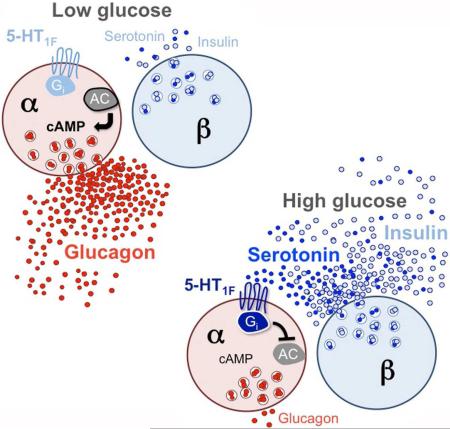
SUMMARY
In the pancreatic islet serotonin is an autocrine signal increasing beta cell mass during metabolic challenges such as those associated with pregnancy or high-fat diet. It is still unclear if serotonin is relevant for regular islet physiology and hormone secretion. Here we show that human beta cells produce and secrete serotonin when stimulated with increases in glucose concentration. Serotonin secretion from beta cells decreases cAMP levels in neighboring alpha cells via 5-HT1F receptors and inhibits glucagon secretion. Without serotonergic input, alpha cells lose their ability to regulate glucagon secretion in response to changes in glucose concentration, suggesting that diminished serotonergic control of alpha cells can cause glucose blindness and the uncontrolled glucagon secretion associated with diabetes. Supporting this model, pharmacological activation of 5-HT1F receptors reduces glucagon secretion and has hypoglycemic effects in diabetic mice. Thus, modulation of serotonin signaling in the islet represents a drug intervention opportunity.
Keywords: Serotonin, beta cell, insulin secretion, alpha cell, glucagon secretion, pancreatic islet, paracrine signal, diabetes
Graphical abstract
eTOC BLURB
Almaça et al. found that serotonin is a paracrine signal released by human pancreatic beta cells to regulate glucagon secretion. Without serotonergic control, alpha cells do not respond appropriately to changes in glucose concentration. Targeting serotonin receptors in alpha cells could be used to reduce glucagon secretion in diabetes.
INTRODUCTION
Serotonin was proposed as a paracrine signal in the pancreatic islet over 40 years ago (Lundquist et al., 1971; Marco et al., 1977; Pontiroli et al., 1978). Serotonin is co-released with insulin (Ekholm et al., 1971; Jaim-Etcheverry and Zieher, 1968; Richmond et al., 1996). Together they play a role in metabolism and maintenance of energy balance, serving as signals for energy sufficiency (Donovan and Tecott, 2013; Ohta et al., 2011). Recent studies renewed interest in serotonin by showing that, during heightened metabolic demand such as pregnancy or high-fat diet feeding, serotonin is produced in the islet and required to maintain glucose homeostasis (Goyvaerts et al., 2015; Kim et al., 2010; Kim et al., 2015; Schraenen et al., 2010). To this end, beta cells increase synthesis of serotonin and secrete it to enhance insulin secretion and stimulate beta cell proliferation via 5-HT2B and 5-HT3 receptors (Bennet et al., 2016; Kim et al., 2010; Kim et al., 2015; Ohara-Imaizumi et al., 2013). Serotonin also promotes insulin granule exocytosis in a receptor-independent manner through serotonylation of small GTPases of Rab family (Paulmann et al., 2009). Numerous studies have thus established serotonin as an autocrine signal increasing beta cell function and mass during insulin resistant states, but only few have examined the impact of serotonin on other islet endocrine cells such as the glucagon secreting alpha cell (Bennet et al., 2015; Marco et al., 1977; Pontiroli et al., 1978).
These recent studies further indicate that serotonin is produced and secreted from beta cells almost exclusively under conditions of increased metabolic demand. Although the machinery for serotonin uptake and release is present in beta cells of several species (Gylfe, 1978; Hellman et al., 1972; Schafer et al., 2013), detecting serotonin secretion from rodent beta cells requires islets to be preloaded in vitro with serotonin or its precursors (Barbosa et al., 1998; Rosario et al., 2008; Smith et al., 1995). This suggests that serotonin may not play a paracrine role in the islet in the normal physiological state. A recent study, however, showed that serotonin is synthesized and secreted at detectable levels from islets of healthy humans (Bennet et al., 2015). This contrast between rodent and human islets is in line with other species differences in islet structure and function (Dolensek et al., 2015; Kim et al., 2009; Stewart et al., 2015) and implies that serotonin has functions in the human islet not predicted by rodent models.
Here we systematically investigated serotonin signaling in the human islet. We determined that serotonin is synthesized and released from beta cells under regular physiological conditions. To test if serotonin is a bona fide paracrine signal, we manipulated endogenous production and secretion of serotonin with pharmacological tools and measured its effects on hormone secretion and intracellular signaling. We identified the receptors mediating these effects. Our results show that beta cell-derived serotonin inhibits glucagon secretion. Serotonin signaling thus contributes to the intricate paracrine network that regulates islet hormone secretion and glucose metabolism.
RESULTS
Human beta cells produce and secrete serotonin under normal physiological conditions
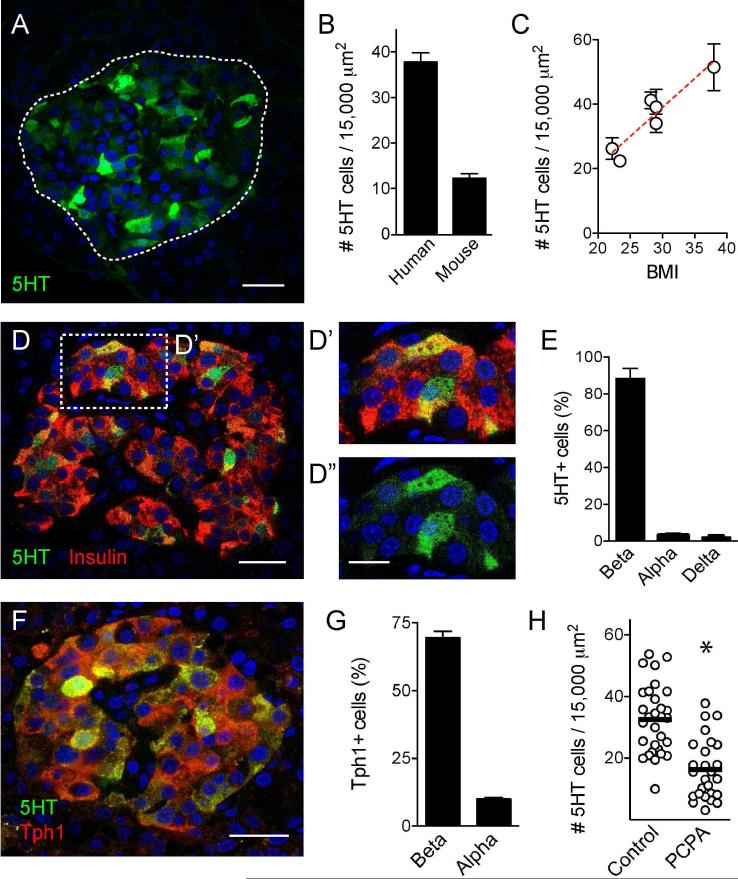
We found that human islets contained at least three times more serotonin-positive cells than mouse islets (5-hydroxytryptamine, 5HT, Figures 1A and 1B). Serotonin immunostaining varied between human pancreases. The number of serotonin-positive cells in human islets correlated positively with donor BMI (r2 = 0.2854, p value < 0.0001; Figure 1C), but not with the gender of the donor (37.8 ± 2 for females and 35 ± 3 for males (p = 0.52, unpaired t-test, n > 23 islets per group). There was a slight negative correlation with donor age (r2 = 0.1337, p = 0.0035, n = 6 individuals with ages between 22 – 54 years old). Most serotonin-positive cells (~ 90%) in human islets were also immunoreactive for insulin (Figure 1D); few (< 10%) expressed glucagon or somatostatin (Figure 1E). To determine whether beta cells produce their own serotonin or take it up from the circulation, we examined the expression of tryptophan hydroxylase 1 (Tph1), the rate-limiting enzyme in serotonin synthesis in the periphery (Walther et al., 2003). Most beta cells (~ 90%) expressed Tph1, the majority of Tph1-positive cells (~70%) were beta cells, and all serotonin-positive cells expressed Tph1 (Figures 1F and 1G). Treating islets with the Tph inhibitor p-Chlorophenylalanine (PCPA) reduced the number of serotonin-positive cells by 45% (Figure 1H).
Figure 1. Beta cells in human pancreatic islets synthesize serotonin.
(A) Z-stack of confocal images of a human islet in a pancreatic section immunostained for serotonin (5HT, green). Dashed line indicates islet.
(B) Estimation of the number of serotonin positive cells per islet area in human and mouse islets. Bars represent average ± SEM.
(C) The number of serotonin-positive cells divided by the islet area shows a positive correlation with donor BMI (r2 = 0.2854, p value < 0.0001).
(D) Confocal image of a human pancreatic islet showing serotonin (green) and insulin (red) immunostaining. Double-labeled cells appear yellow. D’ and D” are higher magnifications of the islet region delimited by box in D, double labeled or single labeled for 5HT.
(E) Quantification of the percentage of serotonin-positive cells also expressing insulin (beta), glucagon (alpha) or somatostatin (delta).
(F) Confocal image of a human pancreatic islet showing serotonin (green) and tryptophan hydroxylase 1 (Tph1, red) immunostaining. All serotonin-positive cells expressed Tph1.
(G) Quantification of the percentage of Tph1-positive cells that are beta or alpha cells.
(H) Quantification of the number of serotonin-positive cells in human islets incubated in culture medium alone or in the presence of the Tph1 inhibitor p-Chlorophenylalanine (PCPA) (10 μM, 2h, 37°C). PCPA treatment significantly reduced the number of serotonin-positive cells. * p value < 0.05 Unpaired Student's t test.
Scale bars represent 20 μm (A, D and F) and 10 μm (D’ and D”).
Serotonin content varied considerably between beta cells, in contrast with the widespread expression of Tph1 (Figure 1). We examined the expression of another enzyme in the serotonin biosynthetic pathway, aromatic amino acid decarboxylase (AADC), and found that it was expressed in most islet cells (Figure S1). Like Tph1, > 90% of beta cells were immunostained for AADC (93 ± 1 %, n = 10 islets, 3 individuals), suggesting that expression of the biosynthetic machinery cannot explain the heterogeneity of serotonin staining. Serotonin uptake into granules requires the vesicular monoamine transporter type 2 (VMAT2), which is expressed in beta cells [see below and also (Anlauf et al., 2003; Schafer et al., 2013)]. Serotonin thus may accumulate only in beta cells that load serotonin into granules via VMAT2. However, most beta cells expressed VMAT2 [see below and (Freeby et al., 2012)]. Therefore, our data cannot explain the uneven levels of serotonin production in the human islet, which are also seen in mouse islets during pregnancy (Goyvaerts et al., 2015).
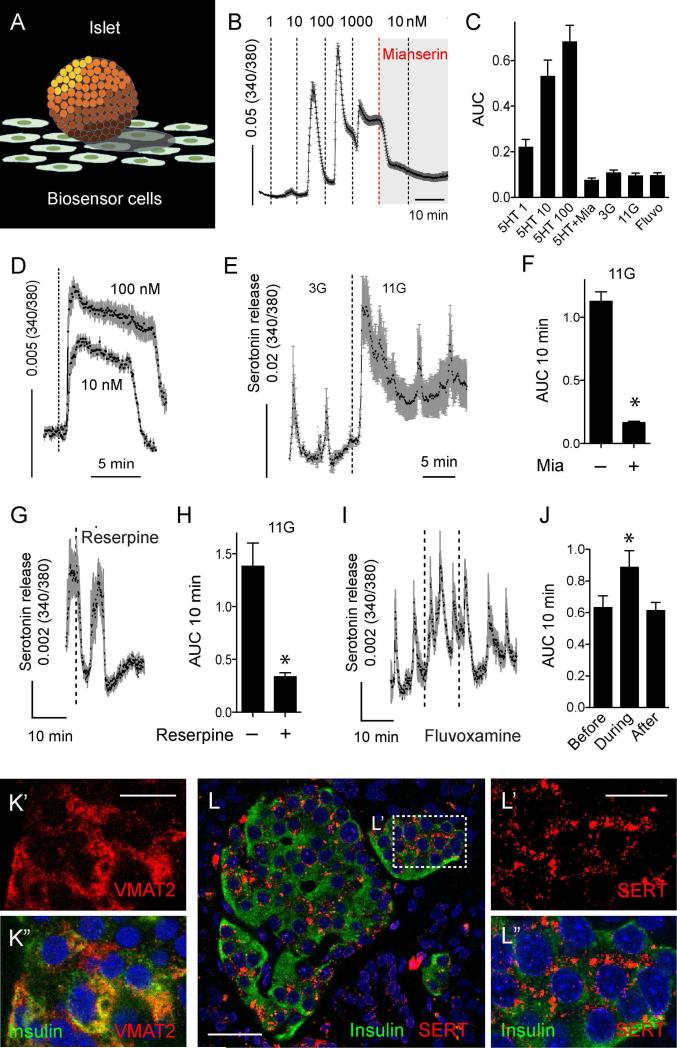
To determine if human beta cells secrete serotonin, we used serotonin biosensor cells [CHO cells expressing serotonin receptor 2C, 5-HT2C (Huang et al., 2005); Figure 2A]. When these cells were loaded with the cytoplasmic free Ca2+ ([Ca2+]i) indicator Fura-2, they showed robust [Ca2+]i responses to serotonin in the nM range, making them ideal for real time detection of serotonin secretion from islets (Figure 2B). Placing isolated human islets on serotonin biosensor cells elicited pulsatile [Ca2+]i responses in 3 mM glucose (Figure 2E). When the glucose concentration was raised to 11 mM, pulsatile [Ca2+]i responses increased to the level equivalent to those elicited by 20 nM serotonin (Figures 2E and S2). These results indicate that human beta cells secrete serotonin at concentrations that activate serotonin receptors [e.g. EC50 = 8 nM for serotonin receptor 5-HT1F; (Adham et al., 1993)]. The glucose-dependence and pulsatile pattern of serotonin release were similar to insulin secretion (Almaca et al., 2015; Satin et al., 2015). Biosensor responses were blocked by the 5-HT2 antagonist mianserin, indicating that serotonin was detected (Figure 2F). In the absence of islets, prolonged exposure to serotonin (10 and 100 nM) did not elicit pulsatile [Ca2+]i responses (Figure 2D). Thus, biosensor [Ca2+]i responses were not inherently pulsatile. Neither glucose nor drugs used to manipulate serotonin uptake and release (see below) induced or altered responses in the biosensor cells in the absence of human islets (Figure 2C). Mouse islets did not release detectable amounts of serotonin in response to glucose (Figure S2), in line with the small number of serotonin-positive cells (Figure 1B).
Figure 2. Human beta cells release serotonin upon stimulation with high glucose and express components of serotonergic machinery.
(A) 5HT biosensors are CHO cells that stably express 5-HT2C receptors and can detect serotonin secretion in real time from islets placed in close proximity. Responses in biosensors are recorded by loading them with fura-2 and imaging [Ca2+]i.
(B) In the absence of human islets, serotonin biosensors respond to direct application of serotonin (1, 10, 100 and 1000 nM), and responses are inhibited by the 5-HT2C receptor antagonist mianserin (Mia, 10 μM).
(C) Quantification (area under the curve (AUC)) of biosensor responses in the absence of human islets to 2 min application of serotonin (1, 10, 100 nM), 10 nM serotonin in the presence of mianserin (5HT+Mia), 10 min incubation in basal (3 mM, 3G) or high (11 mM, 11G) glucose concentration, or in the presence of the serotonin transporter (SERT) inhibitor fluvoxamine (500 nM) in 3G.
(D) [Ca2+]i remains elevated in biosensor cells during prolonged exposure to serotonin solutions (10 and 100 nM) in the absence of islets.
(E) In the presence of human islets, biosensor cells respond to an increase in glucose concentration (from 3 mM to 11 mM). See also Figure S2 for comparison of glucose-induced serotonin secretion from mouse versus human islets.
(F) Biosensor responses to high glucose were inhibited by mianserin (Mia), indicating serotonin secretion upon beta cell activation.
(G) Inhibition of serotonin secretion from human islets with the VMAT2 inhibitor reserpine (100 nM, n = 12 cells, 3 different islet preparations). Reserpine abolished serotonin release in every experiment.
(H) Summary of data from experiments such as those shown in (G) showing AUC values of 10 min recordings before and after reserpine application.
(I) Increase in serotonin levels by application of fluvoxamine (500 nM, n = 21 cells using 3 islet preparations).
(J) Summary of data from experiments such as those shown in (I) showing AUC values of 10 min recordings before, during, and after fluvoxamine application.
(K) Confocal image of a region in a human islet in a pancreatic section immunostained for VMAT2 (red, K’) and insulin (green, merged image in K”).
(L) Confocal image of a human islet in a pancreatic section immunostained for SERT (red) and insulin (green). L’ and L” are higher magnifications of the islet region delimited by box in L, double labeled or single labeled for SERT (merged image in L”).
In all panels bar graphs show mean ± SEM, * p value < 0.05 [Paired Student's t test (F, H) and one-way ANOVA followed by multiple comparisons (J)]. Dashed lines denote drug applications. Scale bars represent 20 μm (L) and 10 μm (K’ and L’).
Manipulating serotonin transporters modulates islet serotonin levels
To explore the mechanisms of serotonin release, we targeted vesicular and membrane transporters for serotonin. Applying the VMAT2 inhibitor reserpine (500 nM) abolished serotonin secretion from human islets (Figures 2G and 2H). The spatiotemporal aspects of serotonin action are limited by uptake from the extracellular space by the membrane serotonin transporter SERT (Claassen et al., 1977). We found that SERT was expressed in beta cells in human islets (Figure 2L). Applying fluvoxamine (500 nM), a selective SERT blocker, significantly increased serotonin secretion from human islets (Figures 2I and 2J). These results indicate that different components of the serotonergic machinery are expressed in beta cells and that these transporters can be targeted pharmacologically to modulate endogenous serotonin levels in human islets.
Exogenous serotonin changes hormone secretion from human islets
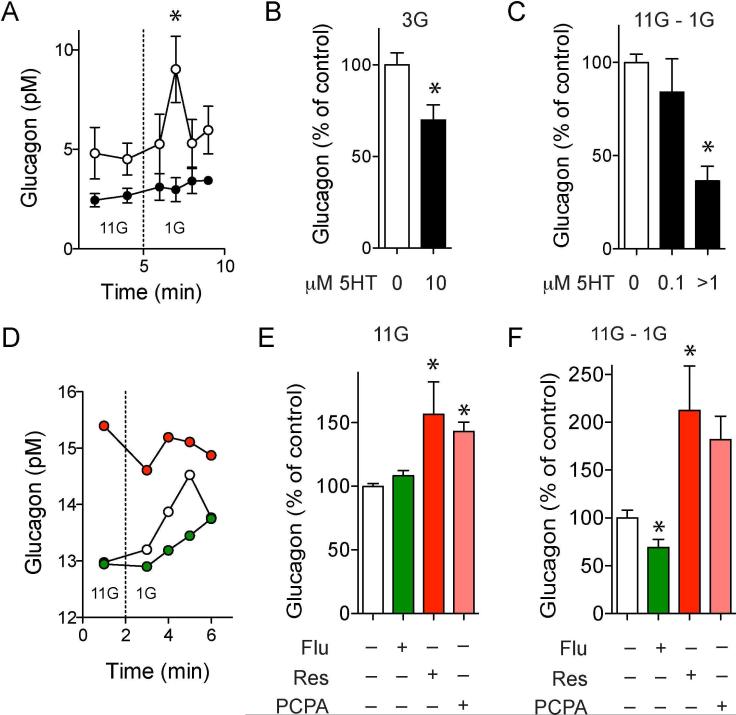
To examine the effects of serotonin signaling in the islet, we first examined how application of serotonin affected hormone secretion. We found that exogenous serotonin decreased somatostatin secretion in a concentration-dependent manner (Figure S3), but had negligible effects on glucose-induced insulin secretion (Figure S3). Serotonin strongly inhibited both stimulated and basal glucagon secretion (Figures 3A, 3B and 3C), in line with studies showing that serotonin inhibits glucagon secretion in mouse, rat, and human islets (Bennet et al., 2015; Marco et al., 1977; Pontiroli et al., 1978). Serotonin also inhibited the release of acetylcholine (Figure S4), a neurotransmitter produced and secreted from alpha cells (Rodriguez-Diaz et al., 2011).
Figure 3. Endogenously released serotonin inhibits glucagon secretion from isolated human islets.
(A) Glucagon secretion stimulated by decreasing glucose from 11 mM to 1 mM is inhibited in the presence of serotonin (10 μM, black circles; n = 3 islet preparations). See also Figure S4 for the effect of serotonin on acetylcholine release from alpha cells.
(B) Effect of serotonin (10 μM) on glucagon levels at 3 mM glucose concentration (n = 3 islet preparations).
(C) Quantification of the total amount of glucagon secreted during the first 10 min after decreasing glucose (area under the curve, AUC) in the presence of different concentrations of exogenous serotonin (n = 3 islet preparations).
(D) Representative traces showing the effect of depleting serotonin with reserpine (red) or of increasing serotonin with fluvoxamine (green) on glucagon secretion. Note that reserpine increases glucagon levels at 11 mM glucose.
(E) Effect of reserpine, fluvoxamine and Tph1 inhibitor PCPA on basal glucagon levels (at 11 mM; n = 6 islet preparations).
(F) Quantification of the total amount of glucagon secreted during the first 10 min after decreasing glucose (AUC) in the presence of reserpine, fluvoxamine or PCPA. Dashed lines indicate the time at which glucose concentration was switched.
Responses are presented as percentage of the respective glucagon response of control columns (100%). For comparisons in B, C, E and F we used one-sample t tests to compare the actual mean to a theoretical mean of 100% (control; * p value < 0.05).
Paracrine serotonin inhibits glucagon secretion from human islets
To examine the role of serotonin as a paracrine signal we manipulated its endogenous levels with PCPA, reserpine, and fluvoxamine (Figures 1H, 2H and 2J). Depleting serotonin from human islets with reserpine increased glucagon levels even in 11 mM glucose, a concentration at which glucagon secretion is minimal (Figures 3D and 3E). In the absence of endogenous serotonin, glucagon secretion stimulated by lowering glucose concentration from 11 mM to 1 mM increased two-fold (Figure 3F). To confirm that the stimulatory effect of reserpine on glucagon secretion was caused by depletion of serotonin, and not of that of other monoamine transmitters (Schafer et al., 2013), we blocked serotonin production with PCPA, the specific inhibitor of Tph1. Similar to reserpine, PCPA treatment increased glucagon levels at high glucose and enhanced glucagon secretion induced by decreasing glucose from 11 mM to 1 mM (Figures 3E and 3F). On the other hand, increasing endogenous serotonin extracellular levels with fluvoxamine inhibited glucagon secretion stimulated by a drop in glucose concentration (Figure 3F).
Expression of serotonin receptors in human islets
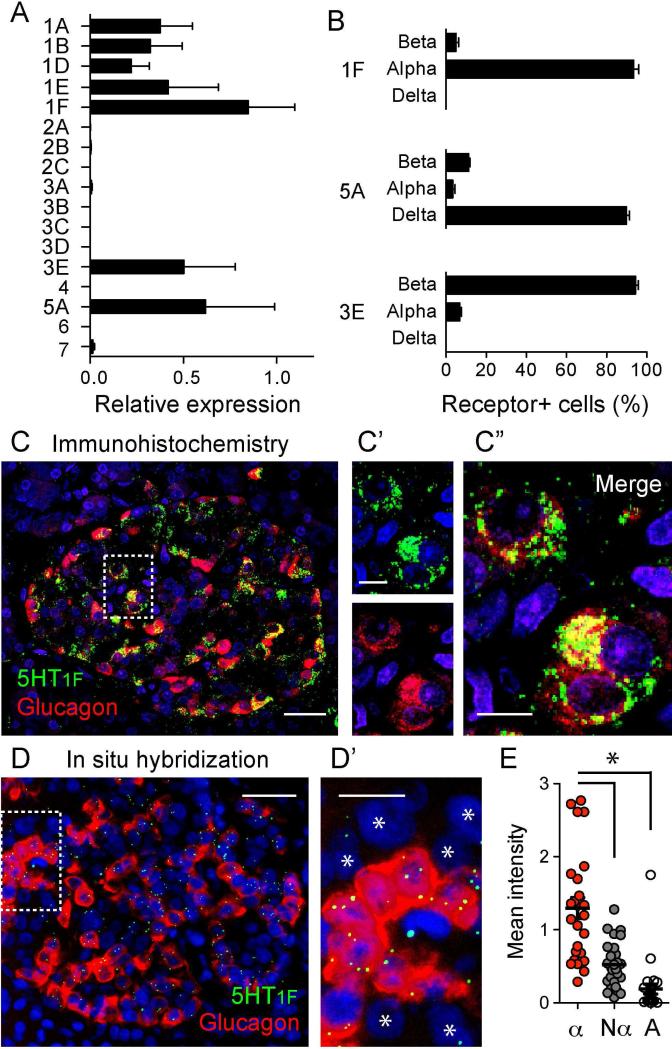
We assayed the expression of 17 different serotonin receptor genes (Oh et al., 2016) in isolated islets by quantitative real-time RT-PCR. Genes encoding members of the Gαi-linked receptor 5-HT1 family, as well as 5-HT5A and 5-HT3E were expressed in human islets (Figure 4A). 5-HT2B and 5-HT3A receptor genes were expressed at lower levels [Figure S5; for 5-HT2B receptors in human and mouse islet beta cells also see (Bennet et al., 2016; Blodgett et al., 2015)]. To localize the receptors expressed at higher levels (5-HT1 family members, 5-HT5A, and 5-HT3E) to particular cell types within the islet we performed immunostaining on human pancreatic sections. Of the different 5-HT3 receptor subunits we detected 5-HT3E subunit immunoreactivity in beta cells (Figures 4B and S5), suggesting that 5-HT3 receptors may be present as heteropentamers in human beta cells (Lummis, 2012).
Figure 4. Alpha cells express 5-HT1F serotonin receptors.
(A) Total RNA was extracted from ~ 400 islets from four different individuals and the expression of 17 serotonin receptor genes was determined by RT-qPCR. The data are normalized to the expression of Rn18s. Each gene was assessed in duplicate. See also Figure S5 for expression of other 5HT receptors.
(B) Quantification of the percentage of 5-HT1F, 5-HT5A, and 5-HT3E receptor-expressing cells that are beta, alpha or delta cells. To determine which cell type expresses the different serotonin receptors, we performed double immunostaining of human pancreatic sections.
(C) Representative confocal image of a human pancreatic section showing an islet immunostained for the serotonin receptor 5-HT1F (green) and glucagon (red). C’ and C” show zoomed images of the region delimited in C (dashed box). Scale bars represent 20 μm (C) and 10 μm (C’ and C”). See also Figure S6 for changes in 5-HT1F expression in type 2 diabetic pancreases.
(D) Frozen human pancreatic sections from 3 donors were analyzed by in situ mRNA hybridization. Shown is a representative epifluorescence image of a human islet probed for the mRNAs encoding 5-HT1F receptor (green) and glucagon (red)
(D’) Zoomed image of the islet region delimited in D (dashed box). Other islet cells (non-alpha cells) are indicated with an asterisk. Scale bars represent 20 μm (D) and 10 μm (D’).
(E) Quantification of the mean fluorescence intensity of 5-HT1F hybridization signal in regions of interest corresponding to alpha cells (glucagon positive, α), non-alpha cells (islet cells negative for glucagon, Nα) and acinar cells (A). Each dot represents the average of 5-HT1F signal for the different regions of interest of each population per islet. *p value < 0.05 (one-way analysis of variance (ANOVA) followed by a Tukey's Multiple Comparison Test, n > 10 islets, 3 donors). These data show that 5-HT1F is mostly expressed in alpha cells.
Most of the immunoreactivity for 5-HT5A receptor (90%) was detected in somatostatin-labeled delta cells (Figures 4B and S5). This receptor couples to decreases in cAMP, which helps explain the inhibitory effects of serotonin on somatostatin secretion (Figure S3). If serotonin inhibits delta cell activity, as we show here, the diminished somatostatin secretion should increase alpha cell activity (Kailey et al., 2012; Schuit et al., 1989). Thus, it is unlikely that the inhibitory effects of serotonin on alpha cells are mediated indirectly via delta cells.
Several members of the 5-HT1 receptor family were detected in blood vessels (Figure S5), but most 5-HT1F immunostaining (>90%) was localized to glucagon-immunoreactive alpha cells (Figures 4B and 4C). About 70% of alpha cells were immunostained for 5-HT1F. Strikingly, the percentage of 5-HT1F immunostained alpha cells decreased to 20% in pancreases from individuals with type 2 diabetes for > 4 years (Figure S6).
To confirm that 5-HT1F is expressed in alpha cells, we performed in situ RNA hybridization using RNAscope technology (Advanced Cell Diagnostics). Using probes against Htr1f and Glucagon we detected fluorescent signals resulting from hybridization of 5-HT1F mRNA molecules within the islet, in particular in glucagon-positive alpha cells (Figures 4D and 4E). Each 5-HT1F mRNA molecule hybridized to a probe appeared as an individual fluorescent dot (Wang et al., 2012). The labeling was eliminated by RNAase treatment, demonstrating the specificity of this signal. The small number of 5-HT1F puncta is expected for the mRNA of a G-protein coupled receptor, which are typically expressed at few copies per cell (Pronin et al., 2014). By contrast, due to the high abundance of glucagon mRNA molecules in alpha cells the hybridization signals merged to occupy the entire cytoplasm. The in situ hybridization results unambiguously confirm that the 5-HT1F receptor is expressed in alpha cells.
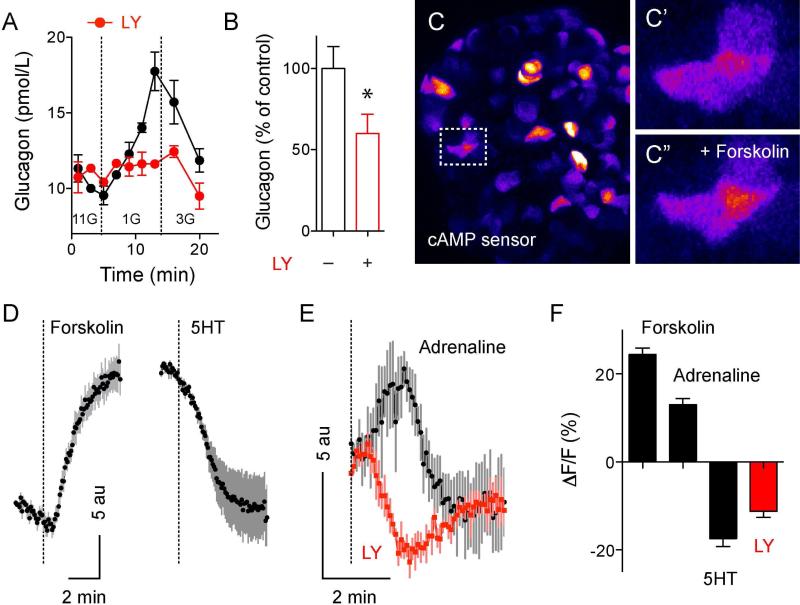
Alpha cells express functional 5-HT1F serotonin receptors
Similarly to serotonin, application of the specific 5-HT1F receptor agonist LY344864 [100 nM; (Phebus et al., 1997)] reduced glucagon secretion from human islets in vitro (Figures 5A and 5B). Because activation of the 5-HT1F receptor is coupled to inhibition of adenylate cyclase (Adham et al., 1993) we hypothesized that serotonin inhibits glucagon secretion by decreasing intracellular cAMP levels in alpha cells. To monitor changes in intracellular cAMP in real time, we infected human islets with a modified baculovirus (BacMam vector) containing a fluorescent cAMP sensor (Montana Molecular; Figure 5C). Raising cAMP levels with forskolin (10 μM) and IBMX (100 μM) increased fluorescence intensity in 70% of the infected cells (Figures 5C, 5D and 5F). By contrast, serotonin (10 μM) or the 5-HT1F receptor agonist LY344864 (100 nM) decreased the fluorescence signal, indicating that serotonin receptor activation reduced intracellular cAMP levels (Figures 5D, 5E and 5F).
Figure 5. Serotonin inhibits glucagon secretion via 5-HT1F receptors and decrease in intracellular cAMP.
(A) Glucagon secretion from human islets stimulated by decreasing glucose concentration from 11 mM to 1 mM is inhibited in the presence of the 5-HT1F receptor agonist LY344864 (LY; 100 nM, red circles).
(B) Quantification of the total amount of glucagon secreted during the first 10 min after decreasing glucose in the presence of LY344864 (n = 6 experiments from 3 islet preparations).
(C) Maximal projection of confocal images of a human islet infected with cAMP green fluorescent sensor (lookup table color scheme was applied). 48h after infection, islet cells show different levels of expression of the cAMP sensor. C’ and C” show zoomed images of the islet region delimited in C (dashed box), before (C’) and 5 min after stimulation with forskolin (10 μM) and IBMX (100 μM) (C”). Fluorescence increases when cAMP levels increase.
(D) Representative tracings of mean fluorescence changes in cells labeled with the cAMP sensor induced by forskolin+IBMX (left) or by 5HT (10 μM; right). Black dots reflect the average response and gray lines the SEM for each time point (n = 10 cells). Dashed lines denote drug applications.
(E) In cells that exhibited adrenaline-induced (10 μM) increase in cAMP, the 5-HT1F receptor agonist LY344864 (LY; 100 nM) induced a decrease in cAMP (n = 5 cells).
(F) Summary of fluorescence changes normalized to baseline fluorescence (%) induced by forskolin+IBMX, adrenaline, 5HT and LY344864 (n = 8-12 cells, 3 coverslips each stimulus).
To identify alpha cells, we took advantage that alpha and beta cells respond differentially to adrenaline (Schuit and Pipeleers, 1986). Adrenaline is a known activator of glucagon secretion in several species (Gromada et al., 1997; Tian et al., 2011; Vieira et al., 2004). A recent study showed that human beta cells express the cAMP-lowering alpha2 adrenergic receptor and that human alpha cells express the cAMP-raising beta2 adrenergic receptor (Blodgett et al., 2015). We observed that in cells in which adrenaline (10 μM) stimulated increases in cAMP levels, the 5-HT1F receptor agonist LY344864 had the opposite effect, that is, it decreased cAMP (Figure 5E). These results indicate that activation of 5-HT1F receptors inhibited glucagon secretion by reducing intracellular cAMP levels in alpha cells.
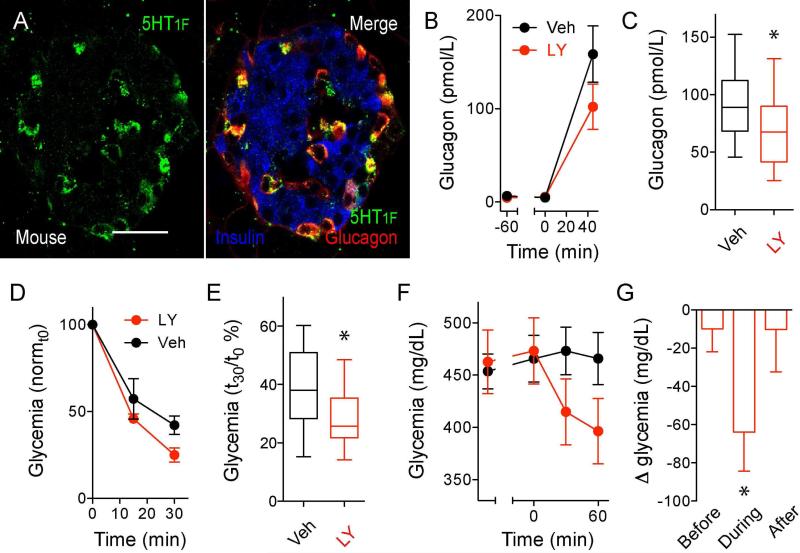
5-HT1F receptors on alpha cells contribute to in vivo glucose homeostasis
To determine the in vivo relevance of serotonin inhibition of glucagon secretion, we performed experiments in mice. 5-HT1F receptor immunostaining was present in mouse alpha cells (Figure 6A). To investigate the role of these receptors, we first stimulated glucagon secretion in C57BL6 mice by inducing hypoglycemia with insulin (1 U/kg body weight). Under these conditions, the 5-HT1F receptor agonist LY344864 [i.v. 1 mg/kg body weight (Phebus et al., 1997)] reduced plasma glucagon levels and exacerbated hypoglycemia (Figures 6B to 6E). In another experiment, in C57Bl/6 mice rendered diabetic with streptozotocin (453 ± 17 mg/dL non-fasting glycemia), a subsequent single injection of LY344864 acutely reduced glycemic levels by ~ 65 mg/dL (Figures 6F and 6G). Similar results were obtained with an outbred mouse strain (127 ± 47 mg/dL reduction in glycemia). These results indicate that in vivo activation of the 5-HT1F receptor controls glucagon secretion and affects glucose homeostasis.
Figure 6. In vivo inhibition of glucagon secretion with a 5-HT1F receptor agonist.
(A) Confocal image of a mouse pancreatic section showing an islet immunostained for the serotonin receptor 5-HT1F (green), glucagon (red), and insulin (blue). 5-HT1F is expressed in alpha cells in mouse islets. Scale bar represent 20 μm.
(B) Increases in glucagon plasma levels stimulated by decreasing glycemia with insulin (1 U/kg) are inhibited in the in the presence of LY344864 (1 mg/kg, i.v.; n = 5 mice per group).
(C) Quantification of data as in B, showing plasma glucagon levels at 45 min after insulin injection (n = 3 experiments with 5 mice per group each experiment). Responses are shown as percentage of the respective glucagon response of vehicle-treated mice. One-sample t test was used to compare the actual mean to a theoretical mean of 100% (vehicle; *p value < 0.05).
(D) Insulin-induced hypoglycemia is exacerbated in the presence of LY344864 (n = 12 mice per group).
(E) Quantification of data as in D, showing glycemia at 30 min after insulin injection (n = 12 mice per group, * p value < 0.05 unpaired Student's t-test).
(F) Changes in glycemia [relative to vehicle-treated mice (control group)] induced by injection of LY344864 (1 mg/kg, i.v.) in mice rendered diabetic with streptozotocin (200 mg/kg, i.v.). LY344864 acutely reduced hyperglycemia in diabetic mice (red symbols; n = 9 mice per group. See also Figure S7 for the effect of LY344864 in diabetic nude mice.
(G) Differences in glycemia (Δ glycemia) between vehicle- or drug-treated diabetic mice before, during the first 1 hour after LY344864 administration, and after (n = 9 mice per group, * p value < 0.05 one-sample t test comparing the actual mean with a theoretical mean of 0).
DISCUSSION
The results presented here firmly establish that serotonin is produced and released by beta cells in islets from non-diabetic, non-pregnant individuals. We show that human alpha cells lose the ability to respond appropriately to changes in glucose when islet serotonin levels are manipulated pharmacologically. In our model, glucose-dependent release of serotonin from the beta cell shapes the secretory profile of the alpha cell and hence helps orchestrate hormone secretion from the human islet.
Our results on human islets contrast with serotonin production in mouse islets, where it occurs during pregnancy and requires placental lactogens and prolactin receptors for the upregulation of Tph1 (Goyvaerts et al., 2015; Kim et al., 2010; Schraenen et al., 2010). Interestingly, > 90% of human beta cells expressed Tph1 and AADC, but only > 20% contained serotonin. Given that most beta cells seem serotonin competent, why do only some beta cells produce serotonin? The availability of the serotonin precursor tryptophan may be limiting, and indeed dietary tryptophan restriction reduces serotonin synthesis in beta cells (Kim et al., 2010). Intracellular tryptophan levels further depend on its degradation by the inducible enzyme indoleamine 2,3-dioxygenase (IDO), which is expressed in a subpopulation of beta cells in the human islet (Sarkar et al., 2007). Whether IDO activity determines serotonin content in beta cells by regulating the availability of tryptophan could be addressed in future studies, for example by inhibiting IDO pharmacologically.
Serotonin has been investigated extensively as an autocrine signal regulating beta cell function and proliferation (Bennet et al., 2016; Goyvaerts et al., 2016; Kim et al., 2010; Kim et al., 2015; Ohara-Imaizumi et al., 2013). By contrast, the effects of serotonin on hormone secretion from other islet endocrine cells have not been systematically investigated. While previous studies reported that exogenous application of serotonin decreases glucagon secretion (Bennet et al., 2015; Marco et al., 1977; Pontiroli et al., 1978), they did not explore the role beta cell-derived serotonin plays in regulating islet hormone secretion. This distinction is crucial for defining serotonin as a bona fide paracrine signal in the islet. By manipulating endogenous serotonin production, release, and uptake in human islets we demonstrate here that local serotonin is a strong paracrine regulator of alpha cell activity.
Serotonin can thus be added to the list of paracrine signals that beta and delta cells secrete to inhibit glucagon secretion when glucose concentration increases [e.g. insulin, Zn2+, GABA and its metabolite gamma-hydroxybutyrate (GHB), ATP, glutamate, somatostatin; reviewed in (Briant et al., 2016; Gromada et al., 2007; Gylfe, 2016)]. The multitude of signals that mediate paracrine inhibition of alpha cells during hyperglycemia suggest that this is an important physiological mechanism that requires redundant signaling pathways. The effects of serotonin on alpha cells, however, differ from those elicited by other beta cell-derived paracrine signals. As we show here, exposure of alpha cells to serotonin induces a rapid and direct decrease in the levels of cAMP, a second messenger important for glucagon secretion (Le Marchand and Piston, 2012; Leclerc et al., 2011; Li et al., 2015). Indeed, low cAMP levels are required for shutting down glucagon secretion in response to rising glucose (Elliott et al., 2015). Other signals use mechanisms that either work on a slower time scale [insulin-dependent translocation of GABAA receptors to the plasma membrane (Xu et al., 2006)], are not glucose-dependent [GABA; (Pizarro-Delgado et al., 2010; Smismans et al., 1997)], or stimulate human alpha cells [ATP (Jacques-Silva et al., 2010); glutamate (Cabrera et al., 2008); Zn2+ (Walker et al., 2011)]. The mechanisms underlying GHB-dependent inhibition of glucagon secretion are still unclear (Li et al., 2013)]. Together, these signals cooperate to inhibit different aspects of alpha cell function, but under conditions in which glucose levels increase serotonin may have the most immediate effects on glucagon secretion.
A salient feature of islet serotonin is that it is required to maintain normoglycemia in insulin resistant states (El-Merahbi et al., 2015; Goyvaerts et al., 2016; Kim et al., 2010; Kim et al., 2015; Ohara-Imaizumi et al., 2013). Our results show that serotonin synthesis is dramatically increased in beta cells of obese individuals, a population with a higher incidence of insulin resistance. In these individuals, enhanced serotonin signaling may allow the islet to cope with higher demand for insulin, presumably by increasing beta cell mass and beta cell responsiveness to glucose (El-Merahbi et al., 2015; Goyvaerts et al., 2016; Kim et al., 2010; Kim et al., 2015; Ohara-Imaizumi et al., 2013). According to our findings, the increased serotonin levels should also reduce secretion of glucagon, a hormone with strong hyperglycemic properties. We therefore propose that increased serotonin input to alpha cells is an additional mechanism that helps maintain glucose homeostasis.
We identified 5-HT1F as the receptor mediating the serotonin effects in alpha cells, in line with recent findings showing mRNA expression in human alpha cells (Blodgett et al., 2015). Upon ligand binding, this receptor signals via Gi to inhibit adenylate cyclase, thus reducing intracellular cAMP levels (Adham et al., 1993). In our hands, in vitro activation of this receptor diminished cAMP levels in alpha cells and inhibited glucagon secretion. We found that the 5-HT1F receptor agonist also reduced glucagon secretion in vivo and had hypoglycemic effects in diabetic mice. Although this agonist may affect serotonin signaling in other tissues, its rapid effects on glucagon secretion suggest that it acts on alpha cell 5-HT1F receptors to modulate glycemia. Our results show a strong reduction in the proportion of 5-HT1F positive alpha cells in individuals with longstanding type 2 diabetes [Figure S6; see also (Bennet et al., 2015)], which suggests that suppression of glucagon secretion by serotonin may be impaired in diabetes.
Because of comorbidity, diabetic patients are frequently treated with selective serotonin reuptake inhibitor (SSRI) antidepressants. While the hypoglycemic effects of SSRIs have been proposed to be beneficial for diabetic patients (Daubresse et al., 1996), SSRIs may also render diabetic patients susceptible to life-threatening hypoglycemia. Indeed, patients on long-term SSRI treatment have poor counter-regulatory responses (Biagetti and Corcoy, 2013; Deeg and Lipkin, 1996; Derijks et al., 2008; Sawka et al., 2000, 2001; Takhar and Williamson, 1999). In our hands, SSRIs increased paracrine serotonin levels within the islet and inhibited glucagon secretion from alpha cells. Whether or not SSRIs suppress glucagon secretion using this mechanism in vivo could be tested by measuring plasma glucagon levels after treatment with SSRIs that only act in the periphery. That could be achieved, for instance, by administering SSRIs together with modulators of the activity of the drug efflux pump P-glycoprotein to prevent them from crossing the blood-brain barrier (O'Brien et al., 2012). In conclusion, serotonergic modulation of alpha cells represents both a therapeutic opportunity and a pharmacological challenge.
EXPERIMENTAL PROCEDURES
We obtained human pancreatic islets from the Integrated Islet Distribution Program (IIDP; Table S1) and human pancreatic tissue samples (from the head of the pancreas) from the Human Islet Cell Processing Facility at the Diabetes Research Institute, University of Miami.
Biosensor cells
Real time measurements of serotonin and acetylcholine secretion were performed using fura-2 AM-loaded Chinese hamster ovary (CHO) cells expressing 5-HT2C receptors and muscarinic M3 receptors, respectively (Huang et al., 2005; Rodriguez-Diaz et al., 2012).
Insulin and glucagon secretion
We measured hormone secretion from isolated human islets with an automated perifusion system. Responses to KCl (25 mM) served as internal reference.
Immunohistochemistry
Human and mouse pancreatic tissues were fixed overnight in 4% PFA, cryoprotected in sucrose and frozen. Sections were incubated with primary antibodies for 24 h in PBS-Triton X-100 (0.3%) with blocking solution. Immunostaining was visualized by using Alexa Fluor conjugated secondary antibodies and cell nuclei stained with DAPI. Slides were mounted with Vectashield mounting medium and imaged on a confocal microscope.
cAMP measurements
Human islets were infected with a baculovirus encoding a cAMP sensor (cADDis; Montana Molecular) for 24h. Islets were imaged 48-72h later on a confocal microscope.
Quantitative real-time RT-PCR
RNA from 400 human islets from 4 donors was extracted and used for cDNA synthesis. Quantitative real-time PCR was performed using the TaqMan system to determine expression of serotonin receptor genes. 18S ribosomal served as reference gene.
In situ RNA hybridization
In situ RNA hybridization of serotonin receptor 5-HT1F and glucagon was performed using RNAscope technology (Advanced Cell Diagnostics) on 10 μm sections of PFA-fixed frozen human pancreatic tissue from 3 donors.
In vivo experiments
C57BL6 mice (male, 8 weeks old, body weight 17 – 23 g) were used. 5-HT1F specific agonist LY344864 was administered at 1 mg/kg with an intravenous injection (Phebus et al., 1997).
Supplementary Material
HIGHLIGHTS.
Human beta cells release serotonin to regulate glucagon secretion
Serotonin lowers cAMP in alpha cells via 5-HT1F receptors
5-HT1F receptor activation reduces hyperglycemia in diabetic mice
Serotonin is a bona fide paracrine signal in the human islet.
ACKNOWLEDGEMENTS
We thank Juan Ricardo Ortiz Meneses and Maikel Rivero for help with immunohistochemistry, Kristin Perez and Simón Caicedo for data analyses, and Kevin Johnson for histological work. This work was funded by NIH grants R56DK084321 (A.C.), R01DK084321 (A.C.), R21ES025673 (A.C.), R01DK1054127 (V.S.), the Diabetes Research Institute Foundation (DRIF); the Juvenile Diabetes Research Foundation (P.O.B.); the Swedish Research Council; the Novo Nordisk Foundation; the Swedish Diabetes Association; the Family Erling-Persson Foundation; the Skandia Insurance Company Ltd; Strategic Research Program in Diabetes at Karolinska Institutet; the Berth von Kantzow's Foundation; VIBRANT Grant FP7-2288933; the Knut and Alice Wallenberg Foundation and Lee Kong Chien School of Medicine, Nanyang Technical University, Singapore and Imperial College, London, UK ERC-2013-AdG 338936-BetaImage. J.A. is a recipient of a postdoctoral fellowship from the American Heart Association (14POST20380499).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
J.A., J.M., and D.M. performed experiments with biosensor cells, hormone assays and ELISAs; J.A. performed immunohistochemistry, RT-PCR and cAMP measurements. A.N.P and V.S. performed in situ hybridization. J.A., A.T. and A.C. conducted in vivo studies. J.A., P.O.B. and A.C. designed the study, analyzed data, and wrote the paper. All authors discussed the results and commented on the manuscript.
REFERENCES
- Adham N, et al. Cloning of another human serotonin receptor (5-HT1F): a fifth 5-HT1 receptor subtype coupled to the inhibition of adenylate cyclase. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:408–412. doi: 10.1073/pnas.90.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almaca J, et al. Spatial and temporal coordination of insulin granule exocytosis in intact human pancreatic islets. Diabetologia. 2015 doi: 10.1007/s00125-015-3747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anlauf M, et al. Expression of the two isoforms of the vesicular monoamine transporter (VMAT1 and VMAT2) in the endocrine pancreas and pancreatic endocrine tumors. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2003;51:1027–1040. doi: 10.1177/002215540305100806. [DOI] [PubMed] [Google Scholar]
- Barbosa RM, et al. Control of pulsatile 5-HT/insulin secretion from single mouse pancreatic islets by intracellular calcium dynamics. The Journal of physiology. 1998;510(Pt 1):135–143. doi: 10.1111/j.1469-7793.1998.135bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet H, et al. Altered serotonin (5-HT) 1D and 2A receptor expression may contribute to defective insulin and glucagon secretion in human type 2 diabetes. Peptides. 2015;71:113–120. doi: 10.1016/j.peptides.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Bennet H, et al. Serotonin (5-HT) receptor 2b activation augments glucose-stimulated insulin secretion in human and mouse islets of Langerhans. Diabetologia. 2016;59:744–754. doi: 10.1007/s00125-015-3847-6. [DOI] [PubMed] [Google Scholar]
- Biagetti B, Corcoy R. Hypoglycemia associated with fluoxetine treatment in a patient with type 1 diabetes. World journal of clinical cases. 2013;1:169–171. doi: 10.12998/wjcc.v1.i5.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blodgett DM, et al. Novel Observations From Next-Generation RNA Sequencing of Highly Purified Human Adult and Fetal Islet Cell Subsets. Diabetes. 2015;64:3172–3181. doi: 10.2337/db15-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briant L, et al. Glucagon secretion from pancreatic alpha-cells. Upsala journal of medical sciences. 2016;121:113–119. doi: 10.3109/03009734.2016.1156789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera O, et al. Glutamate is a positive autocrine signal for glucagon release. Cell metabolism. 2008;7:545–554. doi: 10.1016/j.cmet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen V, et al. Fluvoxamine, a specific 5-hydroxytryptamine uptake inhibitor. British journal of pharmacology. 1977;60:505–516. doi: 10.1111/j.1476-5381.1977.tb07528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubresse JC, et al. Usefulness of fluoxetine in obese non-insulin-dependent diabetics: a multicenter study. Obesity research. 1996;4:391–396. doi: 10.1002/j.1550-8528.1996.tb00247.x. [DOI] [PubMed] [Google Scholar]
- Deeg MA, Lipkin EW. Hypoglycemia associated with the use of fluoxetine. The Western journal of medicine. 1996;164:262–263. [PMC free article] [PubMed] [Google Scholar]
- Derijks HJ, et al. The association between antidepressant use and hypoglycaemia in diabetic patients: a nested case-control study. Pharmacoepidemiology and drug safety. 2008;17:336–344. doi: 10.1002/pds.1562. [DOI] [PubMed] [Google Scholar]
- Dolensek J, et al. Structural similarities and differences between the human and the mouse pancreas. Islets. 2015;7:e1024405. doi: 10.1080/19382014.2015.1024405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan MH, Tecott LH. Serotonin and the regulation of mammalian energy balance. Frontiers in neuroscience. 2013;7:36. doi: 10.3389/fnins.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm R, et al. Monoamines in the pancreatic islets of the mouse. Subcellular localization of 5-hydroxytryptamine by electron microscopic autoradiography. Diabetologia. 1971;7:339–348. doi: 10.1007/BF01219468. [DOI] [PubMed] [Google Scholar]
- El-Merahbi R, et al. The roles of peripheral serotonin in metabolic homeostasis. FEBS letters. 2015;589:1728–1734. doi: 10.1016/j.febslet.2015.05.054. [DOI] [PubMed] [Google Scholar]
- Elliott AD, et al. Somatostatin and insulin mediate glucose-inhibited glucagon secretion in the pancreatic alpha-cell by lowering cAMP. American journal of physiology Endocrinology and metabolism. 2015;308:E130–143. doi: 10.1152/ajpendo.00344.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeby M, et al. Vesicular monoamine transporter, type 2 (VMAT2) expression as it compares to insulin and pancreatic polypeptide in the head, body and tail of the human pancreas. Islets. 2012;4:393–397. doi: 10.4161/isl.22995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyvaerts L, et al. Prolactin receptors and placental lactogen drive male mouse pancreatic islets to pregnancy-related mRNA changes. PloS one. 2015;10:e0121868. doi: 10.1371/journal.pone.0121868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyvaerts L, et al. Serotonin competence of mouse beta cells during pregnancy. Diabetologia. 2016;59:1356–1363. doi: 10.1007/s00125-016-3951-2. [DOI] [PubMed] [Google Scholar]
- Gromada J, et al. Adrenaline stimulates glucagon secretion in pancreatic A-cells by increasing the Ca2+ current and the number of granules close to the L-type Ca2+ channels. The Journal of general physiology. 1997;110:217–228. doi: 10.1085/jgp.110.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J, et al. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocrine reviews. 2007;28:84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- Gylfe E. Association between 5-hydroxytryptamine release and insulin secretion. The Journal of endocrinology. 1978;78:239–248. doi: 10.1677/joe.0.0780239. [DOI] [PubMed] [Google Scholar]
- Gylfe E. Glucose control of glucagon secretion-'There's a brand-new gimmick every year'. Upsala journal of medical sciences. 2016;121:120–132. doi: 10.3109/03009734.2016.1154905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B, et al. Transport and storage of 5-hydroxytryptamine in pancreatic - cells. Biochemical pharmacology. 1972;21:695–706. doi: 10.1016/0006-2952(72)90062-7. [DOI] [PubMed] [Google Scholar]
- Huang YJ, et al. Mouse taste buds release serotonin in response to taste stimuli. Chemical senses. 2005;30(Suppl 1):i39–40. doi: 10.1093/chemse/bjh102. [DOI] [PubMed] [Google Scholar]
- Jacques-Silva MC, et al. ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic beta cell. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6465–6470. doi: 10.1073/pnas.0908935107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaim-Etcheverry G, Zieher LM. Electron microscopic cytochemistry of 5-hydroxytryptamine (5-HT) in the beta cells of guinea pig endocrine pancreas. Endocrinology. 1968;83:917–923. doi: 10.1210/endo-83-5-917. [DOI] [PubMed] [Google Scholar]
- Kailey B, et al. SSTR2 is the functionally dominant somatostatin receptor in human pancreatic beta- and alpha-cells. American journal of physiology Endocrinology and metabolism. 2012;303:E1107–1116. doi: 10.1152/ajpendo.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, et al. Islet architecture: A comparative study. Islets. 2009;1:129–136. doi: 10.4161/isl.1.2.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nature medicine. 2010;16:804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, et al. Functional role of serotonin in insulin secretion in a diet-induced insulin-resistant state. Endocrinology. 2015;156:444–452. doi: 10.1210/en.2014-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Marchand SJ, Piston DW. Glucose decouples intracellular Ca2+ activity from glucagon secretion in mouse pancreatic islet alpha-cells. PloS one. 2012;7:e47084. doi: 10.1371/journal.pone.0047084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc I, et al. AMP-activated protein kinase regulates glucagon secretion from mouse pancreatic alpha cells. Diabetologia. 2011;54:125–134. doi: 10.1007/s00125-010-1929-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, et al. Regulation of glucagon secretion in normal and diabetic human islets by gamma-hydroxybutyrate and glycine. The Journal of biological chemistry. 2013;288:3938–3951. doi: 10.1074/jbc.M112.385682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, et al. Submembrane ATP and Ca2+ kinetics in alpha-cells: unexpected signaling for glucagon secretion. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29:3379–3388. doi: 10.1096/fj.14-265918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lummis SC. 5-HT(3) receptors. The Journal of biological chemistry. 2012;287:40239–40245. doi: 10.1074/jbc.R112.406496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist I, et al. Monoamines in the pancreatic islets of the mouse. 5-hydroxytryptamine as an intracellular modifier of insulin secretion, and the hypoglycaemic action of monoamine oxidase inhibitors. Diabetologia. 1971;7:414–422. doi: 10.1007/BF01212056. [DOI] [PubMed] [Google Scholar]
- Marco J, et al. Inhibition of glucagon release by serotonin in mouse pancreatic islets. Diabetologia. 1977;13:585–588. doi: 10.1007/BF01236311. [DOI] [PubMed] [Google Scholar]
- O'Brien FE, et al. Interactions between antidepressants and P-glycoprotein at the blood-brain barrier: clinical significance of in vitro and in vivo findings. British journal of pharmacology. 2012;165:289–312. doi: 10.1111/j.1476-5381.2011.01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh CM, et al. Serotonin as a New Therapeutic Target for Diabetes Mellitus and Obesity. Diabetes & metabolism journal. 2016;40:89–98. doi: 10.4093/dmj.2016.40.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara-Imaizumi M, et al. Serotonin regulates glucose-stimulated insulin secretion from pancreatic beta cells during pregnancy. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19420–19425. doi: 10.1073/pnas.1310953110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y, et al. Convergence of the insulin and serotonin programs in the pancreatic beta-cell. Diabetes. 2011;60:3208–3216. doi: 10.2337/db10-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmann N, et al. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS biology. 2009;7:e1000229. doi: 10.1371/journal.pbio.1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phebus LA, et al. Characterization of LY344864 as a pharmacological tool to study 5-HT1F receptors: binding affinities, brain penetration and activity in the neurogenic dural inflammation model of migraine. Life sciences. 1997;61:2117–2126. doi: 10.1016/s0024-3205(97)00885-0. [DOI] [PubMed] [Google Scholar]
- Pizarro-Delgado J, et al. Glucose promotion of GABA metabolism contributes to the stimulation of insulin secretion in beta-cells. The Biochemical journal. 2010;431:381–389. doi: 10.1042/BJ20100714. [DOI] [PubMed] [Google Scholar]
- Pontiroli AE, et al. Effects of serotonin, of its biosynthetic precursors and of the anti-serotonin agent metergoline on the release of glucagon and insulin from rat pancreas. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1978;10:200–203. doi: 10.1055/s-0028-1093434. [DOI] [PubMed] [Google Scholar]
- Pronin A, et al. Expression of olfactory signaling genes in the eye. PloS one. 2014;9:e96435. doi: 10.1371/journal.pone.0096435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JE, et al. Calcium- and barium-dependent exocytosis from the rat insulinoma cell line RINm5F assayed using membrane capacitance measurements and serotonin release. Pflugers Archiv : European journal of physiology. 1996;432:258–269. doi: 10.1007/s004240050132. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, et al. Real-time detection of acetylcholine release from the human endocrine pancreas. Nature protocols. 2012;7:1015–1023. doi: 10.1038/nprot.2012.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, et al. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nature medicine. 2011;17:888–892. doi: 10.1038/nm.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario LM, et al. Regulation by glucose of oscillatory electrical activity and 5-HT/insulin release from single mouse pancreatic islets in absence of functional K(ATP) channels. Endocrine journal. 2008;55:639–650. doi: 10.1507/endocrj.k07e-131. [DOI] [PubMed] [Google Scholar]
- Sarkar SA, et al. Induction of indoleamine 2,3-dioxygenase by interferon-gamma in human islets. Diabetes. 2007;56:72–79. doi: 10.2337/db06-0617. [DOI] [PubMed] [Google Scholar]
- Satin LS, et al. Pulsatile insulin secretion, impaired glucose tolerance and type 2 diabetes. Molecular aspects of medicine. 2015;42:61–77. doi: 10.1016/j.mam.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawka AM, et al. Loss of hypoglycemia awareness in an adolescent with type 1 diabetes mellitus during treatment with fluoxetine hydrochloride. The Journal of pediatrics. 2000;136:394–396. doi: 10.1067/mpd.2000.103851. [DOI] [PubMed] [Google Scholar]
- Sawka AM, et al. Loss of awareness of hypoglycemia temporally associated with selective serotonin reuptake inhibitors. Diabetes care. 2001;24:1845–1846. doi: 10.2337/diacare.24.10.1845. [DOI] [PubMed] [Google Scholar]
- Schafer MK, et al. Species-specific vesicular monoamine transporter 2 (VMAT2) expression in mammalian pancreatic beta cells: implications for optimising radioligand-based human beta cell mass (BCM) imaging in animal models. Diabetologia. 2013;56:1047–1056. doi: 10.1007/s00125-013-2847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraenen A, et al. Placental lactogens induce serotonin biosynthesis in a subset of mouse beta cells during pregnancy. Diabetologia. 2010;53:2589–2599. doi: 10.1007/s00125-010-1913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit FC, et al. Sensitivity of rat pancreatic A and B cells to somatostatin. Diabetologia. 1989;32:207–212. doi: 10.1007/BF00265096. [DOI] [PubMed] [Google Scholar]
- Schuit FC, Pipeleers DG. Differences in adrenergic recognition by pancreatic A and B cells. Science (New York, NY) 1986;232:875–877. doi: 10.1126/science.2871625. [DOI] [PubMed] [Google Scholar]
- Smismans A, et al. Nutrient regulation of gamma-aminobutyric acid release from islet beta cells. Diabetologia. 1997;40:1411–1415. doi: 10.1007/s001250050843. [DOI] [PubMed] [Google Scholar]
- Smith PA, et al. A fluorimetric and amperometric study of calcium and secretion in isolated mouse pancreatic beta-cells. Pflugers Archiv : European journal of physiology. 1995;430:808–818. doi: 10.1007/BF00386180. [DOI] [PubMed] [Google Scholar]
- Stewart AF, et al. Human beta-cell proliferation and intracellular signaling: part 3. Diabetes. 2015;64:1872–1885. doi: 10.2337/db14-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takhar J, Williamson P. Hypoglycemia associated with high doses of sertraline and sulphonylurea compound in a noninsulin-dependent diabetes mellitus patient. The Canadian journal of clinical pharmacology = Journal canadien de pharmacologie clinique. 1999;6:12–14. [PubMed] [Google Scholar]
- Tian G, et al. Glucose- and hormone-induced cAMP oscillations in alpha- and beta-cells within intact pancreatic islets. Diabetes. 2011;60:1535–1543. doi: 10.2337/db10-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira E, et al. Involvement of alpha1 and beta-adrenoceptors in adrenaline stimulation of the glucagon-secreting mouse alpha-cell. Naunyn-Schmiedeberg's archives of pharmacology. 2004;369:179–183. doi: 10.1007/s00210-003-0858-5. [DOI] [PubMed] [Google Scholar]
- Walker JN, et al. Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Diabetes, obesity & metabolism. 2011;13(Suppl 1):95–105. doi: 10.1111/j.1463-1326.2011.01450.x. [DOI] [PubMed] [Google Scholar]
- Walther DJ, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science (New York, NY) 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Wang F, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. The Journal of molecular diagnostics : JMD. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu E, et al. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell metabolism. 2006;3:47–58. doi: 10.1016/j.cmet.2005.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.