Abstract
Metastatic prostate cancer is the leading cause of morbidity and mortality in men. In this study, we found that expression level of SFMBT2 is altered during prostate cancer progression and has been associated with the migration and invasion of prostate cancer cells. The expression level of SFMBT2 is high in poorly metastatic prostate cancer cells compared to highly metastatic prostate cancer cells. We also found that SFMBT2 knockdown elevates MMP-2, MMP-3, MMP-9, and MMP-26 expression, leading to increased cell migration and invasion in LNCaP and VCaP cells. SFMBT2 interacts with YY1, RNF2, N-CoR and HDAC1/3, as well as repressive histone marks such as H3K9me2, H4K20me2, and H2AK119Ub which are associated with transcriptional repression. In addition, SFMBT2 knockdown decreased KAI1 gene expression through up-regulation of N-CoR gene expression. Expression of SFMBT2 in prostate cancer was strongly associated with clinicopathological features. Patients having higher Gleason score (≥ 8) had substantially lower SFMBT2 expression than patients with lower Gleason score. Moreover, tail vein or intraprostatic injection of SFMBT2 knockdown LNCaP cells induced metastasis. Taken together, our findings suggest that regulation of SFMBT2 may provide a new therapeutic strategy to control prostate cancer metastasis as well as being a potential biomarker of metastatic prostate cancer.
Keywords: prostate cancer, metastasis, SFMBT2, gene regulation
INTRODUCTION
The number of newly diagnosed prostate cancer patients has decreased slightly, but newly diagnosed patients in the United States is more than 200,000 cases per year [1]. The prostate-specific antigen (PSA) is the most widely used for early detection of prostate cancer, risk classification, and monitoring of the disease although it is not specific for prostate cancer [2, 3]. Therefore, many efforts have been made to identify new biomarkers to increase accuracy of prostate cancer diagnosis, and recently new biomarkers such as SRPK1, CXCL12, and TMPRSS4 have been reported [4–6].
It has been reported that 4% of prostate cancer patients in the United State develop a distant metastasis, but the 5-year survival rate is only 28% compared to 100% in localized and regional disease [7]. Prostate cancer cells are able to infiltrate into the lymphatic system or blood stream, and spread mainly to the liver, lung, lymph nodes and bone [8]. Abnormal control of TGF signaling is known to play an important role in promoting tumor metastasis [9]. Up-regulation of the Akt/mTOR pathway by inactivation of PTEN is also associated with invasion and metastasis of prostate cancer, which has led to development of drugs to inhibit this pathway [10, 11]. In addition, increased expression of matrix metalloproteinases (MMPs) has been shown to be associated with prostate cancer progression and metastasis [11, 12].
Polycomb group (PcG) proteins regulate the expression of developmental genes, and abnormal control of the PcG proteins is known to cause cancer [13, 14]. For example, histone H3K27 methyltransferase EZH2 is upregulated in various types of cancer such as cancer of the breast, colon and prostate [15]. Moreover, EZH2 has received attention as a target for cancer treatment because EZH2-mediated tri-methylation of histone H3K27 results in inactivation of tumor suppressor genes such as PSP94 and p16INK4a [16–18]. Overexpression of the YY1 has been reported in various cancers including that of breast and prostate [19, 20]. YY1 negatively regulates p53 through proteasome-dependent ubiquitination [21]. YY1 also interacts with cell cycle regulators such as cyclin D, c-Myc and Rb, resulting in abnormal cell proliferation [22].
Recently, SFMBT2, another PcG protein [23], was shown to be involved in prostate cancer cell growth. SFMBT2 interacts with YY1 and regulates cell growth through repression of the HOXB13 gene in DU145 prostate cancer cells [24]. SFMBT has an MBT (malignant brain tumor) domain, which is important for gene regulation by recognizing and binding to methylated lysine residue of histone H3 and H4 tails [25]. In fact, MBT domains of Drosophila SFMBT preferentially bind to mono- and di-methylated histone H3K9 and H4K20 peptides, which are associated with transcriptional repression [23, 26]. Human SFMBT2 also binds to methylated lysine residue of histone H3 and H4, which are found in inactive genes, indicating that SFMBT2 may be involved in recognizing repressive hypermethylated histones and maintaining inactive chromatin. Similarly, SFMBT1 forms a complex with LSD1 and CoREST. This complex further induces inactive chromatin and transcriptional repression of replication-dependent histone genes [27].
In this study, we investigated the role of SFMBT2 in metastasis of prostate cancer. Knockdown of SFMBT2 increases prostate cancer cell migration and invasion via direct repression of target genes such as MMP-9, MMP-26, and N-CoR in LNCaP and VCaP cells. In addition, a metastasis suppressor KAI1 gene is regulated indirectly by SFMBT2. Interestingly, expression level of SFMBT2 inversely correlates with Gleason score in prostate cancer patients. Moreover, we found that tail vein or intraprostatic injection of SFMBT2 knockdown LNCaP cells significantly induces metastasis, indicating that SFMBT2 acts as a metastasis suppressor in prostate cancer in vivo.
RESULTS
SFMBT2 regulates cell migration and invasion in LNCaP cells
We found previously that mammalian SFMBT2, a polycomb gene (PcG), regulates cell growth in prostate cancer cells [24]. To further evaluate a possible role of SFMBT2 in prostate cancer progression, we first analyzed expression level of SFMBT2 in various prostate cancer cells lines. The expression level of SFMBT2 was high in normal RWPE-1 prostate cells and poorly metastatic LNCaP prostate cancer cells, but low in highly metastatic prostate cancer cells, such as PC3 and DU145, indicating that SFMBT2 expression is likely related to metastasis (Figure 1A).
Figure 1. SFMBT2-mediated cell migration and invasion in LNCaP cells.
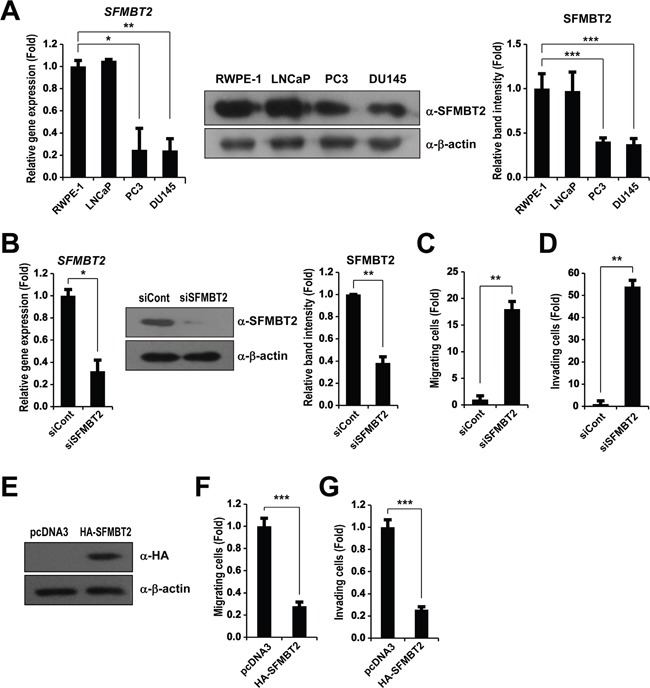
A. Differential expression level of SFMBT2 in normal prostate and prostate cancer cell lines. Transcripts of SFMBT2 and GAPDH were determined by quantitative PCR in RWPE-1, LNCaP, PC3, and DU145 cells (n=3). The cell lysates were immunoblotted with anti-SFMBT2 and anti-β-actin antibodies, respectively (n=3). Western blots were analyzed quantitatively. B. Knockdown of SFMBT2 results in increased cell migration and invasion in LNCaP cells. After control (siCont) or SFMBT2 siRNA (siSFMBT2) were transfected, LNCaP cells were subjected to RNA and protein extraction (n=3). Transcripts of SFMBT2 and GAPDH were determined by quantitative PCR. The cell lysates were immunoblotted with anti-SFMBT2 and anti-β-actin antibodies, respectively. Western blots were analyzed quantitatively. C. After control or SFMBT2 siRNA were transfected, LNCaP cells were subjected to a cell migration assay using a modified Boyden chamber containing uncoated Transwell polycarbonate membrane filters (n=3). The migrated cells stained with cresyl violet were counted. D. After control or SFMBT2 siRNA were transfected, LNCaP cells were subjected to a cell invasion assay using a Biocoat Matrigel invasion chambers (n=3). Invading cells on the membrane stained with cresyl violet were counted. E. PC3 cells were transfected with pcDNA3 or pcDNA3-SFMBT2-HA plasmid (n=3). The cell lysates were immunoblotted with anti-HA and anti-β-actin antibodies, respectively. F, G. After PC3 cells were transfected with pcDNA3 or pcDNA3-SFMBT2-HA plasmid, cell migration assay (n=3) and invasion assay (n=3) were performed. All data represent mean ± S.E.M. Significance values were * P≤0.05, ** P≤0.01 and *** P≤0.005.
We next decided to investigate whether knockdown of SFMBT2 by siRNA affects the cell migration and invasion, which are main features of metastasis, using poorly metastatic LNCaP cells [28, 29]. Quantitative PCR and Western blotting showed SFMBT2 siRNA efficiently down-regulated SFMBT2 expression in LNCaP cells (Figure 1B). When SFMBT2 knockdown LNCaP cells were subjected to cell migration assay using a modified Boyden chamber, we found increased cell migration as compared to control siRNA-transfected cells (Figure 1C). In addition, knockdown of SFMBT2 further resulted in increased cell invasion using a Biocoat Matrigel invasion chamber (Figure 1D). Consistently, we also found that overexpression of SFMBT2 decreases cell migration and invasion in highly metastatic PC3 cells, which expresses low level of SFMBT2 (Figure 1A and 1E-1G). These results suggest that SFMBT2 may function as a negative regulator in cell migration and invasion in prostate cancer cells.
Knockdown of SFMBT2 increases expression and activity of MMP genes
Given that SFMBT2 participates in transcriptional repression, and MMPs are critical for cell migration and invasion through proteolysis of the extracellular matrix [24, 30, 31], we tested whether SFMBT2 regulates MMP gene expression. We examined expression of MMP genes including MMP-2, MMP-3, MMP-7, MMP-9, MMP-13, MMP-14, MMP-15, and MMP-26 that are known to be up-regulated during prostate cancer progression [11]. Among MMPs, we found a significantly increased expression of the MMP-2, MMP-3, MMP-9, and MMP-26 genes in SFMBT2 knockdown LNCaP cells (Figure 2A and Supplementary Figure S1). We also performed experiments using other androgen-dependent prostate cancer VCaP cells [32, 33]. Consistent with the results from LNCaP cells, knockdown of SFMBT2 resulted in increased expression of MMP-2, MMP-3, MMP-9 and MMP-26 genes as well as increases cell migration and invasion in VCaP cells (Supplementary Figure S2).
Figure 2. SFMBT2 regulates expression of matrix metalloproteinase in LNCaP cells.
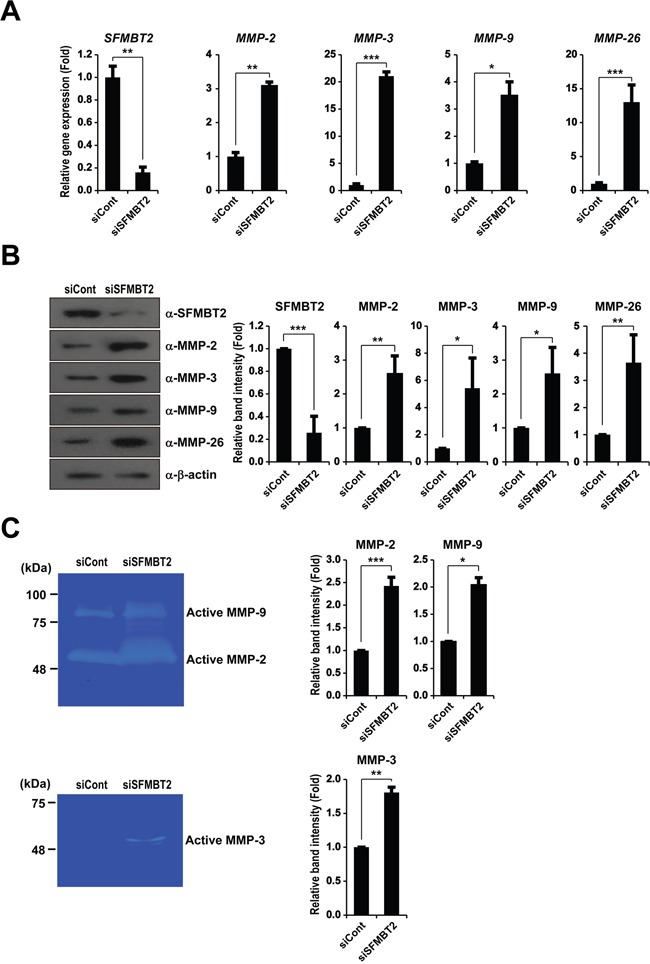
Knockdown of SFMBT2 increases expression of the MMP-2, MMP-3, MMP-9, and MMP-26 gene in LNCaP cells. A. After control or SFMBT2 siRNA were transfected, LNCaP cells were subjected to RNA extraction (n=3). Transcripts of MMP-2, MMP-3, MMP-9, MMP-26, and GAPDH were determined by quantitative PCR. B. The cell lysates were immunoblotted with anti-MMP-2, anti-MMP-3, anti-MMP-9, and anti-MMP-26 antibodies, respectively (n=3). Anti-β-actin was used as a loading control. Western blots were analyzed quantitatively. C. Elevation of enzyme activities of MMP-2, MMP-3, and MMP-9 in SFMBT2 knockdown LNCaP cells. Zymography was performed using the lysates from control and SFMBT2 siRNA-transfected cells (n=3). Equal amounts of the lysate were used for each reaction. Zymography was analyzed quantitatively. All data represent mean ± S.E.M. Significance values were * P≤0.05, ** P≤0.01, and *** P≤0.005.
We further confirmed the up-regulation of MMP expression by Western blot analysis as shown in Figure 2B. Zymography consistently revealed elevated enzyme activity of MMP-2, MMP-9, and MMP-3 in SFMBT2 knockdown LNCaP cells (Figure 2C). Collectively, these results suggest that SFMBT2 may act as a transcriptional repressor of MMP genes to prevent cell migration and invasion in LNCaP and VCaP cells.
Recruitment of SFMBT2, YY1, RNF2, N-CoR, and HDAC1/3 to MMP-9 and MMP-26 gene promoters
In order to study the molecular mechanism underlying SFMBT2-mediated transcriptional repression of MMP genes, we first investigated the interaction of SFMBT2 with YY1 and other cofactors including co-repressors. Since SFMBT2 is found to interact with YY1, which is a mammalian ortholog of Drosophila PHO [24, 34, 35], we further confirmed interaction of SFMBT2 with YY1 in LNCaP cells (Figure 3A). Consistent with a previous report [27], we also found that SFMBT2 interacts with RING1B/RNF2 E3 ubiquitin ligase, which mediates mono-ubiquitination of H2AK119, in LNCaP cells. SFMBT2 interaction with transcriptional co-repressor N-CoR, HDAC1, and HDAC3 were clearly demonstrated (Figure 3A). The MBT domain of the SFMBT family proteins preferentially binds to methylated histone H3K9 and H4K20 peptides [23–26]. We also confirmed interaction of SFMBT2 with repressive histone marks such as di-methylated H3K9 (H3K9me2) and di-methylated H4K20 (H4K20me2) (Figure 3B). Moreover, SFMBT2 was discovered to be associated with mono-ubiquitinated histone H2AK119 (H2AUb), which is found frequently in silenced genes [36], indicating that SFMBT2 may play a role in maintenance of the inactive state of the MMP genes though an association with repressive histone marks in LNCaP cells.
Figure 3. Recruitment of SFMBT2, YY1, RNF2, N-CoR, HDAC1, and HDAC3 to the MMP-9 and MMP-26 gene promoters in LNCaP cells.
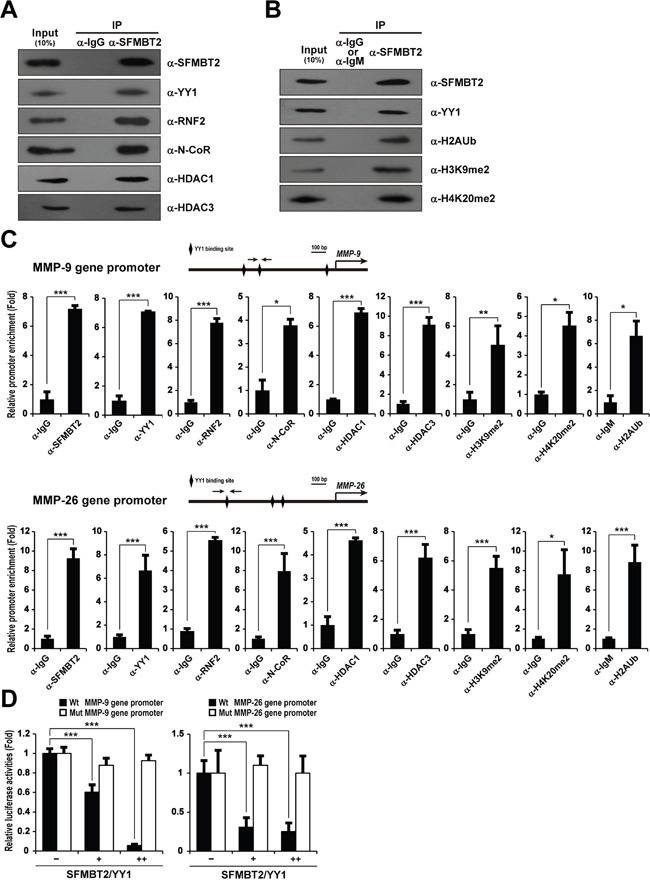
A, B. SFMBT2 interacts with YY1, RNF2, N-CoR, HDAC1, HDAC3, and repressive histone marks. The lysates from LNCaP cells were immunoprecipitated with the anti-SFMBT2 antibody, then immunoblotted with anti-SFMBT2, anti-YY1, anti-RNF2, anti-N-CoR, anti-HDAC1, anti-HDAC3, anti-dimethyl H3K9 (H3K9me2), anti-dimethyl H4K20 (H4K20me2), and anti-mono-ubiquitinated H2AK119 (H2AK119Ub) antibodies, respectively (n=3). Immunoprecipitation with normal IgG or IgM was used as a negative control. C. Enrichment of SFMBT2, YY1, RNF2, N-CoR, HDAC1, and HDAC3 on the MMP-9 and MMP-26 gene promoters in LNCaP cells. A ChIP assay was performed using anti-SFMBT2, anti-YY1, anti-RNF2, anti-N-CoR, anti-HDAC1, anti-HDAC3, anti-H3K9me2, anti-H4K20me2, and anti-H2AK119Ub antibodies, respectively (n=3). The occupancy of each protein was determined by quantitative PCR in MMP-9 and MMP-26 gene promoters encompassing the YY1 binding site using oligonucleotide primers (arrows). ChIP using normal IgG or IgM was performed as a negative control. D. Repression of the MMP-9 and MMP-26 gene promoter reporter activity by over-expression of SFMBT2 and YY1. However, over-expression of SFMBT2 and YY1 does not affect the reporter activity of MMP-9 and MMP-26 gene promoters containing a mutated the YY1 binding site. LNCaP cells were transiently transfected with the human MMP-9 and MMP-26 gene promoter-driven firefly luciferase reporter vector in conjunction with a control Renilla luciferase expression vector (n=3). Expression vectors for SFMBT2 and YY1 were transfected. Reporter activity is represented as fold activation relative to Renilla luciferase activity. All data represent mean ± S.E.M. Significance values were * P≤0.05, ** P≤0.01, and *** P≤0.005.
To investigate recruitment of SFMBT2, YY1, RNF2, N-CoR, HDAC1, and HDAC3 to MMP gene promoters, we first identified putative YY1 binding sites on each genes using in silico bioinformatic analysis (Supplementary Figure S3). We further confirmed YY1 binding site using a series of ChIP assay and mutagenesis. After immunoprecipitation with each of the antibodies, quantitative PCR was performed to amplify the MMP genes' promoter region containing the YY1 binding sites. We found that SFMBT2, YY1, RNF2, N-CoR, HDAC1, and HDAC3 are bound significantly to the MMP-9 and MMP-26 gene promoters (Figure 3C). However, we did not find a significant recruitment of SFMBT2 and YY1 on the MMP-2 and MMP-3 gene promoters, indicating that SFMBT2 may regulate MMP-2 and MMP-3 indirectly (Supplementary Figure S4). H3K9me2 and H4K20me2 were significantly enriched at the MMP-9 and MMP-26 gene promoters. Consistent with recruitment of RNF2, a significant enrichment of H2AK119Ub was also observed at the MMP-9 and MMP-26 gene promoters (Figure 3C). In contrast, we failed to detect a significant enrichment of SFMBT2, YY1, RNF2, N-CoR, HDAC1, and HDAC3 as well as repressive histone marks at the gene promoter region of GAPDH, which is expressed constitutively in LNCaP cells (Supplementary Figure S5). Similarly, when SFMBT2 and YY1 were over-expressed in LNCaP cells carrying MMP-9 and MMP-26 gene promoter reporters, a significant decrease in report activity was observed (Figure 3D). However, we did not observe a significant decreased reporter activity in MMP-9 and MMP-26 gene promoter reporters containing a mutated YY1 binding site (Figure 3D).
SFMBT2 up-regulates KAI1 gene expression through down-regulation of N-CoR
Since the low expression level of the KAI1 has been shown to relate to prostate metastasis [37, 38], we tested whether SFMBT2 regulates KAI1 gene expression. Interestingly, expression of the KAI1 gene was down-regulated in SFMBT2 knockdown LNCaP cells (Figure 4A). Thus, we tested the possibility that SFMBT2 may negatively regulate transcriptional repressors for the KAI1 gene. Among known transcriptional repressors for the KAI1 gene [39], we found up-regulation of N-CoR, TAB2, and NF-κB (p65 and p50) in SFMBT2 knockdown LNCaP cells (Figure 4B). To investigate whether SFMBT2/YY1 directly represses N-CoR, TAB2, p65, and p50 gene expression, we identified functional YY1 binding sites in each gene using in silico bioinformatic analysis (Supplementary Figure S3), ChIP assay, and mutagenesis. Our results indicated that SFMBT2 and YY1 are bound significantly to the N-CoR gene promoter, while we did not observe a significant recruitment of SFMBT2 and YY1 on other genes (Figure 4C and Supplementary Figure S6). We also observed a significant enrichment of RNF2, N-CoR, HDAC1, and HDAC3 as well as repressive histone marks on the N-CoR gene promoter (Figure 4C). Over-expression of SFMBT2 and YY1 repressed reporter activity of the N-CoR gene promoter while reporter activity of the N-CoR gene promoter containing a mutated YY1 binding site did not changed by over-expression of SFMBT2 and YY1 (Figure 4D).
Figure 4. SFMBT2 indirectly regulates expression of KAI1 metastasis suppressor gene through repression of N-CoR gene expression in LNCaP cells.
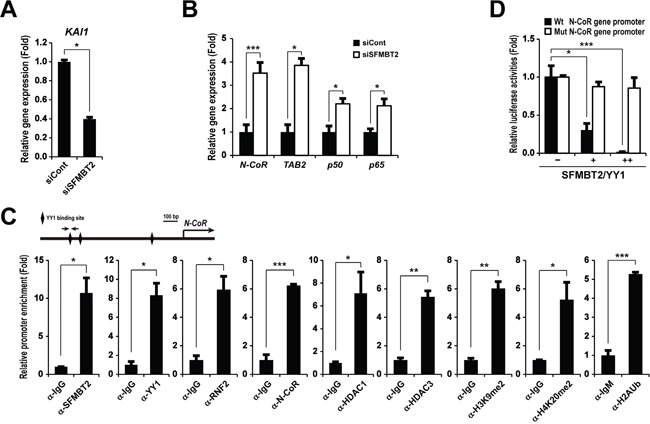
A. Down-regulation of KAI1 gene expression in SFMBT2 knockdown LNCaP cells. Transcripts of KAI1 and GAPDH were determined by quantitative PCR (n=3). B. Up-regulation of transcriptional repressors for KAI1 gene in SFMBT2 knockdown LNCaP cells. Transcripts of N-CoR, TAB2, p50, p65, and GAPDH were determined by quantitative PCR (n=3). C. Enrichment of SFMBT2, YY1, RNF2, N-CoR, HDAC1, HDAC3, di-methyl H3K9, di-methyl H4K20, and mono-ubiquitinated H2AK119 on the N-CoR gene promoter in LNCaP cells. A ChIP assay was performed in LNCaP cells using anti-SFMBT2, anti-YY1, anti-RNF2, anti-N-CoR, anti-HDAC1, anti-HDAC3, anti-H3K9me2, anti-H4K20me2, and anti-H2AK119Ub antibodies, respectively (n=3). The occupancy of each protein was determined by quantitative PCR in the N-CoR gene promoter encompassing the YY1 binding site using oligonucleotide primers (arrows). ChIP using normal IgG or IgM was performed as a negative control. D. Repression of N-CoR gene promoter reporter activity by over-expression of SFMBT2 and YY1. However, over-expression of SFMBT2 and YY1 does not affect reporter activity of the N-CoR gene promoter containing mutated the YY1 binding site. LNCaP cells were transiently transfected with the human N-CoR gene promoter-driven firefly luciferase reporter vector in conjunction with a control Renilla luciferase expression vector (n=3). Expression vectors for SFMBT2 and YY1 were transfected in combination. Reporter activity is represented as fold activation relative to Renilla luciferase activity. All data represent mean ± S.E.M. Significance values were * P≤0.05, ** P≤0.01, and *** P≤0.005.
NF-κB up-regulates MMP-9, MMP-26, and N-CoR gene expression in SFMBT2 knockdown LNCaP cells
It has been reported frequently that YY1 acts as a transcriptional activator and repressor on the same target gene [40–43]. We thus investigated the possibility that YY1 is involved in MMP-9, MMP-26, and N-CoR gene activation in SFMBT2 knockdown LNCaP cells, which were transfected shSFMBT2 stably (shSFMBT2) (Figure 5A). A ChIP assay revealed little to no recruitment of YY1 to the MMP-9, MMP-26, and N-CoR gene promoters (Supplementary Figure S7), indicating that YY1 is not required for MMP-9, MMP-26, and N-CoR gene activation in SFMBT2 knockdown LNCaP cells. Given that NF-κB (p65 and p50) were up-regulated by knockdown of SFMBT2 (Figure 4B), we examined the involvement of NF-κB p65 in MMP-9, MMP-26, and N-CoR gene activation in SFMBT2 knockdown LNCaP cells. As shown in Figure 5B, nuclear localization of p65 was observed in SFMBT2 knockdown LNCaP cells (shSFMBT2) as compared to LNCaP cells transfected with control shRNA stably (shControl). Consistently, knockdown of SFMBT2 resulted in increased phosphorylation of IκB (Figure 5C). We further confirmed our results using BAY 11-7085, an inhibitor of IκBα phosphorylation, to prevent NF-κB activation. BAY 11-7085 treatment attenuated up-regulation of MMP-9, MMP-26, and N-CoR gene expression in SFMBT2 knockdown LNCaP cells (Figure 5D). We also performed ChIP analysis in gene promoters encompassing the NF-κB binding site (Supplementary Figure S3). As expected, recruitment of NF-κB p65 and the p300 co-activator was increased significantly to the MMP-9, MMP-26, and N-CoR gene promoters in SFMBT2 knockdown LNCaP cells as compared to control LNCaP cells (Figure 5E). Enrichment of acetylated H3 to MMP-9, MMP-26, and N-CoR gene promoters was also increased in SFMBT2 knockdown LNCaP cells (Figure 5E).
Figure 5. NF-κB activates MMP-9, MMP-26, and N-CoR gene expression in SFMBT2 knockdown LNCaP cells.
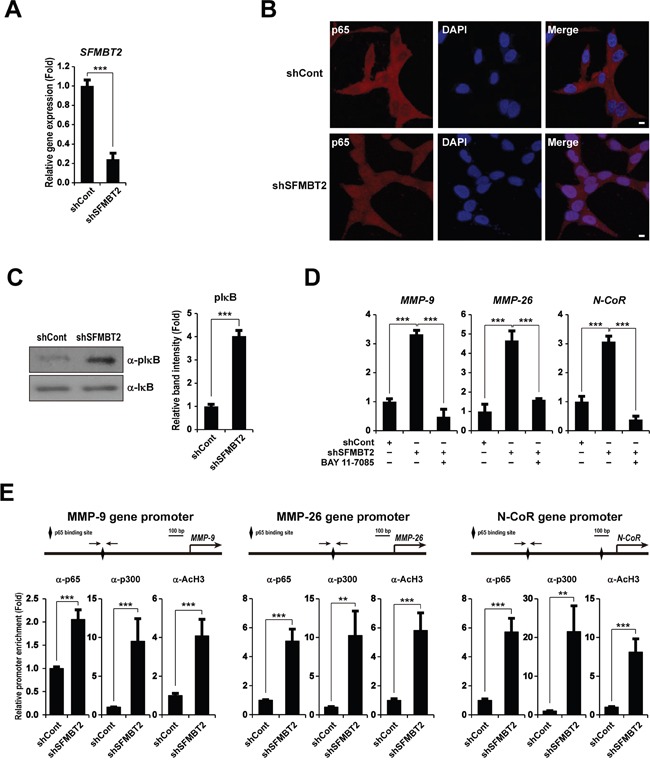
A. After control or SFMBT2 shRNA were transfected stably in LNCaP cells, transcripts of SFMBT2 and GAPDH were determined by quantitative PCR (n=3). B. NF-κB activation in SFMBT2 knockdown LNCaP cells. Knockdown of SFMBT2 results in the nuclear localization of NF-κB p65. Representative photomicrographs of NF-κB p65 in SFMBT2 knockdown LNCaP cells. After control or SFMBT2 shRNA were transfected stably, cells were immunostained with the anti-NF-κB p65 antibody (n=3). Nuclei were identified using DAPI staining. Scale bar, 10 μm. C. Increased phosphorylation of IκB in SFMBT2 knockdown LNCaP cells. After control or SFMBT2 shRNA were transfected stably, lysates were immunoblotted with the anti-IκB and anti-phospho IκB (pIκB) antibodies, respectively (n=3). Western blots were analyzed quantitatively. D. Inactivation of NF-κB by BAY 11-7085 attenuates up-regulation of the MMP-9, MMP-26, and N-CoR genes in SFMBT2 knockdown LNCaP cells. After control or SFMBT2 shRNA were transfected stably, cells were treated with DMSO control or BAY 11-7085. Transcripts of MMP-9, MMP-26, N-CoR, and GAPDH were determined by quantitative PCR (n=3). E. Enrichment of NF-κB p65 and p300 on the MMP-9, MMP-26, and N-CoR gene promoters in SFMBT2 knockdown LNCaP cells. After control or SFMBT2 shRNA were transfected stably, a ChIP assay was performed in LNCaP cells using anti-NF-κB p65, anti-p300, and anti-acetylated H3 antibodies, respectively (n=3). The occupancy of each protein was determined by quantitative PCR in MMP-9, MMP-26, and N-CoR gene promoters encompassing the NF-κB binding site using oligonucleotide primer (arrows). ChIP using normal IgG or IgM was performed as a negative control. All data represent mean ± S.E.M. Significance values were ** P≤0.01 and *** P≤0.005.
SFMBT2 expression in prostate cancer patients
The clinical relevance of SFMBT2 was examined by immunohistochemistry using commercially available prostate cancer tissue arrays (See Materials and Methods). Fifty three samples with various Gleason scores were immunostained using the anti-SFMBT2 antibody (Figure 6A and Supplementary Table S5). In normal prostate tissues, the expression of SFMBT2 was high in 87.5% (7/8 cases) and low in 12.5% (1/8 cases). In contrast, SFMBT2 expression of prostate cancer patient's specimens was low in 60.4 % (32/53 cases), moderate in 32.1% (17/53 cases), and high in 7.5% (4/53 cases) (Supplementary Table S6). Overall, low expression of SFMBT2 appears to be related to prostate cancer. We further analyzed these data to investigate the relationship between expression level of SFMBT2 and Gleason score. We found that 77.42% of analyzed specimens with high Gleason scores of ≥ 8 (24/31 cases) showed low SFMBT2 expression while 22.58% (6+1/31 cases) showed moderate and high SFMBT2 expression (Figure 6A and Supplementary Table S6). In specimens with Gleeson score 4~7, 36.36% (8/22 cases) of specimens showed low SFMBT2 expression while 63.64% (11+3/22 cases) showed moderate and high SFMBT2 expression. These results may suggest that SFMBT2 level inversely correlates with Gleason scores and is related to prognosis of prostate cancer patients such as metastasis and invasion.
Figure 6. SFMBT2 negatively regulates prostate cancer metastasis in vivo.
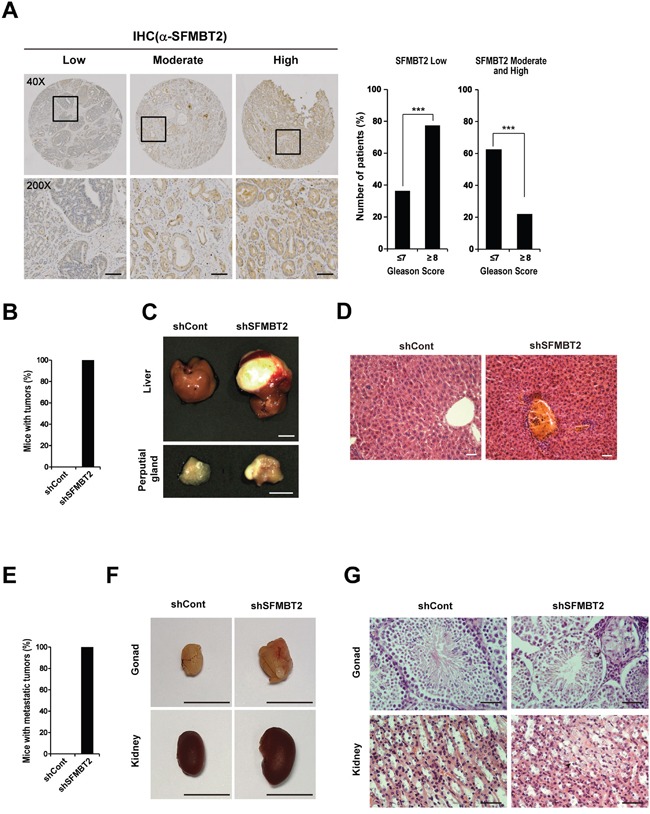
A. Inverse relationship of SFMBT2 expression with Gleason score in prostate cancer. Expression level of SFMBT2 in the specimens with Gleason scores of ≥ 8 was lower than in those with Gleason scores of ≤ 7. Fisher's exact test was used to calculate P values. Immunohistochemical staining of a tissue array from prostate cancer patients was performed using the anti-SFMBT2 antibody. Representative photomicrographs show the low, moderate, and high expression of SFMBT2. Scale bar, 50 μm. B. LNCaP cells (1×106 cells) transfected stably with control (shCont)- or SFMBT2 shRNA (shSFMBT2)-GFP were injected into the tail vein of nude mice, respectively (n=3/group). All mice injected with shSFMBT2-GFP LNCaP cells had tumors. C. Representative photomicrographs show liver and perputial gland tumors in shSFMBT2-GFP LNCaP cells-injected mice at week 15 post injection. Scale bar; 1 cm. D. Infiltration of shSFMBT2-GFP LNCaP cells in the liver. Livers from control (shCont)- or SFMBT2 shRNA (shSFMBT2)-GFP LNCaP cell-injected mice were fixed, sectioned, and stained with H & E. Scale bar, 50 μm. E. LNCaP cells (1×106 cells) transfected stably with control (shCont.)- or SFMBT2 shRNA (shSFMBT2)-GFP were injected into the prostate (dorsal lobe) (n=3/group). All mice injected with shSFMBT2-GFP LNCaP cells had metastatic tumors. F. Representative photomicrographs show gonad and kidney tumors in shSFMBT2-GFP LNCaP cells-injected mice at week 5 post injection. Scale bar; 1 cm. G. Infiltration of shSFMBT2-GFP LNCaP cells in the gonad and kidney. Gonad and kidney from control (shCont)- or SFMBT2 shRNA (shSFMBT2)-GFP LNCaP cell-injected mice were fixed, sectioned, and stained with H & E. Scale bar, 50 μm. All data represent mean ± S.E.M. Significance values were * P≤0.05 and *** P≤0.005.
Down-regulation of SFMBT2 induces tumor metastasis in vivo
In the following experiments, we investigated whether knockdown of SFMBT2 induces tumor metastasis in vivo. We injected stably shSFMBT2-GFP transfected-LNCaP cells, which showed efficient knockdown of SFMBT2, into the tail vein of nude mice (Figure 5A). At 15 weeks after injection, the mice were imaged to detect GFP-positive LNCaP cells. In vivo image analysis revealed that a number of GFP-positive cells increased significantly in all of shSFMBT2-GFP LNCaP cell-injected mice as compared with control mice, which were injected with shControl-GFP LNCaP cells (data not shown). All mice injected with shSFMBT2-GFP LNCaP cells had tumors (Figure 6B). Specifically, we observed tumors containing GFP-positive LNCaP cells in the liver and perputial gland of the shSFMBT2-GFP LNCaP cell-injected mice (Figure 6C and Supplementary Figure S8). Histological analysis of the liver tumors showed infiltration of LNCaP cells (Figure 6D). We also performed intraprostatic injection of stably shSFMBT2-GFP transfected-LNCaP cells. Consistently, we found that knockdown of SFMBT2 induces metastasis (Figure 6E). Metastatic tumors of LNCaP cells expressing shSFMBT2-GFP was observed in the gonad and kidney (Figure 6F and 6G), indicating that knockdown of SFMBT2 promotes prostate cancer metastasis.
DISCUSSION
The mammalian PcG protein SFMBT2 has been shown to play an important role in prostate cancer cell growth through HOXB13 gene regulation via interaction with YY1 [24]. In this study, we further characterized the function of SFMBT2 in prostate cancer metastasis. We showed that the expression level of SFMBT2 is critical for cell migration and invasion, which are fundamental features of prostate cancer malignancy through direct or indirect regulation of MMP-2, MMP-3, MMP-9, MMP-26, N-CoR, and KAI1 gene expression.
It is well known that extracellular matrix (ECM) degradation by MMPs is required for cancer cell migration and invasion [11, 44]. Specifically, increased expression of MMP-2 and MMP-9 has been shown to be associated with prostate cancer progression and metastasis [12, 45]. Other studies also consistently show that inhibition of MMP-2 and MMP-9 expression suppresses the metastatic potential of prostate cancer cells [46, 47]. Although information about MMP-3 is limited regarding prostate cancer progression, it is known that expression of MMP-3 increases in highly metastatic PC3 cells as compared to poorly metastatic LNCaP cells [48]. Invasiveness of PC3 or DU145 cells also seems to be correlated with up-regulation of MMPs including MMP-3 [49–51]. MMP-26 has been shown to activate pro-MMP-9, thereby promoting invasion of prostate cancer cells [52, 53].
Knockdown of SFMBT2 further reveals the dysregulation of the KAI1 metastasis suppressor gene [37, 38]. The expression level of KAI1 is significantly low in invasive prostate tumor as compared to prostate intraepithelial neoplasia (GEO data set: GDS2443). Since SFMBT2 functions as a negative transcriptional regulator, we assumed that SFMBT2 may repress the transcriptional repressor(s) for the KAI1 gene. Among the known transcriptional repressors [39], we found that the N-CoR is repressed by SFMBT2 and YY1 directly. In addition, our results indicate that N-CoR gene expression is regulated by negative feedback, since N-CoR represses its own transcription. Transcriptional repression by SFMBT2 is achieved by interaction with YY1, RNF2, N-CoR, and HDACs at the MMP-9, MMP-26, and N-CoR gene promoters. In fact, YY1 is implicated in prostate cancer development and progression through its regulation of PSA gene expression [54, 55]. We found that E3 ubiquitin ligase RNF2, a PcG protein, is also associated with SFMBT2/YY1 at the promoters. Although the function of RNF2 has not been determined in prostate cancer progression, knockdown of RNF2 by siRNA abolished SNAIL-mediated E-cadherin repression and induced cell migration in pancreatic cancer cells [56].
We assumed that YY1 is also required for up-regulation of MMP-9, MMP-26, and N-CoR gene expression in SFMBT2 knockdown LNCaP cells, since YY1 functions as both a transcriptional activator and a repressor for the same target gene [40–43]. However, ChIP analysis clearly indicated the absence of YY1 but the occupancy of NF-κB on the MMP-9, MMP-26, and N-CoR gene promoters, leading to MMP-9, MMP-26, and N-CoR gene activation in SFMBT2 knockdown LNCaP cells. Our study also shows that NF-κB activation in SFMBT2 knockdown LNCaP cells (Figure 5). Previous studies have consistently demonstrated the requirement of NF-κB for up-regulation of the human MMP-9 gene [57–59]. Although there has been no report thus far on the existence of a functional NF-κB binding site within human MMP-26 and N-CoR gene promoters, we found a putative NF-κB binding site using a promoter reporter and ChIP assays.
Although we do not know how SFMBT2 knockdown results in NF-κB activation at present, recent studies have demonstrated that NF-κB activation maybe associated with metastasis of prostate cancer. NF-κB is activated in highly metastatic prostate cancer cells, such as PC3 and DU145, as compared to poorly metastatic LNCaP cells [59–62]. The nuclear localization of NF-κB was significantly increased in metastatic prostate cancer [60]. Several mechanisms for NF-κB activation have been proposed. For example, LNCaP cells secrete a low level of cytokines, which can activate NF-κB [63, 64]. In addition, genetic alterations such as mutation, amplification, overexpression, and rearrangement of genes involving NF-κB and its signaling also may account for abnormal NF-κB activation in SFMBT2 knockdown LNCaP cells [65–67]. It has been demonstrated that heavy phosphorylation of the IkBα inhibitor by IKK occurs in PC3 and DU145 cells as compared to LNCaP cells [62]. Similarly, we found increased nuclear translocation of NF-κB and phosphorylation of IkBα in SFMBT2 knockdown LNCaP cells (Figure 5A).
The biological significance of SFMBT2 was further confirmed by in vivo metastasis experiments. The tail vein or intraprostatic injection of SFMBT2 knockdown LNCaP cells in mice significantly induces metastasis, indicating that SFMBT2 may inhibit metastasis of prostate cancer in vivo. In addition, expression level of SFMBT2 in prostate cancer patients is negatively correlated with a high Gleason score (≥ 8), which seems closely related to prostate cancer invasion and metastasis [68–71]. Since the follow-up period of the analyzed specimens in this study is an average 28.3 months, we could not test whether expression level of SFMBT2 relates to survival rate. However, it has been found that SFMBT2 levels positively correlate with survival rate in various types of cancer such as breast (GEO accession; GSE21653, P value; 0.0785), colon (GEO accession; GSE17538, P value; 0.068), and astrocytic gliomas (GEO accession; GSE18166, P value; 0.0788). We believe that further studies with larger number of patients are required to support our results.
In conclusion, our study provides evidence that SFMBT2 regulates prostate cancer metastasis either by direct repression of YY1 target genes such as MMP-9, MMP-26, and N-CoR and by indirect regulation of MMP-2, MMP-3, and KAI1 gene expression. Therefore, regulation of SFMBT2 expression or SFMBT2 activity may provide a new therapeutic strategy to suppress cancer cell migration and invasion as well as a potential biomarker in prostate cancer progression.
MATERIALS AND METHODS
RNA interference
LNCaP and VCaP cells were transfected with siRNA against human SFMBT2 (M-026395-01-0005, GE Dharmacon) or control siRNA (sc-37007, Santa Cruz Biotechnology, USA). The efficiency of specific gene knockdown was confirmed with quantitative PCR. Transfection was performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. For stable transfection, LNCaP cells were transfected with GIPZ lentiviral SFMBT2 shRNA containing GFP (RHS4531, GE Dharmacon). GIPZ lentiviral control shRNA (RHS4346, GE Dharmacon) was used as a negative control. Stable cell lines were established by culturing LNCaP cells in media containing 1 μg/ml puromycin. Total 17 clones were obtained. Among them, a cell line showing lowest expression of SFMBT2 was selected by RT-PCR for the further study.
Cell migration and invasion assay
Cell migration assays were performed using modified Boyden Chambers (Transwell, Corning Costar). LNCaP and VCaP cells were starved in serum free medium for 24hr, and then plated into the upper chamber of 24-well Transwell plate with 8μm pore size. Cells were incubated for 24 hr, the migrated cells were fixed, stained with cresyl violet, and counted. For invasion assays, cells were allowed to invade through a matrigel-coated membrane (BD Biosciences). After cells were plated into Transwell plate for 48 hr, cells on the upper side of the membrane were removed and the migrated or invaded cells were fixed, stained with cresyl violet, and counted.
Chromatin immunoprecipitation (ChIP)
ChIP was performed as described previously [24]. Antibodies are described in Supplementary Table S3. Normal IgG (sc-2017, Santa Cruz Biotechnology) or normal IgM (sc-3881, Santa Cruz Biotechnology) was used as a negative control. Quantitative PCR was performed using the primers listed in Supplementary Table S4. The relative proportions of immunoprecipitated fragments were determined using the ΔCt comparative method based on the threshold cycle (Ct) value for each PCR reaction and normalized to input genomic DNA.
Immunohistochemical analysis of SFMBT2 in prostate cancer tissues
Immunohistochemical analysis of prostate cancer tissue arrays (2 mm core diameter) was performed using the Leica BOND MAX autostainer (Leica Biosystems) at the Super Bio Chip Laboratories (Korea). Briefly, slides were deparaffinized at 60°C for 1 hour and then treated with Bond Dewax solution for 3 min at 72°C. Antigen retrieval was performed using Epitomic retrieval solution 2 (pH 9.0) for 20 min at 100°C. After endogenous peroxidases were blocked by incubation with hydrogen peroxide for 5 min, the tissue sections were incubated for 30 min at RT with anti-SFMBT2 antibody (1:150, 730036, Novex). Subsequently, tissue sections were incubated with a polymer-conjugated secondary antibody using the Leica Bond Polymer detection kit. The antigen was visualized with 3, 3′-diaminobenzidine (DAB) solution. The nuclei were counterstained with hematoxylin. The entire fields of tissues array were scanned with Leica BOND-MAX automated imaging system. Intensity values of images from whole tissue were analyzed with ImageJ software (NIH). According to previous report [72], the images were scored based on signal intensity (no staining = 0, weak staining = 1, moderate staining = 2, strong staining = 3) and the extent of stained cells (0% = 0, 1~10% = 1, 11~50% = 2, 51~80% = 3, 81~100% = 4). Scoring was done by two reviewers, who blind to the results. The final score was determined by multiplying the intensity scores with the extent of scores of stained cells. The final scores are in range from 0 to 12. If the score is 0 to 4, it's called “SFMBT2 low”. If the score is 5 to 8, it's called “SFMBT2 moderate”. A score of 8~12 is called “SFMBT2 high”. Fisher's exact test was used to calculate P values.
In vivo metastasis assay
In vivo metastasis assay were performed using mouse tail vein or intraprostatic injection. The committee for experimental animal research at Sogang University approved the animal experiments. Male athymic BALB/c nude mice (5 weeks old, 21 g of average body weight; DBL, Korea) were used for in vivo metastasis experiment with two biological repeats (n=3/group). For tail vein injection, one million LNCaP cells stably transfected with shContol-GFP or shSFMBT2-GFP in 200 μl PBS were injected into tail vein. The mice were imaged for fluorescence using FOBI system (NEO Science). At 15 weeks after injection, mice were dissected and organs were removed and photographed. For intraprostatic injection, male athymic BALB/c nude mice were anesthetized with intraperitoneal injection of 2, 2, 2-tribromoethanol (0.24 μg/g of body weight; Sigma Aldrich) and then placed in a supine position [73]. A midline incision was made in the lower abdomen and the prostate was exteriorized. One million LNCaP cells stably transfected with shContol-GFP or shSFMBT2-GFP in 50 μl PBS were injected to the dorsolateral side of the prostate. The incision was closed with sutures. At 5 weeks after injection, mice were dissected and organs were removed and photographed.
SUPPLEMENTARY MATERIALS FIGURES AND TABLES
Acknowledgments
We thank other member of the laboratory for valuable discussion and technical helps. This work was supported by Basic Science Research Program (2009-0093822, 2013R1A1A1009085) through the National Research Foundation of Korea (NSF) funded by the Ministry of Education, Science and Technology, the Republic of Korea.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction detection and monitoring. Nat Rev Cancer. 2008;8:268–278. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 3.Velonas VM, Woo HH, dos Remedios CG, Assinder SJ. Current Status of Biomarkers for Prostate Cancer. Int J Mol Sci. 2013;14:11034–11060. doi: 10.3390/ijms140611034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mavrou A, Brakspear K, Hamdollah-Zadeh M, Damodaran G, Babaei-Jadidi R, Oxley J, Gillatt DA, Ladomery MR, Harper SJ, Bates DO, Oltean S. Serine-arginine protein kinase 1 (SRPK1) inhibition as a potential novel targeted therapeutic strategy in prostate cancer. Oncogene. 2015;34:4311–4319. doi: 10.1038/onc.2014.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dehghani M, Kianpour S, Zangeneh A, Mostafavi-Pour Z. CXCL12 Modulates Prostate Cancer Cell Adhesion by Altering the Levels or Activities of β1-Containing Integrins. Int J Cell Biol. 2014;2014:981750. doi: 10.1155/2014/981750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi G, Yang X, Dai B, Zhang H, Shen Y, Zhu Y, Zhu Y, Xiao W, Ma C, Wen L, Qin X, Cao D, Ye D. Clinical significance of TMPRSS4 in prostate cancer. Int J Clin Exp Pathol. 2014;7:8053–8058. [PMC free article] [PubMed] [Google Scholar]
- 7.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 8.Disibio G, French SW. Metastatic patterns of cancers: results from a large autopsy study. Arch Pathol Lab Med. 2008;132:931–939. doi: 10.5858/2008-132-931-MPOCRF. [DOI] [PubMed] [Google Scholar]
- 9.Tu WH, Thomas TZ, Masumori N, Bhowmick NA, Gorska AE, Shyr Y, Kasper S, Case T, Roberts RL, Shappell SB, Moses HL, Matusik RJ. The loss of TGF-β signaling promotes prostate cancer metastasis. Neoplasia. 2003;5:267–277. doi: 10.1016/S1476-5586(03)80058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang KD, Ling MT. Targeting drug-resistant prostate cancer with dual PI3K/mTOR inhibition. Curr Med Chem. 2014;21:3048–3056. doi: 10.2174/0929867321666140414100127. [DOI] [PubMed] [Google Scholar]
- 11.Gong Y, Chippada-Venkata UD, Oh WK. Roles of matrix metalloproteinases and their natural inhibitors in prostate cancer progression. Cancers (Basel) 2014;6:1298–1327. doi: 10.3390/cancers6031298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray NP, Reyes E, Tapia P, Badínez L, Orellana N. Differential expression of matrix metalloproteinase-2 expression in disseminated tumor cells and micrometastasis in bone marrow of patients with nonmetastatic and metastatic prostate cancer: theoretical considerations and clinical implications-an immunocytochemical study. Bone Marrow Res. 2012;2012:259351. doi: 10.1155/2012/259351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bracken AP, Helin K. Polycomb group proteins: navigators of lineage pathways led astray in cancer. Nat Rev Cancer. 2009;9:773–784. doi: 10.1038/nrc2736. [DOI] [PubMed] [Google Scholar]
- 14.Tsai HC, Baylin SB. Cancer epigenetics: linking basic biology to clinical medicine. Cell Res. 2011;21:502–517. doi: 10.1038/cr.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauvageau M, Sauvageau G. Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell. 2010;7:299–313. doi: 10.1016/j.stem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beke L, Nuytten M, Van Eynde A, Beullens M, Bollen M. The gene encoding the prostatic tumor suppressor PSP94 is a target for repression by the Polycomb group protein EZH2. Oncogene. 2007;26:4590–4595. doi: 10.1038/sj.onc.1210248. [DOI] [PubMed] [Google Scholar]
- 17.Kondo Y, Shen L, Cheng AS, Ahmed S, Boumber Y, Charo C, Yamochi T, Urano T, Furukawa K, Kwabi-Addo B, Gold DL, Sekido Y, Huang TH, et al. Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nat Genet. 2008;40:741–750. doi: 10.1038/ng.159. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Tong T. FOXA1 antagonizes EZH2-mediated CDKN2A repression in carcinogenesis. Biochem Biophys Res Commun. 2014;453:172–178. doi: 10.1016/j.bbrc.2014.09.092. [DOI] [PubMed] [Google Scholar]
- 19.Begon DY, Delacroix L, Vernimmen D, Jackers P, Winkler R. Yin Yang 1 cooperates with activator protein 2 to stimulate ERBB2 gene expression in mammary cancer cells. J Biol Chem. 2005;280:24428–24434. doi: 10.1074/jbc.M503790200. [DOI] [PubMed] [Google Scholar]
- 20.Seligson D, Horvath S, Huerta-Yepez S, Hanna S, Garban H, Roberts A, Shi T, Liu X, Chia D, Goodglick L, Bonavida B. Expression of transcription factor Yin Yang 1 in prostate cancer. Int J Oncol. 2005;27:131–141. [PubMed] [Google Scholar]
- 21.Sui G, Affar el B, Shi Y, Brignone C, Wall NR, Yin P, Donohoe M, Luke MP, Calvo D, Grossman SR, Shi Y. Yin Yang 1 is a negative regulator of p53. Cell. 2004;117:859–872. doi: 10.1016/j.cell.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Gordon S, Akopyan G, Garban H, Bonavida B. Transcription factor YY1: structure, function, and therapeutic implications in cancer biology. Oncogene. 2006;25:1125–1142. doi: 10.1038/sj.onc.1209080. [DOI] [PubMed] [Google Scholar]
- 23.Klymenko T, Papp B, Fischle W, Köcher T, Schelder M, Fritsch C, Wild B, Wilm M, Müller J. A Polycomb group protein complex with sequence-specific DNA-binding and selective methyl-lysine-binding activities. Genes Dev. 2006;20:1110–1122. doi: 10.1101/gad.377406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee K, Na W, Maeng JH, Wu H, Ju BG. Regulation of DU145 prostate cancer cell growth by Scm-like with four mbt domains 2. J Biosci. 2013;38:105–112. doi: 10.1007/s12038-012-9283-6. [DOI] [PubMed] [Google Scholar]
- 25.Wu S, Trievel RC, Rice JC. Human SFMBT is a transcriptional repressor protein that selectively binds the N-terminal tail of histone H3. FEBS Lett. 2007;581:3289–3296. doi: 10.1016/j.febslet.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimm C, Matos R, Ly-Hartig N, Steuerwald U, Lindner D, Rybin V, Müller J, Müller CW. Molecular recognition of histone lysine methylation by the Polycomb group repressor dSfmbt. EMBO J. 2009;28:1965–1977. doi: 10.1038/emboj.2009.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Bonasio R, Strino F, Kluger Y, Holloway JK, Modzelewski AJ, Cohen PE, Reinberg D. SFMBT1 functions with LSD1 to regulate expression of canonical histone genes and chromatin-related factors. Genes Dev. 2013;27:749–766. doi: 10.1101/gad.210963.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoosein NM, Boyd DD, Hollas WJ, Mazar A, Henkin J, Chung LW. Involvement of urokinase and its receptor in the invasiveness of human prostatic carcinoma cell lines. Cancer Commun. 1991;3:255–264. doi: 10.3727/095535491820873146. [DOI] [PubMed] [Google Scholar]
- 29.Keer HN, Gaylis FD, Kozlowski JM, Kwaan HC, Bauer KD, Sinha AA, Wilson MJ. Heterogeneity in plasminogen activator (PA) levels in human prostate cancer cell lines: increased PA activity correlates with biologically aggressive behavior. Prostate. 1991;18:201–214. doi: 10.1002/pros.2990180303. [DOI] [PubMed] [Google Scholar]
- 30.Bonasio R, Lecona E, Reinberg D. MBT domain proteins in development and disease. Semin Cell Dev Biol. 2010;21:221–230. doi: 10.1016/j.semcdb.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin SC, Kao CY, Lee HJ, Creighton CJ, Ittmann MM, Tsai SJ, Tsai SY, Tsai MJ. Dysregulation of miRNAs-COUP-TFII-FOXM1-CENPF axis contributes to the metastasis of prostate cancer. Nat Commun. 2016;7:11418. doi: 10.1038/ncomms11418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fahrenholtz CD, Rick FG, Garcia MI, Zarandi M, Cai RZ, Block NL, Schally AV, Burnstein KL. Preclinical efficacy of growth hormone-releasing hormone antagonists for androgen-dependent and castration-resistant human prostate cancer. Proc Natl Acad Sci U S A. 2014;111:1084–1089. doi: 10.1073/pnas.1323102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuzmin A, Han Z, Golding MC, Mann MR, Latham KE, Varmuza S. The PcG gene Sfmbt2 is paternally expressed in extraembryonic tissues. Gene Expr Patterns. 2008;8:107–116. doi: 10.1016/j.modgep.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alfieri C, Gambetta MC, Matos R, Glatt S, Sehr P, Fraterman S, Wilm M, Müller J, Müller CW. Structural basis for targeting the chromatin repressor Sfmbt to Polycomb response elements. Genes Dev. 2013;27:2367–2379. doi: 10.1101/gad.226621.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou W, Zhu P, Wang J, Pascual G, Ohgi KA, Lozach J, Glass CK, Rosenfeld MG. Histone H2A monoubiquitination represses transcription by inhibiting RNA polymerase II transcriptional elongation. Mol Cell. 2008;29:69–80. doi: 10.1016/j.molcel.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong JT, Lamb PW, Rinker-Schaeffer CW, Vukanovic J, Ichikawa T, Isaacs JT, Barrett JC. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science. 1995;268:884–886. doi: 10.1126/science.7754374. [DOI] [PubMed] [Google Scholar]
- 38.Dong JT, Suzuki H, Pin SS, Bova GS, Schalken JA, Isaacs WB, Barrett JC, Isaacs JT. Down-regulation of the KAI1 metastasis suppressor gene during the progression of human prostatic cancer infrequently involves gene mutation or allelic loss. Cancer Res. 1996;56:4387–4390. [PubMed] [Google Scholar]
- 39.Baek SH, Ohgi KA, Rose DW, Koo EH, Glass CK, Rosenfeld MG. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-κB and β-amyloid precursor protein. Cell. 2002;110:55–67. doi: 10.1016/s0092-8674(02)00809-7. [DOI] [PubMed] [Google Scholar]
- 40.Park K, Atchison ML. Isolation of a candidate repressor/activator, NF-E1 (YY-1, δ), that binds to the immunoglobulin κ 3′ enhancer and the immunoglobulin heavy-chain μE1 site. Proc Natl Acad Sci U S A. 1991;88:9804–9808. doi: 10.1073/pnas.88.21.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nackley A1, Shea-Eaton W, Lopez D, McLean MP. Repression of the steroidogenic acute regulatory gene by the multifunctional transcription factor Yin Yang 1. Endocrinology. 2002;143:1085–1096. doi: 10.1210/endo.143.3.8668. [DOI] [PubMed] [Google Scholar]
- 42.Weill L, Shestakova E, Bonnefoy E. Transcription factor YY1 binds to the murine β interferon promoter and regulates its transcriptional capacity with a dual activator/repressor role. J Virol. 2003;77:2903–2914. doi: 10.1128/JVI.77.5.2903-2914.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meier RK, Clark BJ. Angiotensin II-dependent transcriptional activation of human steroidogenic acute regulatory protein gene by a 25-kDa cAMP-responsive element modulator protein isoform and Yin Yang 1. Endocrinology. 2012;153:1256–1268. doi: 10.1210/en.2011-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang WG, Sanders AJ, Katoh M, Ungefroren H, Gieseler F, Prince M, Thompson SK, Zollo M, Spano D, Dhawan P, Sliva D, Subbarayan PR, Sarkar M, et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin Cancer Biol. 2015 doi: 10.1016/j.semcancer.2015.03.008. pii: S1044-579X(15)00023-1. [DOI] [PubMed] [Google Scholar]
- 45.Aalinkeel R, Nair BB, Reynolds JL, Sykes DE, Mahajan SD, Chadha KC, Schwartz SA. Overexpression of MMP-9 contributes to invasiveness of prostate cancer cell line LNCaP. Immunol Invest. 2011;40:447–464. doi: 10.3109/08820139.2011.557795. [DOI] [PubMed] [Google Scholar]
- 46.Rah B, Amin H, Yousuf K, Khan S, Jamwal G, Mukherjee D, Goswami A. A novel MMP-2 inhibitor 3-azidowithaferin A (3-azidoWA) abrogates cancer cell invasion and angiogenesis by modulating extracellular Par-4. PLoS One. 2012;7:e44039. doi: 10.1371/journal.pone.0044039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moroz A, Delella FK, Almeida R, Lacorte LM, Fávaro WJ, Deffune E, Felisbino SL. Finasteride inhibits human prostate cancer cell invasion through MMP2 and MMP9 downregulation. PLoS One. 2013;8:e84757. doi: 10.1371/journal.pone.0084757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu L, Wang Z, Li XF, He X, Guan LL, Tuo JL, Wang Y, Luo Y, Zhong HL, Qiu SP, Cao KY. Screening and identification of significant genes related to tumor metastasis and PSMA in prostate cancer using microarray analysis. Oncol Rep. 2013;30:1920–1928. doi: 10.3892/or.2013.2656. [DOI] [PubMed] [Google Scholar]
- 49.Ye L, Sun PH, Martin TA, Sanders AJ, Mason MD, Jiang WG. Psoriasin (S100A7) is a positive regulator of survival and invasion of prostate cancer cells. Urol Oncol. 2013;31:1576–1583. doi: 10.1016/j.urolonc.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 50.Zhu F, Liu P, Li J, Zhang Y. Eotaxin-1 promotes prostate cancer cell invasion via activation of the CCR3-ERK pathway and upregulation of MMP-3 expression. BMC Urol. 2014;31:2049–2054. doi: 10.3892/or.2014.3060. [DOI] [PubMed] [Google Scholar]
- 51.Chen J, Wang Z, Xu D, Liu Y, Gao Y. Aquaporin 3 promotes prostate cancer cell motility and invasion via extracellular signal-regulated kinase 1/2-mediated matrix metalloproteinase-3 secretion. Mol Med Rep. 2015;11:2882–2888. doi: 10.3892/mmr.2014.3097. [DOI] [PubMed] [Google Scholar]
- 52.Zhao YG, Xiao AZ, Newcomer RG, Park HI, Kang T, Chung LW, Swanson MG, Zhau HE, Kurhanewicz J, Sang QX. Activation of pro-gelatinase B by endometase/matrilysin-2 promotes invasion of human prostate cancer cells. J Biol Chem. 2003;278:15056–15064. doi: 10.1074/jbc.M210975200. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto H, Vinitketkumnuen A, Adachi Y, Taniguchi H, Hirata T, Miyamoto N, Nosho K, Imsumran A, Fujita M, Hosokawa M, Hinoda Y, Imai K. Association of matrilysin-2 (MMP-26) expression with tumor progression and activation of MMP-9 in esophageal squamous cell carcinoma. Carcinogenesis. 2004;25:2353–2360. doi: 10.1093/carcin/bgh270. [DOI] [PubMed] [Google Scholar]
- 54.Kashyap V, Bonavida B. Role of YY1 in the pathogenesis of prostate cancer and correlation with bioinformatic data sets of gene expression. Genes Cancer. 2014;5:71–83. doi: 10.18632/genesandcancer.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng Z, Wan M, Cao P, Rao A, Cramer SD, Sui G. Yin Yang 1 regulates the transcriptional activity of androgen receptor. Oncogene. 2009;28:3746–3757. doi: 10.1038/onc.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen J, Xu H, Zou X, Wang J, Zhu Y, Chen H, Shen B, Deng X, Zhou A, Chin YE, Rauscher FJ, 3rd, Peng C, Hou Z. Snail recruits Ring1B to mediate transcriptional repression and cell migration in pancreatic cancer cells. Cancer Res. 2014;74:4353–4363. doi: 10.1158/0008-5472.CAN-14-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yokoo T, Kitamura M. Dual regulation of IL-1 β-mediated matrix metalloproteinase-9 expression in mesangial cells by NF-κ B and AP-1. Am. J. Physiol. 1996;270:F123–F130. doi: 10.1152/ajprenal.1996.270.1.F123. [DOI] [PubMed] [Google Scholar]
- 58.Bond M, Fabunmi RP, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-κB. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- 59.Moon SK, Cha BY, Kim CH. ERK1/2 mediates TNF-α-induced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-κB and AP-1: Involvement of the ras dependent pathway. J Cell Physiol. 2004;198:417–427. doi: 10.1002/jcp.10435. [DOI] [PubMed] [Google Scholar]
- 60.Ismail HA, Lessard L, Mes-Masson AM, Saad F. Expression of NF-κB in prostate cancer lymph node metastases. The Prostate. 2004;58:308–313. doi: 10.1002/pros.10335. [DOI] [PubMed] [Google Scholar]
- 61.Palayoor ST, Youmell MY, Calderwood SK, Coleman CN, Price BD. Constitutive activation of IκB kinase α and NF-κB in prostate cancer cells is inhibited by ibuprofen. Oncogene. 1999;18:7389–7394. doi: 10.1038/sj.onc.1203160. [DOI] [PubMed] [Google Scholar]
- 62.Gasparian AV, Yao YJ, Kowalczyk D, Lyakh LA, Karseladze A, Slaga TJ, Budunova IV. The role of IKK in constitutive activation of NF-κB transcription factor in prostate carcinoma cells. J Cell Sci. 2002;115:141–151. doi: 10.1242/jcs.115.1.141. [DOI] [PubMed] [Google Scholar]
- 63.Baeuerle PA, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 64.Nakajima Y, DelliPizzi AM, Mallouh C, Ferreri NR. TNF-mediated cytotoxicity and resistance in human prostate cancer cell lines. Prostate. 1996;29:296–302. doi: 10.1002/(SICI)1097-0045(199611)29:5<296::AID-PROS4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 65.Rayet B, Gélinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18:6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- 66.Emmerich F, Meiser M, Hummel M, Demel G, Foss HD, Jundt F, Mathas S, Krappmann D, Scheidereit C, Stein H, Dörken B. Overexpression of IκBα without inhibition of NF-κB activity and mutations in the IκBα gene in Reed-Sternberg cells. Blood. 1999;94:3129–3134. [PubMed] [Google Scholar]
- 67.Cabannes E1, Khan G, Aillet F, Jarrett RF, Hay RT. Mutations in the IκBα gene in Hodgkin's disease suggest a tumour suppressor role for IκBα. Oncogene. 1999;18:3063–3070. doi: 10.1038/sj.onc.1202893. [DOI] [PubMed] [Google Scholar]
- 68.Gleason DF. Classification of prostatic carcinoma. Cancer Chemother Rep. 1966;50:125–128. [PubMed] [Google Scholar]
- 69.Gleason DF, The Veterans Administration Cooperative Urological Research Group . Histologic grading and clinical staging of prostatic caricnoma. In: Tannenbaum M, editor. Urologic Pathology: The Prostate. (Chapter 9) Lea & Febiger; Philadelphia: 1977. pp. 171–197. [Google Scholar]
- 70.Glass TR, Tangen CM, Crawford ED, Thompson I. Metastatic carcinoma of the prostate: identifying prognostic groups using recursive partitioning. J Urol. 2003;169:164–169. doi: 10.1016/S0022-5347(05)64059-1. [DOI] [PubMed] [Google Scholar]
- 71.Rusthoven CG, Carlson JA, Waxweiler TV, Yeh N, Raben D, Flaig TW, Kavanagh BD. The prognostic significance of Gleason scores in metastatic prostate cancer. Urol Oncol. 2014;32:707–713. doi: 10.1016/j.urolonc.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 72.Koo CL, Kok LF, Lee MY, Wu TS, Cheng YW, Hsu JD, Ruan A, Chao KC, Han CP. Scoring mechanisms of p16INK4a immunohistochemistry based on either independent nucleic stain or mixed cytoplasmic with nucleic expression can significantly signal to distinguish between endocervical and endometrial adenocarcinomas in a tissue microarray study. J Transl Med. 2009;7:25. doi: 10.1186/1479-5876-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park SI, Kim SJ, McCauley LK, Gallick GE. Pre-clinical mouse models of human prostate cancer and their utility in drug discovery. Curr Protoc Pharmacol. 2010 doi: 10.1002/0471141755.ph1415s51. Chapter 14: Unit 14.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


