Abstract
Use of the alkaline comet assay to assess DNA repair capacity in human populations has been limited by several factors, including lack of methodology for use of unstimulated cryopreserved peripheral blood mononuclear cells (PBMCs), insufficient control of interexperimental variability, and limited analysis of DNA repair kinetics. We show that unstimulated cryopreserved PBMCs can be used in DNA repair studies performed using the comet assay. We have applied data standardization for the analysis of DNA repair capacity using negative and positive internal standards as controls for interexperimental variability. Our standardization procedure also uses negative controls, which provides a way to minimize the interference of interindividual variation in baseline DNA damage levels on DNA repair capacity measurements in populations. DNA repair capacity was assessed in a small human cohort using the parameters described in the literature including initial DNA damage, half-time of DNA repair, and residual DNA damage after 30 and 60 min. We have also introduced new DNA repair capacity parameter, initial rate of DNA repair. There was no difference in DNA repair capacity between fresh and cryopreserved PBMCs when measured by the Olive tail moment and tail DNA. The use of DNA repair capacity parameters in assessment of fast and slow single-strand break repair components is discussed.
INTRODUCTION
The alkaline comet assay is a sensitive and relatively inexpensive technique for the detection of DNA damage and DNA repair (1). One of the major advantages of the alkaline comet assay is the possibility of analyzing DNA damage and repair in individual cells. Furthermore, small numbers of cells are required for this assay, which is particularly advantageous for analyses performed in samples from human populations. The comet assay has been used to assess the genotoxicity of chemicals, environmental exposures to carcinogens, toxins and physical agents both in vitro and in vivo (2, 3). This method was also used to measure DNA repair capacity in live cells (4) and acellular systems (5). In addition, the comet assay has been used in prenatal diagnostic testing for heritable human DNA repair syndromes including xeroderma pigmentosum (XP) and trichothiodystrophy (TTD) (6). Since this method is very sensitive, even minimal changes in DNA damage levels and DNA repair capacity in human populations caused by genetic and demographic variation can be studied. Applications of the alkaline comet assay in biomonitoring include assessing background levels of DNA damage in human populations (7), analysis of nutrient and micronutrient effects on the level of DNA damage (8), examination of DNA damage levels associated with exposure to genotoxic factors (such as cigarette smoke, environmental pollution or chemotherapeutic agents) (9–11), and oxidative stress connected with human pathology (such as infectious disease, diabetes mellitus, cardiovascular disease and hemodialysis in renal failure patients) (12–16). Another epidemiological application of the assay is the determination of interindividual variation in DNA repair capacity (7, 17, 18). Analysis of DNA repair capacity in human populations is an attractive biomarker for clinical investigators, because alterations in several DNA repair pathways are linked with both heritable and sporadically occurring age-associated diseases such as cancer.
Most alkaline comet assay protocols used in biomonitoring employ peripheral blood mononuclear cells (PBMCs) in part because obtaining blood from human subjects is minimally invasive. However, use of PBMCs in epidemiological studies of DNA repair capacity is still challenging due to the technical and logistical difficulties that arise in the use of fresh lymphocytes and the lack of a refined protocol for use with cryopreserved PBMCs. Furthermore, the relatively high interexperimental variability in the comet assay presents difficulty in detecting interindividual variation in response to genotoxic factors (19, 20). It is possible to reduce experimental variation and to detect more subtle differences in DNA repair capacity between individuals by processing negative and positive internal standards in parallel with experimental samples and using these internal standards. Finally, performing a more detailed analysis of DNA repair kinetics may give additional details about variation in DNA repair processes in human populations.
In epidemiological studies, samples are usually collected over an extended period. Depending on the variable analyzed and protocol applied, studies may be performed immediately after taking samples or retrospectively. The most efficient approach for analyzing DNA repair capacity using the comet assay is retrospective, so experiments can be performed in duplicate or triplicate at different times. Cryopreservation at −80°C or in liquid nitrogen has been widely used for human samples in general and PBMCs in particular (18, 21). However, cryopreserved PBMCs were unable to remove H2O2-induced DNA lesions (22). In addition, both treated and untreated cells displayed increasing levels of DNA damage during repair incubation after exposure (22). Therefore, several studies used fresh lymphocytes for DNA repair studies using the alkaline comet assay (23, 24). The disadvantage of using fresh PBMCs is the inability to run independent experiments without obtaining additional blood samples from participants. Visvardis and colleagues demonstrated that PBMC stimulation with PHA-L at a low concentration was required to stimulate repair of H2O2- and γ-radiation-induced DNA damage (25). DNA repair was also detected using the alkaline comet assay in cryopreserved lymphocytes after their 20-h mitogen stimulation in culture conditions in the presence of 2% PHA (18, 26). However, it is possible that lymphocyte stimulation by PHA may influence DNA repair capacity since PHA stimulates T-lymphocyte growth (27). The best alternative to these approaches is the development of an improved procedure for cryopreservation of PBMCs and examination of DNA repair capacity in cryopreserved PBMCs using the comet assay. New procedures should minimize the introduction of DNA damage during cryopreservation and prevent accumulation of DNA damage during repair incubation.
The ability of the comet assay to distinguish subtle variations in DNA repair capacity in human populations depend on the extent of interexperimental variability, since multiple experiments must be performed to accurately screen and analyze DNA damage levels and DNA repair capacity. The source of this interexperimental variability may be multifactorial (19, 20, 28). Perhaps the most important source is small differences in unwinding and electrophoresis conditions during the processing of separate batches of samples because of the variability in the current delivered across the electrophoresis unit and in the composition and temperature of the unwinding/electrophoresis buffer. Additional variability may result from experimental conditions, including slide preparation and room environmental conditions (temperature, humidity and sun exposure). Most of these and other sources of interexperimental variability can be minimized by using internal standards as proposed at the International Workshop on Genotoxicity Testing Procedures (Washington, DC, March 1999) (29).
Repair of single-strand breaks (SSBs) induced by ionizing radiation can be characterized by at least two components. Separate repair components repair different types of DNA damage with various rates (30). To our knowledge, there are no published epidemiological reports showing a population analysis of DNA repair in humans that include the fast and slow components of SSB repair. This approach could provide more details about DNA repair processes in humans.
The aim of this work was to develop an accurate, reproducible and efficient comet assay method for evaluating DNA repair in cryopreserved PBMCs. We showed first that cryopreserved lymphocytes can be used effectively in DNA repair studies using the alkaline comet assay. We minimized interexperimental variability by applying standardization techniques using negative and positive internal standards. Finally, we developed a protocol for estimation of the rate of the fast and slow components of DNA repair in unstimulated cryopreserved PBMCs using data standardization. This protocol includes estimation of the initial rate of DNA repair, a newly introduced DNA repair parameter showing the rate of DNA repair immediately after exposure to a genotoxic agent.
MATERIALS AND METHODS
Culture and Cryopreservation of AG10097 Cells
Cells of the non-malignant lymphoblastoid cell line AG10097 were obtained from the Aging Cell Repository (National Institute of General Medical Sciences, Coriell Repository) and were grown at 37°C in 95% air/5% CO2 in RPMI-1640 medium supplemented with 10% FBS (Invitrogen). For cryopreservation, the cells in exponential phase of growth were collected, suspended in freezing medium (40% RPMI-1640 medium, 50% FBS, 10% dimethyl sulfoxide), and divided into aliquots to obtain samples containing 106 cells in 200 µl of freezing medium. Vials were frozen overnight in isopropanol-containing freezing containers (Nalgene, Rochester, NY) placed at −80°C. Samples were stored in a −140°C freezer.
Isolation and Cryopreservation of Peripheral Blood Mononuclear Cells (PBMCs)
Blood donors were participants in the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study of the National Institute on Aging Intramural Research Program (NIA IRP). The study was reviewed by the Institutional Review Board, and informed consent was obtained from all participants. The cohort in this study consists of two African-American females, four Caucasian females and four Caucasian males ranging in age from 43 to 64 years. Fasting blood samples were collected in 8-ml Vacutainer® heparinized vials (BD, Franklin Lakes, NJ). Blood was transported at room temperature to the laboratory, and the mononuclear cell isolation procedure was performed within 3 h of phlebotomy.
PBMCs were separated by centrifugation of blood diluted with RPMI-1640 medium over Histopaque 1077 (Sigma-Aldrich, St. Louis, MO) at 200g for 15 min. The interphase layer was collected by pipette and was washed in RPMI-1640 medium. The pellet was resuspended to a final volume of 0.68 ml with RPMI-1640 medium. Two 40-µl aliquots were removed, supplemented with 400 µl of complete RPMI-1640 medium (RPMI-1640 with 20% FBS), and placed into a CO2 incubator for 1 and 3.5 h prior to the comet assay. The remaining PBMCs were suspended in 40% RPMI-1640 medium, 50% FBS, and 10% DMSO. Vials were frozen in isopropanol-containing containers placed in a −80°C freezer. Two frozen samples were stored in freezer for 1 and 3.5 h prior to the comet assay. Two fresh and two frozen samples were used to compare DNA repair capacity in fresh and cryopreserved PBMCs.
Measurement of γ-Radiation-Induced DNA Damage in AG10097 Cells
The effect of γ radiation on the level of DNA damage in fresh and cryopreserved AG10097 cells was compared. Fresh AG10097 cells were obtained from an exponentially growing culture. A sample containing 1 × 106 cryopreserved cells was thawed by submersion in a 37°C water bath. Fresh and cryopreserved AG10097 cells were suspended in tubes in 10 ml of complete RPMI-1640 medium (supplemented with 20% FBS and preincubated in an incubator at 95% air/5% CO2). Cells were centrifuged at 200g at 4°C for 15 min, washed and gently resuspended in complete RPMI-1640 medium. Cells were suspended in 0.5% low-melting-point agarose (Invitrogen, Carlsbad, CA) in PBS and spread on fully frosted microscope slides (A. Daigger & Company, Wheeling, IL) precoated with 0.5% normal agarose (Invitrogen). Four sets of samples were prepared. After 20 min of solidification at 4°C, slides were exposed to 137Cs γ radiation on ice at doses ranging from 0–10 Gy at a dose radiation rate of ~1 Gy/min using a Gammacell 40 Exactor 137Cs γ-radiation source (Nordion, Ontario, Canada). After irradiation, slides were immediately placed into cold lysis buffer and processed as described below.
Examination of DNA Repair Capacity in Fresh and Cryopreserved PBMCs
The alkaline comet assay was used to compare DNA repair capacity in fresh and cryopreserved PBMCs from 10 human donors prepared as described above. Two replicate experiments were conducted for each individual. Two sets of samples were prepared for each experiment. Each sample set contained samples for fresh and cryopreserved cells exposed to 0 or 6.3 Gy of γ radiation and allowed to repair for 0, 5, 15, 30 or 60 min. Each set of samples was matched with four negative and four positive internal standards.
Frozen PBMCs samples were thawed in a 37°C water bath. Fresh and cryopreserved PBMCs were washed in complete RPMI-1640 medium (RPMI-1640 medium supplemented with 20% FBS and preincubated overnight in humidified atmosphere of 95% air/5% CO2) and embedded in 0.5% low-melting-point agarose onto fully frosted microscope slides as described for AG10097 cells. Fresh and cryopreserved cells were embedded in two separate gels on each slide. On all slides for the first set, gels with fresh PBMCs were prepared near the frosted slide area and gels with cryopreserved PBMCs farther from this area. For the second set, the location of fresh and cryopreserved cell samples was reversed. After 20 min at 4°C for gel solidification, slides were placed in prewarmed complete RPMI-1640 medium in a CO2 incubator for 30 min (37°C) to allow cells to repair damage induced during gel preparation. Slides were then subjected to 137Cs γ radiation at 6.3 Gy as described above. Unirradiated and irradiated slides were immediately placed into cold lysis buffer (time 0 min) or into complete RPMI-1640 medium and kept for 5, 15, 30 or 60 min in 95% air/5% CO2 at 37°C. DNA repair was stopped by placing the slides in cold lysis buffer.
Internal standard samples were prepared from a single vial of cryopreserved AG10097 cells using the procedure described above. For each experiment done in duplicate, 16 gels with AG10097 cells were arranged on eight slides. Four slides were γ-irradiated with 6.3 Gy (positive internal standards) and the remaining four were not exposed to γ radiation (negative internal standards). Finally, slides were placed into cold lysis buffer.
Alkaline Comet Assay
The comet assay was performed under alkaline conditions following the procedure of Singh et al. (1) with some modifications. AG10097 cells and PBMCs embedded in low-melting-point agarose were lysed overnight at 4°C in lysis buffer (10 mM Tris-HCl, pH 10, 2.5 M NaCl, 100 mM EDTA, 5% DMSO, 1% Triton X-100). The unwinding step was performed for 40 min at 4°C in unwinding/electrophoresis buffer (300 mM NaOH, 1 mM EDTA, pH > 13). Electrophoresis was performed at 8°C for 30 min in unwinding/electrophoresis buffer at an electric-field strength of 0.73 V/cm and a current of 343 mA. The slides were then neutralized with a neutralizing buffer (0.4 Tris-HCl, pH 7.5), rinsed with distilled water, air-dried, stained with 2 µg/ml ethidium bromide, and covered with standard cover slips.
Comets were scored using an Eclipse E-400 fluorescence microscope (Nikon, Japan) attached to a Pulnix video camera (Kinetic Imaging LTD, Liverpool, UK) connected to the image analysis system Komet version 4.0 (Kinetic Imaging LTD). The Olive tail moment, tail DNA and tail length were measured (31, 32).
Mathematical and Statistical Analysis
Radiation-induced DNA damage in AG10097 cells
Four replicate sets of slides were prepared. Fifty comets were selected randomly for each sample. The four most damaged cells per sample were removed to minimize one of the sources of intersample variability: the presence of a minor fraction of cells with extremely high levels of DNA damage. Arithmetic means of the remaining 46 values for each comet assay parameter were calculated and used as indices of the DNA lesions in the sample. Sample mean values representing each γ-radiation dose were compared to identify potential single outlier samples. Data are presented as means and SEM for the three or four samples for each dose. The relationship between the DNA damage level in fresh and cryopreserved AG10097 cells irradiated with 1 to 10 Gy was analyzed by two-way ANOVA. All statistical analyses were performed using Statistica 7.1 (Statsoft Inc., Tulsa, OK).
DNA repair capacity in fresh and cryopreserved PBMCs
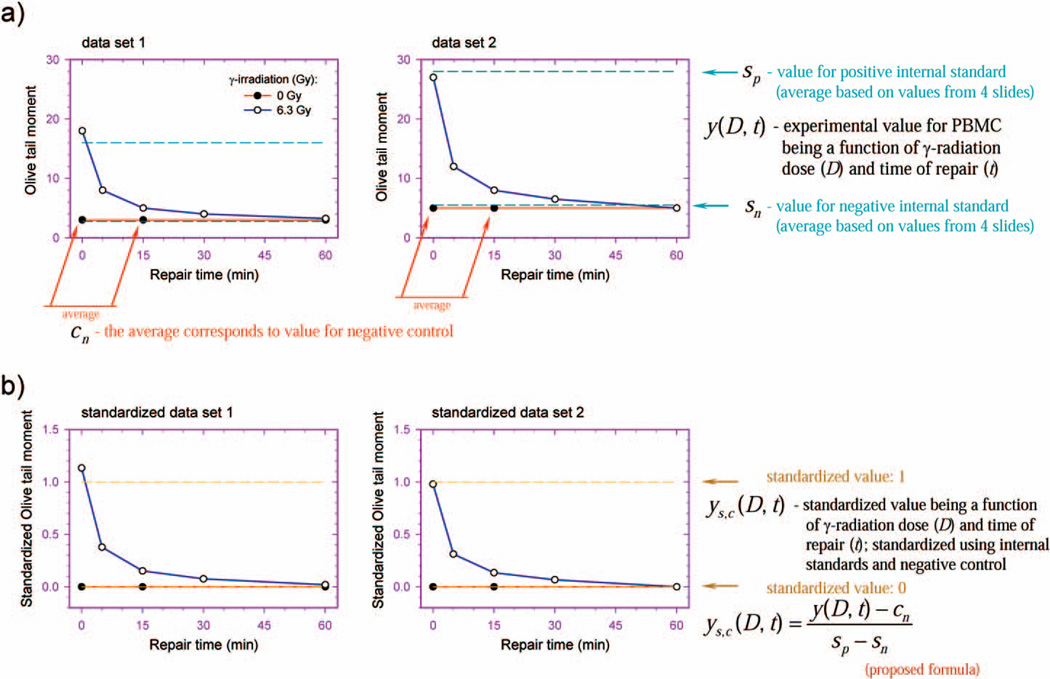
Two independent experiments, each with duplicate samples, were performed for fresh and cryopreserved PBMCs from each individual. Each of four sets of fresh and cryopreserved PBMC samples was exposed to γ radiation and incubated for repair. Each sample set was then processed together with four negative and four positive internal standards through unwinding and electrophoresis steps. Fifty PBMC images and 25 AG10097 cell images per sample were analyzed. Since conditions can differ slightly from electrophoresis to electrophoresis, the use of internal standards may minimize the variations in electrophoresis and experimental conditions. Standardization was performed using the arithmetic mean values for the PBMC samples and internal standards calculated after values corresponding to the four most damaged cells in each sample were removed. The standardization protocol before (Fig. 1A) and after (Fig. 1B) standardization was demonstrated for two model data sets. Negative and positive internal standards (broken blue line) were used to standardize experimental values for irradiated (empty circles) and unirradiated PBMCs (filled circles) (Fig. 1A) using the formula
where ysc(D, t) is standardized value for PBMCs, being a function of γ-radiation dose (D) and time of repair (t); y(D, t) is experimental value for PBMCs, being a function of γ-radiation dose (D) and time of repair (t); sn is the value for the negative internal standard; sp is the value for the positive internal standard; and cn is the value for the negative control being an average value of sample means obtained for unirradiated PBMCs subjected to 0- and 15-min repair incubations. Experimental (not standardized) values (Fig. 1A) obtained from the three or four data sets were used to calculate the means of standardized values for each point. Similarly, standardized values (Fig. 1B) from three or four data sets were used to calculate the means of the standardized values for each point.
FIG. 1.
Proposed new system of internal standards for analysis of DNA repair capacity using the alkaline comet assay. The standardization protocol before (panel a) and after (panel b) standardization is shown for two hypothetical data sets.
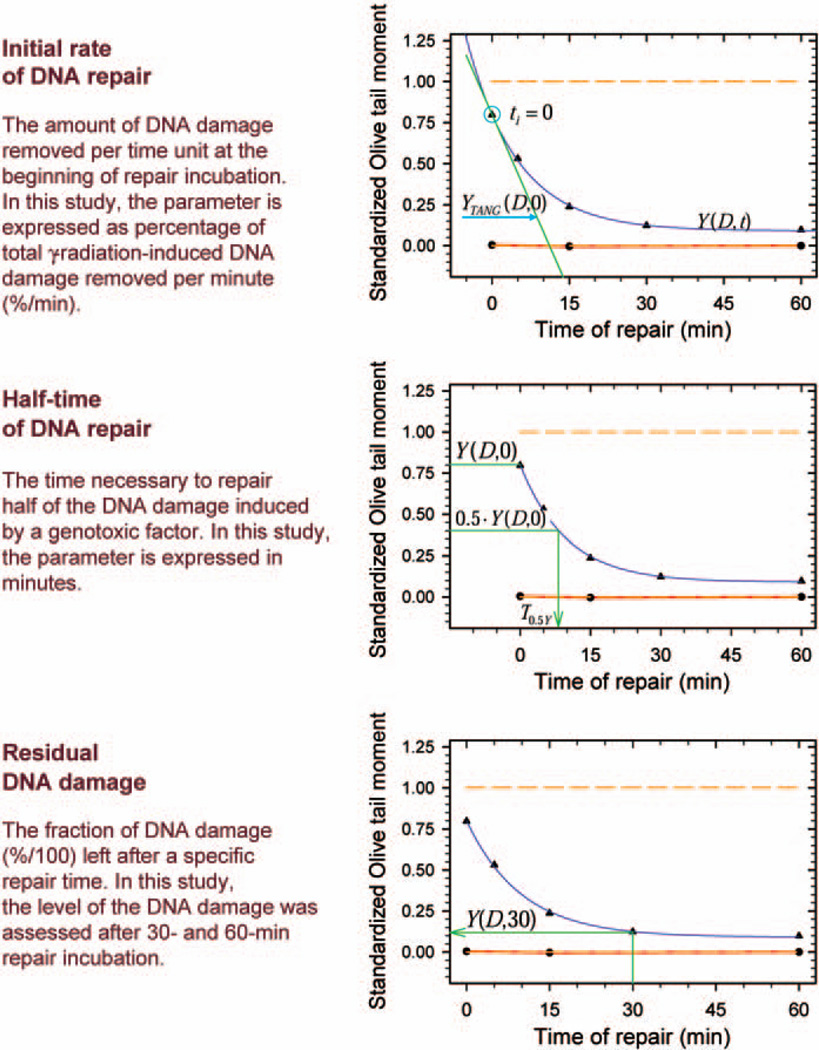
DNA repair kinetics was analyzed in SigmaPlot 8.0 (SPSS Inc., Chicago, IL) and Microsoft Excel 2003. Data on DNA repair kinetics for each individual were graphed in SigmaPlot on separate multiple line-scatter plots. Curves with two exponential terms described by the formula y = y0 + ae−bx + ce−dx were fitted to experimental data showing removal of single-strand breaks in irradiated cells. Exponential curves with two exponential terms described by the formula y = ae−bx + ce−dx were also fitted to standardized data on DNA damage removal in exposed PBMCs. The estimated equation coefficients a, b, c and d corresponding to the equation describing standardized data on kinetics were then used in Excel to assess the following parameters describing DNA repair capacity in the individuals studied: the initial rate of DNA repair, the half-time of DNA repair, and the residual DNA damage after 30 and 60 min (Fig. 2). The initial rate of DNA repair and is the percentage of the total radiation-induced DNA damage removed per minute (%/min) at the beginning of repair incubation. The initial rate of DNA repair was calculated from the slope coefficient of the equation of the straight line tangent to an exponential curve at the point for repair time 0 min:
where v0(DNAr) is the initial rate of DNA repair, A is the slope coefficient of the tangent equation, and a, b, c and d are coefficients of the exponential equation. The half-time of DNA repair is the time in minutes necessary to repair half of the DNA damage induced by a genotoxic factor. Residual DNA damage is the fraction of DNA damage remaining at a particular time after γ irradiation. The values of both parameters were derived directly from the estimated exponential equation.
FIG. 2.
Parameters used to describe DNA repair kinetics in cells.
DNA repair parameters obtained for fresh and cryopreserved PBMCs from 10 individuals were compared using Statistica 7.1. Residual analysis was performed using Statistica to identify outliers. One individual was identified as an outlier and was removed; the remaining nine individuals were taken for further analyses. The significance of the relationship between DNA repair capacity in fresh and cryopreserved PBMCs was analyzed by simple linear regression. Separate analyses were performed for every DNA repair parameter derived using the Olive tail moment as well as tail DNA. We also tested for differences in DNA repair capacity between fresh and cryopreserved PBMCs. For this purpose, we tested whether linear regression lines corresponding to the relationships between DNA repair capacity in fresh (variable x) and in cryopreserved PBMCs (variable y) could be described by the ideal function line y = x, which corresponds to the situation where DNA repair capacity in cryopreserved PBMCs is the same as in fresh PBMCs. To test for departure of the experimental regression line from the ideal line, we compared the experimental F value (Fexp) with the theoretical F distribution at v1 = 2 and v2 = n − v1 degrees of freedom. The experimental F value was derived from the formula
where x is an independent variable (DNA repair capacity in fresh PBMCs), y is a dependent variable (DNA repair capacity in cryopreserved PBMCs), n is the number of measurements, b0, b1 are regression coefficients (elevation, slope), and is the residual variance. The departure of experimental regression lines from the ideal function was tested for all DNA repair parameters.
RESULTS
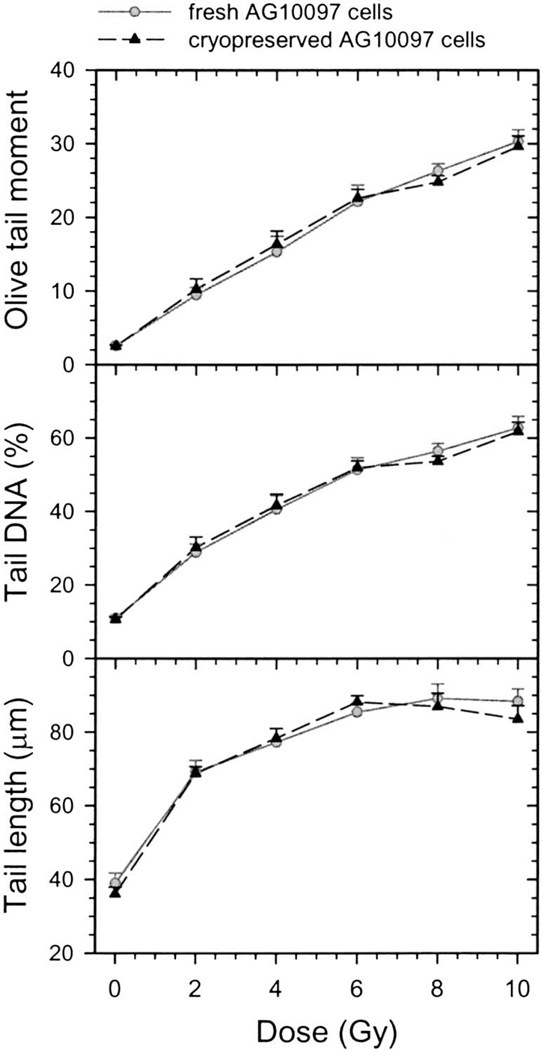
We first examined the dose–effect relationship between γ radiation and the DNA damage level in fresh and cryopreserved AG10097 cells, since we planned to use these cells as internal standards in the study of DNA repair in human populations. Cells embedded in agarose were exposed to 0–10 Gy γ radiation and processed using the comet assay. The DNA damage in cells measured as the Olive tail moment, tail DNA and tail length are shown in Fig. 3. There was no difference between fresh and cryopreserved AG10097 cells in the DNA damage measured by any of three comet assay parameters as analyzed by two-way ANOVA (P > 0.05). Thus cryopreservation did not alter the induction of DNA damage by γ radiation.
FIG. 3.
Effect of γ radiation on DNA damage in fresh and cryopreserved AG10097 lymphoblastoid cells measured by three comet assay parameters: Olive tail moment, tail DNA and tail length. Data are expressed as mean ± SE.
We also analyzed the Olive tail moment, tail DNA and tail length for linearity in the quantification of radiation-induced DNA damage. As presented in Fig. 3, the Olive tail moment data were linear for 0–6 Gy and saturated in the range of 6–10 Gy. The tail DNA data show a slight saturation-related departure from linearity in the dose range 0–6 Gy and a moderate departure at the higher doses. The tail length values increase rapidly between 0–2 Gy and then reach a plateau. In summary, the best results are obtained using the Olive tail moment and the tail DNA. The maximum dose at which no significant departure from linearity appears for these comet assay parameters is approximately 6 Gy.
We examined the usefulness of PHA stimulation in our DNA repair study of PBMCs. To perform these comparative experiments, we used the PHA stimulation method of Visvardis et al. (25). PBMCs embedded in gels were pre-incubated for 30 min in complete RPMI-1640 medium (RPMI-1640 medium + 20% FBS) supplemented with 0–20 µg/ml PHA-L, exposed to γ radiation, and then incubated in medium containing PHA for 5, 15 and 120 min repair. They were processed through the comet assay steps as described in the Materials and Methods. Treatment of PBMCs with PHA-L did not have an effect on DNA repair capacity (data not shown).
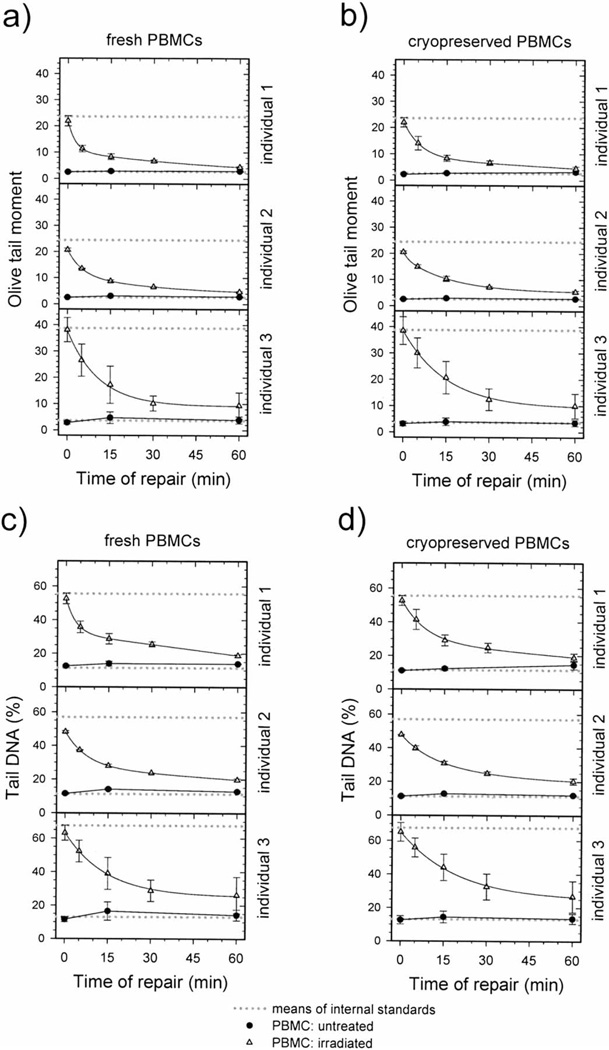
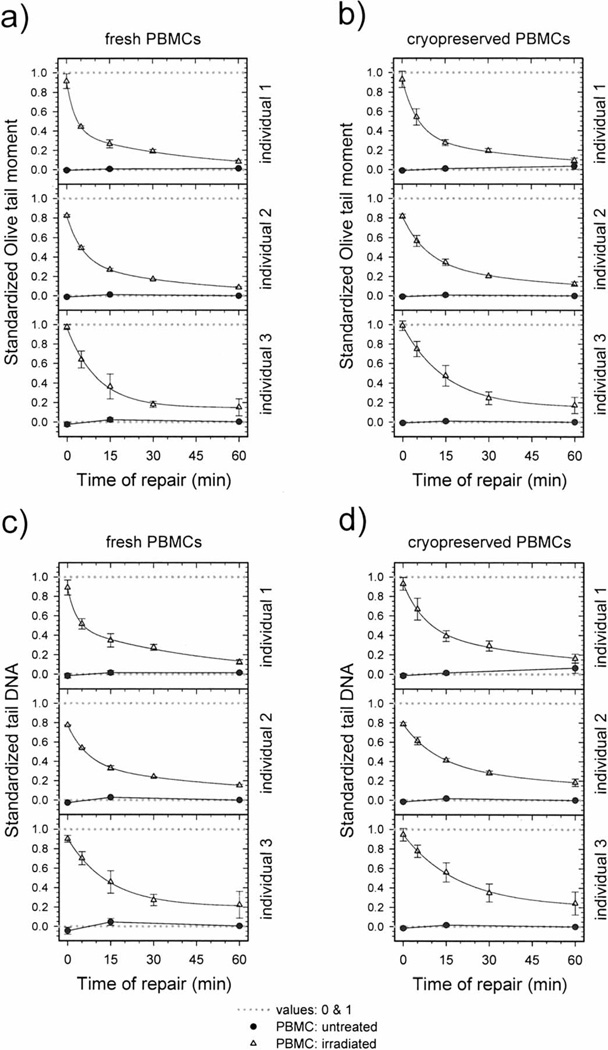
Standardization of DNA repair data for fresh and cryopreserved PBMCs from human participants was performed as described earlier (Fig. 1). Figures 4 and 5 show the DNA repair kinetics obtained from the experimental (not standardized) and standardized data for three individuals. Both figures present data for fresh and cryopreserved PBMCs, and DNA damage levels are expressed as the Olive tail moment or tail DNA. Note that the ranges of experimental values obtained for the level of DNA damage measured in irradiated cells vary among the individuals (Fig. 4). These differences are related mostly to variation in electrophoresis conditions since variations in mean values of negative and positive internal standards are also present. After using data standardization, DNA damage levels for different individuals are more similar, and it is easier to compare the DNA repair kinetics data (Fig. 5).
FIG. 4.
Repair kinetics of DNA damage induced by 6.3 Gy γ radiation in fresh (panels a, c) and cryopreserved (panels b, d) PBMCs taken from three individuals. DNA damage is expressed as Olive tail moment (panels a, b) and tail DNA (panels c, d). A minimum of 138 cells representing three or four replicate samples were analyzed for each experimental point. Data are expressed as means ± SEM. Solid curves: data fitted by the equation y = y0 + ae−bx + ce−dx.
FIG. 5.
Repair kinetics of DNA damage induced by 6.3 Gy γ radiation in fresh (panels a, c) and cryopreserved (panels b, d) PBMCs taken from three individuals. DNA damage is expressed as standardized Olive tail moment (panels a, b) and standardized tail DNA (panels c, d). A minimum of 138 cells representing three or four replicate samples were analyzed for each experimental point. Data are expressed as means ± SEM. Solid curves: data fitted by the equation y = ae−bx + ce−dx; dotted lines at 0 and 1 correspond to negative and positive internal standards.
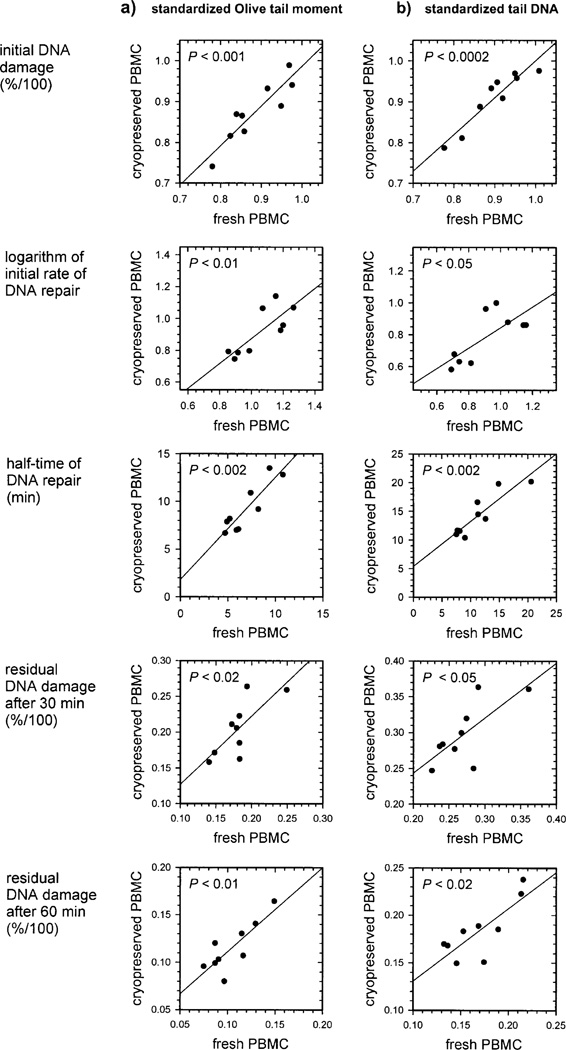
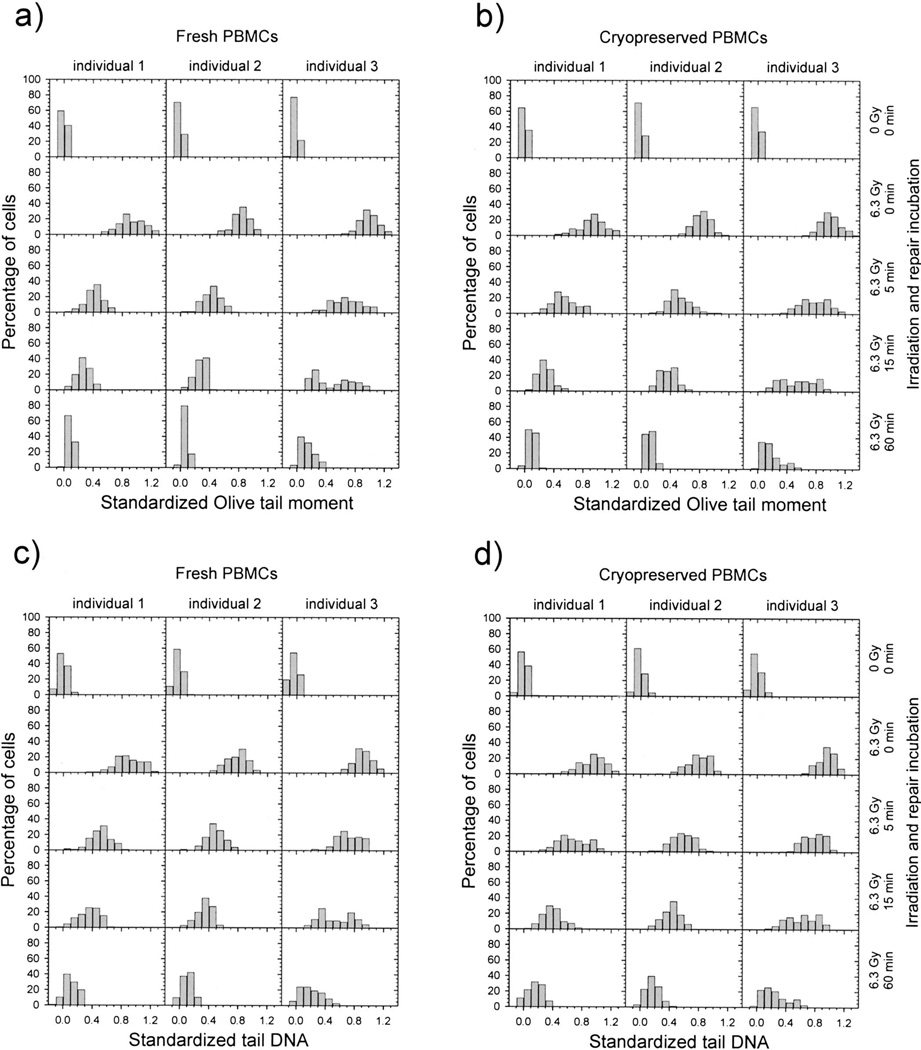
Finally, we compared DNA repair capacity in fresh and cryopreserved PBMCs obtained from nine individuals. We also evaluated whether the Olive tail moment or tail DNA was more useful in assessing DNA repair efficiency (Figs. 5–7). The data for repair kinetics are shown for three individuals in Fig. 5. In Fig. 6, the same data are represented as histograms of the standardized comet assay parameters corresponding to single cells. Individual 1 has the highest DNA repair capacity, and individual 3 has the lowest. Both fresh and cryopreserved PBMCs from individual 1 removed γ-radiation-induced DNA damage faster than cells from individual 3 (Fig. 5). This relationship is more pronounced after 5 and 15 min repair incubation and is substantially less pronounced after 30 and 60 min. Histograms representing 5 and 15 min repair incubation were shifted to the left for individual 1 and were characterized by faster DNA repair capacity (Fig. 6). We also found that the removal of DNA damage in cryopreserved PBMCs is slightly slower than in fresh ones (Fig. 5). Similar relationships between individuals and between fresh and cryopreserved PBMCs were observed for DNA damage measured by the standardized Olive tail moment and standardized tail DNA.
FIG. 7.
Comparison of DNA repair capacity in fresh and cryopreserved human PBMCs. DNA repair capacity was expressed using the indicated parameters. DNA repair parameters were assessed based on DNA damage removal kinetics expressed as the standardized Olive tail moment (panel a) and standardized tail DNA (panel b). Each graph shows the relationship between the parameters obtained for fresh (x axis) and cryopreserved (y axis) PBMCs from nine individuals. Each point corresponds to average data derived from three or four replicate standardized kinetics for a single individual. The relationships were described by linear regression curves and statistical significance by P values.
FIG. 6.
Histograms showing the distribution of standardized Olive tail moment (panels a, b) and standardized tail DNA (panels c, d) in fresh (panels a, c) and cryopreserved (panels b, d) PBMCs. PBMCs were untreated or were exposed to 6.3 Gy γ radiation and then incubated for 0–60 min. The PBMCs were obtained from the same individuals as in Fig. 5. A minimum of 138 cells representing three or four replicate samples were analyzed for each point.
Figure 7 shows a more detailed analysis of the effect of cryopreservation on DNA repair capacity in PBMCs from nine individuals. We also compared different DNA repair parameters (Fig. 2) and standardized comet assay parameters. We found interindividual variation in DNA repair capacity in PBMCs. For example, there is an approximately 2.75-fold interindividual variation in the initial rate of DNA repair and an approximately 2.5-fold variation in the half-time of DNA repair for fresh PBMCs. Moreover, we found a linear correlation between DNA repair capacity for fresh and cryopreserved PBMCs as measured by all the DNA repair parameters examined (Fig. 7a and b). These data showed a slightly higher correlation when DNA repair parameters were estimated using the standardized Olive tail moment rather than the standardized tail DNA. The presence of statistically significant linear relationships between DNA repair parameters obtained for fresh and cryopreserved PBMCs can also indirectly validate the significance of the differences in DNA repair capacity between individuals. The statistical significance of the interindividual variation in DNA repair capacity was confirmed by two-way ANOVA (P < 0.01) for all relationships presented in Fig. 7.
We observed a somewhat lower DNA repair capacity in cryopreserved PBMCs than in fresh PBMCs as measured by the logarithm of the initial rate of DNA repair and the half-time of DNA repair. This conclusion is based on the fact that the departure of the linear regression line from the ideal function line described by the equation y = x, where x is the DNA repair capacity in fresh PBMCs and y is the DNA repair capacity in cryopreserved PBMCs, is present for the initial rate of DNA repair (P < 0.01 and P < 0.001 for standardized Olive tail moment and tail DNA, respectively) and for the half-time of DNA repair (P < 0.02 and P < 0.002). No departure of the experimental regression line from the ideal function was observed for residual DNA damage after 30 and 60 min. The same result was observed using more direct analysis of the kinetics data for particular individuals (Fig. 5). Although the small difference between DNA repair capacity in fresh and cryopreserved PBMCs is present as measured by the initial rate of DNA repair and the half-time of DNA repair, this difference is predictable, and it can be determined by simple linear regression. Thus we can estimate DNA repair efficiency in fresh PBMCs and study the relationships between DNA repair capacity among a cohort of individuals by investigating DNA repair capacity in cryopreserved PBMCs.
DISCUSSION
We have devised a protocol for evaluating DNA repair capacity in cryopreserved PBMCs. We showed that unirradiated cryopreserved PBMCs do not accumulate DNA damage during a 2-h incubation in complete RPMI-1640 medium. These cells were able to remove γ-radiation-induced DNA damage. We also described an improved standardization procedure that facilitates comparison of data from multiple experiments. Finally, we have applied our standardization procedure in comparing DNA repair capacity in fresh and cryopreserved PBMCs. We found that the kinetics of removal of single-strand breaks was consistent between different repeats performed for the same individual and that interindividual variability was higher than variability between replicate sets of samples from the same individual. We show that the DNA repair capacity in cryopreserved PBMCs was comparable to that of fresh PBMCs, suggesting that cryopreserved PBMCs can be used in retrospective analyses of DNA repair capacity in human populations. The consistency of the results obtained for fresh and cryopreserved cells is further proof that the interindividual differences in PBMCs are reproducible. Thus PBMCs isolated from one sample from one individual can be cryopreserved and used for multiple independent retrospective analyses of DNA repair capacity.
In our experiments, PBMCs did not accumulate additional DNA damage during the repair incubation (Fig. 5). Moreover, we were able to detect repair of γ-radiation-induced DNA damage in cryopreserved PBMCs that were not stimulated with mitogens. The DNA repair capacity of unstimulated cryopreserved PBMCs stored for 1 to 5 months was comparable to that found in PHA-stimulated PBMCs (data not shown). Furthermore, we did not observe a decrease in DNA repair capacity with the length of storage of PBMCs at −80°C. Studies by Smith et al. (7) and Chung et al. (33) support our findings, although both measured DNA repair capacity at a single postirradiation time of 10 or 30 min. DNA repair in unstimulated cryopreserved lymphocytes was also detected by Risom and Knudsen using the unscheduled DNA synthesis assay (34).
Our analysis of comet assay parameters for linearity in the quantification of γ-radiation-induced DNA damage showed that the maximum dose at which no significant departure from linearity occurs is approximately 6 Gy for the Olive tail moment and tail DNA (Fig. 3). We used a dose of 6.3 Gy in our studies of DNA repair kinetics, since this dose allowed us to use the entire detection range of our alkaline comet assay protocol and maximized the capacity of the assay to detect the interindividual variation in DNA repair capacity. Comet assay-based studies from other groups using lower radiation doses (for example, 2 Gy) have been reported (24). However, these studies were unable to detect interindividual differences in lymphocytes from healthy cohort groups based on age, sex and smoking history (24). These studies were only able to identify differences in DNA repair capacity between lymphocytes from cancer patients, who have already been shown by numerous techniques to have lower DNA repair capacity, and healthy individuals. These studies may have been successful in identifying defects in DNA repair capacity because the magnitude of the defect is more pronounced in cancer patients (7, 35). Several other studies have used radiation doses in the range of 5–8 Gy (4, 7, 18). It is quite difficult to measure the slow repair component accurately if low γ-radiation doses are used to induce DNA damage. This component is responsible for repair of approximately 5% of γ-radiation-induced DNA damage as measured by the alkaline comet assay (30). Banath, Fushiki and Olive showed that a dose of 8 Gy was appropriate for analysis of the slow repair component in human lymphocytes (4). They were also able to observe interindividual variation in the half-time of SSB rejoining among a small cohort of normal healthy individuals using a γ-radiation dose of 8 Gy. Thus a dose of 6.3 Gy may be considered as appropriate for detecting subtle differences in the efficiency of the fast and slow repair components among human subjects using the alkaline comet assay.
The ability of the comet assay to distinguish subtle variations in DNA repair capacity in a healthy human population may depend on the extent of interexperimental variability. The use of internal standards to reduce interexperimental variability was proposed at the International Workshop on Genotoxicity Testing Procedures (in 1999) (29). Vaghef et al. used mouse lymphocytes as a negative internal standard for correcting experimental comet data. The values of these negative standards were arbitrarily set to 1.0 and were used to standardize the raw comet data obtained from different experiments (36). A more advanced system included use of permanent cultures of untreated K562 human erythroleukemia cells as a negative standard and cells treated with ethyl methane sulfonate (EMS) (2 mM) as a positive internal standard; experimental data were then standardized using an established formula (19). Finally, the negative and positive standards were used as quality control indicators allowing identification of experiments that should be discarded (37, 38).
In this paper, we introduced a new standardization formula, which is based on a simpler formula, ysc(D, t) = [y(D, t) − sn]/(s − sn), derived by De Boeck et al. (19). In the proposed formula, the difference between the separate experimental value ysc(D, t) and the negative control cn is related to an increase in the DNA damage measured in irradiated AG10097 cells (sp − sn). AG10097 cells grow rapidly in suspension, allowing the use of multiple aliquots from the same cell suspension and avoiding the possible induction of DNA damage that may occur in internal standards using cells that grow as monolayers requiring trypsinization or scraping. Adding a negative control Cn to our formula allows the standardized value for unexposed cells to be set to zero. The standardized values for irradiated PBMCs will therefore show the radiation-related increases in DNA damage over the baseline level of DNA damage in unirradiated PBMCs from the same individual. This minimizes interference of interindividual variation in baseline DNA damage levels during measurement of induced DNA damage and ultimately DNA repair capacity in human populations. Thus our standardization procedure provides a way to separate interindividual variation in DNA repair capacity from the interexperimental variation and interindividual differences in DNA damage levels.
A variety of DNA lesions are produced during exposure of cellular DNA to γ radiation including single-strand breaks (SSBs), double-strand breaks (DSBs), AP sites and products of base oxidation. SSBs and DSBs are detected directly by the alkaline comet assay. A DSB is interpreted by the assay as two separate SSBs. AP sites and some other types of DNA lesions are transformed into SSBs at pH greater than 13 during the alkaline unwinding step. Modified bases can also affect the outcome of this assay after their conversion into AP sites and SSBs by DNA repair pathways (39). At least two components of SSB repair kinetics have been identified using alkaline sucrose gradient sedimentation (40), alkali unwinding (41), alkaline elution (42) and the alkaline comet assay (30). The presence of these components is related to the different rates of repair for various types of DNA damage. Most SSBs are repaired very rapidly in most cell types, including PBMCs. They constitute the majority of DNA lesions removed during the first 5–10 min of repair incubation since they are removed by the fast DNA repair component (30). DSBs are generated during γ irradiation at a rate approximately 40 times slower than SSBs and are responsible for a 5% increase in the radiation-induced DNA damage detected by the alkaline comet assay (43). Most DSBs are repaired by the non-homologous end-joining (NHEJ) pathway at rates slightly lower than those at which SSBs are repaired (44). Complex DNA damage, which may include DSBs or multiple SSBs, may constitute a form of damage removed by the slow DNA repair component. It has been estimated that leukocytes will rejoin fewer than 70% of induced DSBs 24 h after exposure to 75 Gy (4). Oxidative base lesions are not detected directly by the comet assay. They are measured indirectly as these lesions are converted transiently into AP sites and SSBs before their complete repair. The half-time of repair for 100 µM H2O2-induced oxidative DNA damage was estimated to be approximately 2 h for formamidopyrimidine-DNA glycosylase (Fpg)-labile sites and about 20 h for endonuclease III-labile sites (39, 45). These transitory lesions may be detected by the assay as damage repaired by slower DNA repair components.
The bi-exponential repair model describes fast and slow DNA repair components and residual non-repairable DNA damage. Both the fast and slow repair components are usually characterized by their half-times and fractions of initial DNA damage repaired. Residual non-repairable DNA damage is the DNA damage that cannot be removed by any of the repair components. The bi-exponential repair model is used in Olive’s laboratory to describe repair of DNA damage detected by the alkaline comet assay (4, 30, 43). Many authors use the simpler monoexponential repair model to describe repair kinetics in irradiated cells (24, 46, 47). The monoexponential model includes information about the amount of the repairable DNA damage fraction, the half-time of their repair, and the value corresponding to the amount of residual non-repairable DNA damage.
Less complex analyses of SSB repair kinetics may involve direct comparison of the curves for DNA damage repair for different cell types, cell lines or individuals without calculating specific parameters that describe DNA repair kinetics (48, 49). Comparison of DNA repair activity can be limited to the assessment of the initial DNA damage and the residual DNA damage measured 10, 15 or 30 min after irradiation (7, 18, 33). The last approach with initial DNA damage and residual DNA damage requires only a small number of samples to assess DNA repair capacity but provides a limited amount of information about DNA repair kinetics.
In our study, we identified a set of DNA repair parameters that permitted us to examine both the fast and slow components of SSB repair (Fig. 2). This set of parameters was optimized for use in an ongoing large epidemiological study. The initial rate of DNA repair is the total of all the repair components responsible for removing different types of repairable DNA lesions immediately after exposure to γ radiation and is largely dependent on the initial rate of the fast component (30). The term “initial rate of DNA repair” has been used differently by others. Blaise and coworkers used the term “initial rate” to describe the average rate of DNA repair during the 15-min period immediately after irradiation (50). The term “initial rate of DNA repair” has also been used to characterize the half-time of DNA repair (51, 52). It has also been referred to as the fast component of DNA repair (53, 54). Other parameters used in our study, the half-time of DNA repair and residual DNA damage after 30 min, are dependent on both the fast and slower DNA repair components. Finally, the residual DNA damage after 60 min is affected mainly by the slower DNA repair kinetics and also by the presence of unrepairable DNA lesions.
We described the kinetics of SSB removal in fresh and frozen PBMCs obtained from nine individuals (Fig. 7). We found that interindividual variation in DNA repair capacity in PBMCs is present in this small human cohort. A statistically significant correlation between DNA repair capacity for fresh and cryopreserved PBMCs was observed for all DNA repair parameters analyzed (Fig. 7). Correlations were stronger for data analyzed using the standardized Olive tail moment compared to standardized tail DNA. Since the logarithm of the initial rate of DNA repair and the half-time of DNA repair are consistent between fresh and cryopreserved PBMCs, it is likely that these parameters will provide a reliable estimate of the rate of the fast SSB repair component in PBMCs. Based on these data, using these measures in future studies of DNA repair in human populations may be beneficial in linking DNA repair capacity to disease risk and treatment response. Application of the newly introduced logarithm of the initial rate of DNA repair together with the other DNA repair parameters may be provide additional insight into interindividual variations in DNA repair processes.
Use of the alkaline comet assay in human epidemiological studies has been hampered by the notions that DNA repair capacity could not be measured accurately in cryopreserved PBMCs and that interexperimental variability prevented accurate assessment of baseline DNA damage levels and DNA repair capacity in studies with large numbers of subjects. Our work hopefully provides a useful technical and methodological approach that will facilitate the retrospective use of comet to correlate individual DNA repair capacity with aging and sporadically occurring age-associated chronic diseases in human populations.
Acknowledgments
We are grateful to Drs. Ngozi Ejiogu, Kamala Foster and Alan B. Zonderman for providing us human samples. We thank Drs. Raymond R. Tice, Simon G. Nyaga, Larry J. Brant, Michael J. Pazin, Myriam Gorospe and Andrew R. Collins for valuable discussions. We also thank to Althaf Lohani for technical assistance. This research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
REFERENCES
- 1.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 2.Trzeciak A, Kowalik J, Malecka-Panas E, Drzewoski J, Wojewodzka M, Iwanenko T, Blasiak J. Genotoxicity of chromium in human gastric mucosa cells and peripheral blood lymphocytes evaluated by the single cell gel electrophoresis (comet assay) Med. Sci. Monit. 2000;6:24–29. [PubMed] [Google Scholar]
- 3.Sekihashi K, Yamamoto A, Matsumura Y, Ueno S, Watanabe-Akanuma M, Kassie F, Knasmuller S, Tsuda S, Sasaki YF. Comparative investigation of multiple organs of mice and rats in the comet assay. Mutat. Res. 2002;517:53–75. doi: 10.1016/s1383-5718(02)00034-7. [DOI] [PubMed] [Google Scholar]
- 4.Banath JP, Fushiki M, Olive PL. Rejoining of DNA single-and double-strand breaks in human white blood cells exposed to ionizing radiation. Int. J. Radiat. Biol. 1998;73:649–660. doi: 10.1080/095530098141906. [DOI] [PubMed] [Google Scholar]
- 5.Dusinská M, Collins A, Kazimí rová A, Barancoková M, Harrington V, Volkovová K, Staruchová M, Horská A, Wsolová L, Krytooulos S. Genotoxic effects of asbestos in humans. Mutat. Res. 2004;553:91–102. doi: 10.1016/j.mrfmmm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 6.Alapetite C, Benoit A, Moustacchi E, Sarasin A. The comet assay as a repair test for prenatal diagnosis of Xeroderma pigmentosum and trichothiodystrophy. J. Invest. Dermatol. 1997;108:154–159. doi: 10.1111/1523-1747.ep12332692. [DOI] [PubMed] [Google Scholar]
- 7.Smith TR, Miller MS, Lohman KK, Case LD, Hu JJ. DNA damage and breast cancer risk. Carcinogenesis. 2003;24:883–889. doi: 10.1093/carcin/bgg037. [DOI] [PubMed] [Google Scholar]
- 8.Collins BH, Horska A, Hotten PM, Riddoch C, Collins AR. Kiwifruit protects against oxidative DNA damage in human cells in vitro. Nutr. Cancer. 2001;39:148–153. doi: 10.1207/S15327914nc391_20. [DOI] [PubMed] [Google Scholar]
- 9.Dusinska M, Dzupinkova Z, Wsolova L, Harrington V, Collins AR. Possible involvement of XPA in repair of oxidative DNA damage deduced from analysis of damage, repair and genotype in a human population study. Mutagenesis. 2006;21:205–211. doi: 10.1093/mutage/gel016. [DOI] [PubMed] [Google Scholar]
- 10.Piperakis SM, Visvardis EE, Sagnou M, Tassiou AM. Effects of smoking and aging on oxidative DNA damage of human lymphocytes. Carcinogenesis. 1998;19:695–698. doi: 10.1093/carcin/19.4.695. [DOI] [PubMed] [Google Scholar]
- 11.Kopjar N, Garaj-Vrhovac V. Application of the alkaline comet assay in human biomonitoring for genotoxicity: a study on Croatian medical personnel handling antineoplastic drugs. Mutagenesis. 2001;16:71–78. doi: 10.1093/mutage/16.1.71. [DOI] [PubMed] [Google Scholar]
- 12.Fujita N, Horiike S, Sugimoto R, Tanaka H, Iwasa M, Kobayashi Y, Hasegawa K, Ma N, Kawanishi S, Kaito M. Hepatic oxidative DNA damage correlates with iron overload in chronic hepatitis C patients. Free Radic. Biol. Med. 2007;42:353–362. doi: 10.1016/j.freeradbiomed.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Domenici FA, Vannucchi MT, Jordao AA, Jr, Meirelles MS, Vannucchi H. DNA oxidative damage in patients with dialysis treatment. Ren. Fail. 2005;27:689–694. doi: 10.1080/08860220500242678. [DOI] [PubMed] [Google Scholar]
- 14.Hannon-Fletcher MP, O’Kane MJ, Moles KW, Weatherup C, Barnett CR, Barnett YA. Levels of peripheral blood cell DNA damage in insulin dependent diabetes mellitus human subjects. Mutat. Res. 2000;460:53–60. doi: 10.1016/s0921-8777(00)00013-6. [DOI] [PubMed] [Google Scholar]
- 15.Collins AR, Gedik CM, Olmedilla B, Southon S, Bellizzi M. Oxidative DNA damage measured in human lymphocytes: large differences between sexes and between countries, and correlations with heart disease mortality rates. FASEB J. 1998;12:1397–1400. [PubMed] [Google Scholar]
- 16.Collins AR, Raslova K, Somorovska M, Petrovska H, Ondrusova A, Vohnout B, Fabry R, Dusinska M. DNA damage in diabetes: correlation with a clinical marker. Free Radic. Biol. Med. 1998;25:373–377. doi: 10.1016/s0891-5849(98)00053-7. [DOI] [PubMed] [Google Scholar]
- 17.Rajeswari N, Ahuja YR, Malini U, Chandrashekar S, Balakrishna N, Rao KV, Khar A. Risk assessment in first degree female relatives of breast cancer patients using the alkaline comet assay. Carcinogenesis. 2000;21:557–561. doi: 10.1093/carcin/21.4.557. [DOI] [PubMed] [Google Scholar]
- 18.Popanda O, Ebbeler R, Twardella D, Helmbold I, Gotzes F, Schmezer P, Thielmann HW, von Fournier D, Haase W, Chang-Claude J. Radiation-induced DNA damage and repair in lymphocytes from breast cancer patients and their correlation with acute skin reactions to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2003;55:1216–1225. doi: 10.1016/s0360-3016(02)04415-2. [DOI] [PubMed] [Google Scholar]
- 19.De Boeck M, Touil N, De Visscher G, Vande PA, Kirsch-Volders M. Validation and implementation of an internal standard in comet assay analysis. Mutat. Res. 2000;469:181–197. doi: 10.1016/s1383-5718(00)00075-9. [DOI] [PubMed] [Google Scholar]
- 20.Fairbairn DW, Olive PL, O’Neill KL. The comet assay: a comprehensive review. Mutat. Res. 1995;339:37–59. doi: 10.1016/0165-1110(94)00013-3. [DOI] [PubMed] [Google Scholar]
- 21.Collins AR, Harrington V, Drew J, Melvin R. Nutritional modulation of DNA repair in a human intervention study. Carcinogenesis. 2003;24:511–515. doi: 10.1093/carcin/24.3.511. [DOI] [PubMed] [Google Scholar]
- 22.Duthie SJ, Pirie L, Jenkinson AM, Narayanan S. Cryopreserved versus freshly isolated lymphocytes in human biomonitoring: endogenous and induced DNA damage, antioxidant status and repair capability. Mutagenesis. 2002;17:211–214. doi: 10.1093/mutage/17.3.211. [DOI] [PubMed] [Google Scholar]
- 23.Muller WU, Bauch T, Streffer C, von Mallek D. Does radiotherapy affect the outcome of the comet assay? Br. J. Radiol. 2002;75:608–614. doi: 10.1259/bjr.75.895.750608. [DOI] [PubMed] [Google Scholar]
- 24.Palyvoda O, Polanska J, Wygoda A, Rzeszowska-Wolny J. DNA damage and repair in lymphocytes of normal individuals and cancer patients: studies by the comet assay and micronucleus tests. Acta Biochim. Pol. 2003;50:181–190. [PubMed] [Google Scholar]
- 25.Visvardis EE, Tassiou AM, Piperakis SM. Study of DNA damage induction and repair capacity of fresh and cryopreserved lymphocytes exposed to H2O2 and gamma-irradiation with the alkaline comet assay. Mutat. Res. 1997;383:71–80. doi: 10.1016/s0921-8777(96)00047-x. [DOI] [PubMed] [Google Scholar]
- 26.Schmezer P, Rajaee-Behbahani N, Risch A, Thiel S, Rittgen W, Drings P, Dienemann H, Kayser KW, Schulz V, Bartsch H. Rapid screening assay for mutagen sensitivity and DNA repair capacity in human peripheral blood lymphocytes. Mutagenesis. 2001;16:25–30. doi: 10.1093/mutage/16.1.25. [DOI] [PubMed] [Google Scholar]
- 27.O’Flynn K, Knott LJ, Russul-Saib M, Abdul-Gaffar R, Morgan G, Beverley PC, Linch DC. CD2 and CD3 antigens mobilize Ca2+ independently. Eur J. Immunol. 1986;16:580–584. doi: 10.1002/eji.1830160521. [DOI] [PubMed] [Google Scholar]
- 28.Chazal M, Roux E, Alapetite C, Roulin C, Moustacchi E, Douki T, Baudouin C, Charveron M, Basset-Seguin N. Interexperimental and interindividual variations of DNA repair capacities after UV-B and UV-C irradiations of human keratinocytes and fibroblasts. Photochem. Photobiol. 2004;79:286–290. doi: 10.1562/ca-03-17.1. [DOI] [PubMed] [Google Scholar]
- 29.Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000;35:206–221. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 30.Olive PL. The role of DNA single- and double-strand breaks in cell killing by ionizing radiation. Radiat. Res. 1998;150:S42–S51. [PubMed] [Google Scholar]
- 31.Kumaravel TS, Jha AN. Reliable comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutat. Res. 2006;605:7–16. doi: 10.1016/j.mrgentox.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Olive PL, Banath JP, Durand RE. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the “comet” assay. Radiat. Res. 1990;122:86–94. [PubMed] [Google Scholar]
- 33.Chang JL, Chen G, Lampe JW, Ulrich CM. DNA damage and repair measurements from cryopreserved lymphocytes without cell culture—a reproducible assay for intervention studies. Environ. Mol. Mutagen. 2006;47:503–508. doi: 10.1002/em.20219. [DOI] [PubMed] [Google Scholar]
- 34.Risom L, Knudsen LE. Use of cryopreserved peripheral mononuclear blood cells in biomonitoring. Mutat. Res. 1999;440:131–138. doi: 10.1016/s1383-5718(99)00019-4. [DOI] [PubMed] [Google Scholar]
- 35.Wei Q, Cheng L, Amos CI, Wang LE, Guo Z, Hong WK, Spitz MR. Repair of tobacco carcinogen-induced DNA adducts and lung cancer risk: a molecular epidemiologic study. J. Natl. Cancer Inst. 2000;92:1764–1772. doi: 10.1093/jnci/92.21.1764. [DOI] [PubMed] [Google Scholar]
- 36.Vaghef H, Nygren P, Edling C, Bergh J, Hellman B. Alkaline single-cell gel electrophoresis and human biomonitoring for genotoxicity: a pilot study on breast cancer patients undergoing chemotherapy including cyclophosphamide. Mutat. Res. 1997;395:127–138. doi: 10.1016/s1383-5718(97)00157-5. [DOI] [PubMed] [Google Scholar]
- 37.Speit G, Witton-Davies T, Heepchantree W, Trenz K, Hoffmann H. Investigations on the effect of cigarette smoking in the comet assay. Mutat. Res. 2003;542:33–42. doi: 10.1016/j.mrgentox.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Tice RR, Nylander-French LA, French JE. Absence of systemic in vivo genotoxicity after dermal exposure to ethyl acrylate and tripropylene glycol diacrylate in Tg.AC (v-Ha-ras) mice. Environ. Mol. Mutagen. 1997;29:240–249. [PubMed] [Google Scholar]
- 39.Collins AR, Dobson VL, Dusinska M, Kennedy G, Stetina R. The comet assay: what can it really tell us? Mutat. Res. 1997;375:183–193. doi: 10.1016/s0027-5107(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 40.Wheeler KT, Wierowski JV. DNA repair kinetics in irradiated undifferentiated and terminally differentiated cells. Radiat. Environ. Biophys. 1983;22:3–19. doi: 10.1007/BF01323757. [DOI] [PubMed] [Google Scholar]
- 41.Dikomey E, Franzke J. Three classes of DNA strand breaks induced by X-irradiation and internal beta-rays. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1986;50:893–908. doi: 10.1080/09553008614551311. [DOI] [PubMed] [Google Scholar]
- 42.vanAnkeren SC, Murray D, Meyn RE. Induction and rejoining of gamma-ray-induced DNA single- and double-strand breaks in Chinese hamster AA8 cells and in two radiosensitive clones. Radiat. Res. 1988;116:511–525. [PubMed] [Google Scholar]
- 43.Olive PL, Banath JP. Induction and rejoining of radiation-induced DNA single-strand breaks: “tail moment” as a function of position in the cell cycle. Mutat. Res. 1993;294:275–283. doi: 10.1016/0921-8777(93)90010-e. [DOI] [PubMed] [Google Scholar]
- 44.Iliakis G, Wang H, Perrault AR, Boecker W, Rosidi B, Windhofer F, Wu W, Guan J, Terzoudi G, Pantelias G. Mechanisms of DNA double strand break repair and chromosome aberration formation. Cytogenet. Genome Res. 2004;104:14–20. doi: 10.1159/000077461. [DOI] [PubMed] [Google Scholar]
- 45.Collins A, Dusinska M, Franklin M, Somorovska M, Petrovska H, Duthie S, Fillion L, Panayiotidis M, Raslova K, Vaughan N. Comet assay in human biomonitoring studies: reliability, validation, and applications. Environ. Mol. Mutagen. 1997;30:139–146. doi: 10.1002/(sici)1098-2280(1997)30:2<139::aid-em6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 46.Wojewodzka M, Kruszewski M, Szumiel I. Effect of signal transduction inhibition in adapted lymphocytes: micronuclei frequency and DNA repair. Int. J. Radiat. Biol. 1997;71:245–252. doi: 10.1080/095530097144111. [DOI] [PubMed] [Google Scholar]
- 47.Zheng H, Olive PL. Reduction of tumor hypoxia and inhibition of DNA repair by nicotinamide after irradiation of SCCVII murine tumors and normal tissues. Cancer Res. 1996;56:2801–2808. [PubMed] [Google Scholar]
- 48.Trenz K, Rothfuss A, Schutz P, Speit G. Mutagen sensitivity of peripheral blood from women carrying a BRCA1 or BRCA2 mutation. Mutat. Res. 2002;500:89–96. doi: 10.1016/s0027-5107(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 49.Alapetite C, Thirion P, de la Rochefordiere A, Cosset JM, Moustacchi E. Analysis by alkaline comet assay of cancer patients with severe reactions to radiotherapy: defective rejoining of radioinduced DNA strand breaks in lymphocytes of breast cancer patients. Int. J. Cancer. 1999;83:83–90. doi: 10.1002/(sici)1097-0215(19990924)83:1<83::aid-ijc16>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 50.Blaise R, Alapetite C, Masdehors P, Merle-Beral H, Roulin C, Delic J, Sabatier L. High levels of chromosome aberrations correlate with impaired in vitro radiation-induced apoptosis and DNA repair in human B-chronic lymphocytic leukaemia cells. Int. J. Radiat. Biol. 2002;78:671–679. doi: 10.1080/09553000110120364. [DOI] [PubMed] [Google Scholar]
- 51.Dunne AL, Price ME, Mothersill C, McKeown SR, Robson T, Hirst DG. Relationship between clonogenic radiosensitivity, radiation-induced apoptosis and DNA damage/repair in human colon cancer cells. Br. J. Cancer. 2003;89:2277–2283. doi: 10.1038/sj.bjc.6601427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dwarkanath BS, Zolzer F, Chandana S, Bauch T, Adhikari JS, Muller WU, Streffer C, Jain V. Heterogeneity in 2-deoxy-d-glucose-induced modifications in energetics and radiation responses of human tumor cell lines. Int. J. Radiat. Oncol. Biol. Phys. 2001;50:1051–1061. doi: 10.1016/s0360-3016(01)01534-6. [DOI] [PubMed] [Google Scholar]
- 53.Losada R, Rivero MT, Slijepcevic P, Goyanes V, Fernandez JL. Effect of Wortmannin on the repair profiles of DNA double-strand breaks in the whole genome and in interstitial telomeric sequences of Chinese hamster cells. Mutat. Res. 2005;570:119–128. doi: 10.1016/j.mrfmmm.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Mendiola-Cruz MT, Morales-Ramirez P. Damage-repair kinetics and early adaptive response induced by gamma rays in murine leukocytes in vivo. Somat. Cell. Mol. Genet. 1999;25:287–299. doi: 10.1023/a:1019964331735. [DOI] [PubMed] [Google Scholar]