Abstract
This study investigated the physicochemical characteristics of black garlic (BG) after different thermal processing steps. Compared with fresh garlic (FG), the moisture content and pH in BG decreased significantly, while the ash content and browning intensity increased during thermal processing. The total mineral and the free sugar contents were significantly higher than that of the BG2 and BG4 samples, respectively. The free sugar content increased by 16-fold in the BG cloves compared with that of FG, while the amino acid content increased during the first stage of thermal processing, and subsequently decreased. The thiosulfinate content in all samples decreased to during thermal processing. The pyruvic acid content initially increased and then decreased during thermal processing. These results contribute to our understanding of the role of thermal processing in the quality formation of BG.
Keywords: black garlic, thermal processing, physicochemical characteristics
INTRODUCTION
Garlic (Allium sativum L.) is a species of the onion genus that has long been used as a culinary seasoning and medical herb (1). Garlic has numerous health benefits and specifically improves digestion. The main components in garlic are organosulfur compounds and bioactive enzymes. Among these, allicin is well known for its pharmacological properties, including anti-bacterial, anti-hyperlipidemic, anti-tumor, and immunoregulatory activity (2). However, the consumption and application of fresh garlic (FG) in foods and medicines is limited due to its characteristic odor, spicy flavor, and tendency to cause an upset stomach.
Black garlic (BG) is a newly processed food prepared by subjecting whole raw garlic to thermal processing at 70~80°C under controlled humidity conditions for 1~3 months without additives (3). This processed food has a fruity taste and is edible in uncooked form. Thermal processing induces many chemical reactions in garlic, such as enzymatic browning and the Maillard reaction, causing its color to change from white and yellow to dark brown. During the heating process, unstable and unpleasant compounds in raw garlic are converted into stable and unflavored compounds. As a result, BG generally has a sweet-sour flavor instead of a pungent odor and taste (4). Moreover, BG does not cause abdominal pain or other gastrointestinal discomport (5), has been reported to have stronger antioxidant activity than FG (6), and shows better efficacy for preventing metabolic diseases and alcoholic hepatotoxicity (7).
In recent years, many studies have been conducted to investigate the bioactive compounds in BG (total phenols; 5-hydroxymethylfurfural) and their functional activities. However, limited information is available regarding changes in the quality of BG during thermal processing. This study aimed to investigate the physicochemical characteristics of BG after different thermal processing steps by analyzing the mineral, free sugar, amino acid, thiosulfinate, and pyruvic acid contents, as well as changes in moisture, ash, browning intensity, and pH to evaluate the effects of thermal processes on garlic. These results might contribute to our understanding of the role of thermal processing on the quality formation of BG.
MATERIALS AND METHODS
Chemicals and reagents
Nitric acid, arabinose, fructose, glucose, sucrose, maltose, o-phthalaldehyde, 2,4 dinitrophenylhydrazine, and pyruvic acid were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Acetonitrile, methanol, and high performance liquid chromatography (HPLC)-grade water were purchased from J.T. Baker (Phillipsburg, NJ, USA). Chemicals and solvents used were of analytical reagent grade.
Sample preparation
Garlic was cultivated in Namhae-gun, Korea. FG bulbs were purchased from the Namhae Bomulsum Agricultural Association (Namhae, Korea) in 2015. BG was produced in a ripening chamber (MBGAM-1500, Minyoung Steel Co., Ltd., Siheung, Korea), without removing the outer layers, using a programmed stepwise heating schedule, as follows: Step 1, 90°C and 100% relative humidity (RH) for 34 h; Step 2, 60°C and 60% RH for 6 h; Step 3, 75°C and 70% RH for 48 h; Step 4, 70°C and 60% RH for 60 h; and Step 5, 65°C and 50% RH for 192 h. Samples of raw garlic gloves, and BG cloves after each of the five steps, were tested in this study, designated as FG, BG1, BG2, BG3, BG4, and BG5, respectively. To prepare garlic powder, FG and BG were peeled, frozen in liquid nitrogen, and immediately freeze-dried. The resulting lyophilized garlic samples were ground with a mortar and pestle, and the resulting powder was stored in sealed plastic bottles at −20°C until analysis.
Determination of moisture content, ash content, browning intensity, and pH
The moisture contents of the garlic samples were determined after each thermal processing step, before freeze-drying, by measuring the weight loss after 12 h at 105°C in a drying oven (8). Garlic samples (4 g) were accurately weighed, placed in a pre-ignited and tared silica crucible, spread in an even layer, and then ignited by gradually increasing the temperature to 500~600°C until the sample turned white, indicating the absence of carbon. The garlic samples were then cooled in a desiccator and weighed. The total ash content was calculated in mg/100 g of sample. To measure pH and browning intensity, 10 g of the garlic sample (before freeze-drying) was blended in 100 mL of distilled water. The pH was measured using a pH meter (S220 SevenCompact™, Mettler-Toledo, Zürich, Switzerland), and the browning intensity was measured at an absorbance of 420 nm using a spectrophotometer (Epoch, BioTek Instruments Inc., Winooski, VT, USA). Garlic samples from different thermal processing steps were diluted with distilled water to obtain a range of absorbance signals.
Determination of mineral content
A garlic powder sample (0.5 g) was put into a burning cup and 15 mL of pure HNO3 was added. The sample was incinerated in a microwave oven at 200°C and the solution diluted to a certain volume with water. Concentrations were determined using inductively coupled plasma atomic emission spectroscopy (ICP-AES) (9). ICP-AES conditions were as follows: instrument, ICP-AES (Varian Medical Systems International AG, Cham, Switzerland); radio frequency power, 0.7~1.5 kW (1.2~1.3 kW for axial); plasma gas flow rate (Ar), 10.5~15 L/min (radial) and 15 L/min (axial); auxiliary Ar, 1.5 L/min; viewing height, 5~12 mm; copy and reading time, 1~5 s (max. 60 s); and copy time, 3 s (max. 100 s).
Determination of free sugars
Garlic powder samples were passed through a Sep-Pak C18 cartridge (Waters Corporation, Milford, MA, USA), preconditioned with methanol (4 mL) and water (10 mL), to remove interfering compounds (10). Before use, residual water in the cartridge was expelled with air. The first 2 mL of sample was discarded, and the next 1 mL was used for analysis after filtration through a 0.45-μm Millipore filter (Merck Millipore, Billerica, MA, USA). The free sugars were determined using an Agilent 1260 infinity quaternary liquid chromatograph (Hewlett Packard, Wilmington, DE, USA). An Agilent quaternary pump connected to a refractive index detector (Hewlett Packard) was used in combination with a Zorbax carbohydrate column (4.6×250 mm i.d., 5 μm particle size, Agilent Technologies, Palo Alto, CA, USA). The mobile phase, consisting of acetonitrile/water (75:25, v/v), was delivered at a flow rate of 2.0 mL/min. The column temperature was 30°C and 1 μL of sample was injected into the HPLC system. Data analysis was performed using the Chemstation software (Hewlett Packard).
Determination of amino acids
Amino acids in the garlic powder samples were determined using an Agilent 1260 infinity quaternary liquid chromatograph (Hewlett Packard) with a multiple wave-length detector operating at 338 nm (excitation=340 nm). Separation was carried out with a Zorbax Eclipse AAA rapid resolution column (150×4.6 mm i.d., 5 μm particle size, Agilent Technologies). The mobile phase, comprising 40 mM Na2HPO4, pH 7.8 (solvent A) and ACN/MeOH/water 45:45:10 (v/v/v) (solvent B) was used with the following linear gradient profile: 0% B (0~1.9 min), 0~57% B (1.9~18.1 min), 57~100% B (18.1~18.8 min), 100% B (18.8~22.3 min), 100~0% B (22.3~23.2 min), and 0% B (23.2~26 min) was applied at a flow rate of 2.0 mL/min. The column was equilibrated for 5 min under initial conditions prior to injection of the next sample. The column temperature was 40°C. Determination of sample amino acid content was facilitated using pre-column derivatization with o-phthalaldehyde, and 0.5 μL portions were injected into the HPLC system. Data analysis was performed using the Chemstation software (Hewlett Packard).
Determination of thiosulfinate
The amount of thiosulfinate in the garlic powder samples was determined by the method of Samaniego-Esguerra et al. (11) with slight modifications. The method is based on the extraction of thiosulfinate into hexane and observation of the solution extinction at 254 nm. A garlic powder sample (0.5 g) was weighed into a 100 mL Erlenmeyer flask and 25 mL of distilled water was added. The sample was reconstituted for 10 min by shaking gently. The mixture was filtered using a white band filter paper. The clear supernatant (5 mL) was measured into a 100 mL Erlenmeyer flask. Thiosulfinate was extracted by adding 10 mL hexane, swirling the mixture gently, and separating the hexane layer. The aqueous layer was returned to the flask, and the remaining thiosulfinate was extracted with 5 mL hexane. The first and second extracts were combined, and the absorbance of the solution was measured at 254 nm. The molar absorptivity of thiosulfinate solution at 254 nm was found to be ɛ=0.014 g/μmol cm according to Samaniego-Esguerra et al. (11). The thiosulfinate content of the hexane solution was calculated using the following equation:
where A is the absorbance, b is the path length (cm), and C is the solution concentration (μmol/g).
Determination of pyruvic acid
A garlic sample (0.5 g, before freeze-drying) was weighed into a 100 mL Erlenmeyer flask, and 15 mL of diluted trichoroacetic acid/water (1:20) was added to inactivate the alliinase enzyme, before blending the mixture in a Vorwerk blender (Vorwerk & Co., Wuppertal, Germany) at maximum speed for 3 min. After maceration for 1 h, the mixture was filtered, and the filtrate was diluted (1: 10) and analyzed for pyruvic acid. Each reaction tube contained 1 mL of diluted filtrate, 1 mL of distilled water, and 1 mL of 2,4-dinitrophenylhydrazine (in 2 M HCl, 125 mg/L). Reaction tubes were vortexed and placed in a water bath at 37°C for 10 min. After the incubation period, 5 mL of 0.6 M NaOH was added, and the tubes were vortexed for 5 min. Pyruvic acid was measured using a spectrophotometer (Epoch, BioTek Instruments Inc.) at 490 nm, and a standard curve was obtained from pure pyruvic acid in water (Sigma Chemical Co.).
Statistical analysis
Each experiment was performed in triplicate. Data are reported as the mean±standard deviation and were analyzed by SPSS (version 20.0, IBM Inc., Armonk, NY, USA). An analysis of variance (ANOVA) and Duncan’s multiple range test were used to determine the significance of differences among the means, and P<0.05 was considered significant.
RESULTS AND DISCUSSION
Main characteristic parameters in garlic after different thermal processing steps
The main characteristic parameters of garlic after the different thermal processing steps are presented in Table 1. The moisture content of garlic cloves was significantly lower than that of FG (P<0.05). The moisture content of BG significantly decreased from 58.48% to 39.03% during thermal processing, when compared with 62.31% for FG (P<0.05). The moisture content was the lowest in BG4 (39.03%). The ash contents of BG4 and BG5 were significantly higher than that of other samples (P<0.05). The ash content increased by 1.5-fold in BG cloves compared with FG. The remarkable increase in the ash contents of BG may be due to the reduced moisture content during thermal processing. Browning intensity increased from 0.14 to 1.93 during thermal processing (P<0.05). The increase was gradual, from 0.14 to 0.79, until BG3, and then sharply increased to 1.93 in BG5. The browning intensity was up to 13-fold higher in the BG cloves than in FG. The UV absorbance at 420 nm is often used as an indicator of the extent to which the Maillard reaction has taken place in foods, representing the final stages of the browning reaction. This intensity is the most convenient measurable index of the Maillard reaction because it can be estimated visually (12). In addition, the pH of BG significantly decreased from 5.27 to 4.01 during thermal processing, compared with 6.29 for FG (P<0.05). This result was in agreement with the report of Shin et al. (13), which showed that the pH of BG decreased during thermal processing. The decrease in pH for heated garlic samples was, in part, associated with the production of browning compounds upon heat treatment during the BG manufacturing process (14).
Table 1.
Main characteristic parameters of garlic after different thermal processing steps
| Parameter | FG | BG1 | BG2 | BG3 | BG4 | BG5 |
|---|---|---|---|---|---|---|
| Moisture (%) | 62.31±0.57a | 58.48±1.18b | 55.52±0.65c | 53.75±0.63d | 39.03±0.01f | 43.06±0.42e |
| Ash (mg/100 g) | 73.59±0.89b | 75.36±0.02b | 76.61±1.95b | 78.40±0.45b | 114.98±8.07a | 114.36±8.65a |
| Browning intensity | 0.14±0.01f | 0.43±0.01e | 0.61±0.01d | 0.79±0.02c | 1.42±0.04b | 1.93±0.04a |
| pH | 6.29±0.06a | 5.27±0.05b | 4.93±0.06c | 4.40±0.05d | 4.01±0.03f | 4.22±0.09e |
Values are mean±SD (n=5).
FG, raw garlic cloves; BG1, black garlic cloves at step 1; BG2, black garlic cloves at step 2; BG3, black garlic cloves at step 3; BG4, black garlic cloves at step 4; BG5, black garlic cloves at step 5.
Different letters (a–f) within a row indicate significant differences at P<0.05.
Determination of mineral contents
The mineral compositions of garlic after different thermal processing steps are presented in Table 2. The mineral profile of garlic showed that it contains potassium as the major mineral with the largest quantity (6,049 mg/100 g), followed by sulfur (1,138 mg/100 g), magnesium (276.47 mg/100 g), sodium (246.34 mg/100 g), and calcium (103.91 mg/100 g). In addition, zinc, potassium, magnesium, iron, manganese, phosphorous, and selenium contents increased, while copper, sodium, calcium, and sulfur contents initially increased, and later decreased, during thermal processing. Potassium, magnesium, and calcium are important for preventing and treating hypertension and their high intake may help decrease coronary heart disease and stroke (15). Furthermore, other minerals, such as phosphorous, iron, zinc, manganese, copper, and selenium were present in low quantities 14.65, 12.31, 7.69, 3.13, 1.95, and 0.45 mg/100 g, respectively. The sum of minerals in each of the garlic samples was the highest in BG2 (1,358.94 mg/100 g). The total mineral content in BG2 was significantly higher than those of the other samples (P<0.05). According to Andreini et al. (16), some transition metals, including iron, zinc, manganese, and copper, are essential for life due to their function as both, structural and catalytic cofactors for proteins. Zinc supplementation in children between 3 months and 5 years of age reduces the frequency and severity of diarrhea and respiratory illnesses (17). Selenium functions as a dietary antioxidant and, thus, has been studied for its possible role in chronic diseases (18).
Table 2.
Mineral composition of garlic after different thermal processing steps
| Minerals (mg/100 g) | FG | BG1 | BG2 | BG3 | BG4 | BG5 | Total1) |
|---|---|---|---|---|---|---|---|
| Cu | 0.27±0.01d | 0.26±0.01d | 0.26±0.01d | 0.34±0.01b | 0.50±0.01a | 0.32±0.01c | 1.95±0.01fg |
| Zn | 1.16±0.03d | 1.22±0.03c | 1.24±0.03c | 1.35±0.03b | 1.45±0.03a | 1.27±0.03c | 7.69±0.03fg |
| K | 907.35±20.63b | 1,051.53±23.92a | 1,020.09±23.19a | 1,028.29±23.38a | 1,024.20±23.29a | 1,018.44±23.17a | 6,049.9±22.93a |
| Na | 22.00±0.50d | 22.39±0.51d | 68.53±1.56a | 49.16±1.12b | 48.75±1.11b | 35.51±0.81c | 246.34±0.94d |
| Mg | 43.23±0.98c | 46.09±1.05b | 46.16±1.05b | 46.25±1.05b | 46.05±1.05b | 48.69±1.11a | 276.47±1.05c |
| Ca | 12.20±0.28d | 18.49±0.42b | 19.62±0.45a | 18.60±0.42b | 19.76±0.45a | 15.24±0.35c | 103.91±0.40e |
| Fe | 1.60±0.04d | 1.99±0.05c | 2.00±0.05c | 2.11±0.05b | 2.34±0.53a | 2.27±0.52a | 12.31±0.21fg |
| Mn | 0.44±0.01b | 0.54±0.01a | 0.53±0.01a | 0.54±0.01a | 0.54±0.01a | 0.54±0.01a | 3.13±0.01fg |
| P | 2.15±0.05d | 2.46±0.06c | 2.37±0.05c | 2.37±0.05c | 2.73±0.06a | 2.57±0.06b | 14.65±0.06f |
| Se | 0.05±0.001d | 0.08±0.002c | 0.01±0.001e | 0.10±0.002b | 0.08±0.002c | 0.13±0.003a | 0.45±0.002g |
| S | 183.05±4.16cd | 192.66±4.38ab | 198.13±4.50a | 195.63±4.45ab | 179.26±4.08d | 189.70±4.31bc | 1,138.43±4.31b |
| Total2) | 1,173.50±2.43f | 1,337.71±2.77c | 1,358.94±2.81a | 1,344.74±2.78b | 1,325.66±2.78d | 1,314.68±2.76e |
Values are mean±SD (n=3).
FG, raw garlic cloves; BG1, black garlic cloves at step 1; BG2, black garlic cloves at step 2; BG3, black garlic cloves at step 3; BG4, black garlic cloves at step 4; BG5, black garlic cloves at step 5.
Different letters (a–g) within a row indicate significant differences at P<0.05.
Sum of individual mineral identified in the garlic at different thermal processing steps.
Sum of each mineral in each of the garlic at different thermal processing steps.
Determination of free sugars
The free sugar content of garlic after different thermal processing steps is presented in Table 3. The free sugar content of garlic during thermal processing showed that fructose was the major sugar in the largest quantity (11,708.01 mg/100 g), followed by sucrose (1,338.11 mg/100 g), glucose (954.64 mg/100 g), arabinose (438.55 mg/100 g), and maltose (197.3 mg/100 g). Moreover, the FG sample exhibited levels of sucrose that were higher than the other sugars, while the levels of fructose were the highest in BG. These results are consistent with those obtained by Atashi et al. (19) and Shin et al. (20), who reported that sucrose was present in the highest quantity, followed by fructose and glucose. The free sugar content of BG4 was significantly higher than that of the other samples (P<0.05). The free sugar content was up to 16-fold higher in the BG cloves than in FG. The free sugar content increased and then decreased during thermal processing (P<0.05). This result is in agreement with Choi et al. (21), who showed that sugar content (e.g., glucose, fructose, sucrose, and maltose) was higher in BG compared with fresh and steamed garlic. Degradation of polysaccharides can occur under acidic and high-temperature conditions (22). In plants, degradation of cell wall polysaccharides causes tissue softening; therefore, the BG texture is more gum-like than that of raw garlic. The decrease in pH and heat treatment during thermal processing could promote further hydrolysis of sucrose to glucose and fructose, which may also be supported by the increase in glucose and fructose contents accompanying the reduced sucrose levels in BG. Moreover, the sweet taste of BG might be related to its increased sugar content.
Table 3.
Free sugar contents of garlic after different thermal processing steps
| Free sugar (mg/100 g) | FG | BG1 | BG2 | BG3 | BG4 | BG5 | Total1) |
|---|---|---|---|---|---|---|---|
| Arabinose | 51.11±5.95d | 18.45±1.70f | 33.04±4.92e | 73.13±6.04c | 148.36±5.66a | 114.46±15.63b | 438.55±6.65d |
| Fructose | 31.40±0.96f | 486.75±11.72e | 1,181.31±27.30d | 2,751.34±22.28c | 3,873.98±22.14a | 3,383.23±44.03b | 11,708.01±21.41a |
| Glucose | 16.65±0.74e | 49.23±2.98d | 53.94±2.80d | 120.91±4.33c | 492.06±42.61a | 221.85±11.53b | 954.64±10.83c |
| Sucrose | 181.72±1.58e | 196.92±3.48d | 289.08±2.89b | 306.89±5.55a | 120.63±4.17f | 242.87±18.05c | 1,338.11±5.95b |
| Maltose | 11.66±0.82e | 3.16±0.36f | 19.60±1.15d | 23.69±1.70c | 91.01±4.13a | 48.18±4.38b | 197.3±2.09e |
| Total2) | 292.54±2.01f | 754.51±4.05e | 1,576.97±7.81d | 3,275.96±7.98c | 4,726.04±15.74a | 4,010.59±18.72b |
Values are mean±SD (n=5).
FG, raw garlic cloves; BG1, black garlic cloves at step 1; BG2, black garlic cloves at step 2; BG3, black garlic cloves at step 3; BG4, black garlic cloves at step 4; BG5, black garlic cloves at step 5.
Different letters (a–f) within a row indicate significant differences at P<0.05.
Sum of individual sugar identified in the garlic at different thermal processing steps.
Sum of each sugar in each of the garlic at different thermal processing steps.
Determination of amino acids
The amino acid contents of garlic after different thermal processing steps are presented in Table 4. The results showed that BG contains tryptophan as the major amino acid in the highest quantity (615.63 mg/100 g), followed by histidine (494.11 mg/100 g), valine (261.40 mg/100 g), glycine (223.02 mg/100 g), arginine (183.81 mg/100 g), and aspartic acid (115.44 mg/100 g). Furthermore, other amino acids, such as leucine, threonine, glutamine, asparagine, cysteine, and lysine, were present in the lowest quantities of 96.24, 73.85, 69.34, 56.50, 41.91, and 17.42 mg/100 g, respectively. The amino acid content increased in the first step (BG1) of thermal processing and decreased again during the later steps of thermal processing. The increased amino acid content, including aspartic acid, threonine, arginine, and leucine, was most likely due to the degradation of proteins or peptides, which may result from enzymatic hydrolysis or non-enzymatic hydrolysis, such as pyrolysis (23). In addition, the pH of BG decreased from 5.27 to 4.01 during thermal processing, compared with 6.29 for FG (Table 1), which may have led to further enzymatic hydrolysis of proteins under acidic conditions. However, asparagine, glutamine, glycine, valine, and tryptophan contents decreased during thermal processing, perhaps caused by reactions with reducing sugars, such as the Maillard reaction. The decreased level of L-tryptophan in BG was likely due to consumption of L-tryptophan in the chemical reaction to produce carboline, which has been reported in aged garlic (24). During this reaction, L-tryptophan reacts with an aldehyde or an α-oxo acid, such as pyruvic acid, produced from the Maillard reaction or from alliin metabolism, via Pictet-Spengler condensation, to form tetrahydro-β-carboline derivatives (24).
Table 4.
Free amino acids content of garlic after different thermal processing steps
| Amino acids (mg/100 g) | FG | BG1 | BG2 | BG3 | BG4 | BG5 | Total1) |
|---|---|---|---|---|---|---|---|
| Aspartic acid | 23.64±1.52c | 40.37±0.92a | 27.59±4.37b | 9.94±0.42e | 13.90±0.55d | – | 115.44±1.56f |
| Glutamic acid | –3) | – | – | – | – | – | – |
| Asparagin | 56.50±1.32a | – | – | – | – | – | 56.50±1.32i |
| Glutamine | 17.35±0.45a | 8.62±0.79c | 14.23±0.95b | 6.83±0.42d | 14.00±0.76b | 8.31±0.91c | 69.34±0.71h |
| Cystine | 2.51±0.14e | 6.62±0.34c | 5.89±0.68c | 4.86±0.27d | 8.50±0.47b | 13.53±1.00a | 41.91±0.48j |
| Histidine | 111.46±1.61b | 55.70±0.72c | 43.69±1.08d | 25.62±0.42e | 230.23±6.43a | 27.41±0.52e | 494.11±2.16b |
| Glycine | 157.75±5.56a | 13.71±0.47c | 13.42±0.59c | 8.65±0.61d | 10.86±0.48cd | 18.63±0.91b | 223.02±1.72d |
| Threonine | 2.17±0.07f | 21.96±0.07a | 18.31±0.12b | 13.47±0.26c | 9.62±0.09d | 8.32±0.49e | 73.85±0.22h |
| Lysine | 3.37±0.10b | 0.55±0.04d | 5.55±0.29a | 3.54±0.82b | 1.99±0.09c | 2.42±0.26c | 17.42±0.32k |
| Arginine | 30.78±2.38c | 43.64±1.87a | 35.46±3.96b | 26.76±2.88c | 21.07±1.64d | 26.10±1.62c | 183.81±2.87e |
| Alanine | – | – | – | – | – | – | – |
| Valine | 216.47±31.53a | 12.95±1.26b | 10.81±0.87b | 8.23±0.20b | 5.82±0.47b | 7.12±0.53b | 261.40±6.97c |
| Tryptophan | 203.03±0.12a | 124.75±3.54b | 108.61±12.77c | 66.54±5.33d | 56.89±11.29d | 55.81±3.44d | 615.63±7.30a |
| Leucine | 18.08±0.24c | 34.23±1.50a | 26.15±2.13b | 17.78±0.53c | – | – | 96.24±1.10g |
| Total2) | 843.11±3.75a | 363.10±1.05c | 309.71±2.53d | 192.22±1.11e | 372.88±2.23b | 167.65±1.08f |
Values are mean±SD (n=5).
FG, raw garlic cloves; BG1, black garlic cloves at step 1; BG2, black garlic cloves at step 2; BG3, black garlic cloves at step 3; BG4, black garlic cloves at step 4; BG5, black garlic cloves at step 5.
Different letters (a–f) within a row indicate significant differences at P<0.05.
Sum of individual sugar identified in the garlic at different thermal processing steps.
Sum of each sugar in each of the garlic at different thermal processing steps.
Not detected (limit of detection: 0.5 mg/100 g).
Determination of thiosulfinate
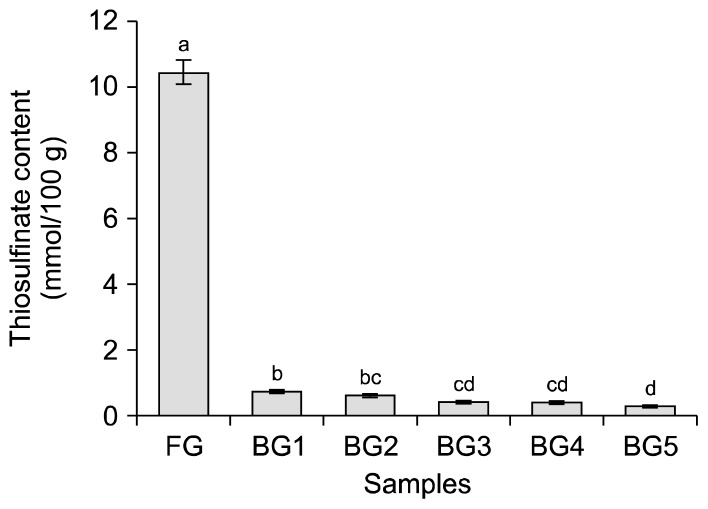
The thiosulfinate contents of garlic samples after different thermal processing steps are presented in Fig. 1. Thiosulfinate is the main flavor substance of FG. The thiosulfinate content decreased in all of the samples during the thermal processing. Therefore, in BG, the offensive odor of FG was removed. The thiosulfinate content of FG was 10.47±0.35 mmol/100 g, similar to the results of Kinalski and Noreña (25). The thiosulfinate content rapidly decreased in the first step of thermal processing (BG1). The thiosulfinate content of BG samples declined to 0.34 mmol/100 g in BG5, significantly lower than that of FG. The amount of thiosulfinate decreased by a 30-fold in BG when compared with FG. Hua and Huang (26) suggested that the heating process reduced the thiosulfinate content due to the Maillard reaction involving fructose, fructan, and other carbohydrates in garlic samples. In addition, Zhang et al. (27) suggested that another probable reason for the decrease of thiosulfinate in the BG sample was its conversion into S-allyl cysteine (SAC), S-allylmercapto-cysteine, arginine, and other undefined compounds, when subjected to thermal processing. Likewise, the alliinase released from cell vacuoles from garlic tissues during thermal processing could degrade thiosulfinate into cytotoxic and odoriferous alkyl alkane-thiosulfinates, such as allicin (28). These results suggest that BG had very little odor, and that allicin was not a significant functional substance in BG.
Fig. 1.
Thiosulfinate contents of garlic samples after different thermal processing steps. Different letters (a–d) above the bars are significantly different at P<0.05, as analyzed by Duncan’s multiple range test. FG, raw garlic cloves; BG1, black garlic cloves at step 1; BG2, black garlic cloves at step 2; BG3, black garlic cloves at step 3; BG4, black garlic cloves at step 4; BG5, black garlic cloves at step 5.
Determination of pyruvic acid
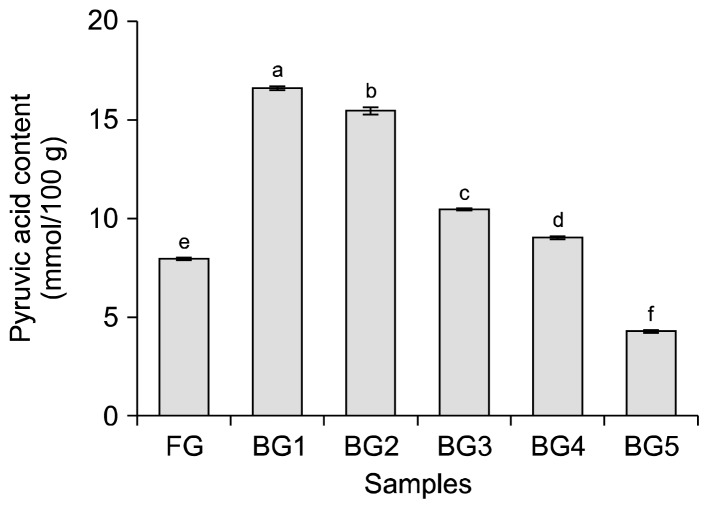
The pyruvic acid contents of garlic after different thermal processing steps are presented in Fig. 2. The proportion of pyruvic acid or thiosulfinate, the products of enzymatic activity of alliinase on the flavor precursor (S-alkyl-L-cysteine sulfoxide) in Allium species, is mainly responsible for the flavor intensity or pungency of this species. The pyruvic acid content increased and then decreased during thermal processing (P<0.05), rapidly increasing to about 16.65 mmol/100 g in the first step of thermal processing (BG1), and then decreasing to 4.25 mmol/100 g in BG5. Gallina et al. (29) reported that the pyruvic acid content of Allium species was highly correlated with their pungency. According to Russo et al. (30), the flavor of Allium species is closely linked to pungency and, thus, to pyruvic acid content. Therefore, these results suggest that the pyruvic acid content is affected by thermal processing, and that the low pyruvic acid content in BG makes the garlic sweeter and less spicy than FG.
Fig. 2.
Pyruvic acid contents of garlic after different thermal processing steps. Different letters (a–f) above the bars are significantly different at P<0.05, as analyzed by Duncan’s multiple range test. FG, raw garlic cloves; BG1, black garlic cloves at step 1; BG2, black garlic cloves at step 2; BG3, black garlic cloves at step 3; BG4, black garlic cloves at step 4; BG5, black garlic cloves at step 5.
In conclusion, the purpose of this study was to investigate mineral, free sugar, amino acid, thiosulfinate, and pyruvic acid contents, and the changes in moisture, ash, browning intensity, and pH to evaluate the effects of thermal processes in garlic. Compared with FG, the moisture content and pH in BG decreased significantly, while the ash content and browning intensity increased during thermal processing. The total mineral and free sugar contents were significantly higher than that of the other samples for the BG2 and BG4 samples. The free sugar content increased by 16-fold in the BG cloves compared with that of FG, while the amino acid content increased during the first stage of thermal processing, and subsequently decreased. The thiosulfinate content in all of the samples decreased during thermal processing. The pyruvic acid content initially increased and then decreased during thermal processing. These results contribute to our understanding of the role of thermal processing in the quality formation of BG.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Butt MS, Sultan MT, Butt MS, Iqbal J. Garlic: nature’s protection against physiological threats. Crit Rev Food Sci Nutr. 2009;49:538–551. doi: 10.1080/10408390802145344. [DOI] [PubMed] [Google Scholar]
- 2.Ye HY, Zhang ZY. Study progress on immune regulation effect of garlic. J Chin Med Pharmacol. 2003;3:54–56. [Google Scholar]
- 3.Kim JH, Nam SH, Rico CW, Kang MY. A comparative study on the antioxidative and anti-allergic activities of fresh and aged black garlic extracts. Int J Food Sci Technol. 2012;47:1176–1182. doi: 10.1111/j.1365-2621.2012.02957.x. [DOI] [Google Scholar]
- 4.Corzo-Martínez M, Corzo N, Villamiel M. Biological properties of onions and garlic. Trends Food Sci Technol. 2007;18:609–625. doi: 10.1016/j.tifs.2007.07.011. [DOI] [Google Scholar]
- 5.Bae SE, Cho SY, Won YD, Lee SH, Park HJ. A comparative study of the different analytical methods for analysis of S-allyl cysteine in black garlic by HPLC. LWT-Food Sci Technol. 2012;46:532–535. doi: 10.1016/j.lwt.2011.11.013. [DOI] [Google Scholar]
- 6.Gorinstein S, Leontowicz M, Leontowicz H, Najman K, Namiesnik J, Park YS, Jung ST, Kang SG, Trakhtenberg S. Supplementation of garlic lowers lipids and increases antioxidant capacity in plasma of rats. Nutr Res. 2006;26:362–368. doi: 10.1016/j.nutres.2006.06.008. [DOI] [Google Scholar]
- 7.Ide N, Lau BHS. Aged garlic extract attenuates intracellular oxidative stress. Phytomedicine. 1999;6:125–131. doi: 10.1016/S0944-7113(99)80047-6. [DOI] [PubMed] [Google Scholar]
- 8.AOAC. Official methods of analysis of AOAC. 17th ed. Association of Official Analytical Chemists; Washionton DC, USA: 2000. Moisture in soft-moist and semi-moist pet foods: Method number 991.02. [Google Scholar]
- 9.Skunjins S. Handbook for ICP-AES (Varian-Vista) Varian Int; Cham, Switzerland: 1998. A short guide to vista series ICP-AES operation; p. 29. [Google Scholar]
- 10.Navarro JM, Martínez VV, Carvajal M. Ammonium, bicarbonate and calcium effects on tomato plants grown under saline conditions. Plant Sci. 2000;157:89–96. doi: 10.1016/S0168-9452(00)00272-7. [DOI] [PubMed] [Google Scholar]
- 11.Samaniego-Esguerra CM, Boag IF, Robertson GL. Kinetics of quality deterioration in dried onions and green beans as a function of temperature and water activity. LWT-Food Sci Technol. 1991;24:53–58. [Google Scholar]
- 12.Yilmaz Y, Toledo R. Antioxidant activity of water-soluble Maillard reaction products. Food Chem. 2005;93:273–278. doi: 10.1016/j.foodchem.2004.09.043. [DOI] [Google Scholar]
- 13.Shin JH, Choi DJ, Chung MJ, Kang MJ, Sung NJ. Changes of physicochemical components and antioxidant activity of aged garlic at different temperatures. J Korean Soc Food Sci Nutr. 2008;37:1174–1181. doi: 10.3746/jkfn.2008.37.9.1174. [DOI] [Google Scholar]
- 14.Bae SE, Cho SY, Won YD, Lee SH, Park HJ. Changes in S-allyl cysteine contents and physicochemical properties of black garlic during heat treatment activity. LWT-Food Sci Technol. 2014;55:397–402. doi: 10.1016/j.lwt.2013.05.006. [DOI] [Google Scholar]
- 15.Houston MC, Harper KJ. Potassium, magnesium, and calcium: their role in both the cause and treatment of hypertension. J Clin Hypertens. 2008;10:3–11. doi: 10.1111/j.1751-7176.2008.08575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM. Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem. 2008;13:1205–1218. doi: 10.1007/s00775-008-0404-5. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal R, Sentz J, Miller MA. Role of zinc administration in prevention of childhood diarrhea and respiratory illnesses: a meta-analysis. Pediatrics. 2007;119:1120–1130. doi: 10.1542/peds.2006-3481. [DOI] [PubMed] [Google Scholar]
- 18.Boosalis MG. The role of selenium in chronic disease. Nutr Clin Pract. 2008;23:152–160. doi: 10.1177/0884533608314532. [DOI] [PubMed] [Google Scholar]
- 19.Atashi S, Akbarpour V, Mashayekhi K, Mousavizadeh SJ. Garlic physiological characteristics from harvest to sprouting in response to low temperature. J Stored Prod Postharvest Res. 2011;2:285–291. [Google Scholar]
- 20.Shin JH, Lee SJ, Jung WJ, Kang MJ, Sung NJ. Physicochemical characteristics of garlic (Allium sativum L.) on collected from the different regions. J Agric Life Sci. 2011;45:103–114. [Google Scholar]
- 21.Choi DJ, Lee SJ, Kang MJ, Cho HS, Sung NJ, Shin JH. Physicochemical characteristics of black garlic (Allium sativum L.) J Korean Soc Food Sci Nutr. 2008;37:465–471. doi: 10.3746/jkfn.2008.37.4.465. [DOI] [Google Scholar]
- 22.Mankarios AT, Jones CFG, Jarvis MC, Threfall DR, Friend J. Hydrolysis of plant polysaccharides and GLC analysis of their constituent neutral sugars. Phytochemistry. 1979;18:419–422. doi: 10.1016/S0031-9422(00)81879-8. [DOI] [Google Scholar]
- 23.Liang T, Wei F, Lu Y, Kodani Y, Nakada M, Miyakawa T, Tanokura M. Comprehensive NMR analysis of compositional changes of black garlic during thermal processing. J Agric Food Chem. 2015;63:683–691. doi: 10.1021/jf504836d. [DOI] [PubMed] [Google Scholar]
- 24.Ichikawa M, Yoshida J, Ide N, Sasaoka T, Yamaguchi H, Ono K. Tetrahydro-β-carboline derivatives in aged garlic extract show antioxidant properties. J Nutr. 2006;136:726S–731S. doi: 10.1093/jn/136.3.726S. [DOI] [PubMed] [Google Scholar]
- 25.Kinalski T, Noreña CPZ. Effect of blanching treatments on antioxidant activity and thiosulfinate degradation of garlic (Allium sativum L.) Food Bioprocess Technol. 2014;7:2152. doi: 10.1007/s11947-014-1282-1. [DOI] [Google Scholar]
- 26.Hua XF, Huang XS. Factors of affecting the allium’s Maillard reaction. Food Sci Tech. 2006;7:156–158. [Google Scholar]
- 27.Zhang M, Lei N, Zhu T, Zhang Z. Thermal processing effects on the chemical constituent and antioxidant activity of S-alk(en)ylcysteine S-oxides (alliin) extract. LWT-Food Sci Technol. 2013;51:309–313. doi: 10.1016/j.lwt.2012.09.024. [DOI] [Google Scholar]
- 28.Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001;131:955S–962S. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 29.Gallina PM, Cabassi G, Maggioni A, Natalini A, Ferrante A. Changes in the pyruvic acid content correlates with phenotype traits in onion clones. Aust J Crop Sci. 2012;6:36–40. [Google Scholar]
- 30.Russo M, di Sanzo R, Cefaly V, Carabetta S, Serra D, Fuda S. Non-destructive flavour evaluation of red onion (Allium cepa L.) ecotypes: an electronic-nose-based approach. Food Chem. 2013;141:896–899. doi: 10.1016/j.foodchem.2013.03.052. [DOI] [PubMed] [Google Scholar]