Abstract
Arousal’s selective effects on cognition go beyond the simple enhancement of emotional stimuli, sometimes enhancing and other times impairing processing of proximal neutral information. Past work shows that arousal impairs encoding of subsequent neutral stimuli regardless of their top-down priority via the engagement of β-adrenoreceptors. In contrast, retrograde amnesia induced by emotional arousal can flip to enhancement when preceding neutral items are prioritized in top-down attention. Whether β-adrenoreceptors also contribute to this retrograde memory enhancement of goal-relevant neutral stimuli is unclear. In this pharmacological study, we administered 40mg of propranolol or 40mg of placebo to healthy young adults to examine whether emotional arousal’s bidirectional effects on declarative memory relies on β-adrenoreceptor activation. Following pill intake, participants completed an emotional oddball task in which they were asked to prioritize a neutral object appearing just before an emotional or neutral oddball image within a sequence of 7 neutral objects. Under placebo, emotional oddballs impaired memory for lower priority oddball+1 objects but had no effect on memory for high priority oddball−1 objects. Propranolol blocked this anterograde amnesic effect of arousal. Emotional oddballs also enhanced selective memory trade-offs significantly more in the placebo than drug condition, such that high priority oddball−1 objects were more likely to be remembered at the cost of their corresponding lower priority oddball+1 objects under arousal. Lastly, those who recalled more high priority oddball−1 objects preceding an emotional versus neutral oddball image showed greater increases in salivary alpha-amylase, a biomarker of noradrenergic system activation, across the task. Together these findings suggest that different noradrenergic mechanisms contribute to the anterograde and retrograde mnemonic effects of arousal on proximal neutral memoranda.
Keywords: locus coeruleus, norepinephrine, arousal, memory, attention, emotion
1. Introduction
Selectivity is at the core of efficient cognitive processing, helping us to prioritize significant information among competing sensory inputs. Years of research demonstrate that emotional experiences dominate this competition for limited mental resources to ensure behaviorally relevant/emotional events are preferentially processed and stored into long-term memory (Dolan, 2002; LaBar & Cabeza, 2006; McGaugh, 2000, 2013). However, this focus on the superiority of emotional memories has led to a blind spot in the emotion-cognition literature. In addition to enhancing processing of emotional stimuli, the effects of arousal also spill over to influence cognitive processing more broadly, sometimes enhancing and other times impairing processing of neutral information appearing just before or after something emotional (Mather & Sutherland, 2011).
One particularly striking example of how emotional arousal influences temporally adjacent neutral stimuli is provided by an oddball paradigm in which a perceptually deviant emotional image is embedded within a sequence of neutral stimuli. Whereas in some studies emotional stimuli enhance memory for preceding neutral items (Anderson et al., 2006; Knight & Mather, 2009), in other studies emotional stimuli impair memory for preceding neutral stimuli (Hurlemann et al., 2005; Hurlemann et al., 2007; Strange et al., 2003). To reconcile these discrepant findings, the arousal-biased competition (ABC) model posits that a momentary increase in arousal amplifies the effects of priority, such that memory of prioritized, important information is enhanced, whereas memory of lower priority information is impaired (Mather & Sutherland, 2011). Fundamentally, this framework builds upon the idea of biased competition in the brain whereby top-down goals or bottom-up perceptual salience help resolve competition among incoming sensory inputs (Beck & Kastner, 2009; Desimone & Duncan, 1995).
To test the ABC hypothesis explicitly, Sakaki et al. (2014) manipulated priority in a visual oddball paradigm by altering the goal-relevance of neutral object images appearing just before (oddball−1 objects) or after (oddball+1 objects) an emotional versus neutral oddball image (Sakaki et al., 2014; Sakaki et al., 2014). As predicted, emotional arousal led to retrograde amnesia for oddball−1 objects when the oddball image was prioritized, whereas prioritizing the neutral oddball−1 image instead led to an emotion-induced retrograde memory enhancement for the object. In contrast, emotional arousal did not benefit memory of neutral oddball+1 objects prioritized in a top-down manner. These contrasting time-dependent effects of arousal suggest that emotion benefits on-going memory processing of already activated representations, but does not facilitate memory for ensuing items even when they are prioritized.
Emerging research lends credence to the idea that emotional arousal amplifies the effects of top-down priority in declarative memory for preceding information but not subsequent information. For instance, hearing a tone conditioned to shock enhances memory consolidation of preceding goal-relevant visual stimuli (Lee et al., 2015). In addition, hearing an emotional sound immediately after seeing an object-scene pair leads to impaired memory for the less salient background scene (Ponzio & Mather, 2014). One oddball study demonstrated that increasing the amount of attention given to neutral items either by reducing the list length or having participants immediately recall versus not recall items at the end of each list, emotion enhanced long-term memory for preceding neutral images (Knight & Mather, 2009). On the other hand, emotion had a weaker effect on long-term memory of subsequent neutral items in the same study. Similarly, when items following oddball pictures are not prioritized by the task instructions, arousing oddballs tend to impair memory of subsequent neutral images (Hurlemann et al., 2005; Schmidt, 2002). Together these findings support the idea that emotional arousal strengthens consolidation of highly activated mental representations, while weakening memory of neutral representations that are either peripheral to the focus of attention or appear just afterward.
Although the ABC model helped reconcile puzzling findings about how arousal shapes cognitive selection processes, the neuromechanism by which arousal amplifies the effects of top-down priority in memory are poorly understood. It is widely recognized that norepinephrine (NE) released in the amygdala under emotional arousal contributes to the superiority of emotional events in attention and memory (Markovic et al., 2014; McGaugh, 2000, 2002; Strange & Dolan, 2004). In particular, numerous studies demonstrate that this NE- and amygdala-dependent enhancement of emotional memory relies on β-adrenergic receptor activation (Cahill et al., 1994; McGaugh, 2013; Strange & Dolan, 2004).
By comparison, it has been less clear how β-adrenoreceptor activation influences memory for intrinsically non-arousing, neutral information. On the one hand, in addition to enhancing processing of emotional stimuli, β-adrenoreceptor activation mediates an emotion-induced retrograde amnesia of inconspicuous neutral stimuli (Hurlemann et al., 2005; Hurlemann et al., 2007; Strange & Dolan, 2004; Strange et al., 2003), suggesting that these receptors can also account for the suppression of low-priority neutral information flanking something emotional. On the other hand, evidence in rodents demonstrates that increasing NE levels in the amygdala can enhance rather than impair memory consolidation of previously learned neutral objects, an effect that is blocked via the administration of the β-adrenoreceptor antagonist propranolol (Barsegyan et al., 2014; Roozendaal et al., 2008). Thus it might not always be the case that β-adrenergic activation leads to emotion-induced memory impairments of neutral representations encoded beforehand.
Previous influential models of noradrenergic modulation of cognition fail to account for the full range of arousal-induced NE effects on memory processes, because they either focus on 1) the selective enhancement of emotionally or motivationally significant stimuli (Aston-Jones & Cohen, 2005; Markovic et al., 2014; McGaugh & Roozendaal, 2002) or 2) the impaired processing of neutral representations occurring before something unexpected and arousing (Bouret & Sara, 2005). To explain how NE mediates arousal’s interaction with goal-directed attention, the Glutamate Amplifies Noradrenergic Effects (GANE) model proposes that the noradrenergic system amplifies the gain of prioritized information processing under arousal irrespective of how priority is instantiated (Mather et al., in press). According to GANE, NE released under arousal modulates mental representations differently as a function of their activation strength, such that NE enhances prioritized inputs even further, while simultaneously suppressing noisy or weak inputs. This selective up-regulation of salient representations is achieved via positive local glutamate-NE feedback loops that generate sufficiently elevated levels of local NE to engage low-affinity β-adrenoreceptors; in turn, β-adrenoreceptor activation potentiates pre- and postsynaptic excitatory activity (Berridge & Waterhouse, 2003) and triggers synaptic plasticity processes that support memory consolidation (Marzo et al., 2009; Salgado et al., 2012; Treviño et al., 2012). At the same time, high glutamatergic activity representing strong inputs should also stimulate local GABAergic activity that inhibits weaker, competing representations (Brown et al., 2005).
In summary, the GANE model shares the view of other theories positing that β-adrenoreceptor activation impairs processing of neutral or inconspicuous stimuli. However, the GANE model’s novel prediction that β-adrenoreceptor activation also facilitates memory consolidation of goal-relevant neutral information has yet to be tested. Thus, the primary aims of this human pharmacological study were to test whether β-adrenoreceptor blockade: 1) abolishes emotion-induced retrograde memory enhancements for preceding goal-relevant stimuli (Sakaki et al., 2014), and 2) abolishes emotion-induced anterograde memory impairments for subsequent inconspicuous neutral stimuli (Hurlemann et al., 2005). We also aimed to determine whether overall noradrenergic system activation, as measured by changes in salivary alpha-amylase across an emotional task (Ditzen et al., 2014), was associated with emotion’s attention-dependent, bidirectional effects on nearby neutral information processing.
To test these hypotheses, we combined the emotional oddball paradigm used in Sakaki et al. (2014) with the administration of 40 mg of propranolol, a β-adrenoreceptor blocker. Our main hypothesis was that, under placebo, emotional oddball images would enhance memory of high priority oddball−1 objects, while impairing memory of less-attended oddball+1 objects. We predicted that β-adrenoreceptor blockade would attenuate this dichotomous influence of emotional oddballs on ongoing versus proactive mnemonic processes.
We also examined the possibility that emotional arousal intensified memory trade-offs between pre- and post-oddball items. According to the arousal-biased competition model, emotional arousal biases the distribution of mental resources towards goal-relevant stimuli, leaving fewer resources available to process less salient stimuli (Mather & Sutherland, 2011). Because mental and energetic resources are limited (Desimone & Duncan, 1995), it is possible that successful encoding of oddball+1 items is contingent on whether or not their corresponding oddball−1 items were subsequently remembered or forgotten. Using a trial-level memory codependency analysis (Strange et al., 2003), we predicted that: 1) participants would be more likely to forget the oddball+1 object when they remembered its corresponding prioritized oddball−1 object on emotional versus neutral oddball trials, and 2) this effect would be diminished by inhibiting β-adrenoreceptors.
2. Methods
2.1 Participants
Thirty-two healthy young adults were recruited from the University of Southern California Psychology Subject Pool to participate in this two-day experiment. All participants provided written informed consent approved by the University of Southern California Health Science Campus Institutional Review Board. Participants were awarded course credits for their participation. Of the enrolled participants, 27 individuals met all of the health screening criteria, ensuring it was safe for them to take the drug. One participant in the placebo condition was excluded due to a script error during the emotional oddball experiment. Thus, a total of 26 participants were included in the final analyses (19 F; Mage = 20 years, SD = 0.25).
Prior to the main experiment session, participants were randomly assigned to either the drug or placebo group using a pre-determined randomization scheme. This resulted in the following drug group assignments: 12 Drug (10 F; Mage = 20.08 years, SD = 0.36) and 14 Placebo (9 F; Mage = 19.9 years, SD = 0.35).
2.2 General Procedure
2.2.1 Health Screening (Day 1)
During the first 30-minute session, participants provided written informed consent and were screened for general health and normal cardiovascular function. Main contraindications of propranolol include: sinus brachycardia, bronchial asthma, diabetes, low blood pressure, depression, problems with circulation, heart disease, pheochromocytoma, and impaired hepatic or renal function. Participants who had a history of any of these conditions were ineligible to participate in the experiment session on Day 2. Additional health-related exclusion criteria included: women who are currently nursing or pregnant; known sensitivity to propranolol or other beta-blockers; psychoactive drug use; a history of smoking; and participants without normal or normal-to-corrected vision and hearing. Blood pressure was measured to ensure that participants did not exhibit hypertension or hypotension according to definitions established by the National Heart Lung and Blood Institute. Of the 32 participants that completed the health screening, 27 were deemed eligible to participate in the main experiment session.
As part of session 1, participants were administered the Center for Epidemiological Studies Depression questionnaire (Radloff, 1977) to assess depression, and the Behavioral Inhibition System and Behavioral Activation System scale (Carver & White, 1994) to assess sensitivity to punishment and reward, respectively.
2.2.2 Experiment Procedure (Day 2)
Participants were randomly assigned to double-blind oral intake of a 40mg single dose of the β-adrenergic receptor antagonist propranolol hydrochloride (N = 12) or a 40mg single dose of vitamin E placebo (N = 14). All pills were compounded by the USC Health Science Campus pharmacy and appeared identical. To reduce variability in salivary alpha-amylase (sAA) levels and control for other factors that might influence performance or sAA concentration (Nater & Rohleder, 2009), participants were instructed to refrain from exercise and eating food within 1 hour, sleep within 2 hours, caffeine within 3 hours, and alcohol within 24 hours of the experiment. All participants complied with these instructions. They were also instructed to remain seated for the entirety of the experiment. All participants’ 4-hr experiment sessions were conducted between the hours of 1pm and 7pm to limit any interaction effects between NE and cortisol on memory (Dickerson & Kemeny, 2004; Hurlemann et al., 2007).
Upon arriving for the 4-hr experiment, participants drank a 10-oz bottle of water and were administered his/her assigned pill. Previous emotion-cognition work shows that propranolol takes approximately 1-2 hours to reach peak plasma concentration (Hurlemann et al., 2010; Strange et al., 2003); thus, we introduced a delay by having participants watch a nature documentary for approximately 70 minutes. The oddball experiment commenced approximately 80 minutes (M = 79 minutes, SD = 6.5 minutes) after pill administration to maximize the memory-altering effects of β-adrenergic blockade. On average, participants finished the emotional oddball task approximately 2 hours and 5 minutes (M = 125 minutes, SD = 6.15 minutes) after pill administration.
In addition to the main oddball task, participants were administered the Positive and Negative Affect Schedule (Watson et al., 1988) at three time points (baseline, pre-task, post-task) to assess changes in positive and negative affect. Potential side effects of the drug were assessed using a 16-item symptoms questionnaire immediately before and immediately after the oddball task. Ratings were made on a scale ranging from 1 = not at all to 7 = a great deal, and included questions related to common side effects of propranolol, such as dizziness, headache, or sensation of numbness in limbs.
2.3 Emotional Oddball Task
2.3.1 Overall Procedure
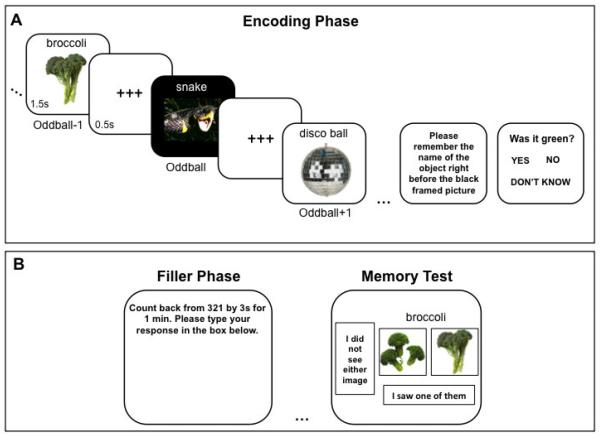
The emotional oddball task was divided into an encoding phase and a two-alternative forced choice recognition memory test (Figure 1).
Figure 1.
A schematic example of a trial from the encoding (A) and filler/memory phase (B) of the emotional oddball task.
2.3.2 Encoding phase
During the encoding phase, participants viewed sequences of seven images that were semantically unrelated. Each of these sequences was composed of six non-oddball photo objects displayed on a white background with no black frame. The other image, the oddball, was perceptually deviant in that it was displayed on a black screen and randomly appears in the 3rd, 4th or 5th position in each sequence. Each image in the sequence also contained an accompanying noun label. The labels were shown above the non-oddballs in black arial font and above the oddball pictures in white arial font. Images were displayed for 1.5 seconds each, with a 500-ms inter inter-stimulus-interval containing a black string of plus signs (+++) displayed on a white background.
Prior to beginning the task, participants were instructed to remember as many neutral objects as possible for a later memory test. Stimulus priority, or goal relevance, was manipulated by instructing participants to try especially hard to memorize the object appearing just before the black-framed oddball image. To increase prioritization of the oddball−1 object and ensure that participants were focusing on the oddball−1 item, they were prompted to report the identity (label) of the oddball−1 target picture at the end of each sequence. Following this response, participants also answered a forced-choice question concerning a perceptual feature of the oddball−1 object. For example, if the oddball−1 image depicted grapes, participants were asked, “Were they green?” and indicated either “Yes” or “No” by key press. This allowed us to test the veracity of the oddball−1 memory representation in working memory and to increase its top-down priority even further. Overall, there were 7 cycles containing 6 sequences each, resulting in a total of 42 individual sequences (1/2 emotional). Each cycle had 3 negative emotional oddball and 3 neutral oddball trials.
2.3.3 Recognition memory test
At the end of each cycle (i.e., after encoding neutral objects from 6 separate sequences), participants were prompted to count backwards from a three-digit number by increments of 3 for one minute. Participants then completed a two-alternative forced choice recognition test containing pairs of old and new items. On each memory trial, participants were presented with two different photographs of the same object side-by-side. Their task was to indicate whether both images were new (not seen previously in any sequence) or to pick the specific image they saw during the encoding phase. Each memory test included 21 image pairs: 6 oddball−1 objects, 6 oddball+1 objects, 6 new object pairs, and 3 fillers (old objects used neither as oddball−1 nor as oddball+1 objects), which were used to motivate participants to try to encode all objects. By testing short-term memory for both oddball−1 and oddball+1 objects, we were able to determine how emotionally arousing oddballs differentially influenced processing of high versus lower priority images. It also enabled us to examine arousal-biased competition effects on a trial-by-trial basis (see Section 2.6.5).
As in Sakaki et al. (2014), this design was chosen so that we could assess the specificity of memory for high and lower priority objects. Each response was coded as a correct response for specific recognition when the participants chose the exact object image from the encoding phase. Old responses assigned to either the exact image or other version of the same object was also obtained as a measure for general recognition memory. To correct for false alarm rates (said “old” when both were new), we analyzed the corrected recognition rate (specific recognition hit rate – false alarm rate).
2.3.4 Stimuli
Oddball stimuli were composed of 21 emotionally negative (Marousal = 6.43, SD = 0.59, Mvalence = 2.39, SD = 0.70) and 21 neutral (Marousal = 3.47, SD = 0.55, Mvalence = 5.30, SD = 0.45) pictures from the International Affective Picture System (Lang et al., 1999). Non-oddball stimuli included 126 pairs of photographs of neutral (i.e., non-arousing) objects obtained from a previous study (Kensinger et al., 2006) and other resources (e.g., the Internet). These images were randomly assigned to the pre- or post-oddball position and further assigned to one of the three conditions (negative, neutral or memory test distracter), which was counterbalanced across participants. One of the object images from each pair was shown during the encoding phase, while the other served as a foil in the memory test. The image that appeared during encoding was counterbalanced across participants. We also included an additional 21 object pairs for fillers. An additional 147 neutral object images were used in the remaining list positions.
2.4. Cardiovascular measurements
To assess the cardiovascular effects of the drug, three measures of blood pressure (systolic/diastolic) and heart rate (beats per minute; BPM) were collected at the following time points relative to pill intake: 0 minutes (baseline), 69 +/− 2 minutes (pre-oddball-task), 125 +/− 6 minutes (post-oddball-task). Cardiac measures were acquired using a Microlife 3MC1-PC Ultimate Automatic Blood Pressure Monitor with Irregular Heartbeat Detection device (China).
Two separate 2 × 3 mixed Analysis of Variance analyses (ANOVAs) were used to analyze drug effects on blood pressure, with Condition (drug vs. placebo) as a between-subjects measure and Time (baseline vs. pre-task vs. post-task) as a repeated-measure. Additionally, two separate follow-up 2 × 2 mixed ANOVAs were used to analyze drug effects on systolic and diastolic blood pressure across the main period of interest, the oddball paradigm, with Condition (drug vs. placebo) as a between-subjects measure and Time (pre-task vs. post-task) as a repeated-measure. Together, these analyses enabled us to not only assess the overall efficacy of the drug but also specifically target its physiological effects during the time that propranolol has been shown to reach peak plasma concentration (Hurlemann et al., 2010).
2.5. Salivary alpha-amylase (sAA) collection and analysis
2.5.1 Saliva samples
Saliva samples were immediately frozen for a minimum of twenty-four hours to allow mucins to precipitate. Prior to the assays, the samples were thawed and centrifuged at 3,000 × g for 15 min to extract particulates from saliva. Clear supernatant was decanted into microtubes.
2.5.2 Salivary alpha-amylase measurement
Alpha-amylase levels were measured using Salimetrics, LLC (State College, PA) enzyme kinetic assay kits and measured optically using Molecular Devices, LLC SpectraMax M3 Multi-mode Microplate Reader (Sunnyvale, CA). Both of these samples were collected using the passive drool method.
2.5.3 Salivary alpha-amylase analysis
To determine the effects of propranolol on central noradrenergic activity, we collected and analyzed two samples of salivary alpha-amylase (sAA), a candidate biomarker of NE release (Ditzen et al., 2014). The first baseline sample was collected immediately prior to the oddball task, whereas the second sample was collected immediately after the oddball task.
The effects of the drug on sAA concentration were assessed using a Time (pre-task vs. post-task) × Condition (drug vs. placebo) mixed ANOVA, with Time as a repeated-measure and Condition as a between-subjects factor. Percent sAA change from pre-task to post-task was used to account for individual differences in emotional versus neutral oddball effects on specific neutral-item memory.
2.6 Memory Analyses
2.6.1 The effects of the drug and emotion on free recall of oddball−1 objects
To assess how effectively participants prioritized object-1 object information in attention and working memory, we examined the effects of the drug and emotional oddballs on the free recall of the identity of the oddball−1 object and one of its perceptual features. Recall performance was probed at the end of each trial (i.e., 7-item sequence of object images). The proportion of trials with correctly recalled information were analyzed using two separate 2 × 2 mixed ANOVAs with Emotion (negative vs. neutral) as a repeated-measure and Condition (drug vs. placebo) as a between-subjects factor. Follow-up Bonferroni-corrected paired t-tests were used to examine the effects of emotion on working memory performance within the drug and placebo groups, separately.
2.6.2 Corrected specific recognition memory analysis
The critical prediction of GANE is that β-adrenoreceptor blockade should attenuate emotional arousal’s dichotomous influence on memory by diminishing an arousal-induced memory enhancement of the high priority, oddball−1 item while preventing arousal-induced suppression of memory for the lower priority, oddball+1 item. To test these hypotheses, we performed two separate 2 (Emotion: negative vs. neutral) × 2 (Condition: drug vs. placebo) mixed ANOVAs on oddball−1 and oddball+1 memory, with Emotion as a repeated measure and Condition as a between-subjects factor. Specific corrected recognition memory performance was operationalized as the hit rate for the exact oddball−1 and oddball+1 object images (participant said “old” and selected the exact object image) minus the false alarm rate (participant said “old” for one of the objects when both objects were “new”).
2.6.3 Corrected general recognition memory analysis
Next, we examined whether emotional oddballs amplified the effects of top-down priority in memory more generally. The dependent measure for general memory was the proportion of trials when participants correctly answered “old” on the memory test, but selected the wrong object image. As before, general recognition memory performance was corrected for false alarm rates.
2.6.4 Trial-by-trial memory codependency analysis
While the corrected recognition memory analysis examines how emotional arousal influences object memory as a function of drug condition, we were most interested in examining arousal-biased competition memory effects on a trial-by-trial basis. Thus, we performed a memory codependency analysis in which we determined whether or not remembering a given oddball−1 object was contingent on remembering its corresponding oddball+1 object.
In this trial-by-trial memory codependency analysis, we calculated the overall frequency of four possible memory outcomes (R = Remembered and F = Forgot): 1) Roddball−1, Foddball+1; 2) Roddball−1, Roddball+1; 3) Foddball−1, Roddball+1; and 4) Foddball−1, Foddball+1. These memory scores were calculated for emotional oddball and neutral oddball trials, separately. We operationalized arousal-biased competition (ABC) memory effects as the following interaction term:
Specifically, these ABC memory scores signify how much more likely participants were to remember the oddball−1 object and forget its corresponding oddball+1 object (selective memory) as opposed to remembering both objects (global memory) on emotional relative to neutral oddball trials.
To examine how β-adrenergic blockade affected this type of memory selectivity under arousal, the ABC memory codependency scores were analyzed using a 2 (Emotion: negative vs. neutral) × 2 (Memory Outcome: RO−1FO+1 vs. RO−1RO+1) × 2 (Condition: drug vs. placebo) mixed ANOVA, with Emotion and Memory Outcome modeled as repeated-measures and Condition modeled as a between-subjects factor. Follow-up Bonferroni-corrected t-tests were used to further examine which memory outcome types were driving any main effects or interactions.
2.7 Association between salivary alpha-amylase change across the task and emotion-related memory difference scores
Previous studies have shown that emotionally arousing images can elicit increases in sAA levels in some, but not all participants (Segal & Cahill, 2009). Thus, to examine whether changes in noradrenergic activity across the task could account for emotion’s effects on oddball−1 object memory, we calculated a sAA percent change score from sample 1 (immediately pre-task) to sample 2 (immediately post-task). These sAA percent change scores were then correlated with corrected specific mean recognition performance for oddball−1 objects and oddball+1 objects.
In two separate analyses, we correlated emotion-induced effects on oddball−1 (high priority) versus oddball+1 (lower priority) specific corrected recognition memory difference scores to account for any individual differences in arousal-biased memory effects. Due to the small sample size, we conducted a Spearman rank correlation coefficient analysis between these measures.
3. Results
3.1 Drug effects on mood and self-reported side effects
Propranolol did not have a significant effect on positive (Drug: M = 24.6, SEM = 1.8; Placebo: M = 23.83, SEM = 1.67) or negative (Drug: M = 11.81, SEM = 1.05; Placebo: M = 12.69, SEM = 0.97) affect (ps > .1), or on any of the symptoms, such as dizziness assessed by questionnaire (ps > .05). This finding indicates that participants did not experience any adverse physical or psychological/affective side effects of the drug or emotional oddball task. In addition, independent samples t-tests revealed that depression levels (Drug: M = 19.33, SEM = 2.41; Placebo: M = 17.71, SEM = 1.45) and sensitivity to punishment (BIS; Drug: M = 20.67, SEM = 1.28; Placebo: M = 20.29, SEM = 1.21) did not significantly differ between the drug and placebo groups (ps > .05).
3.2 Drug effects on blood pressure
Across all participants, systolic blood pressure significantly decreased after pill intake, F(2,23) = 9.32, p = .001, ηp2 = .45, but did not significantly differ between the drug and placebo conditions, F(1,24) = .68, p = .42, ηp2 = .028 (Figure 2). Post hoc t-tests indicated that this main effect of Time was driven by systolic blood pressure being significantly lower immediately before the oddball task (M = 108 mmHg, SEM = 1.86) compared to baseline (M = 115 mmHg, SEM = 1.78; p = .001), t(25) = −4.45, p < .001. There was no significant Time × Condition interaction effect (p > .16). The results from a follow-up 2 (Time: pre-task vs. post-task) × 2 (Condition: drug vs. placebo) mixed ANOVA revealed a non-significant trend towards a significant Time × Condition effect, such that propranolol reduced systolic blood pressure from immediately before to immediately after the oddball task F(1,24) = 2.43, p = .13, ηp2 = .092.
Figure 2.
Changes in systolic (A) and diastolic (B) blood pressure from baseline (intake) to immediately before and after the oddball task. Dark gray bar refers to drug condition, whereas the light gray bar refers to the placebo condition. *p < .05.
Diastolic blood pressure also decreased over time, F(2,22) = 5.53, p = .011, ηp2 = .34, which, like systolic blood pressure, was driven by a large decrease from the baseline (M = 70 mmHg, SEM = 1.23) to the pre-task measurement (M = 66 mmHg, SEM = 1.03; p = .009), t(24) = −3.40, p = .002. There was a significant main effect of Condition, such that the drug group exhibited lower diastolic blood pressure levels than the placebo group, F(1,23) = 4.82, p = .039, ηp2 = .17. The time-by-condition interaction effect on diastolic blood pressure was not significant for the baseline to pre-task period, F(2,22) = 2.5, p = .11, ηp2 = .19.
However, in a subsequent 2 (Time: pre-task vs. post-task) × 2 (Condition: drug vs. placebo) mixed ANOVA examining the later time window, the results revealed that propranolol reduced diastolic blood pressure from immediately before to immediately after the oddball task, F(1,23) = 5.06, p = .034, ηp2 = .18. Thus, propranolol effectively reduced diastolic blood pressure across the time window it has been shown to reach peak plasma concentration (Hurlemann et al., 2010).
3.3 Drug effects on salivary alpha-amylase levels
Compared to placebo, there was a trend such that propranolol administration decreased overall sAA concentration across the oddball task, F(1,24) = 3.3, p = .082, ηp2 = .12, which is consistent with findings that β-adrenoreceptor activation is associated with sAA levels in humans (van Stegeren et al., 2006). There was no significant Time × Condition interaction effect, F(1,24) = 0.11, p = .74, ηp2 = .005, or main effect of Time on sAA concentration, F(1,24) = 2.12, p =.16, ηp2 = .081 (see Figure 3).
Figure 3.
Salivary alpha-amylase (sAA) levels immediately before and immediately after the oddball task by drug condition.
3.4 Working memory accuracy for oddball−1 identity and perceptual features
To determine how effectively participants prioritized the oddball−1 object in working memory (feature binding) and attention, we probed memory for oddball−1 object identity and one of its perceptual features at the end of each trial (Figure 4). A Emotion × Condition mixed ANOVA revealed that emotional oddballs did not significantly influence free recall accuracy for oddball−1 object identity, F(1,24) = 1.86, p = .19, ηp2 = .072. Furthermore, performance was near ceiling for both emotional oddball (M = .97, SEM = .013) and neutral oddball trials (M = .98, SEM = .006). There was no significant interaction (p > .66) or main effect of propranolol (p > .19) on correctly recalling the verbal label of the goal relevant oddball−1 object.
Figure 4.
Yes/no response accuracy for the perceptual detail question about the goal relevant oddball−1 object (A) and free recall accuracy for that object’s identity (B) during the encoding phase of the oddball task.
Likewise, emotion did not significantly affect memory accuracy for perceptual details of the oddball−1 object, F(1,24) = 1.08, p = .31, ηp2 = .043, nor was there a significant interaction with (p > .22) or main effect of propranolol (p > .74). Follow-up one-way ANOVAs revealed that emotion did not significantly affect either of these working memory measures in either drug group independently (ps > .1). This finding that emotion did not affect working memory suggests that arousal did not impair online memory maintenance processes or top-down prioritization of the oddball−1 object.
3.5 Corrected specific recognition memory analysis for oddball−1 and oddball+1 memory
For oddball−1 objects, contrary to our expectation, we did not find a significant effect for memory enhancement under arousal (Sakaki et al., 2014); instead we saw the opposite pattern (i.e., emotion-induced retrograde amnesia for high priority objects), although it was not significant, F(1,24) = 2.11, p = .16, ηp2 = .081. There was no main effect of Condition or Emotion × Condition interaction effect for oddball−1 objects (ps > .33). However, as expected, emotional oddballs significantly impaired memory of the lower priority oddball+1 objects, F(1,24) = 16.09, p = .001, ηp2 = .40. We also found a significant Emotion × Condition interaction effect, F(1,24) = 4.47, p = .045, ηp2 = .16, such that propranolol blocked this emotion-induced anterograde amnesia for oddball+1 objects.
Separate follow-up one-way ANOVAs in each drug group indicated that emotion significantly impaired oddball+1 object memory under placebo, F(1,13) = 29.53, p < .001, ηp2 = .69, but not under propranolol, F(1,11) = 1.22, p = .29, ηp2 = .10. Follow-up t-tests revealed no significant group differences in memory for oddball+1 items following neutral, t(24) = 1.53, p = .14 (placebo > drug), or emotional oddballs t(24) = 0.52, p = .61. This suggests that propranolol primarily blunted the relative anterograde amnestic effects of emotional versus neutral oddballs, despite exerting an ostensibly larger effect on neutral oddball+1 memory on average.
3.6 Corrected general recognition memory analysis for oddball−1 and oddball+1 memory
Next, we examined how emotional oddballs and the drug affected general memory based on object priority (Figure 5B). The results were consistent with the corrected specific recognition memory analysis: Emotion did not significantly impair memory of oddball−1 objects in either drug group (ps > .05), but did significantly impair memory for oddball+1 objects in the placebo group, F(1,13) = 14.33, p = .002, ηp2 = .52. This emotion-induced anterograde amnesia for the oddball+1 objects diminished under propranolol, F(1,11) = 3.96, p = .072, ηp2 = .27, indicating that – as seen for the specific recognition measures – propranolol blunted the memory-impairing effect of emotional arousal on lower priority information. Likewise, follow-up t-tests revealed no significant group differences in general recognition memory for oddball+1 items following neutral, t(24) = 1.34, p = .19 (placebo > drug), or emotional oddballs t(24) = 0.52, p = .61.
Figure 5.
Mean corrected specific recognition and general recognition performance for the objects appearing just before (also high priority) and just after (also lower priority) the emotionally arousing or neutral oddball images. *p < .05; ***p < .001.
To determine whether the effects of the drug and emotional arousal differed based on the specificity of memory (i.e., specific versus general recognition), we performed an exploratory 2 × 2 × 2 mixed ANOVA with Emotion and Memory Type (general vs. specific) as repeated measures and Condition as a between-subjects factor. None of the interactions between Condition and/or Emotion and Memory Type were significant (ps > .1), indicating that the effects of propranolol on emotion-related memory did not differ based on the specificity of the memory trace.
3.8 Trial-by-trial memory codependency analysis
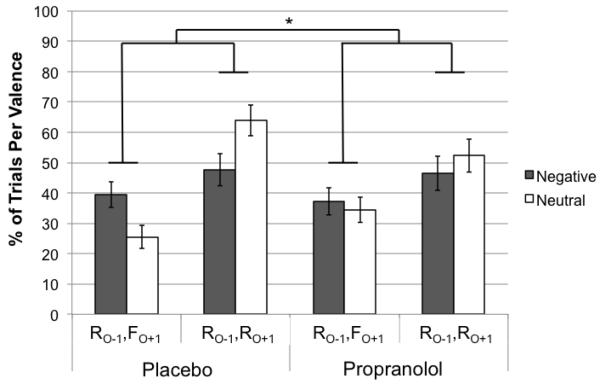
To determine how emotional oddballs influenced memory selectivity on a trial-by-trial basis, we performed a 2 × 2 × 2 mixed ANOVA with Emotion (negative vs. neutral) and Memory Outcome (RO−1FO+1, RO−1RO+1) as repeated measures and Condition (drug vs. placebo) as a between-subjects factor (Figure 6). Overall, participants were more likely to remember both the oddball−1 and oddball+1 object pairs (RO−1RO+1: M = 52.59, SEM = 3.58) than show a selective memory trade-off in favor of the high priority oddball−1 object (RO−1FO+1: M = 34.2, SEM = 2.65), F(1,24) = 9.45, p = .005, ηp2 = .28. However, participants were more likely to remember the high priority oddball−1 object while forgetting the corresponding oddball+1 object when the oddball was emotional, F(1,24) = 15.79, p = .001, ηp2 = .40. Furthermore, the results revealed a significant Emotion × Memory Outcome × Condition interaction effect on trial frequency, F(1,24) = 4.82, p = .038, ηp2 = .17, such that emotion-induced memory trade-offs were more likely to occur in the placebo than propranolol group. There were no other significant main or other interaction effects.
Figure 6.
Results from the trial-by-trial memory codependency analysis. The plot indicates the percentage of trials by valence that participants showed optimal selectivity (remembered oddball−1, but forgot oddball+1) versus more global memory enhancements (remembered both objects) in the drug versus placebo condition. R = Remembered; F = Forgotten; O−1 = oddball−1 object; O+1 = oddball+1 object. *p < .05.
Next, we performed two follow-up 2 (Emotion) × 2 (Memory Outcome) repeated-measures ANOVAs to examine whether emotional arousal significantly enhanced memory trade-offs within the placebo and drug groups, separately. In the placebo group, there were significantly fewer memory trade-offs (RO−1FO+1), compared to more global memory effects (RO−1RO+1), F(1,13) = 10.9, p = .006, ηp2 = .46. As predicted, there was also a significant Emotion × Memory Outcome interaction effect, F(1,13) = 22.07, p < .001 ηp2 = .63, such that oddball−1 objects were more likely to be remembered at the expense of memory for their corresponding oddball+1 objects on emotional versus neutral oddball trials.
In the placebo condition, follow-up paired t-tests indicated that, compared to neutral oddball trials, emotion enhanced selective memory for prioritized objects (i.e., RO−1FO+1 outcome), t(13) = 3.62, p = .003, while reducing the likelihood of remembering both oddball−1 and oddball+1 objects on a given trial (i.e., RO−1RO+1 outcome), t(13) = −5.47, p < .001. However, emotion did not affect memory outcomes for FO−1FO+1 or FO−1RO+1 memory outcome types (ps > .29; see Supplementary Table 1 for all trial frequency means).
In contrast, there were no significant interaction or main effects under β-adrenoreceptor blockade with propranolol (ps > .2). Furthermore, follow-up paired t-tests showed that emotional oddballs did not alter the likelihood of showing any of the four memory codependency outcomes compared to neutral oddballs (ps > .05). Thus, our results raise the possibility that arousal’s divergent effects on high and lower priority stimuli in time may involve an interdependent process facilitated by β-adrenoreceptor activation. Alternatively, such codependencies may have simply resulted from a disproportionately larger influence of the drug and emotional oddballs on oddball+1 object encoding.
3.9 Associations between sAA change across the task and emotional arousal’s influence on prioritized memories
Contrary to one of our main predictions, emotional arousal did not significantly enhance memory for prioritized oddball−1 objects (see Section 3.5). To test the possibility that individual differences could account for the lack of a main effect of emotion, we performed a correlation coefficient analysis between emotion-induced effects on oddball−1 object memory (negative-neutral oddball−1 memory difference scores) and percent sAA concentration change from pre- to post-oddball-task.
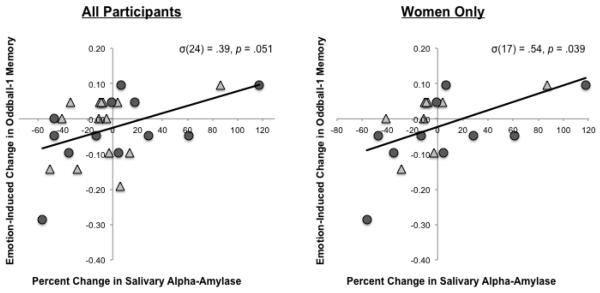
Across all participants, there was a trend towards a positive association between percent sAA change across the task and relatively greater emotion-induced enhancement of the high priority oddball−1 memory trace, σ(24) = .39, p = .051 (Figure 7, left panel). This positive sAA-memory association was significant when only women (the majority of the participants) were examined, σ(17) = .54, p = .028 (Figure 7, right panel).
Figure 7.
Correlation between percent change in salivary alpha-amylase, a marker of noradrenergic activity, across the task and emotion-related retrograde memory effects for the oddball−1, goal-relevant object memoranda across all participants (left panel) and women only (right panel). Triangles = placebo; Circles = propranolol.
Percent sAA change across the task was not significantly correlated with emotion-enhanced oddball−1 memory in either drug condition, separately, for either the whole group or women only (ps > .09). But the association between percent sAA change and emotion-enhanced oddball−1 memory was not significantly quantified (moderated) by drug condition: The interaction between the drug condition and percent sAA change was not significant (p = .49), and the main effects of percent sAA change on emotion-enhanced oddball−1 memory remained similar even after controlling for the effects of drug condition and their interaction with sAA change, F(1,22) = 3.08, p = .09.
Percent sAA change across the task was not significantly associated with emotion-induced amnesia for the oddball+1 object in all participants, σ(24) = .096, p = .64, or in women only, σ(17) = .041, p = .87.1
4. Discussion
In this study, we combined a pharmacological manipulation with an emotional oddball paradigm to test whether β-adrenoreceptor activation mediates arousal’s selective influence on memory for proximal neutral information (Mather et al., in press). Specifically, we were interested in testing two separate hypotheses concerning arousal’s divergent retrograde and anterograde effects on memory selectivity: Emotion-induced activation of β-adrenoreceptors facilitates 1) emotional arousal’s priority-dependent effects on memory consolidation of preceding neutral stimuli, and 2) emotional arousal-induced anterograde amnesia for less-attended, lower priority neutral stimuli.
Contrary to a previous oddball study manipulating the priority of peri-oddball neutral items (Sakaki et al., 2014), emotional arousal did not enhance memory of preceding goal-relevant neutral stimuli under placebo. However, we found that β-adrenergic blockade attenuated the expected emotion-induced memory impairment for relatively less-attended, lower priority neutral images (Hurlemann et al., 2005). When accounting for trial-by-trial memory contingencies, we found that propranolol blocked an emotion-induced trade-off whereby prioritized oddball−1 objects were better recalled at the expense of their corresponding oddall+1 objects. Additionally, a correlation analysis revealed that emotion conferred a memory advantage to goal-relevant objects in those who showed greater increases in sAA across the oddball task. Together these results suggest that broader activation of the noradrenergic system amplifies mnemonic benefits of arousal on top-down prioritized mental representations appearing just beforehand. The finding that the sAA-memory relationship was apparent across both drug groups suggests that such enhancements do not rely exclusively on β-adrenoreceptors; rather, β-adrenoreceptors appear to play a more critical role in mediating the anterograde amnestic effects of arousal on inconspicuous stimuli.
The current results replicate earlier findings that, via β-adrenoreceptor activation, pictorial emotional oddballs proactively impair memory encoding of subsequent neutral pictures (Hurlemann et al., 2005). Past work implicates the amygdala as the critical locus of NE-induced memory deficits for neutral stimuli experienced near something emotional (Strange et al., 2003). The most common interpretation of these emotion-related impairments is that the amygdala selectively modulates cortical and hippocampal activity to favor processing of emotional stimuli (Dolcos et al., 2004; Fastenrath et al., 2014; Kilpatrick & Cahill, 2003; Richardson et al., 2004; Strange & Dolan, 2004; Vuilleumier et al., 2004), thereby leaving fewer resources available to process less salient neutral information. Supporting this hypothesis, bilateral amygdala damage is associated with poorer memory for gist but enhanced memory for visual details of aversive versus neutral photographs (Adolphs et al., 2001), suggesting that this region suppresses information that is peripheral to an emotional event.
Since the observed memory interference for neutral objects occurred in an anterograde fashion, we interpret these arousal effects as emotion impacting more rapid attention and encoding processes rather than consolidation. Indeed, noradrenergic modulation of the amygdala is not only critical for consolidating salient or emotion-laden events but also for modulating initial perception, attention, and encoding processes (Fox et al., 2000; Hamann et al., 1997; Hurlemann et al., 2005; Liddell et al., 2005; Markovic et al., 2014; Vuilleumier, 2005). For example, both β-adrenergic blockade (De Martino et al., 2008) and amygdala lesions (Anderson & Phelps, 2001) reduce the perceptual dominance of emotional stimuli that are presented in close succession to neutral stimuli. Based on these data, our impairment finding may signify a β-adrenergic and amygdala-dependent biasing of attention and encoding resources away from relatively mundane information appearing just after something emotionally significant. Alternatively, emotional arousal’s impairing effects on neutral-item encoding might also be mediated by non-amygdala-related effects of β-adrenoreceptor activation, such as the potentiation of inhibitory signals in sensory cortex (Waterhouse et al., 1980) or enhanced orienting towards oddball stimuli via fronto-parietal network activation (Strange & Dolan, 2007).
We did not replicate the previous finding that emotional oddballs enhance rather than impair memory of preceding neutral items when they are imbued with goal relevance (Sakaki et al., 2014). This lack of an emotion-induced memory benefit for prioritized oddball−1 object images may have resulted from emotionally salient oddballs garnering more attention than the preceding goal-relevant object image. Much evidence indicates that emotional stimuli are rapidly processed and attended to (Fox et al., 2000; Vuilleumier, 2005; Vuilleumier & Schwartz, 2001), especially due to their immediate relevance to survival and wellbeing (Öhman et al., 2001). Thus, insofar as the emotional stimulus was prioritized due to its saliency, it is possible that the goal relevance of the neutral oddball−1 items was not sufficient to overcome strong competition from emotionally significant oddballs. We did not test memory for emotional oddballs, however, since we did not want to risk directing any additional attention, or priority, towards the emotional stimuli.
Interestingly, we found that under placebo, emotional arousal amplified biased competition outcomes such that prioritized oddball−1 objects were better remembered at the expense of memory for their competing oddball+1 object. As expected, β-adrenergic blockade attenuated such enhancements in selectivity under arousal. This finding raises the possibility that β-adrenoreceptor activation is a common mechanism by which arousal simultaneously impacts competitive memory and attention processes between goal-relevant representations and subsequent lower priority neutral information.
In the current design, it is unclear whether such memory contingencies are the product of prioritizing the oddball−1 image since we chose to maximize the experimental power to test potential arousal-related oddball−1 item memory enhancements and oddball+1 item memory impairments and rather than manipulating their level of priority (Sakaki et al., 2014). To dissociate top-down attention-dependent (i.e., priority) from time-dependent (i.e., position) effects of arousal on processing of proximal neutral items, it would be useful to manipulate both the goal relevance of both the oddball+1 and oddball−1 neutral objects in future studies. It is also unclear how emotion interacted with the top-down prioritization of peri-oddball stimuli without assessing memory codependency between emotional oddballs and their surrounding neutral memoranda. However, one previous study using a pictorial oddball paradigm showed that, with slightly longer lists and instructions for participants to recall all items, oddballs were recalled at ceiling rates (Knight & Mather, 2009). Thus, we would expect recall of oddballs to be at ceiling and so not particularly informative, while potentially interfering with top-down prioritization of the oddball−1 object. Furthermore, it is notable that Sakaki et al. (2014) found that whether participants prioritized the emotional oddball image or the oddball−1 object had virtually no effect on memory for the oddball+1 object: Both attention conditions yielded a similar degree of emotion-related memory impairment, suggesting that – regardless of which preceding item garners more attention – on-going memory processes deprive subsequent information of the resources necessary for successful encoding.
One surprising finding was that in our contingency analysis, neutral rather than emotional oddballs predominantly drove both this β-adrenoreceptor-dependent bias in memory selectivity towards top-down prioritized stimuli; likewise, β-adrenergic blockade led to memory impairment for oddball+1 objects on average. These results was unexpected, as the majority of past emotion-cognition studies tend to show more selective effects of arousal-induced NE release on emotional stimuli and their influence on peripheral information processing (Chamberlain et al., 2006; Hurlemann et al., 2005; Segal & Cahill, 2009). Nonetheless, there have been reports of less discriminate effects of arousing events on cognition. For example, β-adrenergic blockade can attenuate both neutral and emotional oddball-evoked activation of a ventral attention brain network known to be heavily modulated by noradrenergic inputs (Corbetta et al., 2008; Strange & Dolan, 2007).
Intriguingly, in prior work that helped inspire the current study, Strange and colleagues (2003) found that propranolol administration was associated with enhanced recall for the neutral word preceding an emotional versus neutral oddball word. Our results were similarly unexpected and imply that β-adrenoreceptor activation during oddball events has a more complex influence on mnemonic and attention processes aren’t limited to the modulatory effects of emotion: We show that when top-down attention is directed elsewhere, the engagement of β-adrenoreceptors can amplify the amnestic anterograde effects of neutral oddballs. How and why this process occurred for neutral rather than emotional oddballs is a mystery and is an important topic for future research. Yet generally these oddball effects are consistent with the idea that unexpected events initiate a “network reset” via phasic NE release that impairs on-going processing and helps re-allocate attentional resources towards new perceptual inputs, thereby enhancing subsequent representations (Bouret & Sara, 2005).
Although β-adrenoreceptor blockade did not affect emotion’s influence on memory for goal-relevant information, an individual differences analysis revealed a positive association between more global noradrenergic activity, as indexed by increased sAA levels across the task, and emotion-induced memory enhancements of goal-relevant objects. This finding accords with previous studies demonstrating that emotional picture-induced increases in sAA are selectively associated with recall of emotional and not neutral images (Segal & Cahill, 2009). Genotyping studies show that carriers of the ADRA2B deletion variant, who purportedly have greater NE availability due to reduced inhibition of noradrenergic signaling, show similar emotional “sparing” effects (Todd et al., 2013) as healthy participants with pharmacologically increased levels of NE (De Martino et al., 2008). Deletion carriers also show greater amygdala and insula activity when viewing negative emotional expressions (Cousijn et al., 2010; Rasch et al., 2009) and greater emotional memory enhancements compared to non-carriers (de Quervain et al., 2007).
Our correlation results are also in line with pharmacological experiments targeting NE’s influence on the attentional blink. For example, pharmacologically increasing NE levels with reboxetine makes emotional stimuli more resistant to blink-related perceptual suppression (De Martino et al., 2008). In the same human pharmacological study, De Martino et al. (2008) found that β-adrenergic blockade reduced T2 sparing for both emotional and neutral stimuli; this emotion-invariant effect of β-adrenergic inhibition contrasts with the more selective arousal effects observed under reboxetine, a selective norepinephrine re-uptake inhibitor (SNRI), suggesting that more generic noradrenergic mechanisms facilitate arousal’s ability to amplify biases in attention and mnemonic processing. More importantly, these findings fit with our observation that elevated sAA across the task was positively associated with arousal-enhanced goal-relevant memory consolidation irrespective of drug condition. We expand on prior NE pharmacological and genotyping studies by showing that the concerted effects of phasic arousal and elevated noradrenergic activity enhance rather than impair memory consolidation of neutral items if those representations are credited as goal relevant.
It is noteworthy that this sAA-related memory effect did not differ based on drug group. If β-adrenergic receptors were blocked by propranolol, how would emotional arousal enhance memory of preceding goal-relevant neutral stimuli? One possibility is that elevating overall NE levels enhanced the phasic effects of arousal on task-focused attention, thereby increasing the selectivity of memory (Aston-Jones & Cohen, 2005; Aston-Jones et al., 1999; Aston-Jones et al., 1994). Similarly, we report that, within the context of elevated noradrenergic activity, arousing oddballs still strengthened high priority memory traces despite β-adrenergic blockade. The mnemonic benefit of elevated NE release on goal-relevant information might therefore involve different adrenoreceptor subtypes. Past work indicates that NE modulates cognitive flexibility and working memory processes in the prefrontal cortex by activating α2-adrenoreceptors (Ramos & Arnsten, 2007; Wang et al., 2007). Such modulation via the engagement of α2-adrenoreceptors may alter the strength of the PFC’s top-down inputs to posterior cortical regions where goal-relevant stimuli are represented (Gazzaley & Nobre, 2012). In contrast, work in animals indicates that β-adrenoreceptors have little influence on prefrontal cortical function, at least when subjects are not under stress (Arnsten, 2000).
Beyond the frontal cortex, α2-adrenoreceptor agonists have been shown to selectively enhance the distribution of blood flow to stimulated sensory regions, thereby supplying the energy necessary to process prioritized, task-relevant inputs (Bekar et al., 2012). Furthermore, in rodents, pairing NE with local visual cortex stimulation enhances the responsiveness of nearby astrocytes, which help facilitate metabolite delivery and synaptic plasticity (Paukert et al., 2014). Blockade of α1-adrenoreceptors reduced this pattern of local astrocytic gain (Paukert et al., 2014). It may be the case, then, that the gain of prioritized information processing under arousal relies on complex interactions between NE and multiple adrenoreceptor subtypes.
There are several limitations in this study that warrant consideration. The sample sizes are modest, so it is difficult to determine whether the lack of an arousal-biased competition effect in memory (Sakaki et al., 2014) was due to insufficient power. Furthermore, this issue limited us from investigating sex differences in emotion’s influence on priority-biased encoding. Previous work shows that women exhibit significantly larger amnestic effects of emotional oddballs on preceding neutral stimuli (Strange et al., 2003). We also did not control for menstrual cycle phase or birth control use, which have been shown to alter emotional memory enhancements induced by elevated noradrenergic activity (Nielsen et al., 2015). Moreover, future pharmacological studies using propranolol should account for participants’ body mass index (BMI) based on evidence of interactions between drug dose size and body mass (Sokol-Hessner et al., 2015). β-adrenoreceptor blockade during memory retrieval can also abolish emotional memory enhancements (Kroes et al., 2010; Murchison et al., 2004). Since participants in this study performed encoding and retrieval during the same session, it is unclear which stages of declarative memory formation were affected by β-adrenergic blockade.
In addition, the reliability of sAA as a biomarker of central noradrenergic activity remains questionable, because its release is sensitive to multiple factors, including saliva flow rate and chewing (Bosch et al., 2011). Nonetheless, we attempted to control for many of these confounds through our strict saliva criteria and conducting the oddball experiment in the afternoon to dissociate the noradrenergic system’s influence on declarative memory from cortisol. The noradrenergic and hypothalamic-pituitary-adrenal (HPA) axis systems are highly interactive, with corticotropin releasing factor stimulating both locus coeruleus and HPA axis activity. However, they have different profiles and are generally assumed to reflect different aspects of the stress response (Ehlert et al., 2005; Granger et al., 2007). At least under stress, the administration of propranolol selectively blunts sAA responses, whereas the administration of metyrapone - a cortisol synthesis blocker – has no significant affect on sAA concentration (Hermans et al., 2011). Thus, we expect that changes in sAA across the task were more related to central noradrenergic signaling rather than HPA axis activation.
An interesting open question is whether the effects of β-adrenoreceptors on goal-relevant memories only emerge after longer periods of consolidation. Accumulated evidence points to a key role of β-adrenoreceptors in long-term memory consolidation of emotional information (Ferry et al., 1999; McGaugh & Roozendaal, 2002; Southwick et al., 2002). Behavioral studies in humans indicate that mnemonic benefit of emotional oddballs on preceding neutral items receiving high attentional weight become apparent after a 1-week delay (Anderson et al., 2006; Knight & Mather, 2009). Likewise, the mnemonic advantage of emotional over neutral stimuli also appears to increase over longer retention intervals (Sharot & Yonelinas, 2008), which may be driven by offline consolidation processes that occur during sleep (Payne et al., 2012; Payne et al., 2008). Endogenous increases in noradrenergic system activity during sleep might also contribute to the preferential retention of prioritized information that is “tagged” by arousal at encoding (Gais et al., 2011; Groch et al., 2011). Future pharmacological studies could examine whether activating β-adrenoreceptors either at encoding or during consolidation affects arousal’s divergent influence on long-term memory of high and lower priority information.
5. Conclusion
The current study replicates key findings that β-adrenergic blockade prevents an emotion-induced anterograde amnesia for relatively less attended stimuli (Hurlemann et al., 2005). Propranolol administration also reduced arousal-enhanced memory selectivity biased towards high priority oddball−1 items and away from their competing oddball+1 items, suggesting that β-adrenoreceptors may modulate arousal-related interference in proactive encoding and consolidation processes. Emotional oddballs did not affect memory of preceding goal-relevant stimuli in either the placebo or beta-blocker groups, but the degree of arousal-biased memory effects for these stimuli was positively correlated with changes in salivary alpha-amylase, a proxy of LC-NE system activity, across the task. Together these results suggest that more generic noradrenergic mechanisms may facilitate arousal’s retrograde memory enhancement of prioritized representations, whereas β-adrenoreceptors play a more selective role in facilitating anterograde amnesia of less salient information under arousal.
Supplementary Material
Supplementary Table 1. Frequency of each trial-by-trial memory outcome by oddball valence (negative vs. neutral), item priority (oddball−1 vs. oddball+1), and drug condition.
Highlights.
Beta-adrenoreceptor blockade attenuated arousal-induced anterograde amnesia
Beta-adrenoreceptor blockade attenuated arousal-enhanced memory selectivity
Salivary alpha-amylase change was correlated with arousal’s retrograde memory effects
This sAA-memory relationship occurred across both the drug and placebo groups
Different noradrenergic mechanisms promote arousal’s bidirectional effects on memory
Acknowledgments
We thank Ringo Huang, Eshed Margalit, Paul Choi, Jolie Cooperman, and Joyce Kim for their assistance with data collection. This project was funded by federal NIH grant R01AG025340 (M.M.), a grant from the European Commission (FP7-PEOPLE-2013-CIG; M.S.), and a University of Southern California Endowed Fellowship (D.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous work suggests that women may be more susceptible to β-adrenergic-dependent impairing effects of arousal on inconspicuous neutral words (Strange et al., 2003). This finding motivated us to re-run all analyses in the women only (n = 19), since including the small number of men in our analyses may have diluted the expected β-adrenergic and emotion-related effects on memory. In women, all of the cardiovascular and sAA analyses yielded consistent results with the whole group. Most of the memory analyses yielded consistent results between the whole group and women-only subgroup, with the exception that, in the mean corrected specific recognition analysis (Section 3.4), propranolol did not affect emotion-induced anterograde amnesia, F(1,17) = 3.11, p = .096, ηp2 = .16. In addition, in the exploratory 2 × 2 × 2 mixed ANOVA with Emotion and Memory Type (general vs. specific) as repeated measures and Condition as a between-subjects factor in Section 3.6, there was a significant Emotion × Memory Type interaction, such that emotional oddballs led to greater anterograde amnesia for specific versus general corrected recognition memory, F(1,17) = 5.32, p = .034, ηp2 = .24. However this interaction effect did not significantly differ between drug groups (p > .27). Lastly, we found that women showed a significant positive association between sAA change across the oddball task and emotion-related retrograde memory effects. For more detail, refer to Section 3.9 and Figure 7B.
6. References
- Adolphs R, Denburg NL, Tranel D. The amygdala's role in long-term declarative memory for gist and detail. Behavioral Neuroscience. 2001;115(5):983–992. doi: 10.1037//0735-7044.115.5.983. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411(6835):305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Wais PE, Gabrieli JDE. Emotion enhances remembrance of neutral events past. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(5):1599–1604. doi: 10.1073/pnas.0506308103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Through the looking glass: differential noradenergic modulation of prefrontal cortical function. Neural Plasticity. 2000;7(1-2):133. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annual Review of Neuroscience. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biological Psychiatry. 1999;46(9):1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. The Journal of Neuroscience. 1994;14(7):4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsegyan A, McGaugh JL, Roozendaal B. Noradrenergic activation of the basolateral amygdala modulates the consolidation of object-in-context recognition memory. Frontiers in Behavioral Neuroscience. 2014;8 doi: 10.3389/fnbeh.2014.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck DM, Kastner S. Top-down and bottom-up mechanisms in biasing competition in the human brain. Vision Research. 2009;49(10):1154–1165. doi: 10.1016/j.visres.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekar LK, Wei HS, Nedergaard M. The locus coeruleus-norepinephrine network optimizes coupling of cerebral blood volume with oxygen demand. Journal of Cerebral Blood Flow and Metabolism. 2012;32:2135–2145. doi: 10.1038/jcbfm.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Research Reviews. 2003;42(1):33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bosch JA, Veerman EC, de Geus EJ, Proctor GB. α-Amylase as a reliable and convenient measure of sympathetic activity: don’t start salivating just yet! Psychoneuroendocrinology. 2011;36(4):449–453. doi: 10.1016/j.psyneuen.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends in Neurosciences. 2005;28(11):574–582. doi: 10.1016/j.tins.2005.09.002. doi: http://dx.doi.org/10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Brown RA, Walling SG, Milway JS, Harley CW. Locus ceruleus activation suppresses feedforward interneurons and reduces beta-gamma electroencephalogram frequencies while it enhances theta frequencies in rat dentate gyrus. J Neurosci. 2005;25(8):1985–1991. doi: 10.1523/JNEUROSCI.4307-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371(6499):702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67(2):319. [Google Scholar]
- Chamberlain SR, Müller U, Blackwell AD, Robbins TW, Sahakian BJ. Noradrenergic modulation of working memory and emotional memory in humans. Psychopharmacology. 2006;188(4):397–407. doi: 10.1007/s00213-006-0391-6. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn H, Rijpkema M, Qin S, van Marle HJ, Franke B, Hermans EJ, Fernández G. Acute stress modulates genotype effects on amygdala processing in humans. Proceedings of the National Academy of Sciences. 2010;107(21):9867–9872. doi: 10.1073/pnas.1003514107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino B, Strange BA, Dolan RJ. Noradrenergic neuromodulation of human attention for emotional and neutral stimuli. Psychopharmacology. 2008;197(1):127–136. doi: 10.1007/s00213-007-1015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ, Kolassa I-T, Ertl V, Onyut PL, Neuner F, Elbert T, Papassotiropoulos A. A deletion variant of the α2b-adrenoceptor is related to emotional memory in Europeans and Africans. Nature Neuroscience. 2007;10(9):1137–1139. doi: 10.1038/nn1945. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Ehlert U, Nater UM. Associations between salivary alpha-amylase and catecholamines–A multilevel modeling approach. Biological Psychology. 2014;103:15–18. doi: 10.1016/j.biopsycho.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298(8):1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42(5):855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Erni K, Hebisch G, Nater U. Effects of yohimibine challenge on salivary alpha-amylase secretion. Psychosomatic Medicine. 2005;67:A92. [Google Scholar]
- Fastenrath M, Coynel D, Spalek K, Milnik A, Gschwind L, Roozendaal B, de Quervain DJ. Dynamic modulation of amygdala–hippocampal connectivity by emotional arousal. The Journal of Neuroscience. 2014;34(42):13935–13947. doi: 10.1523/JNEUROSCI.0786-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between beta- and alpha(1)-adrenoceptors. Journal of Neuroscience. 1999;19(12):5119–5123. doi: 10.1523/JNEUROSCI.19-12-05119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Lester V, Russo R, Bowles RJ, Pichler A, Dutton K. Facial expressions of emotion: Are angry faces detected more efficiently? Cognition & Emotion. 2000;14(1):61–92. doi: 10.1080/026999300378996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Rasch B, Dahmen JC, Sara S, Born J. The memory function of noradrenergic activity in non-REM sleep. Journal of Cognitive Neuroscience. 2011;23(9):2582–2592. doi: 10.1162/jocn.2011.21622. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Nobre AC. Top-down modulation: bridging selective attention and working memory. Trends in Cognitive Sciences. 2012;16(2):129–135. doi: 10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, El-Sheikh M, Gordis EB, Stroud LR. Salivary α-amylase in biobehavioral research. Annals of the New York Academy of Sciences. 2007;1098(1):122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Groch S, Wilhelm I, Diekelmann S, Sayk F, Gais S, Born J. Contribution of norepinephrine to emotional memory consolidation during sleep. Psychoneuroendocrinology. 2011;36(9):1342–1350. doi: 10.1016/j.psyneuen.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Cahill L, McGaugh JL, Squire LR. Intact enhancement of declarative memory for emotional material in amnesia. Learning and Memory. 1997;4(3):301–309. doi: 10.1101/lm.4.3.301. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJF, Ossewaarde L, Henckens M, Qin SZ, van Kesteren MTR, Fernandez G. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334(6059):1151–1153. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Hawellek B, Matusch A, Kolsch H, Wollersen H, Madea B, Dolan RJ. Noradrenergic modulation of emotion-induced forgetting and remembering. Journal of Neuroscience. 2005;25(27):6343–6349. doi: 10.1523/JNEUROSCI.0228-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Matusch A, Hawellek B, Klingmuller D, Kolsch H, Maier W, Dolan RJ. Emotion-induced retrograde amnesia varies as a function of noradrenergic-glucocorticoid activity. Psychopharmacology. 2007;194(2):261–269. doi: 10.1007/s00213-007-0836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Wagner M, Hawellek B, Reich H, Pieperhoff P, Amunts K, Dolan RJ. Amygdala control of emotion-induced forgetting and remembering: Evidence from Urbach-Wiethe disease. Neuropsychologia. 2007;45(5):877–884. doi: 10.1016/j.neuropsychologia.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Walter H, Rehme AK, Kukolja J, Santoro SC, Schmidt C, Maier W. Human amygdala reactivity is diminished by the b-noradrenergic antagonist propanolol. Psychological Medicine. 2010;40:1839–1848. doi: 10.1017/S0033291709992376. doi: http://dx.doi.org/10.1017/S0033291709992376. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Memory for specific visual details can be enhanced by negative arousing content. Journal of Memory and Language. 2006;54(1):99–112. doi: 10.1016/j.jml.2005.05.005. [DOI] [Google Scholar]
- Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage. 2003;20(4):2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Knight M, Mather M. Reconciling findings of emotion-induced memory enhancement and impairment of preceding items. Emotion. 2009;9(6):763–781. doi: 10.1037/a0017281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroes MC, Strange BA, Dolan RJ. β-adrenergic blockade during memory retrieval in humans evokes a sustained reduction of declarative emotional memory enhancement. The Journal of Neuroscience. 2010;30(11):3959–3963. doi: 10.1523/JNEUROSCI.5469-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7(1):54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. The international affective picture system (IAPS): Technical manual and affective ratings. University of Florida, The Center for Research in Psychophysiology; Gainesville, FL: 1999. [Google Scholar]
- Lee TH, Greening SG, Mather M. Encoding on goal-relevant stimuli is strengthened by emotional stimuli in memory. Frontiers in psychology. 2015;6:1173. doi: 10.3389/fpsyg.2015.01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, Williams LM. A direct brainstem–amygdala–cortical ‘alarm’ system for subliminal signals of fear. Neuroimage. 2005;24(1):235–243. doi: 10.1016/j.neuroimage.2004.08.016. doi: http://dx.doi.org.idpproxy.reading.ac.uk/10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Markovic J, Anderson AK, Todd RM. Tuning to the significant: Neural and genetic processes underlying affective enhancement of visual perception and memory. Behavioural Brain Research. 2014;259:229–241. doi: 10.1016/j.bbr.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Marzo A, Bai J, Otani S. Neuroplasticity regulation by noradrenaline in mammalian brain. Current Neuropharmacology. 2009;7(4):286. doi: 10.2174/157015909790031193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Clewett D, Sakaki M, Harley CW. Norepinephrine ignites local hot spots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behavioral and Brain Sciences. doi: 10.1017/S0140525X15000667. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspectives on Psychological Science. 2011;6:114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Memory: A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory consolidation and the amygdala: A systems perspective. Trends in Neurosciences. 2002;25:456–461. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Making lasting memories: Remembering the significant. Proceedings of the National Academy of Sciences. 2013;110(Supplement 2):10402–10407. doi: 10.1073/pnas.1301209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Current Opinion in Neurobiology. 2002;12(2):205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- Murchison CF, Zhang X-Y, Zhang W-P, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117(1):131–143. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- Nater U, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology. 2009;34(4):486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Nielsen SE, Barber SJ, Chai A, Clewett DV, Mather M. Sympathetic arousal increases a negative memory bias in young women with low sex hormone levels. Psychoneuroendocrinology. 2015;62:96–106. doi: 10.1016/j.psyneuen.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130(3):466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Paukert M, Agarwal A, Cha J, Doze VA, Kang JU, Bergles DE. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron. 2014;82(6):1263–1270. doi: 10.1016/j.neuron.2014.04.038. doi: http://dx.doi.org.idpproxy.reading.ac.uk/10.1016/j.neuron.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Chambers AM, Kensinger EA. Sleep promotes lasting changes in selective memory for emotional scenes. Frontiers in Integrative Neuroscience. 2012;6 doi: 10.3389/fnint.2012.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes. Psychological Science. 2008;19(8):781. doi: 10.1111/j.1467-9280.2008.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzio A, Mather M. Hearing something emotional affects memory for what was just seen: How arousal amplifies trade-off effects in memory consolidation. Emotion. 2014;14:1137–1142. doi: 10.1037/a0037983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ramos BP, Arnsten AFT. Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacology and Therapeutics. 2007;113(3):523–536. doi: 10.1016/j.pharmthera.2006.11.006. doi: http://dx.doi.org.idpproxy.reading.ac.uk/10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Spalek K, Buholzer S, Luechinger R, Boesiger P, Papassotiropoulos A, de Quervain D-F. A genetic variation of the noradrenergic system is related to differential amygdala activation during encoding of emotional memories. Proceedings of the National Academy of Sciences. 2009;106(45):19191–19196. doi: 10.1073/pnas.0907425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nature Neuroscience. 2004;7(3):278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiology of Learning and Memory. 2008;90(3):576–579. doi: 10.1016/j.nlm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Fryer K, Mather M. Emotion strengthens high priority memory traces but weakens low priority memory traces. Psychological Science. 2014;25(387-395) doi: 10.1177/0956797613504784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Fryer K, Mather M. Emotion strengthens high-priority memory traces but weakens low-priority memory traces. Psychol Sci. 2014;25(2):387–395. doi: 10.1177/0956797613504784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado H, Kohr G, Trevino M. Noradrenergic 'tone' determines dichotomous control of cortical spike-timing-dependent plasticity. Scientific Reports. 2012;2:7. doi: 10.1038/srep00417. doi: 41710.1038/srep00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SR. Outstanding memories: The positive and negative effects of nudes on memory. Journal of Experimental Psychology-Learning Memory and Cognition. 2002;28(2):353–361. doi: 10.1037//0278-7393.28.2.353. [DOI] [PubMed] [Google Scholar]
- Segal SK, Cahill L. Endogenous noradrenergic activation and memory for emotional material in men and women. Psychoneuroendocrinology. 2009;34(9):1263–1271. doi: 10.1016/j.psyneuen.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Sharot T, Yonelinas AP. Differential time-dependent effects of emotion on recollective experience and memory for contextual information. Cognition. 2008;106(1):538–547. doi: 10.1016/j.cognition.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Sokol-Hessner P, Lackovic SF, Tobe RH, Camerer CF, Leventhal BL, Phelps EA. Determinants of propranolol’s selective effect on loss aversion. Psychological Science. 2015;26(7):1123–1130. doi: 10.1177/0956797615582026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick SM, Davis M, Horner B, Cahill L, Morgan CA, III, Gold PE, Charney DC. Relationship of enhanced norepinephrine activity during memory consolidation to enhanced long-term memory in humans. American Journal of Psychiatry. 2002;159(8):1420–1422. doi: 10.1176/appi.ajp.159.8.1420. [DOI] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. beta-Adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(31):11454–11458. doi: 10.1073/pnas.0404282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. Beta-adrenergic modulation of oddball responses in humans. Behav Brain Funct. 2007;3:29. doi: 10.1186/1744-9081-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Hurlemann R, Dolan RJ. An emotion-induced retrograde amnesia in humans is amygdala- and beta-adrenergic-dependent. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(23):13626–13631. doi: 10.1073/pnas.1635116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd RM, Müller DJ, Lee DH, Robertson A, Eaton T, Freeman N, Anderson AK. Genes for emotion-enhanced remembering are linked to enhanced perceiving. Psychological Science. 2013 doi: 10.1177/0956797613492423. 0956797613492423. [DOI] [PubMed] [Google Scholar]
- Treviño M, Huang S, He K, Ardiles A, De Pasquale R, Guo Y, Kirkwood A. Pull-push neuromodulation of LTP and LTD enables bidirectional experience-induced synaptic scaling in visual cortex. Neuron. 2012;73(3):497–510. doi: 10.1016/j.neuron.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Stegeren A, Rohleder N, Everaerd W, Wolf OT. Salivary alpha amylase as marker for adrenergic activity during stress: Effect of betablockade. Psychoneuroendocrinology. 2006;31(1):137–141. doi: 10.1016/j.psyneuen.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9(12):585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7(11):1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Schwartz S. Emotional facial expressions capture attention. Neurology. 2001;56(2):153–158. doi: 10.1212/wnl.56.2.153. [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Nou E. α2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129(2):397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ. Noradrenergic modulation of somatosensory cortical neuronal responses to lontophoretically applied putative neurotransmitters. Experimental Neurology. 1980;69(1):30–49. doi: 10.1016/0014-4886(80)90141-7. doi: http://dx.doi.org/10.1016/0014-4886(80)90141-7. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Frequency of each trial-by-trial memory outcome by oddball valence (negative vs. neutral), item priority (oddball−1 vs. oddball+1), and drug condition.