Abstract
Around the world, fermentation of foods has been adopted over many generations, primarily due to their commercial significance with enriched flavors and high-profile nutrients. The increasing application of fermented foods is further promoted by recent evidence on their health benefits, beyond the traditionally recognized effects on the digestive system. With recent advances in the understanding of gut-brain interactions, there have also been reports suggesting the fermented food’s efficacy, particularly for cognitive function improvements. These results are strengthened by the proposed biological effects of fermented foods, including neuroprotection against neurotoxicity and reactive oxygen species. This paper reviews the beneficial health effects of fermented foods with particular emphasis on cognitive enhancement and neuroprotective effects. With an extensive review of fermented foods and their potential cognitive benefits, this paper may promote commercially feasible applications of fermented foods as natural remedies to cognitive problems.
Keywords: fermentation, functional food, cognition, neuroprotection, gut-brain axis
INTRODUCTION
Fermentation is a metabolic process, which is induced by a microorganism and characterized by the anaerobic breakdown of carbohydrates to alcohol or organic acids (1). A wealth of literature has focused on the beneficial physiological effects of fermented foods on enteral nutrient absorption and on digestive tract health (2,3). More recently, there has been a considerable increase in research and publications on how the gut microbiota is tied to the host’s immune system, energy metabolism, and even to brain function like stress response (4). Studies which have focused on bidirectional communication between the brain and gut have received attention. There are reports showing that dysbiosis of gut microbiota is associated with anxiety and depression, and administration of probiotics produces anxiolytic- and antidepressant-like activities in animal studies (5–7). Moreover, probiotic-rich diets, either prepared naturally or with industrial fermentation processes, showed positive effects on stress relief and memory enhancement, potentially via gut microbiota improvement (8).
There is much scientific evidence documenting the probiotics’ potential health effect on gut microbiota ecosystems in relation to brain function. Because of their health promoting benefits, probiotics-included diet plans would be an effective strategy to boost one’s health and to manage disease risk (9). Beyond their nutritional value, probiotics hold a great marketing potential because they tend to meet consumer’s demand better than other medicinal products. In other words, probiotics are easy to obtain because most of them are already available with added value from health claims (10). When taken as a part of a balanced diet, they could be contributing factors to disease prevention in a convenient way.
Taken together, a growing body of evidence indicates the potential of fermented foods as functional foods for the brain and cognitive health promotion. However, most of the studies mainly focused on certain functional foods associated with emotion- and stress-related health promotion and overlooked their role in cognition modulation as well as product diversity. Thus, careful investigation of products with a focus on their effects on cognition and neuroprotection was highlighted in this review.
This paper comprehensively reviews the beneficial effects of fermented foods on brain and cognitive function. Literature searches were performed for articles published up to May 2016 on MEDLINE, EMBASE, and the Cochrane Library. The search terms included “fermented”, and “fermentation” in combination with the search terms “cognitive”, “cognition”, “learning”, “memory”, “neuroprotective”, “neuroprotection”, “dementia”, and “Alzheimer”.
The searches from the three electronic databases yielded 312 records after removing duplicate entries. The administration of any fermented product as the target intervention was regarded as eligibility criteria for inclusion. Studies including any standardized outcome measures of neuroprotection, learning, memory, or cognitive function were included. All types of studies were considered for inclusion in the review including clinical trials, animal experimental studies, and in vitro studies using neuronal/glial cell models. In the process, the manuscript included references with no language or site restriction.
Initial screening of titles and abstracts identified 78 potentially relevant references. Of the 78 records retrieved, five studies were excluded because their focus was not on fermented products. Twenty-five studies were excluded as their outcomes were not related to brain or cognitive function. We also excluded one article, which was not available online and other two review articles. As a result, a total of 45 articles were selected and summarized (Table 1).
Table 1.
Studies of fermented foods on brain and cognitive function
| Fermented product | Subject | Measurements | Effects | References |
|---|---|---|---|---|
| Fermented dairy products | ||||
| Camembert cheese extract | Primary microglia cells, N2A cells | Cytokine production, Neurotoxicity | Suppressed microglial TNF-α production; transformation of microglia to anti-inflammatory phenotype; reduced neurotoxicity | 24 |
| Camembert cheese extract | AD model transgenic mice, primary microglia cells | Aβ deposition, cytokine production, neurotrophic factor | Reduction of Aβ accumulation and hippocampal inflammation; enhanced hippocampal neurotrophic factors | 25 |
| Fermented soymilk | PC-12 cells, VaD model rats | Cell viability, MWM | Protective effect on H2O2- and OGD-induced damage in PC-12 cells; Improvement in learning and memory of VaD rats | 26 |
| Fermented milk | ddY mice | Y-maze, NOR | Improvement in scopolamine-induced memory impairment and novel object recognition in mice | 27 |
| Fermented milk | Healthy individuals | Physiological parameters in saliva, plasma, and fecal sample, physical symptoms | Reduced salivary cortisol and plasma L-tryptophan levels; attenuated physical symptoms | 28 |
| Legume- and cereal-based fermented foods | ||||
| Cheonggukjang extract | ICR mice | PA, NOR, AChE, MDA, SOD, NGF | Ameliorated memory defects and neuronal cell death in TMT-treated mice; suppression of AChE activity; activation of the NGF receptor signaling pathway; inhibition of oxidative stress | 29 |
| Cheonggukjang extract | SD rats | PA, MWM, Aβ deposition | Decreased Aβ accumulation, improved cognitive function and glucose regulation in AD diabetic rats | 30 |
| Fermented soybean powder | ICR mice | PA, Y-maze, MWM, AChE | Ameliorated the scopolamine-induced memory impairment and hippocampal BDNF reduction; suppression of AChE activity | 31 |
| Tempeh | Healthy elderly individuals | HVLT | Positive relation between high tempeh consumption and better memory | 32 |
| Red mold rice | IMR32 cells, PC-12 cells, Wistar rats | MWM, PA, cell viability, MDA, SOD, AChE, iNOS, ROS, Aβ deposition | Protection against Aβ-induced neurotoxicity in vitro and in vivo potently ameliorated memory deficit and Aβ accumulation in Aβ-infused rats; suppressed β-secretase activity | 33–35 |
| Red mold rice | Wistar rats | MWM, PA, antioxidant enzymes, ROS, corticosterone | Significant improvement in memory and antioxidant activity in Zn-deficient rats | 36 |
| Red mold rice | SH-SY5Y cells, SD rats | Cell viability, EBST, ROS, MDA, antioxidant enzymes, NO, TNF-α | Neuroprotective effects in the 6-OHDA induced-PD model in vitro and in vivo | 37 |
| Rice vinegar | AD model mice | MWM, antioxidant activity, Aβ deposition | Ameliorated cognitive dysfunction and Aβ accumulation in the AD mice | 41 |
| Fermented plant root products | ||||
| Fermented Codonopsis lanceolata | HEK293 cells, ICR mice | Antioxidant activity, PA, cytotoxicity | Ameliorated the scopolamine-induced memory impairment; Showed antioxidant activity and lowered cytotoxicity | 43 |
| Fermented Codonopsis lanceolata | HT22 cells, ICR mice | Cell viability, MWM, PA, AChE, neurotrophic factor, ROS, antioxidant enzymes, NO | Ameliorated scopolamine-induced memory impairments; neuroprotective effects against glutamate-induced cytotoxicity in vitro suppression of AChE activity and increased BDNF expression in hippocampus; upregulation of antioxidant enzyme’s activity | 44–48 |
| Fermented ginseng | AD model HeLa cells, ICR mice, AD model transgenic mice | PA, MWM, Aβ deposition | Significantly reduced Aβ level in vitro and in vivo ameliorated memory impairment | 50 |
| Fermented ginseng | N2A cells, ApoE knockout mice | Tau, MWM | Inhibited the APP and tau IRES activities; improvement on memory function | 51 |
| Fermented garlic | Wistar rats | MWM, pyramidal neuronal cell number | Increased total number of pyramidal neurons and the spatial memory function in MSG-exposed rats | 52 |
| Fermented yellow onion | HT22 cells | Antioxidant activity, cell viability | Enhances the antioxidative and neuroprotective effect against glutamate-induced neurotoxicity in vitro | 53 |
| Fermented fruits and vegetables | ||||
| Fermented papaya | PC-12 cells, AD model SH-SY5Y cells | Cell viability, antioxidant activity, ROS, iNOS, nNOS, SOD, apoptotic proteins | Reduced oxidative stress-induced cell damage and inflammation; significantly increased cell viability and SOD expression | 54,55 |
| Fermented papaya preparation | Mice | Spontaneous alternation, PA | Significant improvement in scopolamine-induced memory impairment | 56 |
| Fermented papaya | Patients with AD | Urinary 8-OHdG | Decreased urinary 8-OHdG, oxidative stress biomarker in AD patients | 57,58 |
| Fermented grape marc | Human PBMC | Cytokine production, granzyme B, FoxP3 | Increased the release of cytokine and induction of FoxP3; reduced the production of granzyme B | 59 |
| Kimchi extract | ICR mice | PA, MWM, Y-maze, BDNF, pCREB | Prevented memory deficit and increased hippocampal BDNF and pCREB expressions in scopolamine-injected mice | 64 |
| Other fermented plant products | ||||
| Fermented Rhus verniciflua bark | ICR mice | Apoptotic cell death, microglial activation | Attenuated KA-induced neuronal cell death and microglial activation | 66 |
| Fermented tea | Healthy elderly individuals | MMSE | Association between regular consumption of fermented tea and lower risks of cognitive deficiency | 67 |
| Fermented fungi | ||||
| Fermented Ganoderma luciderm extract | SD rats | PA, MWM, hippocampal AChE | Improved scopolamine-induced memory impairment in rats; Lowered hippocampal AChE activity | 68 |
| Fermented Antrodia cinnamomea extract | PC-12 cells | Cell viability, MAPK proteins | Prevented serum deprivation-induced cell apoptosis | 69 |
| Fermented Xylaria nigripes extract | SH-SY5Y cells, BV-2 cells | Cell viability, NO | Protected Aβ-induced injury in SH-SY5Y cells; Inhibitory effect against LPS-stimulated NO production in BV-2 cells | 71 |
| Fermented traditional oriental medicines | ||||
| Fermented Oyaksungisan | HT22 cells | Cell viability, antioxidant activity | Improved antioxidant activity; neuroprotective activity on glutamate-induced neurotoxicity in vitro | 74 |
| Fermented Insampaedok-san | HT22 cells | Cell viability, antioxidant activity | Improved antioxidant activity; neuroprotective activity on glutamate-induced neurotoxicity in vitro | 75 |
| Fermented Chongmyung-tang | ICR mice | Antioxidant activity, PA | Improved scopolamine-induced memory impairment in mice; showed strong antioxidant activity | 77 |
| Fermented Bozhougyiqi-tang | HT22 cells, ICR mice | MWM, PA, cell viability, antioxidant activity | Enhanced neuroprotective effect against glutamate-induced neurotoxicity in vitro; ameliorated the scopolamine-induced memory impairment in mice | 78 |
| Fermented Gumiganghwal-tang | HT22 cells, ICR mice | Cell viability, AChE, ROS, MWM, PA | Enhanced neuroprotective effect against glutamate-induced neurotoxicity in vitro; ameliorated the scopolamine-induced memory impairment in mice; suppression of AChE activity in vitro and in vivo | 79,80 |
| Fermented Hwangryunhaedok-tang | HT22 cells | Cell viability, antioxidant activity | Improved antioxidant activity; neuroprotective activity on glutamate-induced neurotoxicity in vitro | 81 |
| Fermented Sipjeondaebo-tang | C57BL/6 mice | PA, MWM, AChE, ROS, BDNF, pCREB, pAkt | Ameliorated the scopolamine-induced memory impairment in mice; promoted hippocampal neurogenesis and attenuated scopolamine-induced ROS and AChE activity; prevented scopolamine-induced suppression of BDNF | 82 |
AD, Alzheimer’s disease; TNF-α, tumor necrosis factor-alpha; Aβ, β-amyloid; VaD, vascular dementia; MWM, Moris water maze; H2O2, hydrogen peroxide; OGD, oxygen-glucose deprivation; NOR, novel object recognition; PA, passive avoidance; AChE, acetylcholinesterase; MDA, malondialdehyde; SOD, superoxide dismutase; TMT, trimethyltin; NGF, nerve growth factor; SD, Sprague-Dawley; BDNF, brain-derived neurotrophic factor; HVLT, Hopkins verbal learning test; 6-OHDA, 6-hydroxydopamine; PD, Parkinson’s disease; iNOS, inducible nitric oxide synthase; ROS, reactive oxygen species; EBST, elevated body swing test; NO, nitric oxide; ApoE, apolipoprotein E; APP, amyloid precursor protein; IRES, internal ribosome entry site; MSG, monosodium glutamate; nNOS, neuronal nitric oxide synthase; PBMC, peripheral blood mononuclear cells; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; FoxP3, forkhead box P3; MAPK, mitogen-activated protein kinase; LPS, lipopolysaccharide; pCREB, phosphorylated cyclic adenosine monophosphate response element-binding protein; MMSE, mini mental state examination; KA, kanic acid; pAkt, phosphorylated Akt.
EVALUATION OF NEUROPROTECTION AND COGNITIVE FUNCTION
The pathogenesis of cognitive dysfunction is multifaceted and complicated. Cholinergic dysfunction in the brain, including hydrolysis of acetylcholine from increased acetylcholinesterase (AChE) activity, has been suggested to be attributable to this pathogenesis (11,12). Dysregulation of the cholinergic system also disrupts hippocampal neurogenesis by modulating the mechanism involving brain-derived neurotrophic factor (BDNF) and cyclic adenosine monophosphate response element-binding protein (CREB) (11,12). Oxidative stress induces neuronal cell death and may cause cognitive dysfunction. Brain structures supporting memory are highly sensitive to oxidative stress partly due to their high demand for oxygen (13). Another possible cause of cognitive dysfunction is the accumulation of amyloid β (Aβ) and phosphorylated tau protein in the brain (14). Increased Aβ formation and tau hyperphosphorylation are known to cause memory impairments in neurodegenerative diseases such as Alzheimer’s disease (AD) (14). These findings highlight some typical biomarkers for evaluating neuroprotection and neurodegeneration models, and suggest that these could be used as indirect indicators of cognitive function (15, 16).
To mimic neurodegeneration, oxidative injury leading to neuronal death is provoked by neurotoxic chemicals such as glutamate or hydrogen peroxide (H2O2) in cellular models. In animal models, intracerebroventricular injection of Aβ or intraperitoneal injection of scopolamine, a cholinergic antagonist, are widely applied to induce cognitive impairment (17,18). Various biomarkers are examined in these neurodegenerative models before and after treatment of neuroprotective agents. Cell viability, AChE level, neurotrophic factors, expression of antioxidant-related proteins such as superoxide dismutase (SOD), and inflammation indices are commonly measured both in vitro and in vivo to investigate neuroprotective effects (19).
Memory-related performance has been evaluated by established behavioral tests. Morris water maze (MWM) and passive avoidance test are broadly used, while there are numerous tests to assess cognition (20). In clinical studies, cognitive function is usually evaluated by cognitive assessment tools. Neuroprotection in humans can be evaluated by measuring oxidative stress or other biomarkers from peripheral blood and urinary samples (15). In some cases, antioxidant activities such as 2,2-di-phenyl-1-picrylhydrazyl (DPPH) radical scavenging activity and H2O2 scavenging activity can be assessed to identify neuroprotective agents.
THE EFFECTS OF FERMENTED FOODS ON COGNITIVE FUNCTION
Fermented dairy products
Nearly every country has developed traditional fermented dairy products of some type within their farming system. Some of the oldest records suggest that dairy products date back to 9,000 B.C (21). Implied by a long and comprehensive history of dairy products use in many civilizations, consuming fermented dairy products might be considered as an attempted practice of healthy diet choice. Indeed, several epidemiological studies even report that consumption of fermented dairy products reduces a cognitive deficit in the elderly (22,23). Furthermore, there is scientific evidence to indicate that fermented dairy products may have neuroprotective effects (21).
Studies of cells and animals show that fermented dairy products tend to prevent neurotoxicity, indicating its neuroprotective effect. Compounds with camembert cheese extracts suppressed neuronal cell death that was induced by excessive microglia activation (24). In a transgenic mouse model of AD, the accumulation of Aβ in the brain was reduced after feeding camembert cheese extract (25). In addition, production of chemokine and neurotrophic factor in the hippocampus were remarkably increased when the mice were fed with the camembert cheese extract. Dehydroergosterol and oleamide were found as active components of camembert cheese, enhancing microglial anti-inflammatory activity (25).
Soymilk fermented with Lactobacillus plantarum strain exhibited a protective effect on H2O2- and oxygen-glucose deprivation (OGD)-induced damage in PC-12 cells. In addition, oral administration of fermented soymilk extract improved learning and memory in deoxycorticosterone acetate-salt-stimulated rat models of vascular dementia (26).
Calpis sour milk is a Japanese beverage prepared by fermenting skim milk with Lactobacillus helveticus and Saccharomyces cerevisiae. Oral administration of Calpis sour milk whey powder significantly improved scopolamine-induced memory deficits and novel object recognition in mice (27). Fermented milk also reduced plasma L-tryptophan and salivary cortisol levels in healthy subjects who were exposed to stressful situations. In the same study, the rate of subjects experiencing physical symptoms was reduced after consumption of fermented milk for 8 weeks (28).
Legume- and cereal-based fermented foods
Cereals and legumes have been important sources of carbohydrates and proteins. They have been widely consumed in various fermented forms in different parts of the world. These foods contain many bioactive compounds that are absent in unfermented foods. Soybeans and rice are the most popular ingredients used in the preparation of legume- and cereal-based fermented foods (21).
Asian countries developed their own methods to make fermented soybean products. Soy sauce, soybean paste such as miso and doenjang, tempeh, and natto are typical examples of fermented soybean products (21). Neuroprotective effects of fermented soybean have been reported in several animal studies. Cheonggukjang is one of the fermented soybean pastes used in Korean regional cuisine. In an animal study using cheonggukjang extract, a trimethyltin-treated group showed long- and short-term memory loss, whereas groups pretreated with cheonggukjang showed improved memory function in a dose-dependent manner (29). The number of cell death and AChE activity of the hippocampus were decreased in the cheonggukjang-treated group. Expression of nerve growth factor (NGF) and activation of the NGF receptor signaling pathway were also upregulated in the cheonggukjang-treated group. Additionally, the level of SOD activity was enhanced, whereas malondialdehyde was lower in the cheonggukjang-treated group compared to the vehicle-treated group (29). In another study with AD and type 2 diabetes-induced rats, 8-week administration of cheonggukjang extract improved cognitive function and glucose regulation (30). Cheonggukjang is usually prepared with Bacillus strains such as Bacillus licheniformis, Bacillus amylofenices, and Bacillus subtilis. Similarly, fermented soybean powder with Lactobacillus pentosus var. plantarum C29 showed a protective effect against scopolamine-induced memory impairment in mice (31). Scopolamine-induced reduction of hippocampal BDNF expression was reversed, and AChE activity was inhibited after treatment with fermented soybean powder extract (31). A cross-sectional clinical study also reported that fermented soybean products oriented diets have a beneficial effect among this particular population. High consumption of tempeh, a mold-fermented soy product originated from Indonesia, had a positive association with improved memory function in elderly subjects, while tofu showed a negative association when analyzed together (32).
Rice (Oryzae sativa L. Gramineae) is a principle ingredient in Asian foods. Red mold rice (RMR), also known as hongqu (Chinese) or koji (Japanese), is fermented rice which acquired its color from being cultivated with the mold Monascus purpureus. RMR has been used for many centuries to improve its color and flavor, as well as to promote digestion and blood circulation. Lee et al. have reported the neuroprotective effect of RMR ethanol extract in vitro and in vivo (33–35). RMR extract provided strong protection against Aβ40-induced neurotoxicity in PC-12 (35). It rescued cell viability as well as repressed inflammatory responses and oxidative stress. In AD rats infused with Aβ40 into the cerebral ventricle, administration of RMR extract potently reversed the cognitive dysfunction in the memory task and brain damage in the biochemical assay (34). Furthermore, RMR prevented Aβ fibrils formation and decreased Aβ40 accumulation in the hippocampus (34,35). A further study reported that RMR extract treatment suppressed cholesterol-raised β-secretase activity and increased the neuroprotective soluble amyloid precursor protein (APP) R-fragment secretion in vitro and in vivo (33). Other studies revealed that RMR administration has a protective effect in a Zn-deficiency model (36) and Parkinson’s disease model (37). The administration of RMR significantly ameliorated behavioral dysfunction and improved the activity of antioxidant enzymes including glutathione reductase, glutathione peroxidase, SOD, and catalase, which all lead to markedly reduced reactive oxygen species (ROS) production.
Monacolins are the major active functional compounds that are isolated from RMR (21). Monacolin K (also known as lovastatin) is one of the main monacolins that has been found to have various pharmacological activities, including lowering blood cholesterol levels and reducing oxidative damage. Several in vivo studies have indicated that Monacolins K can permeate the blood-brain barrier (38,39). In addition to monacolins, considerable amounts of gamma-aminobutyric acid (GABA) have also been found in RMR, which is the main inhibitory neurotransmitter in the central nervous system (5). RMR also contains multiple functional components derived from the fermentation process (38). The protective effect of RMR might be caused by a synergism among these multiple bioactive components (35,38,40).
Rice vinegar is another fermented rice product which has been cultivated in Asian countries including China, Japan, Korea, and Vietnam. One variation of rice vinegar is Kurozu, a traditional Japanese rice vinegar. It is known that concentrated Kurozu feeding suppresses cognitive dysfunction and brain amyloid accumulation in senescence-accelerated P8 mice (41). Concentrated Kurozu increased mRNA expression of heat shock 70 kDa protein 1A (HSPA1A), a protein that stabilizes proteins against misfolding and aggregation, although the result was ambiguous in mice primary neurons. The expression of HSPA1A may be associated with the decreased accumulation of aggregated proteins in the brain (41).
Fermented plant root products
Several root and tuber crops are particularly fermented to produce foods with dense nutrients in a traditional practice of preservation as fermentation has the advantage of reducing the cyanogen content (21). Recently, a number of studies shifted its focus on the neuroprotective and functional improvement of fermented root products.
Codonopsis lanceolata is a herb mainly found in Asian countries. This plant has been broadly used in traditional medicine. The root of C. lanceolata contains various bioactive ingredients–polyphenols, saponins, alkaloids, steroids, tannins, and triterpenes–collectively; and, these are used to treat a broad range of illnesses–bronchitis, cough, spasm, psychoneurosis, cancer, and inflammation (42). Recent studies have revealed that the fermentation process can improve the functional properties of poor quality C. lanceolata. In the study by He et al. (43), the phenol amounts and DPPH scavenging activities in C. lanceolata extract were significantly increased after probiotic fermentation. Administration of fermented C. lanceolata effectively ameliorated a scopolamine-induced memory deficit in ICR mice. Weon et al. (44) also have reported the cognitive-enhancing effect of steamed and fermented C. lanceolata in animal and biochemical studies. Mice that were orally treated with steamed and fermented C. lanceolata extract (SFCE) exhibited enhanced memory performance compared to mice treated with original C. lanceolata extract in MWM and passive avoidance test. SFCE treatment showed a significant neuroprotective effect against glutamate-induced cell death in HT22 cells (44–47). In biochemical assays, AChE activity was decreased, and the level of CREB phosphorylation and BDNF expression were increased in the hippocampal tissue of scopolamine-treated mice after the administration of fermented C. lanceolata (44). Additionally, treatment with SFCE reduced glutamate-induced ROS accumulation, Ca2+ influx, and nitric oxide (NO) formation in HT22 cells. SFCE upregulated the antioxidant enzyme’s activity, and potentially ameliorated mitochondrial dysfunction by inhibiting Bax and caspase-3 expression in the hippocampal-derived cell line (48).
For thousands of years, ginseng root has been used as an East Asian medicinal herb for various diseases (49). Use of ginseng extract may enhance cognitive and psychomotor functions by stimulating the central nervous system. A study demonstrated that fermented ginseng reversed memory impairment and reduced Aβ accumulation in the AD mouse model (50). Another study reported that NB34, a preparation of fermented Radix notoginseng, can serve in an alike functional manner to memantine, a drug used for the treatment of AD, by reducing the activities of both tau internal ribosome entry site and APP (51).
Garlic and onion have also been recognized for their medicinal properties for centuries. After the feeding of fermented garlic (black garlic) extract, the total number of pyramidal cells in the hippocampus and the spatial memory were enhanced in monosodium glutamate-exposed rats (52). When the hippocampal-derived HT22 cells were treated with fermented yellow onion (Allium cepa) extract, the glutamate-induced neurotoxicity was decreased (53).
Fermented fruits and vegetables
Keeping fruits and vegetables fresh to maintain their nutrient values needs special caution and can become demanding if without a proper refrigerating system. It seems that the practice of fruit and vegetable fermentation may have evolved from ancient times to keep the collected food fresh for longer periods of time, especially when natural resources and harvestings are limited.
Papaya is the fruit of the plant Carcica papaya. It is native to the tropics of the Central and northern South America and is now cultivated in many tropical regions including India, Australia, Malaysia, Indonesia, the Philippines, and Hawaii. Fermented papaya preparation (FPP) has been reported to possess free radical scavenging and antioxidative properties (54). A study in an AD cell model showed that the neurotoxicity of the Aβ can be significantly attenuated by FPP. The expression of SOD was significantly upregulated after FPP treatment, while FPP post-treatment improved cell viability and attenuated the intracellular Ca2+ influx, ROS generation, and NO accumulation (54,55). In a mouse model of AD induced by scopolamine, PS-501, the extract of yeast-fermented papaya, significantly reversed short- and long-term memory impairment (56). There is further evidence to indicate that FPP administration can improve memory functions in human studies. Barbagallo et al. assessed oxidative stress in AD patients by measuring urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG). When FPP was consumed by patients for 6 months, 8-OHdG levels were significantly decreased, with no significant changes in that of the controls (57,58). Several studies suggested that the beneficial effect of FPP is possibly regulated by mitogen-activated protein kinase-mediated signaling pathway or Bax/bcl-2 sensitive pathway (54,55).
Fermented grape marc is the solid remains after the juice has been squeezed from grapes. In a study using human peripheral blood mononuclear cells, fermented grape marc was suggested as a potential therapeutic agent regarding its immunomodulatory activities. Fermented grape marc increased the intracellular content of cytokines and the induction of FoxP3, a biomarker of T regulatory cells while reducing the production of Granzyme B (59).
Kimchi is a long-established Korean traditional food made from green vegetables with various seasonings prepared after the natural fermentation process. Many studies have reported that the concentration of the bioactive components in kimchi changes during fermentation, suggesting its anti-carcinogenic, anti-bacterial, and anti-oxidative properties (60–63). Lactic acid bacteria are the main microorganisms responsible for kimchi fermentation. Lactic acid bacteria isolated from the supernatant of kimchi protected against scopolamine-induced mouse memory deficit as shown by the result of passive avoidance test. Particularly, the strain C29 of several lactic acid bacteria suppressed scopolamine-induced memory deficit related behaviors in Y-maze and MWM tests as well. Furthermore, C29 treatment increased hippocampal BDNF and phosphorylated CREB expressions, which were reduced by scopolamine injection (64).
Other fermented plant products
Rhus verniciflua, also commonly known as Chinese lacquer tree, is a member of the sumac family (Anacardiaceae) of flowering plants, which are native to East Asia. Its value for medicinal applications to treat diabetes mellitus and stomach diseases is underestimated and obscured by its allergic compound–urushinol–which was reported to cause irritation and inflammation (65). To detoxify the stem bark of R. verniciflua, a fermentation process involving mushroom species was applied, and it significantly attenuated allergic-induced responses as well as neuroprotective properties. In ICR mice, R. verniciflua extracts were orally administered for 7 days, followed by an intracerebroventricular injection of kanic acid resulting in apoptotic neuronal cells. Pretreatment with R. verniciflua extracts significantly reduced kanic acid-induced microglial activation and neuronal cell death in the mouse hippocampus (66).
In an epidemiologic study, there was a significant association between total tea consumption and a lower risk of cognitive decline. The effect was most evident in diets with fermented black and oolong teas compared to that of regular green tea (67).
Fermented fungi
Medicinal fungi have long been used for its anticancer, antihepatotoxic, antihypertensive, anti-inflammatory, and antioxidative properties in a practice of traditional Chinese medicine. Several cell culture and animal studies investigated the potential effects of fermented medicinal fungi that would be beneficial for cognitive function.
Ganoderma lucidum is one of the most popular medicinal mushrooms used in China for more than 2,000 years (68). G. lucidum water extracts fermented by lactic acid bacteria significantly enhanced learning memory and cognitive function of scopolamine-induced rats in passive avoidance and MWM test. In parallel with behavioral changes, lower hippocampal AChE activities were apparent in the group treated with fermented G. lucidum extracts in a biochemical test (69).
Antrodia cinnamomea, also known as Antrodia camphorate, is a species of fungus native to Taiwan. It is commonly used to treat various diseases including cancer, cardiovascular disease, and inflammatory reaction. Lu et al. (70) reported that fermented A. cinnamomea extract protects neuron-like PC-12 cells from serum deprivation-induced cytotoxicity in a dose-dependent manner. During the serum deprivation, phosphorylation of extracellular signal-regulated kinase was decreased along with the increase in phosphorylated c-Jun NH2-terminal kinase level and p38, while A. cinnamomea reverses these apoptotic results.
Xylaria nigripes is another medicinal fungus used in traditional Chinese medicine in attempts to resolve sleep, mood, and memory problems. Nineteen natural compounds were isolated from the ethanol extract of fermented X. nigripes. Among the products, compound 17 exhibited neuroprotective effects by attenuating Aβ-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Compound 15 exhibited anti-neuroinflammatory effects by inhibiting lipopolysaccharide-stimulated NO production in BV-2 microglial cells (71).
Fermented traditional oriental medicine
Traditional oriental medicines have been used for thousands of years. Unlike modern drugs, which contain a single active component, traditional oriental medicines consist of multiple components and are based on a multi-target approach (72). The fermentation process converts the components of traditional oriental medicines into active metabolites, hence it enhances their biological activity and improves nutrient absorption (72). Fermentation also reduces the toxicity of traditional oriental medicines. Several studies reported the enhanced biological activity of fermented traditional oriental medicines (5,73).
A Korean traditional herbal formula, Oyaksungisan, has been used to treat rheumatoid arthritis and paralysis. It is composed of twelve herbs including Citrus unshiu peel, Lindera root, Angelica Dahurica root, and Zingiberis rhizoma, which are reported to have anti-cancer effects (74). The fermentation process changed the contents of Oyaksungisan and improved radical scavenging effects. Compared to original Oyaksungisan, treatment with fermented Oyaksungisan showed greater neuroprotective activity on glutamate-stimulated neurotoxicity in HT22 cells (74). Similarly, Insampaedoksan, a traditional oriental medication for antipyretic and anti-inflammatory diseases, exhibited more potent anti-oxidative and neuroprotective activity after fermentation with Lactobacillus (75).
Chongmyung-tang is a Korean herbal medicine, which has been frequently used for the therapy of memory improvement. It consists of three medicinal herbs of Polygalae radix, Acori graminei rhizoma, and Hoelen (76). Nam et al. (77) investigated the antioxidative and memory-enhancing effects of fermented Chongmyung-tang. Both, original Chongmyung-tang and fermented Chongmyung-tang treatments resulted in a significant memory enhancing effect in ICR mice. Both of them also exhibited strong radical scavenging activities. In comparison to the original Chongmyung-tang group, the fermented Chongmyung-tang group showed slightly greater memory function enhancement, although the result was not statistically significant.
The neuroprotective effects of other decocted herbal medicines, which have been traditionally used for the treatment of fatigue or inflammatory diseases, have also been investigated. Fermented Bozhougyiqi-tang (78), Gumiganghwal-tang (79,80), and Hwangryunhaedok-tang (81) showed more potent protective activities against glutamate-stimulated cell death in HT22 cells than the unfermented prescription. Moreover, several studies in scopolamine-treated mice reported that fermented Sipjeondaebo-tang (82), Bozhougyiqi-tang (78), and Gumiganghwal-tang (79,80) significantly ameliorated amnesic behaviors possibly through AChE inhibition. These results suggest that fermentation might improve the pharmacological activities of traditional oriental medicines.
POTENTIAL MECHANISMS OF FERMENTED FOODS
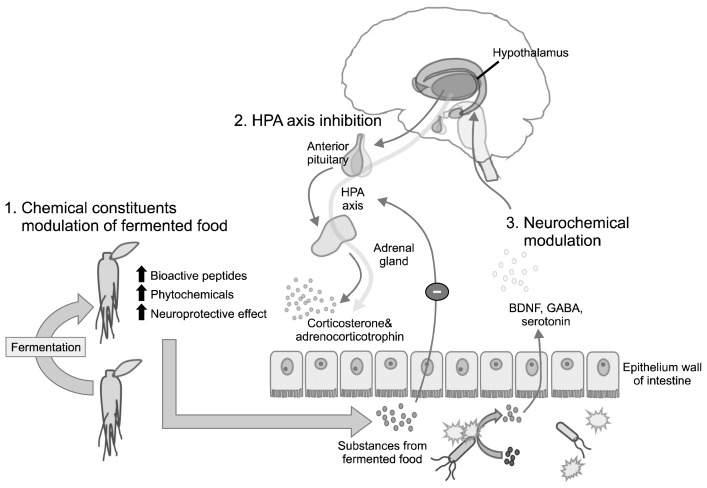
Although the underlying mechanisms of fermented foods on cognitive function is still unclear, emerging evidence indicates that ingestion of fermented food may influence the central nervous system (10). There are several hypotheses for the beneficial effects of fermentation, of which the major ones are described (Fig. 1).
Fig. 1.
Potential mechanisms underlying the efficacy of fermented foods. The figure presents 3 hypotheses through which fermented foods can improve brain and cognitive function. (1) Chemical constituents modulation, (2) HPA axis inhibition, and (3) Neurochemical modulation. HPA, hypothalamic-pituitary-adrenal; BDNF, brain-derived neurotrophic factor; GABA, gamma-aminobutyric acid.
One possible explanation is that fermentation modulates the chemical constituents, improving the activity and bioavailability of the food. As reviewed here, a number of studies have reported the chemical changes such as enriching bioactive peptides and creating phytochemicals during the fermentation process (5). It seems that substances obtained by the incubation of natural foods with microbes enhance their neuroprotective effects. Moreover, the components changed through fermentation may increase bioavailability involving intestinal absorption and utilization of the ingested nutrients within the body (5,21). Our intestinal epithelium is a selectively permeable barrier, preventing the access of harmful substances. The absorption of nutritional components in the intestines is therefore restricted and occasionally the components need to be converted to active forms by bacteria in the intestine (83). For example, a recent study found that short-chain fatty acids, which are fermented products formed by intestinal bacteria, positively influence host metabolism and play a key role in functions of the central nervous system (84). It is also reported that phytochemical absorption and its antioxidative and anti-inflammatory functions can be controlled by resident intestinal microbiota (4,85,86). In another study of germ-free (GF) mice, colonization with microbes was accompanied by marked changes in the transcription of genes associated with nutrient absorption and metabolism (83). These findings suggest that proper fermentation may amplify the beneficial contents of the food, promoting biological function and bioavailability.
In addition to changing constitutive properties, probiotics in fermented foods may also influence brain function via gut microbiota. The human intestine is inhabited by nearly 1014 microorganisms (8). The relation between these microorganisms and their host, which is called commensal intestinal microbiota, begins shortly after birth and remains throughout life. The presence of commensal microbiota is important to the immune system, gastrointestinal homeostasis, and nutrient processing (9). Recently, a growing body of findings revealed that gut microbiota is also critical to the function of the central nervous system, resulting in altered brain function and behaviors (7–9).
The hypothalamic-pituitary-adrenal (HPA) axis is involved in the neurobiology of stress response. It is becoming clear that gut microbiota is significantly associated with the HPA axis. In GF animals, which have no commensal microbiota, restraint stress-induced HPA reactivity with exaggerated corticosterone and adrenocorticotrophin release were compared to the specific pathogen-free controls (87). On the other hand, early development of the gut microbiota reduced the exaggerated HPA axis response of GF mice over their lifetime (87). Feeding of probiotics can also attenuate the HPA-mediated stress responses (88,89). Considering that cognitive deficit is associated with HPA axis hyperactivity, administration of probiotics in fermented foods may improve cognitive function by normalization of HPA activity.
Other potential mechanisms through which fermented foods can influence cognitive function include neurochemical modulation. To date, several studies have provided evidence that neurotransmitter systems are affected by the gut microbiota. For example, reduced expression of hippocampal BDNF, which is important for the survival and differentiation of neurons, was observed in the infected dysbiosis mice model (90) and male GF mice (87,91). Supplementation with probiotics returned BDNF expression to the control level. Alteration in GABA, the main inhibitory neurotransmitter, was also observed. Chronic treatment with the probiotic Lactobacillus rhamnosus changed the expression of certain GABA receptors in a regional-dependent manner, matching the known effects of anxiolytic agents. The changes related to L. rhamnosus treatment were extinguished with vagotomy, indicating a key route of communication from gut to brain (92). Interestingly, some probiotic bacteria are capable of producing GABA from glutamate in culture (93). Other experiments have shown that the administration of probiotics can alter serotonin turnover and related metabolites in the brain (94,95). Neurotransmitters, such as BDNF, glutamate, GABA, and serotonin are reported to be respectively involved in learning and memory. Therefore, it is hypothesized that fermented foods might improve cognitive function by modulating the release of neurotransmitters.
The fermented food’s functional aspect of neuroprotective effects along with the improvement in brain and cognitive function is becoming more evident as studies of animals and humans with positive results are accumulating. The beneficial effects of fermented foods may be due to changes in chemical composition by probiotics and homeostasis in the gut microbiota community.
CONCLUSION
As the mechanisms underlying the beneficial effects of fermented foods are becoming more visible with accumulating results from clinical and animal studies, fermented foods are gaining popularity among consumers for their possible therapeutic and high marketing value. To our knowledge, this paper is the first research to conduct an extensive review on fermented foods and their cognitive enhancing effects, so to be used as a reference when studying the relevance of gut microbiota and fermented foods to brain functionality. Partly due to the paucity of clinical trials in humans using fermented functional foods, quantitative synthesis of these trial results could not be carried out. As more studies are published in the future, it would be possible to conduct a meta-analysis that may be helpful in confirming the beneficial effects of fermented functional foods.
ACKNOWLEDGEMENTS
This work was supported by the SME Tech Convergence Development Project (S2175707) funded by the Small and Medium Business Administration (SMBA, Korea), Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through High Value-added Food Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (116004-2), and Fire Fighting Safety & 119 Rescue Technology Research and Development Program funded by the Ministry of Public Safety and Security (MPSS-Fire Fighting Safety-2016-86). Funders had no role in the design, data collection, interpretation of the materials, manuscript preparation, or decision to publish.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Hutkins RW. Microbiology and technology of fermented foods. 1st ed. Blackwell Publishing; Oxford, UK: 2008. pp. 15–66. [Google Scholar]
- 2.Wang HY, Qi LW, Wang CZ, Li P. Bioactivity enhancement of herbal supplements by intestinal microbiota focusing on ginsenosides. Am J Chin Med. 2011;39:1103–1115. doi: 10.1142/S0192415X11009433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JH, Lee JH, Jin JS. Fermentation of traditional medicine: present and future. Orient Pharm Exp Med. 2012;12:163. doi: 10.1007/s13596-012-0080-4. [DOI] [Google Scholar]
- 4.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 5.Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Bienenstock J, Kunze W, Forsythe P. Microbiota and the gut-brain axis. Nutr Rev. 2015;73(S1):28–31. doi: 10.1093/nutrit/nuv019. [DOI] [PubMed] [Google Scholar]
- 7.Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369–1378. doi: 10.1016/j.psyneuen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Selhub EM, Logan AC, Bested AC. Fermented foods, microbiota, and mental health: ancient practice meets nutritional psychiatry. J Physiol Anthropol. 2014;33:2. doi: 10.1186/1880-6805-33-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasler CM. Functional foods: benefits, concerns and challenges–a position paper from the American Council on Science and Health. J Nutr. 2002;132:3772–3781. doi: 10.1093/jn/132.12.3772. [DOI] [PubMed] [Google Scholar]
- 10.Stanton C, Gardiner G, Meehan H, Collins K, Fitzgerald G, Lynch PB, Ross RP. Market potential for probiotics. Am J Clin Nutr. 2001;73:476s–483s. doi: 10.1093/ajcn/73.2.476s. [DOI] [PubMed] [Google Scholar]
- 11.Bruel-Jungerman E, Lucassen PJ, Francis F. Cholinergic influences on cortical development and adult neurogenesis. Behav Brain Res. 2011;221:379–388. doi: 10.1016/j.bbr.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Collerton D. Cholinergic function and intellectual decline in Alzheimer’s disease. Neuroscience. 1986;19:1–28. doi: 10.1016/0306-4522(86)90002-3. [DOI] [PubMed] [Google Scholar]
- 13.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 14.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain KK. The handbook of biomarkers. 1st ed. Humana Press, Inc.; Totowa, NJ, USA: 2010. pp. 115–396. [DOI] [Google Scholar]
- 16.Jain KK. The handbook of neuroprotection. 1st ed. Humana Press, Inc.; Totowa, NJ, USA: 2011. pp. 281–365. [DOI] [Google Scholar]
- 17.Lehtinen MK, Bonni A. Modeling oxidative stress in the central nervous system. Curr Mol Med. 2006;6:871–881. doi: 10.2174/156652406779010786. [DOI] [PubMed] [Google Scholar]
- 18.Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Cell culture models of oxidative stress and injury in the central nervous system. Curr Neurovasc Res. 2005;2:73–89. doi: 10.2174/1567202052773463. [DOI] [PubMed] [Google Scholar]
- 19.Humpel C. Identifying and validating biomarkers for Alzheimer’s disease. Trends Biotechnol. 2011;29:26–32. doi: 10.1016/j.tibtech.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bushnell PJ. Advanced behavioral testing in rodents: assessment of cognitive function in animals. Curr Protoc Toxicol. 2001;00:11. doi: 10.1002/0471140856.tx1104s00. 4:11.4.1–11.4.34. [DOI] [PubMed] [Google Scholar]
- 21.Farnworth ER. Handbook of fermented functional foods. 2nd ed. CRC Press; Boca Raton, FL, USA: 2008. pp. 1–494. [Google Scholar]
- 22.Camfield DA, Owen L, Scholey AB, Pipingas A, Stough C. Dairy constituents and neurocognitive health in ageing. Br J Nutr. 2011;106:159–174. doi: 10.1017/S0007114511000158. [DOI] [PubMed] [Google Scholar]
- 23.Ozawa M, Ninomiya T, Ohara T, Doi Y, Uchida K, Shirota T, Yonemoto K, Kitazono T, Kiyohara Y. Dietary patterns and risk of dementia in an elderly Japanese population: the Hisayama Study. Am J Clin Nutr. 2013;97:1076–1082. doi: 10.3945/ajcn.112.045575. [DOI] [PubMed] [Google Scholar]
- 24.Ano Y, Kutsukake T, Hoshi A, Yoshida A, Nakayama H. Identification of a novel dehydroergosterol enhancing microglial anti-inflammatory activity in a dairy product fermented with Penicillium candidum. PLoS One. 2015;10:e0116598. doi: 10.1371/journal.pone.0116598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ano Y, Ozawa M, Kutsukake T, Sugiyama S, Uchida K, Yoshida A, Nakayama H. Preventive effects of a fermented dairy product against Alzheimer’s disease and identification of a novel oleamide with enhanced microglial phagocytosis and anti-inflammatory activity. PLoS One. 2015;10:e0118512. doi: 10.1371/journal.pone.0118512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu TH, Chiou J, Tsai TY. Effects of Lactobacillus plantarum TWK10-fermented soymilk on deoxycorticosterone acetate-salt-induced hypertension and associated dementia in rats. Nutrients. 2016;8:260. doi: 10.3390/nu8050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohsawa K, Uchida N, Ohki K, Nakamura Y, Yokogoshi H. Lactobacillus helveticus–fermented milk improves learning and memory in mice. Nutr Neurosci. 2015;18:232–240. doi: 10.1179/1476830514Y.0000000122. [DOI] [PubMed] [Google Scholar]
- 28.Kato-Kataoka A, Nishida K, Takada M, Suda K, Kawai M, Shimizu K, Kushiro A, Hoshi R, Watanabe O, Igarashi T, Miyazaki K, Kuwano Y, Rokutan K. Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef Microbes. 2016;7:153–156. doi: 10.3920/BM2015.0100. [DOI] [PubMed] [Google Scholar]
- 29.Go J, Kim JE, Kwak MH, Koh EK, Song SH, Sung JE, Kim DS, Hong JT, Hwang DY. Neuroprotective effects of fermented soybean products (Cheonggukjang) manufactured by mixed culture of Bacillus subtilis MC31 and Lactobacillus sakei 383 on trimethyltin-induced cognitive defects mice. Nutr Neurosci. 2015;19:247–259. doi: 10.1179/1476830515Y.0000000025. [DOI] [PubMed] [Google Scholar]
- 30.Yang HJ, Kwon DY, Kim HJ, Kim MJ, Jung DY, Kang HJ, Kim DS, Kang S, Moon NR, Shin BK, Park S. Fermenting soybeans with Bacillus licheniformis potentiates their capacity to improve cognitive function and glucose homeostaisis in diabetic rats with experimental Alzheimer’s type dementia. Eur J Nutr. 2015;54:77–88. doi: 10.1007/s00394-014-0687-y. [DOI] [PubMed] [Google Scholar]
- 31.Yoo DH, Kim DH. Lactobacillus pentosus var. plantarum C29 increases the protective effect of soybean against scopolamine-induced memory impairment in mice. Int J Food Sci Nutr. 2015;66:912–918. doi: 10.3109/09637486.2015.1064865. [DOI] [PubMed] [Google Scholar]
- 32.Hogervorst E, Sadjimim T, Yesufu A, Kreager P, Rahardjo TB. High tofu intake is associated with worse memory in elderly indonesian men and women. Dement Geriatr Cogn Disord. 2008;26:50–57. doi: 10.1159/000141484. [DOI] [PubMed] [Google Scholar]
- 33.Lee CL, Kuo TF, Wu CL, Wang JJ, Pan TM. Red mold rice promotes neuroprotective sappalpha secretion instead of Alzheimer’s risk factors and amyloid beta expression in hyperlipidemic Aβ40-infused rats. J Agric Food Chem. 2010;58:2230–2238. doi: 10.1021/jf904027y. [DOI] [PubMed] [Google Scholar]
- 34.Lee CL, Kuo TF, Wang JJ, Pan TM. Red mold rice ameliorates impairment of memory and learning ability in intracerebroventricular amyloid beta-infused rat by repressing amyloid beta accumulation. J Neurosci Res. 2007;85:3171–3182. doi: 10.1002/jnr.21428. [DOI] [PubMed] [Google Scholar]
- 35.Lee CL, Wang JJ, Pan TM. Red mold rice extract represses amyloid beta peptide-induced neurotoxicity via potent synergism of anti-inflammatory and antioxidative effect. Appl Microbiol Biotechnol. 2008;79:829–841. doi: 10.1007/s00253-008-1480-8. [DOI] [PubMed] [Google Scholar]
- 36.Lee BH, Ho BY, Wang CT, Pan TM. Red mold rice promoted antioxidase activity against oxidative injury and improved the memory ability of zinc-deficient rats. J Agric Food Chem. 2009;57:10600–10607. doi: 10.1021/jf902046s. [DOI] [PubMed] [Google Scholar]
- 37.Tseng WT, Hsu YW, Pan TM. The ameliorative effect of Monascus purpureus NTU 568-fermented rice extracts on 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells and the rat model of Parkinson’s disease. Food Funct. 2016;7:752–762. doi: 10.1039/C5FO00976F. [DOI] [PubMed] [Google Scholar]
- 38.Lin CM, Lin YT, Lin RD, Huang WJ, Lee MH. Neuro-cytoprotective effects of aliphatic hydroxamates from Lovastatin, a secondary metabolite from Monascus-fermented red mold rice, in 6-hydroxydopamine (6-OHDA)-treated nerve growth factor (NGF)-differentiated PC12 cells. ACS Chem Neurosci. 2015;6:716–724. doi: 10.1021/cn500275k. [DOI] [PubMed] [Google Scholar]
- 39.Ou HP, Wang MF, Yang SC, Yamamoto S, Wang CCR. Effect of Monascus-fermented products on learning and memory in the SAMP8 mice. J Nutr Sci Vitaminol. 2007;53:253–260. doi: 10.3177/jnsv.53.253. [DOI] [PubMed] [Google Scholar]
- 40.Tseng WT, Hsu YW, Pan TM. Neuroprotective effects of dimerumic acid and deferricoprogen from Monascus purpureus NTU 568-fermented rice against 6-hydroxydopamine-induced oxidative stress and apoptosis in differentiated pheochromocytoma PC-12 cells. Pharm Biol. 2016;54:1434–1444. doi: 10.3109/13880209.2015.1104698. [DOI] [PubMed] [Google Scholar]
- 41.Kanouchi H, Kakimoto T, Nakano H, Suzuki M, Nakai Y, Shiozaki K, Akikoka K, Otomaru K, Nagano M, Matsumoto M. The brewed rice vinegar Kurozu increases HSPA1A expression and ameliorates cognitive dysfunction in aged P8 mice. PLoS One. 2016;11:e0150796. doi: 10.1371/journal.pone.0150796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hossen MJ, Kim MY, Kim JH, Cho JY. Codonopsis lanceolata: a review of its therapeutic potentials. Phytother Res. 2016;30:347–356. doi: 10.1002/ptr.5553. [DOI] [PubMed] [Google Scholar]
- 43.He X, Zou Y, Yoon WB, Park SJ, Park DS, Ahn J. Effects of probiotic fermentation on the enhancement of biological and pharmacological activities of Codonopsis lanceolata extracted by high pressure treatment. J Biosci Bioeng. 2011;112:188–193. doi: 10.1016/j.jbiosc.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Weon JB, Yun BR, Lee J, Eom MR, Ko HJ, Lee HY, Park DS, Chung HC, Chung JY, Ma CJ. Cognitive-enhancing effect of steamed and fermented Codonopsis lanceolata: a behavioral and biochemical study. Evid Based Complement Alternat Med. 2014;2014:319436. doi: 10.1155/2014/319436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weon JB, Lee BH, Yun BR, Lee JW, Lee HY, Park DS, Chung HC, Chung JY, Ma CJ. Memory enhancing effect of Codonopsis lanceolata by high hydrostatic pressure process and fermentation. Korean J Pharmacogn. 2013;44:41–46. [Google Scholar]
- 46.Weon JB, Yun BR, Lee J, Eom MR, Ko HJ, Kim JS, Lee HY, Park DS, Chung HC, Chung JY, Ma CJ. Effect of Codonopsis lanceolata with steamed and fermented process on scopolamine-induced memory impairment in mice. Biomol Ther. 2013;21:405–410. doi: 10.4062/biomolther.2013.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weon JB, Yun BR, Lee J, Eom MR, Kim JS, Lee HY, Park DS, Chung HC, Chung JY, Ma CJ. The ameliorating effect of steamed and fermented Codonopsis lanceolata on scopolamine-induced memory impairment in mice. Evid Based Complement Alternat Med. 2013;2013:464576. doi: 10.1155/2013/464576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weon JB, Yun BR, Lee J, Eom MR, Ko HJ, Lee HY, Park DS, Chung HC, Chung JY, Ma CJ. Neuroprotective effect of steamed and fermented Codonopsis lanceolata. Biomol Ther. 2014;22:246–253. doi: 10.4062/biomolther.2014.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/S0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 50.Kim J, Kim SH, Lee DS, Lee DJ, Kim SH, Chung S, Yang HO. Effects of fermented ginseng on memory impairment and β-amyloid reduction in Alzheimer’s disease experimental models. J Ginseng Res. 2013;37:100–107. doi: 10.5142/jgr.2013.37.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tasi YC, Chin TY, Chen YJ, Huang CC, Lee SL, Wu TY. Potential natural products for Alzheimer’s disease: targeted search using the internal ribosome entry site of tau and amyloid-β precursor protein. Int J Mol Sci. 2015;16:8789–8810. doi: 10.3390/ijms16048789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hermawati E, Sari DC, Partadiredja G. The effects of black garlic ethanol extract on the spatial memory and estimated total number of pyramidal cells of the hippocampus of monosodium glutamate-exposed adolescent male Wistar rats. Anat Sci Int. 2015;90:275–286. doi: 10.1007/s12565-014-0262-x. [DOI] [PubMed] [Google Scholar]
- 53.Yang EJ, Kim SI, Park SY, Bang HY, Jeong JH, So JH, Rhee IK, Song KS. Fermentation enhances the in vitro antioxidative effect of onion (Allium cepa) via an increase in quercetin content. Food Chem Toxicol. 2012;50:2042–2048. doi: 10.1016/j.fct.2012.03.065. [DOI] [PubMed] [Google Scholar]
- 54.Aruoma OI, Hayashi Y, Marotta F, Mantello P, Rachmilewitz E, Montagnier L. Applications and bioefficacy of the functional food supplement fermented papaya preparation. Toxicology. 2010;278:6–16. doi: 10.1016/j.tox.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J, Mori A, Chen Q, Zhao B. Fermented papaya preparation attenuates β-amyloid precursor protein: β-amyloid-mediated copper neurotoxicity in β-amyloid precursor protein and β-amyloid precursor protein Swedish mutation overexpressing SH-SY5Y cells. Neuroscience. 2006;143:63–72. doi: 10.1016/j.neuroscience.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 56.Imao K, Kameyama T, Ukai M. PS-501, fermented papaya preparation, improves scopolamine-induced amnesia in mice. Res Comm Pharm Toxicol. 2001;6:197–204. [Google Scholar]
- 57.Barbagallo M, Marotta F, Dominguez LJ. Oxidative stress in patients with Alzheimer’s disease: effect of extracts of fermented papaya powder. Mediators Inflamm. 2015;2015:624801. doi: 10.1155/2015/624801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barbagallo M, Belvedere M, Di Prima A, Miraglia S, Dominguez LJ. Effetto degli estratti di papaya fermentata sullo stress ossidativo in pazienti con Malattia di Alzheimer. G Gerontol. 2013;61:199–204. [Google Scholar]
- 59.Marzulli G, Magrone T, Kawaguchi K, Kumazawa Y, Jirillo E. Fermented grape marc (FGM): immunomodulating properties and its potential exploitation in the treatment of neurodegenerative diseases. Curr Pharm Des. 2012;18:43–50. doi: 10.2174/138161212798919011. [DOI] [PubMed] [Google Scholar]
- 60.Lee YC. Kimchi: the famous fermented vegetable product in Korea. Food Rev Int. 1991;7:399–415. doi: 10.1080/87559129109540920. [DOI] [Google Scholar]
- 61.Kwak SH, Cho YM, Noh GM, Om AS. Cancer preventive potential of kimchi lactic acid bacteria (Weissella cibaria, Lactobacillus plantarum) J Cancer Prev. 2014;19:253–258. doi: 10.15430/JCP.2014.19.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi IH, Noh JS, Han JS, Kim HJ, Han ES, Song YO. Kimchi, a fermented vegetable, improves serum lipid profiles in healthy young adults: randomized clinical trial. J Med Food. 2013;16:223–229. doi: 10.1089/jmf.2012.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi WY, Park KY. Anticancer effects of organic Chinese cabbage kimchi. J Food Sci Nutr. 1999;4:113–116. [Google Scholar]
- 64.Jung IH, Jung MA, Kim EJ, Han MJ, Kim DH. Lactobacillus pentosus var. plantarum C29 protects scopolamine-induced memory deficit in mice. J Appl Microbiol. 2012;113:1498–1506. doi: 10.1111/j.1365-2672.2012.05437.x. [DOI] [PubMed] [Google Scholar]
- 65.Choi HS, Kim MK, Park HS, Yun SE, Mun SP, Kim JS, Sapkota K, Kim S, Kim TY, Kim SJ. Biological detoxification of lacquer tree (Rhus verniciflua Stokes) stem bark by mushroom species. Food Sci Biotechnol. 2007;16:935–942. [Google Scholar]
- 66.Byun JS, Han YH, Hong SJ, Hwang SM, Kwon YS, Lee HJ, Kim SS, Kim MJ, Chun W. Bark constituents from mushroom-detoxified Rhus verniciflua suppress kainic acid-induced neuronal cell death in mouse hippocampus. Korean J Physiol Pharmacol. 2010;14:279–283. doi: 10.4196/kjpp.2010.14.5.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ng TP, Feng L, Niti M, Kua EH, Yap KB. Tea consumption and cognitive impairment and decline in older Chinese adults. Am J Clin Nutr. 2008;88:224–231. doi: 10.1093/ajcn/88.1.224. [DOI] [PubMed] [Google Scholar]
- 68.Money NP. Are mushrooms medicinal? Fungal Biol. 2016;120:449–453. doi: 10.1016/j.funbio.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 69.Choi YJ, Yang HS, Jo JH, Lee SC, Park TY, Choi BS, Seo KS, Huh CK. Anti-amnesic effect of fermented Ganoderma lucidum water extracts by lactic acid bacteria on scopolamine-induced memory impairment in rats. Prev Nutr Food Sci. 2015;20:126–132. doi: 10.3746/pnf.2015.20.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu MK, Cheng JJ, Lai WL, Lin YJ, Huang NK. Fermented Antrodia cinnamomea extract protects rat PC12 cells from serum deprivation-induced apoptosis: the role of the MAPK family. J Agric Food Chem. 2008;56:865–874. doi: 10.1021/jf072828b. [DOI] [PubMed] [Google Scholar]
- 71.Xiong J, Huang Y, Wu XY, Liu XH, Fan H, Wang W, Zhao Y, Yang GX, Zhang HY, Hu JF. Chemical constituents from the fermented mycelia of the medicinal fungus Xylaria nigripes. Helv Chim Acta. 2016;99:83–89. doi: 10.1002/hlca.201500231. [DOI] [Google Scholar]
- 72.Kim HU, Ryu JY, Lee JO, Lee SY. A systems approach to traditional oriental medicine. Nat Biotechnol. 2015;33:264–268. doi: 10.1038/nbt.3167. [DOI] [PubMed] [Google Scholar]
- 73.Wen YL, Yan LP, Chen CS. Effects of fermentation treatment on antioxidant and antimicrobial activities of four common Chinese herbal medicinal residues by Aspergillus oryzae. J Food Drug Anal. 2013;21:219–226. doi: 10.1016/j.jfda.2013.05.013. [DOI] [Google Scholar]
- 74.Weon JB, Ma JY, Yang HJ, Ma CJ. Neuroprotective activity of fermented Oyaksungisan. Korean J Pharmacogn. 2011;42:22–26. [Google Scholar]
- 75.Ma JY, Ma CJ, Yang HJ, Ma CJ. Quantitative analysis of compounds in fermented Insampaedok-san and their neuroprotective activity in HT22 cells. Nat Prod Sci. 2011;17:58–63. [Google Scholar]
- 76.Lee MR, Yun BS, Oh CJ, Kim BC, Oh HI, Sung CK. Characterization of Korean traditional medicine Chongmyung-tang for cognitive function related to anti-cholinesterases and antioxidant activity. Food Sci Biotechnol. 2011;20:1331. doi: 10.1007/s10068-011-0183-6. [DOI] [Google Scholar]
- 77.Nam JI, Park YW, Jeon H. Memory enhancing and antioxidant properties of fermented Chongmyung-tang. Nat Prod Sci. 2010;16:93–98. [Google Scholar]
- 78.Weon JB, Lee B, Yun BR, Lee J, Ma JY, Ma CJ. Neuroprotective and cognitive enhancing activity of the fermented Bozhougyiqi-Tang. Pharmacogn Mag. 2014;10:249–255. doi: 10.4103/0973-1296.133266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weon JB, Lee J, Eom MR, Jung YS, Ma CJ. Cognitive enhancing effect of the fermented Gumiganghwal-tang on scopolamine-induced memory impairment in mice. Nutr Neurosci. 2016;19:125–130. doi: 10.1179/1476830514Y.0000000152. [DOI] [PubMed] [Google Scholar]
- 80.Yun BR, Weon JB, Lee J, Eom MR, Ma CJ. Neuroprotective effect of the fermented Gumiganghwal-tang. J Biosci Bioeng. 2014;118:235–238. doi: 10.1016/j.jbiosc.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 81.Yang HJ, Weon JB, Lee B, Ma CJ. The alteration of components in the fermented Hwangryunhaedok-tang and its neuroprotective activity. Pharmacogn Mag. 2011;7:207–212. doi: 10.4103/0973-1296.84234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park HR, Lee H, Park H, Cho WK, Ma JY. Fermented Sipjeondaebo-tang alleviates memory deficits and loss of hippocampal neurogenesis in scopolamine-induced amnesia in mice. Sci Rep. 2016;6:22405. doi: 10.1038/srep22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 84.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 85.Renouf M, Guy PA, Marmet C, Fraering AL, Longet K, Moulin J, Enslen M, Barron D, Dionisi F, Cavin C, Williamson G, Steiling H. Measurement of caffeic and ferulic acid equivalents in plasma after coffee consumption: small intestine and colon are key sites for coffee metabolism. Mol Nutr Food Res. 2010;54:760–766. doi: 10.1002/mnfr.200900056. [DOI] [PubMed] [Google Scholar]
- 86.Park EK, Shin J, Bae EA, Lee YC, Kim DH. Intestinal bacteria activate estrogenic effect of main constituents puerarin and daidzin of Pueraria thunbergiana. Biol Pharm Bull. 2006;29:2432–2435. doi: 10.1248/bpb.29.2432. [DOI] [PubMed] [Google Scholar]
- 87.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56:1522–1528. doi: 10.1136/gut.2006.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37:1885–1895. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 90.Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA, Lu J, Khan WI, Corthesy-Theulaz I, Cherbut C, Bergonzelli GE, Collins SM. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112. doi: 10.1053/j.gastro.2010.06.063. e1. [DOI] [PubMed] [Google Scholar]
- 91.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. doi: 10.1053/j.gastro.2011.04.052. e3. [DOI] [PubMed] [Google Scholar]
- 92.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113:411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 94.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiomegut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 95.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43:164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]