Abstract
Purpose
Five-year disease endpoint trajectories are available for every cancer site. In contrast, there are few longitudinal, biobehavioral studies of survivors extending beyond the first or second year following diagnosis. This gap is addressed with stress, depressive symptom, and immunity data from breast cancer patients followed continuously for 5 years.
Methods
Women (N=113) diagnosed and surgically treated for breast cancer and awaiting adjuvant therapy completed self-report measures of stress and depressive symptoms and provided blood for immune assays [natural killer cell cytotoxicity (NKCC) and T cell blastogenesis]. Assessments (12) were repeated every 4 to 6 months for 5 years.
Results
Multiphase linear mixed models show phases of change and identified specific time points of change. Cancer stress shows two distinct phases of decline, with the change point being 12 months. In contrast, a steep decline in depressive symptoms occurs by 7 months, with stable, low levels thereafter. NKCC shows a steady upward trajectory through 18 months and upper limit stability thereafter, whereas there was no reliable trajectory for T cell blastogenesis.
Conclusions
For the first time, trajectories and specific time points of change in biobehavioral data for breast cancer survivors are provided, traced through 5 years. Following diagnosis, the breast survivor experience is one of a co-occurrence of change (recovery) in psychological and innate immunity markers from diagnosis to 18 months, and a pattern of stability (depression, NKCC) or continued improvement (stress) through year 5. These data provide new directions for survivorship care and detail of the biobehavioral trajectory.
Keywords: Stress, immunity, depressive symptoms
Introduction
Many studies have documented the psychological stress of cancer diagnosis and negative changes in biobehavioral responses (1–4). As the number of cancer survivors continues to climb, the need to understand the trajectory of psychological and behavioral responses has been voiced (5–7). When the most critical months (or years) for difficulty are and if difficulties reach some stable level, resolve, or worsen is largely unknown. Understanding the trajectory of host factors, particularly immunity, is needed due to their importance in destruction of malignant cells and surveillance against re-emergence of tumors.
In the short term, stress covaries with negative quality of life, physical symptoms, and treatment morbidities across patients (8,9), and in the long term epidemiologic data show its relationship to cancer mortality (10). In breast cancer, significant declines in stress in the first year with little change through 18 months have been reported (11). Additionally, research suggests a declining pattern of anxiety symptoms from pretreatment to the mid- or end of cancer treatment, with one study demonstrating no change in anxiety at 18 months and again at 8 years (12–17). Symptoms of depression can generate stress (18) and are also associated with heightened risk for cancer death (RR=1.18) (19,20) and all-cause death (RR=1.31) (21,22). Studies of both breast (23) and prostate cancer patients (24) have found low rates (≤6%) of moderate to severe depressive symptoms 2–3 years post diagnosis, contrasting with higher rates found in year one (17,25–28). Heightened stress and depressive symptoms covary with immunity, both cellular (4) and inflammation (29), and declines in stress (11,30) or depressive symptoms (31) relate to improvements.
The effects of cancer treatment on functional immunity have been studied (see Supplementary Materials) using natural killer cell cytotoxicity (NKCC) and/or T-cell blastogenic response to phytohemagglutinin (PHA) or Concanavalin A (ConA) assays. Improvement in NKCC through chemotherapy, with or without radiotherapy, and into the next 6 months has been found (11,32–34), but one study reported a decline in NKCC pre/post radiotherapy (35). Regarding T cell blastogenesis, both declines through 12 months (34) and declines followed by improvement (36) have been reported. When studies compare cancer treatments, reliable differences in these markers are usually not found (33,35,37). An exception has been comparison of taxane versus non-taxane regimens for breast cancer, with higher levels of both NKCC and T cell blastogenesis found in patients additionally receiving taxanes (32). Thus, considering the data across studies, there is suggestive evidence of NKCC recovery in year one, but the trajectory for blastogenesis is unclear. Trajectories thereafter are unknown.
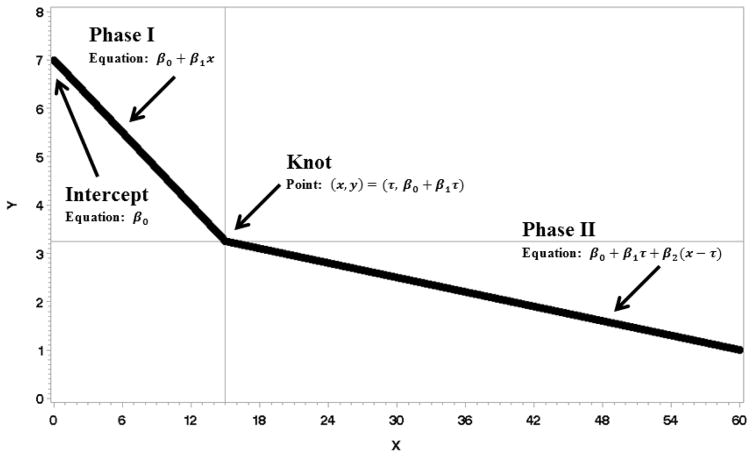
This study aims to address the gap of longitudinal, biobehavioral data for disease free cancer survivors to discover the natural trajectories of stress, depressive symptoms, and immunity. The number of assessments (12) and duration (5 years) enabled the use of a powerful general nonlinear mixed models analysis, providing information on five trajectory elements (see Fig. 1 illustration). First, the initial starting point/baseline level (intercept) of the variable is identified. Second, from the intercept, the rate of change across time (slope for Phase I), be it positive (upward), negative (downward), or zero (no change), is determined. Third, if there is a change in slope, the time point of change (knot) can be empirically determined. Fourth, after the knot, the Phase II slope is determined. These four elements detail the trajectory for “the average person,” but a fifth, the variance of the intercept and slope, provides an indication of any notable variability among individuals in these elements. Lastly, covariates for the trajectory elements were explored. Three areas were identified--demographic, medical, and social--on the basis of evidence for their correlation with psychological and biologic responses (4).
Figure 1.
Example of a linear-linear multiphase function is provided. The intercept is where Phase I intersects the Y axis; its value is β0. Phase I is the line between the intercept and knot; its slope is β1. The two phases meet at a point called the knot. Phase II is the line to right of the knot; its slope is β2.
Method
Patients and procedures
Data come from newly diagnosed Stage II/III breast cancer patients in the assessment-only arm (n=114) of a randomized clinical trial (N=227) testing the effect of a psychological intervention on reducing the risk for recurrence, as described (38–40). Demographic data are displayed in Table 1. The local institutional review board approved the research in accord with an assurances approved by the U.S. Department of Health and Human Service. Accrued from 1994 to 1998, patients were post-surgery/pre-adjuvant therapy. Following informed consent, patients completed a baseline in-person structured interview, self-report questionnaires, a nursing assessment, and a 60 mL blood draw, and were then randomized. Assessment-only and intervention arms did not differ on demographic, stress, depression, immune, disease or treatment characteristics, and other variables (38). Trial outcomes showed intervention arm improvements including reduced negative mood, improved social support and health, and reduced risk for recurrence (38–40). Assessments occurred every 4 months in year one and every 6 months in years 2–5 for patients remaining disease-free.
Table 1.
Demographic, Disease, and Treatment Characteristics of Patients (N=113)
| Characteristics | Mean (SD)/% |
|---|---|
| Age | 50.58 years (SD=10.61) |
| Race (% Caucasian) | 90.3% |
| Partner Status (% Partnered) | 73% |
| Income | $64,580 (SD=84.114) |
| Stage of Disease | |
| Stage II | 92% |
| Stage III | 8% |
| Surgery Type | |
| Modified Radical Mastectomy | 57% |
| Segmental Mastectomy | 43% |
| Adjuvant Therapy Type | |
| Adriamycin | 73% |
| Cyclophosphamide | 100% |
| Taxanes | 20% |
| 5-fluorouracil | 19% |
| Methotrexate | 14% |
| Post-chemotherapy radiotherapy | 51% |
| Tamoxifen | 80% |
| Receiving chemotherapy during: | |
| 0–4 months | 83% |
| 4–8 months | 9% |
| 8–12 months | 0% |
| Receiving radiotherapy during: | |
| 0–4 months | 44% |
| 4–8 months | 9% |
| 8–12 months | 1% |
Measures
Cancer-Specific Stress
The Impact of Events Scale (41) (IES) is a 15-item self-report measure assessing intrusive thoughts and avoidant thoughts and behaviors regarding cancer. A frequency scale ranging from 0 to 5 is used and items summed, ranging from 0 to 75. Internal consistency was 0.87. To reduce patient burden, the IES was not completed at months 42 and 54.
Depressive Symptoms
The 11-item Iowa short form of the Center for Epidemiological Studies Depression Scale (42) (CES-D) was used. Items have a 3-point scale and are summed, ranging from 0 to 22. Internal consistency was 0.73. To reduce patient burden the CESD was not completed at 30, 42 and 54 months.
For the interest of the reader we note that at baseline, two additional questions were included to assess past depression/dysthymia: (a) “In the year prior to your cancer diagnosis, did you have 2 weeks or more during which you felt sad, blue, or depressed, or lost pleasure in things that you usually cared about or enjoyed?” and (b). “Have you had 2 years or more in your life when you felt depressed or sad most days, even if you felt okay sometimes?” For each, “yes” was scored 1 and “no” scored 0. At baseline, 22 people (19%) exceeded the CES-D cutoff, 42 (37%) endorsed (a), and 35 (31%) endorsed (b).
Immunity
Assays for NKCC and T-cell proliferation in response to PHA and ConA were conducted, as detailed (1,38); see also Supplementary Material. All assays were run on fresh samples on the day of the blood draw for all (12) time points. NKCC effector to target (E:T) cell ratios were 100:1, 50:1, and 25:1. T cell proliferation assays used serial dilutions of 2.5, 5.0, and 10.0 cg/mL for each. The T cell flow cytometric staining examined only CD3+ T cells.
Covariates
Measures were as follows: 1) demographic, age (in years); 2) medical, extent of surgery (modified radical vs. segmental mastectomy) and post surgery (baseline) functional status [nurse rated Karnofsky Performance Status, KPS (43)]; and, 3) social, the Social Network Index (44) (SNI) assessed social ties and involvement.
Analytic Strategy
Linear multiphase mixed models were used. Two linear trajectory phases were estimated along with the point where the two phases joined, the knot; see Fig. 1. The knot is the point at which the outcome variable ceases to be predicted by Phase I and begins to be predicted by Phase II. Equation (1.) partially defines a linear multiphase mixed model. The model shows that the ith measure of the jth person is predicted by the first equation when xij < τj and the second equation when xij ≥ τj. The j subscripts on the parameters connote the possibility of random effects of person (individual variability) on each parameter. Multivariate normal random effects were added to the parameters when the data supported them. Though none of the final models supported the use of all four possible random effects; a saturated model is presented in (1.) for completeness.
| 1 |
Models were fit in SAS PROC NLMIXED, selected based on the Akaike Information Criterion (AIC) and represent a single plausible model for describing the data. If a variable exhibited a general skew, a log transformation was done. Missing data were assumed to be missing at random with an ignorable missing-data mechanism. For the NKCC, analyses were first conducted for E:T 25:1, and then for 100:1 and 50:1, with the expectation of replication. The same procedure was used for serial dilutions of 2.5, 5.0, and 10.0 cg/mL for PHA and ConA, again with the expectation of replication.
After selecting a trajectory model, linear covariate effects on each parameter were tested for the covariates age, surgery type, baseline level of the Karnofsky Performance Status (KPS), and the Social Network Index (SNI). Only statistically significant covariate effects are reported. For immunity, only covariates that replicated across at least two E:T ratios or two T cell serial dilutions are reported. If more than one possible combination of parameter-covariate effects were plausible, the combination with lowest AIC is reported.
Results
Stress
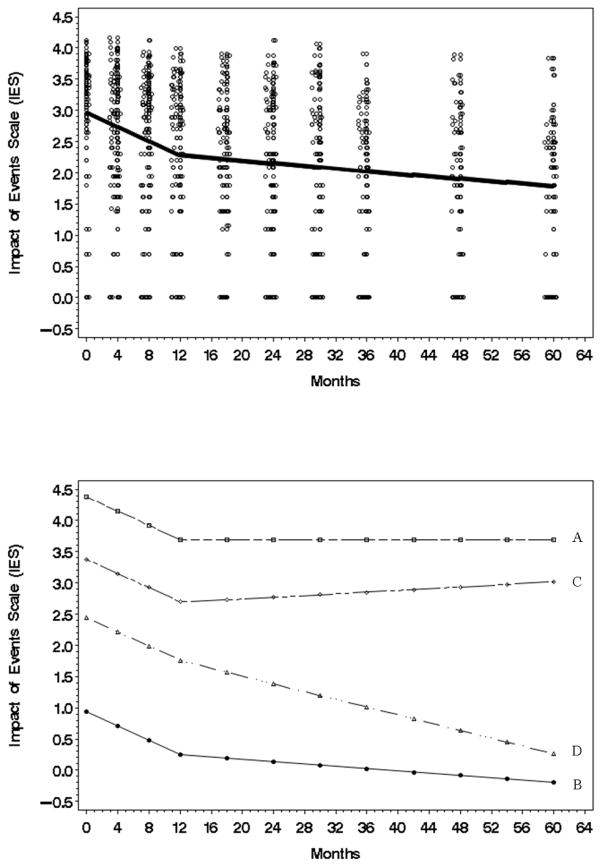
Log-transformed Impact of Events Scale (IES) was used. Regarding the trajectory (Fig 2, top panel), for the average person cancer specific stress showed a steep decrease during Phase I (β̂1 = −0.0566, t = −6.55, p value < .0001 two tail), and thereafter stress continued to decrease, though less steeply, during Phase II (β̂2 = −0.0010, t = −3.16, p value = 0.0021 two tail) [see Fig. 2, top]. The data also revealed that the shift (knot) from Phase I to Phase II occurred at 12 months (τ̂ = 12.2129, t = 5.88, p value < .0001 two tail).
Figure 2.
Top: The estimated Impact of Events Scale (IES) trajectory (natural log) for the average individual (N=113) from initial to 60 month assessment. Data points have been jittered to reduce over plotting. Bottom: A plot (natural log) using the most extreme individual trajectories predicted by the model. A/B trajectories are examples of patients with the highest/lowest intercepts. C/D trajectories are examples of patients with the highest/lowest second phase slopes.
Analyses of individual variability showed two types. The first type of variability was in the baseline (intercept) stress scores, . Illustrated in Fig. 2 (bottom panel), some patients began with higher stress (see intercept for Patient A who had the most extreme, highest, baseline IES score) and others began with lower stress levels (see intercept for Patient B who had the most extreme, lowest, IES score). The second type of variability was in the Phase II slope . For some patients, the Phase II slope was nearly zero (see line for Patient C), indicating stress showed no further decline after 12 months, whereas for other patients stress continued to decline (see Patient D with the steepest phase II slope). Regarding covariates, only social network was significant. On average, the larger a patient’s social network, the lower her stress at baseline (γ̂0 = −.05693, t = −2, p value = .0476 two tail).
Depressive symptoms
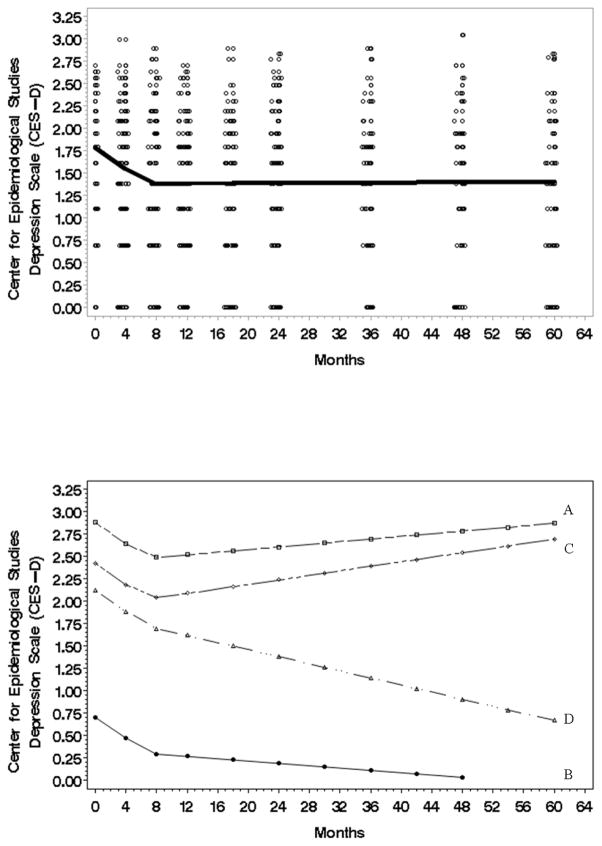
Log-transformed Center for Epidemiological Studies Depression Scale (CESD) data was used. With respect to the trajectory (Fig. 3, top panel) for the average person depressive symptoms declined during Phase I (β̂1 = −.05951, t = −3.13, p value = .0013 two tail), but in Phase II depressive symptoms “leveled out” with no further change (zero slope). The slope shift occurred at 6.7 months (τ̂ = 6.7316, t = 4.02, p value = .0001 two tail). An analysis of individual variability again showed two types of effects. The first type of variability was in the baseline (intercept) scores . Illustrated in Fig. 3 (bottom panel), some patients began with higher levels of depressive symptoms (see intercept for Patient A who had the highest baseline CESD score) and others began with lower depressive symptom levels (see intercept for Patient B who had the lowest CESD score). The second type of variability was in Phase II slopes that showed change after 6.7 months rather than stability . Illustrated in Fig. 3 (bottom panel), some individuals had an increase in depressive symptoms (see Phase II slope for Patient C with the greatest increase), whereas others had depressive symptom declines (see Patient D with the steepest downward slope). No covariates were significant.
Figure 3.
Top: The estimated Center for Epidemiologic Studies-Depression (CESD) scale trajectory (natural log) for the average individual (N=113) from initial to 60 month assessment. Data points have been jittered to reduce over plotting. Bottom: A plot (natural log) using the most extreme individual trajectories predicted by the CESD model. The A/B trajectories are examples of patients with the highest/lowest intercepts. The C/D trajectories are examples of patients with the highest/lowest second phase slopes.
Immunity
Natural Killer Cell Cytotoxicity
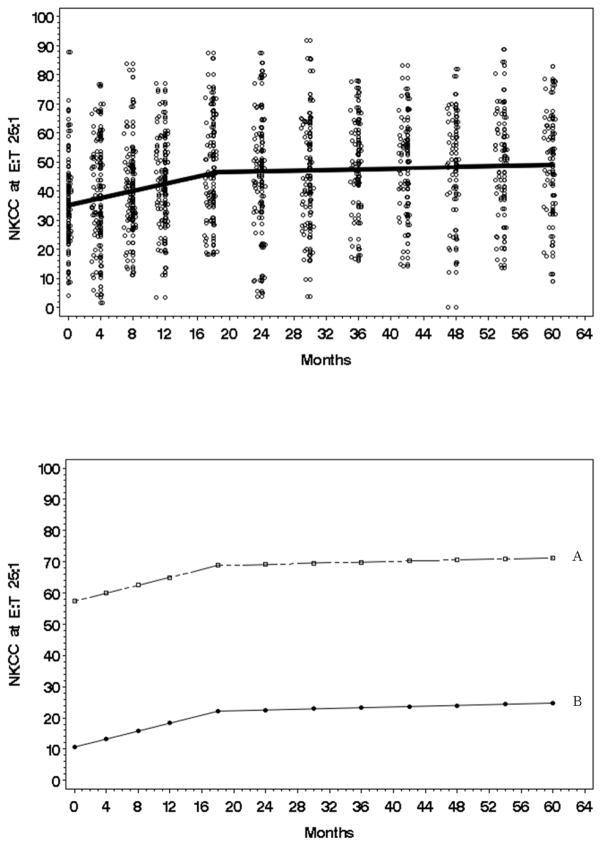
For NKCC, the observed responses were symmetric and unimodal, requiring no transformation. With respect to the trajectory (Fig. 4, top panel), on average NK25 steadily increased in Phase I (β̂1 = 0.6331, t = 4.73, p value < .0001 two tailed), but in Phase II there was no change (zero slope) (β̂2 = 0.0604, t = 1.10, p value = .2735 two tailed). The latter finding is indicative of a ceiling effect in NKCC increase. These effects were replicated at NK50 and NK100. Further, analyses show the slope shift (knot) occurred at 18 months. (τ̂ = 18.00, t = 4.62, p value < .0001). An analysis of individual variability revealed significant effects for the intercept (baseline), . This finding is illustrated in Fig. 4 (bottom panel) with Patient A having the highest baseline NK and Patient B with the lowest baseline NK. While these individuals vary at the baseline, their trajectories are parallel. These effects were replicated at NK50 and NK100.Regarding covariates, age was positively correlated with the intercept, with those older having higher baseline NKCC. For example, at NK50, for every one year increase in age, a person’s baseline NKCC was, on average, 0.29 higher (γ̂0 = 0.29, t = 2.66, p value = 0.009 two tailed). In contrast, KPS impacted the Phase II slope. For example, at NK50, for every one point increase in baseline KPS, the phase two slope increased by .0135 (γ̂2 = 0.0135, t = 2.02, p value = 0.0455 two tailed).
Figure 4.
Top: The estimated natural killer cell cytotoxicity (NKCC) at E:T 25:1 trajectory (raw) for the average individual (N=113) from initial to 60-month assessment. Data points have been jittered to reduce over plotting. Bottom: A plot (raw) using the most extreme individual trajectories predicted by the model. A/B trajectories are examples of patients with the highest/lowest intercepts.
T cell blastogenesis: Con A
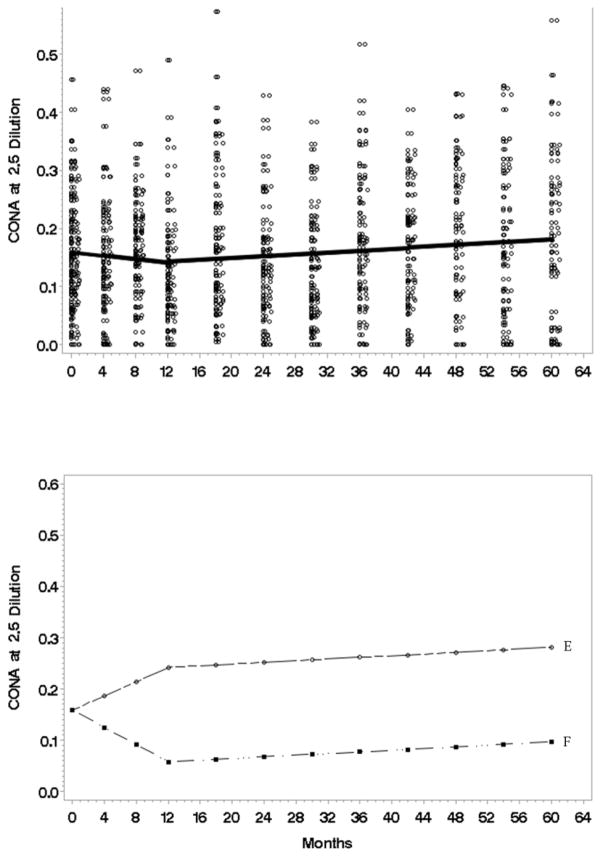
Natural log transformations were used. With respect to the trajectory for the average person (Fig 5, top panel), ConA at 2.5 showed a zero slope in Phase I, and a slight average increase in Phase II (β̂2 = .000807, t = 2.77, p value = .0065 two tail). Analyses revealed a similar trajectory for ConA 5.0 but not 10.0, which could only support a simple line with random slopes. The modest slope shifts (knots) occurred at 12 months (τ̂ = 12, t = 2.51, p value = .0136 two tail).
Figure 5.
Top: The estimated concanavalin A (ConA) at 2.5 serial dilution trajectory (natural log) for the average individual (N=113) from initial to 60 month assessment. Data points have been jittered to reduce over plotting. Bottom: A plot (natural log) using the most extreme individual trajectories predicted by the model. The E/F trajectories are examples of patients having the highest/lowest first phase slopes.
Analyses of individual variability revealed significant effects for Phase I slopes (Fig 5, bottom panel), with some individuals showing an increase in Con A (see Patient E with an increasing Phase I slope) whereas others showing decreasing Con A (see Patient F). No covariates were significant. In summary, there was no average change in ConA in the first year, with the possibility of a slight, increasing trajectory in the later years.
T cell blastogenesis: PHA
Natural log transformations were used. Across serial dilutions, analysis of individual variability showed differences in Phase I slopes , with some patients showing increases in PHA and others decreases. However, in Phase II there was zero slope (no change from month 4.5 to 60). The slope shift (knot) occurred 4.5 months (τ̂ = 4.5409, t = 3.50, p value = .0007). Regarding covariates, older individuals at baseline had a lower average PHA 2.5 (γ̂0 = −.0016, t = −2.8, p value = .0060). In summary, individuals differed in PHA in the early (4–5) months, but there was little detectable change in PHA thereafter. Considering the findings from both blastogenesis assays, the evidence is weak for T cell blastogenesis to vary substantially from post surgery through 5 years in these patients.
Discussion
These data are the first to describe long-term, natural trajectories of cancer-specific stress, depressive symptoms, and immunity for breast cancer survivors. Analyses used multiple time points (12) from diagnosis through to 5 years (a range of 725 to 925 data points per outcome) to determine if phases of change exist, and if so, when shifts in change occurs. The data supported trajectories that are marked by two distinct phases, with estimated time points of the shift from the first to the second phase. If representative of the larger population of patients, we now have estimates of the trajectories for “the average person.” Also, more specific insights may be gained by examining the individual differences among patients.
The cancer specific stress trajectory for the “average” breast cancer patient is one of steady decline, with roughly 60% of the change occurring in the 12 months following diagnosis, with a slow, further decline across the next 4 years. It is notable that the first phase included the months of adjuvant therapy (baseline to 4 months for 90% of the patients) and the 8 months afterwards. Differences among patients in stress have been reported (45) but whether baseline stress modifies the trajectory has been unknown. These analyses revealed that regardless of “starting point” differences, a trajectory in which all patients had the same rate of decline in stress for 12 months fit the data best. After 12 months, trajectories become variable. For some, stress will continue to decline, whereas for others the decline “stalls” with higher stress maintained in the years to follow.
The four assessments in year one enabled discovery of the steep decline in depressive symptoms occurring well before the 12 months previously suggested (17,23,24). Estimates from the data suggest that by 6.7 months the downward trajectory ends, with a stable (low) symptom level for the remaining years. However, individual differences matter in the symptom pattern thereafter. Specifically, after 7 months some lose the gains from Phase I and return (if not exceed) their baseline, whereas others continue with symptom decline. One possibility is that initial high scorers who return to baseline may have a pre-cancer history of depression. The baseline descriptive data showing 19% of the sample exceeded the CES-D cutoff and with more reporting pre cancer dysthymia/depressive symptom histories suggest that a notable percentage was at risk for late, higher depressive symptom levels. Other studies show breast cancer patients with a history of major depressive disorder (MDD) to report increases in depressive symptoms from pre to post chemotherapy in contrast to those with no such history (46), and roughly 50% of cancer patients in MDD treatment trials have been found with a history (pre-cancer) of MDD and/or prior psychiatric treatment (47,48).
There are no long-term data on cancer patients’ NKCC or T cell blastogenic responses. For NKCC, the baseline appeared “low” when compared to the trajectory, which may reflect lingering suppressive effects of the breast cancer itself, the inhibitory effects of surgery, or some combination. Studies from pre to post adjuvant chemo/radiotherapy have shown no changes or ones which reverse (11,33–35). With assessments during and long after the end of chemotherapy and radiotherapy, these data show the post surgery NKCC trajectory to be one of linear recovery---through the 4–8 months of treatment and continuing to 18 months--which may be suggestive of the antitumor role of the NK cell compartment.
NK and T cells have the potential to eliminate cancer cells within tumors and conduct immune surveillance, but do so in fundamentally different ways. Whereas NK cells identify and lyse tumor cells based on the absence of HLA class I molecules (“missing self”), T cells do so with class I-dependent expression of antigenic peptide epitopes that are not expressed by normal cells. Perhaps reflective of these biologic differences, NKCC data were symmetric and unimodal, similar to a bell curve, whereas the T cell blastogenesis data required transformation and was “noisy,” such that detecting a trajectory within and across assays was generally difficult, particularly so with ConA although less so with PHA. This variability likely makes detecting differences difficult (49) though in studies with rigorous controls or randomization significant increases in PHA have been found with cancer (32) and psychological therapies (38). Also, an early study found that patients exhibiting a drop in PHA within 36 months had an increased risk for metastatic disease (36), pointing to the potential importance of the T cell compartment in immune surveillance for recurrent breast cancer.
The research design and methods that enabled this analysis require discussion to evaluate the generalizability of the findings. With the lengthy 5 years of follow up and with a sample at risk for disease progression, missing data due to dropout and participant mortality occurred (See Supplementary Materials). Complete data across 60 months were recorded for N=59 subjects. The analyses assumed that the missing data were missing at random and models were fit to all available data using maximum likelihood. The sample was homogeneous, only regional breast cancer patients, with the timing of accrual insuring that all were post surgery and pre adjuvant therapy at baseline. Valid and reliable psychological measures were used. The psychological trajectories reported may be similar for other newly diagnosed female cancer patients, but their relevance to male patients is not clear. In general, females report higher levels of negative affect and physical symptoms in comparison to men (50), which may result in psychological trajectories in men being more difficult to detect. Regarding gender differences in immunity, they are generally not found in CD3 or NK subsets and when observed, older women (age > 70) have been found with higher NK cell numbers (51,52). Regarding the generality of the immunity trajectories, they did not covary with extent of surgery, and the chemotherapies received by these patients (primarily anthracycline-based) are the same as those in prior immune studies (see Supplementary Materials) and are ones still used to treat breast cancer as well as many others, only NKCC was assessed, and it alone does not capture all of the effector mechanisms for eliminating malignant cells.
The data have clinical relevance, with implications for identifying and providing psychosocial care to those newly diagnosed with breast cancer as well as survivors. Data show the high levels of stress and/or depressive symptoms at diagnosis/treatment are long-term limiting factors. Even with early symptom declines, patients may experience no further improvement in stress and/or return to earlier, higher depressive symptom levels. These patterns underscore the importance of recommendations to screen patients at the time of diagnosis for symptoms of anxiety and depression (53). The data also show that the absolute levels of symptoms at diagnosis/treatment best predict risk, as the analyses explored covariates with a predominance of null effects. Notably, surgery type did not predict subsequent stress or depressive symptoms, nor did income, age, or performance status (KPS). Only social network size covaried with baseline cancer stress, with no effect on the stress trajectory. Thus, patients with higher stress levels are in need of early, evidence based, psychological treatment not only to lower current stress, but to prevent its maintenance and the poorer quality of life which follows (9,11). If psychological treatments are used, they are efficacious in reducing stress, enhancing positive coping and quality of life (54,55), improving health behaviors (56), enhancing biologic responses such as those studied here (38,54), and in some circumstances, reducing risk for recurrence (40) or cancer death (39,57).
In summary, novel data for the biobehavioral trajectories for the modal, disease free, breast cancer survivor are provided from diagnosis/surgery through 5 years. Psychological and innate immune markers (NKCC) improved from diagnosis to 6–18 months, and thereafter gains for the average person were at least stable, if not improving further, for the next four years. However, high levels of stress and/or depressive symptoms at diagnosis/treatment are long-term limiting factors, as after early declines some patients may experience no further improvement in stress and/or rebound to higher depressive symptom levels. The latter scenario is troubling in its duration, continuing through 5 years. However, knowledge of these biobehavioral trajectories will hopefully assist in defining the timing and substance of survivorship care.
Supplementary Material
Statement of Translational Relevance.
The present long-term (5 year) prospective study (N=113) study evaluated the changes that occurred in levels of stress, depression and immune function in patients with stage I–III breast cancer from the time of surgery. Cancer stress showed two distinct phases of decline, with the change point being 12 months. In contrast, a steep decline in depressive symptoms occurred by 7 months, with stable, low levels thereafter. Natural killer cell cytotoxicity showed a steady upward trajectory through 18 months and upper limit stability thereafter, whereas there was no reliable trajectory for T cell blastogenesis. This study is unique in its identification of improvement in immunity, stress, depressive symptoms over a period of five years post-surgery. Behavioral interventions aimed at reducing stress and depression and therapeutic approaches aimed at augmenting the immune response to cancer will benefit from knowledge of these trajectories.
Acknowledgments
Funding: This work was supported by the National Cancer Institute (CA92704, CA144024, CA098133) and the National Institute of Mental Health (MH51487); the American Cancer Society (PBR-89, PBR-89A); the U.S. Army Medical Research Acquisition Activity Breast Cancer Research Program (DAMD 17-94-J-4165, DAMD 17-96-1-6294); and, the Walther Cancer Institute.
Abbreviations
- CES-D
Center for Epidemiological Studies Depression Scale
- ConA
Concanavalin A
- IES
Impact of Events Scale
- NKCC
Natural killer cells cytotoxicity
- PHA
Phytohaemagglutinin
Footnotes
The authors have no conflicts of interest to disclose. This manuscript contains original work that is not currently being considered for publication elsewhere.
References
- 1.Andersen BL, Farrar WB, Golden-Kreutz D, Kutz LA, MacCallum R, Courtney ME, et al. Stress and immune responses after surgical treatment for regional breast cancer. J Natl Cancer Inst. 1998 Jan;90(1):30–6. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiche EV, Nunes SV, Morimoto H. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004 Oct;5(10):617–25. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 3.Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol Bull. 2004 Jul;130(4):601–30. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutgendorf SK, Andersen BL. Biobehavioral approaches to cancer progression and survival: mechanisms and interventions. Am Psychol. 2015 Feb-Mar;70(2):186–97. doi: 10.1037/a0035730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hewitt M, Greenfield S, Stovall E. From cancer patient to cancer survivor: Lost in transition. Washington, D.C: National Academies Press; 2005. [Google Scholar]
- 6.Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008 Jun;112(11 Suppl):2577–92. doi: 10.1002/cncr.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobsen PB, Rowland JH, Paskett ED, Van Leeuwen F, Moskowitz C, Katta S, et al. Identification of key gaps in cancer survivorship research: findings from the American Society of Clinical Oncology Survey. J Oncol Pract. 2016 Mar;12(3):190–3. doi: 10.1200/JOP.2015.009258. [DOI] [PubMed] [Google Scholar]
- 8.Dupont A, Bower JE, Stanton AL, Ganz PA. Cancer-related intrusive thoughts predict behavioral symptoms following breast cancer treatment. Health Psychol. 2014 Feb;33(2):155–63. doi: 10.1037/a0031131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golden-Kreutz DM, Thornton LM, Wells-Di Gregorio S, Frierson GM, Jim HS, Carpenter KM, et al. Traumatic stress, perceived global stress, and life events: prospectively predicting quality of life in breast cancer patients. Health Psychol. 2005 May;24(3):288–96. doi: 10.1037/0278-6133.24.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008 Aug;5(8):466–75. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 11.Thornton LM, Andersen BL, Crespin TR, Carson WE. Individual trajectories in stress covary with immunity during recovery from cancer diagnosis and treatments. Brain Behav Immun. 2007 Feb;21(2):185–94. doi: 10.1016/j.bbi.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantegna G, Petrillo M, Fuoco G, Venditti L, Terzano S, Anchora LP, et al. Long-term prospective longitudinal evaluation of emotional distress and quality of life in cervical cancer patients who remained disease-free 2-years from diagnosis. BMC Cancer. 2013 Jan;13:127. doi: 10.1186/1471-2407-13-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrandina G, Petrillo M, Mantegna G, Fuoco G, Terzano S, Venditti L, et al. Evaluation of quality of life and emotional distress in endometrial cancer patients: a 2-year prospective, longitudinal study. Gynecol Oncol. 2014 Jun;133(3):518–25. doi: 10.1016/j.ygyno.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Hinnen C, Ranchor AV, Sanderman R, Snijders TAB, Hagedoorn M, Coyne JC. Course of distress in breast cancer patients, their partners, and matched control couples. Ann Behav Med. 2008 Oct;36(2):141–8. doi: 10.1007/s12160-008-9061-8. [DOI] [PubMed] [Google Scholar]
- 15.Trudel-Fitzgerald C, Savard J, Ivers H. Evolution of cancer-related symptoms over an 18-month period. J Pain Symptom Manage. 2013 Jun;45(6):1007–18. doi: 10.1016/j.jpainsymman.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Neilson K, Pollard A, Boonzaier A, Corry J, Castle D, Smith D, et al. A longitudinal study of distress (depression and anxiety) up to 18 months after radiotherapy for head and neck cancer. Psychooncology. 2013 Aug;22(8):1843–8. doi: 10.1002/pon.3228. [DOI] [PubMed] [Google Scholar]
- 17.Schroevers M, Ranchor AV, Sanderman R. Adjustment to cancer in the 8 years following diagnosis: a longitudinal study comparing cancer survivors with healthy individuals. Soc Sci Med. 2006 Aug;63(3):598–610. doi: 10.1016/j.socscimed.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Wu SM, Andersen BL. Stress generation over the course of breast cancer survivorship. J Behav Med. 2010 Jun;33(3):250–7. doi: 10.1007/s10865-010-9255-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suppli NP, Johansen C, Christensen J, Kessing LV, Kroman N, Dalton SO. Increased risk for depression after breast cancer: a nationwide population-based cohort study of associated factors in Denmark, 1998–2011. J Clin Oncol. 2014 Dec;32(34):3831–9. doi: 10.1200/JCO.2013.54.0419. [DOI] [PubMed] [Google Scholar]
- 20.Pinquart M, Duberstein P. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010 Nov;40(11):1797–810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009 Nov;115(22):5349–61. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 22.Hjerl K, Andersen EW, Keiding N, Mouridsen HT, Mortensen PB, Jørgensen T. Depression as a prognostic factor for breast cancer mortality. Psychosomatics. 2003 Jan;44(1):24–30. doi: 10.1176/appi.psy.44.1.24. [DOI] [PubMed] [Google Scholar]
- 23.Avis NE, Levine B, Naughton MJ, Case LD, Naftalis E, Van Zee KJ. Age-related longitudinal changes in depressive symptoms following breast cancer diagnosis and treatment. Breast Cancer Res Treat. 2013 May;139(1):199–206. doi: 10.1007/s10549-013-2513-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Punnen S, Cowan JE, Dunn LB, Shumay DM, Carroll PR, Cooperberg MR. A longitudinal study of anxiety, depression and distress as predictors of sexual and urinary quality of life in men with prostate cancer. BJU Int. 2013 Jul;112(2):E67–75. doi: 10.1111/bju.12209. [DOI] [PubMed] [Google Scholar]
- 25.Stanton AL, Rowland JH, Ganz PA. Life after diagnosis and treatment of cancer in adulthood: contributions from psychosocial oncology research. Am Psychol. 2015 Feb-Mar;70(2):159–74. doi: 10.1037/a0037875. [DOI] [PubMed] [Google Scholar]
- 26.Jones SMW, LaCroix AZ, Li W, Zaslavsky O, Wassertheil-Smoller S, Weitlauf J, et al. Depression and quality of life before and after breast cancer diagnosis in older women from the Women’s Health Initiative. J Cancer Surviv. 2015 Dec;9(4):620–9. doi: 10.1007/s11764-015-0438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005 Mar;330(7493):702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krebber AMH, Buffart LM, Kleijn G, Riepma IC, De Bree R, Leemans CR, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121–30. doi: 10.1002/pon.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouchard LC, Antoni MH, Blomberg BB, Stagl JM, Gudenkauf LM, Jutagir DR, et al. Postsurgical depressive symptoms and proinflammatory cytokine elevations in women undergoing primary treatment for breast cancer. Psychosom Med. 2016;78(1):26–37. doi: 10.1097/PSY.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGregor BA, Antoni MH, Boyers A, Alferi SM, Blomberg BB, Carver CS. Cognitive-behavioral stress management increases benefit finding and immune function among women with early-stage breast cancer. J Psychosom Res. 2004 Jan;56(1):1–8. doi: 10.1016/S0022-3999(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 31.Thornton LM, Andersen BL, Schuler TA, Carson WE. A psychological intervention reduces inflammatory markers by alleviating depressive symptoms: secondary analysis of a randomized controlled trial. Psychosom Med. 2009 Sep;71(7):715–24. doi: 10.1097/PSY.0b013e3181b0545c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carson WE, Shapiro CL, Crespin TR, Thornton LM, Andersen BL. Cellular immunity in breast cancer patients completing taxane treatment. Clin Cancer Res. 2004 May 15;10(10):3401–9. doi: 10.1158/1078-0432.CCR-1016-03. [DOI] [PubMed] [Google Scholar]
- 33.Mozaffari F, Lindemalm C, Choudhury A, Granstam-Björneklett H, Lekander M, Nilsson B, et al. Systemic immune effects of adjuvant chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide and/or radiotherapy in breast cancer: a longitudinal study. Cancer Immunol Immunother. 2009 Jan;58(1):111–20. doi: 10.1007/s00262-008-0530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang DH, Weaver MT, Park NJ, Smith B, McArdle T, Carpenter J. Significant impairment in immune recovery after cancer treatment. Nurs Res. 2009 Jan;58(2):105–14. doi: 10.1097/NNR.0b013e31818fcecd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Standish LJ, Torkelson C, Hamill FA, Yim D, Hill-Force A, Fitzpatrick A, et al. Immune defects in breast cancer patients after radiotherapy. J Soc Integr Oncol. 2008 Jan;6(3):110–21. [PMC free article] [PubMed] [Google Scholar]
- 36.Wiltschke C, Krainer M, Budinsky AC, Berger A, Müller C, Zeillinger R, et al. Reduced mitogenic stimulation of peripheral blood mononuclear cells as a prognostic parameter for the course of breast cancer: a prospective longitudinal study. Br J Cancer. 1995 Jun;71(6):1292–6. doi: 10.1038/bjc.1995.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rotstein S, Blomgren H, Petrini B, Wasserman J, Baral E. Long term effects on the immune system following local radiation therapy for breast cancer. I. Cellular composition of the peripheral blood lymphocyte population. Int J Radiat Oncol Biol Phys. 1985 May;11(5):921–5. doi: 10.1016/0360-3016(85)90114-2. [DOI] [PubMed] [Google Scholar]
- 38.Andersen BL, Farrar WB, Golden-Kreutz DM, Glaser R, Emery CF, Crespin TR, et al. Psychological, behavioral, and immune changes after a psychological intervention: a clinical trial. J Clin Oncol. 2004 Sep;22(17):3570–80. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersen BL, Thornton LM, Shapiro CL, Farrar WB, Mundy BL, Yang HC, et al. Biobehavioral, immune, and health benefits following recurrence for psychological intervention participants. Clin Cancer Res. 2010 Jun;16(12):3270–8. doi: 10.1158/1078-0432.CCR-10-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andersen BL, Yang H-C, Farrar WB, Golden-Kreutz DM, Emery CF, Thornton LM, et al. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer. 2008 Dec;113(12):3450–8. doi: 10.1002/cncr.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979 May;41(3):209–18. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. J Aging Health. 1993;5(2):179–93. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 43.Karnofsky D, Burchenal J. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod C, editor. Evaluation of chemotherapeutic agents. New York: Columbia University Press; 1949. pp. 191–205. [Google Scholar]
- 44.Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979 Feb;109(2):186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 45.Heron-Speirs HA, Harvey ST, Baken DM. Moderators of psycho-oncology therapy effectiveness: meta-analysis of therapy characteristics. J Psychosoc Oncol. 2013 Jan;31(6):617–41. doi: 10.1080/07347332.2013.835022. [DOI] [PubMed] [Google Scholar]
- 46.Jim HSL, Small BJ, Minton S, Andrykowski M, Jacobsen PB. History of major depressive disorder prospectively predicts worse quality of life in women with breast cancer. Ann Behav Med. 2012 Jun;43(3):402–8. doi: 10.1007/s12160-011-9333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brothers BM, Yang HC, Strunk DR, Andersen BL. Cancer patients with major depressive disorder: testing a biobehavioral/cognitive behavior intervention. J Consult Clin Psychol. 2011 Apr;79(2):253–60. doi: 10.1037/a0022566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hopko DR, Cannity K, McIndoo CC, File AA, Ryba MM, Clark CG, et al. Behavior therapy for depressed breast cancer patients: predictors of treatment outcome. J Consult Clin Psychol. 2015;83(1):225–31. doi: 10.1037/a0037704. [DOI] [PubMed] [Google Scholar]
- 49.Kosti O, Byrne C, Cocilovo C, Willey SC, Zheng Y-L. Phytohemagglutinin-induced mitotic index in blood lymphocytes: a potential biomarker for breast cancer risk. Breast Cancer Basic Clin Res. 2010 Dec;4:73–83. doi: 10.4137/BCBCR.S6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujita F, Diener E, Sandvik E. Gender differences in negative affect and well-being: the case for emotional intensity. J Pers Soc Psychol. 1991 Sep;61(3):427–34. doi: 10.1037//0022-3514.61.3.427. [DOI] [PubMed] [Google Scholar]
- 51.Al-Attar A, Presnell SR, Peterson CA, Thomas DT, Lutz CT. The effect of sex on immune cells in healthy aging: elderly women have more robust natural killer lymphocytes than do elderly men. Mech Ageing Dev. 2016 Apr;156:25–33. doi: 10.1016/j.mad.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Valiathan R, Ashman M, Asthana D. Effects of aging on the immune system: infants to elderly. Scand J Immunol. 2016 Apr;83(4):255–66. doi: 10.1111/sji.12413. [DOI] [PubMed] [Google Scholar]
- 53.Andersen BL, DeRubeis RJ, Berman BS, Gruman J, Champion VL, Massie MJ, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014 May 20;32(15):1605–19. doi: 10.1200/JCO.2013.52.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGregor BA, Antoni MH. Psychological intervention and health outcomes among women treated for breast cancer: A review of stress pathways and biological mediators. Brain Behav Immun. 2009 Feb;23(2):159–66. doi: 10.1016/j.bbi.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Faller H, Schuler M, Richard M, Heckl U, Weis J, Kuffner R. Effects of psycho-oncologic interventions on emotional distress and quality of life in adult patients with cancer: systematic review and meta-analysis. J Clin Oncol. 2013 Feb;31(6):782–93. doi: 10.1200/JCO.2011.40.8922. [DOI] [PubMed] [Google Scholar]
- 56.Spark LC, Reeves MM, Fjeldsoe BS, Eakin EG. Physical activity and/or dietary interventions in breast cancer survivors: A systematic review of the maintenance of outcomes. J Cancer Surviv. 2013 Mar;7(1):74–82. doi: 10.1007/s11764-012-0246-6. [DOI] [PubMed] [Google Scholar]
- 57.Giese-Davis J, Collie K, Rancourt KMS, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol. 2011 Feb;29(4):413–20. doi: 10.1200/JCO.2010.28.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.