Abstract
Sustained local delivery of single agents and controlled delivery of multiple chemotherapeutic agents are sought for the treatment of brain cancer. A resorbable, multi-reservoir polymer microchip drug delivery system has been against a tumor model. The microchip reservoirs were loaded with 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU). BCNU was more stabile at 37°C within the microchip compared to a uniformly impregnated polymeric wafer (70% intact drug vs. 38%, at 48 hours). The half-life of the intact free drug in the microchip was 11 days, which is a marked enhancement compared to its half-life in normal saline and 10% ethanol (7 and 10 minutes, respectively) (1, 2). A syngeneic Fischer 344 9L gliosarcoma rat model was used to study the tumoricidal efficacy of BCNU delivery from the microchip or homogeneous polymer wafer. A dose-dependent decrease in tumor size was found for 0.17, 0.67, and 1.24 mg of BCNU-microchips. Tumors treated with 1.24 mg BCNU-microchips showed significant tumor reduction (p=0.001) compared to empty control microchips at two weeks. The treatment showed similar efficacy to a polymer wafer with the same dosage. The microchip array of reservoirs allows for delivery of multiple drugs with independent release kinetics and formulations.
Keywords: Drug delivery; biodegradable polymer; microchip; tumor; 1,3-Bis(2-chloroethyl)-1-nitrosourea (BCNU)
1. INTRODUCTION
Brain cancer is still associated with poor prognosis with survival being less than one year when surgical resection is combined with radiation and chemotherapy(3–6). Research is ongoing for sustained intracranial delivery of single agents as well as controlled delivery of combination chemotherapeutics via systemic injection, polymers, pumps, and convection enhanced delivery(7–15). In particular, local controlled treatment of brain tumors with a homogeneous biodegradable polymer, Gliadel® wafer, has been particularly successful in improving survival(16–19). However, only the non-degradable and degradable microchip platform is able to deliver multiple agents in distinct rounds from a single device(20, 21).
The microchip system has a unique array of reservoirs, which enables 1) delivery of multiple drugs, 2) independent modulation of drug release profiles, and 3) several drug formulations in a single implantation procedure, which is not possible with other drug delivery technologies. The microchip has the potential to deliver subsequent doses of therapeutic agents at a later time to prevent or to treat recurrence of neoplasm. Local delivery by microchips can increase the concentration of chemotherapeutic agent at the tumor site while reducing systemic toxicity. This spatial control is particularly beneficial for brain tumors because they rarely spread outside the brain (22).
Delivery of 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU), a commonly used brain cancer chemotherapeutic agent, from a non-degradable microchip was shown to have a dose-dependent inhibiting effect on tumor growth with an efficacy comparable to a subcutaneous injection of an equal dose of the drug(23). This technology was validated in a similar device for long-term repeat dosing by the triggered release of leuprolide (MW 1,205) for up to six months in beagle dogs(24). These devices are not self-contained, however, and require a transcutaneous or wireless interface. A passively actuated, biodegradable controlled release system can avoid the complications of these components in select applications. Our group has previously reported on the behavior of biodegradable microchips that contain an array of drug reservoirs, each sealed by a thin membrane that degrades according to a predetermined schedule to release drug(20). The substrate hydrolyzes after all the doses have been released. Herein, we compare the efficacy of this new device to an existing clinical therapy.
Our studies show that the use of a biodegradable polymer-based microchip containing BCNU can significantly limit tumor growth of 9L gliosarcoma challenge in a rat model. The performance of this new drug delivery device is comparable to a polymer wafer uniformly impregnated with BCNU, similar to the FDA-approved Gliadel® therapy. This study provides the foundation for potentially significant improvements in cancer therapies by means of precise local administration of single or multiple drugs in a predetermined manner from a self-contained degradable microchip.
2. MATERIALS AND METHODS
2.1 Microchip fabrication and packaging
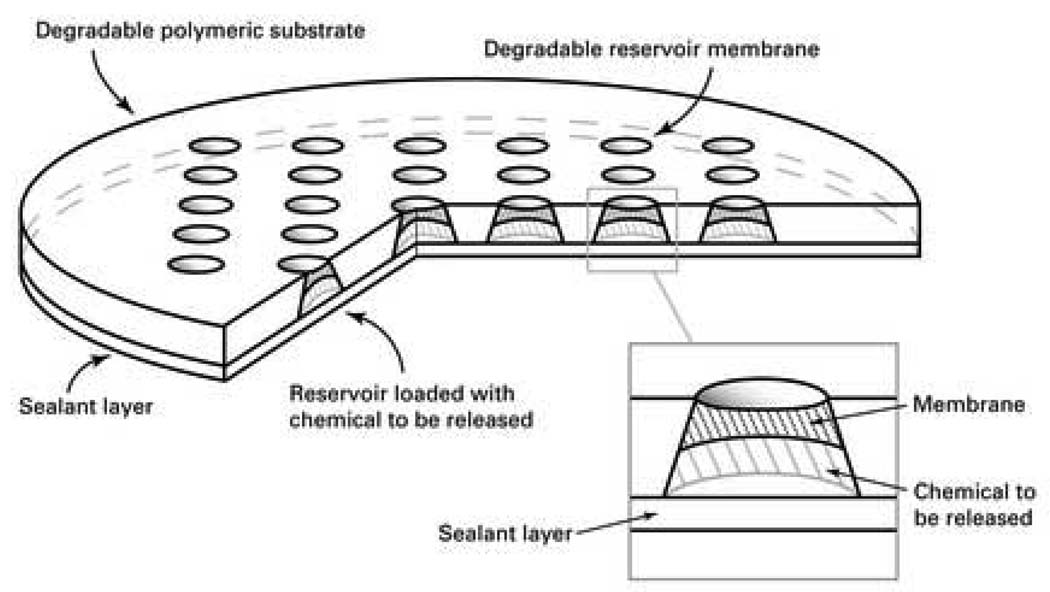
The polymer microchips (Figure 1) were fabricated as previously reported(20). Briefly, purified poly(L-lactic acid) (PLA, MW 194,000; Medisorb 100 L; Alkermes, Cambridge, MA) tablets were compression-molded against a patterned aluminum die and followed by polishing to form the reservoirs. A 12% v/v solution of poly(lactic-co-glycolic) acid (PLGA, MW 11,000; Medisorb 50:50 DL 2A; Alkermes) in 1,1,3,3,-hexafluoro-2-propanol was injected to form a membrane inside each reservoir. The microchip was then dried in a vacuum oven for 2 days at 80°C. A mixture of BCNU (Bristol-Myers Squibb Co., Evansville, IN) and 14C-BCNU (Moravek Biochemicals, Brea, CA) was injected into the microchips using a microinjector (UltraMicroPump II; World Precision Instruments, Sarasota, FL). Each microchip was loaded with a final mass of 0.17, 0.67, or 1.24 mg of BCNU. The final microchip dimensions are approximately 10 mm in diameter and 1 mm in thickness.
Fig. 1.
Schematic showing the polymer microchip. (Reprinted by permission from Macmillan Publishers Ltd: Nature Materials, 2:767–772, 2003.)
Control devices of polymer wafers fabricated from polycarboxyphenoxypropane:sebacic acid (pCPP:SA) were incorporated with 1.24 mg BCNU. Briefly, pCPP:SA in a 20:80 formulation (Guilford Pharmaceuticals, Inc., Baltimore, MD) and BCNU were dissolved together in dichloromethane followed by vacuum desiccation. Polymer disks were then formed by compression molding. Polymers were stored at −80°C prior to implantation. A detailed protocol is described elsewhere (25).
2.2 BCNU Stability and in vitro Release
The stability of BCNU within the multi-reservoir microchips and the uniformly impregnated wafers was studied. BCNU was microinjected into the polymer microchip and then sealed on both sides with pressure sensitive adhesive. Similarly, BCNU was incorporated into the wafers as described above. The microchips were incubated in phosphate buffered saline (PBS) at 37°C in a capped glass vial. BCNU was extracted from the microchip for 3 minutes in 3 ml 20:80 ethanol:water. BCNU was extracted from wafers by dissolving with 99.7:0.3 dichloromethane:acetonitrile. The extracted solutions were immediately assayed using the Bratton-Marshall assay.
Intact BCNU was quantified via a colorimetric assay using the Bratton-Marshall method(26). The assay is very sensitive to only the whole BCNU molecule. One part of the sulfanilamide solution (5 mg/ml in 2M HCl) was added to two parts of each BCNU solution to be tested, sealed, and heated in a water bath at 50°C for 45 minutes. The samples were chilled on ice to room temperature, and 200 ul of the resulting solution and 10 ul of the Bratton-Marshall reagent (3 mg/ml of N-(1-naphthyl)ethylenediamine in deionized water) were transferred to a 96 well plate and the absorbance was read five minutes later at λ=540 nm. The absorbance followed a linear relationship with BCNU concentration from 0 to 50 ug/ml. Samples were diluted in appropriate solvent before performing the assay to be within the linear range as necessary. The percent of intact BCNU was calculated by dividing the amount of intact BCNU as determined from the Bratton-Marshall assay by the initial amount of BCNU as estimated by radioactive-labeling or mass. All stability measurements were performed in triplicate, and the average value was reported.
Microchips were filled with BCNU, sealed and placed into 3 ml PBS at 37°C to determine in vitro release kinetics. Periodically, the PBS was removed and replaced with fresh PBS. The radioactivity of the release medium was measured using a liquid scintillation counter (Packard TriCarb 2200CA; Perkin Elmer Life Sciences, Wellesley, MA) using ScintiSafe Plus 50% scintillation fluid (Fischer Chemicals, Fairlawn, NJ). The radioactivity per timepoint was divided by the total radioactivity loaded and integrated over time to describe the cumulative release profile. The results are reported as the median with error bars representing the 25th and 75th percentiles.
2.3 Animal Experiments
2.3.1 Orthotopic tumor model
Two separate experiments were performed to evaluate the efficacy of BCNU when delivered via the biodegradable microchip. An initial efficacy study examined two dosages of BCNU (0.16 and 0.67 mg) released from the microchip, and a second experiment included a higher dosage of BCNU (1.24mg).
Forty-one F344 Fischer rats underwent implantation of 9L gliosarcoma in the left flank in the first study. The rats were divided into six groups and received one of the following treatments directly beneath the tumor at ten days: 1) a PLGA microchip containing 0.16 mg BCNU (n=8); 2) a PLGA microchip containing 0.67 mg BCNU (n=8); 3) an injection of 0.16 mg BCNU (n=4); 4) an injection of 0.67 mg BCNU (n=4); 5) an empty microchip (n=8); and 6) no treatment (n=9).
Forty F344 rats underwent 9L gliosarcoma flank implantation in the second study. The rats were randomly divided into five groups and received one of the following treatments: 1) a PLGA microchip containing 1.24 mg BCNU (n=8); 2) a pCPP:SA wafer containing 1.24 mg BCNU (n=8); 3) an empty PLGA microchip (n=8); and 4) empty pCPP:SA wafer (n=8); and 5) no treatment (n=8).
The microchips and wafers were sterilized by a gamma radiation dose of 480 Gray from a Cesium-137 source (Mark1-68 irradiator; JLS Shepherd, Glendale, CA) prior to implantation. A 2 mm3 piece of solid 9L glioma, an experimental gliosarcoma syngeneic to the Fischer 344 rat (Brain Tumor Research Center; University of California, San Francisco), was implanted into the left flank of experimental animals as described previously (25). Initial tumor sizes were measured at ten days after tumor implantation, and animals were randomized to experimental groups. The treatment was then placed between the flank muscle and the tumor under aseptic conditions. Microchips were placed with the drug-eluting side facing the tumor. The incision was then closed with sterile autoclips. Tumors were measured three times a week with calipers, and the tumor volume was approximated as an ellipsoid (Eqn. 1). Median tumor volumes versus time were plotted and the delay of tumor growth versus untreated controls was used as the measure of efficacy. The asymmetric, two-tailed Mann-Whitney U test was used to compare tumor sizes at various timepoints. P-values < 0.05 were considered significant.
| (Equation 1) |
2.3.2 Kinetics of drug release
Some animals were individually housed in metabolic cages (Nalgene model # 650-0100, Braintree Scientific Inc., Braintree, MA) to assess drug release by radioactive excretion. Radioactivity measurements were conducted on urine after determining that no radioactivity was detected in the fecal samples. One ml urine samples were taken twice daily and mixed with 5 ml scintillation gel (Ready Gel, Beckman Coulter Inc., Fullerton, CA) in a 7 ml scintillation vial. The radioactivity was measured using a liquid scintillation counter (Beckman Coulter Inc., Fullerton, CA). The amount of radioactivity (nCi) was multiplied by the total volume of urine collected at that time point, and integrated over time to produce the drug recovery curves. Selected explanted microchips were sonicated in methanol and then allowed to soak for 1.5 hours to extract remaining radioactive BCNU. The radioactivity was quantified as above.
2.3.3 Animals
Fischer 344 rats (female, 150~200 g) were obtained from Charles River Laboratories (Wilmington, MA). All animals implanted with radioactive BCNU and two animals from the other groups (serving as controls) were individually housed in metabolic cages, which features a unique funnel and cone design that effectively separates feces and urine into tubes outside the cage. All other animals were housed in standard cages. All animals were given standard rat chow and water ad libitum. Animals housed in metabolic cages were given sugar water (4 tsp/500 ml), which encouraged the animals to drink more and allowed more frequent collection of urine samples. Animals were checked daily for physical or behavioral evidence of toxicity, such as decreased alertness, impaired grooming, or gait disturbances. Animals were housed and treated in accordance with the policies and principles of laboratory care of the Johns Hopkins University School of Medicine Animal Care and Use Committee and the MIT Committee on Animal Care.
3. RESULTS
3.1 Stability of intact BCNU and in vitro Release
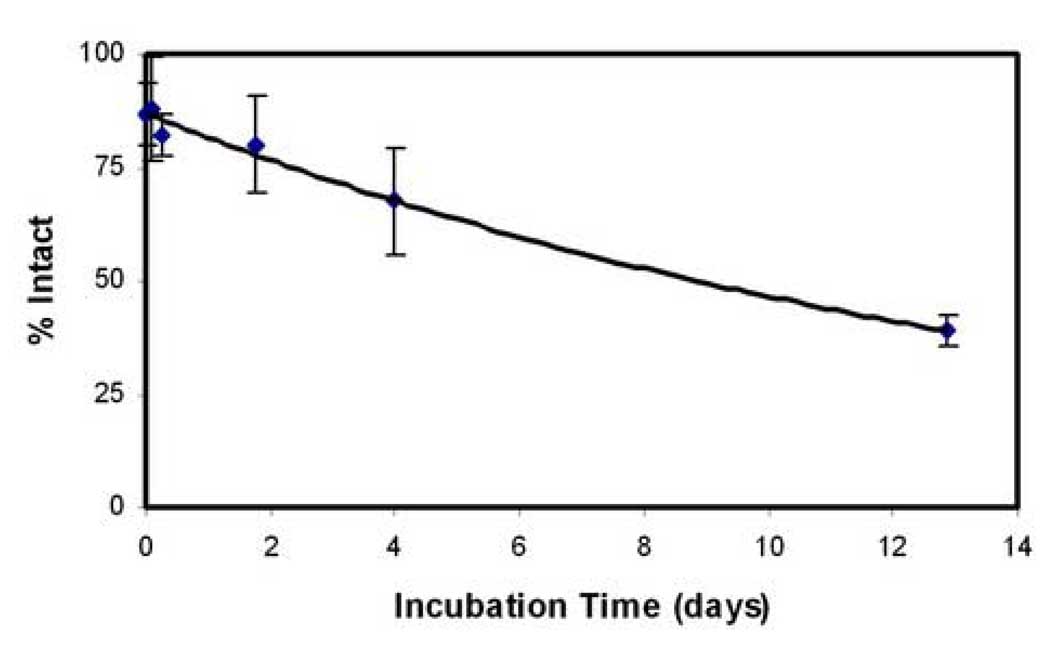
The half-life of BCNU within the microchip measured by the Bratton Marshall assay at 37°C was 11 days (Figure 2). The stability of BCNU in the microchip and wafer was directly compared in a separate experiment. The microchips contained 74% intact BCNU in the microchip and 62% intact BCNU in the wafer after incubation at 37°C for 24 hours when compared to the initial BCNU content. These values drop to 70% and 38% respectively at 48 hours.
Fig. 2.
Intact BCNU in the sealed microchip. The mean ± SD is plotted (n=3). BCNU in the polymer microchip has a half-life at 37°C of 11 days.
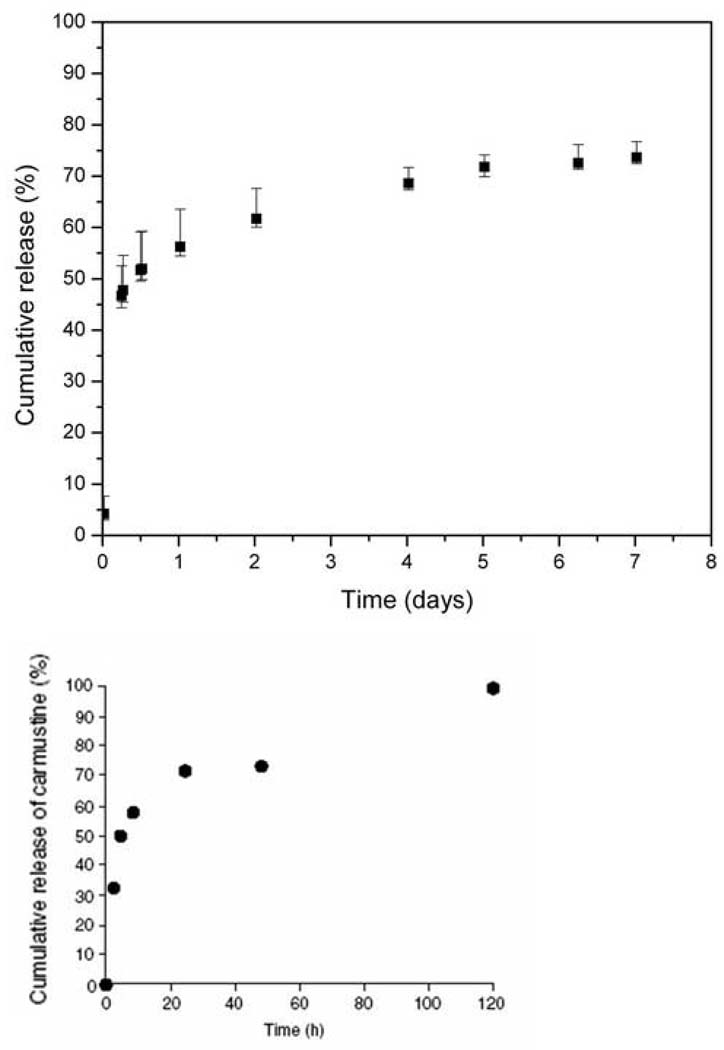
BCNU was released from the microchips in two distinct phases (Figure 3a). Approximately 50% of the BCNU from microchips containing 1.24 mg of BCNU was released during the initial 12 hours. This initial burst was followed by a much slower release of the majority of the remaining contents during the next 5 days.
Fig. 3.
a) Cumulative release of BCNU from microchip into PBS at 37°C (n=6). The median value is reported with the error bars representing the 25th and 75th percentiles.
b) Release of BCNU from pCPP:SA wafers implanted into rat brains. (Reprinted by permission from Elsevier: J Control Release, 42:83–92, 1996.) (Pending)
3.2 In vivo Release of BCNU
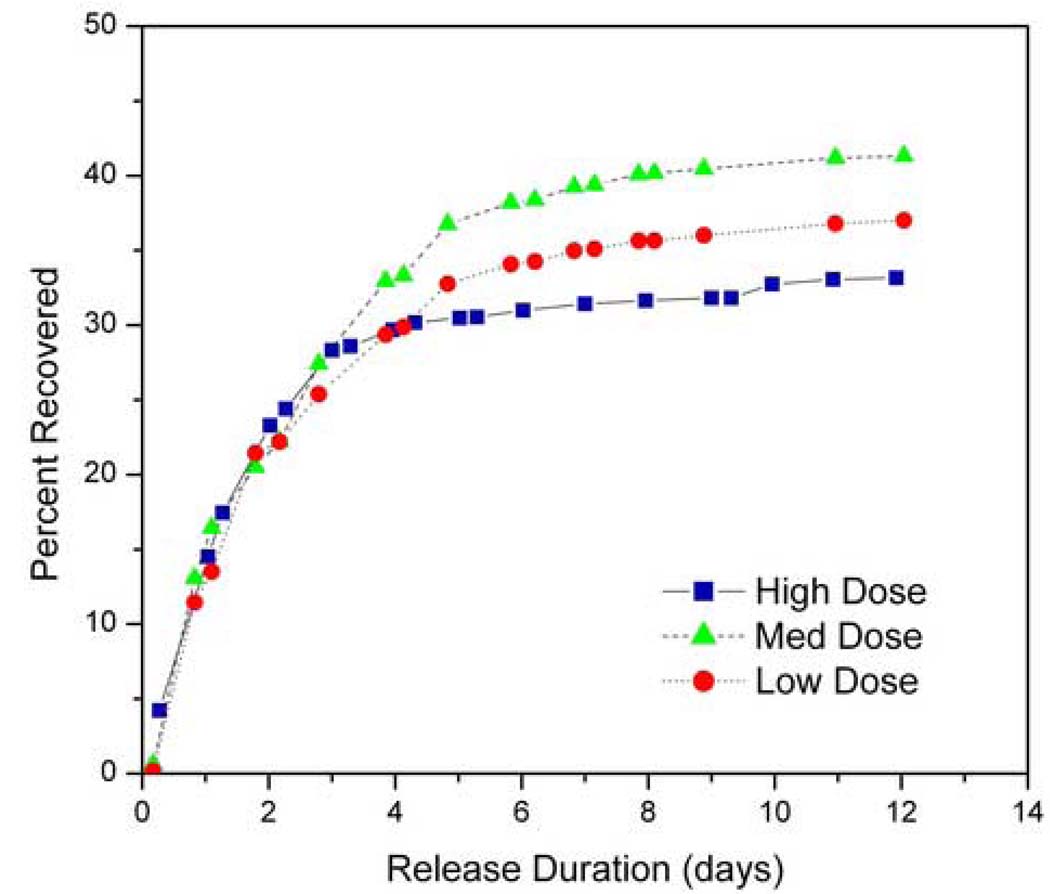
Microchips containing 0.17, 0.67 and 1.24 mg were implanted into the flank of 9L tumor-bearing rats. Animals receiving either no treatment or empty microchips were used as negative controls. The later group was included to account for possible effects due to the polymer platform itself. The urine from all animals in treatment groups (n=8 or 9) and selected animals in negative control groups (both n=2) was collected and the BCNU recovered in the urine was integrated over time (Figure 4 and Table 1). The two control groups showed no radioactivity as expected. The release kinetics from the three BCNU microchip groups were similar, reaching a plateau around day 6. An average of 37, 39, and 33% of the loaded radioactivity was recovered in the urine at 12 days after microchip implantation from the 0.17, 0.67, and 1.24 mg dose microchips, respectively. The urinary recovery shows that BCNU was released immediately upon implantation as was expected from the in vitro data.
Fig. 4.
BCNU release kinetics obtained from quantification of 14C-BCNU excreted in urine after device implantation, normalized by loading amount. There is no statistical difference among three different doses administered (0.17, 0.67, and 1.24 mg). No radioactivity is excreted from the negative controls (not shown).
Table 1.
Urinary recovery of 14C from rats with polymer microchips by Day 12 (n=8 for each group)
| BCNU dosage (mg) | Urinary recovery of 14C (% of initial loading) |
|---|---|
| 0.16* | 37±10 |
| 0.67* | 39±13 |
| 1.24* | 33±8 |
| 1.24** | 44±4 |
Data from dose escalation study
Data from comparison of microchip vs. wafer delivery
The amount of radioactivity recovered from the microchips after sacrifice was approximately 5% of the initial loading for the 0.17 mg dose microchips, and 10% for the 0.67 mg dose microchips. Data for the residual radioactivity in the 1.24 mg microchips were not available, but is presumed to be of the same order. The unaccounted radioactivity may be distributed in various tissues, feces, or expired as CO2(2) .
3.3 Microchip Delivery of BCNU Inhibits 9L Tumor Growth
A study was performed to examine if the BCNU delivered from the passive microchip retained its tumoricidal activity and to observe the effect of this delivery vehicle on tumor growth. The efficacy of three different doses of BCNU from the microchip was tested against the 9L gliosarcoma. Then, a side-by-side comparison of one dose in the microchip was tested against the wafer formulation with the same amount of BCNU.
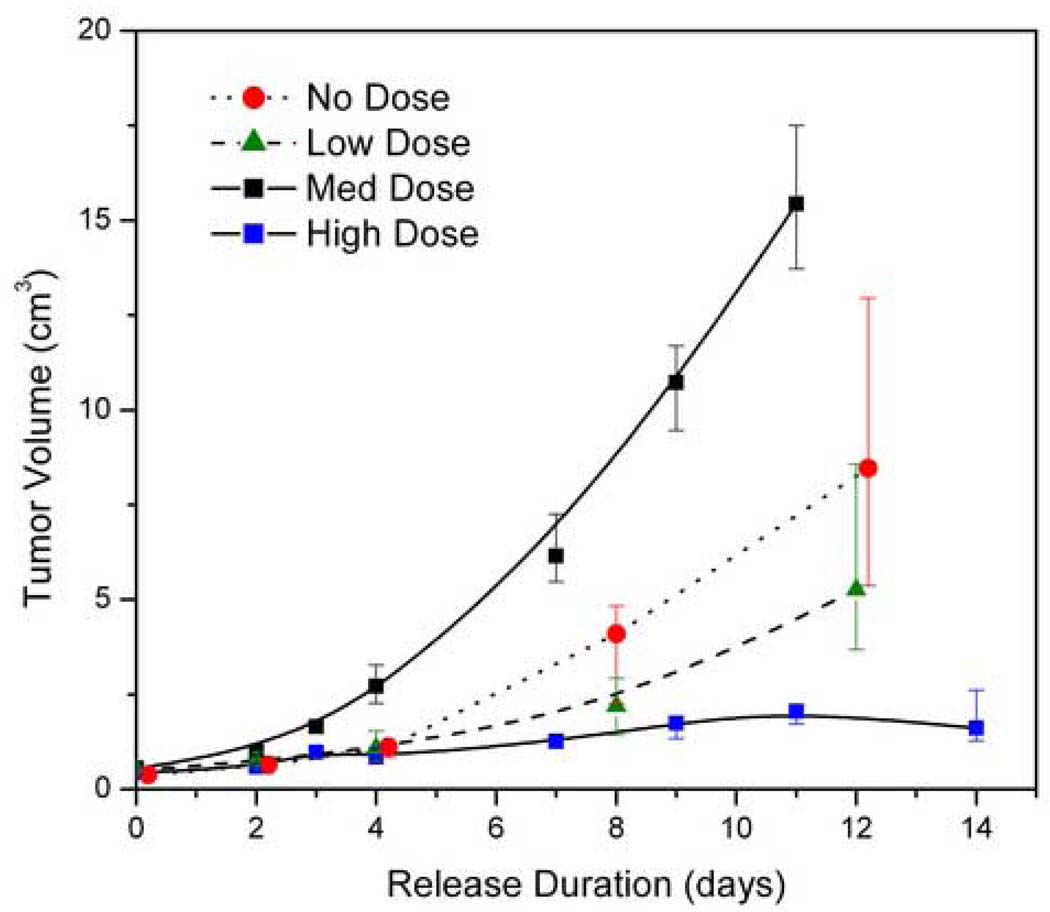
The resulting tumor growth data are shown in Figure 5 for the different treatment groups. No treatment, empty microchips, and empty wafers were used as negative controls to account for possible effects on the tumor growth or growth measurement due to the polymer delivery platform. Overall, the BCNU treatment groups showed a smaller median tumor size than the control groups that received either empty microchips or no treatment. The lowest doses (0.16 mg) showed no significant difference in tumor size from the empty control microchips (p=0.074). The difference was significant, however, for the 0.67 mg dose (p=0.016), and for the 1.24 mg dose (p=0.001), which showed markedly reduced tumor size. Median tumors sizes in treatment groups were compared to empty microchip control at 12 days. Tumors in the low and middle dose groups were 43% and 65% smaller. The tumors receiving high dose microchips were 87% smaller than untreated tumors. The 1.24 mg dose was considered the minimum effective dose and selected for subsequent study.
Fig. 5.
Dose response to BCNU from the polymer microchip. The high and medium doses showed statistical significance from the negative controls (p=0.001 and 0.016 respectively). The tumor reduction from the low dose was not statistically significant (p=0.074). The median values are plotted with error bars representing the 25th and 75th percentiles. Values for the medium dose were offset for clarity.
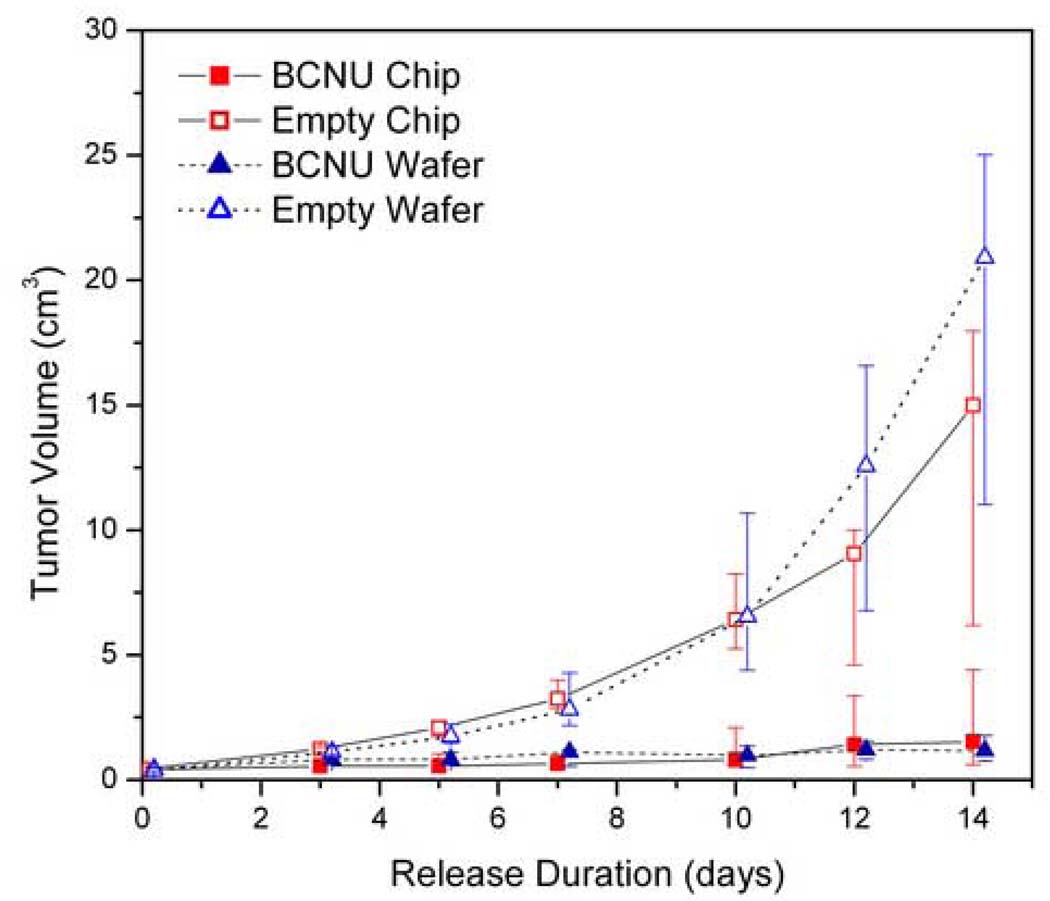
The efficacy of BCNU from the microchip was directly compared to that from a uniformly impregnated wafer (Figure 6). The minimum effective dose of 1.24 mg was microinjected into the polymer microchip or uniformly impregnated throughout the polymer wafers. Negative control groups included blank microchips and blank wafers. The negative control groups showed that the tumor grew by day 14 after microchip implantation to a median of 15.0 and 20.9 cm3 for the blank microchip and the blank wafer, respectively. In contrast, the tumors in the microchip treatment group grew only to a median of 1.5 cm3 and the wafer treatment group to 1.2 cm3. The microchip and the wafer produced significant tumor reduction compared to their respective empty microchip controls (p=0.032 and p=0.001 respectively). There was no significance in the difference in tumor sizes between the two treatment groups (p=0.156). These results show that release of BCNU from the microchip is as effective as release from the wafer.
Fig. 6.
Tumor response to the same loaded dose of BCNU from the polymer microchip and the polyanhydride wafer. Both microchip and wafer showed tumor reduction compared to their empty control (p=0.032 and p=0.001 respectively) and were not statistically different from each other (p=0.156). The median values are plotted with error bars representing the 25th and 75th percentiles. Values were offset for clarity.
4. DISCUSSION
This study demonstrates the in vivo efficacy for cancer treatment of a self-contained degradable microchip that provides precise spatial administration of chemotherapeutics. Specifically, the microchip was used to deliver BCNU against a tumor model and showed significant reduction in tumor growth (Figure 5). Tumors treated with the highest loaded microchip showed marked diminished growth compared to empty control microchips (p=0.001). The microchip was found comparable to that of the pCPP:SA wafer in release profile (Figure 3b) and efficacy in reducing tumor size. This demonstrates the first validation of proper microchip operation and efficacy in an in vivo model. Previously, only the in vitro activity of heparin released from the polymer microchip had been quantified(20), but no in vivo results had been demonstrated. A dose-dependent behavior was observed, with the highest dosage of BCNU delivering the strongest inhibiting effect on the tumor growth.
The release of the chemotherapeutic agent, BCNU, from the microchip starts on day 1 and continues for nearly 2 weeks (Figure 3). BCNU is released quickly from the polymer devices after implantation. This is necessary considering the hydrolytic instability of BCNU. The release profile of BCNU from the microchip is comparable to release from a uniformly impregnated pCPP:SA wafer (Figure 3b), which when released in rat brains at 37°C occurred over a period of approximately 5 days(27). Dang, et al, measured the amount of BCNU recovered from pCPP:SA wafer explants and subtracted that amount from the initial loading to calculate the amount released.
High-pressure liquid chromatography (HPLC) and a colorimetric assay using the Bratton-Marshall reagent can both be used to quantify BCNU (2, 26–28) . The Bratton-Marshall method was preferred for this study to quantify the intact BCNU molecule due to ability to process larger numbers of samples in parallel. Serial measurement of sample as in HPLC was considered undesirable BCNU has a short half-life in aqueous solution. BCNU was shown to have a half-life of 11 days when contained within the microchip at 37°C (Figure 2). This represents a substantial extension of the half-life compared to the free drug molecule in normal saline and 10% ethanol (t1/2= 7 and 10 minutes, respectively)(1, 2). BCNU is also more stable within the microchip compared to the wafer. At 48 hours, the microchip contains nearly twice as much intact BCNU as the wafer (70% and 38% respectively). BCNU acts by alkylating DNA, and it is known that its metabolites also have tumoricidal activity. Free BCNU powder dissolved in cell culture medium has been shown to be cytotoxic to XF498 cells after incubation for up to 12 hours, at which time the intact BCNU molecule is unmeasurable(28). The microchip can release BCNU or its metabolically active degradation products, but for long term, or repeat release, the ability of the microchip to enhance stability of BCNU may also translate to increased effectiveness of other unstable molecules, especially with the use of excipients or stabilizers.
BCNU is reported here to begin release from the 11 kDa PLGA membrane within minutes of incubation in PBS. Previous work from our group has reported release of dextran (MW 70000), human growth hormone (MW 21500), or heparin (MW 6000–20000) from the microchip with the same 11 kDa membrane from between 6 and 17 days in PBS at temperatures from 28–33°C(20). We believe this earlier release may have occurred for several reasons. All release profiles reported here were performed in a controlled 37°C incubator. BCNU is small (MW 214.1) and lipophilic compared to the model compounds used previously. It diffuses through the PLGA membrane and may facilitate swelling and opening of the membrane. This is supported by the observation that the membranes showed a visible swelling as early as a few hours after the BCNU-loaded microchips were submersed in PBS at 37°C, whereas swelling of the membranes in PBS at 28–33°C was observed later to coincide with the released of dextran(29).
The urinary recovery shows BCNU was released on day 1 of implantation (Figure 4) as was expected from the in vitro data. Approximately one third of the initial loading was recovered by day 12 with a majority being released by day 5 (Table 1). The rate of recovery, however, is slower than the rate of release in vitro, attributed to the pharmacokinetics of rodent renal clearance. The total urinary recovery is lower than values reported by other groups (78%, 55%)(30, 31), which used different style metabolic cages, animal models, and administration locations. The recovery reported here is consistent with data from our previous injected control groups and release kinetics measurements(21).
The results from this study show that release of BCNU from the microchip is as effective as release from a clinically approved homogenous polymer matrix. The pCCP:SA wafer with BCNU is widely used to treat brain tumor patients and is commercially available as the Gliadel® Wafer (16, 17). Gliadel® is FDA approved for the treatment of recurrent glioblastoma multiforme and newly diagnosed, high grade malignant gliomas. Six to eight wafers are placed in the cavity that remains after tumor resection, and the BCNU is released as the copolymer degrades. Gliadel® is now used routinely to improve survival in patients with malignant gliomas, but it is capable of delivering only one compound (17–19).
It is expected that delivery of a combination of synergistically acting chemotherapeutic agents can reduce tumor size with fewer side effects beyond that achieved by a monotherapy. Drug resistance and genetic profiling information(32) can be combined with the microchip to customize treatment of tumors. Future studies should exploit combination therapies and the synergistic action of potent combinations using additional anti-cancer drugs, such as temozolomide(33–35) to achieve greater cytotoxicity with lower drug amounts. The use of solid dosage forms allows the microchip to potentially achieve smaller device dimensions and higher drug:polymer ratios than polymer matrix formulations. An optimized reservoir shape and configuration can deliver the same amount of drug with one tenth less polymer. This represents a solid foundation for improvement in treatments of diseases that can benefit from precise administration of multiple drug cocktails from a self-contained degradable microchip.
5. CONCLUSION
BCNU delivered from the polymer microchip inhibited the tumor growth of the 9L glioma in rats. The inhibiting effect on tumor growth was dose-dependent. These results are the first demonstration of in vivo efficacy of a polymer microchip brain cancer therapy. The efficacy validation of this platform as well as important dose response information indicates further evaluation of combination chemotherapy using a polymer platform with multiple reservoirs.
ACKNOWLEDGMENTS
This work was funded by U.S. National Institute of Health grant No. 1 R24 AI47739, the National Science Foundation Graduate Research Fellowship (GK), and a Natural Sciences and Engineering Research Council of Canada Postdoctoral Scholarship (JMK). All in vivo experiments were carried out at the Johns Hopkins Animal Care Facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURE
Michael Cima and Robert Langer are shareholders in MicroCHIPS, Inc., Bedford, Massachusetts. Henry Brem and the Johns Hopkins University are entitled to a share of royalty by MGI Pharma, Inc. on potential sales of some products that are in development. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest polices. Dr. Brem’s laboratory is, and has been, supported by funding from Guilford Pharmaceuticals, Inc., MacroMed, Inc., and MGI Pharma, along with grants from the National Cancer Institute (NCI) and the National Institutes of Health (NIH).
REFERENCES
- 1.Tepe P, Hassenbusch SJ, Benoit R, Anderson JH. BCNU stability as a function of ethanol concentration and temperature. J Neurooncol. 1991;10:121–127. doi: 10.1007/BF00146872. [DOI] [PubMed] [Google Scholar]
- 2.Kari P, McConnell WR, Finkel JM, Hill DL. Distribution of Bratton-Marshall-positive material in mice following intravenous injections of nitrosoureas. Cancer Chemother Pharmacol. 1980;4:243–248. doi: 10.1007/BF00255268. [DOI] [PubMed] [Google Scholar]
- 3.Legler JM, Ries LA, Smith MA, Warren JL, Heineman EF, Kaplan RS, Linet MS. Cancer surveillance series [corrected]: brain and other central nervous system cancers: recent trends in incidence and mortality. J Natl Cancer Inst. 1999;91:1382–1390. doi: 10.1093/jnci/91.16.1382. [DOI] [PubMed] [Google Scholar]
- 4.Gloeckler Ries LA, Reichman ME, Lewis DR, Hankey BF, Edwards BK. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist. 2003;8:541–552. doi: 10.1634/theoncologist.8-6-541. [DOI] [PubMed] [Google Scholar]
- 5.Lagerwaard FJ, Levendag PC. Prognostic factors in patients with brain metastases. Forum (Genova) 2001 Jan-Mar;:27–46. [PubMed] [Google Scholar]
- 6.Surawicz TS, Davis F, Freels S, Laws ER, Jr., Menck HR. Brain tumor survival: results from the National Cancer Data Base. J Neurooncol. 1998;40:151–160. doi: 10.1023/a:1006091608586. [DOI] [PubMed] [Google Scholar]
- 7.Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: a Medical Research Council trial. J Clin Oncol. 2001;19:509–518. doi: 10.1200/JCO.2001.19.2.509. [DOI] [PubMed] [Google Scholar]
- 8.Chae GS, Lee JS, Kim SH, Seo KS, Kim MS, Lee HB, Khang G. Enhancement of the stability of BCNU using self-emulsifying drug delivery systems (SEDDS) and in vitro antitumor activity of self-emulsified BCNU-loaded PLGA wafer. Int J Pharm. 2005;301:6–14. doi: 10.1016/j.ijpharm.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 9.Gallia GL, Brem S, Brem H. Local treatment of malignant brain tumors using implantable chemotherapeutic polymers. J Natl Compr Canc Netw. 2005;3:721–728. doi: 10.6004/jnccn.2005.0042. [DOI] [PubMed] [Google Scholar]
- 10.Giussani C, Carrabba G, Pluderi M, Lucini V, Pannacci M, Caronzolo D, Costa F, Minotti M, Tomei G, Villani R, Carroll RS, Bikfalvi A, Bello L. Local intracerebral delivery of endogenous inhibitors by osmotic minipumps effectively suppresses glioma growth in vivo. Cancer Res. 2003;63:2499–2505. [PubMed] [Google Scholar]
- 11.Hamstra DA, Moffat BA, Hall DE, Young JM, Desmond TJ, Carter J, Pietronigro D, Frey KA, Rehemtulla A, Ross BD. Intratumoral injection of BCNU in ethanol (DTI-015) results in enhanced delivery to tumor--a pharmacokinetic study. J Neurooncol. 2005;73:225–238. doi: 10.1007/s11060-004-5675-2. [DOI] [PubMed] [Google Scholar]
- 12.Lesniak MS, Brem H. Targeted therapy for brain tumours. Nat Rev Drug Discov. 2004;3:499–508. doi: 10.1038/nrd1414. [DOI] [PubMed] [Google Scholar]
- 13.Mardor Y, Rahav O, Zauberman Y, Lidar Z, Ocherashvilli A, Daniels D, Roth Y, Maier SE, Orenstein A, Ram Z. Convection-enhanced drug delivery: increased efficacy and magnetic resonance image monitoring. Cancer Res. 2005;65:6858–6863. doi: 10.1158/0008-5472.CAN-05-0161. [DOI] [PubMed] [Google Scholar]
- 14.Quinn JA, Desjardins A, Weingart J, Brem H, Dolan ME, Delaney SM, Vredenburgh J, Rich J, Friedman AH, Reardon DA, Sampson JH, Pegg AE, Moschel RC, Birch R, McLendon RE, Provenzale JM, Gururangan S, Dancey JE, Maxwell J, Tourt-Uhlig S, Herndon JE, 2nd, Bigner DD, Friedman HS. Phase I trial of temozolomide plus O6-benzylguanine for patients with recurrent or progressive malignant glioma. J Clin Oncol. 2005;23:7178–7187. doi: 10.1200/JCO.2005.06.502. [DOI] [PubMed] [Google Scholar]
- 15.Rhines LD, Sampath P, DiMeco F, Lawson HC, Tyler BM, Hanes J, Olivi A, Brem H. Local Immunotherapy with Interleukin-2 Delivered from Biodegradable Polymer Microspheres Combined with Interstitial Chemotherapy: A Novel Treatment for Experimental Malignant Glioma. Neurosurgery. 2003;52:872–879. doi: 10.1227/01.neu.0000053211.39087.d1. [DOI] [PubMed] [Google Scholar]
- 16.Gliadel (R) Wafer (polifeprosan 20 with Carmustine implant), P. I. Baltimore, MD: Guilford Pharmaceuticals; 2003. [Google Scholar]
- 17.Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G, Muller P, Morawetz R, Schold SC. Placebo-controlled Trial of Safety and Efficacy of Intraoperative Controlled Delivery by Biodegradable Polymers of Chemotherapy for Recurrent Gliomas. Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 18.Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jaaskelainen J, Ram Z. A Phase 3 Trial of Local Chemotherapy with Biodegradable Carmustine (BCNU) Wafers (Gliadel Wafers) in Patients with Primary Malignant Glioma. Neuro-Oncology. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valtonen S, Timonen U, Toivanen P, Kalimo H, Kivipelto L, Heiskanen O, Unsgaard G, Kuurne T. Interstitial Chemotherapy with Carmustine-loaded Polymers for High-grade Gliomas: A Randomized Double-blind Study. Neurosurgery. 1997;41:44–48. doi: 10.1097/00006123-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Grayson ACR, Choi IS, Tyler BM, Wang PP, Brem H, Cima MJ, Langer R. Multi-pulse Drug Delivery from a Resorbable Polymeric Microchip Device. Nature Materials. 2003;2:767–772. doi: 10.1038/nmat998. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Shawgo RS, Tyler B, Henderson PT, Vogel JS, Rosenberg A, Storm PB, Langer R, Brem H, Cima MJ. In vivo release from a drug delivery MEMS device. J Control Release. 2004;100:211–219. doi: 10.1016/j.jconrel.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Giese A, Kucinski T, Knopp U, Goldbrunner R, Hamel W, Mehdorn HM, Tonn JC, Hilt D, Westphal M. Pattern of recurrence following local chemotherapy with biodegradable carmustine (BCNU) implants in patients with glioblastoma. J Neurooncol. 2004;66:351–360. doi: 10.1023/b:neon.0000014539.90077.db. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Ho Duc HL, Tyler B, Williams T, Tupper M, Langer R, Brem H, Cima MJ. In vivo delivery of BCNU from a MEMS device to a tumor model. J Control Release. 2005;106:138–145. doi: 10.1016/j.jconrel.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Prescott JH, Lipka S, Baldwin S, Sheppard NF, Jr., Maloney JM, Coppeta J, Yomtov B, Staples MA, Santini JT., Jr. Chronic, programmed polypeptide delivery from an implanted, multireservoir microchip device. Nat Biotechnol. 2006;24:437–438. doi: 10.1038/nbt1199. [DOI] [PubMed] [Google Scholar]
- 25.Tamargo RJ, Myseros JS, Epstein JI, Yang MB, Chasin M, Brem H. Interstitial Chemotherapy of the 9L Gliosarcoma: Controlled Release Polymers for Drug Delivery in the Brain. Cancer Research. 1993;53:329–333. [PubMed] [Google Scholar]
- 26.Loo TL, Dion RL. Colorimetric Method for the Determination of 1,3-Bis(2-chloroethyl)-1-nitrosourea. Journal of Pharmaceutical Science. 1965;54:809–810. doi: 10.1002/jps.2600540538. [DOI] [PubMed] [Google Scholar]
- 27.Dang W, Daviau T, Ying P, Zhao Y, Nowotnik D, Clow CS, Tyler B, Brem H. Effects of GLIADEL® wafer initial molecular weight on the erosion of wafer and release of BCNU. Journal of Controlled Release. 1996;42:83–92. [Google Scholar]
- 28.Seong H, An TK, Khang G, Choi SU, Lee CO, Lee HB. BCNU-loaded Poly(D,L-lactide-co-glycolide) Wafer and Antitumor Activity Against XF-498 Human CNS Tumor Cells InVitro. International Journal of Pharmaceutics. 2003;251:1–12. doi: 10.1016/s0378-5173(02)00543-4. [DOI] [PubMed] [Google Scholar]
- 29.Grayson ACR, Cima MJ, Langer R. Molecular release from a polymeric microreservoir device: Influence of chemistry, polymer swelling, and loading on device performance. Journal of Biomedical Materials Research Part A. 2004;69A:502–512. doi: 10.1002/jbm.a.30019. [DOI] [PubMed] [Google Scholar]
- 30.DeVita VT, Denham C, Davidson JD, Oliverio VT. The physiological disposition of the carcinostatic 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) in man and animals. Clinical pharmacology and therapeutics. 1967;8:566–577. doi: 10.1002/cpt196784566. [DOI] [PubMed] [Google Scholar]
- 31.Domb A, Rock M, Schwartz J, Perkin C, Yipchuck G, Broxup B, Villemure JG. Metabolic disposition and elimination studies of a radiolabelled biodegradable polymeric implant in the rat brain. Biomaterials. 1994;15:681–688. doi: 10.1016/0142-9612(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 32.Leuraud P, Taillandier L, Medioni J, Aguirre-Cruz L, Criniáere E, Marie Y, Kujas M, Golmard JL, Duprez A, Delattre JY, Sanson M, Poupon MF. Distinct responses of xenografted gliomas to different alkylating agents are related to histology and genetic alterations. Cancer research. 2004;64:4648–4653. doi: 10.1158/0008-5472.CAN-03-3429. [DOI] [PubMed] [Google Scholar]
- 33.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 34.Gururangan S, Cokgor L, Rich JN, Edwards S, Affronti ML, Quinn JA, Herndon JE, 2nd, Provenzale JM, McLendon RE, Tourt-Uhlig S, Sampson JH, Stafford-Fox V, Zaknoen S, Early M, Friedman AH, Friedman HS. Phase I study of Gliadel wafers plus temozolomide in adults with recurrent supratentorial high-grade gliomas. Neuro-oncol. 2001;3:246–250. doi: 10.1093/neuonc/3.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brem S, Tyler B, Li K, Pradilla G, Legnani F, Caplan J, Brem H. Local Delivery of Temozolomide by biodegradable polymers is superior to oral administration in a rodent glioma model. Cancer Chemother Pharmacol. 2007 doi: 10.1007/s00280-006-0407-2. [DOI] [PubMed] [Google Scholar]