Abstract
Three experiments with rats compared the relative ease with which different sets of visual or temporal cues could participate in Pavlovian learning. In Experiment 1, one group was trained to discriminate between visual cues (Light vs Dark), whereas the other group learned to discriminate between temporal cues (early (10 s) versus late (90 s)). Both groups learned to distinguish food-paired from non-paired periods equally well. In Experiment 2, two groups were trained on an ambiguous occasion setting task. For Group Visual, a 2-min Light period signaled that one 10-s auditory conditioned stimulus, CS1, was reinforced with one unconditioned stimulus, US1, but that CS2 was not reinforced; whereas a 2-min dark period signaled that CS1 was not reinforced, but CS2 was reinforced with US2 (i.e., Light: CS1−US1, CS2−; Dark: CS1−, CS2–US2). For Group Temporal, early (10-s) or late (90-s) temporal cues within each of these Light and Dark periods were diagnostic of these contingencies (i.e., Early: CS1–US1, CS2−; Late: CS1−, CS2–US2). Group Visual learned the task, but Group Temporal did not. In Experiment 3 we demonstrated that animals could not solve a related temporal ambiguous occasion setting task in which one visual stimulus signaled that both CSs were reinforced early whereas the other visual stimulus signaled that the CSs were reinforced only late. Contrary to a currently popular information theory approach to timing in Pavlovian learning, these results suggest that overt non-temporal visual stimuli are better incorporated into conditional discrimination learning than are temporal stimuli.
Keywords: Conditional discrimination, differential outcome, interval timing
A fundamental problem for theories of Pavlovian learning continues to be the integration of interval timing and associative processes. Major theories of associative learning have traditionally had little to say about how interval timing works, and major theories of timing have traditionally focused on problems other than those that occupy the attention of association theorists. Exceptions to this general statement exist, but the current state of affairs still lacks a clear consensus on exactly how associative and timing processes might interact.
One notable attempt to bridge these two domains was made by Miller and his colleagues in the context of the “temporal coding hypothesis” (Arcediano & Miller, 2002; Matzel, Held, & Miller, 1988; Miller & Barnet, 1993; Polack, Molet, Miguez, & Miller, 2013). Briefly, this approach suggests that the time between conditioned and unconditioned stimuli (CS, US, respectively) is encoded as part of the underlying association between them. This temporal associative content enables stimuli trained with different temporal parameters to become integrated within a “temporal map” when combined in novel ways. For instance, in a backward conditioning procedure (where US precedes CS) very little evidence of learning to the target CS is commonly revealed. However, if a second order stimulus, CS2, is then sequentially paired with the backward first-order stimulus, CS1, conditioned responding emerges to the second order CS2, in spite of the fact that CS1 itself fails to elicit a conditioned response (Barnet, Cole, & Miller, 1997; see also Arcediano, Escobar, & Miller, 2003; Taylor, Joseph, Zhao, & Balsam, 2014). This result can be understood if the temporal relations become part of the underlying associative circuitry. In particular, CS2 comes to predict CS1 whose temporal relation with the US places it in a favorable position to support responding to CS2.
While this account has led to a number of experiments that have generally produced results in support of the temporal map idea, the notion that the temporal relation between CS and US becomes part of the underlying associative content raises the question of exactly how this can be accomplished within an associative architecture (Miller & Barnet, 1993). Perhaps for this reason, Balsam and Gallistel have suggested a different approach by proposing that when organisms process events within a normal conditioning procedure, those events are directly stored within a temporal memory system (Balsam & Gallistel, 2009; Balsam, Drew, & Gallistel, 2010; Gallistel & Balsam, 2014). Decisions whether or not to respond to a CS are based on various computations performed on this underlying temporal memory structure. For example, if the CS provides information that reduces the overall temporal uncertainty concerning the occurrence of the US, then the CS will provoke conditioned responding (for related accounts see, Gallistel & Gibbon, 2000; Gibbon & Balsam, 1981; Jenkins, Barnes, and Berrara, 1981; Miller & Schachtman, 1985). The model goes on to assert that by storing CS and US events within a temporal memory structure, information about the specific temporal interval between the CS and US is readily available and can be utilized to produce relatively accurate CR timing functions as well as temporal map integrations of the form noted above.
It is important to note that this approach assigns no role to an associative process in explaining temporally controlled behaviors, and, in the extreme, such a view expressly denies that associative learning processes, as traditionally understood, play any role in a complete learning theory (Gallistel & Balsam, 2014). However, while attractive to some, this view that all learning phenomena can be understood within this temporal memory framework is questionable. For one thing, variables traditionally thought to be critical for the establishment of learning, such as reward magnitude or number of conditioning trials, are claimed to be unimportant according to this view (e.g., Gallistel & Balsam, 2014). However, these claims are not well substantiated in the literature (e.g., Gottlieb & Rescorla, 2010; Morris & Bouton, 2006; Rescorla & Wagner, 1972; but see Gottlieb, 2008).
A more conventional approach describes learning in terms of time-independent memory representations of the key relationships between the events (e.g., Delamater, 2012, Harris, Gharaei, & Moore, 2009). For instance, consider a context-based discrimination in which target stimulus X is reinforced and Y nonreinforced in Context A, but these contingencies are switched in Context B. A traditional approach emphasizes the time-independent relationships among the stimuli in terms of the associations that may form among the contexts, the stimuli, and their relationships with reward or non-reward. Time is generally assumed to factor into a traditional associative approach as a necessary condition for the establishment of associative links among events, rather than serving as the basis of how memories of events within the learning situation are encoded.
It seems to us that one question at the heart of this issue is whether the memory structures that support Pavlovian learning are defined entirely in terms of temporal relations (as implied by the temporal information theory approach), or whether time-independent memory structures more accurately describe the learning process (as suggested by a more traditional associative approach). To date, this question has not been adequately addressed in the literature. One way in which this issue can be studied experimentally is by examining the role of temporal information in learning complex conditional relations, similar to the switching task illustrated above. If events are represented in terms of their time of occurrence, then animals should be able to learn a complex conditional discrimination that can be solved with temporal conditional cues, and this ought to be learned at least as well, if not better, than a comparable discrimination that can be solved only with non-temporal conditional cues. Indeed, there are examples of animals utilizing circadian cues or time in session cues to conditionally control performance (e.g., Crystal, 2010; Iordanova, Good, & Honey, 2011; Thorpe & Wilkie, 2006; Zhou & Crystal, 2009), but a direct comparison between the relative ease by which temporal versus non-temporal cues can acquire this conditional function has not been studied.
Consider an ambiguous occasion setting task (Delamater, Kranjec, & Fein, 2010; Holland, 1991; Holland & Reeve, 1991; Nakajima, 1998). In this situation, one target stimulus, CS1, is reinforced when presented along with an occasion setting “feature” stimulus, but not when presented on its own. Conversely, a second target stimulus, CS2, is reinforced when presented by itself, but not when presented along with the same feature stimulus. The feature stimulus in this task is said to become a positive occasion setter for CS1 but a negative occasion setter for CS2, and this is what makes it an “ambiguous” occasion setting stimulus (it has dual positive and negative occasion setting properties). Delamater, Kranjec, and Fein (2010) showed that rats were capable of learning this task in a magazine approach conditioning paradigm, but only when different outcomes were assigned to the two target stimuli (for a theoretical treatment of this effect see also, Delamater, 2012). In this task, 2-min periods of Light were alternated with 2-min periods of Dark. Embedded within each 2-min period were 10-s auditory stimuli (tone or noise), and whether these were reinforced with a US was conditional upon the background visual feature stimulus (i.e., Light: CS1-US1, CS2−; Dark: CS1−, CS2-US2).
This sort of problem lends itself well to a solution in terms of non-temporal memory structure of the following form: CS1 only leads to US1 in the Light, but CS2 only leads to US2 in the Dark. Here, we ask whether temporal cues can be used to solve such a task if they were to function as occasion setting features. Specifically, we compared the relative ease of solving this sort of ambiguous occasion setting task when temporal versus visual cues functioned as occasion setting feature stimuli.
Table 1 illustrates basic experimental designs (used in Experiments 2 and 3) that contrasts ambiguous occasion setting tasks that use visual versus temporal cues as feature stimuli. In all of these tasks, the two auditory target stimuli (CS1, CS2) only occur at 10 s or 90 s after onset of each visual period (Light or Dark). For Group Visual, the visual stimulus functions as the occasion setting feature stimulus, whereas the temporal cues (10 s versus 90 s) are non-informative. What matters is that CS1-US1 pairings occur in the presence of Light, but not Dark, and that CS2-US2 pairings occur in the presence of Dark, but not Light. In contrast, for Group Temporal in both Experiment 2 and 3, the Early or Late temporal cues (10 s versus 90 s) function as the occasion setting stimuli that conditionally predict reinforcement of the CSs. Specifically, in Experiment 2, CS1-US1 pairings occur whenever CS1 is presented Early in either Light or Dark, but not when presented Late in Light or Dark, and CS2-US2 pairings occur whenever CS2 is presented Late, but not Early, in either Light or Dark. Similarly, in Experiment 3 the CSs presented early in the Light are reinforced with their respective USs, but those occurring Late are not reinforced, whereas in the Dark only CSs presented Late are reinforced. In other words, these problems have a similar formal structure but what differs is whether visual or temporal feature stimuli play the diagnostic role of occasion setting stimuli.
Table 1.
Experimental Designs Used in Experiments 1–3
| Visual Discrimination Task (Experiment 1) | Temporal Discrimination Task (Experiment 1) | ||||
| 20 s | 100 s | ||||
|
|
|||||
| Light: | Pellets (Random times) | Light: | Pellet | No Pellets | |
| Dark: | No Pellets | Dark: | Pellet | No Pellets | |
| or | or | ||||
| Light: | No Pellets | Light: | No Pellets | Pellet | |
| Dark: | Pellet (Random times) | Dark: | No Pellets | Pellet | |
| Visual Occasion Setting Task (Experiments 2 & 3) | Temporal Occasion Setting Task (Experiment 2) | ||||
| 10 s | 90 s | 10 s | 90 s | ||
|
|
|
||||
| Light: | CS1-US1 | CS1-US1 | Light: | CS1-US1 | CS1- |
| Light: | CS2− | CS2− | Light: | CS2− | CS2-US2 |
| Dark: | CS1− | CS1− | Dark: | CS1-US1 | CS1− |
| Dark: | CS2-US2 | CS2-US2 | Dark: | CS2− | CS2-US2 |
| Temporal Occasion Setting Task (Experiment 3) | |||||
| 10 s | 90 s | ||||
|
|
|||||
| Light: | CS1-US1 | CS1− | |||
| Light: | CS2-US2 | CS2− | |||
| Dark: | CS1− | CS1-US1 | |||
| Dark: | CS2− | CS2-US2 | |||
Note: Light and Dark stimuli were each 2 min long, and CS1 and CS2 were 10-s auditory stimuli (tone, noise, counterbalanced). US1 and US2 were pellet and liquid sucrose rewards
If events are fundamentally encoded within a temporal memory structure, the temporal version of this task should be easily learned. Indeed, learning the temporal occasion setting problem should be at least as easy as learning the visual occasion setting problem, if not easier, assuming that the animal can distinguish between the 10- and 90-s temporal cues as well as they can distinguish between the Light and Dark visual cues. On the other hand, if events are fundamentally encoded in terms of some non-temporal memory structure (as implied by a traditional associative approach), then the ability to learn these tasks would only depend upon the relative discriminability of the occasion setting stimuli (10 s versus 90 s, and Light versus Dark) because there would be no special status given to temporal or visual cues to participate in learning the problems. The present set of studies explored these ideas. In Experiment 1, we set out to determine the relative discriminability of 10-s (Early) and 90-s (Late) temporal cues compared to Light versus Dark. Experiment 2 assessed the ability of these temporal or visual cues to function as occasion setting stimuli in the task described above. Experiment 3 then explored the ability of temporal cues to function as occasion setting stimuli in a variant of the temporal task used in Experiment 2.
Experiment 1
Before any firm conclusions can be reached concerning the relative ease with which ambiguous occasion setting tasks can be learned on the basis of visual versus temporal feature stimuli, we must first establish that the particular visual and temporal stimuli to be used are equally discriminable to the organism. The aim of this first experiment was to determine if the rats could learn to discriminate between 10-s and 90-s temporal cues at least as well as they could between Light versus Dark in a simple discrimination procedure.
Different groups of rats were trained to associate food with one of the visual or with one of the temporal stimuli (see Table 1). Specifically, for rats in Group Visual, 2-min periods of Light alternated with 2-min periods of Dark, and food was delivered randomly in time within one of these periods (counterbalanced across different sub-groups). Magazine entries during these periods were compared on non-reinforced probe trials to measure the discriminability of these two visual cues. Rats in Group Temporal were also presented with 2-min periods of Light alternating with 2-min periods of Dark, but here food was delivered at a fixed time-point after both Light and Dark onsets. Half the rats received pellets 20 s after onset of Light and Dark, while the other half received it at 100 s. Here too, each session contained non-reinforced probe trials used to compare magazine entries in the 10-s intervals just preceding each of these Early vs Late time-points (i.e., starting at 10 and 90 s following Light or Dark onset). The difference in responding between these two intervals was taken as a measure of the discriminability of the 10- versus 90-s temporal cues.
Method
Subjects
Subjects were 32, experimentally naïve, male (16) and female (16) Long-Evans rats bred at Brooklyn College, but derived from breeders obtained from Charles River Laboratories. The free-feeding body weights varied between 476 and 861 g for males and between 352 and 527 g for females at the beginning of the experiment. Rats were individually housed in wire mesh cages in a colony room that was on a 14-hr light/10-hr dark cycle. Rats were maintained at 85% of their free-feeding body weights by daily supplemental feedings provided following the final experimental session of the day. Subjects were trained and tested during the light phase of their light/dark cycle.
Apparatus
The same apparatus was used as reported in Delamater and Holland (2008). It consisted of two sets of eight identical standard conditioning chambers, each of which was housed in a sound- and light-resistant shell. The conditioning chambers measured 30.5 cm × 24.0 cm × 25.0 cm. Two end walls were constructed of aluminum, and the sidewalls and ceiling were made from clear Plexiglas. The floor consisted of 0.60-cm diameter stainless steel rods spaced 2.0 cm apart. In the center of one end wall 1.2 cm above the grid floor was a recessed food magazine measuring 3.0 × 3.6 × 2.0 cm (length × width × depth). Two 45-mg pellets (TestDiet, MLab rodent tablet) were dropped into the magazine when US delivery was scheduled. In Experiments 2 and 3, a 0.1 ml droplet of a 20% sucrose solution (weight/volume) was delivered through a gravity-feed valve (ASCO Red-Hat valve) directly into a well located at the base of the magazine when this US was scheduled. On the inner walls of the recessed magazine were an infrared emitter and detector enabling the automatic recording of movements inside the magazine. These were located 0.9 cm above the magazine floor and 0.8 cm recessed from the front wall. Located 3.0 cm to the right of the magazine and 8.0 cm above the floor was a response lever (4 cm in width). This lever was not used in these experiments, and access to it was prevented by a sheet metal covering. A 6-W light bulb was mounted on the top portion of the rear wall of the outer chamber, above and towards the back of the conditioning chamber. A speaker was mounted 22 cm behind the front wall of the conditioning chamber (on the same side as the food magazine), and was used to present a white noise stimulus (produced by a Grason-Stadler white noise generator, 12 dB above a background level of 78 dB - C weighting). A second speaker was positioned next to the first speaker and this was used to present a computer-generated 1500 Hz pure tone stimulus (4 dB above background sound levels). These auditory stimuli were used in Experiments 2 and 3 only. The chamber was dark except when the visual stimulus was presented. A fan attached to the outer shell provided cross-ventilation within the shell as well as background noise. All experimental events were controlled and recorded automatically by a Pentium-based PC and interfacing equipment (Alpha Products) located in the same room.
Procedure
The rats were initially magazine trained for two days with the pellet US (as well as with a 20% liquid sucrose US (0.1ml) that was not used in the remainder of this study). On each of the two days, rats were run in a 40-min magazine training session in which one of the USs was randomly presented 20 times within the first 20 min and the second US was randomly presented 20 times in the second half of the session. Each US delivery consisted of two 45-mg pellets. These training sessions occurred in the dark.
Pavlovian Conditioning
Over the next 32 days, all rats received Pavlovian conditioning sessions in which a 2-min Light stimulus strictly alternated with 2 min of Dark. In each 48-min session there were 12 periods of Light alternating with 12 periods of Dark. The visual period with which each session began varied across sessions. Rats were randomly assigned to two groups (n=16 per group) matched on sex. For Group Visual, USs were delivered on a random time 60-s schedule (RT 60) during one of the visually distinct periods, but no USs were delivered during the other period. In this way, food was equally likely to occur throughout the appropriate visual period across each session. However, note that it was possible for a subject to receive two or more US deliveries during a single trial, but there was no constraint forcing a minimum of two US presentations per trial. For one subset of Group Visual, USs were delivered only in Light periods and for a second subset USs were presented only in Dark periods. For subjects in Group Temporal one US was delivered at a fixed time-point following the onset of each visually distinct period. For one subset of Group Temporal, US delivery occurred only 20 s (Early) after the onset of Light and Dark, but never at other times. For a second subset of Group Temporal rats, the US delivery occurred 100 s (Late) after the onset of Light and Dark, but never at other times. Thus, Group Visual rats learned to discriminate Light from Dark, whereas Group Temporal learned to discriminate Early versus Late temporal cues in both Light and Dark periods. These different groups and subgroups were counterbalanced for sex.
Each session consisted of 12 presentations of Light and Dark, but 2 presentations were pseudo-randomly assigned as non-reinforced probe trials during which magazine responding was uncontaminated by the presence of the US. In Group Visual, responding during probe Light and Dark periods was compared to provide a measure of how well the two visually distinct periods could be discriminated. In Group Temporal, on these non-reinforced probe trials, responding was compared between the 10-s intervals preceding the Early (20 s) versus Late (100 s) time-points. For half the subjects US delivery was expected at 20 s, but not 100 s and vice versa for the remaining animals. Thus the difference in responding between these time-points constituted the measure of temporal discrimination between these temporal cues. If discrimination is acquired, then magazine response rates should be greater in the presence of the visual or temporal cue that was previously paired with food pellets than the other corresponding periods. Note that rats in the two groups, Visual and Temporal, were matched in terms of the overall number of USs they received in each training session (i.e., 20).
Statistical Analysis
The data were analyzed using analysis of variance (ANOVA) techniques recommended by Rodger (1974; 1975). Details of the methods can be found in the Appendix, but, briefly, Rodger’s (1975) method entails analyzing data from factorial designs (e.g., with I and J factors) in terms of a one-way design (with I × J levels). Significant F scores are then examined with a set of ν1 linearly independent and mutually orthogonal contrasts selected post-hoc to reveal differences among the various conditions. As a result of these post-hoc analyses two estimates of effect size are computed. First, the method provides an estimate of the noncentrality parameter (Δ) of the non-central F distribution (i.e., the distribution that occurs when the null hypothesis of no group differences is false). This can be thought of as a measure of the overall amount of variation among the population means comprising the conditions being analyzed – numbers larger than 0 reflect increasing amounts of overall variation among the conditions being analyzed. Here, in addition to reporting F scores, we also report our estimate of Δ for each significant F score. The second measure of effect size consists of an estimate of the population means, themselves, implied by the various decisions reached for each of the post-hoc contrasts within the linearly independent set of contrasts. These implied means are computed as a relative measure, μj – μ., where μj refers to a single population mean and μ. refers to the mean of all of the population means. Importantly, these implied means are expressed in σ units (like Cohen’s d (Cohen, 1988)). A difference between two implied means of 1 σ unit, for instance, can be interpreted to reflect a rather large difference between the corresponding population means. Here, in addition to plotting our data in terms of the empirical means, we also report the computed values of the implied population means based on our statistical analysis to provide a more complete quantitative assessment of the various effect sizes for the differences among all of the means that comprise the analysis.
This method of analysis was chosen over others because it is the most comprehensive and powerful ANOVA technique at detecting true effects (see also Rodger & Roberts, 2013). Furthermore, use of these methods can ensure that the expected rate of rejecting true null contrasts in error, Eα, can be fixed by the experimenter and in the present studies the critical value was set to Eα = 0.05. Moreover, with Rodger’s methods the sample sizes used in these studies were chosen to ensure that the expected rate at detecting moderately sized true effects, Eβ, is at least 0.95. All of the statistical techniques used here can be performed with a publically available software package, Simple Powerful Statistics (see also Roberts, 2011), downloadable from the following website: https://sites.google.com/site/spsprogram/home.
Results
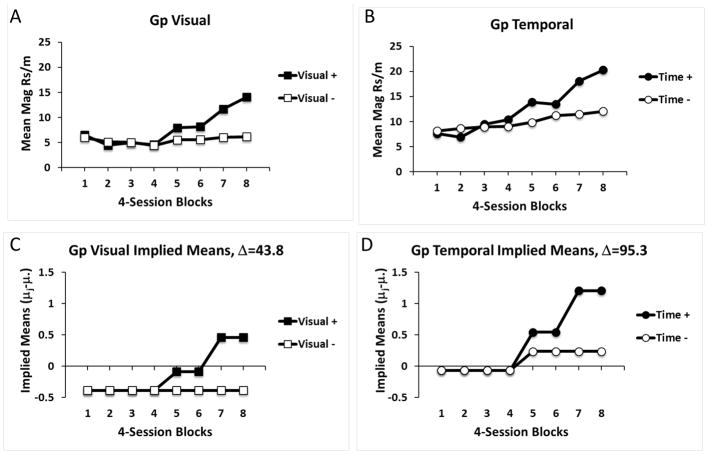
The magazine response data from non-reinforced probe trials in 4-session blocks are presented in Figure 1 for both Group Visual and Group Temporal. The data have been combined over both counterbalanced conditions in each of these groups because there were no differences between them. Responding in Group Visual was averaged across the entire 2-min Light on or Dark periods, where one of these periods (Visual+) was paired with reward and the other period (Visual−) was not. Responding in Group Temporal is displayed in the 10-s intervals from 10–20 s and 90–100 s after Light onset or offset. One of these intervals preceded expected reinforcement (Time+) and the other interval preceded the non-reinforcement interval of interest (Time−). These intervals were of interest because in subsequent studies we were interested in assessing the use of these early and late temporal cues in more complex discrimination procedures. Overall, discriminative responding was revealed in both groups illustrated by greater responding on probe trials for the previously reinforced period than the non-reinforced periods. Importantly, both groups acquired their discrimination at comparable rates. Discriminative responding began to emerge in both groups by the 5th block of training and continued to increase in the 7th and 8th blocks.
Figure 1.
Mean magazine responses per minute on non-reinforced probe trials in Experiment 1 for groups trained on simple Visual (A, light versus dark) or Temporal (B, early versus late) discrimination learning tasks. Responding is shown separately over 4-session blocks of training for reinforced and non-reinforced trials (Visual+, Visual−, Time+, Time−). The lower panels (C, D) μj–μ.) in σ units derived from statistical analysis for reinforced and non-reinforced trial types over training in each group. Δ refers to the estimates of the non-central parameter derived from statistical analysis.
The data were analyzed by performing separate ANOVAs on each group based on a common error term (MSE = 29.803), and this was followed by post-hoc analysis following the procedures of Rodger (1975; 1975). This analysis revealed that discriminative responding developed in both Group Temporal, F(15,450) = 7.38, Δ=95.3, and in Group Visual, F(15,450) = 3.94, Δ =43.8. In addition, there was a significant main effect of Group, F(1,30) = 4.74, MSE = 567.424, Δ =3.4, with Group Temporal responding somewhat higher overall than Group Visual. Further post-hoc tests were evaluated and the analytically derived implied population means resulting from this analysis are presented for each group in the bottom panel of Figure 1. These results confirmed that differential responding began to emerge in each group in blocks 5 and 6, but increased further in blocks 7 and 8 of training. If anything, the absolute level of this discrimination was slightly better in Group Temporal than in Group Visual, as was revealed by a larger Δ score in the former group. However, the overall pattern of discriminative responding was very similar across the two groups, and it can be concluded that with these parameters the temporal discrimination between Early and Late (10- vs 90- s) intervals was at least as good as the visual discrimination between Light and Dark periods.
Discussion
The results from Experiment 1 demonstrate that our rats can discriminate between Light and Dark visual stimuli at least as well as they can distinguish between 10- and 90-s temporal cues. Rats in Group Visual began to respond more to the reinforced than non-reinforced visual stimulus in blocks 5 and 6 of training, with the degree of their differential responding increasing further in blocks 7 and 8. Similarly, rats in Group Temporal also began discriminating between 10- and 90-s temporal cues in blocks 5 and 6 of training and displayed an almost identical increase in differential responding in blocks 7 and 8. If anything, Group Temporal subjects displayed a slightly higher level of differential responding as reflected by a higher Δ value. Nevertheless, it can be concluded from these data that the rats had no more difficulty distinguishing among the 10- and 90-s temporal cues than they had the Light versus Dark cues. This finding is a necessary first step in assessing the relative ease with which these same stimuli can be used to solve ambiguous occasion setting discriminations.
Experiment 2
Table 1 illustrates the experimental design used in Experiment 2. The purpose of this study was to determine if visual and temporal cues could be used with similar success to solve an ambiguous occasion setting task. Group Visual rats learned that CS1 was reinforced with US1 only in the presence, but not the absence of Light, whereas CS2 was reinforced with US2 only in the Dark and never in the Light. Rats in Group Temporal were trained that CS1 was reinforced with US1 only when presented Early (10 s) but never when presented Late (90 s) in either Light or Dark periods, whereas CS2 was reinforced with US2 only when presented Late but never when presented Early in either Light or Dark periods. Magazine entries were recorded 10 s prior to and 10 s during all CS presentations across training, but USs were delivered at the termination of the CS under appropriate conditions. Responding in the presence of CS1 and CS2 was compared between trial conditions in which they were reinforced versus conditions where they were not. This served as a measure of conditional discrimination learning.
It should be noted that the present study introduced a second US to study occasion setting with visual or temporal features. This procedure was deemed necessary because earlier work in the Delamater laboratory showed that rats were unable to learn a visual ambiguous occasion setting problem after 24 sessions of training (with very similar parameters to those employed here) if only a single US was used (unpublished data) or if two USs were used non-differentially (Delamater et al, 2010). Furthermore, Delamater (2012) simulated these differences using a multi-layered connectionist model and suggested that complex occasion setting discriminations might be more easily learned when each of two auditory target stimuli are reinforced with different USs, as opposed to the same US, because such training would effectively increase the perceptual discriminability (i.e., acquired distinctiveness) of the target stimuli. In the present situation, we expect, on a priori grounds, that such differential outcome training could equally help animals trained with either visual or temporal versions of the ambiguous occasion setting tasks studied here.
Method
Subjects
Subjects were 32, experimentally naïve, male (16) and female (16) Long-Evans rats bred at Brooklyn College, but derived from breeders obtained from Charles River Laboratories. The free-feeding body weights varied between 292 and 528 g for males and between 200 to 300 g for females at the beginning of the experiment. The rats were housed in groups of 2–4 in plastic tubs (17 × 8.5 xx 8 in, l × w × h) with wood chip bedding, but otherwise were maintained as in Experiment 1.
Apparatus
The same apparatus was used as in Experiment 1.
Procedure
The rats were initially magazine trained with pellet and sucrose USs. On each of two days, one magazine training session with one US was followed immediately by a second session with the other US. The order was counterbalanced across days. In each session, 20 USs of one kind were delivered according to an RT 60 schedule. Sucrose (0.1 ml of a 20% solution) and pellet (two TestDiet 45 mg grain pellets) USs were delivered to the same food magazine.
Pavlovian Ambiguous Occasion Setting
Over the next 32 days, all rats received ambiguous occasion setting training sessions in which 12, 2-min Light stimulus periods strictly alternated with 12, 2-min Dark periods, as in Experiment 1. During each of these periods 10-s auditory stimuli (i.e., noise and/or tone) were presented starting 10 and 90 s after the onset of either a Light or Dark period. Four combinations of CS presentations were possible during each visually distinct 2-min period: 1) Noise at 10 s and Noise at 90 s, 2) Noise at 10 s and Tone at 90 s, 3) Tone at 10 s and Tone at 90 s, and 4) Tone at 10 s and Noise at 90 s. Thus, there were a total of 8 basic trial types irregularly interspersed within training sessions (these four combinations in Light and also in Dark). Use of this procedure ensured that the outcome of the first auditory stimulus within a 2-min period could not be used to predict the outcome of the second auditory stimulus within that 2-min period.
The experimental design is shown in Table 1. The rats were randomly assigned to two groups (n=16) counterbalanced for sex. In Group Visual Occasion Setting, one of the auditory stimuli, CS1, was reinforced with US1 only in Light periods, but never in Dark periods, whereas the other auditory stimulus, CS2, was reinforced with US2 only in Dark periods, but was never reinforced in Light periods (Light: CS1-US1, CS2−, Dark: CS1−, CS2-US2). Accordingly, Light was a positive occasion setting stimulus for CS1 and a negative occasion setting stimulus for CS2. Which of the two auditory stimuli played the roles of CS1 and CS2 was counterbalanced across rats, as were the specific CS-US assignments.
Rats in Group Temporal were trained with the same formal task structure (see Table 1), except that the specific times (Early; 10 s or Late; 90 s) within the Light and Dark periods (and not the visual stimuli, per se) were the basis for solving the ambiguous occasion setting task (Early: CS1-US1, CS2−; Late: CS1−, CS2-US2). Notice, that in Group Temporal the visual stimuli alone could not be used to solve the discrimination. Conversely, in Group Visual the Early vs Late temporal cues alone could not be used to solve this discrimination.
The main data of interest was magazine responding that occurred during the auditory stimuli on reinforced and non-reinforced trials. Responding during the CS was compared with responding during 10-s Pre-CS periods in order to determine if the occasion setting stimuli elevated CS responding more on reinforced than non-reinforced trials.
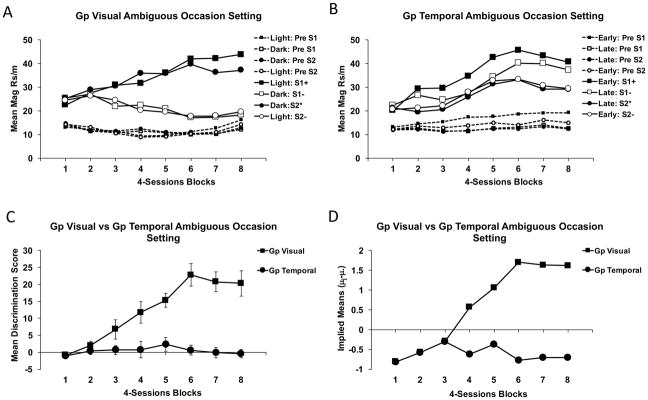
Results
Figure 2 displays mean magazine responses per min across the various reinforced and non-reinforced periods (as well as pre stimulus periods) for the visual (panel A) and temporal (panel B) ambiguous occasion setting tasks used in Experiment 2. It is clear that Group Visual acquired the discrimination quite readily, responding more to CS1 in the presence of the Light than in the Dark and by responding more to CS2 in the Dark than in the Light. It is also clear that Group Temporal failed to learn the discrimination after 32 sessions of training. This group responded similarly to the stimuli on reinforced and non-reinforced trials, but they also showed a tendency to respond more to CS1, the stimulus that was reinforced early in a 2-min period, than to CS2. However, this difference failed to reach significance in a t-test comparing responding to CS1 and CS2 averaged over the final two blocks of training (t(15) = 1.12, p > 0.05). Pre-CS responding across all trial types was generally low and did not differ much among the various trial types or groups, except that Pre-CS responding was slightly higher when CS1 was reinforced early in Group Temporal.
Figure 2.
Mean magazine responding in Experiment 2 during conditioned stimulus (S1, S2) and pre stimulus periods in the light and dark or during early and late intervals over 4-session blocks for the groups trained with visual (A) or temporal (B) ambiguous occasion setting tasks. Responding is shown on trials in which the target stimuli are reinforced (+ or *) or not (−) depending upon which conditional cue is present. Panel C displays the empirical mean +/− SEM discrimination scores across training for each group, and panel D shows the implied mean discrimination scores derived from statistical analysis (as in Figure 1).
For statistical evaluation, we first obtained a measure of conditioned responding for each trial by subtracting responding during the Pre-CS period from the CS period. To assess the magnitude of discriminative responding, discrimination scores were derived by comparing conditioned responding on reinforced versus non-reinforced trials for each block of training. Panel C in Figure 2 shows that Group Visual subjects steadily increased their discriminative performance by this measure across the 8 blocks of training, whereas Group Temporal subjects failed to improve. These data were statistically evaluated by performing separate between group ANOVAs in each block of training using a pooled error estimate (MSE = 76.632) whose degrees of freedom was corrected by Satterthwaite’s procedure (Satterthwaite, 1946). These analyses were then combined with an overall main effect test across the Blocks variable (with further post-hoc tests) and the statistical decisions were then combined across these analyses to derive the implied means using Rodger’s implication formula (Appendix formula 1). These results are presented in panel D of Figure 2.
The two groups differed in blocks 4–8, smallest F(1,101) = 12.54, Δ =11.3, and there was a significant main effect of Blocks, F(7,210) = 16.18, MSE = 42.572, Δ=105.1. The apparent difference between the groups in Block 3, F(1,101) = 3.892, just missed statistical significance (Rodger’s critical F[.05];1,101 = 3.935). Nevertheless, panel D confirms that the two groups clearly differed in blocks 4–8 with the degree of that discriminative performance steadily increasing over training in Group Visual, but not in Group Temporal.
Discussion
The results of Experiment 2 clearly indicate that rats were able to solve the ambiguous occasion setting task if visual stimuli served as conditional feature cues, but not if temporal stimuli played this role. We had earlier observed that rats could readily solve the sort of ambiguous occasion setting problem employed in Group Visual when each target stimulus was associated with different reinforcing outcomes (Delamater et al, 2010). The present study replicates and extends those findings by showing that temporal cues that are equally discriminable as the visual stimuli used here (see Experiment 1) cannot be used to solve (at least with our parameters) a structurally similar ambiguous occasion setting problem. This effect was dramatic. Whereas Group Visual subjects learned the task within 3–4 blocks of training, Group Temporal rats failed to show any level of discriminative responding after 32 training sessions. It may be noted that although we had no a priori reason to suppose that training with differential outcomes would have selective impacts on the development of occasion setting with visual versus temporal feature stimuli, it is possible that the visual feature stimuli derived a stronger benefit than did the otherwise equally discriminable temporal feature stimuli. While we cannot completely refute this possibility, we can think of no evidence to support it either. Further, should such a process be contributing to our findings here, it would surely argue against the primacy of temporal encoding which is what the present study sought to address in the first place. Thus, at present, it would appear as though visual but not temporal cues can be used to solve at least some conditional discrimination learning problems (i.e., ambiguous occasion setting). The next experiment explored this issue further by adopting a modified and perhaps simpler version of our temporal task in an effort to make that problem more solvable.
Experiment 3
The present study compared learning of an ambiguous occasion setting task when visual cues could be used to solve the discrimination or when a combination of visual and temporal cues could be used. Group Visual rats were trained as in Experiment 2. However, for Group Temporal, both auditory CSs were reinforced only when presented Early in one of the visual background stimuli (Light or Dark, counterbalanced) and were reinforced only when presented Late in the presence of the other background stimulus (Dark or Light, counterbalanced). Table 1 illustrates the experimental design. Although the temporal discrimination used here continues to have the structure of a biconditional discrimination problem, the task might be easier to solve than in Experiment 2. In this task each background visual stimulus is uniquely correlated with reinforcement of either CS at one temporal cue, but not the other, theoretically making this problem easier to solve than the task in Experiment 2. Indeed, Iordanova, Good, and Honey (2011) demonstrated that rats could solve a conceptually similar sort of conditional discrimination problem when circadian cues functioned as feature stimuli. If within-session temporal cues could act in a similar manner, then rats may be expected to more readily solve this problem than the one used in Experiment 2.
Method
Subjects
Subjects were 32, experimentally naïve, male (16) and female (16) Long-Evans rats bred at Brooklyn College, but derived from breeders obtained from Charles River laboratories. The free-feeding body weights varied between 361 and 442 g for males and between 205 to 253 g for females at the start of the experiment. Rats were housed and maintained as in Experiment 2.
Apparatus
The same apparatus was used as in the previous experiments.
Procedure
The rats were initially magazine trained as in Experiment 2.
Pavlovian Ambiguous Occasion Setting
All rats were trained on an ambiguous occasion setting task over the next 32 days. Group Visual rats (n=16) were trained exactly as in Experiment 2. Rats in Group Temporal (n=16) were trained using a variant of the temporal occasion setting task run in Experiment 2. Assignments were counterbalanced by sex. In the present experiment, in one subset of Group Temporal rats both auditory CSs were reinforced with their appropriate US whenever they occurred Early, but not Late, during Light periods, and these same CSs were reinforced whenever they occurred Late, but not early, during Dark periods (Light (Early): CS1-US1, CS2-US2; Light (Late): CS1−, CS2−; Dark (Early): CS1−, CS2−; Dark (Late): CS1-US1, CS2-US2). A second subset of rats received the opposite arrangement, where Light signaled that both CSs would be reinforced Late and Dark signaled that they would be reinforced Early. Thus, in this task the conjunction of temporal and visual cues served as occasion setting stimuli.
Once again, magazine responding was recorded in Pre-CS and CS periods in order to assess discriminative responding. In addition, responding in Group Temporal was also recorded in each of the 12, 10-s periods throughout the Light and Dark stimuli to test for systematic changes in responding across time.
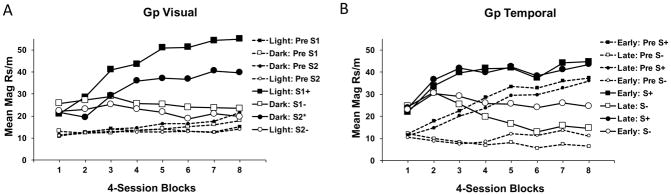
Results
Magazine responding (during Pre-CS and CS periods) across the 8 blocks of training is shown in Figure 3 for Group Visual (panel A) and Group Temporal (panel B). Once again, Group Visual clearly acquired the ambiguous occasion setting discrimination, as demonstrated by greater responding to the CSs when they were reinforced than when not reinforced. In this experiment, Group Visual acquired the positive occasion setting component of the task better than the negative occasion setting component (see also Delamater et al, 2010) as seen by greater differential responding to CS1 in the Light and Dark than to CS2 in the Light and Dark. In addition, Pre-CS responding was low throughout training. Group Temporal, in contrast, clearly did not acquire the discrimination. This group responded more during reinforced versus non-reinforced trials in both the Pre-CS and CS periods similarly. This pattern of responding indicates that Group Temporal subjects learned to anticipate when rewards would occur on the basis of temporal cues alone. However, when the CSs were presented, overall responding was only minimally increased over those levels seen in the Pre-CS period suggesting that any learning to the CSs themselves merely added to the ambient level of responding already elevated by temporal cues.
Figure 3.
Mean magazine responding in Experiment 3 during conditioned stimulus and pre stimulus periods in the light and dark for Group Visual (A) or during early and late intervals for Group Temporal (B) over 4-session blocks of training on the visual or temporal ambiguous occasion setting tasks. Responding is shown separately for each target stimulus (S1, S2) in Group Visual and combined across both stimuli (S) in Group Temporal.
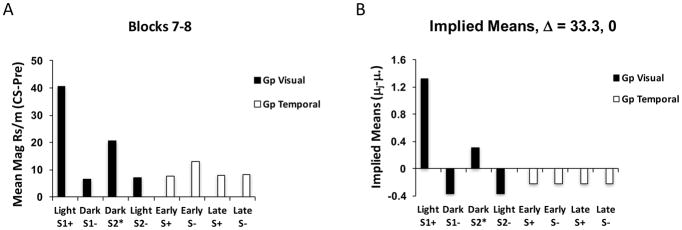
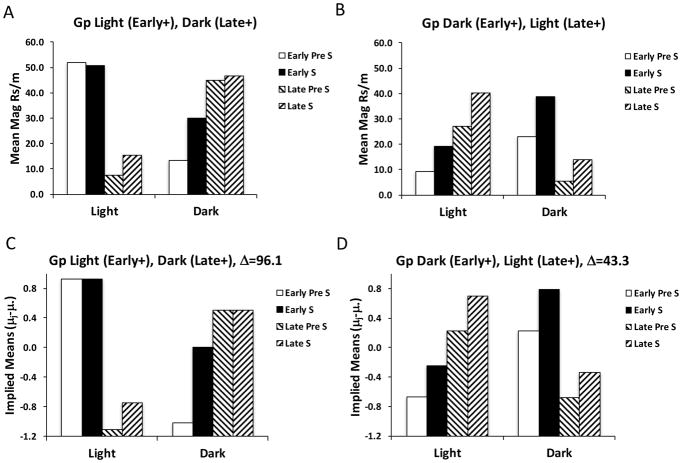
These data were analyzed by first averaging responding across blocks 7 and 8 separately for each of the 4 trial types in each discrimination problem to obtain a measure of asymptotic responding. As in Experiment 1, the difference in responding between the CS and Pre-CS periods was taken to determine to what extent the stimuli differentially elicited conditioned responding (see Figure 4, panel A). Repeated measures ANOVAs were performed on each group, using a pooled error estimate (MSE = 332.183), and this was combined with an overall main effect test of the Groups factor. Group Visual responded differentially across the 4 trial types by the end of training, F(3,90) = 12.37, Δ=33.3, whereas Group Temporal did not. Furthermore, Group Visual responded more, overall, than did Group Temporal, F(1,30) = 6.45, MSE = 448.852, Δ=5.0. Post-hoc analyses were then evaluated and the implied means across the different trial types were derived with Rodger’s implication formula (see Appendix) and the results are shown for both groups in panel B of Figure 4. This analysis confirms the impressions reached above. Importantly, Group Visual responded differentially to the CSs when they were reinforced than when they were not reinforced in this task, the positive occasion setting component (Light: CS1+, Dark: CS1−) was learned better than the negative occasion setting component (Dark: CS2*, Light: CS2−), and Group Temporal failed to differently increase responding during the CS when the auditory target CSs were reinforced versus not reinforced in either the Early or Late temporal cues.
Figure 4.
Data averaged over the final two blocks of training (7 and 8) in Experiment 3 for Group Visual and Group Temporal subjects across the reinforced and nonreinforced trials types (A). In Group Visual, one stimulus (S1) was reinforced in the Light and the other (S2) in the Dark with different reward types (+, *). In Group Temporal, both stimuli (S) were reinforced (+) early in the presence of one background stimulus and late in the presence of the other. Panel B displays the implied means derived from statistical analysis for each group and each trial type (as in Figures 1 and 2).
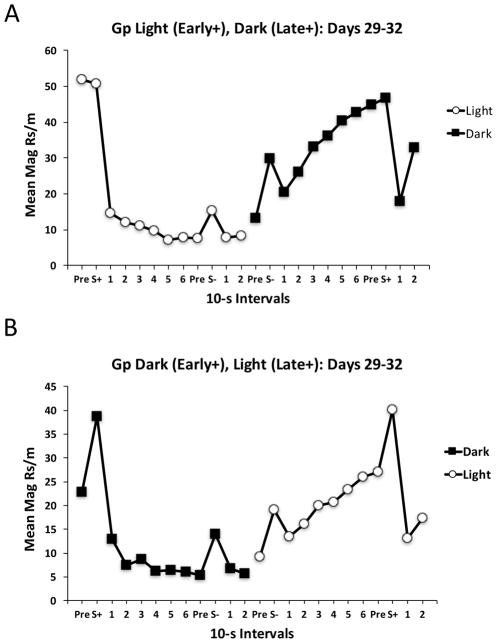
A more detailed examination of the data in Group Temporal was conducted to obtain a more complete picture of the temporal organization of responding across both visually distinct periods. Figure 5 displays responding in 10-s intervals across the Light and Dark periods averaged over days 29–32 of training. The data are shown separately for the sub-group of rats trained with the two CSs signaling reward Early in the Light and Late in the Dark (Gp Light (Early+), Dark (Late+)), and the sub-group trained with the two CSs reinforced Early in the Dark and Late in the Light (Gp Dark (Early+), Light (Late+)). In each of these sub-groups, responding rapidly increased at the onset of either the Light or Dark periods when the CSs were reinforced Early, but then responding rapidly fell and remained low throughout the remaining intervals of that period. In addition, during the visual periods in which the CSs were reinforced Late, responding gradually increased across the entire interval. Responding was also frequently increased transiently over the immediately preceding period when the CSs were presented, but this occurred whether the CS was reinforced (S+) or not (S−). Thus, the subjects had clearly learned to discriminate Early temporal cues within a Light or Dark period from Late temporal cues, but they failed to use these cues to differentially modulate responding to the target CSs as a function of whether they would or would not be reinforced.
Figure 5.
Data from Experiment 3 averaged over the final block of training (days 29–32) is shown separately for the different sub-groups (A, B) trained with the temporal occasion setting task. Mean magazine responses per min is shown in 10-s intervals across the Light and Dark periods. Responding is also shown during the intervals in which the stimuli were reinforced (S+) or not (S−) as well as in the pre stimulus periods (Pre).
These data were statistically evaluated by focusing on responding to the auditory CSs (collapsed over tone and noise), as well as during their Pre-CS periods (similar to the treatment of the data presented in Figure 4), both Early and Late within the Dark and Light periods. These data are displayed separately for the two sub-groups in Figure 6. For both sub-groups, it is clear that responding was higher in both the Pre-CS and CS periods (during both the Dark and Light) when the CSs were reinforced than when they were not reinforced. However, it is also clear that responding to the CSs was not convincingly increased above Pre-CS responding more during reinforced versus non-reinforced trials. Separate repeated measures ANOVAs were performed on these two sub-groups of Group Temporal and revealed significant differences among the various periods for each sub-group, F(7,98) = 15.04, MSE = 179.04, Δ=96.1 for Group Light (Early+), Dark (Late+), and F(7,98) = 7.34, MSE = 179.04, Δ=43.3 for Group Dark (Early+), Light (Late+). Post-hoc analyses were performed on each sub-group and the population means implied by this analysis are displayed in the lower panels of Figure 6. This confirms that subjects responded more within either the Light or Dark periods under conditions when the CS would be reinforced versus non-reinforced. However, reinforced versus non-reinforced difference was similar in the Pre-CS periods as during the CS.
Figure 6.
Mean magazine responses per min in Experiment 3 for the two sub-groups (A, B) of Group Temporal in light and dark periods during stimulus (S) and pre stimulus (Pre) intervals at both early and late time points. The statistically implied means for each sub-group is displayed in the lower set of panels (C, D), as in Figures 1, 2, & 4.
Discussion
The results of Experiment 3 were generally consistent with those found in Experiment 2, and in our previous report (Delamater et al, 2010). Once again, Group Visual rats learned their ambiguous occasion setting discrimination within 3–4 blocks of training. The rats in this experiment learned the positive occasion setting component of the task more rapidly and successfully than the negative occasion setting component of the task (see also Delamater et al, 2010). However, changing the structure of the task for Group Temporal rats did not facilitate learning this biconditional discrimination. These rats learned that reward could occur Early in the presence of one visual background stimulus and Late in the presence of the other (as revealed by differential Pre-CS responding both Early and Late in Light and Dark), but they were unable to use these temporal cues to determine when it was appropriate to respond to the auditory CSs.
This result is a rather striking failure to find conditional control by temporal cues because, as in Experiment 1, this study clearly shows that the rats could easily discriminate between the 10- and 90-s temporal cues used here. In spite of this, when presented with the auditory CSs the rats non-selectively increased their level of magazine responding over their ambient baseline levels. This is consistent with the view that the rats treated the auditory CSs as partially reinforced cues and that the temporal cues were differentially predictive of reward or not in the presence of the different visual background stimuli, but they could not use the temporal cues to modulate responding to the auditory CSs as would be expected of true occasion setting stimuli.
It is worth noting that Group Temporal rats could have learned to ignore the Light and Dark visual cues entirely and learned, instead, to time from the delivery of one reward to the next. Figure 6 shows that this interpretation is unlikely, because once reward was delivered early in a period responding rapidly decreased and remained low throughout the remainder of that interval. Once the background visual stimulus changed (from Light to Dark or vice versa) there was a small increase in responding that was then followed by a gradual increase throughout the remainder of that interval until reward occurred. This suggests that these subjects had not learned to ignore the visual stimuli. Apparently, the rats could use the visual stimuli for the purposes of timing, just as they had done in Experiment 1, but they could not use those temporal cues to occasion set the auditory target CSs.
One potential problem with this interpretation is that, because responding differed so much in the Pre-CS periods before reinforced and non-reinforced trials, differential responding to the CSs would need to be assessed against different “baseline” levels of responding. Thus, it may have been difficult because of a behavioral ceiling for rats to increase further their level of responding during reinforced periods because the Pre-CS level of responding was already so high. Nonetheless, we should point out that, had the rats truly learned to use temporal cues to occasion set the auditory CS-US relations in this study, Pre-CS response levels should have been low throughout the Light and Dark periods. This follows from the fact that the most accurate temporal expectation regarding the arrival of the US would have been estimated from CS onset (only 10 s), not from onset of Light or Dark (20 or 100 s). The fact that Group Temporal rats displayed temporal control during the Pre-CS periods, just highlights the difficulty these rats had in learning the time-based occasion setting discrimination. This interpretation seems more likely given that rats also had such difficulty learning the temporal task in Experiment 2, as well, where there were minimal differences in baseline responding on the different trial types.
General Discussion
The results of the present studies reveal (1) that rats could learn to discriminate 10- and 90-s temporal cues in a simple Pavlovian appetitive task as readily as they could discriminate between 2-min Light from 2-min Dark periods, (2) that these visual cues were more effective than the temporal cues in acquiring the ability to modulate differential responding to 10-s auditory CSs in an ambiguous occasion setting task, and (3) that under conditions where these 10- and 90-s temporal cues clearly acquired control over responding, they failed to modulate responding to (i.e., “occasion set”) the auditory target CSs. These results have important implications for theories of associative learning and temporal control.
These experiments were motivated towards examining a fundamental claim made by one fairly radical theory of learning based on timing principles. Gallistel and Balsam (2014) rejected the traditional notion that associations between events play an explanatory role in Pavlovian learning and suggested, instead, an information theory approach in which animals represent events fundamentally within a temporal memory structure that serves as the basis of conditioned responding. In particular, they assumed that, by encoding events within a temporal memory system, various computations can be performed on those events and conditioned responses will be seen to the CS when it reduces the temporal uncertainty about US occurrence relative to the overall level of temporal uncertainty in the situation. This view contrasts with more traditional associative approaches that often discuss learning in terms of a non-temporal memory structure, e.g., internal representations of the CS and US become associated. The main assumption driving the present studies was that if animals encode events within a temporal memory structure, first and foremost, then it should be relatively easy for them to solve conditional discrimination learning tasks where time itself is a discriminative cue. Further, compared to a conditional discrimination task in which non-temporal cues serve this discriminative function, the temporal task should be at least as easy if not more so. In contrast, from the perspective of a basic associative learning approach there would be no special status given to temporal or non-temporal cues in solving discrimination learning problems. So, to the extent that different temporal and non-temporal cues were equally distinguishable, conditional discrimination tasks depending upon these types of cues should be of similar difficulty.
The results of the present set of studies clearly indicate that rats can distinguish between 10- and 90-s temporal cues as well as they can between Light and Dark non-temporal cues. In spite of this fact, however, the rats very readily learned an ambiguous occasion setting discrimination with visual conditional cues but not with temporal conditional cues (even after 32 training sessions). This rather dramatic difference in learning is problematic for the fundamental assumption of the information theory approach that events are encoded within a temporal memory structure and that this is what constitutes the basis of learned responding. If this were true, then the rats in our temporal occasion setting tasks in Experiments 2 and 3 should have learned at least as rapidly as animals solving the visual cue based occasion setting task. The results of Experiment 3 are especially enlightening in this regard. In that task, rats could have learned that the auditory CSs were reinforced Early during the Light and Late during the Dark periods. However, while the rats responded differentially in the Pre-CS periods in advance of reinforced and non-reinforced target CSs, they failed to use these temporal cues to modulate responding to the CSs themselves. Instead, the rats appeared to learn merely that food rewards would occur Early in the presence of one visual stimulus and Late in the presence of the other (see also Bouton & Garcia-Gutierrez, 2006). Since the time to those rewards was longer than the CS-US interval, the CSs should have substantially reduced any temporal uncertainty regarding the USs’ occurrence. The temporal stimuli should have, therefore, acquired an occasion setting function.
The alternative view, more typical of traditional associative approaches, is that events are represented within a non-temporal memory structure. In this case, conditional relations can be encoded in terms of the associative connections formed among the various time-independent representations of events. For instance, Delamater (2012) and Schmajuk, Lamoureux, and Holland (1998) considered how different multi-layer connectionist networks might be used to explain complex conditional discrimination learning in Pavlovian conditioning (see also, Harris, 2006; Honey & Ward-Robinson, 2002; Pearce, 1994; Wagner & Brandon, 2001). In all of these cases, relatively little is said about how time might factor into the learning process. One common assumption is that time is construed as another type of stimulus (e.g., Buhusi & Schmajuk, 1999; Kehoe, Horne, Macrae, & Horne, 1993; Ludvig, Sutton, & Kehoe, 2012; Staddon & Higa, 1999; Vogel, Brandon, & Wagner, 2003). If time were best construed in this way, we have little reason to suppose in the present studies that our temporal occasion setting discrimination problems should have been more difficult to learn than the visual ones because Experiment 1 revealed that the discriminability of the temporal cues was on par with the visual cues. Thus, our results argue against the claim from a purely associative approach that there should be no special status given to temporal versus non-temporal cues.
Rather, our data are more consistent with an associative model that attempts to integrate interval timing processes within a more modular associative framework (e.g., Church & Broadbent, 1990; Delamater, Desouza, Rivkin, & Derman, 2014; Matell & Meck, 2004). Within this perspective, associative and interval timing processes both co-exist in the brain but do so in different functional “modules.” One of these modules is concerned with traditional associative learning mechanisms and functions to solve the kinds of stimulus selection problems that associative systems are especially good at solving. A different module performs interval timing functions. How the two interact with one another to affect behavior change is a matter of dispute and for further research to clarify (e.g., see McMillan & Roberts, 2013). However, one important point can be made on the basis of our findings. Temporal stimuli are not functionally equivalent to all other, non-abstract, stimuli arising from primary sensory processing systems. We suggest that the associative learning module accepts inputs directly from sensory systems, such that visual and auditory information have no special difficulty in becoming associatively connected within a network that can learn simple and conditional discriminations. However, temporal stimuli are more abstract and do not arise directly from any particular sensory system. These stimuli, therefore, cannot easily become integrated within an associative learning module that solves conditional discrimination problems on the basis of stimulus-stimulus interactions involving temporal cues. This is not to say that interval timing cues cannot ever become integrated within an associative learning module, but, merely, that doing so would be more difficult than when an equally discriminable set of non-temporal sensory cues are used.
There are other examples in the literature where temporal cues clearly have become integrated with other associative information in the control of behavior. For instance, in episodic-like memory studies with non-human animals (Clayton & Dickinson, 1999; Zhou & Crystal, 2009; Honey, Iordanova, & Good, 2014) there is clear evidence that temporal cues are integrated within a memory structure that also includes non-temporal information (e.g., regarding what the event is and where it appears). These studies use circadian cues, however, that are quite different from the types of temporal cues used in interval timing studies more generally. Other work based on “time-place” discrimination learning also reveals strong temporal control over spatially defined instrumental response-reward contingencies (e.g., Thorpe & Wilkie, 2006; Wilkie & Willson, 1992). These studies differ from the present studies in using time within the session as a conditional cue, and these also substantially differ from the within-trial temporal cues employed in the present studies.
In a task more closely related to the present studies, Bouton and his colleagues have shown that the length of the inter-trial interval (ITI) within a session can serve as a conditional cue that modulates responding to a discrete auditory CS paired with food at the end of a long, but not short, ITI. In one study, Bouton and Garcia-Gutierrez (2006; Bouton & Hendrix, 2011) showed that rats responded more to a tone CS that was reinforced with food pellets when it occurred after a 16-min ITI than when it was non-reinforced after a 4-min ITI. They interpreted this result in terms of the 16-min ITI acquiring a positive occasion setting function over the tone CS. Interestingly, a group trained on a similar 4-min versus 1-min ITI task failed to learn this discrimination (but see Bouton & Hendrix, 2011), although the group responded more in Pre-CS periods following a 4-min than a 1-min ITI. They suggested that the ITI cues in this case acquired a simple associative function (predicting food or not) and that this simple associative function blocked conditioning to the tone CS. This finding is consistent with our results in Experiment 3. In that experiment, we observed that Group Temporal rats learned that food would occur Early in the presence of one 2-min visual stimulus and Late in the presence of the other. Apparently, learning to anticipate food reward on the basis of these temporal cues prevented their ability to occasion set the 10-s auditory CS.
The findings of Bouton and Garcia-Gutierrez (2006) and Bouton & Hendrix (2011) supports the view that interval timing cues can sometimes acquire modulatory control over a discrete CS. They do not contradict the present findings because we show here that equivalently discriminable temporal and visual cues acquire modulatory control to different degrees. A further complication in comparing our findings with Bouton and Garcia-Gutierrez (2006) and Bouton and Hendrix (2011) is that our modulation paradigm was a more complex ambiguous occasion setting task. In our situation, Early and Late temporal cues each signaled reinforcement of one CS but non-reinforcement of another. It is noteworthy that, in the Bouton and Garcia-Gutierrez (2006) and Bouton and Hendrix (2011) studies, groups trained with the short ITI signaling reinforcement of the CS and the long ITI signaling non-reinforcement failed to learn the discrimination. In other words, there appears to be an asymmetry in how well short versus long temporal cues can acquire occasion setting properties. This could add to the difficulty that our Group Temporal animals faced in acquiring our ambiguous occasion setting task.
In both of these cases it may be noted that poor occasion setting control by temporal cues may be due to an asymmetry in how Early and Late temporal cues are processed. In particular, a Late interval requires passage through an Early interval, but the reverse is not true (the Late interval is never experienced on the way to an Early interval). This part-whole relationship characteristic of Early versus Late temporal cues may not equally characterize the relationship between Light and Dark visual stimuli. Just how this line of thinking may account for our findings is not clear, but several points are worth making. First, our Light versus Dark discrimination in the abstract consists of discriminating between the presence versus absence of a visual stimulus. However, in practice, the chambers in which our rats were run were merely sound and light attenuating chambers and very likely allowed for some low level of light to enter during Dark periods. Thus, the discrimination was very likely one between high and low levels of illumination and this seems conceptually similar to one between Early and Late temporal cues. Second, the part-whole idea applied to Early and Late temporal cues would suggest that in our ambiguous occasion setting procedure the occasion setting component of the task involving late temporal cues would have been better learned than those involving short temporal cues. However, this was not observed in the present studies. In our studies and in those from Bouton and his colleagues it appears that there are important constraints on when within-trial temporal cues might come to modulate responding to other target CS-US relationships. Learning to time the arrival of reward and using those temporal cues to occasion set other stimuli sometimes appear to be conflicting functions. Third, it is possible that one of the difficulties with our temporal task is related to the fact that a temporal discrimination is based on exposure to a continuous series of temporal cues as they unfold across a trial, whereas the visual discrimination is based only on a comparison of two points on the visual dimension (light vs dark). This could potentially present special difficulties in learning a time-based discrimination. If this conceptual difference between the two tasks posed a special challenge to the animals, however, we would also expect to have seen differences in Experiment 1. In that study, both groups acquired the simple discrimination at comparable rates, and since those discriminations only showed up after approximately 20 training sessions there was plenty of room to have assessed learning rate differences but none appeared. Thus, we think this is an unlikely source of our observed differences in the occasion setting tasks of Experiments 2 and 3.
One may be puzzled by the striking absence of temporal conditional control in the present studies. Other work has clearly shown that complex conditional discrimination tasks can be learned, eventually (e.g., Lin & Honey, 2010). We suspect that in the present circumstance had we continued to train our animals on the temporal tasks animals may have eventually learned them. However, this fact should not detract from our main finding that the visual occasion setting task was much more easily learned. Moreover, it may be possible to find particular parameters with time and visual cues in which temporal occasion setting is at least as good as occasion setting based on visual cues. However, our finding in Experiment 1 is instructive because it demonstrated that simple visual and temporal discriminations using the specific stimuli we used in our occasion setting tasks did not differ. This must be a precondition in order to appropriately assess the relative ease of learning these two types of discriminations. Future research could assess the generality of our findings by finding different equivalently discriminable sets of visual and temporal stimuli.
In summary, the present studies addressed a fundamental assumption of the information theory approach to timing and associative learning (Gallistel & Balsam, 2014) that events within a Pavlovian learning situation are essentially encoded within a temporal memory structure, and this serves as the basis of conditioned responding. This perspective suggests that it should be easy for animals to solve complex conditional discrimination learning tasks where reinforcement or non-reinforcement of CSs is conditional upon different temporal cues. Our findings support the view that temporal cues can acquire simple associative functions in signaling food or its absence, but that such cues cannot easily be used as occasion setting stimuli, i.e., to modulate discrete CSs. Visual cues, in contrast, were equally discriminable to our temporal cues and quite readily acquired conditional control in an ambiguous occasion setting task. The fact that equally discriminable sets of visual and temporal cues acquired modulatory control to different degrees does not fit with the general view that temporal cues have no special status compared to non-temporal cues in Pavlovian learning. We conclude (1) that a simple associative module exists independent of a timing module and functions largely to solve stimulus selection problems involving sometimes rather complex inter-relationships among primary sensory inputs and reward, and (2) that an interval timing module co-exists and gives rise to representations of time that can directly affect expectation of a US but have difficulty interacting with this associative learning module in solving complex discrimination learning problems involving other conditioned stimuli.
Acknowledgments
The research reported here was supported by a National Institute on Drug Abuse and National Institute of General Medical Sciences (034995) grant awarded to ARD. The authors would like to thank Shane Brown, Marina Lyubimova, and Michael Blecher for assisting with the data collection.
Appendix
Rodger’s methods entail reconceptualizing factorial designs (e.g., with I and J factors) in terms of a one-way design (e.g., with I x J levels). Given a significant overall difference among the conditions, experimentally interesting interactions are revealed through post-hoc analyses. When one of the factors is between group and another is within group (as in the present studies), then the analysis consists of repartitioning the sum of squares in either of two ways from the original split plot form of analysis. One method entails repartitioning the repeated J and I x J sum of squares into separate one-way repeated measures analyses performed on each group. These one-way analyses are based on a common pooled error term, which is the same within group error estimate (of σ2(1-ρ)) used in the split plot form. When combining these one-way analyses with the original I main effect analysis then all of the sum of squares are accounted for as in an ordinary between/within split plot analysis but interaction terms are avoided. The second method (used in Experiment 2) entails repartitioning the I and I x J sum of squares into separate between group analyses performed at each level of the repeated measures factor. These are based on a pooled error estimate (of σ2 from the original split plot form). This analysis is then combined with an overall J main effect test to account for all of the sum of squares found in the original split plot form. All statistical tests are evaluated against Rodger’s table of critical F values (Rodger, 1974; 1975), and appropriate post-hoc tests as described below are examined.
Rodger’s methods were chosen over others because of its theoretical completeness in analyzing data and because of the lack of any ambiguity regarding statistical decisions justified by the data. Moreover, because the statistical power of this approach increases with increasing numerator degrees of freedom, rather than decreases as occurs with standard ANOVA methods, this method is the most powerful presently available ANOVA technique at detecting true effects (see also Rodger & Roberts, 2013).
Significant overall F scores were further examined by selecting a set of ν1 linearly independent and mutually orthogonal post-hoc contrasts using Rodger’s contrast formula (Rodger, 1974). Rejected contrasts were assigned a non-zero value expressed in σ units, δ = g σ √Σc2, that reflects how quantitatively different that contrast is from zero. Contrasts not rejected are assigned a value of δ = 0. The reader will note that these values are weighted by a factor, g, that is scaled by the observed size of effect, g = √(ν1 Fh/N) (where Fh is the obtained contrast F). This parameter, g, is analogous to Cohen’s d (Cohen, 1988), but is more general because it applies to all contrasts (not just those that are comparisons between two means). From the set of statistical decisions arising from a given contrast set, the exact quantitative relations between the true population μs can then be calculated with Rodger’s implication formula:
| (1) |
It is important to realize that each contrast set (hCj) with its own set of statistical decisions (i.e., 1δh values) gives rise to one quantitatively unique set of implied population means (expressed in terms of a difference between each true mean from the overall grand mean, μj – μ.).
In addition, based on this analysis one can also calculate the overall amount of non-centrality occurring in the data in the following way.
| (2) |
This value is an estimate of the non-central F distribution’s Δ parameter and reflects the overall amount of variation among the true means justified by the sample data. These values are also reported throughout the manuscript. However, Perlman and Rasmussen (1975) identified a uniformly minimum unbiased estimator of this non-centrality parameter, which produces values that are generally somewhat less than those produced by Equation 2 above. Because Perlman and Rasmussen’s estimator is thought to be a more accurate estimator of Δ, after deriving the population means through Rodger’s method (Equation 1) those values were rescaled in order to bring the overall Δ in line with Perlman and Rasmussen’s (1975) unbiased estimator.
The post-hoc contrast sets chosen for all analyses were comprised of mutually orthogonal and linearly independent contrasts. The specific contrast sets chosen for these analyses together with each contrast’s computed δ values are available by contacting the first author (ARD).
References
- Arcediano F, Miller RR. Some constraints for models of timing: a temporal coding hypothesis perspective. Learning and Motivation. 2002;33:105–123. [Google Scholar]
- Arcediano F, Escobar M, Miller RR. Temporal integration and temporal backward associations in humans and nonhuman subjects. Learning & Behavior. 2003;31:242–256. doi: 10.3758/bf03195986. [DOI] [PubMed] [Google Scholar]
- Balsam PD, Gallistel CR. Temporal maps and informativeness in associative learning. Trends in Neurosciences. 2009;32:73–78. doi: 10.1016/j.tins.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsam PD, Drew MR, Gallistel CR. Time and associative learning. Comparative Cognition and Behaviour Reviews. 2010;5:1–22. doi: 10.3819/ccbr.2010.50001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnet RC, Cole RP, Miller RR. Temporal integration in second-order conditioning and sensory preconditioning. Animal Learning & Behavior. 1997;25:221–233. [Google Scholar]
- Bouton ME, Hendrix MC. Intertrial interval as a contextual stimulus: Further analysis of a novel asymmetry in temporal discrimination learning. Journal of Experimental Psychology: Animal Behavior Processes. 2011;37:79–93. doi: 10.1037/a0021214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Garcıa-Gutierrez A. Intertrial interval as a contextual stimulus. Behavioural Processes. 2006;71:307–317. doi: 10.1016/j.beproc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Schmajuk NA. Timing in simple conditioning and occasion setting: a neural network approach. Behavioural Processes. 1999;45:33–57. doi: 10.1016/s0376-6357(99)00008-x. [DOI] [PubMed] [Google Scholar]
- Church RM, Broadbent HA. Alternative representations of time, number, and rate. Cognition. 1990;37:55–81. doi: 10.1016/0010-0277(90)90018-f. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A. Scrub jays (Aphelocoma coerulescens) remember the relative time of caching as well as the location and content of their caches. Journal of Comparative Psychology. 1999;113:403–416. doi: 10.1037/0735-7036.113.4.403. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Crystal JD. Episodic-like memory in animals. Behavioural Brain Research. 2010;215(2):235–243. doi: 10.1016/j.bbr.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamater AR. On the nature of CS and US representations in Pavlovian learning. Learning & Behavior. 2012;40:1–23. doi: 10.3758/s13420-011-0036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamater AR, Kranjec A, Fein M. Differential outcome effects in Pavlovian biconditional and ambiguous occasion setting tasks. Journal of Experimental Psychology: Animal Behavior Processes. 2010;36:471–481. doi: 10.1037/a0019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamater AR, Desouza A, Rivkin Y, Derman R. Associative and temporal processes: A dual process approach. Behavioural Processes. 2014;101:38–48. doi: 10.1016/j.beproc.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR, Balsam PD. Time to rethink the neural mechanisms of learning and memory. Neurobiology of Learning and Memory. 2014;108:136–144. doi: 10.1016/j.nlm.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR, Gibbon J. Time, rate, and conditioning. Psychological Review. 2000;107:289–344. doi: 10.1037/0033-295x.107.2.289. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Balsam P. Spreading association in time. In: Locurto CM, Terrace HS, Gibbon J, editors. Autoshaping and Conditioning Theory. Academic Press; New York: 1981. [Google Scholar]
- Gottlieb DA. Is the number of trials a primary determinant of conditioned responding. Journal of Experimental Psychology: Animal Behavior Processes. 2008;34:185–201. doi: 10.1037/0097-7403.34.2.185. [DOI] [PubMed] [Google Scholar]
- Gottlieb DA, Rescorla RA. Within-subject effects of number of trials in rat conditioning procedures. Journal of Experimental Psychology: Animal Behavior Processes. 2010;36:217–231. doi: 10.1037/a0016425. [DOI] [PubMed] [Google Scholar]
- Harris JA. Elemental representations of stimuli in associative learning. Psychological Review. 2006;113:584–605. doi: 10.1037/0033-295X.113.3.584. [DOI] [PubMed] [Google Scholar]
- Harris JA, Gharaei S, Moore CA. Representations of single and compound stimuli in negative and positive patterning. Learning & Behavior. 2009;37:230–245. doi: 10.3758/LB.37.3.230. [DOI] [PubMed] [Google Scholar]
- Holland PC. Transfer of control in ambiguous discriminations. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:231–248. doi: 10.1037//0097-7403.17.3.231. [DOI] [PubMed] [Google Scholar]
- Holland PC, Reeve CE. Acquisition and transfer of control by an ambiguous cue. Animal Learning & Behavior. 1991;19:113–124. [Google Scholar]
- Honey RC, Ward-Robinson J. Acquired equivalence and distinctiveness of cues: I. Exploring a neural network approach. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28:378–387. [PubMed] [Google Scholar]
- Honey RC, Iordanova MD, Good M. Associative structures in animal learning: Dissociating elemental and configural processes. Neurobiology of Learning and Memory. 2014;108:96–103. doi: 10.1016/j.nlm.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Iordanova MD, Good M, Honey RC. Configural learning without reinforcement: Integrated memories for what, where and when. Quarterly Journal of Experimental Psychology. 2008;61:1785–1792. doi: 10.1080/17470210802194324. [DOI] [PubMed] [Google Scholar]
- Iordanova MD, Good M, Honey RC. Retrieval-mediated learning involving episodes requires synaptic plasticity in the hippocampus. Journal of Neuroscience. 2011;31:7156–7162. doi: 10.1523/JNEUROSCI.0295-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins HM, Barnes RA, Berrera FJ. Why autoshaping depends on trial spacing. In: Locurto CM, Terrace HS, Gibbon J, editors. Autoshaping and Conditioning Theory. Academic Press; New York: 1981. pp. 255–284. [Google Scholar]
- Kehoe EJ, Horne PS, Macrae M, Horne AJ. Real-time processing of serial stimuli in classical conditioning of the rabbit’s nictitating membrane response. Journal of Experimental Psychology: Animal Behavior Processes. 1993;19:265–283. [PubMed] [Google Scholar]
- Lin TCE, Honey RC. Analysis of the content of configural representations: The role of associatively evoked and trace memories. Journal of Experimental Psychology: Animal Behavior Processes. 2010;36:501–505. doi: 10.1037/a0018348. [DOI] [PubMed] [Google Scholar]
- Ludvig EA, Sutton RS, Kehoe EJ. Evaluating the TD model of classical conditioning. Learning & Behavior. 2012;40:305–319. doi: 10.3758/s13420-012-0082-6. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Cortico-striatal circuits and interval timing: coincidence-detection of oscillatory processes. Cognitive Brain Research. 2004;21:139–170. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Matzel LD, Held FP, Miller RR. Information and expression of simultaneous and backward associations: Implications for contiguity theory. Learning and Motivation. 1988;19:317–344. [Google Scholar]
- McMillan N, Robert WA. Interval timing under variations in the relative validity of temporal cues. Journal of Experimental Psychology: Animal Behavior Processes. 2013;39:334–341. doi: 10.1037/a0032470. [DOI] [PubMed] [Google Scholar]
- Miller RR, Barnet RC. The role of time in elementary associations. Current Directions in Psychological Science. 1993;2:106–111. [Google Scholar]
- Miller RR, Schachtman TR. Conditioning context as an associative baseline: implications for response generation and the nature of conditioned inhibition. In: Miller RR, Spear NE, editors. Information Processing in Animals: Conditioned Inhibition. Erlbaum; Hillsdale, NJ: 1985. [Google Scholar]
- Morris RW, Bouton ME. Effect of unconditioned stimulus magnitude on the emergence of conditioned responding. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:371–385. doi: 10.1037/0097-7403.32.4.371. [DOI] [PubMed] [Google Scholar]
- Nakajima S. Pavlovian feature-ambiguous discrimination in pigeons. In: Schmajuk NA, Holland PC, editors. Occasion Setting: Theory and Data. American Psychological Association; Washington, DC: 1998. pp. 147–165. [Google Scholar]
- Pearce JM. Similarity and discrimination: A selective review and a connectionist model. Psychological Review. 1994;101:587–607. doi: 10.1037/0033-295x.101.4.587. [DOI] [PubMed] [Google Scholar]
- Perlman MD, Rasmussen UA. Some remarks on estimating a noncentrality parameter. Comm Statist. 1975;4:455–468. [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. Appleton-Century-Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- Polack CW, Molet M, Miguez G, Miller RR. Associative structure of integrated temporal relationships. Learning & Behavior. 2013;41:443–454. doi: 10.3758/s13420-013-0119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. Simple, powerful statistics: an instantiation of a better ‘mousetrap’. Journal of Methods & Measurement in the Social Sciences. 2011;2:63–79. [Google Scholar]
- Rodger RS. Multiple contrasts, factors, error rate, and power. British Journal of Mathematical & Statistical Psychology. 1974;27:179–198. [Google Scholar]
- Rodger RS. Setting rejection rate for contrasts selected post hoc when some nulls are false. British Journal of Mathematical and Statistical Psychology. 1975;28:214–232. [Google Scholar]
- Rodger RS, Roberts M. Comparison of power for multiple comparison procedures. Journal of Methods and Measurement in the Social Sciences. 2013;4:20–47. [Google Scholar]
- Schmajuk NA, Lamoureux JA, Holland PC. Occasion setting: A neural network approach. Psychological Review. 1998;105:3–32. doi: 10.1037/0033-295x.105.1.3. [DOI] [PubMed] [Google Scholar]
- Staddon JER, Higa JJ. Time and memory: towards a pacemaker-free theory of interval timing. Journal of the Experimental Analysis of Behavior. 1999;71:215–251. doi: 10.1901/jeab.1999.71-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KM, Joseph V, Zhao AS, Balsam PD. Temporal maps in appetitive Pavlovian conditioning. Behavioural Processes. 2014;101:15–22. doi: 10.1016/j.beproc.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe CM, Wilkie DM. Rats performance on an interval time-place task: Increasing sequence complexity. Learning & Behavior. 2006;34:248–254. doi: 10.3758/bf03192880. [DOI] [PubMed] [Google Scholar]
- Vogel EH, Brandon SE, Wagner AR. Stimulus representation in SOP: II. An application to inhibition of delay. Behavioural Processes. 2003;62:27–48. doi: 10.1016/s0376-6357(03)00050-0. [DOI] [PubMed] [Google Scholar]
- Wagner AR, Brandon SE. A componential theory of Pavlovian conditioning. In: Mowrer RR, Klein SB, editors. Handbook of contemporary learning theories. Mahwah, NJ: Erlbaum; 2001. pp. 23–64. [Google Scholar]
- Wilkie DM, Willson RJ. Time–place learning by pigeons, Columba livia. Journal of the Experimental Analysis of Behavior. 1992;57:145–158. doi: 10.1901/jeab.1992.57-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Crystal JD. Evidence for remembering when events occurred in a rodent model of episodic memory. Proceedings of the National Academy of Sciences. 2009;106:9525–9529. doi: 10.1073/pnas.0904360106. [DOI] [PMC free article] [PubMed] [Google Scholar]