Abstract
Clinical staging improved the possibility of intervening during the psychosis prodrome to limit progression of illness. The current study aimed to validate a novel 4-stage severity-based model with a focus on clinical change over time and risk for conversion to psychosis. One hundred seventy-one individuals at clinical high risk (CHR) for psychosis were followed prospectively (3 ± 1.6 y) as part of the Recognition and Prevention (RAP) program and divided into 4 diagnostic stages according to absence/presence and severity of attenuated positive symptoms. Twenty-two percent of the combined sample recovered (no prodromal symptoms) by study outcome. The negative symptoms only subgroup had the highest symptom stability (70%), but the lowest conversion rate at 5.9%. The subgroup with more severe baseline attenuated positive symptom levels had a higher conversion rate (28%) and a more rapid onset when compared to the moderate attenuated positive symptom subgroup (11%). Finally, the Schizophrenia-Like Psychosis (SLP) subgroup showed low stability (3%), with 49% developing a specific psychotic disorder. The proposed stage model provides a more finely grained classification system than the standard diagnostic approach for prodromal individuals. All 4 stages are in need of early intervention because of low recovery rates. The negative symptom only stage is possibly a separate clinical syndrome, with an increased risk of functional disability. Both subgroups with attenuated positive symptoms are appropriate for studying the mechanisms of psychosis risk, however, individuals with more severe baseline positive symptoms appear better suited to clinical trials. Finally, the SLP category represents an intermediate outcome group appropriate for preventative intervention research but questionable for inclusion in prodromal studies of mechanisms.
Key words: clinical staging, clinical high risk, schizophrenia
Introduction
Despite over 2 decades of preventive research, an understanding of the complex factors preceding the onset of psychosis remains elusive. It has become increasingly evident that one reason for this is that schizophrenia is a cluster of disease entities with multiple causes. As a result, different patterns of early risk factors and attenuated symptoms are likely to define the pre-illness or prodromal period, and disentangling these may hold the keys to both understanding the development and prevention of illness. One way of addressing the issue of heterogeneity is to develop a model mapping out the various clinical stages of illness from earliest signs up to chronic psychosis, thus providing a blueprint for specialized treatment.1–3 However, despite the need for homogeneous and clearly defined stages, the early studies concerned with prevention did not typically differentiate among pre-psychotic phases of illness.4 Instead, the pre-psychotic phase was typically treated as a single clinical entity referred to as the “prodrome.”5–9 The relatively slow progress in uncovering the mechanisms causing psychosis may be at least partly due to the confusion of subtypes within the prodromal phase, a possibility best explored by parsing this phase into more homogeneous subgroups.
Among the handful of early models proposed (eg, Fava and Kellner1 and Lieberman et al10) McGorry’s early clinical staging model provided one of the first attempts to expand upon the pre-psychotic stages.2 In the McGorry et al model,2,3,11–14 the pre-psychotic stage that proceeds a psychotic disorder is made up of several symptom clusters differing in severity and clinical characteristics. The first stage (stage 0) indicates an increased risk, but with no current symptoms (ie, familial high risk). Stage 1 constitutes the current conception of the psychosis prodrome (divided into stage 1a for mild/nonspecific symptoms and stage 1b for moderate sub-threshold symptoms) and stages 2–4, ranging from the first psychotic episode through long-term chronic illness. The standard research definition of the clinical high risk (CHR, also referred to as ultra-high risk or UHR) state includes individuals in stages 1 and 2. The subgroups currently considered to make up the CHR include: (1) Genetic Risk and Deterioration Syndrome (GRD), a trait/state combination of genetic/familial risk or schizotypal personality disorder with functional decline, comparable to McGorry’s stage 1a; (2) Attenuated Positive Symptom Syndrome (APSS), considered an early, subtle form of psychotic-like (lesser intensity) symptoms (McGorry stage 1b); and (3) Brief Intermittent Psychotic Syndrome (BIPS), that are recent, brief, and not seriously disorganizing or dangerous (McGorry stage 2).3,15
The pioneering efforts of McGorry, Yung, and colleagues sparked an interest in prevention that has helped shape the current research field.16 Recent research, however, has suggested that the 3 CHR subgroups may be at different (or mixed) stages of the early illness17 and each may be associated with different levels of risk, with the GRD subgroup having the lowest risk and BIPS having the highest.18 In addition, it is unclear whether each of the 3 subgroups has a different pattern of outcome aside from conversion (eg, stage persistence or symptom recovery), and what the implications are for improving the precision and effectiveness of early intervention.
Cornblatt and colleagues19–21 at the Recognition and Prevention (RAP) Program of the Zucker Hillside Hospital in New York proposed a somewhat different classification system in the late 1990s that was grounded in the theoretical work by Mrazek and Haggerty22 and the neurodevelopment approach proposed by Weinberger.23 Initiated in 1998, the RAP program has been structured according to a theoretical model proposing that schizophrenia is rooted in genetic abnormalities that affect development of basic brain functions. The underlying brain abnormalities are life-long, relatively subtle and provide the necessary but not sufficient foundation for later illness. Examples of the vulnerability factors on the behavioral level are cognitive deficits, social isolation, and school/work problems. On their own, these abnormalities, which typically precede positive symptoms by several years,24 have been shown to lead to various levels of functional disability.25 However, according to this model, when combined with the additional predisposition (likely having an independent genetic etiology) to develop positive symptoms, then psychoses evolve. Therefore, critical to this model is the notion that there are 2 independent processes involved in developing a full-blown psychotic illness. The presence of either one alone can lead to poor long-term prognosis,21,25 but the interaction between these 2 pathways is thought to lead to emergence of full-blown psychosis.
As part of the RAP research strategy, a 4-stage working schema, representing this theoretical model, was then generated, focused on increasing levels of positive symptom severity. In this model, there is one subgroup that represents the nonspecific vulnerability pathway to psychosis, to determine if this is a feasible starting point, or if risk can only be studied in conjunction with positive symptoms. The other 3 subgroups represent the evolution of positive symptoms. Note that negative symptoms run through all 4 stages and positive symptoms show a gradual increase from stages 2 to 4. In contrast to the McGorry model and standard CHR research definitions, there is no subgroup comparable to the GRDs included in the RAP 4-stage model, as familial high risk was viewed as a different clinical entity, especially from an ascertainment perspective, as risk is not based on the patient’s symptom severity but rather on diagnosis of a first degree relative. A further potential confusion in the GRD category is inclusion of schizotypal personality disorder (SPD) as a potential risk factor, also not included in the 4-stage model since this represents a diluted severity and risk for later psychosis that has not been firmly established. As a result, in the 4-stage model, both family history and SPD were treated as dependent variables, rather than selection criteria. As mentioned above, stage 1 consists primarily of negative-type symptoms (eg, social isolation, school failure) and is included as a group with early risk factors but an absence of attenuated positive symptoms and is referred to as CHR−. Stages 2 and 3 follow, characterized by the gradual emergence of positive symptoms, which first appear in mild-to-moderate attenuated form (CHR+Mod; stage 2), and then increase to a severe level (CHR+Sev; stage 3), although still not of psychotic intensity. Stages 2 and 3 are consistent with McGorry stage 1b and with the overall APSS subgroup typically included in North American prodromal studies. Presence of only 1 positive symptom of psychotic intensity is referred to as Schizophrenia-Like Psychosis (or SLPs; stage 4). The SLPs category is characterized both by having only 1 attenuated positive symptom at a severity level of 6 and by failing to meet criteria for any other specific schizophrenia-spectrum or psychotic bipolar disorder. While the standard BIPS prodromal category and the SLPs subgroup are somewhat comparable, the 2 groups are not the same. In the 4-stage model of the RAP program, the SLPs subgroup is considered to be an “intermediate” outcome category: they are no longer strictly prodromal because psychotic level symptoms are already present. In addition, the SLP subgroup is not restricted to less than 1 week of psychotic symptoms that need to be self-limiting, as needed for the BIPS prodromal category, but SLP is akin to psychotic disorder not otherwise specified as per DSM-IV. Therefore, this category is considered a waystation from which evolution continues to more chronic psychotic disorders in many cases and prevention of this process is still considered possible. The sequence of the 4-stage RAP model is therefore CHR−→CHR+Mod→CHR+Sev→SLP→Full-blown specific psychotic disorder. This model highlights the notion that increased symptom severity leads to an increased risk of developing psychosis, with severity varying as a function of initial stage.
The current study aims to explore the clinical validity of the proposed stage model conducted as part of the naturalistic, prospective RAP program in New York. Adolescents and young adults at CHR for psychosis were recruited and classified into 1 of the 4 RAP diagnostic subgroups in order to establish the longitudinal characteristics and broad outcome of each of the 4 stages. To the best of our knowledge, the current assessment represents one of the only evidence based studies to directly test a theoretical stage model of the psychosis prodrome based primarily on symptom severity.26
Specifically, in the present study we aim to examine: (1) The relationship between initial stage (CHR−, CHR+Mod, CHR+Sev, and SLP) and conversion rates over the course of the study with an average follow-up of 3 years; (2) The course of the initial stage diagnosis from baseline to study outcome in terms of remission, improvement, stability, worsening; and (3) The relationship between initial stage diagnosis and medication. Determining the risk of conversion to psychosis as well as stage persistence are important and necessary steps in providing a more complete understanding of the broader outcomes associated with risk for illness. In turn, the stage outcome information can address whether the CHR diagnosis refers to a long-term syndrome. In addition, because prescription of antipsychotic medication has previously been found to be a barometer of symptom severity in CHR individuals27,28 we have included it here as an adjunctive measure. Although not a part of the formal theoretical RAP model, the naturalistic pattern of treatment (based on clinician choice) built into the RAP program is particularly compatible with the use of medication as an indicator of symptom severity.29
Methods
Participants
Participants were recruited to the RAP program during phase I (2000–2006) of the longitudinal study funded by the National Institute of Mental Health since 2000. Patient referrals were made by affiliated outpatient and inpatient psychiatry departments, local mental health providers, school psychologists/counselors, or were self-referred. All procedures were approved by the IRB at the Northwell Health system. Written informed consent (assent from participants<18 y old) was obtained from all participants.
A total of 191 prodromal patients participated in the RAP study in phase I. The RAP criteria overlap in major ways with the Structured Interview for Prodromal Syndromes (SIPS) Criteria of Prodromal Syndromes (COPS). However, RAP selection requires “prodromal” symptom severity levels, but without taking either duration or frequency into account. Research criteria for the RAP CHR categories were based on scores from the Scale of Prodromal Symptoms (SOPS15,30,31): 1) CHR−, 1 or more negative symptoms rated at moderate or greater severity level (and no positive symptoms ≥ 3); Overall CHR+ category based on the presence of 1 or more positive symptoms rated at moderate-to-severe level (3–5 SOPS rating), further subdivided on the basis of severity into 2) CHR+Mod with a total positive symptom score<10; or 3) CHR+Sev with a total positive symptom score≥10; and 4)SLP, 1 positive symptom at a psychotic level of intensity. Subjects in the SLPs category either met a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnosis of psychotic disorder not otherwise specified (PNOS) or brief psychotic disorder (BrPsy) and failed to meet criteria for a diagnosis of schizophrenia based on the following: (1) criteria A, presence of only 1 non-bizarre psychotic symptom; (2) criteria B, presence of sustained adequate role functioning; and/or (3) criteria C, presence of psychotic episodes lasting less than 1 month (in the absence of any psychotropic treatment). Subjects meeting the third criterion, reflecting short duration, all met criteria for BrPsy and were so labeled (see Correll et al32 for details).
Healthy comparison subjects were recruited within the same community and during the same time period as the patients through announcements in local newspapers and advertisements within the health system. Participants between the ages of 12–22 years were included. Exclusion criteria for all participants included: (1) diagnosis of schizophrenia-spectrum or other psychotic disorder (excluding SLPs); (2) non-English speaking; (3) a medical or neurological disorder; (4) Estimated IQ < 70. Healthy controls (CNTLs) with a first-degree relative with a diagnosed Axis I psychotic disorder were also excluded.
Clinical Assessment
Details of the baseline clinical assessment have been reported previously.20 Axis I diagnoses were assessed by the Schedule for Affective Disorders and Schizophrenia for School-Age Children, Epidemiologic Version (K-SADS-E).33 Prodromal symptoms were rated using the SIPS and the companion SOPS.15 Social and role functioning were assessed using the GF:Social and GF:Role scales.34 The SIPS was re-administered approximately 6 months after entry to the RAP Program and regularly every 6 to 9 months, as well as at termination of treatment or conversion to psychosis. For the latter, patients were also re-assessed whenever the study team became aware of a major event potentially indicating clinical worsening/conversion, such as an inpatient admission or patient or caregiver outreach to the program.
Medication Data
Treatment in the RAP program was naturalistic and the decision as to what medications to prescribe was based on the patient’s individual symptoms and the clinician’s best judgment. Medication information was obtained from the patient’s paper or electronic medical record. In the current study, participants had to be on a medication for a minimum of 4 weeks in order for it to be coded as an adequate medication trial. Healthy controls were not taking any psychotropic medication at the baseline assessment.
Clinical Outcome
From the initial sample of 191, follow-up clinical ratings were collected from 171 (89.5%) participants (CHR− = 46; CHR+Mod = 53; CHR+Sev = 39; SLPs = 33), with an average follow-up of 3.0 ± 1.6 years. Data from 92 of these participants (CHR+Mod and CHR+Sev combined) were previously used to develop a prediction algorithm for psychosis.20
Conversion to psychosis in the CHR−, CHR+Mod, and CHR+Sev groups was defined as the presence of a psychotic level positive symptom (score of 6 on the SOPS) for at least 1 week. Conversion in the SLPs was defined as meeting full DSM-IV criteria for schizophreniform, schizophrenia, delusional disorder or bipolar 1 disorder with psychosis.35 The K-SADS-E was used to confirm diagnoses in those participants whose symptoms developed into full psychotic disorders.36
Statistical Analyses
Data analyses were conducted using SPSS (Version 20). Comparisons of demographic and clinical variables were performed with an ANOVA for continuous variables and chi-square analyses for categorical variables.
Aim 1. In order to examine the relationship between initial stage and conversion rates, the Kaplan–Meier method was used to estimate the cumulative rate of conversion for the 4 subgroups.37
Aim 2. In order to evaluate the course of each stage, chi-square analyses were used to evaluate the association between the initial stage assignment and stage at study outcome. Specifically, differences were examined between the subgroups on rates of full stage recovery (no attenuated positive or negative symptoms ≥ 3), stage stability (started and ended the study in the same stage), any improvement (full recovery and remitting to an earlier/previous stage), and remission of attenuated positive symptoms (includes only CHR+Mod, CHR+Sev, and SLPs). Following significant overall chi-square values, adjusted standardized residuals (ASR) were used to determine which subgroup (ie, stage) significantly deviated from the expected distribution (ie, an overrepresentation or underrepresentation). An ASR of ±1.96 was equivalent to P < .05. Phi coefficients (Φ) were used to calculate effect size differences between the subgroups (0.10 = small, 0.30 = medium, 0.50 = large38).
Aim 3. Differences between the 4 subgroups in medication treatment patterns at baseline and during the course of the study were evaluated with chi-square analyses. In addition, McNemar’s test was used to examine changes in medication from baseline to follow-up within each subgroup.39
Results
Baseline Demographic and Clinical Characteristics
Table 1 summarizes baseline demographic and clinical characteristics for the 4 subgroups of CHR subjects and CNTLs. There were no differences between CHR subgroups on any of the demographic variables, except gender, with the CHR− group having a higher than expected proportion of males. Compared to the CNTLs, the CHR subgroups showed no differences in baseline age, education level, handedness, race, ethnicity, socioeconomic status (SES) levels, and estimated premorbid IQ. The CNTL group had significantly higher Current IQ scores compared to the CHR+Sev and SLP groups. At baseline, the SLP group had lower Current IQ scores compared to all the other 3 CHR subgroups.
Table 1.
RAP Phase 1 Sample by Patient Subgroup: Demographic and Clinical Characteristics of Participants at Baseline
| Characteristic | CNTL (n = 68) | CHR− (n = 46) | CHR+Mod (n = 53) | CHR+Sev (n = 39) | SLP (n = 33) | P Value | Post hoc Contrastsa |
|---|---|---|---|---|---|---|---|
| Age, y, mean (SD) | 16.32 (2.62) | 16.00 (2.03) | 15.97 (2.30) | 15.94 (2.02) | 16.25 (2.71) | .89 | — |
| Estimated premorbid IQ, mean (SD) | 110.00 (9.45) | 105.98 (10.79) | 106.32 (10.88) | 103.84 (15.66) | 106.66 (11.13) | .09 | — |
| Estimated current IQ, mean (SD) | 109.94 (13.25) | 101.55 (17.42) | 105.55 (15.33) | 100.49 (16.75) | 97.04 (16.53) | .001 | CNTL>CHR+Sev, SLP |
| Years of education, mean (SD) | 10.52 (2.62) | 9.66 (1.78) | 9.85 (2.2) | 9.54 (2.1) | 9.94 (2.16) | .17 | — |
| Parental SESb, low, no. (%) | 7 (10.9) | 2 (4.5) | 1 (1.9) | 5 (13.2) | 2 (6.5) | .34 | — |
| Gender, no. (%) | |||||||
| Female | 35 (51.5) | 8 (17.4) | 22 (41.5) | 12 (30.8) | 11 (33.3) | <.01 | CNTL> CHR−; CHR+Mod>CHR− |
| Male | 33 (48.5) | 38 (82.6) | 31 (58.5) | 27 (69.2) | 22 (66.7) | ||
| Handedness, right, no. (%) | 60 (88.2) | 41 (89.1) | 46 (86.8) | 32 (82.1) | 25 (75.8) | .43 | — |
| Race, no. (%) | |||||||
| White | 42 (61.8) | 34 (73.9) | 41 (77.4) | 33 (84.6) | 24 (72.7) | .11 | — |
| Ethnic origin | |||||||
| Hispanic, no. (%) | 3 (4.4) | 4 (8.7) | 7 (13.2) | 6 (15.4) | 7 (21.2) | .46 | — |
| Global functioning, mean (SD) | |||||||
| Social | 8.72 (0.91) | 5.39 (1.08) | 6.11 (1.42) | 5.97 (1.55) | 6.04 (1.51) | <.001 | CNTL>CHR−, CHR+Mod, CHR+Sev, SLP |
| Role | 8.71 (1.08) | 5.41 (2.59) | 5.57 (2.13) | 5.67 (1.94) | 6.47 (1.75) | <.001 | CNTL>CHR−, CHR+Mod, CHR+Sev, SLP |
| SIPS score, mean (SD) | |||||||
| Positive | 0.41 (.92) | 2.04 (2.01) | 5.98 (1.9) | 12.62 (2.27) | 15 (4.8) | <.001 | CNTL<CHR−, CHR+Mod, CHR+Sev, SLP; SLP> CHR−, CHR+Mod, CHR+Sev; CHR+Sev>CHR+Mod, CHR−; CHR+Mod> CHR− |
| Negative | 1.18 (1.63) | 16.4 (5.97) | 12.66 (5.38) | 13.99 (6.3) | 12.17 (5.08) | <.001 | CNTL<CHR−, CHR+Mod, CHR+Sev, SLP; CHR >CHR+Mod, SLP |
| Disorganized | 0.45 (1.01) | 4.27 (3.8) | 4.71 (2.96) | 8.15 (4) | 5.17 (3.04) | <.001 | CNTL<CHR−, CHR+Mod, CHR+Sev, SLP; CHR+Sev; CHR+Sev> CHR−, CHR+Mod, SLP |
| General | 0.55 (1.15) | 7.16 (4.75) | 8.03 (4.1) | 9.75 (3.88) | 6.76 (2.84) | <.001 | CNTL<CHR−, CHR+Mod, CHR+Sev, SLP; CHR+Sev; CHR+Sev> CHR−, SLP |
| DSM-IV diagnoses, no. (%) | |||||||
| Mooda | 0 (0.0) | 25 (54.3) | 36 (67.9) | 22 (56.4) | 13 (39.4) | <.001 | CNTL<CHR−, CHR+Mod, CHR+Sev, SLP; CHR+Mod>SLP |
| Anxietyc | 1 (1.15%) | 22 (47.8) | 29 (53.7) | 25 (64.1) | 10 (30.3) | <.001 | CNTL<CHR−, CHR+Mod, CHR+Sev, SLP |
| Substance abused | 0 (0.0) | 2 (4.3) | 5 (9.3) | 4 (10.3) | 3 (9.1) | .11 | — |
Note: SLP, Schizophrenia-Like Psychosis; CNTL, healthy control; CHR, clinical high risk; RAP, Recognition and Prevention; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; NOS, not otherwise specified; SIPS, Structured Interview for Prodromal Syndromes; SES, socioeconomic status.
aDSM-IV defined diagnosis of major depressive disorder, dysthymic disorder, mood disorder NOS, or depressive disorder NOS.
bSocioeconomic status, Hollingshead index (Hollingshead & Redlich40 ), where 1–3 “high” and 4–5 “low”.
cDSM-IV defined diagnosis of panic disorder, posttraumatic stress disorder (PTSD), obsessive-compulsive disorder (OCD), generalized anxiety disorder, anxiety disorder NOS, or phobias including simple phobias and social phobia.
dDSM-IV defined diagnosis of alcohol, amphetamine, cannabis, cocaine, hallucinogen, nicotine, opioid, or polysubstance related substance abuse disorder.
For clinical symptoms, CNTLs had better functioning as seen on the GAF and GF:Social and GF:Role scales compared to all 4 CHR subgroups. In addition, CNTLs had lower levels of SOPS positive, negative, disorganized, and general symptoms compared to all 4 CHR subgroups. The 4 CHR subgroups had comparable levels of functioning at baseline. However, there were overall differences amongst the 4 CHR subgroups on attenuated positive, negative, disorganized, and general symptoms (table 1).
Conversion Rates by Subgroup
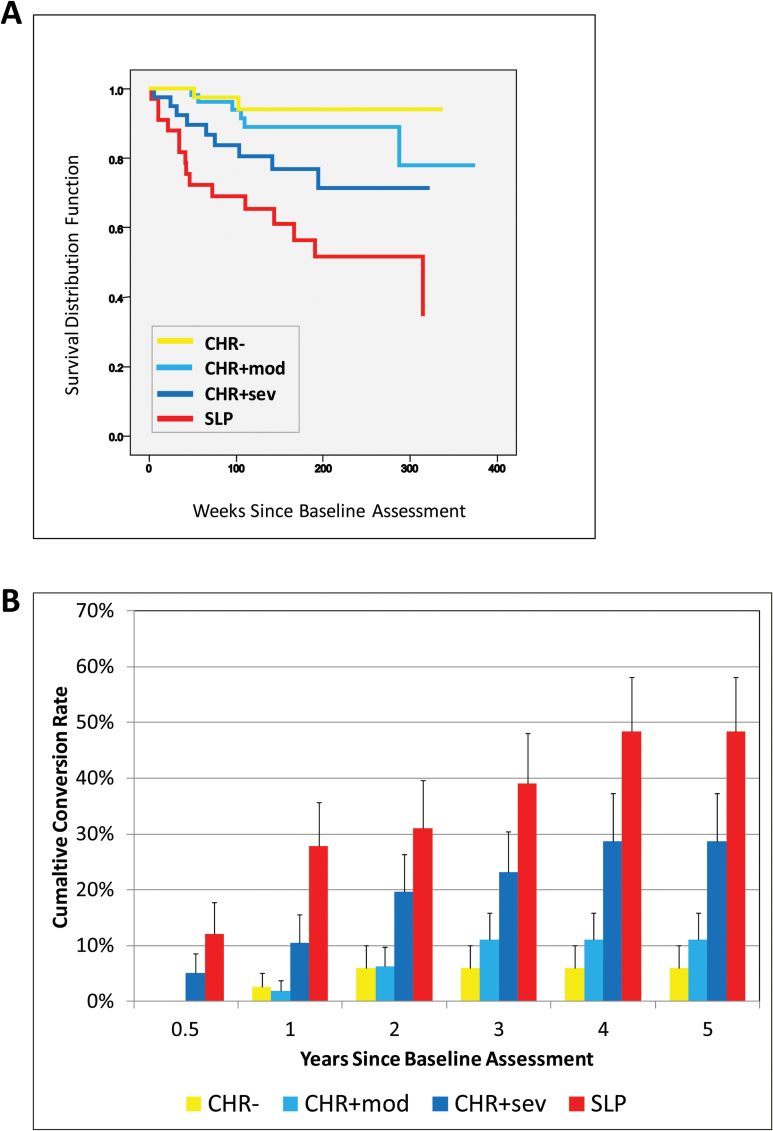
Figure 1A shows Kaplan–Meier estimate of the cumulative survival for each of the 4 CHR subgroups. With each successive stage there was an incremental decrease in the time to conversion (ie, CHR−→CHR+Mod→CHR+Sev→SLP) and an increase in overall conversion rates, suggesting that higher baseline attenuated positive symptom severity increased the overall likelihood of conversion over follow-up. The mean survival time for each subgroup was: CHR−, 6.184 years (SE = 0.198, 95% CI: 5.796–6.573), CHR+Mod, 6.353 years (SE = 0.319, 95% CI: 5.728–6.978); CHR+Sev, 4.918 (SE = 0.369, 95% CI: 4.195–5.640); SLP, 3.822 years (SE = 0.447, 95% CI: 2.946–4.698).
Fig. 1.
Differences in rates of conversion to psychosis among the 4 Recognition and Prevention (RAP) program stages. (A) Kaplan–Meier estimate of the cumulative survival in the 4 RAP clinical high risk (CHR) subgroups (N = 171). (B) Summary of the cumulative conversion rates (SE) by year by subgroup.
As shown in figure 1B, the CHR− and CHR+Mod groups had no conversions during the first 6 months of the study and the rates plateaued after years 2 and 3, respectively. In contrast to the CHR− and CHR+Mod groups, the CHR+Sev and SLP groups had steady increases in the conversion rates starting from the initial 6 months of the study and peaking at year 4.
By the end of year 5, the CHR− group had the lowest overall conversion rate (5.9%, SE = 0.04), which was not significantly different (P = .32) from the rate of the CHR+Mod group (11.1%, SE = .05). However, the CHR− rate was significantly lower than the overall rate of the CHR+Sev (28.7%, SE = 0.086, P = .025) and SLP (48.6%, SE = 0.098, P < .001) groups at the end of year 5. Likewise, the overall CHR+Mod rate was lower than the SLP group (P < .001), but only significant at a trend level from the CHR+Sev group (P = .10), most likely due to a late converter in the CHR+Mod group that occurred after year 5 (figure 1A). At the end of year 5, the SLP group had the highest overall conversion rate at 48.6% and was significantly higher than the CHR− and CHR+Mod groups, but not the CHR+Sev group.
Course of Each Subgroup
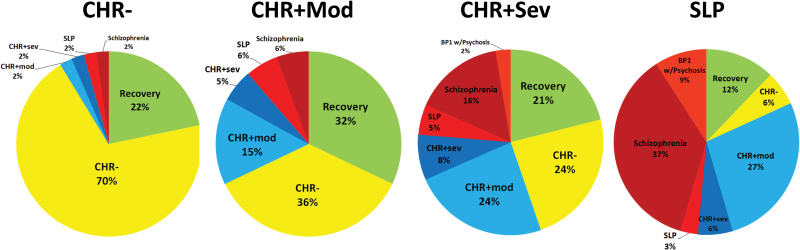
Figure 2 shows the outcome stage as a function of the initial subgroup at baseline. Overall, there was no significant difference in the overall rates of recovery (P = .187). Approximately 22% of all the subgroups achieved full stage recovery with no “prodromal” level attenuated negative or positive symptoms at study outcome. The recovery rates for each subgroup were CHR− at 21.7%, CHR+Mod at 32.1%, CHR+Sev at 21.1%, and SLPs at 12.1%.
Fig. 2.
Final stage at study outcome as a function of the initial stage at study entry (top row). Note: Values are calculated as raw percentages.
In order to determine the stability of each diagnostic subgroup, we calculated the proportion of individuals that started and ended the study at the same stage. There was a significant difference in stability between the stages (P < .001). Starting with the initial CHR− group, each successive stage was associated with less stability. As shown in figure 2, the CHR− group showed the largest amount of stage stability (ASR = 7.9, P < .05), with almost 70% still meeting CHR− status by the end of the study, with large effect size differences between the CHR− compared to CHR+Mod (Φ = 0.55), CHR+Sev (Φ = 0.62), and SLP (Φ = 0.66) groups. In contrast, the CHR+Mod, CHR+Sev, and SLP groups all showed significantly less stability than expected (all P < .05) at 15.1%, 7.9%, and 3.0%, respectively. The effect size differences between the 3 subgroups were all small (CHR+Mod vs SLP, Φ = 0.19; CHR+Mod vs CHR+Sev, Φ = 0.11; SLP vs CHR+Sev,Φ = 0.11).
In addition to stage stability, we also examined whether there was a differential rate of any improvement, which includes full recovery and remitting to an earlier/previous stage (eg, moving from CHR+Mod→CHR−) by initial stage. There was a significant difference in the proportion of subjects that moved to at least 1 earlier stage (P < .001). As noted above, the CHR− group showed the lowest amount of improvement (which is the same as recovery for this subgroup) at 21.7% (P < .001). In comparison, the CHR+Mod (Φ = 0.46), CHR+Sev (Φ = 0.47), and SLP (Φ = 0.31) groups showed high rates of improvement at 67.9%, 68.4%, and 51.5%, respectively.
Individuals in the SLP and CHR+Sev groups may have improved (eg, moved to back to a preceding stage), but may have concluded the study at a stage with attenuated positive symptoms (eg, moving from SLP→CHR+Sev or CHR+Sev→CHR+Mod). Therefore, we also examined differences in the remission of attenuated positive symptoms between the CHR+Mod, CHR+Sev, and SLP groups. There was a significant difference between the 3 groups (P < .001) as the CHR+Mod group showed a greater rate (67.9%) of positive symptom remission when compared to the CHR+Sev (44.7%, Φ = 0.23) and SLP (18.2%, Φ = 0.48) groups.
In terms of specific type of psychosis outcome, 2% of the CHR−, 6% of the CHR+Mod, 16% of the CHR+Sev and 37% of the SLP group developed a schizophrenia-spectrum disorder. Only the SLP and CHR+Sev groups developed bipolar 1 disorder with psychosis, at 9% and 2%, respectively. None of the less severe stages (CHR−, CHR+Mod) developed bipolar disorder (figure 2).
Medication Treatment
As shown in table 2, non-medication rates at baseline were comparable between the 4 CHR subgroups (P = .15), with 55.0% of the overall CHR group not receiving medication. Of the participants already treated with psychotropic medications at baseline, 24.0% were on antidepressants, 25.7% on antipsychotics, 5.3% on anxiolytics, and 4.7% on mood stabilizers. There was an overall group difference in baseline antipsychotics use (P < .001), as SLPs had the highest levels (antipsychotic use for CHR− = 17.4%, CHR+Mod = 17.0%, CHR+Sev = 28.2%, SLP = 57.6%, overall P < .001).
Table 2.
Medication Treatment at Baseline and During the Follow-up Period
| Medication, No. (%) | CHR− (n = 46) | CHR+Mod (n = 53) | CHR+Sev (n = 39) | SLP (n = 33) | P Value |
|---|---|---|---|---|---|
| Baseline | |||||
| No medication | 25 (54.3) | 16 (41.0) | 32 (60.4) | 13 (39.4) | .15 |
| Antipsychotics | 8 (17.4) | 9 (17.0) | 11 (28.2) | 19 (57.6) | <.001 |
| Antidepressants | 12 (26.1) | 15 (28.3) | 13 (33.3) | 4 (12.1) | .21 |
| Anxiolytic | 2 (4.3) | 2 (3.8) | 7 (17.9) | 2 (6.1) | .05 |
| Mood stabilizer | 1 (2.2) | 1 (1.9) | 3 (7.7) | 3 (9.1) | .28 |
| Follow-up | |||||
| No medication | 16 (40.0) | 14 (26.4) | 7 (17.9) | 7 (21.9) | .14 |
| Antipsychotics | 12 (30.0) | 17 (32.1) | 22 (56.4) | 23 (71.9) | <.001 |
| Antidepressants | 21 (52.5) | 33 (62.3) | 25 (64.1) | 15 (46.9) | .38 |
| Anxiolytic | 9 (22.5) | 7 (13.2) | 10 (25.6) | 6 (18.8) | .47 |
| Mood stabilizer | 5 (12.5) | 6 (11.3) | 6 (15.4) | 4 (12.5) | .95 |
Medication rates during the course of the study showed a similar pattern to baseline medication use, with SLPs having the highest rates of antipsychotic usage at 71.9% (overall P < .001). There were no overall differences in the rates of antidepressants, anxiolytics, and mood stabilizers between the 4 subgroups at baseline or over the course of the study (P > .05).
However, there were significant changes in medication rates from baseline to follow-up. A considerably higher percentage of CHR+Mod (P < .001) and CHR+Sev (P < .001) participants received antipsychotics over the course of the follow-up, with increases of 15% and 28%, respectively, relative to baseline. In addition, the CHR+Mod (P < .001), CHR+Sev (P < .001), and SLP (P < .001) groups each had increases in antidepressant use (table 2).
Discussion
The present findings support the validity of a staging model of the psychosis prodrome based on increased clinical severity. Participants in the current study were classified into 1 of 4 CHR subgroups that were defined on the presence and severity of attenuated positive symptoms. Our findings demonstrated that: (1) Risk for conversion was dependent on the starting point in the model, where CHR− (presence of negative symptoms only) had the lowest risk; (2) Defining a CHR+ group based on a relatively high attenuated positive symptom severity (ie, ≥10) yielded a relatively high conversion rate with a more rapid onset, consistent with the assumption of a severity risk factor; (3) The SLP category represents a unique risk group, intermediate between the prodrome and an established psychotic disorder; (4) Recovery (ie, no prodromal symptoms at study outcome) was found for 22% of the sample, regardless of stage; and (5) Persistent illness of varying severity levels appears to characterize the CHR+ subgroups, supporting the proposed long-term syndromal status associated with APSS. Taken together, these findings suggest that merging together subgroups like the CHR+Mod, CHR+Sev, and SLPs (or APSS41 and BIPS42) into a single CHR/UHR research entity will likely confound attempts to identify the mechanisms of the disease as they lay upon different points along the illness trajectory.17 In addition, future time-limited clinical trials should take into account the severity levels of individuals that meet CHR criteria.
The RAP model was partially developed to accommodate the findings from earlier retrospective studies24,43–45 that negative symptoms were experienced by first episode patients several years before the emergence of positive symptoms.24,44–46 The first stage of the model (CHR−) was designed to prospectively study individuals who displayed the negative symptoms characterizing risk for psychosis (without accompanying positive symptoms), with the expectation that some proportion of these individuals would develop psychosis. Contrary to expectation, the CHR− group displayed a low rate of any form of positive symptoms (attenuated or psychotic) at study outcome. Thus, the CHR− group does not seem to be a feasible starting point to study the development of psychosis, as it appears to be too nonspecific. It should be noted, though, that since the average follow-up was 3.0 ± 1.6 years and the mean age of the CHR− group was 16 years old, CHR− subjects may be in an earlier prodromal phase that requires a longer period of follow-up in order to detect additional converters to psychosis. However, other CHR− group characteristics indicate an alternative direction of research. In particular, the CHR− diagnosis is relatively stable, as 70% of the group remained classified as CHR− at study outcome. Therefore, this finding seems to suggest that the CHR− group is a separate heterogeneous clinical entity that is independent of psychotic outcomes and well-suited to the prospective study of mechanisms associated with increased risk of long-term functional disability rather than conversion to psychosis.
The RAP model has since its inception emphasized positive symptom severity as a specific risk factor for psychosis and operationalized the stages according to this assumption. As a result, the overall CHR+ is divided into 2 subgroups, based on severity of total SIPS rated positive symptom scores. Although standard prodromal studies do not differentiate between at-risk subjects with higher vs more moderate attenuated symptoms, the results reported in this study are quite consistent with the assumption of a severity-based risk dimension (moderate vs higher risk). In this study, the CHR+Mod group, with a lower threshold of positive symptom severity (<10), had a conversion rate of 11% after 4 years compared to a higher conversion rate of 28% for the CHR+Sev group. Furthermore, the CHR+Sev group showed a relatively faster rate of conversion, with almost a 20% rate at the end of the 2nd year compared to only a 5% rate in the CHR+Mod group during the same period.
At the end of the severity spectrum, the SLP group was associated with the highest conversion rates and shortest time to conversion. Overall, the SLP group showed no stability (3% of the group retained a SLP rating at outcome), with almost 50% of the group going on to develop a psychotic disorder. In addition, most of the CHR individuals (11%) who developed bipolar disorder with psychotic features were in the SLP group. This outcome suggests that SLPs represent a different risk state than the “prodromal” CHR+ groups. The close similarity between selection criteria further raises questions about the routine inclusion of BIPs in the “high-risk” clinical entity in standard prodromal studies. On the other hand, our findings revealed that almost 50% of the SLPs did not develop a full-blown psychotic disorder and showed improvement to a lower/less severe stage, further suggesting that the SLPs are an intermediate outcome group and may be still appropriate for preventative intervention. Taken together, longer follow-up is needed to determine the extent to which individuals converting to the SLPs category continue to progress to a specific psychotic disorder.
Consistent with previous findings in individuals at CHR,47 almost a quarter of the entire sample recovered and were not experiencing prodromal level attenuated negative or positive symptoms at study outcome. Contrary to the conversion rates, which are a function of starting symptom severity, recovery appears relatively independent of initial stage. Considering that treatment may differentially affect individuals with the most mild (positive) symptoms, this may represent spontaneous recovery or a false positive rate that is broadly applicable to the general CHR category. Conversely, treatment with antipsychotics, antidepressants and other psychotropic medications, occurring in the majority of the CHR sample, could also be related to the observed recovery rates.
Although all the stages showed recovery, a sizable proportion of the 2 CHR+ subgroups (CHR+Mod and CHR+Sev) had persistent residual negative and positive symptoms. While the CHR+Sev group had a higher rate of positive symptom persistence (ie, did not remit over time), an average of 30% of the individuals in the CHR+Mod and CHR+Sev subgroups ended up as CHR− at study outcome. This suggests that negative symptoms may represent a general underlying pathology that does not result from the onset or progression of positive symptoms. Independent of conversion outcome, a major question to be addressed in future studies is the extent to which functional disability is a common theme, with a shared etiology, that runs through all of the stages. Taken together, these findings support the recent notion that the APSS is associated with a high vulnerability for long-term psychopathology, with potentially multiple poor outcomes.48
Treatment Applications
The RAP model was developed to focus on the natural progression of the illness and on developmental risk factors, and, in particular, provides a structure for developing stage-specific interventions. In fact, our naturalistic findings indicate that early treatment of attenuated positive symptoms may be more complex than typically assumed. Rather than designing a single uniform treatment for the CHR syndrome overall, the RAP stage model suggests a more targeted approach, with earlier stages receiving less invasive treatment, later stages more aggressive intervention. The pattern of the naturalistic medication treatment data with antipsychotics, administered according to physician choice, coincided with severity increases across the RAP spectrum, with almost a 40% increase seen from CHR+Mod to the SLP stage, with the SLPs receiving the greatest amount of antipsychotics over the course of the study. On the other hand, the medication data reported here is consistent with previous findings49,50 in showing that treatment with antidepressants may be effective at reducing symptom progression, particularly in some individuals in both the CHR− and CHR+Mod (least severe) groups. It is likely that these less severe subgroups had a high number of false positives with low intensity and possibly fleeting symptoms that were not truly prodromal and were therefore more responsive to antidepressants.
Limitations
Our findings should be interpreted in light of the following potential limitations. First, the first phase of the RAP program provided a range of individual and other psychosocial treatments that were not sufficiently systematic for inclusion in the current analyses. Future studies should therefore explore the relationship between standardized non-pharmacological treatment and stage as well as outcome. Second, further analyses are needed to explore whether there is lawful movement from one stage to another over time, eg, moving from CHR−→CHR+Mod and then from CHR+Mod→CHR+Sev. This will require a longitudinal study with very frequent follow-up contacts to closely track clinical changes. Third, further studies should focus on the impact of changing independent variables, such as functional deficits, on the course of illness. Finally, the proposed model needs to be replicated with longer-term follow-up and comparisons with competing models.
Conclusions
In conclusion, the severity of early symptoms plays a critical role in determining clinical outcome, including the risk of psychosis, time to conversion, and medication treatment. Even when psychosis does not emerge, the more severe the initial attenuated positive symptoms, the more likely those residual symptoms will persist. Negative symptoms and functional deficits are also a major concern in their own right, since these remain over the course of follow-up in a large majority of individuals even in the absence of positive symptoms. Finally, our findings suggest that to further advance our understanding of mechanisms leading to psychosis, the psychosis prodrome will have to be further subdivided into severity based groups that are studied independently.
Funding
Supported by grants MH61523-02 and MH61523-8 from the National Institute of Mental Health (B.A.C.) and the Zucker Hillside Hospital NIMH Advanced Center for Intervention and Services Research for the Study of Schizophrenia MH 074543-01 (John M. Kane, MD). R.E.C. received funding from the Brain and Behavior Research Foundation (NARSAD): Young Investigator Grant 19740 and the Let the Sun Shine Run/Walk, Cold Spring, MN. No funding agency had any role in the collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Acknowledgments
We thank the study participants and their families for their time and effort. The authors also thank Ruth Olsen, BS, and Danielle McLaughlin, MA, for their major long-standing contributions to the RAP program. R.E.C. and A.M.A. report no conflicts. B.A.C. was the original developer of the CPT-IP and has been an advisor for Merck. Over the past 12 months, C.U.C. has been a consultant and/or advisor to or has received honoraria from: Alkermes, Forum, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, Lundbeck, Medavante, Medscape, Otsuka, Pfizer, ProPhase, Sunovion, Supernus, Takeda, and Teva. He has provided expert testimony for Bristol-Myers Squibb, Janssen, and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck and Pfizer. He received grant support from Takeda.
References
- 1. Fava GA, Kellner R. Staging: a neglected dimension in psychiatric classification. Acta Psychiatr Scand. 1993;87:225–230. [DOI] [PubMed] [Google Scholar]
- 2. McGorry P. A treatment-relevant classification of psychotic disorders. Aust N Z J Psychiatry. 1995;29:555–558. [DOI] [PubMed] [Google Scholar]
- 3. McGorry PD, Hickie IB, Yung AR, Pantelis C, Jackson HJ. Clinical staging of psychiatric disorders: a heuristic framework for choosing earlier, safer and more effective interventions. Aust N Z J Psychiatry. 2006;40:616–622. [DOI] [PubMed] [Google Scholar]
- 4. Huber G, Gross G, Schüttler R, Linz M. Longitudinal studies of schizophrenic patients. Schizophr Bull. 1980;6:592–605. [DOI] [PubMed] [Google Scholar]
- 5. Sullivan HS. The onset of schizophrenia. Am J Psychiatry. 1927;84:105–134. [DOI] [PubMed] [Google Scholar]
- 6. Conrad K. Die beginnende Schizophrenie: Versuch einer Gestaltanalyse des Wahns. Stuttgart, Germany: Thieme; 1958. [PubMed] [Google Scholar]
- 7. Kraepelin E. Manic depressive insanity and paranoia. J Nerv Ment Dis. 1921;53:350. [Google Scholar]
- 8. Cameron DE. Early schizophrenia. Am J Psychiatry. 1938;95:567–582. [Google Scholar]
- 9. Bleuler E. Dementia praecox oder Gruppe der Schizophrenien In: Aschaffenburg G, ed Handbuch der Psychiatrie. Spezieller Teil. 4. Abteilung, 1.Hälfte. Leipzig und Wien: Franz Deuticke; 1911. [Google Scholar]
- 10. Lieberman JA, Perkins D, Belger A, et al. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50:884–897. [DOI] [PubMed] [Google Scholar]
- 11. McGorry P, Keshavan M, Goldstone S, et al. Biomarkers and clinical staging in psychiatry. World Psychiatry. 2014;13:211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGorry P, Nelson B. Why we need a transdiagnostic staging approach to emerging psychopathology, early diagnosis, and treatment. JAMA Psychiatry. 2016;13:1–2. [DOI] [PubMed] [Google Scholar]
- 13. McGorry PD, Nelson B, Goldstone S, Yung AR. Clinical staging: a heuristic and practical strategy for new research and better health and social outcomes for psychotic and related mood disorders. Can J Psychiatry. 2010;55:486–497. [DOI] [PubMed] [Google Scholar]
- 14. Agius M, Goh C, Ulhaq S, McGorry P. The staging model in schizophrenia, and its clinical implications. Psychiatr Danub. 2010;22:211–220. [PubMed] [Google Scholar]
- 15. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–715. [DOI] [PubMed] [Google Scholar]
- 16. Fusar-Poli P, Yung AR, McGorry P, van Os J. Lessons learned from the psychosis high-risk state: towards a general staging model of prodromal intervention. Psychol Med. 2014;44:17–24. [DOI] [PubMed] [Google Scholar]
- 17. Cornblatt BA, Carrión RE. Deconstructing the psychosis risk syndrome: moving the field of prevention forward. JAMA Psychiatry. 2016;73:105–106. [DOI] [PubMed] [Google Scholar]
- 18. Fusar-Poli P, Cappucciati M, Borgwardt S, et al. Heterogeneity of psychosis risk within individuals at clinical high risk: a meta-analytical stratification. JAMA Psychiatry. 2016;73:113–120. [DOI] [PubMed] [Google Scholar]
- 19. Cornblatt BA. The New York high risk project to the Hillside recognition and prevention (RAP) program. Am J Med Genet. 2002;114:956–966. [DOI] [PubMed] [Google Scholar]
- 20. Cornblatt BA, Carrion RE, Auther A, et al. Psychosis prevention: a modified clinical high risk perspective from the recognition and prevention (RAP) program. Am J Psychiatry. 2015;172:986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cornblatt BA, Lencz T, Smith CW, Correll CU, Auther AM, Nakayama E. The schizophrenia prodrome revisited: a neurodevelopmental perspective. Schizophr Bull. 2003;29:633–651. [DOI] [PubMed] [Google Scholar]
- 22. Mrazek PJ, Haggerty RJ. Reducing Risks for Mental Disorders: Frontiers for Preventive Intervention Research. Washington, DC: National Academies Press; 1994. [PubMed] [Google Scholar]
- 23. Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. [DOI] [PubMed] [Google Scholar]
- 24. Häfner H, Löffler W, Maurer K, Hambrecht M, an der Heiden W. Depression, negative symptoms, social stagnation and social decline in the early course of schizophrenia. Acta Psychiatrica Scandinavica. 1999;100:105–118. [DOI] [PubMed] [Google Scholar]
- 25. Carrión RE, McLaughlin D, Goldberg TE, et al. Prediction of functional outcome in individuals at clinical high risk for psychosis. JAMA Psychiatry. 2013;70:1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hickie IB, Scott EM, Hermens DF, et al. Applying clinical staging to young people who present for mental health care. Early Interv Psychiatry. 2013;7:31–43. [DOI] [PubMed] [Google Scholar]
- 27. Morrison AP, French P, Walford L, et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomised controlled trial. Br J Psychiatry. 2004;185:291–297. [DOI] [PubMed] [Google Scholar]
- 28. Morrison AP, French P, Parker S, et al. Three-year follow-up of a randomized controlled trial of cognitive therapy for the prevention of psychosis in people at ultrahigh risk. Schizophr Bull. 2007;33:682–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haroun N, Dunn L, Haroun A, Cadenhead KS. Risk and protection in prodromal schizophrenia: ethical implications for clinical practice and future research. Schizophr Bull. 2006;32:166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miller TJ, McGlashan TH, Rosen JL, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. [DOI] [PubMed] [Google Scholar]
- 31. Miller TJ, McGlashan TH, Woods SW, et al. Symptom assessment in schizophrenic prodromal states. Psychiatr Q Winter. 1999;70:273–287. [DOI] [PubMed] [Google Scholar]
- 32. Correll CU, Smith CW, Auther AM, et al. Predictors of remission, schizophrenia, and bipolar disorder in adolescents with brief psychotic disorder or psychotic disorder not otherwise specified considered at very high risk for schizophrenia. J Child Adolesc Psychopharmacol. 2008;18:475–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Orvaschel H, Puig-Antich J. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Epidemiologic Version. Fort Lauderdale, FL: Center for Psychological Studies, Nova Southeastern University; 1994. [Google Scholar]
- 34. Cornblatt BA, Auther AM, Niendam T, et al. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull. 2007;33:688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. American Psychiatric Association. Task Force on DSM-IV. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 36. Chambers WJ, Puig-Antich J, Hirsch M, et al. The assessment of affective disorders in children and adolescents by semistructured interview. Test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Arch Gen Psychiatry. 1985;42:696–702. [DOI] [PubMed] [Google Scholar]
- 37. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 38. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: L. Erlbaum Associates; 1988. [Google Scholar]
- 39. Mc NQ. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika. 1947;12:153–157. [DOI] [PubMed] [Google Scholar]
- 40. Hollingshead AB, Redlich FC. Social Class and Mental Illness. New York, NY: Wiley; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Addington J, Liu L, Perkins D, Carrion RE, Keefe R, Woods SW. The Role of Cognition and Social Functioning as Predictors in the Transition to Psychosis for Youth with Attenuated Psychotic Symptoms. Schizophr Bull. 2017;43:57–63 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fusar-Poli P, Cappucciati M, De Micheli A, et al. Diagnostic and prognostic significance of Brief Limited Intermittent Psychotic Symptoms in individuals at ultra high risk. Schizophr Bull. 2017;43:48–56 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Häfner H. Onset and early course as determinants of the further course of schizophrenia. Acta psychiatrica Scandinavica Supplementum. 2000;102:44–48. [PubMed] [Google Scholar]
- 44. Häfner H, Maurer K, an der Heiden W. ABC Schizophrenia study: an overview of results since 1996. Social Psychiatry Psychiatric Epidemiol. 2013;48:1021–1031. [DOI] [PubMed] [Google Scholar]
- 45. Häfner H, Maurer K, Löffler W, an der Heiden W, Hambrecht M, Schultze-Lutter F. Modeling the early course of schizophrenia. Schizophr Bull. 2003;29:325–340. [DOI] [PubMed] [Google Scholar]
- 46. Murphy BP, Stuart AH, McGorry PD. Duration of untreated negative symptoms and duration of active negative symptoms: proof of concepts. Early Interv Psychiatry. 2008;2:27–33. [DOI] [PubMed] [Google Scholar]
- 47. Addington J, Cornblatt BA, Cadenhead KS, et al. At clinical high risk for psychosis: outcome for nonconverters. Am J Psychiatry. 2011;168:800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carpenter WT. Attenuated psychosis syndrome: need for debate on a new disorder. Psychopathology. 2014;47:287–291. [DOI] [PubMed] [Google Scholar]
- 49. Cornblatt BA, Lencz T, Smith CW, et al. Can antidepressants be used to treat the schizophrenia prodrome? Results of a prospective, naturalistic treatment study of adolescents. J Clin Psychiatry. 2007;68:546–557. [DOI] [PubMed] [Google Scholar]
- 50. Fusar-Poli P, Frascarelli M, Valmaggia L, et al. Antidepressant, antipsychotic and psychological interventions in subjects at high clinical risk for psychosis: OASIS 6-year naturalistic study. Psychol Med. 2015;45:1327–1339. [DOI] [PubMed] [Google Scholar]