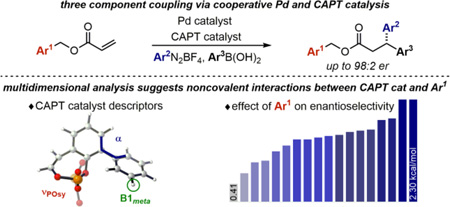
Abstract
The enantioselective 1,1-diarylation of terminal alkenes is reported herein enabled by the combination of palladium catalysis with a chiral anion phase-transfer strategy. The reaction of substituted benzyl acrylates with aryldiazonium salts and arylboronic acids gave the corresponding 3,3-diaryl propanoates in moderate to good yields and high enantioselectivies (up to 98:2 er). Substituents on the benzyl acrylate and chiral anion phase-transfer catalyst significantly affect the enantioselectivity, and multidimensional parameterization identified correlations suggesting structural origins for high stereocontrol.
Graphical Abstract
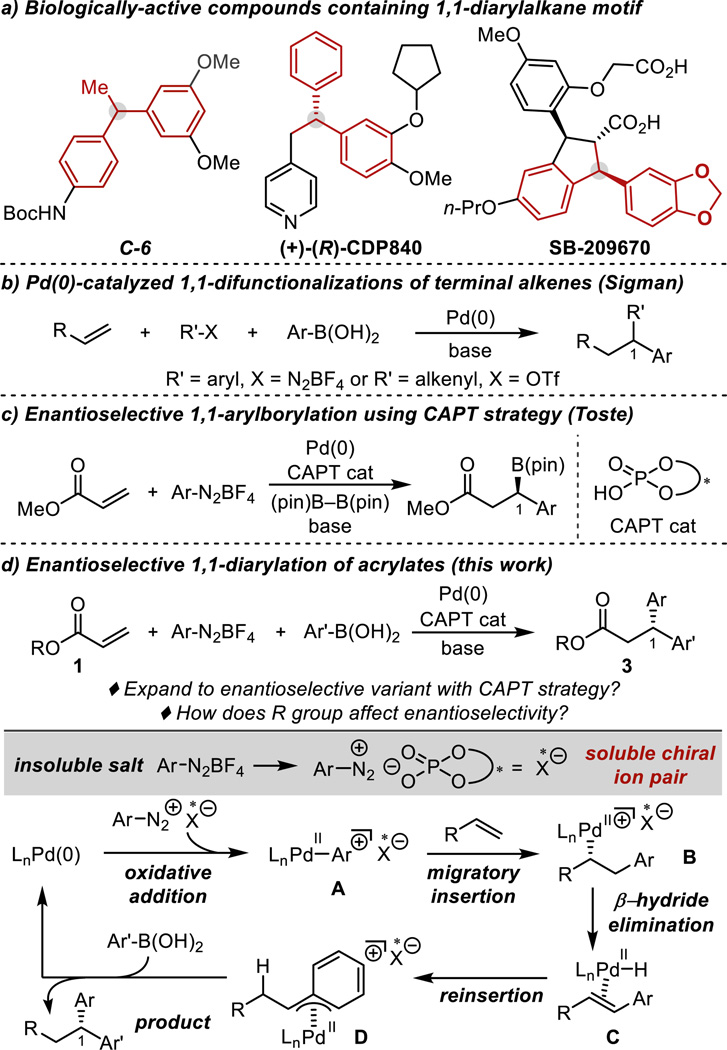
From the perspective of molecular diversity and step economy, palladium-catalyzed difunctionalization reactions of alkenes are a powerful synthetic tool,1 providing rapid access to complex building blocks, such as the 1,1-diarylalkane motif that is widespread in numerous natural products and pharmaceuticals.2 In fact, a high-throughput screen of 1,1-diarylmethines synthesized in our group led to the identification of a compound, C-6, that is selectively active against chemo-resistant breast cancers (Scheme 1a).3 In addition, compounds containing a 1,1-diarylmethine stereogenic center are present in a number of drug targets, like CDP8402b and SB-2096702c (Scheme 1a). Accordingly, developing synthetic methods to access these compounds in a modular and stereocontrolled manner is of great importance, particularly to accelerate the screening process and structure-activity relationship studies in drug discovery.
Scheme 1.
Relevance and overview of the enantioselective 1,1-difunctionalization of alkenes
In this context, our group has focused on the development of Pd(0)-catalyzed three-component coupling of terminal alkenes, arylboronic acids, and aryldiazonium salts or alkenyl triflates to construct 1,1-difunctionalized products (Scheme 1b).4 Despite the significance of enantioenriched 1,1-diarylalkanes, the enantioselective three-component coupling reaction has remained elusive, since the desired process is suppressed in the presence of common P- or N-based chiral ligands.4b To address this problem, the Toste group recently reported the Pd(0)-catalyzed enantioselective 1,1-arylborylation of terminal alkenes using a chiral anion phase-transfer (CAPT) strategy (Scheme 1c).5 We envisioned that this approach would translate well to the enantioselective 1,1-diarylation of alkenes due to the similarity in proposed mechanisms. However, the use of arylboronic acids in place of B2pin2 was projected to involve several additional complications, including solubility issues as well as the potential for direct interactions with the chiral anion.6
Mechanistically, the crucial step in the CAPT approach is the formation of a soluble chiral ion pair between the chiral phosphate anion and an aryldiazonium cation from the corresponding tetrafluoroborate salt that is insoluble under the reaction conditions (Scheme 1d). Oxidative addition of this chiral ion pair by Pd(0) generates cationic Pd-aryl intermediate A that, assuming the chiral anion remains associated, underdoes an enantioselective migratory insertion of the acrylate alkene, generating Pd-alkyl B. In order to form the 1,1-diarylated product, the catalyst migrates to C1 via β-hydride elimination to form alkene C followed by reinsertion to give intermediate D. Stabilized as a π-benzyl species, D undergoes transmetallation with the second coupling partner and reductive elimination, releasing the product. Herein, we present the development of an enantioselective 1,1-diarylation of benzyl acrylates providing 3,3-diarylpropanates7 in high enantioselectivity using a CAPT approach (Scheme 1d). The determination of the best chiral anion and substrate combination was enabled and analyzed using a multi-dimensional parameterization8 tactic revealing key insight into potential remote noncovalent interactions responsible for effective asymmetric catalysis.
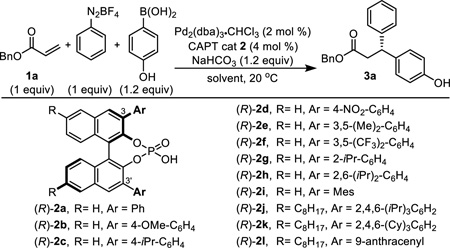
Our efforts towards applying this CAPT strategy in an enantioselective 1,1-diarylation reaction were initiated with the examination of several common BINOL-based chiral phosphoric acids9 (2), in addition to 1a as substrate and phenyldiazonium salt and 4-hydroxyphenyl boronic acid as coupling partners (Table 1). By increasing the torsion of the aryl groups in 2 with substituents at the 2- and 2,6-positions, a significant increase in er was observed compared to 3,5- or 4-substituted catalysts (2a–f), all of which also returned low yields of the desired product (3a). In agreement with this observation, increasing the size of the substituents at the 2,4,6-positions from methyl (2i) to isopropyl (2j) to cyclohexyl (2k) led to increasing er values as well as increased yields. Finally, the extended π-system, 9-anthracenyl (2l), afforded the highest enantioselectivity although slightly lower yields were observed due to a higher percentage of traditional Heck product formation. Control experiments demonstrated that no desired product was observed when hexane was used as solvent (entry 13), and lower enantioselectivity resulted when the reaction was conducted in THF, in which the diazonium salt is soluble (entry 14).
Table 1.
Evaluation of Chiral Anion Phase-Transfer (CAPT) catalysts and solvents.a
 | |||||
|---|---|---|---|---|---|
| entry | CAPT cat | solvent | yield of 3a (%)b | erc | torsion (α)d |
| 1 | 2a | Et2O | 18 | 42.5 : 57.5 | 48° |
| 2 | 2b | Et2O | 12 | 35 : 65 | 48° |
| 3 | 2c | Et2O | 9 | 37.5 : 62.5 | 48° |
| 4 | 2d | Et2O | 19 | 44 : 56 | 48° |
| 5 | 2e | Et2O | 27 | 51.5 : 48.5 | 48° |
| 6 | 2f | Et2O | 14 | 56.5 : 43.5 | 50° |
| 7 | 2g | Et2O | 27 | 60.5 : 39.5 | 69° |
| 8 | 2h | Et2O | 47 | 75.5 : 24.5 | 84° |
| 9 | 2i | Et2O | 40 | 79 : 21 | 82° |
| 10 | 2j | Et2O | 67 | 81 : 19 | 84° |
| 11 | 2k | Et2O | 52 | 84 : 16 | 86° |
| 12 | 2l | Et2O | 47 | 87.5 : 12.5 | 72° |
| 13 | 2l | hexane | 0 | - | |
| 14 | 2l | THF | 39 | 76 : 24 | |
Reactions were performed on 0.1 mmol scale of 1a in solvent (2 mL) at 20 °C for 20 h and were repeated twice.
Yields were determined by 1H NMR of the crude reaction mixture using an internal standard.
Enantiomeric ratios were determined by SFC.
Torsion (α) was calculated using phosphate model system 4.
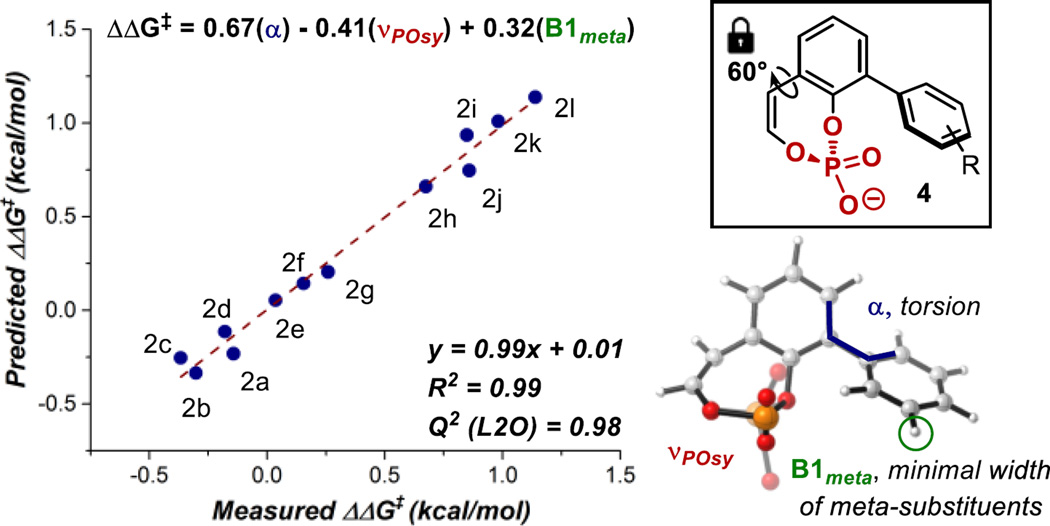
In order to gain further insight into the effect of the chiral anion substitution pattern on enantioselectivity and perhaps predict a more selective CAPT catalyst, we applied our multidimensional parameterization technique, which correlates relevant molecular descriptors to the reaction outcome.8 As a result, an excellent correlation was established, relating the measured enantioselectivity to three parameters derived from a phosphate model system (4, Figure 1). As mentioned previously, chiral anions with 2,6-disubstituted aryl groups resulted in enhanced enantioselectivity, and in general, larger dihedral angles (α) correlate with higher enantioselectivity (Table 1 and Figure S4, R2 = 0.78). The appearance of this term, α, in the multivariate model reinforces the significance of the arene’s orientation towards the phosphate group, conceivably allowing for stabilizing noncovalent interactions with the substrates. Additionally, the symmetric P=O stretch or vPOsy could describe the effect of the aryl ring substituents on the phosphate’s interaction with the diazonium and/or palladium counterions. Finally, Sterimol parameter B1meta represents the minimum width of the meta-substituents of the arenes, suggesting substituent size at this position impacts enantioselectivity.10 While this strategy revealed a potentially predictive model that could identify a more selective CAPT catalyst, an inherent limitation concerns the synthetic effort and accessibility required to examine any predictions. Therefore, we selected the best-performing chiral anion (2l) in terms of er and redirected our efforts towards evaluating another modular component in the reaction: the benzyl acrylate substrate.
Figure 1.
Multidimensional Correlation of Chiral Anion Parameters with Enantioselectivity.
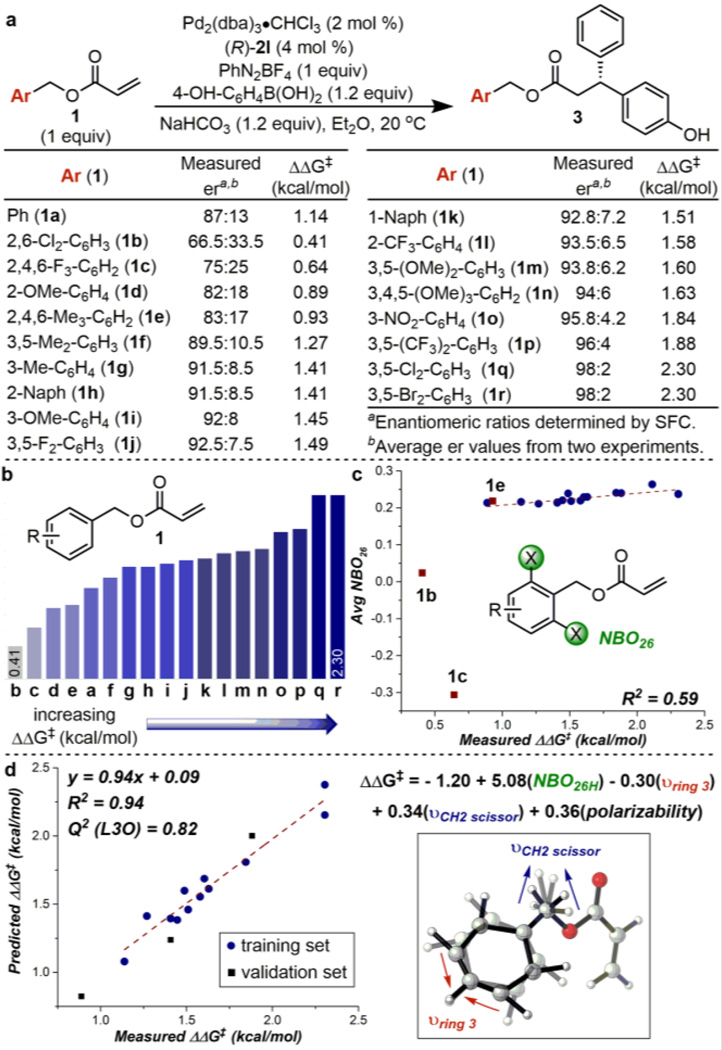
Aligning with our goals to not only optimize this reaction but also understand the influences on enantioselectivity, benzyl acrylates provided an exciting advantage in that a diverse library could easily be generated from the corresponding benzyl alcohols for a broad survey of substituent effects. Therefore, a set of substituted benzyl acrylates was designed to include electron-withdrawing and –donating substitutions in single, multiple and varying positions (Figure 2a,b). After evaluating a number of these benzyl acrylates in combination with phosphate 2l, a significant effect of both the substituents’ identity and position on the arene was revealed (a range of ~2 kcal/mol). As the benzyl group is distal from the site of reaction, these non-intuitive observations support our hypothesis that attractive noncovalent interactions between the benzyl group and the chiral anion are controlling elements in the stereodefining step. The use of substrates 1d (2-OMe), 1f (3,5-Me2), and 1g (3-Me) with electron-donating substituents resulted in similar levels of enantioselectivities to that of benzyl acrylate 1a. Conversely, several additional electron-donating substitutions and two extended-π systems (1h 2-Naph, 1i 3-OMe, 1k 1-Naph, 1m 3,5-(OMe)2, 1n 3,4,5-(OMe)3) exhibited higher enantioselectivities compared to 1a. Adding a substituent at the 4-position did not have a significant effect on the enantiodetermining step (compare 1m and 1n). Finally, good to excellent enantioselectivities were observed when electron-deficient substrates were used (1j 3,5-F2, 1l 2-CF3, 1o 3-NO2, 1p 3,5-(CF3)2, 1q 3,5-Cl2, or 1r 3,5-Br2). Serendipitously, both 1q and 1r resulted in the highest enantioselectivity (98:2 er). In contrast, substrates 1b (2,6-Cl2) and 1c (2,4,6-F3), which contain electronegative atoms at the 2,6 positions, provided the desired product in the lowest er values, emphasizing the positional requirement of a electron withdrawing group on enantioselectivity and thus highlighting the potential for noncovalent interactions between the benzyl group and the CAPT catalyst. In order to understand these diverse effects, additional studies were initiated, focusing on relating the substituents effects to the observed enantioselectivities.
Figure 2.
Evaluation and Correlation of Benzyl Acrylate Substituent Effects on Enantioselectivity.
The same multidimensional parameterization technique was applied to correlate various molecular descriptors of the benzyl acrylates to the measured enantioselectivity. During this process, we observed a trend between the average NBO charge of the atoms at the 2,6-positions and the measured ΔΔG‡ values (Figure 2c).11 Due to the significant difference in charge between a halogen and a hydrogen, we were not surprised to observe that substrates 1b and 1c are outliers in this simple relationship. Nonetheless, this insight could suggest that the 2,6-hydrogens themselves play a role in catalyst recognition, and electron-deficient hydrogens lead to a more selective process.12 Although this parameter could solely represent the electronic contribution from the benzyl acrylates’ substituents, it was also a significant term in the multivariate model, supporting its descriptive power of the benzyl acrylate’s effect on enantioselectivity (1b, 1c, and 1e were removed from the training set, Figure 2d). Combining NBO26H (average NBO charge of the 2,6-hydrogens11) with three additional terms, two stretching frequencies and the acrylate’s mean polarizability, provided an excellent correlation between the measured and predicted ΔΔG‡ values. As IR vibrations are sensitive to changes in electronic nature and mass, νring 3 and νCH2 scissor likely account for these effects resulting from the various benzyl groups’ substituents.13 Finally, we have previously used polarizability as a parameter to propose a substrate-ligand lone pair-π interaction in a Pd-catalyzed redox relay Heck reaction14; therefore, we interpret this variable similarly. Overall, the results from this multidimensional correlation are consistent with our hypothesis that noncovalent interactions are occurring between the benzyl acrylate and the anthracenyl group on the phosphate, such as a π-stacking interaction.15 Additional detailed studies are underway to elucidate the subtle effects on enantioselectivity in this complex system and to identify specific attractive interactions.
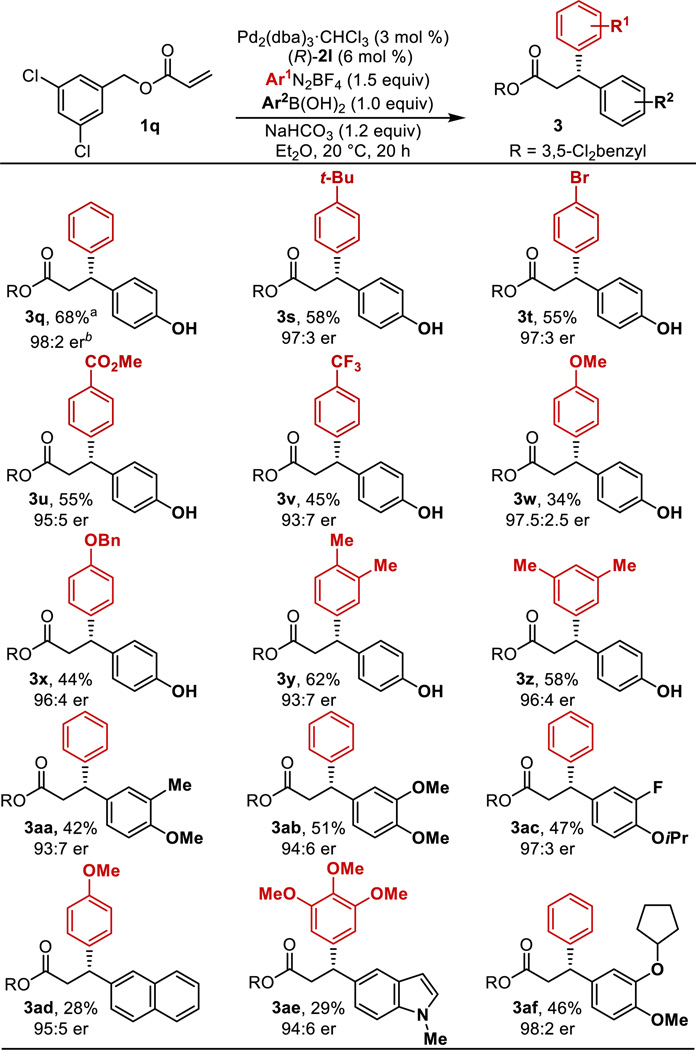
Finally, the scope of the reaction was evaluated using the optimal chiral anion, 2l, and 3,5-dichlorobenzyl acrylate, 1q.16 Overall, excellent enantiomeric ratios are observed in moderate yields. Lower yields are observed due to the competitive formation of the traditional Heck product. Nonetheless, both electron-rich and -poor aryldiazonium salts are well-tolerated under the optimized reaction conditions with electron-withdrawing substituents (3u and 3v) leading to slightly lower enantioselectivities. As boronic acids are known to interact directly with phosphates,6 it was hypothesized that compatibility between these two components may be an issue for the boronic acid scope. This was not foreseen as a detriment since the boronic acid could easily be exchanged with the corresponding diazonium salt. In general, we observed that electron-rich arylboronic acids performed best in this chemistry, leading to higher yields likely due to the increased rate of transmetallation compared to electron-poor arenes. Of note, 2-naphthyl (3ad) and 5-indole (3ae) groups were incorporated in 95:5 and 94:6 er, respectively, albeit in low yields. Additionally, 3af was synthesized in high enantioselectivity (98:2 er), which is a relevant building block en route to the drug target CDP840 (Scheme 1a).2b Although a limitation of this methodology lies in the reaction yields, the modularity of the coupling partners and step economy in this three-component coupling reaction can compensate for these limitations. Additional studies aim to overcome the competitive Heck reaction via exploration of different combinations of benzyl acrylates and chiral phosphoric acids as this pathway is also sensitive to the identity of these two species (Table S1).
In conclusion, we have developed an enantioselective three-component coupling reaction of benzyl acrylates, aryldiazonium salts and arylboronic acids, providing a modular approach to the synthesis of 1,1-diarylated products in high enantioselectivity. The key to a highly selective process was realized by utilizing a chiral anion phase-transfer strategy, wherein enantioselectivity was closely tied to the identity of the CAPT catalyst. Additionally, er values were surprisingly sensitive to the electronic and steric nature as well as the position of substituents on the benzyl acrylate substrate. We applied a multidimensional modeling technique to reveal specific properties of the CAPT catalyst and the acrylate that may be responsible for the observed range in enantioselectivity. These results suggest that attractive noncovalent interactions, such as a π-stacking interaction, between the two components are controlling elements in the enantiodetermining step. Future studies include further exploration of these putative interactions in order to understand the origin of enantioselectivity and facilitate the improvement and expansion of this and other reactions in development.
Supplementary Material
Table 2.
Examination of the aryl diazonium salt and boronic acid scope.
Isolated yields after purification.
Enantiomeric ratios were determined by SFC.
Acknowledgments
The synthetic aspects were supported by National Institute of Health (R01GM063540), and the modeling was supported by NSF (CHE-1361296). E.Y. was supported by a Grant-in-Aid for JSPS Fellows (JSPS KAKENHI, grant number: 26·2447). M.O. thanks the Ermenegildo Zegna Group for a post-doctoral fellowship. We gratefully acknowledge the Center for High Performance Computing (CHPC) at the University of Utah. We would like to thank Anat Milo and Andrew J. Neel for insightful discussions and sharing CAPT catalysts with us.
Footnotes
ASSOCIATED CONTENT
Supporting Information
Experimental procedures and modeling and characterization data can be found in the supporting information. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interests.
REFERENCES
- 1.For reviews, see: Jensen KH, Sigman MS. Org. Biomol. Chem. 2008;6:4083. doi: 10.1039/b813246a. McDonald RI, Liu G, Stahl SS. Chem. Rev. 2011;111:2981. doi: 10.1021/cr100371y.
- 2.For recent reviews, see: Ameen D, Snape TJ. Med. Chem. Commun. 2013;4:893. For examples, see: Alexander RP, Warrellow GJ, Eaton MAW, Boyd EC, Head JC, Porter JR, Brown JA, Reuberson JT, Hutchinson B, Turner P, Boyce B, Barnes D, Mason B, Cannell A, Taylor RJ, Zomaya A, Millican A, Leonard J, Morphy R, Wales M, Perry M, Allen RA, Gozzard N, Hughes B, Higgs G. Bioorganic Med. Chem. Lett. 2002;12:1451. doi: 10.1016/s0960-894x(02)00202-0. Ohlstein EH, Nambi P, Douglas SA, Edwards RM, Gellai M, Lago A, Leber JD, Cousins RD, Gao A, Frazee JS, Peishoff CE, Bean JW, Eggleston DS, Elshourbagy NA, Kumar C, Lee JA, Yue T-L, Louden C, Brooks DP, Weinstock J, Feuerstein G, Poste G, Ruffolo RR, Gleason JG, Elliott JD. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8052. doi: 10.1073/pnas.91.17.8052. Hyttel J, Larsen JJ. J. Neurochem. 1985;44:1615. doi: 10.1111/j.1471-4159.1985.tb08803.x.
- 3.Gligorich K, Vaden R, Shelton D, Wang G, Matsen C, Looper R, Sigman M, Welm B. Breast Cancer Res. 2013;15:R58. doi: 10.1186/bcr3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Liao L, Jana R, Urkalan KB, Sigman MS. J. Am. Chem. Soc. 2011;133:5784. doi: 10.1021/ja201358b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Saini V, Sigman MS. J. Am. Chem. Soc. 2012;134:11372. doi: 10.1021/ja304344h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Saini V, Liao L, Wang Q, Jana R, Sigman MS. Org. Lett. 2013;15:5008. doi: 10.1021/ol4023358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nelson HM, Williams BD, Miró J, Toste FD. J. Am. Chem. Soc. 2015;137:3213. doi: 10.1021/jacs.5b00344. For reviews on chiral ion pairs used in catalysis, see: Phipps RJ, Hamilton GL, Toste FD. Nature Chem. 2012;4:603. doi: 10.1038/nchem.1405. Brak K, Jacobsen EN. Angew. Chem. Int. Ed. 2013;52:534. doi: 10.1002/anie.201205449.
- 6.(a) Zi W, Wang Y-M, Toste FD. J. Am. Chem. Soc. 2014;136:12864. doi: 10.1021/ja507468u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Neel AJ, Milo A, Sigman MS, Toste FD. J. Am. Chem. Soc. 2016;138:3863. doi: 10.1021/jacs.6b00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For examples of catalytic synthetic methods toward enantioenriched 3,3-diaryl propanoates, see: Tolstoy P, Engman M, Paptchikhine A, Bergquist J, Church TL, Leung AW-M, Andersson PG. J. Am. Chem. Soc. 2009;131:8855. doi: 10.1021/ja9013375. Taylor JG, Correia CRD. J. Org. Chem. 2011;76:857. doi: 10.1021/jo102134v. Itoh K, Tsuruta A, Ito J-i, Yamamoto Y, Nishiyama H. J. Org. Chem. 2012;77:10914. doi: 10.1021/jo302357b. Sakuma S, Sakai M, Itooka R, Miyaura N. J. Org. Chem. 2000;65:5951. doi: 10.1021/jo0002184. Paquin J-F, Stephenson CRJ, Defieber C, Carreira EM. Org. Lett. 2005;7:3821. doi: 10.1021/ol051533l. Nishikata T, Kiyomura S, Yamamoto Y, Miyaura N. Synlett. 2008:2487. Luo Y, Carnell AJ. Angew. Chem. Int. Ed. 2010;49:2750. doi: 10.1002/anie.200907033. Xue F, Wang D, Li X, Wan B. Org. Biomol. Chem. 2013;11:7893. doi: 10.1039/c3ob41342j. Miyamura H, Suzuki A, Yasukawa T, Kobayashi S. Adv. Synth. Catal. 2015;357:3815. Xu B, Li M-L, Zuo X-D, Zhu S-F, Zhou Q-L. J. Am. Chem. Soc. 2015;137:8700. doi: 10.1021/jacs.5b05086.
- 8.Sigman MS, Harper KC, Bess EN, Milo A. Acc. Chem. Res. 2016;49:1292. doi: 10.1021/acs.accounts.6b00194. [DOI] [PubMed] [Google Scholar]
- 9.For structure-function analysis of BINOL-derived phosphoric acids, see: Reid JP, Goodman JM. J. Am. Chem. Soc. 2016;138:7910. doi: 10.1021/jacs.6b02825. Reid JP, Simón L, Goodman JM. Acc. Chem. Res. 2016;49:1029. doi: 10.1021/acs.accounts.6b00052.
- 10.(a) Verloop A. In: Drug Design. Ariens EJ, editor. III. Waltham, MA: Academic Press; 1976. p. 133. [Google Scholar]; (b) Verloop A, Tipker J. Pharmacochem. Libr. 1977;2:63. [Google Scholar]; (c) Verloop A, Tipker J. Pharmacochem. Libr. 1987;10:97. [Google Scholar]; (d) Harper KC, Bess EN, Sigman MS. Nature Chem. 2012;4:366. doi: 10.1038/nchem.1297. [DOI] [PubMed] [Google Scholar]
- 11.For substrates 1d, 1k, and 1l the NBO charge of the 2-hydrogen only was considered. For 1e, the average NBO charge of the methyl hydrogens were included since these are most accessible compared to the carbons. Thus, these hydrogens would likely represent a potential site for a noncovalent interaction to occur.
- 12.Hydrogen-bonding interactions of highly polar ortho-C-H bonds in 3,5-bis(CF3)phenyl group in thiourea catalysts are known to be involved in a variety of organocatalytic reactions. For selected examples, see: Okino T, Yasutaka H, Furukawa T, Xu X, Takemoto Y. J. Am. Chem. Soc. 2005;127:119. doi: 10.1021/ja044370p. Berkessel A, Cleemann F, Mukherjee S, Müller TN, Lex J. Angew. Chem. Int. Ed. 2005;44:807. doi: 10.1002/anie.200461442. Tan B, Lu Y, Zeng X, Chua PJ, Zhong G. Org. Lett. 2010;12:2682. doi: 10.1021/ol1007795. Zhang Z, Lippert KM, Hausmann H, Kotke M, Schreiner PR. J. Org. Chem. 2011;76:9764. doi: 10.1021/jo201864e. Lippert KM, Hof K, Gerbig D, Ley D, Hausmann H, Guenther S, Schreiner PR. Eur. J. Org. Chem. 2012;30:5919.
- 13.Milo A, Bess EN, Sigman MS. Nature. 2014;507:210. doi: 10.1038/nature13019. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z-M, Hilton MJ, Sigman MS. J. Am. Chem. Soc. 2016;138:11461. doi: 10.1021/jacs.6b06994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Krenske EH, Houk KN. Acc. Chem. Res. 2013;46:979. doi: 10.1021/ar3000794. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sherrill CD. Acc. Chem. Res. 2013;46:1020. doi: 10.1021/ar3001124. [DOI] [PubMed] [Google Scholar]; (c) Wheeler SE. Acc. Chem. Res. 2013;46:1029. doi: 10.1021/ar300109n. [DOI] [PubMed] [Google Scholar]
- 16.As these substrates demonstrated the same selectivity with the model system, 1q was chosen over 1r due to lower cost.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.