Abstract
The present study was conducted to investigate the ginsenoside profiles of the main root, root hair, and leaf of ginseng in order to demonstrate their possible application in medicine. The total ginsenoside content of the leaf was up to 12 times than that in the main root, and the content of protopanaxadiol groups was higher than that of protopanaxatriol groups in all the samples. The leaf was shown to contain high amounts of ginsenosides Rb3 and Rh1, whereas the main root contained large amounts of ginsenosides Rb1 and Rc. Moreover, Rb2, Rb3, and Rg1 were only detected in the root hair, leaf, and main root, respectively. The ginsenoside Re content of Panax ginseng leaf and root hair was 2.6~4 times higher than that of the main root. Therefore, the results indicate that the ginsenoside content of Panax ginseng is higher in the leaf and root hair, and lower in the main root.
Keywords: Panax ginseng, ginsenosides, different parts
INTRODUCTION
Ginseng is a slow-growing perennial plant of the Panax genus that has been used as a medicinal plant and a functional food for more than 2,000 years (1). The Panax genus has several species, with Asian (Panax ginseng C.A. Meyer) and American (Panax quinquefolius L.) ginsengs being the 2 most commonly used species, although these species differ in chemical composition and bioactivity (2, 3).
Ginsenosides are the main active constituents in Panax ginseng, and the main ginsenosides can be derived from every part of the plant. More than 30 ginsenosides have been isolated from Panax ginseng, most of which contain one of 4 aglycone moieties, i.e., protopanaxadiol (PPD), protopanaxatriol (PPT), ocotillol, or oleanolic acid (4,5). Panax ginseng root is considered the most important part of the plant for medicinal purposes, and most studies on its ginsenosides have focused on the root. However, several studies on the ginsenoside contents of the other parts of Panax ginseng have been reported (6–10), and there are some studies on the variation of ginsenoside structures in different parts of the Panax species (11–14).
Although the most commonly used parts of ginseng are the roots and rhizomes, ginsenosides are also present in the aerial parts of Panax species. However, the underground and aerial parts generally show different ginsenoside profiles. The components and pharmacological properties of ginseng leaf, however, are not completely understood. More interestingly, some reports indicate that the content of active components in the leaf is higher than that in the root (15). However, the leaf is almost completely neglected commercially in comparison to the root, despite there being considerable potential for its use (16). Consequently, the present study is intended to provide a detailed report on the ginsenoside profiles of the main root, root hair, and leaf of ginseng in order to demonstrate their possible application in medicine.
MATERIALS AND METHODS
Chemicals and reagents
The standards of ginsenosides Rg1, Re, Rf, Rb1, Rg2, Rc, Rh1, Rb2, Rb3, Rd, Rg3, and Rh2 were purchased from Ambo Institute (Daejeon, Korea). Acetonitrile and high performance liquid chromatography (HPLC)-grade water were purchased from J.T. Baker (Phillipsburg, NJ, USA). A solid-phase extraction (SPE) column (Bond Elut Plexa Cartridge 6 mL/200 mg, Agilent Technologies, Inc., Santa Clara, CA, USA) was used to purify and concentrate the samples. All other chemicals used were of analytical reagent grade.
Plant material
Approximately 20 kg of six-year-old ginseng plants (Chunpoong cultivar) were obtained from the Gaeseong Ginseng Cooperative Association in Gyeonggi, Korea in late September 2012. The average temperature and precipitation of the area in Gyeonggi throughout the year was 10.5°C and 1,300 mL, respectively. The ginseng was divided into main root, root hair, and leaf, which were freeze-dried and stored at −70°C.
Analysis of ginsenosides
Ultrasonic extraction
A freeze-dried (1.0 g) sample was accurately weighed, placed in a 25 mL conical flask, and 10 mL of a 70% (v/v) ethanol solution was added. The flask was then placed into an ultrasonic extractor for 1 h at 40°C. After the extraction was finished, the extract in the conical flask was cooled to room temperature, filtered, and evaporated on a rotary evaporator at 60°C to remove the ethanol. The residue was then dissolved in 1.0 mL methanol and the solution was filtered through a 0.22 μm filter (17).
SPE column purification
SPE was performed using a Varian Vac Elute SPS 24 vacuum manifold (Varian Inc., Walnut Grove, CA, USA). The SPE cartridge (Bond Elut Plexa Cartridge 6 mL/200 mg, Agilent Technologies, Inc.) was conditioned with 6 mL of methanol and 6 mL of water before introduction of the sample solution. A 1.0 mL aliquot of the sample solution was introduced into the SPE cartridge at a flow rate of 0.1 mL/min. Then, a washing step was performed with 1.0 mL of ultra-pure water at the same flow rate. The retained analytes were eluted with 1.0 mL of methanol at a flow rate of 0.08 mL/min. The eluate solution was filtered through a 0.22 μm filter prior to analysis.
Determination of ginsenosides by HPLC
The ginsenosides were determined by the method of Dong et al. (18) with slight modifications. Analyses were performed using an Agilent 1260 liquid chromatographer (Hewlett Packard, Wilmington, NC, USA) equipped with a quaternary gradient pump and a multiple wavelength detector operating at 203 nm. The samples were separated on a Zorbax Eclipse XDB-C18 column (4.6 mm×150 mm, 5 μm, Agilent Technologies, Inc.) at 35°C with a sample injection volume of 30 μL. The mobile phase was a gradient of water (A) and acetonitrile (B). The following gradient was used: 20% B (0 min), 20% (0~10 min), 32% (10~40 min), 50% (40~55 min), 65% (55~70 min), 90% (70~82 min), and 80% (82~90 min). Data analysis was performed using the Chemstation software (Hewlett Packard). The flow rate of the mobile phase was 0.9 mL/min.
Preparation of standard solutions
Stock solutions containing ginsenosides Rg1, Re, Rf, Rb1, Rg2, Rc, Rh1, Rb2, Rb3, Rd, Rg3, and Rh2 were prepared with methanol. A series of standard operating solutions of different concentrations were obtained by diluting the standard stock solutions.
Statistical analysis
Each experiment was performed in triplicate. The data are reported as the mean±standard deviation and were analyzed by SPSS (version 17.0, IBM Inc., Armonk, NY, USA). Aanalysis of variance (ANOVA) and Duncan’s multiple range test were used to determine the significance of differences among the means, and P<0.05 was considered significant.
RESULTS AND DISCUSSION
SPE purification
SPE is widely used because it can reduce the detection limit of a method significantly through the enrichment of analytes. Furthermore, it is fast, accurate, convenient, and effective. SPE conditions for this process were previously studied in our laboratory, and the optimal conditions were used in these experiments.
Analytical performance of HPLC
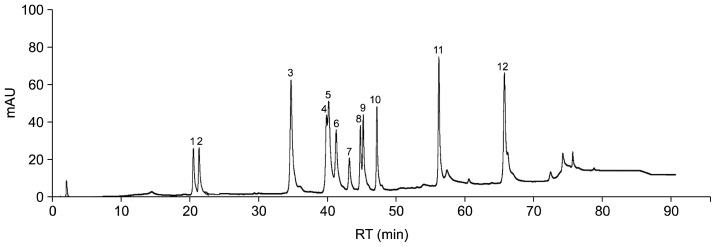
Ginsenosides Rg1, Re, Rf, Rb1, Rg2, Rc, Rh1, Rb2, Rb3, Rd, Rg3, and Rh2 in the extracts were identified by comparison of the retention times with authentic ginsenoside standards obtained from the chromatograms of the mixed standards (Fig. 1). The relationships between the analyte concentrations and measured signals for 12 ginsenosides are listed in Table 1. The linearity of the calibration curves was verified by the correlation coefficients, the results of which are also shown Table 1. The injection precision was obtained by analyzing the peak area variations of 6 injections of a mixture of 12 ginsenoside standards. All the correlation coefficients were high, indicating good linearity. All these data indicate that this method is accurate and sensitive.
Fig. 1.
Typical HPLC chromatogram of 12 ginsenoside standards analyzed at 203 nm. Retention times are presented above the peaks of individual ginsenosides. Identified peaks: Rg1 (1), Re (2), Rf (3), Rb1 (4), Rg2 (5), Rc (6), Rh1 (7), Rb2 (8), Rb3 (9), Rd (10), Rg3 (11), and Rh2 (12).
Table 1.
Calibration curve and concentration range of 12 ginsenosides
| Ginsenosides | Calibration curve1) | r2 | Concentration range (mg/mL) |
|---|---|---|---|
| Rb1 | Y=307.78X+20.9172 | 0.94020 | 0.020~0.050 |
| Rb2 | Y=311.70X−8.6442 | 0.98898 | 0.020~0.050 |
| Rb3 | Y=408.37X−5.3101 | 0.99580 | 0.020~0.050 |
| Rc | Y=222.83X−9.2894 | 0.94847 | 0.025~0.050 |
| Rd | Y=450.26X−10.4182 | 0.98411 | 0.025~0.050 |
| Rg3 | Y=533.92X−4.8735 | 0.99646 | 0.030~0.050 |
| Rh2 | Y=312.60X+13.0997 | 0.97521 | 0.030~0.050 |
| Rg1 | Y=608.43X−1.6284 | 0.96245 | 0.025~0.050 |
| Re | Y=320.16X+2.5602 | 0.91420 | 0.030~0.050 |
| Rf | Y=459.43X−8.5421 | 0.98925 | 0.025~0.050 |
| Rh1 | Y=220.43X−9.2346 | 0.98745 | 0.020~0.050 |
| Rg2 | Y=556.38X−8.2371 | 0.98998 | 0.025~0.050 |
Y and X are the peak area and concentration of the analytes, respectively.
Comparison of ginsenosides in the different parts of Panax ginseng
The different parts of Panax ginseng were studied, and the contents of 12 ginsenosides from the 3 parts were significantly different, as shown in Table 2. The total ginsenoside content of the leaf (3,538.71 mg) was significantly higher than that of the main root (292.87 mg) and the root hair (1,186.72 mg). The total content of ginsenosides in the leaf was up to 12 times higher than that in the main root. Li et al. (19) also found that the ginsenosides levels were much higher in the leaves compared to the roots. Furthermore, the content of PPD groups was higher than that of the PPT groups in all the samples. These results are consistent with those obtained by Shi et al. (13), who reported that the leaf, fine roots, and lateral roots exhibited higher contents of ginsenosides than the main root, and that the content of PPD-type ginsenosides was higher than that of PPT-type ginsenosides. Moreover, Zhang et al. (20) found that the contents of ginsenosides increased with cultivation years, causing a sequential content change of ginsenosides in an organ-specific manner: leaf> rhizome> main root. The leaf contained high amounts of ginsenosides Rb3 and Rh1, whereas the main root contained high amounts of ginsenoside Rb1 and Rc. Compared to the other parts of Panax ginseng, the root hair contained higher amounts of ginsenosides Rb1, Rc, Rh2, Re, Rf, and Rg2. Moreover, Rb2, Rb3, and Rg1 were only detected in the root hair, leaf, and main root, respectively. Kim et al. (21) suggested that the difference of ginsenosides in the parts of Panax ginseng may be due to the movement of ginsenosides between the roots, shoots, and leaves during foliation. Although there have not been any studies investigating the movement of ginsenosides in ginseng, there is evidence for this phenomenon. The leaf contained the highest amount of ginsenoside Rh1 (1,429.33 mg), while the main root and root hair contained the highest amount of ginsenoside Rc (58.72 and 294.13 mg, respectively). The content of ginsenoside Re in Panax ginseng leaf and root hair was 2.6~4 times higher than that of the main root. The results of a previous pharmacological study indicated that ginsenoside Re possesses anti-diabetic activity (6). Based on the results obtained in the present study, Panax ginseng leaf may be beneficial for diabetic patients similar to the other parts of the plant. Rb1, Rc, Re, and Rh1 are the 4 main ginsenosides in the extracts of Panax ginseng root hair. In a previous study, it was found that Re and Rb1 were the main components in American ginseng root, and the quantity of Rg1 was lower (22). In addition, Samukawa et al. (23) reported the distribution of ginsenosides in the different parts of Panax ginseng cultivated in Nagano, Japan. They found that the contents of ginsenosides were higher level in the lateral root, followed by the rhizome, the root hair, and the main root. Therefore, the results indicate that the content of ginsenosides was higher in the leaf and root hair, and lower in the main root than that in the other parts of Panax ginseng.
Table 2.
Content of ginsenosides in the main root, root hair, and leaf of Panax ginseng
| Ginsenoside | Concentrations (mg/100 g of ginseng sample, dry matter basis) | ||
|---|---|---|---|
|
| |||
| Leaf | Main root | Root hair | |
| 20(S)-protopanaxadiol (PPD) groups | |||
| Rb1 | 18.13±0.30c | 57.56±3.34b | 174.10±6.00a |
| Rb2 | –1) | – | 93.22±3.52 |
| Rb3 | 1,150.90±29.73 | – | – |
| Rc | 61.15±3.59b | 58.72±1.70b | 294.13±4.32a |
| Rd | 631.10±26.39a | – | 27.50±1.00b |
| Rg3 | 20.08±2.61a | 21.72±0.58a | 15.47±0.56b |
| Rh2 | 33.47±3.46b | 19.31±0.64c | 52.96±2.15a |
| 20(S)-protopanaxatriol (PPT) groups | |||
| Rg1 | – | 55.10±1.29 | – |
| Re | 144.53±0.90b | 18.54±1.20c | 224.53±1.97a |
| Rf | 32.19±0.81b | 10.46±0.26c | 48.43±5.55a |
| Rh1 | 1,429.33±55.54a | 51.46±3.03c | 193.04±3.14b |
| Rg2 | 17.83±0.59b | – | 63.34±1.34a |
| Total PPD | 1,914.83±11.01a | 157.31±1.57c | 657.38±2.93b |
| Total PPT | 1,623.88±14.46a | 135.56±1.45c | 529.34±3.00b |
| Total content | 3,538.71±12.74a | 292.87±1.51c | 1,186.72±2.97b |
Value are mean±SD (n=3).
Different letters (a–c) within a row indicate significant differences at P<0.05.
Not detected (limit of detection: 5 mg/100 g).
In conclusion, compared to the ginseng root, the ginseng leaf exhibits a higher content of ginsenosides. The market cost of ginseng root, however, is much higher than that of the leaf. Thus, the results of this study indicate that the leaf has a unique composition of ginsenosides and may be their richest source. Utilization of ginseng leaf could greatly reduce the costs associated with ginseng harvesting and manufacturing. More research is required to evaluate the effects of individual ginsenosides on plant growth and defense in order to better understand the physiological role of ginsenosides in the ginseng plant.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Kang KS, Yokozawa T, Kim HY, Park JH. Study on the nitric oxide scavenging effects of ginseng and its compounds. J Agric Food Chem. 2006;54:2558–2562. doi: 10.1021/jf0529520. [DOI] [PubMed] [Google Scholar]
- 2.Chen XG, Liu HY, Lei XH, Fu ZD, Li Y, Tao LH, Han R. Cancer chemopreventive and therapeutic activities of red ginseng. J Ethnopharmacol. 1998;60:71–78. doi: 10.1016/S0378-8741(97)00133-5. [DOI] [PubMed] [Google Scholar]
- 3.Yun TK, Lee YS, Kwon HY, Choi KJ. Saponin contents and anticarcinogenic effects of ginseng depending on types and ages in mice. Zhongguo Yao Li Xue Bao. 1996;17:293–298. [PubMed] [Google Scholar]
- 4.Fuzzati N. Analysis methods of ginsenosides. J Chromatogr B. 2004;812:119–133. doi: 10.1016/S1570-0232(04)00645-2. [DOI] [PubMed] [Google Scholar]
- 5.Fuzzati N, Gabetta B, Jayakar K, Pace R, Peterlongo F. Liquid chromatography-electrospray mass spectrometric identification of ginsenosides in Panax ginseng roots. J Chromatogr A. 1999;854:69–79. doi: 10.1016/S0021-9673(99)00463-X. [DOI] [PubMed] [Google Scholar]
- 6.Attele AS, Zhou YP, Xie JT, Wu JA, Zhang L, Dey L, Pugh W, Rue PA, Polonsky KS, Yuan CS. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–1858. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- 7.Popovich DG, Kitts DD. Generation of ginsenosides Rg3 and Rh2 from North American ginseng. Phytochemistry. 2004;65:337–344. doi: 10.1016/j.phytochem.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Wang HC, Chen CR, Chang CJ. Carbon dioxide extraction of ginseng root hair oil and ginsenosides. Food Chem. 2001;72:505–509. doi: 10.1016/S0308-8146(00)00259-4. [DOI] [Google Scholar]
- 9.Xie JT, Mehendale SR, Wang A, Han AH, Wu JA, Osinski J, Yuan CS. American ginseng leaf: ginsenoside analysis and hypoglycemic activity. Pharmacol Res. 2004;49:113–117. doi: 10.1016/j.phrs.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Yip TT, Lau CN, But PP, Kong YC. Quantitative analysis of ginsenosides in fresh Panax ginseng. Am J Chin Med. 1985;13:77–88. doi: 10.1142/S0192415X85000125. [DOI] [PubMed] [Google Scholar]
- 11.Zhang K, Wang X, Ding L, Li J, Qu CL, Chen LG, Jin HY, Zhang HQ. Determination of seven major ginsenosides in different parts of Panax quinquefolius L. (American ginseng) with different ages. Chem Res Chin Univ. 2008;24:707–711. [Google Scholar]
- 12.Wan JB, Yang FQ, Li SP, Wang YT, Cui XM. Chemical characteristics for different parts of Panax notoginseng using pressurized liquid extraction and HPLC-ELSD. J Pharm Biomed Anal. 2006;41:1596–1601. doi: 10.1016/j.jpba.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 13.Shi W, Wang YT, Li J, Zhang HQ, Ding L. Investigation of ginsenosides in different parts and ages of Panax ginseng. Food Chem. 2007;102:664–668. doi: 10.1016/j.foodchem.2006.05.053. [DOI] [Google Scholar]
- 14.Qu CL, Bai YP, Jin XQ, Wang YT, Zhang K, You JY, Zhang HQ. Study on ginsenosides in different parts and ages of Panax quinquefolius L. Food Chem. 2009;115:340–346. doi: 10.1016/j.foodchem.2008.11.079. [DOI] [Google Scholar]
- 15.Li TSC, Mazza G, Cottrell AC, Gao L. Ginsenosides in roots and leaves of American ginseng. J Agric Food Chem. 1996;44:717–720. doi: 10.1021/jf950309f. [DOI] [Google Scholar]
- 16.Lim JY, Ishiguro K, Kubo I. Tyrosinase inhibitory p-coumaric acid from ginseng leaves. Phytother Res. 1999;13:371–375. doi: 10.1002/(SICI)1099-1573(199908/09)13:5<371::AID-PTR453>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 17.Qu CL, Zhang HQ, Wang JS, Zhang R, Yu SC. Analysis of ginsenosides by SPE-HPLC and its application to quality control. Chem Res Chin Univ. 2010;26:527–531. [Google Scholar]
- 18.Dong H, Bai LP, Wong VK, Zhou H, Wang JR, Liu Y, Jiang ZH, Liu L. The in vitro structure-related anti-cancer activity of ginsenosides and their derivatives. Molecules. 2011;16:10619–10630. doi: 10.3390/molecules161210619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li XG, Yan YZ, Jin XJ, Kim YK, Uddin MR, Kim YB, Bae HH, Kim YC, Lee SW, Park SU. Ginsenoside content in the leaves and roots of Panax ginseng at different ages. Life Sci J. 2012;9:679–683. [Google Scholar]
- 20.Zhang YC, Li G, Jiang C, Yang B, Yang HJ, Xu HY, Huang LQ. Tissue-specific distribution of ginsenosides in different aged ginseng and antioxidant activity of ginseng leaf. Molecules. 2014;19:17381–17399. doi: 10.3390/molecules191117381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YJ, Jeon JN, Jang MG, Oh JY, Kwon WS, Jung SK, Yang DC. Ginsenoside profiles and related gene expression during foliation in Panax ginseng Meyer. J Ginseng Res. 2014;38:66–72. doi: 10.1016/j.jgr.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang A, Wang CZ, Wu JA, Osinski J, Yuan CS. Determination of major ginsenosides in Panax quinquefolius (American ginseng) using high-performance liquid chromatography. Phytochem Anal. 2005;16:272–277. doi: 10.1002/pca.838. [DOI] [PubMed] [Google Scholar]
- 23.Samukawa K, Yamashita H, Matsuda H, Kubo M. Simultaneous analysis of ginsenosides of various ginseng radix by HPLC. Yakugaku Zasshi. 1995;115:241–249. doi: 10.1248/yakushi1947.115.3_241. [DOI] [PubMed] [Google Scholar]