Abstract
Background
Platelets are commonly transfused to critically ill patients. Reports suggest an association between platelet transfusion and infection. However, there is no large study to have determined whether platelet transfusion in critically ill patients is associated with hospital-acquired infection.
Methods
We conducted a multi-centre study using prospectively maintained databases of two large academic intensive care units (ICUs) in Australia. Characteristics of patients who received platelets in ICUs between 2008 and 2014 were compared to those of patients who did not receive platelets. Association between platelet administration and infection (bacteraemia and/or bacteriuria) was modelled using multiple logistic regression and Cox regression, with blood components as time-varying covariates. A propensity covariate adjustment was also performed to verify results.
Results
Of the 18,965 patients included, 2250 (11.9%) received platelets in ICU with a median number of 1 platelet unit (IQR 1–3) administered. Patients who received platelets were more severely ill at ICU admission (mean Acute Physiology and Chronic Health Evaluation III score 65 (SD 29) vs 52 (SD 25), p < 0.01) and had more comorbidities (31% vs 19%, p < 0.01) than patients without platelet transfusion. Invasive mechanical ventilation (87% vs 57%, p < 0.01) and renal replacement therapy (20% vs 4%, p < 0.01) were more frequently administered in patients receiving platelets than in patients without platelets. On univariate analysis, platelet transfusion was associated with hospital-acquired infection in the ICU (7.7% vs 1.4%, p < 0.01). After adjusting for confounders, including other blood components administered, patient severity, centre, year, and diagnosis category, platelet transfusions were independently associated with infection (adjusted OR 2.56 95% CI 1.98–3.31, p < 0.001). This association was also found in survival analysis with blood components as time-varying covariates (adjusted HR 1.85, 95% CI 1.41–2.41, p < 0.001) and when only bacteraemia was considered (adjusted OR 3.30, 95% CI 2.30–4.74, p <0.001). Platelet transfusions remained associated with infection after propensity covariate adjustment.
Conclusions
After adjustment for confounders, including patient severity and other blood components, platelet transfusion was independently associated with ICU-acquired infection. Further research aiming to better understand this association and to prevent this complication is warranted.
Keywords: Platelet transfusion, Hospital-acquired infection, Bacteraemia, Critically ill patients
Background
Platelet (PLT) transfusion is used therapeutically in patients who are bleeding [1], and is also recommended by guidelines [2] to reduce the risk of bleeding in patients with thrombocytopenia (prophylactic transfusion). However, the benefit of prophylactic PLT administration in non-bleeding critically ill patients with thrombocytopenia has been questioned [3, 4]. This is important, as there is some evidence suggesting that PLT administration is associated with adverse effects including infection and sepsis [5, 6]. The association between blood component administration and hospital-acquired infection has been reported mainly for red blood cells (RBC) [7–9]. Nonetheless, this association is probably not restricted to RBC, as fresh frozen plasma (FFP) and PLT transfusion can also cause post transfusion immunomodulation [10].
Platelets play a key role in the inflammatory and immune response, [11] and some authors have hypothesised that PLT transfusion may lead to immunomodulation [10, 12]. Nonetheless, there has been no large study of PLT transfusion in ICU patients to investigate whether PLT administration is associated with an increased risk of developing hospital-acquired infection. Patients admitted to an ICU are likely to be particularly prone to transfusion-mediated immunomodulation [13]. Therefore, we aimed to describe PLT transfusion in a large cohort of critically ill patients and to determine whether PLT transfusion is associated with hospital-acquired infection.
Methods
Patients and study design
This is a retrospective study conducted in the mixed medical-surgical ICUs of two teaching hospitals in Melbourne (Australia). All patients admitted to one of these ICUs between July 2008 and September 2013 were included in the study. The Alfred Hospital (affiliated to Monash University), with a 45-bed ICU capacity, is a tertiary hospital and state-wide referral centre for trauma and heart and lung transplantation; the Austin Hospital (affiliated to the University of Melbourne), with a 20-bed ICU capacity, is a tertiary hospital and referral centre for liver transplantation for the states of Victoria, Tasmania and South Australia. The Research Ethics Committees of both institutions approved the study (LNR13/Austin/233 and Alfred 58/11).
Clinical data
Clinical data were those prospectively extracted from local ICU databases used to collect and submit data to the Australian New Zealand Intensive Care Society (ANZICS) Adult Patient Database (APD). Data were collected on gender, date of birth, comorbidities, ICU and hospital admission and discharge dates, Acute Physiology And Chronic Health Evaluation (APACHE III) score at admission, ICU admission categories, requirement for and dates of initiation and cessation of mechanical ventilation (MV) or renal replacement therapy (RRT), and patient vital status at ICU and hospital discharge.
Transfusion data
Data on all blood components issued and transfused to patients were retrieved from the blood bank laboratory information systems of each hospital. For each PLT unit administered, we recorded the date of transfusion, the type of product (apheresis or pooled), the volume, and the ABO and Rhesus D blood group. Administration of RBC, FFP and cryoprecipitate (CRYO) were also collected. Universal pre-storage leukodepletion was introduced in Australia prior to the study period, and therefore all blood products were pre-storage leukodepleted. Systematic screening of PLT for bacterial contamination was implemented at the same time by the Australian Red Cross Blood Service. PLT transfusion practices in critically ill patients were according to clinician discretion.
The national guidelines in place at the time of the study were the Australian Society of Blood Transfusion (ASBT) guidelines, which recommended prophylactic PLT transfusion at a threshold of 10 to 20 × 109/L in bone marrow failure and a threshold of 50 × 109/L prior to surgery or invasive procedures, [14] and therapeutic PLT transfusion in bleeding patients in whom thrombocytopenia was considered a major contributor factor, when the platelet count was less than 50 × 109/L in the context of massive haemorrhage, or less than 100 × 109/L in the presence of diffuse microvascular bleeding [14]. An observational study conducted in Australia and New Zealand confirmed the compliance with these guidelines in ICU patients during the same study period with only 5% of PLT transfusions that were inappropriate [15]. The updated guidelines published towards the end of the study period specifically for critical care included similar proposed thresholds for prophylactic PLT transfusion in the absence of bleeding (<20 × 109/L) and prior to invasive procedures (<50 × 109/L) [16].
Microbiological data
Microbiological data were retrieved from the microbiology laboratory records (KESTRAL system, www.kestral.com.au, Melbourne, Victoria, Australia) at the Austin hospital, and from a prospectively maintained database by the infectious disease department at the Alfred hospital. Microbiological data included positive blood culture and positive urine culture (defined by the presence of no more than two bacterial or Candida pathogens, and at 105 colony forming units (cfu)/mL or higher [17]). Sample date and pathogens isolated were recorded. When a pathogen known to be a frequent contaminant of microbiological cultures (i.e. coagulase-negative staphylococcus) was isolated, only cases with two or more positive blood cultures with the same pathogen were considered as bloodstream infection.
Statistical analysis
Descriptive statistics are reported as mean (standard deviation) or median (interquartile range) according to data distribution. Hypothesis testing was performed using the chi-square test for categorical variables, Student t test for normally distributed data and Wilcoxon rank-sum test for non-normally distributed data. The relationship between PLT transfusion and ICU-acquired infection was determined by logistic regression with results reported using odds ratios (ORs) (95% confidence interval (CI)). Model discrimination and calibration were determined using the area under the receiver operating characteristic (ROC) curve and the Hosmer-Lemeshow statistic.
Infection was defined as either bacteraemia or bacteriuria occurring in the ICU after 48 hours of ICU stay. Multivariate analysis was performed adjusting for gender, APACHE III score, site, diagnosis category, year, whether the patient received invasive MV or RRT, and whether another type of transfusion (RBC, CRYO, or FFP) had been given prior to the first dose of PLT. Additional sensitivity analysis was performed, adjusting for each patient’s propensity to receive a PLT transfusion; the propensity variable was calculated by using the predicted values of a multiple logistic regression model of platelet transfusion from pre-admission variables including comorbidity variables and the same variables as the multivariate analysis. Cox proportional hazards regression analysis was then performed, with the outcome of time of first infection, and including transfusion variables as time-varying covariates, and gender, APACHE III score, site, diagnosis category, year, and requirement for RRT and MV. Finally, three additional models were then also performed, considering only bacteraemia or bacteriuria as the infectious outcome and considering the number of platelet units transfused as a continuous variable. Propensity assumptions were determined using Schoenfeld residuals. To increase the robustness of our findings, a two-sided p value of 0.01 was considered to indicate significance. Statistical analysis was performed using STATA version 12.1 (StataCorp, TX, USA).
Results
Patient characteristics
A total of 19,101 patients had at least one admission to of the either ICUs over the five and half year study period. Of these, 136 (0.7%) were excluded because of missing data, leaving 18,965 included in the present study. Of the 18,965 patients, 2250 (11.9%) received PLT in the ICU.
Patients’ characteristics and comparisons between patients who received PLT in the ICU and those who did not are displayed in Table 1. Patients who received PLT were younger (59 ± 17 vs 60 ± 17, p = 0.036) and more often male (67.2% vs 63.3%, p < 0.01) than those who did not receive PLT. They had greater illness severity at ICU admission, with an APACHE III score of 65 ± 29 vs 52 ± 25 (p < 0.01) and they more often had comorbidities (31% vs 19%, p < 0.01). Patients who received PLT in the ICU were more often admitted with cardiovascular disease, gastrointestinal (GI) disease, trauma, or haematological disease than those who did not receive PLT. The majority of patients receiving PLT required invasive MV (87% vs 57%, p < 0.01) and one fifth had RRT while in the ICU (20% vs 4%, p < 0.01).
Table 1.
Characteristics of patients with and without platelet (PLT) transfusion in the ICU
| Variables | All patients N = 18,965 | Patients with PLT transfusion N = 2250 | Patients without PLT transfusion N = 16,715 | P value |
|---|---|---|---|---|
| Age, years, mean (SD) | 60 (18) | 59 (17) | 60 (17) | 0.04 |
| Gender, male, n (%) | 12 086 (63.7%) | 1 511 (67.2%) | 10 575 (63.3%) | <0.01 |
| APACHE III score, mean (SD) | 54 (26) | 65 (29) | 52 (25) | <0.01 |
| Comorbidities | ||||
| Any known comorbidity | 3 838 (20%) | 702 (31%) | 3 136 (19%) | <0.01 |
| Cancera | 857 (4.5%) | 192 (8.5%) | 665 (4.0%) | <0.01 |
| Hepatic diseaseb | 674 (3.6%) | 216 (9.6%) | 458 (2.7%) | <0.01 |
| Immunocompromisedc | 1 180 (6.2%) | 311 (13.8%) | 869 (5.2%) | <0.01 |
| IDDM | 511 (2.7%) | 60 (2.7%) | 451 (2.7%) | 0.93 |
| Chronic respiratory disease | 840 (4.4%) | 92 (4.1%) | 748 (4.5%) | 0.40 |
| CVD | 754 (4%) | 137 (6.1%) | 617 (3.7%) | <0.01 |
| Chronic renal failure | 422 (2%) | 59 (2.6%) | 363 (2.2%) | 0.17 |
| Admission diagnosis | ||||
| Cardiovascular | 5 161 (27%) | 701 (31%) | 4 460 (27%) | <0.01 |
| Gastrointestinal | 3 595 (19%) | 581 (26%) | 3 014 (18%) | <0.01 |
| Haematological | 152 (0.8%) | 96 (4.3%) | 56 (0.3%) | <0.01 |
| Neurological | 1 586 (8.4%) | 108 (4.8%) | 1 478 (8.8%) | <0.01 |
| Renal/genitourinary | 1 017 (5.4%) | 108 (4.8%) | 909 (5.4%) | 0.2 |
| Respiratory | 1 467 (7.7%) | 127 (5.6%) | 1 340 (8%) | <0.01 |
| Sepsis | 503 (2.7%) | 73 (3.2%) | 430 (2.6%) | 0.06 |
| Otherd | 2 815 (14.8%) | 82 (3.6%) | 2 733 (16.4%) | <0.01 |
| Trauma | 2 669 (14.1%) | 374 (16.6%) | 2 295 (13.7%) | <0.01 |
| Requirement for MV | 11 531 (61%) | 1 952 (87%) | 9 579 (57%) | <0.01 |
| Requirement for RRT | 1 135 (6%) | 449 (20%) | 686 (4%) | <0.01 |
aCancer group includes myeloma, lymphoma, leukaemia and metastases. bHepatic disease group includes liver cirrhosis, chronic liver disease and hepatic failure. cImmunocompromised includes those listed as immunosuppressed or with immune disease. dOther includes undefined, metabolic, muscle and skin, gynaecologic diseases. PLT platelets, ICU intensive care unit, MV mechanical ventilation, RRT replacement renal therapy, APACHE Acute Physiology And Chronic Health Evaluation, IDDM insulin-dependent diabetes mellitus, CVD chronic vascular diseases, SD standard deviations. P values comparing PLT transfusion group to the no-PLT transfusion group
Transfusion characteristics
PLT units (n = 6012) were transfused over the study period, with a median of 1 PLT unit (IQR 1–3) per patient. Patients with haematology admission diagnosis received more PLTs than the other critically ill patients. The ABO group of the PLT unit transfused was known in 2685 units and was mostly groups O (56%) and A (40%). PLT transfusions were Rhesus-D-positive in 71% of patients. Patients transfused with PLT were also transfused with RBC, FFP and CRYO in 79%, 62% and 33% of cases, respectively. These figures were significantly higher (p < 0.01) than in patients without PLT transfusion (RBC 21%, FFP 6%, cryoprecipitate 0.8%). The first PLT transfusion occurred in the first 24 hours after ICU admission in 82.6% of patients.
PLT transfusion and infections
Overall, 411 patients (2.2%) experienced an ICU-acquired infection in ICU that was either bacteraemia or bacteriuria (Table 2). A much higher proportion of patients who had received platelets developed infection (7.7%) compared to those who had not (1.4%) (p < 0.01). PLT transfusion was associated with occurrence of both bacteraemia and bacteriuria (Table 2). Patterns of pathogens isolated in blood culture and urine culture are displayed in Tables 3 and 4.
Table 2.
Platelet exposure and outcomes
| Variables | All patients N = 18,965 | Patients with PLT transfusion N = 2250 | Patients without PLT transfusion N = 16,715 | P value* |
|---|---|---|---|---|
| Infections, n (%) Any (blood or urine culture) Blood culture Urine culture | 411 (2.2) 178 (0.9) 262 (1.4) | 173 (7.7) 99 (4.4) 89 (4) | 238 (1.4) 79 (0.5) 173 (1) | <0.01 < 0.01 < 0.01 |
| Time to infection, days median (IQR) Any (blood or urine) Blood culture Urine culture | 7.1 (4.2–12) 7.9 (4.5–12) 6.8 (4.1–11.7) | 8.9 (5.1–15.0) 8.4 (5–14) 9.1 (5.2–17) | 6.4 (3.9–10) 6.6 (4.3–12) 6.3 (3.8–10.1) | <0.01 < 0.01 0.25 |
PLT platelets, IQR interquartile range. * P values comparing PLT transfusion group to the no-PLT transfusion group
Table 3.
Microbiological features of positive urine cultures occurring in patients with and without PLT transfusion in the intensive care unit
| Pathogens | Patients with positive UC N = 292 | Patients with positive UC and with PLT transfusion N = 99 | Patients with positive UC without PLT transfusion N = 193 | P value |
|---|---|---|---|---|
| Enterobactericeae Enterococcus | 17 (5.8%) | 43 (43.4%) | 108 (56%) | 0.04 |
| Staphylococcus sp. | 38 (19.5%) | 2 (2%) | 15 (7.8%) | 0.04 |
| Candida sp. | 7 (2.4%) | 3 (3%) | 4 (2.1%) | 0.44 |
| Non-fermentive GNB | 90 (30.8%) | 44 (44.4%) | 46 (23.8%) | < 0.01 |
| Other | 30 (10.3%) | 9 (9.1%) | 21 (10.9%) | 0.63 |
| 2 (0.7%) | 1 (1%) | 1 (0.5%) | 0.56 | |
| UC with >1 microorganism | 25 (8.6%) | 5 (5.1%) | 20 (10.4%) | 0.12 |
PLT platelets, GNB Gram-negative Bacilli; UC urine culture. P values comparing PLT transfusion group to the no-PLT transfusion group
Table 4.
Microbiological features of positive blood cultures in patients with and without PLT transfusion
| Pathogens | Patients with positive blood culture N = 195 | Patients with positive blood culture who had PLT transfusion N = 108 | Patients with positive blood culture without PLT transfusion N = 87 | P value |
|---|---|---|---|---|
| Enterobactericeae | 54 (27.7%) | 28 (26%) | 26 (29%) | 0.54 |
| Enterococcus | 38 (19.5%) | 27 (25%) | 11 (13%) | 0.03 |
| Staphylococcus aureus | 20 (10.3%) | 9 (8.3%) | 11 (12.6%) | <0.01 |
| CNS | 39 (20%) | 21 (19.4%) | 18 (20.7%) | 0.83 |
| Candida | 36 (18.5%) | 26 (24.1%) | 10 (11.5%) | 0.02 |
| Non-fermentive GNB | 16 (8.2%) | 7 (6.5%) | 9 (10.3%) | 0.33 |
| Other | 2 (1%) | 1 (0.9%) | 1 (1.2%) | 0.88 |
| BC with >1 microorganism | 14 (7.2%) | 9 (8.3%) | 5 (5.8%) | 0.49 |
PLT Platelets, CNS coagulase negative Staphylococcus, GNB Gram-negative Bacilli, BC blood culture. P values comparing PLT transfusion group to the no-PLT transfusion group
After adjusting for confounders (gender, APACHE III score, site, diagnosis category, year, whether another type of transfusion had been given prior to the PLT transfusion, MV and/or RRT requirement), PLT transfusion was associated with an increased risk of infection (adjusted odds ratio (OR) 2.56, 95% confidence interval (CI) 1.98–3.31, p < 0.01). The other independent risk factors for infection were female gender, patient severity at admission, requirement for invasive MV, requirement for RRT, admission diagnosis, administration of RBC prior to PLT transfusion and year (Table 5). As there were no significant interactions between PLT transfusion and other variables in the model, there was no evidence to suggest that the relationship between PLT transfusion and infection differed according to any of the covariates considered, including diagnosis category. When adjusting for the propensity to receive platelet transfusion, PLT transfusion remained associated with an increased risk of infection (Appendix).
Table 5.
Independent risk factors for infection in a multivariate analysis
| Variablesa | Adjusted odds ratio* | 95% Confidence interval | P value |
|---|---|---|---|
| PLT | 2.56 | 1.98-3.31 | <0.01 |
| Gender, male | 0.40 | 0.33-0.50 | <0.01 |
| APACHE III score | 1.01 | 1.00-1.01 | <0.01 |
| RBC transfusionb | 1.50 | 1.19-1.90 | <0.01 |
| FFP transfusionb | 0.92 | 0.68-1.24 | 0.57 |
| Cryoprecipitateb | 1.14 | 0.75-1.73 | 0.53 |
| Invasive mechanical ventilation | 3.16 | 2.29-4.36 | <0.01 |
| RRT | 4.75 | 3.56-6.32 | <0.01 |
aOther confounders include centre, year and admission diagnosis. bOnly blood products transfused prior to platelets (PLT) transfusion are considered in the analysis. RBC red blood cells, FFP fresh frozen plasma, RRT renal replacement therapy, APACHE III Acute Physiology And Chronic Health Evaluation III. *Hosmer-Lemeshow p value >0.54, area under the receiver operating characteristic curve 0.83
When considering blood product transfusion (PLT, RBC, FFP or CRYO) as time-varying covariates, PLT administration remained associated with infection (adjusted HR 1.85, 95% CI 1.41–2.41, p < 0.01) (Table 6). When considering bacteraemia and bacteriuria separately, PLT transfusion was still associated with each infection outcome (adjusted OR for bacteraemia 3.30, 95% CI 2.30 − 4.74, p <0.01 and adjusted OR for bacteriuria 2.01, 95% CI 1.44–2.83, p <0.01). There was a dose effect in the association between PLT transfusion and infection (adjusted OR for one PLT unit 1.62, 95% CI 1.11–2.35, p = 0.01; adjusted OR for two PLT units 3.48, 95% CI 2.60–4.68, p < 0.01). Figure 1 shows the survival time analysis for infection in patients with and without PLT and Fig. 2 shows the survival time analysis for bacteraemia in patients with and without PLT.
Table 6.
Independent risk factors for infection in the Cox model
| Variablesa | Adjusted hazard ratio | 95% Confidence interval | P value |
|---|---|---|---|
| PLT transfusion | 1.85 | 1.41-2.41 | <0.01 |
| Gender | 0.43 | 0.35-0.52 | <0.01 |
| APACHE III score | 1.00 | 1.00-1.01 | 0.20 |
| RBCb | 1.02 | 0.79-1.33 | 0.86 |
| FFPb | 0.87 | 0.66-1.14 | 0.32 |
| Cryoprecipitateb | 1.01 | 0.74-1.40 | 0.94 |
| Invasive mechanical ventilation | 0.77 | 0.57-1.06 | 0.11 |
| RRT | 1.33 | 1.03-1.71 | 0.03 |
aOther confounders include centre, year and admission diagnosis. bOnly blood products transfused prior to infection are considered in the analysis. RBC red blood cells, FFP fresh frozen plasma, RRT renal replacement therapy, PLT platelets, APACHE Acute Physiology And Chronic Health Evaluation
Fig. 1.
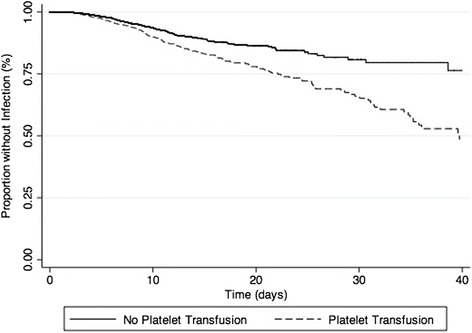
Kaplan-Meier estimates of infection in all patients over 40 days after ICU admission (p < 0.01 by the log-rank test)
Fig. 2.
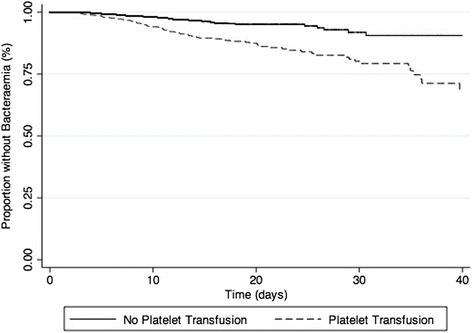
Kaplan-Meier estimates of bloodstream infections in all patients over 40 days after ICU admission (p < 0.01 by the log-rank test) )
Discussion
In this large observational study, PLT transfusion was associated with ICU-acquired infection. This association remained after adjustment for important confounders, including administration of other blood products prior to PLT administration and when considering all blood components as time varying covariates. PLT transfusion was still an independent risk factor for infection when considering separately bacteraemia and bacteriuria as an outcome.
Comparison with the available literature
It has been suggested that there is an association between infections and PLT administration in trauma and post-cardiac-surgery settings [5, 6, 18]. In a post hoc analysis of data from six randomised trials, Spiess et al. found that PLT transfusion was associated with an increased risk of critical adverse events including infection [5]. Nonetheless, the study conducted by Spiess et al. was performed before the widespread use of leukoreduction of blood components and its statistical analysis did not adjust for all other blood components administered.
More recently, Bilgin et al. reported on 1085 cardiac surgery patients in whom the risk of death due to post-surgical infection was independently associated with PLT transfusion [6]. However, the criteria to define infection were not objective, with infection diagnosis based on the “physician’s opinion”. In both studies, microbiology data were not provided. In contrast, there was no association between PLT transfusion and infection or morbidity in other large cohorts of patients who had undergone cardiac surgery [19–21]. Despite these conflicting results, the plausibility of this association is supported by reports showing the independent role of PLT in transfusion-related immunomodulation in animals [10].
Strengths and limitations
To our knowledge, this is the largest available study investigating the association between PLT transfusion and hospital-acquired infections in critically ill patients. Its heterogeneous population and its multi-centre design support the generalizability of our findings. The consistent results with different modelling approaches and the consideration of transfusion of blood components as time-varying to overcome survival bias is another strength that has not been used commonly in similar studies on this topic. The use of prospectively maintained databases of infectious outcomes and microbiology criteria to define infection also reduced bias, which may be introduced if relying on subjective definitions of infection by clinicians.
Nonetheless, our study has some limitations. Its retrospective design did not allow adjustment for relevant but missing data. Although we did perform propensity covariate adjustment to account for the probability of receiving a PLT transfusion, estimation of the propensity score could only be based on the available data. Infections that could not be reliably identified in our data could not be included in the analysis, such as ventilator-associated pneumonia or abdominal infections. Bacteraemia sources were not recorded, making it impossible to classify bacteraemia according to the infection source. There was no information on whether the patient was on antibiotics at the time the microbiology samples were obtained. Such information would have been useful to better understand the relatively low rate of infection in our population. Data were not available on blood components given prior to ICU admission, and infections occurring after ICU discharge. Finally, although we adjusted for important factors known to influence the risk of infection, we cannot exclude the possibility that the observed associations were confounded by unmeasured factors.
Implications of study findings
Our results suggest an association between PLT transfusion and adverse clinical outcomes, and emphasise the importance of avoiding unnecessary transfusions. Avoiding unnecessary transfusion should be a priority and may decrease hospital-acquired infection [3].
Conclusion
In conclusion, we found an independent association between PLT transfusion and the risk of hospital-acquired infections in critically ill patients. This association should be taken into account when transfusing PLT in critically ill patients. Further research to understand this association and to better determine the benefit of PLT in this population is warranted.
Acknowledgements
We thank Ian Meslen for providing transfusion data at the Alfred Hospital and Ben Howden for providing microbiological data at the Austin Hospital.
Funding
This work is part of a research programme ‘Centre of Research Excellence for Patient Blood Management in Critical Illness and Trauma’, which is funded by the Australian National Health and Medical Research Council.
Authors’ contributions
CA, ZM, and RB contributed to the conception of the study and study design. ZM, AF, and MB contributed to the statistical analysis. CA, AF, MB, RB, DP, AC, ZM, CH, AM, and MR contributed to results interpretation. CA, ZM, and AF drafted the manuscript. CA, AF, MB, RB, DP, AC, ZM, CH, AM, and MR revised the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The Research Ethics Committees of both institutions approved the study (LNR13/Austin/233 and Alfred 58/11).
Abbreviations
- APACHE
Acute Physiology And Chronic Health Evaluation
- CI
Confidence interval
- Cryo
Cryoprecipitate
- FFP
Fresh frozen plasma
- HR
Hazard ratio
- ICU
Intensive care unit
- IQR
Interquartile range
- MV
Mechanical ventilation
- OR
Odds ratio
- PLT
Platelets
- RBC
Red blood cells
- RRT
Renal replacement therapy
Appendix
Propensity covariate adjustment
Table 7.
Independent risk factors for infection in a multivariate analysis including propensity covariate adjustment
| Variablesa | Adjusted odds ratio | 95% Confidence interval | P value |
|---|---|---|---|
| PLT | 2.45 | 1.89-3.18 | <0.01 |
| Gender | 0.40 | 0.32-0.49 | <0.01 |
| APACHE III score | 1.00 | 1.01-1.01 | 0.01 |
| RBC transfusionb | 1.39 | 1.08-1.78 | 0.01 |
| FFP transfusionb | 0.69 | 0.46-1.02 | 0.06 |
| Cryoprecipitateb | 0.70 | 0.39-1.28 | 0.25 |
| Invasive mechanical ventilation | 2.89 | 2.07-5.46 | <0.01 |
| RRT | 3.90 | 2.79-5.46 | <0.01 |
| Propensity covariatec | 3.46 | 1.15-10.38 | 0.03 |
aOther confounders include centre, year and admission diagnosis. bOnly blood products transfused prior to platelets (PLT) transfusion are considered in the analysis. cPropensity covariate predicted by gender, site, diagnosis, immune disease, immunosuppressed, chronic liver disease, year, red blood cells (RBC) transfusion, fresh frozen plasma (FFP) transfusion, cryoprecipitate transfusion, invasive mechanical ventilation, renal replacement therapy (RRT), age, leukaemia/myeloma, metastases, hepatic failure, insulin-dependent diabetes. APACHE Acute Physiology And Chronic Health Evaluation
Contributor Information
Cécile Aubron, Phone: +33 298347181, Email: Cecile.aubron@chu-brest.fr, Email: cecile.aubron@monash.edu.
Andrew W. Flint, Email: andrew.flint@monash.edu
Michael Bailey, Email: michael.bailey@monash.edu.
David Pilcher, Email: D.Pilcher@alfred.org.au.
Allen C. Cheng, Email: allen.cheng@monash.edu
Colin Hegarty, Email: colhegarty@hotmail.com.
Antony Martinelli, Email: tony.martinelli@austin.org.au.
Michael C. Reade, Email: m.reade@uq.edu.au
Rinaldo Bellomo, Email: Rinaldo.BELLOMO@austin.org.au.
Zoe McQuilten, Email: zoe.mcquilten@monash.edu.
References
- 1.Arnold DM, Crowther MA, Cook RJ, Sigouin C, Heddle NM, Molnar L, Cook DJ. Utilization of platelet transfusions in the intensive care unit: indications, transfusion triggers, and platelet count responses. Transfusion. 2006;46(8):1286–91. doi: 10.1111/j.1537-2995.2006.00892.x. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, Cipolle MD, Cohn CS, Fung MK, Grossman BJ, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162(3):205–13. doi: 10.7326/M14-1589. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman L, Bercovitz RS, Sholapur NS, Heddle NM, Stanworth SJ, Arnold DM. Platelet transfusions for critically ill patients with thrombocytopenia. Blood. 2014;123(8):1146–51. doi: 10.1182/blood-2013-02-435693. [DOI] [PubMed] [Google Scholar]
- 4.Habr B, Charpentier J, Champigneulle B, Dechartres A, Daviaud F, Geri G, Cariou A, Chiche JD, Mira JP, Pene F. Platelet transfusions in cancer patients with hypoproliferative thrombocytopenia in the intensive care unit. Ann Intensive Care. 2015;5(1):46. doi: 10.1186/s13613-015-0088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiess BD, Royston D, Levy JH, Fitch J, Dietrich W, Body S, Murkin J, Nadel A. Platelet transfusions during coronary artery bypass graft surgery are associated with serious adverse outcomes. Transfusion. 2004;44(8):1143–8. doi: 10.1111/j.1537-2995.2004.03322.x. [DOI] [PubMed] [Google Scholar]
- 6.Bilgin YM, van de Watering LM, Versteegh MI, van Oers MH, Vamvakas EC, Brand A. Postoperative complications associated with transfusion of platelets and plasma in cardiac surgery. Transfusion. 2011;51(12):2603–10. doi: 10.1111/j.1537-2995.2011.03200.x. [DOI] [PubMed] [Google Scholar]
- 7.Rohde JM, Dimcheff DE, Blumberg N, Saint S, Langa KM, Kuhn L, Hickner A, Rogers MA. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA. 2014;311(13):1317–26. doi: 10.1001/jama.2014.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, Fukushima J, Kalil Filho R, Sierra DB, Lopes NH, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304(14):1559–67. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 9.Villanueva C, Colomo A, Bosch A, Concepcion M, Hernandez-Gea V, Aracil C, Graupera I, Poca M, Alvarez-Urturi C, Gordillo J, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 10.Aslam R, Speck ER, Kim M, Freedman J, Semple JW. Transfusion-related immunomodulation by platelets is dependent on their expression of MHC class I molecules and is independent of white cells. Transfusion. 2008;48(9):1778–86. doi: 10.1111/j.1537-2995.2008.01791.x. [DOI] [PubMed] [Google Scholar]
- 11.Jenne CN, Urrutia R, Kubes P. Platelets: bridging hemostasis, inflammation, and immunity. Int J Lab Hematol. 2013;35(3):254–61. doi: 10.1111/ijlh.12084. [DOI] [PubMed] [Google Scholar]
- 12.Stolla M, Refaai MA, Heal JM, Spinelli SL, Garraud O, Phipps RP, Blumberg N. Platelet transfusion - the new immunology of an old therapy. Front Immunol. 2015;6:28. doi: 10.3389/fimmu.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vlaar AP, Wolthuis EK, Hofstra JJ, Roelofs JJ, Boon L, Schultz MJ, Lutter R, Juffermans NP. Mechanical ventilation aggravates transfusion-related acute lung injury induced by MHC-I class antibodies. Intensive Care Med. 2010;36(5):879–87. doi: 10.1007/s00134-010-1802-z. [DOI] [PubMed] [Google Scholar]
- 14.National Health and Medical Research Council (NMHRC) Australian Society of Blood Transfusion (ASBT) Clinical practice guidelines on the use of blood components. Canberra: NHMRC; 2001. [Google Scholar]
- 15.Blood Observational Study-ANZICS-CTG Transfusion practice and guidelines in Australian and New Zealand intensive care units. Intensive Care Med. 2010;36:1138–46. doi: 10.1007/s00134-010-1867-8. [DOI] [PubMed] [Google Scholar]
- 16.National Blood Authorithies . Patient Blood Management Guidelines: Module 4 Critical Care. Canberra: NBA; 2013. p. 2013. [Google Scholar]
- 17.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Bochicchio GV, Napolitano L, Joshi M, Bochicchio K, Shih D, Meyer W, Scalea TM. Blood product transfusion and ventilator-associated pneumonia in trauma patients. Surg Infect (Larchmt) 2008;9(4):415–22. doi: 10.1089/sur.2006.069. [DOI] [PubMed] [Google Scholar]
- 19.Vamvakas EC. Platelet transfusion and postoperative infection in cardiac surgery. Transfusion. 2007;47(2):352–4. doi: 10.1111/j.1537-2995.2007.01115.x. [DOI] [PubMed] [Google Scholar]
- 20.McGrath T, Koch CG, Xu M, Li L, Mihaljevic T, Figueroa P, Blackstone EH. Platelet transfusion in cardiac surgery does not confer increased risk for adverse morbid outcomes. Ann Thorac Surg. 2008;86(2):543–53. doi: 10.1016/j.athoracsur.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 21.Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Meineri M, Wasowicz M, McCluskey SA, Beattie WS. Platelet transfusions are not associated with increased morbidity or mortality in cardiac surgery. Can J Anaesth. 2006;53(3):279–87. doi: 10.1007/BF03022216. [DOI] [PubMed] [Google Scholar]


