Abstract
Growing evidence suggests that transplantation of autologous bone-marrow mononuclear cells (ABMMNCs) can improve the perfusion and contractile function of ischemic myocardium. This procedure could potentially benefit transplant candidates awaiting a donor heart.
To study the safety and feasibility of ABMMNC injection, we performed a prospective, nonrandomized, open-label study in 5 heart transplant candidates with severe ischemic heart failure. Each patient underwent baseline single-photon emission computed tomography, a ramp treadmill protocol, 2-dimensional echocardiography, 24-hour Holter monitoring, and signal-averaged electrocardiography, which were repeated at 2 and 6 months. Transendocardial delivery of ABMMNCs was done with the aid of electromechanical mapping to identify viable myocardium. Each patient received 15 ABMMNC injections of 0.2 cc each. There were no deaths, significant arrhythmias, or other major complications.
The ABMMNC injection reduced the amount of ischemic myocardium (not statistically significant). More important, exercise test results improved significantly. Myocardial volume oxygen consumption increased from 10.6 ± 3 mL/kg/min (baseline) to 16.3 ± 7 mL/kg/min (2 months) and 23 ± 7 mL/kg/min (6 months) (P = 0.0091). In 4 of the 5 cases, this was such an improvement that the patients were no longer eligible for cardiac transplantation. In addition, metabolic equivalents improved from 3.03 ± 0.66 (baseline) to 4.65 ± 1.99 (2 months) and 6.5 ± 2.0 (6 months) (P = 0.0092).
In conclusion, ABMMNC injections were performed safely and resulted in improved exercise capacity. This technique may hold promise as an alternative to medical management in patients with severe ischemic heart failure who are ineligible for conventional revascularization.
Key words: Bone-marrow transplantation; catheter-based transendocardial delivery; coronary angiog-raphy; coronary circulation; electromechanical mapping; endocardium; heart failure, congestive/therapy; heart transplantation; interven-tional cardiology; myocardial ischemia/therapy; stem cell transplantation; tomography, emission-computed, single-photon; transplantation, autologous; ventricular function, left
Coronary artery disease remains the leading cause of morbidity and mortality in the Western world.1 In a growing number of patients, coronary artery disease progresses to end-stage heart failure without further options for revascularization. When optimal standard therapy offers limited survival, cardiac transplantation is indicated for these patients.2 However, transplantation is hindered by the scarcity of donor organs.3 This shortage has led to the development of alternative therapies, such as implantation of a ventricular assist device (VAD) as a bridge to transplantation and therapy tailored to achieve certain hemodynamic goals. Nevertheless, because VAD implantation can involve substantial morbidity, including infectious, hemorrhagic, and thromboembolic complications,4 this approach has its own limitations.
Within the past few years, researchers have made many advances in their understanding of stem-cell biology and have found growing evidence that cell transplantation can improve the perfusion and contractile function of ischemic myocardium.5–12 We recently reported the initial safety and feasibility of autologous bone-marrow mononuclear cell (ABMMNC) injection into areas of ischemic myocardium in patients with end-stage ischemic heart failure.13 Short-term follow-up studies have shown improved perfusion, as measured by single-photon emission computed tomography (SPECT), and increased left ventricular ejection fractions (LVEFs), as evaluated by 2-dimensional (2-D) echocardiography. Therefore, ABMMNC injection could potentially benefit transplant candidates awaiting a donor heart. To assess the safety and feasibility of ABMMNC injection, we studied a subgroup of patients who were on the waiting list for heart transplantation.
Patients and Methods
Patient Population
This prospective, nonrandomized, open-label study focused on 5 patients with severe ischemic heart failure and no remaining options for standard revascularization who were awaiting cardiac transplantation. All 5 patients were receiving the maximally tolerated degree of medical therapy, and none were enrolled in a cardiac rehabilitation program. The following inclusion criteria were required for enrollment: 1) chronic coronary artery disease with a reversible perfusion defect as revealed by SPECT; 2) an LVEF of <0.40 as evaluated by echocardiography or left ventricular angiography; 3) ineligibility for percutaneous or surgical revascularization as determined by coronary arteriography; ineligibility for surgical or percutaneous revascularization as determined by 2 expert committees (a surgical committee comprising 2 cardiovascular surgeons and a noninvasive cardiologist, and an interventional committee comprising 2 interventional cardiologists and 1 noninvasive cardiologist); and 4) signed informed consent. In addition, all 5 patients were actively listed for a cardiac transplant and had a myocardial volume oxygen consumption (mVO2) of <14 mL/kg/min. Patients were excluded from the study if they met any of the criteria listed in Table I.
TABLE I. Exclusion Criteria
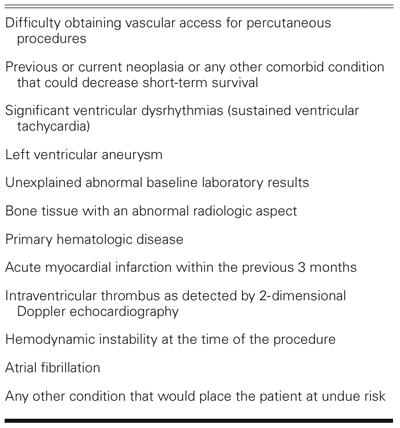
The ethics committee of Pro-Cardiaco Hospital (Rio de Janeiro) and the Brazilian National Research Ethics Council approved the study protocol.
Methods
Baseline Evaluation. In all patients, baseline evaluation included exercise stress tests, a ramp treadmill protocol,14 24-hour Holter monitoring, 2-D Doppler echocardiography, dipyridamole SPECT perfusion scanning, and signal-averaged electrocardiography. All patients also underwent serum C-reactive protein (CRP) and white blood cell (WBC) evaluation.
Bone-Marrow Aspiration and Isolation of Mononuclear Cells. Approximately 4 hours before the cell injection procedure, 50 mL of bone marrow was aspirated from the patient's posterior iliac crest with the aid of local anesthesia. Bone-marrow mononuclear cells were isolated by means of a density gradient on Ficoll-Paque™ Plus (Amersham Biosciences; Amersham, UK). The mononuclear cells were exhaustively washed with heparinized saline solution containing 5% human serum albumin. After being filtered through 100-μm nylon mesh to remove cell aggregates, the cells were resuspended in saline solution with 5% human serum albumin for injection. A small fraction of the cell suspension was used for cell counting and viability testing with trypan blue exclusion. Cell viability was shown to be consistently above 95%. Cultures of the clinically used cell preparations were negative for bacterial and fungal organisms.
Transendocardial Delivery of Autologous Bone-Marrow Mononuclear Cells. Patients were taken to the cardiac catheterization laboratory approximately 1 hour before the anticipated arrival of the bone-marrow cells from the laboratory. Left-sided heart catheterization with biplane left ventricular angiography was performed. Subsequently, electromechanical mapping of the left ventricle was performed as previously described, with use of the NOGA™ Cardiac Navigation System (Biosense Webster, Inc.; Diamond Bar, Calif).15 The general treatment area was selected by matching the area identified as ischemic by previous SPECT perfusion imaging. The electromechanical map was then used to target the specific treatment area by identifying viable myocardium (unipolar voltage, ≥ 6.9 mV) within the general region. Areas with decreased mechanical activity (local linear shortening <12%, indicating hibernating myocardium) were preferred.
The NOGA injection catheter was prepared by adjusting the needle extension at 0° and 90° flex and by using 0.1 cc of ABMMNCs to fill the needle's dead space. The injection catheter tip was passed across the aortic valve and into the target area. Each injection site was carefully evaluated before the cells were injected. Before each injection of cells into the left ventricular wall, the following safety criteria had to be met to ensure intramyocardial delivery: 1) perpendicular positioning of the catheter relative to the left ventricular wall; 2) excellent loop stability (<4); 3) maximal needle extension of 6 mm; and 4) the presence of a premature ventricular contraction on extension of the needle into the myocardium. Each patient received 15 ABMMNC injections of 0.2 cc each.
Follow-Up Evaluations at 2 and 6 Months. At 2 and 6 months, all patients underwent noninvasive follow-up evaluations consisting of a repeat dipyridamole SPECT perfusion scan, ramp treadmill protocol, 2-D echocardiography, 24-hour Holter monitoring, and signal-averaged electrocardiography. In addition, all patients underwent repeat serum CRP and WBC evaluation.
Dipyridamole stress and resting SPECT imaging were performed using the same stress procedure at baseline and at follow-up as previously described.13 The results were interpreted by a blinded, experienced observer. The ramp treadmill protocol has been previously described.16 In brief, the predicted mVO2 was used to tailor the patient workload. The treadmill speed was initially 0.5 mph, and the inclination was 0 to 10% with a planned duration of 10 minutes of exercise. Holter monitoring and signal-averaged electrocardiography were performed according to previously described standard protocols.17
Statistical Analysis. Continuous variables are presented as mean and standard deviation, and categorical variables are expressed as percentages. Because each patient was studied at baseline, 2 months, and 6 months, differences among the 3 time periods were assessed with a 2-way analysis of variance (ANOVA) technique appropriate for a randomized complete block design. Categorical variables were assessed with a χ2 test. A P value of ≤0.05 was considered significant.
Results
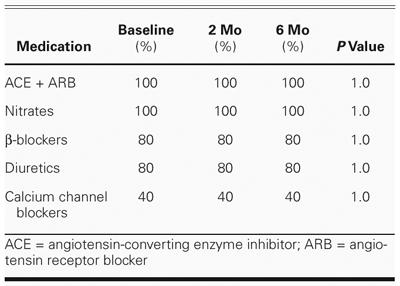
Table II lists the demographic characteristics of the patient population. All patients received unchanged medical therapy throughout the study period (Table III).
TABLE II. Demographic Characteristics of the 5 Patients

TABLE III. Percentage of Patients Receiving Selected Cardiac Medications at Baseline, 2-Month, and 6-Month Follow-Up Evaluation
Safety Data. There were no deaths or major periprocedural complications. One patient had transient pulmonary edema that was easily reversed with loop diuretics after the procedure. No postprocedural pericardial effusions were seen on 2-D Doppler echocardiography. Each patient was discharged on the 3rd hospital day in accordance with the protocol. No sustained arrhythmias were associated with the injection procedures, nor did any significant arrhythmias occur while the patients were hospitalized. Holter monitoring showed no significant difference in the number or percentage of premature ventricular contractions or sustained ventricular arrhythmias at baseline, 2 months, and 6 months after the procedure (Table IV). Similarly, there was no significant difference in the signal-averaged electrocardiographic results at baseline, 2 months, and 6 months. Although the WBC count was not significantly different at those same time intervals, the CRP level varied significantly over time (P = 0.04). The CRP was modestly elevated at 6 months (compared with the 2-month level) but was unchanged from baseline (P >0.05).
TABLE IV. Safety Data: Laboratory, Holter Monitor, and Signal-Averaged Electrocardiographic Results

Noninvasive Follow-Up Evaluations at 2 and 6 Months. When baseline values were compared with 2-month follow-up values, the exercise test results were found to have improved significantly: the mVO2 increased from 10.6 ± 3 to 16.3 ± 7 mL/kg/min (P = 0.0091) (Fig. 1), and metabolic equivalents (METs) increased from 3.03 ± 0.66 to 4.65 ± 1.99 (Fig. 2). Moreover, a continued significant increase in the exercise test variables was documented at 6 months: the mVO2 increased from 16.3 ± 7 to 23 ± 7 mL/kg/min (P = 0.0091), and METs increased from 4.65 ± 1.99 to 6.5 ± 2.0 (P = 0.0092).
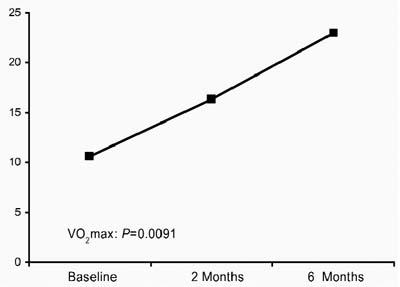
Fig. 1 Myocardial volume oxygen consumption (mVOM/2) at baseline, 2 months, and 6 months.
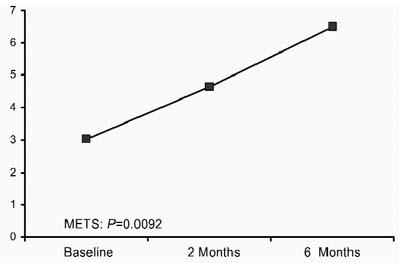
Fig. 2 Metabolic equivalents (METS) at baseline, 2 months, and 6 months.
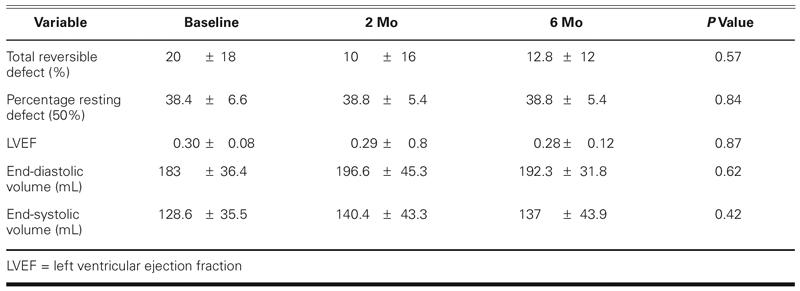
Echocardiographic data revealed that end-systolic volume, end-diastolic volume, and LVEF did not change significantly from baseline to 2 or 6 months (Table V). Likewise, nuclear perfusion imaging studies showed similar amounts of total reversible defect and percentages of resting defects with 50% activity (scar) at baseline, 2 months, and 6 months (Table V).
TABLE V. Results of Noninvasive Follow-Up Evaluation at 2 and 6 Months
Discussion
Our study describes, for the 1st time, 6-month follow-up results after ABMMNC transplantation with the use of transendocardial injections in heart transplant candidates. The results suggest that the injection of ABMMNCs is safe and improves exercise capacity when viable ischemic areas of myocardium are targeted.
Infarct-related heart failure remains a major cause of morbidity and mortality.1 After a myocardial infarction involving substantial injury, native angiogenesis is rarely sufficient to restore tissue vascularity. As a result, chronically ischemic (but viable) myocardium may persist in association with varying degrees of scar tissue. Despite the lack of further revascularization options, patients with ischemic heart failure who are listed for heart transplantation may retain a considerable amount of viable myocardium. In these viable areas, the underlying physiologic processes allow restoration of myocardial function if perfusion is improved. Therefore, in theory, the use of angiogenesis to improve the perfusion of viable areas could be effective in this challenging patient population.
The initial evidence suggests that bone-marrow-derived progenitor cells may participate in the natural healing processes after tissue injury and are recruited from the bone marrow once vascular trauma ensues.18,19 In addition, there is evidence that subsets of bone marrow cells can differentiate into cardiomyocytes and endothelial cells.19,20 Therefore, theoretically, bone-marrow-derived progenitor cell transplantation could offer an alternative therapy for ischemic heart disease by contributing to myocardial tissue replacement, neo-angiogenesis, or both. Accordingly, in the present study, we used electromechanical mapping technology to deliver bone-marrow-derived progenitor cells to areas of viable myocardium. This technology has been widely confirmed to be accurate for identifying and delineating scarred and viable myocardium and for differentiating degrees of infarct transmurality.15 In this way, the technology offers a theoretical benefit over surgical or intracoronary approaches, because the viability of the site can be determined before each injection. Furthermore, one can avoid the potential ischemia provoked by coronary manipulation. Specifically, many sites targeted in this study were in epicardial vascular beds that were totally occluded, making intracoronary delivery impossible. In addition, transendocardial delivery seemed safer for these chronically ill, high-risk patients, because it precluded the surgical risks of morbidity and mortality.
In this unique study population, all the patients were awaiting heart transplantation and, therefore, had an mVO2 of <14 mL/kg/min and a mean LVEF of 0.30. This fact may lend increased significance to our findings, since other studies described in the literature have involved lower-risk patients. In the present study, ABMMNC injection reduced the amount of ischemic myocardium, although not to a statistically significant degree. More important, the mVO2 improved significantly from baseline to 2 months, and the improvement continued at the 6-month follow-up evaluation (P = 0.0091). At that time, 4 of the 5 study patients had such an improved mVO2 that they were no longer eligible for cardiac transplantation. This finding is important, because the mVO2 has considerable prognostic value in heart failure patients: those with an mVO2 of <14 mL/kg/min have a far worse prognosis than do those with a higher mVO2.21 Exercise capacity is also linked directly to quality of life.
Left ventricular assistance has been used clinically as a bridge to cardiac transplantation.2 Recently, this approach has been shown to provide more of a survival benefit than does optimal medical therapy for patients with end-stage heart failure who are ineligible for heart transplantation.4 However, the use of left ventricular assist devices is hampered by high long-term costs22 and associated complications.23 In the present study, no infectious or inflammatory complications were associated with the procedure; WBC counts and serum CRP levels remained unchanged from baseline to 6-month follow-up. Despite concerns over the potential for malignant arrhythmias, no such arrhythmias were recorded on Holter studies, and the total number and percentage of premature ventricular contractions were unchanged after cell transplantation. Signal-averaged electrocardiographic results also remained unchanged.
In this initial safety and feasibility study, we introduced a new therapeutic modality into a complicated clinical setting involving heart transplant candidates. Despite the small number of patients treated, the limitations associated with subgroup analysis, and the potential placebo effect seen in phase I trials, we believe that ABMMNC transplantation may show promise as an alternative to medical management in these patients. The ABMMNC injections were performed safely and resulted in improved exercise capacity at the 6-month follow-up evaluation. Further investigation in larger, double-blind, randomized clinical trials is warranted.
Footnotes
Address for reprints: Emerson C. Perin, MD, PhD, 6624 Fannin, Suite 2220, Houston, TX 77030
E-mail: eperin@crescentb.net
*Co-principal investigators for the study, Catheter-Based Transendocardial Delivery of Autologous Bone-Marrow-Derived Mononuclear Cells in Patients Listed for Heart Transplantation.
References
- 1.American Heart Association. 2001 Heart and Stroke Statistical Update. Dallas, Tex: American Heart Association; 2000. [[{thij}<rf id='r1-6'>1. <urf>American Heart Association. 2001 Heart and Stroke Statistical Update. Dallas, Tex: American Heart Association; 2000.</urf></rf> ]] BOO!
- 2.Hunt SA, Frazier OH. Mechanical circulatory support and cardiac transplantation. Circulation 1998;97:2079–90. [[{thij}<rf id='r2-6'>2. <urf>Hunt SA, Frazier OH. Mechanical circulatory support and cardiac transplantation. Circulation 1998;97:2079–90.</urf></rf> ]] BOO! [DOI] [PubMed]
- 3.International Society for Heart and Lung Transplantation. ISHLT Transplant Registry Quarterly Reports. Addison (TX): International Society for Heart and Lung Transplantation; 2001. [[{thij}<rf id='r3-6'>3. <urf>International Society for Heart and Lung Transplantation. ISHLT Transplant Registry Quarterly Reports. Addison (TX): International Society for Heart and Lung Transplantation; 2001.</urf></rf> ]] BOO!
- 4.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med 2001;345:1435–43. [[{thij}<rf id='r4-6'>4. <urf>Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med 2001;345:1435–43.</urf></rf> ]] BOO! [DOI] [PubMed]
- 5.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001;410:701–5. [[{thij}<rf id='r5-6'>5. <urf>Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001;410:701–5.</urf></rf> ]] BOO! [DOI] [PubMed]
- 6.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001;7:430–6. [[{thij}<rf id='r6-6'>6. <urf>Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001;7:430–6.</urf></rf> ]] BOO! [DOI] [PubMed]
- 7.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JL, Uchida S, Masuda H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 2001;103:634–7. [[{thij}<rf id='r7-6'>7. <urf>Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JL, Uchida S, Masuda H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 2001;103:634–7.</urf></rf> ]] BOO! [DOI] [PubMed]
- 8.Tomita S, Mickle DA, Weisel RD, Jia ZQ, Tumiati LC, Allidina Y, et al. Improved heart function with myogenesis and angiogenesis after autologous porcine bone marrow stromal cell transplantation. J Thorac Cardiovasc Surg 2002; 123:1132–40. [[{thij}<rf id='r8-6'>8. <urf>Tomita S, Mickle DA, Weisel RD, Jia ZQ, Tumiati LC, Allidina Y, et al. Improved heart function with myogenesis and angiogenesis after autologous porcine bone marrow stromal cell transplantation. J Thorac Cardiovasc Surg 2002; 123:1132–40.</urf></rf> ]] BOO! [DOI] [PubMed]
- 9.Tse HF, Kwong YL, Chan JK, Lo G, Ho CL, Lau CP. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet 2003;361:47–9. [[{thij}<rf id='r9-6'>9. <urf>Tse HF, Kwong YL, Chan JK, Lo G, Ho CL, Lau CP. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet 2003;361:47–9.</urf></rf> ]] BOO! [DOI] [PubMed]
- 10.Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet 2003;361:45–6. [[{thij}<rf id='r10-6'>10. <urf>Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet 2003;361:45–6.</urf></rf> ]] BOO! [DOI] [PubMed]
- 11.Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 2002;106:1913–8. [[{thij}<rf id='r11-6'>11. <urf>Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 2002;106:1913–8.</urf></rf> ]] BOO! [DOI] [PubMed]
- 12.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation 2002;106:3009–17. [[{thij}<rf id='r12-6'>12. <urf>Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation 2002;106:3009–17.</urf></rf> ]] BOO! [DOI] [PubMed]
- 13.Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation 2003;107:2294–302. [[{thij}<rf id='r13-6'>13. <urf>Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation 2003;107:2294–302.</urf></rf> ]] BOO! [DOI] [PubMed]
- 14.Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the Ameri can College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol 2002;40:1531–40. [[{thij}<rf id='r14-6'>14. <urf>Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the Ameri can College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol 2002;40:1531–40.</urf></rf> ]] BOO! [DOI] [PubMed]
- 15.Perin EC, Silva GV, Sarmento-Leite R, Sousa AL, Howell M, Muthupillai R, et al. Assessing myocardial viability and infarct transmurality with left ventricular electromechanical mapping in patients with stable coronary artery disease: validation by delayed-enhancement magnetic resonance imaging. Circulation 2002;106:957–61. [[{thij}<rf id='r15-6'>15. <urf>Perin EC, Silva GV, Sarmento-Leite R, Sousa AL, Howell M, Muthupillai R, et al. Assessing myocardial viability and infarct transmurality with left ventricular electromechanical mapping in patients with stable coronary artery disease: validation by delayed-enhancement magnetic resonance imaging. Circulation 2002;106:957–61.</urf></rf> ]] BOO! [DOI] [PubMed]
- 16.American College of Sports Medicine, Franklin BA, Whaley MH, Howley ET. ACSM's guidelines for exercise testing and exercise prescription. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2000. [[{thij}<rf id='r16-6'>16. <urf>American College of Sports Medicine, Franklin BA, Whaley MH, Howley ET. ACSM's guidelines for exercise testing and exercise prescription. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2000.</urf></rf> ]] BOO!
- 17.Savard P, Rouleau JL, Ferguson J, Poitras N, Morel P, Davies RF, et al. Risk stratification after myocardial infarction using signal-averaged electrocardiographic criteria adjusted for sex, age, and myocardial infarction location. Circulation 1997;96:202–13. [[{thij}<rf id='r17-6'>17. <urf>Savard P, Rouleau JL, Ferguson J, Poitras N, Morel P, Davies RF, et al. Risk stratification after myocardial infarction using signal-averaged electrocardiographic criteria adjusted for sex, age, and myocardial infarction location. Circulation 1997;96:202–13.</urf></rf> ]] BOO! [DOI] [PubMed]
- 18.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999; 85:221–8. [[{thij}<rf id='r18-6'>18. <urf>Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999; 85:221–8.</urf></rf> ]] BOO! [DOI] [PubMed]
- 19.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol 2003;23:1185–9. [[{thij}<rf id='r19-6'>19. <urf>Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol 2003;23:1185–9.</urf></rf> ]] BOO! [DOI] [PubMed]
- 20.Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, et al. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation 2003;107:1024–32. [[{thij}<rf id='r20-6'>20. <urf>Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, et al. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation 2003;107:1024–32.</urf></rf> ]] BOO! [DOI] [PubMed]
- 21.Roul G, Moulichon ME, Bareiss P, Gries P, Sacrez J, Germain P, et al. Exercise peak VO2 determination in chronic heart failure: is it still of value? Eur Heart J 1994;15:495–502. [[{thij}<rf id='r21-6'>21. <urf>Roul G, Moulichon ME, Bareiss P, Gries P, Sacrez J, Germain P, et al. Exercise peak VO2 determination in chronic heart failure: is it still of value? Eur Heart J 1994;15:495–502.</urf></rf> ]] BOO! [DOI] [PubMed]
- 22.Moskowitz AJ, Rose EA, Gelijns AC. The cost of long-term LVAD implantation. Ann Thorac Surg 2001;71(3 Suppl): S195-204. [[{thij}<rf id='r22-6'>22. <urf>Moskowitz AJ, Rose EA, Gelijns AC. The cost of long-term LVAD implantation. Ann Thorac Surg 2001;71(3 Suppl): S195-204.</urf></rf> ]] BOO! [DOI] [PubMed]
- 23.Malani PN, Dyke DB, Pagani FD, Chenoweth CE. Nosocomial infections in left ventricular assist device recipients. Clin Infect Dis 2002;34:1295–300. [[{thij}<rf id='r23-6'>23. <urf>Malani PN, Dyke DB, Pagani FD, Chenoweth CE. Nosocomial infections in left ventricular assist device recipients. Clin Infect Dis 2002;34:1295–300.</urf></rf> ]] BOO! [DOI] [PubMed]


