Abstract
Early detection of cancers is challenging for lack of specific biomarkers. Adiponectin is an adipokine predominantly derived from adipocytes and hypoadiponectinemia has been reported to associate with risk of many types of cancers. However, available evidence is controversial. Some studies show that increased adiponectin levels correlate with cancer risk. Therefore, we performed a meta-analysis of the association between circulating adiponectin levels and cancer development. A systematic search of PubMed, EMBASE, Wiley Online Library and Cochrane Library was conducted for eligible studies involving circulating adiponectin and malignancies from inception to August 8, 2015. Standard mean differences (SMDs) with 95% confidence intervals (95% CIs) were calculated by use of a random-effect model. Funnel plot and Egger's linear regression test were conducted to examine the risk of publication bias. 107 studies were included with 19,319 cases and 25,675 controls. The pooled analysis indicated that circulating adiponectin levels were lower in patients with various cancers than in controls, with a pooled SMD of −0.334 μg/ml (95% CI, −0.465 to −0.203, P = 0.000). No evidence of publication bias was observed. Circulating high molecular weight adiponectin levels were also lower in cancer patients than in controls, with a pooled SMD of −0.502 μg/ml (95% CI, −0.957 to −0.047, P = 0.000). This meta-analysis provides further evidence that decreased adiponectin levels is associated with risk of various cancers. Hypoadiponectinemia may represent a useful biomarker for early detection of cancers.
Keywords: adiponectin, malignancy, biomarker, diagnosis, meta-analysis
INTRODUCTION
Cancer, a major cause of human mortality, has been a worldwide public health problem. A variety of factors such as genetic lesions, environmental aspect and increasing adoption of unhealthy lifestyle are considered as crucial causes of cancer [1]. Among them, obesity is an important factor contributing to the occurrence and development of malignancies. According to the literature in 2005, 396 and 937 million people suffer from obese and overweight worldwide, respectively [2]. Epidemiological research reveals that obesity increases the risk of cancer with evidence that obese women have 50% higher incidence rate than normal weight women [3]. In the process of obesity, dysregulated circulating hormones and growth factors may play an important role in carcinogenesis [4]. Among them, aberrant adiponectin concentration is reported to be a vital link between obesity and cancer.
Adiponectin, firstly discovered by Scherer et al. in 1995, is an adipokine predominantly produced by adipocytes with the monomeric subunit containing 244 amino-acids in human and circulates abundantly in plasma [5, 6]. Three bioactive forms of adiponectin are produced after post-transcriptional process known as trimeric low molecular weight (90 kD, LMW), hexameric medium molecular weight (180 kD, MMW) and oligomeric high molecular weight (> 400 kD, HMW) adiponectin. Among them, HMW-adiponectin is the dominant form in plasma and has the most biological activity than the other two isoforms [7]. Adiponectin mainly acts on two seven-transmembrane adiponectin receptors, AdipoR1 and AdipoR2. Besides, T-cadherin is also responsible for mediating the role of adiponectin in certain tissues [8, 9]. Adiponectin exerts pleiotropic functions in human health such as anti-inflammation, anti-atherosclerosis, and anti-angiogenesis. It also has the properties of insulin-sensitizing and balancing glucose and lipid metabolism in various cells [10]. A number of studies reveal that circulating adiponectin levels decrease in metabolic syndrome, whereas overexpression of it can counteract metabolic dysfunctions [10]. Besides, increased weight reduces the plasma adiponectin level and decreased weight upregulates circulating adiponectin level [11].
It was first reported that circulating adiponectin level was lower in patients with breast cancer in 2003 [12]. Since then, the most clinical studies have indicated that hypoadiponectinemia is associated with risk of various cancers including prostate, endometrial, and colorectal cancers [13–16]. In addition, adiponectin has anti-proliferative and pro-apoptotic effects on cultured cancer cell lines [17, 18]. These results suggest that adiponectin might be an important regulator in carcinogenesis and progression of cancers. However, unchanged or increased circulating adiponectin levels in pancreatic and hepatocellular carcinoma are also reported [19, 20]. Therefore, understanding the exact role of adiponectin in cancer may offer a novel target in tumor diagnosis and therapeutic strategy. In order to gain a more explicit and evidence-based conclusion on the association between circulating adiponectin levels and carcinogenesis, we conducted a comprehensive meta-analysis of current available studies.
RESULTS
Literature selection
The initial comprehensive search yielded 1486 articles, of which 235 articles were excluded for duplication. Then 997 studies were ruled out because of apparent irrelevance after reading titles and/or abstracts. The remaining 254 studies were included for full-text reading, of which 151 studies were removed for one of the following reasons: (i) reviews, comments or letters (n = 37); (ii) shared population (n = 13); (iii) no report of adiponectin levels and/or SDs for both patients and controls or there was not enough information to calculate them (n = 25); (iv) not case-control study (n = 76). 4 additional studies were included from checking the references list. Finally, 107 studies met the inclusion criteria and were used for further analysis [12, 13, 15, 16, 20–112]. The flow diagram of this selection process was showed in Figure 1.
Figure 1. Flow diagram of the included studies.
Study characteristics
Among the 107 studies, a total of 25,675 controls and 19,319 cases were enrolled until August, 2015. Geographic regions were various, among which 46 studies from Asia, 39 studies from Europe, 19 studies from America, and 3 studies from Africa. 16 types of malignancies were investigated in this meta-analysis, with digestive system cancers accounting for the largest percentage (43 studies); other types included: breast cancer (20 studies), prostate cancer (13 studies), endometrial carcinoma (11 studies), lung cancer (5 studies), renal cancer (3 studies), acute leukemia (3 studies), non-Hodgkin's lymphoma (3 studies), Hodgkin's lymphoma (1 study), multiple myeloma (2 studies), melanoma (1 study), thyroid cancer (1 study), and tongue cancer (1 study). Circulating samples included serum (65 studies) and plasma (37 studies), while 5 studies did not mention the exact one. Most researches provided the mean concentrations of circulating adiponectin levels and the SDs of them. SDs from 11 studies were calculated based on the sample size and P values. 96 studies had NOS scores greater than 6 along with 11 studies had scores of 5. The main characteristics of eligible articles were listed in Table 1.
Table 1. Characteristics of all the included studies in the meta-analysis.
| Author | Year | Type | Country | Ethnicity | Sample | Mean age (Case/control) | Number (Case/control) | Study design | Assay method | Assay source | Study quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Petridou et al. | 2006 | Acute leukemia | Greece/USA | Caucasian | Serum | NR | 201/201 | Case-control | RIA | Beth Israsel Deaconess Medical Center | 8 |
| Moschovi et al. | 2010 | Acute leukemia | Greece | Caucasian | Plasma | 4.3/5.2 | 9/9 | Prospective case-control | Other | Linco Research | 7 |
| Aref et al. | 2013 | Acute leukemia | Egypt | African | Serum | 42.8/49.1 | 80/20 | Case-control | Elisa | R&D Systems | 5 |
| Miyoshi et al. | 2003 | Breast cancer | Japan | Asian | Serum | 54.0/52.8 | 102/100 | Case-control | Elisa | NR | 7 |
| Mantzoros et al. | 2004 | Breast cancer | Greece | Caucasian | Serum | NR | 174/167 | Case-control | RIA | Beth Israsel Deaconess Medical Center | 8 |
| Chen et al. | 2006 | Breast cancer | Taiwan | Asian | Serum | 49.9/48.9 | 100/100 | Case-control | RIA | Linco Research | 7 |
| Korner et al. | 2007 | Breast cancer | Greece | Caucasian | Serum | 62.5/55.6 | 74/76 | Case-control | RIA | ALPCO Diagnostics | 7 |
| Kang et al. | 2007 | Breast cancer | Korea | Asian | Serum | 47.4/47.8 | 41/43 | Case-control | Elisa | AdipoGen | 7 |
| Hou et al. | 2007 | Breast cancer | China | Asian | Serum | 48/49 | 80/50 | Case-control | Elisa | R&D Systems | 6 |
| Tworoger et al. | 2007 | Breast cancer | USA | Caucasian | Blood | 57.1/58.1 | 1166/1575 | Nested case-control | RIA | Linco Research | 7 |
| Tworoger et al. | 2007 | Breast cancer | USA | Caucasian | Blood | 45.4/45.1 | 311/621 | Nested case-control | RIA | Linco Research | 7 |
| Hancke et al. | 2010 | Breast cancer | Switzerland | Caucasian | Serum | 59.5/49.0 | 159/41 | Case-control | Elisa | BioVendor Laboratory Medicine | 6 |
| Cust et al. | 2009 | Breast cancer | Sweden | Caucasian | Plasma | 52.5/NR | 561/561 | Case-control | RIA | Linco Research | 7 |
| Shahar et al. | 2010 | Breast cancer | Malaysia | Asian | Serum | 47.3/46.2 | 70/138 | Case-control | Elisa | Linco Research | 7 |
| Dalamaga et al. | 2011 | Breast cancer | Greece | Caucasian | Serum | 61.5/62.8 | 102/102 | Case-control | Elisa | Avibion | 7 |
| Al Khaldi et al. | 2011 | Breast cancer | Kuwait | Asian | Plasma | 49/60 | 60/68 | Case-control | Elisa | Linco Research | 7 |
| Touvier et al. | 2013 | Breast cancer | France | Caucasian | Plasma | 49.2/51.5 | 218/436 | Nested case-control | Elisa | R&D Systems | 9 |
| Gulcelik et al. | 2012 | Breast cancer | Turkey | Asian | Serum | 51.4/52.4 | 83/40 | Case-control | Elisa | B-Bridge International Inc. | 7 |
| Al Awadhi et al. | 2012 | Breast cancer | Kuwait | Asian | Plasma | 50.3/50.7 | 144/77 | Case-control | Elisa | Linco Research | 7 |
| Alokail et al. | 2013 | Breast cancer | Saudi Arabia | Asian | Serum | 46.4/43.1 | 56/53 | Case-control | Other | Luminex Corporation | 7 |
| Ollberding et al. | 2013 | Breast cancer | USA | Caucasian | Serum | 67.8/67.8 | 706/706 | Nested case-control | Elisa | R&D Systems | 8 |
| Gross et al. | 2013 | Breast cancer | USA | Caucasian | Plasma | 62.6/62.5 | 272/272 | Case-control | Elisa | ALPCO Diagnostics | 7 |
| Minatoya et al. | 2014 | Breast cancer | Japan | Asian | Serum | NR | 66/66 | Case-control | Other | SRL | 7 |
| Gulcelik et al. | 2012 | Colon cancer | Turkey | Asian | Serum | 52.1/52.4 | 27/40 | Case-control | Elisa | B-Bridge International Inc. | 7 |
| Otake et al. | 2005 | Colorectal adenoma | Japan | Asian | Plasma | 59.0/58.0 | 51/52 | Case-control | Elisa | Otsuka Pharmaceutical | 8 |
| Fukumoto et al. | 2008 | Colorectal adenoma | Japan | Asian | Plasma | NR | 656/648 | Case-control | Elisa | Otsuka Pharmaceutical | 7 |
| Kumor et al. | 2009 | Colorectal adenoma | Poland | Caucasian | Serum | 62.4/60.1 | 37/25 | Case-control | Elisa | R&D Systems | 7 |
| Erarslan et al. | 2009 | Colorectal adenoma | Turkey | Asian | Plasma | 63.0/59.0 | 31/50 | Case-control | Elisa | RayBio | 8 |
| Nakajima et al. | 2010 | Colorectal adenoma | Japan | Asian | Plasma | 66.8/66.7 | 72/72 | Case-control | Elisa | Otsuka Pharmaceutical | 7 |
| Otake et al. | 2010 | Colorectal adenoma | Japan | Asian | Plasma | 65.1/67.9 | 47/26 | Case-control | Elisa | Otsuka Pharmaceutical | 7 |
| Yamaji et al. | 2010 | Colorectal adenoma | Japan | Asian | Plasma | NR | 778/735 | Case-control | Elisa | Sekisui Medical | 6 |
| Danese et al. | 2013 | Colorectal adenoma | Italy | Caucasian | Serum | 63.0/59.5 | 40/40 | Case-control | Elisa | Mediagnost | 7 |
| Wei et al. | 2005 | Colorectal cancer | USA | Caucasian | Plasma | 66.6/66.5 | 179/356 | Nested case-control | RIA | Linco Research | 8 |
| Stocks et al. | 2008 | Colorectal cancer | Sweden | Caucasian | Plasma | 59.7/NR | 306/595 | Nested case-control | Elisa | R&D Systems | 6 |
| Guadagni et al. | 2009 | Colorectal cancer | Italy | Caucasian | Serum | 63.0/59.0 | 90/30 | Case-control | Elisa | BioVendor Laboratory Medicine | 8 |
| Kumor et al. | 2009 | Colorectal cancer | Poland | Caucasian | Serum | 58.6/60.1 | 36/25 | Case-control | Elisa | R&D Systems | 7 |
| Erarslan et al. | 2009 | Colorectal cancer | Turkey | Asian | Plasma | 57.0/59.0 | 23/50 | Case-control | Elisa | RayBio | 8 |
| Nakajima et al. | 2010 | Colorectal cancer | Japan | Asian | Plasma | 63.7/63.5 | 115/115 | Case-control | Elisa | Otsuka Pharmaceutical | 7 |
| Otake et al. | 2010 | Colorectal cancer | Japan | Asian | Plasma | 66.7/67.9 | 51/26 | Case-control | Elisa | Otsuka Pharmaceutical | 7 |
| Kemik et al | 2010 | Colorectal cancer | Turkey | Asian | Serum | 43.5/40.4 | 126/38 | Case-control | RIA | Linco Research | 7 |
| Gonullu et al. | 2010 | Colorectal cancer | Turkey | Asian | Serum | 56.6/51.0 | 36/37 | Case-control | Elisa | BioSource | 8 |
| Catalan et al. | 2011 | Colorectal cancer | Spain | Caucasian | Plasma | 66.0/44.0 | 11/18 | Case-control | Elisa | R&D Systems | 8 |
| Chen et al. | 2012 | Colorectal cancer | China | Asian | Plasma | 61.9/58.3 | 165/102 | Case-control | Elisa | Adlitteram Diagnostic Laboratories. Inc. | 7 |
| Touvier et al. | 2012 | Colorectal cancer | France | Caucasian | Plasma | 51.8/52.1 | 50/100 | Nested case-control | Elisa | R&D Systems | 9 |
| Aleksandrova et al. | 2012 | Colorectal cancer | Germany | Caucasian | Serum | 58.3/58.3 | 1206/1206 | Case-control | Elisa | ALPCO Diagnostics | 9 |
| Song et al. | 2013 | Colorectal cancer | USA | Caucasian | Plasma | 61.9/61.9 | 616/1205 | Case-control | Elisa | ALPCO Diagnostics | 9 |
| Cust et al. | 2007 | Endometrical carcinoma | UK | Caucasian | Plasma | 56.9/56.9 | 284/548 | Nested case-control | Elisa | R&D Systems | 8 |
| Soliman et al. | 2006 | Endometrical carcinoma | USA | Caucasian | Serum | 66.6/61.2 | 117/238 | Case-control | Elisa | R&D Systems | 5 |
| Ashizawa et al. | 2010 | Endometrical carcinoma | Japan | Asian | Serum | 59.9/57.5 | 146/150 | Case-control | RIA | Linco Research | 8 |
| Dossus et al. | 2013 | Endometrical carcinoma | Germany | Caucasian | Serum | 57.7/57.7 | 233/446 | Case-control | Elisa | R&D Systems | 8 |
| Friedenreich et al. | 2012 | Endometrical carcinoma | USA | Caucasian | Serum | 59/59 | 514/962 | Case-control | Elisa | ALPCO Diagnostics | 9 |
| Luhn et al. | 2013 | Endometrical carcinoma | USA | Caucasian | Serum | NR | 167/327 | Nested case-control | RIA | Linco Research | 8 |
| Erdogan et al. | 2013 | Endometrical carcinoma | Turkey | Asian | Serum | 56.6/49.7 | 60/70 | Case-control | Elisa | eBioscience | 6 |
| Ma et al. | 2013 | Endometrical carcinoma | China | Asian | Serum | 53.2/53.3 | 206/310 | Case-control | Elisa | Bender MedSystems | 9 |
| Dallal et al. | 2013 | Endometrical carcinoma | USA | Caucasian | Serum | 67.4/67.5 | 62/124 | Nested case-control | Elisa | Millipore | 8 |
| Mihu et al. | 2013 | Endometrical carcinoma | Romania | Caucasian | Serum | 60.2/58.5 | 44/44 | Case-control | Elisa | R&D Systems | 6 |
| Ohbuchi et al. | 2014 | Endometrical carcinoma | Japan | Asian | Serum | 61.2/58.1 | 43/62 | Case-control | Elisa | Daiichi Co. Ltd. | 8 |
| Diao et al. | 2009 | Esophageal cancer | China | Asian | Plasma | 58.0/49.0 | 43/33 | Case-control | Elisa | Adlitteram Diagnostic Laboratories. Inc. | 6 |
| Nakajima et al. | 2010 | Esophageal cancer | Japan | Asian | Blood | 63.6/63.6 | 117/117 | Case-control | Elisa | Otsuka Pharmaceutical | 6 |
| Yildirim et al. | 2009 | Esophageal cancer | Turkey | Asian | Serum | 64/61 | 62/30 | Case-control | Elisa | Avibion | 6 |
| Ishikawa et al. | 2005 | Gastric cancer | Japan | Asian | Plasma | 64.2/59.3 | 75/52 | Case-control | Elisa | Otsuka Pharmaceutical | 6 |
| Nakajima et al. | 2009 | Gastric cancer | Japan | Asian | Blood | 61.0/60.8 | 156/156 | Case-control | Elisa | Otsuka Pharmaceutical | 8 |
| Seker et al. | 2010 | Gastric cancer | Turkey | Asian | Plasma | 60.0/38.6 | 40/43 | Case-control | Elisa | Linco Research | 5 |
| Diakowska et al. | 2014 | Gastroesophageal cancer | Poland | Caucasian | Serum | 60.0/58.0 | 85/60 | Case-control | Elisa | R&D Systems | 7 |
| Kotani et al. | 2009 | Hepatacellular carcinoma | Japan | Asian | Serum | 63.5/62.7 | 59/334 | Nested case-control | Elisa | Daiichi Co. Ltd. | 8 |
| Liu et al. | 2009 | Hepatacellular carcinoma | Taiwan/China | Asian | Serum | 50.7/53.8 | 120/116 | Case-control | Elisa | B-Bridge International Inc. | 5 |
| Sumie et al. | 2011 | Hepatacellular carcinoma | Japan | Asian | Serum | 67.4/61.2 | 97/97 | Case-control | Elisa | EikenChenical Co. Ltd. | 7 |
| Sadik et al | 2012 | Hepatacellular carcinoma | Egypt | African | Serum | 58.9/55.7 | 69/121 | Case-control | Elisa | Assaypro | 7 |
| Chen et al. | 2012 | Hepatacellular carcinoma | Taiwan/China | Asian | Serum | 52.4/52.2 | 65/165 | Case-control | RIA | Linco Research | 6 |
| Khattab et al. | 2012 | Hepatacellular carcinoma | Egypt | African | Plasma | 43.9/42.9 | 147/320 | Case-control | Other | Linco Research | 5 |
| Chen et al. | 2014 | Hepatacellular carcinoma | Taiwan/China | Asian | Plasma | NR | 185/373 | Nested case-control | Elisa | B-Bridge International Inc. | 8 |
| Petridou et al. | 2010 | Hodgkin lymphoma | Greece | Caucasian | Serum | 11.5/11.2 | 75/75 | Case-control | RIA | Linco Research | 7 |
| Jamieson et al. | 2004 | Lung cancer | UK | Caucasian | Serum | 64.0/65.0 | 20/13 | Case-control | RIA | Linco Research | 7 |
| Karapanagiotou et al. | 2008 | Lung cancer | Greece | Caucasian | Serum | 64.2/55.5 | 101/51 | Case-control | Elisa | BioVendor | 6 |
| Petridou et al. | 2007 | Lung cancer | Greece | Caucasian | Serum | NR | 85/170 | Case-control | RIA | Beth Israsel Deaconess Medical Center | 8 |
| Gulen et al. | 2012 | Lung cancer | Turkey | Asian | Serum | 65.6/63.5 | 63/25 | Case-control | Elisa | BioVendor | 7 |
| Kerenidi et al. | 2013 | Lung cancer | Greece | Caucasian | Serum | 62.9/NR | 80/40 | Case-control | Elisa | Linco Research | 7 |
| Antoniadis et al. | 2011 | Melanoma | Greece/Canada | Caucasian | Serum | 52.7/53.3 | 55/165 | Case-control | RIA | Beth Israsel Deaconess Medical Center | 8 |
| Dalamaga et al. | 2009 | Multiple myeloma | Greece/Canada | Caucasian | Serum | NR | 73/73 | Case-control | Elisa | Avibion | 8 |
| Hofmann et al. | 2012 | Multiple myeloma | USA | Caucasian | Plasma | NR | 174/348 | Case-control | Elisa | R&D Systems | 7 |
| Pamuk et al. | 2006 | Non-Hodgkin's lymphoma | Turkey | Asian | Serum | 63.2/58.5 | 28/17 | Case-control | Elisa | OtsukaCo.Ltd | 5 |
| Petridou et al. | 2009 | Non-Hodgkin's lymphoma | Greece | Caucasian | Serum | 8.8/8.8 | 121/121 | Case-control | RIA | NR | 7 |
| Conroy et al. | 2013 | Non-Hodgkin's lymphoma | USA | Caucasian | Plasma | 70.0/70.0 | 272/541 | Nested case-control | Elisa | R&D Systems | 7 |
| Chang et al. | 2007 | Pancreatic cancer | Taiwan/China | Asian | Serum | 64.6/49.5 | 72/290 | Case-control | Elisa | R&D Systems | 8 |
| Dalamaga et al. | 2009 | Pancreatic cancer | Greece | Caucasian | Serum | 69.0/70.1 | 81/81 | Case-control | RIA | Linco Research | 7 |
| Solomon et al. | 2008 | Pancreatic cancer | USA | Caucasian | Serum | 58.0/58.0 | 311/510 | Case-control | Elisa | Millipore | 8 |
| Krechler et al. | 2011 | Pancreatic cancer | Czech Republic | Caucasian | Plasma | 51.9/64.5 | 64/64 | Case-control | RIA | DRG Inc. | 8 |
| Grote et al. | 2012 | Pancreatic cancer | Germany | Caucasian | Serum | 58.0/60.0 | 452/452 | Nested case-control | Other | R&D Systems | 8 |
| Bao et al. | 2013 | Pancreatic cancer | USA | Caucasian | Plasma | NR | 468/1080 | Nested case-control | Elisa | ALPCO Diagnostics | 8 |
| Goktas et al. | 2005 | Prostate cancer | Turkey | Asian | Plasma | 65.8/62.2 | 30/36 | Case-control | RIA | Linco Research | 8 |
| Goktas et al. | 2005 | Prostate cancer | Turkey | Asian | Plasma | 65.8/65.0 | 30/41 | Case-control | RIA | Linco Research | 8 |
| Baillargeon et al. | 2006 | Prostate cancer | USA | Caucasian | Serum | 63.5/63.2 | 125/125 | Nested case-control | Other | Luminex | 7 |
| Michalakis et al. | 2007 | Prostate cancer | Greece | Caucasian | Serum | 74.0/64.0 | 75/150 | Case-control | RIA | Linco Research | 5 |
| Michalakis et al. | 2007 | Prostate cancer | Greece | Caucasian | Serum | 74.0/70.0 | 75/75 | Case-control | RIA | Linco Research | 5 |
| Housa et al. | 2008 | Prostate cancer | Czech Republic | Caucasian | Serum | 63.6/70.5 | 43/25 | Case-control | RIA | Linco Research | 5 |
| Grosman et al. | 2010 | Prostate cancer | Argentina | Caucasian | Serum | NR | 25/25 | Case-control | RIA | Linco Research | 7 |
| Li et al. | 2010 | Prostate cancer | USA | Caucasian | Plasma | 59.0/58.6 | 620/599 | Nested case-control | RIA | Linco Research | 7 |
| Dhillon et al. | 2011 | Prostate cancer | USA | Caucasian | Plasma | 57.9/57.5 | 1286/1267 | Nested case-control | RIA | Linco Research | 8 |
| Lopez Fontana et al. | 2011 | Prostate cancer | Argentina | Caucasian | Serum | 63.8/64.9 | 35/35 | Case-control | Elisa | Linco Research | 6 |
| Al Khaldi et al. | 2011 | Prostate cancer | Kuwait | Asian | Plasma | 59.0/60.0 | 14/68 | Case-control | Elisa | Linco Research | 7 |
| Touvier et al. | 2013 | Prostate cancer | France | Caucasian | Plasma | 54.9/51.5 | 156/1024 | Nested case-control | Elisa | R&D Systems | 9 |
| Tewari et al. | 2013 | Prostate cancer | India | Asian | Blood | 66.5/65.7 | 95/95 | Case-control | Other | NR | 5 |
| Spyridopoulos et al. | 2012 | Renal cancer | Greece | Caucasian | Serum | 61.5/60.7 | 60/236 | Case-control | RIA | Beth Israsel Deaconess Medical Center | 8 |
| Liao et al. | 2013 | Renal cancer | Finland/USA | Caucasian | Serum | 57/57 | 273/273 | Nested case-control | Elisa | Millipore | 9 |
| Liao et al. | 2013 | Renal cancer | Canada/USA | Caucasian | Serum | NR | 768/917 | Case-control | Elisa | Millipore | 9 |
| Mitsiades et al. | 2011 | Thyroid cancer | USA | Caucasian | Serum | 51.2/55.4 | 175/107 | Case-control | RIA | Beth Israsel Deaconess Medical Center | 5 |
| Guo et al. | 2013 | Tongue cancer | China | Asian | Serum | 57.2/52.7 | 59/50 | Case-control | Elisa | Adipobiotech | 8 |
Abbreviations: NR, not reported; Elisa, enzyme-linked immunosorbent assay; RIA, radioimmunoassay.
Circulating adiponectin levels and carcinogenesis
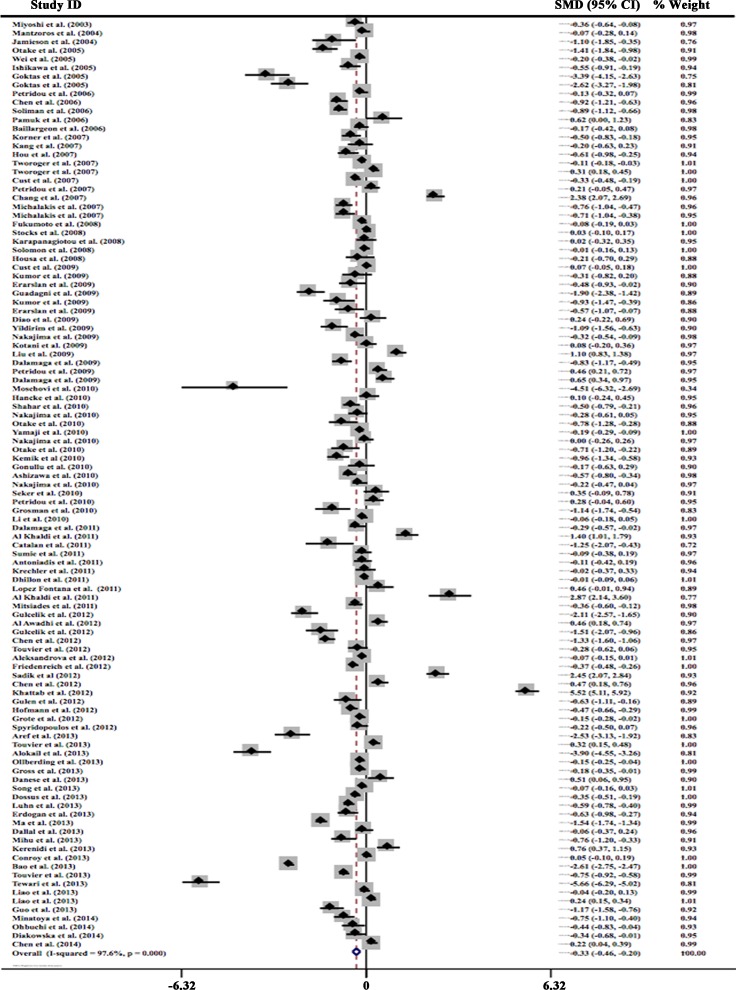
Data from 107 studies were analyzed in a random-effect model to compare circulating adiponectin levels in people with different cancers and controls. Results showed that circulating adiponectin levels in cancer cases were significantly lower than in the controls with a pooled SMD of −0.334 μg/ml (95% CI, −0.465 to −0.203, P = 0.000). Statistically significant amount of heterogeneity was observed across these studies (I2 = 97.6%, P < 0.0001), so subgroup analysis was carried out next. These results were presented in Figure 2.
Figure 2. Forest plot of studies in circulating total adiponectin and cancer risk.
The combined SMD and 95% CIs were calculated through a random-effect model.
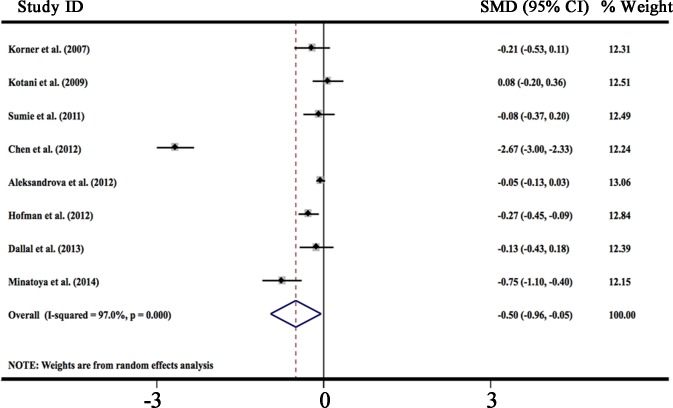
HMW-adiponectin is the dominant form of adiponectin in plasma and correlates with cardiovascular disease, insulin resistance, and obesity [7, 113, 114]. But few studies have evaluated the relationship between circulating HMW-adiponectin levels and cancer risk. We analyzed data from 8 studies in a random-effect model to compare circulating HMW-adiponectin levels in people with different cancers [33, 56, 58, 72, 83, 94, 107, 108]. Results showed that circulating HMW-adiponectin levels in cancer cases were significantly lower than in the controls with a pooled SMD of −0.502 μg/ml (95% CI, −0.957 to −0.047, P = 0.000), which is consistent with the results derived from total adiponectin levels. Statistically significant amount of heterogeneity was observed across these studies (I2 = 97.0%, P < 0.0001). These results were presented in Figure 3.
Figure 3. Forest plot of studies in circulating high molecular weight adiponectin and cancer risk.
The combined SMD and 95% CIs were calculated through a random-effect model.
Subgroup analysis and meta-regression
Stratified subgroup analysis was performed to evaluate the potential sources of heterogeneity including ethnicity, cancer type, study design, blood sample, assay method, study size, study quality and mean age of cancer patients (Table 2). Lower levels of circulating adiponectin were observed in both Asian (SMD −0.555, 95% CI, −0.812 to −0.298) and Caucasian people (SMD −0.269, 95% CI, −0.400 to −0.138). Similar results were also presented in people with breast (SMD −0.334, 95% CI, −0.543 to −0.126), colorectal (SMD −0.496, 95% CI, −0.653 to −0.339), endometrial (SMD −0.594, 95% CI, −0.825 to −0.363), prostate (SMD −0.892, 95% CI, −1.345 to −0.438), thyroid (SMD −0.358, 95% CI, −0.601 to −0.116), tongue (SMD −1.172, 95% CI, −1.580 to −0.764), gastroesophageal (SMD −0.278, 95% CI, −0.553 to −0.004) cancer, multiple myeloma (SMD −0.621, 95% CI, −0.966 to −0.276), and acute leukemia (SMD −0.594, 95% CI, −0.825 to −0.363). Notably, circulating adiponectin levels were higher in the patients with hepatocellular cancer than in controls among 7 studies included (SMD 1.385, 95% CI, 0.240 to 2.530).
Table 2. Subgroup analysis of the relationships between circulating adiponectin levels and study characteristics.
| Characteristics | Number of studies | Number (Case/control) | SMD | 95% CI | Heterogeneity (I2) |
|---|---|---|---|---|---|
| Ethnicity | |||||
| Caucasian | 58 | 14178/19758 | −0.269 | −0.400 to −0.138 | 96.8% |
| Asian | 46 | 4845/5456 | −0.555 | −0.812 to −0.298 | 97.1% |
| African | 3 | 296/461 | 1.821 | −2.201 to 5.843 | 99.6% |
| Cancer Types | |||||
| Acute leukemia | 3 | 290/230 | −2.236 | −4.418 to −0.054 | 97.3% |
| Multiple myeloma | 2 | 247/421 | −0.621 | −0.966 to −0.276 | 69.7% |
| Breast cancer | 20 | 4545/5292 | −0.334 | −0.543 to −0.126 | 95.5% |
| Colorectal cancers | 23 | 4749/5591 | −0.496 | −0.653 to −0.339 | 91.3% |
| Endometrial cancer | 11 | 1876/3281 | −0.594 | −0.825 to −0.363 | 92.8% |
| Prostate cancer | 13 | 2609/3565 | −0.892 | −1.345 to −0.438 | 97.9% |
| Thyroid cancer | 1 | 175/107 | −0.358 | −0.601 to −0.116 | NA |
| Tongue cancer | 1 | 59/50 | −1.172 | −1.580 to −0.764 | NA |
| Hepatocellular cancer | 7 | 742/1526 | 1.385 | 0.240 to 2.530 | 99.2% |
| Gastroesophageal cancer | 7 | 578/491 | −0.278 | −0.553 to −0.004 | 78.1% |
| Hodgkin lymphoma | 1 | 75/75 | 0.28 | −0.041 to 0.602 | NA |
| Non-Hodgkin lymphoma | 3 | 421/679 | 0.316 | −0.048 to 0.68 | 79.7% |
| Lung cancer | 5 | 349/299 | −0.085 | −0.58 to 0.409 | 87.1% |
| Melanoma | 1 | 55/165 | −0.112 | −0.418 to 0.193 | NA |
| Pancreatic cancer | 6 | 1448/2477 | 0.037 | −1.207 to 1.281 | 99.6% |
| Renal cancer | 3 | 1101/1426 | 0.021 | −0.246 to 0.288 | 86.6% |
| Study Design | |||||
| Case-control study | 86 | 11965/14210 | −0.346 | −0.505 to −0.188 | 97.2% |
| Nested case-control study | 21 | 7354/11465 | −0.290 | −0.553 to −0.026 | 98.5% |
| Blood samples | |||||
| Serum | 65 | 9171/11101 | −0.335 | −0.483 to −0.186 | 95.8% |
| Plasma | 37 | 8303/12010 | −0.238 | −0.497 to 0.022 | 98.6% |
| NR | 5 | 1845/2564 | −1.072 | −1.775 to −0.369 | 98.8% |
| Assay methods | |||||
| RIA | 29 | 6190/7587 | −0.316 | −0.459 to −0.172 | 93.0% |
| Elisa | 71 | 12179/16968 | −0.266 | −0.426 to −0.106 | 97.4% |
| Others | 7 | 950/1120 | −1.305 | −3.113 to 0.502 | 99.5% |
| Study size | |||||
| ≥100 patients | 48 | 16057/21437 | −0.135 | −0.299 to-0.030 | 98.3% |
| <100 patients | 59 | 3262/4238 | −0.549 | −0.825 to −0.273 | 96.5% |
| Study quality | |||||
| ≥6 | 96 | 16352/22425 | −0.334 | −0.465 to −0.203 | 97.3% |
| <6 | 11 | 2967/3250 | −0.267 | −0.700 to 0.165 | 98.2% |
| Patients’ age (mean) | |||||
| ≥60 | 44* | 4770/6414* | −0.489 | −0.689 to −0.288 | 95.6% |
| <60 | 47* | 9782/12935* | −0.194 | −0.383 to −0.004 | 97.7% |
Abbreviations: NA, not assessable.
There are 91 studies with 14,552 cases and 19,349 controls reported the mean age of cancer patients.
Additionally, adiponectin was significantly lower in patients who used serum as test samples (SMD −0.335, 95% CI, −0.483 to −0.186), and in 37 studies who used plasma as testing samples, 26 studies showed the inverse relation of adiponectin to cancer risk. Assay method (radioimmunoassay or enzyme-linked immunosorbent assay) did not affect the results that circulating adiponectin was lower in cancer patients with pooled SMD of −0.316 and −0.266. Study size (more or less than 100 patients) did not change the result of estimated SMD either (SMD −0.135, 95% CI, −0.299 to −0.030; SMD −0.549, 95% CI, −0.825 to −0.273, respectively). Besides, no matter the mean age of cancer patients is older or younger than 60 years, decreased adiponectin levels were still exist in cancer patients (SMD −0.489, 95% CI, −0.689 to −0.288; SMD −0.194, 95% CI, −0.383 to −0.004, respectively).
Next we performed meta-regression to evaluate the effect of the above factors on the estimate of SMD. In meta-regression, none of the examined factors, such as ethnicity, cancer type, study design, blood sample, assay method, study size, study quality and mean age of cancer patients was proved to be significant contributing factors.
Sensitivity analysis
Sensitivity analysis was performed by excluding one study at a time and calculating the pooled SMDs for the remaining studies. It was found that the combined SMDs were similar to one another and statistically significant. None of the studies influence the pooled results substantially in this analysis (Table 3).
Table 3. The pooled SMDs and 95% CIs of the included studies through sensitivity analysis.
| Study omitted | Estimate | 95% CI |
|---|---|---|
| Miyoshi et al. (2003) | −0.33362126 | −.46549249 to −.20175007 |
| Jamieson et al. (2004) | −0.32790136 | −.45929646 to −.19650623 |
| Mantzoros et al. (2004) | −0.3366529 | −.46877834 to −.20452745 |
| Goktas et al. (2005) | −0.31018904 | −.44067159 to −.1797065 |
| Goktas et al. (2005) | −0.31467217 | −.4453963 to −.18394804 |
| Wei et al. (2005) | −0.33543056 | −.46779135 to −.20306975 |
| Otake et al. (2005) | −0.32370359 | −.45491788 to −.19248928 |
| Ishikawa et al. (2005) | −0.33179379 | −.4634814 to −.20010617 |
| Petridou et al. (2006) | −0.33611172 | −.4683494 to −.20387407 |
| Baillargeon et al. (2006) | −0.33559188 | −.46757615 to −.2036076 |
| Chen et al. (2006) | −0.32800215 | −.45950019 to −.1965041 |
| Pamuk et al. (2006) | −0.34167045 | −.47312284 to −.21021806 |
| Soliman et al. (2006) | −0.32821506 | −.45972851 to −.19670163 |
| Cust et al. (2007) | −0.33429027 | −.46701819 to −.20156233 |
| Korner et al. (2007) | −0.33221245 | −.4639549 to −.20046999 |
| Kang et al. (2007) | −0.33505732 | −.46672907 to −.20338558 |
| Tworoger et al. (2007) | −0.33802846 | −.47386777 to −.20218913 |
| Tworoger et al. (2007) | −0.3405844 | −.47277904 to −.20838977 |
| Chang et al. (2007) | −0.35825887 | −.48626736 to −.2302504 |
| Michalakis et al. (2007) | −0.32968622 | −.46133375 to −.19803868 |
| Michalakis et al. (2007) | −0.33015183 | −.46179458 to −.19850905 |
| Hou et al. (2007) | −0.33114624 | −.46280947 to −.19948301 |
| Petridou et al. (2007) | −0.33918613 | −.47100937 to −.20736288 |
| Fukumoto et al. (2008) | −0.33727011 | −.47093907 to −.20360115 |
| Solomon et al. (2008) | −0.3375347 | −.47031215 to −.20475723 |
| Housa et al. (2008) | −0.33494586 | −.46656513 to −.2033266 |
| Karapanagiotou et al. (2008) | −0.337192 | −.46895465 to −.20542936 |
| Stocks et al. (2008) | −0.33803195 | −.47080877 to −.20525511 |
| Dalamaga et al. (2009) | −0.34321341 | −.47464448 to −.21178232 |
| Dalamaga et al. (2009) | −0.32902971 | −.46060005 to −.19745934 |
| Petridou et al. (2009) | −0.34161058 | −.47322133 to −.2099998 |
| Kotani et al. (2009) | −0.33791459 | −.46976718 to −.20606196 |
| Guadagni et al. (2009) | −0.31941918 | −.45032975 to −.18850861 |
| Kumor et al. (2009) | −0.32856214 | −.46004361 to −.1970806 |
| Erarslan et al. (2009) | −0.33168712 | −.46326634 to −.20010787 |
| Kumor et al. (2009) | −0.3339898 | −.4655939 to −.2023856 |
| Erarslan et al. (2009) | −0.33251002 | −.46413583 to −.20088424 |
| Cust et al. (2009) | −0.33854863 | −.47171903 to −.20537826 |
| Nakajima et al. (2009) | −0.33416247 | −.4662253 to −.20209965 |
| Diao et al. (2009) | −0.33897933 | −.47058302 to −.2073756 |
| Yildirim et al. (2009) | −0.3268407 | −.45826575 to −.19541568 |
| Li et al. (2009) | −0.34737712 | −.47806501 to −.21668923 |
| Moschovi et al. (2010) | −0.31926209 | −.45011383 to −.18841037 |
| Hancke et al. (2010) | −0.33801222 | −.46974581 to −.20627865 |
| Seker et al. (2010) | −0.34004903 | −.47163537 to −.2084627 |
| Nakajima et al. (2010) | −0.33507797 | −.46703228 to −.20312366 |
| Petridou et al. (2010) | −0.33972663 | −.47141987 to −.20803338 |
| Grosman et al. (2010) | −0.32690996 | −.45831883 to −.19550107 |
| Li et al. (2010) | −0.3373847 | −.47090244 to −.20386696 |
| Nakajima et al. (2010) | −0.3372006 | −.46912611 to −.2052751 |
| Otake et al. (2010) | −0.3304036 | −.46196187 to −.19884537 |
| Kemik et al (2010) | −0.3278496 | −.45933568 to −.19636351 |
| Gonullu et al. (2010) | −0.3353405 | −.46698609 to −.20369488 |
| Nakajima et al. (2010) | −0.33436027 | −.46614832 to −.20257224 |
| Ashizawa et al. (2010) | −0.33154425 | −.4634259 to −.19966258 |
| Shahar et al. (2010) | −0.3222657 | −.4640207 to −.20043245 |
| Otake et al. (2010) | −0.3297922 | −.46132797 to −.19825645 |
| Yamaji et al. (2010) | −0.33636427 | −.47040036 to −.20232819 |
| Antoniadis et al. (2011) | −0.3360277 | −.46786067 to −.2041948 |
| Dhillon et al. (2011) | −0.33877328 | −.47420669 to −.20333987 |
| Al Khaldi et al. (2011) | −0.3581396 | −.48865175 to −.22762746 |
| Sumie et al. (2011) | −0.33623996 | −.46812296 to −.20435697 |
| Catalan et al. (2011) | −0.3270525 | −.4584052 to −.19569978 |
| Dalamaga et al. (2011) | −0.33432293 | −.46621501 to −.20243084 |
| Al Khaldi et al. (2011) | −0.34966454 | −.48045498 to −.21887414 |
| Krechler et al. (2011) | −0.33685401 | −.46860847 to −.20509957 |
| Mitsiades et al. (2011) | −0.33370164 | −.46567562 to −.20172767 |
| Lopez Fontana et al. (2011) | −0.3409504 | −.47248313 to −.20941767 |
| Spyridopoulos et al. (2012) | −0.33505121 | −.46693206 to −.20317033 |
| Hofmann et al. (2012) | −0.33265379 | −.46483427 to −.2004733 |
| Gulen et al. (2012) | −0.33108562 | −.46266881 to −.1995024 |
| Sadik et al (2012) | −0.35871589 | −.48782459 to −.2296071 |
| Chen et al. (2012) | −0.32368076 | −.45458078 to −.1927807 |
| Gulcelik et al. (2012) | −0.32346013 | −.45471603 to −.19220424 |
| Aleksandrova et al. (2012) | −0.33816311 | −.47353563 to −.20279059 |
| Friedenreich et al. (2012) | −0.33428073 | −.46782497 to −.20073651 |
| Touvier et al. (2012) | −0.3405067 | −.47251758 to −.20849583 |
| Gulcelik et al. (2012) | −0.31726849 | −.44793424 to −.18660273 |
| Al Awadhi et al. (2012) | −0.34153 | −.47313255 to −.2099274 |
| Grote et al. (2012) | 0.33630246 | −.46936187 to −.20324306 |
| Chen et al. (2012) | −0.34161338 | −.47320184 to −.2100249 |
| Khattab et al. (2012) | −0.38207525 | −.50227177 to −.2618787 |
| Dossus et al. (2013) | −0.33401754 | −.46652448 to −.20151059 |
| Bao et al. (2013) | −0.30136451 | −.41626969 to −.18645933 |
| Guo et al. (2013) | −0.3258861 | −.45724642 to −.1945257 |
| Touvier et al. (2013) | −0.33433828 | −.46610662 to −.2025699 |
| Liao et al. (2013) | −0.33713764 | −.46957946 to −.20469585 |
| Liao et al. (2013) | −0.3403554 | −.47350773 to −.20720309 |
| Conroy et al. (2013) | −0.33808553 | −.47072488 to −.2054462 |
| Touvier et al. (2013) | −0.3296572 | −.46145904 to −.19785538 |
| Kerenidi et al. (2013) | −0.3439351 | −.4753255 to −.21254471 |
| Danese et al. (2013) | −0.34142008 | −.47294763 to −.20989256 |
| Song et al. (2013) | −0.33765575 | −.47181916 to −.20349233 |
| Luhn et al. (2013) | −0.33139816 | −.46341154 to −.19938481 |
| Ma et al. (2013) | −0.32076678 | −.45034227 to −.1911912 |
| Dallal et al. (2013) | −0.33651593 | −.4683443 to −.20468754 |
| Alokail et al. (2013) | −0.30377382 | −.43343174 to −.17411587 |
| Ollberding et al. (2013) | −0.33671638 | −.47059336 to −.20283943 |
| Gross et al. (2013) | −0.33571425 | −.46818775 to −.20324075 |
| Aref et al. (2013) | −0.31502652 | −.4457356 to −.18431742 |
| Tewari et al. (2013) | −0.28841972 | −.41580069 to −.16103874 |
| Erdogan et al. (2013) | −0.33102214 | −.46268752 to −.19935676 |
| Mihu et al. (2013) | −0.3298324 | −.46139839 to −.1982664 |
| Chen et al. (2014) | −0.33950841 | −.47162384 to −.20739301 |
| Ohbuchi et al. (2014) | −0.33285877 | −.46454135 to −.20117618 |
| Minatoya et al. (2014) | −0.32982665 | −.46143582 to −.19821748 |
| Diakowska et al. (2014) | −0.33376125 | −.46553349 to −.20198898 |
| Combined | −0.33375105 | −.46467104 to −.20283107 |
Publication bias
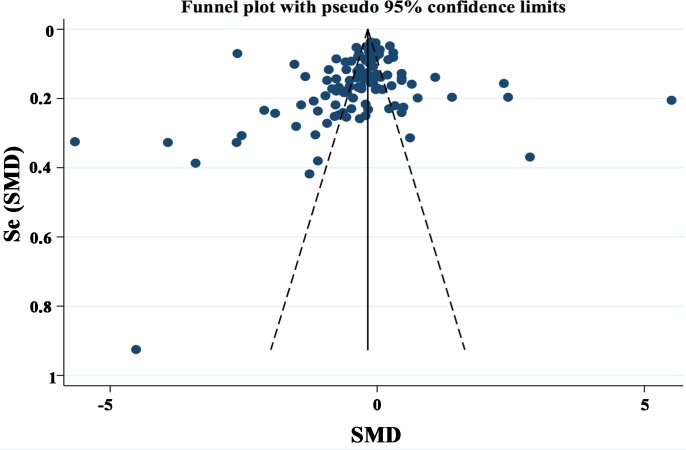
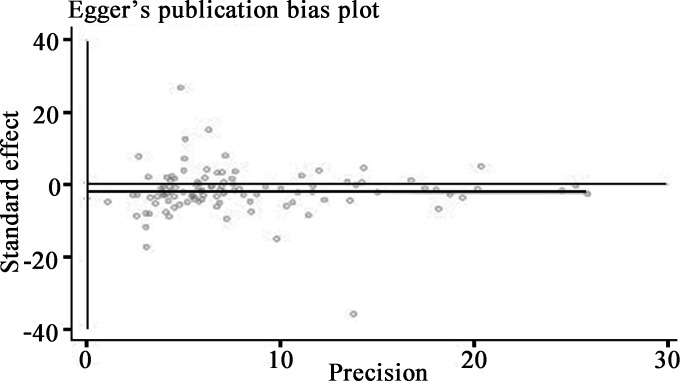
Publication bias was assessed by funnel plot and Egger's regression test. Funnel plot shapes demonstrated a marginally asymmetrical distribution (Figure 4), accordingly we performed further analysis with Egger's test. The tested result (Figure 5) showed no evidence of publication bias (P = 0.123).
Figure 4. Funnel plot of lower adiponectin expression and cancer risk.
Circles indicate included studies.
Figure 5. Egger's linear regression test for publication bias detection.
DISCUSSION
By integrating 107 studies, our meta-analysis revealed that lower circulating adiponectin levels were associated with higher risk of cancers. Despite the existence of heterogeneity, the disparity of adiponectin levels between malignant individuals and controls reveals the potential ability of adiponectin to serve as a biomarker for early detection of cancers.
Aberrant adiponectin secretion is associated with tumor progression, metastasis and overall prognosis. Two previous meta-analysis indicated that lower adiponectin levels were associated with higher risk of breast cancer, colorectal cancer and colorectal adenoma [115, 116]. By synthesizing 107 studies involving 19,319 cases with different malignancies, the present meta-analysis estimate the inverse association between circulating adiponectin levels and cancer risk. Moreover, through subgroup analysis, we identified that this inverse relation of adiponectin to cancer risk might be more meaningful in breast, colon, endometrial, prostate, and gastroesophageal cancers. Besides, adiponectin levels tend to decrease as tumor stage increases in gastric cancer [62]. Kang et al. also indicate that breast cancer patients with less than the median adiponectin levels are easy to develop lymph node metastasis [82]. Low adiponectin level is the independent predictor of unfavorable prognosis in colorectal cancer [117]. These findings demonstrate that adiponectin is not only associated with cancer risk, but also correlated with tumor progression. Additionally, in our included 107 studies, 8 studies evaluated the relationship between circulating levels of adiponectin subtypes and cancer risk. The changing trend of total adiponectin was almost same with the three adiponectin subtypes in cancer patients, especially with HMW-adiponectin, that it is inversely associated with cancer risk.
Circulating adiponectin levels are affected by various factors, including inflammatory, dietary, hormonal, genetic, and medicine. One of possible explanations for decreased adiponectin levels in malignancies is the sustained inflammatory status of cancer patients leads to the increased proinflammatory cytokines such as TNF-α and IL-6, which are all reported to suppress adiponectin transcription and translation in adipocyte cell line [118, 119]. Besides, in obesity-related cancers, adiponectin may control its own production through a negative feedback loop during the development of obesity [120]. Moreover, dietary with lower intake of fiber and magnesium can also reduce circulating adiponectin levels [121].
However, elevated adiponectin levels are also reported in hepatocellular carcinoma. Since adiponectin is mainly degraded in the liver and adiponectin levels are elevated in advanced disease including cirrhosis and virus-related cancer [61, 122]. One possible explanation for increased adiponectin level in hepatocellular carcinoma might be due to deteriorated hepatic metabolism resulted from repeated necroinflammation and regeneration. Besides, conflicting results also exist in clinical studies of pancreatic cancer that both higher and lower adiponectin levels are reported to be associated with cancer risk [45, 50]. After reviewing the pancreatic cancer studies with higher levels of adiponectin, we found that almost half of them were accompanied with jaundice [45]. Since cholestasis would lead to the chronic liver deterioration, it is possible that increased adiponectin levels might be due to the reduced degradation.
The peripheral functions of adiponectin are mainly mediated through AdipoR1 and AdipoR2. The expression levels of AdipoRs vary between malignant tissues and their peritumoral normal counterparts. The upregulation of AdipoR1 and AdipoR2 are reported in gastric carcinoma [123], whereas decreased in prostate cancer tissues compared with the nonmalignant tissues [36]. Increased expression of AdipoRs may be the response of reduced circulating as well as local adiponectin levels and reduced expression suggests that the sensitivity of AdipoRs to adiponectin is decreased in tumor tissues. Yabushita et al. indicate that poor expression of AdipoR1 is associated with tumor invasion and lymph node metastasis, as well as poor prognosis in endometrial cancer patients [124]. A study of non-small cell lung cancer also indicates that patients with higher expression of AdipoR1 have longer overall survival and AdipoR2 expression is inversely correlated with tumor size [125]. Those findings further illustrate the protective role of adiponectin as well as AdipoRs and shed light on exploiting them for cancer therapy. Recently, AdipoRs agonist called 355ADP is identified and might represent a new strategy to replace low adiponectin level in cancer [126].
Despite the inverse correlation between adiponectin and various cancers, the underlying mechanisms of adiponectin in potential cancer suppression are still need to elucidate. Adiponectin decreases low density lipoprotein (LDL) receptor expression in breast cancer cells through promoting autophagic flux and inhibits LDL-cholesterol-induced tumor cell proliferation [127]. Adiponectin induces the phosphorylation of p53, a tumor suppressor, which renders cell cycle arrest and apoptosis in cancer cell lines [128]. Adiponectin also inhibits leptin-induced metastasis by downregulating JAK/STAT3 pathway, displaying an inverse correlation with cancer development [129]. In contrast, adiponectin promotes the angiogenesis in human chondrosarcoma by increasing vascular endothelial growth factor-A expression [130]. It is also reported to exert anti-apoptotic effects on pancreatic cancer cells through activation of AMPK/Sirtuin-1 signaling pathway [131]. Taken together, adiponectin might play a complicated role in carcinogenesis and progression of cancers.
Our study has some limitations that need to be addressed when interpreting the results. The significant heterogeneity was observed among the studies thus the conclusion should be more conservative. Although stratified analysis was conducted, none of the factors including ethnicity, cancer type, study design, blood sample, assay method, study size, study quality, and mean age of cancer patients were confirmed to contributing factors. Some possible reasons may partially explain this heterogeneity. Adiponectin levels are changed along with the tumor development. The tumor type, size, histological grade, and lymph node metastasis are the possible contributors caused heterogeneity. It is difficult for us to acquire the detailed information from the included studies. Besides, the subjects were from different regions and the lifestyle combined with diet was varied, which might influence the level of adiponectin. Since adiponectin is mainly secreted from adipose tissue, variables such as age, hormone receptor expression, menopausal status and BMI could contribute to the secretion and those factors were not fully deliberated for the complexity of tumor environment.
CONCLUSIONS
In summary, the present study shows significant difference in circulating adiponectin levels between patients with malignancies and controls. Low circulating adiponectin level is associated with increased cancer risk, which suggests that adiponectin may serve as a potential biomarker for early detection of cancers considering its abundance in blood. Thorough understanding the roles of adiponectin and its receptors in the progression of cancers is helpful to cancer screening and promote individualized treatment.
MATERIALS AND METHODS
Search strategy
Based on the standard guidelines, a systematic search of English literature from Cochrane library, Wiley online library, PubMed was conducted to retrieve eligible studies until August 8, 2015. Searching terms included Medical Subject Heading (Mesh) and free text words “adiponectin”, “ADPN”, “Acrp 30”, “AdipoQ”, “GBP 28” or “apM1” in combination with “neoplasm”, “cancer”, “carcinoma”, “malignancy” or “tumor”. Furthermore, we manually searched references of relevant studies to add potential research to this meta-analysis.
Inclusion and exclusion criteria
Studies were included if they met the following criteria: (i) full text case-control studies published in peer-reviewed journals evaluating the relationship between circulating adiponectin concentration and carcinogenesis; (ii) all cases were diagnosed as cancer by pathological biopsy or other medical methods with blood sample obtained before any therapies and all the controls were people without any cancers. (iii) circulating adiponectin level and standard deviation (SD) of it were provided or there were enough information to estimate them. Reviews, letters or animal experiments were excluded and articles without key information to carry on further analysis were also beyond consideration. Meanwhile, if replicated patient cohort was published in different studies, only the most recent or complete one was chosen. Since all the studies included were acquired from literature, ethics committee approval was not needed.
Data extraction
Based on the checklist of MOOSE (Meta-analysis Of Observational Studies in Epidemiology) [132], two reviewers (Tai W and Peng Y) extracted the following data independently from eligible studies: the last name of first author, year of publication, geographic region, ethnicity, tumor type, study design, sample type, adiponectin assay method, number of patients and controls, assay source, mean ± SD of adiponectin concentration. Disagreement was resolved by discussion until the two reviewers reached a consensus.
Quality assessment of included studies
Two reviewers (Tai W and Peng Y) independently assessed the quality of each included study according to the Newcastle-Ottawa Quality Assessment Scale (NOS) [133] ranges from 0 to 9 stars. Studies with more than 6 stars were considered as high-quality studies. Any disagreement was resolved by discussion and reevaluation.
Statistical analysis
We acquired the mean ± SD of circulating adiponectin levels from cases and controls through three ways. The most accurate method was extracted them from the original research directly. However, a few studies presented the results as median values or standard error. In that case, we regarded median value as mean value considering the large sample size and calculated the SD value by using standard error and population number. If necessary, we contacted the author for detailed information. Standard mean differences (SMDs) and the corresponding 95% confidence intervals (CIs) of circulating adiponectin were calculated for all the eligible studies. Cochran's Q-test was performed to test the heterogeneity of included studies and P < 0.05 was considered statistically significant. Higgins I-squared statistic was applied to offer evidence of heterogeneity with I2 > 50% suggesting significant heterogeneity. The pooled SMD and 95% CI was calculated using a fixed-effects model if the heterogeneity was not significant, otherwise a random-effect model was employed and subgroup analyses and meta-regression were adopted to detect the potential cause of heterogeneity.
Sensitivity analysis was executed to detect the robustness of the results. Publication bias was evaluated by use of funnel plot and Egger's linear regression test. The Stata 13.0 software (Stata Corporation, College Station, TX, USA) was used to perform all the statistical analysis. All P values were two-sided.
Footnotes
CONFLICTS OF INTEREST
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial conflict with any materials discussed in the paper. All authors declare that there is no conflict of interest regarding the publication of this work.
GRANT SUPPORT
This study was supported by the National Natural Science Foundation of China (No. 81472527) and National Supporting Program for Science and Technology of China (No. 2014BAI04B06).
REFERENCES
- 1.Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133–1145. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 2.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 4.Trevellin E, Scarpa M, Carraro A, Lunardi F, Kotsafti A, Porzionato A, Saadeh L, Cagol M, Alfieri R, Tedeschi U, Calabrese F, Castoro C, Vettor R. Esophageal adenocarcinoma and obesity: peritumoral adipose tissue plays a role in lymph node invasion. Oncotarget. 2015;6:11203–11215. doi: 10.18632/oncotarget.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 6.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 7.Fujimatsu D, Kotooka N, Inoue T, Nishiyama M, Node K. Association between high molecular weight adiponectin levels and metabolic parameters. J Atheroscler Thromb. 2009;16:553–559. doi: 10.5551/jat.1073. [DOI] [PubMed] [Google Scholar]
- 8.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 9.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab. 2013;2:133–141. doi: 10.1016/j.molmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu YM, Lacorte JM, Viguerie N, Poitou C, Pelloux V, Guy-Grand B, Coussieu C, Langin D, Basdevant A, Clement K. Adiponectin gene expression in subcutaneous adipose tissue of obese women in response to short-term very low calorie diet and refeeding. J Clin Endocrinol Metab. 2003;88:5881–5886. doi: 10.1210/jc.2003-030886. [DOI] [PubMed] [Google Scholar]
- 12.Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, Matsuzawa Y, Noguchi S. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9:5699–5704. [PubMed] [Google Scholar]
- 13.Goktas S, Yilmaz MI, Caglar K, Sonmez A, Kilic S, Bedir S. Prostate cancer and adiponectin. Urology. 2005;65:1168–1172. doi: 10.1016/j.urology.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 14.Gu C, Qu Y, Zhang G, Sun L, Zhu Y, Ye D. A single nucleotide polymorphism in ADIPOQ predicts biochemical recurrence after radical prostatectomy in localized prostate cancer. Oncotarget. 2015;6:32205–32211. doi: 10.18632/oncotarget.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soliman PT, Wu D, Tortolero-Luna G, Schmeler KM, Slomovitz BM, Bray MS, Gershenson DM, Lu KH. Association between adiponectin, insulin resistance, and endometrial cancer. Cancer. 2006;106:2376–2381. doi: 10.1002/cncr.21866. [DOI] [PubMed] [Google Scholar]
- 16.Fukumoto J, Otake T, Tajima O, Tabata S, Abe H, Mizoue T, Ohnaka K, Kono S. Adiponectin and colorectal adenomas: self defense forces health study. Cancer Sci. 2008;99:781–786. doi: 10.1111/j.1349-7006.2008.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dieudonne MN, Bussiere M, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2006;345:271–279. doi: 10.1016/j.bbrc.2006.04.076. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Wen K, Han X, Liu R, Qu Q. Adiponectin mediates antiproliferative and apoptotic responses in endometrial carcinoma by the AdipoRs/AMPK pathway. Gynecol Oncol. 2015;137:311–320. doi: 10.1016/j.ygyno.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Pezzilli R, Barassi A, Corsi MM, Morselli-Labate AM, Campana D, Casadei R, Santini D, Corinaldesi R, D'Eril GM. Serum leptin, but not adiponectin and receptor for advanced glycation end products, is able to distinguish autoimmune pancreatitis from both chronic pancreatitis and pancreatic neoplasms. Scand J Gastroenterol. 2010;45:93–99. doi: 10.3109/00365520903358907. [DOI] [PubMed] [Google Scholar]
- 20.Chen MJ, Yeh YT, Lee KT, Tsai CJ, Lee HH, Wang SN. The promoting effect of adiponectin in hepatocellular carcinoma. J Surg Oncol. 2012;106:181–187. doi: 10.1002/jso.23059. [DOI] [PubMed] [Google Scholar]
- 21.Mitsiades N, Pazaitou-Panayiotou K, Aronis KN, Moon HS, Chamberland JP, Liu X, Diakopoulos KN, Kyttaris V, Panagiotou V, Mylvaganam G, Tseleni-Balafouta S, Mantzoros CS. Circulating adiponectin is inversely associated with risk of thyroid cancer: in vivo and in vitro studies. J Clin Endocrinol Metab. 2011;96:E2023–2028. doi: 10.1210/jc.2010-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spyridopoulos TN, Dessypris N, Antoniadis AG, Gialamas S, Antonopoulos CN, Katsifoti K, Adami HO, Chrousos GP, Petridou ET. Insulin resistance and risk of renal cell cancer: a case-control study. Hormones (Athens) 2012;11:308–315. doi: 10.14310/horm.2002.1359. [DOI] [PubMed] [Google Scholar]
- 23.Liao LM, Weinstein SJ, Pollak M, Li Z, Virtamo J, Albanes D, Chow WH, Purdue MP. Prediagnostic circulating adipokine concentrations and risk of renal cell carcinoma in male smokers. Carcinogenesis. 2013;34:109–112. doi: 10.1093/carcin/bgs322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao LM, Schwartz K, Pollak M, Graubard BI, Li Z, Ruterbusch J, Rothman N, Davis F, Wacholder S, Colt J, Chow WH, Purdue MP. Serum leptin and adiponectin levels and risk of renal cell carcinoma. Obesity (Silver Spring) 2013;21:1478–1485. doi: 10.1002/oby.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petridou E, Mantzoros CS, Dessypris N, Dikalioti SK, Trichopoulos D. Adiponectin in relation to childhood myeloblastic leukaemia. Br J Cancer. 2006;94:156–160. doi: 10.1038/sj.bjc.6602896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moschovi M, Trimis G, Vounatsou M, Katsibardi K, Margeli A, Damianos A, Chrousos G, Papassotiriou I. Serial plasma concentrations of adiponectin, leptin, and resistin during therapy in children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2010;32:e8–13. doi: 10.1097/MPH.0b013e3181b8a50c. [DOI] [PubMed] [Google Scholar]
- 27.Aref S, Ibrahim L, Azmy E, Al Ashary R. Impact of serum adiponectin and leptin levels in acute leukemia. Hematology. 2013;18:198–203. doi: 10.1179/1607845412Y.0000000059. [DOI] [PubMed] [Google Scholar]
- 28.Petridou ET, Dessypris N, Panagopoulou P, Sergentanis TN, Mentis AF, Pourtsidis A, Polychronopoulou S, Kalmanti M, Athanasiadou-Piperopoulou F, Moschovi M. Adipocytokines in relation to Hodgkin lymphoma in children. Pediatr Blood Cancer. 2010;54:311–315. doi: 10.1002/pbc.22294. [DOI] [PubMed] [Google Scholar]
- 29.Pamuk GE, Turgut B, Demir M, Vural O. Increased adiponectin level in non-Hodgkin lymphoma and its relationship with interleukin-10. Correlation with clinical features and outcome. J Exp Clin Cancer Res. 2006;25:537–541. [PubMed] [Google Scholar]
- 30.Petridou ET, Sergentanis TN, Dessypris N, Vlachantoni IT, Tseleni-Balafouta S, Pourtsidis A, Moschovi M, Polychronopoulou S, Athanasiadou-Piperopoulou F, Kalmanti M, Mantzoros CS. Serum adiponectin as a predictor of childhood non-Hodgkin's lymphoma: a nationwide case-control study. J Clin Oncol. 2009;27:5049–5055. doi: 10.1200/JCO.2008.19.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conroy SM, Maskarinec G, Morimoto Y, Franke AA, Cooney RV, Wilkens LR, Goodman MT, Hernadez BY, Le Marchand L, Henderson BE, Kolonel LN. Non-hodgkin lymphoma and circulating markers of inflammation and adiposity in a nested case-control study: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2013;22:337–347. doi: 10.1158/1055-9965.EPI-12-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalamaga M, Karmaniolas K, Panagiotou A, Hsi A, Chamberland J, Dimas C, Lekka A, Mantzoros CS. Low circulating adiponectin and resistin, but not leptin, levels are associated with multiple myeloma risk: a case-control study. Cancer Causes Control. 2009;20:193–199. doi: 10.1007/s10552-008-9233-7. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann JN, Liao LM, Pollak MN, Wang Y, Pfeiffer RM, Baris D, Andreotti G, Lan Q, Landgren O, Rothman N, Purdue MP. A prospective study of circulating adipokine levels and risk of multiple myeloma. Blood. 2012;120:4418–4420. doi: 10.1182/blood-2012-06-438606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antoniadis AG, Petridou ET, Antonopoulos CN, Dessypris N, Panagopoulou P, Chamberland JP, Adami HO, Gogas H, Mantzoros CS. Insulin resistance in relation to melanoma risk. Melanoma Res. 2011;21:541–546. doi: 10.1097/CMR.0b013e32834b0eeb. [DOI] [PubMed] [Google Scholar]
- 35.Baillargeon J, Platz EA, Rose DP, Pollock BH, Ankerst DP, Haffner S, Higgins B, Lokshin A, Troyer D, Hernandez J, Lynch S, Leach RJ, Thompson IM. Obesity, adipokines, and prostate cancer in a prospective population-based study. Cancer Epidemiol Biomarkers Prev. 2006;15:1331–1335. doi: 10.1158/1055-9965.EPI-06-0082. [DOI] [PubMed] [Google Scholar]
- 36.Michalakis K, Williams CJ, Mitsiades N, Blakeman J, Balafouta-Tselenis S, Giannopoulos A, Mantzoros CS. Serum adiponectin concentrations and tissue expression of adiponectin receptors are reduced in patients with prostate cancer: a case control study. Cancer Epidemiol Biomarkers Prev. 2007;16:308–313. doi: 10.1158/1055-9965.EPI-06-0621. [DOI] [PubMed] [Google Scholar]
- 37.Housa D, Vernerova Z, Heracek J, Prochazka B, Cechak P, Kuncova J, Haluzik M. Adiponectin as a potential marker of prostate cancer progression: studies in organ-confined and locally advanced prostate cancer. Physiol Res. 2008;57:451–458. doi: 10.33549/physiolres.931156. [DOI] [PubMed] [Google Scholar]
- 38.Grosman H, Fabre B, Mesch V, Lopez MA, Schreier L, Mazza O, Berg G. Lipoproteins, sex hormones and inflammatory markers in association with prostate cancer. Aging Male. 2010;13:87–92. doi: 10.3109/13685530903410617. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Stampfer MJ, Mucci L, Rifai N, Qiu W, Kurth T, Ma J. A 25-year prospective study of plasma adiponectin and leptin concentrations and prostate cancer risk and survival. Clin Chem. 2010;56:34–43. doi: 10.1373/clinchem.2009.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al Khaldi RM, Al Mulla F, Al Awadhi S, Kapila K, Mojiminiyi OA. Associations of single nucleotide polymorphisms in the adiponectin gene with adiponectin levels and cardio-metabolic risk factors in patients with cancer. Dis Markers. 2011;30:197–212. doi: 10.3233/DMA-2011-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhillon PK, Penney KL, Schumacher F, Rider JR, Sesso HD, Pollak M, Fiorentino M, Finn S, Loda M, Rifai N, Mucci LA, Giovannucci E, Stampfer MJ, Ma J. Common polymorphisms in the adiponectin and its receptor genes, adiponectin levels and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2618–2627. doi: 10.1158/1055-9965.EPI-11-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez Fontana CM, Maselli ME, Perez Elizalde RF, Di Milta Monaco NA, Uvilla Recupero AL, Lopez Laur JD. Leptin increases prostate cancer aggressiveness. J Physiol Biochem. 2011;67:531–538. doi: 10.1007/s13105-011-0098-y. [DOI] [PubMed] [Google Scholar]
- 43.Tewari R, Rajender S, Natu SM, Goel A, Dalela D, Goel MM, Tondon P. Significance of obesity markers and adipocytokines in high grade and high stage prostate cancer in North Indian men - a cross-sectional study. Cytokine. 2013;63:130–134. doi: 10.1016/j.cyto.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Touvier M, Fezeu L, Ahluwalia N, Julia C, Charnaux N, Sutton A, Mejean C, Latino-Martel P, Hercberg S, Galan P, Czernichow S. Association between prediagnostic biomarkers of inflammation and endothelial function and cancer risk: a nested case-control study. Am J Epidemiol. 2013;177:3–13. doi: 10.1093/aje/kws359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang MC, Chang YT, Su TC, Yang WS, Chen CL, Tien YW, Liang PC, Wei SC, Wong JM. Adiponectin as a potential differential marker to distinguish pancreatic cancer and chronic pancreatitis. Pancreas. 2007;35:16–21. doi: 10.1097/MPA.0b013e3180547709. [DOI] [PubMed] [Google Scholar]
- 46.Stolzenberg-Solomon RZ, Weinstein S, Pollak M, Tao Y, Taylor PR, Virtamo J, Albanes D. Prediagnostic adiponectin concentrations and pancreatic cancer risk in male smokers. Am J Epidemiol. 2008;168:1047–1055. doi: 10.1093/aje/kwn221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dalamaga M, Migdalis I, Fargnoli JL, Papadavid E, Bloom E, Mitsiades N, Karmaniolas K, Pelecanos N, Tseleni-Balafouta S, Dionyssiou-Asteriou A, Mantzoros CS. Pancreatic cancer expresses adiponectin receptors and is associated with hypoleptinemia and hyperadiponectinemia: a case-control study. Cancer Causes Control. 2009;20:625–633. doi: 10.1007/s10552-008-9273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krechler T, Zeman M, Vecka M, Macasek J, Jachymova M, Zima T, Zak A. Leptin and adiponectin in pancreatic cancer: connection with diabetes mellitus. Neoplasma. 2011;58:58–64. doi: 10.4149/neo_2011_01_58. [DOI] [PubMed] [Google Scholar]
- 49.Grote VA, Rohrmann S, Dossus L, Nieters A, Halkjaer J, Tjonneland A, Overvad K, Stegger J, Chabbert-Buffet N, Boutron-Ruault MC, Clavel-Chapelon F, Teucher B, Becker S, Montonen J, Boeing H, Trichopoulou A, et al. The association of circulating adiponectin levels with pancreatic cancer risk: a study within the prospective EPIC cohort. Int J Cancer. 2012;130:2428–2437. doi: 10.1002/ijc.26244. [DOI] [PubMed] [Google Scholar]
- 50.Bao Y, Giovannucci EL, Kraft P, Stampfer MJ, Ogino S, Ma J, Buring JE, Sesso HD, Lee IM, Gaziano JM, Rifai N, Pollak MN, Cochrane BB, Kaklamani V, Lin JH, Manson JE, et al. A prospective study of plasma adiponectin and pancreatic cancer risk in five US cohorts. J Natl Cancer Inst. 2013;105:95–103. doi: 10.1093/jnci/djs474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jamieson NB, Brown DJ, Michael Wallace A, McMillan DC. Adiponectin and the systemic inflammatory response in weight-losing patients with non-small cell lung cancer. Cytokine. 2004;27:90–92. doi: 10.1016/j.cyto.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 52.Petridou ET, Mitsiades N, Gialamas S, Angelopoulos M, Skalkidou A, Dessypris N, Hsi A, Lazaris N, Polyzos A, Syrigos C, Brennan AM, Tseleni-Balafouta S, Mantzoros CS. Circulating adiponectin levels and expression of adiponectin receptors in relation to lung cancer: two case-control studies. Oncology. 2007;73:261–269. doi: 10.1159/000127424. [DOI] [PubMed] [Google Scholar]
- 53.Karapanagiotou EM, Tsochatzis EA, Dilana KD, Tourkantonis I, Gratsias I, Syrigos KN. The significance of leptin, adiponectin, and resistin serum levels in non-small cell lung cancer (NSCLC) Lung Cancer. 2008;61:391–397. doi: 10.1016/j.lungcan.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 54.Gulen ST, Karadag F, Karul AB, Kilicarslan N, Ceylan E, Kuman NK, Cildag O. Adipokines and systemic inflammation in weight-losing lung cancer patients. Lung. 2012;190:327–332. doi: 10.1007/s00408-011-9364-6. [DOI] [PubMed] [Google Scholar]
- 55.Kerenidi T, Lada M, Tsaroucha A, Georgoulias P, Mystridou P, Gourgoulianis KI. Clinical significance of serum adipokines levels in lung cancer. Med Oncol. 2013;30:507. doi: 10.1007/s12032-013-0507-x. [DOI] [PubMed] [Google Scholar]
- 56.Kotani K, Wakai K, Shibata A, Fujita Y, Ogimoto I, Naito M, Kurozawa Y, Suzuki H, Yoshimura T, Tamakoshi A. Serum adiponectin multimer complexes and liver cancer risk in a large cohort study in Japan. Asian Pac J Cancer Prev. 2009;10(Suppl):87–90. [PubMed] [Google Scholar]
- 57.Liu CJ, Chen PJ, Lai MY, Liu CH, Chen CL, Kao JH, Chen DS. High serum adiponectin correlates with advanced liver disease in patients with chronic hepatitis B virus infection. Hepatol Int. 2009;3:364–370. doi: 10.1007/s12072-008-9111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sumie S, Kawaguchi T, Kuromatsu R, Takata A, Nakano M, Satani M, Yamada S, Niizeki T, Torimura T, Sata M. Total and high molecular weight adiponectin and hepatocellular carcinoma with HCV infection. PLoS One. 2011;6:e26840. doi: 10.1371/journal.pone.0026840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khattab MA, Eslam M, Mousa YI, Ela-adawy N, Fathy S, Shatat M, Abd-Aalhalim H, Kamal A, Sharawe MA. Association between metabolic abnormalities and hepatitis C-related hepatocellular carcinoma. Ann Hepatol. 2012;11:487–494. [PubMed] [Google Scholar]
- 60.Sadik NA, Ahmed A, Ahmed S. The significance of serum levels of adiponectin, leptin, and hyaluronic acid in hepatocellular carcinoma of cirrhotic and noncirrhotic patients. Hum Exp Toxicol. 2012;31:311–321. doi: 10.1177/0960327111431091. [DOI] [PubMed] [Google Scholar]
- 61.Chen CL, Yang WS, Yang HI, Chen CF, You SL, Wang LY, Lu SN, Liu CJ, Kao JH, Chen PJ, Chen DS, Chen CJ. Plasma adipokines and risk of hepatocellular carcinoma in chronic hepatitis B virus-infected carriers: a prospective study in taiwan. Cancer Epidemiol Biomarkers Prev. 2014;23:1659–1671. doi: 10.1158/1055-9965.EPI-14-0161. [DOI] [PubMed] [Google Scholar]
- 62.Ishikawa M, Kitayama J, Kazama S, Hiramatsu T, Hatano K, Nagawa H. Plasma adiponectin and gastric cancer. Clin Cancer Res. 2005;11:466–472. [PubMed] [Google Scholar]
- 63.Nakajima TE, Yamada Y, Hamano T, Furuta K, Gotoda T, Katai H, Kato K, Hamaguchi T, Shimada Y. Adipocytokine levels in gastric cancer patients: resistin and visfatin as biomarkers of gastric cancer. J Gastroenterol. 2009;44:685–690. doi: 10.1007/s00535-009-0063-5. [DOI] [PubMed] [Google Scholar]
- 64.Seker M, Bilici A, Sonmez B, Ustaalioglu BB, Gumus M, Gozu H, Sargin M, Orcun A, Gezen C, Eser M, Bildik N, Salepci T. The association of serum adiponectin levels with histopathological variables in gastric cancer patients. Med Oncol. 2010;27:1319–1323. doi: 10.1007/s12032-009-9382-x. [DOI] [PubMed] [Google Scholar]
- 65.Diakowska D, Markocka-Maczka K, Szelachowski P, Grabowski K. Serum levels of resistin, adiponectin, and apelin in gastroesophageal cancer patients. Dis Markers. 2014;2014:619649. doi: 10.1155/2014/619649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diao Y, Li H, Li H, Zhou Y, Ma Q, Wang Y, Li D. Association of serum levels of lipid and its novel constituents with the different stages of esophageal carcinoma. Lipids Health Dis. 2009;8:48. doi: 10.1186/1476-511X-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yildirim A, Bilici M, Cayir K, Yanmaz V, Yildirim S, Tekin SB. Serum adiponectin levels in patients with esophageal cancer. Jpn J Clin Oncol. 2009;39:92–96. doi: 10.1093/jjco/hyn143. [DOI] [PubMed] [Google Scholar]
- 68.Nakajima TE, Yamada Y, Hamano T, Furuta K, Oda I, Kato H, Kato K, Hamaguchi T, Shimada Y. Adipocytokines and squamous cell carcinoma of the esophagus. J Cancer Res Clin Oncol. 2010;136:261–266. doi: 10.1007/s00432-009-0657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cust AE, Kaaks R, Friedenreich C, Bonnet F, Laville M, Lukanova A, Rinaldi S, Dossus L, Slimani N, Lundin E, Tjonneland A, Olsen A, Overvad K, Clavel-Chapelon F, Mesrine S, Joulin V, et al. Plasma adiponectin levels and endometrial cancer risk in pre- and postmenopausal women. J Clin Endocrinol Metab. 2007;92:255–263. doi: 10.1210/jc.2006-1371. [DOI] [PubMed] [Google Scholar]
- 70.Ashizawa N, Yahata T, Quan J, Adachi S, Yoshihara K, Tanaka K. Serum leptin-adiponectin ratio and endometrial cancer risk in postmenopausal female subjects. Gynecol Oncol. 2010;119:65–69. doi: 10.1016/j.ygyno.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 71.Friedenreich CM, Langley AR, Speidel TP, Lau DC, Courneya KS, Csizmadi I, Magliocco AM, Yasui Y, Cook LS. Case-control study of markers of insulin resistance and endometrial cancer risk. Endocr Relat Cancer. 2012;19:785–792. doi: 10.1530/ERC-12-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dallal CM, Brinton LA, Bauer DC, Buist DS, Cauley JA, Hue TF, Lacroix A, Tice JA, Chia VM, Falk R, Pfeiffer R, Pollak M, Veenstra TD, Xu X, Lacey JV., Jr Obesity-related hormones and endometrial cancer among postmenopausal women: a nested case-control study within the B~FIT cohort. Endocr Relat Cancer. 2013;20:151–160. doi: 10.1530/ERC-12-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dossus L, Lukanova A, Rinaldi S, Allen N, Cust AE, Becker S, Tjonneland A, Hansen L, Overvad K, Chabbert-Buffet N, Mesrine S, Clavel-Chapelon F, Teucher B, Chang-Claude J, Boeing H, Drogan D, et al. Hormonal, metabolic, and inflammatory profiles and endometrial cancer risk within the EPIC cohort--a factor analysis. Am J Epidemiol. 2013;177:787–799. doi: 10.1093/aje/kws309. [DOI] [PubMed] [Google Scholar]
- 74.Erdogan S, Sezer S, Baser E, Gun-Eryilmaz O, Gungor T, Uysal S, Yilmaz FM. Evaluating vaspin and adiponectin in postmenopausal women with endometrial cancer. Endocr Relat Cancer. 2013;20:669–675. doi: 10.1530/ERC-13-0280. [DOI] [PubMed] [Google Scholar]
- 75.Luhn P, Dallal CM, Weiss JM, Black A, Huang WY, Lacey JV, Jr, Hayes RB, Stanczyk FZ, Wentzensen N, Brinton LA. Circulating adipokine levels and endometrial cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2013;22:1304–1312. doi: 10.1158/1055-9965.EPI-13-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma Y, Liu Z, Zhang Y, Lu B. Serum leptin, adiponectin and endometrial cancer risk in Chinese women. J Gynecol Oncol. 2013;24:336–341. doi: 10.3802/jgo.2013.24.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mihu D, Ciortea R, Mihu CM. Abdominal adiposity through adipocyte secretion products, a risk factor for endometrial cancer. Gynecol Endocrinol. 2013;29:448–451. doi: 10.3109/09513590.2012.752452. [DOI] [PubMed] [Google Scholar]
- 78.Ohbuchi Y, Suzuki Y, Hatakeyama I, Nakao Y, Fujito A, Iwasaka T, Isaka K. A lower serum level of middle-molecular-weight adiponectin is a risk factor for endometrial cancer. Int J Clin Oncol. 2014;19:667–673. doi: 10.1007/s10147-013-0603-0. [DOI] [PubMed] [Google Scholar]
- 79.Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, Papadiamantis Y, Markopoulos C, Spanos E, Chrousos G, Trichopoulos D. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89:1102–1107. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 80.Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo FC, Fu OY, Chen HY, Hou MF, Yuan SS. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237:109–114. doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 81.Hou WK, Xu YX, Yu T, Zhang L, Zhang WW, Fu CL, Sun Y, Wu Q, Chen L. Adipocytokines and breast cancer risk. Chin Med J (Engl) 2007;120:1592–1596. [PubMed] [Google Scholar]
- 82.Kang JH, Yu BY, Youn DS. Relationship of serum adiponectin and resistin levels with breast cancer risk. J Korean Med Sci. 2007;22:117–121. doi: 10.3346/jkms.2007.22.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Korner A, Pazaitou-Panayiotou K, Kelesidis T, Kelesidis I, Williams CJ, Kaprara A, Bullen J, Neuwirth A, Tseleni S, Mitsiades N, Kiess W, Mantzoros CS. Total and high-molecular-weight adiponectin in breast cancer: in vitro and in vivo studies. J Clin Endocrinol Metab. 2007;92:1041–1048. doi: 10.1210/jc.2006-1858. [DOI] [PubMed] [Google Scholar]
- 84.Tworoger SS, Eliassen AH, Kelesidis T, Colditz GA, Willett WC, Mantzoros CS, Hankinson SE. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab. 2007;92:1510–1516. doi: 10.1210/jc.2006-1975. [DOI] [PubMed] [Google Scholar]
- 85.Cust AE, Stocks T, Lukanova A, Lundin E, Hallmans G, Kaaks R, Jonsson H, Stattin P. The influence of overweight and insulin resistance on breast cancer risk and tumour stage at diagnosis: a prospective study. Breast Cancer Res Treat. 2009;113:567–576. doi: 10.1007/s10549-008-9958-8. [DOI] [PubMed] [Google Scholar]
- 86.Hancke K, Grubeck D, Hauser N, Kreienberg R, Weiss JM. Adipocyte fatty acid-binding protein as a novel prognostic factor in obese breast cancer patients. Breast Cancer Res Treat. 2010;119:367–367. doi: 10.1007/s10549-009-0577-9. [DOI] [PubMed] [Google Scholar]
- 87.Shahar S, Salleh RM, Ghazali AR, Koon PB, Mohamud WN. Roles of adiposity, lifetime physical activity and serum adiponectin in occurrence of breast cancer among Malaysian women in Klang Valley. Asian Pac J Cancer Prev. 2010;11:61–66. [PubMed] [Google Scholar]
- 88.Dalamaga M, Karmaniolas K, Papadavid E, Pelekanos N, Sotiropoulos G, Lekka A. Elevated serum visfatin/nicotinamide phosphoribosyl-transferase levels are associated with risk of postmenopausal breast cancer independently from adiponectin, leptin, and anthropometric and metabolic parameters. Menopause. 2011;18:1198–1204. doi: 10.1097/gme.0b013e31821e21f5. [DOI] [PubMed] [Google Scholar]
- 89.Al Awadhi SA, Al Khaldi RM, Al Rammah T, Kapila K, Mojiminiyi OA. Associations of adipokines & insulin resistance with sex steroids in patients with breast cancer. Indian J Med Res. 2012;135:500–505. [PMC free article] [PubMed] [Google Scholar]
- 90.Gulcelik MA, Colakoglu K, Dincer H, Dogan L, Yenidogan E, Gulcelik NE. Associations between adiponectin and two different cancers: breast and colon. Asian Pac J Cancer Prev. 2012;13:395–398. doi: 10.7314/apjcp.2012.13.1.395. [DOI] [PubMed] [Google Scholar]
- 91.Alokail MS, Al-Daghri N, Abdulkareem A, Draz HM, Yakout SM, Alnaami AM, Sabico S, Alenad AM, Chrousos GP. Metabolic syndrome biomarkers and early breast cancer in Saudi women: evidence for the presence of a systemic stress response and/or a pre-existing metabolic syndrome-related neoplasia risk? BMC Cancer. 2013;13:54. doi: 10.1186/1471-2407-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gross AL, Newschaffer CJ, Hoffman-Bolton J, Rifai N, Visvanathan K. Adipocytokines, inflammation, and breast cancer risk in postmenopausal women: a prospective study. Cancer Epidemiol Biomarkers Prev. 2013;22:1319–1324. doi: 10.1158/1055-9965.EPI-12-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ollberding NJ, Kim Y, Shvetsov YB, Wilkens LR, Franke AA, Cooney RV, Maskarinec G, Hernandez BY, Henderson BE, Le Marchand L, Kolonel LN, Goodman MT. Prediagnostic leptin, adiponectin, C-reactive protein, and the risk of postmenopausal breast cancer. Cancer Prev Res (Phila) 2013;6:188–195. doi: 10.1158/1940-6207.CAPR-12-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Minatoya M, Kutomi G, Shima H, Asakura S, Otokozawa S, Ohnishi H, Akasaka H, Miura T, Mori M, Hirata K. Relation of serum adiponectin levels and obesity with breast cancer: a Japanese case-control study. Asian Pac J Cancer Prev. 2014;15:8325–8330. doi: 10.7314/apjcp.2014.15.19.8325. [DOI] [PubMed] [Google Scholar]
- 95.Otake S, Takeda H, Suzuki Y, Fukui T, Watanabe S, Ishihama K, Saito T, Togashi H, Nakamura T, Matsuzawa Y, Kawata S. Association of visceral fat accumulation and plasma adiponectin with colorectal adenoma: evidence for participation of insulin resistance. Clin Cancer Res. 2005;11:3642–3646. doi: 10.1158/1078-0432.CCR-04-1868. [DOI] [PubMed] [Google Scholar]
- 96.Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst. 2005;97:1688–1694. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- 97.Stocks T, Lukanova A, Johansson M, Rinaldi S, Palmqvist R, Hallmans G, Kaaks R, Stattin P. Components of the metabolic syndrome and colorectal cancer risk; a prospective study. Int J Obes (Lond) 2008;32:304–314. doi: 10.1038/sj.ijo.0803713. [DOI] [PubMed] [Google Scholar]
- 98.Erarslan E, Turkay C, Koktener A, Koca C, Uz B, Bavbek N. Association of visceral fat accumulation and adiponectin levels with colorectal neoplasia. Dig Dis Sci. 2009;54:862–868. doi: 10.1007/s10620-008-0440-6. [DOI] [PubMed] [Google Scholar]
- 99.Guadagni F, Roselli M, Martini F, Spila A, Riondino S, D'Alessandro R, Del Monte G, Formica V, Laudisi A, Portarena I, Palmirotta R, Ferroni P. Prognostic significance of serum adipokine levels in colorectal cancer patients. Anticancer Res. 2009;29:3321–3327. [PubMed] [Google Scholar]
- 100.Kumor A, Daniel P, Pietruczuk M, Malecka-Panas E. Serum leptin, adiponectin, and resistin concentration in colorectal adenoma and carcinoma (CC) patients. Int J Colorectal Dis. 2009;24:275–281. doi: 10.1007/s00384-008-0605-y. [DOI] [PubMed] [Google Scholar]
- 101.Gonullu G, Kahraman H, Bedir A, Bektas A, Yucel I. Association between adiponectin, resistin, insulin resistance, and colorectal tumors. Int J Colorectal Dis. 2010;25:205–212. doi: 10.1007/s00384-009-0828-6. [DOI] [PubMed] [Google Scholar]
- 102.Kemik O, Sumer A, Kemik AS, Hasirci I, Purisa S, Dulger AC, Demiriz B, Tuzun S. The relationship among acute-phase response proteins, cytokines and hormones in cachectic patients with colon cancer. World J Surg Oncol. 2010;8:85. doi: 10.1186/1477-7819-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nakajima TE, Yamada Y, Hamano T, Furuta K, Matsuda T, Fujita S, Kato K, Hamaguchi T, Shimada Y. Adipocytokines as new promising markers of colorectal tumors: adiponectin for colorectal adenoma, and resistin and visfatin for colorectal cancer. Cancer Sci. 2010;101:1286–1291. doi: 10.1111/j.1349-7006.2010.01518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Otake S, Takeda H, Fujishima S, Fukui T, Orii T, Sato T, Sasaki Y, Nishise S, Kawata S. Decreased levels of plasma adiponectin associated with increased risk of colorectal cancer. World J Gastroenterol. 2010;16:1252–1257. doi: 10.3748/wjg.v16.i10.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamaji T, Iwasaki M, Sasazuki S, Tsugane S. Interaction between adiponectin and leptin influences the risk of colorectal adenoma. Cancer Res. 2010;70:5430–5437. doi: 10.1158/0008-5472.CAN-10-0178. [DOI] [PubMed] [Google Scholar]
- 106.Catalan V, Gomez-Ambrosi J, Rodriguez A, Ramirez B, Silva C, Rotellar F, Hernandez-Lizoain JL, Baixauli J, Valenti V, Pardo F, Salvador J, Fruhbeck G. Up-regulation of the novel proinflammatory adipokines lipocalin-2, chitinase-3 like-1 and osteopontin as well as angiogenic-related factors in visceral adipose tissue of patients with colon cancer. J Nutr Biochem. 2011;22:634–641. doi: 10.1016/j.jnutbio.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 107.Aleksandrova K, Boeing H, Jenab M, Bueno-de-Mesquita HB, Jansen E, van Duijnhoven FJ, Fedirko V, Rinaldi S, Romieu I, Riboli E, Romaguera D, Westphal S, Overvad K, Tjonneland A, Boutron-Ruault MC, Clavel-Chapelon F, et al. Total and high-molecular weight adiponectin and risk of colorectal cancer: the European Prospective Investigation into Cancer and Nutrition Study. Carcinogenesis. 2012;33:1211–1218. doi: 10.1093/carcin/bgs133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen MW, Ye S, Zhao LL, Wang SY, Li YX, Yu CJ, Xie HJ, Wang YM. Association of plasma total and high-molecular-weight adiponectin with risk of colorectal cancer: an observational study in Chinese male. Med Oncol. 2012;29:3129–3135. doi: 10.1007/s12032-012-0280-2. [DOI] [PubMed] [Google Scholar]
- 109.Touvier M, Fezeu L, Ahluwalia N, Julia C, Charnaux N, Sutton A, Mejean C, Latino-Martel P, Hercberg S, Galan P, Czernichow S. Pre-diagnostic levels of adiponectin and soluble vascular cell adhesion molecule-1 are associated with colorectal cancer risk. World J Gastroenterol. 2012;18:2805–2812. doi: 10.3748/wjg.v18.i22.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Danese E, Minicozzi AM, Montagnana M, De Manzoni G, Lippi G, Guidi GC. Lack of an association between circulating adiponectin levels and risk of colorectal adenoma. Clin Lab. 2013;59:211–214. doi: 10.7754/clin.lab.2012.120717. [DOI] [PubMed] [Google Scholar]
- 111.Song M, Zhang X, Wu K, Ogino S, Fuchs CS, Giovannucci EL, Chan AT. Plasma adiponectin and soluble leptin receptor and risk of colorectal cancer: a prospective study. Cancer Prev Res (Phila) 2013;6:875–885. doi: 10.1158/1940-6207.CAPR-13-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guo XH, Wang JY, Gao Y, Gao M, Yu GY, Xiang RL, Li L, Yang NY, Cong X, Xu XY, Li SL, Peng X, Wu LL. Decreased adiponectin level is associated with aggressive phenotype of tongue squamous cell carcinoma. Cancer Sci. 2013;104:206–213. doi: 10.1111/cas.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Inoue T, Kotooka N, Morooka T, Komoda H, Uchida T, Aso Y, Inukai T, Okuno T, Node K. High molecular weight adiponectin as a predictor of long-term clinical outcome in patients with coronary artery disease. Am J Cardiol. 2007;100:569–574. doi: 10.1016/j.amjcard.2007.03.062. [DOI] [PubMed] [Google Scholar]
- 114.Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, Imai Y, Nagai R, Kadowaki T. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29:1357–1362. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
- 115.Ye J, Jia J, Dong S, Zhang C, Yu S, Li L, Mao C, Wang D, Chen J, Yuan G. Circulating adiponectin levels and the risk of breast cancer: a meta-analysis. Eur J Cancer Prev. 2014;23:158–165. doi: 10.1097/CEJ.0b013e328364f293. [DOI] [PubMed] [Google Scholar]
- 116.An W, Bai Y, Deng SX, Gao J, Ben QW, Cai QC, Zhang HG, Li ZS. Adiponectin levels in patients with colorectal cancer and adenoma: a meta-analysis. Eur J Cancer Prev. 2012;21:126–133. doi: 10.1097/CEJ.0b013e32834c9b55. [DOI] [PubMed] [Google Scholar]
- 117.Ferroni P, Palmirotta R, Spila A, Martini F, Raparelli V, Fossile E, Mariotti S, Del Monte G, Buonomo O, Roselli M, Guadagni F. Prognostic significance of adiponectin levels in non-metastatic colorectal cancer. Anticancer Res. 2007;27:483–489. [PubMed] [Google Scholar]
- 118.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 119.Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J, Paschke R. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2003;301:1045–1050. doi: 10.1016/s0006-291x(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 120.Bauche IB, Ait El Mkadem S, Rezsohazy R, Funahashi T, Maeda N, Miranda LM, Brichard SM. Adiponectin downregulates its own production and the expression of its AdipoR2 receptor in transgenic mice. Biochem Biophys Res Commun. 2006;345:1414–1424. doi: 10.1016/j.bbrc.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 121.Qi L, Rimm E, Liu S, Rifai N, Hu FB. Dietary glycemic index, glycemic load, cereal fiber, and plasma adiponectin concentration in diabetic men. Diabetes Care. 2005;28:1022–1028. doi: 10.2337/diacare.28.5.1022. [DOI] [PubMed] [Google Scholar]
- 122.Tietge UJ, Boker KH, Manns MP, Bahr MJ. Elevated circulating adiponectin levels in liver cirrhosis are associated with reduced liver function and altered hepatic hemodynamics. Am J Physiol Endocrinol Metab. 2004;287:E82–89. doi: 10.1152/ajpendo.00494.2003. [DOI] [PubMed] [Google Scholar]
- 123.Barresi V, Grosso M, Giuffre G, Tuccari G, Barresi G. The expression of adiponectin receptors Adipo-R1 and Adipo-R2 is associated with an intestinal histotype and longer survival in gastric carcinoma. J Clinical Pathol. 2009;62:705–709. doi: 10.1136/jcp.2009.066175. [DOI] [PubMed] [Google Scholar]
- 124.Yabushita H, Iwasaki K, Obayashi Y, Wakatsuki A. Clinicopathological roles of adiponectin and leptin receptors in endometrial carcinoma. Oncol Lett. 2014;7:1109–1117. doi: 10.3892/ol.2014.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Abdul-Ghafar J, Oh SS, Park SM, Wairagu P, Lee SN, Jeong Y, Eom M, Yong SJ, Jung SH. Expression of adiponectin receptor 1 is indicative of favorable prognosis in non-small cell lung carcinoma. Tohoku J Exp Med. 2013;229:153–162. doi: 10.1620/tjem.229.153. [DOI] [PubMed] [Google Scholar]
- 126.Otvos L, Jr, Haspinger E, La Russa F, Maspero F, Graziano P, Kovalszky I, Lovas S, Nama K, Hoffmann R, Knappe D, Cassone M, Wade J, Surmacz E. Design and development of a peptide-based adiponectin receptor agonist for cancer treatment. BMC Biotechnol. 2011;11:90. doi: 10.1186/1472-6750-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu J, Xu A, Lam KS, Wong NS, Chen J, Shepherd PR, Wang Y. Cholesterol-induced mammary tumorigenesis is enhanced by adiponectin deficiency: role of LDL receptor upregulation. Oncotarget. 2013;4:1804–1818. doi: 10.18632/oncotarget.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 129.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 130.Lee HP, Lin CY, Shih JS, Fong YC, Wang SW, Li TM, Tang CH. Adiponectin promotes VEGF-A-dependent angiogenesis in human chondrosarcoma through PI3K, Akt, mTOR, and HIF-alpha pathway. Oncotarget. 2015;6:36746–36761. doi: 10.18632/oncotarget.5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang B, Cheng X, Wang D, Peng M, Xue Z, Da Y, Zhang N, Yao Z, Li M, Xu A, Zhang R. Adiponectin promotes pancreatic cancer progression by inhibiting apoptosis via the activation of AMPK/Sirt1/PGC-1alpha signaling. Oncotarget. 2014;5:4732–4745. doi: 10.18632/oncotarget.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 133.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]