Abstract
Gamma‐aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the brain, and plays a key role in brain development. However, the in vivo levels of brain GABA in early life are unknown. Using edited MRS, in vivo GABA can be detected as GABA+ signal with contamination of macromolecule signals. GABA+ is evaluated as the peak ratio of GABA+/reference compound, for which creatine (Cr) or water is typically used. However, the concentrations and T 1 and T 2 relaxation times of these references change during development. Thus, the peak ratio comparison between neonates and children may be inaccurate. The aim of this study was to measure in vivo neonatal brain GABA+ levels, and to investigate the dependency of GABA levels on brain region and age. The basal ganglia and cerebellum of 38 neonates and 12 children were measured using GABA‐edited MRS. Two different approaches were used to obtain GABA+ levels: (i) multiplying the GABA/water ratio by the water concentration; and (ii) multiplying the GABA+/Cr by the Cr concentration. Neonates exhibited significantly lower GABA+ levels compared with children in both regions, regardless of the approach employed, consistent with previous ex vivo data. A similar finding of lower GABA+/water and GABA+/Cr in neonates compared with children was observed, except for GABA+/Cr in the cerebellum. This contrasting finding resulted from significantly lower Cr concentrations in the neonate cerebellum, which were approximately 52% of those of children. In conclusion, care should be taken to consider Cr concentrations when comparing GABA+/Cr levels between different‐aged subjects.
Keywords: gamma‐aminobutyric acid (GABA), human study, MEGA‐PRESS, neonates, normal brain, spectroscopic quantitation
Abbreviations
- BG
basal ganglia
- Cho
choline
- Cr
creatine
- Cr‐ref
creatine reference approach
- FWHM
full width at half maximum
- GABA
gamma‐aminobutyric acid
- LB
line broadening
- MM
macromolecule
- Off
selective inversion pulses applied at 7.5 ppm
- On
selective inversion pulses applied at 1.9 ppm
- T1
longitudinal relaxation time
- T2
transverse relaxation time
- VOI
volume of interest
- Wat‐ref
water reference approach
1. INTRODUCTION
Gamma‐aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the brain. During early life, the function of GABA in the central nervous system switches from excitatory to inhibitory.1, 2 However, despite the importance of GABA in the brain, the levels of in vivo GABA during brain development have not been reported. In vivo GABA signals can be detected using edited MRS. This signal is termed GABA+, as the GABA 3 ppm signal that appears in edited MRS spectra can contain macromolecule (MM) signals.3, 4, 5, 6 GABA+ signals can also be evaluated by the peak area ratio of GABA+ to a reference compound such as Cr, N‐acetylaspartate, or water.7 However, the concentrations and longitudinal‐ and transverse‐relaxation times (T 1 and T 2) of these reference compounds change during development.8, 9 Thus, use of these methods for assessment of neonatal GABA levels may be inaccurate.
The aim of the present study was to obtain in vivo data on neonatal brain GABA levels in the basal ganglia (BG) and cerebellum, and to investigate the dependency of GABA levels on brain region and age. We provide a method for obtaining neonatal brain GABA levels using a clinical 3 T MR scanner, Gannet (a batch‐analysis tool for GABA‐edited MRS data10), and in‐house software.
2. METHODS
2.1. Human subjects
This study was approved by the institutional ethical review board of our Children's Medical Center, where all clinical data used in this study were acquired. Informed written consent was obtained from the subjects and/or legal guardian. In our center, single‐voxel MRS was performed routinely for diagnosis in patients receiving brain MRI examination. MR examination was performed in neonates because of neurological symptoms, suspicion of organic disease, or routine evaluation before discharge. MEGA‐PRESS for GABA data collection was performed from 2013 in our center. The data acquisition period for this study was September 2013 to December 2015.
A final 38 neonates who met the following criteria were enrolled: no major anomaly or radiological findings at the time of the clinical examination until their latest examination, and MRS data of a certain standard (see GABA+ signal quantification for more detail). Neonatal clinical findings were evaluated by an experienced pediatric radiologist and a board‐certificated neonatologist. Out of 38 neonatal subjects, there were 28 preterm infants (12 boys, 16 girls; gestational age 23–36 weeks; studied at a postconceptional age of 35–41 weeks) and 10 term infants (six boys, four girls; gestational age 37–41 weeks; studied at a postconceptional age of 38–43 weeks). As controls, 12 normal volunteer children (one boy, 11 girls; mean 10.2 ± 3.6 years, range 6–16 years) were included.
2.2. Proton MRI and MRS
All MR investigations were performed on a clinical 3 T MR scanner (MAGNETOM Verio; Siemens, Erlangen, Germany) with bore diameter of 70 cm. RF signal transmission and reception were performed with a whole‐body coil and a 32‐channel head RF coil receiver (inner diameter 22 cm), respectively. The excitation frequency was set to the proton resonance of water (4.7 ppm, 123.24 MHz). Before the MR examination, shimming was performed automatically using a vendor‐provided shim tool. The subject was placed inside the magnet in the horizontal supine position. During the MR examination, the neonates were wrapped in vacuum‐type immobilization bags (CFI Medical Solutions, Fenton, MI, USA) to prevent excessive head movement and protect their hearing. Heart rate and transcutaneous oxygen saturation were monitored continuously with a pulse oximeter (Nonin, Plymouth, MN, USA). Sedation with thiopental (2–10 mg/kg body weight) was used for all neonatal subjects. T 1‐weighted (3D MPRAGE: T E/T R/T I 2.14/1570/800 ms; FOV 150 × 120 mm2; matrix 192 × 192; thickness 1 mm; observation bandwidth 61.4 kHz) or T 2‐weighted (FSE: T E/T R 123/5000 ms; FOV 150 × 120 mm2; matrix 256 × 232; thickness 2 mm; observation bandwidth 51.5 kHz) MR images obtained for clinical diagnosis were used as localizers. As routine examination, the single‐voxel MRS data by PRESS sequence (T E/T R 30/5000 ms),11 both with and without a water suppression pulse (bandwidth 50 Hz), were obtained. For GABA signal detection, the Siemens prototype MEGA‐PRESS sequence12 was used (T E/T R 69/1500 ms; bandwidth 1200 Hz; 512 data points; 128 (64 × 2) averages), with selective inversion pulses applied at 1.9 ppm (On) and at 7.5 ppm (Off). The volumes of interest (VOIs) of the BG were 3.7–7.6 and 12.8–19.2 mL in neonates and children, respectively, and those of the cerebellum were 3.7–9.4 and 12.4–37.4 mL, respectively (Figure 1).
Figure 1.
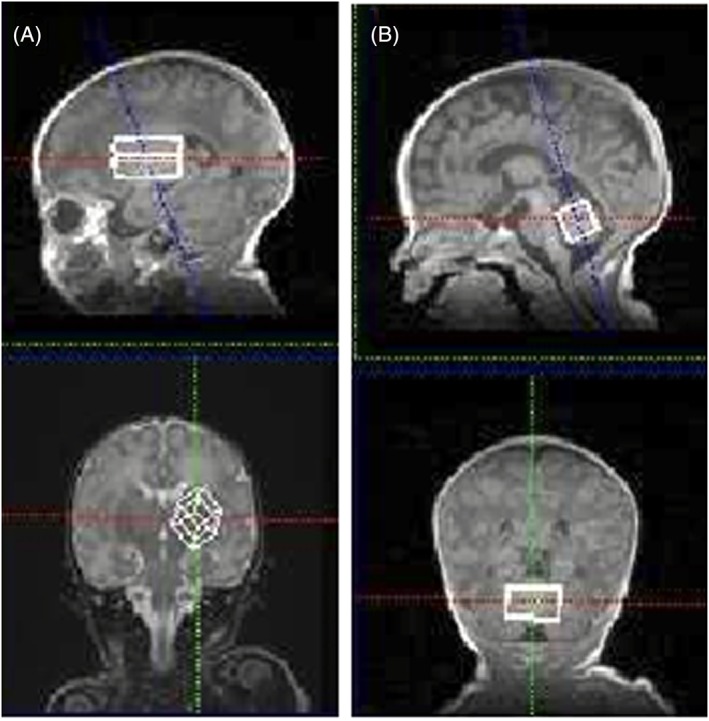
Example T 1‐weighted images (3D: T E/T R/T I 2.14/1570/800 ms) used for VOI selection of the BG (volume 4.4 mL) A, and cerebellum (3.7 mL) B, in a neonate (postconceptional age of 39 weeks)
2.3. T 1 and T 2 values for metabolites and water
The T 1 and T 2 values of each metabolite or water were as follows: T 1 neonates, GABA, Cr, choline (Cho), and water =1490, 1660, 1180, 2220 ms, respectively; T 1 children, GABA, Cr, Cho, and water =1310, 1460, 1300, and 1760 ms, respectively; T 2 neonates, GABA, MM, Cr, Cho, and water =150, 40, 199, 384, and 130 ms, respectively; T 2 children, GABA, MM, Cr, Cho, and water =88, 40, 116, 248, and 70 ms, respectively.13, 14, 15, 16 All values for children were taken from those of adults, as the metabolite concentrations are largely unchanged from 5 years of age.17, 18 As we could not find metabolite T 1 and T 2 at 3 T in neonates, the values of Cr and Cho were taken from a 2.4 T reference,13 while GABA values were obtained by multiplying adult GABA values by the Cr relaxation time ratios of neonates to adults.14
2.4. GABA+ signal quantification
Using the Gannet software10 running in MATLAB (MathWorks, Natick, MA, USA), the time domain data (On and Off) were processed into edited spectra. The process included multiplying the exponential line of 3 Hz as a window function, zero‐filling to 32 k data points, fast Fourier transform, phase and baseline correction, and subtraction of Off from On spectra to make the edited spectrum. Using in‐house software running in MATLAB, the peak areas of GABA+ of edited spectra, Cr of Off spectra, and unsuppressed water from the spectra by PRESS were measured. For the Cr 3.0 ppm peak fitting, the Cho 3.2 ppm peak was also fitted. GABA+ was considered to consist of bimodal GABA and MM peaks. In the least square method, the best fitting of Lorentzian line shapes to the peaks was investigated. The full width at half maximum (FWHM) of the Lorentzian peak was defined as
| (1) |
where is the natural line width of a peak that has T 2, LB is line broadening due to magnetic field inhomogeneity, and 3 Hz is the exponential window function value. A first‐order slope and baseline offset were also considered for the calculation (Figure 2).
Figure 2.
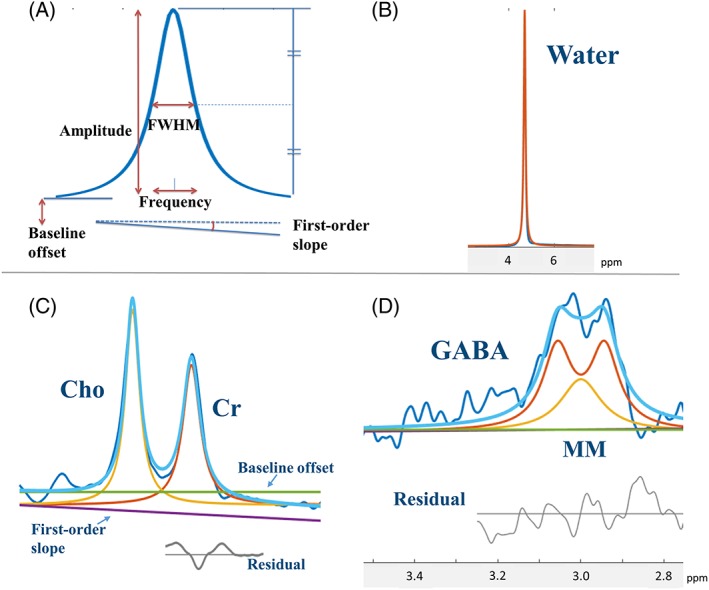
MRS peak quantification using model spectra. A, To fit each MRS signal, a model Lorentzian line with five variable parameters was used. B‐D, The peak fittings of water B, Cho and Cr C, and GABA and MMs D
We assumed that the field inhomogeneity was constant during one MRS data scan. Thus, the same LB value was applied for GABA, MM, Cr, and Cho. The LB value was determined during the Cr and Cho peak fitting by changing it, and the final value was used for GABA and MM peak fitting as a fixed value. Only MRS data with an LB of 10 Hz or less were included in this study.
Two different approaches were used to obtain GABA+. In the first approach, the water signal was used as a reference, and GABA+/water was multiplied by the water concentration (Wat‐ref):
| (2) |
where Reg is a region (neonatal BG, neonatal cerebellum, child BG, or child cerebellum), N is the data number of the region, the first and second exponential equations are the correction factors for the peaks decreased by T 1 and T 2, respectively, and the water concentrations were published values of 48.9 and 44.4 M for neonates and children, respectively.19 The second approach used the Cr signal as a reference (Cr‐ref):
| (3) |
Cr concentration was calculated from PRESS data of an independent MRS study. The Cr proton peak was compared with that of unsuppressed water, with the same values for water concentrations as for Wat‐ref. Finally, the GABA+ values were normalized (n‐GABA+) by dividing by the GABA+ value in the neonatal BG region.
2.5. Statistical analysis
All statistical analyses were performed using statistical software (SPSS; IBM, Chicago, IL, USA). The Mann–Whitney U test was used for comparisons of GABA+ levels between the different ages and regions, and for Cr concentrations between the different ages. A p value less than 0.05 was considered statistically significant.
3. RESULTS
In the present study, 47 MR spectra (36 BG, 11 cerebellum) from a total of 38 neonates were used. One data set that met the inclusion criteria but showed an incorrect GABA+/Cr value of 1.17 was excluded. The GABA+/water ratios of the neonates were significantly lower than those of the children in both the BG and cerebellum (p < 0.001 for both, Figure 3A). The GABA+/Cr ratios were significantly lower in the neonates than the children in the BG (p = 0.001), but were higher in the cerebellum of neonates compared with children (p = 0.006) (Figure 3B). For Cr concentrations, there were significant age group differences between neonates and children in both the BG and cerebellum (p < 0.001 for both). In particular, the Cr concentration in the cerebellum in children was approximately twice that in neonates (6.3 versus 12.1 mM, respectively, Table 1).
Figure 3.
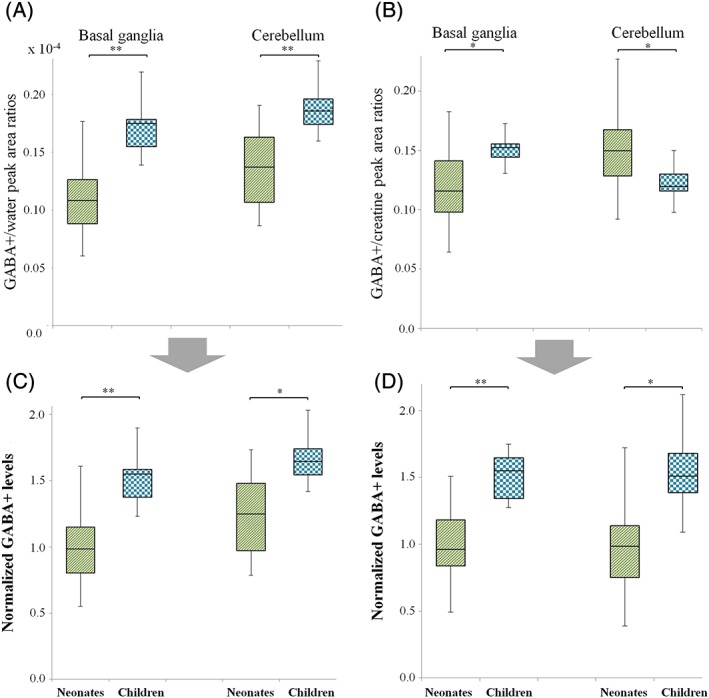
Box and whisker plots of in vivo brain GABA+ levels in the BG and cerebellum. A, B, The peak area ratios of GABA+ to water A, and GABA+ to Cr B. C, D, The GABA+ levels normalized by water C, and Cr D. *p < 0.01, **p < 0.001
Table 1.
Cr concentrations in the brain of neonates and children
| BG | Cerebellum | |
|---|---|---|
| Neonates | 8.0 ± 0.9 (n = 36) | 6.3 ± 1.9 (n = 11) |
| Children | 9.5 ± 0.8 (n = 10) | 12.1 ± 2.0 (n = 12) |
| p values | <0.001 | <0.001 |
Data are shown in mM (mean ± standard deviation).
The normalized GABA+ levels were significantly lower in neonates than in children in both regions, regardless of the approach employed (Wat‐ref, p < 0.001 and p = 0.001 for BG and cerebellum, respectively, Figure 3C; Cr‐ref, p < 0.001 and p = 0.001, respectively, Figure 3D). In intragroup comparisons, there were no differences in GABA+ levels between the BG and cerebellum, except for neonates using Wat‐ref (p = 0.009).
3.1. Discussion
A key finding of the present study was that neonates displayed significantly lower in vivo brain GABA+ levels than children in both the BG and cerebellum. Previous ex vivo studies of postmortem brains and cerebrospinal fluid also reported significant increases in GABA concentrations with age.20, 21 Therefore, it is likely that in vivo GABA concentrations in the BG and cerebellum are lower in neonates compared with children, and that they increase with development.
The higher GABA+/Cr ratio in the neonatal cerebellum compared with that in children was likely due to the 52% lower Cr concentrations in neonates compared with children. Therefore, care should be taken regarding Cr concentrations when comparing GABA+/Cr levels between different‐aged subjects. Cr‐ref also requires the water concentration to calculate the Cr concentration. Compared with Cr‐ref, Wat‐ref is simpler and may be clinically useful for neonatal GABA+ evaluation.
The contribution of MM to the age‐related change in GABA+ signal between the neonates and children is unclear. However, two studies of teenage to adult subjects found no significant correlations between MM peak contribution and age,22, 23 while our data suggest that the MM contribution would need to increase by 20–50% from neonates to children to produce similar GABA concentrations. Thus, we believe that our data reflect a real increase in GABA in the brain from neonates to children.
In both approaches used to measure GABA+, chemical shift displacements of the water peaks occurred owing to the slice selection gradients,24 i.e., the water peak displacement against GABA+ in Wat‐ref, and against Cr in Cr‐ref. For a VOI of 20 mm3, the water peak displacement against GABA+ in Wat‐ref was approximately 1.8, 6.2, and 6.2 mm in the x, y, and z directions, respectively, while the water peak displacement against Cr in Cr‐ref was approximately 1.4, 4.6, and 4.6 mm in the x, y, and z directions, respectively. The major metabolites by PRESS showed normal concentrations when using water as an internal reference, suggesting that the adjacent regions of the VOI had a tissue composition similar to that within the VOI. Therefore, the displacement error in our study was likely to be small.
In the present study, the neonatal subjects were carefully selected clinically and radiologically, and have since been followed up by medical doctors at our center. As of September 2016, the subjects reached corrected ages of 10 months to 3 years. If a subject showed evidence of genetic abnormalities or a reduced developmental quotient during follow‐up examinations, then their GABA data were excluded.
Generally, the decrease in MRS signals for metabolites and water due to longitudinal and transverse relaxations are considered, except for the situation with very short T E and long T R. In the present study, the signal intensities were corrected using published T 1 and T 2 values. As these published values may be not exactly the same as for our subjects, the corrected values may have introduced errors. Nevertheless, without correction of T 1 and T 2 in Equations (2) and (3), the differences of GABA+ levels between neonates and children were approximately 8% smaller than with corrected values, and significant differences were still observed in both regions (p ≤ 0.022). Therefore, our data reliably indicate that GABA+ levels were lower in neonates.
4. CONCLUSIONS
We assessed in vivo neonatal brain GABA+ levels using edited MRS. In both the BG and cerebellum, the GABA+ levels of neonates were significantly lower than those of children, consistent with previous ex vivo data. When comparing GABA+/Cr levels between different age groups, care should be taken regarding Cr concentrations of the subjects. Furthermore, the chemical shift displacements of water should be considered in the evaluation of the GABA+/water and Cr/water values. Future studies of age‐related MM contribution rates and the relaxation times of metabolites/water are required to improve the accuracy of measurement of GABA+ levels.
ACKNOWLEDGMENTS
The authors thank Dr Yasuhiko Tachibana for technical support, Mr Masahiko Sato, Mr Kohki Kusagiri, Mr Yasutake Muramoto, and Yuichi Suzuki for help during acquisition of MR images and spectroscopy data, and Ms Akemi Suzuki and Ms Kazumi Sato for assistance with data analysis. This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers 26461843 and 15K09943, and by the Kanagawa Municipal Hospital Pediatric Research Fund.
Tomiyasu, M. , Aida, N. , Shibasaki, J. , Umeda, M. , Murata, K. , Heberlein, K. , Brown, M. A. , Shimizu, E. , Tsuji, H. , and Obata, T. (2017) In vivo estimation of gamma‐aminobutyric acid levels in the neonatal brain, NMR in Biomedicine, 30, e3666. doi: 10.1002/nbm.3666.
REFERENCES
- 1. Ben‐Ari Y, Khalilov I, Kahle KT, Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist. 2012;18(5):467–486. [DOI] [PubMed] [Google Scholar]
- 2. Kilb W. Development of the GABAergic system from birth to adolescence. Neuroscientist. 2012;18(6):613–630. [DOI] [PubMed] [Google Scholar]
- 3. Edden RA, Puts NA, Barker PB. Macromolecule‐suppressed GABA‐edited magnetic resonance spectroscopy at 3 T. Magn Reson Med. 2012a;68(3):657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Near J, Simpson R, Cowen P, Jezzard P. Efficient gamma‐aminobutyric acid editing at 3 T without macromolecule contamination: MEGA‐SPECIAL. NMR Biomed. 2011;24(10):1277–1285. [DOI] [PubMed] [Google Scholar]
- 5. Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma‐aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90(12):5662–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Waddell KW, Avison MJ, Joers JM, Gore JC. A practical guide to robust detection of GABA in human brain by J‐difference spectroscopy at 3 T using a standard volume coil. Magn Reson Imaging. 2007;25(7):1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puts NA, Edden RA. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog Nucl Magn Reson Spectrosc. 2012;60:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kreis R, Hofmann L, Kuhlmann B, Boesch C, Bossi E, Huppi PS. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 2002;48(6):949–958. [DOI] [PubMed] [Google Scholar]
- 9. Tomiyasu M, Aida N, Endo M, et al. Neonatal brain metabolite concentrations: an in vivo magnetic resonance spectroscopy study with a clinical MR system at 3 Tesla. PLoS One. 2013;8(11):e82746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: a batch‐processing tool for the quantitative analysis of gamma‐aminobutyric acid‐edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40(6):1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bottomley PA. Spatial localization in NMR spectroscopy in vivo . Ann N Y Acad Sci. 1987;508:333–348. [DOI] [PubMed] [Google Scholar]
- 12. Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11(6):266–272. [DOI] [PubMed] [Google Scholar]
- 13. Brooksbank BW, Atkinson DJ, Balazs R. Biochemical development of the human brain. III. Benzodiazepine receptors, free gamma‐aminobutyrate (GABA) and other amino acids. J Neurosci Res. 1982;8(4):581–594. [DOI] [PubMed] [Google Scholar]
- 14. Edden RA, Intrapiromkul J, Zhu H, Cheng Y, Barker PB. Measuring T2 in vivo with J‐difference editing: application to GABA at 3 tesla. J Magn Reson Imaging. 2012b;35(1):229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mlynarik V, Gruber S, Moser E. Proton T 1 and T 2 relaxation times of human brain metabolites at 3 Tesla. NMR Biomed. 2001;14(5):325–331. [DOI] [PubMed] [Google Scholar]
- 16. Puts NA, Barker PB, Edden RA. Measuring the longitudinal relaxation time of GABA in vivo at 3 tesla. J Magn Reson Imaging. 2013;37(4):999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993;30(4):424–437. [DOI] [PubMed] [Google Scholar]
- 18. Pouwels PJ, Brockmann K, Kruse B, et al. Regional age dependence of human brain metabolites from infancy to adulthood as detected by quantitative localized proton MRS. Pediatr Res. 1999;46(4):474–485. [DOI] [PubMed] [Google Scholar]
- 19. Williams LA, Gelman N, Picot PA, et al. Neonatal brain: regional variability of in vivo MR imaging relaxation rates at 3.0 T—initial experience. Radiology 2005;235(2):595–603. [DOI] [PubMed] [Google Scholar]
- 20. Casado M, Molero M, Sierra C, Garcia‐Cazorla A, Ormazabal A, Artuch R. Analysis of cerebrospinal fluid gamma‐aminobutyric acid by capillary electrophoresis with laser‐induced fluorescence detection. Electrophoresis. 2014;35(8):1181–1187. [DOI] [PubMed] [Google Scholar]
- 21. Kok RM, Howells DW, van den Heuvel CC, Guerand WS, Thompson GN, Jakobs C. Stable isotope dilution analysis of GABA in CSF using simple solvent extraction and electron‐capture negative‐ion mass fragmentography. J Inherit Metab Dis. 1993;16(3):508–512. [DOI] [PubMed] [Google Scholar]
- 22. Hofmann L, Slotboom J, Boesch C, Kreis R. Characterization of the macromolecule baseline in localized 1H‐MR spectra of human brain. Magn Reson Med. 2001;46(5):855–863. [DOI] [PubMed] [Google Scholar]
- 23. Mader I, Seeger U, Karitzky J, Erb M, Schick F, Klose U. Proton magnetic resonance spectroscopy with metabolite nulling reveals regional differences of macromolecules in normal human brain. J Magn Reson Imaging. 2002;16(5):538–546. [DOI] [PubMed] [Google Scholar]
- 24. Smith AS, Weinstein MA, Hurst GC, DeRemer DR, Cole RA, Duchesneau PM. Intracranial chemical‐shift artifacts on MR images of the brain: observations and relation to sampling bandwidth. Am J Roentgenol. 1990;154(6):1275–1283. [DOI] [PubMed] [Google Scholar]


