Abstract
We prospectively compared closure and complication rates in 91 children with secundum atrial septal defects: 44 (mean age, 8.1 ± 4.7 years) were treated surgically and 47 (mean age, 10.1 ± 4.9 years) were treated by percutaneous Amplatzer® septal occluder. Complications were classified as mild, moderate, or severe. The closure rate was similar in the 2 groups: 42/44 children (95.5%) in the surgical group versus 46/47 patients in the device group (97.5%). Mild complications were observed in 17/44 patients in the surgical group vs 2/47 in the device group; moderate, 11/44 in the surgical vs 1/47 in the device group; and severe, 2/44 in the surgical group vs none in the device group. Blood products were administered to 18 patients in the surgical group and to 1 patient in the device group P < 0.001).
Transcatheter closure of secundum atrial septal defects with the Amplatzer device has the advantage of fewer complications, shorter hospitalization, and reduced need of blood products. Nonetheless, the surgeon's ability to close any atrial septal defect regardless of its size or location remains an important advantage of surgery.
Key words: Adolescent; child; echocardiography; heart catheterization; heart septal defects, atrial/therapy/surgery; human; postoperative complica-tions; prostheses and implants
Secundum atrial septal defects (ASDs) account for 10% of congenital heart disease in neonates at birth and as much as 30% to 40% in adults.1 In the adults, the incidence of late complications (for example, rhythm disturbances) increases. Surgical repair of ASDs is a well established procedure and is very safe, with a negligible mortality rate.2 Transcatheter occlusion of an ASD was first described by King and colleagues in 1976.3 Although satisfactory results have been reported with transcatheter occlusion of these defects with a number of devices,4 only one of them has received Food and Drug Administration approval: the Amplatzer® atrial septal occluder (AGA Medical Corporation; Golden Valley, Minn). This device was first introduced into clinical practice by Masura and colleagues in 1997,5 and our experience with this device was published in 2003.6 Only a few studies have compared transcatheter occlusion with surgical repair, and these have included both children and adults.7–9 We performed a prospective comparison study with a group of children from a single institution, treated by surgery or an Amplatzer device, with relatively long follow-up.
Patients and Methods
From February 1998 through October 2000, 91 consecutive children from 2 to ≤18 years of age underwent closure of isolated ASDs: 44 by surgery and 47 by Amplatzer septal occluder. The ASDs were hemodynamically significant in all patients, with a pulmonary-to-systemic flow ratio >1.5:1 and dilatation of the right atrium and ventricle. All children were in sinus rhythm before treatment and were evaluated by standard transthoracic echocardiography (TTE) with a Sonos 2000 ultrasound system (Hewlett-Packard Medical [acquired by Philips Medical Systems; Andover, Mass]) before treatment, before discharge, after 3 months, after 1 year, and every year thereafter. Suitability for nonsurgical closure and technique of transcatheter closure of ASDs with the Amplatzer occluders has been previously described.4–6 Generally, for percutaneous closure, an adequate rim of the ASD was necessary (except for the anterior rim toward the aorta), as was an ASD that was not excessively large. In children who preliminarily qualified for device implantation, transesophageal echocardiography (TEE) and balloon sizing of the ASD were performed during heart catheterization. Four children were disqualified from interventional treatment (the ASDs were too large for currently available devices).
Both treatment methods (surgical and Amplatzer device closure) were presented to the parents of children participating in the study, and informed consent was obtained from the parents. The investigation was performed in compliance with human studies guidelines.
Surgery was performed in patients who had ASDs that were unsuitable for transcatheter closure and in 3 children whose parents preferred surgery. The ASD size (measured by TTE) was larger in the surgical group (mean, 16.0 ± 6.5 mm; median, 15 mm; range, 5–28 mm) than in the device group (mean, 10.8 ± 3.2 mm; median, 10.0 mm; range, 7–19 mm) (P <0.001). Surgical closure with the use of cardiopulmonary bypass was performed by suture alone in 38 patients and with a pericardial patch in 6. A standard median sternotomy was used in all patients. Both venae cavae were typically cannulated through the right atrium: to the superior vena cava via the right atrial appendage, and to the inferior vena cava close to the vessel. All operations were performed on a beating heart, without aortic cross-clamping or administration of cardioplegic solution.
With slight modifications to the system of Galal and colleagues,10 we classified complications after ASD closure as mild, moderate, or severe. Mild complications included small pericardial effusions, headaches, 1st-degree atrioventricular (AV) block, and atrial rhythm disturbances; moderate complications included pneumonia and atelectasis, paroxysmal supraventricular tachycardia, and AV junctional rhythm; and severe complications included bleeding that required reoperation, and transient neurologic events.
For all patients, medical records were reviewed, including follow-up examinations, through October 2003.
Statistical analyses were performed using the Student's t-test and χ2 tests. A P value of <.05 was considered significant.
Results
Forty-four children underwent surgery at a mean age of 8.1 ± 4.7 years (median, 6.5 years; range, 2.3–16.9 years), and 47 underwent Amplatzer occluder implantation at a mean age of 10.1 ± 4.9 years (median, 9.9 years; range, 2.3–17.7 years) (P <0.05). No death occurred in either group. The mean hospital stay in the surgically treated children was 7.5 ± 3.1 days (median, 7.0 days; range, 4–22 days) days versus 2.2 ± 1.1 days (median, 2.0 days; range, 1–15 days) in the device group (P <0.001). The closure rate at discharge was similar between groups: 42 of 44 (95.5%) in the surgical group versus 46 of 47 (97.9%) in the device group. All residual shunts were trivial; with time, a residual shunt disappeared in 1 patient from the device group.
Follow-up in all patients continued through October 2003 (mean follow-up, 3.9 ± 0.9 years; median, 3.8 years; range, 2.9–5.4 years). Mild complications were observed in 17 of 44 children in the surgical group (12 had pericardial effusion and 5 had 1st-degree AV block, atrial rhythm disturbances, or both) (Fig. 1). In the device group, 2 of 47 children had mild complications (both had transient headaches during the first 2 months after the procedure). Moderate complications occurred in 11 of 44 children in the surgical group (8 children had transient nodal rhythm, supraventricular tachycardia, or both, and 3 had pneumonia or atelectasis). One of 47 patients in the device group, aged 17.7 years, experienced a moderate complication: on the 1st day after implantation of the Amplatzer device, supraventricular tachycardia occurred. The condition was treated successfully by intravenous injection of verapamil. Severe complications were observed in 2 children in the surgical group: one had bleeding that required reoperation, and the other (with infant cerebral paralysis) had transient hemiparesis, probably induced by cardiopulmonary bypass. No severe complications occurred in the device group.
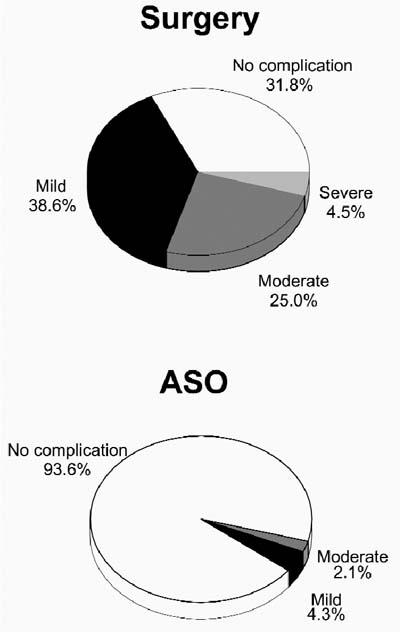
Fig. 1 Percentage of complications after closure of atrial septal defects by surgery (top) and transcatheter Amplatzer atrial septal occluder (ASO, bottom).
Blood products (fresh-frozen plasma for volume replacement or packed erythrocytes for anemia treatment) were administered to 18 patients in the surgical group and to 1 patient in the device group (P <0.001). In the patient from the device group—a 3-year-old child—the reason for blood transfusion was blood loss from a failed sheath. All together, complications occurred in 30 of 44 patients (68.2%) in the surgical group versus 3 of 47 patients (6.4%) in the device group (P <0.05). In the surgical group, rhythm disturbances persisted during follow-up in 2 children (AV junctional rhythm in both patients, one with short supraventricular tachycardia).
Discussion
In this study of surgical versus device closure in patients with secundum ASD, an important advantage was the young age of the participating patients. Atrial arrhythmias (fibrillation and flutter) develop progressively with age, and there is little evidence that late surgical correction prevents their development.11 Preoperative arrhythmias are present in more than 50% of patients who are over 35 years of age at the time of surgery. After ASD surgical repair, early postoperative arrhythmias—particularly sinus node dysfunction—occur in 30% to 75% of patients.12 (In our study, arrhythmias occurred in 30% of surgical patients, in contrast to 2% of the patients treated with the Amplatzer device). Like other authors,9 we found a lower complication rate in children treated with the Amplatzer occluder. Most postsurgical problems (such as small pericardial effusions and junctional rhythm) did not affect our patients' overall condition and disappeared with time. On the other hand, the transient rhythm disturbances observed during the early postoperative period could be predictive of serious arrhythmias some years after surgery.13 Recently, we also found that, 1 month after ASD closure, autonomic heart control (heart rate variability) was more impaired after surgery than after interventional treatment.14
More than half of the children in our study had ASDs that were suitable for transcatheter closure with the Amplatzer septal occluder. Closure of ASDs with this device was a comparable alternative to surgery, and residual shunts were rare, trivial, and of no hemodynamic significance. Another important finding from our study was that parents almost invariably preferred transcatheter closure of the ASDs for their children, despite the absence of long-term experience with this new treatment. The most severe complication of ASD closure with the Amplatzer occluder is embolization of the device. In our series of 181 implants, embolization occurred in 1 adult patient.6 The reason for this complication may have been underestimation of the size of the ASD, which again emphasizes the fundamental role of the echocardiographic examination before and during the procedure. Some patients who undergo treatment with the Amplatzer device experience transient headaches; this sequela occurred in 2 children in our study. The reason for this event is still unknown.15
Limitations of this study include the lack of randomization and the fact that the device group included older children with smaller ASDs. Patient selection probably remains the key to achieving good results with both methods of treatment.
Conclusions
Transcatheter closure of ASDs with Amplatzer devices has the advantage of fewer complications, shorter hospitalizations, reduced need for blood products, less discomfort for patients, and no incisional scar. It is our opinion that, in selected children, surgical closure of secundum ASDs is no longer necessary. Nevertheless, the surgeon's ability to close any ASD, regardless of anatomy, remains an important advantage of surgery.
Footnotes
Address for reprints: Dr. Jacek Bialkowski, Chief, Congenital Heart Diseases & Pediatric Cardiology Dept., Silesian Center for Heart Diseases, ul. Szpitalna 2, 41800 Zabrze, Poland
E-mail: jabi_med@poczta.onet.pl
References
- 1.Hoffman JI, Christianson R. Congenital heart disease in a cohort of 19,502 births with long-term follow-up. Am J Cardiol 1978;42:641–7. [DOI] [PubMed]
- 2.Rigby ML. The era of transcatheter closure of atrial septal defects. Heart 1999;81:227–8. [DOI] [PMC free article] [PubMed]
- 3.King TD, Thompson SL, Steiner C, Mills NL. Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA 1976;235:2506–9. [PubMed]
- 4.Masura J, Gavora P, Formanek P, Hijazi ZM. Transcatheter closure of secundum atrial septal defects using the new self-centering amplatzer septal occluder: initial human experience. Cathet Cardiovasc Diagn 1997;42:388–93. [DOI] [PubMed]
- 5.Baskett RJ, Tancock E, Ross DB. The gold standard for atrial septal defect closure: current surgical results, with an emphasis on morbidity. Pediatr Cardiol 2003;24:444–7. [DOI] [PubMed]
- 6.Bialkowski J, Kusa J, Szkutnik M, Kalarus Z, Banaszak P, Bermudez-Canete R, et al. Percutaneous catheter closure of atrial septal defect. Short-term and mid-term results [in Spanish]. Rev Esp Cardiol 2003;56:383–8. [DOI] [PubMed]
- 7.Berger F, Vogel M, Alexi-Meskishvili V, Lange PE. Comparison of results and complications of surgical and Amplatzer device closure of atrial septal defects. J Thorac Cardiovasc Surg 1999;118:674–80. [DOI] [PubMed]
- 8.Durongpisitkul K, Soongswang J, Laohaprasitiporn D, Nana A, Sriyoschati S, Ponvilawan S, et al. Comparison of atrial septal defect closure using amplatzer septal occluder with surgery. Pediatr Cardiol 2002;23:36–40. [DOI] [PubMed]
- 9.Thompson JD, Aburawi EH, Watterson KG, Van Doorn C, Gibbs JL. Surgical and transcatheter (Amplatzer) closure of atrial septal defects: a prospective comparison of results and cost. Heart 2002;87:466–9. [DOI] [PMC free article] [PubMed]
- 10.Galal MO, Wobst A, Halees Z, Hatle L, Schmaltz AA, Khougeer F, et al. Peri-operative complications following surgical closure of atrial septal defect type II in 232 patients—a baseline study. Eur Heart J 1994;15:1381–4. [DOI] [PubMed]
- 11.Brecker SJ. Atrial septal defect. In: Redington A, Shore D, Oldershaw P, editors. Congenital heart disease in adults: a practical guide. London: WB Saunders; 1994. p. 103–10.
- 12.Vatter VL. Postoperative arrhythmias after surgery for congenital heart defects. Cardiol Rev 1994;2:83–7.
- 13.Meijboom F, Hess J, Szatmari A, Utents EM, McGhie J, Deckers JW, et al. Long-term follow-up (9 to 20 years) after surgical closure of atrial septal defect at a young age. Am J Cardiol 1993;72:1431–4. [DOI] [PubMed]
- 14.Bialkowski J, Karwot B, Szkutnik M, Sredniawa B, Chodor B, Zeifert B, et al. Comparison of heart rate variability between surgical and interventional closure of atrial septal defect in children. Am J Cardiol 2003;92:356–8. [DOI] [PubMed]
- 15.Bourdages M, Piette E, Dahdah N, Miro J. Incidence of headaches after ASD percutaneous catheter closure with Amplatzer device [abstract]. Catheter Cardiovasc Interv 2002;7:106.


