Abstract
Bacteria use redox-sensitive transcription factors to coordinate responses to redox stress. The [2Fe-2S] cluster-containing transcription factor SoxR is particularly tuned to protect cells against redox-active compounds (RACs). In enteric bacteria, SoxR is paired with a second transcription factor, SoxS, that activates downstream effectors. However, SoxS is absent in non-enteric bacteria, raising questions as to how SoxR functions. Here, we first show that SoxR of Acinetobacter oleivorans displayed similar activation profiles in response to RACs as did its homolog from Escherichia coli but controlled a different set of target genes, including sinE, which encodes an endoribonuclease. Expression, gel mobility shift, and mutational analyses indicated that sinE is a direct target of SoxR. Redox potentials and permeability of RACs determined optimal sinE induction. Bioinformatics suggested that only a few γ- and β-proteobacteria might have SoxR-regulated sinE. Purified SinE, in the presence of Mg2+ ions, degrades rRNAs, thus inhibiting protein synthesis. Similarly, pretreatment of cells with RACs demonstrated a role for SinE in promoting persistence in the presence of antibiotics that inhibit protein synthesis. Our data improve our understanding of the physiology of soil microorganisms by suggesting that both non-enteric SoxR and its target SinE play protective roles in the presence of RACs and antibiotics.
Keywords: antibiotics, bacteria, biofilm, endoribonuclease, Escherichia coli (E. coli), oxidative stress, reactive oxygen species (ROS), Acinetobacter, SoxR, transcriptional regulation
Introduction
Oxidative stress is an unavoidable consequence of aerobic and anaerobic metabolism when cells are exposed to O2. Bacteria have evolved to harbor defense mechanisms against various reactive oxygen species (ROS).4 Bacteria protect themselves by activating ROS-sensing transcription factors, including OxyR and PerR, that detect hydrogen peroxide (H2O2) using cysteine residues and iron, respectively, and SoxR, which responds to superoxide or redox-active compounds (RACs) using a [2Fe-2S] cluster (1–3). Bacteria can produce many natural RACs, such as pyocyanin (PYO), actinorhodin, and plumbagin (PL), that act as antibiotics, toxic compounds, and quorum signals (4). RACs can generate toxic doses of ROS inside cells by mediating electron transfer from redox enzymes to O2. When Escherichia coli is exposed to RACs, SoxR is activated by the oxidation of its [2Fe-2S] cluster, and it subsequently induces the transcription of soxS (5–8). SoxS then induces the transcription of numerous genes involved in the repair of oxidatively damaged enzymes and DNA and the elimination of ROS (9–12).
However, the E. coli-SoxRS paradigm does not apply to non-enteric bacteria because of the absence of SoxS and no induction of many known E. coli SoxRS regulon (13–18). Surprisingly, activated SoxR can directly induce several genes (lpxC, aroF, sodA, and mgtA) in E. coli by binding their promoters without soxS (16). SoxR can be activated directly by RACs rather than by superoxide, as evidenced by the fact that RACs can oxidize the [2Fe-2S]1+ cluster of SoxR even under anaerobic conditions (1, 2, 12). Activated SoxR can induce several genes that specifically defend against RACs, not superoxide. Pseudomonas aeruginosa and Streptomyces coelicolor excrete RACs (pyocyanin and actinorhodin, respectively) that can activate their native SoxR regulons (14, 17, 18). In both bacteria, SoxR appears to regulate the synthesis and excretion of RACs (3, 19). Enteric bacteria have presumably acquired soxR through horizontal gene transfer to defend themselves by recognizing RACs that other non-enteric bacteria excrete (3, 10). Consistent with this speculation, E. coli SoxR can sense a broader spectrum of RACs than P. aeruginosa and S. coelicolor (19, 20). SoxR proteins from E. coli, P. aeruginosa, and S. coelicolor show differential sensitivity to RACs, probably due to distinct redox potentials and membrane permeability of RACs (19–21). Thus, the emerging views of new SoxR activation and its role are intriguing; however, only a few species of bacteria have been investigated, and the functions of SoxR-controlled genes remain largely uncharacterized. Additional characterizations of SoxR and its regulon in non-enteric bacteria are required to provide new perspectives on the evolutionary fine-tuning of SoxR regulation.
Bacteria need to cope with ROS generated during aerobic metabolism, by RACs from competing neighbors, or during host infection. Superoxide and hydrogen peroxide can also be generated photochemically from natural organic matter and in aqueous solutions of aromatic compounds (22, 23). Although Acinetobacter species, which belong to a class of γ-proteobacteria, are widely distributed in nature, and their pathogenic species are of great concern for human disease, the genetics and physiology of Acinetobacter species are poorly explored. Previously, we showed that diesel-degrading Acinetobacter oleivorans DR1, originally isolated from rice paddies, experienced oxidative stress during n-hexadecane degradation and exposure to antibiotics (24–27). Aerobic biodegradation of hydrocarbon compounds can generate ROS, which affects bacterial cell physiology (28, 29). Severe growth defects in the Acinetobacter oxyR mutant grown in hexadecane-amended conditions indicated that OxyR is important for the elimination of ROS during hexadecane biodegradation (30). It was recently reported that SoxR from Acinetobacter baumannii AYE can bind the promoter regions of two genes, abuO and abeD, which encode a TolC-like outer membrane protein and an RND-type membrane transporter, respectively (31, 32). Our bioinformatics study suggested that Acinetobacter species, including A. oleivorans DR1, possess a SoxR binding site in the promoter regions of several genes, including a gene coding an L-PSP endoribonuclease, which is one of the five known categories of gene products controlled by SoxR in non-enteric bacteria (33). However, the molecular mechanisms of SoxR and its function and regulation of downstream genes have not been characterized in Acinetobacter species.
The goal of this study was to expand knowledge of the SoxR regulon from enteric to non-enteric bacteria. In this study, we characterized the physiological roles of SoxR and the SoxR regulon in A. oleivorans. Our data provide the first evidence that a novel SoxR-controlled L-PSP endoribonuclease shows ribonuclease activity and inhibits protein synthesis by binding and degrading rRNAs.
Results
Functional Characterization of A. oleivorans SoxR
The genome of A. oleivorans DR1 harbors a SoxR homolog (AOLE_12135, 154 amino acids; referred to as AoSoxR) with 62.7, 55.6, and 68.0% amino acid identities with SoxR of E. coli (EcSoxR), P. aeruginosa PAO1 (PaSoxR), and Pseudomonas putida KT240 (PpSoxR), respectively. The soxR gene contains four conserved cysteine residues (CX2CXCX5C) that anchor the [2Fe-2S] center and two residues, Arg-56 and Trp-92, which are known to be important for redox signaling activity (33). These sequence analyses indicated that AoSoxR may function similarly to EcSoxR. The algorithm blastp (protein-protein BLAST) found no soxS homolog in A. oleivorans.
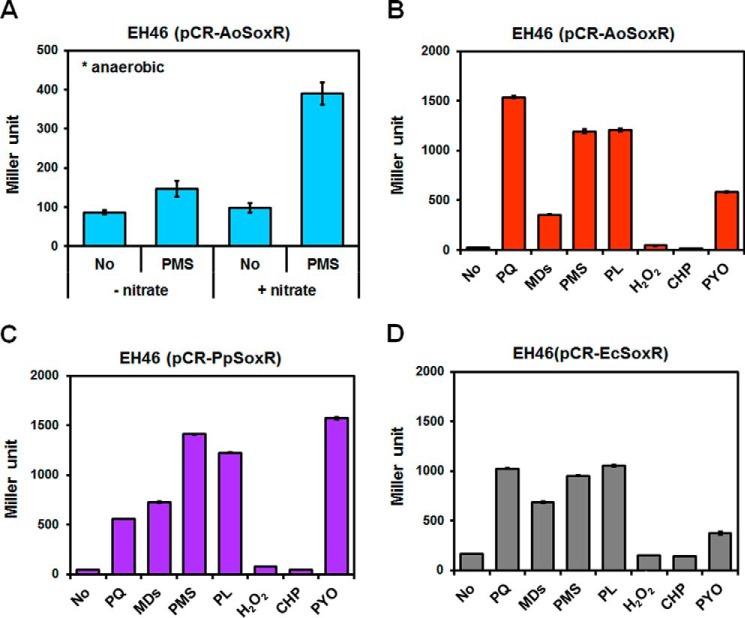
We examined the functionality of AoSoxR by cross-complementation of an E. coli soxR mutant EH46 (ΔsoxRS, soxS′:lacZ). AoSoxR in E. coli could be activated directly by phenazine methosulfate (PMS) even in the absence of O2, and this activation increased when nitrate, an alternative anaerobic electron acceptor, was supplied to the cells (Fig. 1A). The addition of other RACs, such as methyl viologen (paraquat (PQ)), PMS, and PL, resulted in high levels of AoSoxR-dependent E. coli soxS promoter expression (Fig. 1B). A 10-fold lower concentration of PYO, a natural RAC produced by P. aeruginosa, also activated AoSoxR very efficiently. The water-soluble form of menadione, menadione sodium bisulfite (MDs), appeared not to be effective in activating AoSoxR. The same SoxR activation profile was not observed with PpSoxR in E. coli (Fig. 1C). AoSoxR responded much more strongly to PQ than to PYO, whereas the opposite was true for PpSoxR. A similar degree of EcSoxR activation with PQ, PMS, and PL was found (Fig. 1D). Neither H2O2 nor cumene hydrogen peroxide (CHP) activated any SoxRs (Fig. 1). Consistent with another report (19), our data showed that AoSoxR is functional and that different SoxRs have profiles with differential sensitivities toward RACs because of their different redox potentials.
FIGURE 1.
Functional identification of SoxR. Shown is complementation of an E. coli soxR mutant with AoSoxR (A and B), PpSoxR (C), and EcSoxR (D). A, strain EH46 (pCR-AoSoxR) was grown anaerobically in LB or LB/nitrate medium. Cells in the exponential phase of growth were treated with 50 μm PMS for 2 h. The level of soxS′::lacZ reporter expression was measured by β-galactosidase activity using o-nitrophenyl-β-d-galactopyranoside as a substrate. B, C, and D, strains were grown aerobically in LB medium. The same concentration (0.1 mm) of PQ, MDs, PMS, PL, H2O2, and CHP and 0.01 mm PYO were used to treat exponentially growing cells. After incubation for 1 h, the expression of soxS′::lacZ was quantified by measuring β-galactosidase activity. All data show the average of three replicates, and error bars indicate S.D.
Protection by SoxR against High Concentrations of RACs
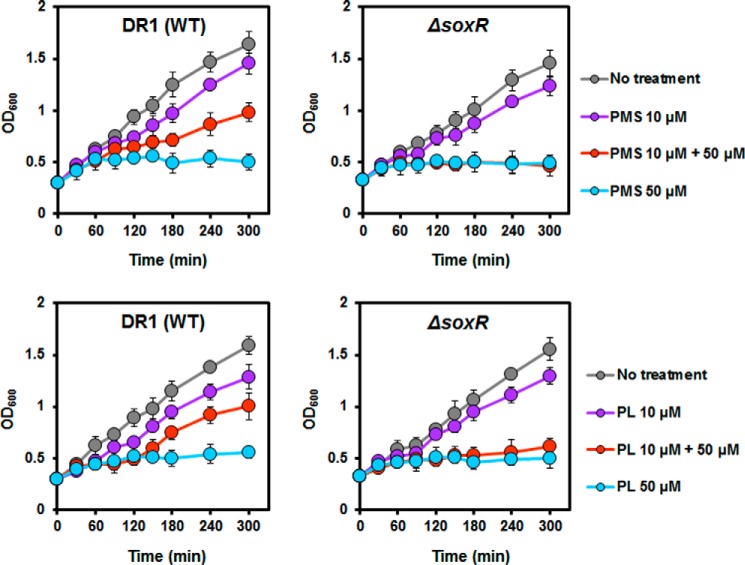
A slight growth defect was observed in soxR mutant strains cultured with and without RACs compared with wild type (Fig. 2). We speculated that pretreatment of cells with RAC could activate AoSoxR and the SoxR regulon, which provides a protective effect against toxic RACs. To test this hypothesis, we measured the growth of the wild type and soxR mutant strains in the presence of RACs. Exponentially growing wild type and soxR mutant cells were treated with lower concentrations of PMS and PL for 30 min, and then higher concentrations of PMS and PL were added. No activation effect of SoxR with low concentrations of RACs was observed in the soxR mutant (Fig. 2 (red circle) and supplemental Fig. S1A). Our data provide evidence that activation of AoSoxR by RACs gives rise to resistance to high concentrations of RACs.
FIGURE 2.
SoxR protection against high concentrations of PMS and PL. Cells were inoculated into nutrient medium, and the cultures were incubated at 30 °C. When the cultures reached the exponential phase, low concentrations of RACs (10 μm) were added. After 30 min of incubation, a higher concentration (50 μm) of the same compounds was added to culture. Cell growth was measured at A600. The graphs show the average of three replicates, and the error bars indicate S.D.
Transcriptome Analysis under PQ and PMS Treatments
To identify genes that were up-regulated and down-regulated in response to RACs and possible AoSoxR target genes, total RNA sequencing was performed using cells grown to the exponential phase (A600 ∼0.4) and then exposed to PQ (1 mm) and PMS (0.2 mm) for 15 min. Several known E. coli SoxRS target genes, such as fumC, sodA, fpr, acnA, and nfsB, and other oxidative stress defense genes, such as katG, ahpC, and trx, were induced (supplemental Table S1 and Table 1). However, many genes responding to RACs appear not to be directly associated with superoxide detoxification because these oxidative stress-related genes are known to be involved in H2O2 detoxification. Transcriptome data also suggested that genes categorized as functioning in other metabolic processes are important for detoxifying RACs: glyoxylate bypass (aceA, aceB), inorganic or organic ion transports (chrA, mntH, zntA), ABC transporters (artQ, hisP, glnQ), amino acid metabolism and transporters (ansP, argT), and sulfur metabolism (msuD, sbp, cysW, cysE, ssuD) (supplemental Table S2). Lower levels of expression were found, mainly of genes associated with ribosome biogenesis and structure, motility, and chemotaxis (supplemental Table S2). Whereas PQ and PMS up-regulated 16.5 and 22.1% of genes (640 of 3874 and 856 of 3874) 2-fold, respectively, two RACs increased the expression of only 8% (309 of 3874) of genes in common. These data indicated that exposure to each RAC leads to both common and unique cellular responses.
TABLE 1.
Gene expression profiling of oxidative stress-related genes with 1 mm PQ or 200 μm PMS treatment for 15 min compared with no treatment of cells in nutrient broth during the exponential phase
EX, exponential phase.
| Gene | Product | -Fold change (PQ/EX) | -Fold change (PMS/EX) |
|---|---|---|---|
| AOLE_01750 (sodC) | Cu/Zn-superoxide dismutase | 1.60 | 0.23 |
| AOLE_05305 (sodA) | Superoxide dismutase | 1.95 | 1.51 |
| AOLE_14380 (oxyR) | Hydrogen peroxide-inducible gene activator | 2.44 | 1.17 |
| AOLE_09800 | Catalase | 2.04 | 5.78 |
| AOLE_11770 (katE) | Catalase | 2.52 | 1.38 |
| AOLE_12755 | Catalase | 1.29 | 1.13 |
| AOLE_17390 (katG) | Catalase | 1.57 | 9.24 |
| AOLE_13380 (ahpC) | Peroxiredoxin | 2.14 | 5.09 |
| AOLE_11385 (ahpF) | Alkyl hydroperoxide reductase subunit F | 0.57 | 8.69 |
| AOLE_15340 (trxB) | Thioredoxin-disulfide reductase | 1.19 | 3.64 |
| AOLE_13410 (ahpF) | Alkyl hydroperoxide reductase subunit F | 0.57 | 7.62 |
| AOLE_12135 (soxR) | Redox-sensitive transcriptional activator SoxR | 3.61 | 1.78 |
| AOLE_19220 | Thiol-disulfide isomerase and thioredoxin | 1.51 | 2.21 |
| AOLE_14365 | Rubredoxin | 1.78 | 1.57 |
| AOLE_02585 | Thioredoxin 2 | 2.56 | 1.72 |
| AOLE_14370 | Rubredoxin-NAD+ reductase | 3.27 | 1.99 |
| AOLE_07375 (recA) | Recombinase A | 1.47 | 1.79 |
| AOLE_08120 | Glutaredoxin-like protein | 1.26 | 1.52 |
| AOLE_07635 | Thioredoxin | 0.34 | 2.16 |
| AOLE_00700 (rimK) | Glutathione synthetase | 1.26 | 2.59 |
| AOLE_05015 | Lactoylglutathione lyase | 2.13 | 0.77 |
| AOLE_06195 | Hydroxyacylglutathione hydrolase | 3.65 | 0.83 |
| AOLE_00075 (gst) | Glutathione S-transferase | 0.71 | 4.52 |
| AOLE_11225 (gst) | Glutathione S-transferase | 0.87 | 4.43 |
| AOLE_11605 (gst) | Glutathione S-transferase | 1.71 | 3.51 |
| AOLE_13455 (gst) | Glutathione S-transferase | 1.89 | 3.63 |
| AOLE_16495 (gst) | Glutathione S-transferase | 1.47 | 2.40 |
| AOLE_18655 (gst) | Glutathione S-transferase | 1.92 | 4.62 |
Identification of AoSoxR Target Genes
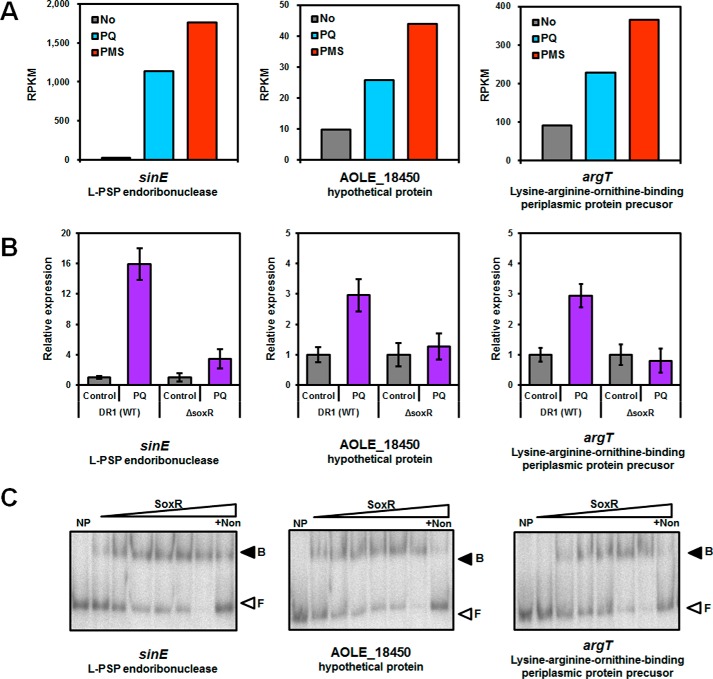
To identify SoxR-regulated genes, we used the A. oleivorans genome and transcriptome data indicating possible SoxR binding sites (24, 31–33). Although five possible candidate promoters (AOLE_16635 encoding a putative L-PSP endoribonucleases, AOLE_18450 encoding a membrane protein, argT, scpA, and hisZ) were found using a putative SoxR binding site, two of them (scpA and hisZ) were not induced by RACs. These first three genes were up-regulated by PQ and PMS treatment (Fig. 3A). AOLE_16635 is one of the genes that is most highly expressed by PQ and PMS (42- and 65-fold, respectively, supplemental Table S2). Interestingly, the AOLE_16635 gene belongs to one of five gene categories that SoxR might control in non-enteric bacteria (33): dehydrogenases, oxygenases, putative L-PSP endoribonucleases, methyl/acetyl transferases, and exporters. The first three of these genes were selected, and expression was confirmed by quantitative reverse transcriptase-PCR (qRT-PCR). RAC-induced expression of these three genes diminished in the soxR mutant (Fig. 3B). The qRT-PCR analysis also confirmed that the genes scpA and hisZ were not induced by RACs (data not shown). Induction of genes encoding a putative L-PSP endoribonucleases (hereafter referred to as sinE (SoxR-induced endoribonuclease)) in the soxR mutant was reduced but not completely abolished (Fig. 3B) with PQ. However, expression analysis of sinE in the presence of other RACs (PMS, PL, and MDs) confirmed that sinE expression is controlled by SoxR (supplemental Fig. S1B), even if some proportion of sinE expression is SoxR-independent.
FIGURE 3.
Gene expression analysis obtained from RNA-seq and qRT-PCR and binding of SoxR to the promoter region of target genes. A, comparison of gene expression patterns obtained from the RNA-seq analysis of exponentially growing wild type cells and PQ- or PMS-treated cells. The y axis shows RPKM values of each sample. B, relative expression of SoxR-controlled genes in the wild type and soxR mutant by qRT-PCR. The cells were grown to the exponential phase (A600 ∼0.4) and treated with paraquat (1 mm) for 15 min. All data represent the average of three replicates, and the error bars indicate S.D. C, binding of SoxR to the promoter region of target genes. Lane 1, free DNA (no protein); lane 2, purified SoxR, 3 nm; lane 3, purified SoxR, 7 nm; lane 4, purified SoxR, 13 nm; lane 5, purified SoxR, 25 nm; lane 6, purified SoxR, 40 nm; lane 7, purified SoxR, 70 nm; lane 8, purified SoxR, 70 nm with non-probing DNA. The nonspecific competitor poly(dI-dC) was added to all binding reactions. Protein-DNA complexes (B) and free DNA probes (F) are indicated with filled and open arrowheads, respectively.
To demonstrate direct regulation of three candidate genes by SoxR, we overproduced and purified SoxR. AoSoxR protein aggregation was observed during experiments. To enhance the solubility and stability of SoxR, appropriate additives (glycine betaine, proline, l-arginine, dipotassium phosphate, and cetyltrimethylammonium bromide) were used for purification steps. More soluble and stable SoxR proteins were successfully purified in the presence of glycine betaine (supplemental Fig. S2A). Purified SoxR exhibited a red-brown color and the absorption spectrum showed the characteristics of [2Fe-2S] proteins (34, 35). These could not be observed using purified SoxR with the reducing agent β-mercaptoethanol (supplemental Fig. S2B). To investigate whether SoxR binds to the promoter regions of the target genes, we conducted an electrophoretic mobility shift assay (EMSA) using DNA probes (PsinE, PAOLE_18450, and PargT) with purified SoxR protein. SoxR retarded the migration of the three DNA probes, suggesting that the SoxR binding site might lie in promoter-specific regions of the three target genes (Fig. 3C). To ensure that the binding of SoxR to the DNA probe was specific, the nonspecific competitor poly(dI-dC) was added to all binding reactions. The addition of excess unlabeled probe abolished SoxR binding to the labeled fragments. This finding suggests that AoSoxR regulates the expression of the sinE, AOLE_18450, and argT genes by binding directly to their promoter regions.
Effective Concentration Range of RACs for the Induction of sinE
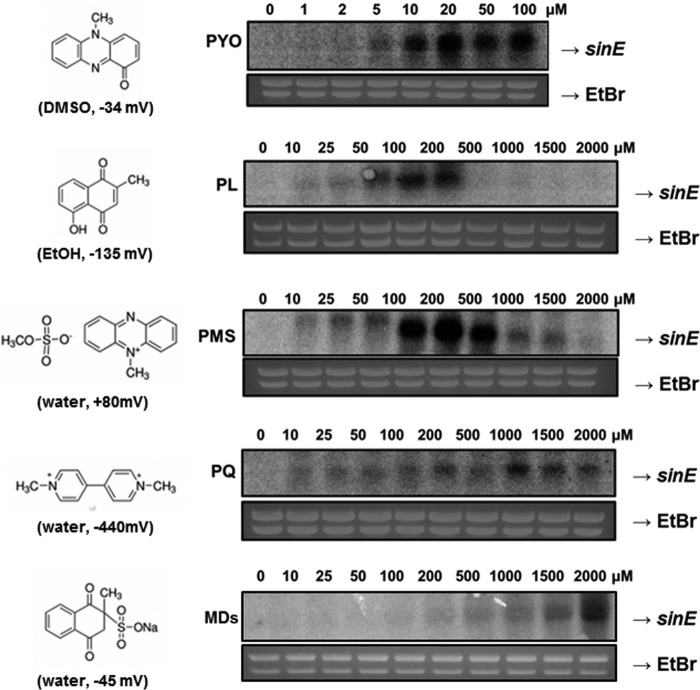
To understand the activation of AoSoxR for the induction of sinE expression, the effective concentration ranges of diverse RACs were determined (Fig. 4). AoSoxR can be activated easily by PYO and PL, which may pass through cell membranes because of their non-polarity and small size. As little as 5 μm PYO, a natural RAC produced by P. aeruginosa, induced sinE maximally at 20 μm. Another natural RAC, PL, led to expression of sinE in a narrow range of concentrations, with maximal expression at 10–200 μm. Interestingly, other RACs, such as PMS, PQ, and MDs, have a broad concentration range, and the maximal activation level increased from 100 to 500 μm for PMS, 1000 μm for PQ, and 2000 μm for MDs (Fig. 4). These three RACs are charged and highly water-soluble. The redox potentials of all RACs individually could not explain the differential sensitivity of AoSoxR to various RACs. Unlike ScSoxR, which could not sense PQ (20), AoSoxR was activated by PQ in a wide concentration range, with maximal expression between 1 and 1.5 mm (Fig. 4). It is unclear why high concentrations of PL and PMS treatment were not sufficient to induce sinE gene. This pattern was also reported when S. coelicolor was exposed to high concentrations of RACs (20). High concentrations of these chemicals may impose transcriptional defects, or they may trigger the degradation of the SoxR Fe-S cluster beyond its functional oxidized form.
FIGURE 4.
Ranges of RAC concentrations for AoSoxR activation. Exponentially growing cells (A600 ∼0.4) were treated with various concentrations of RACs for 15 min. To evaluate SoxR activation, its direct target gene (sinE) was selected and subjected to gene expression analysis. The solvents and reduction potentials of the RACs are indicated in parentheses.
SinE Functioning as a Ribonuclease and Inhibiting Protein Synthesis
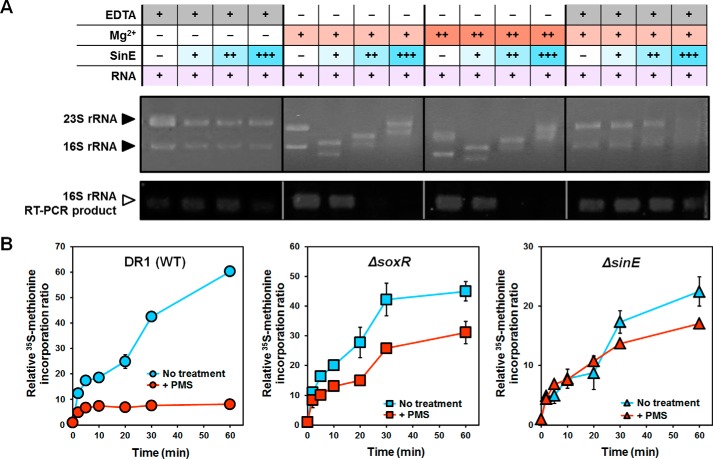
The protein sequences of L-PSP endoribonucleases are highly conserved in prokaryotes (supplemental Table S3). However, the function of L-PSP endoribonucleases, including SinE, has not been characterized in bacteria. SinE homologs with consensus SoxR binding sites in their promoter regions were found in only a few proteobacteria (supplemental Fig. S3). To determine the function of SinE, we overproduced and purified SinE (supplemental Fig. S2C). A previous mammalian L-PSP study demonstrated that L-PSP cleaves phosphodiester bonds in the single-stranded regions of the 5′- or 3′-32P-labeled 5S rRNA (36). Total RNA was extracted from A. oleivorans cells and reacted with purified SinE. SinE could bind rRNA and degrade rRNA in the presence of Mg2+ (Fig. 5A). Our RT-PCR analysis targeting 16S rRNA failed when total RNAs were treated only with SinE and Mg2+, confirming that SinE degrades rRNA with Mg2+. This rRNA degradation capability of SinE was lost when a metal chelator, EDTA, was added to the mixture, showing that SinE requires Mg2+ for its activity (Fig. 5A).
FIGURE 5.
Function of SinE as a ribonuclease and inhibition of protein synthesis. A, in vitro RNase activity of SinE. Total RNA (1.5 μg) isolated from A. oleivorans DR1 was incubated with SinE (8–40 μm) and MgCl2 (2.5–5 mm) for 1 h at 30 °C. Reaction mixtures were loaded onto denaturing agarose gels and then stained with ethidium bromide to visualize 23S and 16S rRNA. EDTA (50 mm) was used to inhibit RNase activity. To compare residual 16S rRNA after reaction with SinE, semiquantitative real-time PCR was conducted. B, comparison of protein synthesis between the wild type and the soxR and sinE mutant under PMS treatment. The cells were grown to exponential phase (A600 ∼0.2) in 10-fold diluted nutrient medium and treated with PMS (10 μm). To measure protein synthesis, cells were labeled with 5 μCi/ml [35S]methionine. At different time intervals, each sample was mixed with liquid mixture and measured in a scintillation counter. Radioactivity was normalized by the A600 and expressed as a relative ratio, taking the radioactivity of 0 min samples to be 1. All data show the average of three replicates, and the error bars indicate S.D.
We hypothesized that the RNase activity of SinE might affect protein synthesis because rRNA degradation ability of SinE could give rise to low translation efficiency. Our previous transcriptome analyses also showed that genes involved in translation, including those that contribute to ribosome structure and biogenesis, are repressed upon exposure to RACs, which might be due to SoxR-induced SinE activity. To test this hypothesis, a protein synthesis assay was performed using [35S]methionine. The addition of PMS inhibited [35S]methionine incorporation partly because sinE was induced by PMS treatment (Fig. 5B). However, when either soxR or sinE was deleted, the inhibition of protein synthesis by PMS treatment was not as severe as that of wild type cells (Fig. 5B). Taken together, SoxR-induced SinE could affect protein synthesis in the presence of PMS due to its ribonuclease activity, which brought our attention to examining the role of SinE under RACs because inhibition of protein synthesis could induce cell survival against RACs or antibiotics (37, 38).
Resistance by SoxR-regulated SinE against RACs and Antibiotics
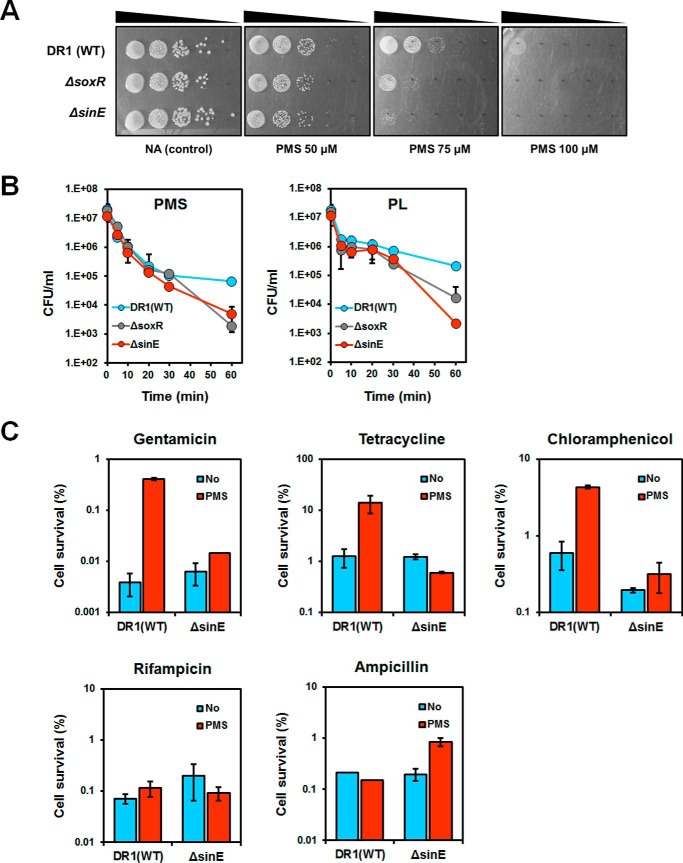
We have shown that activation of AoSoxR has a protective effect against RACs (Fig. 2). Additionally, the soxR and sinE mutants showed growth defects when cultured with RACs on agar plates (Fig. 6A) and greater sensitivity to RACs (PMS and PL) than wild type cells in liquid media (Fig. 6B). We considered that induction of SinE might be important for persistence in the presence of various protein synthesis-inhibiting antibiotics because those might not work when cells stop protein synthesis. We compared the survival of cells with or without induction of SinE in the presence of different antibiotics. Interestingly, PMS-pretreated cells showed increased numbers of persisters in the presence of gentamicin, tetracycline, and chloramphenicol (Fig. 6C). However, no significant changes were observed in the presence of other classes of antibiotics, including ampicillin and rifampicin. Thus, SoxR-regulated SinE plays an important role in cell survival by generating persisters upon exposure to high concentrations of protein synthesis-inhibiting antibiotics. We found that the sinE mutant showed a mucoid phenotype (supplemental Fig. S4A). The genome of A. oleivorans DR1 contains exopolysaccharide (EPS) operons: poly-N-acetyl glucosamine operons and the K locus (39). The expression of genes involved in the EPS complex was increased by >3.0-fold in the sinE mutant (supplemental Fig. S4B). Thus, the sinE mutant might increase EPS production to protect itself under stresses.
FIGURE 6.
Comparison of RAC and antibiotic sensitivity in the wild type and soxR and sinE mutants. A, PMS sensitivity assay. Cells were grown overnight in nutrient medium and subsequently diluted 100-fold. The cells reached the mid-exponential phase (A600 ∼0.6), and serially diluted cells were spotted on nutrient agar with or without PMS. B, survival of cells after treatment with PMS or PL. Exponential phase cells were harvested, and ∼107 cells/ml were inoculated into fresh PBS (5 ml) containing 100 μm PMS or PL. At each time point, the cells were harvested and washed in PBS. The number of viable cells was determined by counting the cfu. C, effect of induced SinE on antibiotic resistance. PMS (25 μm) was added to cells in the exponential phase for 30 min. Cells were washed twice to remove PMS and inoculated into fresh nutrient broth with antibiotics (gentamicin, 5 μg/ml; tetracycline, 5 μg/ml; chloramphenicol, 100 μg/ml; ampicillin, 1000 μg/ml; rifampicin, 10 μg/ml). After 3 h of incubation, the number of viable cells was determined by counting the cfu. All data show the average of three replicates, and the error bars indicate S.D.
Discussion
Notably, different RACs have their own optimal effective concentrations and different cellular response times for sinE induction. SoxR-binding sites in the promoter region of soxS from E. coli and other known target genes from P. aeruginosa, S. coelicolor, and A. oleivorans DR1 are similar to one another (17, 18, 20, 33). However, the activation behavior of SoxRs may differ across bacterial phyla. It has been suggested that SoxR from enteric bacteria can sense a wider range of RACs than those from P. aeruginosa and S. coelicolor because enteric SoxR is more easily oxidized by RACs because of a difference in redox potential (19). Singh et al. (20) demonstrated that both kinetic reactivity of the iron-sulfur cluster and redox potential in SoxR are important for determining the differential selectivity of SoxR when bacteria are exposed to oxidants. PaSoxR and EcSoxR can sense a larger number of RACs than SoxR from S. coelicolor (ScSoxR). In this study, AoSoxR was activated by all tested RACs, although it responded slowly to PQ and MDs (Figs. 1 and 4). This finding indicates that AoSoxR may have a low redox potential compared with ScSoxR or show a similar redox potential as EcSoxR and PaSoxR, based on the activation profiles of each SoxR (19–21). Recently, it was reported that specific amino acid residues in SoxR determine redox potential and differential sensitivity to RACs (19, 21). The corresponding region containing critical residues in AoSoxR is 128LEQCP132 (underlining indicates two important residues); this region may act as a sensor for determining the differential sensitivities and physiological responses observed in E. coli, P. aeruginosa, and S. coelicolor (19, 21). Our findings also indicate that the oxidation and activation behaviors differed among SoxRs from A. oleivorans, P. putida, and E. coli according to the types and concentrations of RACs. The differential sensitivity of each SoxR may be an evolutionary consequence that reflects the various habitats of microorganisms.
The level of soxS′::lacZ expression by AoSoxR in response to PQ and MDs found in the E. coli reporter assay was different from the sinE induction pattern observed in the Northern blotting assay (Figs. 1B and 4). The gradual induction of sinE reached its maximum at 1 mm during a 15-min exposure to PQ (Fig. 4), and the expression of sinE became greater when PQ exposure time increased (supplemental Fig. S5A). However, AoSoxR in E. coli was dramatically activated at 0.1 mm PQ by a 1-h treatment (Fig. 1B). These data indicated that it takes a different amount of time for PQ, a charged compound, to enter into different cells. MDs at 0.1 mm activated AoSoxR highly in E. coli, in contrast to the lack of effect observed in the Northern blotting assay (Fig. 4). These differences might result from the number of soxR genes because a high copy number of plasmids was apparent when AoSoxR was expressed in E. coli. When a high copy number of soxR was introduced into A. oleivorans, MDs activated SoxR even at low concentrations that did not activate the wild type strain (supplemental Fig. S5B), indicating that the number of SoxR in cells gives rise to its sensitivity to RACs. It is worth noting that different RACs induce the expression of soxS to different degrees (Table 1), which might reflect the different sensitivities of each SoxR to RACs in other microorganisms.
A comprehensive bioinformatics study of bacterial genomes revealed that non-enteric bacteria, which lack the SoxS homolog, contained SoxR binding sites on the promoter regions of five categorized genes: dehydrogenases, oxygenases, transporters, L-PSP endoribonucleases, and putative acetyl- or methyltransferases (33). Although L-PSP endoribonuclease contains SoxR-binding sites in its promoter region in α- and β-proteobacteria and non-enteric γ-proteobacteria (33) (supplemental Table S3), the regulation of L-PSP endoribonuclease by SoxR and its function have not been characterized in bacteria. L-PSP endoribonuclease is a member of the YjgF/YER057c/UK114 family of proteins present in bacteria, archaea, and eukaryotes and defines the pfam 01042 group (supplemental Table S3). The protein sequences of L-PSP endoribonucleases are highly conserved in prokaryotes, indicating that the protein may be involved in a basic cellular process (36). However, SinE homologs with consensus SoxR binding sites in their promoter regions were found in only a few proteobacteria (supplemental Fig. S3 and supplemental Table S3). The SinE homolog (PP_3522) of P. putida KT2440 (PpSinE), which increased 2.43-fold in the presence of PQ, might have the SoxRbox in its promoter region, but there was no difference in the expression of the SinE homolog (PA4173) from P. aeruginosa (PaSinE) because the SoxR binding site in the promoter region of PA4173 was absent, as determined in our previous transcriptome analyses (NCBI GEO site under accession number GSE78230 (1 mm PQ treatment in P. aeruginosa) and GSE34409/GSE34410 (1 mm PQ treatment in P. putida KT2440)). This implies that PpSinE might be controlled by SoxR, whereas PaSinE is not. Interestingly, the SinE homolog is located next to the soxR gene in β-proteobacteria, which strongly indicates that their sinE genes might also be regulated by SoxR (supplemental Fig. S3). The L-PSP protein causes the disaggregation of polysomes when added to a rabbit reticulocyte cell-free system (36). This effect is comparable with the inhibition of translation by the heme-regulated eukaryotic initiation factor 2α kinase (40). [α-32P]UTP-labeled mRNA incubated with the L-PSP protein provides direct evidence that the protein is an endoribonuclease (36). L-PSP cleaved phosphodiester bonds in the single-stranded regions of the 5′- or 3′-32P-labeled 5S rRNA (36). These activities are responsible for translation inhibition. Vercruysse et al. (41) suggested that L-PSP endoribonuclease is non-essential, based on the availability of mutants, but that it plays a role in mRNA metabolism.
The stability of numerous transcripts can be regulated by RNase E via degradosome assemblage (42). There were no significant differences in the expression of RNase E (encoded by AOLE_17430) with the two RAC treatments and the presence or absence of SoxR (supplemental Fig. S6A). Among the 22 genes coding for proteins annotated as ribonuclease in A. oleivorans DR1, sinE was exclusively induced by the two RACs, and its expression was soxR-dependent. The expression of sinE was up-regulated 2.8-fold in the presence of soxR in our transcriptome analysis of the wild type and soxR mutant harboring the SinE overexpression vector with PMS treatment (supplemental Table S4). SinE may be involved in transcript stability, similar to RNase E, under RAC-originated oxidative stress. To identify other SinE-targeted mRNA, multiple transcriptome analyses were performed. However, we failed to find an additional target. Density plots showing the distribution of reads per kilobase of transcript per million mapped sequence reads (RPKM) values obtained from each condition showed similar patterns (supplemental Fig. S6B), suggesting that SinE might have only a few targets. If SinE degraded a broad spectrum of mRNA, the RPKM distributions would have been different from each other. Among differentially expressed genes in our transcriptome analysis, we found 121 genes that were expressed at a low level in wild type cells harboring the SinE overexpression vector compared with the soxR mutant containing the same vector with PMS treatment (supplemental Table S4). These genes showing low levels of expression may also show reduced transcript levels by SoxR-induced SinE with RAC treatment.
The SoxRS system in E. coli controls over 100 genes whose products can repair redox stress-induced intracellular damage and reestablish redox homeostasis (43). Numerous SoxRS regulons function to eliminate RACs from the intracellular space in a variety of ways (12, 19), such as by blocking, exporting, and chemically modifying redox stress compounds (12). Some parts of the SoxRS regulon can supplement the NADPH pools and repair cell damage, such as inactivated enzymes and DNA lesions (12, 44). SoxR in non-enteric bacteria can activate its regulon directly without the secondary transcription factor SoxS. The PaSoxR and ScSoxR regulons consist of membrane transporters and monooxygenase (14, 17, 18, 45). Both regulons are induced by endogenously generated RACs (phenazine produced by P. aeruginosa and actinorhodin produced by S. coelicolor), indicating that SoxR evolved to detect metabolites and process them for transport or removal (19). The role of AoSoxR is presumably to protect cells from RACs released by other microbes in the environment or Acinetobacter itself even if secondary metabolites of Acinetobacter have not been examined. Our transcriptome analysis showed that the expression of AOLE_16640 (encoding a major facilitator superfamily protein) was increased in the presence of either PQ or PMS (34- and 22-fold, respectively); 33 genes related to transport systems were up-regulated >2.0-fold by RACs (supplemental Table S2). Among them, AOLE_16640 (co-transcribed with sinE) and AOLE_05515 were directly regulated by activated SoxR. It was recently reported that the abuO gene encoding a TolC-like outer membrane protein and the abeD gene encoding a RND-type membrane transporter of A. baumannii AYE were directly regulated by SoxR (31, 32). A putative outer membrane protein (AOLE_18125) that showed 94% amino acid identity with AbuO was up-regulated 4.1-fold with PMS treatment; however, there was no difference in the presence of PQ in our transcriptome analyses. A multidrug resistance protein (AOLE_03935) corresponding to the abeD gene in A. oleivorans DR1 (96.5% amino acid identity) was not expressed in the presence of RACs. Several oxygenases that may modify RACs showed 10-fold increased expression with RAC treatment (supplemental Table S2). When A. oleivorans DR1 was exposed to exogenous RACs or when endogenous redox-active secondary products accumulated, these highly expressed transport systems and oxygenases may have contributed to reducing the effects of redox stress. Although there are differences in the SoxR regulon members among organisms, mitigation of RAC-originated stress may be the ultimate goal of SoxR or SoxRS regulation.
SinE may play a critical role in the response to RACs and antibiotics. We found that the sinE mutant, which was sensitive to RACs and antibiotics, showed a mucoid phenotype and higher expression of genes involved in EPS production (supplemental Fig. S4). SinE could degrade rRNA and inhibit protein synthesis in the presence of RAC. Arrested protein synthesis driven by SinE might contribute to cell protection by inducing temporal increase of cell survival under RACs stress. The comprehensive approaches used in this study suggest that SinE is a SoxR-induced endoribonuclease and that it can play important roles in protecting against toxic chemicals and reducing redox stress in A. oleivorans DR1. However, RNase specificity of SinE and its additional targets remain to be solved so that their roles in cell protection under RAC stress may be understood. Our results contribute to an understanding of the physiology and ecology of soil microorganisms used for bioremediation processes in which stress induced by RACs is an important challenge.
Experimental Procedures
Bacterial Strains, Plasmids, and Culture Conditions
Bacterial strains and plasmids used in this study are shown in supplemental Table S5. A. oleivorans DR1 was grown at 30 °C in nutrient broth with aeration via shaking. E. coli was grown at 37 °C in Luria-Bertani (LB) medium. When required, antibiotics were added at the following concentrations: 50 μg/ml kanamycin, 20 μg/ml tetracycline, and 100 μg/ml ampicillin. Growth was monitored by measuring A600 of cultures using a biophotometer (Eppendorf, Hamburg, Germany). The complete genome sequence of A. oleivorans DR1 can be found in GenBankTM (accession no. CP002080). A. oleivorans DR1 was deposited in the Korea Collection for Type Cultures (accession number 23045) and the Japan Collection of Microorganisms (accession number 16667).
Chemical Treatments
The following chemicals were purchased from Sigma: PQ, MDs, PMS, PL, PYO, and CHP. H2O2 was purchased from Junsei. PQ, MDs, PMS, and H2O2 were dissolved in distilled water. PL and CHP were dissolved in ethanol. PYO was dissolved in dimethyl sulfoxide. Solvent effects can generally be ignored because they are not significantly affected by changes in the solvent.
Construction of the E. coli soxR Mutants Expressing AoSoxR, PpSoxR, or EcSoxR
For the complementation analysis in E. coli, SoxR regions from A. oleivorans DR1, P. putida, and E. coli were amplified using primers (supplemental Table S5; pCR2.1 DR1 SoxR-F/R for AoSoxR amplification, pCR2.1 P. putida SoxR-F/R for PpSoxR amplification, pCR2.1 E. coli SoxR-F/R for EcSoxR amplification) and cloned into the pCR2.1-TOPO vector (Invitrogen). The constructed vectors were transformed into E. coli strain EH46 (ΔsoxRS, soxS′::lacZ) for β-galactosidase assays.
Construction of Mutants and the Overexpression Strain
The internal region of the soxR gene was amplified using the DR1 soxR SC-F and R primers. The PCR product for the soxR mutant was digested using EcoRI and KpnI restriction enzymes. Each fragment was subsequently inserted into a pVIK112 vector via ligation (46). The constructed plasmids were then transformed into E. coli S17–1λpir, and pVIK112-soxR was transformed into A. oleivorans DR1. The soxR mutant strain was confirmed by PCR.
To inactivate the sinE gene, a kanamycin cassette (Km) was inserted. The regions upstream and downstream of the sinE gene were amplified using sinE (AOLE_16635)-NDK1-2/NDK2 and sinE (AOLE_16635)-CDK1/CDK2-2 primer pairs, respectively. The amplified fragments were cloned into pCR2.1-TOPO, creating pCR2.1-sinE. The Km cassette from pUC4K (47) was subcloned into the BamHI cloning site of pCR2.1-sinE, forming pCR2.1-sinE::Km. The plasmid was digested with EcoRI/KpnI to isolate the sinE::Km cartridge, which was ligated to pCVD442 (48), generating pCVD442-sinE::Km. This constructed plasmid was introduced by electroporation into E. coli S17–1λpir. pCVD442-sinE::Km was transformed into A. oleivorans DR1. The sinE mutant strain was confirmed by both PCR verification and sequencing of the PCR product. Full-length soxR and sinE with their own promoter regions were obtained from A. oleivorans DR1 using the soxR OE-F/R PCR primer pair and sinE (AOLE_16635) OE-F/R PCR primer pair. The PCR product was digested and then ligated into pRK415, yielding pRK415-soxR and pRK415-sinE. The constructed plasmids were introduced into A. oleivorans DR1.
β-Galactosidase Assay
To measure complementation in an E. coli soxR mutant, we conducted a β-galactosidase assay. EH46 strains were grown in LB medium to an A600 of 0.3 and then treated with chemicals for 1 h at 37 °C. β-Galactosidase activity was measured using o-nitrophenyl-β-d-galactopyranoside as a substrate (49). β-Galactosidase activity by EH46 harboring pCR2.1-TOPO (empty vector) is not shown in Fig. 1 because there was no difference between the untreated and treated conditions (range of Miller units: 27–35).
RNA Extraction, Library Construction, and Sequencing
The cells were grown to the exponential phase (A600 ∼0.4) and treated with PQ (1 mm) or PMS (0.2 mm) for 15 min. A. oleivorans DR1 and the soxR mutant harboring the pRK415-sinE plasmid were treated with 50 μm PMS during the exponential phase. Total RNA was isolated from 5 ml of cells using the RNeasy minikit (Qiagen) according to the manufacturer's instructions. All procedures for RNA sequencing and alignment of the transcriptome were conducted by Chunlab (Seoul, South Korea). The RNA was subjected to a subtractive Hyb-based rRNA removal process using the MICROBExpress bacterial mRNA enrichment kit (Ambion). Following this process, a library was constructed as described previously (50). RNA sequencing was performed with two runs of the Illumina genome analyzer IIx to generate single-ended 100-bp reads. Genome sequence and annotation information of A. oleivorans DR1 were obtained from the NCBI database (accession number NC_014259.1). Quality-filtered reads were aligned to the reference genome sequence using CLC Genomics Workbench version 6.5.1 (CLC bio). Mapping was based on a minimal length of 100 bp with an allowance of up to two mismatches. Relative transcript abundance was measured in RPKM (51). The genes showing a -fold change (RPKM of DR1-PQ or PMS/RPKM of DR1-exp) >2.0 and <0.5 were regarded as up-regulated and down-regulated genes, respectively. Mapped reads were visualized using the BamView and Artemis programs (52). RNA-seq data were deposited in the NCBI Gene Expression Omnibus site under accession numbers GSE44347 (grown to the exponential and stationary phase in NB medium) and GSE70356 (1 mm PQ and 0.2 mm PMS treatment).
Determination of the Consensus SoxR-binding Sequence of Acinetobacter Species
To identify AoSoxR target genes, we determined the consensus SoxR-binding sequence from the statistically predicted SoxR homolog-binding sequences of the Acinetobacter species A. baumannii ATCC 17978, A. baumannii AYE, A. baumannii, and Acinetobacter sp. ADP1(31–33). The Acinetobacter SoxR-binding consensus sequence was constructed as 5′-TTGACYTCAASTKAASTTKARSTTKS-3′ (where Y indicates C or T; S indicates G or C; K indicates G or T; and R indicates G or A). We searched for the consensus sequence of Acinetobacter SoxR binding sites in the complete genome of A. oleivorans DR1.
Gene Expression Analysis by qRT-PCR
Total RNA was isolated from 5 ml of cells using an RNeasy minikit according to the manufacturer's instructions. cDNA was synthesized from 1 μg of RNA with primers for the target gene and used as a template for qRT-PCR. The PCR mixture contained 12.5 μl of iQ SYBR Green Supermix (Bio-Rad), 1 μl of each primer (0.5 μm), and 2 μl of cDNA in a total volume of 25 μl. The PCR conditions were 95 °C for 3 min, followed by 40 cycles of 45 s at 95 °C, 45 s at 60 °C, and 45 s at 72 °C. To normalize the expression of each gene, the expression level of 16S rDNA was quantified with primers used previously (53). The results were determined from triplicate experiments.
Protein Overexpression and Purification
The SoxR and SinE regions were amplified, and the fragment was cloned into the pET-28a(+) vector. The constructed vector was conjugated to E. coli Top10 and transformed into E. coli BL21 (DE3) for overexpression. The cells were induced by adding 0.5 mm isopropyl β-d-1-thiogalactopyranoside for 3 h at 30 °C. Protein purification was conducted as described previously (30). All purification steps were performed at 4 °C using an FPLC system (AKTA FPLC, Unicorn version 4.0, Amersham Biosciences) with a nickel-nitrilotriacetic acid column (1 ml; HisTrap, Amersham Biosciences). SDS-PAGE was performed using 12% polyacrylamide gels to determine the level of expression and for purification. Absorbance spectra were recorded with a Mecasys Optizen Pop UV-visible spectrophotometer.
EMSA
The EMSA was conducted as described previously (30). PCR products were dephosphorylated and labeled with [γ-32P]ATP and T4 polynucleotide kinase. Reaction mixtures (20-μl final volume each) containing ∼3 fmol of DNA probe, protein, and loading buffer in 5× binding buffer (50 mm Tris, pH 7.5, 10 mm MgCl2, 50% glycerol (v/v), 10 mm DTT, and 375 mm KCl) were analyzed by electrophoresis on 5% polyacrylamide gels in a 0.5× Tris borate/EDTA buffer (1.1 m Tris, 900 mm borate, 25 mm EDTA, pH 8.3). To ensure that the binding of protein to the DNA probe was specific, the nonspecific competitor poly(dI-dC) (1 μg) and non-probing DNA (5-fold molar excesses) were added to binding reactions.
Gene Expression Analysis by Northern Blotting
Total RNA was isolated using an RNeasy kit according to the manufacturer's instructions. A Northern blotting analysis was then performed as described previously (54). RNA concentrations were estimated by measuring the absorbance at 260 nm. Samples of total RNA (2.5 μg) were loaded onto denaturing agarose gels containing 0.25 m formaldehyde, separated, and then stained with ethidium bromide to visualize 23S and 16S rRNA. The fractionated RNA was transferred to nylon membranes (Schleicher and Schuell, Dassell, Germany) using a TurboBlotter (Schleicher and Schuell). The amount of mRNA was determined by hybridizing the membrane with a specific 32P-labeled probe (Takara, Tokyo, Japan) prepared by PCR amplification with the respective primer pairs. Autoradiography was conducted using an IP plate (Fujifilm, Japan) and a multiplex bio-imaging system (Fujifilm, Tokyo, Japan).
In Vitro RNase Assay
An RNase assay was conducted as described by Vercruysse et al. (41). Reactions were carried out in 50 mm Tris-Cl (pH 7.5) in a 20-μl volume. Total RNA (1.5 μg) isolated from A. oleivorans DR1 was incubated with purified SinE (8–40 μm) and MgCl2 (2.5–5 mm) for 1 h at 30 °C. Reaction mixtures were loaded onto denaturing agarose gels and stained with ethidium bromide to visualize 23S and 16S rRNA. EDTA (50 mm) was used to inhibit RNase activity. To compare residual 16S rRNA after the reaction with SinE, semiquantitative RT-PCR was conducted.
Measurement of Total Protein Synthesis Rates
The cells were grown to the exponential phase (A600 ∼0.4) and treated with PMS (25 μm) for 30 min. These cells were collected by centrifugation and resuspended in MSB medium without methionine. To measure protein synthesis, these cells were labeled with 5 μCi of [35S]methionine (PerkinElmer Life Sciences). At different time intervals, each sample was mixed with liquid mixture, and the intensity was measured with a scintillation counter. Radioactivity was expressed as a relative ratio, taking the radioactivity of 0 min samples to be 1.
Susceptibility Tests
Wild type and mutant strains were grown overnight in nutrient broth and subsequently diluted 100-fold. The cells reached mid-exponential phase (A600 ∼0.6), and serially diluted cells were spotted on nutrient agar with or without antibiotics and PMS. For the survival test, 100 μm PMS or PL was used. Exponential phase cells were harvested and washed two times with PBS (pH 7.4). Approximately 107 cells/ml were inoculated into fresh PBS (5 ml) containing PMS or PL. At each time point, the cells were harvested and washed in PBS. The number of viable cells was determined by counting the cfu.
Author Contributions
J. K. and W. P. designed and coordinated the study. J. K. and C. P. performed the experiments and collected the data. J. K. wrote the first complete draft of the manuscript. J. A. I. and W. P. provided substantial modifications, and all authors contributed to and approved the final version of the manuscript.
Supplementary Material
This work was supported by National Research Foundation of Korea (NRF) Grant NRF-2014R1A2A2A05007010 funded by the Korean government (MSIP) (to W. P.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.

This article contains supplemental Figs. S1–S6 and Tables S1–S5.
- ROS
- reactive oxygen species
- RAC
- redox-active compound
- PYO
- pyocyanin
- PL
- plumbagin
- PMS
- phenazine methosulfate
- PQ
- paraquat
- MDs
- menadione sodium bisulfite
- CHP
- cumene hydrogen peroxide
- EPS
- exopolysaccharide
- RPKM
- reads per kilobase of transcript per million mapped sequence reads
- qRT-PCR
- quantitative RT-PCR
- RNA-seq
- RNA sequencing
- L-PSP
- liver perchloric acid-soluble protein.
References
- 1. Dietrich L. E., and Kiley P. J. (2011) A shared mechanism of SoxR activation by redox-cycling compounds. Mol. Microbiol. 79, 1119–1122 [DOI] [PubMed] [Google Scholar]
- 2. Gu M., and Imlay J. A. (2011) The SoxRS response of Escherichia coli is directly activated by redox-cycling drugs rather than by superoxide. Mol. Microbiol. 79, 1136–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Imlay J. A. (2015) Transcription factors that defend bacteria against reactive oxygen species. Annu. Rev. Microbiol. 69, 93–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okegbe C., Sakhtah H., Sekedat M. D., Price-Whelan A., and Dietrich L. E. (2012) Redox eustress: roles for redox-active metabolites in bacterial signaling and behavior. Antioxid. Redox Signal. 16, 658–667 [DOI] [PubMed] [Google Scholar]
- 5. Ding H., Hidalgo E., and Demple B. (1996) The redox state of the [2Fe-2S] clusters in SoxR protein regulates its activity as a transcription factor. J. Biol. Chem. 271, 33173–33175 [DOI] [PubMed] [Google Scholar]
- 6. Hidalgo E., Ding H., and Demple B. (1997) Redox signal transduction: mutations shifting [2Fe-2S] centers of the SoxR sensor-regulator to the oxidized form. Cell 88, 121–129 [DOI] [PubMed] [Google Scholar]
- 7. Ding H., and Demple B. (2000) Direct nitric oxide signal transduction via nitrosylation of iron-sulfur centers in the SoxR transcription activator. Proc. Natl. Acad. Sci. U.S.A. 97, 5146–5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pomposiello P. J., and Demple B. (2000) Identification of SoxS-regulated genes in Salmonella enterica serovar typhimurium. J. Bacteriol. 182, 23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martin R. G., and Rosner J. L. (2001) The AraC transcriptional activators. Curr. Opin. Microbiol. 4, 132–137 [DOI] [PubMed] [Google Scholar]
- 10. Martin R. G., and Rosner J. L. (2003) Analysis of microarray data for the marA, soxS, and rob regulons of Escherichia coli. Methods Enzymol. 370, 278–280 [DOI] [PubMed] [Google Scholar]
- 11. Pomposiello P. J., and Demple B. (2001) Redox-operated genetic switches: the SoxR and OxyR transcription factors. Trends Biotechnol. 19, 109–114 [DOI] [PubMed] [Google Scholar]
- 12. Imlay J. A. (2013) The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat. Rev. Microbiol. 11, 443–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kobayashi K., and Tagawa S. (2004) Activation of SoxR-dependent transcription in Pseudomonas aeruginosa. J. Biochem. 136, 607–615 [DOI] [PubMed] [Google Scholar]
- 14. Palma M., Zurita J., Ferreras J. A., Worgall S., Larone D. H., Shi L., Campagne F., and Quadri L. E. (2005) Pseudomonas aeruginosa SoxR does not conform to the archetypal paradigm for SoxR-dependent regulation of the bacterial oxidative stress adaptive response. Infect. Immun. 73, 2958–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park W., Peña-Llopis S., Lee Y., and Demple B. (2006) Regulation of superoxide stress in Pseudomonas putida KT2440 is different from the SoxR paradigm in Escherichia coli. Biochem. Biophys. Res. Commun. 341, 51–56 [DOI] [PubMed] [Google Scholar]
- 16. Seo S. W., Kim D., Szubin R., and Palsson B. O. (2015) Genome-wide reconstruction of OxyR and SoxRS transcriptional regulatory networks under oxidative stress in Escherichia coli K-12 MG1655. Cell Rep. 12, 1289–1299 [DOI] [PubMed] [Google Scholar]
- 17. Dela Cruz R., Gao Y., Penumetcha S., Sheplock R., Weng K., and Chander M. (2010) Expression of the Streptomyces coelicolor SoxR regulon is intimately linked with actinorhodin production. J. Bacteriol. 192, 6428–6438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shin J. H., Singh A. K., Cheon D. J., and Roe J. H. (2011) Activation of the SoxR regulon in Streptomyces coelicolor by the extracellular form of the pigmented antibiotic actinorhodin. J. Bacteriol. 193, 75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sheplock R., Recinos D. A., Mackow N., Dietrich L. E., and Chander M. (2013) Species-specific residues calibrate SoxR sensitivity to redox-active molecules. Mol. Microbiol. 87, 368–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh A. K., Shin J. H., Lee K. L., Imlay J. A., and Roe J. H. (2013) Comparative study of SoxR activation by redox-active compounds. Mol. Microbiol. 90, 983–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee K. L., Singh A. K., Heo L., Seok C., and Roe J. H. (2015) Factors affecting redox potential and differential sensitivity of SoxR to redox-active compounds. Mol. Microbiol. 97, 808–821 [DOI] [PubMed] [Google Scholar]
- 22. Draper W. M., and Crosby D. G. (1983) Photochemical generation of superoxide radical anion in water. J. Agric. Food. Chem. 31, 734–737 [Google Scholar]
- 23. Garg S., Rose A. L., and Waite T. D. (2011) Photochemical production of superoxide and hydrogen peroxide from natural organic matter. Geochim. Cosmochim. Acta 75, 4310–4320 [Google Scholar]
- 24. Jung J., Baek J. H., and Park W. (2010) Complete genome sequence of the diesel-degrading Acinetobacter species strain DR1. J. Bacteriol. 192, 4794–4795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jung J., Noh J., and Park W. (2011) Physiological and metabolic responses for hexadecane degradation in Acinetobacter oleivorans DR1. J. Microbiol. 49, 208–215 [DOI] [PubMed] [Google Scholar]
- 26. Kim J., Noh J., and Park W. (2013) Insight into norfloxacin resistance of Acinetobacter oleivorans DR1: target gene mutation, persister, and RNA-Seq analyses. J. Microbiol. Biotechnol. 23, 1293–1303 [DOI] [PubMed] [Google Scholar]
- 27. Heo A., Jang H. J., Sung J. S., and Park W. (2014) Global transcriptome and physiological responses of Acinetobacter oleivorans DR1 exposed to distinct classes of antibiotics. PLoS One 9, e110215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Denef V. J., Patrauchan M. A., Florizone C., Park J., Tsoi T. V., Verstraete W., Tiedje J. M., and Eltis L. D. (2005) Growth substrate- and phase-specific expression of biphenyl, benzoate, and C1 metabolic pathways in Burkholderia xenovorans LB400. J. Bacteriol. 187, 7996–8005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Denef V. J., Klappenbach J. A., Patrauchan M. A., Florizone C., Rodrigues J. L., Tsoi T. V., Verstraete W., Eltis L. D., and Tiedje J. M. (2006) Genetic and genomic insights into the role of benzoate-catabolic pathway redundancy in Burkholderia xenovorans LB400. Appl. Environ. Microbiol. 72, 585–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim J., Cho Y., Jang I. A., and Park W. (2015) Molecular mechanism involved in the response to hydrogen peroxide stress in Acinetobacter oleivorans DR1. Appl. Microbiol. Biotechnol. 99, 10611–10626 [DOI] [PubMed] [Google Scholar]
- 31. Srinivasan V. B., Vaidyanathan V., and Rajamohan G. (2015) AbuO, a TolC-like outer membrane protein of Acinetobacter baumannii, is involved in antimicrobial and oxidative stress resistance. Antimicrob. Agents Chemother. 59, 1236–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Srinivasan V. B., Venkataramaiah M., Mondal A., and Rajamohan G. (2015) Functional characterization of AbeD, an RND-type membrane transporter in antimicrobial resistance in Acinetobacter baumannii. PLoS One 10, e0141314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dietrich L. E., Teal T. K., Price-Whelan A., and Newman D. K. (2008) Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science 321, 1203–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hidalgo E., and Demple B. (1994) An iron-sulfur center essential for transcriptional activation by the redox-sensing SoxR protein. EMBO J. 13, 138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hidalgo E., Bollinger J. M. Jr, Bradley T. M., Walsh C. T., and Demple B. (1995) Binuclear [2Fe-2S] clusters in the Escherichia coli SoxR protein and role of the metal centers in transcription. J. Biol. Chem. 270, 20908–20914 [DOI] [PubMed] [Google Scholar]
- 36. Morishita R., Kawagoshi A., Sawasaki T., Madin K., Ogasawara T., Oka T., and Endo Y. (1999) Ribonuclease activity of rat liver perchloric acid-soluble protein, a potent inhibitor of protein synthesis. J. Biol. Chem. 274, 20688–20692 [DOI] [PubMed] [Google Scholar]
- 37. Kwan B. W., Valenta J. A., Benedik M. J., and Wood T. K. (2013) Arrested protein synthesis increases persister-like cell formation. Antimicrob. Agents Chemother. 57, 1468–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leszczynska D., Matuszewska E., Kuczynska-Wisnik D., Furmanek-Blaszk B., and Laskowska E. (2013) The formation of persister cells in stationary-phase cultures of Escherichia coli is associated with the aggregation of endogenous proteins. PLoS One 8, e54737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jang I. A., Kim J., and Park W. (2016) Endogenous hydrogen peroxide increases biofilm formation by inducing exopolysaccharide production in Acinetobacter oleivorans DR1. Sci. Rep. 6, 21121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen J. J., Crosby J. S., and London I. M. (1994) Regulation of heme-regulated eIF-2 α kinase and its expression in erythroid cells. Biochimie 76, 761–769 [DOI] [PubMed] [Google Scholar]
- 41. Vercruysse M., Köhrer C., Davies B. W., Arnold M. F., Mekalanos J. J., RajBhandary U. L., and Walker G. C. (2014) The highly conserved bacterial RNase YbeY is essential in Vibrio cholerae, playing a critical role in virulence, stress regulation, and RNA processing. PLoS Pathog. 10, e1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Uppal S., Akkipeddi V. S., and Jawali N. (2008) Posttranscriptional regulation of cspE in Escherichia coli: involvement of the short 5′-untranslated region. FEMS Microbiol. Lett. 279, 83–91 [DOI] [PubMed] [Google Scholar]
- 43. Pomposiello P. J., Bennik M. H., and Demple B. (2001) Genome-wide transcriptional profiling of the Escherichia coli responses to superoxide stress and sodium salicylate. J. Bacteriol. 183, 3890–3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giró M., Carrillo N., and Krapp A. R. (2006) Glucose-6-phosphate dehydrogenase and ferredoxin-NADP(H) reductase contribute to damage repair during the soxRS response of Escherichia coli. Microbiology 152, 1119–1128 [DOI] [PubMed] [Google Scholar]
- 45. Naseer N., Shapiro J. A., and Chander M. (2014) RNA-Seq analysis reveals a six-gene SoxR regulon in Streptomyces coelicolor. PLoS One 9, e106181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kalogeraki V. S., and Winans S. C. (1997) Suicide plasmids containing promoterless reporter genes can simultaneously disrupt and create fusions to genes of diverse bacteria. Gene 188, 69–75 [DOI] [PubMed] [Google Scholar]
- 47. Oka A., Sugisaki H., and Takanami M. (1981) Nucleotide sequence of the kanamycin resistance transposon Tn903. J. Mol. Biol. 147, 217–226 [DOI] [PubMed] [Google Scholar]
- 48. Philippe N., Alcaraz J. P., Coursange E., Geiselmann J., and Schneider D. (2004) Improvement of pCVD442, a suicide plasmid for gene allele exchange in bacteria. Plasmid 51, 246–255 [DOI] [PubMed] [Google Scholar]
- 49. Miller J. H. (1992) A Short Course in Bacterial Genetics, pp. 72–74, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 50. Yi H., Cho Y. J., Won S., Lee J. E., Jin Yu H., Kim S., Schroth G. P., Luo S., and Chun J. (2011) Duplex-specific nuclease efficiently removes rRNA for prokaryotic RNA-seq. Nucleic Acids Res. 39, e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mortazavi A., Williams B. A., McCue K., Schaeffer L., and Wold B. (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5, 621–628 [DOI] [PubMed] [Google Scholar]
- 52. Carver T., Böhme U., Otto T. D., Parkhill J., and Berriman M. (2010) BamView: viewing mapped read alignment data in the context of the reference sequence. Bioinformatics 26, 676–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Watanabe K., Kodama Y., and Harayama S. (2001) Design and evaluation of PCR primers to amplify bacterial 16S ribosomal DNA fragments used for community fingerprinting. J. Microbiol. Methods 44, 253–262 [DOI] [PubMed] [Google Scholar]
- 54. Kim J., and Park W. (2013) Indole inhibits bacterial quorum sensing signal transmission by interfering with quorum sensing regulator folding. Microbiology 159, 2616–2625 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.