Summary
Progressive neuronal cell loss in a small subset of brainstem and mesencephalic nuclei and widespread aggregation of the α-synuclein protein in the form of Lewy bodies and Lewy neurites are neuropathological hallmarks of Parkinson’s disease. Most cases occur sporadically, but mutations in several genes, including α-synuclein, are associated with disease development. The mechanisms driving neurodegeneration remain unknown, hence limiting therapeutic strategies aimed at blocking neuronal death. This review describes current evidence for a predominant role of α-synuclein in the pathogenesis of PD, as well as some of the most promising α-synuclein-based strategies currently in development for this incurable neurodegenerative disorder.
Keywords: Parkinson’s disease, α-synuclein, therapeutic strategies, pipeline, clinical trials
Introduction
Over the past two decades, a myriad of studies have suggested a significant pathogenic role of α-synuclein (α-syn) in both familial and sporadic forms of Parkinson’s disease (PD). PD belongs to the synucleinopathies, which includes dementia with Lewy bodies (DLB) and multiple system atrophy (MSA). PD is the second most common neurodegenerative disorder affecting 1 to 3% of the population over the age of 50 1 and over 5 million people worldwide 2. Classical motor signs of PD include bradykinesia, rigidity, resting tremor and gait disturbance with postural instability 3, primarily attributed to the dramatic loss of dopamine (DA)-containing neurons in the substantia nigra pars compacta (SNpc). In addition to DA neuronal cell loss, another pathological hallmark of PD is the presence of intraneuronal proteinaceous cytoplasmic inclusions, named Lewy bodies (LB), and dystrophic Lewy neurites (LN) that also contain α-syn deposits. The mechanisms leading to the formation and the pathogenic significance of these inclusions remain unknown.
To date, there is no existing neuroprotective or neurorestorative therapy for treating this chronic disorder. However, two key discoveries that occurred 17 years ago have dramatically impacted PD research: i) the identification of mutations in the gene encoding for α-syn (SNCA) in families with PD 4, 5 and ii) the demonstration that α-syn is a major component of Lewy pathology 4, 5. Despite our increasing understanding of PD pathogenesis, the exact mechanisms of the progressive DA cell loss in the SNpc remain to be unraveled. Here, we review recent advances in understanding the pivotal role of α-syn in PD and stress the need to focus therapeutic development on this target.
Implication of α-syn in the disease process
α-Synuclein and Parkinson’s disease
In 1912, F.J.H. Lewy first described the intraneuronal proteinaceous cytoplasmic inclusions which became the histopathological hallmark of PD 6. Several years after this discovery, K.N. Tretiakoff named them the “Lewy Body”. In the early 90’s, α-syn was identified as the precursor of the non-amyloid component (NAC) of Alzheimer’s disease (AD) amyloid plaques 7-9. The first link between α-syn and PD appeared in 1997 with the identification of point mutations in the SNCA gene in familial forms of PD (PARK1 locus) 4. Polymeropoulos and colleagues identified the SNCA gene coding for α-syn on chromosome 4q21-q23 and described A53T as the first point mutation causing autosomal-dominant PD 4. Six missense mutations in SNCA are now associated with autosomal dominant PD: p.A53T, p.A30P, p.E46K, p.H50Q, p.G51D, p.A53E 4, 10-15 (Figure 1). These mutations are extremely rare with only a few families identified for each mutation. While the p.A30P mutation induces a clinical picture close to sporadic disease, p.A53T, p.E46K, p.H50Q and the newly identified p.G51D and p.A53E mutations are characterized by an earlier onset of parkinsonism with rapid disease progression and additional clinical features such as hallucinations, dementia, pyramidal tract impairment and autonomic failure 4, 10-12, 15. Neuropathological reports on autopsies of PD patients with p.A53T, p.A30P, p.E46K and p.G51D mutations described dopaminergic cell loss with extensive synucleinopathy in several brain regions 10, 12, 16, 17. The subsequent identification of families with duplication or triplication of the SNCA gene (PARK4 locus) strengthened the link between α-syn and PD, and indicated that increased levels of even the wild-type protein alone can cause the disease 18, 19. The clinical phenotype of patients with SNCA triplication (i.e. early onset parkinsonism with dementia) is more severe than in those with SNCA duplication (i.e. close to idiopathic PD) suggesting a dose-dependent relationship between disease severity and SNCA gene dosage. The common genetic variability at the SNCA locus is a robust risk factor for disease.
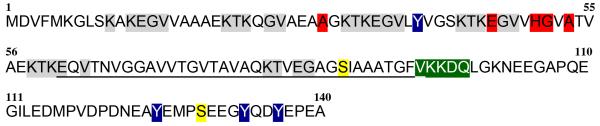
Figure 1. Primary structure of human α-synuclein (UniProtKB/Swiss-Prot: P37840).
Clinical Mutations (A53T, A30P, E46K, H50Q, G51D, A53E) are indicated in red. Amphipathic N-terminal region contains six imperfect lysine-rich highly conserved motif repeats (KTKEGV), which involve in binding of lipids, marked in grey. Central hydrophobic region contains non-amyloid beta component (NAC) sequence from residue 61 to 95 is underlined. Two major phosphorylation sites (Ser87 and Ser129) are colored in yellow. CMA recognition sites are marked in green. Nitration sites (Y39, Y125, Y133, Y136) are colored in blue.
α-Synuclein structure and function
Although the physiological function of α-syn remains to be fully elucidated, α-syn is implicated in modulating synaptic activity through regulation of synaptic-vesicle release (recently reviewed in 20). α-Syn, a 14kDa protein (other isoforms however exist), is a member of a small family of three proteins: α-, β- (β-syn) and γ-synuclein (γ-syn) 2. Three different domains can be defined in the 14kDa isoform of α-syn: i) an N-terminal domain (residues 1-60), ii) a central NAC domain (residues 61-95), and iii) a C-terminal domain (residues 96-140) (Figure 1). The N-terminal domain is characterized by repetitions of the four lysine-rich highly conserved motif “KTK(E/Q)GV”, similar to lipid-binding motifs in amphipathic helical domains of lipoproteins 21. All known clinical mutations are present in this region, emphasizing the importance of this domain in the aggregation of α-syn. The NAC domain has a high content in hydrophobic amino acids, responsible for the aggregation-prone properties, while the C-terminal end is characterized by a high content in proline, aspartate and glutamate residues. α- and β-syn have a high sequence identity (around 90%) in the amino-terminal domain, while the NAC region of α-syn specifically contains a 12 amino acid motif (residues 71-82: “VTGVTAVAQKTV”) that is a key element in the α-syn aggregation process, in particular its fibrillation (accumulation of β-sheet-rich aggregates) 22.
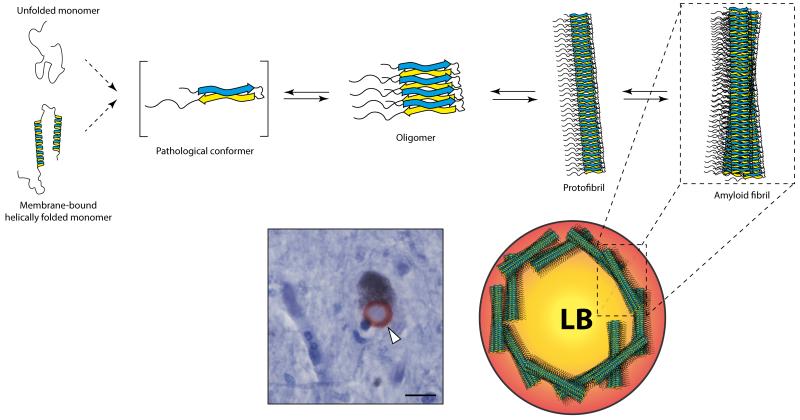
The native state of α-syn is extensively debated. While some reported that α-syn purified from human cells is a helically folded tetramer, others found that α-syn predominantly exist as an unfolded monomer 8, 23-26. Taken together, these studies suggest that α-syn exists under various conformational shapes and oligomeric states in a dynamic equilibrium, modulated by factors either accelerating or inhibiting fibrillation (Figure 2). Disease-related mutations impact the aggregation dynamics 27-29. The identification and characterization of the toxic α-syn species remain incomplete. Two hypotheses have been proposed: toxic species could be (i) amyloid-like insoluble fibrils, notably found in LB, or (ii) soluble, prefibrillar intermediates, such as oligomers or protofibrils (Figure 2). Several groups have sifted through the different states of α-syn aggregation and thoroughly examined the functional consequences of aggregate-associated toxicity. Winner and colleagues generated mutants unable to form fibrils while keeping an oligomeric state, and showed enhanced toxicity in vivo 30. Increasing evidence from both in vitro and in vivo studies has corroborated that oligomeric species are the most relevant 26, 29-32 and suggests that LB may be protective and represent a form of aggresome 33. While the different oligomeric types exist in a dynamic equilibrium and slowly convert into fibrils, Lashuel and coworkers recently proposed that annular oligomers are not on the pathway leading to amyloid formation and are therefore potentially toxic 2. This result, if confirmed, suggests that stabilization of the amyloid pathway might be of therapeutic interest.
Figure 2. Schematic summary of α-synuclein aggregation pathway.
The panel shows that α-syn exists under various conformational shapes. α-Syn exists as at least two structural isoforms: a natively unfolded monomer and a helix-rich membrane-bound form. Both isoforms may undergo dramatic structural changes resulting in the formation of β-sheet rich assemblies. From in vitro studies, it is clear that α-syn behaves in a dynamic equilibrium where monomer can aggregate first into several types of small oligomeric species that can be stabilized by β-sheet interactions and then into higher molecular weight insoluble protofibrils and may polymerize into amyloidogenic fibrils resembly those found in Lewy Body (LB). However, the mechanism governing the fundamental conformational change of normal monomeric α-syn to a pathological, disease-associated form, remains unknown. Photomicrograph illustrates one synuclein stained-mesencephalic LB (in red) in a neuromelanin-positive neuron from sporadic PD patient indicated by the white arrow. Scale bar: 5μm.
α-syn oligomeric species bind to lipids and increase mitochondrial, lysosomal and vesicular membrane permeability, a common feature of aggregation-prone proteins 32, 34-37. Increasing membrane permeability leads to calcium influx, ion homeostasis disruption and cell death through caspase-3 activation 32. Oligomers, but not monomers, can inhibit synaptic vesicle docking 38.
Several post-translational modifications (PTMs) of α-syn were characterized and their presence noted in LB pathology (for in-depth review see 21). First, phosphorylation is the best-studied PTMs. Several studies characterized two sites of phosphorylation in α-syn, Ser87 and especially Ser129, which is phosphorylated in brains from PD patients 39, 40 as well as in several in vivo and in vitro models of PD 41. It remains unclear whether phosphorylation of α-syn impacts the fibrillation process 42. Among the different PTMs identified, Ser129 phosphorylated (pS129) α-syn is thought to be the dominant form of α-syn in LB 41. Consistent with these findings, a recent unbiased top down proteomics study quantified various α-syn forms in the frontal cortex from PD cases and controls and demonstrated the presence of pS129 α-syn only in SDS-insoluble (LB-enriched) fraction of brain tissue 43. However, the total amount of pS129 α-syn was quite variable between cases and represented a minor fraction of unphosphorylated α-syn, raising the question around the validity of pS129 α-syn as a therapeutic target. Second, nitration of α-syn at residues Y39, Y125 and Y133 has been characterized in brains from patients with synucleinopathies 44. Third, oxidation of α-syn could occur by way of oxidized derivatives of DA leading to a decrease in fibril formation and a subsequent increase in protofibril accumulation 45. Both nitration and oxidation decrease the propensity of α-syn to form fibrils and stabilize oligomers, and might thus enhance toxicity 46, 47. Truncated α-syn species have also been found in LB 41, 43. Truncation typically occurs in the disordered C-terminal third of the protein, most frequently at or near position 121. Truncation is associated with an increased propensity of α-syn to form fibrils in vitro and with increased toxicity in overexpressing flies and rats 48, 49. However, no compelling data correlate truncated α-syn levels and clinicopathology 43. Additionally, we still lack direct in vivo evidence that inhibition of α-syn-cleaving proteases decreases cell death. Indeed, the physiological or pathological significance of α-syn cleavage remains equivocal. While most studies focus on the N-terminal peptide (the longest one) produced by cleavage, future studies should also consider the C-terminal peptide.
α-Synuclein degradation
Increased levels of aggregated α-syn in PD suggest that defective protein handling and clearance contribute to its pathogenesis. It is now established that α-syn is degraded both by the ubiquitin-proteasome system (UPS) and by the autophagy/lysosomal pathway (ALP) 50-52. α-syn contains a chaperone-mediated autophagy (CMA) recognition motif 95VKKDQ99 (KFERQ like) allowing interaction with cytosolic-hsc70 and translocation into the lysosome through the lysosome-associated membrane receptor protein, LAMP2a 52. Interestingly, mutant (p.A30P and p.A53T) and DA-modified α-syn, unlike wild-type α-syn, fail to be released within the lysosomal lumen after binding to LAMP2a, hence clogging this autophagy translocation machinery and leading to the accumulation of CMA-clients, at least in vitro 52, 53. Additionally, α-syn by itself may compromise macroautophagy 54, 55. In vivo evidence suggests that normal soluble α-syn is primarily degraded by the UPS while more complex conformations, including aggregates are disposed of by the ALP 56. Consistent with these observations, pathogenic depletion in proteasome components and lysosomes (i.e. loss of catalytic activity and decrease in lysosome number) has been observed in sporadic PD brains and in both toxic and genetic rodent models supporting the idea that defects in protein quality-control mechanism contribute to PD pathogenesis 57, 58. A vicious cycle thus occurs in which protein accumulation impairs clearance of the protein thus promoting further accumulation ultimately leading to neurodegeneration.
The properties of mutant and post-translationally modified α-syn species suggest that α-syn-mediated toxicity could occur through several distinct pathways (Figure 3). Apart from its localization at presynaptic terminals, α-syn accumulation has also been associated with endoplasmic reticulum (ER) stress 59 and several organelle defects, e.g. mitochondrion or lysosome. Localization at the mitochondria-associated ER membrane has been recently reported 60. Both WT and mutant forms of α-syn may impact mitochondrial morphology 60. The aforementioned cellular defects could also be related to ageing, that remains the most prominent risk factor for PD 61. Ageing itself leads to mitochondrial and lysosomal impairments, altered calcium metabolism and production of reactive oxygen species 61. Combination of ageing and PD alterations could lead to a cellular stress condition that interferes with intracellular clearance pathways, favor α-syn aggregation and contribute to the extracellular release of α-syn.
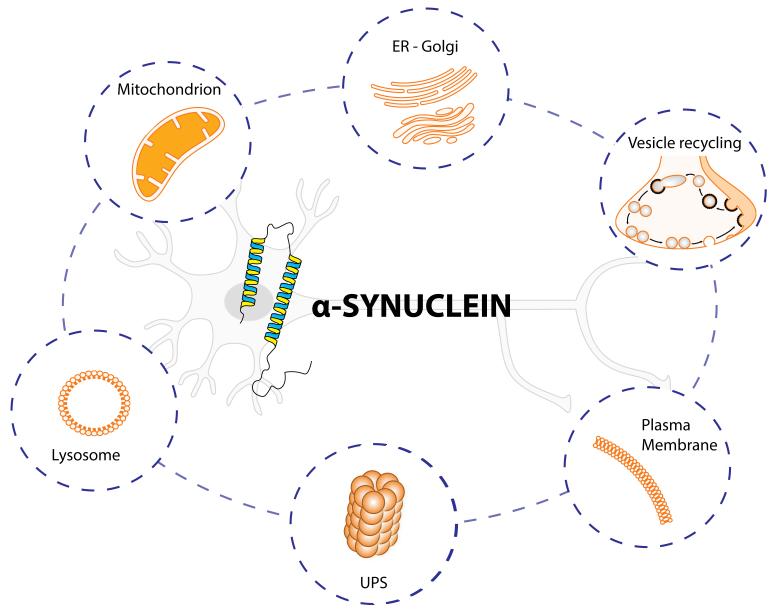
Figure 3. Schematic summary of established interactions between α-synuclein and cellular components.
The figure highlights six different intracellular pathways affected by α-synuclein (α-syn). The protein α-syn is enriched at the pre-synaptic terminals of almost all types of neurons in the brain, where it participates in the vesicle recycling, thereby modulating synaptic function. α-syn can be degraded by the ubiquitin-proteasome system (UPS) and inside the lysosomes. α-syn interacts strongly with membranes, such as plasma membrane and mitochondrion. If misfolded, α-syn forms distinct structures that are prone to aggregation, first into oligomers, then into larger structures. It is now believed that α-syn oligomers are the toxic form that may impair basic neuronal processes, such as ER-Golgi trafficking, lysosome and UPS functions, reduced mitochondrial activity and alter the plasma membrane through the pore/perforations that can dysregulate calcium and cation homeostasis.
α-Synuclein spreading
In 2008, three independent studies reported that embryonic mesencephalic neurons grafted into the striatum of three PD patients, out of 8 investigated, develop LB-like structures (α-syn, ubiquitin and Thioflavin-S positive) in grafted neurons, 11, 14 and 16 years after brain surgery 62-64. This observation suggested a “host-to-the-graft” transmission of LB pathology in the human brain and led to the speculation that cell-to-cell transmission of abnormal α-syn species contribute to PD pathogenesis. Relevant to this concept is the fact that PD patients exhibit α-syn-positive deposits in different brain regions, which, in some instances, are interconnected 65. A variety of evidence now suggests that α-syn pathology initiates in the periphery (possibly gastro-intestinal system) with subsequent spread to the central nervous system. However, whether such an anatomical pattern reflects some sort of caudo-rostral transmission of abnormal α-syn species or merely a caudo-rostral gradient of neuronal sensitivity thresholds for developing α-syn-positive deposits remains to be established.
The exact mechanism underlying the initiation of α-syn misfolding and aggregation in a recipient cell remains unknown. In vivo studies have added a further piece to the puzzle with the observation that intracerebral inoculation of α-syn-derived from purified LB taken from the SNpc of PD patients 66 or in vitro recombinant pffs of α-syn 67 can promote LB-like pathology in host neurons of recipient animals. Other reviews on α-syn spreading are available that cover more examples that we can here 68, but we will pinpoint the unsolved questions that should be answered.
Evidence piles up to strengthen the concept that α-syn may self-propagate, thereby contributing to the progression and extension of PD. In this context, the existence of toxic vs. non-toxic α-syn strains could underlie the differences in disease propagation between individuals, cell types or synucleinopathies 69. However, it has not been yet demonstrated whether the pathological conversion of endogenous α-syn triggered by PD-derived material or recombinant α-syn pffs may actually occur directly through a seeding prion process or rather indirectly as a general response to cellular stress. In addition, besides any potential pathogenic effect of intraneuronal α-syn, extracellular pathological α-syn is able to activate a deleterious microglial response that may also contribute to overall cell death and extension of the PD pathological process 70. It also remains to determine what initiates the misfolding of α-syn and a prion-like cascade. Potential causes include genetic mutations and duplication/triplication (in rare instances), impaired clearance, or exposure to toxins or infectious agents. The latter has attracted much interest, as olfactory filaments are the only nerves directly exposed to the exterior environment. Terminals of the dorsal motor nucleus of the vagus nerve reside in the gastric mucosa just microns from the lumen. As these structures appear to be the first areas of the brain involved in the PD process in at least some patients, it is easy to imagine that they could readily be exposed to toxic or infectious agents. Finally, it is possible that misfolding of α-syn occurs randomly, and the initiation of a prion cascade is a function of multiple factors that might contribute differentially in different individuals. Among several mechanisms, α-syn oligomers are thought to be the toxic species and the cause of the neurodegenerative process. Further, these oligomers would spread throughout the brain and induce α-syn pathology in interconnected structures. The toxicity of these pathogenic forms of α-syn, which remains to be unequivocally demonstrated, could be exerted through different intertwined mechanisms involving both cell autonomous (e.g. ion homeostasis perturbation, disruption of the mitochondrial network and/or impaired proteostasis) (Figure 3) and non-cell autonomous (e.g. neuro-inflammation) pathways. In line with the latter, aggregated and oxidatively modified α-syn holds interesting immunological features possibly involved in lethal neuro-immune interactions. In innate immunity, the Toll-like Receptor 2 has been identified as a major microglial receptor for neuron-released oligomeric α-syn and the ensuing inflammatory reaction 70. Among the mechanisms involved in the neurotoxic microglial-associated innate immune response, the oxidative burst generated by these brain macrophages is central and most likely implicated in the post-translational modification of α-syn through nitration of Y125 and Y133 residues 71. Interestingly, nitrated α-syn, unlike the native protein, can escape immune tolerance and generate a deleterious T helper cell response directed toward dopaminergic neurons as shown by immunization and passive transfer experiments in mice 72. Although infiltrated T cell have been identified in the brain of PD patients, it remains to be proven that this adaptive immune response is related to pathological α-syn antigens 73. Overall, it is tempting to speculate that any approaches targeting α-syn aggregation or propagation would indirectly impact on these immune responses but other, more direct, immunotherapeutics may also be considered.
Where do we come from and where are we going?
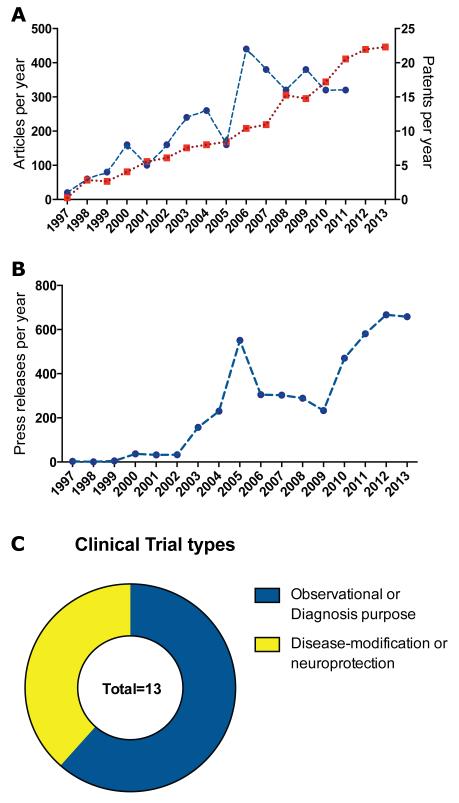
α-Syn-related publications have consistently grown since its relationship to PD was discovered in 1997 to reach 446 articles published in 2013 (Figure 4A). Interestingly, 50% of the articles were published in the last five years (Figure 4A). The increase in scientific publications is associated with an increasing α-syn-related patent deposit rate (Figure 4A). Since 1994, a total of 176 patent families have been filed, with an average of 20 patents per year in the last 3 years (Figure 4A). The majority of early patents were for diagnosis purposes and/or drug screening against α-syn. More recently, α-syn-related patents refer to anti-aggregation compounds, gene-silencing approaches and clearance strategies (Table 1), holding still a basic science status (Table 1). Conversely, a few have entered the pipeline but have not yet moved to clinical trials, suggesting that α-syn will be one of the main foci for biomarkers identification and disease-modifying strategies in the near future (Table 2). So far, 13 active α-syn-focused clinical trials are ongoing funded mostly by academic or non-industrial partners (Figure 4C and Table 3). Beside the increasing number of scientific publications and patents dealing with α-syn, the increasing number of economic press releases in large media underlines the rising interest for therapeutic strategies targeting α-syn (Figure 4B).
Figure 4. Analysis of α-synuclein-related publications, patents and press releases per year.
(A) The emergence of the α-syn research field as measured by the number of scientific articles per year (in red) is accompanied by patents deposition (in blue). No gap between the two curves is observed. The patent (blue) line stops in 2011 since we used the priority date, which is closely equivalent to the deposition date (the patent will only become public 18 months after deposit). Literature search was conducted in PubMed, as well in Scopus® (Elsevier) and Web of Science® (Thomson Reuters) databases with MeSH terms and/or keywords in title, and abstract fields. Between 2127 and 5026 documents were retrieved on the subject depending on the database and the type of documents (article, review, notes, proceedings…) from 1997 to 2013. The patent analysis was run in the world wide collection of INPADOC family patents using Orbit® (Questel) patent research platform through a boolean search combining keywords in different topic field (title, abstract & claims) and International Patent sub-class Code (IPC) A61P-025/16 for antiparkinsonian drugs. All subsequent analyses were performed on patent family, i.e. a set of patent applications with the same priority date in different countries related to the same invention. Since 1997, and up to December 31th, 2013, we identified 176 patent families filed worldwide (in blue). (B) Press release in large media per year. The curve tendency fits with scientific publications and patent deposits. For the analysis, we used a collection of 3576 documents retrieved from Scopus for which we extracted citations count and compared to trends of press releases in large media by searching Factiva® (Dow Jones) database. Two peaks emerge, a first in 2005 with the post-genomic revolution and the interference RNA technology breakthrough, and a second one in 2010 with the support of the Michael J. Fox Foundation (MJFF) for candidate compounds and the launch of clinical trials (e.g. Affiris). (C) Of 1313 studies in the field of PD, only 13 open studies are associated with the “synuclein” keyword and 8 are actually biomarker studies. We divided clinical trials in two categories, observational or for diagnosis purpose (n=8) and disease modifying or neuroprotective strategies (n=5). Data were collected from the Food and Drug Administration’s clinical trials database (clinicaltrials.gov). Clinical trials, which were terminated, completed or of which the status was unknown were excluded. Abbreviations: NIH: national institute of health; α-syn: α-synuclein.
Table 1. Patents focusing on α-synuclein that have not been announced in the pipeline.
α-syn: α-synuclein; PD: Parkinson’s disease; AD: Alzheimer’s disease; MSA: Multiple System Atrophy; DLB: Dementia with Lewy Body; GBA: Glucocerebrosidase gene name; ß-syn: ß-synuclein; Aß: amyloid-beta; siRNA: small interfering RNA; miRNA: microRNA; RNAi : RNA interference.
| Patent publication # | Year of publication | Assignee | Main claims |
|---|---|---|---|
| WO2013127918 | 2013 | Pharnext (Issy Les Moulineaux, France) | Use of acamprosate, baclofen, cinalcet, mexiletine, sulfasoxazol, torasemide to delay PD |
| WO2013063516 | 2013 | Neotop Bioscience (Dublin, Ireland) | New antibody panel for PD and other Lewy Body diseases |
| WO2013020368 | 2013 | Hong Kong university (Hong Kong, China) |
Rhodiola rosea extract (e.g. rosavin) as an α-syn oligomerization inhibitor |
| WO2012170899 | 2012 | Prothera (Reno, USA) | Protease or peptidase (prolyl oligopeptidase) for PD patients with GBA mutations |
| WO2012068405 | 2012 | ISIS Pharmaceuticals (Carlsbad, USA) | New oligonucleotide library to decrase α-syn expression |
| WO2011107544 | 2011 | Dr. Rentschlzer Holding (Laupheim, Deutchland) | Antibody library targeting α-syn for PD and synucleinopathies |
| WO2011056222 | 2011 | University of Pittsburg (Pittsburg, USA) | Anti-protein aggregate compound |
| WO2010129791 | 2010 |
University Of Medicine & Dentistry of New Jersey
(Somerset, USA) |
Use of miRNA to decrase α-syn expression |
| WO2010103515 | 2010 | Tel Aviv University (Tel-Aviv, Israel) | Use of ß-syn derived peptides to decrease α-syn aggregation |
| WO2010094090 | 2010 |
Katholleke University Leuven
(Leuven, Belgium) & University of Graz (Gras, Austria) |
Sodium/hydrogen exchange type-1 transport system inhibitors against α-syn toxicity |
| WO2010060073 | 2010 | Tel Aviv University (Tel-Aviv, Israel) | Bacteriophage-based therapy for synucleinopathy |
| WO2010037135 | 2010 |
University Of California
(Oakland, USA) & Rosalind Franklin University of Medicine (Chicago, USA) |
Increasing clearance of protein aggregates by virus-mediated expression of chimeric polypeptide |
| WO2009086306 | 2009 | Whitehead Institute for Biomedical Research (Cambridge, USA) | List of target genes involved in α-syn toxicity |
| WO2009020624 | 2009 | University of Alabama (Tuscaloosa, USA) | SURF, SEC22 and Acyl CoA Oxidase alterations and modulations for PD |
| WO2008157425 | 2008 | University of California (Oakland, USA) | Use of polypeptide to slow protein aggregation in PD, AD, MSA, DLB |
| WO2008002465 | 2008 | Feinstein Institute For Medical Research (Manhasset, USA) | Guanylhydrazone compounds to prevent Aß and α-syn aggregation |
| WO2007135426 | 2007 | Isis Innovation (Oxford, GB) | Agents (RNAi, siRNA,DNA, ribozyme) to decrease α-syn expression levels |
| WO2006124892 | 2006 | Whitehead Institute for Biomedical Research (Cambridge, USA) | List of target genes involved in α-syn toxicity |
| WO2006091964 | 2006 | University of Alabama (Tuscaloosa, USA) | Methods for identification and targeting of genes involved in α-syn aggregation |
| WO2006073734 | 2006 |
Whitehead Institute for Biomedical Research
(Cambridge, USA) & University of Missouri (Missouri, USA) |
List of target genes involved in α-syn toxicity |
| WO2006039253 | 2006 | Children"s Memorial Hospital (Chicago, USA) | siRNA to decrease α-syn expression |
| WO0020020 | 2000 | University of California (La Jolla, USA) | Use of ß-syn as a dominant-negative effect to decrease α-syn aggregation |
Table 2. List of compounds targeting either directly or indirectly α-synuclein which are in the pipeline but not yet translated to clinical trials.
Compounds listed here are still in preclinical development. For clarity purpose, we excluded the two compounds that are already in clinical development: PD01 and PRX002. Targets, mechanism of action, PCT numbers are indicated when information was available. α-syn: α-synuclein; GCase: glucocerebrosidase; GAIM: general amyloid interaction motif; IgG: immunoglobulin G; PTC: patent cooperation treaty.
| Drug Name | Sponsor | Target Name | Mechanisms of action | PCT or priority patent |
|---|---|---|---|---|
| AT-3375 |
Amicus Therapeutics
(Cranbury, USA) |
GCase | Pharmacological chaperone that increases GCase enzyme activity |
WO2007137237, WO2007150064, WO2010118282 |
| PD-0805 |
BioArctic Neuroscience
(Stockholm, Sweden) |
α-syn | Antibody against “toxic” forms of α-syn | WO2009133521 |
| EDP-32 |
BioLineRx
(Jerusalem, Israel) |
α-syn | Aggregation inhibitor | WO2010103515 |
| NI-202 |
Neurimmune Therapeutics
(Schlieren, Switzerland) |
α-syn | Antibody | WO2010069603 |
| NPT-088 |
NeuroPhage
(Cambridge, USA) |
α-syn, Tau, Aß | Second generation of NPT-001; human IgG1 fusion protein containing GAIM |
|
| NPT-001 |
NeuroPhage
(Cambridge, USA) |
α-syn, Tau, Aß | Bacteriophage M13 capsid with GAIM recognizing and remodeling misfolded protein aggregates |
|
| NLF-1233 |
nLife Therapeutics
(Granada, Spain) |
α-syn | RNAi | WO2014064257, WO2011131693 |
| PBT-434 |
Prana Biotechnology
(Parkville, Australia) |
Unspecified | Metal (iron) attenuating compound | |
| USP14 inhibitors |
Proteostasis Therapeutics
(Cambridge, USA) |
Ubiquitin specific peptidase 14 |
Proteasome activity enhancer | |
| ReS9-S7 |
reMYND
(Leuven, Belgium) |
Unspecified | Facilitates α-syn degradation | |
| ReS12-S |
reMYND
(Leuven, Belgium) |
α-syn | Chemical, synthetic | WO2009019295 |
| SIG-1012 |
Signum Biosciences
(Monmouth Junction, USA) |
Protein phosphatase 2 (formerly 2A) |
α-syn phosphorylation enhancer | |
| G2 PD |
TauRx Pharmaceuticals
(Singapore, Republic of Singapore) |
Unspecified | Compound targeting misfolded α-syn |
Table 3. Active clinical trials testing new emergent drugs and/or targets for PD treatment.
PD: Parkinson’s disease; α-syn: α-synuclein; MSA: multiple system atrophy: UMSARS-ME: motor examination (ME) of the Unified MSA Rating Scale
| ClinicalTrials.gov identifiers |
Title | Primary outcome measures |
|---|---|---|
| NCT01885494 | AFF008E: Observational Phase 1b Follow-up Extension Study for Patients With PD After Immunization With AFFITOPE® PD01A |
Tolerability and Safety |
| NCT02046434 |
Phenylbutyrate As a Treatment for Abnormal Accumulation of Brain Protein in PD |
Levels of α-syn in blood plasma |
| NCT02095171 | Single Ascending Dose Study of PRX002 in Healthy Subjects | Safety, tolerability and pharmacokinetics |
| NCT02157714 | Multiple Ascending Dose Study of PRX002 in Patients With PD | Safety, tolerability and pharmacokinetics |
| NCT02008721 | Progression Rate of MSA Under EGCG Supplementation as Anti-Aggregation-Approach |
UMSARS – ME |
Strategies to combat α-synuclein toxicity
α-syn aggregation is now considered as a major pathogenic process in PD and offers several targets for preventing α-syn toxicity 74. While perhaps premature, several clinical trials focusing on α-syn in PD have been initiated (Table 3), and numerous additional approaches - often reflecting collaboration between industry and academia - are moving forward.
Increasing protein clearance
Given the relationship between α-syn burden and pathology, the obvious first move would be to reduce its expression, through. two strategies: (i) reducing the synthesis or (ii) increasing the clearance. Silencing of SNCA in adult animals using small hairpin RNA leads to contrasting results: degeneration 75, 76 and inflammation activation 77 have been reported in rodent models while no effects were reported in squirrel monkey 78. Several explanations could underlie the apparent inconsistency, including the fact that experiments involved different animal species (rodent vs. primates) and may be dependent upon the extent and/or duration of α-syn deficiency: i.e. after partial and temporary α-syn reduction in adult RNAi-treated animals versus lifelong ablation of α-syn in mutant mice. One another possibility is that toxic RNA silencing effects might result from saturation of endogenous RNAi machinery by high RNAi levels, leading to interference of miRNA processing.
Increasing α-syn degradation by enhancing proteasomal or lysosomal activity represents another therapeutic possibility. Recently, 1-[1-(4-fluorophenyl)-2,5-dimethylpyrrol-3-yl]-2-pyrrolidin-1-ylethanone, IU1 (a small molecule identified in a high-throughput screening) was shown to enhance proteasomal function associated with increased clearance of tau 79. This new molecular area should be considered in future studies for PD. As discussed above, α-syn is degraded under pathological conditions by the ALP and relevant to PD, α-syn-mediated impairment of CMA activates macroautophagy in vitro 80. Several studies have reported successful neuroprotection with strategies aiming at increasing autophagy in in vitro and in vivo models of PD 57, 81-83 (Table 4). For instance, overexpression of the transcription factor EB 82, LAMP2a 83 or Beclin-1 81 were able to provide neuroprotection in rat models based on the overexpression of human α-syn or in a transgenic mouse model of PD 81-83. Most of the in vivo work is based, however, on viral-mediated overexpression calling for further demonstration in complementary models (Box 1).
Table 4. Effects of gene expression strategies and pharmacological compounds in experimental in vivo models of Parkinson’s disease.
In regards to experimental design, preclinical studies can be sorted in four groups depending on the lag time between the induction of the pathology and the administration of the neuroprotective compound. The therapeutic compound can be administered: (I) prophylactically, (II) concomitantly or (III) after onset of symptoms. The fourth group (IV) is based on the use of transgenic animals. In most cases, the therapeutic compound is administered to adult transgenic animals, although rare examples of delivery after onset of symptoms exist (Anle138b for instance). AAV, adeno-associated virus; CMA, chaperone-mediated autophagy; TFEB, transcription factor EB; LAMP2a, lysosome-associated membrane protein 2 type a; α-syn, α-synuclein; mTOR, mammalian target of rapamycin; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PLK2, polo-like kinase 2; PP2A, Protein phosphatase-2A ; Thy1, THYmocyte differentiation antigen 1 ; Hsp70, heat shock protein 70; Hsp104, heat shock protein 104; LV, lentivirus ; Snca, synuclein gene nomenclature ; PDGFβ, platelet-derived growth factor-β; Pffs: α-syn preformed fibrils.
| Target | Mechanism of action | Animal Model | Main outcome | Experimental design |
References |
|---|---|---|---|---|---|
| Autophagic gene expression | |||||
| TFEB | Enhancement of lysosomal biogenesis |
Rat AAV2/6 - α-syn |
Neuroprotection Decreases α-syn pathology |
II | 123 |
| LAMP2a | Boosting of CMA function |
Rat AAV2/6 - α-syn |
Neuroprotection Decreases α-syn pathology |
II | 124 |
| Beclin 1 | Enhancement of autophagy |
Mice PDGFß- α-syn |
Decreases α-syn pathology | IV | 122 |
| Rat AAV2/6 - α-syn |
Neuroprotection Decrease α-syn pathology |
II | 123 | ||
| Autophagy-enhancing drug | |||||
| Rapamycin | Inhibition of mTOR | Mice - MPTP | Neuroprotection | I | 87 |
| Temsirolimus | Rapamycin analog | Rat AAV2/6 - α-syn |
Neuroprotection Decreases α-syn pathology |
III | 123 |
| Trehalose | Enhancement of autophagy |
Mice Parkin deleted / tau overexpressing |
Neuroprotection | IV | 127 |
| Modulation of protein phosphorylation | |||||
| PLK2 | Phosphorylation of α- syn at S129 |
Rat AAV2/6 - α-syn |
Neuroprotection Decreases α-syn pathology |
II | 134 |
|
PP2A
(Pharmacological activation) |
Dephosphorylation of α- syn at S129 |
Mice Thy1- α-syn |
Restores motor behaviour Decreases α-syn pathology and glial activation |
IV | 136 |
| Inhibition of α-syn truncation | |||||
|
Calpastatin
(Overexpression of inhibitor) |
Truncation inhibitor | Mice Thy1-A30P- α-syn |
Decreases α-syn pathology and glial activation | IV | 138 |
| Disaggregation of α-syn | |||||
|
Hsp70
(Overexpression) |
Molecular chaperone | Fly- α-syn | Neuroprotection | IV | 139 |
|
Hsp70
(Pharmacological activation) |
Molecular chaperone | Fly - α-syn | Neuroprotection | IV | 141 |
| HSP104 | Molecular chaperone | Rat LV-A30P - α-syn |
Neuroprotection Decreases α-syn pathology |
II | 63 |
| Anle138b | Oligomer modulator | Mice Rotenone Thy1 - A30P - α-syn |
Decreases behavioral impairments Decreases α-syn pathology Increases in disease-free survival |
IV - III | 144 & 148 |
| CRL01 | Molecular tweezer | Zebrafish - α-syn | Increases survival Decreases α-syn pathology |
IV | 145 |
| KYP-2047 | Prolyl oligopeptidase inhibitor |
Mice Thy1-A30P - α-syn Sncatm(A30P) |
Fiber protection Decreases α-syn pathology |
IV | 146 & 147 |
| α-syn antibodies | |||||
| 9E4 | Passive immunization | Mice PDGFß - α-syn |
Decreases α-syn pathology | IV | 149 |
| AFFITOPE® technology | Active immunization | Mice Thy1 - α-syn PDGFß - α-syn |
Improves behavioral impairments Decreases α-syn pathology and glial activation Induce inflammatory response |
IV | 150 |
| Syn303 | Passive immunization | Mice Pffs induced degeneration |
Reduces motor dysfunction Decreases dopaminergic cell loss and α-syn pathology |
II - III | 157 |
Textbox 1. Clinically driven experimental designs for target validation and drug development.
Currently, there are many α-syn-based models of PD developed from simple to more complex organisms. They contributed to a better understanding of the function of α-syn, etiology and pathogenesis of PD. While none of them reproduce the full pathology, all share some similarities with the disease. In this regard, the use of a set of complementary models seems mandatory to fully validate a new therapeutic target.
As illustrated in Table 4, most preclinical studies use an experimental design in which the therapeutic compound is administered either prophylactically or concomitantly with the induction of pathology. This raises the question of the relevance regarding the progressive nature of PD. Patients can be treated only after the onset of symptoms and diagnosis (usually around 30%-50% of dopaminergic cell loss). Hence, there is a crucial need for clinically driven experiments to validate drug candidates. Ideally, these experiments should fulfill four criteria 119, 120. First, the chosen model should represent the progressive nature of PD, thus avoiding the use of acute toxic models. One cannot ignore the progressive nature of the disease, as some drugs, such as minocycline, were deleterious only in chronic models. Second, the therapeutic compound should be administered after symptom onset and once degeneration has started. Third, the therapeutic compound should be tested in complementary animal models (pathogenic and etiologic). Fourth, final proof of efficacy should be obtained in the non-human primate model of PD, as neuronal physiology is knowingly different between primate and rodents. Although it is impossible to remove all doubts before testing a drug in early phases in patients, our ethical obligation is to use the most relevant preclinical validation method. Hence, to increase the chance of successful clinical trials, we must use a combination of relevant models associated with a clinically driven experimental design to test neuroprotective compounds in preclinical investigations.
Small autophagy-activating molecules, such as rapamycin, hold promise for rapid translation to patients. This compound is a FDA-approved macrolide that inhibits the activity of the mammalian target of rapamycin (mTOR). It efficiently enhances autophagy in vivo 57, 84. However, long-term use of rapamycin is associated with off-target effects (such as interstitial pneumonitis, high levels of triglycerides, reduced wound healing and anemia – some of them are attributed to its immunosuppressant properties that might increase the risk for cancer), which may preclude its chronic use for PD 85. The disaccharide trehalose is a mTOR-independent activator of autophagy, which also attenuated neuropathology abnormalities in several models of neurodegenerative disorders including PD 86-88. The exact connection between trehalose and autophagy as well as the mechanisms underlying its neuroprotective effect remain unknown. Two conclusions can however be drawn from the studies with autophagy-activating molecules 81-83. First, identification of specific and safe compounds boosting specifically the ALP could be a successful strategy. Second, in light of the recent success in terms of safety and tolerability with lentiviral- and AAV-based approaches 89, 90, gene therapy designed to enhance UPS and lysosomal function should be considered.
Acting on α-syn post-translational modifications
Another strategy is to dampen the PTMs associated with the pathological forms of α-syn. Selecting the most suitable PTM remains challenging in light of the conflicting results obtained with the still popular α-syn phosphorylation at S129. While hyperphosphorylation of α-syn is present in the brain of PD patients, its levels are very low 43. Furthermore, overexpression of a kinase known to phosphorylate at S129, polo-like kinase 2 (PLK2) or its yeast ortholog CDC5, is neuroprotective in several in vitro, yeast, nematode and rat models of PD 91. At odds with these studies, Lee and colleagues reported that pharmacological activation of phosphoprotein phosphatase 2 (PP2A) dephosphorylates α-syn and decreases the α-syn burden in a mouse transgenic model of PD 92. Moreover, overexpression of G-protein-coupled receptor kinase 6, another α-syn kinase, accelerates degeneration in a rat model of PD 93 and preventing phosphorylation at S129 prevents neurotoxicity, while increasing the numbers of large inclusion bodies in transgenic flies 94. Further studies are therefore needed to completely unravel the pathological significance of S129-phosphorylation and to define its relevance for therapeutic intervention. Regarding the truncated forms of α-syn, recent results suggest that the overexpression of a calpain-specific inhibitor reduces α-syn pathology in the p.A30P mouse transgenic model 95. Surprisingly, the opposite, e.g. calpain activity enhancement, did not worsen α-syn pathology 95. Again, additional studies on both postmortem tissues and animal models are needed to elucidate the relevance of truncated α-syn in PD pathogenesis, identify the relevant proteases, and thereby determine the validity of targeting α-syn cleaving proteases for treatment of PD.
Targeting α-syn aggregation
Inhibiting α-syn aggregation remains an extremely attractive target for drug development. Several groups have focused on the disaggregation pathway, ad hoc for this purpose. In yeast, this pathway is composed of several molecular chaperones, in particular heat-shock proteins (Hsp) 40 and 70. Hsp40 and 70 are found in LB 96 and overexpression or pharmacological activation of Hsp70 protects from α-syn toxicity in in vitro and fly models of PD, notably through a decrease in oligomers 96-98. Since oligomeric forms are the suspected toxic species, formation of stable fibrils might be an interesting strategy to prevent cell death. An intense research effort is required to fully understand the toxicity of α-syn oligomers. Identifying aggregation inhibitors from compound library screens should be moved to the forefront. Only few have been developed so far and all were reported to efficiently provide neuroprotection; these include in vitro agents, such as EGCG 99 and in vivo agents, such as anle138b 100 (3-(1,3-benzodioxol-5-yl)-5-(3- bromophenyl)-1H-pyrazole), CLR01 101 and a prolyl oligopeptidase inhibitor, KYP2047 102, 103 (Table 4). Anle138b has shown neuroprotective properties in prion-infected mice, in two neurotoxic PD models and one genetic model of PD 100, 104. The combination of these models, sharing similarities with the various aspects of the human condition, represents a singular validation plan that deserves further examination (Box 1). These encouraging results stress the need for a better understanding of α-syn aggregation and represent a potentially fruitful series of targets for therapeutic development.
A currently “hot” strategy is the use of antibodies that target α-syn, similar to what has been tried during the last decade in the AD field. Several groups have reported neuroprotection after passive (based on the use of antibodies against the protein) or active (vaccination-based approach using full-length protein or short peptides) immunization in transgenic mouse models of PD 105-107. These reports have led pharmaceutical companies such as Roche to start a phase I clinical trial based on the use of a monoclonal antibody directed against α-syn (PRX002, initially developed by Elan Pharmaceuticals, patent#US7910333 – see Table 3 for NCT number). Active immunization with Affitope PD01 (Affiris, patent #WO2009103105) was found safe in a first pilot study in 32 PD patients (see Table 4 and http://www.affiris.at/press_releases/PD01A_MJFF_E.pdf). PD03, from Affitope, will soon be evaluated in patients with PD within the European SYMPATH consortium (http://www.sympath-project.eu/DL/sympath_factsheet_EN.pdf). Both studies include several exploratory efficacy outcome measures. Although passive and active immunization approaches are fascinating, several key questions remain to be answered. First, most studies were performed in transgenic mice in which human α-syn expression is restricted to the brain (PDGFß- and Thy1-A30P-synuclein transgenic mice), while the majority of α-syn is found on the membrane of red blood cells 108. It is therefore important to investigate in early clinical studies how antibodies react with peripheral α-syn and whether unbound antibodies could gain access to the brain compartment at sufficient levels. Second, α-syn is a cytosolic protein and LB are intraneuronal inclusions. How antibodies would recognize the intracellular protein and promote its intracellular degradation is unknown but the strategy might halt transcellular α-syn propagation. Surprisingly, passive immunization against α-syn activate autophagy 106. Antibodies might thus trigger non-selective autophagy, thus leading to the clearance of α-syn through a non α-syn-related mechanism. Indeed autophagy might be unselectively activated as part of an innate immune response against pathogens, here the presence of antibodies 109; thus leading to the clearance of α-syn through a non-α-syn-related mechanism. The use of intrabodies, that are gene-engineered antibodies specifically built for acting intracellularly, might be more specific 110. Finally, the development of conformational antibodies, i.e. antibodies recognizing the structure of the peptide not simply the sequence, might be an interesting way to target specific oligomers. In this regard, a recent study using the pffs-based model of PD demonstrated that immunotherapy with antibodies specifically targeting misfolded α-syn is blocking the entrance and propagation of α-syn in neurons, and prevents the development of neuropathological abnormalities in the brain 111. Altogether strategies aiming at decreasing α-syn aggregation either by disaggregation, stabilizing the amyloid pathway or immunization might be of therapeutic interest for PD. However, one must consider that the precise α-syn species to target remains unclear and that there is a theoretical concern that some α-syn species might be protective and that their removal could accelerate the disease process.
Additional strategies
In light of recent data suggesting spreading properties of α-syn, a better knowledge of the underlying mechanisms might provide therapeutic opportunities. First, as discussed above, nucleation appears to be a critical step in α-syn aggregate formation and therefore the identification of the structure of these “seeds” would be a huge asset. The two key components of spreading are secretion and uptake. Several pathways seem to be involved in α-syn transfer from cell to cell, including endocytosis, direct membrane penetration, trans-synaptic dissemination, exosome-mediated transfer and receptor-dependent uptake (reviewed elsewhere 2).
Lessons learned from clinical trials in AD
Two humanised monoclonal anti-amyloid β (Aβ) plaque antibodies were ineffective in halting cognitive decline after 72 weeks (bapineuzumab) and 78 weeks (solanezumab) of treatment.114 and 115 Although these clinical trials did not reach their primary endpoints, a decrease in Aβ plaques was identified by PET.116 An explanation might be that therapeutic strategies for Alzheimer"s disease should take into account both tau and Aβ deposits because the density of cortical tau aggregates correlates with cognitive decline.117 A second explanation might be that patients had Alzheimer"s disease that was too advanced to hope for recovery, underlining the importance of testing neuroprotective strategies at an early stage. Roughly 25% of clinically diagnosed patients enrolled in the two trials were amyloid negative, as established by PET imaging or CSF Aβ levels. This finding underscores the need for an objective diagnostic biomarker for selection of patients with Parkinson"s disease: a tracer for α-synuclein pathology will be crucial to further drug development. This finding also raises a major conceptual question: can we ultimately reverse α-synuclein lesions in Parkinson"s disease? At least one study118 has provided positive results. Although no overt neurodegeneration was reported in the mouse model of dementia with Lewy bodies used in this study, suppression of α-synuclein expression decreased the already established synucleinopathy.118 This strategy should be now tested in a model that leads to frank neurodegeneration (panel).
How to translate preclinical findings to clinical trials in PD patients?
Primary outcomes of previous disease-modification or neuroprotection trials in PD were based on clinical measures. Owing to the slow rate of progression of motor symptoms in PD, observed differences between treatment groups were small and a matter of considerable debate despite innovative concepts such as the "delayed-start" design 1. Therefore, efforts to improve study design including the development of objective surrogate markers will be critical for future success.
It was recently suggested that MSA and idiopathic REM sleep behavioral disorder (iRBD) may be good models for proof-of-concept studies with compounds targeting α-syn 117. MSA is a rapidly progressing disorder leading to severe motor disability within a few years 118. The fast deterioration of MSA clinical outcomes increases the sensitivity to change over time and should allow detecting disease-modifying effects more rapidly than in PD. Clinical trial duration should therefore be shorter in MSA than in PD, and this is a strong advantage when developing a drug, especially in the early stage. Crucial milestones have been reached for successfully conducting clinical intervention trials in MSA patients and survival may be used as primary outcome 117. Thirty percent of patients with iRBD develop a defined synucleinopathy at 3 years, rising to 66% at 7.5 years. Stratification with prodromal markers of PD (e.g. deficits in olfaction, color vision and cognition, as well as autonomic dysfunction and subtle motor impairment) further increases the risk of conversion up to 65% at 3 years. Thus, the conversion to defined synucleinopathy may be used in stratified cohorts of iRBD patients for testing compounds very early in the neurodegenerative process.
Concluding remarks
α-syn bears an unquestionable role in PD pathogenesis. Experimental research supports several therapeutic strategies to combat α-syn toxicity with promising preclinical results. However, an understanding of clinicopathologic relationship of various α-syn forms in PD patients is just emerging and needs to be strengthened further. Additionally, we need to establish biomarkers (imaging, biochemical, genomic) of α-syn pathology in live patients and their progression over time in order to conduct biomarker-aided clinical trials of novel therapeutics. The current pipeline of interventions targeting α-syn appears to be wide-ranging but with the usual amount of risk associated with novel approaches. In conclusion, we believe that targeting α-syn for treating PD seems to be a suitable direction to adopt but further preclinical studies are needed to de-risk therapeutic targets and trials.
Supplementary Material
Acknowledgements
The University of Bordeaux and the Centre National de la Recherche Scientifique provided infrastructural support. This work was supported by a grant from the Fondation pour la Recherche Médicale (to B.D.); by Agence Nationale de la Recherche Grants ANR-12-BSV4-0001-01 and LABEX BRAIN ANR-10-LABX-43 (to E.B.); ANR-13-BSV1-0013-01 and “Investissements d’avenir” program ANR-10-IAIHU-06 (to S.H.), by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Department of Health and Human Services; project ZO1 AG000949 (to A.S.), and Fondo de Investigación Sanitaria-Instituto de Salud Carlos III, Spain (M.V.). M.B. is a recipient of an MESR fellowship (France).
Footnotes
Contributions
EB, WGM, BD and MB were responsible for the conception of the article. BD, MB and PG did the literature review. PG created the tables. BD and MB created the figures. BD, MB, EB, WGM wrote the first draft article. BD, MB, PG, SP, MV, SH, AS, CWO, KMM, EB, GAP and WGM critically revised the entire manuscript and approved the final version.
Declaration of interests
Dr. Dehay reports grants from Framework Program 7 European Commission and Fondation de la Recherche Médicale outside the submitted work. Mr Bourdenx, Dr. Gorry, Dr. Przedborski, Dr. Hunot, Dr. Singleton, Dr. Merchant and Dr. Petsko have nothing to disclose. Dr. Vila reports grants from Fondo de Investigación Sanitaria - Instituto de Salud Carlos III (Spain), Programa de Consorcios Estratégicos Nacionales en Investigación Técnica-CENIT, INNPACTO program, European Regional Development Fund (EU) outside the submitted work. In addition, Dr. Vila has an issued patent entitled “compositions and methods for the treatment of Parkinson’s disease by the selective delivery of oligonucleotide molecules to specific neuron types”. Dr. Bezard reports personal fees from Motac neuroscience Ltd UK. Dr. Bezard is a shareholder of Motac Holding UK, and Plenitudes SARL France, CROs respectively validating at preclinical stage therapeutic strategies for movement and cognitive disorders and providing management consultancy, i.e. with no relationship to the submitted work. Dr. Bezard reports grants from Michael J Fox Foundation USA, Fondation de France, Agence Nationale de la recherche France, Framework Program 7 European Commission, Cariplo Foundation, China Science Fund China, Medical Research Council UK, France Parkinson, Institut de Recherche en santé du Canada, outside the submitted work. Dr. Meissner is an advisory board member of Advisory Board member of ANM GmbH, Cologne, Germany and Novartis France, activities outside the submitted work.. Dr. Meissner reports grants from he University Hospital Bordeaux, the PSP and PD patients association, the French Ministry of Health, the National Research Agency (ANR) and the European Community, all outside the submitted work. Dr. Meissner received fees from UCB, Novartis, TEVA/Lundbeck, GSK and CMA Québec for teaching lectures and travel grants from Affiris, Novartis, GSK, TEVA/Lundbeck. Dr. Olanow reports being a shareholder of Clintrex (USA), a CRO designing clinical trials on behalf of for-profit and non-profit organizations, i.e. with no relationship to the submitted work. Dr. Olanow received personal fees from Abbvie, from Novartis/Orion, Teva/Lundbeck, and Ceregene for consulting outside the submitted work. Dr. Olanow is an executive committee member of the Michael J Fox Foundation and the World Parkinson Congress.
REFERENCES
- 1.Meissner WG, et al. Priorities in Parkinson"s disease research. Nat Rev Drug Discov. 2011;10:377–93. doi: 10.1038/nrd3430. [DOI] [PubMed] [Google Scholar]
- 2.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14:38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jankovic J. Parkinson"s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–76. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 4.Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson"s disease. Science. 1997;276:2045–7. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 5.Spillantini MG, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 6.Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol. 2013;9:13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- 7.Ueda K, et al. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:11282–6. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr. NACP, a protein implicated in Alzheimer"s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–15. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 9.Iwai A, et al. The precursor protein of non-A beta component of Alzheimer"s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–75. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 10.Lesage S, et al. G51D alpha-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann Neurol. 2013;73:459–71. doi: 10.1002/ana.23894. [DOI] [PubMed] [Google Scholar]
- 11.Kruger R, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson"s disease. Nat Genet. 1998;18:106–8. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 12.Zarranz JJ, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–73. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 13.Appel-Cresswell S, et al. Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson"s disease. Mov Disord. 2013;28:811–3. doi: 10.1002/mds.25421. [DOI] [PubMed] [Google Scholar]
- 14.Proukakis C, et al. A novel alpha-synuclein missense mutation in Parkinson disease. Neurology. 2013;80:1062–4. doi: 10.1212/WNL.0b013e31828727ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasanen P, et al. A novel alpha-synuclein mutation A53E associated with atypical multiple system atrophy and Parkinson"s disease-type pathology. Neurobiol Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Seidel K, et al. First appraisal of brain pathology owing to A30P mutant alpha-synuclein. Ann Neurol. 2010;67:684–9. doi: 10.1002/ana.21966. [DOI] [PubMed] [Google Scholar]
- 17.Kotzbauer PT, et al. Fibrillization of alpha-synuclein and tau in familial Parkinson"s disease caused by the A53T alpha-synuclein mutation. Exp Neurol. 2004;187:279–88. doi: 10.1016/j.expneurol.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Chartier-Harlin MC, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson"s disease. Lancet. 2004;364:1167–9. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 19.Singleton AB, et al. alpha-Synuclein locus triplication causes Parkinson"s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 20.Bendor JT, Logan TP, Edwards RH. The function of alpha-synuclein. Neuron. 2013;79:1044–66. doi: 10.1016/j.neuron.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rochet JC, Hay BA, Guo M. Molecular insights into Parkinson"s disease. Prog Mol Biol Transl Sci. 2012;107:125–88. doi: 10.1016/B978-0-12-385883-2.00011-4. [DOI] [PubMed] [Google Scholar]
- 22.Giasson BI, Murray IV, Trojanowski JQ, Lee VM. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem. 2001;276:2380–6. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, et al. A soluble alpha-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci U S A. 2011;108:17797–802. doi: 10.1073/pnas.1113260108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartels T, Choi JG, Selkoe DJ. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–10. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gould N, et al. Evidence of native alpha-synuclein conformers in the human brain. J Biol Chem. 2014;289:7929–34. doi: 10.1074/jbc.C113.538249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fauvet B, et al. alpha-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem. 2012;287:15345–64. doi: 10.1074/jbc.M111.318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–20. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 28.Conway KA, et al. Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson"s disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A. 2000;97:571–6. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karpinar DP, et al. Pre-fibrillar alpha-synuclein variants with impaired beta-structure increase neurotoxicity in Parkinson"s disease models. EMBO J. 2009;28:3256–68. doi: 10.1038/emboj.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winner B, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci U S A. 2011;108:4194–9. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cremades N, et al. Direct observation of the interconversion of normal and toxic forms of alpha-synuclein. Cell. 2012;149:1048–59. doi: 10.1016/j.cell.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danzer KM, et al. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27:9220–32. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka M, et al. Aggresomes formed by alpha-synuclein and synphilin-1 are cytoprotective. J Biol Chem. 2004;279:4625–31. doi: 10.1074/jbc.M310994200. [DOI] [PubMed] [Google Scholar]
- 34.Volles MJ, Lansbury PT., Jr. Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson"s disease-linked mutations and occurs by a pore-like mechanism. Biochemistry. 2002;41:4595–602. doi: 10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
- 35.Quist A, et al. Amyloid ion channels: a common structural link for protein-misfolding disease. Proc Natl Acad Sci U S A. 2005;102:10427–32. doi: 10.1073/pnas.0502066102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Rooijen BD, Claessens MM, Subramaniam V. Membrane Permeabilization by Oligomeric alpha-Synuclein: In Search of the Mechanism. PLoS One. 2010;5:e14292. doi: 10.1371/journal.pone.0014292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freeman D, et al. Alpha-synuclein induces lysosomal rupture and cathepsin dependent reactive oxygen species following endocytosis. PLoS One. 2013;8:e62143. doi: 10.1371/journal.pone.0062143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi BK, et al. Large alpha-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking. Proc Natl Acad Sci U S A. 2013;110:4087–92. doi: 10.1073/pnas.1218424110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okochi M, et al. Constitutive phosphorylation of the Parkinson"s disease associated alpha-synuclein. J Biol Chem. 2000;275:390–7. doi: 10.1074/jbc.275.1.390. [DOI] [PubMed] [Google Scholar]
- 40.Fujiwara H, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–4. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 41.Anderson JP, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–52. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 42.Paleologou KE, et al. Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein. J Biol Chem. 2008;283:16895–905. doi: 10.1074/jbc.M800747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kellie JF, et al. Quantitative measurement of intact alpha-synuclein proteoforms from post-mortem control and Parkinson"s disease brain tissue by intact protein mass spectrometry. Sci Rep. 2014;4:5797. doi: 10.1038/srep05797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giasson BI, et al. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–9. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 45.Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr. Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–9. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 46.Norris EH, Giasson BI, Ischiropoulos H, Lee VM. Effects of oxidative and nitrative challenges on alpha-synuclein fibrillogenesis involve distinct mechanisms of protein modifications. J Biol Chem. 2003;278:27230–40. doi: 10.1074/jbc.M212436200. [DOI] [PubMed] [Google Scholar]
- 47.Yamin G, Uversky VN, Fink AL. Nitration inhibits fibrillation of human alpha-synuclein in vitro by formation of soluble oligomers. FEBS Lett. 2003;542:147–52. doi: 10.1016/s0014-5793(03)00367-3. [DOI] [PubMed] [Google Scholar]
- 48.Periquet M, Fulga T, Myllykangas L, Schlossmacher MG, Feany MB. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J Neurosci. 2007;27:3338–46. doi: 10.1523/JNEUROSCI.0285-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ulusoy A, Febbraro F, Jensen PH, Kirik D, Romero-Ramos M. Co-expression of C-terminal truncated alpha-synuclein enhances full-length alpha-synuclein-induced pathology. Eur J Neurosci. 2010;32:409–22. doi: 10.1111/j.1460-9568.2010.07284.x. [DOI] [PubMed] [Google Scholar]
- 50.Bennett MC, et al. Degradation of alpha-synuclein by proteasome. J Biol Chem. 1999;274:33855–8. doi: 10.1074/jbc.274.48.33855. [DOI] [PubMed] [Google Scholar]
- 51.Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003;278:25009–13. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- 52.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–5. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 53.Martinez-Vicente M, et al. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118:777–88. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winslow AR, et al. alpha-Synuclein impairs macroautophagy: implications for Parkinson"s disease. J Cell Biol. 2010;190:1023–37. doi: 10.1083/jcb.201003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanik SA, Schultheiss CE, Volpicelli-Daley LA, Brunden KR, Lee VM. Lewy body-like alpha-synuclein aggregates resist degradation and impair macroautophagy. J Biol Chem. 2013;288:15194–210. doi: 10.1074/jbc.M113.457408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ebrahimi-Fakhari D, et al. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of alpha-synuclein. J Neurosci. 2011;31:14508–20. doi: 10.1523/JNEUROSCI.1560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dehay B, et al. Pathogenic lysosomal depletion in Parkinson"s disease. J Neurosci. 2010;30:12535–44. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH. Alterations in lysosomal and proteasomal markers in Parkinson"s disease: relationship to alpha-synuclein inclusions. Neurobiol Dis. 2009;35:385–98. doi: 10.1016/j.nbd.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 59.Bellucci A, et al. Induction of the unfolded protein response by alpha-synuclein in experimental models of Parkinson"s disease. J Neurochem. 2011;116:588–605. doi: 10.1111/j.1471-4159.2010.07143.x. [DOI] [PubMed] [Google Scholar]
- 60.Guardia-Laguarta C, et al. alpha-Synuclein is localized to mitochondria-associated ER membranes. J Neurosci. 2014;34:249–59. doi: 10.1523/JNEUROSCI.2507-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–94. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson"s disease. Nat Med. 2008;14:504–6. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 63.Li JY, et al. Lewy bodies in grafted neurons in subjects with Parkinson"s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–3. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 64.Mendez I, et al. Dopamine neurons implanted into people with Parkinson"s disease survive without pathology for 14 years. Nat Med. 2008;14:507–9. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Braak H, et al. Staging of brain pathology related to sporadic Parkinson"s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 66.Recasens A, et al. Lewy body extracts from Parkinson disease brains trigger alpha-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol. 2014;75:351–62. doi: 10.1002/ana.24066. [DOI] [PubMed] [Google Scholar]
- 67.Luk KC, et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–53. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Recasens A, Dehay B. Alpha-synuclein spreading in Parkinson"s disease. Front Neuroanat. 2014;8:159. doi: 10.3389/fnana.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bousset L, et al. Structural and functional characterization of two alpha-synuclein strains. Nat Commun. 2013;4:2575. doi: 10.1038/ncomms3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim C, et al. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirsch EC, Hunot S. Neuroinflammation in Parkinson"s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–97. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 72.Benner EJ, et al. Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS One. 2008;3:e1376. doi: 10.1371/journal.pone.0001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brochard V, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–92. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schapira AH, Olanow CW, Greenamyre JT, Bezard E. Slowing of neurodegeneration in Parkinson"s disease and Huntington"s disease: future therapeutic perspectives. Lancet. 2014;384:545–55. doi: 10.1016/S0140-6736(14)61010-2. [DOI] [PubMed] [Google Scholar]
- 75.Gorbatyuk OS, et al. In vivo RNAi-mediated alpha-synuclein silencing induces nigrostriatal degeneration. Mol Ther. 2010;18:1450–7. doi: 10.1038/mt.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khodr CE, et al. An alpha-synuclein AAV gene silencing vector ameliorates a behavioral deficit in a rat model of Parkinson"s disease, but displays toxicity in dopamine neurons. Brain Res. 2011;1395:94–107. doi: 10.1016/j.brainres.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khodr CE, Becerra A, Han Y, Bohn MC. Targeting alpha-synuclein with a microRNA-embedded silencing vector in the rat substantia nigra: positive and negative effects. Brain Res. 2014;1550:47–60. doi: 10.1016/j.brainres.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCormack AL, et al. Alpha-synuclein suppression by targeted small interfering RNA in the primate substantia nigra. PLoS One. 2010;5:e12122. doi: 10.1371/journal.pone.0012122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee BH, et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature. 2010;467:179–84. doi: 10.1038/nature09299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xilouri M, Vogiatzi T, Vekrellis K, Park D, Stefanis L. Abberant alpha-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy. PLoS One. 2009;4:e5515. doi: 10.1371/journal.pone.0005515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spencer B, et al. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson"s and Lewy body diseases. J Neurosci. 2009;29:13578–88. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Decressac M, et al. TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein toxicity. Proc Natl Acad Sci U S A. 2013;110:E1817–26. doi: 10.1073/pnas.1305623110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xilouri M, et al. Boosting chaperone-mediated autophagy in vivo mitigates alpha-synuclein-induced neurodegeneration. Brain. 2013;136:2130–46. doi: 10.1093/brain/awt131. [DOI] [PubMed] [Google Scholar]
- 84.Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson"s disease. J Neurosci. 2010;30:1166–75. doi: 10.1523/JNEUROSCI.3944-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bove J, Martinez-Vicente M, Vila M. Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci. 2011;12:437–52. doi: 10.1038/nrn3068. [DOI] [PubMed] [Google Scholar]
- 86.Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem. 2007;282:5641–52. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- 87.Rodriguez-Navarro JA, et al. Trehalose ameliorates dopaminergic and tau pathology in parkin deleted/tau overexpressing mice through autophagy activation. Neurobiol Dis. 2010;39:423–38. doi: 10.1016/j.nbd.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 88.Castillo K, et al. Trehalose delays the progression of amyotrophic lateral sclerosis by enhancing autophagy in motoneurons. Autophagy. 2013;9:1308–20. doi: 10.4161/auto.25188. [DOI] [PubMed] [Google Scholar]
- 89.Marks WJ, Jr., et al. Gene delivery of AAV2-neurturin for Parkinson"s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:1164–72. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- 90.Palfi S, et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson"s disease: a dose escalation, open-label, phase 1/2 trial. Lancet. 2014;383:1138–46. doi: 10.1016/S0140-6736(13)61939-X. [DOI] [PubMed] [Google Scholar]
- 91.Oueslati A, Schneider BL, Aebischer P, Lashuel HA. Polo-like kinase 2 regulates selective autophagic alpha-synuclein clearance and suppresses its toxicity in vivo. Proc Natl Acad Sci U S A. 2013;110:E3945–54. doi: 10.1073/pnas.1309991110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee KW, et al. Enhanced phosphatase activity attenuates alpha-synucleinopathy in a mouse model. J Neurosci. 2011;31:6963–71. doi: 10.1523/JNEUROSCI.6513-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sato H, et al. Authentically phosphorylated alpha-synuclein at Ser129 accelerates neurodegeneration in a rat model of familial Parkinson"s disease. J Neurosci. 2011;31:16884–94. doi: 10.1523/JNEUROSCI.3967-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen L, Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci. 2005;8:657–63. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- 95.Diepenbroek M, et al. Overexpression of the calpain-specific inhibitor calpastatin reduces human alpha-Synuclein processing, aggregation and synaptic impairment in [A30P]alphaSyn transgenic mice. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson"s disease. Science. 2002;295:865–8. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 97.Klucken J, Shin Y, Masliah E, Hyman BT, McLean PJ. Hsp70 Reduces alpha-Synuclein Aggregation and Toxicity. J Biol Chem. 2004;279:25497–502. doi: 10.1074/jbc.M400255200. [DOI] [PubMed] [Google Scholar]
- 98.Auluck PK, Bonini NM. Pharmacological prevention of Parkinson disease in Drosophila. Nat Med. 2002;8:1185–6. doi: 10.1038/nm1102-1185. [DOI] [PubMed] [Google Scholar]
- 99.Bieschke J, et al. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Proc Natl Acad Sci U S A. 2010;107:7710–5. doi: 10.1073/pnas.0910723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wagner J, et al. Anle138b: a novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson"s disease. Acta Neuropathol. 2013;125:795–813. doi: 10.1007/s00401-013-1114-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prabhudesai S, et al. A novel "molecular tweezer" inhibitor of alpha-synuclein neurotoxicity in vitro and in vivo. Neurotherapeutics. 2012;9:464–76. doi: 10.1007/s13311-012-0105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Savolainen MH, et al. The beneficial effect of a prolyl oligopeptidase inhibitor, KYP-2047, on alpha-synuclein clearance and autophagy in A30P transgenic mouse. Neurobiol Dis. 2014;68C:1–15. doi: 10.1016/j.nbd.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Myohanen TT, et al. A prolyl oligopeptidase inhibitor, KYP-2047, reduces alpha-synuclein protein levels and aggregates in cellular and animal models of Parkinson"s disease. Br J Pharmacol. 2012;166:1097–113. doi: 10.1111/j.1476-5381.2012.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Levin J, et al. The oligomer modulator anle138b inhibits disease progression in a Parkinson mouse model even with treatment started after disease onset. Acta Neuropathol. 2014;127:779–80. doi: 10.1007/s00401-014-1265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bae EJ, et al. Antibody-aided clearance of extracellular alpha-synuclein prevents cell-to-cell aggregate transmission. J Neurosci. 2012;32:13454–69. doi: 10.1523/JNEUROSCI.1292-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Masliah E, et al. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS One. 2011;6:e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 107.Mandler M, et al. Next-generation active immunization approach for synucleinopathies: implications for Parkinson"s disease clinical trials. Acta Neuropathol. 2014;127:861–79. doi: 10.1007/s00401-014-1256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fuchs J, et al. Genetic variability in the SNCA gene influences alpha-synuclein levels in the blood and brain. FASEB J. 2008;22:1327–34. doi: 10.1096/fj.07-9348com. [DOI] [PubMed] [Google Scholar]
- 109.Pareja ME, Colombo MI. Autophagic clearance of bacterial pathogens: molecular recognition of intracellular microorganisms. Front Cell Infect Microbiol. 2013;3:54. doi: 10.3389/fcimb.2013.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou C, Przedborski S. Intrabody and Parkinson"s disease. Biochim Biophys Acta. 2009;1792:634–42. doi: 10.1016/j.bbadis.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tran HT, et al. Alpha-synuclein immunotherapy blocks uptake and templated propagation of misfolded alpha-synuclein and neurodegeneration. Cell Rep. 2014;7:2054–65. doi: 10.1016/j.celrep.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salloway S, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer"s disease. N Engl J Med. 2014;370:322–33. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Doody RS, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer"s disease. N Engl J Med. 2014;370:311–21. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 114.Rinne JO, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer"s disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9:363–72. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- 115.Lee VM, Brunden KR, Hutton M, Trojanowski JQ. Developing therapeutic approaches to tau, selected kinases, and related neuronal protein targets. Cold Spring Harb Perspect Med. 2011;1:a006437. doi: 10.1101/cshperspect.a006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lim Y, et al. alpha-Syn suppression reverses synaptic and memory defects in a mouse model of dementia with Lewy bodies. J Neurosci. 2011;31:10076–87. doi: 10.1523/JNEUROSCI.0618-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fernagut PO, et al. Multiple system atrophy: a prototypical synucleinopathy for disease-modifying therapeutic strategies. Neurobiol Dis. 2014;67:133–9. doi: 10.1016/j.nbd.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 118.Fanciulli A, Wenning GK. Multiple-system atrophy. N Engl J Med. 2015;372:249–63. doi: 10.1056/NEJMra1311488. [DOI] [PubMed] [Google Scholar]
- 119.Bezard E. Neuroprotection for Parkinson"s disease: a call for clinically driven experimental design. Lancet Neurol. 2003;2:393. doi: 10.1016/s1474-4422(03)00432-0. [DOI] [PubMed] [Google Scholar]
- 120.Bezard E, Yue Z, Kirik D, Spillantini MG. Animal models of Parkinson"s disease: limits and relevance to neuroprotection studies. Mov Disord. 2013;28:61–70. doi: 10.1002/mds.25108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.