Abstract
Background
Plasmodium ookinete surface proteins as post-fertilization target antigens are potential malaria transmission-blocking vaccine (TBV) candidates. Putative secreted ookinete protein 25 (PSOP25) is a highly conserved ookinete surface protein, and has been shown to be a promising novel TBV target. Here, we further investigated the TBV activities of the full-length recombinant PSOP25 (rPSOP25) protein in Plasmodium berghei, and characterized the potential functions of PSOP25 during the P. berghei life-cycle.
Methods
We expressed the full-length P. berghei PSOP25 protein in a prokaryotic expression system, and developed polyclonal mouse antisera and a monoclonal antibody (mAb) against the recombinant protein. Indirect immunofluorescence assay (IFA) and Western blot were used to test the specificity of antibodies. The transmission-blocking (TB) activities of antibodies were evaluated by the in vitro ookinete conversion assay and by direct mosquito feeding assay (DFA). Finally, the function of PSOP25 during Plasmodium development was studied by deleting the psop25 gene.
Results
Both polyclonal mouse antisera and anti-rPSOP25 mAb recognized the PSOP25 proteins in the parasites, and IFA showed the preferential expression of PSOP25 on the surface of zygotes, retorts and mature ookinetes. In vitro, these antibodies significantly inhibited ookinetes formation in an antibody concentration-dependent manner. In DFA, mice immunized with the rPSOP25 and those receiving passive transfer of the anti-rPSOP25 mAb reduced the prevalence of mosquito infection by 31.2 and 26.1%, and oocyst density by 66.3 and 63.3%, respectively. Genetic knockout of the psop25 gene did not have a detectable impact on the asexual growth of P. berghei, but significantly affected the maturation of ookinetes and the formation of midgut oocysts.
Conclusions
The full-length rPSOP25 could elicit strong antibody response in mice. Polyclonal and monoclonal antibodies against PSOP25 could effectively block the formation of ookinetes in vitro and transmission of the parasites to mosquitoes. Genetic manipulation study indicated that PSOP25 is required for ookinete maturation in P. berghei. These results support further testing of the PSOP25 orthologs in human malaria parasites as promising TBV candidates.
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-016-1932-4) contains supplementary material, which is available to authorized users.
Keywords: Plasmodium berghei, PSOP25, Ookinete, Transmission-blocking vaccine
Background
Malaria remains one of the most prevalent tropical infectious diseases and is endemic in nearly 95 countries and territories around the world, with estimated > 3.2 billion people being at risk. In 2015, there were approximately 214 million new malaria cases resulting in 438,000 deaths, ~ 80% of which occurred in Africa [1]. Currently, due to the spread of insecticide-resistant mosquitoes and multidrug-resistant parasites, major malaria control efforts including vector control and chemotherapy are becoming increasingly ineffective [2–4]. These trends highlight the need for developing an integrated malaria control strategy to eliminate malaria transmission. A transmission-blocking vaccine (TBV) targeting the sexual stages of the Plasmodium has the potential to reduce malaria transmission and prevent the spread of resistant parasites. It is predicted that TBV administration can reduce child mortality even in areas of high endemicity [5]. Additionally, TBV can slow down the spread of mutant parasites, which will prolong the effective lives of antimalarial drugs and vaccines [6]. Mathematical models further predict that TBVs will be an effective tool for malaria elimination [7].
TBV is designed to target the Plasmodium antigens expressed during sexual development or Anopheles midgut proteins that interact with sexual stages and allow ookinetes to traverse the Anopheles midgut epithelial cells. Research on TBVs has led to the identification and experimental validation of several potential TBV candidates, but only a few including Pfs48/45 [8, 9], Pfs230 [10, 11] and Pfs25 [12] in P. falciparum, and Pvs25 and Pvs28 in P. vivax [13], have been found effective in blocking parasite transmission. Investigations on the two 6-cysteine domain protein family members, Pfs48/45 and Pfs230, have shown that anti-Pfs48/45 monoclonal and polyclonal antibodies in experimental animals can effectively inhibit the transmission of P. falciparum to mosquitoes [9, 14, 15], while Pfs230-raised antibodies are sufficient to block development of the oocysts and competent to induce complement-dependent transmission-blocking (TB) activity [11]. Furthermore, antibodies against both Pfs48/45 and Pfs230 have been detected in natural infections, thereby bringing the potential to boost and/or enhance antibody titers with TBVs against these antigens [16]. Unlike pre-fertilization proteins, post-fertilization antigens are expressed solely after the formation of the zygotes within the mosquito midgut. Concealed from the host’s immune system, these antigens have limited diversity among the parasite populations [17, 18]. The major ookinete surface protein Pfs25 is a well-characterized 25-kDa glycosyl-phosphatidylinositol (GPI)-anchored protein with four epidermal growth factor-like domains. Pfs25 is involved in adhesion of ookinete and plays an important role in subsequent penetration of the mosquito midgut [19, 20]. Mouse antiserum against native Pfs25 [21], heterologously expressed Pfs25, or the P. vivax ortholog Pvs25 proteins can effectively inhibit parasite development in mosquitoes [22–24]. Though Pfs25 and Pvs25 provide evidence for the efficacy of post-fertilization antigens in TBVs, more TBV candidate antigens and higher levels of TB activities are needed for an effective deployable vaccine.
With efforts for identifying new TBV candidates, we have recently identified a post-fertilization antigen PSOP25 (PBANKA_111920) in the rodent parasite Plasmodium berghei. Psop25 encodes a 350 amino acid (aa) protein with a signal peptide, and the native protein is predicted to be 40 kDa. Psop25 transcript is highly expressed in ookinetes and occupied in the 99th percentile in the transcriptome of ookinetes [25]. Ookinete-specific expression of this protein was confirmed in our previous study [26]. Antisera from mice immunized with a partial PSOP25 domain (aa 45–245), which included ten predicted antibody epitopes, inhibited ookinete formation by 53.0% in in vitro ookinete cultures. Mosquitoes fed on this partial PSOP25 domain-immunized mice also resulted in modestly decreased oocyst prevalence (25.0%) and significantly reduced oocyst densities (64.3%) [26], suggesting that PSOP25 could be a new promising target for TBVs. Here we set out to further investigate the TBV activities of the full-length PSOP25 protein in P. berghei, and characterize the functions of PSOP25 by genetic knockout (KO).
Methods
Mice, parasites and mosquitoes
Female BALB/c mice (six- to eight-week-old; Beijing Animal Institute, Beijing, China) were used for all animal experiments. P. berghei (ANKA strain 2.34) and Δpsop25 lines (psop25 gene knockout line) were maintained in mice and used for challenge infection. Adult Anopheles stephensi mosquitoes of the Hor strain were fed with 10% (w/v) glucose solution and maintained in an insectary with a surrounding of 50–80% relative humidity, at 25 °C.
Expression and purification of rPSOP25
For the expression of full-length PSOP25, a psop25 fragment encoding aa 25–350 (excluding the signal peptide) was amplified from P. berghei genomic DNA with psop25-F and psop25-R primers (Additional file 1: Table S1). The psop25 fragment and the prokaryotic expression vector pET30a (+) (Novagen, Darmstadt, Germany) were digested with restriction enzymes NdeI and HindIII, then ligation was performed using the Ligation High Kit (Toyobo, Osaka, Japan). The recombinant plasmid was transformed in Escherichia coli BL-21 (Novagen, Darmstadt, Germany) and the His-tagged recombinant PSOP25 (rPSOP25) was expressed at 20 °C for 12 h after induction with 1 mM isopropyl-β-D-thiogalactopyranoside (Sigma-Aldrich, St. Louis, USA). For protein purification, cultures were harvested and lysed using binding buffer containing 10 mM imidazole, 300 mM NaCl and 50 mM sodium phosphate (pH 8.0) and treated by sonication (15 cycles of 20 s pulses and 30 s intervals). The soluble rPSOP25 was purified by Ni-NTA His-Bind Superflow (Novagen, Darmstadt, Germany), according to the manufacturer’s instructions. Purified rPSOP25 was extensively desalted in 0.1 M phosphate buffered saline (PBS, pH 7.4) overnight at 4 °C, and then analyzed by SDS-PAGE.
Animal immunization and monoclonal antibody (mAb) production
To obtain polyclonal antisera against rPSOP25, a group of six female BALB/c mice were subcutaneously immunized with the purified protein (50 μg/mouse) emulsified in complete Freund’s adjuvant (Sigma-Aldrich, St. Louis, USA), which has been used to produce high-titer antibodies [27]. Subsequently, the mice were given two booster immunizations of 25 μg of rPSOP25 at 3-week intervals with the rPSOP25 protein emulsified in incomplete Freund’s adjuvant (Sigma-Aldrich, St. Louis, USA). A group of negative control mice (n = 6) were immunized with PBS and same adjuvant formulations. For the final bleed, mouse blood was collected at 10 days after the final immunization by cardiac puncture and the antisera were obtained after the blood had clotted at room temperature. Antisera from individual mice were mixed together and used in the subsequent trials.
For monoclonal antibody (mAb) production, rPSOP25-immunized BALB/c mice were obtained as described above, then the spleen cells of the immunized mice were extracted and fused with Sp2/0-Ag14 myeloma cells to produce the anti-PSOP25 mAb [28]. The fused hybridoma cells were generated using the traditional polyethylene glycol method, and then selected in the hypoxanthine-aminopterin-thymidine medium. The antibodies were screened by indirect antibody capture enzyme-linked immunosorbent assay (ELISA). The IgG fractions were prepared by ammonium sulfate precipitation, and then purified on a Protein A column (ThermoFisher Scientific, Waltham, USA), according to the manufacturer’s instructions. The mAb isotype was determined by using the SBA Clonotyping™ System-HRP (Southern Biotechnology Associates, Birmingham, USA).
ELISA
Antibody titers to rPSOP25 were determined by ELISA on day 14, 35 and 52 after the first immunization as previously described [26]. Briefly, 96-well plates were coated overnight with purified rPSOP25 at 4 °C, and blocked with blocking buffer (0.05% Tween 20 in 0.1 M PBS, 1% bovine serum albumin, pH 7.4) for 2 h at 37 °C. The plates were then washed twice with PBS-T (0.05% Tween 20 in 0.1 M PBS, pH 7.4) and incubated with pooled mouse anti-rPSOP25 sera (1:200 dilution) in blocking buffer at 37 °C for 2 h. After two washes, the wells were incubated for 2 h at 37 °C with a 1:5000 dilution of HRP-conjugated goat anti-mouse IgG antibody (Invitrogen, Waltham, USA). After five final washes, tetramethyl benzidine (Amresco, Solon, USA) was added and the reaction was stopped by 2 mM H2SO4. The absorbance at 490 nm was measured with an ELISA plate reader.
For estimating the end point titer of immunized mice, sera from all mice in each immunization and control group were pooled and diluted from 1:200 to 1:204800 in a blocking buffer and incubated at 37 °C for 2 h. The end point titers of the total IgG corresponded to the highest dilution at which the OD490 value was higher than the cut-off value, which was defined as the mean of the pooled negative control antisera + 3 × standard deviation [29].
Ookinete enrichment and lysate preparation
The enrichment of ookinetes was referred to a modified protocol [30]. Briefly, 1.2 mg phenylhydrazine (Sigma-Aldrich, St. Louis, USA) in 0.9% NaCl were intraperitoneally (i.p.) injected into BALB/c mice 3 days before P. berghei infection. These treated mice were then i.p. injected with 5 × 106 P. berghei-infected red blood cells (iRBCs) to initiate the blood-stage infection. Parasitemia was allowed to reach approximately 1–3% at three days post-infection (p.i.), when the mice were anesthetized. After removal of the white blood cells, the infected blood was diluted 1:10 with the ookinete culture medium (100 mg/l neomycin, 50 mg/l streptomycin, 50 mg/l penicillin, 20% (v/v) FBS, and 1 mg/l heparin in RPMI 1640, pH 8.3) in a petri dish and maintained at 19 °C for 24 h. The culture was then diluted in 45 ml of 0.17 M NH4Cl on ice for 10 min to lyse erythrocytes. After a wash with 0.1 M PBS, ookinetes were separated on a 62% (v/v) Nycodenz/PBS cushion by centrifugation (1300× g) for 25 min at 25 °C, treated with 0.15% saponin and washed once with 0.1 M PBS. The ookinete lysate was prepared by resuspending the ookinetes in 2% SDS containing 1% Triton X-100 and 1 × protease inhibitor cocktail (Roche, Castle Hill, Australia) for 30 min at room temperature.
Western blot
The parasite antigens (10 μg) or purified rPSOP25 (500 ng) were subjected to electrophoresis under reducing or non-reducing conditions using a 10% SDS-PAGE gel and electro-transferred to PVDF membrane (Bio-Rad, Hercules, USA). Western blot was performed essentially as described [26]. Primary antibodies were the pooled mouse anti-rPSOP25 serum (1:200) or anti-rPSOP25 mAb (1:1000), and HRP-conjugated goat anti-mouse IgG antibodies (1:10,000) (Invitrogen, Waltham, USA) were used as the secondary antibodies. Pbs21 mAb (clone 13.1, 1:1000) was included as a positive control [31]. The sera (1:200) obtained from mice immunized with the PBS-adjuvant formulations were used as the negative control. The blot was developed using an ECL Western Blotting Kit (ThermoFisher Scientific, Waltham, USA).
Indirect immunofluorescence assay (IFA)
Parasites containing asexual stages, gametocytes, zygotes and ookinetes of P. berghei were fixed on slides [32]. Anti-rPSOP25 mAb (1:500) or Pbs21 mAb (clone 13.1, 1:500, positive control) or negative control sera (1:500) was incubated in 5% skim milk and labeled with FITC-conjugated goat anti-mouse IgG (1:500; Invitrogen, Waltham, USA) at 37 °C for 1 h. After staining of nuclei with 4′, 6-diamidino-2-phenylindole (DAPI; Invitrogen, Waltham, USA), the slides were examined under Olympus BX53 (Olympus Corporation, Center Valley, USA) and the images were processed using Adobe Photoshop (Adobe Systems Inc., San Jose, USA).
Quantification of TB activities
For the in vitro assay, phenylhydrazine pre-treated mice were infected as described above. On day 3 p.i., parasitemia was determined, and 10 μl of blood were taken from appropriate hosts and added to 90 μl ookinete culture medium containing anti-rPSOP25 serum or negative control mouse serum at final dilutions of 1:5, 1:10, and 1:50. Additionally, anti-rPSOP25 mAb was added to the ookinete culture at 10, 5 and 1 μg/100 μl (concentration of mAb was 0.5 μg/μl) of ookinete culture, respectively. Ookinete cultures were incubated at 19 °C for 24 h and the ookinete conversion rates were determined as described previously [26, 32].
For direct mosquito feeding assays (DFA), experimental mice (n = 3) were immunized with rPSOP25 and negative control mice (n = 3) were immunized with the PBS-adjuvant formulations as described above. Ten days after the final immunization, mice were infected i.p. with 5 × 106 P. berghei ANKA iRBC. For the antibody transfer experiment, three normal mice were injected intravenously with 150 μg of anti-rPSOP25 mAb/mouse 1 h before mosquito feeding. Four-day-old female A. stephensi mosquitoes (starved for 12 h) were allowed to feed on rPSOP25 immunized mice or antibody-transferred mice for 30 min. Engorged mosquitoes were maintained in an insectary at 21 °C and 70% relative humidity. Ten days after feeding, ~ 30 mosquitoes were dissected, and midguts were stained with 0.5% mercurochrome (Sigma-Aldrich, St. Louis, USA) to count the number of oocysts per midgut.
Generation of psop25 KO parasites
To knock out all the protein-coding sequence of the psop25 gene, the target vector containing an hdhfr selection cassette was used (kindly provided by plasmoGEM, vector design ID, PbGEM-042760; http://plasmogem.sanger.ac.uk/). Before P. berghei transfection, vector DNA was digested by NotI followed by ethanol precipitation. The linearized plasmid (10 μg) was electroporated into purified schizonts using the Nucleofector device as described previously [32]. After transfection, the complete parasite suspension was injected intravenously via the tail vein into mice. Following a 24 h incubation period, infected mice were treated for 3–4 days with pyrimethamine (Sigma-Aldrich, St. Louis, USA) via drinking water (70 μg/ml). Infected blood was collected and confirmed by integration-specific PCR (Additional file 1: Table S1). Monoclonal parasite lines were then obtained by limiting dilution.
Phenotypic analysis of the Δpsop25 line
To study whether deletion of psop25 affected parasite growth, five phenylhydrazine-treated mice were inoculated i.p. with either 5 × 106 wildtype (WT) or Δpsop25 iRBCs. For each genotype, blood smears were used to monitor daily parasitemia, gametocytemia (mature gametocytes per 100 RBCs), and the gametocyte sex ratio (female: male ratio) [33]. To quantify male gamete exflagellation, 10 μl of P. berghei-infected blood collected from mouse tail vein on day 3 p.i. were added into 90 μl of ookinete culture medium and incubated for 15 min at 25 °C, and the exflagellation centers were counted as previously described [32]. At the same time, ookinetes were cultured in vitro as described above [34], and the ookinete conversion rates were determined by IFA using a Pbs21 mAb [35]. For oocyst quantifications, mice at 3 days p.i. with each parasite line were fed to starved A. stephensi mosquitoes for 30 min [36, 37]. Ten days after feeding, ~ 30 fed mosquitoes from each genotype were dissected for counting of the number of oocysts per infected mosquito and to determine the prevalence and intensity of infection.
Statistical analysis
Parasitemia, gametocytemia, gametocyte sex ratio and ookinete conversion rates between groups were analyzed by the Student’s t-test, using GraphPad Prism software. The prevalence of infection (proportion of infected mosquito) was analyzed by the Fisher’s exact test, while the intensity (number of oocysts per midgut) was analyzed by the Mann-Whitney U-test [38], using SPSS version 17.0. P-values less than 0.05 were considered statistically significant.
Results
The full-length rPSOP25 is immunogenic
A PSOP25 fragment which corresponded to aa 25–350 excluding the signal peptide was expressed in E. coli. This fragment included 14 predicted B cell epitopes [39] (Additional file 2: Figure S1). The purified rPSOP25 protein had a molecular size of ~ 36.5 kDa from SDS-PAGE analysis, which was consistent with the predicted size of PSOP25 protein (Fig. 1a). To determine the immunogenicity of purified rPSOP25, we performed ELISA using pooled serum generated from mice immunized with the recombinant protein. The results showed that immunization with rPSOP25 induced strong antibody responses as compared to the negative control; the rPSOP25-specific IgG titers increased continuously during the course of vaccination (Student’s t-test: t (10) = 35.13, P < 0.0001; Fig. 1b). The antisera collected 10 days after the final immunization with rPSOP25 reached a titer of 1:1024000 (Fig. 1c). Meanwhile, an anti-rPSOP25 mAb was produced from a selected hybridoma line, which was determined to be the IgG1 isotype (Additional file 2: Figure S2).
Fig. 1.
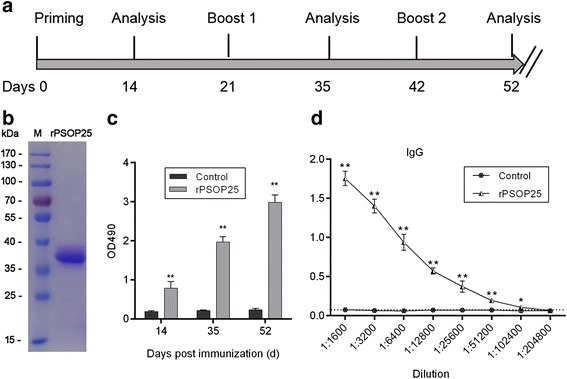
a Mouse immunization and analysis scheme. b rPSOP25 was purified from E. coli and analyzed on a 10% SDS-PAGE gel. c Antibody titers in immunized mice during immunization, experimental mice were immunized with rPSOP25 formulated in Freund’s adjuvant; control mice were immunized with only Freund’s adjuvant and 0.1 M PBS. The data represent two separate experiments. Error bar shows mean ± standard deviation (SD). SD indicates the assay variance. **P < 0.01 (Student’s t-test). d Anti-rPSOP25 total IgG titer at 10 days after the final immunization analyzed by ELISA. Mean of control antisera + 3 × SD is shown by the broken lines. IgG titers correspond to the last dilution of the anti-rPSOP25 sera where in OD490 values were above the cut-off values. Cut-off value was defined as that of the pooled sera from control mice. The experiment was performed three times. Error bar shows mean ± SD. *P < 0.05, **P < 0.01 (Student’s t-test). Abbreviations: M, molecular weight marker; rPSOP25, purified rPSOP25 under reducing conditions
Anti-rPSOP25 antisera and mAb recognize the ookinete proteins
The specificity of the pooled anti-rPSOP25 antisera and mAb was determined by Western blot against the rPSOP25 or protein lysate from ookinetes. On Western blots, both the antisera and mAb detected the 36.5 kDa rPSOP25 (Fig. 2a) and a band of approximately 40 kDa in the lysate of purified ookinetes under reducing conditions (Fig. 2a) and non-reducing conditions (Additional file 2: Figure S3), which is close to the predicted size of PSOP25. In a previous study, IFA using antisera raised against a partial domain of the PSOP25 protein indicated that PSOP25 is expressed on the surface of ookinetes [26]. Consistently, IFA with the anti-rPSOP25 mAb using zygotes, retorts and ookinetes without membrane permeabilization revealed strong fluorescence of the parasite body, which agrees with the surface localization of PSOP25 (Fig. 2b).
Fig. 2.
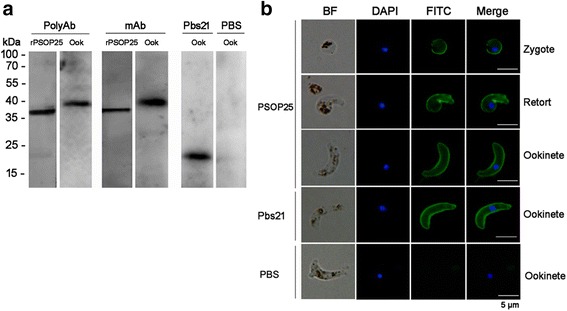
a Western blot analysis of purified rPSOP25 protein and P. berghei ookinete (Ook) lysates with anti-rPSOP25 sera (PolyAb) and anti-rPSOP25 mAb. Lysates were subjected to electrophoresis under reducing conditions by SDS-PAGE. Pbs21 mAb was used as positive control; a control mouse serum (PBS) was used as negative control. b IFA was performed on zygote, retort and ookinete at different time points of ookinete culture using anti-rPSOP25 mAb (green - FITC). Positive control - Pbs21 mAb, negative control - a control mouse serum (PBS). Nuclei were labelled with DAPI (blue). BF, bright field. Scale-bars: 5 μm
Antibodies against PSOP25 show obvious TB activities
Anti-rPSOP25 antisera and mAb were used in ookinete conversion assay to study the TB activity of the antibodies against PSOP25. When incubated with pooled mouse anti-rPSOP25 antisera or mAb, ookinete conversion was inhibited in a dose-dependent manner. In ookinete cultures supplemented with the pooled immune sera at 1:5, 1:10 and 1:50 dilutions, ookinete conversion rates were reduced by 62.5% (Student’s t-test: t (4) = 22.52, P < 0.0001), 47.9% (Student’s t-test: t (4) = 21.44, P < 0.0001), and 22.5% (Student’s t-test: t (4) = 9.11, P = 0.0008), respectively (Fig. 3a). Compared with the control sera, ookinete conversion rates in cultures with mAb added at 10, 5 and 1 μg/100 μl were reduced by 71.6% (Student’s t-test: t (4) = 32.04, P < 0.0001), 60.8% (Student’s t-test: t (4) = 27.84, P < 0.0001) and 32.0% (Student’s t-test: t (4) = 18.60, P < 0.0001), respectively (Fig. 3a).
Fig. 3.
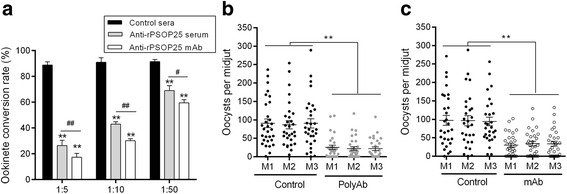
a TB activities of anti-rPSOP25 serum and anti-rPSOP25 mAb on P. berghei ookinete formation in vitro. Anti-rPSOP25 serum, anti-rPSOP25 mAb, or normal mouse serum (control) were diluted at 1:5, 1:10 and 1:50, respectively. Means were representative of three independent experiments. Error bar shows mean ± SD. ** indicate significant difference compared with the control sera (P < 0.01). # indicate significant difference between anti-rPSOP25 serum and mAb group (P < 0.05), ## P < 0.01 (Student’s t-test). b Direct mosquito feeding assay to assess the TB activity of polyclonal antisera in rPSOP25-immunized mice (3 mice per group). c Passive antibody transfer experiment to assess the TB activity of the anti-PSOP25 mAb. For b and c, mosquito midguts were dissected at ten days post-infection, the number of oocysts was counted under a microscopy. The data are collated from three experiments. The mean number of oocysts and the SEM in each group are shown. **P < 0.01 (Mann-Whitney U-test)
To further examine the TB effect of anti-rPSOP25 antibodies in vivo, mice were immunized with rPSOP25 or passively transferred with the mAb and used in DFA. Ten days after feeding, mosquitoes were dissected and midgut oocysts were counted. Mosquitoes fed on the rPSOP25-immunized mice showed a 31.2% reduction in the prevalence of oocysts, as compared to the control groups. The mean prevalence was 68.7% in mosquitoes fed on the rPSOP25-immunized mice, whereas it was 99.9% in mosquitoes fed on the control mice (Fisher’s exact test: OR = 40.72, 95% CI = 5.38–307.91, P < 0.001; Fig. 3b, Table 1). Moreover, mosquitoes fed on rPSOP25-immunized mice revealed a 66.3% reduction in oocyst density compared to the control group (Mann-Whitney U-test: Z = -8.32, P < 0.0001; Fig. 3b, Table 1). Similarly, mosquitoes fed on the mice passively transferred with the mAb against PSOP25 had a 26.1% reduction in the prevalence of oocysts (Fisher’s exact test: OR = 16.46, 95% CI = 3.76–72.13, P < 0.001; Fig. 3b, Table 1) and a 63.3% reduction in density of oocysts (Mann-Whitney U-test: Z = -6.97, P < 0.0001; Fig. 3b, Table 1).
Table 1.
Evaluation of TB effect of anti-rPSOP25 serum and mAb in mosquito feeding assays
| Immunization groupsa | Monoclonal antibody transfer groupsb | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control mice | rPSOP25 immunized mice | Control mice | mAb transferred mice | |||||||||
| M1 | M2 | M3 | M1 | M2 | M3 | M1 | M2 | M3 | M1 | M2 | M3 | |
| No. of mosquitoes infected/dissected | 30/30 | 30/30 | 29/30 | 20/28 | 20/30 | 19/28 | 28/29 | 27/28 | 28/28 | 22/30 | 21/29 | 20/29 |
| Prevalence of infection (%)c | 100 | 100 | 99.7 | 71.4 | 66.7 | 67.9 | 96.6 | 96.4 | 100 | 73.3 | 72.4 | 69.0 |
| Mean prevalence (%) | 99.9 | 68.7* | 97.7 | 71.6* | ||||||||
| Reduction in prevalence (%)d | 31.2 | 26.1 | ||||||||||
| Oocyst intensitye | 90.7 | 86.8 | 90.5 | 25.2 | 22.1 | 21.7 | 97.1 | 96.3 | 94.3 | 29.8 | 34.2 | 33.7 |
| SEMf | 11.58 | 11.17 | 12.50 | 5.890 | 5.232 | 5.138 | 13.47 | 13.66 | 11.82 | 5.462 | 6.373 | 6.800 |
| Mean oocyst intensity | 89.3 | 23.0* | 95.9 | 32.6* | ||||||||
| Reduction in oocyst intensity (%)g | 66.3 | 63.3 | ||||||||||
aTB activity assay was carried out using rPSOP25-immunized mice
bTB activity assay was carried out using BALB/C mice transferred with the anti-PSOP25 mAb
cThe prevalence of infection was calculated by the number of mosquitoes with oocysts/total mosquitoes dissected in each group × 100%
dThe percent reduction of prevalence was calculated as % mean prevalence control – % mean prevalence PSOP25
eMean number of oocysts per mosquito midgut
fStandard error of the mean
gThe percent reduction in oocyst intensity was calculated as (mean oocyst intensity control – mean oocyst intensity PSOP25)/mean oocyst intensity control × 100%
*P < 0.001 for comparisons between the experimental group and the control group
PSOP25 is required for the maturation of ookinetes
To determine the function of PSOP25 during Plasmodium development, a psop25 gene KO line (Δpsop25) was generated in P. berghei (Fig. 4a) [40]. Genotypes of the cloned pyrimethamine-resistant parasites were confirmed by integration-specific PCR (Fig. 4b). To determine if psop25 gene knockout led to any deficiencies in parasite development, we compared parasitemia, gametocytemia and gametocyte sex ratio between groups of BALB/c mice infected with 5 × 106 Δpsop25 or WT P. berghei parasites. Consistent with no expression of the PSOP25 protein in asexual erythrocytic stages, Δpsop25 had no evident effect on asexual parasitemia (Fig. 5a). In addition, on day 3 p.i., gametocytemia and gametocyte sex ratio did not differ significantly between the WT parasite and the Δpsop25 line (Fig. 5b, c). However, mean male gamete exflagellation events were slightly but significantly reduced in the Δpsop25 line, as compared to the WT parasites (Student’s t-test: t (10) = 4.01, P = 0.0024; Fig. 5d). Furthermore, in vitro ookinete cultures established from parasites at day 3 p.i. showed that ookinete conversion rate was significantly reduced in the Δpsop25 line, although the mature ookinetes in the Δpsop25 line appeared morphologically normal (data not shown). Using reverse-transcriptase-PCR, we determined that there was no psop25 expression in the ookinetes of the Δpsop25 line, further confirming that psop25 was deleted (data not shown). In the WT line, the zygote, retort, and ookinete conversion rates was 2.0, 8.8 and 87.74%, respectively. Whereas in the Δpsop25 line, 13.3 and 41.0% parasites progressed to the zygote and retort stages, respectively, further maturation to ookinetes was reduced by 60.9% (Student’s t-test: t (4) = 31.69, P < 0.0001; Fig. 5e), indicating that PSOP25 might play a crucial role in ookinete maturation. The oocyst density was reduced to 29.7 per mosquito midgut in those fed on the Δpsop25 parasites as compared to 96.9 in WT parasites, reflecting a 69.4% reduction (Mann-Whitney U-test: Z = -4.25, P < 0.0001; Fig. 5f, Table 2).
Fig. 4.
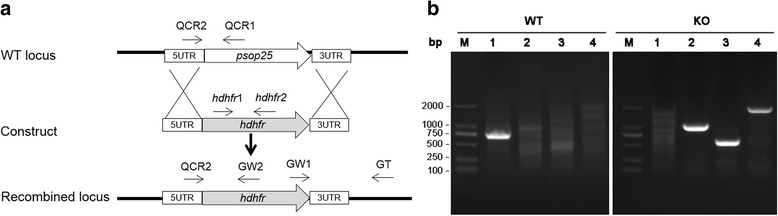
a Schematic representation of the WT locus, the construct used for transfection and the recombined locus with psop25 replaced with the hdhfr cassette. Primers QCR1, QCR2, GW2, hdhfr1, hdhfr2, GW1 and GT used for diagnostic PCR of the WT locus or knockout are marked. b Lane 1: primers QCR1 + QCR2 (696 bp) are used for diagnostic PCR of the WT locus. Lanes 2, 3 and 4 are PCR products from primers GW2 + QCR2 (1,002 bp), hdhfr1 + hdhfr2 (561 bp), GW1 + GT (1,831 bp) for PCR verification of psop25 KO, respectively
Fig. 5.
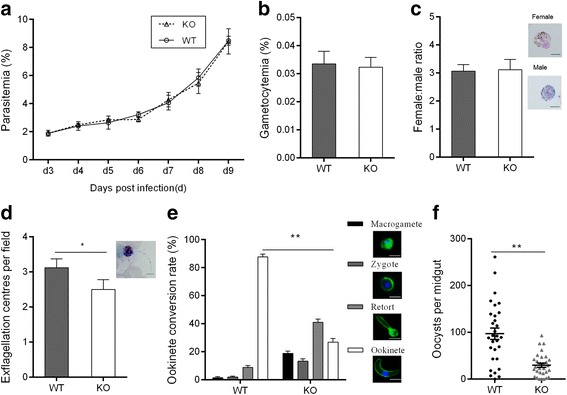
a Average parasitemia was calculated in mice after infection with the wild-type (WT) or Δpsop25 parasites (n = 5). b Gametocytemia in mice infected with WT or Δpsop25 parasites (n = 3). c Female: male gametocyte ratios of WT or Δpsop25 parasites (n = 3). d Exflagellation of WT and Δpsop25 microgametes (n = 3), *P < 0.05 (Student’s t-test). e Ookinete conversion rates in vitro of WT and Δpsop25 parasites. For c, d and e, characteristic morphologies of parasites are shown on the right (n = 3), **P < 0.01 (Student’s t-test). f Oocyst number per midgut in mosquitoes 10 days after feeding on mice infected with the WT and the Δpsop25 parasites. The horizontal bar shows the mean number of oocysts per midgut in mosquito (± SEM). **P < 0.01 (Mann-Whitney U-test). For a-f, all the data are representative of three separate experiments. Scale-bars: 5 μm
Table 2.
Oocyst number per midgut in mosquitoes 10 days after feeding on mice infected with the WT and the Δpsop25 parasites
| No. of mosquitoes (infected/dissected) | Prevalence of infection (%)a | Mean oocyst (IQR)b | SEMc | Reduction in oocyst intensity (%)d | P | |
|---|---|---|---|---|---|---|
| Wild type | 29/30 | 99.7 | 96.93 (43.00–139.8) | 12.01 | ||
| Δpsop25 | 27/30 | 90 | 29.67 (10.25–43.25) | 4.481 | 67.26 | < 0.001 |
aThe prevalence of infection was calculated by the number of mosquitoes with oocysts/total mosquitoes dissected in each group × 100%
bIQR, inter-quartile range
cStandard error of the mean
dThe percent reduction in oocyst intensity was calculated as (mean oocyst intensity WT – mean oocyst intensity Δpsop25)/mean oocyst intensity WT × 100%
Discussion
Disrupting the parasite life-cycle to prevent the disease from being transmitted to other individuals represents a key component of an integrated malaria control strategy [41]. Despite investigations on several TBV antigens over the last 40 years, there is still a need to discover new TBV vaccine candidates for malaria elimination purpose [42]. In our previous study, we evaluated the transmission-blocking activities of a partial 200 aa PSOP25 domain [26]. Here we expressed the full-length rPSOP25 protein and raised polyclonal antisera as well as mAb for this protein, which were found to possess effective TB activities in an in vitro ookinete formation assay and in vivo DFA.
Antibody concentrations against TBV candidates, as measured by conventional ELISA, have been shown to be associated with the effectiveness of TB activities in mosquito membrane feeding assays [19, 43]. In our previous study, the 200 aa PSOP25 fragment including ten predicted antibody epitopes had elicited obvious antibody responses, and immunized mouse antisera produced TB activities with 25% reduction in prevalence and 64.3% reduction in oocyst density [26]. The full-length PSOP25 is predicted to contain four additional antibody epitopes, and the full-length rPSOP25 indeed induced high antibody titers in mice. In parallel comparison experiments, mosquitoes fed on mice immunized with the full-length and partial rPSOP25 showed similar levels (~60%) of reduction in oocyst density as compared to those fed on control mice. However, there was a greater degree of reduction in oocyst prevalence in mosquitoes fed on mice immunized with the full-length protein (31.2%) as compared to that in mosquitoes fed on mice immunized with the partial rPSOP25 (25%) [26]. The TB activities of PSOP25 were comparable to those of PSOP12 [44], PSOP7 and PSOP26 [26] in the reduction of oocyst density and infection prevalence in DFA. Furthermore, monoclonal antibodies have been a valuable tool for the characterization of TBVs [45, 46]. Previous studies have explored passive transfer of transmission blocking mAbs (e.g. Pbs21 mAb clone 13.1) for TB activities [31, 47]. In this study, an IgGl-type mAb against PSOP25 significantly inhibited the development of ookinetes and oocyst when administered through passive transfer prior to mosquito feeding, and the TB activities were comparable to those from the full-length rPSOP25 immunization group. Passive immunization with TB mAbs may be of additional values as an intervention in specific circumstances, including malaria epidemic settings [48, 49].
Transmission-blocking strategies require improved knowledge of the basic biology of the parasite [50]. Recent efforts in genomics, transcriptomics and proteomics have revealed a large number of molecules that may play key roles in ookinete development [51–53]. Further, screening for novel vaccine candidates based on gene KO and phenotypic analysis will undoubtedly yield valuable information regarding the cell biology of the ookinetes [52]. Several ookinete proteins which play various roles in midgut colonization have been characterized, including the GPI-anchored P25 and P28 proteins [20, 54], circumsporozoite TRAP-related protein (CTRP) [55, 56], Plasmodium von Willebrand factor A domain-related protein (WARP) [57], secreted ookinete apical protein (SOAP) [55, 58], and the recently described putative secreted ookinete proteins (PSOPs) [52]. Here, we generated a Δpsop25 line, and detected slightly reduced exflagellation activity of male gametocytes, but significantly reduced ookinete conversion rate in vitro. Whereas most Δpsop25 parasites progressed normally to zygote and retort stages, further maturation to ookinetes was retarded, which resulted in a 60.9% reduction in the number of ookinetes as compared to the WT parasite. This phenotype shows some similarity with that of psop2 knockout parasites, which appeared morphologically normal but showed reduced in vivo ookinete numbers [52]. The blockade in ookinete maturation in the Δpsop25 parasite was further reflected in the reduction of oocyst density in DFA. The oocysts per mosquito midgut in those fed on the Δpsop25 parasites was reduced by 69.4%, like that with the Δpsop9 line [52]. Given that other PSOP proteins such as PSOP26 showed ookinete surface localization [26] and there is a possibility that these surface proteins interact, it would be interesting to determine whether psop25 disruption could affect the expression of other PSOP proteins.
Conclusions
This study confirmed that the full-length recombinant protein of a newly identified TBV candidate PSOP25 expressed in ookinetes of the rodent parasite P. berghei could also elicit a strong antibody response in mice. Both polyclonal mouse antisera and mAb against this protein recognized the surface of zygotes, retorts and ookinetes and possessed similar TB activities as the polyclonal antisera generated against the truncated version of this protein. Genetic KO study indicated that PSOP25 in P. berghei is required for ookinete formation and maturation. Collectively, PSOP25 is an excellent TBV candidate targeting the post-fertilization stages, and further assessment of TB activities in P. falciparum and P. vivax are warranted.
Acknowledgements
We are grateful to Ms. Jun Liu for technical support and to Dr. Hiroyuki Matsuoka for providing the Pbs21 mAb clone 13.1. We thank plasmoGEM for kindly providing the target vector PbGEM-042760 (http://plasmogem.sanger.ac.uk/).
Funding
This study was supported by the National Institutes of Health grants R01AI099611 and R01AI104946, and the National Natural Science Foundation of China 81471978.
Availability of data and materials
The data supporting the conclusions of this article are included within the article.
Authors’ contributions
YC and LC conceived the study and helped draft the manuscript. EL, GBH and TT helped with the bioinformatics analysis and drafted the manuscript. WZ carried out the rPSOP25 protein expression, mAb development, TB activity studies of PSOP25 and drafted the manuscript. FL and YH carried out function studies by genetic knockout, statistical analysis. QL and QF participated in the antibodies specificity detection and statistical analysis. All authors contributed to the writing of the manuscript, read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Animal use was carried out according to the guidelines of the animal ethics committee of China Medical University.
Abbreviations
- CTRP
Circumsporozoite TRAP-related protein
- ELISA
Enzyme-linked immunosorbent assay
- GPI
Glycosyl-phosphatidylinositol
- i.p.
Intraperitoneally
- IFA
Indirect immunofluorescence assay
- iRBCs
Infected red blood cells
- KO
Knockout
- mAb
Monoclonal antibody
- PBS
Phosphate-buffered saline
- PBS-T
0.05% Tween 20 in phosphate-buffered saline
- PSOPs
Putative secreted ookinete proteins
- RBCs
Red blood cells
- SOAP
Secreted ookinete apical protein
- TBS-T
0.1% Tween 20 in Tris-buffered saline
- TBV
Transmission-blocking vaccine
- WARP
von Willebrand factor A domain-related protein
- WT
Wildtype
Additional files
Primer information and sequences. (DOCX 15 kb)
Predicted B cell epitopes of the PSOP25 protein (http://tools.iedb.org/bcell). Below is the protein domain architecture of PSOP25 with signal peptide highlighted in red, low complexity in pink, and transmembrane region in blue. Figure S2. The isotype of anti-rPSOP25 mAb was identified by ELISA using by the SBA Clonotyping™ System-HRP. The data represent two separate experiments. Error bar shows mean + standard deviation. Figure S3. Western blot analysis of P. berghei ookinete lysates with anti-rPSOP25 sera (PolyAb) and anti-rPSOP25 mAb. Lysates were subjected to electrophoresis under non-reducing conditions by SDS-PAGE. Pbs21 mAb was used as positive control; a control mouse serum (PBS) was used as negative control. (ZIP 2413 kb)
Contributor Information
Wenqi Zheng, Email: zhengwenqi2011@163.com.
Fei Liu, Email: skyliu00@sina.com.
Yiwen He, Email: 845209605@qq.com.
Qingyang Liu, Email: liuqingyang1987@163.com.
Gregory B. Humphreys, Email: gbh109@psu.edu
Takafumi Tsuboi, Email: tsuboi@ccr.ehime-u.ac.jp.
Qi Fan, Email: fanqi2002@yahoo.com.
Enjie Luo, Email: enjieluo2011@163.com.
Yaming Cao, Email: ymcao@mail.cmu.edu.cn.
Liwang Cui, Email: luc2@psu.edu.
References
- 1.WHO. World malaria report. 2015. http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/.
- 2.Miotto O, Almagro-Garcia J, Manske M, Macinnis B, Campino S, Rockett KA, et al. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat Genet. 2013;45(6):648–655. doi: 10.1038/ng.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505(7481):50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, Alonso PL, del Portillo HA. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9(9):555–566. doi: 10.1016/S1473-3099(09)70177-X. [DOI] [PubMed] [Google Scholar]
- 5.Smith TA, Leuenberger R, Lengeler C. Child mortality and malaria transmission intensity in Africa. Trends Parasitol. 2001;17(3):145–149. doi: 10.1016/S1471-4922(00)01814-6. [DOI] [PubMed] [Google Scholar]
- 6.Kaslow DC. Transmission-blocking vaccines. Chem Immunol. 2002;80:287–307. doi: 10.1159/000058850. [DOI] [PubMed] [Google Scholar]
- 7.Eckhoff P. Mathematical models of within-host and transmission dynamics to determine effects of malaria interventions in a variety of transmission settings. Am J Trop Med Hyg. 2013;88(5):817–827. doi: 10.4269/ajtmh.12-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Outchkourov NS, Roeffen W, Kaan A, Jansen J, Luty A, Schuiffel D, et al. Correctly folded Pfs48/45 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in mice. P Natl Acad Sci USA. 2008;105(11):4301–4305. doi: 10.1073/pnas.0800459105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh SK, Roeffen W, Andersen G, Bousema T, Christiansen M, Sauerwein R, et al. A Plasmodium falciparum 48/45 single epitope R0.6C subunit protein elicits high levels of transmission blocking antibodies. Vaccine. 2015;33(16):1981–1986. doi: 10.1016/j.vaccine.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald NJ, Nguyen V, Shimp R, Reiter K, Herrera R, Burkhardt M, et al. Structural and Immunological Characterization of Recombinant 6-Cysteine Domains of the Plasmodium falciparum Sexual Stage Protein Pfs230. J Biol Chem. 2016;291(38):19913–19922. doi: 10.1074/jbc.M116.732305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tachibana M, Wu Y, Iriko H, Muratova O, MacDonald NJ, Sattabongkot J, et al. N-terminal prodomain of Pfs230 synthesized using a cell-free system is sufficient to induce complement-dependent malaria transmission-blocking activity. Clin Vaccine Immunol. 2011;18(8):1343–1350. doi: 10.1128/CVI.05104-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SM, Wu CK, Plieskatt J, McAdams DH, Miura K, Ockenhouse C, King CR. Assessment of Pfs25 expressed from multiple soluble expression platforms for use as transmission-blocking vaccine candidates. Malar J. 2016;15(1):405. doi: 10.1186/s12936-016-1464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaurio RA, Pacheco MA, Cornejo OE, Durrego E, Stanley CE, Jr, Castillo AI, et al. Evolution of the Transmission-Blocking Vaccine Candidates Pvs28 and Pvs25 in Plasmodium vivax: Geographic Differentiation and Evidence of Positive Selection. PLoS Negl Trop Dis. 2016;10(6):e0004786. doi: 10.1371/journal.pntd.0004786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roeffen W, Theisen M, van de Vegte-Bolmer M, van Gemert G, Arens T, Andersen G, et al. Transmission-blocking activity of antibodies to Plasmodium falciparum GLURP.10C chimeric protein formulated in different adjuvants. Malar J. 2015;14:443. doi: 10.1186/s12936-015-0972-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miura K, Stone WJ, Koolen KM, Deng B, Zhou L, van Gemert GJ, et al. An inter-laboratory comparison of standard membrane-feeding assays for evaluation of malaria transmission-blocking vaccines. Malar J. 2016;15:463. doi: 10.1186/s12936-016-1515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones S, Grignard L, Nebie I, Chilongola J, Dodoo D, Sauerwein R, et al. Naturally acquired antibody responses to recombinant Pfs230 and Pfs48/45 transmission blocking vaccine candidates. J Infect. 2015;71(1):117–127. doi: 10.1016/j.jinf.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Sinden RE. A proteomic analysis of malaria biology: integration of old literature and new technologies. Int J Parasitol. 2004;34(13–14):1441–1450. doi: 10.1016/j.ijpara.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Stone WJ, Dantzler KW, Nilsson SK, Drakeley CJ, Marti M, Bousema T, et al. Naturally acquired immunity to sexual stage P. falciparum parasites. Parasitology. 2016;143(2):187–198. doi: 10.1017/S0031182015001341. [DOI] [PubMed] [Google Scholar]
- 19.Vermeulen AN, Ponnudurai T, Beckers PJ, Verhave JP, Smits MA, Meuwissen JH. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med. 1985;162(5):1460–1476. doi: 10.1084/jem.162.5.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomas AM, Margos G, Dimopoulos G, van Lin LH, de Koning-Ward TF, Sinha R, et al. P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions. Embo J. 2001;20(15):3975–3983. doi: 10.1093/emboj/20.15.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tirawanchai N, Winger LA, Nicholas J, Sinden RE. Analysis of immunity induced by the affinity-purified 21-kilodalton zygote-ookinete surface antigen of Plasmodium berghei. Infect Immun. 1991;59(1):36–44. doi: 10.1128/iai.59.1.36-44.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregory JA, Li F, Tomosada LM, Cox CJ, Topol AB, Vinetz JM, et al. Algae-produced Pfs25 elicits antibodies that inhibit malaria transmission. PLoS One. 2012;7(5):e37179. doi: 10.1371/journal.pone.0037179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar R, Angov E, Kumar N. Potent malaria transmission-blocking antibody responses elicited by Plasmodium falciparum Pfs25 expressed in Escherichia coli after successful protein refolding. Infect Immun. 2014;82(4):1453–1459. doi: 10.1128/IAI.01438-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blagborough AM, Musiychuk K, Bi H, Jones RM, Chichester JA, Streatfield S, et al. Transmission blocking potency and immunogenicity of a plant-produced Pvs25-based subunit vaccine against Plasmodium vivax. Vaccine. 2016;34(28):3252–3259. doi: 10.1016/j.vaccine.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otto TD, Bohme U, Jackson AP, Hunt M, Franke-Fayard B, Hoeijmakers WA, et al. A comprehensive evaluation of rodent malaria parasite genomes and gene expression. BMC Biol. 2014;12:86. doi: 10.1186/s12915-014-0086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng W, Kou X, Du Y, Liu F, Yu C, Tsuboi T, et al. Identification of three ookinete-specific genes and evaluation of their transmission-blocking potentials in Plasmodium berghei. Vaccine. 2016;34(23):2570–2578. doi: 10.1016/j.vaccine.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter RL, Lal AA. Copolymer adjuvants in malaria vaccine development. Am J Trop Med Hyg. 1994;50(4 Suppl):52–58. doi: 10.4269/ajtmh.1994.50.52. [DOI] [PubMed] [Google Scholar]
- 28.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 29.Chowdhury DR, Angov E, Kariuki T, Kumar N. A potent malaria transmission blocking vaccine based on codon harmonized full length Pfs48/45 expressed in Escherichia coli. PLoS One. 2009;4(7):e6352. doi: 10.1371/journal.pone.0006352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinden RE, Hartley RH, Winger L. The development of Plasmodium ookinetes in vitro: an ultrastructural study including a description of meiotic division. Parasitology. 1985;91(Pt 2):227–244. doi: 10.1017/S0031182000057334. [DOI] [PubMed] [Google Scholar]
- 31.Winger LA, Tirawanchai N, Nicholas J, Carter HE, Smith JE, Sinden RE. Ookinete antigens of Plasmodium berghei. Appearance on the zygote surface of an Mr 21 kD determinant identified by transmission-blocking monoclonal antibodies. Parasite Immunol. 1988;10(2):193–207. doi: 10.1111/j.1365-3024.1988.tb00214.x. [DOI] [PubMed] [Google Scholar]
- 32.Kou X, Zheng W, Du F, Liu F, Wang M, Fan Q, et al. Characterization of a Plasmodium berghei sexual stage antigen PbPH as a new candidate for malaria transmission-blocking vaccine. Parasit Vectors. 2016;9(1):190. doi: 10.1186/s13071-016-1459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guttery DS, Poulin B, Ramaprasad A, Wall RJ, Ferguson DJ, Brady D, et al. Genome-wide functional analysis of Plasmodium protein phosphatases reveals key regulators of parasite development and differentiation. Cell Host Microbe. 2014;16(1):128–140. doi: 10.1016/j.chom.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janse CJ, Mons B, Rouwenhorst RJ, Van der Klooster PF, Overdulve JP, Van der Kaay HJ. In vitro formation of ookinetes and functional maturity of Plasmodium berghei gametocytes. Parasitology. 1985;91(Pt 1):19–29. doi: 10.1017/S0031182000056481. [DOI] [PubMed] [Google Scholar]
- 35.Reininger L, Billker O, Tewari R, Mukhopadhyay A, Fennell C, Dorin-Semblat D, et al. A NIMA-related protein kinase is essential for completion of the sexual cycle of malaria parasites. J Biol Chem. 2005;280(36):31957–31964. doi: 10.1074/jbc.M504523200. [DOI] [PubMed] [Google Scholar]
- 36.Doi M, Tanabe K, Tachibana S, Hamai M, Tachibana M, Mita T, et al. Worldwide sequence conservation of transmission-blocking vaccine candidate Pvs230 in Plasmodium vivax. Vaccine. 2011;29(26):4308–4315. doi: 10.1016/j.vaccine.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hills T, Srivastava A, Ayi K, Wernimont AK, Kain K, Waters AP, et al. Characterization of a new phosphatase from Plasmodium. Mol Biochem Parasit. 2011;179(2):69–79. doi: 10.1016/j.molbiopara.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Blagborough AM, Yoshida S, Sattabongkot J, Tsuboi T, Sinden RE. Intranasal and intramuscular immunization with Baculovirus Dual Expression System-based Pvs25 vaccine substantially blocks Plasmodium vivax transmission. Vaccine. 2010;28(37):6014–6020. doi: 10.1016/j.vaccine.2010.06.100. [DOI] [PubMed] [Google Scholar]
- 39.Kolaskar AS, Tongaonkar PC. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990;276(1–2):172–174. doi: 10.1016/0014-5793(90)80535-Q. [DOI] [PubMed] [Google Scholar]
- 40.Janse CJ, Franke-Fayard B, Mair GR, Ramesar J, Thiel C, Engelmann S, et al. High efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol Biochem Parasitol. 2006;145(1):60–70. doi: 10.1016/j.molbiopara.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Goodman AL, Blagborough AM, Biswas S, Wu Y, Hill AV, Sinden RE, et al. A viral vectored prime-boost immunization regime targeting the malaria Pfs25 antigen induces transmission-blocking activity. PLoS One. 2011;6(12):e29428. doi: 10.1371/journal.pone.0029428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.mal ERACGoV A research agenda for malaria eradication: vaccines. PLoS Med. 2011;8(1):e1000398. doi: 10.1371/journal.pmed.1000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikolaeva D, Draper SJ, Biswas S. Toward the development of effective transmission-blocking vaccines for malaria. Expert Rev Vaccines. 2015;14(5):653–680. doi: 10.1586/14760584.2015.993383. [DOI] [PubMed] [Google Scholar]
- 44.Sala KA, Nishiura H, Upton LM, Zakutansky SE, Delves MJ, Iyori M, et al. The Plasmodium berghei sexual stage antigen PSOP12 induces anti-malarial transmission blocking immunity both in vivo and in vitro. Vaccine. 2015;33(3):437–445. doi: 10.1016/j.vaccine.2014.11.038. [DOI] [PubMed] [Google Scholar]
- 45.Vermeulen AN, Roeffen WF, Henderik JB, Ponnudurai T, Beckers PJ, Meuwissen JH. Plasmodium falciparum transmission blocking monoclonal antibodies recognize monovalently expressed epitopes. Dev Biol Stand. 1985;62:91–97. [PubMed] [Google Scholar]
- 46.Carter R. Transmission blocking malaria vaccines. Vaccine. 2001;19(17–19):2309–2314. doi: 10.1016/S0264-410X(00)00521-1. [DOI] [PubMed] [Google Scholar]
- 47.Ranawaka GR, Fleck SL, Blanco AR, Sinden RE. Characterization of the modes of action of anti-Pbs21 malaria transmission-blocking immunity: ookinete to oocyst differentiation in vivo. Parasitology. 1994;109(Pt 4):403–411. doi: 10.1017/S0031182000080653. [DOI] [PubMed] [Google Scholar]
- 48.Sauerwein RW. Malaria transmission-blocking vaccines: the bonus of effective malaria control. Microbes Infect. 2007;9(6):792–795. doi: 10.1016/j.micinf.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 49.Sauerwein RW, Bousema T. Transmission blocking malaria vaccines: Assays and candidates in clinical development. Vaccine. 2015;33(52):7476–7482. doi: 10.1016/j.vaccine.2015.08.073. [DOI] [PubMed] [Google Scholar]
- 50.Tewari R, Straschil U, Bateman A, Bohme U, Cherevach I, Gong P, et al. The systematic functional analysis of Plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe. 2010;8(4):377–387. doi: 10.1016/j.chom.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall N, Karras M, Raine JD, Carlton JM, Kooij TW, Berriman M, et al. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science. 2005;307(5706):82–86. doi: 10.1126/science.1103717. [DOI] [PubMed] [Google Scholar]
- 52.Ecker A, Bushell ES, Tewari R, Sinden RE. Reverse genetics screen identifies six proteins important for malaria development in the mosquito. Mol Microbiol. 2008;70(1):209–220. doi: 10.1111/j.1365-2958.2008.06407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lal K, Prieto JH, Bromley E, Sanderson SJ, Yates JR, 3rd, Wastling JM, et al. Characterisation of Plasmodium invasive organelles; an ookinete microneme proteome. Proteomics. 2009;9(5):1142–1151. doi: 10.1002/pmic.200800404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sinden RE, Billingsley PF. Plasmodium invasion of mosquito cells: hawk or dove? Trends Parasitol. 2001;17(5):209–212. doi: 10.1016/S1471-4922(01)01928-6. [DOI] [PubMed] [Google Scholar]
- 55.Nacer A, Underhill A, Hurd H. The microneme proteins CTRP and SOAP are not essential for Plasmodium berghei ookinete to oocyst transformation in vitro in a cell free system. Malar J. 2008;7:82. doi: 10.1186/1475-2875-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramakrishnan C, Dessens JT, Armson R, Pinto SB, Talman AM, Blagborough AM, et al. Vital functions of the malarial ookinete protein, CTRP, reside in the A domains. Int J Parasitol. 2011;41(10):1029–1039. doi: 10.1016/j.ijpara.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuda M, Yano K, Tsuboi T, Torii M, von Willebrand CY, Factor A. von Willebrand Factor A domain-related protein, a novel microneme protein of the malaria ookinete highly conserved throughout Plasmodium parasites. Mol Biochem Parasitol. 2001;116(1):65–72. doi: 10.1016/S0166-6851(01)00304-8. [DOI] [PubMed] [Google Scholar]
- 58.Dessens JT, Siden-Kiamos I, Mendoza J, Mahairaki V, Khater E, Vlachou D, et al. SOAP, a novel malaria ookinete protein involved in mosquito midgut invasion and oocyst development. Mol Microbiol. 2003;49(2):319–329. doi: 10.1046/j.1365-2958.2003.03566.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusions of this article are included within the article.


