Abstract
We evaluated transmyocardial laser revascularization (TMLR) with coronary artery bypass grafting (CABG) versus CABG alone for severe coronary artery disease involving ≥1 myocardial region unsuited for CABG.
At 4 centers, 44 consecutive patients were randomized for CABG+TMLR (n = 23) or CABG alone (n = 21). Operative and in-hospital mortality and morbidity rates were monitored. Clinical status was evaluated at hospital discharge, 1 year, and 4 years. Success was characterized by relief of angina and freedom from repeat revascularization and death.
Preoperatively, 20 patients (47%) were at high risk. The CABG technique, number of grafts, and target vessels were similar in both groups. Patients undergoing CABG+ TMLR received 25 ± 11 laser channels. Their ≤30-day mortality was 13% (3/23) compared with 28% (6/21) after CABG alone (P = 0.21). There were no significant intergroup differences in the number of intraoperative or in-hospital adverse events.
The follow-up period was 50.3 ± 17.8 months for CABG alone and 48.1 ± 16.8 months for CABG+TMLR. Both groups had substantially improved angina and functional status at 1 and 4 years, with no significant differences in cumulative 4-year mortality. The incidence of repeat revascularization was 24% after CABG alone versus none after CABG+ TMLR (P < 0.05). The 4-year event-free survival rate was 14% versus 39%, respectively (P < 0.064).
In conclusion, CABG+TMLR appears safe and poses no additional threat for high-risk patients. Improved overall success and repeat revascularization rates may be due to better perfusion of ischemic areas not amenable to bypass. Further studies are warranted to determine whether these trends are indeed significant.
Key words: Angina pectoris/classification/surgery, cohort studies, coronary artery bypass, coronary disease/surgery/mortality, laser surgery/instrumentation/methods/mortality, lasers/therapeutic use, myocardial revascularization, survival analysis, treatment outcome
Despite immense progress in the treatment of coronary artery disease, diffuse coronary atherosclerosis remains a clinical challenge for cardiologists and cardiac surgeons. In patients with severe multivessel coronary artery disease and concomitant impaired left ventricular function, traditional coronary artery bypass grafting (CABG) can be associated with significant mortality and morbidity.1
Complete revascularization of 3 or more stenotic vessels in high-risk patients is independently associated with reduced mortality and symptom-free survival.2 At 6 years, the survival rate is 69% for patients with grafts to all 3 major coronary vessels versus 45% in patients with only 2 bypass grafts. However, in patients with broadly diffuse severe stenoses, complete revascularization is not always possible. These patients tend to have persistent postoperative angina and other cardiac events.3,4
In several clinical trials,5,6 transmyocardial laser revascularization (TMLR) as sole therapy has been shown to be effective in improving angina in patients who were not otherwise candidates for surgical or percutaneous transluminal intervention. In TMLR patients treated with the CO2 laser, Frazier and associates7 demonstrated improved relative subendocardial blood flow through the ischemic myocardial region on positron emission tomography (PET) scans. In a multicenter, randomized, controlled trial consisting of 192 patients at 12 sites throughout the United States, cardiac perfusion was significantly improved in the TMLR recipients but deteriorated in the medically managed patients.8 Three groups9–11 have used TMLR successfully as an adjunct to CABG in cohorts similar to the one described here. Unfortunately, from a scientific viewpoint, it is hard to isolate the efficacy of TMLR from that of CABG when combined interventions are undertaken in the absence of a control group. In addition, the long-term effect of TMLR as an adjunct to CABG has not previously been studied.
The present prospective, controlled study was undertaken to evaluate the perioperative and late efficacy of CABG+TMLR versus CABG alone, so that a cause-effect relationship could be established between adjunctive use of the laser and at least the immediate perioperative outcome with respect to cardiogenic shock, the need for mechanical circulatory support, and death. Moreover, this study was designed to identify and compare the long-term clinical outcome after CABG+TMLR versus CABG alone.
Patients and Methods
Trial Design and Patient Characteristics
This prospective, controlled, 1:1 randomized, multicenter trial involved 44 patients at 4 U.S. centers (see Appendix). All of the patients required myocardial revascularization for severe, multistenotic coronary artery disease. All had at least 1 myocardial region that was not initially considered amenable to direct revascularization with an arterial or venous bypass graft, as determined by preoperative coronary angiography. After entering the study, patients were randomized into 2 groups. The CABG+TMLR group underwent TMLR with a CO2 laser (PLC Medical Systems Inc.; Franklin, Mass) as an adjunct to CABG. The CABG group received traditional CABG alone. The study protocol was approved by the U.S. Food and Drug Administration and by each hospital's institutional review board. Preoperatively, each patient underwent clinical examination, angiography, and evaluation of medical history and coexisting risk factors. Each patient's baseline ejection fraction was determined with multigated equilibrium (99m) Tc radionuclide cineangiography, echocardiography, or cardiac catheterization. All patients were evaluated according to the Cleveland Clinic risk stratification score (CCRSS),1 which preoperatively estimates the risk of morbidity and mortality in patients scheduled to undergo CABG.
When feasible, the patient and the referring cardiologist were blinded with respect to the type of treatment. At the surgeons' discretion, the CABG+TMLR patients who were found to have bypassable vessels in the TMLR-targeted area at the time of surgery could be switched to the CABG group.
Study Endpoints
The objective of the study was to compare the 2 groups with respect to perioperative and late mortality and morbidity and with respect to major adverse cardiac events (lack of angina relief, need for an additional revascularization procedure, and death).
Timing of the Evaluations
Evaluations were performed before surgery, at hospital discharge, at 1 year, and at 4 years. Patients who died, who needed additional surgical intervention, or who were lost to follow-up were considered eligible for evaluation until the time of the disqualifying event.
Operative Procedure
The TMLR procedure was performed with a high-powered CO2 laser, which can create 1-mm transmyocardial channels on the beating heart in less than 100 msec at a pulse energy of 15 to 80 J. The channels were placed in the left ventricular free wall, approximately 1 to 2 cm2 apart. Transesophageal echocardiography was used to verify transmural vaporization of the laser energy.
Coronary artery bypass grafting was performed according to standard protocol at each investigational site. The TMLR procedure was completed before heparinization and initiation of the CABG procedure. The preoperative medical regimen, including antianginal agents, was reinstituted postoperatively at the discretion of the investigator or referring physician.
Statistical Analysis
All statistical tests were performed at a 5% significance level and at 80% power, using Microsoft Excel spreadsheet or SAS software (SAS Institute, Inc.; Cary, NC) on a personal computer. Standard or paired t-testing was used to compare continuous variables. The χ2, 2-sided exact Kruskal-Wallis, marginal homogeneity, and Fisher exact tests were used to compare categorical variables.
Results
Preoperative Results
The study was performed from December 1996 through June 1998 and originally included 51 patients whose angina was classified as Canadian Cardiovascular Society class 3 or 4. Twenty-four of these patients were assigned to the CABG group, and the remaining 27 patients were assigned to the CABG+TMLR group.
Three CABG patients and 4 CABG+TMLR patients subsequently withdrew from the study: of the 3 CABG patients, the first died before surgery, the second was withdrawn after CABG because of a protocol violation, and the third was withdrawn before surgery for an unknown reason. Of the 4 CABG+TMLR patients, 3 underwent complete revascularization with CABG alone after direct, intraoperative visualization of the coronary arteries revealed suitable target vessels originally deemed inoperable by preoperative coronary angiography (they were not included in our evaluation of the CABG group), and 1 patient underwent emergency CABG when the right ventricle was accidentally entered before TMLR could be performed.
The remaining 44 patients had similar characteristics (Table I). Each had refractory angina despite long-term maximal medical therapy. Twelve (57%) of the 21 patients in the CABG group had experienced a previous myocardial infarction that was subsequently associated with congestive heart failure, and 12 had previously undergone conventional CABG or interventional balloon angioplasty, with or without stenting. Thirteen (57%) of the 23 patients in the CABG+ TMLR group had experienced a previous myocardial infarction, and 18 patients (78%) had undergone previous revascularization procedures. The left ventricu-lar ejection fraction was 0.48 ± 0.10 in CABG patients and 0.45 ± 0.11 in CABG+TMLR patients. In each group, 14% of the patients needed intraaortic balloon pump (IABP) support preoperatively.
TABLE I. Baseline Patient Characteristics, Medical History, Risk Factors, and Risk Score
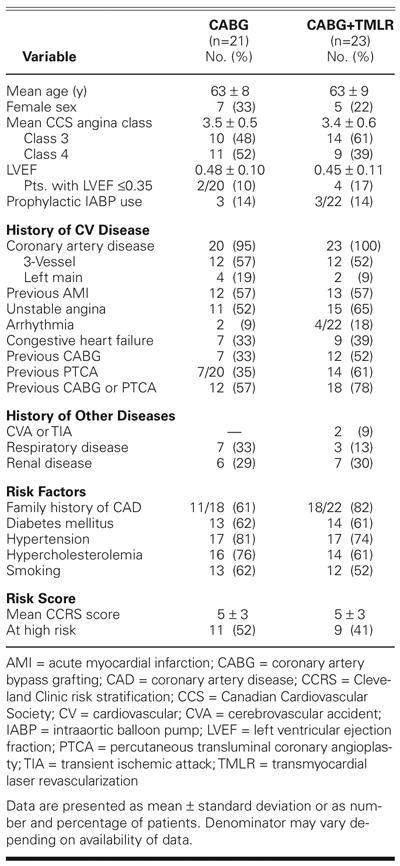
In both groups, more than half of the patients were smokers, and most had diabetes mellitus, hypertension, and hypercholesterolemia. Arrhythmias, as well as cerebrovascular, respiratory, or renal disease, were also present. The overall CCRSS was 5 ± 3. Of the total population of 44 patients, 20 (47%) were considered at high risk (CCRSS, ≥5). More CABG patients (52%) than CABG+TMLR patients (41%) were at high risk (P = NS).
Perioperative Results
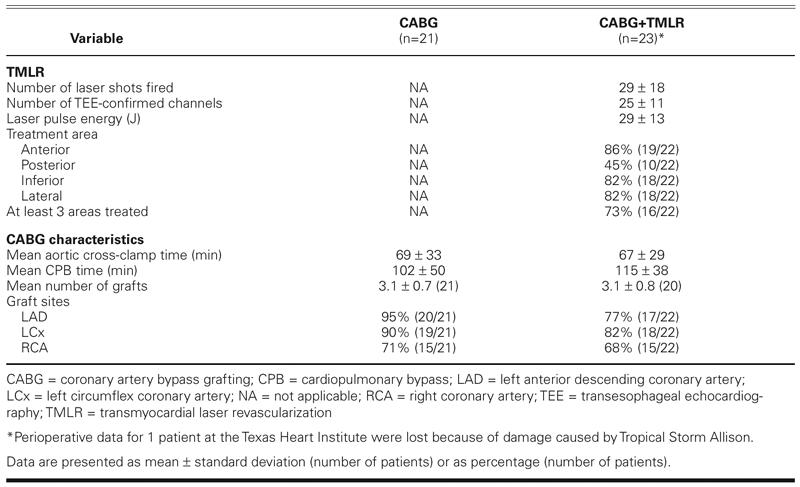
The mean duration of cardiopulmonary bypass was 102 ± 50 minutes for the CABG group and 115 ± 38 minutes for the CABG+ TMLR group (P = 0.29) (Table II). The mean aortic cross-clamp time was 69 ± 33 minutes for the CABG group and 67 ± 29 minutes for the CABG+TMLR group. In the latter group, the distribution of the laser channels was similar in the anterior, lateral, and inferior areas of the left ventricle. In contrast, fewer laser channels were created in the posterior region of the ventricle. The left anterior descending artery was revascularized in 95% of the CABG patients but in only 77% of the CABG+TMLR group.
TABLE II. Characteristics of Revascularization Treatment
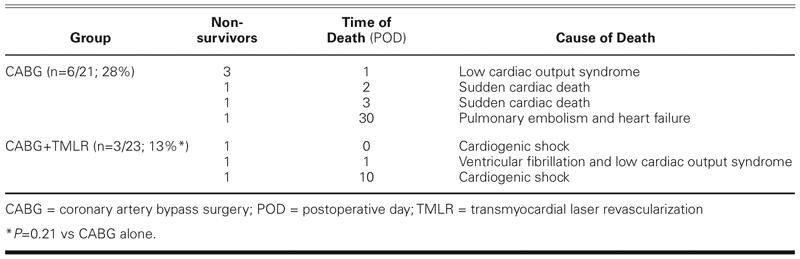
The in-hospital mortality was 28% (6/21) for the CABG group and 13% (3/23) for the CABG+TMLR group (P = 0.21) (Table III). Postoperatively, 3 CABG patients developed a low cardiac output syndrome related to progressive heart failure and died within 24 hours despite extensive pharmacologic support and placement of a left ventricular assist device. Two other patients died of sudden cardiac arrest on days 2 and 3. One patient had a pulmonary embolism, which caused severe right-sided heart failure and death at 30 days. In the CABG+TMLR group, 2 patients developed cardiogenic shock immediately after the operation and died on days 0 and 10. One other patient had ventricular fibrillation on the 1st postoperative day, developed a low cardiac output syndrome that was refractory to therapy, and died the same day.
TABLE III. Perioperative (≤30-Day) Mortality: Times and Causes of Death
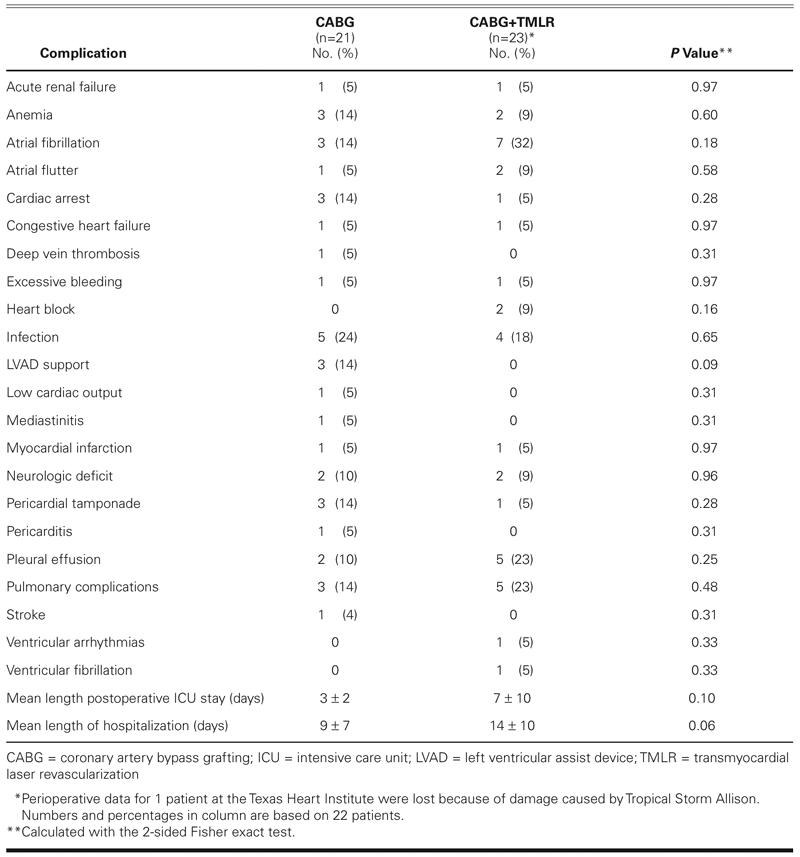
Perioperatively, 1 patient in each group had an acute myocardial infarction, diagnosed on the basis of electrocardiography and postoperative cardiac enzyme levels (Table IV). Postoperatively, atrial fibrillation was the most common complication in the CABG+ TMLR group. Cardiac arrest occurred in 3 patients in the CABG group (14%) versus 1 patient in the CABG+TMLR group (5%). The incidence of infection, excessive bleeding, and pulmonary or renal complications was comparable in both groups. Patients in the CABG group showed a trend toward a shorter stay both in the intensive care unit (3 ± 2 vs 7 ± 10 days; P = 0.10) and in the hospital (9 ± 7 vs 14 ± 10 days; P = 0.06).
TABLE IV. Perioperative (≤30-Day) Morbidity
Late Follow-Up Results
Other than the 6 in-hospital deaths, the CABG group had no mortality during the 1st postoperative year. During the 1st few months after surgery, 1 CABG+TMLR patient had recurrent heart failure that was ultimately refractory to all life-saving attempts, and this patient died at 5 months (Table V). Another CABG+TMLR patient had a complicated postoperative course but was discharged from the hospital after 17 days. Seven months later, he died at home, presumably of cardiac causes. There was no significant intergroup difference in mortality rate at 1 year.
TABLE V. Late Mortality (≤30 Days)
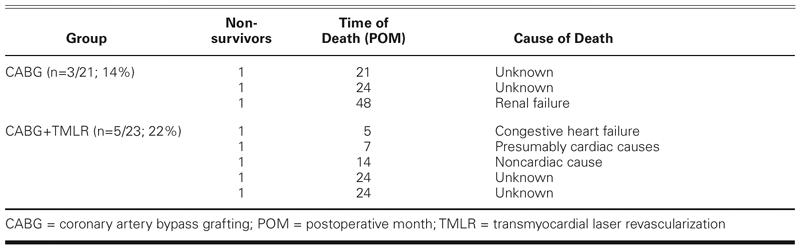
Within the 1st postoperative year, 5 patients in the CABG group underwent angioplasty for persistent angina and bypass graft stenosis. During the immediate postoperative period, 1 of these patients underwent thrombectomy of a radial artery graft supplying the left anterior descending coronary artery. In the 3rd, 6th, 13th, and 14th months, respectively, the other 4 patients required PTCA for stenotic lesions that had developed in the native coronary arteries or bypass grafts and were causing recurrent ischemia and angina. In neither group did any patient need repeat revascularization after 14 months. Three patients in the CABG group died during the 21st, 24th, and 48th postoperative months (Table V). The cause of death was renal failure in 1 patient and unknown in the other 2 patients. Similarly, 3 patients in the CABG+TMLR group died: 1 in the 14th month and 2 in the 24th month. The cause of death was noncardiac in 1 patient (lupus erythematosus) and unknown in the other 2 cases. The late mortality rate was not significantly different between the 2 groups.
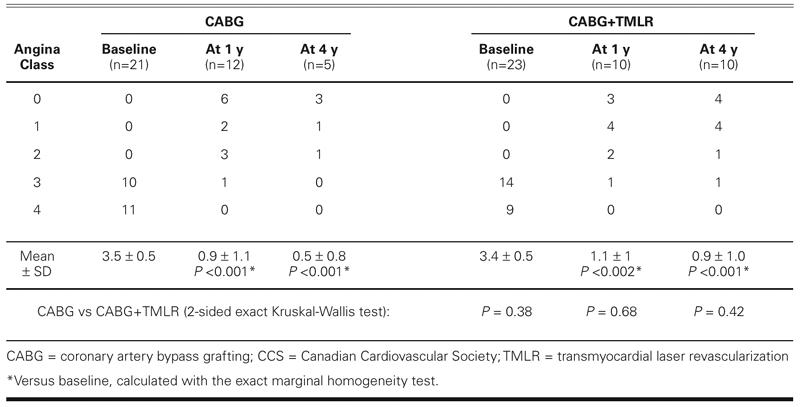
Compared with the baseline level, the angina class improved significantly in both groups at the 1- and 4- year follow-up evaluations (Table VI). Two patients in each group reported little or no relief from angina at 1 year. Two CABG patients and 1 CABG+TMLR patient reported no relief from angina during the late follow-up period. At the end of the study, there was no significant intergroup difference.
TABLE VI. Angina Status at Follow-Up (CCS Classification)
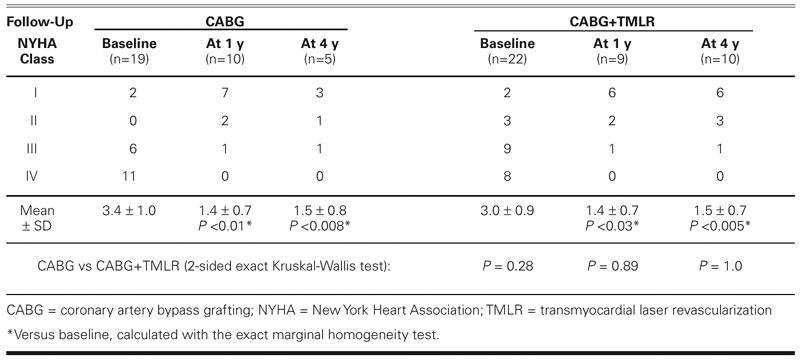
In both groups, the New York Heart Association functional class at 1 and 4 years improved significantly over baseline levels (Table VII). There were no significant intergroup differences in New York Heart Association functional class.
TABLE VII. Heart Failure Status at Follow-Up (NYHA Functional Class)
The long-term follow-up period lasted for 50.3 ± 17.8 months (range, 21–66 months) for the CABG group and 48.1 ± 16.8 months (range, 14–68 months) for the CABG+TMLR group. At 4 years, overall treatment success, defined as freedom from death, repeat revascularization, and continued or recurrent angina, was 14% (3/21) in the CABG group and 39% (9/23) in the CABG+TMLR group (P < 0.064) (Table VIII and Fig. 1).
TABLE VIII. Follow-Up Results at 1 and 4 Years
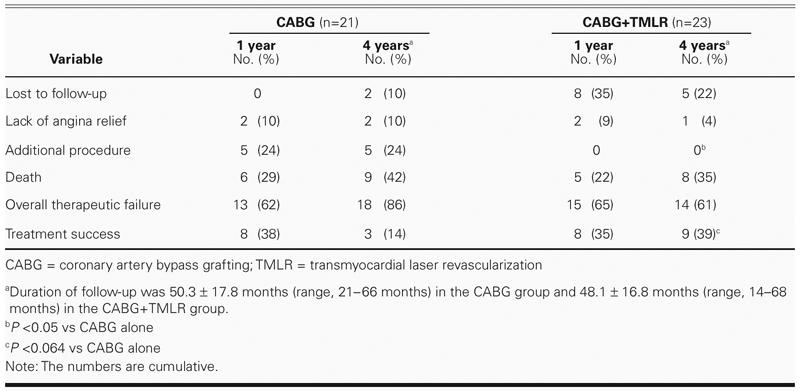
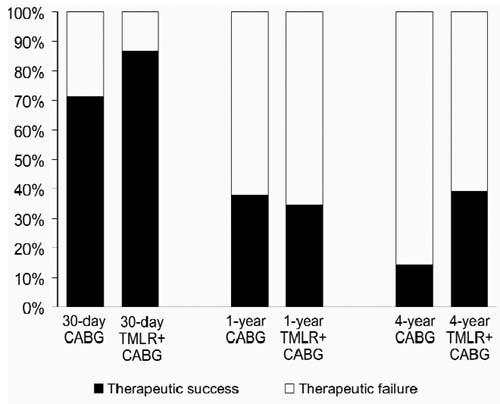
Fig. 1 Event-free survival at 4 years.
Discussion
We evaluated the efficacy of TMLR as an adjunct to CABG in 23 patients who had distal, diffuse coronary artery disease not amenable to optimal revascularization by conventional means. Our control group comprised 21 patients with similar angiographic anatomy and comorbidities who underwent CABG alone. More than two thirds of the patients in both groups had experienced at least 1 previous myocardial infarction, followed by CABG or PTCA. According to the CCRSS, almost half of the patients in both groups were at high surgical risk. The perioperative mortality was lower in the CABG+TMLR group than in the CABG alone group (13% vs 28%; P = 0.21). Despite the lack of a significant intergroup difference in this small series of patients, the lower mortality rate in the CABG+TMLR group appears to be a promising trend. The slightly superior perioperative effect of adjunctive TMLR may be attributable to the creation of acutely patent channels that have been reported to improve myocardial perfusion in ischemic areas not amenable to CABG.8 Short- and long-term channel patency has previously been demonstrated histologically and ventriculographically in both animals and human beings.12–14
The perioperative mortality in our CABG+TMLR group (3/23; 13%) was lower than the 20% mortality predicted by the CCRSS for patients with a similar preoperative risk. Previously, Allen and colleagues15 had reported a randomized, multicenter trial in which TMLR with a Ho:YAG laser was performed in combination with CABG. The 1.5% perioperative mortality rate in their treatment group was also less than the 6.3% predicted by the risk-assessment method used in their study (Parsonnet risk-stratification). In contrast to Allen's results, however, the overall perioperative mortality rates in both of our patient groups were considerably higher. This difference may be related to our patients' higher risk scores, which resulted from their substantial comorbidities, including higher rates of previous CABG or PTCA, renal disease, and preoperative IABP support for impaired left ventricular function. In another recently published meta-study, 390 patients underwent CABG+TMLR because of diseased vessels that could not be grafted for technical reasons. Those patients had similar rates of operative deaths and acute adverse events compared with patients who underwent CABG (n = 39,454) (4.9% vs 4.1% and 18.1% vs 15.5%, respectively).16 Although the mortality and morbidity rates in both groups were lower than those of our patients, detailed risk stratification in that study was not described. According to the authors, the 30-day mortality rate was 4.2% after 2,475 CABG+TMLR operations.16 This was lower than the mortality rate in our patients who received the same treatment. In our patients, however, the occurrence of preoperative risk factors such as diabetes, angina class, heart failure, previous myocardial infarction, and unstable angina rates was substantially higher.
In each of our treatment groups, the baseline Canadian Cardiovascular Society angina class and the New York Heart Association functional class compared with the respective 1- and 4-year follow-up results showed significant improvement. There was no intergroup difference in either of these classifications or in the overall distribution of angina classes. The improvement in the CABG+TMLR group was consistent with the results of earlier studies of TMLR as sole therapy in which patients experienced a dramatic clinical improvement of more than 2 classes up to 5 years postoperatively.17 The placebo effect has been proposed as a possible mechanism to account for the beneficial effects associated with TMLR17,18 or percutaneous myocardial laser revascularization (PMLR).19 In our study, however, because the patients were blinded to treatment for up to 1 year postoperatively, the long-term outcome was unlikely to have been influenced by the placebo effect, which is not expected to last beyond 1 year after the procedure. However, in the adjunctive setting, the beneficial effects can be attributed to the bypass surgery performed in both groups. Nevertheless, the difference between the CABG+TMLR and CABG-only groups with respect to the overall failure rate (defined as recurrent angina, repeat revascularization, and/or death) neared significance in our study (CABG, 86%; CABG+TMLR, 61%; P < 0.064). In this context, it is quite remarkable that, when CABG+TMLR was used to treat diffuse coronary artery disease, the overall success rate improved significantly at a mean of 48 months postoperatively. In their TMLR study involving the Ho:YAG laser, Allen and coworkers15 observed a nonsignificant trend toward fewer overall treatment failures in the CABG+TMLR group during the 1-year follow-up period, but they did not consider the incidence of repeat revascularization at that interval. In our study, the repeat revascularization rate at 4 years was zero for the CABG+TMLR group and 24% for the CABG group (P < 0.05).
The mechanisms that underlie these results remain controversial.20–22 Because the Ho:YAG laser lends itself to percutaneous applications, numerous clinical trials have been conducted to test the efficacy of PMLR; most of them have yielded negative results with respect to procedural risk and morbidity.23–25 Caution must be used, however, in comparing PMLR results to those of TMLR, because the CO2 and Ho:YAG lasers have completely different histologic and arrhythmogenic properties.20,26 Therapeutic options for patients with symptomatic diffuse coronary artery disease are very limited and do not substantially improve either cardiac function or quality of life. With the introduction of TMLR, however, another potential therapeutic tool was added to this limited arsenal. Combining TMLR with conventional CABG might possibly improve survival and provide relief of angina in this high-risk population.
Appendix
The following centers and principal investigators participated in this study:
Texas Heart Institute, Houston, Texas. O.H. Frazier, MD (23 patients)
Washington Heart at Washington Hospital Center, Washington, DC. Steven W. Boyce, MD (18 patients)
Heart Institute at Columbia Audubon Hospital, Louisville, Kentucky. Allan M. Lansing, MD (5 patients)
Rush-Presbyterian-St. Luke's Medical Center, Chicago, Illinois. Robert J. March, MD (5 patients)
Footnotes
Address for reprints: Kamuran A. Kadipasaoglu, PhD, Cardiovascular Research Laboratories, Texas Heart Institute, MC 3–268, 6770 Bertner Avenue, Houston, TX 77030
E-mail: kamurank@heart.thi.tmc.edu
References
- 1.Higgins TL, Estafanous FG, Loop FD, Beck GJ, Blum JM, Paranandi L. Stratification of morbidity and mortality outcome by preoperative risk factors in coronary artery bypass patients. A clinical severity score [published erratum appears in JAMA 1992;268:1860]. JAMA 1992;267:2344–8. [PubMed]
- 2.Bell MR, Gersh BJ, Schaff HV, Holmes DR Jr, Fisher LD, Alderman EL, et al. Effect of completeness of revascularization on long-term outcome of patients with three-vessel disease undergoing coronary artery bypass surgery. A report from the Coronary Artery Surgery Study (CASS) Registry. Circulation 1992;86:446–57. [DOI] [PubMed]
- 3.Jones EL, Craver JM, Guyton RA, Bone DK, Hatcher CR Jr, Riechwald N. Importance of complete revascularization in performance of the coronary bypass operation. Am J Cardiol 1983;51:7–12. [DOI] [PubMed]
- 4.Jones EL, Weintraub WS. The importance of completeness of revascularization during long-term follow-up after coronary artery operations. J Thorac Cardiovasc Surg 1996;112: 227–37. [DOI] [PubMed]
- 5.Horvath KA, Cohn LH, Cooley DA, Crew JR, Frazier OH, Griffith BP, et al. Transmyocardial laser revascularization: results of a multicenter trial with transmyocardial laser revascularization used as sole therapy for end-stage coronary artery disease. J Thorac Cardiovasc Surg 1997;113:645–54. [DOI] [PubMed]
- 6.Allen KB, Dowling RB, Fudge TL, Schoettle GP, Selinger SL, Gangahar DM, et al. Comparison of transmyocardial revascularization with medical therapy in patients with refractory angina. N Engl J Med 1999;341:1029–36. [DOI] [PubMed]
- 7.Frazier OH, Cooley DA, Kadipasaoglu KA, Pehlivanoglu S, Lindenmeir M, Barasch E, et al. Myocardial revascularization with laser. Preliminary findings. Circulation 1995;92(9 Suppl):II58–65. [DOI] [PubMed]
- 8.Frazier OH, March RJ, Horvath KA. Transmyocardial revascularization with a carbon dioxide laser in patients with end-stage coronary artery disease. N Engl J Med 1999;341: 1021–8. [DOI] [PubMed]
- 9.Trehan N, Mishra Y, Mehta Y, Jangid DR. Transmyocardial laser as an adjunct to minimally invasive CABG for complete myocardial revascularization. Ann Thorac Surg 1998; 66:1113–8. [DOI] [PubMed]
- 10.Vincent JG, Bardos P, Kruse J, Maass D. End stage coronary disease treated with the transmyocardial CO2 laser revascularization: a chance for the ‘inoperable’ patient. Eur J Cardiothorac Surg 1997;11:888–94. [DOI] [PubMed]
- 11.Stamou SC, Boyce SW, Cooke RH, Carlos BD, Sweet LC, Corso PJ. One-year outcome after combined coronary artery bypass grafting and transmyocardial laser revascularization for refractory angina pectoris. Am J Cardiol 2002;89: 1365–8. [DOI] [PubMed]
- 12.Perin EC, Dohmann HJ, Dohmann HF, de Mattos ND, Carvalho LA. Laser channels after percutaneous transmyocardial revascularization. Circulation 1999;99:2218. [DOI] [PubMed]
- 13.Cooley DA, Frazier OH, Kadipasaoglu KA, Pehlivanoglu S, Shannon RL, Angelini P. Transmyocardial laser revascularization. Anatomic evidence of long-term channel patency. Tex Heart Inst J 1994;21:220–4. [PMC free article] [PubMed]
- 14.Horvath KA, Smith WJ, Laurence RG, Schoen FJ, Appleyard RF, Cohn LH. Recovery of viability of an acute myocardial infarct after transmyocardial laser revascularization. J Am Coll Cardiol 1995;25:258–63. [DOI] [PubMed]
- 15.Allen KB, Dowling RB, DelRossi AJ, Realyvasques F, Lefrak EA, Pfeffer TA, et al. Transmyocardial laser revascularization combined with coronary artery bypass grafting: a multicenter, blinded, prospective, randomized, controlled trial. J Thorac Cardiovasc Surg 2000;119:540–9. [DOI] [PubMed]
- 16.Peterson ED, Kaul P, Kaczmarek RG, Hammill BG, Armstrong PW, Bridges CR, et al. From controlled trials to clinical practice: monitoring transmyocardial revascularization use and outcomes. J Am Coll Cardiol 2003;42:1611–6. [DOI] [PubMed]
- 17.Horvath KA, Aranki SF, Cohn LH, March RJ, Frazier OH, Kadipasaoglu KA, et al. Sustained angina relief 5 years after transmyocardial laser revascularization with a CO2 laser. Circulation 2001;104(12 Suppl 1):I81–4. [DOI] [PubMed]
- 18.Schofield PM, Sharples LD, Caine N, Burns S, Tait S, Wistow T, et al. Transmyocardial laser revascularisation in patients with refractory angina: a randomised controlled trial [published erratum appears in Lancet 1999;353(9165):1714]. Lancet 1999;353(9152):519–24. [DOI] [PubMed]
- 19.Gray TJ, Burns SM, Clarke SC, Tait S, Sharples LD, Caine N, Schofield PM. Percutaneous myocardial laser revascularization in patients with refractory angina pectoris. Am J Cardiol 2003;91(6):661–6. [DOI] [PubMed]
- 20.Kadipasaoglu KA, Frazier OH. Transmyocardial laser revascularization: effect of laser parameters on tissue ablation and cardiac perfusion. Semin Thorac Cardiovasc Surg 1999;11: 4–11. [DOI] [PubMed]
- 21.Spanier T, Smith CR, Burkhoff D. Angiogenesis: a possible mechanism underlying the clinical benefits of transmyocardial laser revascularization. J Clin Laser Med Surg 1997;15: 269–73. [DOI] [PubMed]
- 22.Kwong KF, Schuessler RB, Kanellopoulos GK, Saffitz JE, Sundt TM 3rd. Nontransmural laser treatment incompletely denervates canine myocardium. Circulation 1998(19 Suppl):II67–72. [PubMed]
- 23.Leon MB, Baim DS, Moses JW, Laham RJ, Knopf W, Reisman M, et al. A randomized blinded clinical trial comparing percutaneous laser myocardial revascularization (using Biosense LV mapping) vs placebo in patients with refractory coronary ischemia [abstract]. Circulation 2000;102(18 Suppl 2):II565.
- 24.Burkhoff D, Schmidt S, Schulman SP, Myers J, Resar J, Becker LC, et al. Transmyocardial laser revascularisation compared with continued medical therapy for treatment of refractory angina pectoris: a prospective randomised trial. ATLANTIC Investigators. Angina Treatments-Lasers and Normal Therapies in Comparison. Lancet 1999;354(9182): 885–90. [DOI] [PubMed]
- 25.Oesterle SN, Sanborn TA, Ali N, Resar J, Ramee SR, Heuser R, et al. Percutaneous transmyocardial laser revascularisation for severe angina: the PACIFIC randomised trial. Potential Class Improvement From Intramyocardial Channels. Lancet 2000;356(9243):1705–10. [DOI] [PubMed]
- 26.Kadipasaoglu KA, Sartori M, Masai T, Cihan HB, Clubb FJ Jr, Conger JL, Frazier OH. Intraoperative arrhythmias and tissue damage during transmyocardial laser revascularization. Ann Thorac Surg 1999;67(2):423–31. [DOI] [PubMed]


