes” or “no” to the question “Any psychotic disorder?” and specified the psychotic symptoms. This rating is based on all available information at the time of interview. There are no universally acclaimed criteria for defining a psychotic bipolar subtype. Previous studies have used the definition of having hallucinations and/or delusions during an affective episode.10 Studies of healthy subjects have shown that 10%–28% of the normal population have experienced hallucinations and/or delusions.21,22 In line with this notion, one would expect that some of the reported psychotic symptoms in a bipolar material are not attributable to the bipolar illness. Some subjects also had hallucinations and delusions during an affective episode but were using cannabis or amphetamine at the time. Therefore we defined psychosis as having hallucinations, delusions or disorganized behavior during an affective episode which was not drug-induced, and that these symptoms were severe enough to be classified as psychotic by a psychiatrist. The patients reporting their only lifetime experience of psychotic symptoms after drug use or during a euthymic state (n = 25) were excluded from further analysis.
Healthy Control Subjects
A matched cohort of 102 healthy control subjects was recruited from the general population (supplementary material).
Acquisition and Pre-processing of MRI Data
MRI scans were acquired at the Karolinska MR Research Centre from January 2006 to December 2011 after the clinical interview. The median time difference in days between clinical examination and scanning was 206 (range 2–1084 d). Medical chart records were reviewed to secure that no case had a psychotic mood episode in the interval between clinical examination and scanning. No subject was, however, reclassified after this review. Sagittal T1 weighted, axial and coronal T2 weighted and axial fluid attenuation inversion recovery (FLAIR) T2 weighted scans were acquired for examination by a senior radiologist to control for clinically significant anatomical abnormalities. A total of 170 patients and 102 controls were scanned (General Electric Signa Excite 1.5T). Two patients with hydrocephalus and one patient with Wilson’s disease were excluded from further analyses and diverted to neurological follow-up.
All subjects were examined in the same scanner and with the same protocol, using an 8-channel head coil. 1.8mm slices of coronal images were acquired using a 3-dimensional spoiled gradient echo recall sequence (3D-SPGR) with the following parameters: time to repetition = 21.0ms, echo time = 6ms, number of excitations = 1, flip angle = 30°, acquisition matrix = 256×256×124. The calibration filter for the scanner was changed during the study, resulting in differences in the intensity distribution between subjects scanned with different filters. There was no significant difference in distribution of subjects scanned with the old (SCIC) and new (PURE) filters in the PBD (31/54) and NPBD (23/59) groups (Fisher’s exact test, 2-tailed, P = .25), still this potentially confounding factor has been taken into account in the statistical analyses by adding a binary covariate.
The pre-processing and analysis of images were done in Matlab version 7.14.0.739 (www.mathworks.com/products/matlab/) using SPM12 statistical parametric mapping software (www.fil.ion.ucl.ac.uk/spm/). The method of VBM is characterized by a number of mathematical steps for pre-processing, which previously has been described in detail.23,24 The 3D-SPGR series of images were manually reoriented to the anterior–posterior commissure (AC-PC) line, all by the same investigator. Images were then segmented into gray and white matter images in native space. Rigidly aligned images were also created, enabling the use of DARTEL.25 Using DARTEL, a common template based on all gray and white aligned images, and a flow field for each subject, were created. This template and the flow fields were then used to warp, modulate, and normalize all the gray matter images to Montreal Neurological Institute (MNI)-space. Modulation was used to preserve local volumes. All options for estimating and writing the Statistical Parametric Maps (SPM’s) were set to default except for the option to preserve amount instead of concentrations when normalizing; in this process SPM’s are resliced to a voxel size of 1.5×1.5×1.5mm. Since both cortical and subcortical gray matter is included in the analysis, the normalized images were smoothed with an 8×8 × 8mm full width at half maximum Gaussian smoothing kernel.
Statistical Analyses of MRI-Data
The smoothed images were sorted into 2 groups based on the presence of a previous psychotic episode. The PBD and NPBD groups were then compared with respect to local gray matter volumes using a 2-sample T-test, assuming unequal variance and adding age, sex, and scanner filter as covariates of no interest. We assumed unequal variance because we could not presuppose equal variance in all voxels. The 2 groups differed markedly in the prevalence of comorbid attention deficit hyperactivity disorder (ADHD) (psychosis: 16%, no-psychosis: 38%), a diagnosis that has previously been associated with altered gray matter volume.26 Therefore, we corrected for ADHD by introducing this diagnosis as a covariate of no interest to lower the risk of false positive findings. Also, the 2 groups differed in frequency of subjects with current or previous treatment with antipsychotics and lithium; drugs that may have an impact on brain volume.27 We corrected for this by adding the accumulated number of months that a subject had taken any antipsychotic, (since volumetric effect is associated with long-term antipsychotic treatment), and current lithium treatment, (since volumetric effects are rapid28 and may be artifacts caused by that lithium reduces the T1 relaxation time in gray matter29), as covariates of no interest. Age and antipsychotics were modeled as continuous variables and the others as binary variables. The covariates were not centered. Proportional global normalization using total intracranial volume, calculated using the “Tissue Volumes” utility in SPM12,30 was applied to correct for differences in brain size. An optimized mask, using the Masking Toolbox and the method described by Ridgway et al,31 was created from the smoothed images to exclude non-gray tissue from the analyses.
Statistical significance was set to Family-wise error (FWE)-corrected voxel level P < .05.
The clusters of significant volumetric difference between PBD and NPBD subjects where then used to create a second mask. This mask was applied in the analyses of PBD and NPBD subjects compared to healthy controls, to determine if the volumetric differences are caused by increase or reduction of gray matter volume in either group.
Results
Demographic data are presented in table 1. Of the 85 subjects with a history of psychosis, 40 had experienced hallucinations and 77 had delusions; only 8 subjects had experienced hallucinations without delusions. No subject was diagnosed with psychosis by only having a disorganized behavior. As expected, few patients in the psychosis group had bipolar II disorder. The prevalence of comorbid ADHD was significantly higher in the no-psychosis group. There were no other significant differences between the 2 groups concerning somatic or psychiatric comorbidity.
Table 1.
Demographics, Medication, Family History, and Comorbidities
| Psychosis n = 85 | No Psychosis n = 82 | P | Controls n = 102 | |
|---|---|---|---|---|
| Males | 30 (35%) | 34 (41%) | .43 | 56 (50%) |
| Females | 55 (65%) | 48 (59%) | .43 | 56 (50%) |
| BP1 | 69 (81%) | 16 (20%) | <.00001 | 0 |
| BP2 | 16 (19%) | 66 (80%) | <.00001 | 0 |
| Lithium, current use | 53 (62%) | 34 (41%) | .0085 | 0 |
| Antidepressants, current use | 32 (38%) | 49 (60%) | .0053 | 0 |
| Antipsychotics, current use | 33 (39%) | 11 (13%) | .0002 | 0 |
| Antipsychotics, lifetime months use, mean ± SD | 7±16 | 4±12 | .11 | 0 |
| Anticonvulsants, current use | 28 (33%) | 23 (28%) | .51 | 0 |
| Age, mean ± SD | 37±13 | 40±13 | .20 | 39±15 |
| First episode, age, mean ± SD | 22±9 | 21±11 | .54 | n/a |
| Illness duration, mean ± SD | 16±10 | 19±14 | .068 | n/a |
| Lifetime manic episodes,a mean ± SD | 3±3 | 3±6 | .97 | 0 |
| 9 y education | 14 (16%) | 12 (15%) | .83 | 1 (1%) |
| 12 y education | 25 (29%) | 22 (27%) | .73 | 36 (36%) |
| University education | 46 (54%) | 48 (59%) | .64 | 61 (61%) |
| CGI, median (min–max) | 5 (2–7) | 4 (2–6) | <.00001 | n/a |
| First degree relative with bipolar disorder | 16 (19%) | 11 (13%) | .40 | 0 |
| Any relative bipolar disorder | 23 (27%) | 21 (26%) | .86 | 0 |
| First degree with schizophrenia | 2 (3%) | 1 (1%) | 1 | 0 |
| Any relative schizophrenia | 6 (7%) | 6 (7%) | 1 | 0 |
| Any suicide attempt | 39 (46%) | 23 (28%) | .025 | 0 |
| Attention deficit hyperactivity disorder | 14 (16%) | 31 (38%) | .0028 | 0 |
| Panic disorder | 20 (24%) | 28 (34%) | .17 | 0 |
| Social phobia | 13 (15%) | 18 (22%) | .32 | 0 |
| Obsessive compulsive disorder | 11(13%) | 11 (13%) | 1 | 0 |
| Generalized anxiety disorder | 11 (13%) | 15 (18%) | .40 | 0 |
| Anorexia | 8 (9%) | 8 (10%) | 1 | 0 |
| Bulimia | 9 (11%) | 9 (11%) | 1 | 0 |
| Alcohol dependence | 14 (16%) | 13 (16%) | 1 | 0 |
| Drug abuse | 11 (13%) | 5 (7%) | .19 | 0 |
| Body Mass Index, mean ± SD | 26±4 | 24±4 | .057 | 24±3 |
Note: BD1, bipolar 1 disorder; BD2, bipolar 2 disorder; CGI, clinical global impression. Fisher’s exact test, 2-tailed, was used for frequencies, Student’s 2-sided T-test for continuous variables and Mann-Whitney U-test for ordinal data.
aBipolar I subjects only.
Patients with previous psychotic episodes were more likely to be treated with lithium or antipsychotics. Patients without psychosis were more commonly treated with antidepressants.
The PBD subjects had significantly higher ratings on lifetime Clinical Global Impression (CGI) scale and were more likely to have attempted suicide.
Voxel-based Morphometry Analyses
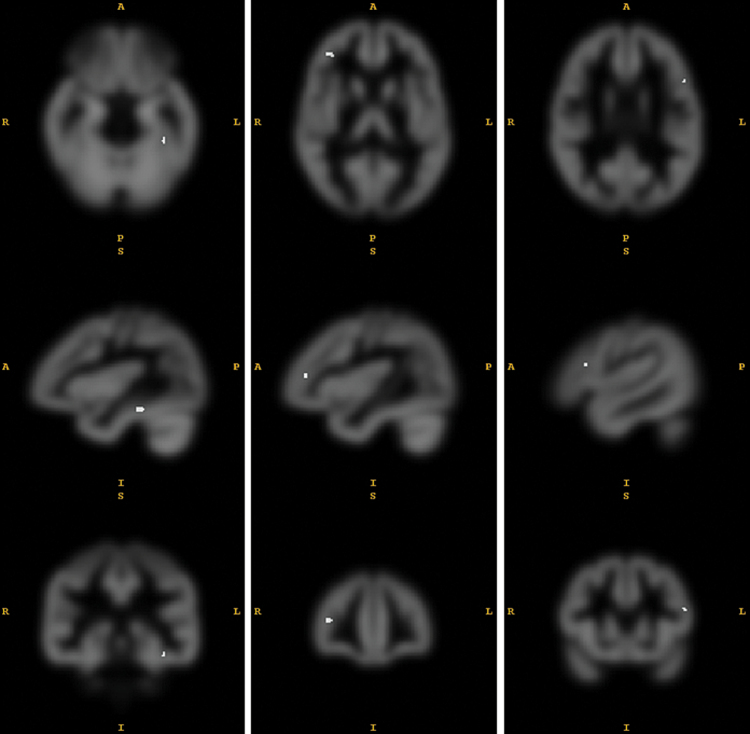
The VBM analysis revealed 4 clusters of decreased gray matter in the psychosis group compared to the no-psychosis group (table 2). In the left fusiform gyrus, peak voxel [x = −38 y = −33 z = −20] MNI-space, there was a cluster of 18 voxels, P = .010, T = 5.07 that survived the correction for multiple comparisons (FWE P < .05 threshold; figure 1). Thirty-three contiguous voxels in the right DLPFC, peak voxel [x = 39,y = 42, z = 11], FWE-corrected P = .014, T = 4.98. This corresponds to the right middle frontal gyrus, on the border between Brodmann area BA46 and BA10. Also, 2 small clusters in the left inferior frontal gyrus and the right parieto-occipital area had significantly smaller volume. There was no area of larger gray matter volume in the psychosis group.
Table 2.
Regions of Smaller Gray Matter Volume in Patients With a History of Psychosis Compared to Patients With no History of Psychosis
| Region | MNI-Coordinates x y z | Cluster Size (Voxels) | Peak Voxel P, FWE-Corrected | T | Effect Size Cohen’s d |
|---|---|---|---|---|---|
| Left fusiform gyrus | −38 −33 −20 | 18 | .010 | 5.07 | 0.79 |
| Right dorsolateral prefrontal cortex | 39 42 11 | 33 | .014 | 4.98 | 0.78 |
| Left inferior frontal gyrus, pars triangularis | −51 18 21 | 9 | .033 | 4.59 | 0.74 |
| Right parieto-occipital area | 59 −59 14 | 2 | .046 | 4.66 | 0.73 |
Note: FWE, Family-wise error; MNI, Montreal Neurological Institute.
Fig. 1.
Areas of smaller gray matter volume in patients with a history of psychosis, P < .05 family-wise error (FWE)-corrected on voxel level. Peak voxels [x = −38 y = −33 z = −20], [x = 39 y = 42 z = 11] and [x = −51 y = 18 z = 21] Montreal Neurological Institute (MNI)-space. 327.5 resels. Full width at half maximum (FWHM) [x = 10.6 y = 10.4 z = 10.1] voxels.
To determine whether the volumetric differences are caused by a decreased volume in the psychosis group or an increased volume in the no-psychosis group, we analyzed the 2 groups in comparison with healthy controls: (1) no-psychosis subjects vs healthy controls; (2) psychosis subjects vs healthy controls. In these 2 post hoc analyses, we did not want to confound the results with potential gray matter volume alterations associated to BD per se. We therefore used the significant clusters from the main analysis as an explicit mask, containing 62 voxels. Age, sex, and scanner filter were entered as covariates, and total intracranial volume was used for proportional global normalization. Regions of interest were defined by the mask, and the level of significance was set to voxel-level FWE-corrected P < .05. Results showed that the psychosis group had significantly smaller gray matter volume in the fusiform gyrus, the DLPFC and the inferior frontal gyrus (table 3). By contrast, no significant difference was found when comparing the no-psychosis group with controls. The clusters contained voxels of both larger and smaller volume than healthy controls but the largest effect was towards larger volume in the NPBP-group.
Table 3.
Difference in Gray Matter Volume in the 3 Clusters of Significant Volume Difference Between PBD and NPBD Subjects
| Peak Voxel MNI- Space (x y z) | P FWE- Corrected | T | Cluster Size (Voxels With P < .05) | Effect Size (Cohens d) | |
|---|---|---|---|---|---|
| PBD < HC | |||||
| Left Fusiform | −38 −36 −21 | 2.7×10−7 | 6.38 | 11 | 0.94 |
| Right DLPFC | 41 42 9 | 2.6×10−8 | 6.95 | 27 | 1.03 |
| Left Inferior frontal | −53 20 20 | 1.3×10−7 | 5.86 | 7 | 0.86 |
| NPBD > HC | |||||
| Left Fusiform | −38 −36 −18 | .58 | 0.95 | None | 0.14 |
| Right DLPFC | 42 42 12 | .63 | 0.83 | None | 0.12 |
| Left Inferior frontal | −53 20 20 | .43 | 1.27 | None | 0.19 |
Note: PBD, psychosis bipolar disorder; NPBD, no-psychosis bipolar disorder; HC, healthy controls; DLPFC, dorsolateral prefrontal cortex.
The diagnostic criteria incur a high level of covariance between psychotic symptoms and bipolar subtype I. One study has shown smaller prefrontal gray matter volume in bipolar I compared to bipolar II subjects.32 Since this difference is in the area of one of our findings, we added bipolar subtype as a binary covariate of no interest in a post hoc analysis. There was still a significant prefrontal volume difference in [x = 39,y = 42, z = 12], FWE-corrected P = .011, T = 5.04, between the psychosis and no-psychosis group.
Discussion
To elucidate the potential anatomical brain correlate of psychosis, this study compared gray matter volume in euthymic bipolar patients with or without previous psychotic episodes, and compared the results with a healthy control group. The main findings were clusters of significantly smaller volume in the left fusiform gyrus and the right prefrontal cortex (BA10/46) in the psychosis group, compared with the no-psychosis group. These clusters were smaller in the psychosis group compared with healthy controls, whereas the no-psychosis bipolar group did not differ from controls. This suggests that gray matter decrease in these areas are specifically associated with psychosis rather than with BD per se. We have previously reported that the lifetime number of manic episodes is associated with smaller gray matter volume in the DLPFC.15 In the present study, there was no significant difference in the number of manic episodes between the psychosis and no-psychosis groups.
Core functions of the prefrontal cortex pertain to the supervision of automatic behaviour, integration of sensory inputs, spatial organization, and coordination of sensory and emotional efference.33 The prefrontal cortex also evaluates feedback from a performed action and integrates successful actions into the memory.33,34 A malfunction of the prefrontal cortex may cause deficits with respect to working memory, verbal fluency, set-shifting, psychomotor speed, attention, and also cause delusions.35 Recently, the DLPFC has been functionally implicated in psychosis by differences in prediction error signaling36,37 and in prodromal psychosis.38 One proposed role of this brain region is to evaluate information from other cortical areas, elicited by somatosensory stimuli, and reject those interpretations that are unreal, a sort of “belief evaluation”.39 Hence, the function, structure, and connectivity of the prefrontal cortex are important in relation to the development of psychosis. A reduced gray matter volume in BA10 has previously been reported in patients with schizophrenia compared to controls40 and has been associated with poor outcome.41 Also, reduced volume in this area is associated with impaired metacognitive accuracy in healthy subjects.42 BA10 was though not identified as a key area in schizophrenia in a large-scale meta-analysis of brain structure.43 The “gateway hypothesis” of the rostral prefrontal cortex44 suggests that BA10 is involved in coordinating stimulus-independent and stimulus-oriented cognition. According to this notion, BA10 is a gateway between the mental life influenced by perceptual stimuli and the self-generated mental life that is stimulus independent. Investigators have therefore speculated that a malfunctioning gateway-system increases the risk of developing delusions.45,46
Several studies have shown associations between volume reduction in the adjacent BA46 and both schizophrenia and BD. There is compelling evidence that the BA46 is associated with psychosis. The 2-factor model of delusions presented by Coltheart, based on studies of patients with delusions secondary to cortical atrophy or ischaemia,39 suggests that delusions are formed from a misinterpreted sensory input in a first area, after which a second area fails to reject the faulty interpretation. The second area is suggested to lie within the rostral DLPFC,39 which gains support from fMRI studies of prediction error tasks showing that subjects with delusions fail to activate this area.36,37 Our finding agrees with these studies and adds a morphometric association that could partly explain why delusional patients fail to activate the DLPFC in the fMRI-studies. Still, the PBD patients have no psychotic symptoms in-between mood episodes. In line with the 2-factor model, this suggests that malfunction in other brain areas, causing mania or depression, requires increased top-down regulation from the rostral DLPFC to preserve the ability of reality testing.
Two previous studies have shown that bipolar patients with delusions have a smaller gray matter volume in the DLPFC.11,12 Still, delusions are only one of the symptoms of psychosis and a delusional patient does not necessarily present with hallucinations and/or disorganized behavior. The common factor for these 3 psychotic symptoms is the individual’s inability to identify them as false or inappropriate, and we suggest that this can be attributed to a malfunction of the DLPFC. We bind this reduction in DLPFC to patients with the whole range of psychotic symptoms vs no psychotic symptoms, and thereby generalize the notion that a reduction in DLPFC is associated with all psychoses.
We also found that the PBD group demonstrated a smaller gray matter volume in the left fusiform gyrus. This region has previously proved to be smaller in schizophrenia47 and to be negatively correlated with positive symptoms.48 Interestingly, the cortex is thinner in this area in people with non-clinical auditory hallucinations, not fulfilling criteria for schizotypal personality disorder or schizophrenia,49 compared with controls. Framed in the 2-factor model discussed above, the fusiform gyrus might be the area where an auditory hallucination is generated. A functioning DLPFC would, however, reject this hallucination as false and cause a nonclinical hallucination, whereas a psychotic hallucination would require dysfunction in both the fusiform gyrus and the DLPFC. This may suggest that patients with a premorbid low gray matter volume in the fusiform gyrus and DLPFC are susceptible to develop psychotic symptoms in mania or depression.
Finally, we found that the psychosis group had a smaller gray matter volume in the left inferior frontal gyrus. This is in line with a recent mega-analysis of patients with schizophrenia that showed smaller volume in the inferior frontal cortex compared to controls.50 Even though this study do not convey which symptom of schizophrenia that might be associated with this volume reduction, another direct comparison between schizophrenic patients with and without hallucinations demonstrated less volume in the left inferior frontal gyrus in the group with hallucinations,51 which might suggest that that the volume reduction is coupled to psychotic symptoms rather than schizophrenia per se. Moreover, a study of amphetamine induced psychosis implicated smaller volume in this area as a marker for psychosis proneness.52
Hence, all 3 areas of smaller gray matter volume that we found associated with psychosis have previously been structurally associated with hallucinations and/or delusions.
Strengths and Limitations
Strengths of this well-powered study include the meticulously characterized patients and controls examined in the same scanner. However, potential confounding effects of medication cannot be excluded but cannot be tested properly on cross-sectional data due to confounding by indication. The prevalence of lithium and antipsychotics users was higher, and antidepressant users lower, in the psychosis group. However, since lithium in several studies has been reported to increase prefrontal gray matter volume,4,28,29 this imbalance would rather have introduced false negative results and is unlikely to explain the findings of this study. The effects of antipsychotics and antidepressants on brain structure are not fully ascertained although antipsychotic treatment has been associated with discrete gray matter reduction.27 To evaluate the effects of the potential confounders, we performed a post hoc multiple regression analysis with the peak voxel value in [x = −38,y = −33, z = −20] as the dependent variable and age, gender, scanner filter, ADHD, bipolar subtype, CGI-score, illness duration, antidepressants use, lithium use, the accumulated months of antipsychotic use and suicide attempts as regressors (supplementary material). We found significant effects for scanner filter, lithium treatment, and antipsychotics treatment. These parameters were corrected for in the VBM analyses. The effect of antipsychotics may be a confound by indication, ie, subjects with a low volume in the left fusiform gyrus may be more likely to have psychosis and hence more likely to be treated with antipsychotics. The effect of lithium was larger volume in treated subjects.
A limitation is the slice thickness in the MRI-scans. This decreases sensitivity through partial volume effects. There is though a trade-off between slice thickness and the scanning time. By keeping the scanning time short we were able to acquire images with no motion artifacts, which increases sensitivity through reduced noise.
This is a cross-sectional study of euthymic patients with BD, ie, no subject has psychotic symptoms when scanned. Little is known about moodstate-dependent changes in gray matter volume, but brain plasticity may confound the results. We did not have ratings of the severity of the psychotic episodes; a factor that could increase the sensitivity of the analyses. However, these limitations decrease sensitivity and are unlikely to cause false positive results. Since only 8 subjects in our sample had a history of hallucinations without accompanying delusions, we did not have enough power to detect differences attributable to the specific psychotic symptoms. Finally, we did not have complete data on handedness, why the lateralization of findings should be interpreted with caution.
Conclusions
We found reduced cortical volume in 3 separate regions in bipolar patients with a history of psychosis, compared to bipolar patients with no history of psychosis. Although the neurobiological mechanism underlying psychotic symptoms most certainly is multifactorial, one of the determining factors could hence be a reduction of gray matter volume in the rostral DLPFC. This finding, taken together with fMRI findings discussed above, can prove to be useful in clinical research on the treatment of psychosis. Future studies could eg, evaluate if metacognitive training of psychotic patients and exercises in identifying prediction errors can increase DLPFC volume and decrease psychotic symptoms.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This research was supported by grants from the Swedish Medical Research Council (K2011-61X-14647-09-3, K2010-61X-21569-01-1, and K2010-61P-21568-01-4), the Swedish foundation for Strategic Research, the Swedish Brain foundation, and the Swedish Federal Government under the LUA/ALF agreement (ALF 20100305, ALFGBG-142041). None of these agencies had a role in study design, in the collection, analysis or interpretation of data, in the writing of the report or in the decision to submit the article for publication.
Supplementary Material
Acknowledgment
The authors report no conflicts of interest.
References
- 1. Arnone D, Cavanagh J, Gerber D, et al. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry. 2009;195:194–201. [DOI] [PubMed] [Google Scholar]
- 2. Fornito A, Yücel M, Patti J, et al. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res. 2009;108:104–113. [DOI] [PubMed] [Google Scholar]
- 3. Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res. 2010;117:1–12. [DOI] [PubMed] [Google Scholar]
- 4. Hallahan B, Newell J, Soares JC, et al. Structural magnetic resonance imaging in bipolar disorder: an international collaborative mega-analysis of individual adult patient data. Biol Psychiatry. 2011;69:326–335. [DOI] [PubMed] [Google Scholar]
- 5. Ivleva EI, Bidesi AS, Keshavan MS, et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170:1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kempton MJ, Salvador Z, Munafò MR, et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675–690. [DOI] [PubMed] [Google Scholar]
- 7. Filipovic BR, Djurovic B, Marinkovic S, et al. Volume changes of corpus striatum, thalamus, hippocampus and lateral ventricles in posttraumatic stress disorder (PTSD) patients suffering from headaches and without therapy. Cent Eur Neurosurg. 2011;72:133–137. [DOI] [PubMed] [Google Scholar]
- 8. Canuso CM, Bossie CA, Zhu Y, et al. Psychotic symptoms in patients with bipolar mania. J Affect Disord. 2008;111:164–169. [DOI] [PubMed] [Google Scholar]
- 9. Perugi G, Akiskal HS, Micheli C, et al. Clinical characterization of depressive mixed state in bipolar-I patients: Pisa-San Diego collaboration. J Affect Disord. 2001;67:105–114. [DOI] [PubMed] [Google Scholar]
- 10. Strasser HC, Lilyestrom J, Ashby ER, et al. Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biol Psychiatry. 2005;57:633–639. [DOI] [PubMed] [Google Scholar]
- 11. Tost H, Ruf M, Schmäl C, et al. Prefrontal-temporal gray matter deficits in bipolar disorder patients with persecutory delusions. J Affect Disord. 2010;120:54–61. [DOI] [PubMed] [Google Scholar]
- 12. Radaelli D, Poletti S, Gorni I, et al. Neural correlates of delusion in bipolar depression. Psychiatry Res. 2014;221:1–5. [DOI] [PubMed] [Google Scholar]
- 13. Sarrazin S, Poupon C, Linke J, et al. A multicenter tractography study of deep white matter tracts in bipolar I disorder: psychotic features and interhemispheric disconnectivity. JAMA Psychiatry. 2014;71:388–396. [DOI] [PubMed] [Google Scholar]
- 14. Edmiston EE, Wang F, Kalmar JH, et al. Lateral ventricle volume and psychotic features in adolescents and adults with bipolar disorder. Psychiatry Res. 2011;194:400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ekman CJ, Lind J, Ryden E, et al. Manic episodes are associated with grey matter volume reduction - a voxel-based morphometry brain analysis. Acta Psychiatr Scand. 2010;122:507–515. [DOI] [PubMed] [Google Scholar]
- 16. Sachs GS, Thase ME, Otto MW, et al. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol Psychiatry. 2003;53:1028–1042. [DOI] [PubMed] [Google Scholar]
- 17. Guy W. (ed). ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Heath, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration; 1976. [Google Scholar]
- 18. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. [DOI] [PubMed] [Google Scholar]
- 19. Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 20. World Health Organisation. ICD-10 Classifications of Mental and Behavioural Disorder: Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerland: World Health Organisation; 1992. [Google Scholar]
- 21. Kendler KS, Gallagher TJ, Abelson JM, et al. Lifetime prevalence, demographic risk factors, and diagnostic validity of nonaffective psychosis as assessed in a US community sample. The National Comorbidity Survey. Arch Gen Psychiatry. 1996;53:1022–1031. [DOI] [PubMed] [Google Scholar]
- 22. Poulton R, Caspi A, Moffitt TE, et al. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 2000;57:1053–1058. [DOI] [PubMed] [Google Scholar]
- 23. Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. [DOI] [PubMed] [Google Scholar]
- 24. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. [DOI] [PubMed] [Google Scholar]
- 25. Ashburner J, Friston KJ. Diffeomorphic registration using geodesic shooting and Gauss-Newton optimisation. Neuroimage. 2011;55:954–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seidman LJ, Biederman J, Liang L, et al. Gray matter alterations in adults with attention-deficit/hyperactivity disorder identified by voxel based morphometry. Biol Psychiatry. 2011;69:857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ho BC, Andreasen NC, Ziebell S, et al. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Monkul ES, Matsuo K, Nicoletti MA, et al. Prefrontal gray matter increases in healthy individuals after lithium treatment: a voxel-based morphometry study. Neurosci Lett. 2007;429:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cousins DA, Aribisala B, Nicol Ferrier I, et al. Lithium, gray matter, and magnetic resonance imaging signal. Biol Psychiatry. 2013;73:652–657. [DOI] [PubMed] [Google Scholar]
- 30. Malone IB, Leung KK, Clegg S, et al. Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. Neuroimage. 2015;104:366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ridgway GR, Omar R, Ourselin S, et al. Issues with threshold masking in voxel-based morphometry of atrophied brains. Neuroimage. 2009;44:99–111. [DOI] [PubMed] [Google Scholar]
- 32. Ha TH, Ha K, Kim JH, et al. Regional brain gray matter abnormalities in patients with bipolar II disorder: a comparison study with bipolar I patients and healthy controls. Neurosci Lett. 2009;456:44–48. [DOI] [PubMed] [Google Scholar]
- 33. Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. [DOI] [PubMed] [Google Scholar]
- 34. Royall DR, Lauterbach EC, Cummings JL, et al. Executive control function: a review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2002;14:377–405. [DOI] [PubMed] [Google Scholar]
- 35. Arnsten AF. Prefrontal cortical network connections: key site of vulnerability in stress and schizophrenia. Int J Dev Neurosci. 2011;29:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Corlett PR, Honey GD, Aitken MR, et al. Frontal responses during learning predict vulnerability to the psychotogenic effects of ketamine: linking cognition, brain activity, and psychosis. Arch Gen Psychiatry. 2006;63:611–621. [DOI] [PubMed] [Google Scholar]
- 37. Corlett PR, Murray GK, Honey GD, et al. Disrupted prediction-error signal in psychosis: evidence for an associative account of delusions. Brain. 2007;130:2387–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fusar-Poli P, McGuire P, Borgwardt S. Mapping prodromal psychosis: a critical review of neuroimaging studies. Eur Psychiatry. 2012;27:181–191. [DOI] [PubMed] [Google Scholar]
- 39. Coltheart M. The neuropsychology of delusions. Ann N Y Acad Sci. 2010;1191:16–26. [DOI] [PubMed] [Google Scholar]
- 40. Mitelman SA, Brickman AM, Shihabuddin L, et al. A comprehensive assessment of gray and white matter volumes and their relationship to outcome and severity in schizophrenia. Neuroimage. 2007;37:449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mitelman SA, Canfield EL, Newmark RE, et al. Longitudinal assessment of gray and white matter in chronic schizophrenia: a Combined Diffusion-Tensor and Structural Magnetic Resonance Imaging Study. Open Neuroimag J. 2009;3:31–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fleming SM, Weil RS, Nagy Z, et al. Relating introspective accuracy to individual differences in brain structure. Science. 2010;329:1541–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Palaniyappan L, Balain V, Liddle PF. The neuroanatomy of psychotic diathesis: a meta-analytic review. J Psychiatr Res. 2012;46:1249–1256. [DOI] [PubMed] [Google Scholar]
- 44. Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn Sci. 2007;11:290–298. [DOI] [PubMed] [Google Scholar]
- 45. Garrett M, Silva R. Auditory hallucinations, source monitoring, and the belief that “voices” are real. Schizophr Bull. 2003;29:445–457. [DOI] [PubMed] [Google Scholar]
- 46. Harrington L, Langdon R, Siegert RJ, et al. Schizophrenia, theory of mind, and persecutory delusions. Cogn Neuropsychiatry. 2005;10:87–104. [DOI] [PubMed] [Google Scholar]
- 47. Koenders L, Machielsen MW, van der Meer FJ, et al. Brain volume in male patients with recent onset schizophrenia with and without cannabis use disorders. J Psychiatry Neurosci. 2015;40:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Song J, Han DH, Kim SM, et al. Differences in gray matter volume corresponding to delusion and hallucination in patients with schizophrenia compared with patients who have bipolar disorder. Neuropsychiatr Dis Treat. 2015;11:1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van Lutterveld R, van den Heuvel MP, Diederen KM, et al. Cortical thickness in individuals with non-clinical and clinical psychotic symptoms. Brain. 2014;137:2664–2669. [DOI] [PubMed] [Google Scholar]
- 50. Gupta CN, Calhoun VD, Rachakonda S, et al. Patterns of Gray Matter Abnormalities in Schizophrenia Based on an International Mega-analysis. Schizophr Bull. 2015;41:1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van Tol MJ, van der Meer L, Bruggeman R, et al. Voxel-based gray and white matter morphometry correlates of hallucinations in schizophrenia: the superior temporal gyrus does not stand alone. Neuroimage Clin. 2014;4:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aoki Y, Orikabe L, Takayanagi Y, et al. Volume reductions in frontopolar and left perisylvian cortices in methamphetamine induced psychosis. Schizophr Res. 2013;147:355–361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.