Abstract
We present the case of a patient in whom a previously undetected anomalous origin of the circumflex coronary artery caused myocardial ischemia and led to positive myocardial scintigraphic results. Subsequent coronary angiography showed that the left circumflex coronary artery arose from the right coronary ostium—an anomaly that has been associated with chest discomfort—without atherosclerotic lesions. The peripheral distribution of the left circumflex artery was normal.
We describe the clinical and angiographic findings in our patient and discuss the relationship between coronary artery anomalies and ischemia.
Key words: Coronary angiography, coronary vessel anomalies, myocardial ischemia, scintigraphy
Coronary artery anomalies are found in 0.6% to 1.55% of patients who undergo coronary angiography,1–5 and the increasing use of diagnostic coronary angiography is uncovering even more such abnormalities. Indeed, most coronary anomalies are found incidentally during coronary arteriography. Although these anomalies are present at birth, relatively few manifest symptoms in children. Anomalous origin of a coronary artery does not generally lead to myocardial ischemia.
The origin of the left circumflex coronary artery (LCx) from the right sinus of Valsalva is one of the most common anatomic variations of the coronary artery circulation.1–6 Although this anomaly is classified as benign and asymptomatic, it can cause myocardial ischemia without atherosclerosis.7 Herein, we report a case of anomalous origin of the LCx coronary artery as a rare cause of myocardial ischemia.
Case Report
In May 2003, a 73-year-old man presented at the department of chest diseases of our institution with dyspnea and chest discomfort on exertion, which he had been experiencing for 3 years. He was diagnosed with chronic obstructive pulmonary disease. The following month, he was referred to our clinic due to chest pain. He had no family history of ischemic heart disease and no known coronary risk factors. Upon detailed examination, he was diagnosed as having chronic stable angina pectoris. An electrocardiogram showed slow-rate atrial fibrillation without significant ST-segment changes. An echocardiogram showed a dilated left ventricle, mild global hypokinetic walls with a reduced ejection fraction (about 0.50), and no congenital cardiac anomaly. A conventional exercise stress test could not be performed due to osteoarthritis in the patient's knees; instead, we scheduled myocardial perfusion scintigraphy. A single photon emission computed tomographic (SPECT) study was performed with 20 mCi of technetium-99m methoxyisobutylisonitrile (Tc-99m MIBI) after a pharmacologic stress test with dobutamine and again with the patient at rest. On the images taken after the stress, hypoperfusion was seen in the mid and basal segments of the inferoseptal region of the heart. Perfusion of these segments returned to normal on the images taken with the patient at rest (Fig. 1). These disturbances suggested ischemic changes induced by the test. Therefore, we performed left heart catheterization; coronary angiography revealed coronary arteries that were free of lesions. The LCx artery was found to arise from the right coronary ostium and to course behind the aorta (Figs. 2 and 3). The peripheral distribution of the LCx was normal. Given this evidence, we surmised that the patient's symptoms of ischemia were caused by the anomalous coronary artery. We gave him a β-blocker and nitrate therapy, and he was free of chest pain when we last saw him in May 2004.
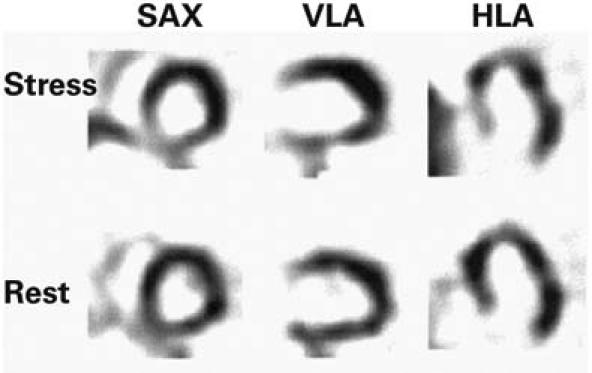
Fig. 1 Tomographic scans during stress myocardial scintigraphy, in the short-axis (SAX), vertical long-axis (VLA), and horizontal long-axis (HLA) views, show perfusion defects in the mid and basal inferoseptal segments. The resting SAX, VLA, and HLA views show the perfusion after its return to normal.
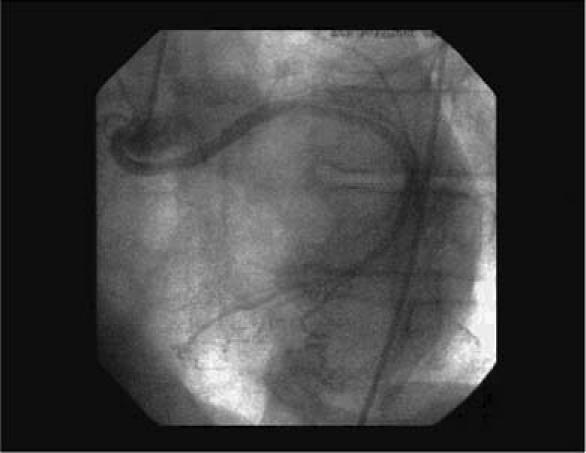
Fig. 2 Coronary angiogram in the left anterior oblique view shows the circumflex coronary artery originating in the right coronary ostium and following a retroaortic course to its normal distribution area.
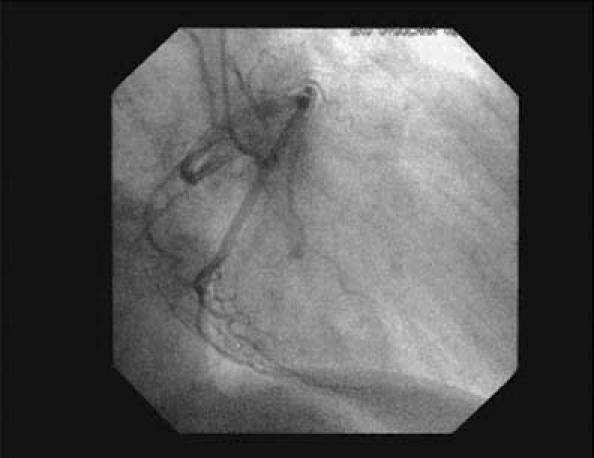
Fig. 3 Coronary angiogram in the right anterior oblique view shows the origin of the circumflex artery in the right sinus of Valsalva.
Discussion
The origin of the LCx from the right sinus of Valsalva is one of the most common coronary artery anomalies; the incidence ranges from 0.20% to 0.71%.1–5 The anomalous LCx originates from a separate orifice in the right sinus of Valsalva. It courses behind the aorta to the left part of the atrioventricular sulcus, where it resumes its usual configuration. Although this anomaly is classified as benign and asymptomatic, it can cause myocardial ischemia, and a few cases of sudden death, myocardial infarction, and angina pectoris in the absence of atherosclerotic lesions have been reported.7–9 These manifestations could be due to repeated compression of the anomalous artery by a dilated aortic root or to unusual angling as a result of the retroaortic course of the LCx, which can compress the coronary ostium and restrict blood flow.10 Myocardial ischemia should be ruled out before a coronary anomaly is considered benign. One of the tests most often used for this purpose is the thallium exercise stress test. However, this test is not sufficiently sensitive to show myocardial perfusion defects, which has been demonstrated in patients with LCx anomalies who have positive conventional stress test results but negative thallium stress test results.8,11 When we performed scintigraphy with Tc-99m MIBI in our patient, the area of ischemia was shown to be in the distribution region of the LCx.
Not all patients who have coronary anomalies have symptoms of ischemia. Our patient's symptoms occurred only with maximal exercise. We believe that his chest discomfort was caused by the pressure of the aorta on the LCx as it coursed around the aortic root. During effort, the root of the aorta widens due to increased blood flow. In our patient, these conditions exerted additional pressure on the LCx; therefore, blood flow through the LCx artery could not meet the increased demand of blood flow for the myocardium, and ischemia occurred.
Surgery was not chosen for this patient, because of his advanced age and the absence of severe ischemia. We achieved a satisfactory result with medical treatment, including a β-blocker and nitrate therapy. Because the symptoms completely resolved after β-blocker treatment, we did not need to confirm improvement with a repeat nuclear stress test. In their study investigating coronary artery anomalies leading to sudden death, Taylor and colleagues7 found that coronary artery anomalies coursing between the aorta and the pulmonary arteries caused a significant portion of deaths in patients under 35 years of age. Nevertheless, the age and the clinical course of our patient directed us toward conservative treatment. In elderly patients, a calcified and rigid aortic root can cause myocardial ischemia by compressing an anomalous coronary artery, as it did in our patient. We conclude that the anomalous origin of the left circumflex coronary artery is not always benign. As in most cases, treatment for such a condition should be tailored to the individual patient.
Footnotes
Address for reprints: Mustafa Aydin, MD, Zonguldak Karaelmas Universitesi Tip Fakultesi, Kardiyoloji Anabilimdali, Kozlu, 67600 Zonguldak, Turkey
E-mail: drmustafaaydin@hotmail.com
References
- Kimbiris D, Iskandrian AS, Segal BL, Bemis CE. Anomalous aortic origin of coronary arteries. Circulation 1978; 58:606–5. [DOI] [PubMed]
- 2.Sheldon WC, Hobbs RE, Millit D, Raghavan PV, Moodie DS. Congenital variations of coronary artery anatomy. Cleve Clin Q 1980;47:126–30.
- 3.Donaldson RM, Raphael M, Radley-Smith R, Yacoub MH, Ross DN. Angiographic identification of primary coronary anomalies causing impaired myocardial perfusion. Cathet Cardiovasc Diagn 1983;9:237–29. [DOI] [PubMed]
- 4.Wilkins CE, Betancourt B, Mathur VS, Massumi A, De Castro CM, Garcia E, Hall RJ. Coronary artery anomalies: a review of more than 10,000 patients from the Clayton Cardiovascular Laboratories. Tex Heart Inst J 1988;15:166–73. [PMC free article] [PubMed]
- 5.Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn 1990;21:28–40. [DOI] [PubMed]
- 6.Angelini P. Normal and anomalous coronary arteries: definitions and classification. Am Heart J 1989;117: 418–34. [DOI] [PubMed]
- 7.Taylor AJ, Byers JP, Cheitlin MD, Virmani R. Anomalous right or left coronary artery from the contralateral coronary sinus: “high-risk” abnormalities in the initial coronary artery course and heterogeneus clinical outcomes. Am Heart J 1997;133:428–35. [DOI] [PubMed]
- 8.Piovesana P, Corrado D, Verlato R, Lafisca N, Mantovani N, DiMarco A, Pantaleoni A. Morbidity associated with anomalous origin of the left circumflex coronary artery from the right aortic sinus. Am J Cardiol 1989;63:762–3. [DOI] [PubMed]
- 9.Corrado D, Penelli T, Piovesana P, Thiene G. Anomalous origin of the left circumflex coronary artery from the right aortic sinus of Valsalva and sudden death. Cardiovasc Pathol 1994;3:269–71. [DOI] [PubMed]
- 10.Roberts WC. Major anomalies of coronary arterial origin seen in adulthood. Am Heart J 1986;111:941–63. [DOI] [PubMed]
- 11.Molajo AO, Bray CL, Prescott MC, Testa HJ. Thallium-201 myocardial imaging in patients with angina pectoris and anomalous aortic origin of the circumflex coronary artery. Int J Cardiol 1988;18:371–81. [DOI] [PubMed]


