Abstract
The pioneering work by Patrick H. O’Farrell established two-dimensional gel electrophoresis as one of the most important high-resolution protein separation techniques of modern biochemistry (Journal of Biological Chemistry 1975, 250, 4007–4021). The application of two-dimensional gel electrophoresis has played a key role in the systematic identification and detailed characterization of the protein constituents of skeletal muscles. Protein changes during myogenesis, muscle maturation, fibre type specification, physiological muscle adaptations and natural muscle aging were studied in depth by the original O’Farrell method or slightly modified gel electrophoretic techniques. Over the last 40 years, the combined usage of isoelectric focusing in the first dimension and sodium dodecyl sulfate polyacrylamide slab gel electrophoresis in the second dimension has been successfully employed in several hundred published studies on gel-based skeletal muscle biochemistry. This review focuses on normal and physiologically challenged skeletal muscle tissues and outlines key findings from mass spectrometry-based muscle proteomics, which was instrumental in the identification of several thousand individual protein isoforms following gel electrophoretic separation. These muscle-associated protein species belong to the diverse group of regulatory and contractile proteins of the acto-myosin apparatus that forms the sarcomere, cytoskeletal proteins, metabolic enzymes and transporters, signaling proteins, ion-handling proteins, molecular chaperones and extracellular matrix proteins.
Keywords: difference in-gel electrophoresis, isoelectric focusing, mass spectrometry, muscle fiber type, muscle plasticity, muscle proteomics, muscular atrophy, polyacrylamide gel electrophoresis, protein separation, skeletal muscle
1. Introduction
Efficient protein separation is a prerequisite for a variety of bioanalytical applications [1]. Physicochemical parameters such as size, charge and solubility of individual polypeptides have been extensively exploited to develop sophisticated techniques for the isolation of specific protein species. Electro-focusing methods and one-dimensional gel electrophoresis (GE) are long established methods of protein biochemistry. Conventional isoelectric focusing (IEF) separates proteins by differences in their isoelectric point (pI) whereby a pH gradient along the length of a gel provides the support system for protein migration until they reach their pI-value with no net charge [2]. In contrast, one-dimensional gel electrophoresis in the presence of an anionic detergent, such as sodium dodecyl sulfate (SDS), is based on the separation of denatured and structurally linearized polypeptides within complex protein mixtures. Electrophoretic mobility patterns mostly depend on size differences of the denatured molecules due to the introduction of an overall negative charge [3,4,5]. However, since many protein species exhibit a similar net charge or relative molecular mass, one-dimensional gel bands are often heterogeneous in composition. Once it became clear that biochemical techniques focusing on only one parameter have relatively limited separation capacity, alternative approaches were attempted. Especially the sequential usage of two independent methods promised the separation of proteins at higher resolution. The technical realization of this ground-breaking concept was the beginning of a new era in protein biochemistry. The combined property of the pI-value of a protein and its molecular size following denaturation was successfully exploited in the development of standardized two-dimensional gel electrophoresis (2D-GE). Historical and technical aspects of combined gel electrophoretic approaches have been extensively reviewed [6,7,8].
Patrick H. O’Farrell’s work set the scene for high-resolution gel electrophoresis [9]. Many other laboratories developed similar approaches or modified the original gel electrophoretic method to adapt this technique to other analytical applications [10,11,12,13]. Both, protein biochemistry and the more recently established field of mass spectrometry (MS)-based proteomics have heavily depended on the 2D-GE method in the past, making this method one of the most commonly employed standard technique of protein separation. In addition, gel-free approaches and the usage of 1D-GE systems are frequently used for the proteomic analysis of complex tissues. The most crucial capabilities of the 2D-GE technique are the simultaneous resolution of thousands of distinct protein species within the same gel system and the reliable determination of their relative molecular mass and pI-value, as well as their relative quantity. Especially the application of the 2D-GE method for the efficient separation of different protein isoforms with dynamic post-translational modifications (PTM) has made outstanding contributions in analytical biochemistry. In contrast to other large-scale separation approaches such as liquid chromatography (LC), individual protein spots are visualized in 2D gels so that their status in relation to fragmentation and modification can be directly accessed. Furthermore, gel-separated and -embedded proteins are relatively stable and can be safely stored for long periods of time prior to further analysis [14,15,16,17].
This article focuses on the application of the 2D-GE method in basic myology and discusses the enormous scientific impact of this method on recent proteomic studies of normal and physiologically challenged skeletal muscle tissues. This includes the systematic cataloguing of the protein constituents of different contractile fiber types and the findings from surveys of proteome-wide changes during physiological adaptations. Comparative proteomics has also played a key role in the pathobiochemical evaluation of global changes in diseased skeletal muscles [18]. However, this topic is not addressed in this article on the biochemistry of normal, adapting and aging skeletal muscles. Several extensive reviews on the pathoproteomics of neuromuscular disorders have outlined the various proteomic techniques used in the determination of molecular and cellular mechanisms that underlie common muscle diseases [19,20,21,22]. The critical examination of how MS-based proteomics can be used for the systematic identification and biochemical characterization of novel protein biomarkers, which may be exploitable for the future design of improved predictive, diagnostic, prognostic and/or therapy-monitoring assays, has also recently been reviewed [23]. Below sections give an overview of major proteomic studies that have employed 2D-GE methods and analyzed fiber type specification and protein changes during muscle development, fiber type transformation, exercise-induced adaptations, hypoxia-associated alterations, disuse-related muscular atrophy and skeletal muscle aging.
2. Two-Dimensional Gel Electrophoresis of Skeletal Muscle Proteins
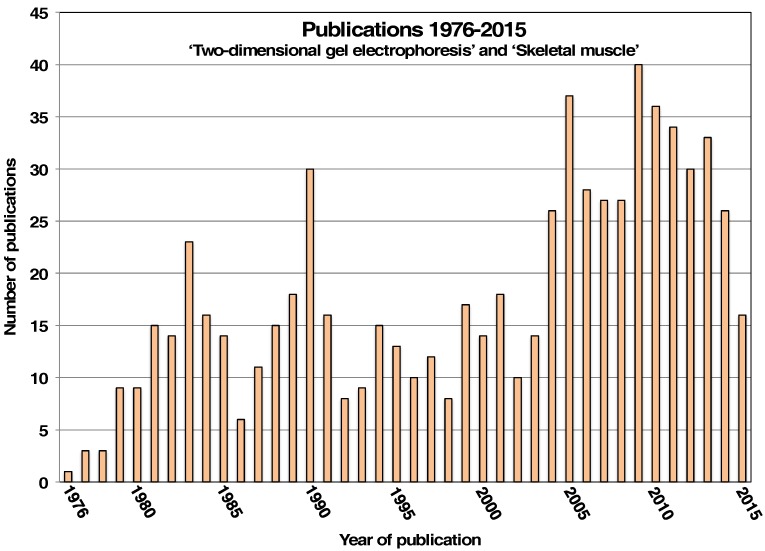
The 2D-GE technique is a frequently used and highly reliable bioanalytical method for the systematic assessment of skeletal muscle tissues. Following tissue homogenization under optimized conditions and in the presence of a suitable protease inhibitor cocktail, a large portion of skeletal muscle proteins can be extracted without major complications due to proteolytic degradation and then separated by 2D-GE. The application of the original O’Farrell method [9] or modified and optimized gel electrophoretic techniques [24] played an essential role in the systematic identification and thorough characterization of the protein components that form the functional basis of skeletal muscle contractility. The PubMed databank of the US National Library of Medicine contains nearly 33,000 entries with the keyword “two-dimensional gel electrophoresis” of which over 600 publications are in relation to the combined key words “two-dimensional gel electrophoresis” and “skeletal muscle”, including from the year 2001 onwards nearly 200 papers using MS-based proteomics [25]. Figure 1 summarizes in a histogram the number of published papers per year on this topic since 1976 and shows an increase in the usage of the 2D-GE method after the incorporation of MS-based proteomics for the routine analysis of skeletal muscle tissues since 2004. Over the last 4 decades, the combined usage of IEF in the first dimension and SDS-PAGE in the second dimension has been successfully employed to identify and characterize several thousand muscle-associated or muscle-derived protein species. These muscle protein species belong to the diverse group of regulatory and contractile proteins of the acto-myosin apparatus that forms the sarcomere, cytoskeletal proteins, metabolic enzymes and transporters, signaling proteins, ion-handling proteins, molecular chaperones, extracellular matrix proteins and myokines.
Figure 1.
Summary of the number of publication entries with the keywords “two-dimensional gel electrophoresis” and “skeletal muscle” registered with the PubMed databank of the US National Library of Medicine ranging from 1976 to 2015.
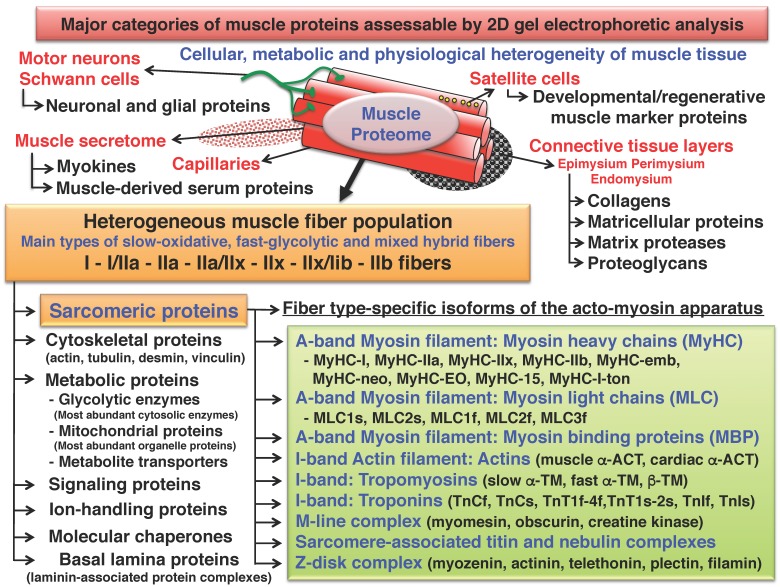
As outlined in Figure 2, conventional biochemical approaches and more recently established MS-based proteomic methods have integrated the 2D-IEF/SDS-PAGE technique as a highly suitable method for detailed investigations into skeletal muscle proteins. It is important to mention that skeletal muscle tissues are heterogeneous in their composition and highly dynamic in their response to cellular, metabolic or physiological challenges [26]. The main contractile units of an individual skeletal muscle are presented by diverse fiber populations, consisting usually of slow-oxidative, intermediate fast-glycolytic/oxidative and fast-glycolytic cell types, as well as mixed hybrid fibers. This relates to the histochemically well-defined fiber types I, IIa, IIx and IIb, and the hybrid fibers I/IIa, IIa/IIx and IIx/IIb [27]. The internationally agreed nomenclature of muscle fiber types is based on the distribution of myosin heavy chain (MyHC) isoforms [28]. Besides contractile fibers, muscle tissue contains motor neurons with their extensive myelin sheets, an elaborate network of capillaries, satellite cells and multiple layers of connective tissue, including the epimysium, perimysium and endomysium [29]. Biochemical studies using homogenized tissue preparations have to take into account this cellular heterogeneity of skeletal muscles [21], as well as the presence of actively secreted and passively released fiber-derived proteins that constitute the muscle secretome [30].
Figure 2.
Overview of protein classes from skeletal muscle tissue that can be separated by routine two-dimensional gel electrophoresis using isoelectric focusing in the first dimension and sodium dodecyl sulfate polyacrylamide slab gel electrophoresis in the second dimension. Abbreviations used: ACT, actin; MBP, myosin binding protein; MLC, myosin light chain; MyHC, myosin heavy chain; TM, tropomyosin; Tn, troponin.
Prior to the publication of the original O’Farrell method [9], 2D-GE studies on skeletal muscle employed SDS-PAGE in both dimensions with differing gel concentrations. These early attempts could only separate a few distinct protein spots from muscle ribosomes [31] and the microsomal fraction from skeletal muscles [32]. However, following the publication of high-resolution 2D-IEF/SDS-PAGE methods [9,10,11,12], this new and more refined approach was quickly adapted in the field of basic and applied myology [24]. Initial studies included the analysis of major structural and regulatory proteins of muscle fibers [33,34], the evaluation of human muscle biopsy specimens [35,36] and the identification of contractile protein isoforms in single skeletal muscle fibers [37,38]. In the pre-proteomic era of 2D-GE biochemistry [39], the technique was extensively applied to the detailed analysis of subunit structures and isoform expression patterns of major skeletal muscle proteins and their changes during development, fiber adaptations, contractile fatigue and denervation, as reviewed by Bárány et al. [40]. From 1995 onwards, gel-based surveys and 2D-GE databases became an integral part of skeletal muscle proteomics [41]. Protein changes during myogenesis, muscle maturation, fibre type specification, physiological muscle adaptations, muscle regeneration and natural muscle aging were studied in depth by the original O’Farrell method or slightly modified gel electrophoretic techniques. Below sections review the application of the 2D-IEF/SDS-PAGE method in modern proteomics and its modifications for comparative studies using fluorescent dyes. Included are descriptions of the systematic cataloging of the assessable skeletal muscle proteome, and the comparative proteomic profiling of muscle plasticity in relation to neuromuscular activity versus disuse atrophy.
3. Cataloguing of the Skeletal Muscle Proteome Using Two-Dimensional Gel Electrophoresis
MS-based muscle proteomics was instrumental in the identification of several thousand individual protein isoforms following gel electrophoretic separation [21,42,43]. General technical aspects of the most frequently employed 2D-GE methods in the proteomic profiling of crude tissue extracts, subcellular fractions or isolated protein complexes have been extensively discussed and reviewed [15,17,44,45,46]. Over the last few years, several excellent methods books have been published that focus on specific aspects of the many modifications used in routine 2D-GE and proteome analysis protocols. These comprehensive collections of detailed method descriptions have been edited by experts in the field, including Link [47], Reinders and Sickmann [48], Cramer and Westermeier [49], Kurien and Scofield [50], and Marengo and Robotti [51]. In-gel staining methods and routinely used detection technologies for studying 2D protein spot patterns have also been extensively described and critically examined in numerous publications [52,53,54,55,56,57,58,59,60]. The rapidly moving field of MS methodology analyzing peptides obtained from the proteolytic digestion of proteins is the subject of many excellent articles that outline in detail the many instruments and approaches available in modern proteomics research [61,62,63,64]. These optimized gel-based protein separation approaches, highly reliable pre-electrophoretic or in-gel staining techniques and sophisticated MS methods for the unequivocal identification of individual protein species have been extensively used in skeletal muscle proteomics [20,21,22]. In relation to skeletal muscle biochemistry and proteomics, detailed step-by-step descriptions of tissue sample preparation, protein extraction, protein solubilization, IEF, SDS-PAGE, pre-electrophoretic protein labeling, post-electrophoretic protein staining and subsequent MS analysis of proteins of interest following controlled proteolytic degradation have been published in comprehensive methods papers [65,66,67,68].
In the first volume of the journal Proteomics, launched in 2001, publications by Hochstrasser and colleagues [69] and Dunn and co-workers [70] set the scene for 2D gel-based skeletal muscle proteomics. Their initial studies identified over 70 proteins each from normal mouse and rat skeletal muscle homogenates, including many sarcoplasmic, metabolic and myofibrillary proteins such as various myosin subunits, actin isoforms, regulatory sarcomeric proteins, glycolytic enzymes, mitochondrial proteins and molecular chaperones [69,70]. The application of the 2D-GE method in combination with MS technology for cataloging mouse skeletal muscle was part of extending the SWISS-2DPAGE database to include proteomic maps of major types of tissue [69]. A variety of databases exist for the image comparison of 2D gels, the identification of internet-based gel images, the cataloguing of results from systematic 2D-GE analyses and the integration of electrophoretic and mass spectral data from proteomic analyses, including World-2DPAGE Constellation SWISS-2DPAGE, WEB P.A.G.E, Flicker, GELBANK, Open2Dprot Project, LECB 2-D PAGE Gel Images Data Sets, 2DWG, ProteomeWeb, PHProteomicsDB and pProRep [71,72,73,74,75,76,77,78]. In 2001, the new field of skeletal muscle proteomics was further developed by the systematic identification of sarcoplasmic proteins from several hake species [79] and the mass spectrometric characterization of bovine myosin light chain MLC1f polymorphism following 2D-GE separation [80]. Potential technical shortcomings of 2D-GE for the comprehensive separation of highly complex protein mixtures have often been discussed in the past and compared to the perceived superiority of LC-based methods [17,81,82]. In our opinion, both protein separation techniques should be seen as complementary proteomic methods and be used in combination to achieve the maximum coverage of the assessable proteome of a particular biological specimen.
In the case of skeletal muscle fibers, approximately half of the protein constituents belong to the sarcomere units that are made up of large numbers of isoforms of myosin heavy chains (MyHC), myosin light chains (MLC), actins (ACT), troponins (TN) and tropomyosins (TM). These contractile and regulatory protein species are routinely identified by gel-based proteomics [43], demonstrating the usefulness of 2D-GE for the classification of muscle types and fiber specification. Metabolic enzymes are also highly abundant in muscle tissues and straightforwardly assessable by gel-based proteomics [83]. Prior to outlining the many advantages and bioanalytical applications of 2D-GE in skeletal muscle proteomics in subsequent sections, the below listing highlights certain issues that may hamper gel-based methods and other types of analyses. The most frequently encountered biological and technical complications (and some alternative approaches to avoid these potentially limiting factors) are:
Possible under-estimation of particular types of proteins, including highly hydrophobic proteins, very high-molecular-mass proteins and low-copy-number proteins. Changing gel conditions, the introduction of suitable pre- and post-fractionation steps, as well as higher sensitivity detection protocols can often overcome some of these technical limitations [84,85,86,87].
Hypothetical under-representation or 2D streaking of proteins with extreme pI-values, which however depends heavily on the particular IEF conditions employed in the first dimensional separation step. Often very acidic protein species form vertical streaking patterns at the pH 3 region and very basic proteins at the pH 11 region. To at least partially overcome this problem, the usage of narrow-range immobilized pH gradients can be applied for zooming in on protein species that do not fall into the commonly applied range of approximately pI 3 to 11 [88,89,90,91]. In addition, combining the findings from several different IEF gels in the first dimension with slightly overlapping pI-values can be advantageous for producing more comprehensive protein coverage [15,92,93].
Potentially restricted separation of complex protein mixtures with greatly differing molecular masses using routine 2D-GE approaches. Often the usage of large-scale gels, optimized gradient SDS-PAGE slab gel systems in the second dimension and the reduction of sample complexity can overcome some of these technical problems and be used to cover protein species that do not fall into in the routinely analyzed range of approximately 10 to 250 kDa [67,94].
Latent cross-contamination of individual 2D protein spots through highly abundant polypeptides that are dragged throughout the 2D gel system due to their exceedingly high density. These abnormal electrophoretic mobility patterns of particular proteins cause a certain degree of 2D streaking, which can be minimized by (i) decreasing the total amount of protein loading; (ii) using very large gel systems with a higher discriminatory capacity and/or (iii) applying optimized pre-fractionation techniques to decisively decrease sample complexity [95,96,97,98]. Artifacts can be kept to a minimum using 50 to 200 μg of total protein in first dimension gels. Lower protein concentrations usually result in weak staining patterns. Comparative studies with fluorescent dyes give optimum results with approximately 50 μg of protein per sample.
Potential discrepancies between the findings from the densitometric scanning of gel images and the MS-based protein identification in case of a heterogeneous composition of a single 2D protein spot. For example, if a protein spot contains more than one protein species and the most abundant protein is not as susceptible to digestion as the low-copy-number proteins in its vicinity, then the concentration change of this 2D protein spot (as determined by densitometric scanning) may be misleading. However, this analytical complication is a relatively rare occurrence and the use of simple post-fractionation approaches and/or independent verification of gel-based proteomic data by immunoblotting surveys or immunofluorescence microscopical analysis can effectively assess the rate of these kinds of analytical discrepancies [21].
In relation to the analysis of skeletal muscle preparations by 2D-GE, the above listed technical and biological issues may complicate especially the routine analysis of the many very large proteins present in the neuromuscular system, such as titin (3700 kDa), nebulin (800 kDa), obscurin (720 kDa), the ryanodine receptor Ca2+-release channel (565 kDa) and dystrophin (427 kDa). However, GE methods can routinely detect fragments of these high-molecular-mass proteins. Many integral or membrane-associated muscle proteins are under-represented in 2D gels and their detailed proteomic analysis has to be carried out with enrichment methods prior to 2D-GE analysis, supplementing LC methods and/or alternative 1D gradient gel systems [99,100,101,102]. The most highly abundant proteins in muscle homogenates are ACTs, MyHCs, MLCs, TMs and TNs. Depending on the overall loading capacity of a particular 2D gel, the high density of some isoforms of these sarcomeric proteins can cause a select amount of cross-contamination in particular regions of a 2D-GE system [67,103].
Despite these bioanalytical limitations, the many technical advantages of the 2D-GE approach far outweigh the potential shortcomings of this extensively used protein separation method, as listed below:
Extremely reliable protein separation system that can be routinely used in large-scale and high-throughput proteomic surveys. Multi-gel systems using large buffer tanks can run a considerable number of 2D gels in parallel making this approach both cost-effective and highly reproducible for systematic biochemical studies [15,16,17].
Staining of 2D gels with highly sensitive dyes ranging from colloidal Coomassie Blue to silver stains to a variety of fluorescent dyes can visualize a wide dynamic range of proteins of differing abundance [56,57,58,104].
Technical provision of a bioanalytical platform that is ideally suited for the subsequent identification of specific protein isoforms and their PTMs [46,47,48]. Many in-gel staining or labeling methods can specifically highlight PTMs, such as enzyme-conjugated lectin labeling or Pro-Q Emerald staining for glycosylation or the fluorescent Pro-Q Diamond dye for phosphorylation [58,105,106,107].
Direct visualization of proteins of interest as discrete 2D spots, enabling the exact evaluation of the characteristic combination of the pI-value and relative molecular mass of a particular protein subunit or isoform. This provides a unique analytical advantage over simpler 1D gel systems that display heterogeneous protein bands or LC methods. Often MS data from LC-based analyses do not given efficient information on sequence coverage to unequivocally determine whether a fully intact protein species or fragments have been detected. In contrast, proteomic data from the analysis of distinct 2D-GE spots can be directly correlated with the electrophoretic mobility and thereby the relative molecular mass of the protein of interest [21].
Since potential discrepancies between the mass spectrometric identification of a protein and its position in a 2D gel in relation to its pI-value and/or molecular mass can be easily assessed, the rate of false positive protein hits can be conveniently measured and swiftly eliminated from the final list of altered protein species. Additional analyses can then determine whether an abnormal or unexpected electrophoretic mobility pattern is due to protein degradation, protein clustering or a technical artifact caused by 2D streaking and cross-contamination [18].
Rapid and quantitative analyses of paired protein samples can be conducted. An example of an extremely powerful comparative 2D-GE method is the fluorescence 2D-DIGE technique [108] that eliminates gel-to-gel variations by the differential pre-electrophoretic labeling of protein fractions and the subsequent separation on the same 2D gel followed by image analysis [109]. See below section for details on the DIGE method and its application in skeletal muscle proteomics.
Since the beginning of the new millennium, several thousand muscle-associated or muscle-derived protein species and subgroups with particular PTMs have been identified and used to establish the skeletal muscle proteome. A large proportion of the comprehensive cataloguing of total muscle tissue extracts from various species and subtypes of muscle was carried out by 2D-IEF/SDS-PAGE [69,70,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126]. Subcellular fractions of skeletal muscles have also been studied by 2D-GE and MS analysis [127], including nuclei [128], mitochondria [129,130,131,132], the contractile apparatus [133], cytosol [128] and the muscle secretome [134,135]. In addition, 1D-GE and on-membrane digestion has been used to characterize the sarcolemma [99] and sarcoplasmic reticulum [101] from skeletal muscle preparations. Major classes of PTMs, such as muscle protein nitration, glycosylation and phosphorylation were determined by 2D-GE methodologies [105,106,136,137]. These gel-based proteomic cataloguing studies were supplemented with data from alternative GE methods and a large number of LC-based proteomic investigations [138,139,140,141,142,143,144,145,146,147,148,149,150] to fully comprehend the enormous complexity of the muscle proteome [21]. Details of major 2D gel-based studies for the establishment of the skeletal muscle proteome are listed in Table 1. Within this large cohort of skeletal muscle proteins, fiber type-specific expression patterns of a few hundred muscle proteins have been established by 2D-GE analysis, confirming the molecular and cellular heterogeneity between predominantly fast-twitching and slow-twitching muscles [113,114,115,116,117,118]. This important topic of skeletal muscle physiology and the major changes that occur during fiber transitions is discussed in the below section on comparative proteomics.
Table 1.
Major 2D-IEF/SDS-PAGE-based proteomic studies of normal skeletal muscle tissues.
| Proteomic Analysis | Tissue and Species | References |
|---|---|---|
| Human 2D gel reference maps | Human vastus lateralis and laryngeal muscle | Gelfi et al. [111]; Li et al. [112]; Kovalyova et al. [124] |
| Human fast versus slow muscle fibre type specification | Normal human deltoideus and vastus lateralis muscles | Capitanio et al. [115] |
| Mouse 2D gel reference maps | Normal mouse gastrocnemius and quadriceps muscle | Sanchez et al. [69]; Raddatz et al. [121] |
| Mouse fast versus slow muscle fibre type specification | Normal and kyphoscoliotic mouse soleus and vastus lateralis muscles | Le Bihan et al. [117] |
| Rat 2D gel reference map | Normal rat skeletal muscle from the abdominal wall | Yan et al. [70] |
| Rat fast versus slow muscle fibre type specification | Normal rat soleus, gastrocnemius and extensor digitorum longus muscles | Okumura et al. [116]; Gelfi et al. [118] |
| Rabbit 2D gel reference map | Rabbit gastrocnemius muscle | Almeida et al. [122] |
| Bovine 2D gel reference maps | Bovine semitendinosus muscle | Bouley et al. [113]; Chaze et al. [119] |
| Pig fast versus slow muscle fibre type specification | Normal pig longissimus dorsi and soleus muscles | Kim et al. [114] |
| Pufferfish and killifish 2D gel reference maps | Skeletal muscles from Takifugu rubripes and Fundulus grandis | Lu et al. [125]; Abbaraju et al. [126] |
| Mitochondrial 2D gel maps | Subsarcolemmal and intermyofibrillar mitochondria from various rat muscles | Reifschneider et al. [129]; O’Connell et al. [130]; Lombardi et al. [131]; Ferreira et al. [132] |
| Contractile apparatus 2D gel map | Enriched acto-myosin apparatus from rat gastrocnemius muscle | Gannon et al. [133] |
| Cytosol and nucleus 2D gel map | Nucleus and cytosolic fraction from mouse gastrocnemius and soleus muscles | Vitorino et al. [128] |
| Muscle secretome 2D gel maps | Seretome from cultured muscle cells | Gajendran et al. [134]; Hartwig et al. [135] |
| 2D PTM gel maps of protein glycosylation | Rat leg skeletal muscles | O’Connell et al. [105]; Cieniewski-Bernard et al. [137] |
| 2D PTM gel map of protein phosphorylation | Rat gastrocnemius muscle | Gannon et al. [106,133] |
| 2D PTM gel map of protein nitration | Rat leg skeletal muscles | Kanski et al. [136] |
4. Comparative Skeletal Muscle Proteomics Using Two-Dimensional Gel Electrophoresis
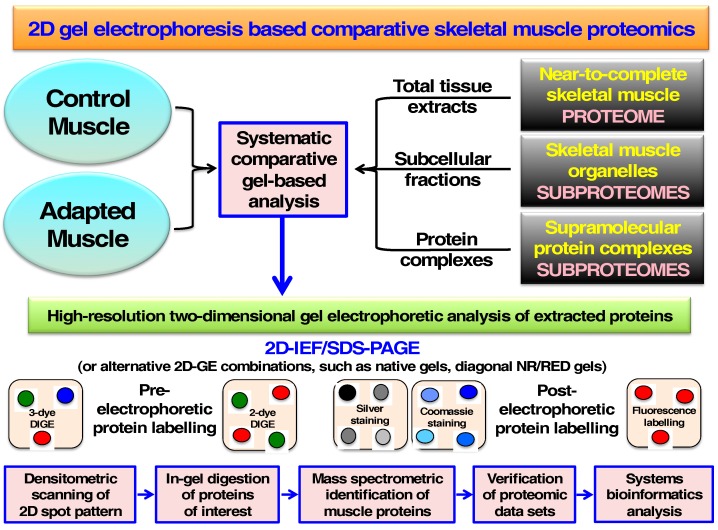
MS-based muscle proteomics has focused on the systematic determination of protein changes during myogenesis, fiber transformation, exercise-induced adaptations, hypoxia, disuse atrophy and muscle aging. A general proteomic workflow used frequently in comparative skeletal muscle proteomics is outlined in Figure 3.
Figure 3.
Overview of proteomic approaches routinely used in the profiling of skeletal muscle proteins following separation by two-dimensional gel electrophoresis. Abbreviations used: DIGE, difference in-gel electrophoresis; GE, gel electrophoresis; IEF, isoelectric focusing; NR/RED, non-reducing/reducing; PAGE, polyacrylamide gel electrophoresis.
Comparative proteomic studies have been carried out with (i) total crude tissue extracts representing the assessable and near-to-complete skeletal muscle proteome; (ii) subcellular fractions enriched in specific organelles thereby representing distinct subproteomes; and (iii) isolated supramolecular protein complexes. For the systematic comparative analysis, protein mixtures were separated by high-resolution 2D-GE using usually IEF and SDS-PAGE, but also alternative combinations such as native gels or diagonal non-reducing/reducing gel systems. Protein visualization was performed with both pre-electrophoretic and post-electrophoretic labeling techniques. 2D spot patterns were routinely scanned by densitometry and proteins of interest then identified by in-gel digestion and MS analysis of the resulting peptide populations. The verification of proteomic hits was usually carried out by immunoblot analysis, immunofluorescence microscopy and enzyme assays.
4.1. Proteome Signature of Skeletal Muscle Development
Skeletal muscle development is a highly complex cell biological process that is associated with considerable alterations in protein expression patterns. Substantial proteome-wide changes during the phenotypic conversions of myoblasts into post-mitotic myotubes were confirmed by MS studies [151]. Embryonic and adult myogenesis is regulated by a variety of factors, including the transcription factors PAX3 and PAX7 during the initial induction of mesodermal precursor cells and during regenerative processes following muscle injury. Wnt glycoproteins and the myogenic regulatory factors MyoD, Myf5, MRF4 and myogenin play a key role during the segmentation into somites and the formation of the primary myotome [152]. The fusion of myogenic cells results in the formation of multi-nucleated myofibers, which is followed by innervation and muscle maturation. Electro-stimulation of the motor unit system results in the expression of a variety of distinct maturation markers and muscle-specific proteins, including the junctional acetylcholine receptor and the membrane cytoskeletal protein dystrophin. The extremely high level of regenerative capacity of adult muscle fibers is due to the presence of inducible satellite cells, which represent myogenic stem cells that become activated during reactive cycles of fiber regeneration [153]. Skeletal muscle tissue mass is regulated by a complex network of anabolic and catabolic mechanisms and this process includes crosstalk between a variety of regulatory pathways [154]. A key intracellular signaling molecule with differential effects on neuromuscular loading is the transcriptional co-activator PGC-1α (peroxisome proliferator-activated receptor gamma co-activator 1-alpha). Crucial regulatory systems include the TGFβ (transforming growth factor) and myostatin signaling pathway, the NFκb (nuclear factor kappa) and inflammatory cytokines pathway, the IGF1-PI3K-Akt-mTOR (insulin-like growth factor 1-phosphatidylinositol-3-kinase-serine/threonine protein kinase PKB-mammalian target of rapamycin) pathway, the autophagy lysosome, the ubiquitin proteasome, acetylating enzymes and myogenic regulatory factors [155].
The complexity of cell biological mechanism involved in myoblast activation and fiber maturation can be conveniently studied in a systematic fashion by muscle proteomics. Large-scale MS studies of developing muscle fibers promise to establish the regulatory hierarchy of myogenesis and skeletal muscle regeneration. 2D gel-based analyses of skeletal muscle development have involved the proteomic profiling of C2C12 muscle cell culture models with a focus on myoblast differentiation and myotube formation [156,157] and postnatal muscle growth [158,159]. In addition, 1D-GE approaches were used to study changes in the skeletal muscle secretome during myogenesis [160,161,162]. The phenotypic conversion of mono-nucleated myoblasts into differentiated and multi-nucleated myotubes was shown to be associated with considerable changes in highly regulated muscle proteins involved in intercellular signal transduction, intracellular signaling systems, the maintenance of cell shape, the regulation of cell proliferation and apoptosis, as well as protein folding, stabilization and degradation in relation to the cellular stress response [156,157]. Proteomics also confirmed complex changes during the early postnatal period. The developing rat tibialis anterior [158] and porcine longissimus dorsi muscle [159] showed a time-dependent increase in contractile proteins and drastic alterations in metabolic enzymes, cytoskeletal proteins, molecular chaperones and signal transduction factors. An interesting negative regulator of muscle growth is myostatin, a secreted differentiation factor that belongs to the TGF-β superfamily [163]. Semitendious muscles from Belgium Blue bulls that lack myostatin were shown to be characterized by a higher proportion of fast-twitch glycolytic fibers with alterations in contractile protein isoform expression patterns and an increase in myosin binding protein MBP-H [164,165].
4.2. Muscle Plasticity and Fiber Type Specification
The physiological regulation of contractile fiber size, fiber type distribution and skeletal muscle mass is closely linked to neuromuscular activity levels. A variety of molecular networks are involved in skeletal muscle plasticity and fibre type specification [155]. Physiological adaptations to changed functional demands and fiber type formation is intimately related to cytosolic Ca2+-levels and the activation of the Ca2+-calmodulin/calcineurin system. This pathway is involved in the dephosphorylation and subsequent translocation of NFAT (nuclear factor of activated T-cells) into the fibre nucleus, which triggers NFAT-mediated remodeling of muscle gene transcription. Importantly, the activation of the mitogen-activated protein kinase MAPK is a Ca2+-dependent process [166]. Thus, Ca2+-homeostasis plays not only a central role in the regulation of the excitation-contraction-relaxation cycle and skeletal muscle metabolism, but also influences fiber type specification and muscle adaptations. Adult skeletal muscles are composed of a mixture of slow-twitching fibers, fast-twitching fibers and hybrid fibers. In cell biological terms, the functional and transitional status of an individual skeletal muscle is characterized by the dynamic ratio of slow-to-fast-to-hybrid fibers [167]. The particular mixture of fibers determines the ability of an individual skeletal muscle to generate force. It also influences its susceptibility to contractile fatigue and its properties in relation to shortening kinetics.
Distinct populations of contractile fibers can be distinguished by their cell biological properties (tissue color, cellular diameter, capillary density, mitochondrial content), physiological characteristics (contraction time, power output, relaxation time, relative resistance to fatigue) and biochemical status (aerobic versus anaerobic activities, ratio of oxidative versus glycolytic enzymes, myoglobin levels, triglyceride storage, glycogen levels) [27]. These specific properties of predominantly fast versus slow fibers are reflected by discrete differences in muscle protein density and protein isoform expression patterns. The differential biochemical profile of slow versus fast fibers was confirmed by extensive gel-based proteomic studies that have clearly established several hundred fiber type-specific protein isoforms, including contractile proteins, cytoskeletal proteins, metabolic enzymes, signaling proteins and ion-handling proteins. Major comparative studies of slow versus fast muscles are listed in Table 1 and include the 2D-GE analysis of human deltoideus versus vastus lateralis muscles [115], pig longissimus dorsi versus soleus muscles [114], rat soleus, gastrocnemius and extensor digitorum longus muscles [116,118] and mouse soleus versus vastus lateralis muscles [117], as well as the subcellular study of the nucleus and cytosol from mouse gastrocnemius versus soleus muscles [128].
Future studies on muscle plasticity can build on these extensive proteomic 2D-GE maps and determine how chronic neuronal, mechanical or metabolic changes may affect the fiber type distribution during physiological muscle conditioning or pathophysiological insults. In general, complex work load-related signaling mechanisms are involved in interrelated pathways with common molecular mediators that induce skeletal muscle hypertrophy versus disuse-related muscular atrophy [154]. The overall process includes neuronal electro-stimulation patterns, mechanical stimulation, stretch-induced fiber alterations, the influence of shear stress and the effects of gravity exposure, as well as hormonal and metabolic factors. The various signals from these varied physiological stimulations present enhanced versus reduced neuromuscular loading and are efficiently transduced into muscle fibers [155]. The cell biological integration alters the response of individual motor units. Thus, neuronal activities, metabolite concentration, oxygen levels, the degree of cellular stress and substrate signaling are key factors that influence changes in the muscle phenotype.
4.3. Exercise-Induced Proteome Signature
The effects of enhanced physical activity have been extensively studied in humans and animal models by 2D-GE analysis to determine large-scale adaptations in skeletal muscle and establish time-dependent tissue changes during exercise [168]. Biological issues that have to be taken into account are the intensity of exercise, the training mode, the overall neuromuscular load, the exercise regimen and the specific skeletal muscle types under investigation [169]. Combined transcriptomic, metabolomic and proteomic approaches promise to identify the detailed regulatory mechanisms underlying molecular and cellular changes during and following physical training. Initial proteomic studies suggest that a large variety of protein alterations reinforce the establishment of power performance versus the endurance phenotype [170]. The findings from previous physiological and biochemical studies of exercise-related protein changes were confirmed by proteomics. The exercise-induced proteome signature is influenced by the type and duration of exercise and especially involves the regulation of glycolytic and mitochondrial protein synthesis, and changes in the isoform expression pattern of contractile proteins. Besides the protein systems involved in ATP generation and contractile force, oxygen and metabolite delivery are crucial factors, as well as components that maintain the cellular stress response and anti-oxidant capacity of adapting fibers. Proteomics corroborated the physiological concept that a single bout of intensive exercise affects cytosolic Ca2+-release and triggers increased amino acid metabolism. In contrast, chronic endurance training is clearly related to enhanced mitochondrial metabolism and an up-regulation of proteins involved in the citric acid cycle and oxidative phosphorylation [171].
Proteomic profiling of exercise has included the evaluation of protein changes in various human muscles [172,173,174,175,176,177] and established animal models of different training regimes [178,179,180,181,182,183,184,185,186,187,188]. The individual investigations are listed in Table 2. Human exercise studies have focused on (i) the effects of interval training on the vastus lateralis muscle [172]; (ii) changes in the mitochondrial proteome from vastus lateralis muscle following extended periods of endurance training [173]; (iii) the response of soleus and vastus lateralis muscles to vibration exercise countermeasures to prevent muscular atrophy in lower limbs due to long-term bed rest [174,175]; (iv) the effects of acute or repeated eccentric exercises on rectus femoris muscle [176] and (v) exercise-induced muscle damage and inflammation in vastus lateralis muscle following extensive downhill running [177]. Distinct adaptive responses were identified for key muscle proteins, including changes in the ATP synthase and succinate dehydrogenase following interval training [172], a glycolytic-to-oxidative enzyme shift during endurance training [173], altered MyHCs and oxidative enzymes following vibration exercise [174,175], changes in glycolytic enzymes and myosins in response to repeated eccentric exercises [176], and altered expression levels of actin, desmin and calsequestrin in damaged muscles [177].
Table 2.
Major 2D-IEF/SDS-PAGE-based proteomic studies of myogenesis, skeletal muscle adaptations, physical activity and muscle aging.
| Proteomic Analysis | Skeletal Muscle Tissue | References |
|---|---|---|
| Postnatal development | Rat tibialis anterior and porcine longissimus dorsi muscle | Sun et al. [158]; Xu et al. [159] |
| Myoblast differentiation and myotube formation | C2C12 cell culture model | Tannu et al. [156]; Casadei et al. [157] |
| Interval training | Human vastus lateralis muscle | Holoway et al. [172] |
| Endurance training | Human vastus lateralis muscle | Egan et al. [173] |
| Vibration exercise during long-term bed rest | Human soleus and vastus lateralis | Moriggi et al. [174]; Salanova et al. [175] |
| Repeated eccentric exercises | Human rectus femoris muscle | Hody et al. [176] |
| Downhill running-induced muscle damage | Human vastus lateralis muscle | Malm and Yu [177] |
| Various types of animal endurance training | Rat plantaris, gastrocnemius, tibialis anterior, soleus and epitrochlearis muscles; and horse vastus lateralis muscle | Burniston [178]; Guelfi et al. [181]; Yamaguchi et al. [182]; Gandra et al. [183]; Magherini et al. [185]; Bouwman et al. [186] |
| One bout of an exhaustive exercise | Rat gastrocnemius muscle | Gandra et al. [184] |
| Endurance training following gene doping | Various mouse leg muscles | Macedo et al. [187] |
| Chronic low-frequency electro-stimulation | Rabbit tibialis anterior muscle | Donoghue et al. [179,180] |
| High-capacity versus low-capacity runners | Rat soleus muscles | Burniston et al. [188] |
| Myostatin-related muscle hypertrophy | Belgium Blue bulls semitendious muscle lacking myostatin | Bouley et al. [164]; Keady et al. [165] |
| Hypoxia-induced muscle adaptations | Zebrafish, rat and human vastus lateralis muscle | Bosworth et al. [191]; De Palma et al. [192]; Vigano et al. [193]; Levett et al. [194] |
| Disuse atrophy due to neuromuscular unloading, immobilization or denervation | Rat soleus, tibialis anterior, laryngeal and gastrocnemius muscles | Isfort et al. [196,197,198]; Seo et al. [199]; Moriggi et al. [200]; Ferreira et al. [201]; Basco et al. [202]; Wang et al. [203]; Li et al. [204]; Sato et al. [205] |
| Skeletal muscle aging | Various aged rat skeletal muscles, including the gastrocnemius muscle | O’Connell et al. [105,216]; Gannon et al. [106,133] Kanski et al. [136]; Feng et al. [239]; Doran et al. [217,220]; Piec et al. [240]; Capitanio et al. [221,223] |
| Sarcopenia of old age | Various aged human skeletal muscles, including the vastus lateralis muscle | Gelfi et al. [219]; Staunton et al. [222] |
The proteomic signature of increased neuromuscular loading in animals was evaluated in response to (i) moderate intensity endurance training in rat plantaris muscle [178]; (ii) 14 and 60 days of chronic low-frequency stimulation of rabbit tibialis anterior muscle [179,180]; (iii) high intensity swimming in rat gastrocnemius and epitrochlearis muscles [181,182]; (iv) treadmill endurance overtraining in rat gastrocnemius muscle [183]; (v) one bout of an exhaustive exercise in rat gastrocnemius muscle [184]; (vi) training effects on protein carbonylation in rat tibialis anterior and soleus muscles [185]; (vii) different stages of endurance training in horse vastus lateralis muscle [186]; (viii) endurance training in mouse leg muscles with insulin-like growth factor-mediated gene doping [187]; and (ix) high-capacity versus low-capacity running in rat soleus muscle [188]. Endurance training was shown to be clearly related to shifts from glycolytic metabolism to mitochondrial bioenergetics [176,181,182,183,187]. The chronic activation of motor units by electro-stimulation caused a drastic increase of markers of oxidative metabolism and a transformation of contractile proteins to slower isoforms [179,180]. Interestingly, the comparative 2D-GE analysis of soleus muscles from rats that were artificially selected as either high- or low-capacity runners identified protein disulfide isomerase PDIA3 as a key component that is associated with the aerobic capacity of slow skeletal muscles [188].
4.4. Hypoxia-Related Muscle Adaptations
Although contractile fibers are metabolically robust and remarkably resistant to short periods of oxygen deprivation, very strenuous exercise or physical activity during chronic exposure at altitude cause an adaptive response in the skeletal musculature [189]. A chronic decrease in oxygen levels causes a bioenergetic challenge to metabolically active muscle tissue and is associated with muscular atrophy due to a transient activation of proteolysis and an mTOR-related inhibition of muscle protein synthesis, as well as an overproduction of reactive oxygen species and the stabilization of the oxygen-sensitive hypoxia-inducible factor HIF-1α [190]. Proteomic studies have confirmed hypoxia-induced muscle adaptations using both animal models of oxygen deprivation, i.e., zebrafish in hypoxic tanks [191] and rats in hypoxic chambers [192], and human biopsy specimens from vastus lateralis muscle during adaptations to different periods of hypoxia [193,194]. The response to chronic hypoxia is clearly associated with an inhibition of fatty acid oxidation and a decreased expression of proteins involved in the citric acid cycle and oxidative phosphorylation, as well as moderate alterations in the glycolytic pathway [191,192,193,194]. The hypoxia-related loss of muscle mass and decrease in fiber area is probably an adjustment to impaired oxygen diffusion, and the apparent oxidative-to-glycolytic shift in fiber metabolism represents an optimization of bioenergetic processes during exposure to environmental hypoxia.
4.5. Proteome-Wide Changes during Disuse Atrophy
A variety of physiological or pathophysiological conditions may lead to muscular atrophy. Prolonged episodes of muscle disuse or neuromuscular unloading are related to traumatic nerve crush, complete denervation, exposure to microgravity, extended bed rest in the severely ill, various forms of immobilization or a general lack of physical activity as for example seen in the elderly. Muscular atrophy has severe effects on the musculature triggering a net loss of skeletal muscle protein mass and contractile strength. In general, muscular atrophy is associated with a drastic decrease in protein synthesis and concomitant increase in the rates of protein breakdown, as well as slow-to-fast transitions in contractile kinetics and an oxidative-to-glycolytic shift in fiber metabolism [195]. A considerable number of gel-based studies have used animal models of denervation, immobilization or extended periods of muscle disuse to determine proteome-wide changes during muscular atrophy [196,197,198,199,200,201,202,203,204,205]. These studies have confirmed the general tendency of atrophying skeletal muscles to undergo a stepwise conversion to faster contractile properties and increased glycolytic metabolism. The proteomic profiling of rat muscles following denervation [196], hindlimb suspension [197] or immobilization by the pin-heel method [198] showed increases in carbonic anhydrase CA3, enolase and fast MLC, as well as decreases in the fatty acid binding protein and slow MLC. As described in the proteomic studies listed in Table 2, neuromuscular unloading is clearly associated with a slow-to-fast transformation process [199,200,201,202,203] and complete denervation triggers extensive isoform switching in slow versus fast isoforms of the contractile proteins MyHC, MLC, TN and TM [204,205]. As already mentioned in above section on exercise proteomics, vibration training can be beneficial to counteract muscular atrophy triggered by extended periods of bed rest [174,175]. The proteomic profiling of disuse atrophy following chronic bed rest has revealed decreases of type I fibers and an increase of hybrid fibers in human soleus and vastus lateralis muscles. An oxidative-to-glycolytic shift in skeletal muscle metabolism was confirmed by the identification of elevated levels of glycogen phosphorylase and glycolytic enzymes, and a concomitant decrease in mitochondrial enzymes involved in oxidative phosphorylation [175].
4.6. Sarcopenia of Old Age
The regenerative capacity of senescent fibers is greatly compromised causing a natural decline in contractile strength during skeletal muscle aging [206]. This functional decline of the neuromuscular system is related to both a gradual loss in skeletal muscle mass and fiber type shifting due to a higher susceptibility of aged type II fibres to muscular atrophy [207]. Sarcopenia of old age has been recognized as a major contributor to frailty in the elderly [208] and affects a large portion of the general population over 75 years of age [209]. The pathophysiological mechanisms of sarcopenia are believed to be highly complex resulting in a multi-factorial etiology. Recent proteomic studies have established extensive changes in the skeletal muscle proteome during the natural aging process [210,211,212], whereby gel-based proteomics has been instrumental in the initial cataloguing of the aged muscle proteome [213,214,215]. Skeletal muscle aging is closely associated with persistent impairments of the peripheral nervous system, which trigger excitation-contraction uncoupling and pathophysiological cycles of denervation and faulty reinnervation. Besides reduced neuronal stimulation, many other factors influence the senescent muscle phenotype, such as chronic inflammation, oxidative stress, hormonal imbalances, lipotoxicity, impaired capillary blood flow, decreased protein synthesis and reduced numbers of inducible satellite cells. Increased stress levels in aged fibres were shown by the proteomic establishment of elevated levels of a variety of molecular chaperones, including the small heat shock proteins cvHsp and αB-crystallin [216,217].
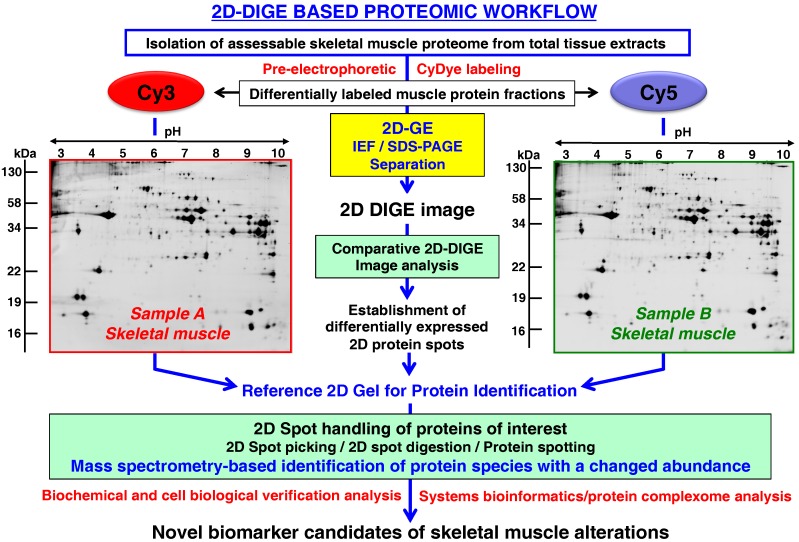
A frequently used 2D-GE method in comparative proteomics is represented by fluorescence difference in-gel electrophoresis (DIGE) [108,109,218] and has also been applied to studying sarcopenia of old age [219,220,221,222,223]. The DIGE method was originally described by Minden and colleagues [224] and can be used with differential fluorescent 2-dye or 3-dye systems for minimal or saturation protein labeling prior to 2D-GE [225,226,227]. Figure 4 gives an overview of the routine DIGE analysis of two differing skeletal muscle specimens.
Figure 4.
Fluorescence two-dimensional difference in-gel electrophoretic analysis of aging skeletal muscle (Sample A: Young muscle; Sample B: Aged muscle). Abbreviations used: DIGE, difference in-gel electrophoresis; GE, gel electrophoresis; IEF, isoelectric focusing; PAGE, polyacrylamide gel electrophoresis.
Detailed technical aspects of the DIGE technique for the comparative proteomic analysis of skeletal muscle tissues is outlined in several method papers [65,66,68,228,229]. The development of optimized 2D software analysis tools [230] has boosted the accuracy of quantitative investigations of multiple protein samples on the same 2D gel [231]. The parallel analysis of differentially and pre-electrophoretically labeled proteomes on the same gel greatly reduces gel-to-gel variations, making the DIGE method an excellent comparative tool of analytical protein biochemistry [232,233]. In the field of skeletal muscle proteomics, pre-electrophoretically labeled and 2D-GE separated proteins usually account for a representative proportion of components from the contractile apparatus, structural cytoskeleton, basal lamina, excitation-contraction coupling complex, major metabolic pathways, ion handling systems and regulatory mechanisms [228,234]. For the routine 2D-DIGE analysis of the skeletal muscle proteome, usually 50 μg protein aliquots from individual fractions are differentially labeled with Cy2, Cy3 or Cy5 dyes [65,66,68].
Changes in the abundance of mitochondrial enzymes [128,129,235] and an altered oxidative status of redox-sensitive proteins have been shown to occur in aged muscles [236,237,238]. PTM changes have been reported in phosphoproteins of the contractile apparatus [106,133] and glycoproteins of metabolic pathways [105]. In addition, age-dependent protein nitration and carbonylation appear to play a role in skeletal muscle aging [136,239]. Extensive 2D-GE studies of contractile proteins have confirmed fast-to-slow transitions during both human and animal muscle aging, including a variety of fast and slow MyHC and MLC isoforms [100,133,219,220,221,222,223,240]. These studies demonstrate the bioanalytical robustness and technical suitability of 2D-GE techniques for the efficient separation of the many isoforms of contractile proteins. Proteomic findings support the pathobiochemical concept that age-related fiber transitions towards a slower contractile phenotype are not a primary process, but are based on secondary mechanisms associated with a preferential decline in faster twitching type II fibres [207,214]. This also agrees with the observed glycolytic-to-oxidative shift during muscle aging. Although mitochondrial metabolism is impaired in senescent fibers, the higher susceptibility of glycolytic type II fibers to muscular atrophy appears to cause an overall shift to more oxidative bioenergetics in a slower twitching fiber population from aged skeletal muscles with a drastic decrease in tissue mass [235].
Gel-based meat proteomics has been used to establish reference maps of bovine muscle [113,119], rabbit muscle [122] and pig muscle [114], as well as for studying tissue growth [164,165,241,242] and post mortem changes in bovine, porcine and chicken muscles [243,244,245,246,247,248]. The findings from meat proteomics are important for maximizing muscle growth, meat production, slaughter methods, muscle-to-meat processing and meat storage [249,250,251].
5. Conclusions
Over the last 40 years, protein separation has been carried out by sophisticated 2D-GE techniques and established this method as a highly suitable and versatile approach for the systematic analysis and characterization of the skeletal muscle proteome. In basic and applied myology, the application of the original O’Farrell method or slightly modified versions has resulted in the cataloging of several thousand distinct muscle protein isoforms and the identification of hundreds of fiber type-specific protein species. Gel-based proteomic studies have established a variety of protein changes in physiologically challenged skeletal muscles, including the effects of myogenesis, exercise, regeneration, hypoxia, prolonged disuse and natural aging. In this regard, the 2D-GE method has played an essential role in modern muscle biology and the systematic identification of the molecular components that form the functional units of contractility and adaptability to changed functional demands. The extensive usage of 2D-GE has been instrumental in the establishment of the highly dynamic skeletal muscle proteome signature that is characterized by an extremely diverse population of protein species. Crucial issues in muscle tissue proteomics are optimum protein extraction and efficient protein separation prior to MS analysis. Currently no single protein biochemical method is capable of separating all of the molecular species that constitute the skeletal muscle proteome. The considerable differences in charge, size, solubility and abundance may result in the under-representation of specific subtypes of peptides and proteins. Independent of the specific separation approach, such as gel-based techniques or liquid chromatography, it is currently not possible to cover the entire range of muscle protein isoforms in large-scale and high-throughput analyses. Technical limitations of the 2D-GE method may especially affect the proteomic analysis of very high-molecular-mass proteins, integral membrane proteins and low-abundance proteins. Although liquid chromatography has the advantage of being able to efficiently separate highly hydrophobic proteins, this method cannot routinely determine the characteristic combination of the relative molecular mass and the pI-value of a specific protein of interest. Hence, the direct visualization of muscle proteins as discrete 2D spots can be useful to clearly determine whether smaller fragments or a full-length protein have been identified by proteomics. Therefore, using a combination of different biochemical techniques with overlapping separation capabilities for dissimilar subtypes of proteins would be the best way to cover the majority of muscle protein species in comprehensive proteomic studies. With the rapid advances in MS technology, it can be expected that future proteomic investigations will establish an even more refined understanding of the interactions between regulatory proteins, contractile elements, cytoskeletal proteins, extracellular matrix proteins, metabolic enzymes, signaling complexes, ion-handling complexes and molecular chaperones to form the structural and functional basis of the neuromuscular system.
Acknowledgments
The author’s laboratory was supported by grants from Muscular Dystrophy Ireland, the Health Research Board, the Deutsche Duchenne Stiftung aktion benni & co e.V., the Irish Higher Education Authority and the Hume Scholarship programme of Maynooth University. The authors wish to thank their many national and international colleagues for their continued support of our skeletal muscle proteomics project.
Abbreviations
The following abbreviations are used in this manuscript:
| 2D | two-dimensional |
| ACT | actin |
| DIGE | difference in-gel electrophoresis |
| GE | gel electrophoresis |
| Hsp | heat shock protein |
| IEF | isoelectric focusing |
| LC | liquid chromatography |
| MBP | myosin binding protein |
| MLC | myosin light chain |
| MS | mass spectrometry |
| MyHC | myosin heavy chain |
| PAGE | polyacrylamide gel electrophoresis |
| p I | isoelectric point |
| PTM | post-translational modification |
| SDS | sodium dodecyl sulfate |
| TM | tropomyosin |
| TN | troponin |
Author Contributions
S.M., P.D. and K.O. discussed this review article in detail and wrote it collectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Righetti P.G. Bioanalysis: Its past, present, and some future. Electrophoresis. 2004;25:2111–2127. doi: 10.1002/elps.200305808. [DOI] [PubMed] [Google Scholar]
- 2.Rible H. Historical and theoretical aspects of isoelectric focusing. Ann. N. Y. Acad. Sci. 1973;209:11–22. doi: 10.1111/j.1749-6632.1973.tb47515.x. [DOI] [PubMed] [Google Scholar]
- 3.Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J. Biol. Chem. 1969;244:4406–4412. [PubMed] [Google Scholar]
- 4.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 5.Chrambach A., Rodbard D. Polyacrylamide gel electrophoresis. Science. 1971;172:440–451. doi: 10.1126/science.172.3982.440. [DOI] [PubMed] [Google Scholar]
- 6.Kubota K., Kosaka T., Ichikawa K. Combination of two-dimensional electrophoresis and shotgun peptide sequencing in comparative proteomics. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005;815:3–9. doi: 10.1016/j.jchromb.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 7.Friedman D.B., Hoving S., Westermeier R. Isoelectric focusing and two-dimensional gel electrophoresis. Methods Enzymol. 2009;463:515–540. doi: 10.1016/S0076-6879(09)63030-5. [DOI] [PubMed] [Google Scholar]
- 8.Klose J. From 2-D electrophoresis to proteomics. Electrophoresis. 2009;30(Suppl. 1):S142–S149. doi: 10.1002/elps.200900118. [DOI] [PubMed] [Google Scholar]
- 9.O’Farrell P.H. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 10.Klose J. Protein mapping by combined isoelectric focusing and electrophoresis of mouse tissues. A novel approach to testing for induced point mutations in mammals. Humangenetik. 1975;26:231–243. doi: 10.1007/BF00281458. [DOI] [PubMed] [Google Scholar]
- 11.Scheele G.A. Two-dimensional gel analysis of soluble proteins. Charaterization of guinea pig exocrine pancreatic proteins. J. Biol. Chem. 1975;250:5375–5385. [PubMed] [Google Scholar]
- 12.Iborra F., Buhler J.M. Protein subunit mapping. A sensitive high resolution method. Anal. Biochem. 1976;74:503–511. doi: 10.1016/0003-2697(76)90232-3. [DOI] [PubMed] [Google Scholar]
- 13.Anderson L., Anderson N.G. High resolution two-dimensional electrophoresis of human plasma proteins. Proc. Natl. Acad. Sci. USA. 1977;74:5421–5425. doi: 10.1073/pnas.74.12.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klose J., Kobalz U. Two-dimensional electrophoresis of proteins: An updated protocol and implications for a functional analysis of the genome. Electrophoresis. 1995;16:1034–1059. doi: 10.1002/elps.11501601175. [DOI] [PubMed] [Google Scholar]
- 15.Görg A., Weiss W., Dunn M.J. Current two-dimensional electrophoresis technology for proteomics. Proteomics. 2004;4:3665–3685. doi: 10.1002/pmic.200401031. [DOI] [PubMed] [Google Scholar]
- 16.Rabilloud T., Chevallet M., Luche S., Lelong C. Two-dimensional gel electrophoresis in proteomics: Past, present and future. J. Proteom. 2010;73:2064–2077. doi: 10.1016/j.jprot.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira B.M., Coorssen J.R., Martins-de-Souza D. 2DE: The phoenix of proteomics. J. Proteom. 2014;104:140–150. doi: 10.1016/j.jprot.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 18.Doran P., Donoghue P., O’Connell K., Gannon J., Ohlendieck K. Proteomic profiling of pathological and aged skeletal muscle fibres by peptide mass fingerprinting (Review) Int. J. Mol. Med. 2007;19:547–564. doi: 10.3892/ijmm.19.4.547. [DOI] [PubMed] [Google Scholar]
- 19.Ohlendieck K. Proteomics of skeletal muscle differentiation, neuromuscular disorders and fiber aging. Expert Rev. Proteom. 2010;7:283–296. doi: 10.1586/epr.10.2. [DOI] [PubMed] [Google Scholar]
- 20.Gelfi C., Vasso M., Cerretelli P. Diversity of human skeletal muscle in health and disease: Contribution of proteomics. J. Proteom. 2011;74:774–795. doi: 10.1016/j.jprot.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 21.Ohlendieck K. Skeletal muscle proteomics: Current approaches, technical challenges and emerging techniques. Skelet. Muscle. 2011;1:6. doi: 10.1186/2044-5040-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dowling D., Murphy S., Ohlendieck K. Proteomic profiling of muscle fibre type shifting in neuromuscular diseases. Expert Rev. Proteom. 2016;13:783–799. doi: 10.1080/14789450.2016.1209416. [DOI] [PubMed] [Google Scholar]
- 23.Ohlendieck K. Proteomic identification of biomarkers of skeletal muscle disorders. Biomark. Med. 2013;7:169–186. doi: 10.2217/bmm.12.96. [DOI] [PubMed] [Google Scholar]
- 24.Giometti C.S. Muscle protein analysis by two-dimensional gel electrophoresis. Crit. Rev. Clin. Lab. Sci. 1982;18:79–109. doi: 10.3109/10408368209082590. [DOI] [PubMed] [Google Scholar]
- 25.US National Library of Medicine, National Institutes of Health PubMed Site. [(accessed on 15 August 2016)]; Available online: http://www.ncbi.nlm.nih.gov/pubm.
- 26.Pette D. The adaptive potential of skeletal muscle fibers. Can. J. Appl. Physiol. 2002;27:423–448. doi: 10.1139/h02-023. [DOI] [PubMed] [Google Scholar]
- 27.Schiaffino S., Reggiani C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 28.Pette D., Staron R.S. Myosin isoforms, muscle fiber types, and transitions. Microsc. Res. Tech. 2000;50:500–509. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Frontera W.R., Ochala J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015;96:183–195. doi: 10.1007/s00223-014-9915-y. [DOI] [PubMed] [Google Scholar]
- 30.Weigert C., Lehmann R., Hartwig S., Lehr S. The secretome of the working human skeletal muscle—A promising opportunity to combat the metabolic disaster? Proteom. Clin. Appl. 2014;8:5–18. doi: 10.1002/prca.201300094. [DOI] [PubMed] [Google Scholar]
- 31.Sherton C.C., Wool I.G. A comparison of the proteins of rat skeletal muscle and liver ribosomes by two-dimensional polyacrylamide gel electrophoresis. Observations on the partition of proteins between ribosomal subunits and a description of two acidic proteins in the large subunit. J. Biol. Chem. 1974;249:2258–2267. [PubMed] [Google Scholar]
- 32.Barrett E.J., Headon D.R. Two-dimensional polyacrylamide gel electrophoresis of rabbit skeletal muscle microsomal proteins. FEBS Lett. 1975;51:12112–12115. doi: 10.1016/0014-5793(75)80867-2. [DOI] [PubMed] [Google Scholar]
- 33.Izant J.G., Lazarides E. Invariance and heterogeneity in the major structural and regulatory proteins of chick muscle cells revealed by two-dimensional gel electrophoresis. Proc. Natl. Acad. Sci. USA. 1977;74:1450–1454. doi: 10.1073/pnas.74.4.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zechel K. Localization of the charge differences in the actins of rabbit skeletal muscle and chicken gizzard by two-dimensional gel electrophoretic analysis of tryptic fragments. Hoppe Seylers Z. Physiol. Chem. 1979;360:777–782. doi: 10.1515/bchm2.1979.360.1.777. [DOI] [PubMed] [Google Scholar]
- 35.Giometti C.S., Anderson N.G., Anderson N.L. Muscle protein analysis. I. High-resolution two-dimensional electrophoresis of skeletal muscle proteins for analysis of small biopsy samples. Clin. Chem. 1979;25:1877–1884. [PubMed] [Google Scholar]
- 36.Giometti C.S., Danon M.J., Anderson N.G. Human muscle proteins: Analysis by two-dimensional electrophoresis. Neurology. 1983;33:1152–1156. doi: 10.1212/WNL.33.9.1152. [DOI] [PubMed] [Google Scholar]
- 37.Billeter R., Heizmann C.W., Howald H., Jenny E. Analysis of myosin light and heavy chain types in single human skeletal muscle fibers. Eur. J. Biochem. 1981;116:389–395. doi: 10.1111/j.1432-1033.1981.tb05347.x. [DOI] [PubMed] [Google Scholar]
- 38.Billeter R., Heizmann C.W., Reist U., Howald H., Jenny E. Two-dimensional peptide analysis of myosin heavy chains and actin from single-typed human skeletal muscle fibers. FEBS Lett. 1982;139:45–48. doi: 10.1016/0014-5793(82)80483-3. [DOI] [PubMed] [Google Scholar]
- 39.O’Farrell P.H. The pre-omics era: The early days of two-dimensional gels. Proteomics. 2008;8:4842–4852. doi: 10.1002/pmic.200800719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bárány K., Bárány M., Giometti C.S. Polyacrylamide gel electrophoretic methods in the separation of structural muscle proteins. J. Chromatogr. A. 1995;698:301–332. doi: 10.1016/0021-9673(94)01189-L. [DOI] [PubMed] [Google Scholar]
- 41.Dunn M.J. Quantitative two-dimensional gel electrophoresis: From proteins to proteomes. Biochem. Soc. Trans. 1997;25:248–254. doi: 10.1042/bst0250248. [DOI] [PubMed] [Google Scholar]
- 42.Ohlendieck K. Proteomic profiling of skeletal muscle plasticity. Muscles Ligaments Tendons J. 2012;1:119–126. [PMC free article] [PubMed] [Google Scholar]
- 43.Holland A., Ohlendieck K. Proteomic profiling of the contractile apparatus from skeletal muscle. Expert Rev. Proteom. 2013;10:239–257. doi: 10.1586/epr.13.20. [DOI] [PubMed] [Google Scholar]
- 44.López J.L. Two-dimensional electrophoresis in proteome expression analysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007;849:190–202. doi: 10.1016/j.jchromb.2006.11.049. [DOI] [PubMed] [Google Scholar]
- 45.Rabilloud T., Lelong C. Two-dimensional gel electrophoresis in proteomics: A tutorial. J. Proteom. 2011;74:1829–18241. doi: 10.1016/j.jprot.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 46.Rogowska-Wrzesinska A., Le Bihan M.C., Thaysen-Andersen M., Roepstorff P. 2D gels still have a niche in proteomics. J. Proteom. 2013;88:4–13. doi: 10.1016/j.jprot.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 47.Link A.J. Methods in Molecular Biology. Volume 112. Humana Press; Totowa, NJ, USA: 1999. 2-D Proteome Analysis Protocols; pp. 1–601. [Google Scholar]
- 48.Reinders J., Sickmann A. Methods in Molecular Biology. Volume 564. Humana Press; Totowa, NJ, USA: 2009. Proteomics Methods and Protocols; pp. 1–431. [Google Scholar]
- 49.Cramer R., Westermeier R. Methods in Molecular Biology. Volume 854. Humana Press; Totowa, NJ, USA: 2012. Difference Gel Electrophoresis (DIGE) Methods and Protocols; pp. 1–401. [Google Scholar]
- 50.Kurien B.T., Scofield R.H. Methods in Molecular Biology. Volume 869. Humana Press; Totowa, NJ, USA: 2012. Protein Electrophoresis Methods and Protocols; pp. 1–648. [Google Scholar]
- 51.Marengo E., Robotti E. Methods in Molecular Biology. Volume 1384. Humana Press; Totowa, NJ, USA: 2016. 2D PAGE Map Analysis Methods and Protocols; pp. 1–331. [Google Scholar]
- 52.Patton W.F. Detection technologies in proteome analysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;771:3–31. doi: 10.1016/S1570-0232(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 53.Westermeier R., Marouga R. Protein detection methods in proteomics research. Biosci. Rep. 2005;25:19–32. doi: 10.1007/s10540-005-2845-1. [DOI] [PubMed] [Google Scholar]
- 54.Miller I., Crawford J., Gianazza E. Protein stains for proteomic applications: Which, when, why? Proteomics. 2006;6:5385–5408. doi: 10.1002/pmic.200600323. [DOI] [PubMed] [Google Scholar]
- 55.Riederer B.M. Non-covalent and covalent protein labeling in two-dimensional gel electrophoresis. J. Proteom. 2008;71:231–244. doi: 10.1016/j.jprot.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 56.Weiss W., Weiland F., Görg A. Protein detection and quantitation technologies for gel-based proteome analysis. Methods Mol. Biol. 2009;564:59–82. doi: 10.1007/978-1-60761-157-8_4. [DOI] [PubMed] [Google Scholar]
- 57.Gauci V.J., Wright E.P., Coorssen J.R. Quantitative proteomics: Assessing the spectrum of in-gel protein detection methods. J. Chem. Biol. 2011;4:3–29. doi: 10.1007/s12154-010-0043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurien B.T., Scofield R.H. A brief review of other notable protein detection methods on acrylamide gels. Methods Mol. Biol. 2012;869:617–620. doi: 10.1007/978-1-61779-821-4_56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panfoli I., Calzia D., Santucci L., Ravera S., Bruschi M., Candiano G. A blue dive: From ‘blue fingers’ to ‘blue silver’. A comparative overview of staining methods for in-gel proteomics. Expert Rev. Proteom. 2012;9:627–634. doi: 10.1586/epr.12.63. [DOI] [PubMed] [Google Scholar]
- 60.Butt R.H., Coorssen J.R. Coomassie blue as a near-infrared fluorescent stain: A systematic comparison with Sypro Ruby for in-gel protein detection. Mol. Cell. Proteom. 2013;12:3834–3850. doi: 10.1074/mcp.M112.021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yates J.R., Ruse C.I., Nakorchevsky A. Proteomics by mass spectrometry: Approaches, advances, and applications. Annu. Rev. Biomed. Eng. 2009;11:49–79. doi: 10.1146/annurev-bioeng-061008-124934. [DOI] [PubMed] [Google Scholar]
- 62.Altelaar A.F., Heck A.J. Trends in ultrasensitive proteomics. Curr. Opin. Chem. Biol. 2012;16:206–213. doi: 10.1016/j.cbpa.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 63.Cox J., Mann M. Quantitative, high-resolution proteomics for data-driven systems biology. Annu. Rev. Biochem. 2011;80:273–299. doi: 10.1146/annurev-biochem-061308-093216. [DOI] [PubMed] [Google Scholar]
- 64.Faini M., Stengel F., Aebersold R. The Evolving Contribution of Mass Spectrometry to Integrative Structural Biology. J. Am. Soc. Mass Spectrom. 2016;27:966–974. doi: 10.1007/s13361-016-1382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lewis C., Doran P., Ohlendieck K. Proteomic analysis of dystrophic muscle. Methods Mol. Biol. 2012;798:357–369. doi: 10.1007/978-1-61779-343-1_20. [DOI] [PubMed] [Google Scholar]
- 66.Gelfi C., De Palma S. 2D DIGE analysis of protein extracts from muscle tissue. Methods Mol. Biol. 2012;854:155–168. doi: 10.1007/978-1-61779-573-2_11. [DOI] [PubMed] [Google Scholar]
- 67.Reed P.W., Densmore A., Bloch R.J. Optimization of large gel 2D electrophoresis for proteomic studies of skeletal muscle. Electrophoresis. 2012;33:1263–1270. doi: 10.1002/elps.201100642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carberry S., Ohlendieck K. Gel electrophoresis-based proteomics of senescent tissues. Methods Mol. Biol. 2013;1048:229–246. doi: 10.1007/978-1-62703-556-9_17. [DOI] [PubMed] [Google Scholar]
- 69.Sanchez J.C., Chiappe D., Converset V., Hoogland C., Binz P.A., Paesano S., Appel R.D., Wang S., Sennitt M., Nolan A., et al. The mouse SWISS-2D PAGE database: A tool for proteomics study of diabetes and obesity. Proteomics. 2001;1:136–163. doi: 10.1002/1615-9861(200101)1:1<136::AID-PROT136>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 70.Yan J.X., Harry R.A., Wait R., Welson S.Y., Emery P.W., Preedy V.R., Dunn M.J. Separation and identification of rat skeletal muscle proteins using two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2001;1:424–434. doi: 10.1002/1615-9861(200103)1:3<424::AID-PROT424>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 71.Appel R.D., Sanchez J.C., Bairoch A., Golaz O., Miu M., Vargas J.R., Hochstrasser D.F. SWISS-2DPAGE: A database of two-dimensional gel electrophoresis images. Electrophoresis. 1993;14:1232–1238. doi: 10.1002/elps.11501401185. [DOI] [PubMed] [Google Scholar]
- 72.Hoogland C., Mostaguir K., Sanchez J.C., Hochstrasser D.F., Appel R.D. SWISS-2DPAGE, ten years later. Proteomics. 2004;4:2352–2356. doi: 10.1002/pmic.200300830. [DOI] [PubMed] [Google Scholar]
- 73.Lemkin P.F. The 2DWG meta-database of two-dimensional electrophoretic gel images on the Internet. Electrophoresis. 1997;18:2759–2773. doi: 10.1002/elps.1150181510. [DOI] [PubMed] [Google Scholar]
- 74.Lemkin P.F., Thornwall G. Flicker image comparison of 2-D gel images for putative protein identification using the 2DWG meta-database. Mol. Biotechnol. 1999;12:159–172. doi: 10.1385/MB:12:2:159. [DOI] [PubMed] [Google Scholar]
- 75.Babnigg G., Giometti C.S. ProteomeWeb: A web-based interface for the display and interrogation of proteomes. Proteomics. 2003;3:584–600. doi: 10.1002/pmic.200300396. [DOI] [PubMed] [Google Scholar]
- 76.Babnigg G., Giometti C.S. GELBANK: A database of annotated two-dimensional gel electrophoresis patterns of biological systems with completed genomes. Nucleic Acids Res. 2004;32:D582–D585. doi: 10.1093/nar/gkh089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pernet P., Bruneel A., Baudin B., Vaubourdolle M. PHProteomicDB: A module for two-dimensional gel electrophoresis database creation on personal web sites. Genom. Proteom. Bioinform. 2006;4:134–136. doi: 10.1016/S1672-0229(06)60024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Laukens K., Matthiesen R., Lemière F., Esmans E., Onckelen H.V., Jensen O.N., Witters E. Integration of gel-based proteome data with pProRep. Bioinformatics. 2006;22:2838–2840. doi: 10.1093/bioinformatics/btl487. [DOI] [PubMed] [Google Scholar]
- 79.Piñeiro C., Vázquez J., Marina A.I., Barros-Velázquez J., Gallardo J.M. Characterization and partial sequencing of species-specific sarcoplasmic polypeptides from commercial hake species by mass spectrometry following two-dimensional electrophoresis. Electrophoresis. 2001;22:1545–1552. doi: 10.1002/1522-2683(200105)22:8<1545::AID-ELPS1545>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 80.Clarke A.J., Knight C., Bass J., Cooper G.J. Identification and characterization of a bovine myosin light chain-1 fast polymorphism. Proteomics. 2001;1:1495–1502. doi: 10.1002/1615-9861(200111)1:12<1495::AID-PROT1495>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 81.Brewis I.A., Brennan P. Proteomics technologies for the global identification and quantification of proteins. Adv. Protein Chem. Struct. Biol. 2010;80:1–44. doi: 10.1016/B978-0-12-381264-3.00001-1. [DOI] [PubMed] [Google Scholar]
- 82.Roepstorff P. Mass spectrometry based proteomics, background, status and future needs. Protein Cell. 2012;3:641–647. doi: 10.1007/s13238-012-2079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ohlendieck K. Proteomics of skeletal muscle glycolysis. Biochim. Biophys. Acta. 2010;1804:2089–2101. doi: 10.1016/j.bbapap.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 84.Kashino Y., Harayama T., Pakrasi H.B., Satoh K. Preparation of membrane proteins for analysis by two-dimensional gel electrophoresis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007;849:282–292. doi: 10.1016/j.jchromb.2006.10.067. [DOI] [PubMed] [Google Scholar]
- 85.Tan S., Tan H.T., Chung M.C. Membrane proteins and membrane proteomics. Proteomics. 2008;8:3924–3932. doi: 10.1002/pmic.200800597. [DOI] [PubMed] [Google Scholar]
- 86.Sadowski P.G., Groen A.J., Dupree P., Lilley K.S. Subcellular localization of membrane proteins. Proteomics. 2008;8:3991–4011. doi: 10.1002/pmic.200800217. [DOI] [PubMed] [Google Scholar]
- 87.Zheng Y.Z., Foster L.J. Biochemical and proteomic approaches for the study of membrane microdomains. J. Proteom. 2009;72:12–22. doi: 10.1016/j.jprot.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 88.Manadas B., English J.A., Wynne K.J., Cotter D.R., Dunn M.J. Comparative analysis of OFFGel, strong cation exchange with pH gradient, and RP at high pH for first-dimensional separation of peptides from a membrane-enriched protein fraction. Proteomics. 2009;9:5194–5198. doi: 10.1002/pmic.200900349. [DOI] [PubMed] [Google Scholar]
- 89.Corbett J.M., Dunn M.J., Posch A., Görg A. Positional reproducibility of protein spots in two-dimensional polyacrylamide gel electrophoresis using immobilised pH gradient isoelectric focusing in the first dimension: An interlaboratory comparison. Electrophoresis. 1994;15:1205–1211. doi: 10.1002/elps.11501501182. [DOI] [PubMed] [Google Scholar]
- 90.Westbrook J.A., Yan J.X., Wait R., Welson S.Y., Dunn M.J. Zooming-in on the proteome: Very narrow-range immobilised pH gradients reveal more protein species and isoforms. Electrophoresis. 2001;22:2865–2871. doi: 10.1002/1522-2683(200108)22:14<2865::AID-ELPS2865>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 91.Zuo X., Speicher D.W. Comprehensive analysis of complex proteomes using microscale solution isoelectrofocusing prior to narrow pH range two-dimensional electrophoresis. Proteomics. 2002;2:58–68. doi: 10.1002/1615-9861(200201)2:1<58::AID-PROT58>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 92.Görg A., Drews O., Lück C., Weiland F., Weiss W. 2-DE with IPGs. Electrophoresis. 2009;30(Suppl. 1):S122–S132. doi: 10.1002/elps.200900051. [DOI] [PubMed] [Google Scholar]
- 93.Staunton L., Jockusch H., Wiegand C., Albrecht T., Ohlendieck K. Identification of secondary effects of hyperexcitability by proteomic profiling of myotonic mouse muscle. Mol. Biosyst. 2011;7:2480–2489. doi: 10.1039/c1mb05043e. [DOI] [PubMed] [Google Scholar]
- 94.Jarrold B., DeMuth J., Greis K., Burt T., Wang F. An effective skeletal muscle prefractionation method to remove abundant structural proteins for optimized two-dimensional gel electrophoresis. Electrophoresis. 2005;26:2269–2278. doi: 10.1002/elps.200410367. [DOI] [PubMed] [Google Scholar]
- 95.Nordhoff E., Egelhofer V., Giavalisco P., Eickhoff H., Horn M., Przewieslik T., Theiss D., Schneider U., Lehrach H., Gobom J. Large-gel two-dimensional electrophoresis-matrix assisted laser desorption/ionization-time of flight-mass spectrometry: An analytical challenge for studying complex protein mixtures. Electrophoresis. 2001;22:2844–2855. doi: 10.1002/1522-2683(200108)22:14<2844::AID-ELPS2844>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 96.Righetti P.G., Castagna A., Herbert B., Candiano G. How to bring the “unseen” proteome to the limelight via electrophoretic pre-fractionation techniques. Biosci. Rep. 2005;25:3–17. doi: 10.1007/s10540-005-2844-2. [DOI] [PubMed] [Google Scholar]
- 97.Vercauteren F.G., Arckens L., Quirion R. Applications and current challenges of proteomic approaches, focusing on two-dimensional electrophoresis. Amino Acids. 2007;33:405–414. doi: 10.1007/s00726-006-0460-5. [DOI] [PubMed] [Google Scholar]
- 98.Groen A.J., Lilley K.S. Proteomics of total membranes and subcellular membranes. Expert Rev. Proteom. 2010;7:867–878. doi: 10.1586/epr.10.85. [DOI] [PubMed] [Google Scholar]
- 99.Lewis C., Ohlendieck K. Mass spectrometric identification of dystrophin isoform Dp427 by on-membrane digestion of sarcolemma from skeletal muscle. Anal. Biochem. 2010;404:197–203. doi: 10.1016/j.ab.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 100.Donoghue P., Staunton L., Mullen E., Manning G., Ohlendieck K. DIGE analysis of rat skeletal muscle proteins using nonionic detergent phase extraction of young adult versus aged gastrocnemius tissue. J. Proteom. 2010;73:1441–1453. doi: 10.1016/j.jprot.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 101.Staunton L., Ohlendieck K. Mass spectrometric characterization of the sarcoplasmic reticulum from rabbit skeletal muscle by on-membrane digestion. Protein Pept. Lett. 2012;19:252–263. doi: 10.2174/092986612799363208. [DOI] [PubMed] [Google Scholar]
- 102.Ohlendieck K. On-Membrane Digestion Technology for Muscle Proteomics. J. Membr. Sep. Technol. 2013;2:1–12. doi: 10.6000/1929-6037.2013.02.01.1. [DOI] [Google Scholar]
- 103.Carberry S., Zweyer M., Swandulla D., Ohlendieck K. Comparative proteomic analysis of the contractile-protein-depleted fraction from normal versus dystrophic skeletal muscle. Anal. Biochem. 2014;446:108–115. doi: 10.1016/j.ab.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 104.Gauci V.J., Padula M.P., Coorssen J.R. Coomassie blue staining for high sensitivity gel-based proteomics. J. Proteom. 2013;90:96–106. doi: 10.1016/j.jprot.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 105.O’Connell K., Doran P., Gannon J., Ohlendieck K. Lectin-based proteomic profiling of aged skeletal muscle: Decreased pyruvate kinase isozyme M1 exhibits drastically increased levels of N-glycosylation. Eur. J. Cell Biol. 2008;87:793–805. doi: 10.1016/j.ejcb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 106.Gannon J., Staunton L., O’Connell K., Doran P., Ohlendieck K. Phosphoproteomic analysis of aged skeletal muscle. Int. J. Mol. Med. 2008;22:33–42. doi: 10.3892/ijmm.22.1.33. [DOI] [PubMed] [Google Scholar]
- 107.Agrawal G.K., Thelen J.J. A high-resolution two dimensional Gel- and Pro-Q DPS-based proteomics workflow for phosphoprotein identification and quantitative profiling. Methods Mol. Biol. 2009;527:3–19. doi: 10.1007/978-1-60327-834-8_1. [DOI] [PubMed] [Google Scholar]
- 108.Minden J.S., Dowd S.R., Meyer H.E., Stühler K. Difference gel electrophoresis. Electrophoresis. 2009;30(Suppl. 1):S156–S161. doi: 10.1002/elps.200900098. [DOI] [PubMed] [Google Scholar]
- 109.Arentz G., Weiland F., Oehler M.K., Hoffmann P. State of the art of 2D DIGE. Proteom. Clin. Appl. 2015;9:277–288. doi: 10.1002/prca.201400119. [DOI] [PubMed] [Google Scholar]
- 110.Isfort R.J. Proteomic analysis of striated muscle. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;771:155–165. doi: 10.1016/S1570-0232(02)00056-9. [DOI] [PubMed] [Google Scholar]
- 111.Gelfi C., De Palma S., Cerretelli P., Begum S., Wait R. Two-dimensional protein map of human vastus lateralis muscle. Electrophoresis. 2003;24:286–295. doi: 10.1002/elps.200390025. [DOI] [PubMed] [Google Scholar]
- 112.Li Z.B., Lehar M., Braga N., Westra W., Liu L.H., Flint P.W. Study of human laryngeal muscle protein using two-dimensional electrophoresis and mass spectrometry. Proteomics. 2003;3:1325–1334. doi: 10.1002/pmic.200300454. [DOI] [PubMed] [Google Scholar]
- 113.Bouley J., Chambon C., Picard B. Mapping of bovine skeletal muscle proteins using two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2004;4:1811–1824. doi: 10.1002/pmic.200300688. [DOI] [PubMed] [Google Scholar]
- 114.Kim N.K., Joh J.H., Park H.R., Kim O.H., Park B.Y., Lee C.S. Differential expression profiling of the proteomes and their mRNAs in porcine white and red skeletal muscles. Proteomics. 2004;4:3422–3428. doi: 10.1002/pmic.200400976. [DOI] [PubMed] [Google Scholar]
- 115.Capitanio D., Viganò A., Ricci E., Cerretelli P., Wait R., Gelfi C. Comparison of protein expression in human deltoideus and vastus lateralis muscles using two-dimensional gel electrophoresis. Proteomics. 2005;5:2577–2586. doi: 10.1002/pmic.200401183. [DOI] [PubMed] [Google Scholar]
- 116.Okumura N., Hashida-Okumura A., Kita K., Matsubae M., Matsubara T., Takao T., Nagai K. Proteomic analysis of slow- and fast-twitch skeletal muscles. Proteomics. 2005;5:2896–2906. doi: 10.1002/pmic.200401181. [DOI] [PubMed] [Google Scholar]
- 117.Le Bihan M.C., Hou Y., Harris N., Tarelli E., Coulton G.R. Proteomic analysis of fast and slow muscles from normal and kyphoscoliotic mice using protein arrays, 2-DE and MS. Proteomics. 2006;6:4646–4661. doi: 10.1002/pmic.200500746. [DOI] [PubMed] [Google Scholar]
- 118.Gelfi C., Viganò A., De Palma S., Ripamonti M., Begum S., Cerretelli P., Wait R. 2-D protein maps of rat gastrocnemius and soleus muscles: A tool for muscle plasticity assessment. Proteomics. 2006;6:321–340. doi: 10.1002/pmic.200501337. [DOI] [PubMed] [Google Scholar]
- 119.Chaze T., Bouley J., Chambon C., Barboiron C., Picard B. Mapping of alkaline proteins in bovine skeletal muscle. Proteomics. 2006;6:2571–2575. doi: 10.1002/pmic.200500452. [DOI] [PubMed] [Google Scholar]
- 120.Chiu K.H., Huang H.W., Mok H.K. Differential proteome analysis of hagfish dental and somatic skeletal muscles. Mar. Biotechnol (N.Y.) 2007;9:689–700. doi: 10.1007/s10126-007-9020-6. [DOI] [PubMed] [Google Scholar]
- 121.Raddatz K., Albrecht D., Hochgräfe F., Hecker M., Gotthardt M. A proteome map of murine heart and skeletal muscle. Proteomics. 2008;8:1885–1897. doi: 10.1002/pmic.200700902. [DOI] [PubMed] [Google Scholar]
- 122.Almeida A.M., Campos A., van Harten S., Cardoso L.A., Coelho A.V. Establishment of a proteomic reference map for the gastrocnemius muscle in the rabbit (Oryctolagus cuniculus) Res. Vet. Sci. 2009;87:196–199. doi: 10.1016/j.rvsc.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 123.Addis M.F., Tanca A., Pagnozzi D., Rocca S., Uzzau S. 2-D PAGE and MS analysis of proteins from formalin-fixed, paraffin-embedded tissues. Proteomics. 2009;9:4329–4339. doi: 10.1002/pmic.200900010. [DOI] [PubMed] [Google Scholar]
- 124.Kovalyova M.A., Kovalyov L.I., Toropygin I.Y., Shigeev S.V., Ivanov A.V., Shishkin S.S. Proteomic analysis of human skeletal muscle (m. vastus lateralis) proteins: Identification of 89 gene expression products. Biochemistry. 2009;74:1239–1252. doi: 10.1134/S0006297909110108. [DOI] [PubMed] [Google Scholar]
- 125.Lu J., Zheng J., Liu H., Li J., Chen H., Chen K. Protein profiling analysis of skeletal muscle of a pufferfish, Takifugu rubripes. Mol. Biol. Rep. 2010;37:2141–2147. doi: 10.1007/s11033-009-9684-2. [DOI] [PubMed] [Google Scholar]
- 126.Abbaraju N.V., Boutaghou M.N., Townley I.K., Zhang Q., Wang G., Cole R.B., Rees B.B. Analysis of tissue proteomes of the Gulf killifish, Fundulus grandis, by 2D electrophoresis and MALDI-TOF/TOF mass spectrometry. Integr. Comp. Biol. 2012;52:626–635. doi: 10.1093/icb/ics063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ohlendieck K. Organelle proteomics in skeletal muscle biology. J. Integr. OMICS. 2012;2:27–38. doi: 10.5584/jiomics.v2i2.111. [DOI] [Google Scholar]
- 128.Vitorino R., Ferreira R., Neuparth M., Guedes S., Williams J., Tomer K.B., Domingues P.M., Appell H.J., Duarte J.A., Amado F.M. Subcellular proteomics of mice gastrocnemius and soleus muscles. Anal. Biochem. 2007;366:156–169. doi: 10.1016/j.ab.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Reifschneider N.H., Goto S., Nakamoto H., Takahashi R., Sugawa M., Dencher N.A., Krause F. Defining the mitochondrial proteomes from five rat organs in a physiologically significant context using 2D blue-native/SDS-PAGE. J. Proteome Res. 2006;5:1117–1132. doi: 10.1021/pr0504440. [DOI] [PubMed] [Google Scholar]
- 130.O’Connell K., Ohlendieck K. Proteomic DIGE analysis of the mitochondria-enriched fraction from aged rat skeletal muscle. Proteomics. 2009;9:5509–5524. doi: 10.1002/pmic.200900472. [DOI] [PubMed] [Google Scholar]
- 131.Lombardi A., Silvestri E., Cioffi F., Senese R., Lanni A., Goglia F., de Lange P., Moreno M. Defining the transcriptomic and proteomic profiles of rat ageing skeletal muscle by the use of a cDNA array, 2D- and Blue native-PAGE approach. J. Proteom. 2009;72:708–721. doi: 10.1016/j.jprot.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 132.Ferreira R., Vitorino R., Alves R.M., Appell H.J., Powers S.K., Duarte J.A., Amado F. Subsarcolemmal and intermyofibrillar mitochondria proteome differences disclose functional specializations in skeletal muscle. Proteomics. 2010;10:3142–3154. doi: 10.1002/pmic.201000173. [DOI] [PubMed] [Google Scholar]
- 133.Gannon J., Doran P., Kirwan A., Ohlendieck K. Drastic increase of myosin light chain MLC-2 in senescent skeletal muscle indicates fast-to-slow fibre transition in sarcopenia of old age. Eur. J. Cell Biol. 2009;88:685–700. doi: 10.1016/j.ejcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 134.Gajendran N., Frey J.R., Lefkovits I., Kuhn L., Fountoulakis M., Krapfenbauer K., Brenner H.R. Proteomic analysis of secreted muscle components: Search for factors involved in neuromuscular synapse formation. Proteomics. 2002;2:1601–1615. doi: 10.1002/1615-9861(200211)2:11<1601::AID-PROT1601>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 135.Hartwig S., Raschke S., Knebel B., Scheler M., Irmler M., Passlack W., Muller S., Hanisch F.G., Franz T., Li X., et al. Secretome profiling of primary human skeletal muscle cells. Biochim. Biophys. Acta. 2014;1844:1011–1017. doi: 10.1016/j.bbapap.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 136.Kanski J., Alterman M.A., Schöneich C. Proteomic identification of age-dependent protein nitration in rat skeletal muscle. Free Radic. Biol. Med. 2003;35:1229–1239. doi: 10.1016/S0891-5849(03)00500-8. [DOI] [PubMed] [Google Scholar]
- 137.Cieniewski-Bernard C., Bastide B., Lefebvre T., Lemoine J., Mounier Y., Michalski J.C. Identification of O-linked N-acetylglucosamine proteins in rat skeletal muscle using two-dimensional gel electrophoresis and mass spectrometry. Mol. Cell. Proteom. 2004;3:577–585. doi: 10.1074/mcp.M400024-MCP200. [DOI] [PubMed] [Google Scholar]
- 138.Maughan D.W., Henkin J.A., Vigoreaux J.O. Concentrations of glycolytic enzymes and other cytosolic proteins in the diffusible fraction of a vertebrate muscle proteome. Mol. Cell. Proteom. 2005;4:1541–1549. doi: 10.1074/mcp.M500053-MCP200. [DOI] [PubMed] [Google Scholar]
- 139.Picariello G., De Martino A., Mamone G., Ferranti P., Addeo F., Faccia M., Spagnamusso S., Di Luccia A. Proteomic study of muscle sarcoplasmic proteins using AUT-PAGE/SDS-PAGE as two-dimensional gel electrophoresis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006;833:101–108. doi: 10.1016/j.jchromb.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 140.Forner F., Foster L.J., Campanaro S., Valle G., Mann M. Quantitative proteomic comparison of rat mitochondria from muscle, heart, and liver. Mol. Cell. Proteom. 2006;5:608–619. doi: 10.1074/mcp.M500298-MCP200. [DOI] [PubMed] [Google Scholar]
- 141.Højlund K., Yi Z., Hwang H., Bowen B., Lefort N., Flynn C.R., Langlais P., Weintraub S.T., Mandarino L.J. Characterization of the human skeletal muscle proteome by one-dimensional gel electrophoresis and HPLC-ESI-MS/MS. Mol. Cell. Proteom. 2008;7:257–267. doi: 10.1074/mcp.M700304-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Parker K.C., Walsh R.J., Salajegheh M., Amato A.A., Krastins B., Sarracino D.A., Greenberg S.A. Characterization of human skeletal muscle biopsy samples using shotgun proteomics. J. Proteome Res. 2009;8:3265–3277. doi: 10.1021/pr800873q. [DOI] [PubMed] [Google Scholar]
- 143.Lefort N., Yi Z., Bowen B., Glancy B., De Filippis E.A., Mapes R., Hwang H., Flynn C.R., Willis W.T., Civitarese A., et al. Proteome profile of functional mitochondria from human skeletal muscle using one-dimensional gel electrophoresis and HPLC-ESI-MS/MS. J. Proteom. 2009;72:1046–1060. doi: 10.1016/j.jprot.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Højlund K., Bowen B.P., Hwang H., Flynn C.R., Madireddy L., Geetha T., Langlais P., Meyer C., Mandarino L.J., Yi Z. In vivo phosphoproteome of human skeletal muscle revealed by phosphopeptide enrichment and HPLC-ESI-MS/MS. J. Proteome Res. 2009;8:4954–4965. doi: 10.1021/pr9007267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Malik Z.A., Cobley J.N., Morton J.P., Close G.L., Edwards B.J., Koch L.G., Britton S.L., Burniston J.G. Label-Free LC-MS Profiling of Skeletal Muscle Reveals Heart-Type Fatty Acid Binding Protein as a Candidate Biomarker of Aerobic Capacity. Proteomes. 2013;1:290–308. doi: 10.3390/proteomes1030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Burniston J.G., Connolly J., Kainulainen H., Britton S.L., Koch L.G. Label-free profiling of skeletal muscle using high-definition mass spectrometry. Proteomics. 2014;14:2339–2344. doi: 10.1002/pmic.201400118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu Z., Du X., Deng J., Gu M., Hu H., Gui M., Yin C.C., Chang Z. The interactions between mitochondria and sarcoplasmic reticulum and the proteome characterization of mitochondrion-associated membrane from rabbit skeletal muscle. Proteomics. 2015;15:2701–2704. doi: 10.1002/pmic.201400493. [DOI] [PubMed] [Google Scholar]
- 148.Murgia M., Nagaraj N., Deshmukh A.S., Zeiler M., Cancellara P., Moretti I., Reggiani C., Schiaffino S., Mann M. Single muscle fiber proteomics reveals unexpected mitochondrial specialization. EMBO Rep. 2015;16:387–395. doi: 10.15252/embr.201439757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rakus D., Gizak A., Deshmukh A., Wiśniewski J.R. Absolute quantitative profiling of the key metabolic pathways in slow and fast skeletal muscle. J. Proteome Res. 2015;14:1400–1411. doi: 10.1021/pr5010357. [DOI] [PubMed] [Google Scholar]
- 150.Deshmukhm A.S., Murgia M., Nagaraj N., Treebak J.T., Cox J., Mann M. Deep proteomics of mouse skeletal muscle enables quantitation of protein isoforms, metabolic pathways, and transcription factors. Mol. Cell. Proteom. 2015;14:841–853. doi: 10.1074/mcp.M114.044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Le Bihan M.C., Barrio-Hernandez I., Mortensen T.P., Henningsen J., Jensen S.S., Bigot A., Blagoev B., Butler-Browne G., Kratchmarova I. Cellular Proteome Dynamics during Differentiation of Human Primary Myoblasts. J. Proteome Res. 2015;14:3348–3361. doi: 10.1021/acs.jproteome.5b00397. [DOI] [PubMed] [Google Scholar]
- 152.Buckingham M., Rigby P.W. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell. 2014;28:225–238. doi: 10.1016/j.devcel.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 153.Dayanidhi S., Lieber R.L. Skeletal muscle satellite cells: Mediators of muscle growth during development and implications for developmental disorders. Muscle Nerve. 2014;50:723–732. doi: 10.1002/mus.24441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Egerman M.A., Glass D.J. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014;49:59–68. doi: 10.3109/10409238.2013.857291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hoppeler H. Molecular networks in skeletal muscle plasticity. J. Exp. Biol. 2016;219:205–213. doi: 10.1242/jeb.128207. [DOI] [PubMed] [Google Scholar]
- 156.Tannu N.S., Rao V.K., Chaudhary R.M., Giorgianni F., Saeed A.E., Gao Y., Raghow R. Comparative proteomes of the proliferating C(2)C(12) myoblasts and fully differentiated myotubes reveal the complexity of the skeletal muscle differentiation program. Mol. Cell. Proteom. 2004;3:1065–1082. doi: 10.1074/mcp.M400020-MCP200. [DOI] [PubMed] [Google Scholar]
- 157.Casadei L., Vallorani L., Gioacchini A.M., Guescini M., Burattini S., D’Emilio A., Biagiotti L., Falcieri E., Stocchi V. Proteomics-based investigation in C2C12 myoblast differentiation. Eur. J. Histochem. 2009;53:261–268. doi: 10.4081/ejh.2009.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sun H., Zhu T., Ding F., Hu N., Gu X. Proteomic studies of rat tibialis anterior muscle during postnatal growth and development. Mol. Cell. Biochem. 2009;332:161–171. doi: 10.1007/s11010-009-0186-2. [DOI] [PubMed] [Google Scholar]
- 159.Xu Y., Qian H., Feng X., Xiong Y., Lei M., Ren Z., Zuo B., Xu D., Ma Y., Yuan H. Differential proteome and transcriptome analysis of porcine skeletal muscle during development. J. Proteom. 2012;75:2093–2108. doi: 10.1016/j.jprot.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 160.Chan X.C., McDermott J.C., Siu K.W. Identification of secreted proteins during skeletal muscle development. J. Proteome Res. 2007;6:698–710. doi: 10.1021/pr060448k. [DOI] [PubMed] [Google Scholar]
- 161.Henningsen J., Rigbolt K.T., Blagoev B., Pedersen B.K., Kratchmarova I. Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol. Cell. Proteom. 2010;9:2482–2496. doi: 10.1074/mcp.M110.002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chan C.Y., Masui O., Krakovska O., Belozerov V.E., Voisin S., Ghanny S., Chen J., Moyez D., Zhu P., Evans K.R., et al. Identification of differentially regulated secretome components during skeletal myogenesis. Mol. Cell. Proteom. 2011;10:M110.004804. doi: 10.1074/mcp.M110.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sharma M., McFarlane C., Kambadur R., Kukreti H., Bonala S., Srinivasan S. Myostatin: Expanding horizons. IUBMB Life. 2015;67:589–600. doi: 10.1002/iub.1392. [DOI] [PubMed] [Google Scholar]
- 164.Bouley J., Meunier B., Chambon C., De Smet S., Hocquette J.F., Picard B. Proteomic analysis of bovine skeletal muscle hypertrophy. Proteomics. 2005;5:490–500. doi: 10.1002/pmic.200400925. [DOI] [PubMed] [Google Scholar]
- 165.Keady S.M., Kenny D.A., Ohlendieck K., Doyle S., Keane M.G., Waters S.M. Proteomic profiling of bovine M. longissimus lumborum from Crossbred Aberdeen Angus and Belgian Blue sired steers varying in genetic merit for carcass weight. J. Anim. Sci. 2013;91:654–665. doi: 10.2527/jas.2012-5850. [DOI] [PubMed] [Google Scholar]
- 166.Gehlert S., Bloch W., Suhr F. Ca2+-dependent regulations and signaling in skeletal muscle: From electro-mechanical coupling to adaptation. Int. J. Mol. Sci. 2015;16:1066–1095. doi: 10.3390/ijms16011066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Blaauw B., Schiaffino S., Reggiani C. Mechanisms modulating skeletal muscle phenotype. Compr. Physiol. 2013;3:1645–1687. doi: 10.1002/cphy.c130009. [DOI] [PubMed] [Google Scholar]
- 168.Burniston J.G., Hoffman E.P. Proteomic responses of skeletal and cardiac muscle to exercise. Expert Rev. Proteom. 2011;8:361–377. doi: 10.1586/epr.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Petriz B.A., Gomes C.P., Rocha L.A., Rezende T.M., Franco O.L. Proteomics applied to exercise physiology: A cutting-edge technology. J. Cell. Physiol. 2012;227:885–898. doi: 10.1002/jcp.22809. [DOI] [PubMed] [Google Scholar]
- 170.Ohlendieck K. Proteomics of exercise-induced skeletal muscle adaptations. OA Sports Med. 2013;1:3. doi: 10.13172/2053-2040-1-1-565. [DOI] [Google Scholar]
- 171.Padrão A.I., Ferreira R., Amado F., Vitorino R., Duarte J.A. Uncovering the exercise-related proteome signature in skeletal muscle. Proteomics. 2016;16:816–830. doi: 10.1002/pmic.201500382. [DOI] [PubMed] [Google Scholar]
- 172.Holloway K.V., O’Gorman M., Woods P., Morton J.P., Evans L., Cable N.T., Goldspink D.F., Burniston J.G. Proteomic investigation of changes in human vastus lateralis muscle in response to interval-exercise training. Proteomics. 2009;9:5155–5174. doi: 10.1002/pmic.200900068. [DOI] [PubMed] [Google Scholar]
- 173.Egan B., Dowling P., O’Connor P.L., Henry M., Meleady P., Zierath J.R., O’Gorman D.J. 2-D DIGE analysis of the mitochondrial proteome from human skeletal muscle reveals time course-dependent remodelling in response to 14 consecutive days of endurance exercise training. Proteomics. 2011;11:1413–1428. doi: 10.1002/pmic.201000597. [DOI] [PubMed] [Google Scholar]
- 174.Moriggi M., Vasso M., Fania C., Capitanio D., Bonifacio G., Salanova M., Blottner D., Rittweger J., Felsenberg D., Cerretelli P., et al. Long term bed rest with and without vibration exercise countermeasures: Effects on human muscle protein dysregulation. Proteomics. 2010;10:3756–3774. doi: 10.1002/pmic.200900817. [DOI] [PubMed] [Google Scholar]
- 175.Salanova M., Gelfi C., Moriggi M., Vasso M., Viganò A., Minafra L., Bonifacio G., Schiffl G., Gutsmann M., Felsenberg D., et al. Disuse deterioration of human skeletal muscle challenged by resistive exercise superimposed with vibration: Evidence from structural and proteomic analysis. FASEB J. 2014;28:4748–4763. doi: 10.1096/fj.14-252825. [DOI] [PubMed] [Google Scholar]
- 176.Hody S., Leprince P., Sergeant K., Renaut J., Croisier J.L., Wang F., Rogister B. Human muscle proteome modifications after acute or repeated eccentric exercises. Med. Sci. Sports Exerc. 2011;43:2281–2296. doi: 10.1249/MSS.0b013e318222edf3. [DOI] [PubMed] [Google Scholar]
- 177.Malm C., Yu J.G. Exercise-induced muscle damage and inflammation: Re-evaluation by proteomics. Histochem. Cell Biol. 2012;138:89–99. doi: 10.1007/s00418-012-0946-z. [DOI] [PubMed] [Google Scholar]
- 178.Burniston J.G. Changes in the rat skeletal muscle proteome induced by moderate-intensity endurance exercise. Biochim. Biophys. Acta. 2008;1784:1077–1086. doi: 10.1016/j.bbapap.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 179.Donoghue P., Doran P., Dowling P., Ohlendieck K. Differential expression of the fast skeletal muscle proteome following chronic low-frequency stimulation. Biochim. Biophys. Acta. 2005;1752:166–176. doi: 10.1016/j.bbapap.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 180.Donoghue P., Doran P., Wynne K., Pedersen K., Dunn M.J., Ohlendieck K. Proteomic profiling of chronic low-frequency stimulated fast muscle. Proteomics. 2007;7:3417–3430. doi: 10.1002/pmic.200700262. [DOI] [PubMed] [Google Scholar]
- 181.Guelfi K.J., Casey T.M., Giles J.J., Fournier P.A., Arthur P.G. A proteomic analysis of the acute effects of high-intensity exercise on skeletal muscle proteins in fasted rats. Clin. Exp. Pharmacol. Physiol. 2006;33:952–957. doi: 10.1111/j.1440-1681.2006.04470.x. [DOI] [PubMed] [Google Scholar]
- 182.Yamaguchi W., Fujimoto E., Higuchi M., Tabata I. A DIGE proteomic analysis for high-intensity exercise-trained rat skeletal muscle. J. Biochem. 2010;148:327–333. doi: 10.1093/jb/mvq073. [DOI] [PubMed] [Google Scholar]
- 183.Gandra P.G., Valente R.H., Perales J., Pacheco A.G., Macedo D.V. Proteomic profiling of skeletal muscle in an animal model of overtraining. Proteomics. 2012;12:2663–2667. doi: 10.1002/pmic.201200137. [DOI] [PubMed] [Google Scholar]
- 184.Gandra P.G., Valente R.H., Perales J., Pacheco A.G., Macedo D.V. Proteomic analysis of rat skeletal muscle submitted to one bout of incremental exercise. Scand. J. Med. Sci. Sports. 2012;22:207–216. doi: 10.1111/j.1600-0838.2010.01235.x. [DOI] [PubMed] [Google Scholar]
- 185.Magherini F., Abruzzo P.M., Puglia M., Bini L., Gamberi T., Esposito F., Veicsteinas A., Marini M., Fiorillo C., Gulisano M., et al. Proteomic analysis and protein carbonylation profile in trained and untrained rat muscles. J. Proteom. 2012;75:978–992. doi: 10.1016/j.jprot.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 186.Bouwman F.G., van Ginneken M.M., Noben J.P., Royackers E., de Graaf-Roelfsema E., Wijnberg I.D., van der Kolk J.H., Mariman E.C., van Breda E. Differential expression of equine muscle biopsy proteins during normal training and intensified training in young standardbred horses using proteomics technology. Comp. Biochem. Physiol. Part D Genom. Proteom. 2010;5:55–64. doi: 10.1016/j.cbd.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 187.Macedo A., Moriggi M., Vasso M., De Palma S., Sturnega M., Friso G., Gelfi C., Giacca M., Zacchigna S. Enhanced athletic performance on multisite AAV-IGF1 gene transfer coincides with massive modification of the muscle proteome. Hum. Gene Ther. 2012;23:146–157. doi: 10.1089/hum.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Burniston J.G., Kenyani J., Gray D., Guadagnin E., Jarman I.H., Cobley J.N., Cuthbertson D.J., Chen Y.W., Wastling J.M., Lisboa P.J., et al. Conditional independence mapping of DIGE data reveals PDIA3 protein species as key nodes associated with muscle aerobic capacity. J. Proteom. 2014;106:230–245. doi: 10.1016/j.jprot.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Flueck M. Plasticity of the muscle proteome to exercise at altitude. High Alt. Med. Biol. 2009;10:183–193. doi: 10.1089/ham.2008.1104. [DOI] [PubMed] [Google Scholar]
- 190.Favier F.B., Britto F.A., Freyssenet D.G., Bigard X.A., Benoit H. HIF-1-driven skeletal muscle adaptations to chronic hypoxia: Molecular insights into muscle physiology. Cell. Mol. Life Sci. 2015;72:4681–4696. doi: 10.1007/s00018-015-2025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bosworth C.A., Chou C.W., Cole R.B., Rees B.B. Protein expression patterns in zebrafish skeletal muscle: Initial characterization and the effects of hypoxic exposure. Proteomics. 2005;5:1362–1371. doi: 10.1002/pmic.200401002. [DOI] [PubMed] [Google Scholar]
- 192.De Palma S., Ripamonti M., Vigano A., Moriggi M., Capitanio D., Samaja M., Milano G., Cerretelli P., Wait R., Gelfi C. Metabolic modulation induced by chronic hypoxia in rats using a comparative proteomic analysis of skeletal muscle tissue. J. Proteome Res. 2007;6:1974–1984. doi: 10.1021/pr060614o. [DOI] [PubMed] [Google Scholar]
- 193.Vigano A., Ripamonti M., De Palma S., Capitanio D., Vasso M., Wait R., Lundby C., Cerretelli P., Gelfi C. Proteins modulation in human skeletal muscle in the early phase of adaptation to hypobaric hypoxia. Proteomics. 2008;8:4668–4679. doi: 10.1002/pmic.200800232. [DOI] [PubMed] [Google Scholar]
- 194.Levett D.Z., Viganò A., Capitanio D., Vasso M., De Palma S., Moriggi M., Martin D.S., Murray A.J., Cerretelli P., Grocott M.P., et al. Changes in muscle proteomics in the course of the Caudwell Research Expedition to Mt. Everest. Proteomics. 2015;15:160–171. doi: 10.1002/pmic.201400306. [DOI] [PubMed] [Google Scholar]
- 195.Carmeli E., Aizenbud D., Rom O. How Do Skeletal Muscles Die? An Overview. Adv. Exp. Med. Biol. 2015;861:99–111. doi: 10.1007/5584_2015_140. [DOI] [PubMed] [Google Scholar]
- 196.Isfort R.J., Hinkle R.T., Jones M.B., Wang F., Greis K.D., Sun Y., Keough T.W., Anderson N.L., Sheldon R.J. Proteomic analysis of the atrophying rat soleus muscle following denervation. Electrophoresis. 2000;21:2228–2234. doi: 10.1002/1522-2683(20000601)21:11<2228::AID-ELPS2228>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 197.Isfort R.J., Wang F., Greis K.D., Sun Y., Keough T.W., Farrar R.P., Bodine S.C., Anderson N.L. Proteomic analysis of rat soleus muscle undergoing hindlimb suspension-induced atrophy and reweighting hypertrophy. Proteomics. 2002;2:543–550. doi: 10.1002/1615-9861(200205)2:5<543::AID-PROT543>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 198.Isfort R.J., Wang F., Greis K.D., Sun Y., Keough T.W., Bodine S.C., Anderson N.L. Proteomic analysis of rat soleus and tibialis anterior muscle following immobilization. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;769:323–332. doi: 10.1016/S1570-0232(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 199.Seo Y., Lee K., Park K., Bae K., Choi I. A proteomic assessment of muscle contractile alterations during unloading and reloading. J. Biochem. 2006;139:71–80. doi: 10.1093/jb/mvj007. [DOI] [PubMed] [Google Scholar]
- 200.Moriggi M., Cassano P., Vasso M., Capitanio D., Fania C., Musicco C., Pesce V., Gadaleta M.N., Gelfi C. A DIGE approach for the assessment of rat soleus muscle changes during unloading: Effect of acetyl-l-carnitine supplementation. Proteomics. 2008;8:3588–3604. doi: 10.1002/pmic.200701176. [DOI] [PubMed] [Google Scholar]
- 201.Ferreira R., Vitorino R., Neuparth M.J., Appell H.J., Duarte J.A., Amado F. Proteolysis activation and proteome alterations in murine skeletal muscle submitted to 1 week of hindlimb suspension. Eur. J. Appl. Physiol. 2009;107:553–563. doi: 10.1007/s00421-009-1151-1. [DOI] [PubMed] [Google Scholar]
- 202.Basco D., Nicchia G.P., Desaphy J.F., Camerino D.C., Frigeri A., Svelto M. Analysis by two-dimensional Blue Native/SDS-PAGE of membrane protein alterations in rat soleus muscle after hindlimb unloading. Eur. J. Appl. Physiol. 2010;110:1215–1224. doi: 10.1007/s00421-010-1592-6. [DOI] [PubMed] [Google Scholar]
- 203.Wang F., Zhang P., Liu H., Fan M., Chen X. Proteomic analysis of mouse soleus muscles affected by hindlimb unloading and reloading. Muscle Nerve. 2015;52:803–811. doi: 10.1002/mus.24590. [DOI] [PubMed] [Google Scholar]
- 204.Li Z.B., Lehar M., Samlan R., Flint P.W. Proteomic analysis of rat laryngeal muscle following denervation. Proteomics. 2005;5:4764–4776. doi: 10.1002/pmic.200401329. [DOI] [PubMed] [Google Scholar]
- 205.Sato Y., Shimizu M., Mizunoya W., Wariishi H., Tatsumi R., Buchman V.L., Ikeuchi Y. Differential expression of sarcoplasmic and myofibrillar proteins of rat soleus muscle during denervation atrophy. Biosci. Biotechnol. Biochem. 2009;73:1748–1756. doi: 10.1271/bbb.90085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Budui S.L., Rossi A.P., Zamboni M. The pathogenetic bases of sarcopenia. Clin. Cases Miner. Bone Metab. 2015;12:22–26. doi: 10.11138/ccmbm/2015.12.1.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lexell J. Human aging, muscle mass, and fiber type composition. J. Gerontol. A Biol. Sci. Med. Sci. 1995;50:11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 208.Landi F., Calvani R., Cesari M., Tosato M., Martone A.M., Bernabei R., Onder G., Marzetti E. Sarcopenia as the Biological Substrate of Physical Frailty. Clin. Geriatr. Med. 2015;31:367–374. doi: 10.1016/j.cger.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 209.Peterson S.J., Braunschweig C.A. Prevalence of Sarcopenia and Associated Outcomes in the Clinical Setting. Nutr. Clin. Pract. 2016;31:40–48. doi: 10.1177/0884533615622537. [DOI] [PubMed] [Google Scholar]
- 210.Chaves D.F., Carvalho P.C., Lima D.B., Nicastro H., Lorenzeti F.M., Siqueira-Filhom M., Hirabara S.M., Alves P.H., Moresco J.J., Yates J.R., III, et al. Comparative proteomic analysis of the aging soleus and extensor digitorum longus rat muscles using TMT labeling and mass spectrometry. J. Proteome Res. 2013;12:4532–4546. doi: 10.1021/pr400644x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gueugneau M., Coudy-Gandilhon C., Gourbeyre O., Chambon C., Combaret L., Polge C., Taillandier D., Attaix D., Friguet B., et al. Proteomics of muscle chronological ageing in post-menopausal women. BMC Genom. 2014;15:1165. doi: 10.1186/1471-2164-15-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Théron L., Gueugneau M., Coudy C., Viala D., Bijlsma A., Butler-Browne G., Maier A., Béchet D., Chambon C. Label-free quantitative protein profiling of vastus lateralis muscle during human aging. Mol. Cell. Proteom. 2014;13:283–294. doi: 10.1074/mcp.M113.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Doran P., Donoghue P., O’Connell K., Gannon J., Ohlendieck K. Proteomics of skeletal muscle aging. Proteomics. 2009;9:989–1003. doi: 10.1002/pmic.200800365. [DOI] [PubMed] [Google Scholar]
- 214.Ohlendieck K. Proteomic Profiling of Fast-To-Slow Muscle Transitions during Aging. Front Physiol. 2011;2:105. doi: 10.3389/fphys.2011.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Baraibar M.A., Gueugneau M., Duguez S., Butler-Browne G., Bechet D., Friguet B. Expression and modification proteomics during skeletal muscle ageing. Biogerontology. 2013;14:339–352. doi: 10.1007/s10522-013-9426-7. [DOI] [PubMed] [Google Scholar]
- 216.O'Connell K., Gannon J., Doran P., Ohlendieck K. Proteomic profiling reveals a severely perturbed protein expression pattern in aged skeletal muscle. Int. J. Mol. Med. 2007;20:145–153. doi: 10.3892/ijmm.20.2.145. [DOI] [PubMed] [Google Scholar]
- 217.Doran P., Gannon J., O’Connell K., Ohlendieck K. Aging skeletal muscle shows a drastic increase in the small heat shock proteins alphaB-crystallin/HspB5 and cvHsp/HspB7. Eur. J. Cell Biol. 2007;86:629–640. doi: 10.1016/j.ejcb.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 218.Timms J.F., Cramer R. Difference gel electrophoresis. Proteomics. 2008;8:4886–4897. doi: 10.1002/pmic.200800298. [DOI] [PubMed] [Google Scholar]
- 219.Gelfi C., Vigano A., Ripamonti M., Pontoglio A., Begum S., Pellegrino M.A., Grassi B., Bottinelli R., Wait R., Cerretelli P. The human muscle proteome in aging. J. Proteome Res. 2006;5:1344–1353. doi: 10.1021/pr050414x. [DOI] [PubMed] [Google Scholar]
- 220.Doran P., O’Connell K., Gannon J., Kavanagh M., Ohlendieck K. Opposite pathobiochemical fate of pyruvate kinase and adenylate kinase in aged rat skeletal muscle as revealed by proteomic DIGE analysis. Proteomics. 2008;8:364–377. doi: 10.1002/pmic.200700475. [DOI] [PubMed] [Google Scholar]
- 221.Capitanio D., Vasso M., Fania C., Moriggi M., Viganò A., Procacci P., Magnaghi V., Gelfi C. Comparative proteomic profile of rat sciatic nerve and gastrocnemius muscle tissues in ageing by 2-D DIGE. Proteomics. 2009;9:2004–2020. doi: 10.1002/pmic.200701162. [DOI] [PubMed] [Google Scholar]
- 222.Staunton L., Zweyer M., Swandulla D., Ohlendieck K. Mass spectrometry-based proteomic analysis of middle-aged vs. aged vastus lateralis reveals increased levels of carbonic anhydrase isoform 3 in senescent human skeletal muscle. Int. J. Mol. Med. 2012;30:723–733. doi: 10.3892/ijmm.2012.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Capitanio D., Vasso M., De Palma S., Fania C., Torretta E., Cammarata F.P., Magnaghi V., Procacci P., Gelfi C. Specific protein changes contribute to the differential muscle mass loss during ageing. Proteomics. 2016;16:645–656. doi: 10.1002/pmic.201500395. [DOI] [PubMed] [Google Scholar]
- 224.Unlü M., Morgan M.E., Minden J.S. Difference gel electrophoresis: A single gel method for detecting changes in protein extracts. Electrophoresis. 1997;18:2071–2077. doi: 10.1002/elps.1150181133. [DOI] [PubMed] [Google Scholar]
- 225.Alban A., David S.O., Bjorkesten L., Andersson C., Sloge E., Lewis S., Currie I. A novel experimental design for comparative two-dimensional gel analysis: Two-dimensional difference gel electrophoresis incorporating a pooled internal standard. Proteomics. 2003;3:36–44. doi: 10.1002/pmic.200390006. [DOI] [PubMed] [Google Scholar]
- 226.Viswanathan S., Unlü M., Minden J.S. Two-dimensional difference gel electrophoresis. Nat. Protoc. 2006;1:1351–1358. doi: 10.1038/nprot.2006.234. [DOI] [PubMed] [Google Scholar]
- 227.Minden J.S. Two-dimensional difference gel electrophoresis. Methods Mol. Biol. 2012;869:287–304. doi: 10.1007/978-1-61779-821-4_24. [DOI] [PubMed] [Google Scholar]
- 228.Malm C., Hadrevi J., Bergström S.A., Pedrosa-Domellöf F., Antti H., Svensson M., Frängsmyr L. Evaluation of 2-D DIGE for skeletal muscle: Protocol and repeatability. Scand. J. Clin. Lab. Investig. 2008;68:793–800. doi: 10.1080/00365510802277464. [DOI] [PubMed] [Google Scholar]
- 229.Carberry S., Zweyer M., Swandulla D., Ohlendieck K. Application of fluorescence two-dimensional difference in-gel electrophoresis as a proteomic biomarker discovery tool in muscular dystrophy research. Biology. 2013;2:1438–1464. doi: 10.3390/biology2041438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tonge R., Shaw J., Middleton B., Rowlinson R., Rayner S., Young J., Pognan F., Hawkins E., Currie I., Davison M. Validation and development of fluorescence two-dimensional differential gel electrophoresis proteomics technology. Proteomics. 2001;1:377–396. doi: 10.1002/1615-9861(200103)1:3<377::AID-PROT377>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 231.Marouga R., David S., Hawkins E. The development of the DIGE system: 2D fluorescence difference gel analysis technology. Anal. Bioanal. Chem. 2005;382:669–678. doi: 10.1007/s00216-005-3126-3. [DOI] [PubMed] [Google Scholar]
- 232.Karp N.A., Kreil D.P., Lilley K.S. Determining a significant change in protein expression with DeCyder during a pair-wise comparison using two-dimensional difference gel electrophoresis. Proteomics. 2004;4:1421–1432. doi: 10.1002/pmic.200300681. [DOI] [PubMed] [Google Scholar]
- 233.Karp N.A., Lilley K.S. Maximising sensitivity for detecting changes in protein expression: Experimental design using minimal CyDyes. Proteomics. 2005;5:3105–3115. doi: 10.1002/pmic.200500083. [DOI] [PubMed] [Google Scholar]
- 234.Doran P., Martin G., Dowling P., Jockusch H., Ohlendieck K. Proteome analysis of the dystrophin-deficient MDX diaphragm reveals a drastic increase in the heat shock protein cvHSP. Proteomics. 2006;6:4610–4621. doi: 10.1002/pmic.200600082. [DOI] [PubMed] [Google Scholar]
- 235.Staunton L., O’Connell K., Ohlendieck K. Proteomic Profiling of Mitochondrial Enzymes during Skeletal Muscle Aging. J. Aging Res. 2011;2011:908035. doi: 10.4061/2011/908035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ibebunjo C., Chick J.M., Kendall T., Eash J.K., Li C., Zhang Y., Vickers C., Wu Z., Clarke B.A., Shi J., et al. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol. Cell. Biol. 2013;33:194–212. doi: 10.1128/MCB.01036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lourenço Dos Santos S., Baraibar M.A., Lundberg S., Eeg-Olofsson O., Larsson L., Friguet B. Oxidative proteome alterations during skeletal muscle ageing. Redox Biol. 2015;5:267–274. doi: 10.1016/j.redox.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.McDonagh B., Sakellariou G.K., Smith N.T., Brownridge P., Jackson M.J. Redox proteomic analysis of the gastrocnemius muscle from adult and old mice. Data Brief. 2015;4:344–348. doi: 10.1016/j.dib.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Feng J., Xie H., Meany D.L., Thompson L.V., Arriaga E.A., Griffin T.J. Quantitative proteomic profiling of muscle type-dependent and age-dependent protein carbonylation in rat skeletal muscle mitochondria. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:1137–1152. doi: 10.1093/gerona/63.11.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Piec I., Listrat A., Alliot J., Chambon C., Taylor R.G., Bechet D. Differential proteome analysis of aging in rat skeletal muscle. FASEB J. 2005;19:1143–1145. doi: 10.1096/fj.04-3084fje. [DOI] [PubMed] [Google Scholar]
- 241.Teltathum T., Mekchay S. Proteome changes in Thai indigenous chicken muscle during growth period. Int. J. Biol. Sci. 2009;5:679–685. doi: 10.7150/ijbs.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kim N.K., Lee S.H., Cho Y.M., Son E.S., Kim K.Y., Lee C.S., Yoon D., Im S.K., Oh S.J., Park E.W. Proteome analysis of the m. longissimus dorsi between fattening stages in Hanwoo steer. BMB Rep. 2009;42:433–438. doi: 10.5483/BMBRep.2009.42.7.433. [DOI] [PubMed] [Google Scholar]
- 243.Jia X., Hildrum K.I., Westad F., Kummen E., Aass L., Hollung K. Changes in enzymes associated with energy metabolism during the early post mortem period in longissimus thoracis bovine muscle analyzed by proteomics. J. Proteome Res. 2006;5:1763–1769. doi: 10.1021/pr060119s. [DOI] [PubMed] [Google Scholar]
- 244.Jia X., Ekman M., Grove H., Faergestad E.M., Aass L., Hildrum K.I., Hollung K. Proteome changes in bovine longissimus thoracis muscle during the early postmortem storage period. J. Proteome Res. 2007;6:2720–2731. doi: 10.1021/pr070173o. [DOI] [PubMed] [Google Scholar]
- 245.Di Luca A., Elia G., Hamill R., Mullen A.M. 2D DIGE proteomic analysis of early post mortem muscle exudate highlights the importance of the stress response for improved water-holding capacity of fresh pork meat. Proteomics. 2013;13:1528–1544. doi: 10.1002/pmic.201200145. [DOI] [PubMed] [Google Scholar]
- 246.Canto A.C., Suman S.P., Nair M.N., Li S., Rentfrow G., Beach C.M., Silva T.J., Wheeler T.L., Shackelford S.D., Grayson A., et al. Differential abundance of sarcoplasmic proteome explains animal effect on beef Longissimus lumborum color stability. Meat Sci. 2015;102:90–98. doi: 10.1016/j.meatsci.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 247.Franco D., Mato A., Salgado F.J., López-Pedrouso M., Carrera M., Bravo S., Parrado M., Gallardo J.M., Zapata C. Tackling proteome changes in the longissimus thoracis bovine muscle in response to pre-slaughter stress. J. Proteom. 2015;122:73–85. doi: 10.1016/j.jprot.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 248.Yang H., Xu X.L., Ma H.M., Jiang J. Integrative analysis of transcriptomics and proteomics of skeletal muscles of the Chinese indigenous Shaziling pig compared with the Yorkshire breed. BMC Genet. 2016;17:80. doi: 10.1186/s12863-016-0389-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Paredi G., Raboni S., Bendixen E., de Almeida A.M., Mozzarelli A. “Muscle to meat” molecular events and technological transformations: The proteomics insight. J. Proteom. 2012;75:4275–4289. doi: 10.1016/j.jprot.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 250.D’Alessandro A., Zolla L. Meat science: From proteomics to integrated omics towards system biology. J. Proteom. 2013;78:558–577. doi: 10.1016/j.jprot.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 251.Longo V., Lana A., Bottero M.T., Zolla L. Apoptosis in muscle-to-meat aging process: The omic witness. J. Proteom. 2015;125:29–40. doi: 10.1016/j.jprot.2015.04.023. [DOI] [PubMed] [Google Scholar]