Kir6.2-containing KATP channels are prominent in pancreatic β cells, and gain-of-function mutations in these channels are the most common cause of human neonatal diabetes mellitus. Remedi et al. find that Kir6.1 subunits are also present in pancreatic KATP channels and that gain-of-function mutations can also cause impaired glucose tolerance and insulin secretion.
Abstract
Gain-of-function (GOF) mutations in the pore-forming (Kir6.2) and regulatory (SUR1) subunits of KATP channels have been identified as the most common cause of human neonatal diabetes mellitus. The critical effect of these mutations is confirmed in mice expressing Kir6.2-GOF mutations in pancreatic β cells. A second KATP channel pore-forming subunit, Kir6.1, was originally cloned from the pancreas. Although the prominence of this subunit in the vascular system is well documented, a potential role in pancreatic β cells has not been considered. Here, we show that mice expressing Kir6.1-GOF mutations (Kir6.1[G343D] or Kir6.1[G343D,Q53R]) in pancreatic β cells (under rat-insulin-promoter [Rip] control) develop glucose intolerance and diabetes caused by reduced insulin secretion. We also generated transgenic mice in which a bacterial artificial chromosome (BAC) containing Kir6.1[G343D] is incorporated such that the transgene is only expressed in tissues where Kir6.1 is normally present. Strikingly, BAC-Kir6.1[G343D] mice also show impaired glucose tolerance, as well as reduced glucose- and sulfonylurea-dependent insulin secretion. However, the response to K+ depolarization is intact in Kir6.1-GOF mice compared with control islets. The presence of native Kir6.1 transcripts was demonstrated in both human and wild-type mouse islets using quantitative real-time PCR. Together, these results implicate the incorporation of native Kir6.1 subunits into pancreatic KATP channels and a contributory role for these subunits in the control of insulin secretion.
Introduction
In the pancreatic β cell, ATP-sensitive K+ (KATP) channels play a critical role in coupling membrane excitability to glucose-stimulated insulin secretion, and thereby maintaining blood glucose in a narrow range. Gain-of-function (GOF) mutations in the pore-forming Kir6.2 subunit or in the regulatory sulfonylurea receptor 1 (SUR1) subunit have been identified as the commonest cause of human neonatal diabetes mellitus (NDM; Gloyn et al., 2004b; Vaxillaire et al., 2004; Sperling, 2006; Hamilton-Shield, 2007; Polak and Cavé, 2007). Normally, increased glucose metabolism elevates intracellular [ATP]/[ADP] ratio inhibiting KATP channels, leading to membrane depolarization, opening of voltage-dependent Ca2+ channels, and an increase in intracellular [Ca2+], which, in turn, triggers insulin secretion (Nichols, 2006). Antidiabetic sulfonylurea drugs promote insulin secretion by binding to the SUR1 subunit of the KATP channel, independently of the metabolic state of the cell (Ashcroft and Gribble, 1999). Sulfonylurea drugs have been widely used in type 2 diabetes treatment and, recently, successfully used to treat NDM patients with KATP-GOF mutations (Pearson et al., 2006).
There are two Kir6 subunit–encoding genes (KCNJ8, Kir6.1 and KCNJ11, Kir6.2). Kir6.2 is a major component of pancreatic β cell KATP channels: we previously demonstrated development of profound neonatal diabetes in mice constitutively expressing Kir6.2-GOF mutations in pancreatic β cells (Koster et al., 2000; Remedi et al., 2009). These mice rapidly develop severe diabetes followed by the development of secondary consequences of systemic diabetes, including marked loss of insulin content and β cell mass (Girard et al., 2009; Remedi et al., 2009; Wang et al., 2014), consequences that are completely avoided by maintenance of normoglycemia at disease onset (Remedi et al., 2009, 2011; Benninger et al., 2011) and dramatically reversed by intensive insulin therapy (Wang et al., 2014).
Kir6.1 was originally cloned from the pancreas (Inagaki et al., 1995), but neither the presence of Kir6.1-containing KATP channels in the β cell nor anycontribution to insulin secretion has been established. Given the high sequence similarity between Kir6.1 and Kir6.2, and based on the development of severe diabetes in Kir6.2-GOF mice (Girard et al., 2009; Remedi et al., 2009), we hypothesized that pancreatic expression of GOF mutations in the Kir6.1 subunit of the channel would also decrease insulin secretion. To probe this, we generated transgenic mice expressing Kir6.1 mutations under Cre-recombinase control to specifically express the transgene in the tissue of interest. Single mutant Kir6.1[G343D], double mutant Kir6.1[G343D,Q53R], and WT Kir6.1 transgenic mice (Li et al., 2013) were crossed with rat-insulin-promoter Cre mice (Rip-Cre [Herrera, 2002]) to generate pancreatic β cell–specific Rip-Kir6.1[GD], Rip-Kir6.1[GD,QR], and Rip-Kir6.1[WT] mice, respectively. Single mutant Rip-Kir6.1[GD] mice show glucose intolerance, and double mutant Rip-Kir6.1[GD,QR] mice develop as severe diabetes as do Rip-Kir6.2-GOF transgenic mice. We also expressed Kir6.1 mutants in bacterial artificial chromosomes (BACs) in order to express the transgene only in tissues where the Kir6.1 gene is normally present. Strikingly, BAC-Kir6.1[GD] mice show similar glucose intolerance to Rip-Kir6.1[GD] mice. The results have important implications for the molecular basis of pancreatic KATP channels and for the potential role of KCNJ8 (Kir6.1) gene variants in insulin secretory control and the development of diabetes.
Materials and methods
Human islets and study approval
Isolated cadaver-derived human islets were obtained from the Integrated Islet Distribution Program (IIDP) sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases and the JDRF-sponsored Islets for Research Program at Washington University (JDRF-31-2008-382). The IIDP uses only cadaver donors that have consented to research. The Washington University Medical Center (WUMC) Human Studies Committee (HSC) Institutional Review Board (IRB) approved all studies involving the use of isolated cadaver-derived human islets (approval number: 93-0068). The IRB exempted the study from HIPAA compliance based on the regulatory definition of human subject. Review date: 7/8/2004; review committee: 08 MRCR. Human islet donor data: four independent organ donors, two males and two females; mean age 35 yr, mean purity 85.8%, and mean viability 92.9%.
Quantitative real-time PCR (qRT-PCR) analysis
Mouse islets were isolated and immediately processed for RNA isolation. Upon arrival, human islets were collected by centrifugation, cleaned, and hand-selected in CMRL media under a stereomicroscope (SMZ745 system; Nikon) and immediately processed for RNA isolation to perform qRT-PCR. Cellular RNA was isolated using the RNeasy Mini kit (QIAGEN), and DNA was removed using DNase1 RNase-free solution (QIAGEN). cDNA was prepared from RNA using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems), and qRT-PCR was performed using StepOne software 2.3 and TaqMan primers (Applied Biosystems). Actin was used as a reference gene.
Generation of CX1-Kir6.1[WT], CX1-Kir6.1[GD], CX1-Kir6.1[GD,QR], BAC-Kir6.1[WT], and BAC-Kir6.1[GD] transgenic mice
Inducible Kir6.1-GOF transgenes were generated by subcloning WT, Kir6.1[Gly343Asp] (G343D), and Kir6.1[Gly343Asp,Gln53Arg] (G343D,Q53R)-poly-A cDNAs downstream of the CX-1 promoter in the pBS-CX1-LEL vector (a gift from G. Owens, University of Virginia, Charlottesville, VA), which contains the chicken β-actin (CX1) promoter, followed by the eGFP coding region (including a stop codon), flanked by two loxP sites, to create CX1-Kir6.1[WT], CX1-Kir6.1[G343D], and CX1-Kir6.1[G343D,Q53R] transgenic constructs (Li et al., 2013). 6–11 founder mice carrying (1) Kir6.1[WT], Kir6.1[G343D], or Kir6.1[G343D,Q53R] were generated and bred to homogeneity by multiple (6×) backcrosses to C57BL/6J mates as previously described (Li et al., 2013). These mice were crossed with rat-insulin-promoter Cre (Rip-Cre) mice to generate pancreatic β cell–specific Rip-Kir6.1[GD], Rip-Kir6.1[GD,QR], and Rip-Kir6.1[WT] mice.
A mouse BAC containing the Kir6.1 genomic locus (BAC ID# RP23-159E3) was used to generate the Kir6.1[WT] and Kir6.1[G343D] BAC transgenes via galK-based homologous recombination in bacteria. Positive clones from the first round of recombineering were subjected to a second round of recombineering to remove the galK gene, leaving behind only the desired mutations. Positive recombinants were identified via directional PCR, restriction digest, and sequencing. Mutant and WT BAC DNA were prepared using a modified QIAGEN maxi-prep, which was dot dialyzed on 0.1 µM EMD Millipore filters using BAC injection buffer. The Washington University Transgenic Vectors Core generated the Kir6.1[WT] and Kir6.1[G343D] BAC transgenes, and microinjection services were provided by the Washington University Mouse Genetics Core. Kir6.1−/− (Kir6.1 knockout [Kir6.1KO]) mice, originally generated by Miki et al. (2002), were obtained as a gift from S. Seino (Kobe University Graduate School of Medicine, Kobe, Japan).
Animal study approval
All procedures complied with the standards for the care and use of animal subjects as stated in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication No. 85-23, revised 1996).
Blood glucose
Tail blood was assayed for glucose content using a Glucometer Elite Xl meter (Bayer Corp). The limit of detection was 600 mg/dl, and glucose at or above this level was recorded as 600 mg/dl but considered to be a lower limit of the true value.
Glucose tolerance test (GTT)
Intraperitoneal GTT was performed in 12-h fasted 7-wk-old mice by injection of a bolus of glucose (1.5 g/kg body weight). Blood was taken at different times and assayed for glucose content as above.
Islet isolation
8-wk-old mice were anesthetized with 0.2 ml isoflurane and killed by cervical dislocation. The bile duct was cannulated and perfused with Hank’s balanced salt solution (Sigma-Aldrich) containing collagenase (0.3 mg/ml, Collagenase Type XI; Sigma-Aldrich). Pancreata were removed and digested for 5 min at 37°C, hand shaken, and washed three times in cold Hanks’ solution. Islets were isolated by hand under a dissecting SMZ745 microscope, and pooled islets were maintained overnight in CMRL-1066 (5.6 mM glucose) culture medium (GIBCO) supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 µg/ml streptomycin.
Insulin secretion
After overnight incubation in low glucose (5.6 mM) CMRL-1066 medium, islets (10 per well in 12-well plates) were preincubated in glucose-free CMRL-1066 plus 3 mM glucose and then incubated for 60 min at 37°C in CMRL-1066 plus different glucose concentrations, 1 µM glibenclamide, or 30 mM KCl, as indicated. After the incubation period, the medium was removed and assayed for insulin release. Insulin secretion was measured using a Rat Insulin radioimmunoassay according to manufacturer’s procedure (RIA; EMD Millipore; Remedi et al., 2009). Plasma insulin was measured using Singulex Erenna method (similar to insulin enzyme-linked immunosorbent assay) at the Core Laboratory for Clinical Studies, Washington University in St. Louis. Experiments were repeated in triplicate.
Isolated islet fluorescence imaging
Freshly isolated islets from 8-wk-old mice were imaged for eGFP, a reporter of transgene expression, using an inverted florescent microscope (LSM 510 laser-scanning confocal microscope; ZEISS). Images were taken with 20× objectives using the same settings for both control and transgenic islets.
86Rb+ efflux experiments
Isolated islets were preincubated for 6 h with 86Rb+ (rubidium chloride 1.5 mCi/ml; GE Healthcare). Loaded islets were placed in microcentrifuge tubes (30 per group) and washed twice with RPMI-1640 medium at 37°C (Sigma-Aldrich). 86Rb+ efflux was assayed by replacing the bathing solution with Ringer’s solution with metabolic inhibitor (MI), with or without 1 µM glibenclamide. MI solution contained 2.5 mg/ml oligomycin and 1 mM 2-deoxyglucose, together with 10 mM tetraethylammonium to block voltage-gated K+ channels and 10 µM nifedipine to block Ca2+ entry. The other solutions were different glucose concentrations (1, 7, and 20 mM glucose). The bathing solution was replaced with fresh solution every 5 min over a 50-min period and counted in a scintillation counter. 86Rb efflux in the presence of glucose and drugs were fit by a single exponential, in which the rate constant (k) is proportional to the K+ (Rb+)-conductance in the islet membranes.
Statistics
Data are presented as mean ± SEM. Differences among groups were tested using unpaired t test to assess significance. Differences were assumed to be significant in each case if P < 0.05, and nonsignificant differences are not indicated in the figures.
Results
Kir6.1 is expressed in mouse and human pancreatic islets
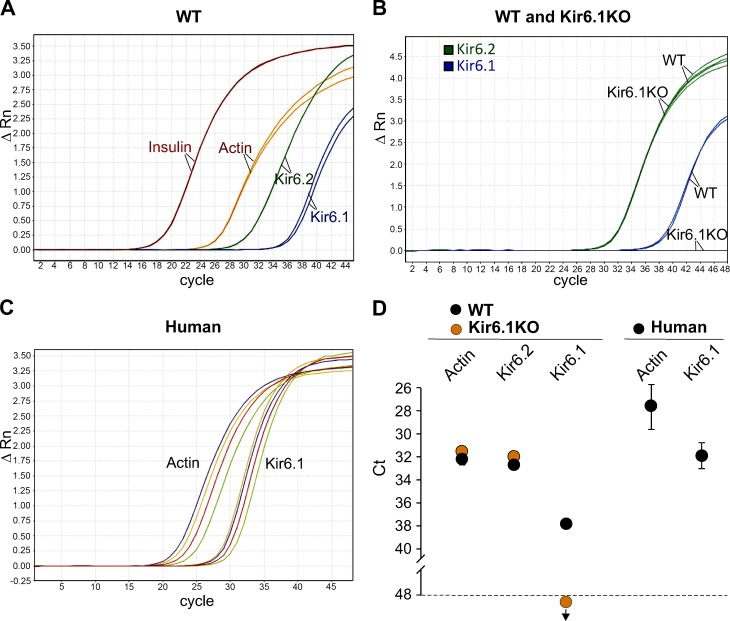
Although Kir6.1 subunits are prominent components of vascular KATP channels (Flagg et al., 2010), the cDNA was originally cloned from pancreatic islets (Inagaki et al., 1995), but there was little consideration of the presence of this subunit in insulin-secreting cells. The latter studysuggest that Kir6.1 is naturally expressed in islets, raising the possibility that Kir6.1 subunits might contribute to native islet KATP function. WT and Kir6.1KO mouse islets were subjected to qRT-PCR. As predicted, robust Kir6.1 signals, in addition to Kir6.2 signals, were detected in mouse WT islets (Fig. 1 A), but there was no Kir6.1 signal detectable (at up to 50 cycles) in Kir6.1KO islets (Fig. 1 B), confirming the validity of the Kir6.1 signal in WT islets. Expression of Kir6.2 was not different between WT and Kir6.1KO islets (Fig. 1 B). Importantly, human islets obtained from cadaveric organ donors also showed a robust Kir6.1 transcript level (Fig. 1 C). Thus, the Kir6.1 subunit of the KATP channel is natively present in both mouse and human pancreatic islets.
Figure 1.
Kir6.1 transcript is present in primary human and mouse islets. qRT-PCR in mouse and human islets. (A and B) Representative traces of transcript amplification versus cycle number from mouse islets. (A) Insulin is represented in red, Kir6.2 in green, and Kir6.1 in blue. Actin was used as housekeeping gene and is shown in orange. Same color lines are duplicates from the same sample. (B) Comparative amplification traces for Kir6.2 (green) and Kir6.1 (blue) genes in WT and Kir6.1KO islets (n = 5 mice of each genotype, each sample was run in duplicates). Kir6.2 transcripts are present at similar levels in both WT and Kir6.1KO islets, but Kir6.1 transcript is only detected in WT islets and not in Kir6.1KO islets. (C) Each color represents traces for Kir6.1 transcript and the corresponding actin transcript in islets from independent human cadaveric organ donors. (D) Mean crossing threshold (Ct) for transcripts from mouse and human islets. Data represent mean ± SEM, n = 4 independent batches of human islets and n = 5 mice from each genotype. Samples were run in duplicates.
β cell expression of ATP-insensitive Kir6.1-containing KATP channels
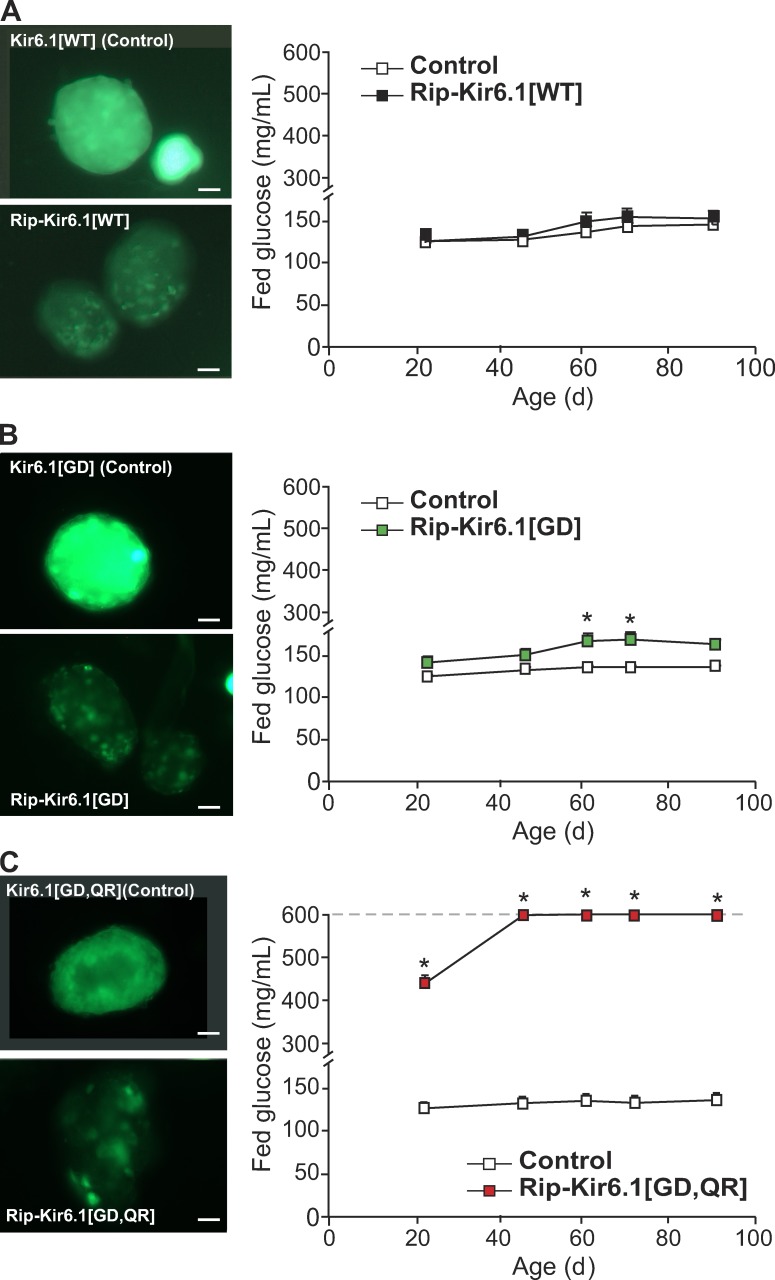
To study the effects of Kir6.1 subunits of the KATP channel in vivo, we have generated novel mice that express either the Kir6.1 WT (Kir6.1[WT]) or a Kir6.1-GOF, single mutant G343D (Kir6.1[GD]) or double mutant G343D/Q53R (Kir6.1[GD,QR]) transgenes under Cre-recombinase control (Li et al., 2013). Global Cx1 Kir6.1[WT], Kir6.1[GD], and Kir6.1[GD,QR] mice express GFP fluorescence ubiquitously in the whole body, but after tissue-specific Cre-recombination, these mice lose fluorescence only in the tissue of interest (Li et al., 2013). Specific expression in pancreatic β cells was achieved by crossing Kir6.1 transgenic mice with Rip-Cre mice. Fig. 2 shows marked loss of fluorescence in pancreatic islets from double transgenic Rip-Kir6.1[WT] (A), Rip-Kir6.1[GD] (B), and Rip-Kir6.1[GD,QR] (C; as an indication of transgene expression), compared with bright fluorescent islets from single Kir6.1[WT], Kir6.1[GD], and Kir6.1[GD,QR] transgenic mice (lacking recombination and absence of transgene expression). Littermate control mice in all experiments below include pooled WT, single transgenic Rip-Cre, and single transgenic Kir6.1[GD] or Kir6.1 [GD,QR] mice. In each case, no significant differences were found between these genotypes when independently tested.
Figure 2.
Increased blood glucose in Kir6.1-GOF mice. (A–C, left) Representative intrinsic GFP fluorescence in freshly isolated islets from transgenic mice. High fluorescence in transgenic islets indicates the presence of the Kir6.1 transgene (Kir6.1[x], Control), and loss of fluorescence in transgenic β cells within islets after Cre-recombination (Rip-Kir6.1[x]) indicates actual expression of the transgene specifically in these cells. Bars, 10 µm. (right) Fed blood glucose over time after weaning in Rip-Kir6.1[WT] (A, black), single mutant Rip-Kir6.1[GD] (B, green), and double mutant Rip-Kir6.1[GD,QR] (C, red) with respect to their control littermates (white). Data represent mean ± SEM, n = 8–10 animals in each group. Significant differences (*, P < 0.05) between transgenic and control littermate mice are indicated (t test).
Diabetes in Kir6.1-GOF mutant mice
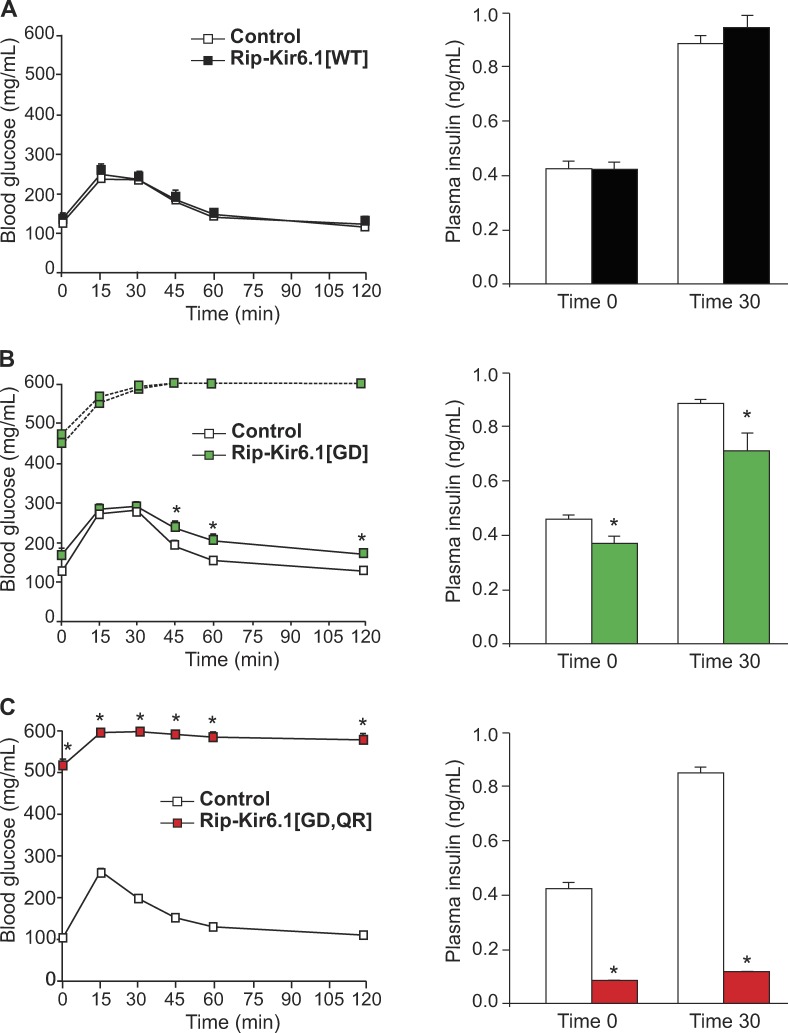
GOF mutations in the Kir6.1 subunit of the KATP channel result in either glucose intolerance (Rip-Kir6.1[GD]) or frank diabetes (Rip-Kir6.1[GD,QR]), mimicking the consequences of weak or more significant expression of Kir6.2-GOF transgenes (Koster et al., 2000, 2005; Remedi et al., 2009). 7-wk-old double transgenic Rip-Kir6.1[GD] mice showed higher fed glucose than littermate control mice (Fig. 2 B). They also demonstrate increased fasting blood glucose and a mildly impaired glucose tolerance, accompanied by reduced circulating plasma insulin after glucose challenge with respect to control littermates (Fig. 3 B). Importantly, however, 2 out of 10 Rip-Kir6.1[GD] mice showed diabetes with dramatically high fasting blood glucose levels and a marked impairment of glucose tolerance (Fig. 2 B). Strikingly, all Rip-Kir6.1[GD,QR] mice developed severe diabetes (Fig. 2 C) with markedly impaired glucose tolerance, as the result of significantly reduced plasma insulin levels in response to glucose challenge (Fig. 3 C). Importantly, however, and as expected, overexpression of WT Kir6.1 subunits in Rip-Kir6.1[WT] mice did not result in increased blood glucose or glucose intolerance (Figs. 2 A and 3A), suggesting that impaired glucose tolerance and diabetes are promoted by Kir6.1-GOF and are not an artifactual consequence of overexpressed transgenic protein levels per se.
Figure 3.
Glucose tolerance in Kir6.1 transgenic mice. (A–C) GTT on 12-h fasted (left) and plasma insulin (right) in Rip-Kir6.1[WT] (A, black), Rip-Kir6.1[GD] (B, green), and Rip-Kir6.1[GD,QR] (C, red) and control littermates at 7 wk of age. Blood glucose concentration versus time after injection of 1.5 g/kg glucose. Plasma insulin was determined in fasted mice (time 0) and 30 min after glucose challenge during GTT. Data represent mean ± SEM, n = 8–10 animals in each group. For Rip-Kir6.1[GD], 2 of 10 animals, with fasting blood glucose >300 mg/dl, are shown individually (dashed line), and the remaining 8 of 10 animals, with fasting blood glucose <200 mg/dl, are shown as mean data (solid line). Significant differences (*, P < 0.05) between transgenic and control littermate mice are indicated (t test).
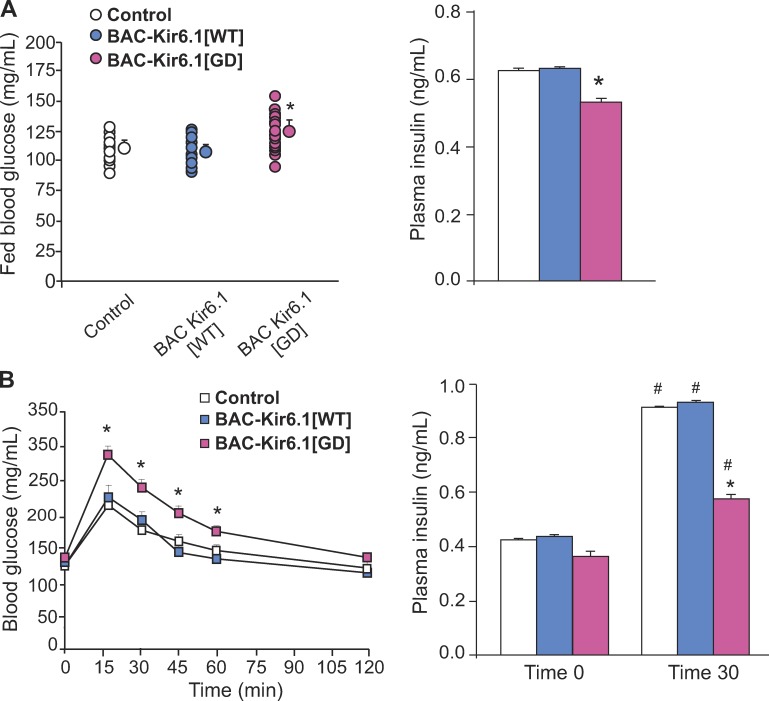
We have also generated BAC Kir6.1 transgenic (BAC-Kir6.1[GD]) and WT (BAC-Kir6.1[WT]) mice (see Materials and methods). Because the BAC constructs are expected to contain the native regulatory elements of the Kir6.1 gene, with no exogenous promoter, the transgene should be expressed under native promoter control, and hence only in tissues where the Kir6.1 subunit is normally present. Strikingly, BAC-Kir6.1[GD] mice show higher blood glucose levels, reduced circulating insulin in fed conditions (Fig. 4 A), and impaired glucose tolerance with reduced plasma insulin at 30 min, compared with BAC-Kir6.1[WT] mice (Fig. 4 B). The similar phenotype of BAC-Kir6.1[GD] mice to Rip-Kir6.1[GD] mice implicates native Kir6.1 in controlling glucose tolerance and, as shown below, most likely by regulating β cell secretion directly. Given the likely low level of Kir6.1 in the β cell, under normal conditions, WT Kir6.1 is unlikely to play a major role, and we detected no significant effects of Kir6.1KO on blood glucose (Kir6.1KO: 108 ± 2.02 mg/dl and WT: 119 ± 2.59 mg/dl), glucose tolerance, or KATP conductance assessed by Rb efflux (not depicted).
Figure 4.
Fed blood glucose and glucose tolerance in BAC-Kir6.1 mice. (A) Fed blood glucose (left) and plasma insulin (right) in BAC-Kir6.1[WT] (blue), BAC-Kir6.1[GD] (pink), and control littermate (white) mice. (B) Glucose (left) and insulin levels (right) at time 0 (fasting) and 30 min after glucose challenge in BAC-Kir6.1[WT] (blue), BAC-Kir6.1[GD] (pink), and control littermate (white) mice. Data represent mean ± SEM, n = 10–15 animals in each group. Significant differences (*, P < 0.05) between transgenic and control littermate mice under the same condition and (#, P < 0.05) on the same genotype mice under different conditions are indicated. Nonsignificant differences are not indicated.
Kir6.1-GOF mouse islets show decreased responsivity to glucose challenge but maintained KCl response
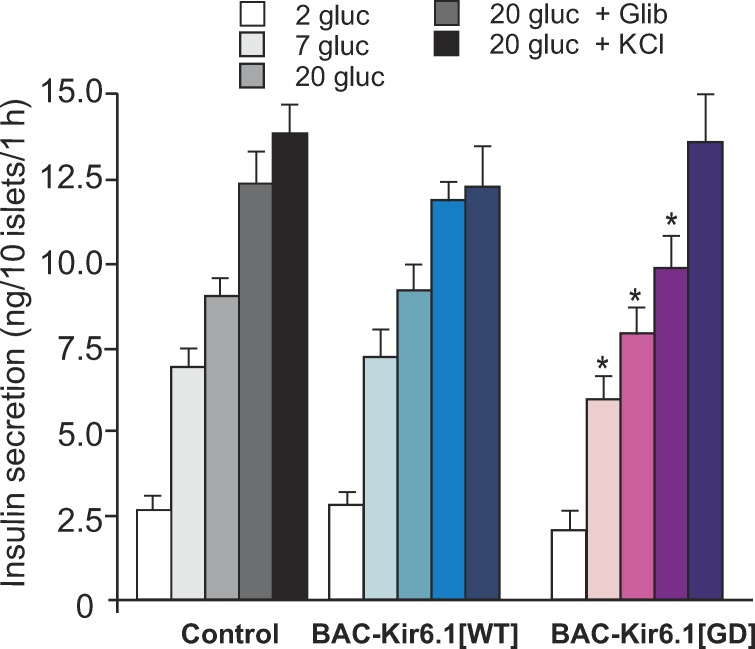
Consistent with hyperglycemia in BAC-Kir6.1[GD] mice shown above, there was also a modest but significant reduction in glucose-dependent insulin secretion from isolated BAC-Kir6.1[GD] islets, with respect to controls (Fig. 5). Importantly, however, BAC-Kir6.1[GD] still responded to glibenclamide, although with an smaller response, and responded with similar maximal secretion in response to K+ depolarization (KCl; Fig. 5), indicating no marked impairment of secretory processes downstream of the electrical signal. As expected, BAC-Kir6.1[WT] mice showed similar glucose- and glibenclamide-dependent secretion to control littermate mice (Fig. 5).
Figure 5.
Impaired glucose-dependent insulin secretion in BAC-Kir6.1[GD] transgenic mice. Glucose-dependent insulin secretion on isolated islets from BAC-Kir6.1[GD] and BAC-Kir6.1[WT] mice. Batches of 10 islets were statically preincubated in low glucose and then incubated in various glucose concentrations, or in glibenclamide, or in KCl for 1 h. Data represent mean ± SEM, n = 4–5 mice in each group. Significant differences (*, P < 0.05) between transgenic and control littermates under the same condition are indicated (t test).
Kir6.1-GOF islets exhibit a reduction in total KATP channel density but sensitivity to glibenclamide drugs
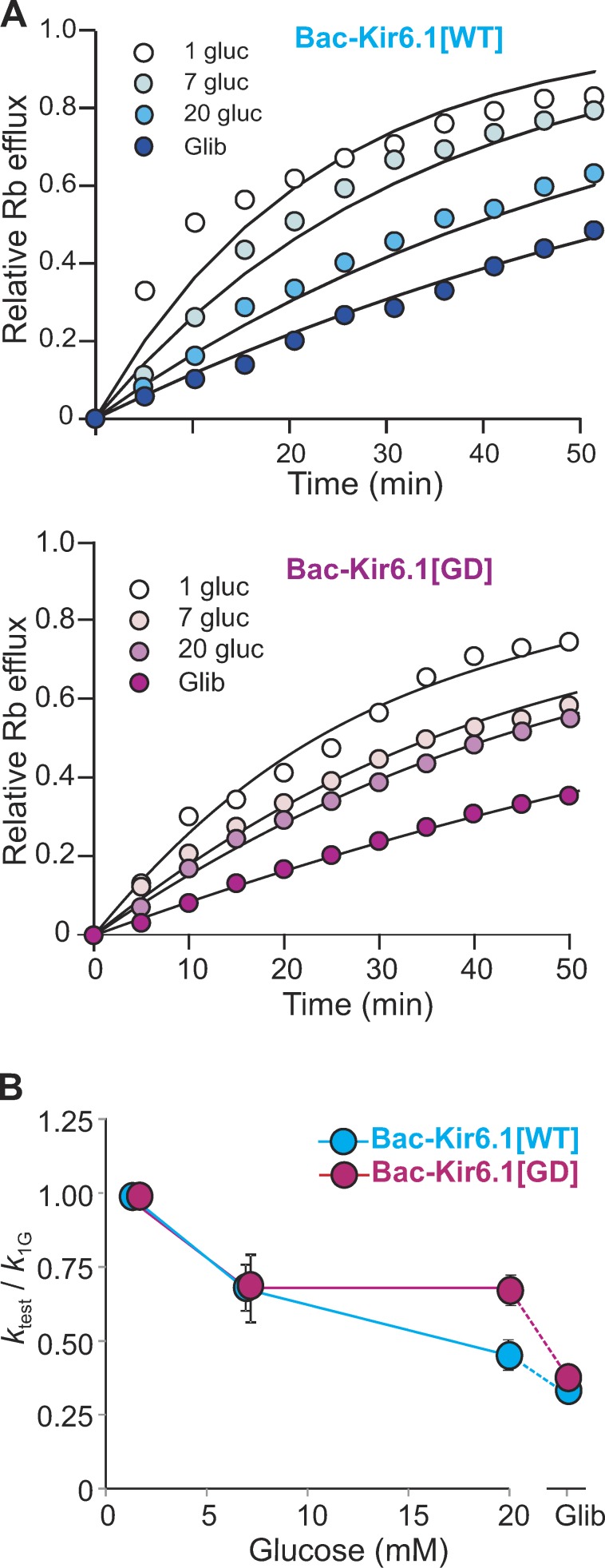
K conductance in intact BAC-Kir6.1[WT] and BAC-Kir6.1[GD] transgenic was assessed by 86Rb+ efflux in the presence of different [glucose] and glibenclamide. Fig. 6 A shows representative sample fluxes from both genotypes. Fluxes were fit by single exponentials and reciprocal rate constants (normalized to 1 mM glucose) are plotted in Fig. 6 B. In BAC-Kir6.1[WT] islets, the flux is glucose sensitive, being almost completely reduced to basal (i.e., flux in glibenclamide) in the presence of 20 mM glucose. In contrast, the BAC-Kir6.1[GD] flux is markedly less glucose sensitive, with substantial glibenclamide-sensitive (i.e., KATP-dependent) flux still present even at 20 mM glucose (Fig. 6 B). Interestingly, the absolute maximum rate constant (in 1 mM glucose) was slightly lower in BAC-Kir6.1[GD] islets (0.029 ± 0.010 min−1) than in control islets (0.042 ± 0.004 min−1), which may reflect reduced maximal KATP conductance as a result of lower single channel current in Kir6.1-containing channel pores (Nichols, 2006).
Figure 6.
Reduced glucose sensitivity of KATP conductance in Kir6.1-GOF islets. (A) Glucose- and glibenclamide-dependent Rb+ effluxes from BAC-Kir6.1[WT] (blue) and BAC-Kir6.1[GD] (pink) islets. (B) Rate constant for Rb+ efflux at each condition (ktest), normalized to rate constant in 1 mM glucose (k1G), in BAC-Kir6.1[WT] and BAC-Kir6.1[GD] islets. Data represent mean ± SEM, n = 4 mice (n > 50 islets per sample) in each group.
Discussion
Kir6.1-containing channels are present in both human and mouse islets: Implications for human diabetes
Although Kir6.1 subunits (encoded by KCNJ8) are prominent components of vascular KATP channels (Flagg et al., 2010), the Kir6.1 cDNA was originally cloned from pancreatic islets (Inagaki et al., 1995), yet there has been little subsequent consideration of any role or even the likely presence of this subunit in insulin-secreting cells. Our expression analysis shows that Kir6.1 transcript is clearly present in both human and mouse islets (Fig. 1) and, importantly, that there is no Kir6.1 transcript detected in Kir6.1KO mouse islets and no effect of Kir6.1 deletion on Kir6.2 transcript levels (Fig. 1). Other experiments (supplementary data in Pagliuca et al. [2014]) have also found KCNJ8 gene expression in human β cells isolated from cadaveric donors and in β cells generated from human pluripotent stem cells. Furthermore, transcriptome data from 10 human cadaveric organ donors show that KCNJ8 (Kir6.1, ranked 5,410) was second only to KCNJ11 (Kir6.2, ranked 3,486) as the highest expressed Kir subunit in β cells (genes ranked in descending order according to magnitude of expression, i.e., insulin was ranked 1; Segerstolpe et al., 2016). Together, these studies highlight the presence of Kir6.1 in pancreatic β cells and raise the exciting possibility that alterations in Kir6.1 expression or channel properties could affect insulin secretion and thereby play a modulatory role in human diabetes.
Diabetes induced by GOF mutation in the Kir6.1 subunit of the KATP channel
In the pancreatic β cell, KATP channels are the critical link between glucose metabolism and insulin secretion, highlighted by the fact that GOF mutations in the pore-forming Kir6.2 and regulatory SUR1 subunits of the KATP channel (KATP-GOF) are the main cause of human NDM (Gloyn et al., 2004a,b; Vaxillaire et al., 2004; Massa et al., 2005). Mice expressing Kir6.2-GOF mutations specifically in pancreatic β cells reiterate key features of NDM (Koster et al., 2000; Girard et al., 2009; Remedi et al., 2009). Here, we have shown that pancreatic expression of a severely ATP-insensitive Kir6.1 mutation in Rip-Ki6.1[GD,QR] mice also results in reduced circulating plasma insulin and development of severe diabetes, whereas expression of a mildly ATP-insensitive mutation in Rip-Kir6.1[GD] mice leads to development of glucose intolerance, comparable with the phenotypes of strong and mild GOF mutations in Kir6.2 in mice and humans (Koster et al., 2000, 2006; Gloyn et al., 2004b; Flanagan et al., 2007; Remedi et al., 2009, 2011; Villareal et al., 2009).
Our data show that deliberate expression of Kir6.1-GOF mutations in β cells can result in their ready incorporation into functional β cell KATP channels and hence in the modulation of insulin secretion. One potential caveat is that transgenic expression of mutant subunit proteins may result in gross islet abnormalities, but the fact that no significant differences were found between Rip-Kir6.1[WT] mice and littermate control mice (Figs. 2 A and 3A) suggests no major abnormalities as a result of transgene overexpression per se. More importantly, BAC-Kir6.1-GOF transgenic mice (but not BAC-Kir6.1[WT] transgenic mice), which express the transgene under control of the native promoter, also show higher glucose levels and reduced circulating plasma insulin in fed conditions (Fig. 4 A). BAC-Kir6.1-GOF show glucose intolerance with decreased insulin secretion 30 min after glucose challenge (Fig. 4 B) and a specific reduction in glucose-sensitive insulin secretion in vitro (Fig. 5). The overall KATP conductance in BAC-Kir6.1-GOF transgenic islets exhibits reduced glucose sensitivity, with substantial glibenclamide-sensitive Rb flux still present at high (20 mM) [glucose] (Fig. 6). These results imply that not only are Kir6.1 subunits normally expressed in the islet but that GOF mutations can reduce the glucose dependence of KATP conductance and hence of insulin secretion.
Diabetic mice and patients with KATP-GOF mutations respond to sulfonylurea drugs
The high sensitivity of Kir6.2/SUR1 channels to sulfonylurea inhibition has led to a dramatic change in therapy for NDM, with most patients successfully transferring from injected insulin therapy to sulfonylurea tablets (Hattersley and Ashcroft, 2005; Pearson et al., 2006; Masia et al., 2007; Wambach et al., 2010) or even being initiated on sulfonylurea therapy (Wambach et al., 2010; Marshall et al., 2015). However, many Kir6.2-GOF mutations also exhibit reduced sensitivity to sulfonylureas (Koster et al., 2005), which may contribute to the generally observed requirement for relatively high doses of sulfonylureas in treatment of NDM patients (Hattersley and Ashcroft, 2005; Pearson et al., 2006). The involvement of Kir6.1 subunits in β cell KATP channels has potentially important implications for KATP pharmacologies in the treatment of diabetes and the possibility that isoform-specific blockers of these channels (Wellman et al., 1999) may need to be considered in the future. Although no mutations in the Kir6.1-encoding gene (KCNJ8) located on chromosome 12p12 have yet been identified in diabetic patients, multiple studies have suggested linkage to diabetes mapping to chromosome 12p (Ng et al., 2004; Wiltshire et al., 2004). Further studies will be necessary to confirm any linkage between Kir6.1 and glucose intolerance or diabetes.
Acknowledgments
We are extremely grateful to Dr. Pedro Herrera (Department of Genetic Medicine and Development, University of Geneva Medical School, Geneva, Switzerland) for providing us with Rip-Cre mice and Dr. Susumu Seino for providing us with Kir6.1KO mice. We are also grateful to Dr. Michael McDaniel and Mrs. Connie Marshall (Department of Pathology and Immunology, Washington University School of Medicine) for kindly providing human islets for this study.
This work was supported by National Institutes of Health grants DK098584 to M.S. Remedi and DK109407 to C.G. Nichols. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare no competing financial interests.
Angus C. Nairn served as editor.
Footnotes
Abbreviations used:
- BAC
- bacterial artificial chromosome
- GOF
- gain-of-function
- GTT
- glucose tolerance test
- KO
- knockout
- NDM
- neonatal diabetes mellitus
- qRT-PCR
- quantitative real-time PCR
References
- Ashcroft F.M., and Gribble F.M.. 1999. ATP-sensitive K+ channels and insulin secretion: their role in health and disease. Diabetologia. 42:903–919. 10.1007/s001250051247 [DOI] [PubMed] [Google Scholar]
- Benninger R.K., Remedi M.S., Head W.S., Ustione A., Piston D.W., and Nichols C.G.. 2011. Defects in beta cell Ca2+ signalling, glucose metabolism and insulin secretion in a murine model of KATP channel-induced neonatal diabetes mellitus. Diabetologia. 54:1087–1097. 10.1007/s00125-010-2039-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagg T.P., Enkvetchakul D., Koster J.C., and Nichols C.G.. 2010. Muscle KATP channels: recent insights to energy sensing and myoprotection. Physiol. Rev. 90:799–829. 10.1152/physrev.00027.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan S.E., Patch A.M., Mackay D.J., Edghill E.L., Gloyn A.L., Robinson D., Shield J.P., Temple K., Ellard S., and Hattersley A.T.. 2007. Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes. 56:1930–1937. (published erratum appears in Diabetes 2008. 57:523) 10.2337/db07-0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard C.A., Wunderlich F.T., Shimomura K., Collins S., Kaizik S., Proks P., Abdulkader F., Clark A., Ball V., Zubcevic L., et al. 2009. Expression of an activating mutation in the gene encoding the KATP channel subunit Kir6.2 in mouse pancreatic beta cells recapitulates neonatal diabetes. J. Clin. Invest. 119:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloyn A.L., Cummings E.A., Edghill E.L., Harries L.W., Scott R., Costa T., Temple I.K., Hattersley A.T., and Ellard S.. 2004a Permanent neonatal diabetes due to paternal germline mosaicism for an activating mutation of the KCNJ11 gene encoding the Kir6.2 subunit of the β-cell potassium adenosine triphosphate channel. J. Clin. Endocrinol. Metab. 89:3932–3935. 10.1210/jc.2004-0568 [DOI] [PubMed] [Google Scholar]
- Gloyn A.L., Pearson E.R., Antcliff J.F., Proks P., Bruining G.J., Slingerland A.S., Howard N., Srinivasan S., Silva J.M.C.L., Molnes J., et al. 2004b Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N. Engl. J. Med. 350:1838–1849. 10.1056/NEJMoa032922 [DOI] [PubMed] [Google Scholar]
- Hamilton-Shield J.P. 2007. Overview of neonatal diabetes. Endocr. Dev. 12:12–23. [DOI] [PubMed] [Google Scholar]
- Hattersley A.T., and Ashcroft F.M.. 2005. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 54:2503–2513. 10.2337/diabetes.54.9.2503 [DOI] [PubMed] [Google Scholar]
- Herrera P.L. 2002. Defining the cell lineages of the islets of Langerhans using transgenic mice. Int. J. Dev. Biol. 46:97–103. [PubMed] [Google Scholar]
- Inagaki N., Tsuura Y., Namba N., Masuda K., Gonoi T., Horie M., Seino Y., Mizuta M., and Seino S.. 1995. Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues, including pancreatic islets, pituitary, skeletal muscle, and heart. J. Biol. Chem. 270:5691–5694. 10.1074/jbc.270.11.5691 [DOI] [PubMed] [Google Scholar]
- Koster J.C., Marshall B.A., Ensor N., Corbett J.A., and Nichols C.G.. 2000. Targeted overactivity of β cell KATP channels induces profound neonatal diabetes. Cell. 100:645–654. 10.1016/S0092-8674(00)80701-1 [DOI] [PubMed] [Google Scholar]
- Koster J.C., Remedi M.S., Dao C., and Nichols C.G.. 2005. ATP and sulfonylurea sensitivity of mutant ATP-sensitive K+ channels in neonatal diabetes: implications for pharmacogenomic therapy. Diabetes. 54:2645–2654. 10.2337/diabetes.54.9.2645 [DOI] [PubMed] [Google Scholar]
- Koster J.C., Remedi M.S., Masia R., Patton B., Tong A., and Nichols C.G.. 2006. Expression of ATP-insensitive KATP channels in pancreatic beta-cells underlies a spectrum of diabetic phenotypes. Diabetes. 55:2957–2964. 10.2337/db06-0732 [DOI] [PubMed] [Google Scholar]
- Li A., Knutsen R.H., Zhang H., Osei-Owusu P., Moreno-Dominguez A., Harter T.M., Uchida K., Remedi M.S., Dietrich H.H., Bernal-Mizrachi C., et al. 2013. Hypotension due to Kir6.1 gain-of-function in vascular smooth muscle. J. Am. Heart Assoc. 2:e000365 10.1161/JAHA.113.000365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B.A., Green R.P., Wambach J., White N.H., Remedi M.S., and Nichols C.G.. 2015. Remission of severe neonatal diabetes with very early sulfonylurea treatment. Diabetes Care. 38:e38–e39. 10.2337/dc14-2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masia R., De Leon D.D., MacMullen C., McKnight H., Stanley C.A., and Nichols C.G.. 2007. A mutation in the TMD0-L0 region of sulfonylurea receptor-1 (L225P) causes permanent neonatal diabetes mellitus (PNDM). Diabetes. 56:1357–1362. 10.2337/db06-1746 [DOI] [PubMed] [Google Scholar]
- Massa O., Iafusco D., D’Amato E., Gloyn A.L., Hattersley A.T., Pasquino B., Tonini G., Dammacco F., Zanette G., Meschi F., et al. Early Onset Diabetes Study Group of the Italian Society of Pediatric Endocrinology and Diabetology . 2005. KCNJ11 activating mutations in Italian patients with permanent neonatal diabetes. Hum. Mutat. 25:22–27. 10.1002/humu.20124 [DOI] [PubMed] [Google Scholar]
- Miki T., Suzuki M., Shibasaki T., Uemura H., Sato T., Yamaguchi K., Koseki H., Iwanaga T., Nakaya H., and Seino S.. 2002. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat. Med. 8:466–472. 10.1038/nm0502-466 [DOI] [PubMed] [Google Scholar]
- Ng M.C., So W.Y., Lam V.K., Cockram C.S., Bell G.I., Cox N.J., and Chan J.C.. 2004. Genome-wide scan for metabolic syndrome and related quantitative traits in Hong Kong Chinese and confirmation of a susceptibility locus on chromosome 1q21-q25. Diabetes. 53:2676–2683. 10.2337/diabetes.53.10.2676 [DOI] [PubMed] [Google Scholar]
- Nichols C.G. 2006. KATP channels as molecular sensors of cellular metabolism. Nature. 440:470–476. 10.1038/nature04711 [DOI] [PubMed] [Google Scholar]
- Pagliuca F.W., Millman J.R., Gürtler M., Segel M., Van Dervort A., Ryu J.H., Peterson Q.P., Greiner D., and Melton D.A.. 2014. Generation of functional human pancreatic β cells in vitro. Cell. 159:428–439. 10.1016/j.cell.2014.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson E.R., Flechtner I., Njølstad P.R., Malecki M.T., Flanagan S.E., Larkin B., Ashcroft F.M., Klimes I., Codner E., Iotova V., et al. Neonatal Diabetes International Collaborative Group . 2006. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N. Engl. J. Med. 355:467–477. 10.1056/NEJMoa061759 [DOI] [PubMed] [Google Scholar]
- Polak M., and Cavé H.. 2007. Neonatal diabetes mellitus: a disease linked to multiple mechanisms. Orphanet J. Rare Dis. 2:12 10.1186/1750-1172-2-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remedi M.S., Kurata H.T., Scott A., Wunderlich F.T., Rother E., Kleinridders A., Tong A., Brüning J.C., Koster J.C., and Nichols C.G.. 2009. Secondary consequences of β cell inexcitability: Identification and prevention in a murine model of KATP-induced neonatal diabetes mellitus. Cell Metab. 9:140–151. 10.1016/j.cmet.2008.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remedi M.S., Agapova S.E., Vyas A.K., Hruz P.W., and Nichols C.G.. 2011. Acute sulfonylurea therapy at disease onset can cause permanent remission of KATP-induced diabetes. Diabetes. 60:2515–2522. 10.2337/db11-0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstolpe Å., Palasantza A., Eliasson P., Andersson E.M., Andréasson A.C., Sun X., Picelli S., Sabirsh A., Clausen M., Bjursell M.K., et al. 2016. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab. 24:593–607. 10.1016/j.cmet.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling M.A. 2006. The genetic basis of neonatal diabetes mellitus. Pediatr. Endocrinol. Rev. 4:71–75. [PubMed] [Google Scholar]
- Vaxillaire M., Populaire C., Busiah K., Cavé H., Gloyn A.L., Hattersley A.T., Czernichow P., Froguel P., and Polak M.. 2004. Kir6.2 mutations are a common cause of permanent neonatal diabetes in a large cohort of French patients. Diabetes. 53:2719–2722. 10.2337/diabetes.53.10.2719 [DOI] [PubMed] [Google Scholar]
- Villareal D.T., Koster J.C., Robertson H., Akrouh A., Miyake K., Bell G.I., Patterson B.W., Nichols C.G., and Polonsky K.S.. 2009. Kir6.2 variant E23K increases ATP-sensitive K+ channel activity and is associated with impaired insulin release and enhanced insulin sensitivity in adults with normal glucose tolerance. Diabetes. 58:1869–1878. 10.2337/db09-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambach J.A., Marshall B.A., Koster J.C., White N.H., and Nichols C.G.. 2010. Successful sulfonylurea treatment of an insulin-naïve neonate with diabetes mellitus due to a KCNJ11 mutation. Pediatr. Diabetes. 11:286–288. 10.1111/j.1399-5448.2009.00557.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., York N.W., Nichols C.G., and Remedi M.S.. 2014. Pancreatic β cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab. 19:872–882. 10.1016/j.cmet.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman G.C., Barrett-Jolley R., Köppel H., Everitt D., and Quayle J.M.. 1999. Inhibition of vascular KATP channels by U-37883A: A comparison with cardiac and skeletal muscle. Br. J. Pharmacol. 128:909–916. 10.1038/sj.bjp.0702868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltshire S., Frayling T.M., Groves C.J., Levy J.C., Hitman G.A., Sampson M., Walker M., Menzel S., Hattersley A.T., Cardon L.R., and McCarthy M.I.. 2004. Evidence from a large U.K. family collection that genes influencing age of onset of type 2 diabetes map to chromosome 12p and to the MODY3/NIDDM2 locus on 12q24. Diabetes. 53:855–860. 10.2337/diabetes.53.3.855 [DOI] [PubMed] [Google Scholar]