Abstract
Myocardial infarction and other cardiovascular events constitute the leading causes of death in dialysis-dependent, end-stage renal disease patients. Due to growth in the elderly population, the number of uremic patients who need surgical revascularization is likely to increase. Whether or not coronary artery bypass grafting is safe for patients on long-term dialysis remains a great concern.
We retrospectively reviewed all cases of elective or urgent isolated coronary artery bypass grafting in our hospital, from 1 January 1998 through 31 March 2003, and identified 23 consecutive patients with dialysis-dependent renal disease (Group D). Twenty-two of them were on hemodialysis, and 1 was on peritoneal dialysis; the mean duration of dialysis was 19.2 ± 22.5 months. We chose 69 matched non-dialysis patients who underwent bypass grafting in 2001 to serve as our control group (ND). Preoperative, operative, and postoperative data on these patients were compared.
Group D consisted of 14 men and 9 women with a mean age of 63.8 ± 9.9 years, and the mean number of distal anastomoses was 3.5 ± 1.2. There were no significant differences between the 2 groups in preoperative factors, intubation time, intensive care unit stay, major complications, and 30-day mortality. However, uremic patients had a greater tendency to bleed, longer postoperative hospital stays, and more late deaths.
We conclude that under a well-prepared dialysis program and meticulous perioperative management, coronary artery bypass grafting can be performed in dialysis-dependent patients, with increased but acceptable perioperative morbidity and mortality risks.
Key words: Cardiopulmonary bypass; coronary artery bypass; coronary disease/complications; kidney failure, chronic; off-pump coronary artery bypass; postoperative care; preoperative care; renal dialysis; retrospective studies
Dialysis treatment is one of the great technical successes of 20th-century medicine; it provides the chance of prolonging life in end-stage renal disease (ESRD) patients. The worldwide prevalence of treated ESRD continues to climb: in countries with established ESRD treatment programs, the prevalence across all age groups varies from 6% to 16% and is highest in Japan and Taiwan.1 Taiwan has the 2nd highest prevalence of ESRD in the world and has the highest prevalence in all age groups above 20 years (>3,000 per million persons aged 45 and older).1 Because of the growing numbers of elderly patients who are entering dialysis programs today, the number of dialysis patients who will require myocardial revascularization is expected to increase. There are many complications in treating ischemic heart disease in dialysis patients, such as poor general condition before surgery, poor water and electrolyte control, calcified aorta and peripheral arterial trees, and the necessity for simultaneous operations. Coronary artery bypassing grafting (CABG) has become the standard treatment for ESRD patients with coronary artery disease because it has been shown, in this subset, to yield better overall and angina-free survival than does percutaneous transluminal coronary angioplasty (PTCA).2,3 Nevertheless, management of the water–electrolyte balance in these patients remains an important issue. Through close cooperation with our staff nephrologists and the use of ultrafiltration, we performed routine dialysis protocols and integrated surgery into the patient's routine schedule. The aim of this study was to review our management policies and examine our 5-year surgical experience with CABG in patients on long-term dialysis.
Patients and Methods
Twenty-three consecutive, dialysis-dependent, ESRD patients (14 men and 9 women, with a mean age of 63.8 ± 9.9 years) underwent elective, isolated CABG at our institution from 1 January 1998 through 31 March 2003. We retrospectively reviewed all these patients' demographic and clinical data, including coronary risk factors, comorbidities, preoperative cardiac and angiographic profiles, operative data, postoperative complications, and results; this study group we called our D (dialysis) group. Our ND (non-dialysis) control group comprised 69 matched non-dialysis patients (61 men and 8 women, with a mean age of 63.8 ± 11.2 years) who had undergone CABG in 2001 and who matched Group D in age and operative method. Patients who underwent emergent CABG or concomitant surgery of the heart valves or other vessels, or major general surgery, were excluded from both study groups.
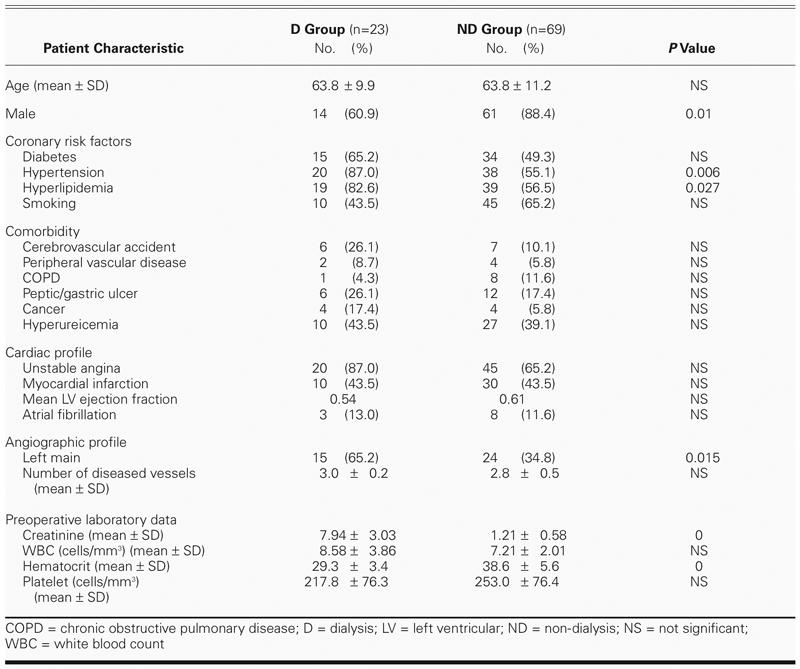
The demographic data are set forth in Table I. Preoperatively, 22 of the 23 (96%) patients in Group D were on hemodialysis, and the remaining patient was on peritoneal dialysis; the duration of dialysis ranged from 1 to 81 months (mean, 19.2 ± 22.5 months). The causes of ESRD were diabetic nephropathy in 14 patients (61%), glomerulonephritis in 1 (4%), polycystic kidney disease in 1 (4%), and unknown origin in 7 (30%). In Group ND, the male-to-female ratio was significantly higher. Group D had significantly more frequent histories of hypertension, hyperlipidemia, and left main disease. Anemia was more commonly observed in Group D, although the difference did not reach statistical significance.
TABLE I. Demographics and Preoperative Factors of the Patients
Six patients (26%) in Group D and 16 patients (23%) in Group ND underwent off-pump coronary artery bypass (OPCAB) grafting, and the others in both groups received CABG under conventional cardiopulmonary bypass. The cardiopulmonary bypass operations were performed under cardioplegia at moderate hypothermia (28–32 °C), with use of a roller pump (flow rate, 1.2–2.2 L/min/m2) and membrane oxygenator. We used topical cardiac cooling and infused a blood hyperkalemic cardioplegic solution by the antegrade or retrograde approach, or both.
From late 2000, we gradually increased the number of OPCAB procedures. We used apex suction (Starfish™ 2, Medtronic Inc.; Minneapolis, Minn; or Pyramid Positioner, Estech, Inc.; Danville, Calif) to elevate the apex of the heart. Stabilization of the target arteries was accomplished by a cardiac wall stabilization platform (Octopus III stabilizer, Medtronic Inc.; CTS tissue stabilizer, Cardiothoracic Systems Inc.; Cupertino, Calif; or OPVACR Synergy Stabilizers, Estech, Inc.).
All patients received a 2nd-generation cephalosporin (Cefmetazole), 1 g intravenously for antimicrobial prophylaxis 30 minutes before surgery and again postoperatively, every 6 hours for 48 hours.
Dialysis Program. To simplify our hemodialysis and postoperative nursing care plan, we performed dialysis in accordance with the patients' previous routine. We scheduled hemodialysis on the day before surgery, in accordance with the usual schedules for 22 of the 23 patients in Group D. Intraoperative ultrafiltration was performed in patients on cardiopulmonary bypass, but not in OPCAB patients. Postoperatively, Group D patients underwent their usual hemodialysis on the 2nd postoperative day. One patient, who had been in a peritoneal dialysis program for several years, underwent his usual continuous ambulatory peritoneal dialysis on the day before surgery. In the operative theater, he also received intraoperative ultrafiltration during cardiopulmonary bypass, and he then underwent peritoneal dialysis again in the surgical intensive care unit during recovery.
Statistical Analysis. Statistical analysis was performed using Student's t-test for continuous variables, and the χ2 test or Fisher's exact test for categorical variables. Continuous variables were expressed as mean ± SD. Discrete variables were presented as percentages. A P value of less than 0.05 was considered significant. All statistical analyses were performed using SPSS 10.0 (SPSS, Inc.; Chicago, Ill).
Results
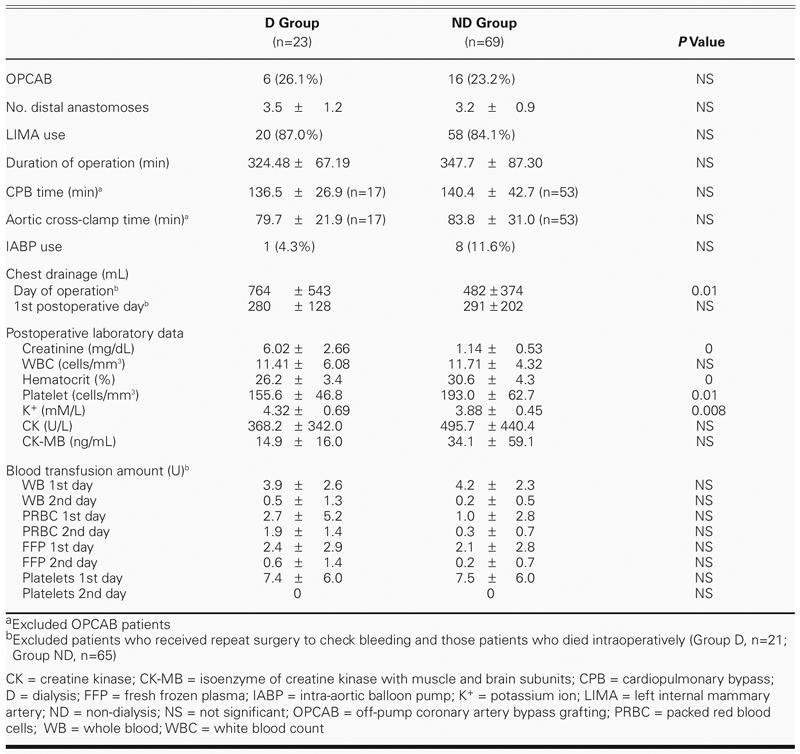
Operative data are shown in Table II. Roughly the same percentages of patients in groups D and ND underwent OPCAB (around 25%) and left internal mammary artery (LIMA) grafting (about 85%). Operative times (from skin incision to skin closure), cardiopulmonary bypass times (from start of bypass to off pump), aortic cross-clamp times, intra-aortic balloon pump use, postoperative leukocyte levels, and cardiac enzyme changes (data obtained immediately after surgery) were not significantly different between groups D and ND. Excluding those patients (2 in Group D and 4 in Group ND) who either died on the table or underwent reoperation for massive bleeding, Group D had higher drainage output from chest tubes and higher platelet consumption than did Group ND, but they did not need more blood transfusions. In our hospital, the indications for blood transfusion were low hemoglobin levels (below 10 gm/dL), severe bleeding, and substantial chest tube drainage.
TABLE II. Operative Factors
The postoperative profiles are shown in Table III. There were no significant differences between the 2 groups in mean duration of mechanical ventilation or in length of stay in intensive care. Group D, however, required significantly longer postoperative stays than did Group ND. No obvious differences in major complications were noted between the 2 groups, except for poor leg-wound healing and cellulitis, which was more common in Group D and was the chief reason for that group's longer postoperative stays. Although 2 patients in Group D and 1 patient in Group ND underwent reoperation due to persistent bleeding or tamponade, the statistical difference was not significant. Sternal infection was defined as a superficial sternal wound with pus or infection in need of wet dressing; mediastinitis was defined as deep sternal infection in need of re-exploration and additional surgical intervention; postoperative neurologic complications included seizure and acute cerebral infarction as shown by magnetic resonance imaging; arrhythmia was defined as any atrial fibrillation, or atrial or ventricular premature contractions recorded on charts or requiring extra medication.
TABLE III. Postoperative Profile
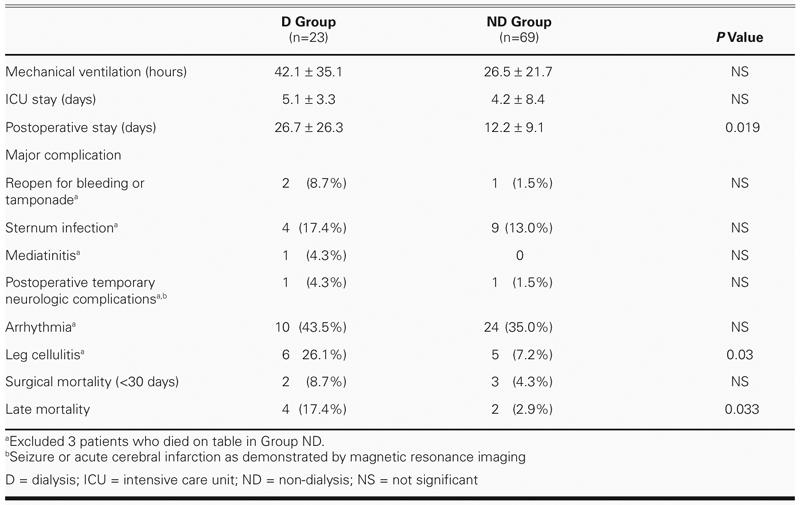
Early Mortality. The 30-day mortality rates of groups D and ND were 8.7% and 4.3%, respectively (not significant), while the late mortality rates were 17.4% and 2.9%, respectively (P = 0.033). In Group D, there were 2 in-hospital deaths within 30 days: 1 OPCAB patient, discharged on postoperative day 8, died on day 15 of unknown causes at home and was unresponsive to resuscitation at the emergency room; and the other, a pump patient, died unexpectedly on postoperative day 12 of sudden sustained ventricular tachycardia, during hemodialysis. In Group ND, all 3 deaths within 30 days occurred on the table: 1 pump patient was allergic to protamine, which resulted in failure of cardiac pumping and failure to resuscitate; and 2 patients who experienced sudden ventricular tachycardia during OPCAB died after their surgeries even after conversion to extracorporeal membrane oxygenation.
Late Mortality. In Group D, 4 patients died after 30 postoperative days. The 1st patient died on postoperative day 46 of severe mediastinitis, the 2nd died on postoperative day 118 of sudden sustained ventricular tachycardia, the 3rd died 14 months postoperatively of peritonitis associated with continuous ambulatory peritoneal dialysis, and the last died 32 months postoperatively of bowel ischemia with severe sepsis. In Group ND, there were 2 late deaths: 1 patient died 3 months postoperatively of cholecystitis with hepatic-renal encephalopathy, and the other died at home 16 months postoperatively of an unknown cause unrelated to the heart.
Discussion
Although dialysis prolongs the lifespan and life quality of patients with ESRD, people who undergo dialysis still have only an overall 5-year survival rate of 55% to 60%.4,5 Cardiovascular complications are the leading cause of death in patients with ESRD; these account for 47% to 54% of deaths in patients who are on maintenance dialysis.6,7 The higher incidence of coronary artery disease in this patient population can be attributed to the presence of comorbid conditions that include hypertension, hyperlipidemia, renal anemia, fluid overload by arteriovenous shunt, heterotopic calcification due to secondary hyperparathyroidism, and abnormal carbohydrate metabolism that leads to accelerated atherosclerosis.2,8 Because of the growing number of elderly patients who are entering dialysis programs, the number of dialysis patients who require myocardial revascularization is expected to increase. Both PTCA and CABG are technically feasible in patients with ESRD, and PTCA yields better immediate survival rates; however, higher recurrent angina and restenosis rates limit the long-term benefits of PTCA, so CABG remains the preferred therapy in such patients.2,3 Whether coronary artery bypass grafting can be performed safely in ESRD patients, and how, have remained questions of great concern in recent years.
Fluid overload and pulmonary congestion related to cardiopulmonary bypass, anemia, and the bleeding tendency in these patients are sources of worry for most surgeons. In addition, the dialysis program is an important issue for ESRD patients. Because over-dialysis has not been found to be harmful, some surgeons and nephrologists may even prescribe intensive perioperative dialysis in all dialysis patients who undergo CABG.9 Some recommend dialysis more than 24 hours before CABG, others recommend dialysis as close to the procedure as possible, while still others propose intraoperative dialysis, or place a peritoneal catheter at the time of operation and initiate peritoneal dialysis immediately upon arrival in intensive care.10,11 Some authors still suggest avoiding dialysis immediately after the operation,10 in order to minimize dialysis-associated hypotension during recovery.
Our ESRD patients who underwent CABG on cardiopulmonary bypass received intraoperative ultra-filtration almost until the weaning process, and cooperation with our nephrology teams enabled us to set our dialysis protocols on the days before and after surgery. Although patients who underwent OPCAB did not receive ultrafiltration, we minimized fluid infusion as much as possible during surgery and postoperative care, and applied the same dialysis protocols on the days before and after surgery. When performed in accordance with these dialysis protocols, CABG appears to be safe and not to impose an extra load on nephrologists, who must care for a large number of renal patients, many of whom are not on dialysis.
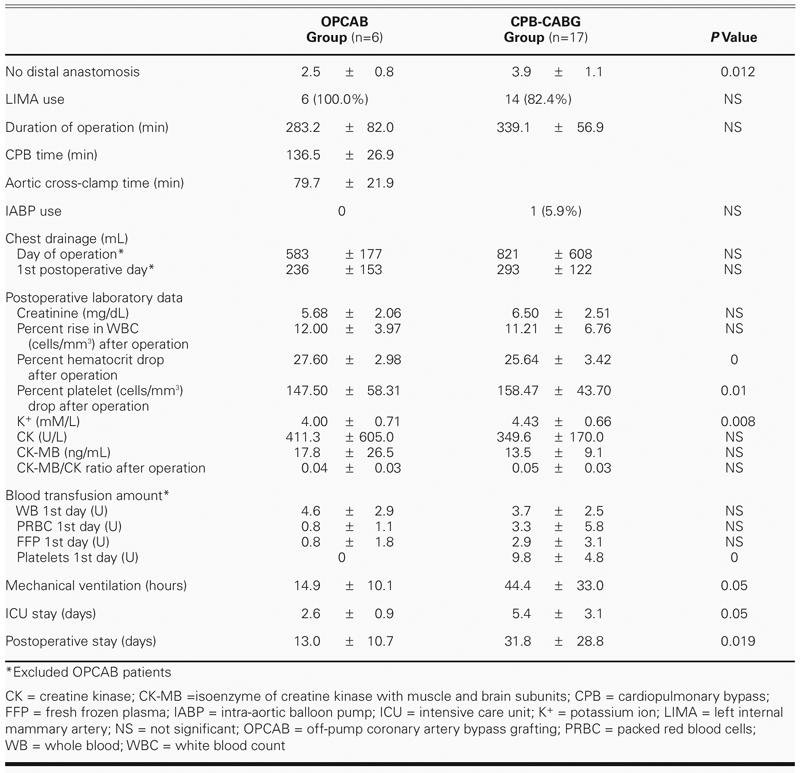
Coronary artery bypass grafting is the preferred approach in the treatment of severe coronary disease in patients with ESRD, because it improves their cardiac symptoms, quality of life, and overall functional status.2,4,12 However, long-term survival remains relatively limited, and the hospital mortality and morbidity rates are substantially high, when these patients are compared with CABG patients who have normal renal function. Many investigators have observed that additional complications, such as wound infection, delayed tamponade, and cerebrovascular events, occur in dialysis patients,4,12,13 but our experience indicates that complications, in general, do not differ from those of non-dialysis patients, except for poor healing of the leg. In comparison with the 9% to 12.9% early mortality rates in recently published studies of dialysis patients who underwent elective CABG,5,14 our 8.7% 30-day mortality rate seems acceptable, although it is still slightly higher than that of the control group. Can OPCAB improve the surgical results in high-risk uremia patients? We found, in both our D and ND groups, that OPCAB results did not differ from conventional cardiopulmonary bypass results in regard to early mortality and morbidity. This runs slightly counter to the findings of Papadimitriou and colleagues,10 who concluded that avoidance of cardiopulmonary bypass might be of some benefit in chronic dialysis patients. In the early course of our learning curve in performing OPCAB, 2 patients in the ND group died due to sudden ventricular tachycardia during heart elevation, even after conversion to cardiopulmonary bypass via extracorporeal membrane oxygenation. After that, we sometimes used the intra-aortic balloon pump for temporary heart support before beginning surgery, used magnesium sulfate (MgSO4) during the operation, and always took great care in elevating the heart; now OPCAB is much safer. In sum, we believe that OPCAB needs much support from anesthetists and that intra-aortic balloon pumping can make OBCAB surgery much safer. Although OPCAB did not reduce the incidence of blood transfusion requirements or of postoperative leukocytosis in our uremia patients (Table IV), it reduced postoperative intubation time, improved patients' postoperative condition, and reduced length of stay in intensive care and in the wards. So we still suggest that OPCAB can be useful in the treatment of coronary artery disease in dialysis patients, except in those who are hemodynamically unstable; but more cases are needed to demonstrate this conclusively.
TABLE IV. Operative Factors Comparison in End-Stage Renal Disease Patients
Whether dialysis-dependent uremia patients should receive complete revascularization is a question of great concern for most surgeons.7 We aggressively revascularized our dialysis-dependent uremia patients (3.5 ± 1.2 distal anastomoses on average), and the late mortality rate in our D group exceeded that of our ND group. However, the causes of excess death were not cardiovascular in origin, and for quality-of-life reasons we still perform complete revascularization when possible.
Conclusion
Patients on chronic dialysis who require CABG constitute a surgical challenge, for their condition is still associated with high rates of early and late death. However, good preparation, adherence to a routine dialysis program, and meticulous perioperative management can reduce the risk to an acceptable level.
Acknowledgments
We thank Prof. Jeng-Jung Huang and his team for their kind cooperation and for their excellent contribution to the management of the dialysis patients.
Footnotes
Address for reprints: Yu-Jen Yang, MD, PhD, Department of Surgery, National Cheng Kung University Hospital, 138 Sheng-Li Road, Tainan 704, Taiwan, Republic of China
E-mail: kcd56@mail.ncku.edu.tw
References
- 1.U.S. Renal Data System, USRDS 2002 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health; National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda (MD); 2002.
- 2.Koyanagi T, Nishida H, Kitamura M, Endo M, Koyanagi H, Kawaguchi M, et al. Comparison of clinical outcomes of coronary artery bypass grafting and percutaneous transluminal coronary angioplasty in renal dialysis patients. Ann Thorac Surg 1996;61:1793–6. [DOI] [PubMed]
- 3.Agirbasli M, Weintraub WS, Chang GL, King SB, Guyton RA, Thompson TD, et al. Outcome of coronary revascularization in patients on renal dialysis. Am J Cardiol 2000; 86:395–9. [DOI] [PubMed]
- 4.Hirose H, Amano A, Takahashi A, Ozaki S, Nagano N. Coronary artery bypass grafting for hemodialysis-dependent patients. Artif Organs 2001;25:239–47. [PubMed]
- 5.Endo M. Coronary artery bypass grafting in patients on chronic hemodialysis in Japan. Artif Organs 2001;25:237–8. [PubMed]
- 6.Eagle KA, Guyton RA, Davidoff R, Ewy GA, Fonger J, Gardner TJ, et al. ACC/AHA Guidelines for Coronary Artery Bypass Graft Surgery: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1991 Guidelines for Coronary Artery Bypass Graft Surgery). American College of Cardiology/American Heart Association. J Am Coll Cardiol 1999;34:1262–347. [DOI] [PubMed]
- 7.Frenken M, Krian A. Cardiovascular operations in patients with dialysis-dependent renal failure. Ann Thorac Surg 1999;68:887–93. [DOI] [PubMed]
- 8.Gelsomino S, Da Col P, Frassani R, Morocutti G, Muzzi R, Bassi F, et al. Coronary artery bypass grafting in patients with dialysis-dependent renal failure: ten-year results. Ital Heart J 2001;2:379–83. [PubMed]
- 9.Okada H, Tsukamoto I, Sugahara S, Nakamoto H, Oohama K, Yamashita Y, et al. Does intensive perioperative dialysis improve the results of coronary artery bypass grafting in haemodialysed patients? Nephrol Dial Transplant 1999;14:771–5. [DOI] [PubMed]
- 10.Papadimitriou LJ, Marathias KP, Alivizatos PA, Michalis A, Palatianos GM, Stavridis GT, et al. Safety and efficacy of off-pump coronary artery bypass grafting in chronic dialysis patients. Artif Organs 2003;27:174–80. [DOI] [PubMed]
- 11.Hamada Y, Kawachi K, Nakata T, Takano S, Tsunooka N, Sato M, et al. Cardiac surgery in patients with end-stage renal disease. Utility of continuous ambulatory peritoneal dialysis. Jpn J Thorac Cardiovasc Surg 2001;49:99–102. [DOI] [PubMed]
- 12.Owen CH, Cummings RG, Sell TL, Schwab SJ, Jones RH, Glower DD. Coronary artery bypass grafting in patients with dialysis-dependent renal failure. Ann Thorac Surg 1994;58:1729–33. [DOI] [PubMed]
- 13.Khaitan L, Sutter FP, Goldman SM. Coronary artery bypass grafting in patients who require long-term dialysis. Ann Thorac Surg 2000;69:1135–9. [DOI] [PubMed]
- 14.Gelsomino S, Morocutti G, Masullo G, Cheli G, Poldini F, Da Broi U, Livi U. Open heart surgery in patients with dialysis-dependent renal insufficiency. J Card Surg 2001;16:400–7. [DOI] [PubMed]


