Abstract
Many neurons degenerate after injuries resulting from overstimulation, drugs, genetic mutations, and aging. Although several growth factors and neurotrophins delay degeneration and promote regrowth of neural processes, the role of fibroblast growth factor 8 (FGF8) in mammalian spiral ganglion neurons (SGN) neurite outgrowth has not been examined. This study develops and uses SGN cell cultures suitable for experimental analysis, it investigates whether FGF8a and FGF8b isoforms affect the neurite outgrowth from SGN cultured in vitro. We found that both FGF8a and FGF8b promoted the outgrowth of neurites from cultured SGN. This response is mediated by FGF receptors and involves the activation of IκBα-mediated NFκB signaling pathway. These findings suggest that, besides its morphogenetic role during development, FGF8 may have trophic functions in the adult which are relevant to regeneration.
Keywords: FGF8 isoforms, regeneration, trophic function, morphogen, development
1. INTRODUCTION
Fibroblast growth factor 8 (FGF8) is a member of the FGF family. It plays a pivotal role in the patterning of the brain during embryogenesis (Crossley and Martin 1995; Suzuki-Hirano and Shimogori 2009). Unlike other FGFs, a relatively large number of FGF8 isoforms has been described, which are products of alternative splicing (Crossley and Martin 1995; Olsen et al. 2005). Eight FGF8 isoforms have been found in the mouse (FGF8a-h) and four in humans (FGF8a, b, e and f). FGF8a and FGF8b have 100% sequence homology between mouse and human (Sunmonu et al. 2010). FGF8 elicits its effects by the activation of four different subtypes of tyrosine kinase FGF receptors, named FGFR1 through FGFR4 (Zhang et al. 2006). The FGF8a and FGF8b structures and their receptor-binding affinity properties have been well characterized (Olsen et al., 2005). The single residue phenylalanine 32 (F32) from the alternatively spliced N-terminal region of FGF8b confers on it a higher binding affinity than FGF8a toward the “c” isoforms of their receptors FGFR1–3 and FGFR4 (Olsen et al., 2005).
Previous studies in vitro have implicated other FGFs and neurotrophins in migration, differentiation, neurite outgrowth (Hossain et al. 2008; Hossain and Morest 2000; Zhou et al. 1996), survival (Pirvola et al. 1995) and regeneration of primary auditory neurons (Wang and Green 2011; Wei et al. 2007). The role of FGF8 in the nervous system, and particularly in the auditory system, has received less attention. For these reasons we have used the auditory system as a model to investigate the function of FGF8.
The cochlear spiral ganglion neurons (SGN) convey sensory information representing environmental sounds from the hair cells in the cochlea to the cochlear nucleus in the brain. SGN degenerate after injuries resulting from noise, drugs, genetic mutations, and aging (Bao and Ohlemiller 2010; White et al. 2000). Since SGN are the primary cells in a pathway linked to the development and maintenance of language, learning, communication and social interaction, it would be advantageous especially to arrest this pathology and promote regrowth of SGN. Since growth factors and neurotrophins can delay degeneration of SGN and other neurons, and promote regrowth of neural processes (Gillespie and Shepherd 2005; Klimaschewski et al. 2004; Miller et al. 2007; Sapieha et al. 2003), we investigated whether FGF8 promoted the growth of neurites from dissociated SGN cultured in vitro, and which signaling pathway might be involved. These cultures allowed accurate measurements to be made of individual neurites growing from the neurons.
In the auditory system, FGF8 is required during development for otic placode induction, pillar cell differentiation and the patterning of the organ of Corti (Jacques et al. 2007; Ladher et al. 2005; Leger and Brand 2002; Pirvola et al. 2000). It acts by activating FGFR1, FGFR2 or FGFR3 receptors, which are expressed in the cochlea during development (Hayashi et al. 2007; Jacques et al. 2007; Leger and Brand 2002; Oelling et al. 1995). FGF8 also functions as an axon guidance cue during CNS development (Irving et al. 2002). One could predict, therefore, that FGF8 may have a role in re-establishing synaptic connections after hearing loss (Webber and Raz 2006). For example, FGF8 may participate in axonal regeneration by promoting regrowth of SGN processes, since it promotes neurite outgrowth in explants of chicken vestibular and auditory neurons (Fantetti and Fekete 2011).
Based on the functions of FGF8 discussed above, we hypothesize that FGF8 may promote neurite outgrowth from mammalian SGN, a function that has not yet been investigated. The present study focuses on whether FGF8a and FGF8b isoforms could function as trophic factors in the mouse cochlea. Using dissociated mouse SGN cultured in vitro, we determined if these isoforms affect neurite outgrowth, and the possible receptors and signaling pathways involved.
2. RESULTS
2.1 DCX staining of the neurites
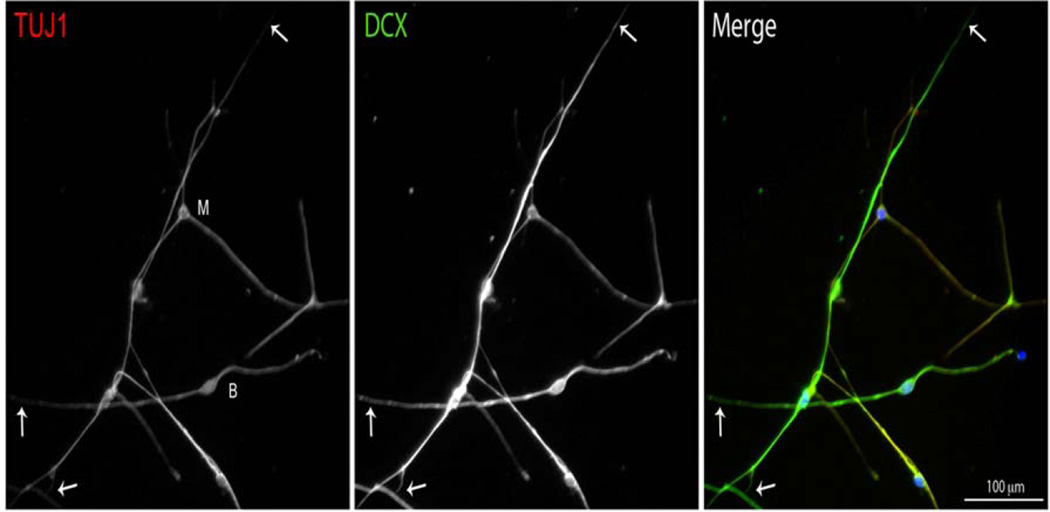
To measure the length of the neurites of SGN in vitro, we used two neuronal markers. In addition to the widely used neuronal marker directed against β-tubulin III (TUJ1), we used the microtubule-associated protein doublecortin (DCX), because of its distribution at the ends of neuritic processes (Friocourt et al. 2003). We found that, although both antibodies are specific for neurons, staining both the soma and the neurites of the SGN, the anti-DCX antibody stains the neurites out to their ends, which are labeled faintly or unlabeled with the anti-TUJ1 (Fig. 1). Therefore we used anti-DCX in the subsequent experiments to visualize neurites in vitro.
Fig. 1.
Localization of TUJ1 (βIII-Tubulin) and DCX in cultured neurons derived from P8 mouse cochlea. Each of these neuronal markers labels both the soma and the neurites of SGN in culture. DCX, however, extends into the distal parts of the neurites (arrows), farther than TUJ1. M (multipolar cell body), B (bipolar cell body).
As revealed by TUJ1 and DCX immunostaining (Figs. 1 and 3), we observed bipolar and multipolar SGN, which are typical of dissociated SGN (Whitlon et al., 2006) grown in culture and immature SGN (Hossain et al., 2008).
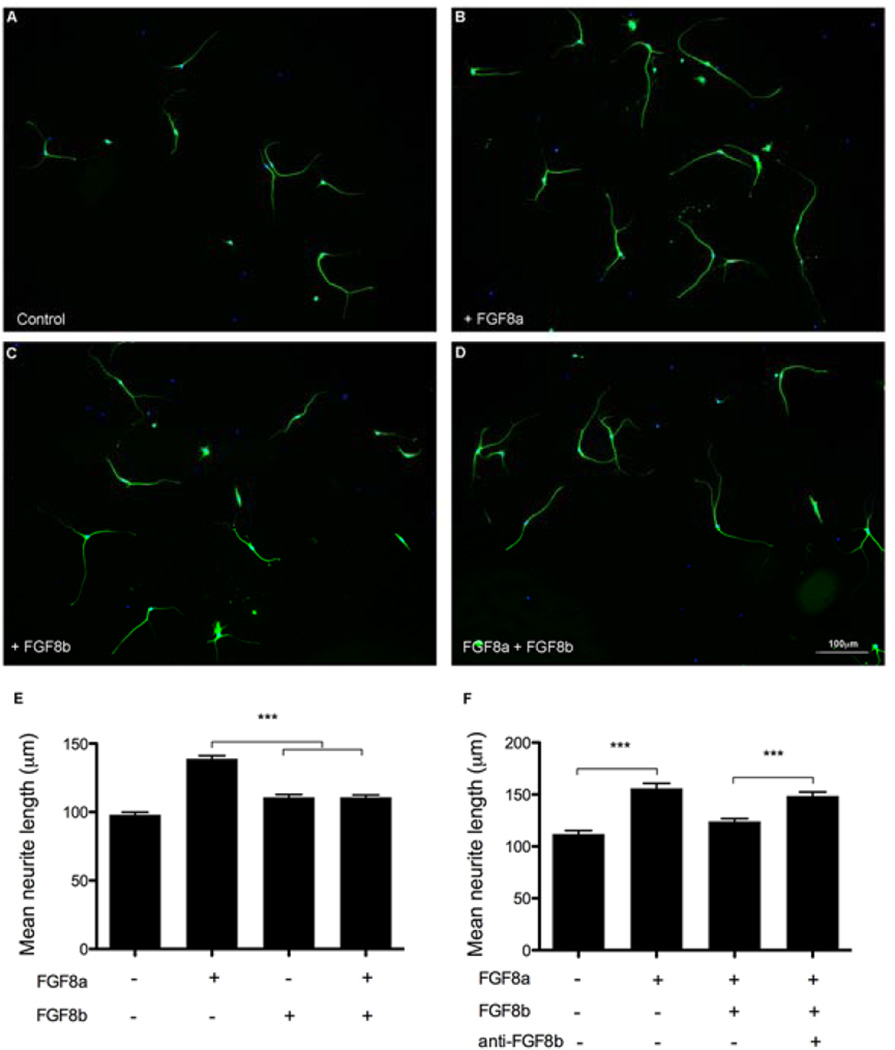
Fig. 3.
FGF8b blocks the FGF8a-promoted neurite outgrowth. Representative images are shown of cultured SGN with control medium (A), 250 ng/ml of FGF8a (B), 5 ng/ml of FGF8b (C) or both FGF8a and FGF8b (D). FGF8a and FGF8b independently promote neurite outgrowth to different extents, whereas when they are combined, only the effect of FGF8b is apparent (E). When FGF8b is neutralized by pre-incubation with anti-FGF8b, and then both FGF8a and the neutralized FGF8b are added to the medium, the effect of FGF8a is observed again (F). Results are expressed as mean ± SEM of neurite length; n = 50 to 80 neurites per condition from three independent experiments. Differences between treatments were determined by ANOVA followed by Tukey’s multiple comparison post-hoc test (***p < 0.001).
2.2. Survival of neurons with the treatments
We evaluated the effect of treatments on cell survival by counting the number of SGN per mm2. We found no significant differences between treatments (Table 1). This result rules out the possibility that the effect of treatments on neurite outgrowth can be attributed to differences in survival as reflected in the number of neurons.
Table 1.
The treatments did not affect neuronal survival. Neurons were stained with anti-DCX antibody 24 hrs after plating. Cultures were maintained in the absence of serum (control) and the presence of FGF8a (250 ng/ml), FGF8b (5 ng/ml), BAY11-7082 (125 nM) or PD173074 (100 nM). Cell counts are from independent micrographs (n) taken at 20×. Differences were evaluated with a one-way ANOVA (F= 0.8885, P= 0.4738, df = 4).
| Treatment | Neurons per mm2 (a) | n |
|---|---|---|
| Control | 9 ± 0.7 | 20 |
| FGF8a | 9 ± 0.7 | 22 |
| FGF8b | 10 ± 0.8 | 23 |
| BAY11-7082 | 11 ± 1.0 | 18 |
| PD173074 | 9 ± 0.8 | 20 |
Survival (labeled neurons),
Mean ± SE
2.3 FGF8a and FGF8b effects on SGN neurite outgrowth
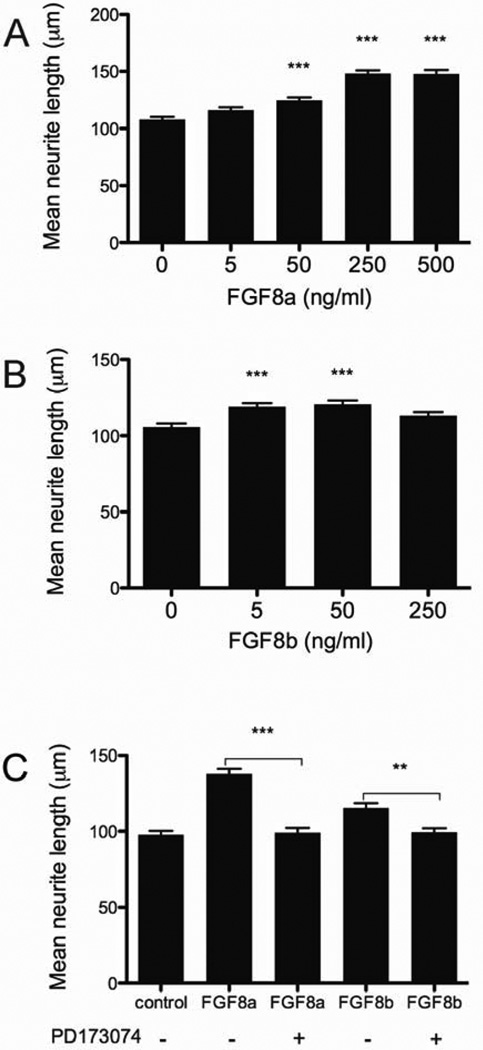
We tested the effects of FGF8a or FGF8b in vitro on the neurite outgrowth from primary auditory neurons. Although neither isoform had an effect on the survival of the SGN (Table 1), we found a 39.2 ± 3.1 (mean ± SE) percent increase in the length of neurites in cultures treated with 250 ng/ml of FGF8a, compared to untreated controls; at higher doses the FGF8a did not promote any further increase in the length of neurites (Fig. 2A). FGF8b promoted a 15.3 ± 2.8 (mean ± SE) percent increase of neurite outgrowth when it was present at 5 or 50 ng/ml. This stimulation was only 6.9 ± 2.3 (mean ± SE) percent when FGF8b was used at 250 ng/ml (Fig. 2B).
Fig. 2.
FGF8a and FGF8b promote cultured SGN-neurite outgrowth in a dose-dependent manner through FGF receptors. Neurite outgrowth was evaluated 20 hours after addition of FGFs (total of 24 hours). The maximum outgrowth was observed in the presence of 250 or 500 ng/ml of FGF8a (A) and 5 or 50 ng/ml of FGF8b (B). This effect was prevented by the FGF receptor inhibitor PD173074 (C). When used, the inhibitor (100nM) was added 15 minutes before the FGF8. Results are expressed as mean ± SEM of neurite length; n = 50 to 80 neurites per condition in each of three experiments. Differences were determined by ANOVA; comparison between treatments was determined by Tukey’s multiple comparison post-hoc test. In A and B, asterisks denote differences from the controls (***p < 0.001, **p < 0.05).
2.4 Effect of FGFR inhibitor on FGF8-stimulated neurite outgrowth
To determine if FGF8 receptors mediated the FGF8-stimulation of neurite outgrowth, we tested the effect of the FGF receptor inhibitor PD173074 (Bansal et al. 2003; Skaper et al. 2000). By exposing the cultures to 100 nM PD173074 and either FGF8a (250 ng/ml) or FGF8b (5 ng/ml), the FGF8-stimulated neurite outgrowth was suppressed (Fig. 2C). PD173074 did not have any additional effect on FGF8-stimulated neurite outgrowth when present at 200 nM (data not shown). In addition, the inhibitor had no discernible effect on SGN survival (Table 1) or on the morphology of the cell bodies. These findings suggest that FGF receptor activity may be required for FGF8-stimulated neurite outgrowth.
2.5 Interaction of FGF8a and FGF8b
To evaluate whether FGF8a or FGF8b isoforms have a synergistic or competitive action, we exposed the SGN cultures to both FGF8a and FGF8b at concentrations of 250 and 5 ng/ml, respectively. Despite the higher concentration of FGF8a, its effect on neurite outgrowth (Figs. 3B and E) was suppressed in the presence of FGF8b (Figs. 3D and E). In contrast, the effect of FGF8b remained the same, whether it was present alone or together with FGF8a (Figs. 3C, D and E). These findings are consistent with a higher affinity of FGF8b for the receptor, as reported previously (Olsen et al. 2006), and it suggests that FGF8b can competitively interfere with the action of FGF8a.
Therefore in another test, we first pre-incubated the FGF8b with the anti-FGF8b antibody, to neutralize its activity. Then we exposed the cultures to FGF8a, the combination of FGF8a and FGF8b, or the combination of FGF8a and the neutralized FGF8b. The neutralized FGF8b had no significant effect on the action of FGF8a on neurite outgrowth (Fig.3F). This suggested that FGF8a can exert its full effect on neurite outgrowth only when FGF8b is absent.
2.6 Effect of NFκB pathway inhibitor on FGF8a-neurite outgrowth
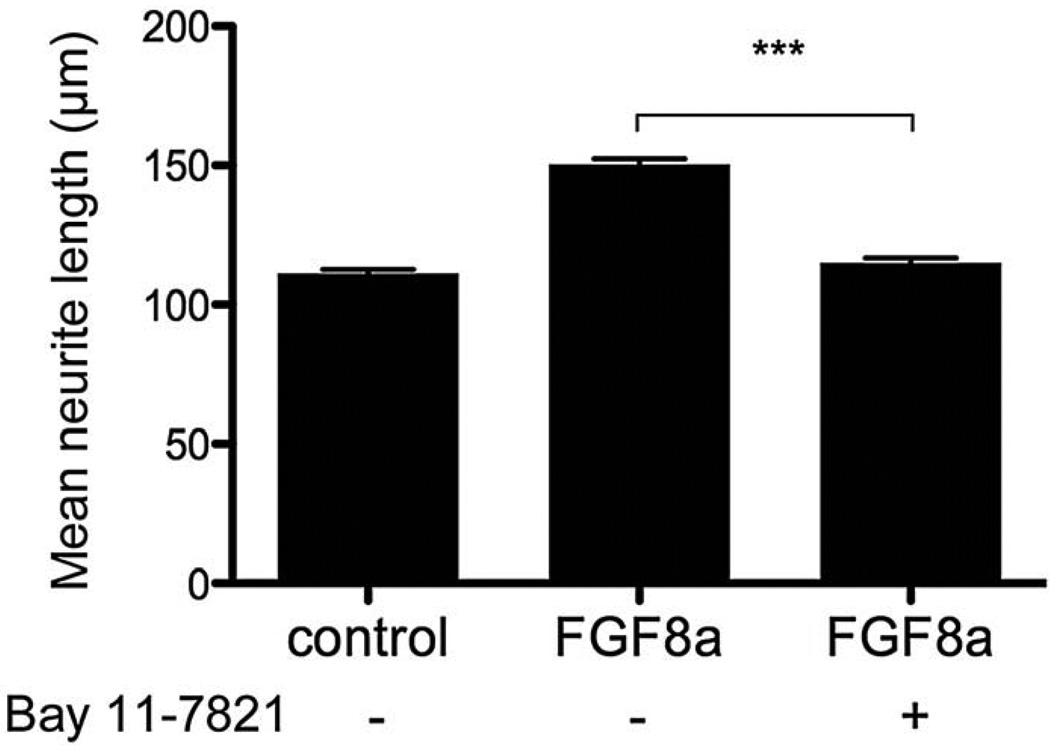
FGF receptors exert their effects by activating one or more signal transduction pathways (Reuss and von Bohlen und Halbach, 2003). Our preliminary studies investigated whether ERK, PI3K and NFκB pathways are involved in the FGF8-promoted neurite outgrowth. Our data indicated that inhibitors of the ERK (U0126, 50 nM) and PI3K (Wortmannin, 10 nM) pathways did not modify the FGF8-promoted neurite outgrowth (data not shown). By contrast, the NFκB inhibitor, BAY11-7082, suppressed the FGF8-neurite promoted outgrowth (Fig 4). In addition, the inhibitor had no discernible effect on SGN survival (Table 1). This suggests that FGF8 leads to neurite outgrowth by promoting the IκBα phosphorylation, hence activating the NFκB signaling pathway.
Fig. 4.
The FGF8a-promoted neurite outgrowth is prevented by an NFκB inhibitor. The NFκB inhibitor, BAY11-7082, prevents the FGF8a-promoted neurite outgrowth. The inhibitor (125 nM) was added 15 minutes before the FGF8a (250 ng/ml). Results are expressed as mean ± SEM of neurite length; n = 50 to 80 neurites per condition from three independent experiments. Differences between treatments were determined by ANOVA followed by Tukey’s multiple comparison post-hoc test (***p < 0.001).
3. DISCUSSION
One of the current models to test the regenerative capacity of primary auditory neurons is a cell culture of dissociated SGN. Although exposing such cultures to several growth factors facilitates regeneration by promoting neurite outgrowth (Hossain and Morest 2000; Vieira et al. 2007; Wei et al. 2007), the role of FGF8 has not been studied previously.
In this study, we report a trophic action of FGF8a and FGF8b, two of the four homolog FGF8 splice forms found in human and mouse (Sunmonu et al. 2010). In cell cultures of SGN, both isoforms promote neurite outgrowth without affecting neuronal survival. However, FGF8a promotes twice the growth of neurite length compared to FGF8b. FGF8b seems to be predominant and to compete with FGF8a. For example, when the isoforms are both present, neurite outgrowth resembles that observed with FGF8b alone, regardless of the higher concentration of FGF8a. In addition, this competition is prevented by the neutralization of FGF8b. These findings are consistent with the biological role of FGF8 alternative splicing in modulating the binding affinity of FGF8a and FGFb toward certain isoforms of the FGFR (Olsen et al. 2006). The crystal structure of FGF8b in complex with FGFR2c revealed that the higher FGFR affinity of FGF8b compared to FGF8a is due to the interaction between residue F32 from the N-terminal region of FGF8b and the D3 hydrophobic groove of FGFR2c. Surface plasmon resonance analysis confirmed that FGF8a binds markedly more weakly than FGF8b to FGFRc isoforms as a result of the F32-D3 interaction. The mutation of F32 in FGF8b functionally converts it into FGF8a by reducing the receptor-binding affinity, thus modifying morphological and gene expression patterning abilities during mid-hindbrain development (Olsen et al. 2006). In the present report the presumed higher binding of FGF8b may be interfering with the FGF8a-promoted neurite outgrowth by competing for the receptor but leading to a different cellular response, not evident and out of the scope of this study.
We found that staining with the DCX antibody is a useful tool to reveal the length of the neurites of cultured SGN. Staining with anti-TUJ1, an antibody for β-tubulin III, a component of neuronal microtubules, was poor or absent in distal regions of the neurite, limiting the visualization of the extent of neurites. This finding suggests that the distribution of β-tubulin III appears later than 24 hours (when our cultures were harvested) at the distal processes of SGN, consistent with other cultured neurons in which this isotype of β-tubulin is not incorporated into microtubules during early neurite outgrowth (Ferreira and Caceres, 1992). This result reflects the influence of culture conditions on the distribution and/ or expression of cytoskeletal proteins. For instance in Whitlon et al. (2006) the use of BDNF, NT3, LIF and serum, the time in vitro after plating (42 hours) and the presence of limbus tissue on their SGN cultures may have some effect on the maturation of neuronal processes, as reflected in the distribution and/ or expression of β-tubulin III. The same is true for the Vieira et al. (2007) experiments, where the SGN cultures were maintained four days in vitro. Both reports found immunoreactivity of SGN for anti-TUJ1 antibody.
DCX is a microtubule-associated protein (Gleeson et al. 1999); its localization at the tips of growing neuronal processes suggests that it may also participate in neurite extension or regulate some adhesion molecules for axonal guidance (Friocourt et al. 2003). The present staining of DCX in cultured SGN demonstrates its distribution in the soma, the proximal neurite, and in the ends of growing neuronal processes. The expression of DCX in SGN may reflect a role in maintaining the morphology (Koizumi et al. 2006) of adult SGN.
FGF8-promoted neurite outgrowth requires one or more FGFRs, as the FGF8a and FGF8b-promoted neurite outgrowth was blocked by the FGFR inhibitor, PD173074. This inhibitor blocks FGFR1 and FGFR3-mediated activities (Miyake et al. 2010; Skaper et al. 2000) without interfering with FGF/FGFR downstream signaling or with other tyrosine kinase receptors (Bansal et al. 2003; Skaper et al. 2000). Taken together, these findings imply that FGFR1 or FGFR3 or both subtypes are present in cultured SGN, as suggested previously (Hossain and Morest 2000; Wei et al. 2007). However, we cannot rule out an effect of cell adhesion molecules in our model, since recent reports indicate that FGF2 promotes neurite outgrowth by enhancing the interaction between FGFR1 and NCAM (Allodi et al. 2013). Additionally, NCAM and N-cadherin are expressed in the growing tip of neurites (Doherty and Walsh 1996), and both molecules involve FGFR activation to induce neurite outgrowth (Doherty and Walsh 1996; Hansen et al. 2008). Thus the interaction with these noncanonical co-receptors should be considered as part of the complex signaling through FGF receptors.
The bFGF-promoted FGFRs activity has been shown to up-regulate protein expression through NFκB activation (Sigala et al. 2010); and transcriptional control mechanisms are important in regulation of axon growth and regeneration (Gutierrez et al. 2008; Moore and Goldberg 2011). Consistent with this, our findings suggest that the activation of the NFκB signaling pathway is required for FGF8-promoted neurite outgrowth. In this study, the inhibitor BAY11-7082, which prevents IκBα phosphorylation and hence NFκB activation, blocked the FGF8-promoted neurite outgrowth from cultured SGN. This inhibitor was reported to be as efficient as a genetic blockade of NFκB activation (Widera et al. 2006). A possible explanation for these findings is that upon FGFR stimulation the IκBα-mediated NFκB signaling pathway is activated to promote neurite outgrowth. Additional evidence, consistent with this conclusion, suggests that NFκB plays a neuroprotective role, as mutant mice deficient in NFκB experience accelerated age-related degeneration of SGN and increased susceptibility to noise-induced hearing loss (Lang et al. 2006).
In summary, we demonstrate here that FGF8a and FGF8b promote SGN neurite outgrowth in vitro. This response required activation of FGF receptors and the NFκB signaling pathway. Future studies will be required to reveal its functional significance.
4. MATERIALS AND METHODS
4.1 Animals and tissue preparation
ICR-CD1 mice were obtained from Charles River Laboratories. Eight to 10 day old mice were used for SGN cell cultures. The University of Connecticut Health Center Animal Care Committee approved the use of the animals in this study. Every effort was made to minimize the suffering of animals and to limit their number. To make SGN cell cultures, mice were decapitated before the temporal bone was dissected and treated as described below.
4.2 Spiral ganglion cell culture and treatments
The protocol used was based on those of Vieira et al. (2007) and Whitlon et al. (2006). Each cochlea was isolated from the temporal bone; the spiral ligament and the organ of Corti were removed. The modiolus was collected in Hibernate-A medium, containing 2% B27 (Invitrogen) and 0.1% penicillin-streptomycin (Invitrogen). The tissue was digested by adding collagenase I (125 µg/ml; Sigma), dispase (1 mg/ml; Stem Cell) and DNase I (300 µg/ml; Sigma) for 10 minutes at 37° C and triturating gently. The enzymatic digestion was terminated by diluting the enzymes with a 10-fold volume of Hibernate-A medium and a 3 min centrifugation at 200 ×g. The dissociated cells were plated in 2% B27 Neurobasal medium on coverslips in 4-well plates (Nalge Nunc International; the culture area was 1.9 cm2 per well) previously coated with poly-D-lysine (0.1 mg/ml overnight; Sigma) and laminin (10 µg/ml two hours; Invitrogen) at a density of two cochleas per plate (There are around 8000 neurons per ganglion, according to Whitlon et al. (2006)). Cultures were maintained in a humidified 5% CO2 incubator at 37° C. In preliminary experiments the viability and apoptosis of our cultures were determined using the Annexin V-FITC apoptosis detection kit (Calbiochem) and analyzed by flow cytometery. We found that after three days of culture without serum the percentage of living neurons was 9.1 % ± 0.9 % (mean ± standard error), comparable with the Whitlon et al. (2006) experiments in the absence of serum.
After allowing 2 to 3 hours for cell attachment, recombinant mouse proteins, rmFGF8a, rmFGF8b (R&D Systems) or both were added to the indicated concentrations and the cultures were incubated for a further 20–22 hours. When used, inhibitors (100 nM PD 173074 or 125 nM BAY11-7082; Tocris) were added 15 minutes before the FGF8 proteins, in 0.001 – 0.005 % DMSO, which had no effect on neurite outgrowth when compared to controls. In some experiments, we neutralized the activity of rmFGF8b by pre-incubating it with the anti-FGF8b antibody (R&D Systems) for 30 minutes, before it was added to the cell culture.
4.3 Immunolabeling
After culture for 24 hours, the cells were fixed for 20 minutes with 4% paraformaldehyde in PB and rinsed with 0.9 % NaCl in PB (PBS). The cells were then incubated in blocking buffer, containing 10% normal goat serum, 1% BSA and 0.3% triton X-100 in PB, for 1 hour at room temperature. Primary antibodies in blocking buffer were added to the cells and incubated overnight at 4°C or 2 hours at room temperature. We used rabbit anti-DCX (1:500; Santa Cruz) and mouse anti-TUJ1 (1:1000; Covance) as neuronal markers. After rinsing well, the cells were incubated in secondary antibodies conjugated with Alexa Fluor® 488 or 568 (Invitrogen). Nuclei were visualized by using the fluorescent DNA stain bisBenzimide H 33258 (Hoechst).
4.4 Imaging and measurements
Images of immunostained cultured cells were captured on a Zeiss Axiovert 200M microscope equipped with an X-Cite 120Q fluorescence illumination system (Lumen Dynamics Group Inc.) and Axiovision software for image acquisition. Cells were located by first inspecting the stained cellular nuclei. Then images of anti-DCX and/or anti-TUJ1 staining were captured by photographing 20× fields throughout the culture area on the coverslip. The captured images were used to analyze neurite length. Neurite length of all the neurons bearing neurites (more than 50 individual neurons per condition) was determined from at least three independent experiments. We used the free-hand line segment tool in Image J software (National Institutes of Health) to measure the longest process extending from the soma. To evaluate the effect of treatments on cell survival, the number of labeled neurons per micrograph was counted and normalized to 1 mm2.
4.5 Statistical analysis
Statistical analyses were performed using one-way ANOVA (GraphPad 5.0b Prism). Each experiment was performed in triplicate. Individual comparisons were determined using Dunnet’s or Tukey’s multiple comparison post hoc tests. Data are presented as mean ± SEM and means were considered significantly different if P≤0.05.
Highlights.
We report that FGF8a and FGF8b promote spiral ganglion neuron (SGN) neurite growth in vitro
This action is mediated by FGF receptors
FGF8b competitively interferes with the action of FGF8a
The NFκB signaling pathway is required for SGN neurite outgrowth
The DCX antibody reveals the distal neurites of cultured SGN.
Acknowledgments
Contract grant sponsor: NIH
Contract grant number: 5R01-DC000127
SGH is grateful to Julia Gross, for helping her to learn how to dissect spiral ganglion neurons, and Steven Kempe, for instruction in assembling micrographic images.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allodi I, Casals-Diaz L, Santos-Nogueira E, Gonzalez-Perez F, Navarro X, Udina E. Fgf-2 low molecular weight selectively promotes neuritogenesis of motor neurons in vitro. Mol Neurobiol. 2013;47:770–781. doi: 10.1007/s12035-012-8389-z. [DOI] [PubMed] [Google Scholar]
- Bansal R, Magge S, Winkler S. Specific inhibitor of FGF receptor signaling: FGF-2-mediated effects on proliferation, differentiation, and MAPK activation are inhibited by PD173074 in oligodendrocyte-lineage cells. J Neurosci Res. 2003;74(4):486–493. doi: 10.1002/jnr.10773. [DOI] [PubMed] [Google Scholar]
- Bao J, Ohlemiller KK. Age-related loss of spiral ganglion neurons. Hear Res. 2010;264(1–2):93–97. doi: 10.1016/j.heares.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P, Walsh FS. CAM-FGF Receptor Interactions: A Model for Axonal Growth. Mol Cell Neurosci. 1996;8:99–111. doi: 10.1006/mcne.1996.0049. [DOI] [PubMed] [Google Scholar]
- Fantetti KN, Fekete DM. Members of the BMP, Shh and FGF morphogen families promote chicken statoacoustic ganglion neurite outgrowth and neuron survival in vitro. Dev Neurobiol. 2011 doi: 10.1002/dneu.20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Caceres A. Expression of the class III beta-tubulin isotype in developing neurons in culture. J Neurosci Res. 1992;32:516–529. doi: 10.1002/jnr.490320407. [DOI] [PubMed] [Google Scholar]
- Friocourt G, Koulakoff A, Chafey P, Boucher D, Fauchereau F, Chelly J, Francis F. Doublecortin functions at the extremities of growing neuronal processes. Cereb Cortex. 2003;13(6):620–626. doi: 10.1093/cercor/13.6.620. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Shepherd RK. Clinical application of neurotrophic factors: the potential for primary auditory neuron protection. Eur J Neurosci. 2005;22(9):2123–2133. doi: 10.1111/j.1460-9568.2005.04430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23(2):257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Gutierrez H, O'Keeffe GW, Gavalda N, Gallagher D, Davies AM. Nuclear factor kappa B signaling either stimulates or inhibits neurite growth depending on the phosphorylation status of p65/RelA. J Neurosci. 2008;28(33):8246–8256. doi: 10.1523/JNEUROSCI.1941-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SM, Berezin V, Bock E. Signaling mechanisms of neurite outgrowth induced by the cell adhesion molecules NCAM and N-cadherin. Cell Mol Life Sci. 2008;65:3809–3821. doi: 10.1007/s00018-008-8290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Cunningham D, Bermingham-McDonogh O. Loss of Fgfr3 leads to excess hair cell development in the mouse organ of Corti. Dev Dyn. 2007;236(2):525–533. doi: 10.1002/dvdy.21026. [DOI] [PubMed] [Google Scholar]
- Hossain WA, D'Sa C, Morest DK. Interactive roles of fibroblast growth factor 2 and neurotrophin 3 in the sequence of migration, process outgrowth, and axonal differentiation of mouse cochlear ganglion cells. J Neurosci Res. 2008;86(11):2376–2391. doi: 10.1002/jnr.21685. [DOI] [PubMed] [Google Scholar]
- Hossain WA, Morest DK. Fibroblast growth factors (FGF-1, FGF-2) promote migration and neurite growth of mouse cochlear ganglion cells in vitro: immunohistochemistry and antibody perturbation. J Neurosci Res. 2000;62(1):40–55. doi: 10.1002/1097-4547(20001001)62:1<40::AID-JNR5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Irving C, Malhas A, Guthrie S, Mason I. Establishing the trochlear motor axon trajectory: role of the isthmic organiser and Fgf8. Development. 2002;129(23):5389–5398. doi: 10.1242/dev.00117. [DOI] [PubMed] [Google Scholar]
- Jacques BE, Montcouquiol ME, Layman EM, Lewandoski M, Kelley MW. Fgf8 induces pillar cell fate and regulates cellular patterning in the mammalian cochlea. Development. 2007;134(16):3021–3029. doi: 10.1242/dev.02874. [DOI] [PubMed] [Google Scholar]
- Klimaschewski L, Nindl W, Feurle J, Kavakebi P, Kostron H. Basic fibroblast growth factor isoforms promote axonal elongation and branching of adult sensory neurons in vitro. Neuroscience. 2004;126(2):347–353. doi: 10.1016/j.neuroscience.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Higginbotham H, Poon T, Tanaka T, Brinkman BC, Gleeson JG. Doublecortin maintains bipolar shape and nuclear translocation during migration in the adult forebrain. Nat Neurosci. 2006;9(6):779–786. doi: 10.1038/nn1704. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Wright TJ, Moon AM, Mansour SL, Schoenwolf GC. FGF8 initiates inner ear induction in chick and mouse. Genes Dev. 2005;19(5):603–613. doi: 10.1101/gad.1273605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang H, Schulte BA, Zhou D, Smythe N, Spicer SS, Schmiedt RA. Nuclear factor kappaB deficiency is associated with auditory nerve degeneration and increased noise-induced hearing loss. J Neurosci. 2006;26(13):3541–3550. doi: 10.1523/JNEUROSCI.2488-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger S, Brand M. Fgf8 and Fgf3 are required for zebrafish ear placode induction, maintenance and inner ear patterning. Mech Dev. 2002;119(1):91–108. doi: 10.1016/s0925-4773(02)00343-x. [DOI] [PubMed] [Google Scholar]
- Miller JM, Le Prell CG, Prieskorn DM, Wys NL, Altschuler RA. Delayed neurotrophin treatment following deafness rescues spiral ganglion cells from death and promotes regrowth of auditory nerve peripheral processes: effects of brain-derived neurotrophic factor and fibroblast growth factor. J Neurosci Res. 2007;85(9):1959–1969. doi: 10.1002/jnr.21320. [DOI] [PubMed] [Google Scholar]
- Miyake M, Ishii M, Koyama N, Kawashima K, Kodama T, Anai S, Fujimoto K, Hirao Y, Sugano K. 1-tert-butyl-3-[6-(3,5-dimethoxy-phenyl)-2-(4-diethylamino-butylamino)-pyrido[2,3 -d]pyrimidin-7-yl]-urea (PD173074), a selective tyrosine kinase inhibitor of fibroblast growth factor receptor-3 (FGFR3), inhibits cell proliferation of bladder cancer carrying the FGFR3 gene mutation along with up-regulation of p27/Kip1 and G1/G0 arrest. J Pharmacol Exp Ther. 2010;332(3):795–802. doi: 10.1124/jpet.109.162768. [DOI] [PubMed] [Google Scholar]
- Moore DL, Goldberg JL. Multiple transcription factor families regulate axon growth and regeneration. Dev Neurobiol. 2011;71(12):1186–1211. doi: 10.1002/dneu.20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oellig C, Pirvola U, Taylor L, Elde R, Hokfelt T, Pettersson RF. Acidic FGF and FGF receptors are specifically expressed in neurons of developing and adult rat dorsal root ganglia. Eur J Neurosci. 1995;7(5):863–874. doi: 10.1111/j.1460-9568.1995.tb01073.x. [DOI] [PubMed] [Google Scholar]
- Olsen SK, Li JY, Bromleigh C, Eliseenkova AV, Ibrahimi OA, Lao Z, Zhang F, Linhardt RJ, Joyner AL, Mohammadi M. Structural basis by which alternative splicing modulates the organizer activity of FGF8 in the brain. Genes Dev. 2006;20(2):185–198. doi: 10.1101/gad.1365406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Spencer-Dene B, Xing-Qun L, Kettunen P, Thesleff I, Fritzsch B, Dickson C, Ylikoski J. FGF/FGFR-2(IIIb) signaling is essential for inner ear morphogenesis. J Neurosci. 2000;20(16):6125–6134. doi: 10.1523/JNEUROSCI.20-16-06125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss B, von Bohlen und Halbach O. Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res. 2003;313(2):139–157. doi: 10.1007/s00441-003-0756-7. [DOI] [PubMed] [Google Scholar]
- Sapieha PS, Peltier M, Rendahl KG, Manning WC, Di Polo A. Fibroblast growth factor-2 gene delivery stimulates axon growth by adult retinal ganglion cells after acute optic nerve injury. Mol Cell Neurosci. 2003;24(3):656–672. doi: 10.1016/s1044-7431(03)00228-8. [DOI] [PubMed] [Google Scholar]
- Sigala F, Savvari P, Liontos M, Sigalas P, Pateras IS, Papalampros A, Basdra EK, Kolettas E, Kotsinas A, Papavassiliou AG, Gorgoulis VG. Increased expression of bFGF is associated with carotid atherosclerotic plaques instability engaging the NF-kappaB pathway. J Cell Mol Med. 2010;14(9):2273–2280. doi: 10.1111/j.1582-4934.2010.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD, Kee WJ, Facci L, Macdonald G, Doherty P, Walsh FS. The FGFR1 inhibitor PD 173074 selectively and potently antagonizes FGF-2 neurotrophic and neurotropic effects. J Neurochem. 2000;75(4):1520–1527. doi: 10.1046/j.1471-4159.2000.0751520.x. [DOI] [PubMed] [Google Scholar]
- Sunmonu NA, Li K, Li JY. Numerous isoforms of Fgf8 reflect its multiple roles in the developing brain. J Cell Physiol. 2010 doi: 10.1002/jcp.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki-Hirano A, Shimogori T. The role of Fgf8 in telencephalic and diencephalic patterning. Semin Cell Dev Biol. 2009;20(6):719–725. doi: 10.1016/j.semcdb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Vieira M, Christensen BL, Wheeler BC, Feng AS, Kollmar R. Survival and stimulation of neurite outgrowth in a serum-free culture of spiral ganglion neurons from adult mice. Hear Res. 2007;230(1–2):17–23. doi: 10.1016/j.heares.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Wang Q, Green SH. Functional role of neurotrophin-3 in synapse regeneration by spiral ganglion neurons on inner hair cells after excitotoxic trauma in vitro. J Neurosci. 2011;31(21):7938–7949. doi: 10.1523/JNEUROSCI.1434-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber A, Raz Y. Axon guidance cues in auditory development. Anat Rec A Discov Mol Cell Evol Biol. 2006;288(4):390–396. doi: 10.1002/ar.a.20299. [DOI] [PubMed] [Google Scholar]
- Wei D, Jin Z, Jarlebark L, Scarfone E, Ulfendahl M. Survival, synaptogenesis, and regeneration of adult mouse spiral ganglion neurons in vitro. Dev Neurobiol. 2007;67(1):108–122. doi: 10.1002/dneu.20336. [DOI] [PubMed] [Google Scholar]
- White JA, Burgess BJ, Hall RD, Nadol JB. Pattern of degeneration of the spiral ganglion cell and its processes in the C57BL/6J mouse. Hear Res. 2000;141(1–2):12–18. doi: 10.1016/s0378-5955(99)00204-x. [DOI] [PubMed] [Google Scholar]
- Whitlon DS, Ketels KV, Coulson MT, Williams T, Grover M, Edpao W, Richter CP. Survival and morphology of auditory neurons in dissociated cultures of newborn mouse spiral ganglion. Neuroscience. 2006;138(2):653–662. doi: 10.1016/j.neuroscience.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Widera D, Mikenberg I, Kaus A, Kaltschmidt C, Kaltschmidt B. Nuclear Factor-kappaB controls the reaggregation of 3D neurosphere cultures in vitro. Eur Cell Mater. 2006;11:76–84. doi: 10.22203/ecm.v011a08. discussion 85. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281(23):15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]