Abstract
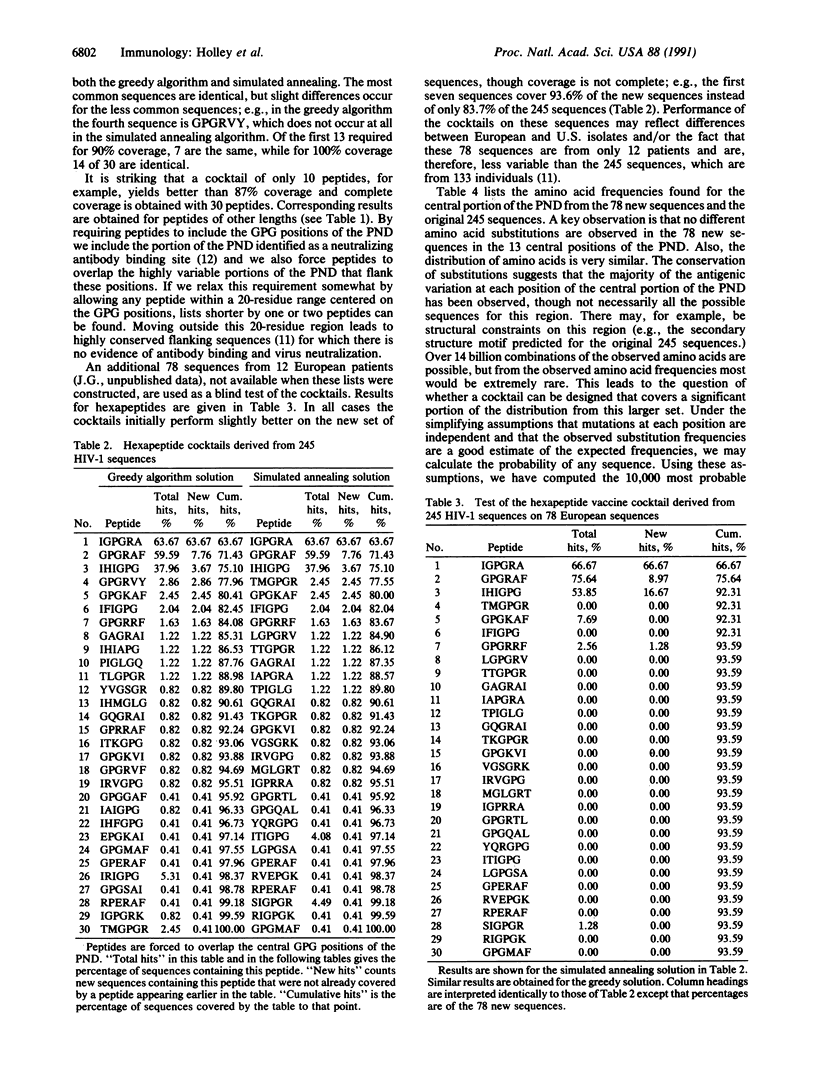
Sequences of the principal neutralizing determinant (PND) of the external envelope protein, gp120, from 245 isolates of human immunodeficiency virus type 1 are analyzed. The minimal set of peptides that would elicit antibodies to neutralize a majority of U.S. and European isolates of human immunodeficiency virus type 1 is determined with the assumption that peptides of a given length including the central Gly-Pro-Gly triad are required. In spite of the hypervariability of the PND, 90% of these 245 sequences include peptides from a set of 7 pentapeptides, 13 hexapeptides, or 17 heptapeptides. Tests of these peptide sets on 78 additional PND sequences show that 95% are covered by the 7 pentapeptides, 94% by the 13 hexapeptides, and 86% by the 17 heptapeptides. To anticipate variants not yet observed, single amino acid mutation frequencies from the 245 isolates are used to calculate an expanded set of the 10,000 most probable PND sequences. These sequences cover 86% of the total distribution expected for the central portion of the PND. Peptide lists derived from this expanded set when tested on the 78 additional sequences show that 7 pentapeptides cover 95%, 13 hexapeptides cover 94%, and 17 heptapeptides cover 94%. These results suggest that peptide cocktails of limited size with the potential to cover a large fraction of PND sequence variation may be feasible vaccine candidates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon R., Shapira M., Jacob C. O. Synthetic vaccines. J Immunol Methods. 1983 Jul 29;61(3):261–273. doi: 10.1016/0022-1759(83)90220-x. [DOI] [PubMed] [Google Scholar]
- Berman P. W., Gregory T. J., Riddle L., Nakamura G. R., Champe M. A., Porter J. P., Wurm F. M., Hershberg R. D., Cobb E. K., Eichberg J. W. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990 Jun 14;345(6276):622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- Devash Y., Calvelli T. A., Wood D. G., Reagan K. J., Rubinstein A. Vertical transmission of human immunodeficiency virus is correlated with the absence of high-affinity/avidity maternal antibodies to the gp120 principal neutralizing domain. Proc Natl Acad Sci U S A. 1990 May;87(9):3445–3449. doi: 10.1073/pnas.87.9.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Nara P. L., Schleif W. A., Lewis J. A., Davide J. P., Lee D. R., Kessler J., Conley S., Matsushita S., Putney S. D. Antibody-mediated in vitro neutralization of human immunodeficiency virus type 1 abolishes infectivity for chimpanzees. J Virol. 1990 Aug;64(8):3674–3678. doi: 10.1128/jvi.64.8.3674-3678.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getzoff E. D., Geysen H. M., Rodda S. J., Alexander H., Tainer J. A., Lerner R. A. Mechanisms of antibody binding to a protein. Science. 1987 Mar 6;235(4793):1191–1196. doi: 10.1126/science.3823879. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Tainer J. A., Rodda S. J., Mason T. J., Alexander H., Getzoff E. D., Lerner R. A. Chemistry of antibody binding to a protein. Science. 1987 Mar 6;235(4793):1184–1190. doi: 10.1126/science.3823878. [DOI] [PubMed] [Google Scholar]
- Goudsmit J., Debouck C., Meloen R. H., Smit L., Bakker M., Asher D. M., Wolff A. V., Gibbs C. J., Jr, Gajdusek D. C. Human immunodeficiency virus type 1 neutralization epitope with conserved architecture elicits early type-specific antibodies in experimentally infected chimpanzees. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4478–4482. doi: 10.1073/pnas.85.12.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., LaRosa G. J., Profy A. T., Bolognesi D. P., Herlihy W. C., Putney S. D., Matthews T. J. Broadly neutralizing antibodies elicited by the hypervariable neutralizing determinant of HIV-1. Science. 1990 Dec 14;250(4987):1590–1593. doi: 10.1126/science.1703322. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., McDanal C., Ross K. L., Eckler L. I., Jellis C. L., Profy A. T., Rusche J. R., Bolognesi D. P., Putney S. D. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6768–6772. doi: 10.1073/pnas.86.17.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick S., Gelatt C. D., Jr, Vecchi M. P. Optimization by simulated annealing. Science. 1983 May 13;220(4598):671–680. doi: 10.1126/science.220.4598.671. [DOI] [PubMed] [Google Scholar]
- LaRosa G. J., Davide J. P., Weinhold K., Waterbury J. A., Profy A. T., Lewis J. A., Langlois A. J., Dreesman G. R., Boswell R. N., Shadduck P. Conserved sequence and structural elements in the HIV-1 principal neutralizing determinant. Science. 1990 Aug 24;249(4971):932–935. doi: 10.1126/science.2392685. [DOI] [PubMed] [Google Scholar]
- Lerner R. A. Antibodies of predetermined specificity in biology and medicine. Adv Immunol. 1984;36:1–44. doi: 10.1016/s0065-2776(08)60898-6. [DOI] [PubMed] [Google Scholar]
- Matsushita S., Robert-Guroff M., Rusche J., Koito A., Hattori T., Hoshino H., Javaherian K., Takatsuki K., Putney S. Characterization of a human immunodeficiency virus neutralizing monoclonal antibody and mapping of the neutralizing epitope. J Virol. 1988 Jun;62(6):2107–2114. doi: 10.1128/jvi.62.6.2107-2114.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palker T. J., Clark M. E., Langlois A. J., Matthews T. J., Weinhold K. J., Randall R. R., Bolognesi D. P., Haynes B. F. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche J. R., Javaherian K., McDanal C., Petro J., Lynn D. L., Grimaila R., Langlois A., Gallo R. C., Arthur L. O., Fischinger P. J. Antibodies that inhibit fusion of human immunodeficiency virus-infected cells bind a 24-amino acid sequence of the viral envelope, gp120. Proc Natl Acad Sci U S A. 1988 May;85(9):3198–3202. doi: 10.1073/pnas.85.9.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. A., Ting R., Langlois A. J., Weinhold K. J., Lyerly H. K., Javaherian K., Matthews T. J. Characteristics of a neutralizing monoclonal antibody to the HIV envelope glycoprotein. AIDS Res Hum Retroviruses. 1988 Jun;4(3):187–197. doi: 10.1089/aid.1988.4.187. [DOI] [PubMed] [Google Scholar]
- Stanfield R. L., Fieser T. M., Lerner R. A., Wilson I. A. Crystal structures of an antibody to a peptide and its complex with peptide antigen at 2.8 A. Science. 1990 May 11;248(4956):712–719. doi: 10.1126/science.2333521. [DOI] [PubMed] [Google Scholar]
- Thomas E. K., Weber J. N., McClure J., Clapham P. R., Singhal M. C., Shriver M. K., Weiss R. A. Neutralizing monoclonal antibodies to the AIDS virus. AIDS. 1988 Feb;2(1):25–29. doi: 10.1097/00002030-198802000-00004. [DOI] [PubMed] [Google Scholar]



