Abstract
Background
We investigated choroidal thicknesses at five sites in two siblings (four eyes) with nanophthalmos using swept-source optical coherence tomography.
Case presentation
Case 1, a 51-year-old Japanese female with high hyperopia (Right: +20.5 Dioptors, Left: +19.5 Dioptors), had axial lengths of 15.6 mm in both eyes. Case 2, a 55-year-old Japanese male with high hyperopia (Right: +22.5 Dioptors, Left: +22.8 Dioptors), had axial lengths of 14.8 and 14.7 mm in the right and left eyes, respectively. Choroidal thickness was measured at five sites in each eye using swept-source optical coherence tomography; subfoveal, nasal, temporal, superior and inferior (the 4 non-subfoveal sites were measured 3000 µm from the fovea).
Conclusion
The mean choroidal thickness was 355.8 ± 63.6 μm at the subfoveal, 466.3 ± 85.1 μm at the nasal, 274.8 ± 77.2 μm at the temporal, 396.8 ± 54.6 μm at the superior, and 480.8 ± 66.8 μm at the inferior (mean ± standard deviation) site. Choroidal thickness was maximal at the inferior site. The choroid was thinnest, in diminishing order, at the nasal, superior, subfoveal and temporal sites.
Keywords: Axial length, Choroidal thickness, Hyperopia, Nanophthalmos, Optical coherence tomography
Background
Nanophthalmos is a rare condition without other ocular or systemic anomalies, in which axial length (AL) is less than 20 mm and the eye volume is about two-thirds less than normal [1]. Nanophthalmos is associated with high hyperopia, narrow palpebral fissures, enophthalmos, shallow anterior chamber and scleral wall thickening. It can cause complications such as angle-closure glaucoma and uveal effusion [1]. Posterior segment findings in nanophthalmic cases reportedly include papillomacular folds, dilated vessels, cysts, pseudopapilledema, and thickened choroid [2, 3]. Genetic mutations are associated with nanophthalmos according to some reports, which described associations with the serine protease gene (PRSS56) and membrane frizzled-related protein gene (MFRP) [4, 5].
The choroid is the vascular layer containing pigmented tissues, and is located between the sclera and the retinal pigment epithelium (RPE). The choroid, which is essential for retinal function, provides oxygen and nourishment to the outer layer of the retina.
Recently, swept source-optical coherence tomography imaging (SS-OCT) has been used to evaluate choroidal changes. SS-OCT is known to have greater scanning speed and a longer wavelength (1050 nm), enabling deeper penetration through the RPE into the choroid, than other modalities. Hirata et al. reported choroidal thickness (CT) and volume at different sites in normal subjects using SS-OCT [6]. Demircan et al. detected subfoveal choroid thickening using enhanced depth imaging optical coherence tomography in patients with nanophthalmos [3]. There are many reports describing CT at the fovea itself, as well as at sites inferior, nasal, superior, and temporal to the fovea [6–8]. However, no studies have used SS-OCT to measure CT at different sites in nanophthalmos cases. The purposes of this study were to measure CT at different sites in two siblings with nanophthalmos and to compare the findings of our cases and those of normal eyes.
Case presentation
Case 1: A 51-year-old Japanese female with nanophthalmic eyes. An objective refraction test (ORT) revealed high hyperopia in both eyes (Right: +20.5 Diopters (D), Left: +19.5 D). AL was 15.6 mm in both eyes.
Case 2: A 55-year-old Japanese male, the brother of Case 1, with nanophthalmic eyes. ORT revealed high hyperopia in both eyes (Right: +22.5 D, Left: +22.8 D). AL was 14.8 mm in the right and 14.7 mm in the left eye.
The AL was measured using an intraocular lens master (Carl Zeiss, Germany). Retinal folds at the macula and dilated veins were detected in all 4 eyes. SS-OCT revealed retinal folds at the fovea and a thickened choroid in all 4 eyes (Fig. 1).
Fig. 1.
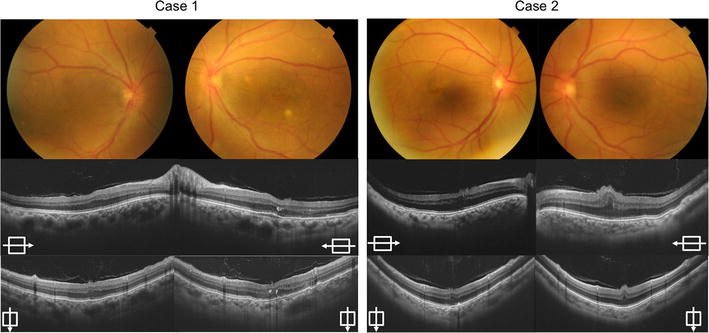
The color fundus photographs and swept-source optical coherence tomography images of both cases. The color fundus photographs of Case 1 and Case 2 show macular folds and dilated vessels (top). The swept source-optical coherence tomography images reveal macular folds and thickened choroid at the subfoveal point in all four eyes. The horizontal arrows indicate horizontal images through the fovea (middle). The vertical arrows indicate vertical images through the fovea (bottom)
CT was measured manually at the fovea and 4 other sites (nasal, temporal, superior and inferior to the subfoveal measurement point) 3000 µm from the foveal site in each eye using SS-OCT (DRI OCT-1 “Atlantis”; Topcon Medical Inc., Tokyo, Japan) (Fig. 2). CT was measured manually with a built-in caliper (H.K.), according to Rhaman et al. [8]. CT was measured from the bottom of the hyper-reflective line of the RPE to the chorioscleral border at each measurement point. The mean CT values at each site in the four eyes were analyzed.
Fig. 2.
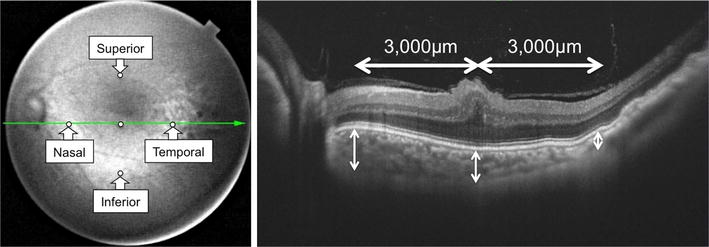
The infrared image (left) and the swept source-optical coherence tomography image (right) from Case 2 (left). The horizontal line is through the fovea on the infrared image. White circles indicate the fovea and the other four sites (nasal, temporal, superior and inferior, each measured 3000 µm from the fovea). (Right) Choroidal thickness was measured from the bottom of the hyper-reflective line of the retinal pigment epithelium to the chorioscleral border at each measurement point
Conclusions
The mean CT measurements are shown in Table 1. CT was maximal at the inferior site. The second largest CT value was measured at the nasal site, followed in order by the superior, subfoveal and temporal sites, with the latter being the thinnest portion of the choroid.
Table 1.
Average choroidal thickness at five sites
| Site | Choroidal thickness (mean ± standard deviation) µm |
|---|---|
| Inferior | 480.8 ± 66.8 |
| Nasal | 466.3 ± 85.1 |
| Superior | 396.8 ± 54.6 |
| Subfoveal | 355.8 ± 63.6 |
| Temporal | 274.8 ± 77.2 |
There are reports describing CT at the nasal site as being less than that at the other 4 sites (subfoveal, temporal, inferior and superior) in healthy subjects [6, 7]. In healthy Japanese subjects, the mean CT at the fovea was reported to be 202.6 ± 83.5 µm, the mean CT at the nasal measurement point 152.5 ± 71.1 µm [7]. In our cases, however, the mean CT at the inferior and nasal sites were greater than those at the same sites in healthy subjects. The CT values at the subfoveal and nasal sites were also greater than those at the same sites in healthy Japanese subjects, serving as controls.
The ALs in the four eyes of our two subjects were equivalent to those seen in nanophthalmos cases examined between the postnatal period and 2 years of age [9]. Furthermore, in our cases, ultrasonic B-mode imaging revealed thickened sclera and short AL. Therefore, it was suspected that development of the sclera in these four eyes had stopped during this early period of life. It has been hypothesized that retinal folds are molded by normal retinal development with arrested scleral development [10]. This hypothesis may apply to our cases.
Primordial tissues of the choroid and sclera are comprised of mesenchymal cells around an optic cup. From the 6-week embryonic stage, differentiation into choroid and sclera starts via induction of the RPE. Therefore, we theorized that development of the choroid had stopped in these siblings with nanophthalmos, since the primordial tissues of the choroid and sclera were the same. If the eyeball develops normally, the choroid may extend from the disc in the temporal direction, and the nasal site is thinner in normal eyes. In these four eyes, however, the nasal site near the disc was thickened due to poor extension of the eyeball.
It is easy to obtain non-invasive OCT images, even in relatively young patients. Salchow et al. reported only 4 (3.3%) of 121 subjects younger than 18 years to be uncooperative [11]. Based on only one horizontal OCT image, we can surmise that strong hyperopia is present with a short AL, if the OCT image has findings indicating that the nasal choroid is thicker than the temporal choroid, there are papillomacular folds and non-inflammatory cells are present in the vitreous cavity.
The major limitation of this study is the small number of patients. Also, we were not able to perform a genetic analysis in these cases. Further study is needed to clarify the features of and genetic contributions to nanophthalmos.
In conclusion, CT was maximal at the inferior site. The choroid was thinnest, in diminishing order of CT measurement values, at the nasal, superior, subfoveal and temporal sites. Unlike normal eyes, the nasal site of the choroid in these four nanophthalmic eyes was thickened, presumably due to arrest of choroidal and scleral development.
Authors’ contributions
HK and AS conceived this study, and participated in its conception and design, as well as data acquisition. HK and AS wrote the manuscript. RM, AK, and MY critically revised the manuscript for important intellectual content. HK, AS, RM, AK, and MY gave final approval of the version to be published. All authors read and approved the final manuscript.
Acknowledgements
The authors have no competing interests to declare. Nihon University School of Medicine provided financial support for this study. Neither the sponsor nor any other funding organization had a role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Data for this study is available as additional documents and can be downloaded through the link provided in the end of this manuscript (Figs. 1, 2).”
Ethics approval and consent to participate
All procedures adhered to the tenets of the Declaration of Helsinki.
Consent for publication
Written informed consent was obtained from both patients for publication of this Case Report and any accompanying images.
Abbreviations
- AL
axial length
- CT
choroidal thickness
- D
diopters
- ORT
objective refraction test
- SS-OCT
swept source-optical coherence tomography
- RPE
retinal pigment epithelium
Contributor Information
Hiroyuki Kaneko, Email: henryhiroyuki1214@gmail.com.
Ari Shinojima, Phone: +81-3-3293-1711, Email: ariearly7@gmail.com.
Mori Ryusaburo, Email: ryu-m@sa2.so-net.ne.jp.
Akiyuki Kawamura, Email: kw-eye-c@xb3.so-net.ne.jp.
Mitsuko Yuzawa, Email: yuzawa.mitsuko@nihon-u.ac.jp.
References
- 1.Duke-Elder S. Normal and abnormal development: congenital deformities system of ophthalmology. St Louis: Mosby; 1964. [Google Scholar]
- 2.Nowilaty SR, Mousa A, Ghazi NG. The posterior pole and papillomacular fold in posterior microphthalmos: novel spectral-domain optical coherence tomography findings. Ophthalmology. 2013;120:1656–1664. doi: 10.1016/j.ophtha.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Demircan A, Altan C, Osmanbasoglu OA. Subfoveal choroidal thickness measurements with enhanced depth imaging optical coherence tomography in patients with nanophthalmos. Br J Ophthalmol. 2014;3:345–349. doi: 10.1136/bjophthalmol-2013-303465. [DOI] [PubMed] [Google Scholar]
- 4.Orr A, Dubé M, Zenteno J, Jiang H, Asselin G, Evans SC. Mutations in a novel serine protease PRSS56 in families with nanophthalmos. Mol Vis. 2011;17:1850–1861. [PMC free article] [PubMed] [Google Scholar]
- 5.Wasmann RA, Wassink-Ruiter JS, Sundin OH. Novel membrane frizzled-related protein gene mutation as cause of posterior microphthalmia resulting in high hyperopia with macular folds. Acta Ophthalmol. 2014;92:276–281. doi: 10.1111/aos.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirata M, Tsujikawa A, Matsumoto A. Macular choroidal thickness and volume in normal subjects measured by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:4971–4978. doi: 10.1167/iovs.11-7729. [DOI] [PubMed] [Google Scholar]
- 7.Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y. Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci. 2014;51:2173–2176. doi: 10.1167/iovs.09-4383. [DOI] [PubMed] [Google Scholar]
- 8.Rahman W, Chen FK, Yeoh J, Patel P, Tufail A, Da Cruz L. Repeatability of manual subfoveal choroidal thickness measurements in healthy subjects using the technique of enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:2267–2271. doi: 10.1167/iovs.10-6024. [DOI] [PubMed] [Google Scholar]
- 9.Majima A. Microphthalmos and its pathogenic classification. Nihon Ganka Gakkai Zasshi. 1994;12:1180–1200. [PubMed] [Google Scholar]
- 10.Boynton JR, Purnell EW. Bilateral microphthalmos without microcornea associated with unusual papillomacular retinal folds and high hyperopia. Am J Ophthalmol. 1975;79:820–826. doi: 10.1016/0002-9394(75)90743-6. [DOI] [PubMed] [Google Scholar]
- 11.Salchow DJ, Oleynikov YS, Chiang MF, Kennedy-Salchow SE, Langton K, Tsai JC, Al-Aswad LA. Retinal nerve fiber layer thickness in normal children measured with optical coherence tomography. Ophthalmology. 2006;113:786–791. doi: 10.1016/j.ophtha.2006.01.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this study is available as additional documents and can be downloaded through the link provided in the end of this manuscript (Figs. 1, 2).”


