Abstract
A preclinical study demonstrated anti‐proliferative and apoptotic effect of propranolol on multiple myeloma (MM) cell. Clinical studies suggested that beta‐blocker (BB) might impact the prognosis of breast, prostate, colorectal, ovarian, lung, and skin cancer. This retrospective study evaluated the effect of BB in MM disease‐specific survival (DSS) and overall survival (OS). Among 1,971 newly diagnosed MM patients seen at Mayo Clinic between 1995 and 2010, usage of BB and other cardiac (or antihypertensive) medications were abstracted. Cumulative incidence function and Kaplan–Meier method were used to estimate 5‐year cumulative incidence rate (CIR) of MM death and OS rate, respectively. Nine hundred and thirty (47.2%) patients had no intake of cardiac medications; 260 (13.2%) used BB alone; 343 (17.4%) used both BB/non‐BB cardiac medications; and 438 (22.2%) had non‐BB cardiac drugs. Superior MM DSS was observed in BB only users, compared to patients without any cardiac drugs ( , 0.53, 95% confidence interval [CI], 0.42–0.67, P adj.<0.0001) and non‐BB cardiac drugs users ( , 0.49, 95% CI, 0.38–0.63, P adj.<0.0001). Patients on both BB and other cardiac drugs showed superior DSS than non‐cardiac drugs users ( , 0.54, 95% CI, 0.44–0.67, P adj.<0.0001) and non‐BB cardiac drug users. ( , 0.50, 95% CI, 0.40–0.62, P adj.<0.0001). MM DSS did not differ between BB users with and without other cardiac drugs (P adj.=0.90). Multivariable analysis showed the same pattern for OS. In patients with MM, BB intake is associated with a reduced risk of disease‐specific death and overall mortality in comparison to non‐BB or no use of cardiac drugs. Am. J. Hematol. 92:50–55, 2017. © 2016 Wiley Periodicals, Inc.
Introduction
Preclinical studies have demonstrated that beta‐blocker (BB) could inhibit multiple cellular activities such as cell proliferation, invasion, migration, angiogenesis, and tumor immune response that are involved in cancer progression and metastasis by interfering with the β‐adrenergic receptor signaling pathway 1. Recently a number of clinical observational studies tested the hypothesis that BB treatment might impact the prognosis of breast, prostate, colorectal, ovarian, lung, and skin cancer 2, 3, 4, 5, 6, 7, 8, 9, 10, 11. However the findings were inconsistent 1 and the possible impact of BB on cancer outcomes remains controversial. In vitro, the non‐selective BB, propranolol, has anti‐proliferative and apoptotic activity on a multiple myeloma (MM) cell line 12. In the absence of outcome data related to BB use in MM, we conducted this study to investigate the association between BB use and survival outcomes in MM patients.
Methods
Patients
This study was approved by the Mayo Clinic Institutional Review Board. Only patients who had provided prior consent for research directed access to their medical records were included. We excluded patients with coexisting amyloidosis, those without information related to the use of cardiac (or antihypertensive) drugs or MM therapies, and those without follow up or survival information in the medical records. Data extracted from the Mayo Clinic electronic medical records included date of birth, gender, date of diagnosis, initial diagnostic data of radiologic, histopathological and laboratory tests, performance status (PS), International Staging System (ISS), cardiac history and medications including BB usage documented for a minimum of 3 months at any time after diagnosis of MM (if any), cytogenetic and fluorescent in situ hybridization (FISH) risk strata based on Mayo Stratification of Myeloma and Risk‐Adapted Therapy (mSMART) 13, stem cell transplant, MM therapies, date of death or last follow up and cause of death. Of 2104 newly diagnosed MM patients seen at Mayo Clinic, Rochester between 1995 and 2010 within 90 days of diagnosis, 1,971 patients met all inclusion criteria. These patients were categorized into four groups based on their cardiac or antihypertensive medication intake history: patients without any cardiac (or antihypertensive) medications; patients taking BB alone (e.g., metoprolol, atenolol, carvedilol, propranolol); those who only took non‐BB cardiac (or antihypertensive) drugs (e.g., ACE inhibitor, calcium channel blocker, angiotensin II receptor blocker, diuretic); and patients on BB and other non‐BB cardiac medications.
Statistical analysis
The MM disease‐specific survival (DSS) and overall survival (OS) were the primary endpoints. OS was defined as the time from the date of diagnosis to the date of death due to all causes, and living patients were censored at the last follow‐up date. The causes of death were identified and categorized as due to MM, cardiac disease, and other reasons. DSS was defined as the time from diagnosis to death caused by MM. Cardiac and other deaths were the competing events for MM cause‐specific deaths. The demographics, disease characteristics of patients, and chemotherapies administered were summarized by median (range) and frequency (percentage) for continuous and categorical factors, respectively, per BB intake groups. These patient characteristics were compared among the BB usage groups using Kruskal Wallis test or Chi‐Squared test as appropriate. Cumulative incidence functions and Kaplan–Meier method were used to estimate the 5‐year cumulative incidence rate (CIR) of MM death and OS rate, respectively. In univariate analysis, OS were compared among BB intake groups by log‐rank test. To account for the competing risks, DSS were compared among groups by Gray's test. Adjusted cause‐specific hazard ratio ( ) and hazard ratio (HRadj.) was estimated using Cox proportional hazard model for OS and DSS, respectively, adjusting for demographics, disease characteristics, diagnosis year, and various chemotherapies. Interaction test was performed to explore whether MM therapies influenced the association between BB usage and outcomes. All statistical analyses were carried out in SAS 9.4 software (SAS Institute, Cary, NC). Statistical significance was inferred at P‐value < 0.05 for all comparisons.
Results
Among this group of 1,971 patients, 930 (47.2%) had no cardiac medication intake (either no cardiovascular history or borderline hypertension without treatment requirements); 260 (13.2%) used a BB for a minimum of 3 months any time after diagnosis of MM; 343 (17.4%) had intake of both BB (≥ 3 months) and non‐BB cardiac medications; and 438 patients (22.2%) only took other categories of non‐BB cardiac drugs. The indications for cardiac medications included hypertension, coronary artery disease, congested heart failure, cardiac dysrhythmia, valvular heart disease, pulmonary hypertension, angina, and myocardial infarct. These four groups of BB users and nonusers were compared with regard to clinical characteristics as age, gender, year of diagnosis, ISS stage, PS, mSMART classification, and MM therapies (Table 1). There were no statistically significant differences in gender, PS and mSMART classification between the four groups. However, the patients who did not take cardiac medications were younger compared to those who took BB, BB plus non‐BB cardiac medications, and other non‐BB cardiac medications (median age 60, 64, 68 and 68 years old, respectively; P < 0.0001), were less likely to present with ISS stage 3 disease (17.9% vs. 25.1%, 36.9%, and 30.4% respectively; P < 0.0001), and more likely to be diagnosed before year of 2000 (25.8% vs. 14.2%, 11.4%, 20.8% respectively; P < 0.0001). Patients in the BB only group had higher use of bortezomib (P = 0.001), lenalidomide (P = 0.025), pomalidomide (P = 0.0144), ixazomib (P = 0.0368), and autologous stem cell transplant (ASCT) (P < 0.0001).
Table 1.
Characteristics Comparison Among Cardiac Medication Usage Groups
|
No cardiac medications (N = 930) |
Beta‐blocker (N = 260) |
Beta‐blocker and other cardiac medications (N = 343) |
Non‐beta‐blocker cardiac medications (N = 438) |
Total (N = 1971) |
P value | |
|---|---|---|---|---|---|---|
| Age at diagnosis | <0.0001a | |||||
| N | 930 | 260 | 343 | 438 | 1971 | |
| Median | 60.0 | 64.0 | 68.0 | 68.0 | 64.0 | |
| Range | (22.0–91.0) | (37.0–92.0) | (32.0–94.0) | (29.0–92.0) | (22.0–94.0) | |
| Gender | 0.3164a | |||||
| Female | 394 (42.4%) | 98 (37.7%) | 129 (37.6%) | 173 (39.5%) | 794 (40.3%) | |
| Male | 536 (57.6%) | 162 (62.3%) | 214 (62.4%) | 265 (60.5%) | 1177 (59.7%) | |
| mSMART | 0.4016a | |||||
| Standard Risk | 271 (62.9%) | 107 (58.8%) | 116 (56.3%) | 117 (60.9%) | 611 (60.4%) | |
| Intermediate Risk | 110 (25.5%) | 45 (24.7%) | 54 (26.2%) | 44 (22.9%) | 253 (25.0%) | |
| High Risk | 50 (11.6%) | 30 (16.5%) | 36 (17.5%) | 31 (16.1%) | 147 (14.5%) | |
| Missing | 499 | 78 | 137 | 246 | 960 | |
| International Staging System | <0.0001a | |||||
| I | 330 (37.9%) | 92 (36.7%) | 90 (27.2%) | 117 (28.2%) | 629 (33.7%) | |
| II | 385 (44.2%) | 96 (38.2%) | 119 (36.0%) | 172 (41.4%) | 772 (41.3%) | |
| III | 156 (17.9%) | 63 (25.1%) | 122 (36.9%) | 126 (30.4%) | 467 (25.0%) | |
| Missing | 59 | 9 | 12 | 23 | 103 | |
| Performance Score | 0.1287a | |||||
| 0 | 360 (41.6%) | 108 (43.5%) | 126 (39.1%) | 167 (41.6%) | 761 (41.4%) | |
| 1 | 332 (38.4%) | 91 (36.7%) | 107 (33.2%) | 140 (34.9%) | 670 (36.5%) | |
| 2+ | 173 (20.0%) | 49 (19.8%) | 89 (27.6%) | 94 (23.4%) | 405 (22.1%) | |
| Missing | 65 | 12 | 21 | 37 | 135 | |
| Diagnosis Year | <0.0001a | |||||
| 1995–1999 | 240 (25.8%) | 37 (14.2%) | 39 (11.4%) | 91 (20.8%) | 407 (20.6%) | |
| 2000–2004 | 291 (31.3%) | 77 (29.6%) | 108 (31.5%) | 144 (32.9%) | 620 (31.5%) | |
| 2005–2010 | 399 (42.9%) | 146 (56.2%) | 196 (57.1%) | 203 (46.3%) | 944 (47.9%) | |
| Pomalidomide | 0.0144a | |||||
| No | 861 (92.6%) | 229 (88.1%) | 311 (90.7%) | 414 (94.5%) | 1815 (92.1%) | |
| Yes | 69 (7.4%) | 31 (11.9%) | 32 (9.3%) | 24 (5.5%) | 156 (7.9%) | |
| Lenalidomide | 0.0250a | |||||
| No | 578 (62.2%) | 147 (56.5%) | 205 (59.8%) | 295 (67.4%) | 1225 (62.2%) | |
| Yes | 352 (37.8%) | 113 (43.5%) | 138 (40.2%) | 143 (32.6%) | 746 (37.8%) | |
| Thalidomide | 0.2777a | |||||
| No | 668 (71.8%) | 189 (72.7%) | 262 (76.4%) | 331 (75.6%) | 1450 (73.6%) | |
| Yes | 262 (28.2%) | 71 (27.3%) | 81 (23.6%) | 107 (24.4%) | 521 (26.4%) | |
| Stem cell transplant | <0.0001a | |||||
| No | 480 (51.6%) | 116 (44.6%) | 204 (59.5%) | 288 (65.8%) | 1088 (55.2%) | |
| Yes | 450 (48.4%) | 144 (55.4%) | 139 (40.5%) | 150 (34.2%) | 883 (44.8%) | |
| Alkylator | 0.2767a | |||||
| No | 457 (50.7%) | 114 (44.4%) | 167 (49.7%) | 216 (51.6%) | 954 (49.9%) | |
| Yes | 444 (49.3%) | 143 (55.6%) | 169 (50.3%) | 203 (48.4%) | 959 (50.1%) | |
| Missing | 29 | 3 | 7 | 19 | 58 | |
| Anthracycline | <0.0001a | |||||
| No | 694 (77.0%) | 215 (83.7%) | 295 (87.8%) | 371 (88.5%) | 1575 (82.3%) | |
| Yes | 207 (23.0%) | 42 (16.3%) | 41 (12.2%) | 48 (11.5%) | 338 (17.7%) | |
| Missing | 29 | 3 | 7 | 19 | 58 | |
| Bortezomib | 0.0010a | |||||
| No | 677 (75.1%) | 167 (65.0%) | 257 (76.5%) | 328 (78.3%) | 1429 (74.7%) | |
| Yes | 224 (24.9%) | 90 (35.0%) | 79 (23.5%) | 91 (21.7%) | 484 (25.3%) | |
| Missing | 29 | 3 | 7 | 19 | 58 | |
| Carfilzomib | 0.4177a | |||||
| No | 882 (97.9%) | 251 (97.7%) | 328 (97.6%) | 415 (99.0%) | 1876 (98.1%) | |
| Yes | 19 (2.1%) | 6 (2.3%) | 8 (2.4%) | 4 (1.0%) | 37 (1.9%) | |
| Missing | 29 | 3 | 7 | 19 | 58 | |
| Ixazomib | 0.0368a | |||||
| No | 887 (98.4%) | 246 (95.7%) | 328 (97.6%) | 413 (98.6%) | 1874 (98.0%) | |
| Yes | 14 (1.6%) | 11 (4.3%) | 8 (2.4%) | 6 (1.4%) | 39 (2.0%) | |
| Missing | 29 | 3 | 7 | 19 | 58 | |
Kruskal Wallis
Chi‐Squared.
At the time of this analysis, 1,345 patients were died. The estimated median follow up for those alive was 74.3 months. Figure 1 shows the cumulative Incidence curves for MM‐specific death. Significant differences in cumulative incidence rates (CIR) of MM‐specific death overtime were shown among four BB intake groups by Gray's test, accounting for competing risks of cardiac and other deaths. Patients who took only BB had the lowest 5‐year CIR of MM‐specific death 23.5%, [95% confidence interval (CI], 18.8–29.4%], followed by patients who took both BB and other cardiac drugs (CIR, 31.9%, 95% CI, 27.2–37.4%) and those without any documentation of cardiac (or antihypertensive) drugs use (CIR, 41.3, 95% CI, 38.2–44.7%). The patients who took non‐BB cardiac medications had the highest 5‐year CIR of MM‐specific death (49.9%, 95% CI, 45.3–54.9%). Figure 2 shows the Kaplan–Meier estimates of OS. Log‐rank test shows significant difference in OS among four BB intake groups (P <0.0001). The patients who took only BB had highest 5‐year OS rate at 66.0% (95% CI, 60.5–72.1%) and the patients who took non‐BB cardiac medications had lowest 5‐year OS rate at 39.3% (95% CI, 34.9–44.2%). The patients who took BB plus other cardiac drugs and who had no cardiac drugs had similar 5‐year OS rate, 49.6% and 50.0%, respectively (Supplementary Information Table 1).
Figure 1.
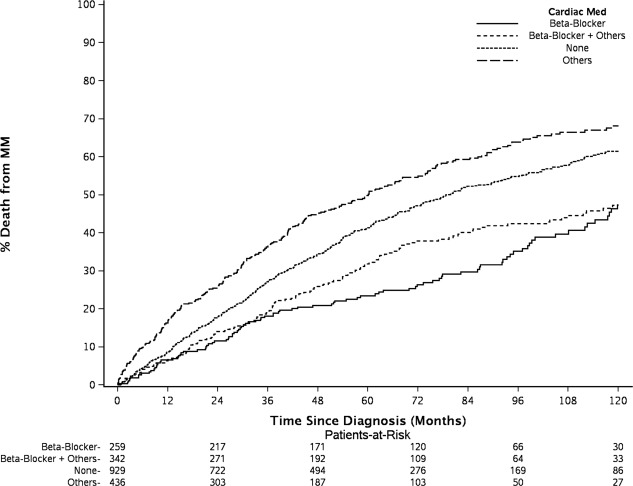
Significant differences in cumulative incidence rates (CIR) from multiple myeloma (MM)‐specific death were seen among beta‐blockers (BB) intake groups. The patients who took only BB had the lowest 5‐year CIR (23.5%), followed by those taking both BB and other cardiac drugs (31.9%), no cardiac drugs use (41.3%), and other non‐BB cardiac medications (49.9%).
Figure 2.
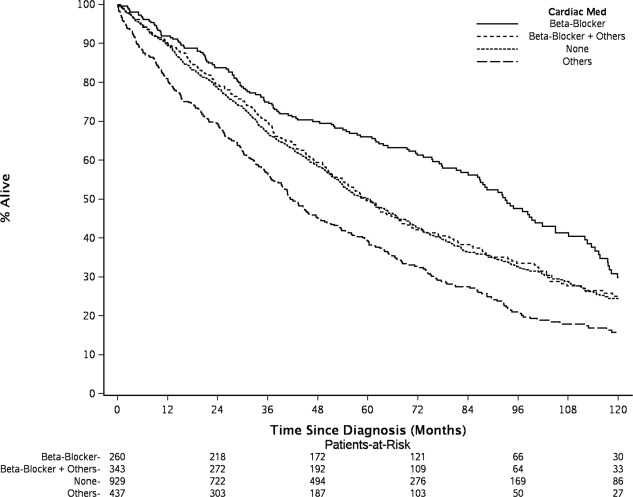
The patients who took only BB had highest 5‐year OS rate at 66.0%, and non‐BB cardiac medications users had lowest 5‐year OS rate at 39.3%. The patients who took BB plus other cardiac drugs and who had no cardiac drugs had similar 5‐year OS rate, 49.6% and 50.0%, respectively.
Cytogenetic and FISH stratification is an important prognostic factor, but the data were unavailable in some patients especially for those who were diagnosed in early years. We first performed a multivariable (MV) analysis to adjust for age, gender, year of diagnosis, performance score, and MM therapies for all patients. An additional analysis with a smaller subset of patients who had cytogenetic/FISH performed (n = 915) was also done. Despite the reduced sample size, the MV analyses results were consistent irrespective of the high‐risk FISH, that is, deletion 17p, t(14;16), or t(14;20). Therefore, we only presented the adjusted association estimates based on the MV model without FISH risk in Table 2. Both superior MM DSS and OS were observed for patients taking BB only compared to those taking non‐BB cardiac drugs (DSS: , 0.49, 95% CI, 0.38–0.63, P adj.<0.0001; OS: HRadj., 0.62, 95% CI, 0.50–0.76, P adj.<0.0001), and compared to those with no record of cardiac drugs (DSS: , 0.53, 95% CI, 0.42–0.67, P adj.<0.0001; OS: HRadj., 0.67, 95% CI, 0.55–0.81, P adj.<0.0001). The patients who received both BB and other cardiac drugs also had superior MM DSS than those who did not use cardiac drugs ( , 0.54, 95% CI, 0.44–0.67, P adj.<0.0001) and those who used non‐BB cardiac drugs ( , 0.50, 95% CI, 0.40–0.62, P adj.<0.0001). The same pattern was observed for OS. There are no difference in MM DSS (P adj.=0.90) and OS (P adj.=0.36) between BB users who took other cardiac drugs or not.
Table 2.
Multivariable Model Comparing Overall Survival and MM Disease‐Specific Survival Among Beta‐Blocker Intake Groups.a
| MM disease‐specific Death | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cardiac med | Event/total |
Cause‐specific hazard ratio (95% CI) |
P‐value |
Cause‐specific hazard ratio (95% CI) |
P‐value |
Cause‐specific hazard ratio (95% CI) |
P‐value |
Cause‐specific hazard ratio (95% CI) |
P‐value |
| Beta‐blocker | 90/238 | Reference | – | 0.98 (0.75–1.29) | 0.8979 | 0.49 (0.38–0.63) | <0.0001 | 0.53 (0.42–0.67) | <0.0001 |
| Beta‐blocker + others | 127/311 | 1.02 (0.77–1.34) | 0.8979 | Reference | – | 0.50 (0.40–0.62) | <0.0001 | 0.54 (0.44–0.67) | <0.0001 |
| Others | 237/377 | 2.03 (1.59–2.60) | <0.0001 | 1.99 (1.60–2.48) | <0.0001 | Reference | – | 1.08 (0.92–1.28) | .3278 |
| None | 444/812 | 1.87 (1.49–2.36) | <0.0001 | 1.84 (1.50–2.26) | <0.0001 | 0.92 (0.78–1.08) | 0.3278 | Reference | – |
| Overall Survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cardiac med | Event/total |
Hazard ratio (95% CI) |
P‐value |
Hazard ratio (95% CI) |
P‐value |
Hazard ratio (95% CI) |
P‐value | ||
| Beta‐blocker | 134/238 | Reference | – | 0.90 (0.72–1.13) | 0.3617 | 0.62 (0.50–0.76) | <0.0001 | 0.67 (0.55–0.81) | <0.0001 |
| Beta‐blocker + others | 204/311 | 1.11 (0.89–1.38) | 0.3617 | Reference | – | 0.68 (0.57–0.82) | <0.0001 | 0.74 (0.62–0.87) | 0.0004 |
| Others | 287/377 | 1.62 (1.32–2.00) | <0.0001 | 1.47 (1.22–1.76) | <0.0001 | Reference | – | 1.08 (0.93–1.25) | 0.2912 |
| None | 532/812 | 1.50 (1.24–1.82) | <0.0001 | 1.35 (1.14–1.60) | 0.0004 | 0.92 (0.80–1.07) | 0.2912 | Reference | – |
Adjusting for age, sex, PS, ISS, diagnosis year, and all chemotherapy agents. When mSMART risk category of cytogenetic and FISH was included additionally, the results are consistent with what were presented in the table (data not shown).
Of note, in multivariable analysis adjusting for prognostic factors, the improved survival outcome from BB was independent of MM therapies. We further evaluated whether various chemotherapies impacted the observed association between BB usage and survival. The interaction tests between four cardiac medication usage groups and each MM treatment agent was not significant in either univariate or multivariate analyses (Supplementary Information Table 2). The improved survival benefit of BB was independent and additional to the effect of MM therapies.
Discussion
This study was designed to evaluate the prognostic association of BB usage with survival among patients with MM. We found that the use of BB was associated with longer OS and DSS in MM patients even after adjustment for prognostic factors, suggesting that BB usage was independently associated with better MM prognosis. Our findings are concordant with the emerging evidence suggesting that BB reduce cancer progression and metastasis 1. Preclinical studies have suggested that the β‐adrenergic signaling pathway regulates multiple developmental process in cell proliferation, differentiation and migration 1. Epinephrine and norepinephrine are released during stress response, and activate β‐adrenergic receptors (β‐ARs) 1. Activation of β‐adrenergic signaling results in promotion of inflammation by induction of pro‐inflammatory cytokine interleukin 6 (IL‐6), overexpression of vascular endothelial growth factor (VEGF) leading to angiogenesis—a critical process for tumor growth and progression 1, enhancing tumor cells to invade the extracellular matrix 14, and decreased sensitivity to apoptosis of cancer cells 15. Because BB blocks the action of catecholamine on β‐ARs, these agents have been explored, both in vitro and in vivo, and found to have antitumor effect in ovarian, breast, colon, prostate, pancreatic and small cell lung cancer 16, 17, 18, 19, 20, 21. In MM, bone marrow angiogenesis is stimulated by the malignant plasma cells in the bone marrow microenvironment 22. Bone marrow angiogenesis, driven by several angiogeneic factors including VEGF and IL‐6 23, has been associated with disease aggressiveness of myeloma and disease progression from monoclonal gammopathy of undetermined significance (MGUS) to MM 24. In vitro studies have shown that production and up regulation of VEGF and IL‐6 are inhibited by BB 25, 26. A recent in vitro experiment also suggested that BB affects NF‐κB signaling in MM cells by regulating the expression levels of genes involved in upstream and downstream pathways 12. BB caused an increase of apoptotic Bcl‐10 gene, a decrease of antiapoptotic Bcl‐2 gene, an increase in IL‐10– a known factor to suppress NF‐κB–and an increase of tumor necrosis factor receptor superfamily member 10b(TNFRSF10B), which is an important transducer of apoptosis 12. The NF‐κB signaling pathway is a main target of MM treatment 12, and is one of the major pathways by which proteasome inhibitors effect MM cell death 27. It is through these pathways that BB may impact MM survival.
Our observation adds to the growing evidence of improved survival among oncology patients receiving BB including non‐small cell lung cancer 2, ovarian cancer 3, 4 and colorectal cancer 5; prostate (reduced cancer specific mortality) 6, 7 and breast cancer 8, 9; and melanoma 10, 11. Other positive studies have shown increased response to chemotherapy in patients with neuroblastoma 28, better response to radiation therapy in meningioma 29, and decreased risk of progression in melanoma 10, 11. The evidence is conflicting since a few other studies have demonstrated no association between BB use and prognosis for ovarian cancer 30, 31, prostate cancer 32, breast cancer 33, melanoma 34, 35, and lung cancer 36. A systemic review and meta‐analysis of BB use in 46,265 breast cancer patients from 11 papers concluded that there is an improvement of cancer specific survival and disease free survival associated with BB use, but no association of survival outcome and the use of angiotensin receptor blockers and angiotensin‐converting enzyme inhibitors 37. Another meta‐analysis of the impact of BB on cancer survival was based on studies published between 1993 and 2013. In this study, the authors identified a total of 181 citations from MEDLINE and Embase database and included 12 studies on different types of non‐hematologic cancer, resulting in a total of 18 comparisons obtained from 20,898 subjects. The meta‐analysis demonstrated that BB use was associated with prolonged overall survival and disease‐free survival. Further analysis according to cancer type suggested significant effect in breast, ovarian and lung cancer, but not in prostate cancer and melanoma 38. Our study provides the first clinical evidence demonstrating overall and disease specific survival benefit associated with the use of BB in MM patients.
Limitations of this study include its retrospective nature and the lack of adequate information regarding the duration and the dosage of BBs. The majority of the patients had their cardiac medications managed by the local providers. Many patients were already on BB with unknown durations and dosage adjustments prior to their evaluation from MM standpoint and continued BB after last seen at Mayo Clinic. These shortcomings limited the ability to investigate the correlation of cumulative BB dosage on MM survival outcome, and will require prospective studies to confirm the role of BB in MM patients.
Authorship Contributions
Yi L Hwa: Prepared and submitted IRB application; contributed to conception and study; retrospectively reviewed all medical records and collected data; contributed to acquisition and data interpretation; drafted and critically revised the manuscript.
Shi Q: Conducted data analysis and prepared all figures and tables; critically revised the manuscript
Lacy MQ, Gertz M, Buadi F, Rajkumar SV, Kumar S, Kapoor P, Go RS, Leung N, Hayman ST, Lin Y, Lust JA, Dingli D, Russell SJ, Gonsalves WI: Contributed to acquisition and mentoring the process; critically revised the manuscript.
Dispenzieri A: Contributed to conception and study design; supervised the entire study process and provided detailed advice; mentored the project; contributed to acquisition and data interpretation; critically revised the manuscript.
Supporting information
Supporting Information Tables.
Acknowledgment
Predolin Foundation and Robert A. Kyle Hematologic Malignancies Program.
Conflicts of interests: The authors declare no competing financial interests.
References
- 1. Ji Y, Chen S, Xiao X, et al. Beta‐blockers: a novel class of antitumor agents. Onco Targets Ther 2012;5:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang HM, Liao ZX, Komaki R, et al. Improved survival outcomes with the incidental use of beta‐blockers among patients with non‐small‐cell lung cancer treated with definitive radiation therapy. Ann Oncol 2013;24:1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diaz ES, Karlan BY, Li AJ. Impact of beta blockers on epithelial ovarian cancer survival. Gynecol Oncol 2012;127:375–378. [DOI] [PubMed] [Google Scholar]
- 4. Schmidt SA, Schmidt M. Beta‐blockers and improved survival from ovarian cancer: New miracle treatment or another case of immortal person‐time bias? Cancer 2016;122:324–325. [DOI] [PubMed] [Google Scholar]
- 5. Jansen L, Hoffmeister M, Arndt V, et al. Stage‐specific associations between beta blocker use and prognosis after colorectal cancer. Cancer 2014;120:1178–1186. [DOI] [PubMed] [Google Scholar]
- 6. Grytli HH, Fagerland MW, Fossa SD, et al. Association between use of beta‐blockers and prostate cancer‐specific survival: a cohort study of 3561 prostate cancer patients with high‐risk or metastatic disease. Eur Urol 2014;65:635–641. [DOI] [PubMed] [Google Scholar]
- 7. Grytli HH, Fagerland MW, Fossa SD, et al. Use of beta‐blockers is associated with prostate cancer‐specific survival in prostate cancer patients on androgen deprivation therapy. Prostate 2013;73:250–260. [DOI] [PubMed] [Google Scholar]
- 8. Powe DG, Voss MJ, Zanker KS, et al. Beta‐blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 2010;1:628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Melhem‐Bertrandt A, Chavez‐Macgregor M, Lei X, et al. Beta‐blocker use is associated with improved relapse‐free survival in patients with triple‐negative breast cancer. J Clin Oncol 2011;29:2645–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lemeshow S, Sorensen HT, Phillips G, et al. beta‐Blockers and survival among Danish patients with malignant melanoma: a population‐based cohort study. Cancer Epidemiol Biomarkers Prev 2011;20:2273–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Giorgi V, Grazzini M, Gandini S, et al. Treatment with beta‐blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med 2011;171:779–781. [DOI] [PubMed] [Google Scholar]
- 12. Kozanoglu I, Yandim MK, Cincin ZB, et al. New indication for therapeutic potential of an old well‐known drug (propranolol) for multiple myeloma. J Cancer Res Clin Oncol 2013;139:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar SK, Mikhael JR, Buadi FK, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk‐Adapted Therapy (mSMART) consensus guidelines. Mayo Clin Proc 2009;84:1095–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang EV, Sood AK, Chen M, et al. Norepinephrine up‐regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)−2, and MMP‐9 in nasopharyngeal carcinoma tumor cells. Cancer Res 2006;66:10357–10364. [DOI] [PubMed] [Google Scholar]
- 15. Sastry KS, Karpova Y, Prokopovich S, et al. Epinephrine protects cancer cells from apoptosis via activation of cAMP‐dependent protein kinase and BAD phosphorylation. J Biol Chem 2007;282:14094–14100. [DOI] [PubMed] [Google Scholar]
- 16. Kim‐Fuchs C, Le CP, Pimentel MA, et al. Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta‐adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun 2014;40:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lang K, Drell TLt, Lindecke A, et al. Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int J Cancer 2004;112:231–238. [DOI] [PubMed] [Google Scholar]
- 18. Sood AK, Bhatty R, Kamat AA, et al. Stress hormone‐mediated invasion of ovarian cancer cells. Clin Cancer Res 2006;12:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sloan EK, Priceman SJ, Cox BF, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res 2010;70:7042–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masur K, Niggemann B, Zanker KS, et al. Norepinephrine‐induced migration of SW 480 colon carcinoma cells is inhibited by beta‐blockers. Cancer Res 2001;61:2866–2869. [PubMed] [Google Scholar]
- 21. Schuller HM, Cole B. Regulation of cell proliferation by beta‐adrenergic receptors in a human lung adenocarcinoma cell line. Carcinogenesis 1989;10:1753–1755. [DOI] [PubMed] [Google Scholar]
- 22. Vacca A, Ria R, Reale A, et al. Angiogenesis in multiple myeloma. Chem Immunol Allergy 2014;99:180–196. [DOI] [PubMed] [Google Scholar]
- 23. Jakob C, Sterz J, Zavrski I, et al. Angiogenesis in multiple myeloma. Eur J Cancer 2006;42:1581–1590. [DOI] [PubMed] [Google Scholar]
- 24. Rajkumar SV, Mesa RA, Fonseca R, et al. Bone marrow angiogenesis in 400 patients with monoclonal gammopathy of undetermined significance, multiple myeloma, and primary amyloidosis. Clin Cancer Res 2002;8:2210–2216. [PubMed] [Google Scholar]
- 25. Madden KS, Szpunar MJ, Brown EB. Beta‐adrenergic receptors (beta‐AR) regulate VEGF and IL‐6 production by divergent pathways in high beta‐AR‐expressing breast cancer cell lines. Breast Cancer Res Treat 2011;130:747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park SY, Kang JH, Jeong KJ, et al. Norepinephrine induces VEGF expression and angiogenesis by a hypoxia‐inducible factor‐1alpha protein‐dependent mechanism. Int J Cancer 2011;128:2306–2316. [DOI] [PubMed] [Google Scholar]
- 27. Ganten TM, Koschny R, Haas TL, et al. Proteasome inhibition sensitizes hepatocellular carcinoma cells, but not human hepatocytes, to TRAIL. Hepatology 2005;42:588–597. [DOI] [PubMed] [Google Scholar]
- 28. Pasquier E, Street J, Pouchy C, et al. Beta‐blockers increase response to chemotherapy via direct antitumour and anti‐angiogenic mechanisms in neuroblastoma. Br J Cancer 2013;108:2485–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pintea B, Kinfe TM, Baumert BG, et al. Earlier and sustained response with incidental use of cardiovascular drugs among patients with low‐ to medium‐grade meningiomas treated with radiosurgery (SRS) or stereotactic radiotherapy (SRT). Radiother Oncol 2014;111:446–450. [DOI] [PubMed] [Google Scholar]
- 30. Eskander R, Bessonova L, Chiu C, et al. Beta blocker use and ovarian cancer survival: A retrospective cohort study. Gynecol Oncol 2012;127:S21. [Google Scholar]
- 31. Heitz F, du Bois A, Harter P, et al. Impact of beta blocker medication in patients with platinum sensitive recurrent ovarian cancer‐a combined analysis of 2 prospective multicenter trials by the AGO Study Group, NCIC‐CTG and EORTC‐GCG. Gynecol Oncol 2013;129:463–466. [DOI] [PubMed] [Google Scholar]
- 32. Cardwell CR, Coleman HG, Murray LJ, et al. Beta‐blocker usage and prostate cancer survival: a nested case‐control study in the UK Clinical Practice Research Datalink cohort. Cancer Epidemiol 2014;38:279–285. [DOI] [PubMed] [Google Scholar]
- 33. Cardwell CR, Coleman HG, Murray LJ, et al. Beta‐blocker usage and breast cancer survival: a nested case‐control study within a UK Clinical Practice Research Datalink cohort. Int J Epidemiol 2013;42:1852–1861. [DOI] [PubMed] [Google Scholar]
- 34. McCourt C, Coleman HG, Murray LJ, et al. Beta‐blocker usage after malignant melanoma diagnosis and survival: a population‐based nested case‐control study. Br J Dermatol 2014;170:930–938. [DOI] [PubMed] [Google Scholar]
- 35. Livingstone E, Hollestein LM, van Herk‐Sukel MP, et al. Beta‐blocker use and all‐cause mortality of melanoma patients: results from a population‐based Dutch cohort study. Eur J Cancer 2013;49:3863–3871. [DOI] [PubMed] [Google Scholar]
- 36. Cata JP, Villarreal J, Keerty D, et al. Perioperative beta‐blocker use and survival in lung cancer patients. J Clin Anesth 2014;26:106–117. [DOI] [PubMed] [Google Scholar]
- 37. Raimondi S, Botteri E, Munzone E, et al. Use of beta‐blockers, angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers and breast cancer survival: Systematic review and meta‐analysis. Int J Cancer 2016;139:212–219. [DOI] [PubMed] [Google Scholar]
- 38. Choi CH, Song T, Kim TH, et al. Meta‐analysis of the effects of beta blocker on survival time in cancer patients. J Cancer Res Clin Oncol 2014;140:1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Tables.


