Abstract
Short linear motifs (SLiM) are short peptides that facilitate protein function and protein-protein interactions. Viruses utilize these motifs to enter into the host, interact with cellular proteins, or egress from host cells. Studying functional motifs may help to predict protein characteristics, interactions, or the putative cellular role of a protein. In virology, it may reveal aspects of the virus tropism and help find antiviral therapeutics. This review highlights the recent understanding of functional motifs utilized by viruses. Special attention was paid to the function of proteins harboring these motifs, and viruses encoding these proteins. The review highlights motifs involved in (i) immune response and post-translational modifications (e.g., ubiquitylation, SUMOylation or ISGylation); (ii) virus-host cell interactions, including virus attachment, entry, fusion, egress and nuclear trafficking; (iii) virulence and antiviral activities; (iv) virion structure; and (v) low-complexity regions (LCRs) or motifs enriched with residues (Xaa-rich motifs).
Keywords: clathrin endocytosis, low-complexity repeats, ubiquitylation, agnoprotein, APOBEC, pentraxin, PDZ domain, retinoblastoma, inhibitor of apoptosis (IAP), transposition
1. Introduction
Interactions between viral and cellular proteins are required for virus entry, replication, or egress from the cell. These interactions are facilitated by peptide sequences, so-called domains or motifs [1,2]. These sequences could be either (i) short linear motifs (SLiM), 3–11 residues, e.g., RGD; (ii) structural motifs or domains, about 30 residues, e.g., tetratricopeptide repeat (TPR), zinc finger or ankyrin; or (iii) they may contain a repeated residue(s) (e.g., Leu-rich, SR-rich, AR-rich or PEST-rich motifs). The consensus motif follows the PROSITE pattern [3]. The consensus is formed of a regular expression pattern, e.g., Px(2)[ED]. In the pattern, a single-letter amino acid abbreviation is indicated. The alternative (degenerated) residues in a position are bracketed, while “x” letter denotes any residue in the position. The number between parentheses refers to the number of occurrences of a residue.
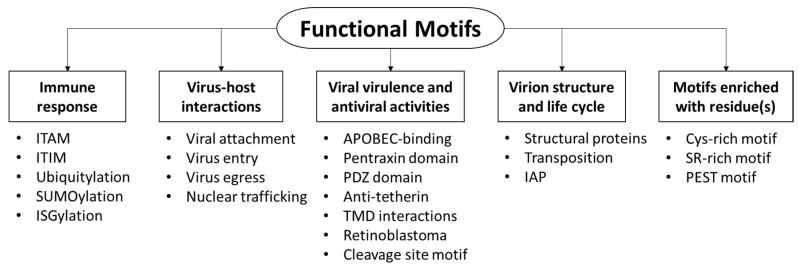
Viruses utilize a number of functional motifs to attach and enter into host cells, or interact with cellular proteins. This article aims to review the current understanding of motifs utilized by viruses for fruitful infection, highlighting the function of motifs and/or proteins harboring these motifs, in an attempt to classify the motifs based on the molecular function of the harboring proteins. The motifs can be classified into five main categories (Figure 1): (i) motifs that mediate immune response; (ii) virus-host interactions, including entry and cellular trafficking; (iii) virulence and antiviral activities, which may disturb cellular processes; (iv) virion structure; and (v) motifs enriched with residues.
Figure 1.
Five categories of motifs were reviewed, based on function of proteins harboring the motif.
2. Motif Involved in Immune Response and post-translational modification processes
Immune response. B and T cells employ two types of receptors with positive and negative regulators, the so-called immunoreceptor tyrosine-based activation motif (ITAM) and the immunoreceptor tyrosine-based inhibition motif (ITIM), respectively [4]. These receptors are responsible for immune response and signal transduction in immune cells. They bear either ITAM (Yxx[LI]x6–8Yxx[LI]) or ITIM ([SIVL]xYxx[IVL]) motifs. The dendritic cell (DC) immunoreceptor (DCIR), a C-type lectin receptor expressed on DCs, acts as an attachment factor for human immunodeficiency virus type 1 (HIV-1) [5]. DCIR contains ITIM, which binds to the Glu-Pro-Ser (EPS) motif. Chemical inhibitors directed against this motif prevent attachment of HIV-1 to DCs.
Post-translational modification processes. Cellular processes, such as ubiquitylation, SUMOylation and ISGylation, require particular motifs for proteins to bind and initiate them. In adenoviruses, protein VI recruits Nedd4 E3 ubiquitin ligases by the PPxY motif, facilitating its ubiquitylation [6]. The SLQxLA, VxHxMY, HCCH (Hx5Cx17–18Cx3–5H) and PPLP motifs in the viral infectivity factor (Vif) protein bind to Cullin5, ElonginB and C, inducing protein polyubiquitination and proteasome-mediated degradation [7,8,9,10].
SUMOylation is a post-translational modification process by which small protein (SUMO, small ubiquitin-related modifier) binds to a wide range of cellular proteins, modifying their functions by adding a bulky moiety, and promoting particular protein-protein interactions [11,12]. SUMOylation of substrates is initiated by the binding of SUMO with lysine residue in the SUMOylation consensus motif, φKx[DE], where φ denotes large hydrophobic residues (F, I, L or V). It is noteworthy that the SUMO motif is not the exclusive motif for SUMOylation, and the SUMO substrate can be modified in different sites, such as the SxS (φφxSxS[DE][DE][DE]) and [VI]x[VI][VI] motifs [12,13,14]. A number of viruses (including herpesviruses and hepatitis C virus, HCV) were able to trigger SUMOylation-dependent mechanisms by recruiting E2 and E3 ubiquitin ligases [15,16,17,18]. SUMO was suggested to play roles in the nuclear localization of viral cargo [19], suggesting their roles in virus replication [17]. Notably, the sentrin-specific proteases (SENPs) family are SUMO proteases, which are able to detach SUMOs from their substrates [20]. Interfering with the proteins involved in (de-)SUMOylation processes via SENPs was suggested as a potential technique for developing an antiviral agent [17,18,21].
Viral proteins, such as paramyxovirus C and V proteins, mouse cytomegalovirus (CMV) pM27, and Kaposi's sarcoma-associated herpesvirus K3, K5 and viral interferon regulatory factor 3, can inhibit signal transduction and activators of transcription (STAT) or major histocompatibility complex [22,23,24,25,26,27,28,29,30]. These interactions downregulate the interferon (IFN) pathway, regulate the expression of interferon-stimulated genes (ISGs), and suppress both cytokine-mediated immunity and anti-viral defense [22]. Similar mechanisms were suggested for equine herpesvirus-1 [31], hepatitis E virus [32], and hepatitis B virus [33].
ISG15, a ubiquitin-like interferon-stimulated protein, is stimulated by interferon or viral infection [34,35]. ISG15 is cytokine-like protein that promotes antiviral immune response. On mice, ISG15 expression reduces Sindbis virus replication and clearance in multiple organs, and attenuates infection [34]. Further evidence shows that Novirhabdovirus, Birnavirus and Iridovirus infection could be inhibited by the over-expression of zebrafish ISG15 in EPC cells [36,37]. On the other hand, ISG15 conjugates with the substrate protein through its conserved LRLRGG consensus sequence, leading to antiviral response [35]. Mutations of glycine residues (LRAA) destabilize this conjugation [36]. However, evidence shows that the fish ISG15 homolog can promote an antiviral immune response, even in unconjugated form [37].
3. Motifs Required for Virus Attachment, Entry, Trafficking, and Egress
3.1. Viral Receptors
Viruses utilize receptors and co-receptors to attach and enter into host cells. HIV attaches to one or two co-receptors, CCR5 or CXCR4, to enter cells [38,39,40,41,42,43,44,45]. The conserved GPG[RQ] motif in the crown of the third variable loop region of the gp120 protein is crucial for virus attachment [43,44,45,46,47]. In adenovirus (Adv), it is suggested that the KKTK motif in Adv2 and Adv5 fiber shaft attaches to heparin sulfate proteoglycans to start the infection [48,49]. A mutation in KKTK affects Adv5 tropism. Further investigations show that the KKTK motif in Adv-C is important for post-entry steps [50,51]. Virus lacking the KKTK motif efficiently infects liver cells in vivo.
Integrin-binding. Integrins are cell surface adhesion molecules composed of α and β subunits. They are expressed by a variety of cells and can be utilized by microbes [49,52]. Integrins interact with the conserved Arg-Gly-Asp (RGD) motif of the adenovirus penton base, which promote endocytosis and endosomal escape, as reviewed in [53,54]. Several reports suggest the ability of viruses to evolve mechanisms by which they utilize RGD-like motifs (RGG or GGG), as reviewed in [55] or the potential integrin-binding motif YGD motif [56] to enter into host cells. Moreover, the SDI motif in glycoprotein H (gH) of equine herpes viruses 1 and 4 may bind to integrins [57]. Foot-and-mouth disease virus (FMDV) VP1 capsid protein harbors the RGDLxxL sequence, which is required for binding to cellular integrins [58]. The two Leu residues stabilize the interaction and play roles in determining integrin specificity. Nonetheless, in the absence of RGD, DLxxL, KGD or KGE is employed for the attachment to cellular receptors [58].
3.2. Virus Entry
3.2.1. Endocytosis
The 3a protein encoded by severe acute respiratory syndrome–associated coronavirus (SARS-CoV) functions as an ion channel protein [59]. It harbors the Yxxφ motif, which is necessary for endocytosis, intracellular trafficking, and surface transport of SARS-CoV. Sodium taurocholate co-transporting polypeptide (NTCP) at the plasma membrane is a receptor for hepatitis B and D viruses (HBV and HDV) [60]. Endocytosis of HBV and HDV is regulated by the dileucine motif (222LL223) and the phosphorylation of T225 and S226 in NTCP [61]. Moreover, PPxY is required for Adv5 entry and cellular microtubule-dependent trafficking [6].
3.2.2. Clathrin Endocytosis
The clathrin-coated vesicles recruit soluble clathrin by adaptor proteins (APs) AP-1 (in the trans-Golgi network) and AP-2 (at the cell surface). The clathrin-binding motifs of APs bind to the N-terminal domain of clathrin. Two clathrin-binding motifs were defined: clathrin-box, which conforms to sequence LφXφ[DE] or L[LI][DEN][LF][DE], and W-box, which conforms to sequence PWxxW [62]. Moreover, the µ subunit of AP1 recognizes two sorting signals, a tyrosine-based Yxxφ motif and an acidic dileucine motif, [ED]xxxL[LI] [63]. HIV-1 viral protein unique (Vpu) hijacks AP-1 and antagonizes BST2 via YxYxxφ, [63]. AP-1 reroutes BST2 to the lysozyme and mediates the endo-lysosomal degradation of BST2. Similar mechanisms were described in HIV Nef, which hijacks clathrin AP-1 and interacts with the major histocompatibility complex (MHC-1) [64,65]. This interaction is stabilized by (PxxP)3 repeats and directs MHC-I to the endo-lysosomal pathway.
3.2.3. Virus Fusion
The short motif mediates interaction with other proteins leading to virus fusion and entry. For example, the fusion protein encoded by the Newcastle disease virus (NDV) harbors LL and Yxxφ motifs in the cytoplasmic tail and plays a role in viral fusion, replication and pathogenesis [66,67]. Moreover, interferon-induced transmembrane (IFITM) proteins inhibit virus entry and cell-cell fusion of several viruses, including coronavirus, HIV-1, influenza and Ebola viruses [68]. The KRxx (dibasic residues) motif in the C-terminal of IFITM-1 modulates a species-specific antiviral sorting signal against viruses by controlling protein subcellular localization, while IFITM-3 interacts with AP2 through its Yxxφ sorting motif at the N-terminus [69,70,71].
3.3. Virus Egress from the Cell
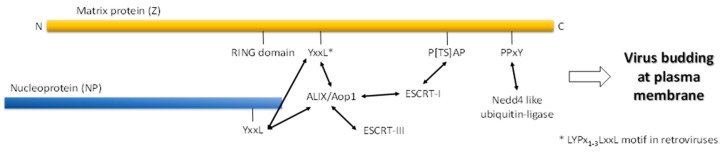
Viruses recruit endosomal sorting complexes required for the transport (ESCRT) pathway to egress from the cell, which leads to virus budding and initiating new infection, as reviewed in [72,73,74,75,76]. The pathway is mediated by several molecular interactions between proteins through late (l)-domain motifs (P[TS]AP, PPxY, YxxL, and φPxV) (Figure 2) [67,77,78]. These motifs mediate binding to ESCRT, which leads to the budding and release of viruses, including a number of retroviruses, arenaviruses and paramyxoviruses. In the absence of the PPPY motif, LYPxnL in the gag protein serves as an alternative motif that recruits ESCRT machinery for the release and replication of retroviruses [79,80], while in Ebola virus, these interactions are mediated by 7PTAP10, 10PPEY13 and 18YPxn[LI]26 [81]. First, proteins harboring the PPxY, LYPxnL or PTAP motifs interact with Nedd4, Alix and Tsg101 proteins, respectively. Then, these interactions trigger ESCRT machinery and the release of the virus by budding [82]. Interestingly, archaeal ESCRT could be involved in the egress of Sulfolobus turreted icosahedral virus by forming virus-associated pyramid structures on the cell membrane of Sulfolobus Archaea, as reviewed in [83]. Due to the crucial role of these motifs, several attempts were suggested for developing antiviral therapeutic agents targeting these motifs and/or the proteins harboring them [78,81]. Targeting l-domain-dependent recruitment of host Nedd4 and Tsg101 shows depletion of viral egress for a number of RNA viruses, including vesicular stomatitis, rabies viruses, and hepatitis E virus [84,85].
Figure 2.
A schematic diagram of arenavirus late-domain motifs and their role in interaction with cellular proteins leading to virus budding and egress from the cell [67].
3.4. Nuclear Trafficking
The trafficking of a protein into or from the nucleus is orchestrated by two motifs: (i) nuclear export signal (NES), which regulates proteins export from the nucleus to the cytoplasm; and (ii) the nuclear localization sequence (NLS) motif, which imports proteins into the nucleus [86,87]. The canonical NES consensus motif is LxxxLxxLxL, but L can be replaced by I, V, F or M [88], whereas the NLS motifs are classified into six classes (as seen below in Table 1 and Table S1) [89]. Interestingly, the first NLS was discovered in SV40 Large T-antigen with the monopartite PKKKRKV sequence [90,91,92]. The nucleoprotein of influenza B virus (BNP) harbors a conserved 44KRxR47 motif, and a mutation on the K or R residue results in the disruption or failure of nuclear import and localization, suggesting that the motif is a NLS sequence [93,94].
Table 1.
List of pattern of functional motifs and the function of the protein harboring them. 1
| Function of Protein Containing the Pattern | Pattern Motif | References |
|---|---|---|
| 6-cysteine motif, degradation of chitin and chitotriose | Cx13–20Cx5–6Cx9–19Cx10–14Cx4–14C | [95,96] |
| Adenovirus fiber flexibility motif | KLGxGLxF[DN] and KxGGLxF[DN] | [50] |
| Agnoprotein function, productive viral infection | L[FL][VI]F[VIL]LE[LF]LLxF and Qxx[IML]xx[FY] | [97,98,99] |
| Agnoprotein—NLS | RRRRx5Rx4RK | [100] |
| Binding of virus proteins to retinoblastoma protein, gene expression and virus replication | LxCxE and [LI]xCx[DE] | [101,102,103,104,105,106,107,108,109] |
| Binding to ESCRT, paramyxoviruses budding | φPxV | [79] |
| Binding to integrins and viral attachment to cellular receptors | RGD, DLxxL, LDV, RGDLxxL, SDI, KGD and KGE | [53,54,55,56,57,58] |
| Budded virions production and nucleocapsid assembly | Cx5CxnHx6C (C2HC zinc finger) | [95,96] |
| Clathrin-binding motifs, clathrin-box | LφXφ[DE], L[LI][DEN][LF][DE] and PWxxW | [62] |
| Cleavage motif of Newcastle disease virus | [GE][KR]Q[GE]RL and [RK]RQ[RK]RF | [110] |
| Cleavage site for Influenza A virus hemagglutinin | KKKRGLF, [QE][ST]RGLF, Rx[RK]RGLF, RxRRGLF and RxxRGLF | [111] |
| Enhance virion-release, anti-tetherin activity | DSGxxS | [112,113] |
| Helix-Helix Interactions | AxxxAxxxAxxxW and VxxxIxxLxxxL | [114,115] |
| Heparan sulfate-binding motif, post-internalization steps of adenovirus | KKTK, or bbxb and bbbxxb | [48,49,50,51] |
| HIV neutralization by human antibodies | GPG[RQ] | [43,44,45,46,47] |
| HIV release, interfering with tetherin function | [GD]DIWK | [113] |
| Induction of cellular-malignant transformation by Kaposin, activation of cap-dependent translation, and HIV retrotransposition | LxxLL | [116,117,118,119,120,121,122] |
| IAP, block the apoptosis | Gx2Yx4Dx3Cx2Cx6Wx9Hx6–10C, Cx2Cx9–39Cx1–3Hx2–3Cx2Cx4–48Cx2C and A[KITV][AEP][FEISY] | [123,124,125] |
| Interact with clathrin adaptor protein | PxxP and YxYxxΦ | [63,64,65] |
| ISGylation, antiviral response | LRGG and LRLRGG | [35,36] |
| ITAM motif | Yxx[LI]x6–8Yxx[LI] | [4] |
| ITIM motif | [SIVL]xYxx[IVL] | [4] |
| Necessary for endocytosis, intracellular trafficking, interact with clathrin APs, and promotes viral spread, fusion and replication | YxxΦ | [64,65] |
| Nuclear export signal (NES), regulates protein export to nucleus from cytoplasm | [LIVFM]x2–3[LIVFM]x[LIVFM] and LxxxLxxLxL | [88] |
| NLS motifs | i: KR[KR]R and K[KR]RK ii: [PR]xxKR{DE}[KR] iii: KRx[WFY]xxAF iv: [RP]xxKR[KR]{DE} v: LGKR[KR][WFY] Bipartite: KRx10–12K[KR][KR] and KRx10–12K[KR]X[KR] |
[89,93,94] |
| Pentraxin domain, pathogen recognition, host defense, and antiviral response | HxCx[ST]WxS | [126,127] |
| Protein folding, Rossmann folds motifs, and bind FAD or NAD(P) | Gx3G, Gx3[GA] and Gx1-2GxxG | [128] |
| Protein interaction and thiol-disulfide transfer | CxxC and CxxxC | [129,130,131] |
| Proton transport, channel function, and transmembrane domain | HxxxW | [132] |
| Recruits ESCRT pathway, and mediates viral budding and release | YxxL, P[TS]AP and LYPxL | [67,77,79] |
| Regulation by interaction of retrovirus Vif with APOBEC, cullin5, elongin, and E3 ligase | PPLP, SLQxLA, VxHxMY, HCCH, YYxW, DPD, YxxL, YRHHY, EDRW, DRMR, TGERxW, LGxGxxIxW, WxSLVK, W[HKN]SLVK, VxIPLx4-5L, VxIPLx4-5Lxφx2YwxL, SL[VI]x4Yx9Y and T[DEQ]x5Adx2[IL] | [7,8,9,10,133,134,135,136,137,138,139,140,141,142,143,144] |
| Sorting signal, anti-tetherin | ExxxLV | [145] |
| SUMOylation—SUMO binding to substrate | φφxSxS[DE][DE][DE], φKx[DE] and [VI]x[VI][VI] | [12,13] |
| Ubiquitylation, interaction with Nedd4 E3 ubiquitin ligases, recruit ESCRT pathway, and mediates virus entry, cellular microtubule-dependent trafficking, budding, and release | PPxY | [6,67,77] |
1 Degenerate residues are bracketed, braces refer to the excluded residues (i.e., any residues except those between braces), “x” means any residue, b refers to basic residues (H, K or R), “φ” denotes large hydrophobic residues (F, I, L or V), and the number of recurrence is indicated after residues.
Agnoprotein
Agnoprotein (agnosis means unknown in Latin) is a regulatory protein encoded by some polyomaviruses, including the BK virus (BKV, named after the isolation from patient, initials B.K.), JC virus (JCV, John Cunningham virus) and simian vacuolating virus 40 (SV40) [100]. The exact function is unknown, but it is reported to have role in viral DNA replication and transcription, which requires an FIL-rich motif (L[FL][VI]F[VIL]LE[LF]LLxF) at the N-terminus [97,98]. Moreover, it may facilitate nuclear egress by interacting with heterochromatin protein 1 at the nuclear envelope [146]. Interactions with proliferating cell nuclear antigen (PCNA) lead to the inhibition of PCNA-dependent DNA synthesis and the reduction of cell proliferation [99]. The PCNA-interacting protein box (PIP motif, Qxx[IML]xx[FY]) is shared with most of the PCNA-interacting proteins. Although JCV, BKV and SV40 agnoproteins harbor PIP-like consensus (QR[LI][FL][IV]F), several regions could be involved in the interaction [99]. The agnoproteins contain a l-rich and KR-rich motif (such as RRRRx5Rx4RK), which may represent a classic NES and NLS, respectively [100]. Ironically, although agnoproteins contain NES and NLS motifs, most of the known agnoproteins localize in the cytoplasm and/or are perinuclear [100], and their nuclear trafficking needs to be elucidated.
4. Viral Virulence
4.1. APOBEC-Binding Motifs
The “Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like” (APOBEC) proteins are crucial for the editing of cytosine to uracil bases during reverse transcription (mRNA editing), as reviewed in [8,133,147,148]. Three proteins, APOBEC-3C, 3F and 3G (A3C, A3F and A3G), exhibit potent antiviral activity by inhibiting retroviruses, including HIV replication, reverse transcription and DNA integration into the host genome [147]. Vif proteins encoded by HIV and simian immunodeficiency virus (SIV) bind to E3 ubiquitin ligase, cullin5 and elongin, leading to A3 ubiquitination and proteasomal degradation [8,9,10,133,134,148]. By this mechanism, retroviruses can suppress A3 antiretroviral activity [133]. These interactions are mediated by a number of motifs, including the YRHHY, PPLP, DRMR, and T[DEQ]x5Adx2[IL] motifs, whereas other motifs were also reported (Table 1) [133,134,135,136,137,138,139,140,141,142,143,144,149].
4.2. Pentraxin Domain
The Pentraxin superfamily are pattern recognition receptors, which include long pentraxin-3 and the short serum amyloid P component and C reactive protein. They have a diverse role in inflammation, host defense and antiviral response [126,127]. These proteins are characterized by a pentameric structure and the pentraxin domain (HxCx[ST]WxS). The hemagglutinin (HA) glycoprotein of influenza A virus recognizes sialic acid on pentraxin-3, resulting in virus neutralization [150]. Further analysis suggests that this interaction is critical for productive viral infection [151].
4.3. The PDZ Domain
PDZ is an abbreviation for post-synaptic density protein (PSD95), Drosophila disc large tumor suppressor (Dlg1), and zonula occludens-I protein (zo-1). The canonical PDZ domains harbor the conserved carboxylate-binding loop motif groove ([RK]xxx[GSTF]φGφ) between αB and βB structural elements [152]. It mediates protein-protein interaction, phosphorylation and regulates cellular signaling, including transport and ion channel signaling, as reviewed in [152]. It also mediates interactions between cytoplasmic proteins and tight junction proteins, which can be used by viruses to enter into host cells, as reviewed in [153,154]. PDZ domains are classified into three classes based on the C-terminus recognition sequence motif of their target proteins: the class I domain, which recognizes the [ST]xφ motif; the class II domain, which recognizes the φxφ motif; and the class III domain, which recognizes the [DE]xφ motif.
The human papillomavirus (HPV) E6 protein targets PDZ domain–containing proteins, which are regulated by protein phosphorylation and protein kinase signaling pathways, as shown in Figure 3 [155,156]. Influenza A virus NS1 contains PDZ domain–binding motif (ESEV and RSKV motifs in the NS1 of avian and human influenza viruses, respectively). A mutation in ESEV affects the PI3K/Akt pathway, interactions of NS1 with scaffolding proteins and the virulence of avian H5N1 influenza viruses [157]. Tax1 is another PDZ-binding motif containing oncoprotein, encoded by Human T-cell leukemia virus (HTLV-1) [158]. The Tax1 protein is involved in various functions, including interaction with proteins (it harbors PDZ) involved in cell signaling, such as transcription factors (cAMP response element-binding protein), nuclear factors (NF-κB), chromatin-modifying enzymes, GTPases and kinases (MAPK). These signal cascades may lead to the inhibition of cell cycle progression, and DNA repair, as reviewed in [158] and [159]. Tax1 acts as a transcriptional activator by activating PI3K-Akt and NF-κB pathways, which induce transformation, continued cell cycle progression and resisting apoptosis [159,160], and may induce CD83 expression on T cells [161].
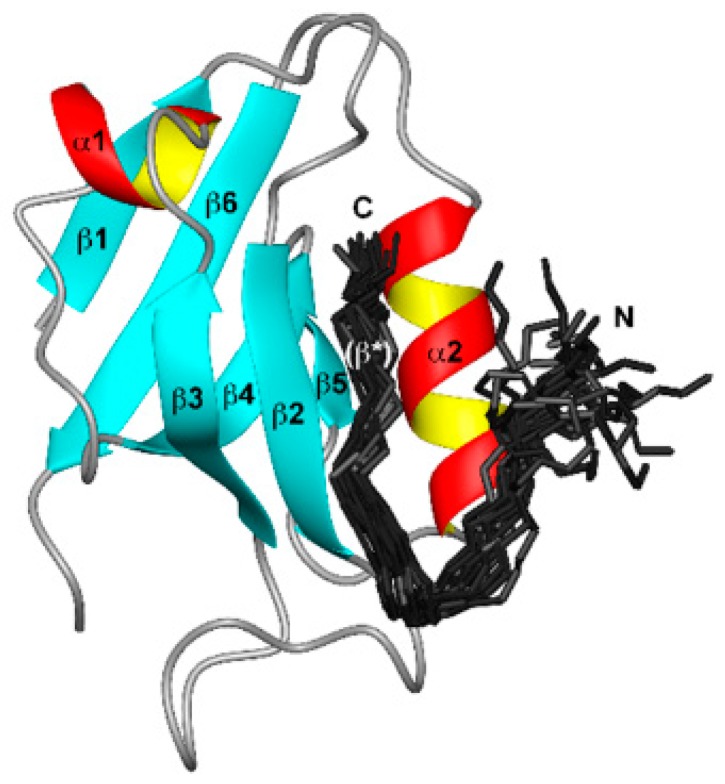
Figure 3.
Binding of HPV E6 to the second PDZ domain (PDZ2) from the human homologue of the Drosophila discs large tumor suppressor protein (hDlg). E6 (150 residues) consists of two zinc-binding domains (Cx2Cx29Cx2C). The bundle of 20 best E6 structures (residues 141 to 151, dark grey). Adopted and modified from [156], published under Creative Commons Attribution license.
4.4. Anti-Tetherin Activity
Tetherin (bone marrow stromal antigen 2, BST2) is a cellular protein inhibiting virus release and has antiviral activity. HIV-1 Vpu enhances the release of viral particles from infected cells by counteracting human tetherin [162]. The ExxxLV motif in the second α-helix has been shown to be required for tetherin degradation and virion release from CD4+ T cells [145]. Mutation of the motif (which is conserved in most HIV-1 clades) inhibits the ESCRT-dependent degradation of Vpu-tetherin complex [145]. This transmembrane interaction is required for Vpu interactions with APs [163]. Two other domains in Vpu (Yxxφ and DSGxxS) could mediate anti-tetherin activity [112], whereas the [GD]DIWK motif in monkey BST2, but not in human, is required for interaction with HIV-1 Vpu [113].
4.5. Transmembrane Domain (TMD) Interactions
Viral proteins can interact with cellular proteins through TMDs to counteract innate immune response. These interactions are mediated by motifs. HIV-1 Vpu can antagonize tetherin within the lipid bilayer, with α-helical TMDs of both proteins [114]. The conservation of the Ax3Ax3Ax3W and Vx3IxxLx3L motifs in HIV Vpu and primate BST2, respectively, suggests their putative role in TMD interaction [114,115]. Also, the GxxxG motif is identified for protein-protein, transmembrane-helix and helix-helix interactions [164,165]. Mutation in the 125GxxxG129 motif in the second transmembrane segments of the NS4B protein may influence protein-folding and interactions, and the replication of engineered HCV-JFH1 [166]. Another example is the influenza virus M2 ion channel protein, which is vital for replication and proton transport [167,168]. M2 has a transmembrane domain, which harbors the conserved HxxxW motif, where H and W are involved in the protein’s channel function. Similarly, the p7 protein encoded by HCV is a viroporin that harbors the HxxxW conserved motif and can transport protons [132].
4.6. Retinoblastoma (Rb or pRb)
The Rb encoded by humans is involved in protein-protein interactions, gene expression, cell division and acts as a tumor suppressor. Interaction between oncogenic protein and Rb leads to the phosphorylation and inactivation of Rb, and the progression of cancer. Viral oncoproteins can utilize the conserved Rb-binding motif (LxCxE) on viral proteins to bind to Rb, modulate gene expression, and cause tumor growth. Examples of Rb-binding proteins are as the following: (i) human CMV UL97 serine-threonine kinase [101]; (ii) Polyomaviruses large and small T antigen oncoproteins, which interact with tumor suppressor proteins, and Merkel cell polyomavirus (MCPyV) large T antigen, which harbors LxCxE and NLS (RKRK) motifs (essential for replication) [102,103,104,105,106]; (iii) White spot syndrome virus IE1 and WSV056 that regulate cell cycle progression [107]; (iv) Adenovirus E1A [108]; and (v) HPV E7 [109]. Furthermore, Rb-related protein (RBR) in plants is involved in protein-protein interactions and gene expression [169]. The geminiviruses replication factor AL1 interacts with RBR to modulate host gene expression and DNA replication machinery. It is noteworthy that the LxCxE motif is not the exclusive Rb-binding motif, for instance AL1 does not harbor the LxCxE motif, but recruits helix 4 to bind to plant RBR [169].
4.7. Cleavage Site Motif
The viral protein precursor is cleaved by cellular proteases (e.g., matriptase or furin) into active protein form. Among the examples, NDV fusion glycoprotein (F protein) is encoded as an inactive precursor, which is cleaved proteolytically, into two bisulfide-linked polypeptides [170,110]. This cleavage determines the strain type, either lentogenic (avirulent), mesogenic (intermediate) or velogenic (virulent). The consensus sequence of the F protein cleavage site of lentogenic is 112[GE][KR]Q[GE]Rα↓L117, while the site of velogenic and mesogenic strains is 112[RK]RQ[RK]R↓F117 [110]. Moreover, the F protein mediates virus entry and fusion with the cell membrane for most avian paramyxoviruses type 9 (APMV-9) strains. Recent reports show that the F protein cleavage site sequence is not a major determinant of pathogenicity and virulence of APMV-7 in chickens [171], and other regions of the F protein could modulate virus virulence [172]. In influenza A virus, the cleavage site of HA is Rx[RK]R↓GLF in highly pathogenic avian influenza virus H5N1, while RxxR↓, RxRR↓, and KKKR↓ are also reported [111]. The R and K can be replaced by non-basic residues, such as [QE][ST]R↓GLF.
5. Motifs Essential for Virion Structure and Life Cycle (Usually Unique to Virus Families)
5.1. Motifs Involved in Structural Proteins
Adenoviruses bear short and/or long fibers. The fiber consists of a shaft and knob. Analysis of Adv fibers showed that the Adv-D fiber shaft bears fiber flexibility motifs KLGxGLxF[DN] and KxGGLxF[DN], which may have roles in interactions with host cells [50].
5.2. Transposition
Kaposin is an oncoprotein that transforms cells in culture and induces tumor formation. Expression and transforming activity of Kaposin A protein is determined by the LxxLL motif [116,117], whereas LQQLL in HIV-1 viral protein of regulation (Vpr) is required for retrotransposition [118,119]. Also, LxxLL and PDZ protein-binding domains are important for the HPV16 E6 protein to interact with the p53 protein [120,173,174,175]. The interaction then activates mTORC1 (rapamycin complex 1) signaling, kinase phosphorylation, translation initiation factor and cap-dependent translation. Therefore, HPV16 E6 protein is correlated with HPV-induced oncogenesis and could be considered as a future therapeutic against HPV-induced cancers [120,121]. Further evidence shows that E6 proteins lacking the LxxLL motif can interact with p53 [122].
5.3. Inhibitor of Apoptosis (IAP) Family Proteins (Apoptosis Suppressors)
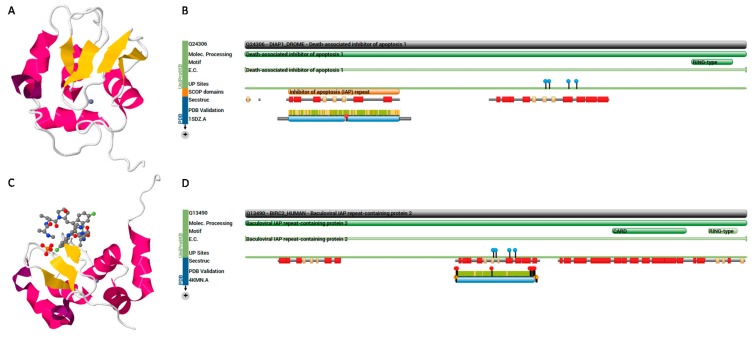
IAP is encoded by virus members of eight families: Ascoviridae, Asfarviridae, Baculoviridae, Hytrosaviridae, Iridoviridae, Malacoherpesviridae, Nudiviridae, and subfamily Entomopoxvirinae of family Poxviridae [176]. Baculoviruses block apoptosis by encoding IAPs, which are characterized by the presence of one or more baculoviral IAP repeat (BIR) domains, except for Deltabaculovirus [177,178]. The core component of BIR is a Cys/His motif (Gx2Yx4Dx3Cx2Cx6Wx9Hx6–10C) that coordinates a single zinc ion; however, about two-thirds of the human IAP proteins harbor a C-terminus RING domain (40–60 amino acids), with consensus Cx2Cx9–39Cx1–3Hx2–3Cx2Cx4–48Cx2C [123]. IAP (70 amino acids) mediates protein-protein interactions essential for anti-apoptotic potential [124] by binding to the IAP-binding motif (A[KITV][AEP][FEISY]) (Figure 4, Table S1) [125].
Figure 4.
(A) Structure of death-associated inhibitor of apoptosis 1 (DIAP1) protein of Drosophila melanogaster (PDB ID: 1SDZ, Uniprot ID: Q24306) [179]; (B) protein features show that it belongs to the IAP family, and contains two BIR repeats and a RING-type zinc finger; (C) structure of baculoviral IAP repeat-containing protein 2 (BIRC2) of human (PDB ID: 4KMN, Uniprot ID: Q13490); (D) protein features show that it contains three BIR repeats, a CARD domain and a RING-type zinc finger. The figures adopted from PDB and Uniprot.
6. Motifs Enriched with Residues (Xaa-Rich Motifs) and Low-Complexity Regions
Low-complexity regions (LCRs) are repeats or extensions of one or more residue(s), which could be flanked or interrupted by other residues [180,181,182,183]. Few structural and functional data are available on LCRs, because they may not crystallize easily [181,182,183]. However, they may play roles in protein-protein interactions [183]. In bibliography, there is another type of sequences, which are not referred to as LCRs. They are referred to as Xaa-rich or X-rich motifs, where “X” or “Xaa” refers to any amino acid. They are enriched with residue(s), which may not be repeated, but are flanked by other residues. These alternative residues enrich the structure of x-rich motifs. G-rich residues could be considered as an example, such as GxxxG, [VI]xGxGxxG or (Gx1–3Gx1–3G). They can be detected in oxidoreductases and may mediate binding to FAD or NAD [128]. Also, the KR-rich motif (such as RKRK and RRRRx5Rx4RK) is an example which may represent a classic NLS [100]. The functions and structures of these sequences deserve to be elucidated by future studies.
6.1. Cys-Rich Motifs
Thioredoxins (trx) belong to the oxidoreductase superfamily, and harbor thioredoxin fold, which is a four-stranded β-sheet surrounded by three α-helices. It reduces thiol groups during thiol-disulfide exchange [184,185,186]. The trx fold first was discovered in bacteria, then found in eukaryotes. The family harbors a conserved CxxC active site motif, which is a signature for the family and thiol-disulfide reactions. CxxC and CxxxC motifs have roles in poxvirus A16 protein interaction and thiol-disulfide transfer during cytoplasmic redox pathway [129]. Moreover, the CxxC motif in the HTLV-1 envelope-fusion protein (env) mediates disulfide isomerization and, hence, promotes viral fusion and infection [130]. CxxxC in Respiratory syncytial virus G protein contributes to virus pathogenicity by binding to the CX3CR1 receptor on host cells [131]. Blocking CX3CR1 with antibodies reduces infection and triggers the immune response.
Proteins containing the chitin-binding domain, or the 6-cysteine motif, Cx13–20Cx5–6Cx9–19Cx10–14Cx4–14C, are able to degrade chitin and chitotriose. Other proteins have antimicrobial activity and are associated with immune response against pathogens. Ac83 and ha83 proteins encoded by baculoviruses harbor putative C2HC zinc finger (Cx5CxnHx6C) and 6-cysteine motifs, respectively, and have a role in budded virion production and nucleocapsid assembly [95,96]. A zinc finger domain is also characterized in the large T antigen of polyomaviruses, including SV40 [106,187]. Large T antigen (LTag) contains four conserved domains, the J domain, the origin-binding domain (OBD), the zinc-binding domain, and the AAA+ ATPase domains. The J domain may have a role in viral DNA replication, OBD may contribute to DNA replication and binding to transcription factors, and ATPase has enzymatic activities to support the required energy, while the zinc finger domain is responsible for the oligomerization of LTag forming hexamers [106,187].
6.2. SR-Rich Motif
These LCR motifs are found in a number of viral proteins, which suggests their role in virus replication [188]. Among these proteins are: (1) SSRSSSRSRGNSR in SARS-CoV nucleocapsid protein; (2) RSNSRSRSRSRSRSR and SRSKSRARSQSR in turkey and human astrovirus capsid protein, respectively; (3) SSRYSSTSRERSRLSR in Marburg virus L protein; and (4) RSISRDKTTTDYRSSRS in the minor nucleoprotein of Ebola virus.
6.3. PEST Motif
This is a peptide sequence which is rich in Pro (P), Glu (E), Ser (S) and Thr (T). It acts as a signal peptide for protein degradation. The motif is required for binding between the HPV16 E7 protein with human interferon regulatory factor-9 [189]. The PEST motif was predicted in HBV proteins and mouse norovirus non-structural protein; however, the exact role in infection is unknown and may not be necessary for the infection process [190,191].
7. Concluding Remarks and Future Perspective
This article reviews the functional motifs utilized by viruses. These motifs are required for productive virus infection. The patterns and functions of motifs were highlighted, aiming to present an insight into motifs and their patterns. The proteins harboring these motifs, as well as viruses encoding these proteins, were also highlighted. The motifs were divided into five main groups according to their cellular function during the virus replication cycle (Figure 1, and as summarized in Table 1).
It worth emphasizing that viruses may use multiple motifs for one process. They might be able to evolve mechanisms to utilize alternative motifs in the absence of the primary one. For example, (i) SUMO-binding to substrate [12,13]; (ii) RGD-like motifs (RGG or GGG) [55]; and (iii) the LxCxE motif is not the exclusive Rb-binding motifs [169]. Moreover, the consensus pattern is not the absolute measure for the protein functions. Although the motif might fulfill the pattern consensus, it could not perform the function. Other factors could influence the function. For example, the NTCP harbors two LL motifs, (136LL137) and (222LL223), but the second motif was shown to be more effective in regulating endocytosis [61], which could be due to the phosphorylation of the adjacent T225 and S226 residues. The 125GxxxG129 motif in the second transmembrane segments of the NS4B protein, but not 143GxxxG147 in the third segments, is required for HCV replication [166].
These motifs mediate interactions and molecular processes within host cells. Therefore, an increasing amount of evidence suggests that motifs can be considered as potential targets for therapeutic agents. These attempts include (i) interfering with post-translational modification processes by SENPs proteases [17,18,21]; (ii) motifs mediating the ESCRT pathway (P[TS]AP, PPxY and KATN) as anti-filovirus therapeutic agents [78,81,84,85]; (iii) inhibiting Vif-mediated degradation of antiretroviral A3 [147,133]; (iv) HPV16 E6 protein acting against HPV-induced oncogenesis [120,121]. Moreover, targeting and counteracting proteins (motifs) involved in entry could lead to an efficient therapeutic strategy [192], whereas targeting cellular processes may lead to increased cytotoxicity.
It is also important to emphasize that studying functional motifs would benefit from the prediction of protein characteristics, cellular interactions or the putative role of a protein. The link between functional motifs and protein functional analysis and/or prediction should be established by future research. Moreover, these studies may assist in characterizing virus tropism and studying emerging viruses (zoonotic viruses) capable of infecting humans [56,193]. Since these motifs are subjected to evolutionary modifications, it is of interest to study lateral gene transfer between species or strains as well as evolutionary events occurring in proteins. Also, it is important to study functional and molecular modifications accompanying insertion into or mutation of the motifs within proteins. On the other hand, the numbers of newly isolated viruses were expanded over last years, particularly giant viruses, which harbor proteins of unknown functions. This expansion requires efforts by future research to predict protein functions, which could be achieved by in silico determination of sequence characteristics and prediction of structural and functional sites in the sequences prior to designing further experiments.
Acknowledgments
The author would like to thank editors and reviewers for the valuable comment.
Supplementary Materials
The following is available online at www.mdpi.com/2227-7382/4/1/3/s1. Table S1: IAP and nuclear trafficking motifs.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kadaveru K., Vyas J., Schiller M.R. Viral infection and human disease—Insights from minimotifs. Front. Biosci. 2008;13:6455–6471. doi: 10.2741/3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Via A., Uyar B., Brun C., Zanzoni A. How pathogens use linear motifs to perturb host cell networks. Trends Biochem. Sci. 2015;40:36–48. doi: 10.1016/j.tibs.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Sigrist C.J., de Castro E., Cerutti L., Cuche B.A., Hulo N., Bridge A., Bougueleret L., Xenarios I. New and continuing developments at PROSITE. Nucleic Acids Res. 2013;41:D344–D347. doi: 10.1093/nar/gks1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrow A.D., Trowsdale J. You say ITAM and I say ITIM, let’s call the whole thing off: The ambiguity of immunoreceptor signalling. Eur. J. Immunol. 2006;36:1646–1653. doi: 10.1002/eji.200636195. [DOI] [PubMed] [Google Scholar]
- 5.Lambert A.A., Azzi A., Lin S.X., Allaire G., St-Gelais K.P., Tremblay M.J., Gilbert C. Dendritic cell immunoreceptor is a new target for anti-AIDS drug development: Identification of DCIR/HIV-1 inhibitors. PLoS ONE. 2013;8:e67873. doi: 10.1371/journal.pone.0067873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wodrich H., Henaff D., Jammart B., Segura-Morales C., Seelmeir S., Coux O., Ruzsics Z., Wiethoff C.M., Kremer E.J. A capsid-encoded PPxY-motif facilitates adenovirus entry. PLoS Pathog. 2010;6:e1000808. doi: 10.1371/journal.ppat.1000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H., Lv G., Zhou X., Li Z., Liu X., Yu X.F., Zhang W. Requirement of HIV-1 Vif C-terminus for Vif-CBF-β interaction and assembly of CUL5-containing E3 ligase. BMC Microbiol. 2014;14 doi: 10.1186/s12866-014-0290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wissing S., Galloway N.L., Greene W.C. HIV-1 Vif versus the APOBEC3 cytidine deaminases: An intracellular duel between pathogen and host restriction factors. Mol. Asp. Med. 2010;31:383–397. doi: 10.1016/j.mam.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans S.L., Schon A., Gao Q., Han X., Zhou X., Freire E., Yu X.F. HIV-1 Vif N-terminal motif is required for recruitment of Cul5 to suppress APOBEC3. Retrovirology. 2014;11 doi: 10.1186/1742-4690-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng Y., Baig T.T., Love R.P., Chelico L. Suppression of APOBEC3-mediated restriction of HIV-1 by Vif. Front. Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geiss-Friedlander R., Melchior F. Concepts in sumoylation: A decade on. Nat. Rev. Mol. Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- 12.Enserink J.M. Sumo and the cellular stress response. Cell Div. 2015;10 doi: 10.1186/s13008-015-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickey C.M., Wilson N.R., Hochstrasser M. Function and regulation of sumo proteases. Nat. Rev. Mol. Cell Biol. 2012;13:755–766. doi: 10.1038/nrm3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong R., Wang A. SCE1, the SUMO-conjugating enzyme in plants that interacts with NIb, the RNA-dependent RNA polymerase of Turnip mosaic virus, is required for viral infection. J. Virol. 2013;87:4704–4715. doi: 10.1128/JVI.02828-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varadaraj A., Mattoscio D., Chiocca S. SUMO Ubc9 enzyme as a viral target. IUBMB Life. 2014;66:27–33. doi: 10.1002/iub.1240. [DOI] [PubMed] [Google Scholar]
- 16.Wimmer P., Schreiner S. Viral mimicry to usurp ubiquitin and sumo host pathways. Viruses. 2015;7:4854–4872. doi: 10.3390/v7092849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang P.C., Kung H.J. SUMO and KSHV replication. Cancers. 2014;6:1905–1924. doi: 10.3390/cancers6041905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L., Li S., Li Y., Duan X., Liu B., McGilvray I. Ubiquitin-like protein modifiers and their potential for antiviral and anti-hcv therapy. Expert Rev. Proteom. 2013;10:275–287. doi: 10.1586/epr.13.15. [DOI] [PubMed] [Google Scholar]
- 19.Wilson V.G., Rangasamy D. Viral interaction with the host cell sumoylation system. Virus Res. 2001;81:17–27. doi: 10.1016/S0168-1702(01)00365-3. [DOI] [PubMed] [Google Scholar]
- 20.Drag M., Salvesen G.S. DeSUMOylating enzymes—SENPs. IUBMB Life. 2008;60:734–742. doi: 10.1002/iub.113. [DOI] [PubMed] [Google Scholar]
- 21.Madu I.G., Li S., Li B., Li H., Chang T., Li Y.J., Vega R., Rossi J., Yee J.K., Zaia J., et al. A Novel Class of HIV-1 Antiviral Agents Targeting HIV via a SUMOylation-Dependent Mechanism. Sci. Rep. 2015;5 doi: 10.1038/srep17808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuchs S.Y. Ubiquitination-mediated regulation of interferon responses. Growth Factors. 2012;30:141–148. doi: 10.3109/08977194.2012.669382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcin D., Marq J.B., Strahle L., le Mercier P., Kolakofsky D. All four Sendai virus C proteins bind Stat1, but only the larger forms also induce its mono-ubiquitination and degradation. Virology. 2002;295:256–265. doi: 10.1006/viro.2001.1342. [DOI] [PubMed] [Google Scholar]
- 24.Ulane C.M., Horvath C.M. Paramyxoviruses SV5 and HPIV2 assemble STAT protein ubiquitin ligase complexes from cellular components. Virology. 2002;304:160–166. doi: 10.1006/viro.2002.1773. [DOI] [PubMed] [Google Scholar]
- 25.Ulane C.M., Kentsis A., Cruz C.D., Parisien J.P., Schneider K.L., Horvath C.M. Composition and assembly of STAT-targeting ubiquitin ligase complexes: Paramyxovirus V protein carboxyl terminus is an oligomerization domain. J. Virol. 2005;79:10180–10189. doi: 10.1128/JVI.79.16.10180-10189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trilling M., Le V.T., Fiedler M., Zimmermann A., Bleifuss E., Hengel H. Identification of DNA-damage DNA-binding protein 1 as a conditional essential factor for cytomegalovirus replication in interferon-gamma-stimulated cells. PLoS Pathog. 2011;7:e1002069. doi: 10.1371/journal.ppat.1002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q., Means R., Lang S., Jung J.U. Downregulation of gamma interferon receptor 1 by Kaposi’s sarcoma-associated herpesvirus K3 and K5. J. Virol. 2007;81:2117–2127. doi: 10.1128/JVI.01961-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boname J.M., Lehner P.J. What has the study of the K3 and K5 viral ubiquitin E3 ligases taught us about ubiquitin-mediated receptor regulation? Viruses. 2011;3:118–131. doi: 10.3390/v3020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt K., Wies E., Neipel F. Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor 3 inhibits gamma interferon and major histocompatibility complex class ii expression. J. Virol. 2011;85:4530–4537. doi: 10.1128/JVI.02123-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brulois K., Toth Z., Wong L.Y., Feng P., Gao S.J., Ensser A., Jung J.U. Kaposi’s sarcoma-associated herpesvirus K3 and K5 ubiquitin E3 ligases have stage-specific immune evasion roles during lytic replication. J. Virol. 2014;88:9335–9349. doi: 10.1128/JVI.00873-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarkar S., Balasuriya U.B., Horohov D.W., Chambers T.M. Equine herpesvirus-1 suppresses type-I interferon induction in equine endothelial cells. Vet. Immunol. Immunopathol. 2015;167:122–129. doi: 10.1016/j.vetimm.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Huang F., Yang C., Yu W., Bi Y., Long F., Wang J., Li Y., Jing S. Hepatitis E virus infection activates signal regulator protein alpha to down-regulate type I interferon. Immunol. Res. 2015 doi: 10.1007/s12026-015-8729-y. [DOI] [PubMed] [Google Scholar]
- 33.Jegaskanda S., Ahn S.H., Skinner N., Thompson A.J., Ngyuen T., Holmes J., de Rose R., Navis M., Winnall W.R., Kramski M., et al. Downregulation of interleukin-18-mediated cell signaling and interferon gamma expression by the hepatitis B virus e antigen. J. Virol. 2014;88:10412–10420. doi: 10.1128/JVI.00111-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lenschow D.J., Giannakopoulos N.V., Gunn L.J., Johnston C., O’Guin A.K., Schmidt R.E., Levine B., Virgin H.W.T. Identification of interferon-stimulated gene 15 as an antiviral molecule during Sindbis virus infection in vivo. J. Virol. 2005;79:13974–13983. doi: 10.1128/JVI.79.22.13974-13983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morales D.J., Lenschow D.J. The antiviral activities of ISG15. J. Mol. Biol. 2013;425:4995–5008. doi: 10.1016/j.jmb.2013.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langevin C., van der Aa L.M., Houel A., Torhy C., Briolat V., Lunazzi A., Harmache A., Bremont M., Levraud J.P., Boudinot P. Zebrafish ISG15 exerts a strong antiviral activity against RNA and DNA viruses and regulates the interferon response. J. Virol. 2013;87:10025–10036. doi: 10.1128/JVI.01294-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W., Zhang M., Xiao Z.Z., Sun L. Cynoglossus semilaevis ISG15: A secreted cytokine-like protein that stimulates antiviral immune response in a LRGG motif-dependent manner. PLoS ONE. 2012;7:e44884. doi: 10.1371/journal.pone.0044884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang C., Parrish N.F., Wilen C.B., Li H., Chen Y., Pavlicek J.W., Berg A., Lu X., Song H., Tilton J.C., et al. Primary infection by a human immunodeficiency virus with atypical coreceptor tropism. J. Virol. 2011;85:10669–10681. doi: 10.1128/JVI.05249-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choe H., Farzan M., Sun Y., Sullivan N., Rollins B., Ponath P.D., Wu L., Mackay C.R., LaRosa G., Newman W., et al. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/S0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 40.Michael N.L., Nelson J.A., KewalRamani V.N., Chang G., O’Brien S.J., Mascola J.R., Volsky B., Louder M., White G.C., II, Littman D.R., et al. Exclusive and persistent use of the entry coreceptor CXCR4 by human immunodeficiency virus type 1 from a subject homozygous for CCR5 Δ32. J. Virol. 1998;72:6040–6047. doi: 10.1128/jvi.72.7.6040-6047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berger E.A., Murphy P.M., Farber J.M. Chemokine receptors as HIV-1 coreceptors: Roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 42.Huang C.C., Tang M., Zhang M.Y., Majeed S., Montabana E., Stanfield R.L., Dimitrov D.S., Korber B., Sodroski J., Wilson I.A., et al. Structure of a V3-containing HIV-1 gp120 core. Science. 2005;310:1025–1028. doi: 10.1126/science.1118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haqqani A.A., Tilton J.C. Entry inhibitors and their use in the treatment of HIV-1 infection. Antivir. Res. 2013;98:158–170. doi: 10.1016/j.antiviral.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Henrich T.J., Kuritzkes D.R. HIV-1 entry inhibitors: Recent development and clinical use. Curr. Opin. Virol. 2013;3:51–57. doi: 10.1016/j.coviro.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Perez J., Staropoli I., Azoulay S., Heinrich J.T., Cascajero A., Colin P., Lortat-Jacob H., Arenzana-Seisdedos F., Alcami J., Kellenberger E., et al. A single-residue change in the HIV-1 V3 loop associated with maraviroc resistance impairs CCR5 binding affinity while increasing replicative capacity. Retrovirology. 2015;12 doi: 10.1186/s12977-015-0177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fouchier R.A., Groenink M., Kootstra N.A., Tersmette M., Huisman H.G., Miedema F., Schuitemaker H. Phenotype-associated sequence variation in the third variable domain of the human immunodeficiency virus type 1 gp120 molecule. J. Virol. 1992;66:3183–3187. doi: 10.1128/jvi.66.5.3183-3187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Catasti P., Bradbury E.M., Gupta G. Structure and polymorphism of HIV-1 third variable loops. J. Biol. Chem. 1996;271:8236–8242. doi: 10.1074/jbc.271.14.8236. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y., Bergelson J.M. Adenovirus receptors. J. Virol. 2005;79:12125–12131. doi: 10.1128/JVI.79.19.12125-12131.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma A., Li X., Bangari D.S., Mittal S.K. Adenovirus receptors and their implications in gene delivery. Virus Res. 2009;143:184–194. doi: 10.1016/j.virusres.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Darr S., Madisch I., Hofmayer S., Rehren F., Heim A. Phylogeny and primary structure analysis of fiber shafts of all human adenovirus types for rational design of adenoviral gene-therapy vectors. J. Gen. Virol. 2009;90:2849–2854. doi: 10.1099/vir.0.014514-0. [DOI] [PubMed] [Google Scholar]
- 51.Di Paolo N.C., Kalyuzhniy O., Shayakhmetov D.M. Fiber shaft-chimeric adenovirus vectors lacking the KKTK motif efficiently infect liver cells in vivo. J. Virol. 2007;81:12249–12259. doi: 10.1128/JVI.01584-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cusack S. Adenovirus complex structures. Curr. Opin. Struct. Biol. 2005;15:237–243. doi: 10.1016/j.sbi.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Marvin S.A., Wiethoff C.M. Emerging roles for ubiquitin in adenovirus cell entry. Biol. Cell. 2012;104:188–198. doi: 10.1111/boc.201100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hendrickx R., Stichling N., Koelen J., Kuryk L., Lipiec A., Greber U.F. Innate immunity to adenovirus. Hum. Gene Ther. 2014;25:265–284. doi: 10.1089/hum.2014.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang G., Wang Y., Shang Y., Zhang Z., Liu X. How foot-and-mouth disease virus receptor mediates foot-and-mouth disease virus infection. Virol. J. 2015;12 doi: 10.1186/s12985-015-0246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robinson C.M., Zhou X., Rajaiya J., Yousuf M.A., Singh G., DeSerres J.J., Walsh M.P., Wong S., Seto D., Dyer D.W., et al. Predicting the next eye pathogen: Analysis of a novel adenovirus. MBio. 2013;4:e00595–e00512. doi: 10.1128/mBio.00595-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Azab W., Lehmann M.J., Osterrieder N. Glycoprotein H and α4β1 integrins determine the entry pathway of alphaherpesviruses. J. Virol. 2013;87:5937–5948. doi: 10.1128/JVI.03522-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berryman S., Clark S., Kakker N.K., Silk R., Seago J., Wadsworth J., Chamberlain K., Knowles N.J., Jackson T. Positively charged residues at the five-fold symmetry axis of cell culture-adapted foot-and-mouth disease virus permit novel receptor interactions. J. Virol. 2013;87:8735–8744. doi: 10.1128/JVI.01138-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Minakshi R., Padhan K. The YXXΦ motif within the severe acute respiratory syndrome coronavirus (SARS-CoV) 3a protein is crucial for its intracellular transport. Virol. J. 2014;11 doi: 10.1186/1743-422X-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan H., Zhong G., Xu G., He W., Jing Z., Gao Z., Huang Y., Qi Y., Peng B., Wang H., et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife. 2012;1:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stross C., Kluge S., Weissenberger K., Winands E., Haussinger D., Kubitz R. A dileucine motif is involved in plasma membrane expression and endocytosis of rat sodium taurocholate cotransporting polypeptide (NTCP) Am. J. Physiol. Gastrointest. Liver Physiol. 2013;305:G722–G730. doi: 10.1152/ajpgi.00056.2013. [DOI] [PubMed] [Google Scholar]
- 62.Lemmon S.K., Traub L.M. Getting in touch with the clathrin terminal domain. Traffic. 2012;13:511–519. doi: 10.1111/j.1600-0854.2011.01321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jia X., Weber E., Tokarev A., Lewinski M., Rizk M., Suarez M., Guatelli J., Xiong Y. Structural basis of HIV-1 Vpu-mediated BST2 antagonism via hijacking of the clathrin adaptor protein complex 1. eLife. 2014;3:e02362. doi: 10.7554/eLife.02362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wonderlich E.R., Williams M., Collins K.L. The tyrosine binding pocket in the adaptor protein 1 (AP-1) mu1 subunit is necessary for Nef to recruit AP-1 to the major histocompatibility complex class I cytoplasmic tail. J. Biol. Chem. 2008;283:3011–3022. doi: 10.1074/jbc.M707760200. [DOI] [PubMed] [Google Scholar]
- 65.Collins D.R., Collins K.L. HIV-1 accessory proteins adapt cellular adaptors to facilitate immune evasion. PLoS Pathog. 2014;10:e1003851. doi: 10.1371/journal.ppat.1003851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Samal S., Khattar S.K., Paldurai A., Palaniyandi S., Zhu X., Collins P.L., Samal S.K. Mutations in the cytoplasmic domain of the newcastle disease virus fusion protein confer hyperfusogenic phenotypes modulating viral replication and pathogenicity. J. Virol. 2013;87:10083–10093. doi: 10.1128/JVI.01446-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.El Najjar F., Schmitt A.P., Dutch R.E. Paramyxovirus glycoprotein incorporation, assembly and budding: A three way dance for infectious particle production. Viruses. 2014;6:3019–3054. doi: 10.3390/v6083019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li K., Jia R., Li M., Zheng Y.M., Miao C., Yao Y., Ji H.L., Geng Y., Qiao W., Albritton L.M., et al. A sorting signal suppresses IFITM1 restriction of viral entry. J. Biol. Chem. 2015;290:4248–4259. doi: 10.1074/jbc.M114.630780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bailey C.C., Zhong G., Huang I.C., Farzan M. IFITM-family proteins: The cell’s first line of antiviral defense. Annu. Rev. Virol. 2014;1:261–283. doi: 10.1146/annurev-virology-031413-085537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jia R., Xu F., Qian J., Yao Y., Miao C., Zheng Y.M., Liu S.L., Guo F., Geng Y., Qiao W., et al. Identification of an endocytic signal essential for the antiviral action of IFITM3. Cell. Microbiol. 2014;16:1080–1093. doi: 10.1111/cmi.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Melvin W.J., McMichael T.M., Chesarino N.M., Hach J.C., Yount J.S. IFITMS from mycobacteria confer resistance to influenza virus when expressed in human cells. Viruses. 2015;7:3035–3052. doi: 10.3390/v7062759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schuh A.L., Audhya A. The escrt machinery: From the plasma membrane to endosomes and back again. Crit. Rev. Biochem. Mol. Biol. 2014;49:242–261. doi: 10.3109/10409238.2014.881777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCullough J., Colf L.A., Sundquist W.I. Membrane fission reactions of the mammalian ESCRT pathway. Annu. Rev. Biochem. 2013;82:663–692. doi: 10.1146/annurev-biochem-072909-101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meng B., Lever A.M. Wrapping up the bad news: HIV assembly and release. Retrovirology. 2013;10 doi: 10.1186/1742-4690-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weissenhorn W., Poudevigne E., Effantin G., Bassereau P. How to get out: ssRNA enveloped viruses and membrane fission. Curr. Opin. Virol. 2013;3:159–167. doi: 10.1016/j.coviro.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hurley J.H. Escrts are everywhere. EMBO J. 2015;34:2398–2407. doi: 10.15252/embj.201592484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Votteler J., Sundquist W.I. Virus budding and the ESCRT pathway. Cell Host Microbe. 2013;14:232–241. doi: 10.1016/j.chom.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolff S., Ebihara H., Groseth A. Arenavirus budding: A common pathway with mechanistic differences. Viruses. 2013;5:528–549. doi: 10.3390/v5020528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dilley K.A., Gregory D., Johnson M.C., Vogt V.M. An LYPSL late domain in the gag protein contributes to the efficient release and replication of Rous sarcoma virus. J. Virol. 2010;84:6276–6287. doi: 10.1128/JVI.00238-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Erpapazoglou Z., Walker O., Haguenauer-Tsapis R. Versatile roles of k63-linked ubiquitin chains in trafficking. Cells. 2014;3:1027–1088. doi: 10.3390/cells3041027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han Z., Madara J.J., Liu Y., Liu W., Ruthel G., Freedman B.D., Harty R.N. ALIX rescues budding of a double PTAP/PPEY L-domain deletion mutant of Ebola VP40: A role for ALIX in Ebola virus egress. J. Infect. Dis. 2015;212(Suppl. 2):S138–S145. doi: 10.1093/infdis/jiu838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sette P., Jadwin J.A., Dussupt V., Bello N.F., Bouamr F. The ESCRT-associated protein Alix recruits the ubiquitin ligase Nedd4-1 to facilitate HIV-1 release through the LYPXnL l domain motif. J. Virol. 2010;84:8181–8192. doi: 10.1128/JVI.00634-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quemin E.R., Quax T.E. Archaeal viruses at the cell envelope: Entry and egress. Front. Microbiol. 2015;6 doi: 10.3389/fmicb.2015.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han Z., Lu J., Liu Y., Davis B., Lee M.S., Olson M.A., Ruthel G., Freedman B.D., Schnell M.J., Wrobel J.E., et al. Small-molecule probes targeting the viral PPxY-host Nedd4 interface block egress of a broad range of RNA viruses. J. Virol. 2014;88:7294–7306. doi: 10.1128/JVI.00591-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kenney S.P., Wentworth J.L., Heffron C.L., Meng X.J. Replacement of the hepatitis E virus ORF3 protein PxxP motif with heterologous late domain motifs affects virus release via interaction with TSG101. Virology. 2015;486:198–208. doi: 10.1016/j.virol.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cohen S., Au S., Pante N. How viruses access the nucleus. Biochim. Biophys. Acta. 2011;1813:1634–1645. doi: 10.1016/j.bbamcr.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 87.Fay N., Pante N. Nuclear entry of DNA viruses. Front. Microbiol. 2015;6 doi: 10.3389/fmicb.2015.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.La Cour T., Kiemer L., Molgaard A., Gupta R., Skriver K., Brunak S. Analysis and prediction of leucine-rich nuclear export signals. Protein Eng. Des. Sel. 2004;17:527–536. doi: 10.1093/protein/gzh062. [DOI] [PubMed] [Google Scholar]
- 89.Kosugi S., Hasebe M., Matsumura N., Takashima H., Miyamoto-Sato E., Tomita M., Yanagawa H. Six classes of nuclear localization signals specific to different binding grooves of importin alpha. J. Biol. Chem. 2009;284:478–485. doi: 10.1074/jbc.M807017200. [DOI] [PubMed] [Google Scholar]
- 90.Kalderon D., Roberts B.L., Richardson W.D., Smith A.E. A short amino acid sequence able to specify nuclear location. Cell. 1984;39:499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- 91.Lanford R.E., Butel J.S. Construction and characterization of an SV40 mutant defective in nuclear transport of T antigen. Cell. 1984;37:801–813. doi: 10.1016/0092-8674(84)90415-X. [DOI] [PubMed] [Google Scholar]
- 92.Howley P.M., Livingston D.M. Small DNA tumor viruses: Large contributors to biomedical sciences. Virology. 2009;384:256–259. doi: 10.1016/j.virol.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wanitchang A., Narkpuk J., Jongkaewwattana A. Nuclear import of influenza B virus nucleoprotein: Involvement of an N-terminal nuclear localization signal and a cleavage-protection motif. Virology. 2013;443:59–68. doi: 10.1016/j.virol.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 94.Sherry L., Smith M., Davidson S., Jackson D. The N terminus of the influenza B virus nucleoprotein is essential for virus viability, nuclear localization, and optimal transcription and replication of the viral genome. J. Virol. 2014;88:12326–12338. doi: 10.1128/JVI.01542-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu S., Wang W., Wang Y., Yuan M., Yang K. The baculovirus core gene ac83 is required for nucleocapsid assembly and per os infectivity of autographa californica nucleopolyhedrovirus. J. Virol. 2013;87:10573–10586. doi: 10.1128/JVI.01207-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu H., Xu J., Liu Q., Liu T.X., Wang D. Ha83, a chitin binding domain encoding gene, is important to helicoverpa armigera nucleopolyhedrovirus budded virus production and occlusion body assembling. Sci. Rep. 2015;5 doi: 10.1038/srep11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saribas A.S., White M.K., Safak M. JC virus agnoprotein enhances large T antigen binding to the origin of viral DNA replication: Evidence for its involvement in viral DNA replication. Virology. 2012;433:12–26. doi: 10.1016/j.virol.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sami Saribas A., Abou-Gharbia M., Childers W., Sariyer I.K., White M.K., Safak M. Essential roles of Leu/Ile/Phe-rich domain of JC virus agnoprotein in dimer/oligomer formation, protein stability and splicing of viral transcripts. Virology. 2013;443:161–176. doi: 10.1016/j.virol.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gerits N., Johannessen M., Tummler C., Walquist M., Kostenko S., Snapkov I., van Loon B., Ferrari E., Hubscher U., Moens U. Agnoprotein of polyomavirus BK interacts with proliferating cell nuclear antigen and inhibits DNA replication. Virol. J. 2015;12 doi: 10.1186/s12985-014-0220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gerits N., Moens U. Agnoprotein of mammalian polyomaviruses. Virology. 2012;432:316–326. doi: 10.1016/j.virol.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gill R.B., Frederick S.L., Hartline C.B., Chou S., Prichard M.N. Conserved retinoblastoma protein-binding motif in human cytomegalovirus UL97 kinase minimally impacts viral replication but affects susceptibility to maribavir. Virol. J. 2009;6 doi: 10.1186/1743-422X-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wendzicki J.A., Moore P.S., Chang Y. Large T and small T antigens of Merkel cell polyomavirus. Curr. Opin. Virol. 2015;11:38–43. doi: 10.1016/j.coviro.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garneski K.M., DeCaprio J.A., Nghiem P. Does a new polyomavirus contribute to Merkel cell carcinoma? Genome Biol. 2008;9 doi: 10.1186/gb-2008-9-6-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cheng J., Rozenblatt-Rosen O., Paulson K.G., Nghiem P., DeCaprio J.A. Merkel cell polyomavirus large T antigen has growth-promoting and inhibitory activities. J. Virol. 2013;87:6118–6126. doi: 10.1128/JVI.00385-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Borchert S., Czech-Sioli M., Neumann F., Schmidt C., Wimmer P., Dobner T., Grundhoff A., Fischer N. High-affinity Rb binding, p53 inhibition, subcellular localization, and transformation by wild-type or tumor-derived shortened Merkel cell polyomavirus large T antigens. J. Virol. 2014;88:3144–3160. doi: 10.1128/JVI.02916-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.An P., Saenz Robles M.T., Pipas J.M. Large T antigens of polyomaviruses: Amazing molecular machines. Annu. Rev. Microbiol. 2012;66:213–236. doi: 10.1146/annurev-micro-092611-150154. [DOI] [PubMed] [Google Scholar]
- 107.Ran X., Bian X., Ji Y., Yan X., Yang F., Li F. White spot syndrome virus IE1 and WSV056 modulate the G1/S transition by binding to the host retinoblastoma protein. J. Virol. 2013;87:12576–12582. doi: 10.1128/JVI.01551-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Berk A.J. Recent lessons in gene expression, cell cycle control, and cell biology from adenovirus. Oncogene. 2005;24:7673–7685. doi: 10.1038/sj.onc.1209040. [DOI] [PubMed] [Google Scholar]
- 109.Lee J.O., Russo A.A., Pavletich N.P. Structure of the retinoblastoma tumour-suppressor pocket domain bound to a peptide from HPV E7. Nature. 1998;391:859–865. doi: 10.1038/36038. [DOI] [PubMed] [Google Scholar]
- 110.Zamarin D., Palese P. Oncolytic newcastle disease virus for cancer therapy: Old challenges and new directions. Future Microbiol. 2012;7:347–367. doi: 10.2217/fmb.12.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Garten W., Braden C., Arendt A., Peitsch C., Baron J., Lu Y., Pawletko K., Hardes K., Steinmetzer T., Bottcher-Friebertshauser E. Influenza virus activating host proteases: Identification, localization and inhibitors as potential therapeutics. Eur. J. Cell. Biol. 2015;94:375–383. doi: 10.1016/j.ejcb.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 112.Sauter D. Counteraction of the multifunctional restriction factor tetherin. Front. Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yoshida T., Koyanagi Y., Strebel K. Functional antagonism of rhesus macaque and chimpanzee BST-2 by HIV-1 Vpu is mediated by cytoplasmic domain interactions. J. Virol. 2013;87:13825–13836. doi: 10.1128/JVI.02567-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Skasko M., Wang Y., Tian Y., Tokarev A., Munguia J., Ruiz A., Stephens E.B., Opella S.J., Guatelli J. HIV-1 Vpu protein antagonizes innate restriction factor BST-2 via lipid-embedded helix-helix interactions. J. Biol. Chem. 2012;287:58–67. doi: 10.1074/jbc.M111.296772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jafari M., Guatelli J., Lewinski M.K. Activities of transmitted/founder and chronic clade B HIV-1 Vpu and a C-terminal polymorphism specifically affecting virion release. J. Virol. 2014;2014 doi: 10.1128/JVI.03472-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tomkowicz B., Singh S.P., Lai D., Singh A., Mahalingham S., Joseph J., Srivastava S., Srinivasan A. Mutational analysis reveals an essential role for the LXXLL motif in the transformation function of the human herpesvirus-8 oncoprotein, kaposin. DNA Cell Biol. 2005;24:10–20. doi: 10.1089/dna.2005.24.10. [DOI] [PubMed] [Google Scholar]
- 117.Flaveny C., Reen R.K., Kusnadi A., Perdew G.H. The mouse and human Ah receptor differ in recognition of LXXLL motifs. Arch. Biochem. Biophys. 2008;471:215–223. doi: 10.1016/j.abb.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kino T., Gragerov A., Kopp J.B., Stauber R.H., Pavlakis G.N., Chrousos G.P. The HIV-1 virion-associated protein vpr is a coactivator of the human glucocorticoid receptor. J. Exp. Med. 1999;189:51–62. doi: 10.1084/jem.189.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Iijima K., Okudaira N., Tamura M., Doi A., Saito Y., Shimura M., Goto M., Matsunaga A., Kawamura Y.I., Otsubo T., et al. Viral protein R of human immunodeficiency virus type-1 induces retrotransposition of long interspersed element-1. Retrovirology. 2013;10 doi: 10.1186/1742-4690-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ganti K., Broniarczyk J., Manoubi W., Massimi P., Mittal S., Pim D., Szalmas A., Thatte J., Thomas M., Tomaic V., et al. The human papillomavirus E6 PDZ binding motif: From life cycle to malignancy. Viruses. 2015;7:3530–3551. doi: 10.3390/v7072785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Manzo-Merino J., Thomas M., Fuentes-Gonzalez A.M., Lizano M., Banks L. HPV E6 oncoprotein as a potential therapeutic target in HPV related cancers. Expert Opin. Ther. Targets. 2013;17:1357–1368. doi: 10.1517/14728222.2013.832204. [DOI] [PubMed] [Google Scholar]
- 122.Stutz C., Reinz E., Honegger A., Bulkescher J., Schweizer J., Zanier K., Trave G., Lohrey C., Hoppe-Seyler K., Hoppe-Seyler F. Intracellular analysis of the interaction between the human papillomavirus type 16 E6 oncoprotein and inhibitory peptides. PLoS ONE. 2015;10:e0132339. doi: 10.1371/journal.pone.0132339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Budhidarmo R., Day C.L. IAPs: Modular regulators of cell signalling. Semin. Cell Dev. Biol. 2015;39:80–90. doi: 10.1016/j.semcdb.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 124.Pohl C., Jentsch S. Ernst Schering Foundation Symposium Proceedings. Springer; Berlin, Germany; Heidelberg, Germany: 2008. Regulation of apoptosis and cytokinesis by the anti-apoptotic E2/E3 ubiquitin-ligase bruce; pp. 115–126. [DOI] [PubMed] [Google Scholar]
- 125.Berthelet J., Dubrez L. Regulation of apoptosis by inhibitors of apoptosis (IAPs) Cells. 2013;2:163–187. doi: 10.3390/cells2010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moalli F., Jaillon S., Inforzato A., Sironi M., Bottazzi B., Mantovani A., Garlanda C. Pathogen recognition by the long pentraxin PTX3. J. Biomed. Biotechnol. 2011;2011 doi: 10.1155/2011/830421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ng W.C., Tate M.D., Brooks A.G., Reading P.C. Soluble host defense lectins in innate immunity to influenza virus. J. Biomed. Biotechnol. 2012;2012 doi: 10.1155/2012/732191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kleiger G., Eisenberg D. GXXXG and GXXXA motifs stabilize FAD and NAD(P)-binding Rossmann folds through C(alpha)-H... O hydrogen bonds and van der Waals interactions. J. Mol. Biol. 2002;323:69–76. doi: 10.1016/S0022-2836(02)00885-9. [DOI] [PubMed] [Google Scholar]
- 129.Satheshkumar P.S., Olano L.R., Hammer C.H., Zhao M., Moss B. Interactions of the vaccinia virus A19 protein. J. Virol. 2013;87:10710–10720. doi: 10.1128/JVI.01261-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li K., Zhang S., Kronqvist M., Wallin M., Ekstrom M., Derse D., Garoff H. Intersubunit disulfide isomerization controls membrane fusion of human T-cell leukemia virus Env. J. Virol. 2008;82:7135–7143. doi: 10.1128/JVI.00448-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chirkova T., Boyoglu-Barnum S., Gaston K.A., Malik F.M., Trau S.P., Oomens A.G., Anderson L.J. Respiratory syncytial virus g protein cx3c motif impairs human airway epithelial and immune cell responses. J. Virol. 2013;87:13466–13479. doi: 10.1128/JVI.01741-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Meshkat Z., Audsley M., Beyer C., Gowans E.J., Haqshenas G. Reverse genetic analysis of a putative, influenza virus M2 HXXXW-like motif in the p7 protein of hepatitis C virus. J. Viral Hepat. 2009;16:187–194. doi: 10.1111/j.1365-2893.2008.01064.x. [DOI] [PubMed] [Google Scholar]
- 133.Salter J.D., Morales G.A., Smith H.C. Structural insights for HIV-1 therapeutic strategies targeting Vif. Trends Biochem. Sci. 2014;39:373–380. doi: 10.1016/j.tibs.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.He Z., Zhang W., Chen G., Xu R., Yu X.F. Characterization of conserved motifs in HIV-1 Vif required for APOBEC3G and APOBEC3F interaction. J. Mol. Biol. 2008;381:1000–1011. doi: 10.1016/j.jmb.2008.06.061. [DOI] [PubMed] [Google Scholar]
- 135.Dang Y., Wang X., Zhou T., York I.A., Zheng Y.H. Identification of a novel WxSLVK motif in the N terminus of human immunodeficiency virus and simian immunodeficiency virus Vif that is critical for APOBEC3G and APOBEC3F neutralization. J. Virol. 2009;83:8544–8552. doi: 10.1128/JVI.00651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen G., He Z., Wang T., Xu R., Yu X.F. A patch of positively charged amino acids surrounding the human immunodeficiency virus type 1 Vif SLVx4Yx9Y motif influences its interaction with APOBEC3G. J. Virol. 2009;83:8674–8682. doi: 10.1128/JVI.00653-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dang Y., Davis R.W., York I.A., Zheng Y.H. Identification of 81LGxGxxIxW89 and 171EDRW174 domains from human immunodeficiency virus type 1 Vif that regulate APOBEC3G and APOBEC3F neutralizing activity. J. Virol. 2010;84:5741–5750. doi: 10.1128/JVI.00079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Russell R.A., Pathak V.K. Identification of two distinct human immunodeficiency virus type 1 Vif determinants critical for interactions with human APOBEC3G and APOBEC3F. J. Virol. 2007;81:8201–8210. doi: 10.1128/JVI.00395-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yu X., Yu Y., Liu B., Luo K., Kong W., Mao P., Yu X.F. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302:1056–1060. doi: 10.1126/science.1089591. [DOI] [PubMed] [Google Scholar]
- 140.Marin M., Rose K.M., Kozak S.L., Kabat D. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat. Med. 2003;9:1398–1403. doi: 10.1038/nm946. [DOI] [PubMed] [Google Scholar]
- 141.Pery E., Rajendran K.S., Brazier A.J., Gabuzda D. Regulation of APOBEC3 proteins by a novel YXXL motif in human immunodeficiency virus type 1 Vif and simian immunodeficiency virus SIVagm Vif. J. Virol. 2009;83:2374–2381. doi: 10.1128/JVI.01898-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huthoff H., Malim M.H. Identification of amino acid residues in APOBEC3G required for regulation by human immunodeficiency virus type 1 Vif and virion encapsidation. J. Virol. 2007;81:3807–3815. doi: 10.1128/JVI.02795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Luo K., Xiao Z., Ehrlich E., Yu Y., Liu B., Zheng S., Yu X.F. Primate lentiviral virion infectivity factors are substrate receptors that assemble with cullin 5-E3 ligase through a HCCH motif to suppress APOBEC3G. Proc. Natl. Acad. Sci. USA. 2005;102:11444–11449. doi: 10.1073/pnas.0502440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Paul I., Cui J., Maynard E.L. Zinc binding to the HCCH motif of HIV-1 virion infectivity factor induces a conformational change that mediates protein-protein interactions. Proc. Natl. Acad. Sci. USA. 2006;103:18475–18480. doi: 10.1073/pnas.0604150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kueck T., Neil S.J. A cytoplasmic tail determinant in HIV-1 Vpu mediates targeting of tetherin for endosomal degradation and counteracts interferon-induced restriction. PLoS Pathog. 2012;8:e1002609. doi: 10.1371/journal.ppat.1002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Okada Y., Suzuki T., Sunden Y., Orba Y., Kose S., Imamoto N., Takahashi H., Tanaka S., Hall W.W., Nagashima K., et al. Dissociation of heterochromatin protein 1 from lamin B receptor induced by human polyomavirus agnoprotein: Role in nuclear egress of viral particles. EMBO Rep. 2005;6:452–457. doi: 10.1038/sj.embor.7400406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Moris A., Murray S., Cardinaud S. AID and APOBECs span the gap between innate and adaptive immunity. Front. Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Harris R.S., Dudley J.P. Apobecs and virus restriction. Virology. 2015;479–480:131–145. doi: 10.1016/j.virol.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pery E., Sheehy A., Nebane N.M., Brazier A.J., Misra V., Rajendran K.S., Buhrlage S.J., Mankowski M.K., Rasmussen L., White E.L., et al. Identification of a novel HIV-1 inhibitor targeting Vif-dependent degradation of human APOBEC3G protein. J. Biol. Chem. 2015;290:10504–10517. doi: 10.1074/jbc.M114.626903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Reading P.C., Bozza S., Gilbertson B., Tate M., Moretti S., Job E.R., Crouch E.C., Brooks A.G., Brown L.E., Bottazzi B., et al. Antiviral activity of the long chain pentraxin PTX3 against influenza viruses. J. Immunol. 2008;180:3391–3398. doi: 10.4049/jimmunol.180.5.3391. [DOI] [PubMed] [Google Scholar]
- 151.Job E.R., Bottazzi B., Short K.R., Deng Y.M., Mantovani A., Brooks A.G., Reading P.C. A single amino acid substitution in the hemagglutinin of H3N2 subtype influenza a viruses is associated with resistance to the long pentraxin PTX3 and enhanced virulence in mice. J. Immunol. 2014;192:271–281. doi: 10.4049/jimmunol.1301814. [DOI] [PubMed] [Google Scholar]
- 152.Lee H.J., Zheng J.J. PDZ domains and their binding partners: Structure, specificity, and modification. Cell Commun. Signal. 2010;8 doi: 10.1186/1478-811X-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Torres-Flores J.M., Arias C.F. Tight junctions go viral! Viruses. 2015;7:5145–5154. doi: 10.3390/v7092865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Erlendsson S., Madsen K.L. Membrane binding and modulation of the PDZ domain of PICK1. Membranes. 2015;5:597–615. doi: 10.3390/membranes5040597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Delury C.P., Marsh E.K., James C.D., Boon S.S., Banks L., Knight G.L., Roberts S. The role of protein kinase a regulation of the E6 PDZ-binding domain during the differentiation-dependent life cycle of human papillomavirus type 18. J. Virol. 2013;87:9463–9472. doi: 10.1128/JVI.01234-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mischo A., Ohlenschlager O., Hortschansky P., Ramachandran R., Gorlach M. Structural insights into a wildtype domain of the oncoprotein E6 and its interaction with a PDZ domain. PLoS ONE. 2013;8:e62584. doi: 10.1371/journal.pone.0062584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fan S., Macken C.A., Li C., Ozawa M., Goto H., Iswahyudi N.F., Nidom C.A., Chen H., Neumann G., Kawaoka Y. Synergistic effect of the PDZ and p85β-binding domains of the NS1 protein on virulence of an avian H5N1 influenza A virus. J. Virol. 2013;87:4861–4871. doi: 10.1128/JVI.02608-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Boxus M., Twizere J.C., Legros S., Dewulf J.F., Kettmann R., Willems L. The HTLV-1 Tax interactome. Retrovirology. 2008;5 doi: 10.1186/1742-4690-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Higuchi M., Fujii M. Distinct functions of HTLV-1 Tax1 from HTLV-2 Tax2 contribute key roles to viral pathogenesis. Retrovirology. 2009;6 doi: 10.1186/1742-4690-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cherian M.A., Baydoun H.H., Al-Saleem J., Shkriabai N., Kvaratskhelia M., Green P., Ratner L. Akt pathway activation by human T-cell leukemia virus type 1 Tax oncoprotein. J. Biol. Chem. 2015;290:26270–26281. doi: 10.1074/jbc.M115.684746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tanaka Y., Mizuguchi M., Takahashi Y., Fujii H., Tanaka R., Fukushima T., Tomoyose T., Ansari A.A., Nakamura M. Human T-cell leukemia virus type-I Tax induces the expression of CD83 on T cells. Retrovirology. 2015;12 doi: 10.1186/s12977-015-0185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gonzalez M.E. Vpu protein: The viroporin encoded by HIV-1. Viruses. 2015;7:4352–4368. doi: 10.3390/v7082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kueck T., Foster T.L., Weinelt J., Sumner J.C., Pickering S., Neil S.J. Serine phosphorylation of HIV-1 Vpu and its binding to tetherin regulates interaction with clathrin adaptors. PLoS Pathog. 2015;11:e1005141. doi: 10.1371/journal.ppat.1005141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mueller B.K., Subramaniam S., Senes A. A frequent, GxxxG-mediated, transmembrane association motif is optimized for the formation of interhelical Cα-H hydrogen bonds. Proc. Natl. Acad. Sci. USA. 2014;2014 doi: 10.1073/pnas.1319944111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Langosch D., Teese M.G. The role of GxxxG motifs in transmembrane domain interactions. Biochemistry. 2015;54:5125–5135. doi: 10.1021/acs.biochem.5b00495. [DOI] [PubMed] [Google Scholar]
- 166.Han Q., Aligo J., Manna D., Belton K., Chintapalli S.V., Hong Y., Patterson R.L., van Rossum D.B., Konan K.V. Conserved GXXXG- and S/T-like motifs in the transmembrane domains of NS4B protein are required for hepatitis C virus replication. J. Virol. 2011;85:6464–6479. doi: 10.1128/JVI.02298-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ma C., Soto C.S., Ohigashi Y., Taylor A., Bournas V., Glawe B., Udo M.K., Degrado W.F., Lamb R.A., Pinto L.H. Identification of the pore-lining residues of the BM2 ion channel protein of influenza B virus. J. Biol. Chem. 2008;283:15921–15931. doi: 10.1074/jbc.M710302200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pielak R.M., Chou J.J. Influenza M2 proton channels. Biochim. Biophys. Acta. 2011;1808:522–529. doi: 10.1016/j.bbamem.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Arguello-Astorga G., Lopez-Ochoa L., Kong L.J., Orozco B.M., Settlage S.B., Hanley-Bowdoin L. A novel motif in geminivirus replication proteins interacts with the plant retinoblastoma-related protein. J. Virol. 2004;78:4817–4826. doi: 10.1128/JVI.78.9.4817-4826.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.De Leeuw O.S., Koch G., Hartog L., Ravenshorst N., Peeters B.P. Virulence of newcastle disease virus is determined by the cleavage site of the fusion protein and by both the stem region and globular head of the haemagglutinin-neuraminidase protein. J. Gen. Virol. 2005;86:1759–1769. doi: 10.1099/vir.0.80822-0. [DOI] [PubMed] [Google Scholar]
- 171.Xiao S., Khattar S.K., Subbiah M., Collins P.L., Samal S.K. Mutation of the F-protein cleavage site of avian paramyxovirus type 7 results in furin cleavage, fusion promotion, and increased replication in vitro but not increased replication, tissue tropism, or virulence in chickens. J. Virol. 2012;86:3828–3838. doi: 10.1128/JVI.06765-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Heiden S., Grund C., Roder A., Granzow H., Kuhnel D., Mettenleiter T.C., Romer-Oberdorfer A. Different regions of the newcastle disease virus fusion protein modulate pathogenicity. PLoS ONE. 2014;9:e113344. doi: 10.1371/journal.pone.0113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Oh K.J., Kalinina A., Park N.H., Bagchi S. Deregulation of EIF4E: 4E-BP1 in differentiated human papillomavirus-containing cells leads to high levels of expression of the E7 oncoprotein. J. Virol. 2006;80:7079–7088. doi: 10.1128/JVI.02380-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Spangle J.M., Munger K. The human papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling and increases protein synthesis. J. Virol. 2010;84:9398–9407. doi: 10.1128/JVI.00974-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Spangle J.M., Ghosh-Choudhury N., Munger K. Activation of cap-dependent translation by mucosal human papillomavirus E6 proteins is dependent on the integrity of the LXXLL binding motif. J. Virol. 2012;86:7466–7472. doi: 10.1128/JVI.00487-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Clem R.J. Viral IAPs, then and now. Semin. Cell Dev. Biol. 2015;39:72–79. doi: 10.1016/j.semcdb.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 177.Darding M., Meier P. IAPs: Guardians of RIPK1. Cell Death Differ. 2012;19:58–66. doi: 10.1038/cdd.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fuentes-Gonzalez A.M., Contreras-Paredes A., Manzo-Merino J., Lizano M. The modulation of apoptosis by oncogenic viruses. Virol. J. 2013;10 doi: 10.1186/1743-422X-10-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yan N., Wu J.W., Chai J., Li W., Shi Y. Molecular mechanisms of DrICE inhibition by DIAP1 and removal of inhibition by Reaper, Hid and Grim. Nat. Struct. Mol. Biol. 2004;11:420–428. doi: 10.1038/nsmb764. [DOI] [PubMed] [Google Scholar]
- 180.Huntley M.A., Golding G.B. Simple sequences are rare in the protein data bank. Proteins. 2002;48:134–140. doi: 10.1002/prot.10150. [DOI] [PubMed] [Google Scholar]
- 181.Haerty W., Golding G.B. Low-complexity sequences and single amino acid repeats: Not just “junk” peptide sequences. Genome. 2010;53:753–762. doi: 10.1139/g10-063. [DOI] [PubMed] [Google Scholar]
- 182.Luo H., Nijveen H. Understanding and identifying amino acid repeats. Brief. Bioinform. 2014;15:582–591. doi: 10.1093/bib/bbt003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kumari B., Kumar R., Kumar M. Low complexity and disordered regions of proteins have different structural and amino acid preferences. Mol. Biosyst. 2015;11:585–594. doi: 10.1039/C4MB00425F. [DOI] [PubMed] [Google Scholar]
- 184.Berndt C., Lillig C.H., Holmgren A. Thioredoxins and glutaredoxins as facilitators of protein folding. Biochim. Biophys. Acta. 2008;1783:641–650. doi: 10.1016/j.bbamcr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 185.Hanschmann E.M., Godoy J.R., Berndt C., Hudemann C., Lillig C.H. Thioredoxins, glutaredoxins, and peroxiredoxins—Molecular mechanisms and health significance: From cofactors to antioxidants to redox signaling. Antioxid. Redox Signal. 2013;19:1539–1605. doi: 10.1089/ars.2012.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Galligan J.J., Petersen D.R. The human protein disulfide isomerase gene family. Hum. Genom. 2012;6 doi: 10.1186/1479-7364-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rose P.E., Schaffhausen B.S. Zinc-binding and protein-protein interactions mediated by the polyomavirus large T antigen zinc finger. J. Virol. 1995;69:2842–2849. doi: 10.1128/jvi.69.5.2842-2849.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tylor S., Andonov A., Cutts T., Cao J., Grudesky E., van Domselaar G., Li X., He R. The SR-rich motif in SARS-CoV nucleocapsid protein is important for virus replication. Can. J. Microbiol. 2009;55:254–260. doi: 10.1139/W08-139. [DOI] [PubMed] [Google Scholar]
- 189.Antonsson A., Payne E., Hengst K., McMillan N.A. The human papillomavirus type 16 E7 protein binds human interferon regulatory factor-9 via a novel PEST domain required for transformation. J. Interferon Cytokine Res. 2006;26:455–461. doi: 10.1089/jir.2006.26.455. [DOI] [PubMed] [Google Scholar]
- 190.Lepere-Douard C., Trotard M., le Seyec J., Gripon P. The first transmembrane domain of the hepatitis B virus large envelope protein is crucial for infectivity. J. Virol. 2009;83:11819–11829. doi: 10.1128/JVI.01026-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Borin B.N., Tang W., Nice T.J., McCune B.T., Virgin H.W., Krezel A.M. Murine norovirus protein NS1/2 aspartate to glutamate mutation, sufficient for persistence, reorients side chain of surface exposed tryptophan within a novel structured domain. Proteins. 2013;82:1200–1209. doi: 10.1002/prot.24484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Teissier E., Penin F., Pecheur E.I. Targeting cell entry of enveloped viruses as an antiviral strategy. Molecules. 2011;16:221–250. doi: 10.3390/molecules16010221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Smith I., Wang L.F. Bats and their virome: An important source of emerging viruses capable of infecting humans. Curr. Opin. Virol. 2013;3:84–91. doi: 10.1016/j.coviro.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.