Abstract
Ewing's sarcoma is a rare and aggressive malignancy. In the present study, tumor from a patient with a Ewing's sarcoma with cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B) loss and FUS-ERG fusion was implanted in the right chest wall of nude mice to establish a patient-derived orthotopic xenograft (PDOX) model. The aim of the present study was to determine efficacy of cyclin-dependent kinase 4/6 (CDK4/6) and insulin-like growth factor-1 receptor (IGF-1R) inhibitors on the Ewing's sarcoma PDOX. The PDOX models were randomized into the following groups when tumor volume reached 50 mm3: G1, untreated control; G2, doxorubicin (DOX) (intraperitoneal (i.p.) injection, weekly, for 2 weeks); G3, CDK4/6 inhibitor (palbociclib, PD0332991, per oral (p.o.), daily, for 14 days); G4, IGF-1R inhibitor (linsitinib, OSI-906, p.o., daily, for 14 days). Tumor growth was significantly suppressed both in G3 (palbociclib) and in G4 (linsitinib) compared to G1 (untreated control) at all measured time points. In contrast, DOX did not inhibit tumor growth at any time point, which is consistent with the failure of DOX to control tumor growth in the patient. The results of the present study demonstrate the power of the PDOX model to identify effective targeted molecular therapy of a recalcitrant DOX-resistant Ewing's sarcoma with specific genetic alterations. The results of this study suggest the potential of PDOX models for individually-tailored, effective targeted therapy for recalcitrant cancer.
Keywords: palbociclib, linsitinib, patient-derived orthotopic xenograft, PDOX, Ewing's sarcoma
INTRODUCTION
Sarcomas are a heterogeneous group of connective tissue malignancies of mesenchymal origin. They comprise approximately 1 percent of adult and 15 percent of pediatric malignancies. The most frequent site of primary sarcoma is the extremity, followed by the retroperitoneum and trunk. The most common site of metastasis is the lung. However, sarcoma can metastasize to almost any location or organ. There are over 50 known histological sub-types of sarcoma. Ewing's sarcoma is a rare malignancy in which EWS-FLI1 is considered to be the causal translocation for 90% of cases [1]. Treatment for Ewing's sarcoma uses surgery, radiation, and chemotherapy but with poor outcome. Novel more effective treatment is necessary for this recalcitrant disease [2–7].
Palbociclib (PD0332991), a CDK4/6 inhibitor, has shown treatment efficacy for ovarian cancer, glioblastoma, and chordoma cell lines with CDKN2A loss [8–10]. Recently, palbociclib treatment efficacy for a patient with metastatic breast cancer with CDKN2A loss has been described. A clinical trial in patients with liposarcoma with CDK4 amplification showed promising efficacy of palbociclib treatment [11].
Linsitinib (OSI-906) is a kinase inhibitor of both insulin receptor (IR) and insulin growth factor receptors (IGF-1R) [12]. Linsitinib was previously used to treat osteosarcoma cells and Ewing's sarcoma cells [13]. Linsitinib is being tested in a Phase III trial in adrenocortical carcinoma and in a Phase I/II clinical trial in ovarian cancer [14].
Clinically-relevant mouse models of sarcomas would permit evaluation of targeted molecular individualized therapy based on the genetic alternations of the patient's tumor. Our laboratory pioneered the patient-derived orthotopic xenograft (PDOX) nude mouse model with the technique of surgical orthotopic implantation (SOI). Our laboratory has developed PDOX models of all major tumor types including pancreatic [15–19], breast [20], ovarian [21], lung [22], cervical [23], colon [24–26] and stomach cancer [27] as well as mesothelioma [28] and sarcoma [6 29–31].
Recently, a patient tumor with high-grade undifferentiated pleomorphic soft-tissue sarcoma from a striated muscle was grown orthotopically in the right biceps femoris muscle of nude mice to establish a PDOX model. Tumor-targeting S. typhimurium A1-R followed by DOX eradicated the PDOX tumor in our laboratory [6].
A PDOX nude mouse model of follicular dendritic-cell sarcoma (FDCS) was also established in the biceps muscle of nude mice in our laboratory. The FDCS PDOX was resistant to both doxorubicin (DOX), as well as to NVP-BEZ235, dactolisib (BEZ), an experimental agent which is a dual pan-phosphoinositide 3-kinase-mammalian target of rapamycin inhibitor. However, in contrast to DOX and BEZ, the FDCS PDOX was sensitive to tumor-targeting Salmonella typhimurium A1-R [31].
In the present study, a Ewing's sarcoma patient with both FUS-ERG fusion [1, 32] and CDKN2A/B loss was studied. No patient with both these genetic alterations has been previously reported. Therefore, CDK4/6- and IGF-1R-inhibitors (Figure 1) were tested on this patient's tumor in the PDOX model (Figure 2).
Figure 1. Schematic representation of Palbociclib (PD0332991, CDK4/6 inhibitor) and Linsitinib (OSI-906, IGF-1R inhibitor) blockade.
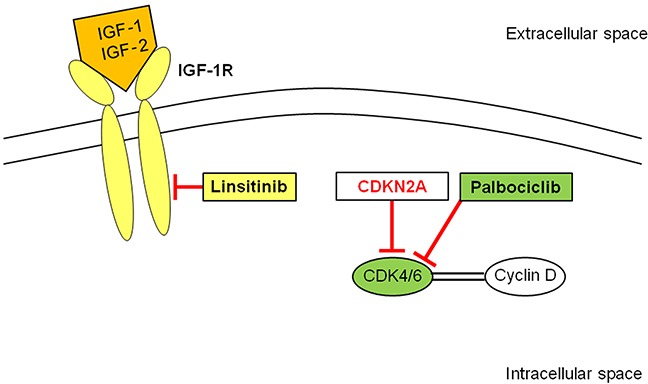
CDK4/6 forms a complex with cyclin D which activates a cascade resulting in cell proliferation. Palbociclib inhibits CDK4/6 that is activated by the loss of CDKN2A. Linsitinib blocks IGF-1R which is activated by its ligands, IGF-1 or IGF-2, resulting in apoptosis blockade, and cell proliferation. IGF-1R: insulin-like growth factor-1 receptor; CDK: cyclin-dependent kinase.
Figure 2. Establishment of Ewing's sarcoma PDOX model.
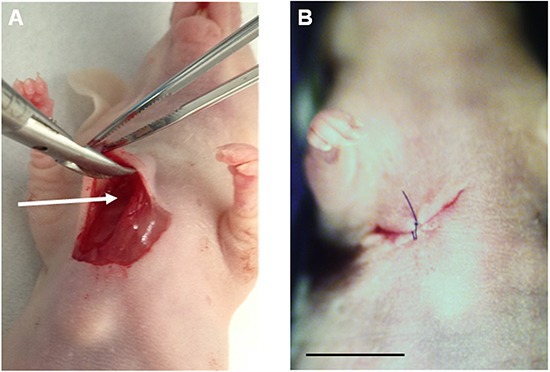
A. After making a skin incision on the right chest wall of a nude mouse, the space between the pectoral muscle and intercostal muscle (arrow) was expanded. A 4 mm3 fragment of the patient tumor was implanted orthotopically into the space. B. The pectoral muscle and the skin were closed with a 6-0 nylon suture. Scale bar: 10 mm.
RESULTS AND DISCUSSION
Genetic alterations in the patient's tumor
Gene expression profiling (Foundation Medicine, Cambridge, MA) of the patient tumor revealed genetic alteration of CDKN2A/B loss and FUS-ERG fusion.
Comparison of histology of original patient tumor and PDOX model
Histological analysis of the patient's tumor demonstrated an infiltrative proliferation of small round blue cells with round-to-avoid hyperchomatic nuclei, scanty eosinophilic-to-clear cytoplasm, and diffuse, membranous CD99 immunoreactivity. The PDOX tumor had a similar histomorphologic appearance similar to the original biopsy (Figure 3).
Figure 3. Histological comparison between patient original tumors and a PDOX tumor.
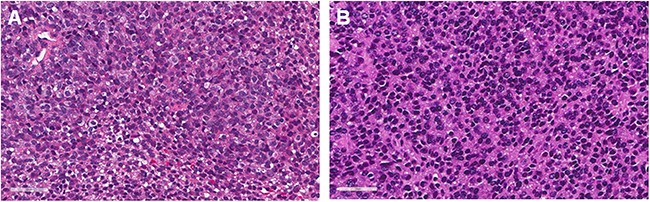
A. H & E staining of the resected patient original tumor and B. H & E staining of the untreated PDOX tumor. Scale bars: 50 μm.
Palbociclib (PD0332991, CDK4/6 inhibitor) and Linsitinib (OSI-906, IGF-1R inhibitor) significantly inhibited tumor growth in the Ewing's sarcoma PDOX model
The PDOX models were randomized into the following groups when tumor volume reached 50 mm3: G1, untreated control; G2, DOX (intraperitoneal (i.p.) injection, weekly, for 2 weeks); G3, CDK4/6 inhibitor (palbociclib, PD0332991, per oral (p.o.), daily, for 14 days); G4, IGF-1R inhibitor (linsitinib, OSI-906, p.o., daily, for 14 days). DOX did not inhibit tumor growth, which is consistent with the failure of DOX to control tumor growth in the patient. Palbociclib significantly inhibited tumor growth compared to untreated control from day 8 to 22 (Figure 4, P > 0.01 at day-22). Linsitinib also significantly inhibited tumor growth compared to control from day 4 to 22 (Figure 4, P > 0.01 at day-22). On day 22, tumor volume was as follows: untreated control (G1) (209.8 ± 48.5 mm3); DOX (G2) (175.7 ± 79.9 mm3); palbociclib (G3) (74.3 ± 47.9 mm3); and linsitinib (G4) (101.0 ± 20.3 mm3).
Figure 4. Palbociclib (PD0332991, CDK4/6 inhibitor) and linsitinib (OSI-906, IGF-1R inhibitor) significantly inhibited tumor growth in a Ewing's sarcoma PDOX model.
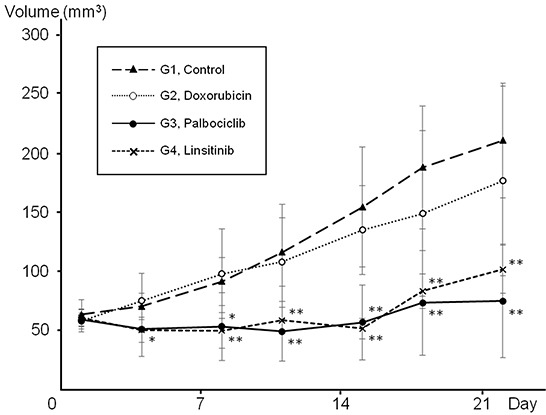
Line graph shows tumor volume at each time point. Palbociclib significantly inhibited tumor growth compared to untreated control from day 8 to 22. Linsitinib also significantly inhibited tumor growth compared to control from day 4 to 22. In contrast, doxorubicin did not inhibit tumor growth at any time point. *P < 0.05, **P < 0.01 compared to untreated control. Error bars: ± 1 SD. CDK: cyclin-dependent kinase; IGF-1R: insulin-like growth factor-1 receptor; PDOX: patient-derived orthotopic xenograft.
Since CDKN2A, a tumor suppressor gene, suppresses CDK4/6 which stimulates cancer cells to cycle, loss of CDKN2A can result in increased cancer-cell proliferation. Incidence of CDKN2A loss in tumors of Ewing's sarcoma was reported to be relatively frequent with 11.2%. Palbociclib (PD0332991), a CDK4/6 inhibitor, had shown treatment efficacy for ovarian cancer, glioblastoma, and chordoma cell lines with CDKN2A loss [8–10]. Recently, palbociclib treatment efficacy for a patient with metastatic breast cancer with CDKN2A loss has been described [34]. A clinical trial in patients with liposarcoma with CDK4 amplification showed promising efficacy of palbociclib treatment [11]. Palbociclib treatment efficacy in the present study is likely through suppression of the CDK4/6 pathway which was activated by CDKN2A loss (Figure 1).
The findings of CDKN2A/B loss offered a clue that this tumor may be succeptible to CDK4/6 inhibition, which is normally inhibited by CDKN2A/B and suggests that the cell cycle is a viable target in Ewing's sarcoma [34, 35].
The patient's tumor was characterized by a FUS-ERG fusion gene as well as the CDKN2A/B loss. Ewing's sarcoma is a rare small round blue-cell tumor that is mostly characterized by translocations involving EWSR1 and members of the ETS transcription factor family. In recent years, Ewing's sarcoma like small round blue cell tumors have been characterized by non-canonical translocations including CIC-DUX4 [36] and FUS-ERG [32, 37]. It is felt that these non-canoncial Ewing's sarcomas make up a portion of the group of atypical Ewing's sarcomas. Because of the rarity of FUS-ERG in Ewing's sarcomas, the prognosis, treatment and molecular biology of this genetic alteration is poorly understood. The development of PDOX models will help to better study and define this disease.
Currently there are no therapies that have been developed that reliably inhibit ERG fusion proteins. This rare FUS-ERG transgene has also been noted in ALL and AML and found to modulate the retinoic acid pathway in AML [38]. In addition, Cironi et. al. [39] demonstrated that this fusion gene upregulated IGF-1 in AML indicating that this may be a candidate target for diseases with the FUS-ERG transgenes. Other studies in leukemia point to a possible role for PIM1 and MAPK inhibitors for malignancies with ERG transgenes [40]. The IGF-1R pathway promotes proliferation and prevents apoptosis of cancer cells through activation of phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways [41]. Consistent expression of IGF-1R was observed both in Ewing's sarcoma cell lines and patient tissue [42, 43]. In a Phase II study for patients with Ewing's sarcoma, measurable treatment response to an IGF-1R inhibitor was demonstrated [42]. In the present study, the IGF-R inhibitor linsitinib essentially arrested the Ewing's sarcoma (Figure 4), suggesting further study and use of this inhibitor in sarcoma.
CONCLUSIONS
We report here significant efficacy of CDK4/6 and IGF-1R inhibitors on a rare Ewing's sarcoma with FUS-ERG fusion and CDKN2A/B loss in a PDOX model. The results can be used to tailor further treatment for this patient.
Until 2003, all of the reported Ewing tumors involved rearrangement of the EWS gene, at 22q12, with an ETS-family transcription factor. In 2003, four Ewing's tumor cases were reported that had joined chromosome bands 16p11 and 21q22. Detailed genetic analysis of two of the cases demonstrated how this rearrangement resulted in the fusion of the FUS gene at 16p11 to the ERG gene at 21q22 [32].
The fusion proteins could possibly comprise the transactivation domain of FUS and the DNA-binding domain of transcription factor ERG; thus FUS/ERG may be an aberrant transcription factor [32].
The four Ewing's tumor cases previously described and the present case may represent a novel type of Ewing's tumor in which 22q12 is not rearranged, and the primary translocation involves chromosomes 16 and 21 [32].
Cironi et al [39] observed in transfection studies that FUS/ERG activated the IGF-1 promoter and induced IGF-1 expression. This is consistent with the results of the present study that targeting the IGF-IR inhibited the Ewing sarcoma PDOX with the FUS/ERG fusion.
Palbociclib, a small-molecule inhibitor of cyclin-dependent kinases 4 and 6 [8–10], was recently approved by the FDA for HER2-negative breast cancer. CDKN2A (p16) is a negative regulator of CDK 4 and 6. Previously a patient with metastatic estrogen receptor- positive, HER2-negative breast cancer with CDKN2A loss responded to palbociclib, as briefly described above, which is consistent with the results of the present study, where palbociclib inhibited the Ewing's sarcoma PDOX, which lost CDKN2A, by targeting CDK4/6 [33].
In a previous study from our laboratory, fluorophore-conjugated IGF-1R antibodies selectively visualized metastatic colon cancer [44]. Future studies will use the IGF-IR antibodies to detect metastasis in PDOX models of Ewing's sarcoma to enable determination of efficacy of molecular inhibitors on metastasis as well as the primary tumor.
Previously developed concepts and strategies of highly selective tumor targeting can take advantage of molecular targeting of tumors, including tissue-selective therapy which focuses on unique differences between normal and tumor tissues [45–50].
MATERIALS AND METHODS
Mice
Athymic nu/nu female nude mice (AntiCancer Inc., San Diego, CA), 4–6 weeks old, were used in this study. All animal studies were conducted with an AntiCancer Institutional Animal Care and Use Committee (IACUC)-protocol specifically approved for this study and in accordance with the principals and procedures outlined in the National Institutes of Health Guide for the Care and Use of Animals under Assurance Number A3873-1. In order to minimize any suffering of the animals the use of anesthesia and analgesics were used for all surgical experiments. Animals were anesthetized by subcutaneous injection of a 0.02 ml solution of 20 mg/kg ketamine, 15.2 mg/kg xylazine, and 0.48 mg/kg acepromazine maleate. The response of animals during surgery was monitored to ensure adequate depth of anesthesia. The animals were observed on a daily basis and humanely sacrificed by CO2 inhalation when they met the following humane endpoint criteria: severe tumor burden (more than 20 mm in diameter), prostration, significant body weight loss, difficulty breathing, rotational motion and body temperature drop. Animals were housed in a barrier facility on a high efficiency particulate arrestance (HEPA)-filtered rack under standard conditions of 12-hour light/dark cycles. The animals were fed an autoclaved laboratory rodent diet.
Patient-derived tumor
The Ewing's sarcoma tumor from the right chest wall of a female patient was resected by JY in the Department of Surgery, University of California, Los Angeles (UCLA). Written informed consent was provided by the patient, and the Institutional Review Board (IRB) of UCLA approved this experiment. Neoadjuvant chemotherapy with DOX, vincristine, and cyclophosphamide, was previously administered to the patient.
Establishment of PDOX model of soft-tissue Ewing's sarcoma
A fresh tumor tissue sample from the Ewing's sarcoma from the right chest wall was obtained and transported immediately to the laboratory of AntiCancer on ice. The sample was minced into 5 mm fragments. The tumor fragments were implanted subcutaneously in nude mice. Seven weeks later, the implanted tumors grew to approximately 10 mm in diameter. The established tumors were harvested and minced into 4 mm3 fragments. Mice were anethesized with the ketamine mixture. A 10 mm skin incision was made on the right chest wall. The PDOX model was established by implanting a single tumor fragment orthotopically into the layer between the pectoral muscle and intercostal muscle in the right chest wall of the nude mouse. The wound was closed with a 6-0 nylon suture (Ethilon, Ethicon, Inc., NJ) (Figure 2).
Treatment study design in the PDOX model of sarcoma
Experimental protocol: G1: untreated control (n = 6); G2: treated with doxorubicin (i.p., 3 mg/kg, weekly, 2 weeks, n = 6); G3: treated with palbociclib (p.o., 100 mg/kg, daily, 14 days, n = 6); G4: treated with linsitinib (p.o., 25 mg/kg, daily, 14 days, n = 6). When tumor volume reached 50 mm3, treatment started. Tumor length and width were measured twice in a week. Tumor volume was calculated with the following formula: Tumor volume (mm3) = length (mm) × width (mm) × width (mm) × 1/2. Data are presented as mean ± SD. Tumor growth inhibition was defined as following equation reported by Stebbing et al. [51]: Tumor growth inhibition (%) = (1 − (T22 − T1) / (C22 − C1)) × 100. T1, average treated tumor volume on day 1; T22, average treated tumor volume on day 22; C1, average control tumor volume on day 1; C22, average control tumor volume on day 22. All treated mice were followed up until day 22.
Histological examination
Fresh tumor samples were fixed in 10% formalin and embedded in paraffin before sectioning and staining. Tissue sections (5 μm) were deparaffinized in xylene and rehydrated in an ethanol series. Hematoxylin and eosin (H&E) staining was performed according to standard protocol. Histological examination was performed with a BHS system microscope (Olympus Corp., Tokyo, Japan). Images were acquired with INFINITY ANALYZE software (Lumenera Corporation, Ottawa, Canada) [6, 31].
Statistical analysis
SPSS statistics version 21.0 was used for all statistical analyses (IBM, New York City, NY, USA). Significant differences for continuous variables were determined using the Mann-Whitney U test. Line graphs expressed average and error bar showed SD. A probability value of P ≤0.05 was considered statistically significant.
Footnotes
CONFLICTS OF INTEREST
Y.Z. is an employee of AntiCancer Inc. T.M., T.K., K.I., K.K. and R.M.H. are unsalaried associates of AntiCancer Inc. R.M.H. is an unsalaried associate of PDOX Inc. There are no other competing financial interests.
DEDICATION
This paper is dedicated to the memory of A. R. Moossa, M.D., and Sun Lee, M.D.
REFERENCES
- 1.Chen S, Deniz K, Sung YS, Zhang L, Dry S, Antonescu CR. Ewing sarcoma with ERG gene rearrangements: A molecular study focusing on the prevalence of FUS-ERG and common pitfalls in detecting EWSR1-ERG fusions by FISH. Genes Chromosomes Cancer. 2016;55:340–9. doi: 10.1002/gcc.22336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen TO, West RB. Translating gene expression into clinical care: sarcomas as a paradigm. J Clin Oncol. 2010;28:1796–1805. doi: 10.1200/JCO.2009.26.1917. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen TO, West RB, Linn SC, Alter O, Knowling MA, O'Connell JX, Zhu S, Fero M, Sherlock G, Pollack JR, Brown PO, Botstein D, van de Rijn M. Molecular characterization of soft tissue tumours: a gene expresstion study. The Lancet. 2002;359:1301–7. doi: 10.1016/S0140-6736(02)08270-3. [DOI] [PubMed] [Google Scholar]
- 4.Segal NH, Pavlidis P, Antonescu CR, Maki RG, Noble WS, DeSantis D, Woodruff JM, Lewis JJ, Brennan MF, Houghton AN, Cordon-Cardo C. Classification and subtype prediction of adult soft tissue sarcoma by functional genomics. Am J Pathol. 2003;163:691–700. doi: 10.1016/S0002-9440(10)63696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nystrom LM, Reimer NB, Reith JD, Dang L, Zlotecki RA, Scarborough MT, Gibbs CP., Jr Multidisciplinary management of soft tissue sarcoma. ScientificWorldJournal. 2013;28:852462. doi: 10.1155/2013/852462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murakami T, DeLong J, Eilber FC, Zhao M, Zhang Y, Zhang N, Singh A, Russell T, Deng S, Reynoso J, Quan C, Hiroshima Y, Matsuyama R, Chishima T, Tanaka K, Bouvet M, Chawla S, Endo I, Hoffman RM. Tumor-targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft PDOX model. Oncotarget. 2016;7:12783–90. doi: 10.18632/oncotarget.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borden EC, Baker LH, Bell RS, Bramwell V, Demetri GD, Eisenberg BL, Fletcher CD, Fletcher JA, Ladanyi M, Meltzer P, O'sullivan B, Parkinson DR, Pisters PW, Saxman S, Singer S, Sundaram M, van Oosterom AT, Verweij J, Waalen J, Weiss SW, Brennan MF. Soft tissue sarcomas of adults: state of the translational science. Clin Ca Res. 2003;9:1941–56. [PubMed] [Google Scholar]
- 8.Konecny GE, Winterhoff B, Kolarova T, Qi J, Manivong K, Dering J, Yang G, Chalukya M, Wang HJ, Anderson L, Kalli KR, Finn RS, Ginther C, Jones S, Velculescu VE, Riehle D, Cliby WA, Randolph S, Koehler M, Hartmann LC, Slamon DJ. Expression of p16 and retinoblastoma determines response to CDK4/6 inhibition in ovarian cancer. Clin Cancer Res. 2011;17:1591–602. doi: 10.1158/1078-0432.CCR-10-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cen L, Carlson BL, Schroeder MA, Ostrem JL, Kitange GJ, Mladek AC, Fink SR, Decker PA, Wu W, Kim JS, Waldman T, Jenkins RB, Sarkaria JN. p16-Cdk4-Rb axis controls sensitivity to a cyclin-dependent kinase inhibitor PD0332991 in glioblastoma xenograft cells. Neuro Oncol. 2012;14:870–81. doi: 10.1093/neuonc/nos114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Witzleben A, Goerttler LT, Marienfeld R, Barth H, Lechel A, Mellert K, Böhm M, Kornmann M, Mayer-Steinacker R, von Baer A, Schultheiss M, Flanagan AM, Möller P, Brüderlein S, Barth TF. Preclinical Characterization of Novel Chordoma Cell Systems and Their Targeting by Pharmocological Inhibitors of the CDK4/6 Cell-Cycle Pathway. Cancer Res. 2015;75:3823–31. doi: 10.1158/0008-5472.CAN-14-3270. [DOI] [PubMed] [Google Scholar]
- 11.Dickson MA, Tap WD, Keohan ML, D'Angelo SP, Gounder MM, Antonescu CR, Landa J, Qin LX, Rathbone DD, Condy MM, Ustoyev Y, Crago AM, Singer S, Schwartz GK. Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. J Clin Oncol. 2013;31:2024–8. doi: 10.1200/JCO.2012.46.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulvihill MJ, Cooke A, Rosenfeld-Franklin M, Buck E, Foreman K, Landfair D, O'Connor M, Pirritt C, Sun Y, Yao Y, Arnold LD, Gibson NW, Ji QS. Discovery of OSI-906: a selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor. Future Med Chem. 2009;1:1153–71. doi: 10.4155/fmc.09.89. [DOI] [PubMed] [Google Scholar]
- 13.Garofalo C, Manara MC, Nicoletti G, Marino MT, Lollini PL, Astolfi A, Pandini G, Lopez-Guerrero JA, Schaefer KL, Belfiore A, Picci P, Scotlandi K. Efficacy of and resistance to anti-IGF-1R therapies in Ewing's sarcoma is dependent on insulin receptor signaling. Oncogene. 2011;30:2730–40. doi: 10.1038/onc.2010.640. [DOI] [PubMed] [Google Scholar]
- 14.Kuijjer ML, Peterse EF, van den Akker BE, Briaire-de Bruijn IH, Serra M, Meza-Zepeda LA, Myklebost O, Hassan AB, Hogendoorn PC, Cleton-Jansen AM. IR/IGF1R signaling as potential target for treatment of high-grade osteosarcoma. BMC Cancer. 2013;13:245. doi: 10.1186/1471-2407-13-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically from histologically intact patient specimens. Proc Natl Acad Sci USA. 1992;89:5645–5649. doi: 10.1073/pnas.89.12.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiroshima Y, Zhao M, Maawy A, Zhang Y, Katz MH, Fleming JB, Uehara F, Miwa S, Yano S, Momiyama M, Suetsugu A, Chishima T, Tanaka K, Bouvet M, Endo I, Hoffman RM. Efficacy of Salmonella typhimurium A1-R versus chemotherapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX) J Cell Biochem. 2014;115:1254–1261. doi: 10.1002/jcb.24769. [DOI] [PubMed] [Google Scholar]
- 17.Hiroshima Y, Maawy A, Zhang Y, Murakami T, Momiyama M, Mori R, Matsuyama R, Katz MH, Fleming JB, Chishima T, Tanaka K, Ichikawa Y, Endo I, Hoffman RM, Bouvet M. Metastatic recurrence in a pancreatic cancer patient derived orthotopic xenograft (PDOX) nude mouse model is inhibited by neoadjuvant chemotherapy in combination with fluorescence-guided surgery with an anti-CA 19-9-conjugated fluorophore. PLOS ONE. 2014;9:e114310. doi: 10.1371/journal.pone.0114310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiroshima Y, Zhang Y, Murakami T, Maawy AA, Miwa S, Yamamoto M, Yano S, Sato S, Momiyama M, Mori R, Matsuyama R, Chishima T, Tanaka K, Ichikawa Y, Bouvet M, Endo I, Zhao M, Hoffman RM. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograph (PDOX) and cell line mouse models. Oncotarget. 2014;5:12346–12357. doi: 10.18632/oncotarget.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiroshima Y, Maawy AA, Katz MH, Fleming JB, Bouvet M, Endo I, Hoffman RM. Selective efficacy of zoledronic acid on metastasis in a patient-derived orthotopic xenograph (PDOX) nude-mouse model of human pancreatic cancer. J Surg Oncol. 2015;111:311–315. doi: 10.1002/jso.23816. [DOI] [PubMed] [Google Scholar]
- 20.Fu X, Le P, Hoffman RM. A metastatic-orthotopic transplant nude-mouse model of human patient breast cancer. Anticancer Res. 1993;13:901–904. [PubMed] [Google Scholar]
- 21.Fu X, Hoffman RM. Human ovarian carcinoma metastatic models constructed in nude mice by orthotopic transplantation of histologically-intact patient specimens. Anticancer Res. 1993;13:283–286. [PubMed] [Google Scholar]
- 22.Wang X, Fu X, Hoffman RM. A new patient-like metastatic model of human lung cancer constructed orthotopically with intact tissue via thoracotomy in immunodeficient mice. Int J Cancer. 1992;51:992–995. doi: 10.1002/ijc.2910510626. [DOI] [PubMed] [Google Scholar]
- 23.Hiroshima Y, Zhang Y, Zhang M, Maawy A, Mii S, Yamamoto M, Uehara F, Miwa S, Yano S, Murakami T, Momiyama M, Chishima T, Tanaka K, Ichikawa Y, Bouvet M, Murata T, Endo I, Hoffman RM. Establishment of a patient-derived orthotopic xenograph (PDOX) model of HER-2-positive cervical cancer expressing the clinical metastatic pattern. PLOS ONE. 2015;10:e0117417. doi: 10.1371/journal.pone.0117417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu X, Besterman JM, Monosov A, Hoffman RM. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci USA. 1991;88:9345–9349. doi: 10.1073/pnas.88.20.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metildi CA, Kaushal S, Luiken GA, Talamini MA, Hoffman RM, Bouvet M. Fluorescently-labeled chimeric anti-CEA antibody improves detection and resection of human colon cancer in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Surg Oncol. 2014;109:451–458. doi: 10.1002/jso.23507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiroshima Y, Maawy A, Metildi CA, Zhang Y, Uehara F, Miwa S, Yano S, Sato S, Murakami T, Momiyama M, Chishima T, Tanaka K, Bouvet M, Endo I, Hoffman RM. Successful fluorescence-guided surgery on human colon cancer patient-derived orthotopic xenograft mouse models using a fluorophore-conjugated anti-CEA antibody and a portable imaging system. J Laparoendosc Adv Surg Tech A. 2014;24:241–247. doi: 10.1089/lap.2013.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furukawa T, Kubota T, Watanabe M, Kitajima M, Fu X, Hoffman R.M. Orthotopic transplantation of histologically intact clinical specimens of stomach cancer to nude mice: correlation of metastatic sites in mouse and individual patient donors. Int. J Cancer. 1993;53:608–612. doi: 10.1002/ijc.2910530414. [DOI] [PubMed] [Google Scholar]
- 28.Astoul P, Wang X, Colt HG, Boutin C, Hoffman RM. A patient-like human malignant pleural mesothelioma nude-mouse model. Oncology Reports. 1996;3:483–7. [PubMed] [Google Scholar]
- 29.Hiroshima Y, Zhang Y, Zhang N, Uehara F, Maawy A, Murakami T, Mii S, Yamamoto M, Miwa S, Yano S, Momiyama M, Mori R, Matsuyama R, Chishima T, Tanaka K, Ichikawa Y, Bouvet M, Endo I, Hoffman RM. Patient-derived orthotopic xenograft (PDOX) nude mouse model of soft-tissue sarcoma more closely mimics the patient behavior in contrast to the subcutaneous ectopic model. Anticancer Res. 2015;35:697–701. [PubMed] [Google Scholar]
- 30.Hiroshima Y, Zhao M, Zhang Y, Zhang N, Maawy A, Murakami T, Mii S, Uehara F, Yamamoto M, Miwa S, Yano S, Momiyama M, Mori R, Matsuyama R, Chishima T, Tanaka K, Ichikawa Y, Bouvet M, Endo I, Hoffman RM. Tumor-targeting Salmonella typhimurium A1-R arrests a chemo-resistant patient soft-tissue sarcoma in nude mice. PLOS ONE. 2015;10:e0134324. doi: 10.1371/journal.pone.0134324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiyuna T, Murakami T, Tome Y, Kawaguchi K, Igarashi K, Zhang Y Zhao M, Li Y, Bouvet M, Kanaya F, Singh A, Dry S, Eilber FC, Hoffman RM. High efficacy of tumor-targeting Salmonella typhimurium A1-R on a doxorubicin- and dactolisib-resistant follicular dendritic-cell sarcoma in a patient-derived orthotopic xenograft nude mouse model. Oncotarget. 2016;7:33046–54. doi: 10.18632/oncotarget.8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shing DC, McMullan DJ, Roberts P, Smith K, Chin SF, Nicholson J, Tillman RM, Ramani P, Cullinane C, Coleman N. FUS/ERG gene fusions in Ewing's tumors. Cancer Res. 2003;63:4568–76. [PubMed] [Google Scholar]
- 33.Gao J, Adams RP, Swain SM. Does CDKN2A loss predict palbociclib benefit? Curr Oncol. 2015;22:e498–501. doi: 10.3747/co.22.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kowalewski AA, Randall RL, Lessnick SL. Cell Cycle Deregulation in Ewing's sarcoma pathogenesis. Sarcoma. 2011;2011:59870. doi: 10.1155/2011/598704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovar H, Auinger A, Jug G, Aryee D, Zoubek A, Salzer-Kuntschik M, Gadner H. Narrow spectrum of infrequent p53 mutations and absence of MDM2 amplification in Ewing tumours. Oncogene. 1993;8:2683–90. [PubMed] [Google Scholar]
- 36.Italiano A, Sung YS, Zhang L, Singer S, Maki RG, Coindre JM, Antonescu CR. High prevalence of CIC fusion with double-homeobox (DUX4) transcription factors in EWSR1-negative undifferentiated small blue round cell sarcomas. Genes Chromosomes Cancer. 2012;51:207–18. doi: 10.1002/gcc.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berg T, Kalsaas AH, Buechner J, Busund LT. Ewing sarcoma-peripheral neuroectodermal tumor of the kidney with a FUS-ERG fusion transcript. Cancer Genet Cytogenet. 2009;194:53–7. doi: 10.1016/j.cancergencyto.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Sotoca AM, Prange KH, Reijnders B, Mandoli A, Nguyen LN, Stunnenberg HG, Martens JH. The oncofusion protein FUS-ERG targets key hematopoietic regulators and modulates the all-trans retinoic acid signaling pathway in t(16;21) acute myeloid leukemia. Oncogene. 2016;35:1965–76. doi: 10.1038/onc.2015.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cironi L, Riggi N, Provero P, Wolf N, Suvà ML, Suvà D, Kindler V, Stamenkovic I. IGF1 is a common target gene of Ewing's sarcoma fusion proteins in mesenchymal progenitor cells. PLOS ONE. 2008;3:e2634. doi: 10.1371/journal.pone.0002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldberg L, Tijssen MR, Birger Y, Hannah RL, Kinston SJ, Schütte J, Beck D, Knezevic K, Schiby G, Jacob-Hirsch J, Biran A, Kloog Y, Marcucci G, Bloomfield CD, Aplan PD, Pimanda JE, Göttgens B, Izraeli S. Genome-scale expression and transcription factor binding profiles reveal therapeutic targets in transgenic ERG myeloid leukemia. Blood. 2013;122:2694–703. doi: 10.1182/blood-2013-01-477133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tognon CE, Sorensen PH. Targeting the insulin-like growth factor 1 receptor (IGF1R) signaling pathway for cancer therapy. Expert Opin Ther Targets. 2012;16:33–48. doi: 10.1517/14728222.2011.638626. [DOI] [PubMed] [Google Scholar]
- 42.Fleuren ED, Versleijen-Jonkers YM, Boerman OC, van der Graaf WT. Targeting receptor tyrosine kinases in osteosarcoma and Ewing sarcoma: current hurdles and future perspectives. Biochim Biophys Acta. 2014;1845:266–76. doi: 10.1016/j.bbcan.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Asmane I, Watkin E, Alberti L, Duc A, Marec-Berard P, Ray-Coquard I, Cassier P, Decouvelaere AV, Ranchère D, Kurtz JE, Bergerat JP, Blay JY. Insulin-like growth factor type 1 receptor (IGF-1R) exclusive nuclear staining: a predictive biomarker for IGF-1R monoclonal antibody (Ab) therapy in sarcomas. Eur J Cancer. 2012;48:3027–35. doi: 10.1016/j.ejca.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Park J, Murakami T, Lee J Zhang Y, Hoffman RM, Bouvet M. Fluorescent-antibody targeting of insulin-like growth factor-1 receptor visualizes metastatic human colon cancer in orthotopic mouse models. PLOS ONE. 2016;11:e0146504. doi: 10.1371/journal.pone.0146504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blagosklonny MV. Matching targets for selective cancer therapy. Drug Discov Today. 2003;8:1104–7. doi: 10.1016/s1359-6446(03)02806-x. [DOI] [PubMed] [Google Scholar]
- 46.Blagosklonny MV. Teratogens as anti-cancer drugs. Cell Cycle. 2005;4:1518–21. doi: 10.4161/cc.4.11.2208. [DOI] [PubMed] [Google Scholar]
- 47.Blagosklonny MV. Treatment with inhibitors of caspases, that are substrates of drug transporters, selectively permits chemotherapy-induced apoptosis in multidrug-resistant cells but protects normal cells. Leukemia. 2001;15:936–41. doi: 10.1038/sj.leu.2402127. [DOI] [PubMed] [Google Scholar]
- 48.Blagosklonny MV. Target for cancer therapy: proliferating cells or stem cells. Leukemia. 2006;20:385–91. doi: 10.1038/sj.leu.2404075. [DOI] [PubMed] [Google Scholar]
- 49.Apontes P, Leontieva OV, Demidenko ZN, Li F, Blagosklonny MV. Exploring long-term protection of normal human fibroblasts and epithelial cells from chemotherapy in cell culture. Oncotarget. 2011;2:222–33. doi: 10.18632/oncotarget.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blagosklonny MV. Tissue-selective therapy of cancer. Br J Cancer. 2003;89:1147–51. doi: 10.1038/sj.bjc.6601256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stebbing J, Paz K, Schwartz GK, Wexler LH, Maki R, Pollock RE, Morris R, Cohen R, Shankar A, Blackman G, Harding V, Vasquez D, Krell J, Zacharoulis S, Ciznadija D, Katz A, Sidransky D. Patient-derived xenografts for individualized care in advanced sarcoma. Cancer. 2014;120:2006–15. doi: 10.1002/cncr.28696. [DOI] [PMC free article] [PubMed] [Google Scholar]


