Abstract
Background
Among the different serotypes of Streptococcus pneumoniae, serotype 3 has received global attention. We report the fatal case of a 76-year-old Japanese man who had an invasive pneumococcal disease associated with pneumonia caused by serotype 3 S. pneumoniae.
Case presentation
The patient had a history of hypertension, laryngeal cancer, chronic obstructive pulmonary disease, and type 2 diabetes mellitus. Following a cerebral arteriovenous malformation hemorrhage, he underwent surgery to remove the hematoma and began rehabilitation. On day 66 of hospitalization, he suddenly developed a fever, and coarse crackles and wheezes were heard in his right lung. A diagnosis of hospital-acquired aspiration pneumonia was made, and initial treatment with piperacillin/tazobactam was started. Teicoplanin was added after S. pneumoniae was isolated from the blood culture, however, the patient died 5 days later. The S. pneumoniae detected in the sputum smear was serotype 3, showed mucoid colonies and susceptibility to penicillins, cephalosporins, carbapenems, and levofloxacin, but resistance to erythromycin.
Conclusion
We experienced a fatal case of pneumonia caused by mucoid serotype 3 S. pneumoniae with a thick capsule. Serotype 3-associated pneumonia may develop a wider pulmonary infiltrative shadow, a prolonged therapeutic or hospitalization course, and a poor outcome. Careful observation and intervention are required, and the use of additional antibiotics or intravenous immunoglobulins should be considered in such cases. Pneumococcal immunization is also an important public health measure to minimize the development of severe infections caused by serotype 3 strains.
Keywords: Invasive Pneumococcal disease, IPD, Mucoid, Streptococcus pneumoniae, Serotype 3
Background
More than 95 different antigenic serotypes of Streptococcus pneumoniae are known. Owing to a thicker capsule, greater virulence, and higher mortality rate compared to other strains [1, 2], serotype 3 S. pneumoniae has received global attention. We report a case of invasive pneumococcal disease (IPD) associated with pneumonia caused by serotype 3 S. pneumoniae, with a dramatic clinical course.
Case presentation
A 76-year-old Japanese man with a history of hypertension, laryngeal cancer, chronic obstructive pulmonary disease (COPD), and type 2 diabetes mellitus developed a cerebral arteriovenous malformation hemorrhage and was hospitalized at Aichi Medical University Hospital, Japan. His vaccination history was unknown. Case characteristics and laboratory data on the first visit are summarized in Table 1. Following surgery for removal of the hematoma, he began rehabilitation and was encouraged to engage in early postoperative ambulation. In March, 2015, on the 66th day of hospitalization, he developed a sudden fever and exhibited a sharp decline in oxygenation.
Table 1.
Patient characteristics and the first visit laboratory test findings
| Patient characteristics | |
| Height | 168.0 cm |
| Body weight | 67.4 kg |
| Body mass index | 23.9 kg/m2 |
| Hematological test | |
| White blood cell count | 7700/µL |
| Neutrophil 86.0% | |
| Lymphocyte 11.0% | |
| Monocyte 2.0% | |
| Red blood cell count | 441 × 104/µL |
| Hemoglobin | 13.1 g/dL |
| Platelet count | 27.6 × 104/µL |
| Blood gas | |
| pH | 7.182 |
| pCO2 | 53.9 mmHg |
| pO2 | 118.9 mmHg |
| HCO3 − | 19.5 mmol/L |
| Lactate | 91.6 mg/dL |
| Biochemical test | |
| Blood urea nitrogen | 20.3 mg/dL |
| Creatinine | 1.01 mg/dL |
| Estimated glomerular filtration rate | 55 mL/min/1.73m2 |
| Sodium | 126 mEq/L |
| Potassium | 4.8 mEq/L |
| Chloride | 90 mEq/L |
| Total bilirubin | 0.14 mg/dL |
| Aspartate aminotransferase | 57 IU/L |
| Alanine aminotransferase | 31 IU/L |
| Alkaline phosphatase | 427 IU/L |
| Lactate dehydrogenase | 402 IU/L |
| γ-glutamyl transpeptidase | 152 IU/L |
| Cholinesterase | 123 IU/L |
| Creatine phosphokinase | 31 IU/L |
| Albumin | 2.5 g/dL |
| C-reactive protein | 16.26 mg/dL |
| Procalcitonin | 13.81 ng/mL |
At the onset of fever, the patient’s vital signs were as follows: body temperature, 37.8 °C; blood pressure, 84/41 mmHg; heart rate, 107/min; respiration rate, 30/min; and SpO2, 82% (room air). Blood gas analysis (room air) showed pH 7.538, 25.7 mmHg, 47.6 mmHg, HCO3 − 21.4 mmol/L, and lactate 38.9 mg/dL. His level of consciousness was I-2 on the Japan Coma Scale. Physical examination showed coarse crackles and wheezes in the right lung. Based on chest radiography (Fig. 1) and computed tomography images (Fig. 2), hospital-acquired aspiration pneumonia was diagnosed.
Fig. 1.
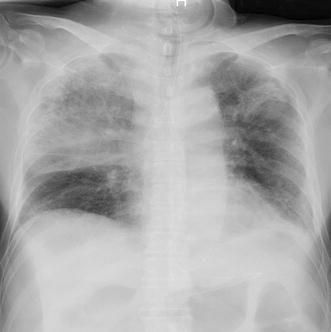
Chest radiography (decubitus) image at the onset of fever
Fig. 2.

Chest computed tomography images at the onset of fever
Piperacillin/tazobactam 4.5 g was administered three times daily as initial treatment (Fig. 3). Streptococcus pneumoniae infection was suspected based on a rapid identification test using a sputum smear, and a strain of S. pneumoniae was isolated from the blood culture sampled at the onset of fever. The patient was admitted to the intensive care unit and teicoplanin was added to his treatment regimen. However, his SpO2 and respiratory rate continued to be unstable. After 5 days of concomitant teicoplanin administration, the patient died.
Fig. 3.
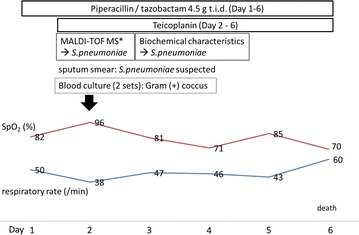
Clinical course of the present case, diagnosed as an invasive pneumococcal disease with pneumonia. Asterisk denotes matrix-assisted laser desorption/ionization-time of flight mass spectrometry
Streptococcus pneumoniae detected in the smear and the morphologic characteristics of the colonies on blood agar are shown in Fig. 4. The isolate was mucoid serotype strain 3, with a thick capsule. Antibiotic susceptibility to penicillins, cephalosporins, carbapenems, and levofloxacin was good, with resistance observed only to a macrolide (erythromycin) (Table 2).
Fig. 4.
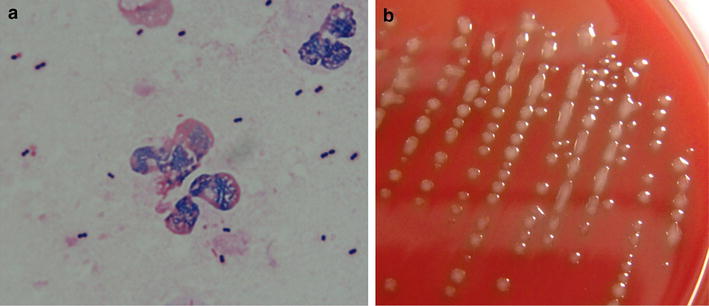
Sputum smear (a) and colonies (b) of the isolate from the patient showing serotype 3 Streptococcus pneumoniae. Capsule is stained pale pink (a). Mucoid colonies on blood agar are not dimpled (b)
Table 2.
Antibiotic susceptibility of the isolated Streptococcus pneumoniae
| MIC (µg/mL)a | S/I/Rb | |
|---|---|---|
| Penicillin G | ≤0.063 | S |
| Ampicillin | ≤0.25 | S |
| Cefotaxime | ≤0.5 | S |
| Cefepime | ≤0.5 | S |
| Imipenem | ≤0.5 | S |
| Meropenem | ≤0.125 | S |
| Levofloxacin | ≤1 | S |
| Erythromycin | 2 | R |
| Sulfamethoxazole/trimethoprim | ≤0.25 | S |
aMinimum inhibitory concentration
bDefined as “susceptible”, “intermediate” or “resistant” based on the Clinical And Laboratory Standards Institute standards M100-S25
Conclusions
Streptococcus pneumoniae is encapsulated, which is highly important for its virulence. In particular, serotype 3 strains are reported to be heavily encapsulated compared to other serotypes [1, 2], and tend to form mucoid colonies [3]. These features are related to its high virulence as they protect the bacteria from phagocytosis, inhibit opsonization by complement, and allow it to escape the neutrophil extracellular traps.
Mucoid serotype 3 is the second most common isolate in adult IPD cases. It is reported to be more common in adults with pneumonia, sepsis, and empyema/pleuritis, but not meningitis. In a previous study from Japan, among isolates from 43 adult fatal cases, serotype 3 has remained dominant without significant changes over time [4]. Community-acquired pneumonia caused by mucoid-type pneumococcus is reported to develop a wider infiltrative shadow, higher treatment failure rate, and a longer treatment period or hospitalization than the non-mucoid type [5].
The serotypes of the strains in children and adults are different. An increased prevalence of serotype 3 S. pneumoniae among children was reported in one region of Japan after introduction of the 7-valent pneumococcal conjugate vaccine (PCV7) [6]. National surveillance of pediatric patients after the 13-valent pneumococcal conjugate vaccine (PCV13) introduction in Japan showed that the prevalence rates of serotype 3 were 0.8% and 8.5% in IPD and non-IPD patients, respectively, in 2014 [7]. Serotype 3 was not dominant overall, and there was no significant difference in its prevalence rate between 2012 (PCV7 era, 3.7%) and 2014 (PCV13 era, 3.8%).
Serotype 3 has been reported to be dominant among case isolates in adult pneumococcal pneumonia. The Adult Pneumonia Study Group-Japan investigated etiologic factors at four community-based hospitals in four prefectures from September 2011 through January 2013. Of 100 S. pneumoniae isolates, serotype 3 was the most dominant (22%), followed by serotypes 11A (10%) and 19F (8%) [8]. In a report on the annual changes in the prevalence of each serotype in lower respiratory samples of adult pneumococcal pneumonia patients from 2011 to 2013, serotype 3 was continuously isolated from 15% or more patients, while the frequency of serotypes 19F, 23F, and 4 decreased annually [9]. Serotype 3 is one of the remaining dominant serotypes in other countries and appears to be more important in older adults on a global level [10]. It should be noted that the prevalence of serotype 3 has not decreased despite higher-valent vaccine introduction.
An outbreak of pneumococcal pneumonia caused by S. pneumoniae serotype 3 was reported in a nursing home unit at a local hospital in Kanagawa, Japan, in 2013 [11]. Among 31 residents, 27 (87%) had been vaccinated for influenza in the 2012–13 season, but only 2 (7%) among them had been immunized with the 23-valent polysaccharide pneumococcal vaccine (PPSV23). In total, ten confirmed cases of pneumonia and 16 influenza-like illness (ILI) cases were identified. In the same period, 6 of 28 (attack rate 21%) staff members presented with ILI, but none developed pneumonia. All six S. pneumoniae isolates showed identical pulsed-field gel electrophoresis patterns and were susceptible to penicillins, cephalosporins, carbapenems, and vancomycin, and were resistant to erythromycin and clindamycin. All pneumonia patients were hospitalized and none had been vaccinated with PPSV23. Shiramoto et al. reported that the immunogenicity of PCV13 and PPSV23, measured as opsonophagocytic activity titer, for serotype 3 were both lower than that for the other serotypes, suggesting lower vaccination efficacy [12]. Another study reported that the effectiveness of the PCV13 vaccine for serotype 3 was not significant [13]. Although we cannot conclude that the relatively poor efficacy of vaccination is the only reason for the dominance of serotype 3 after the introduction of the higher-valent vaccine, we should take into account the possible variability of immunogenicity depending on the serotype.
The Advisory Committee on Immunization Practices (ACIP) recommended in 2014 that all adults ≥65 years of age should receive PCV13, followed by PPSV23 [14, 15]. This 2-step vaccination approach is intended to maximize the efficacy of pneumococcal vaccination. Initial PCV13 induces acquired T cell memory function, and wider serotype coverage is induced by the subsequent PPSV23 [16]. In Japan, both PPSV23 and PCV13 have been used in the elderly to prevent pneumococcal infections since the approval of extended use of PCV13 in June 2014. The national immunization program launched in October 2014 for those aged ≥65 years only subsidized PPSV23 [17]. Widespread adoption of ACIP recommendations would potentially improve the efficacy of pneumococcal immunization.
Addressing the antibacterial susceptibility, Okade et al. reported that 100% of the 42 serotype 3 strains in their study cohort had penicillin binding protein gene (pbp) mutations and macrolide resistance genes [6]. Minimum inhibitory concentrations (MICs) of penicillins are still usually low, even with the pbp mutations. The S. pneumoniae isolate in the present case was susceptible to penicillins, but resistant to a macrolide (erythromycin). However, the in vivo–in vitro paradox of macrolides has recently been reported. For example, many azithromycin-resistant pneumococcal pneumonia cases have successfully been treated using azithromycin alone [18]. A case of severe community-acquired pneumonia due to mucoid S. pneumoniae, that was macrolide-resistant and penicillin-susceptible, was also cured by additional azithromycin administration [16]. Based on the antibiotic susceptibility pattern of S. pneumoniae, penicillins are the first treatment choices for infections caused by serotype 3 strains. Macrolides can be considered for concomitant administration in cases in where penicillins do not show sufficient efficacy.
Athlin et al. investigated the relationship between S. pneumoniae serotype and immunoglobulin (Ig) titer in community-acquired pneumococcal pneumonia patients [19]. Higher Ig titer ratios were observed in patients infected with serotypes with a thin capsule and medium/high invasive potential (including 1, 7F, 4, 9 N, 9 V, and 14) than in patients infected with serotypes with a thick capsule and low invasive potential (including 3, 6B, 19A, 19F, and 23F). Low Ig titer ratios (<1) were predominantly found in patients infected with serotypes with a thick capsule. According to a report by Hennezel et al. combination therapy with intravenous immunoglobulin (IVIG) and ampicillin was effective in a mouse model of invasive pneumonia caused by serotype 3 S. pneumoniae [20]. Based on these results, adding IVIG to the antibiotic therapy regimen may be an option in severe cases of IPD, such as the present case.
As the patient’s immunization status was unclear in the present case, the effectiveness of PPSV23 or PCV13 against serotype 3 S. pneumoniae could not be evaluated. The case was further complicated by a history of hypertension, laryngeal cancer, COPD, type 2 diabetes mellitus, and cerebral arteriovenous malformation hemorrhage. In addition, the patient’s nutritional status was not good, as indicated by the low serum albumin level. These factors possibly influenced the disease outcome. However, in severe cases caused by serotype 3 S. pneumoniae with a poor treatment response, as the present case, any available treatment measures such as the concomitant use of antibiotics and adding IVIG to the regimen should be considered. Raising awareness and promoting effective pneumococcal immunization would be other potential strategies to minimize the risk of serotype 3 infections.
We experienced a case of nosocomial pneumonia caused by mucoid serotype 3 S. pneumoniae with a fulminant course. The isolate was susceptible to all tested antibiotics other than erythromycin, but the case was not managed successfully. Serotype 3 isolates tend to have a thick capsule, and infected patients often have severe and refractory IPD. In particular, pneumonia due to serotype 3, which tends to form mucoid colonies, may develop a wider pulmonary infiltrative shadow, a prolonged therapeutic or hospitalization period, and a poor outcome. Careful observation and intervention are required throughout the clinical course in such patients. Although the complicated background influenced the current patient’s outcome, additional use of other antibiotics or immunoglobulins could be considered as therapeutic options when the pathogen is serotype 3 or another mucoid pneumococcus. In addition, effective and appropriate immunization for S. pneumoniae is an important public health measure to minimize the development of severe infections caused by serotype 3 strains.
Authors’ contributions
NS, YY, JH, DS, HS, NN, YK and HM contributed to the documentation. All authors read and approved the final manuscript.
Acknowledgements
None.
Competing interests
Naomi Sugimoto is an employee of Pfizer Japan Inc.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Consent for publication
Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images.
Ethics approval and consent to participate
This case report was approved by Ethical Committee of the Aichi Medical University Hospital.
Abbreviations
- IPD
invasive pneumococcal disease
- COPD
chronic obstructive pulmonary disease
- PCV7
7-valent pneumococcal conjugate vaccine
- PCV13
13-valent pneumococcal conjugate vaccine
- PPSV23
23-valent polysaccharide pneumococcal vaccine
- ILI
influenza-like illness
- ACIP
Advisory Committee on Immunization Practices
- pbp (gene)
penicillin binding protein
- MIC
minimum inhibitory concentration
- IVIG
intravenous immunoglobulin
Contributor Information
Naomi Sugimoto, Email: naomi.sugimoto@pfizer.com.
Yuka Yamagishi, Email: y.yamagishi@mac.com.
Jun Hirai, Email: j.eichi419@gmail.com.
Daisuke Sakanashi, Email: saka74d@aichi-med-u.ac.jp.
Hiroyuki Suematsu, Email: suematsu.hiroyuki.375@mail.aichi-med-u.ac.jp.
Naoya Nishiyama, Email: n.naoyaso@gmail.com.
Yusuke Koizumi, Email: ykoizumi@aichi-med-u.ac.jp.
Hiroshige Mikamo, Phone: +81-561-61-1842, Email: mikamo@aichi-med-u.ac.jp.
References
- 1.Weinberger DM, Harboe ZB, Sanders EA, Ndiritu M, Klugman KP, Rückinger S, et al. Association of serotype with risk of death due to pneumococcal pneumonia: a meta-analysis. Clin Infect Dis. 2010;51:692–699. doi: 10.1086/655828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grabenstein JD, Musey LK. Differences in serious clinical outcomes of infection caused by specific pneumococcal serotypes among adults. Vaccine. 2014;32:2399–2405. doi: 10.1016/j.vaccine.2014.02.096. [DOI] [PubMed] [Google Scholar]
- 3.Hammerschmidt S, Wolff S, Hocke A, Rosseau S, Müller E, Rohde M. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect Immun. 2005;73:4653–4667. doi: 10.1128/IAI.73.8.4653-4667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiba N. Current status of invasive pneumococcal diseases and the preventive pneumococcal vaccines in Japan. Jpn J Chemother. 2011;59:561–572. [Google Scholar]
- 5.Kawasaki S, Aoki N. A case of severe community-acquired pneumonia caused by mucoid type Streptococcus pneumoniae. Ann Jpn Resp Soc. 2015;4:303–308. [Google Scholar]
- 6.Okade H, Funatsu T, Eto M, Furuya Y, Mizunaga S, Nomura N, et al. Impact of the pneumococcal conjugate vaccine on serotype distribution and susceptibility trends of pediatric non-invasive Streptococcus pneumoniae isolates in Tokai, Japan over a 5-year period. J Infect Chemother. 2014;20:423–428. doi: 10.1016/j.jiac.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Nakano S, Fujisawa T, Ito Y, Chang B, Suga S, Noguchi T, et al. Serotypes, antimicrobial susceptibility, and molecular epidemiology of invasive and non-invasive Streptococcus pneumoniae isolates in paediatric patients after the introduction of 13-valent conjugate vaccine in a nationwide surveillance study conducted in Japan in 2012–2014. Vaccine. 2016;34:67–76. doi: 10.1016/j.vaccine.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Morimoto K, Suzuki M, Ishifuji T, Yaegashi M, Asoh N, Hamashige N, et al. The burden and etiology of community-onset pneumonia in the aging Japanese population: a multicenter prospective study. PLoS ONE. 2015 doi: 10.1371/journal.pone.0122247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akata K, Chang B, Yatera K, Kawanami T, Yamasaki K, Naito K, et al. Distribution and annual changes in Streptococcus pneumoniae serotypes in adult Japanese patients with pneumonia. J Infect Chemother. 2015;21:723–728. doi: 10.1016/j.jiac.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Hausdorff WP, Hanage WP. Interim results of an ecological experiment-conjugate vaccination against the pneumococcus and serotype replacement. Hum Vaccin Immunother. 2016;12:358–374. doi: 10.1080/21645515.2015.1118593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuroki T, Ishida M, Suzuki M, Furukawa I, Ohya H, Watanabe Y, et al. Outbreak of Streptococcus pneumoniae serotype 3 pneumonia in extremely elderly people in a nursing home unit in Kanagawa, Japan, 2013. J Am Geriatr Soc. 2014;62:1197–1198. doi: 10.1111/jgs.12863. [DOI] [PubMed] [Google Scholar]
- 12.Shiramoto M, Hanada R, Juergens C, Shoji Y, Yoshida M, Ballan B, et al. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine compared to the 23-valent pneumococcal polysaccharide vaccine in elderly Japanese adults. Hum Vaccin Immunother. 2015;11:2198–2206. doi: 10.1080/21645515.2015.1030550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews NJ, Waight PA, Burbidge P, Pearce E, Roalfe L, Zancolli M, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014;14:839–846. doi: 10.1016/S1473-3099(14)70822-9. [DOI] [PubMed] [Google Scholar]
- 14.Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2014;63:822–825. [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi M, Bennett NM, Gierke R, Almendares O, Moore MR, Whitney CG, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2015;64:944–947. doi: 10.15585/mmwr.mm6434a4. [DOI] [PubMed] [Google Scholar]
- 16.Namkoong H, Ishii M, Funatsu Y, Kimizuka Y, Yagi K, Asami T, et al. Theory and strategy for pneumococcal vaccines in the elderly. Hum Vaccin Immunother. 2016;12:336–343. doi: 10.1080/21645515.2015.1075678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoshi SL, Kondo M, Okubo I. Economic evaluation of immunisation programme of 23-valent pneumococcal polysaccharide vaccine and the inclusion of 13-valent pneumococcal conjugate vaccine in the list for single-dose subsidy to the elderly in Japan. PLoS ONE. 2015 doi: 10.1371/journal.pone.0139140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohno S, Tateda K, Kadota J, Fujita J, Niki Y, Watanabe A, et al. Contradiction between in vitro and clinical outcome: intravenous followed by oral azithromycin therapy demonstrated clinical efficacy in macrolide-resistant pneumococcal pneumonia. J Infect Chemother. 2014;20:199–207. doi: 10.1016/j.jiac.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Athlin S, Kaltoft M, Slotved HC, Herrmann B, Holmberg H, Konradsen HB, et al. Association between serotype-specific antibody response and serotype characteristics in patients with pneumococcal pneumonia, with special reference to degree of encapsulation and invasive potential. Clin Vaccine Immunol. 2014;21:1541–1549. doi: 10.1128/CVI.00259-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Hennezel L, Ramisse F, Binder P, Marchal G, Alonso JM. Effective combination therapy for invasive pneumococcal pneumonia with ampicillin and intravenous immunoglobulins in a mouse model. Antimicrob Agents Chemother. 2001;45:316–318. doi: 10.1128/AAC.45.1.316-318.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.


