Abstract
Aims
Differentiation between early-phase arrhythmogenic right ventricular cardiomyopathy (ARVC) and right ventricular outflow tract (RVOT)-ventricular tachycardia (VT) can be challenging, and correct diagnosis is important. We compared electrocardiogram (ECG) parameters and morphological right ventricular (RV) abnormalities and investigated if ECG and cardiac imaging can help to discriminate early-phase ARVC from RVOT-VT patients.
Methods and results
We included 44 consecutive RVOT-VT (47 ± 14 years) and 121 ARVC patients (42 ± 17 years). Of the ARVC patients, 77 had definite ARVC and 44 had early-phase ARVC disease. All underwent clinical examination, ECG, and Holter monitoring. Frequency of premature ventricular complexes (PVC) was expressed as percent per total beats/24 h (%PVC), and PVC configuration was recorded. By echocardiography, we assessed indexed RV basal diameter (RVD), indexed RVOT diameter, and RV and left ventricular (LV) function. RV mechanical dispersion (RVMD), reflecting RV contraction heterogeneity, was assessed by speckle-tracking strain echocardiography. RV ejection fraction (RVEF) was assessed by cardiac magnetic resonance imaging (CMR). Patients with early-phase ARVC had lower %PVC by Holter and PVC more frequently originated from the RV lateral free wall (both P < 0.001). RVD was larger (21 ± 3 vs. 19 ± 2 mm, P < 0.01), RVMD was more pronounced (22 ± 15 vs. 15 ± 13 ms, P = 0.03), and RVEF by CMR was decreased (41 ± 8 vs. 49 ± 4%, P < 0.001) in early-phase ARVC vs. RVOT-VT patients.
Conclusion
Patients with early-phase ARVC had structural abnormalities with lower RVEF, increased RVD, and pronounced RVMD in addition to lower %PVC by Holter compared with RVOT-VT patients. These parameters can help correct diagnosis in patients with unclear phenotypes.
Keywords: ARVC, RVOT-VT, cardiac imaging, ventricular arrhythmias
Introduction
The right ventricular outflow tract (RVOT) is the most common site of origin for idiopathic ventricular tachycardias (VT) and frequent premature ventricular complexes (PVC) in patients with structurally normal hearts, known as RVOT-VT. RVOT-VT is supposed to be a relatively benign condition,1 with generally well-tolerated ventricular arrhythmias. However, the RVOT area may also be origin of VT in patients with arrhythmogenic right ventricular cardiomyopathy (ARVC),2 an inherited cardiomyopathy predisposing to ventricular arrhythmias, biventricular dysfunction, and sudden cardiac death and therefore a far from a benign condition.3,4 Regardless of the underlying process, VT from the RVOT area with a left bundle branch block and inferior axis is common in both conditions, and to distinguish between idiopathic RVOT-VT and early stages of ARVC is challenging. The treatment and prognosis, however, differ substantially, and an incorrect diagnosis may be devastating. Although patients with RVOT-VT commonly have structurally normal ventricles, frequent PVC may cause myocardial remodelling with subsequent reduced function and dilatation. These secondary and often reversible structural changes in patients with RVOT-VT further complicate the discrimination to early-phase ARVC,5 but a comprehensive comparison between these entities has not been previously performed. Furthermore, patients in the early stages of ARVC often have only subtle or even non-detectable structural changes, despite the risk of arrhythmias.6 Risk of arrhythmias in overt ARVC7 is reported to originate from fibro-fatty replacement of myocardium forming the substrate for VT. In early phases of ARVC, other mechanisms may be involved including disruptive electrical conduction8 in addition to assumed early fibrosis. In contrast, monomorphic origin of PVC and VT in RVOT-VT is thought to be a result of triggered activity.9,10
Diagnostic accuracy in ARVC was improved by the ARVC 2010 Task Force Criteria (TFC), classifying ARVC diagnosis in possible, borderline, and definite ARVC and including the echocardiographic parameters right ventricular (RV) morphology and function, RVOT diameter, and RV fractional area change.2 The discrimination of ARVC from RVOT-VT is particularly challenging in the early phases of ARVC patients. We aimed to compare findings from electrocardiogram (ECG) and morphological RV abnormalities including new echocardiographic strain parameters, to distinguish patients with early-phase ARVC from RVOT-VT. We hypothesized that sensitive imaging parameters can detect subtle myocardial changes in early phases of ARVC that are relevant to distinguish early-phase ARVC from RVOT-VT.
Methods
Study patients
In this cross-sectional study, consecutive RVOT-VT patients were recruited from Oslo University Hospital, Norway. The diagnosis of RVOT-VT was based on a clinical phenotype with monomorphic VT or monomorphic PVC originating from the RVOT region with documented left bundle branch block and inferior axis.11 Furthermore, a diagnosis of RVOT-VT required maximal exercise test negative for ischaemia or coronary angiography without significant stenosis and no mutations in ARVC associated genes identified by genetic testing.11
Consecutive ARVC patients were recruited from Oslo University Hospital, Norway (n = 105) and Lund University Hospital, Sweden (n = 16). A subset of these patients (n = 108) have been previously reported.12 The 2010 TFC were used for diagnosis and classification of ARVC disease.2
All patients underwent clinical examination, and we recorded medical history, use of antiarrhythmic medications, and occurrence of ventricular arrhythmias. Ventricular arrhythmias were defined as syncope with assumed arrhythmic origin, documented non-sustained/sustained VT, and aborted cardiac arrests. Treatments such as radio frequency ablation, implantable cardioverter defibrillator implantation, and cardiac transplantation were recorded.
Written informed consent was given by all study participants. The study complied with the Declaration of Helsinki and was approved by the Regional Committees for Medical Research Ethics in Sweden and Norway.
Twelve-lead. electrocardiogram, signal-averaged ECG, and 24-h Holter monitoring
Twelve-lead ECG was obtained, and the configuration of PVC was analysed according to their likely site of origin as described previously by Zhang et al.13 Signal-averaged ECG was performed using a MAC® 5000-analysing system (GE Medical Systems, Milwaukee, WI, USA). Time domain analysis was obtained in the band-pass filter 40–250 Hz. Late potentials were considered present if ≥1 of the following parameters were abnormal: total filtered QRS duration ≥114 ms, the low amplitude (<40 µV) late signal duration ≥38 ms, and the last (40 ms) QRS root-mean-square voltage ≤20 µV. Twenty-four-hour Holter monitoring was performed using Schiller medilog®AR4 and AR4+ to assess VT and frequency of PVC in percent of total heart beats in 24 h (%PVC).
Echocardiographic studies
Patients underwent echocardiographic examination (Vivid 7 or Vivid E9, GE Vingmed, Horten, Norway). Data were digitally stored for off-line analysis (EchoPac®, GE Vingmed). Echocardiographic analyses were performed blinded to clinical data. We assessed RVOT diameter from the parasternal short axis and RV basal diameter (RVD) (both indexed by body surface area), RV fractional area change (RV FAC), and tricuspid annular plane systolic excursion (TAPSE) from the four-chamber view. Strain analyses were performed by 2D speckle-tracking echocardiography. RV strain by speckle-tracking technique was traced from the four-chamber view focusing on the right ventricle.14 Peak negative longitudinal strains from the RV free wall segments were averaged as RV strain (Figure 1). Time-to-peak longitudinal strain was defined as the time from onset of Q/R on ECG to peak negative longitudinal RV strain.15 RV mechanical dispersion was defined as the standard deviation of time-to-peak strain from RV free wall segments. From the left ventricle (LV), we assessed end-diastolic diameter (LVEDD), end-diastolic volume and end-systolic volume (all indexed by body surface area), ejection fraction (LVEF) by the modified Simpson's biplane method, and cardiac output.14 LV strain was traced from the three apical views. LV global longitudinal strain (LVGLS) and LV mechanical dispersion were calculated similarly to RV from 16 LV segments.16,17 Echocardiographic parameters were considered abnormal according to current guidelines.2,14,18
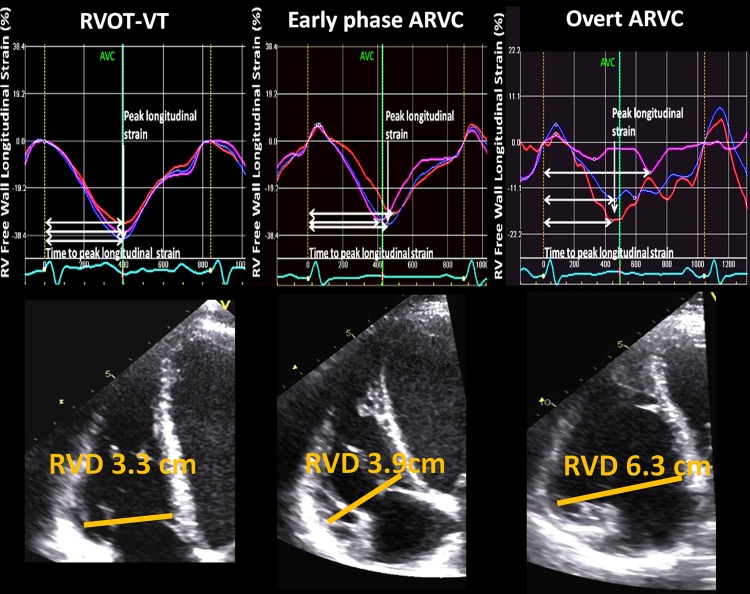
Figure 1.
Echocardiographic longitudinal strain curves from an RVOT patient, an early-phase ARVC patient, and an ARVC patient with overt disease. Upper panels: RV free wall longitudinal strain curves from the apical RV-focused four-chamber view in patients with RVOT-VT (left panel), early-phase ARVC (mid-panel), and overt ARVC (right panel). Vertical white arrow indicates the amplitudes of RV strain curves, which were calculated as the average peak negative longitudinal strain in three RV free wall segments. Horizontal white arrows indicate time-to-peak strain, defined as time from onset of Q/R on the ECG to peak negative longitudinal strain. The standard deviation of time-to-peak strain in the same three RV segments was defined as RV mechanical dispersion, reflecting contraction inhomogeneity. Patients with early-phase ARVC (mid-panel) showed more pronounced RV mechanical dispersion compared with RVOT-VT patients (left panel). Lower panels: Measures of RV basal diameters in these patients. Patients with early-phase ARVC (mid-panel) had larger diameters compared with patients with RVOT-VT (left panel). ARVC, arrhythmogenic right ventricular cardiomyopathy; AVC, aortic valve closure; RV, right ventricle; RVD, RV basal diameter; RVOT, right ventricular outflow tract; VT, ventricular tachycardia.
Cardiac magnetic resonance imaging
Cardiac magnetic resonance imaging (CMR) was performed in a subset of patients (n = 80), in a 1.5 Tesla unit (Magnetom Sonata, Vision Plus or Avanto Siemens, Erlangen, Germany) using a phased array body coil. The RV free wall was imaged by axial and sagittal breath-hold T1-weighted turbo spin echo technique. Standard long- and short-axis breath-hold cine sequences (fast imaging with steady-state free precession, trueFISP) were obtained to cover both ventricles.19 RV and LV volumes and ejection fractions were calculated by summation of the luminal areas of the short-axis images at end-diastole and end-systole, including the RV and LV outflow tracts, but excluding the papillary muscles.20 Post-processing analyses were performed using Qmass® MR 6.1.6 software (Medis Medical Imaging Systems, Leiden, the Netherlands). Inter- and intraobserver analyses for RVEF were performed in 10 patients.
Genetic analyses
Genomic DNA was isolated from peripheral blood from a subset of RVOT-VT patients and from ARVC index patients and their family members. The individual exons with flanking intron sequences of the genes plakophilin-2 (PKP2), desmocollin-2 (DSC2), desmoglein-2 (DSG2), desmoplakin (DSP), and 29 of the 105 exons of the ryanodine receptor-2 (RYR2) gene were sequenced by polymerase chain reaction amplification in combination with direct sequencing. Index patients with confirmed pathogenic mutations in these genes were defined as ARVC mutation positive, and mutation-positive family members were included by cascade genetic screening.
Statistical analyses
Data were presented as mean ± standard deviation. Differences between groups were assessed by χ2 test and Fisher's exact test for categorical variables and unpaired Student's t-test for continuous variables (SPSS 20.0, Inc., Chicago, IL). Logistic regression was used to find predictors of early-phase ARVC. Parameters with P-values <0.05 from univariable analyses were included in multivariable analyses. C-statistics were calculated by receiver operating characteristic (ROC) curves to assess the parameters' ability to differentiate between early-phase ARVC and RVOT-VT patients. The value closest to the upper left corner of the ROC curve was defined as giving optimal sensitivity and specificity. Two-sided P-values <0.05 were considered significant.
Results
Clinical characteristics
In all, 177 patients were screened for participation in the study, 56 patients with suspected RVOT-VT and 121 ARVC subjects. Patients with suspected RVOT-VT were referred to our hospital for clinical workup and eventual radio frequency catheter ablation. Clinical workup showed that 12 (21%) of these patients had a different aetiology of ventricular arrhythmias, 7 had definite ARVC by 2010 TFC, 3 had LVOT-VT, 1 had ischaemic heart disease, and 1 had dilated cardiomyopathy, and they were excluded from the RVOT-VT group. The RVOT-VT population therefore included 44 patients (Table 1). Of these, 26 (59%) were genetically tested for ARVC-related mutations with negative results.
Table 1.
Clinical characteristics in 44 RVOT-VT and 121 ARVC patients
| RVOT-VT (n = 44) | Total ARVC (n = 121) | P-value vs. RVOT-VT | Early-phase ARVC (n = 44) | P-value vs. RVOT-VT | |
|---|---|---|---|---|---|
| Age at diagnosis (years) | 47 ± 14 | 42 ± 17 | 0.08 | 39 ± 17 | 0.01 |
| Male gender (n) | 13 (30%) | 69 (57%) | <0.01 | 22 (50%) | 0.08 |
| BSA (m2) | 1.9 ± 0.2 | 1.9 ± 0.2 | 0.88 | 1.9 ± 0.2 | 0.86 |
| Heart rate (bpm) | 66 ± 13 | 63 ± 13 | 0.19 | 68 ± 13 | 0.61 |
| Syncope (n) | 25 (61%) | 44 (44%) | 0.06 | 12 (29%) | <0.01 |
| Ventricular arrhythmia (n) (TFC) | 29 (69%) | 74 (62%) | 0.43 | 9 (21%) | <0.001 |
| Sustained VT (n) | 5 (14%) | 65 (55%) | <0.001 | 5 (12%) | 1.00 |
| Non-sustained VT (n) | 24 (67%) | 9 (8%) | <0.001 | 4 (9%) | <0.001 |
| ACA (n) | 0 (0%) | 13 (13%) | 0.01 | 1 (2%) | 0.48 |
| RFA (n) | 22 (50%) | 45 (43%) | 0.42 | 4 (10%) | <0.001 |
| ICD (n) | 4 (9%) | 61 (50%) | <0.001 | 6 (14%) | 0.50 |
| SAECG ≥1 pathological value (TFC) | 16 (46%) | 50 (55%) | 0.35 | 12 (34%) | 0.33 |
| %PVC by Holter | 18.6 ± 15.3 | 1.9 ± 6.5 | <0.001 | 1.5 ± 7.7 | <0.001 |
| Beta-blocker (n) | 42 (98%) | 67 (65%) | <0.001 | 11 (26%) | <0.001 |
| Amiodarone (n) | 2 (5%) | 26 (25%) | <0.01 | 1 (2%) | 1.00 |
| ARVC-related mutation (n) TFC | 0 (0%) | 82 (75%) | <0.001 | 37 (84%) | <0.001 |
Mean ± SD, P by Student's t-test.
ACA, aborted cardiac arrest; ARVC, arrhythmogenic right ventricular cardiomyopathy; BSA, body surface area; ICD, implantable cardioverter defibrillator; PVC, premature ventricular complexes; RFA, radio frequency ablation; RV, right ventricle; RVOT, right ventricular outflow tract; SAECG, signal-averaged electrocardiogram; TFC, ARVC 2010 Task Force Criteria; VT, ventricular tachycardia.
We included 121 ARVC patients, 73 (60%) were index patients, and 48 (40%) were mutation-positive family members. Definite ARVC was diagnosed in 77 (64%), and 44 (36%) had early-phase ARVC.
Comparison between RVOT-VT patients and the total ARVC population
RVOT-VT patients were more frequently female compared with the total ARVC population (P < 0.01) (Table 1). Ventricular arrhythmias occurred in 29 (69%) RVOT-VT and in 74 (62%) ARVC patients (P = 0.43) (Table 1). Aborted cardiac arrest occurred in 13 patients, all of whom had ARVC. RVOT-VT patients had less frequently sustained VT (P < 0.001), but more frequently non-sustained VT (P < 0.001) and more frequent PVC during Holter monitoring (P < 0.001) compared with ARVC patients (Table 1). Parameters obtained by signal-averaged ECG were less pathological in RVOT-VT patients compared to ARVC (all P < 0.05).
Imaging results
By echocardiography, 44 (100%) RVOT-VT and 119 (98%) ARVC patients could be evaluated. A subset of 23 (52%) RVOT-VT patients and 57 (47%) ARVC patients had assessable CMR examinations (P = 0.39).
All RV diameters were within normal range in the RVOT-VT patients compared with increased RV diameters in the total ARVC population (all P < 0.05) (Table 2). Furthermore, RV function was decreased both by echocardiography and by CMR, and RV mechanical dispersion was pronounced in ARVC patients (all P < 0.001), whereas RV function was in the lower normal range in RVOT-VT patients (Tables 2 and 3). LV function was reduced by echocardiographic LVEF and LVGLS, and LV mechanical dispersion was more pronounced in ARVC compared with RVOT-VT (Table 2).
Table 2.
Echocardiographic parameters in 44 RVOT-VT and 119 ARVC patients
| RVOT-VT (n = 44) | Total ARVC (n = 119) | P-value vs. RVOT-VT | Early-phase ARVC (n = 43) | P-value vs. RVOT- VT | |
|---|---|---|---|---|---|
| RV | |||||
| RV wall motion abnormality (TFC) | 4 (9%) | 50 (42%) | <0.001 | 3 (7%) | 1.00 |
| RVOT diameter (mm/m2) (TFC) | 17 ± 3 | 19 ± 4 | <0.01 | 18 ± 3 | 0.17 |
| RVD (mm/m2) | 19 ± 2 | 23 ± 4 | <0.001 | 21 ± 3 | <0.01 |
| RV FAC (%) (TFC) | 46 ± 5 | 38 ± 11 | <0.001 | 46 ± 7 | 0.96 |
| TAPSE (mm) | 22 ± 4 | 18 ± 5 | <0.001 | 20 ± 4 | 0.09 |
| RV strain (%) | −27.1 ± 5.0 | −22.7 ± 7.3 | <0.001 | −26.4 ± 5.3 | 0.53 |
| RVMD (ms) | 15 ± 11 | 32 ± 29 | <0.001 | 22 ± 15 | 0.03 |
| LV | |||||
| Cardiac output (L/min) | 4.4 ± 1.2 | 3.8 ± 1.2 | <0.01 | 4.2 ± 1.1 | 0.52 |
| LVEDD (mm/m2) | 27 ± 3 | 27 ± 3 | 0.44 | 27 ± 4 | 0.97 |
| LVEDV (mL/m2) | 61 ± 11 | 58 ± 17 | 0.29 | 58 ± 11 | 0.16 |
| LVESV (mL/m2) | 26 ± 7 | 27 ± 14 | 0.61 | 25 ± 6 | 0.53 |
| LVEF (%) | 57 ± 5 | 54 ± 8 | 0.03 | 58 ± 4 | 0.85 |
| LVGLS (%) | −21.3 ± 2.7 | −18.5 ± 3.7 | <0.001 | −19.9 ± 2.5 | 0.02 |
| LVMD (ms) | 39 ± 12 | 54 ± 27 | <0.01 | 40 ± 11 | 0.49 |
Mean ± SD, P by Student's t-test.
ARVC, arrhythmogenic right ventricular cardiomyopathy; LV, left ventricle; LVEDD, indexed left ventricular end-diastolic diameter; LVEDV, indexed left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVGLS, left ventricular global longitudinal strain; LVESV, indexed left ventricular end-systolic volume; LVMD, left ventricular mechanical dispersion; RV, right ventricle; RVD, indexed right ventricular basal diameter; RVFAC, right ventricular fractional area change; RVMD, RV mechanical dispersion; RVOT, indexed right ventricular outflow tract; TAPSE, tricuspid annular plane systolic excursion; TFC, ARVC 2010 Task Force Criteria.
Table 3.
CMR parameters in 23 RVOT-VT and 57 ARVC patients
| Clinical categories | RVOT-VT (n = 23) | ARVC total (n = 57) | P-value vs. RVOT-VT | Early-phase ARVC (n = 19) | P-value vs. RVOT-VT |
|---|---|---|---|---|---|
| Male gender (n) | 8 (35%) | 32 (56%) | 0.08 | 10 (53%) | 0.25 |
| Age at diagnosis (years) | 44 ± 14 | 42 ± 16 | 0.76 | 36 ± 17 | 0.14 |
| BSA (m2) | 1.9 ± 0.2 | 1.9 ± 0.2 | 0.65 | 1.9 ± 0.2 | 0.94 |
| RVEDV (mL/m2) (TFC) | 75 ± 17 | 88 ± 36 | 0.09 | 69 ± 19 | 0.36 |
| RVESV (mL/m2) | 38 ± 9 | 56 ± 33 | <0.01 | 40 ± 12 | 0.43 |
| RVEF (%) (TFC) | 49 ± 4 | 39 ± 12 | <0.001 | 41 ± 8 | <0.001 |
| LVEDV (mL/m2) | 74 ± 21 | 74 ± 18 | 0.90 | 69 ± 17 | 0.51 |
| LVESV (mL/m2) | 35 ± 14 | 37 ± 13 | 0.59 | 34 ± 10 | 0.78 |
| LVEF (%) | 53 ± 6 | 51 ± 8 | 0.21 | 52 ± 7 | 0.39 |
Mean ± SD, P by Student's t-test.
ARVC, arrhythmogenic right ventricular cardiomyopathy; BSA, body surface area; CMR, cardiac magnetic resonance imaging; LVEDV, indexed left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, indexed left ventricular end-systolic volume; RVEDV, indexed right ventricular end-diastolic volume; RVEF, right ventricular ejection fraction; RVESV; indexed right ventricular end-systolic volume; RVOT, right ventricular outflow tract; TFC, ARVC 2010 Task Force Criteria.
Intra- and interobserver intraclass correlation for RVEF by CMR was 0.96 (95% CI 0.86–0.99) and 0.92 (95% CI 0.66–0.98), respectively.
Comparison between patients with RVOT-VT and with early-phase ARVC
Of the 44 early-phase ARVC patients, 35 (80%) were mutation-positive family members and 2 (4%) were mutation-positive index patients. The remaining 7 (16%) were index patients with borderline (n = 6) and possible (n = 1) ARVC. These 7 patients had experienced ventricular arrhythmias suggestive of ARVC, in addition to having pathologic signal-averaged ECG (n = 5), RV dysfunction or fatty infiltration by CMR (n = 4), and dilated RVD (n = 4); hence, their phenotypes were considered clinically suggestive of early-phase ARVC.
Early-phase ARVC patients were younger than the RVOT-VT patients at diagnosis (P = 0.01) and had less frequently ventricular arrhythmias and PVC (Table 1). Lower %PVC by Holter was a strong and independent marker of early-phase ARVC (Table 1) [adjusted odds ratio (OR) 0.77 (95% CI 0.64–0.92) P < 0.01]. A %PVC of <2 discriminated optimally between early-phase ARVC and RVOT-VT with an AUC of 0.93 (95% CI 0.86–1.00) by C-statistics. ECG morphologies of the PVC were available in 43 RVOT-VT patients and in 9 patients with early-phase ARVC (Table 4). In patients with RVOT-VT, site of origin of PVC was mainly the septal part of the RVOT (98%), while only 22% of patients with early-phase ARVC had this origin (P < 0.001). In early-phase ARVC, 56% had origin of PVC in the RV free wall and 22% had PVC origin in the LV.
Table 4.
Results from analysis of ECG pattern of PVC/VT in early-phase ARVC vs. RVOT-VT to identify the site/focus of arrhythmia according to the ECG algorithm of Zhang et al.13
| Site of origin of arrhythmia according to ECG pattern1 | RVOT-VT (n = 43) | Early-phase ARVC (n = 9) | P-value |
|---|---|---|---|
| RVOT free wall | 1 (2%) | 5 (56%) | <0.001 |
| RVOT septum | 42 (98%) | 2 (22%) | <0.001 |
| LV | 0 (0%) | 2 (22%) | 0.03 |
P by Fisher's exact test.
ARVC, arrhythmogenic right ventricular cardiomyopathy; ECG, electrocardiogram; LV, left ventricle; PVC, premature ventricular complexes; RV, right ventricle; RVOT, right ventricular outflow tract; VT, ventricular tachycardia.
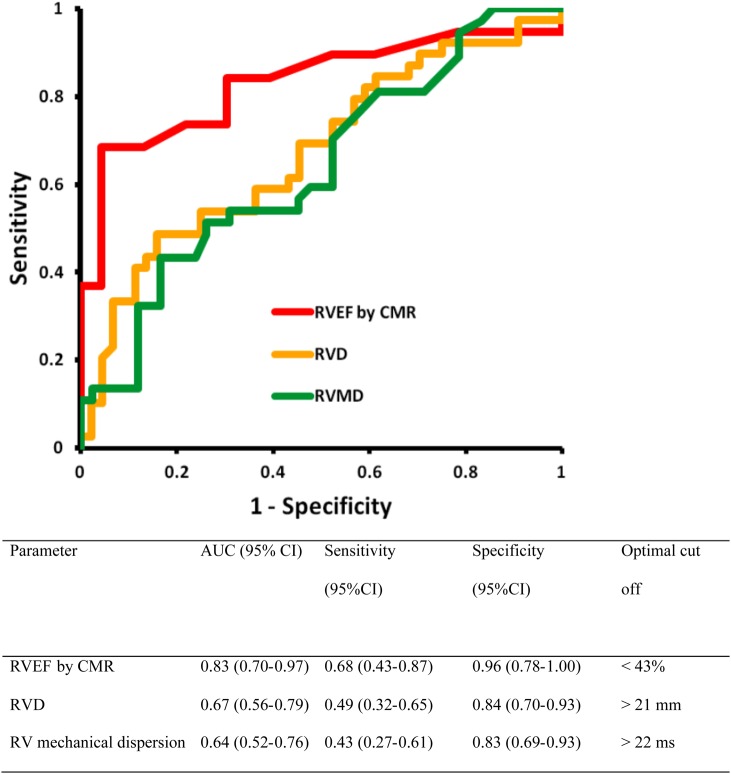
RVD, RV mechanical dispersion, LVGLS, and RVEF by CMR were markers of early-phase ARVC (all P < 0.05 compared with RVOT-VT, Tables 2 and 3). In multivariable analysis, RVD and RV mechanical dispersion were the only independent imaging predictors of early-phase ARVC (Table 5). RVEF <43%, RVD >21 mm, and RV mechanical dispersion >22 ms had optimal ability to discriminate between RVOT-VT and ARVC (Figure 2).
Table 5.
Multivariable analysis of parameters to predict the status of early-phase ARVC (n = 44) vs. RVOT-VT (n = 44)
| Parameter | OR | 95% CI | P-value |
|---|---|---|---|
| Age at diagnosis (years) | 0.96 | 0.90–1.03 | 0.23 |
| RVEF by CMR (%) | 0.88 | 0.76–1.02 | 0.09 |
| RVD (mm/m2) | 2.29 | 1.10–4.78 | 0.03 |
| RV mechanical dispersion (ms) | 1.09 | 1.00–1.18 | 0.04 |
| LVGLS (%) | 1.41 | 0.84–2.34 | 0.19 |
ARVC, arrhythmogenic right ventricular cardiomyopathy; CI, confidence interval; CMR, cardiac magnetic resonance imaging; LVGLS, left ventricular global longitudinal strain; RVD, indexed right ventricular basal diameter; RVEF, right ventricular ejection fraction; RVOT, right ventricular outflow tract; VT, ventricular tachycardia.
Figure 2.
ROC curves for the ability of parameters to discriminate between 44 patients with early-phase ARVC and 44 RVOT-VT patients. RVEF by CMR and RVD and RV mechanical dispersion by echocardiography had good ability to discriminate early-phase ARVC from RVOT-VT patients. ARVC, arrhythmogenic right ventricular cardiomyopathy; AUC, area under the curve; CI, confidence interval; CMR, cardiac magnetic resonance imaging; RVD, indexed right ventricular basal diameter; RVEF, right ventricular ejection fraction; RVMD, right ventricular mechanical dispersion.
Discussion
This study presents a new approach to compare early-phase ARVC from RVOT-VT patients. Early-phase ARVC patients had less PVC, slightly enlarged RV diameter, more dispersed RV contraction, and reduced RV function by CMR compared with RVOT-VT patients. These findings indicate that myocardial function is affected also in early-phase ARVC disease and that analyses of both structural and functional changes can help to discriminate early-phase ARVC from RVOT-VT. In patients with an unclear phenotype, the number of PVC and a few imaging parameters may help discrimination between early-phase ARVC and RVOT-VT patients.
Clinical characteristics and ventricular arrhythmias
Current workup in ARVC and RVOT-VT patients includes clinical, electrocardiographic (ECG), echocardiographic, and CMR studies, and genetic analyses may be indicated in suspected ARVC cases. The differentiation between ARVC and RVOT-VT is obvious when the ARVC phenotype is fully expressed. As expected, our total ARVC population had more severe disease, indicated by more frequent aborted cardiac arrest and clearly pathological structural and functional cardiac changes. However, differentiation between RVOT-VT and early-phase ARVC remains challenging. Interestingly, one-fifth of the referred RVOT-VT patients screened for participation in our study were excluded due to other aetiology of their arrhythmias, and more than half of these fulfilled the 2010 TFC for definite ARVC.
Early-phase ARVC patients were younger than the RVOT-VT patients, a result of the early detection of ARVC mutation positive by family screening. Furthermore, early-phase ARVC patients had less frequent PVC and arrhythmias, with <2%PVC in early-phase ARVC compared with 18%PVC in RVOT-VT patients and less frequent nsVT. The relatively low number of PVC in early-phase ARVC and the frequent origin of PVC in the lateral free wall have also been reported by others9,13,21,22 and emphasize that RVOT-VT is most likely in patients with very frequent monomorphic PVC originating from the septal part of the RVOT. Importantly, severe arrhythmias were more prevalent in ARVC compared with RVOT-VT, and all aborted cardiac arrests occurred only in ARVC patients, highlighting the different prognosis of these conditions.
Structural and functional findings
All RV imaging parameters used in the ARVC 2010 TFC2 were pathological in ARVC patients compared with low normal values in RVOT-VT patients. However, only RV diameter and RV mechanical dispersion were independent markers of early-phase ARVC disease. RV mechanical dispersion has previously been reported as a marker of arrhythmias in ARVC,23 and this study showed that mechanical dispersion can also distinguish between early-phase ARVC and RVOT-VT. Mechanical dispersion may have the ability to detect subtle changes in contraction and might be explained by diffuse cell necrosis causing electrical and myocardial remodelling in ARVC in contrast to RVOT-VT.
LV function by global longitudinal strain was reduced in early-phase ARVC compared with RVOT-VT patients, indicating subtle biventricular affection also in early stages of ARVC.12,23
In this study, RVEF by CMR was the best imaging parameter to discriminate between early-phase ARVC and RVOT-VT. However, RVEF is included in the ARVC 2010 TFC and was used as a primary parameter to differentiate between RVOT-VT and ARVC patients, and the impact of RVEF may therefore be overestimated. Some previous studies have suggested that the majority of RVOT-VT patients have structurally normal hearts by CMR,24,25 while others found subtle RV abnormalities by CMR which were associated with worse outcome.26 However, evaluation of a patient's diagnosis should not rely on one single parameter, and we propose the combined use of CMR and echocardiographic parameters to discriminate early-phase ARVC from RVOT-VT. The echocardiographic parameters can be of help to select patients for further CMR studies and in centers where CMR is not widely available.
Clinical implications
One-fifth of referred RVOT-VT patients were re-diagnosed, and more than half of these had definite ARVC reflecting that discrimination between these diseases is challenging. Our findings indicate that patients with no structural abnormalities and very frequent PVC with appropriate morphology were more likely to have RVOT-VT than early-phase ARVC. Patients with a moderate number of PVC (in our study <2%PVC/24 h) and suspected RVOT-VT should be evaluated by the ARVC 2010 TFC to detect those fulfilling definite ARVC criteria to avoid overlooking the more severe ARVC disease. The majority of our patients with early-phase ARVC were genetic positive, and therefore we had a genetic gold standard for our diagnosis. However, 50% of ARVC patients in general are reported to be genetic negative or with variants of uncertain significance. In these patients and in patients with unclear phenotypes, the number of PVC and a few CMR and echocardiographic parameters may help the discrimination between early-phase ARVC and RVOT-VT patients.
Limitations
This study had a cross-sectional design, and future studies should follow both ARVC and RVOT-VT patients for a longer period of time to explore overlap between the diagnoses and deterioration in cardiac function. Although we excluded all patients with definite ARVC criteria from our RVOT-VT population, we cannot exclude that patients diagnosed with RVOT-VT in our study will develop criteria for ARVC in future. Only a subset of patients (n = 80, 48%) had an appropriate CMR study, which may have influenced our results. However, equal proportions of early-phase ARVC and RVOT-VT patients had CMR studies with no significant differences in characteristics (Table 3).
Conclusions
As expected, the total ARVC population had more severe structural pathology compared with RVOT-VT patients. In early-phase ARVC, %PVC was the most powerful marker to distinguish ARVC from RVOT-VT, and >2%PVC per 24 h was suggestive of RVOT-VT diagnosis. By imaging, early-phase ARVC patients had slightly lower RV function, more pronounced RV mechanical dispersion, and larger RV diameter compared with RVOT-VT patients. Careful cardiac imaging may help to correctly diagnose early-phase ARVC and RVOT-VT patients and aid treatment decisions.
Funding
This work was supported by the South-Eastern Norway Regional Health Authority and the Center for Cardiological Innovation, and funded by the Research Council of Norway, Simon Fougner Hartmanns family foundation, and research grants from governmental funding of clinical research within the Swedish National Health Service and the Swedish Heart-Lung Foundation. Funding to pay the Open Access publication charges for this article was provided by Center for Cardiological Innovation funded by the Norwegian Research Council.
Conflict of interest: none declared.
References
- 1.Viskin S, Rosso R, Rogowski O, Belhassen B. The ‘short-coupled’ variant of right ventricular outflow ventricular tachycardia: a not-so-benign form of benign ventricular tachycardia? J Cardiovasc Electrophysiol 2005;16:912–6. [DOI] [PubMed] [Google Scholar]
- 2.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010;121:1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy in Lancet. Lancet 2009;373:1289–300. [DOI] [PubMed] [Google Scholar]
- 4.Corrado D, Wichter T, Link MS, Hauer RN, Marchlinski FE, Anastasakis A et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Circulation 2015;132:441–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takemoto M, Yoshimura H, Ohba Y, Matsumoto Y, Yamamoto U, Mohri M et al. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol 2005;45:1259–65. [DOI] [PubMed] [Google Scholar]
- 6.te Riele AS, James CA, Rastegar N, Bhonsale A, Murray B, Tichnell C et al. Yield of serial evaluation in at-risk family members of patients with ARVD/C. J Am Coll Cardiol 2014;64:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhonsale A, Groeneweg JA, James CA, Dooijes D, Tichnell C, Jongbloed JD et al. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers. Eur Heart J 2015;36:847–55. [DOI] [PubMed] [Google Scholar]
- 8.Corrado D, Basso C, Pilichou K, Thiene G. Molecular biology and clinical management of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart 2011;97:530–9. [DOI] [PubMed] [Google Scholar]
- 9.Lerman BB. Mechanism, diagnosis, and treatment of outflow tract tachycardia. Nat Rev Cardiol 2015;12:597–608. [DOI] [PubMed] [Google Scholar]
- 10.Kim RJ, Iwai S, Markowitz SM, Shah BK, Stein KM, Lerman BB. Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J Am Coll Cardiol 2007;49:2035–43. [DOI] [PubMed] [Google Scholar]
- 11.Buxton AE, Waxman HL, Marchlinski FE, Simson MB, Cassidy D, Josephson ME. Right ventricular tachycardia: clinical and electrophysiologic characteristics. Circulation 1983;68:917–27. [DOI] [PubMed] [Google Scholar]
- 12.Saberniak J, Hasselberg NE, Borgquist R, Platonov PG, Sarvari SI, Smith HJ et al. Vigorous physical activity impairs myocardial function in patients with arrhythmogenic right ventricular cardiomyopathy and in mutation positive family members. Eur J Heart Fail 2014;16:1337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang F, Chen M, Yang B, Ju W, Chen H, Yu J et al. Electrocardiographic algorithm to identify the optimal target ablation site for idiopathic right ventricular outflow tract ventricular premature contraction. Europace 2009;11:1214–20. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–71. [DOI] [PubMed] [Google Scholar]
- 15.Haugaa KH, Goebel B, Dahlslett T, Meyer K, Jung C, Lauten A et al. Risk assessment of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy by strain echocardiography. J Am Soc Echocardiogr 2012;25:667–73. [DOI] [PubMed] [Google Scholar]
- 16.Edvardsen T, Haugaa KH. Imaging assessment of ventricular mechanics. Heart 2011;97:1349–56. [DOI] [PubMed] [Google Scholar]
- 17.Haugaa KH, Amlie JP, Berge KE, Leren TP, Smiseth OA, Edvardsen T. Transmural differences in myocardial contraction in long-QT syndrome: mechanical consequences of ion channel dysfunction. Circulation 2010;122:1355–63. [DOI] [PubMed] [Google Scholar]
- 18.Badano LP, Miglioranza MH, Edvardsen T, Colafranceschi AS, Muraru D, Bacal F et al. European Association of Cardiovascular Imaging/Cardiovascular Imaging Department of the Brazilian Society of Cardiology recommendations for the use of cardiac imaging to assess and follow patients after heart transplantation. Eur Heart J Cardiovasc Imaging 2015;16:919–48. [DOI] [PubMed] [Google Scholar]
- 19.Edvardsen T, Gerber BL, Garot J, Bluemke DA, Lima JA, Smiseth OA. Quantitative assessment of intrinsic regional myocardial deformation by Doppler strain rate echocardiography in humans: validation against three-dimensional tagged magnetic resonance imaging. Circulation 2002;106:50–6. [DOI] [PubMed] [Google Scholar]
- 20.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixit S, Gerstenfeld EP, Callans DJ, Marchlinski FE. Electrocardiographic patterns of superior right ventricular outflow tract tachycardias: distinguishing septal and free-wall sites of origin. J Cardiovasc Electrophysiol 2003;14:1–7. [DOI] [PubMed] [Google Scholar]
- 22.Bhonsale A, James CA, Tichnell C, Murray B, Madhavan S, Philips B et al. Risk stratification in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. Circ Arrhythm Electrophysiol 2013;6:569–78. [DOI] [PubMed] [Google Scholar]
- 23.Sarvari SI, Haugaa KH, Anfinsen OG, Leren TP, Smiseth OA, Kongsgaard E et al. Right ventricular mechanical dispersion is related to malignant arrhythmias: a study of patients with arrhythmogenic right ventricular cardiomyopathy and subclinical right ventricular dysfunction. Eur Heart J 2011;32:1089–96. [DOI] [PubMed] [Google Scholar]
- 24.Markowitz SM, Weinsaft JW, Waldman L, Petashnick M, Liu CF, Cheung JW et al. Reappraisal of cardiac magnetic resonance imaging in idiopathic outflow tract arrhythmias. J Cardiovasc Electrophysiol 2014;25:1328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tandri H, Bluemke DA, Ferrari VA, Bomma C, Nasir K, Rutberg J et al. Findings on magnetic resonance imaging of idiopathic right ventricular outflow tachycardia. Am J Cardiol 2004;94:1441–5. [DOI] [PubMed] [Google Scholar]
- 26.Aquaro GD, Pingitore A, Strata E, Di BG, Molinaro S, Lombardi M. Cardiac magnetic resonance predicts outcome in patients with premature ventricular complexes of left bundle branch block morphology. J Am Coll Cardiol 2010;56:1235–43. [DOI] [PubMed] [Google Scholar]