Abstract
First, we describe the hallmark contributions of Irv Gottesman’s pioneering scholarship for schizophrenia research including concepts of polygenicity, gene × environment interactions, epigenetics and the endophenotype concept. Gottesman and colleagues’ twin studies showed that genes, not social factors, mediate schizophrenia risk. He then showed that schizophrenia is highly polygenic. Next, he introduced the concept of epigenetics into schizophrenia research. Gottesman then introduced the quantitative endophenotype concept. Endophenotypes are laboratory-based measures that show deficits in schizophrenia patients and lesser deficits in their first degree “unaffected” relatives and are viewed as being more proximal to genes and having a simpler genetic architecture than are “fuzzy” qualitative diagnostic disorders. Endophenotypes offer an exciting path to gene discovery, neural circuits, genetic architecture and new treatment pathways of schizophrenia and related psychotic disorders. Second, we were asked to discuss 2 of many endophenotype Consortia and related studies, in order to illustrate the impact of Gottesman’s work. We describe the Consortium on the Genetics of Schizophrenia (COGS) exploring neurocognitive and neurophysiological endophenotypes in family and case-control studies. Association, linkage, sequencing and epigenetic studies are described. The Bipolar and Schizophrenia Network for Intermediate Phenotypes (BSNIP) uses an array of endophenotypes including brain imaging in studies across the psychosis dimension, allowing for dimensional analyses. BSNIP results have led to the concept of biotypes, advancing the field. Irv Gottesman was imaginatively prescient in generating novel insights and predicting many major issues which challenge schizophrenia researchers who still use his concepts to guide current research approaches.
Key words: genes, biomarkers, Gottesman, endophenotypes, schizophrenia
Introduction
We were asked by the Editors to discuss 2 broad areas for this review. First, to review Irv Gottesman’s protean contributions to schizophrenia research. And Second, to illustrate how Gottesman’s endophenotype concept (one of his many innovative ideas) has guided the scientific pursuits of the 2 Consortia that we represent.
Irv Gottesman’s seminal contributions to schizophrenia research, focusing on behavioral genetics and advancing our understanding of the neurobiological basis of schizophrenia, have guided the field’s understanding of schizophrenia genomics. His protean interests and insights extend across many areas. For example, he identified that schizophrenia was a polygenic non-Mendelizing disorder.1 Subsequently, Gottesman introduced the concept of epigenetics to the world of schizophrenia research.2 This is important because, eg, epigenetic “marks” in DNA change how genes are expressed in the DNA to RNA to protein mantra of modern genetics. Gottesman also pioneered the use of endophenotypes: laboratory-based heritable biomarkers which show quantitative deficits in people with schizophrenia and intermediate deficits in their first degree “unaffected” relatives compared with normal control subjects.3–5 Gottesman made a compelling argument that endophenotypes offer a unique and productive platform for understanding the genomics of schizophrenia and other complex neuropsychiatric brain disorders. The endophenotype concept is complementary to large scale agnostic case control studies since the neurobiology and heritability of endophenotypes is partially or largely known. Cumulatively, Gottesman’s intellectual contributions stand as a monumental and transformative series of achievements which have presaged many of the major findings and approaches in the field of schizophrenia and neuropsychiatric research and have guided multiple generations of researchers. Here we describe Gottesman’s concepts of polygenicity, gene × environment (G × E), and epigenetics. Then we focus on endophenotypes and were asked to describe 2 (Consortium on the Genetics of Schizophrenia [COGS] and Bipolar and Schizophrenia Network for Intermediate Phenotypes [BSNIP]) of many endophenotype Consortia in order to illustrate the depth and breadth of Irv Gottesman’s influence on the field of schizophrenia research.
Polygeneity and G × E Interactions
After leaving his lectureship at Harvard, Gottesman joined Elliot Slator and James Shields at the Institute of Psychiatry in London. This allowed him and Shields access to the Maudsley Twin Register and helped transform the field of schizophrenia research. In 1972 Irv Gottesman collaborated with James Shields in the book “Schizophrenia and Genetics: A Twin Study Vantage Point”.6 The authors concluded that a series of twin and family studies led to the conclusion that schizophrenia was not the result of “poor parenting” or social miscommunication patterns transferred from parent to child, but was rather a genetically mediated neurobiological disorder. Gottesman was among the first people to understand that schizophrenia, unlike simple Mendelian disorders such as Huntington’s Disease, is a common complex disorder and its genomic basis is highly polygenic, not just a socially transmitted problem nor even just a single gene disorder with variable penetrance.1,3,7 Thus, polygenic loading scores, now so widely used in schizophrenia research, are a key to understanding the complex pattern of vulnerability (diathesis) that is conferred by both common heritable variants and de novo mutations. Polygeneity has its current expression in many recent studies such as the Psychiatric Genetics Consortium (PGC) which identified 108 loci associated with schizophrenia.8 In addition, Gottesman’s construct of quantitative endophenotypes (see below) allows us to understand the functional neurobiological deficits produced by these many genes of relatively small effect size on risk. In addition, Gottesman championed the concept of G × E interactions in creating a fuller understanding the etiology of schizophrenia. In his work, Gottesman used verbatim commentaries from people with schizophrenia and their family members which humanized this devastating clinical no fault brain disorder. The use of heartfelt first person accounts contributed to ongoing attempts to de-stigmatize schizophrenia and other Serious Mental Illnesses (SMI). In summarizing this work, Todd Gould stated in Schizophrenia Research Forum (7/7/16)9 that “Gottesman’s models predicted a scenario in which environmental factors interact with genetic predispositions over the course of development to produce behavioral phenotypes. This model parsimoniously explained that certain behavioral phenotypes may aggregate, but not segregate within families”.10 This has preceded the current enthusiasm in the field for looking at complex G × E interactions. Thus, using the powerful vantage point of twin and family studies, even absent genotyping, Gottesman solidified our understanding that schizophrenia is a common complex polygenic disorder (cf Gould,11 Neale and Sklar10). The identification of these many schizophrenia risk genes continues to this day in the context of increasingly large scale, often agnostic attempts to identify the genetic diathesis contributing to schizophrenia vulnerability. In complementary fashion, use of deeply (endo)phenotyped smaller samples of very well characterized patients and family members using the “neurobiologically informed” endophenotype strategy utilized in other medical disorders such as hypertension or hyperlipidemia, adds to our still “early” and evolving knowledge of schizophrenia genetics (Braff and Braff12).
Epigenetics
A next major contribution of Irv Gottesman was introducing the concept of epigenetics into the world of schizophrenia research. In his book written with Shields, “Schizophrenia the Epigenetic Puzzle,” (1982) Gottesman once again broke new ground in introducing the concept of “epigenetic control” to schizophrenia research.2 In its simplest form, epigenetics means “on top of” genes. As research has evolved following Gottesman’s introduction of this concept, we have come to understand that, in addition to genes, the environment can actually change the manner in which genes are structured and expressed. These gene-changing environmental events are multifaceted and include the Dutch Hunger Winter and other neurodevelopmentally “timed” environmental insults to the developing brain. These environmental events and vectors involve biological impacts and the resulting functionally damaging changes in DNA that occur via processes such as methylation and histone modification that distort the normal DNA to RNA to protein cascade of events.13–15 Gottesman was one of the first scientists to understand that epigenetics was an emerging and important concept for understanding schizophrenia genetics. Thus, we are not necessarily fated to have a life trajectory determined by heritable genes at birth, but rather both in utero and postpartum, the environment impinges on DNA and its expression throughout the life cycle. From a series of relevant studies, we now understand that methylation and other environmental epigenetic events that can change the pattern of gene expression in schizophrenia patients. This has led to a plethora of studies that reach out to more fully understand how, where and when epigenetic events occur and how they affect brain function in schizophrenia (eg, Montano et al,13 Aberg et al,14 Hannon et al,15 Cromby et al,16 Karsli-Ceppioglu17). A detailed analysis of schizophrenia epigenetics, while important, is beyond the scope of this review, so the interested reader is referred to Gottesman’s 1982 book and the references in the above sentence, a first pass through an extensive, rapidly expanding and fascinating literature.
Endophenotypes
It is important to note that entire reviews and volumes have been devoted to Gottesman’s concepts of polygenicity and epigenetics. This review was crafted to (mostly) discuss the endophenotype concept in schizophrenia research. Having broken new ground in the field of polygenicity and epigenetics, Gottesman understood that unraveling the complex genomic basis of the fuzzy and qualitative diagnosis of schizophrenia would be a daunting task. How to make the task simpler, more parsimonious and more neurobiologically meaningful? To do this Gottesman introduced the concept of endophenotypes into the schizophrenia literature (cf Gottesman and Shields3; Gottesman and Gould4). Endophenotypes simplify the search for “schizophrenia risk genes” and also elucidate their clinical significance. This will be explicated in some detail by us below based on our 2 Consortia studies: COGS and BSNIP (2 of many such studies). Endophenotypes are quantitative biomarkers which cannot be observed by the naked eye, but rather are laboratory-based measures and because these neurocognitive, neurophysiological, brain imaging, metabolic and other measures are quantitative biomarkers, we can assess the degree to which schizophrenia patients have key neurobiological deficits and the level of association of these hallmark schizophrenia endophenotype deficits to schizophrenia risk genes (Gottesman and Gould4). A key here is that, unlike the qualitative and clinically useful but fuzzy diagnosis of schizophrenia in the Diagnostic and Statistical Manual (DSM), endophenotypes are quantitative measures (eg, list learning scores on the CVLT,18 percent prepulse inhibition of the startle reflex (PPI),19,20 or cortical thickness by brain region). It is important to note that these quantitative measures connect to other major areas of medicine, where quantitative measures such as blood pressure blood sugar levels and lipid levels can be used with gene associations and polygenic risk scores in order to understand the level and functional significance of the genetic contributions to the underlying neuropathology of disease.
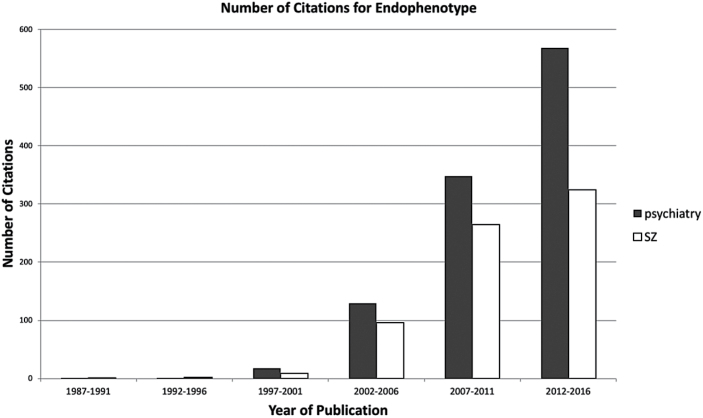
Individuals with schizophrenia have endophenotype deficits across many key heritable domains making them ideally compatible with concepts such as the Research Domain Criteria and thus “bridge genomic complexity and disorder herterogeneity.”21 In addition, their first degree “unaffected” relatives have quantitative neurobiological scores intermediate between schizophrenia patients and normal controls. Gottesman posited that these endophenotype deficits co-segregate with the illness. There are a number of other characteristics of endophenotypes (cf Gottesman and Gould4), but the key to this important concept is that it opens psychiatric research to quantitative measurements and appropriate statistical modeling of what is amiss in the behavioral “output” of the brains of schizophrenia patients.5 The use of endophenotypes to understand schizophrenia has led to a vastly increasing literature, as investigators from many countries and many Consortia and individual laboratories have carefully utilized endophenotypes to understand the behavioral, biomarker and genomic basis of schizophrenia (figure 1). In addition, endophenotypes are especially valuable because the neural circuit basis of heritable endophenotypes such as working memory can be understood via human and functional brain imaging and the use of animal models where neural circuit dysfunction can be induced with animal model lesions and other biological and social/environmental manipulations can be used.20 This has led to vast growth of endophenotype research (figure 1). One COGS endophenotype, PPI (sensorimotor gating) now has 7800 references since its introduction into the neuropathological literature in 1978 in Enoch Calloway’s laboratory at UCSF,19 although the term “endophenotype” is not always in the titles of related articles (figure 1).
Fig. 1.
Number of endophenotype citations have increased since 1987. This figure shows the current dramatically increasing number of citations for “endophenotypes and psychiatry” and “endophenotypes and schizophrenia.”
Two (Out of Many) Examples of the Endophenotype Approach: The Fruits of Gottesman’s Endophenotype Concept
As an exemplar of the importance of endophenotypes, and major subject of this commentary, we will elucidate what has been found by our 2 endophenotype Consortia: COGS and BSNIP study. We were specifically asked by the Editors to (briefly) review some of Irv Gottesman’s many contributions to the field of schizophrenia and behavioral genetics research (see above). We were also tasked with illustrating how Gottesman’s endophenotype concept (one of his many innovative ideas) and his direct input to us has guided the specific lines of pursuit of COGS and BSNIP. We now proceed to the second of these 2 tasks.
COGS and its Study Group*
About 15 years ago, with the support and encouragement of Irv Gottesman (eg Braff, Freedman, Schork, Gottesman5), the COGS-1, a 7-site family study was started (cf Schizophrenia Bulletin Special Issue 2007).22 The driving force here was that endophenotypes are more proximal to genes than is the qualitative or “fuzzy” construct of schizophrenia. Thus, on the gene to clinical phenotype expression pathway, endophenotypes are viewed as quantitative measures which are amenable to quantitative analysis and are “closer” to genes and also have a simpler genetic architecture. While this assumption of relative schizophrenia endophenotype genetic simplicity compared with schizophrenia itself might be questioned, it would be unlikely to be questioned by anyone who has administered the Wisconsin Card Sorting Test (an endophenotype) to a person with schizophrenia and then spent 2 hours of detailed and challenging and complex clinical interviewing to arrive at a DSM diagnosis. The relative complexity of the schizophrenia diagnostic label, vs the simplicity of the Wisconsin Card Sorting Test (WCST) quantitative output, has profound face and now construct validity. The WCST endophenotype is clearly “simpler” than the fuzzy qualitative diagnostic label of schizophrenia on a face validity basis, and the now its simpler (but still polygenic) underpinnings are being identified, confirming Gottesman’s strong inference based reasoning.23,24
One advantage of the COGS-1 design is that families were obtained via an affected proband who had both parents (a “trio”) and at least 1 unaffected full sib available for extensive (8–12 h) testing of neurocognitive and neurophysiological domains on the 12 primary measures that had been already identified by us and others as endophenotypes (cf Special Issue Schizophrenia Bull 200722). Trios allow for the identification of de novo vs common heritable mutations.25 Subsequently, in a Special Issue of Schizophrenia Research (2015)26 we reported on the behavioral profiles of the more extensive COGS-2 case control sample and extended our original COGS-1 behavioral data on the neurocognitive and neurophysiological deficits and the factor analytic derived patterns of these deficits.27 In the genetic context, we have reported on association, linkage and methylation studies that illuminate the genetic basis of schizophrenia using the endophenotype strategy as proposed by Gottesman.13,23–25,28 For both COGS-1 and COGS-2 samples (a total of 4076 extensively characterized deeply phenotyped subjects), associations using the PGC Psychchip for Genome-wide Association Analysis analysis and sequencing (eg, Gulsuner et al 2013)25 is done and/or proceeding as is biotype analysis (see below) pioneered by our BSNIP colleagues.
The COGS 1 and 2 studies have identified the neurocognitive and neurophysiological endophenotype deficits that occur in schizophrenia probands and unaffected family members (cf Schiz Res Special Issue 2015).26 Association studies of the COGS genes and the 12 primary endophenotypes that were selected a priori based on the extant literature have linked up these endophenotype deficits to both individual genes and to a 42 gene glutamate related network which has an ERBB4-NRGL hub.23,24
It is important to note that these 2 genes are intimately related to glutamate neurotransmission as well as other synaptic processes and are highly pleiotropic with significant associations to 9 of 12 COGS primary endophenotypes. These endophenotypes also reflect domains identified by, and in some cases isomorphic with, the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) study and FDA as new antipsychotic treatment targets. These findings buttress the importance of glutamate dysregulation in schizophrenia and also open a portal for the use of glutamate agonist drugs in schizophrenia treatment. This is a substantial boost to the concept of biomarker and genomic selection (ie, precision-based medicine) of schizophrenia patients for clinical trials. Also in the first COGS sequencing study done with our colleagues at University of Washington,25 we have reported on de novo gene mutations using trios from COGS 1 which are related to a network of genes that guide neurodevelopmentally important prefrontal cortical function. These findings are all being followed up and extended in the original and new samples. We have also looked at epigenetic marks and found an excess of methylation events in these endophenotype deficit burdened patients in collaboration with our colleagues at Johns Hopkins University.13 We have also formed” TOSCA” a Consortium 3 of Consortia of COGS with the Project Among African-Americans to Explore Risks for Schizophrenia (PAARTNERS)29 and the Multiplex Multigenerational Investigation of Schizophrenia (MGI)30 in order to leverage the use of some common measures (ie, The University of Pennsylvania Computerized Neurocognitive Battery) used across the 3 family and case control deep endophenotyping Consortia.
A major advantage of the endophenotype strategy employed by COGS, (Schiz Bull Special Issue 2007),22 BSNIP (see below), PAARTNERS,29 MGI,30 Psychosis Endophenotype International Consortium (PEIC), the NIMH/Lieber group31 and many others (eg, COGENT)32 is that we were able to better understand the genetic architecture and neural circuit basis of these quantitative endophenotype deficits associated with genetic mutations in schizophrenia via human brain imaging studies and extensive animal model studies of underlying genes, and neural circuitry, illuminating the neural circuit basis of some (but not all) endophenotype deficits (eg Swerdlow, Braff, Geyer Psychopharmacology 2016).20 The promise of endophenotypes is that we will progressively be able to better understand the genetic architecture and neural circuit basis of schizophrenia-linked deficits with future research. In addition, in conjunction with other ongoing endophenotype studies we can better understand the relationship of these endophenotype deficits (and their associated damaging genetic variations) to real-world functional outcome. This real-world functional domain is very important when we start thinking of genomic deficits and how they may relate to a generation of new biological and psychosocial treatments, since ultimately we need to create a bridge between genes and better real world function via precision guided medicine.
BSNIP and its Study Group**
Again, as encouraged and modeled by Irv Gottesman, the BSNIP was formed to characterize the dimension of psychosis neurobiologically, using a dense collection (well over 50) of broadly accepted endophenotypes, much like those included in COGS.33 The several psychosis diagnoses studied together included schizophrenia (SzP), schizoaffective (SAD), and psychotic bipolar disorder (PBP), disorders which were already known to have overlapping endophenotypes and genetic associations.34 BSNIP is a 5-site network which aimed originally, to develop endophenotypic markers to associate conventional psychosis diagnosis with biomarkers, to advantage diagnosis, discovery in genetic research and to develop the basis for novel treatment targets.33 Conceptually, this project identified a dimension of “psychotic disorders” at its outset, intended to identify markers of the individual psychoses in a unified study, where endophenotypic assessment was standardized and scored equivalently. Theoretically, results from this kind of a study could have helped distinguish between psychosis diagnoses where the “fuzzy” DSM boundaries obscured clear classification or where symptom patterns evolved over time. What we learned over the course of the clinical characterization is that psychosis diagnoses which are developed from DSM criteria based on phenomenology do not coincide with entities developed based on endophenotypic measures closely enough to utilize endophenotypes.35
The BSNIP multisite study relied on nearly 2500 representative individuals with psychosis, their relatives and healthy comparators. The endophenotype scoring was centralized to an appropriate expert. Inter-site reliability and training for biomarker collection was established. The early data showed that individuals with schizophrenia and schizoaffective diagnoses were neurobiologically indistinguishable. All proband groups showed reduced psychosocial function from healthy comparators, as did relative groups. Curiously, lifetime suicidality was high in all psychosis groups, rivaling what is observed in bipolar and depression populations. And, the family histories of the probands were complex, with individual proband family trees showing virtually equal numbers of “pure” (single diagnosis) and “mixed” (SzP and PBP) diagnoses.33
Once the individual endophenotypes were analyzed in the overall BSNIP group, it became clear that diagnostic distinctions did not capture individual biological markers nor limit biological heterogeneity. Cognition (total BACS score) varied by extent but not type of impairment across conventional diagnoses,36 as did oculomotor smooth pursuit and antisaccade performance,37 and regional brain volume.38 This suggests a severity continuum but not a distinct diagnosis-based organization across the endophenotypes. It was the original hypothesis that there was considerable overlap between the psychoses diagnoses with respect to phenotypic markers. However, single endophenotypes fell weakly onto conventional diagnoses. This outcome suggested 2 assumptions which dictated a different forward direction for study: either (1) that the conventional diagnoses, strongly grounded in phenomenology, are primary and binding and the endophenotypic characteristics generate less confidence; or (2) that the endophenotypic brain characteristics capture stronger quantifiable traits to use in characterizing brain diseases and should stand further study to see if and where they might over-ride phenomenology traits.39 Organizing disease units around the endophenotypic characteristics of the probands, generated highly biologically interesting outcomes,40 which are now being tested in BSNIP2. BSNIP2 investigators are exploring the hypothesis that endophenotypes are more informative characteristics of brain function then clinically derived phenomenology and are worthy of pursuit on the hypothesis that endophenotypes will create more biologically homogeneous constructs for molecular discovery. It took a battery rather than a single biomarker to distinguish psychosis groups clearly.35 BSNIP outcomes, interpretation and forward hypotheses represent an extensive embodiment of Irv Gottesman’s proposal about endophenotypes. They could be used to segment psychosis conditions, relying more closely on biological brain measures than on the conventional diagnoses. We postulate, that this endophenotype approach provides a unique key to understanding psychotic disorders and their molecular basis.
Where could this Gottesman-inspired approach lead? One possibility is that endophenotypic observations made over a population of persons in the psychosis dimension are a first approximation needed to describe the neurobiological characteristics of broader psychiatric brain disorders, a model for further discovery research. Reasoning from this single study, BSNIP produced a proof of principle that rational neurobiologically constructed groups of psychotic individuals can vary independently of their overall diagnosis. Like diseases in other areas of medicine, the endophenotypic measures could be more important for understanding the underlying biology of the illness than the phenomenological presentation. After all, it is the quantification of hematocrit, not the level of patient fatigue, which is the monitor for anemia and key to pathophysiology—although fatigue is important for patient care. Once it is clear which endophenotypes mark critical disease characteristics, it will be those measures that we use to assess disease severity and monitor for disease response to treatment and use as drug targets in discovery research. And, for genetic discovery, mutations might lead to pathology in brain circuits and in synaptic function for which endophenotypes might serve as guides.
BSNIP will be able to make genetic and epigenetic contributions to these cross-diagnostic endophenotypic features soon, and will establish the stability of these endophenotypic-defined batteries and proband clusters and enlarge the proband groups for genetically-relevant discovery with ongoing research. The articulation and modeling of the use of endophenotypes, in its application to the study of brain disorders in its broadest sense, is a unique contribution which can be credited to Irv Gottesman’ prescient scientific insights. It is not only a conduit to take current disease categories and make them more precise, but to potentially reconfigure categories using endophenotypes as an organizational tool, which could be an important and critical part of the Gottesman legacy. Further, the neurobiology of the endophenotypes is known, so these pieces of information can be used to understand the neurobiology which mediates psychotic symptoms.
Conclusion
Cumulatively, Irv Gottesman’s seminal contributions to schizophrenia research blaze a profoundly insightful and generative path: from polygenicity to G × E interactions to epigenetics to the important concept of quantitative endophenotypes that is the major focus of this review. Gottesman understood that the challenges of the polygenicity of schizophrenia and environmental contributions to risk created a conundrum. With so many genes requiring perhaps tens of thousands of subjects for identification of schizophrenia risk loci of very small effect size, the field needs novel integrative concepts. One such concept is polygenic risk scores as well as gene networks which allow us to integrate the effects of many genes of relatively small effect size on schizophrenia risk. He also proposed the quantitative endophenotype strategy in order to inform the challenging gene to phene pathways in schizophrenia. A key in the use of endophenotypes is the identification of quantitative endophenotype associated gene networks where common and rare highly penetrant variants and epigenetic marks all may perturb function such as glutamate neurotransmission and associated neurocognitive, neurophysiological and brain imaging quantitative endophenotypes. Integrating these gene networks with their corresponding neural circuits allows us to assess the gene and gene network “output” expressed as quantitative endophenotypes. Gottesman understood that ultimately GWAS and sequencing studies of schizophrenia will need to link up with laboratory-based quantitative endophenotype measures and clinical real world function to “close the loop” on gene to phene pathways. Endophenotypes, lying closer to genes on the gene to phene pathway and related closely to new MATRICS identified antipsychotic treatment targets and to real world function, will undoubtedly continue to be very important in understanding and developing pharmacological and behavioral treatments for schizophrenia. The seminal work begun by Irv Gottesman reinforced by his warm collegiality and wonderful insights, continues to inform and energize the field of schizophrenia research as others continue the work he started.
Funding
VA San Diego Healthcare System (MIRECC VISN-22) and National Institutes of Health/National Institute of Mental Health (NIH/NIMH) (grant #MH065571) to D.L.B.; NIH/NIMH (grants #MH077851, MH076690, MH096890, MH102656) and the Simmons Foundation to C.A.T.
Acknowledgments
*COGS Collaborating PIs: David L. Braff, Monica E. Calkins, Michael F. Green, Tiffany A. Greenwood, Raquel E. Gur, Ruben C. Gur, Laura C. Lazzeroni, Gregory A. Light, Keith H. Nuechterlein, Allen D. Radant, Larry J. Seidman, Larry J. Siever, Jeremy M. Silverman, William S. Stone, Catherine A. Sugar, Neal R. Swerdlow, Debby W. Tsuang, Ming T. Tsuang, Bruce I. Turetsky, Kristin S. Cadenhead, Dorcas J. Dobie, Robert Freedman, Nicholas J. Schork. **BSNIP Collaborating PIs: Brett Clementz; Elliot Gershon; Matcheri Keshavan; Godfrey Pearlson; John Sweeney; Carol Tamminga; Gunvant Thaker. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Gottesman II, Shields J. A polygenic theory of schizophrenia. Proc Natl Acad Sci U S A. 1967;58:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gottesman II, Shields J, Hanson DR. Schizophrenia, the epigenetic puzzle. New York, NY: Cambridge University Press; 1982:258. [Google Scholar]
- 3. Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122:15–30. [DOI] [PubMed] [Google Scholar]
- 4. Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. [DOI] [PubMed] [Google Scholar]
- 5. Braff DL, Freedman R, Schork NJ, Gottesman II. Deconstructing schizophrenia: an overview of the use of endophenotypes in order to understand a complex disorder. Schizophr Bull. 2007;33:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gottesman II, Shields J. Schizophrenia and Genetics; a Twin Study Vantage Point. New York, NY: Academic Press, Inc; 1972:433. [Google Scholar]
- 7. Farmer AE, McGuffin P, Gottesman II. Twin concordance for DSM-III schizophrenia. Scrutinizing the validity of the definition. Arch Gen Psychiatry. 1987;44:634–641. [DOI] [PubMed] [Google Scholar]
- 8. Ripke S, Neale B, Corvin A, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gould T. Irving I. Gottesman, Giant of Schizophrenia Research: 1930–2016. Schizophrenia Research Forum. July 7, 2016 http://www.schizophreniaforum.org/news/irving-i-gottesman-giant-schizophrenia-research-1930-2016 Accessed July 7, 2016.
- 10. Neale BM, Sklar P. Genetic analysis of schizophrenia and bipolar disorder reveals polygenicity but also suggests new directions for molecular interrogation. Curr Opin Neurobiol. 2015;30:131–138. [DOI] [PubMed] [Google Scholar]
- 11. Gould TD. Irving I. Gottesman (1930–2016): The multifactorial threshold model of complex phenotypes mediated by endophenotype strategies [published online ahead of print September 28, 2016]. Genes Brain Behav. doi:10.1111/gbb.12345. [DOI] [PubMed] [Google Scholar]
- 12. Braff L, Braff DL. The neuropsychiatric translational revolution: still very early and still very challenging. JAMA Psychiatry. 2013;70:777–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Montano C, Taub M, Jaffe A, et al. Association of DNA methylation differences with schizophrenia in an epigenome-wide association study. JAMA Psychiatry. 2016;73:506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aberg KA, McClay JL, Nerella S, et al. Methylome-wide association study of schizophrenia: identifying blood biomarker signatures of environmental insults. JAMA Psychiatry. 2014;71:255–264. doi:10.1001/jamapsychiatry.2013.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hannon E, Dempster E, Viana J, et al. An integrated genetic-epigenetic analysis of schizophrenia: evidence forco-localization of genetic associations and differential DNA methylation. Genome Biol. 2016;17:176. doi:10.1186/s13059-016-1041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cromby J, Chung E, Papadopoulos D, Talbot C. Reviewing the epigenetics of schizophrenia [published online ahead of print August 25, 2016]. J Ment Health. [DOI] [PubMed] [Google Scholar]
- 17. Karsli-Ceppioglu S. Epigenetic mechanisms in psychiatric diseases and epigenetic therapy [published online ahead of print September 4, 2016]. Drug Dev Res. doi:10.1002/ddr.21340. [DOI] [PubMed] [Google Scholar]
- 18. Stone WS, Mesholam-Gately RI, Braff DL, et al. California Verbal Learning Test-II performance in schizophrenia as a function of ascertainment strategy: comparing the first and second phases of the Consortium on the Genetics of Schizophrenia (COGS). Schizophr Res. 2015;163:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. [DOI] [PubMed] [Google Scholar]
- 20. Swerdlow NR, Braff DL, Geyer MA. Sensorimotor gating of the startle reflex: what we said 5 years ago, what has happened since then, and what comes next [published online ahead of print August 18, 2016]. J Psychopharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Insel T, Cuthbert B. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry. 2009;66:988–989. [DOI] [PubMed] [Google Scholar]
- 22. Braff DL, ed. 2007 Special Issue Schizophrenia Bulletin. Special Theme: The Use of Endophenotypes to Deconstruct and Understand the Genetic Architecture, Neurobiology, and Guide Future Treatments of the Group of Schizophrenias. Schizophr Bull. 2007;33: 19–104.17035358 [Google Scholar]
- 23. Greenwood TA, Lazzeroni LC, Murray SS, et al. Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am J Psychiatry. 2011;168:930–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Greenwood TA, Light GA, Swerdlow NR, Radant AD, Braff DL. Association analysis of 94 candidate genes and schizophrenia-related endophenotypes. PLoS One. 2012;7:e29630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gulsuner S, Wash T, Watts AC, et al. ; Consortium on the Genetics of Schizophrenia (COGS), PAARTNERS Study Group Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal profrontal cortical network. Cell. 2013;154:518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Braff DL, ed. Schizophrenia Research Special Issue 2015 Endophenotypes in Schizophrenia. Schizophr Res. 2015;163:1–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seidman LJ, Hellemann G, Nuechterlein KH, et al. Factor structure and heritability of endophenotypes in schizophrenia: findings from the Consortium on the Genetics of Schizophrenia (COGS-1). Schizophr Res. 2015;163:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Greenwood TA, Swerdlow NR, Gur RE, et al. Genome-wide linkage analyses of 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am J Psychiatry. 2013;170:521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aliyu MH, Calkins ME, Swanson CL, Jr, et al. ; PAARTNERS Study Group Project among African-Americans to explore risks for schizophrenia (PAARTNERS): recruitment and assessment methods. Schizophr Res. 2006;87:3–44. [DOI] [PubMed] [Google Scholar]
- 30. Gur RE, Nimgaonkar VL, Almasy L, et al. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry. 2007;164:813–819. [DOI] [PubMed] [Google Scholar]
- 31. Tan H-Y, Callicott JH, Weinberger DR. Intermediate phenotypes in schizophrenia genetics redux: is it a no brainer? Mol Psychiatry. 2008;13:233–238. [DOI] [PubMed] [Google Scholar]
- 32. Lencz T, Knowles E, Davies G, et al. Molecular genetic evidence for overlap between general cognitive ability and risk for schizophrenia: a report from the Cognitive Genomics consorTium (COGENT). Mol Psychiatry. 2014;19:168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tamminga CA, Ivleva EI, Keshavan MS, et al. Clinical phenotypes of psychosis in Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170:1263–1274. [DOI] [PubMed] [Google Scholar]
- 34. Ivleva EI, Morris DW, Moates AF, Suppes T, Thaker GK, Tamminga CA. Genetics and intermediate phenotypes of the schizophrenia–bipolar disorder boundary. Neurosci Biobehav Rev. 2010;34:897–921. [DOI] [PubMed] [Google Scholar]
- 35. Clementz BA, Sweeney JA, Hamm JP, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173:373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hochberger WC, Hill SK, Nelson CL, et al. Unitary construct of generalized cognitive ability underlying BACS performance across psychotic disorders and in their first-degree relatives. Schizophr Res. 2016;170:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lencer R, Sprenger A, Reilly JL, et al. Pursuit eye movements as an intermediate phenotype across psychotic disorders: evidence from the B-SNIP study. Schizophr Res. 2015;169:326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ivleva EI, Bidesi AS, Keshavan MS, et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170:1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Keshavan MS, Clementz BA, Pearlson GD, Sweeney JA, Tamminga CA. Reimagining psychoses: an agnostic approach to diagnosis. Schizophr Res. 2013;146:10–16. [DOI] [PubMed] [Google Scholar]
- 40. Tamminga CA, Pearlson GD, Stan AD, et al. Strategies for advancing disease definition using biomarkers and genetics: the bipolar and schizophrenia network for intermediate phenotypes. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. In Press. [DOI] [PubMed] [Google Scholar]