Abstract
The increasing use of strategies incorporating nanoscale zero valent iron (nZVI) for soil and groundwater in situ remediation is raising some concerns regarding the potential adverse effects nZVI could have on indigenous microbial communities and ecosystem functioning. This review provides an overview of the current literature pertaining to the impacts of nZVI applications on microbial communities. Toxicity studies suggest that cell membrane disruption and oxidative stress through the generation of Fe2+ and reactive oxygen species by nZVI are the main mechanisms contributing to nZVI cytotoxicity. In addition, nZVI has been shown to substantially alter the taxonomic and functional composition of indigenous communities. However, because the physico-chemical conditions encountered in situ highly modulate nZVI toxicity, a better understanding of the environmental factors affecting nZVI toxicity and transport in the environment is of primary importance in evaluating the ecological consequences that could result from a more extensive use of nZVI.
Keywords: Nanoscale zerovalent iron, Environmental impact, Toxicity mechanisms, Oxidative stress, Microbial community, nZVI transport, In situ remediation
Graphical abstract
1- Introduction
Nanoscale zero valent iron (nZVI) is the most commonly used nanomaterial in Europe and in the United States for soil and groundwater remediation. More recently, its use for wastewater remediation (Fu et al., 2014; Grieger et al., 2010; Mueller et al., 2012), and anaerobic digestion process enhancement (Carpenter et al., 2015; Hu et al., 2015) has also received growing attention. Due to its reduced size, nZVI has a higher reactivity towards a broad range of contaminants, including halogenated compounds, nitrate, phosphate, polycyclic aromatic hydrocarbons, and heavy metals (Fu et al., 2014; Mueller et al., 2012; Tosco et al., 2014; Wei-xian, 2003), and a higher mobility compared to its microscale counterpart. In addition, its application does not require excavation as highly concentrated nZVI slurries are directly injected underground, at or near the source of contamination. Consequently, nZVI is regarded as a promising remediation strategy suitable to a broad range of applications and environments. However, microorganisms, which are key players in many fundamental ecosystem processes, are the first exposed to nZVI particles. Therefore, potential issues related to long-term alteration of ecosystem functioning have to be considered and a thorough evaluation of the effect of nZVI on indigenous microorganisms is needed before further in situ deployment of nZVI remediation strategies. In the last decade, toxicity studies revealed that nZVI could exert some degree of toxicity towards microbial species and the effects of nZVI at the cellular and community levels are progressively being elucidated. In view of the abundant published literature on the topic, this review provides an updated overview of the potential impacts of the in situ deployment of nZVI on microbial communities (summarized in Figure 1). As characteristics and behavior of nZVI in the environment are essential for assessing its effects on microbial communities, a first section succinctly covers the main processes involved in nZVI chemistry. In a second section, toxicity studies conducted on microbial species are reviewed, and both the mechanisms likely mediating nZVI cytotoxicity, and the cellular defenses set off to counteract nZVI toxicity are presented. The following section covers the potential impacts of nZVI on the structure and ecological functions of microbial communities. Finally, based on current and potential future in situ applications of nZVI treatments, the transport and the possible routes of nZVI from injection points to non-target environments are considered.
Figure 1. nZVI oxidation, cytotoxicity, cellular defense mechanisms mediated by nZVI, and potential routes of nZVI in the natural environment.
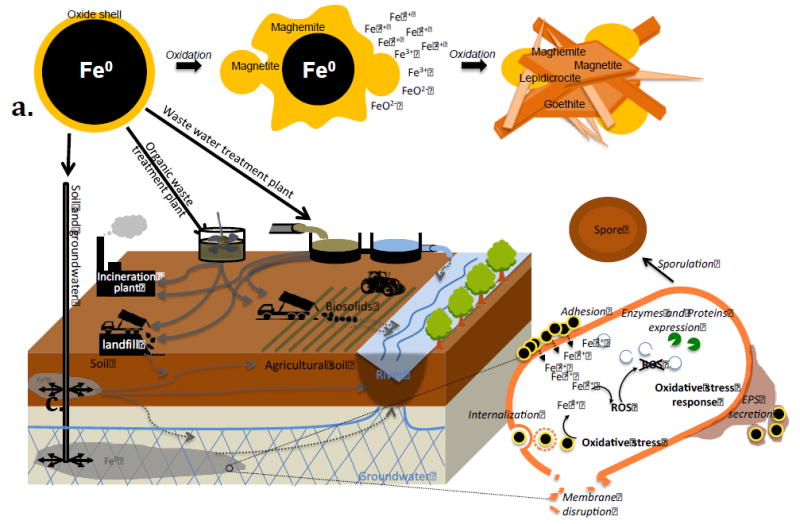
Illustration of (a.) nZVI oxidation process, (b.) bacterial toxicity and defense mechanisms, and (c.) potential routes followed by nZVI in the environment. Plain grey arrows represent deliberate injection or amendment of nZVI, and deliberate transport of material potentially containing nZVI. Dashed grey arrows represent potential non-deliberate transport of nZVI in non-target environments. Abbreviations: EPS, Extracellular polymeric substances; ROS, Reactive oxygen species.
2- Chemical behavior of nZVI in situ
2-1 Release of soluble iron and reactive oxygen species
Even under highly controlled anaerobic conditions and despite the different protocols existing to synthesize nZVI, particle surface oxidation always occurs to some degree and results in the formation of a nanoparticle with a Fe0 core surrounded by an outer layer of iron oxide of at least 3 nm in thickness. Because kinetics of the initial stages of Fe0 oxidation are fast, once introduced into the environment the outer oxide layer of the nanoparticle thickens relatively quickly (Crane and Scott, 2012). Ferrous (Fe2+) and ferric (Fe3+) irons, known to have cytotoxic effects, are initially released near the nanoparticle surface and progressively oxidize to form Fe(II) and Fe(III) oxides. This reaction evolves until the Fe0 core is completely oxidized. The specific oxidation reactions of Fe0 to soluble iron species are listed below for anaerobic (Eq. 1) and aerobic (Eq. 2 and 3) conditions.
| (Eq. 1) |
| (Eq. 2) |
| (Eq. 3) |
Because the domain of stability for Fe3+ is narrower than for Fe2+, under most environmental conditions, Fe2+ will be preferentially released upon nZVI injection. Fe2+ oxidation kinetics will vary greatly depending on environmental conditions and dictate the concentration of Fe2+ close to the injection point. The rate of Fe2+ oxidation can be influenced by various abiotic factors, including concentration in anionic species, dissolved oxygen, or other oxidants such as environmental pollutants. For instance, concentration in anionic species such as Cl- in groundwater will reduce Fe2+ oxidation rate, resulting in a higher accumulation of Fe2+ (Adeleye et al., 2013).
Fe2+oxidation can also take place when hydrogen peroxide (H2O2) is present, such as inside a biological cell, resulting in the release of hydroxyl hydrogen radical (OH.) (Eq. 4), superoxide (O2-) (Eq.5), or ferryl ion (FeO2+) (Eq. 6; Ševců et al., 2011) through Fenton reaction. These highly reactive oxygen species (ROS) play a significant role in nZVI cytotoxicity.
| (Eq. 4) |
| (Eq. 5) |
| (Eq. 6) |
2-2 Formation of insoluble iron oxide
Because Fe2+ and Fe3+ are only stable under specific environmental conditions, they are temporarily present in the environment, but ultimately oxidize to insoluble iron species such as Fe3O4 (maghemite) (Eq. 7), Fe3O4 (magnetite) (Eq. 8), FeOOH (lepidocrocite) (Eq. 9 and 10), or goethite (Eq. 11).
| (Eq. 7) |
| (Eq. 8) |
| (Eq. 9) |
| (Eq. 10) |
| (Eq. 11) |
High oxidation rates (e.g., oxic condition) will favor the formation of lepidocrocite (Eq. 9 and 10) or goethite (Eq. 11) (Kumar et al., 2014a). However, at lower oxidation rates (e.g., anoxic condition), soluble iron dehydroxylation will occur prior to oxidation, favoring the formation of crystalline green rusts, followed by magnetite as the end product of nZVI oxidation (Eq. 8) (Drever, 1988; Stumm and Morgan, 1996). The newly formed structures have been described as micronic aggregates of sphere, needle or board-shaped, composed of magnetite, lepidocrocite and goethite, respectively, with individual size between 20 nm and 1 μm (Greenlee et al., 2012; Su et al., 2013).
Kumar et al. (2014a), however, observed the formation of additional nanoparticles < 20 nm at the oxide layer surface of nZVI particles after 35 days under anaerobic conditions. Although never observed, detachment of oxide nanoparticles during the distortion of the oxide shell structure could potentially occur (Kumar et al., 2014a), and represent a potential hazard to microorganisms.
2-3 Effect of nZVI particle synthesis method and stabilization
Despite the numerous existing protocols, nZVI synthesis is mostly performed by chemical iron reduction. Synthesis parameters such as reduction rate, reagent concentrations (i.e., [Fe3+]/[BH4] ratio) and concentration of nZVI nuclei (i.e., [Fe3+]), are known to greatly influence nZVI morphology, crystallinity, and specific surface area (i.e., size) (Hwang et al., 2011). Smaller nZVI particles usually result from synthesis under high reduction rate, and high reductant and nuclei concentration, while nZVI poor crystallinity is more associated to high nuclei concentration (Hwang et al., 2011). Other synthesis conditions including pH, temperature, the use of ethanol or additional cations have also been shown to influence nZVI characteristics as well as its reactivity (Song et al, 2005). Therefore nZVI reactivity, hence toxicity, will likely depend on the synthesis methods and conditions used to produce the nanoparticles. In addition, because bare nZVI has low colloidal stability (Lowry and Casman, 2009; Tratnyek and Johnson, 2006), especially under commonly encountered environmental conditions (Saleh et al., 2008), nZVI particles synthetized for in situ remediation are typically coated with stabilizers. Polyacrylic acid, polyaspartate, chitosan, sodium oleate, and carboxymethyl cellulose coatings have been reported to significantly improve the colloidal stability of nZVI, reducing particle adhesion to mineral surfaces, and increasing nZVI mobility and overall reactivity (Phenrat et al., 2008; Saleh et al., 2007; Sirk et al., 2009). Although laboratory observations in columns of sand showed that the stabilizing effects of nZVI surface treatments could be maintained for an extended period of time compared to bare nZVI (Kim et al., 2009), biotic and abiotic degradation processes occurring in situ may progressively remove nanoparticle coating, modifying nZVI behavior and overall toxicity.
3- nZVI cytotoxicity
3-1 Toxicity studies on bacterial and fungal species
In the last decade, an increasing amount of studies evaluating the toxicity of nZVI on bacterial and fungal species have been conducted (Table 1). These studies primarily used in vitro toxicity assays to measure cell viability, cell growth, cell integrity, or biological activity of various microbial species exposed to nZVI, with exposure times ranging from 5 mins to 42 days, and nZVI concentrations ranging for 1 to 10,000 mg/l (Table 1). While a few studies reported no effect of nZVI, most of them found a strong to severe negative effect on cell viability, integrity and activity. Exposure to the highest nZVI concentrations tested did not lead to any toxic effects towards Klebiella planticola or Klebiella oxytoca (Fajardo et al., 2012; Saccà et al., 2013), but resulted in a severe toxic effect on Bacillus nealsoni (Fajardo et al., 2012). Bacillus subtilis, however, was found to be more resistant to nZVI toxicity than Escherichia coli (Chen et al., 2011) or Pseudomonas fluorescens (Diao and Yao, 2009), and P. putida displayed a lower sensitivity to nZVI than E. coli (Chaithawiwat et al., 2016). Within the same genus, differences in resistance were also reported. B. cereus displayed a lower resistance to nZVI toxicity compared to B. nealsoni (Fajardo et al., 2013, 2012), and while nZVI only had a transient toxic effect on Pseudomonas stutzeri (Saccà et al., 2014a), P. fluorescens exposed to lower doses was completely inactivated (Diao and Yao, 2009). Chaithawiwat et al. (2016) showed that different strains within the same species could also display differential sensitivity to nZVI. Additionally, they demonstrated that bacterial cells in stationary growth phase presented lower sensitivity to nZVI compare to cells harvested in lag or exponential phase, likely because rpoS, a transcriptional factor regulating various genes involved in cell stress response, was naturally more expressed during stationary growth (Battesti et al., 2011; Chaithawiwat et al., 2016; Kidarsa et al., 2013).
Table 1.
Summary of in vitro toxicity studies conducted on bacterial and fungal species.
| Species tested | nZVI characteristics |
nZVI concentration |
Experimental conditions |
Measured biological parameters |
Methods | Effect | Reference |
|---|---|---|---|---|---|---|---|
| Klebsiella planticola (Gram -) | nZVI (NANOFER 25S*) | 1,000-10,000 mg/l | 0.2-24 h-exposure, no shaking, aerobic | Cell viability | CFU assay | No effect on viability and activity. | Fajardo et al., 2012 |
| < 50 nm | Biological activity | O2 consumption | Attachment of nZVI to cell surface. | ||||
| Cell integrity | TEM/SEM | No significant cell damage. | |||||
| Klebsiella oxytoca (Gram -) | nZVI (NANOFER 25S*) | 1,000-10,000 mg/l | 0.2-24 h-exposure, no shaking, aerobic | Cell viability | CFU / Liquid-to-plate | No bactericidal and negligible bacteriostatic effects. | Sacca et al., 2013 |
| 80-120 nm | Cell integrity | ||||||
| Protein transcription level | TEM/SEM | Attachment of nZVI to cell surface without significant cell damage. | |||||
| 2D-electrophoresis/MALDI-TOF mass spectrometry | Overproduction of tryptophanase. | ||||||
| Pseudomonas fluorescens (Gram -) | Home-made bare-nZVI | 100-10,000 mg/l | 5 min-exposure, vigorous shaking, aerobic/anaerobic | Cell viability | CFU assay | Complete inactivation at all concentration tested. | Diao and Yao, 2009 |
| 20-30 nm | Cell integrity | SEM | Iron precipitate coating on cell surface. | ||||
| Pseudomonas putida (Gram -) | nZVI (Toda Kogyo Corp., Japan) | 1,000 mg/l | 5-60 min-exposure, mixing, aerobic | Cell viability | CFU assay | More severe toxicity on exponential and decline growth phases. | Chaithawiwat et al., 2016 |
| 10-70 nm | Toxic effect is strain-dependent. | ||||||
| Pseudomonas stutzeri (Gram -) | nZVI (NANOFER 25S*) | 1,000-10,000 mg/l | 0.2-48 h-exposure, aerobic | Cell viability | CFU assay | Slight toxicity only after 0.2 h-exposure. | Sacca et al., 2014a |
| < 50 nm | Gene expression level (pykA, gyrA, katB) | RT-qPCR | No effect on activity. | ||||
| Protein transcription level | 2D SDS-PAGE | Increase of oxidative stress response. | |||||
| Cell integrity | TEM | Down-regulation of membrane transport proteins. Up-regulation of oxidative stress response proteins. | |||||
| Attachment on nZVI on cell surface. | |||||||
| Alcaligene (Gram -) | Home-made bare-nZVI, chitosan-nZVI, sodium-oleate-nZVI ~80 nm | 650 mg/l | 1-9 d-exposure, shaking, aerobic | Biological activity | Total RNA measurement | Decrease of activity in the first 2 days. | An et al., 2010 |
| Agrobacterium sp.(Gram -) | Home-made bare-nZVI, CMC-nZVI | 100-250 mg/l | 1-6 h-exposure, shaking, aerobic | Cell viability | CFU assay | Significant toxicity (53.2 and 5.3% survival after 1 h-exposure to 100 mg/l CMC- and bare-nZVI, respectively). | Zhou et al., 2014 |
| 80-120 nm | Cell integrity | TEM | Drastic damage of cell membrane. | ||||
| Agrobacterium sp. PH-08 | nZVI (NaBond, China) | 100-10,000 mg/l | 1-6 h-exposure, shaking, aerobic | Cell viability | CFU assay | 11.4 and 32% decrease in cell viability after 6 h-exposure to 100 and 1,000 mg/l nZVI, respectively. | Le et al., 2014 |
| 36.3 ± 2.3 nm | Gene expression level (Catechol dioxygenase) | RT-qPCR | Only slight effect on degradation capabilities. | ||||
| Cell integrity | Propidium Iodine | Attachment of nZVI cell surface without disruption or internalization. | |||||
| TEM/SEM | |||||||
| Escherichia coli (Gram -) | nZVI (Toda Kogyo Corp., Japan) | 1,000 mg/l | 5-60 min-exposure, mixing, aerobic | Cell viability | CFU assay | More severe toxicity on exponential and decline growth phases. | Chaithawiwat et al., 2016 |
| 10-70 nm | Toxic effect is strain-dependent. | ||||||
| Escherichia coli | Home-made bare-nZVI | 7-700 mg/l | 1 h-exposure, aerobic | Cell viability | CFU assay | Severe toxicity (~75% inactivation at ≥70 mg/l nZVI). | Auffan et al., 2008 |
| 320 ± 30 nm | |||||||
| Escherichia coli | Bare nZVI (Toda kogyo Corp., Japan) | 1-2,000 mg/l | 5-60 min-exposure, shaking, aerobic/anaerobic | Cell viability | CFU assay | 1.8 and 5.2-log inactivation for 7 and 28% Fe0 nZVI, respectively. <0.2-log inactivation with coated nZVI after 1h-exposure. | Li et al., 2010 |
| PSS-nZVI | TEM | Attachment on nZVI on cell surface. | |||||
| PA-nZVI | |||||||
| NOM-nZVI | |||||||
| Escherichia coli | Home-made bare nZVI | 1.2-110 mg/l | 2-60 min-exposure, mixing, aerobic/anaerobic | Cell viability | CFU assay | Severe toxicity under de-aerated. | Lee et al., 2008 |
| 10-80 nm | Cell integrity | TEM | Slight toxicity under aerated conditions. | ||||
| Significant cell damage. | |||||||
| Escherichia coli | nZVI (Toda kogyo Corp., Japan) | 1,000 mg/l | 1-4 h-exposure, shaking, aerobic | Cell viability | CFU assay | Severe toxicity (60%, 70%, ~100% inactivation after 1, 2, and 4 h-exposure, respectively). | Chen et al. 2011 |
| 50 nm-5 μm | |||||||
| Escherichia coli | Home-made bare-nZVI, electrosprayed nZVI | 5,000 mg/l | 5 min-exposure, vortexing, aerobic | Cell viability | CFU assay | 80% inactivation. | Chen et al., 2012 |
| 20-30 nm | |||||||
| Escherichia coli | Home-made bare-nZVI | 1.2-110 mg/l | 1 h-exposure, mixing, aerobic/anaerobic | Cell viability | Live/Dead viability | Serious damage of cell membrane and respiratory activity under de-aerated conditions, but not under air-saturated conditions. | Kim et al., 2010 |
| 10-80 nm | |||||||
| Sphingomonas sp. PH-07 (Gram -) | Home-made bare – nZVI | 500-10,000 mg/l | 4 d-exposure, shaking, aerobic | Cell viability | CFU assay | Grow normally up to 2,500 mg/l nZVI. | Y.-M. Kim et al., 2012 |
| SSA: 33.2 ± 1.2 m2.g- | Does not growth > 5,000 mg/l . | ||||||
| Bacillus nealsonii (Gram +) | nZVI (NANOFER 25S*) | 1,000-10,000 mg/l | 0.2-24 h-exposure, no shaking, aerobic | Cell viability | CFU assay | Slight and high toxicity at 5,000 and 10,000 mg/l respectively. | Fajardo et al., 2012 |
| < 50 nm | Cell integrity | O2 consumption | Reduced metabolic activity. | ||||
| TEM/SEM | Cell content leakage. | ||||||
| Bacillus subtilis | nZVI (Toda kogyo Corp., Japan) | 1,000 mg/l | 1-4 h-exposure, shaking, aerobic | Cell viability | CFU assay | Strong toxicity (20%, 70%, ~1000% inactivation after, 1, 2, and 4 h-exposure, respectively). | Chen et al., 2011 |
| 50 nm-5 μm | |||||||
| Bacillus subtilis | Home-made bare-nZVI | 100-10,000 mg/l | 5 min-exposure, vigorous shaking, aerobic | Cell viability | CFU assay | Strong bactericidal effect (100, 95, and 80% inactivation at 100, 1,000, and 10,000 mg/l nZVI, respectively). | Diao and Yao, 2009 |
| 20-30 nm | Cell integrity | SEM | Massive needle-shaped coating on cell surface. | ||||
| Bacillus cereus (Gram +) | nZVI (NANOFER 25S*) | 1,000-10,000 mg/l | 0.2-24 h-exposure, no shaking, aerobic | Cell viability | CFU assay | Negative effect on cell viability, but no effect after 48 h-exposure. | Fajardo et al., |
| < 50 nm | Protein transcription level | 2-D2D SDS-PAGE | Up-regulation of oxidative stress response proteins. Down-regulation of cell wall and motility proteins. | ||||
| Gene expression level (pykA, gyrA, katB, nirS, narG) | RT-qPCR | No change in genes expression level. | |||||
| Cell integrity | TEM | Early sporulation. | |||||
| Paracoccus sp. YF1 (Gram -) | Home-made bare-nZVI | 50-1,000 mg/l | 20 h-exposure, shaking, aerobic | Nitrate removal | UV-spectrophotometry | 50 mg.l-1 nZVI promoted and >50 mg/l decreased growth and nitrate removal. | Jiang et al., 2015 |
| Cell growth | Spectrometry (OD600nm) | Attachment of nZVI to cell surface. | |||||
| Cell integrity | SEM | ||||||
| Paracoccus sp. YF1 | Home-made bare-nZVI | 1,000 mg/l | 20 h-exposure, shaking, | Nitrate removal | UV-spectrophotometry | Decrease of cell growth and nitrate removal. | Jiang et al., 2013 |
| 20-80 nm | Cell growth | Spectrometry (OD600nm) | Attachment of nZVI to cell surface. | ||||
| Cell integrity | SEM | ||||||
| Paracoccus sp. YF1 | Home-made bare nZVI | 50-1,000 mg/l | 24 h-exposure, shaking, aerobic/anaerobic | Nitrate removal | UV-spectrophotometry | Initial retardation in cell growth and nitrate removal. | Liu et al., 2014 |
| 20-80 nm | Cell growth | Spectrometry (OD600nm) | Attachment of nZVI to cell surface. | ||||
| Cell integrity | SEM | ||||||
| Saccharomyces cerevisiae (Ascomycetous yeast) | nZVI (Skyspring Nanomaterials Inc., USA) | 100-1,000 mg/l | 20 h-exposure, shaking, aerobic | Biological activity Cell integrity | O2 consumption | Low overall toxicity. | Otero-Gonzales et al., 2013 |
| 40-60 nm | Toxicity yeast assay/Flow cytometry | Negligible cell damage. | |||||
| Aspergilus versicolor (Ascomycetous | Home-made bare-nZVI | 100-10,000 mg/l | 5 min-exposure, vigorous shaking, aerobic | Cell viability | CFU assay | No toxic effect. | Diao and Yao, 2009 |
| 20-30 nm | Cell integrity | SEM | Yellow-brown coating observed on cell surface. | ||||
| Trametes versicolor (Basidiomycetous Fungi) | nZVI (Sun innovation, Inc., USA) | 0.1 mM Fe0 | 42 d-exposure, shaking, aerobic | Cell growth | Dry weight | No effect on growth. | Shah et al., 2010 |
| 25 nm | Cell activity | Lignocellulolytic and Cellulolytic enzyme production | Decrease of cellulolytic enzymes production. |
Abbreviations: CFU, colony forming unit; CMC, carboxyl methyl cellulose; NOM, natural organic mater; PA, poly aspartate; PSS, polystyrene sulfonate; RT-qPCR, reverse transcription-quantitative PCR; SEM, scanning electron microscopy; SSA, surface specific area; TEM, transmission electron microscopy; MALDI-TOF, matrix-assisted laser desorption ionization tandem time-of-flight; SDS-PAGE sodium dodecyl sulftate-polyacrylamide gel electrophoresis. GirA, katB, narG, nirS, and pykA genes encode for gyrase (DNA replication enzyme), catalase (cellular detoxification enzyme), nitrate reductase, nitrite reductase, and pyruvate kinase (glycolysis enzyme), respectively.
NANOFER 25S: nZVI coated with polyethylene glycol sorbitant monostearate with a percentage Fe0 of 14-18% (NANOIRON s.r.o. , Czech Republic).
In comparison, fungal species seem to display a much higher tolerance to nZVI toxicity (Diao and Yao, 2009; Otero-González et al., 2013; Shah et al., 2010). Concentration up to 10,000 mg/l did not significantly affect the growth of Aspergillus versicolor (Diao and Yao, 2009), and although Trametes versicolor cellulolytic enzyme production, and Saccharomyces cerevisiae metabolism decreased in the presence of nZVI, their viability was not significantly affected (Otero-González et al., 2013; Shah et al., 2010).
3-2 Toxicity mechanisms
Most of the literature suggests that cell membrane disruption and oxidative stress through the generation of Fe2+ and ROS by nZVI are likely the main mechanisms contributing to nZVI toxicity. In the majority of studies that used electron microscopy to evaluate damage to cell integrity, precipitation of nZVI or iron oxide on the cell wall or inside the bacterial cell was commonly observed (Table 1), suggesting that direct contact of nZVI with bacterial cell is required for nZVI to exert toxicity. Based on these observations, several mechanisms have been hypothesized. The high reducing power of nZVI may denature lipopolysaccharides, and electron and ionic membrane transport proteins, compromising the permeability of the membrane, and facilitating the entrance of toxic Fe2+ into the cell (Lee et al., 2008). Once internalized, Fe2+ could react with the H2O2 produced in mitochondria (i.e., through Fenton reaction), and form highly reactive oxygen species such as OH., O2, or FeO2+, leading to oxidative stress and subsequent cell death. Alternatively, nZVI could also complex with lipoteichoic acids, major constituents of the cell wall of Gram+ bacteria, or the anionic structures of the cell wall of certain bacterial species could stimulate the precipitation of iron oxides. Both these reactions could obstruct the porins of the cell outer membrane and prevent nutrient uptake (Chen et al., 2012; Diao and Yao, 2009). Although iron oxide accumulation was also observed on the cell wall of the fungus Aspergillus versicolor, its growth was not affected (Diao and Yao, 2009). This is likely due to the fact that fungi have an extremely rigid chitinous cell wall, acting as an extra shield against nZVI toxicity (Diao and Yao, 2009; Otero-González et al., 2013; Shah et al., 2010).
Another often-suggested mechanism contributing to nZVI toxicity involves the generation of highly reactive oxygen species, which accumulate in the cell environment and denature macromolecules including lipids, proteins, and nucleic acids, damaging intracellular structures and eventually leading to cell death. Oxidative stress could be directly caused by internalized nZVI, or indirectly through the release of toxic Fe2+ by nZVI adsorbed onto the cell, leading to a local increase in Fe2+ and penetration through the damaged membrane (Auffan et al., 2008), leading to oxidative stress and subsequent cell death as described above. Under aerated conditions, Fe2+ oxidizes more rapidly than it would under anaerobic conditions. Therefore, the contribution of Fe2+ to nZVI toxicity is higher under anaerobic conditions than under aerobic conditions (Kim et al., 2010). Significant inactivation of E. coli, mediated by Fe2+ was previously observed under de-aerated conditions (Lee et al., 2008), while no bactericidal effect was observed under aerated conditions.
Both mechanisms hypothesized for nZVI toxicity to bacteria imply a contact between ZVI nanoparticles and bacteria. Upon contact, redox reactions between iron and cell material may occur and/or reactive oxygen species that damage cells may be produced. The observed rate of inactivation will therefore be function of these intrinsic reaction rates and the rate of nZVI attachment to bacteria. If the intrinsic reaction in either case is assumed to be first order described by a rate constant, kint, the overall rate constant for the reaction, kT, can be described as sequential processes of transport, attachment and reaction (Barton et al., 2015). Transport is described by a collision rate kernel, β, which describes nanoparticle transport to the vicinity of a bacterium. Attachment reflects the relative affinity αNB of nanoparticles of concentration N for bacteria of concentration B. The observed apparent rate of the reduction reaction, kT is related to the rates of heteroaggregation between nZVI and bacteria as:
| (Eq. 12) |
Surface treatments that increase mobility of nZVI may also decrease the affinity of nZVI for bacteria. Thus, it is possible that increasing nanoparticle mobility for better dispersal at contaminated sites will also decrease adverse effects on bacteria at these sites.
3-3 Cellular defenses against nZVI toxicity
As toxicity mechanisms of nZVI are being elucidated, resistance mechanisms or adaptative stress responses set off by bacterial cells to counteract nZVI toxicity are also being unraveled. Bacillus species respond to nZVI toxicity by forming spores (Fajardo et al., 2013, 2012). The appearance of a septum across B. cereus cells, which initiates the process of endospore formation, was observed after exposure to nZVI (Fajardo et al., 2013). This observation was supported by proteomic analyses that revealed a down regulation of flagellin, a structural protein of the flagellum, and phosphoglucosamine mutase, an enzyme catalyzing the synthesis of cell wall peptidoglycans and membrane lipopolysaccharides, likely preparing the cell to enter sporulation (Fajardo et al., 2013). The formation of spores in response to nZVI toxicity is likely a defense mechanism providing the cell with an extra protective barrier (i.e., hard coating surrounding the cell), preventing direct contact between nZVI and the bacteria. Another study by (Saccà et al., 2014a) showed that B. stutzeri exposed to nZVI down regulated the production of several porins and transporters proteins which play essential roles in nutrient and iron uptake through the cellular membrane, likely preventing iron to enter the cell and cause intracellular oxidative damages. Klesbiella oxycota’s high resistance to nZVI toxicity was attributed to the overproduction of tryptophanase, an enzyme converting tryptophan to indole, a signaling molecule produced under stressing environmental conditions and use to induce sporulation within the bacterial population (Saccà et al., 2013).
The production of extracellular polymeric substances [2] by certain bacterial species, such as Agrobacterium sp. and Sphingomonas sp. could also be one of the defense mechanisms developed by microbial species to mitigate nZVI toxic effects (Kim et al., 2012; Le et al., 2014). Excreted EPS adhere to nZVI and bacterial surface, limiting the contact between the two (Kim et al., 2012; Le et al., 2014). EPS are known to promote cell aggregation and adhesion in biofilms and flocs, protecting the cells against unfavorable environmental conditions. EPS also enhances communication between cells within the biofilm and floc formations, facilitating enzyme and metabolite secretion (Wingender et al., 1999).
Finally, oxidative stress response is the most often-cited defense mechanism triggered by nZVI toxicity. By using a strain of E. coli lacking superoxide dismutase, a superoxide-scavenging enzyme that transforms highly reactive O2- into O2 or H2O2, Auffan et al. (2008) demonstrated that oxidative stress response was involved in E. coli resistance to nZVI. In the absence of superoxide dismutase, O2- would accumulate into the cell and denature macromolecules and intracellular structures. Catalase, an enzyme encoding by the katB gene, and also involved in cell ROS detoxification, catalyzes the transformation of H2O2 to H2O and O2. This enzyme was overexpressed when P. stutzeri was exposed to nZVI (Saccà et al., 2014a, 2014b), suggesting that an oxidative stress response induced by H2O2 was involved in this case. In B. cereus, however, katB expression levels did not change after nZVI exposure, suggesting that other reactive oxygen species than H2O2 were involved nZVI toxicity, or that B. cereus quick entrance in sporulation prevented the cell to experience oxidative stress (Fajardo et al., 2013). Kim et al. (2010) also suggested that the primary ROS implicated in nZVI-induced oxidative stress in E. coli were OH. and FeO2+ rather than O2- and H2O2. Proteomics analyses revealed that the defense mechanisms triggered by P. stutzeri involved the up-regulation of several other enzymes participating in oxidative stress response and iron homeostasis. For instance, the observed up regulation of delta-aminolevulinic acid dehydratase, an enzyme directly involved in cellular oxidative stress response, likely acted as an iron scavenger (Saccà et al., 2014a). Chaperonins and heat shock proteins, which are known to play a role when cells are exposed to various stressors, were also up regulated as well as several superoxide dismutases. In B. cereus, the up-regulation of dehydrogenases, implicated in ATP production, might have provided the additional energy required by the cell to enter in sporulation. In the same species, thioredoxin, a protein involved in oxidative stress response was also over produced (Fajardo et al., 2013). Transcriptomic and proteomic approaches are progressively shedding light on the complex enzymatic machinery involved in nZVI toxicity resistance and have proven extremely valuable in deciphering some of the mechanisms involved nZVI toxicity and cellular defenses.
3-4 Parameters influencing nZVI toxicity
3-4-1 nZVI dosage and exposure time
In general, toxic effects of nZVI towards bacterial species tend to increase with increasing nZVI concentrations (Auffan et al., 2008; Fajardo et al., 2012; Kim et al., 2012; Le et al., 2014). One study, however, showed that after 2 hours, 10,000 mg/l nZVI was less toxic to B. cereus than 1,000 mg/l, and that after 24 h, significant toxicity was still observed but only at the lowest concentration (Fajardo et al., 2013). nZVI aggregation is an important parameter to take into consideration when evaluating nZVI toxicity. nZVI aggregation rate will increase with increasing concentration, which will result in the formation of larger nanoparticle aggregates that ultimately sediment more rapidly from suspension (Lowry and Casman, 2009; Tratnyek and Johnson, 2006; Phenrat et al. 2007), resulting in reduced overall cytotoxicity.
Another important factor to consider in assessing nZVI toxicity is exposure time. For instance the toxicity of nZVI observed after 10 minutes on P. stutzeri faded after extended exposure times (Saccà et al., 2014a). Similarly, Alcaligenes eutrophus RNA content rapidly decreased during the first two days of incubation with nZVI. In the following days, however, growth gradually started again (An et al., 2010). In these particular studies, nZVI oxidation rather than sedimentation likely caused the decrease or loss of nZVI toxicity. Therefore, nZVI toxicity increases with increasing concentration but aggregation and oxidation are likely to mitigate the toxicity effects of nZVI if excessive concentrations and longer exposure times are applied.
3-4-2 Effect of insoluble iron oxides
Upon injection into the environment, nZVI will ultimately oxidize. The speed and type of iron oxides formed mainly depend on the concentration of dissolved oxygen. Under aerobic conditions, the lifetime of nZVI (i.e., until nZVI reaches complete oxidation) can be as short as 2 hours, while under anaerobic conditions nZVI can take several months to completely oxidize (Li et al., 2010). The Fe0 content of nZVI gradually decreases with oxidation. E. coli exposed for 60 days to aged nZVI containing 20%, 7%, and 0% Fe0 (i.e., completely oxidized) displayed 5 log-, 2 log-inactivation, and no inactivation, respectively (Li et al., 2010), suggesting that nZVI toxicity decreases with decreasing nZVI Fe0 content, hence oxidation. Depending on the progression of nZVI oxidation, the proportion of the formed magnetite, maghemite and lepidocrocite varies. Exposure of P. fluorescens, B. subtilis, and the fungus A. versicolor to nZVI completely oxidized to FeOOH (lepidocrocite) did not result in any toxic effects even at concentrations up to 10,000 mg/l (Diao and Yao, 2009). Similar results were observed with Agrobacterium sp. (Zhou et al., 2014). At low concentrations (≤100 mg/l), magnetite was even found to promote denitrification by Paracoccus sp. by acting as a slow-release electron donor (Jiang et al., 2013; Liu et al., 2014). The toxicity of nZVI decreases with oxidation and ceases when the particle is completely oxidized because of the loss of electronic or ionic transfers at its surface (Auffan et al., 2009). Although never observed, it has been suggested that the potential detachment of oxide nanoparticles from aged nZVI surface could occur (Kumar et al., 2014a). While Auffan et al. (2008), and Otero-González et al. (2013) showed no toxic effect of maghemite nanoparticles on either E. coli. or S. cerevisiae, in another study by Azam et al. (2012), the growth of E. coli, Pseudomonas aeruginosa, Staphylococcus aureus and B. subtilis was inhibited by maghemite nanoparticles at concentrations as low as 65 mg/l. Magnetite nanoparticles also presented some degree of toxicity towards E coli at concentrations above 700 mg/l (Auffan et al., 2008). Therefore, even after nZVI passivation the secondary formation of iron oxide nanoparticles could also present some degree of toxicity towards microbial life.
3-4-3 Coating and presence of natural organic mater
Although a majority of toxicity studies have been conducted with bare-nZVI, nZVI particles used for in situ remediation are typically coated, and the type of coatings used to enhance nZVI particle colloidal stability has also been found to modulate nZVI toxicity. Chitosan and sodium oleate coatings displayed less toxicity towards Alcaligenes eutrophus than unmodified nZVI (An et al., 2010). Synthetic polymers such as polystyrene sulfonate, polyaspartate, or olefin maleic acid remarkably decreased nZVI toxicity towards E. coli and Dehalococcoides spp. (Li et al., 2010; Xiu et al., 2010a). A microcosm study even showed that polyaspartate coatings stimulated microbial growth in aquifer samples (Kirschling et al., 2010). Unlike bare-nZVI, these coated nanoparticles did not attach to the cells, suggesting that some coating stabilizers may limit the adhesion of nanoparticles to bacterial cells, likely by increasing electrostatic repulsions between the two (Li et al., 2010). However, although carboxy-methyl cellulose (CMC)-stabilized nZVI also had a reduced toxicity compared to bare-nZVI, it significantly adhered to Agrobacterium sp. cell surface but without causing any damages to the membrane. A potential explanation for the observed reduced toxicity despite the physical contact between CMC-coated nanoparticles and bacterial cells, is that CMC might act as a hydroxyl radical scavenger, protecting the cell from oxidative stress (Zhou et al., 2014). Certain nZVI dispersants such as polyacrylic acid however, appeared to have a slight adverse effect on a TCE-degrading microbial community (Chang et al., 2014).
Natural organic matter (NOM) and humic acids, which are found in natural environments at high concentrations, have also been shown to attenuate nZVI toxicity. Chen et al., (2011) reported a mitigation of the bactericidal toxicity of nZVI towards E. coli and B. subtilis in the presence of humic acids as they adsorbed onto nZVI particles and bacterial cells, minimizing direct contact between the two. In agreement with these results, Li et al., (2010) showed that the presence of NOM reduced nZVI toxicity towards E. coli. Although nZVI with synthetic coating showed a higher reduced toxicity than NOM-nZVI, the same electric repulsion forces were involved in reducing nZVI cytotoxic effects. Therefore, the type of coating material and the presence of NOM are also factors to take into consideration when evaluating nZVI toxicity.
4- Effect on complex microbial communities
4-1 Impact of nZVI on community structure
Although valuable knowledge resulted from in vitro toxicity studies, given the complex microbial interactions existing in natural habitats and the high influence of environmental conditions, nZVI toxic effects are expected to be different in situ than in vitro. An increasing number of studies assessing the effect of nZVI on complex microbial communities from natural and engineered environments such as aquifer sediments, river water, soil, and activated sludge have been conducted (Table 2). These studies have integrated traditional microbial ecology and molecular techniques, often using a microcosm experimental setup, hence taking into account the interaction of nZVI with the microbial component and the environmental matrix.
Table 2.
Summary of studies conducted on the effect of nZVI on microbial communities.
| Community studied | nZVI characteristics | nZVI concentration | Experimental conditions | Measured biological parameters | Methods | Effect | Reference |
|---|---|---|---|---|---|---|---|
| Agricultural land | nZVI (NANOFER 25S*) | 34 g Fe0/kg | 72 h-exposure, shaking, aerobic. | Community composition | FISH | Significant shifts in community composition. | Fajardo et al., 2012 |
| < 50 nm | Gene quantification and expression level (nirS, narG, gyrA) | RT-qPCR | No effect on denitrifying populations and gene expression level. | ||||
| Brownfield soil | nZVI (NANOFER 25S*) | 100 g/kg | 13 d-exposure. | Community composition | FISH | Increase in β- and decrease in ε-proteobacteria. | Fajardo et al., 2015 |
| Gene expression level (nirS, narG, KatB, pykA) | RT-qPCR | Overexpression of katB, and underexpression of pykA. No change in narG and nir S expression. | |||||
| Arable land sandy, loam, and clay soil | Home-made CMC-nZVI | 0.27 g Fe0/kg | 4 month-exposure, 15°C, dark. | Community composition | PLFA profiles | Community composition shifts higher in sandy soil. Shift in community functionality only in clay soil. | Pawlett et al., 2013 |
| 10 ± 5.2 nm | Community functional profile | MSIR | Reduction of Gram – bacteria and AMF. | ||||
| Microbial biomass | Fumigation-extraction | ||||||
| Arable land soil | PAA-nZVI (Golder Associates Inc., USA) | 10 g/kg | 28 d-exposure, 25°C, dark | Community composition | DGGE | Shifts in Community composition. | Tilston et al., 2013 |
| 12.5 ±0.3 nm | Chloroaromatic mineralizing microbial population activity | 14C-based MPN technique ([14C]-UL-2,4-D substrate) | Chloroaromatic biodegradation activity reduced. | ||||
| Loamy-sand, and loam soil | nZVI (NANOFER 25S*) | 17 g/kg | 7 d-exposure, 21 ±0.5°C, dark. | Community composition | FISH | No major change in community composition. | Sacca et al., 2014b |
| <50 nm | Gene expression level (katB, and pykA) | RT-qPCR | Overexpression of katB and pykA. | ||||
| Aquifer sediment | PAA-nZVI, bare-nZVI (Toda Kogyo Corp., Japan) | 1,500 mg/l | 250 d-exposure, shaking, 23 ±2°C, dark. | Community composition | DGGE | Significant shift in microbial community composition. | Kirschling et al., 2010 |
| 27.5 nm | Microbial population dynamics (bacterial and archaeal 16S rDNA, dsrA gene) | qPCR | Increase of SRB and methanogens. | ||||
| Aquifer sediment | nZVI (Toda Kogyo Corp., Japan) | 500-3,000 mg/l | 130 d-exposure, 16±2°C, dark, anaerobic | Community composition | 16S environmental cloning-sequencing | Shifts in microbial community. | Kumar et al., 2014 |
| 70-100 nm | |||||||
| Activated sludge | Home-made bare-nZVI | 20-200 mg/l | 9 h-exposure, SBR, anaerobic/aerobic | Community composition | Pyrosequencing | No drastic changes in community composition. | Wu et al., 2013 |
| SSA: 20.71 m2.g-1 | nZVI cytotoxicity | Intracellular | ROS increased with increasing nZVI doses. | ||||
| Microbial activity | ROS and LDH | No effect of nZVI on LDH release. | |||||
| Sludge surface integrity | ATP cellular content | ATP content decreased with increasing nZVI doses. | |||||
| SEM/EDS | Slight damage of sludge surface at 200 mg/l. | ||||||
| Activated sludge | nZVI (NANOFER 25S*) | 0.1 20 mg/l | 56 d-exposure, SBR, 20°C | Community composition | Pyrosequencing | No significant shifts in community composition. | Ma et al., 2015 |
| 46 ± 10 nm |
Abbreviations: AMF, arbuscular mycorrhizal Fungi; CMC, carboxyl methyl cellulose; DGGE, denaturing gradient gel electrophoresis; EDS, energy dispersive X-ray spectroscopy; FISH, fluorescent in situ hybridization; LDH, lactate dehydrogenase; MSIR, multiple substrate-induced respiration; PAA, polyacrylic acid; PLFA, phospholipid fatty acid; ROS, reactive oxygen species ; RT-qPCR, reverse transcription-quantitative PCR; SBR, sequencing batch reactor; SEM, scanning electron microscopy; SRB, sulfate-reducing bacteria. GirA, katB, narG, nirS, and pykA genes encode for gyrase (DNA replication enzyme), catalase (cellular detoxification enzyme), nitrate reductase, nitrite reductase, and pyruvate kinase (glycolysis enzyme), respectively.
NANOFER 25S: nZVI coated with polyethylene glycol sorbitant monostearate with a percentage Fe0 of 14-18% (NANOIRON s.r.o. , Czech Republic).
In aquifer sediments, Kirschling et al., (2010) reported significant shifts in eubacterial diversity that were still apparent 250 days after nZVI addition. Kumar et al. (2014b) also reported a shift in the microbial community structure of aquifer sediments from a community dominated by Acidithiobacillus ferrooxidans and Sulfobacillus-related species to a community dominated by Clostridium and Sporotalea propionica-related species 130 days after nZVI addition. These results suggest that nZVI is likely to have long-term impacts on aquifer indigenous microbial community structure. In soil, nZVI was also reported to trigger drastic shifts in microbial community structure (Tilston et al., 2013). In a sandy clay loam, Fajardo et al. (2012) reported an increase in α-proteobacteria and Archaea and a decrease in β- and γ-proteobacteria abundances. In a different soil however, they noted an increase in β- and a decrease in ε-proteobacteria (Fajardo et al., 2015). Saccà et al. (2014b) who compared the effect of nZVI on the community structure of two different soils reported a decrease in Cytophaga-Flavo-bacteria and Firmicutes, and an increase in Actinobacteria in a loamy soil, whereas in a soil with higher sand content a decrease in α-proteobacteria and β-proteobacteria was observed. Using phospholipid fatty acid (PLFA) profiles, Pawlett et al. (2013) showed that the soil microbial biomass of Gram– bacteria and arbuscular mycorrhizal fungi decreased as an effect of nZVI, and that shifts in community structure were more important in sandy soil than in clay. Therefore, soil texture and organic matter content seem to greatly influence the effect of nZVI on microbial communities.
In nitrifying activated sludge reactors amended with a sequential increase of nZVI from 0.2 to 20 mg/l over 56 days, pyrosequencing approaches revealed a slight shift in microbial communities exposed to nZVI (Ma et al., 2015). In activated sludge, shifts in microbial community composition increased with nZVI dosage from 20 to 200 mg/l (Wu et al., 2013), confirming the dose-dependent effect of nZVI. Once introduced into the system, nZVI reduces the oxidation-reduction potential (ORP), increases the pH of the environmental medium, and produces cathodic H2 (Eq. 1), creating new environmental conditions that are more favorable for certain bacterial taxa than for others (Kischling et al., 2010; Kumar et al., 2014b). Therefore, in both a direct (i.e., toxicity effect) and indirect way (i.e., by inducing environmental physic-chemical changes), nZVI could select for certain bacterial species over others, hence inducing in a shift in microbial community composition.
Microbial communities display critical functions involved in global nutrient cycling and because some bacterial species are functionally redundant, a shift in taxonomic composition does not necessarily translate in functional losses. Therefore, only looking at changes in community taxonomic composition gives an incomplete picture of the effect of nZVI on ecosystems. Some of the studies surveyed in the next section focus on specific functional communities of interest involved in nitrogen cycling, methanogenesis, sulfate reduction, and dehalogenation. Although most of these biological processes were studied in the context of wastewater treatment, results of these studies are also applicable to other natural ecosystems where these microbial processes occur.
4-2 Impact of nZVI on microbial functions
4-2-1 Nitrogen removal
Because nZVI generates a large quantity of H2 under anaerobic conditions (Eq. 1), which could stimulate hydrogen-utilizing denitrifiers and hence accelerate the rate of biological denitrification, nZVI treatment used in combination with biotic denitrification is expected to improve denitrification rates in engineered water clean-up systems (Liu et al., 2014; Shin and Cha, 2008). A few studies assessing nZVI toxicity towards hydrogenotrophic denitrifyers were conducted and although a slight toxicity towards the hydrogenotrophic denitrifying bacterium Parracoccus sp. was observed at concentration close to 1,000 mg/l, the addition of 50 mg/l nZVI stimulated Paracoccus sp. denitrification rate and cell growth (Jiang et al., 2013; Liu et al., 2014). Alcaligenes eutrophus, which display a higher resistance to metallic ions, was much more resistant to nZVI toxicity (An et al., 2009; 2010), and presented an increased nitrate removal activity with increasing nZVI concentrations up to 700 mg/l (An et al., 2009). The primary goal of these studies though, was to develop a system combining nZVI-based nitrate removal and biological denitrification in order to increase biological denitrification and limit the amount of ammonium generated through the abiotic reduction of nitrate by nZVI. However, while some denitrifying bacterial cultures were positively affected by nZVI in terms of denitrification rates (Shin and Cha, 2008; An et al., 2009), most studies conducted on complex microbial communities showed that nZVI addition did not substantially affect the overall denitrification process. Although the gradual addition of nZVI in a lab-scale nitrifying sequencing batch reactor resulted in a shift of the overall community structure, nitrate removal did not increase (Ma et al., 2015). In soil, Fajardo et al. (2012; 2015), who quantified the expression of functional denitrifying genes, narG and nirS, encoding nitrate and nitrite reductase, respectively, did not detect any effect of nZVI on nitrate removal. Similarly, in activated sludge, although the metabolic activity of the whole microbial community seemed to decrease with increasing doses of nZVI, no change in nitrate removal was detected (Wu et al., 2013).
Anaerobic ammonium oxidation (anammox) is another key biological process utilized for wastewater treatment. Under anaerobic conditions, anammox bacteria convert ammonium to nitrogen gas while using nitrite as an electron acceptor. Because of the obvious advantages of this process for water cleanup purposes, studies using nZVI in order to increase ammonium-removal yields were conducted. The widespread application of this process, however, is still hindered by a relatively long reactor startup time and the loss of a substantial fraction of the annamox bacterial biomass during reactor operation. Ren et al. (2015) showed that the addition of nZVI in an anammox upflow anaerobic sludge blanket reactor reduced startup time by 33.3%, and led to higher ammonium removal efficiencies. Indeed, nZVI, as a powerful reductant, consumed dissolved oxygen and generated ammonium via abiotic nitrate reduction, creating the favorable conditions for the faster growth of annamox bacteria. In addition, nZVI likely stimulated the secretion of EPS by anammox bacteria, promoting their aggregation in flocs and preventing their washout of the reactor (Ren et al., 2015).
4-2-2 Methanogenesis
nZVI has also been shown to increase methanogenesis and its use has been considered as a promising strategy to improve yield and quality of biogas production during anaerobic digestion. The fast release of H2 by nZVI under anaerobic conditions favors the growth of hydrogenotrophic methanogens, which convert H2 and CO2 to CH4. Thus, in addition to enhance CH4 production, this process increases the quality and value of the biogas by fixing CO2. Although substantial enhancement in CH4 production in the range of 28 to 61% (Carpenter et al., 2015; Hu et al., 2015; Su et al., 2013) is usually achieved in the presence of nZVI, one study showed that high concentration of nZVI could also be detrimental to methanogens. Indeed, at higher nZVI concentrations, the faster release of hydrogen gas increases the hydrogen gas partial pressure at levels that favor hydrogenotrophic anaerobic processes, such as homoacetogenesis, over methanogenesis. In addition, the higher concentration of Fe2+ that might result from the addition of higher concentration of nZVI could inhibit methanogenesis (Yang et al., 2013).
4-2-3 Sulfate reduction
Biological sulfate-reduction is one of the most efficient ways to treat heavy metal-laden environments. In this approach, the production of sulfide, a by-product of biological sulfate reduction, leads to the sequestration of heavy metals through the formation of metal-sulfide precipitates. The use of nZVI in the context of heavy metal decontamination has been considered as an approach to enhance sulfate reduction, hence increase heavy metal sequestration. The decrease in dissolved oxygen and ORP, as well as the generation of H2 that can be used as an electron donor by sulfate reducing bacteria (SRB), is expected to create favorable conditions for the growth of SRB. In addition, abiotic heavy metal removal by nZVI could also occur through surface adsorption and subsequent precipitation. Kumar et al. (2014b) who investigated the effect of nZVI amendment on sulfate reduction, however, found that sulfate reduction was completely inhibited at nZVI concentrations above 500 mg/l. In agreement with these results, Barnes et al. (2010) found that doses above 300 mg/l led to a complete inhibition of sulfate reduction. On the contrary, Kirschling et al. (2010), who used quantitative PCR to measure SRB from aquifer material, reported an increase of the SRB population upon addition of 1,500 mg/l nZVI. The inconsistencies between these results are likely due to the synthrophic or competitive relationships existing between functional groups within the microbial community, as several functional groups compete for the cathodic H2 generated by nZVI.
4-2-4 De-halogenation
In situ nZVI deployment may also affect indigenous microbial communities whose biodegradative potential could contribute to natural contaminant remediation. Therefore looking at the effects of nZVI on these particular communities is extremely relevant when assessing long-term impacts. As it does for biological denitrification, methanogenesis, and sulfate reduction, the cathodic H2 generated by nZVI could serve as an electron donor for halorespirers, hence stimulating biological degradation of halogenated compounds. Laboratory-scale experiments, however, reported that TCE-degrading microbial activity was increasingly inhibited by increasing nZVI concentrations (Barnes et al., 2010; Xiu et al., 2010a, 2010b). nZVI coating, however, was shown to mitigate the negative effect of nZVI on degrading communities (Xiu et al., 2010a, 2010b). In line with these results, Tilston et al. (2013) showed that a chloroaromatic mineralizing population size and activity were reduced by the addition of nZVI, and even though population size recovered after 7 days, biological degradation remained inhibited. In a dioxin-contaminated soil, the addition of nZVI did not stimulate degradation, but promoted methanogenesis instead (Binh et al., 2015). Methanogens have shown to outcompete dechlorinators for the use of H2 when higher H2 concentrations and higher ORP are encountered. In this study, the dose of nZVI concentration used might have been too high, resulting in an excessive release of H2 and fast increase of ORP which favored methanogens over dechlorinators (Binh et al., 2015; Xiu et al., 2010a, 2010b).
It should be noted, however, that the laboratory studies described above may not adequately reproduce the conditions encountered in situ. Indeed, a pilot-scale CMC-nZVI injection performed on a site contaminated with chlorinated organic compounds resulted in the successful degradation of chlorinated compound accompanied by a significant increase of Dehalococcoides spp. at the injection point and along the nZVI flow path (Kocur et al., 2015). This suggests that environmental variables such as soil texture and organic matter content, as well as nanoparticle coating, likely mitigate the negative effect of nZVI on in situ degrading-microbial communities.
5- Fate of nZVI in the environment
5-1 Transport
Understanding nZVI transport is essential to evaluate the potential of nZVI to travel from injection points to untargeted environmental compartments, and to assess the microbial community exposure to nZVI. While migration with relative ease from injection points may result in the dilution of nZVI, no transport will create hotspots of nZVI concentration close to the injection point. To date, most studies on nZVI migration have been performed in the context of groundwater and soil remediation, and have focused on the ability of nZVI to migrate within a contaminated plume. After injection, nZVI mobility is influenced by many interrelated environmental parameters, including ionic strength and composition, pH, O2 concentration, presence of natural organic matter, and hydraulic conductivity of the environmental medium, as well as intrinsic properties of the nZVI particles such as surface coating, size, and concentration (Illés and Tombácz, 2006; Saleh et al., 2008; Gottschalk et al., 2010; Gottschalk and Nowack, 2011; Mueller et al., 2013; Mueller and Nowack, 2008). Although in groundwater systems, aggregation and adhesion to environmental particle surface are the primary factors limiting nZVI transport (Dong and Lo, 2013; Illés and Tombácz, 2006; Jung et al., 2014; Kocur et al., 2013; Petosa et al., 2010; Yin et al., 2012), high nZVI injection rates and high porosity of the aquifer material will enhance nZVI mobility (Kanel and Choi, 2007; Saleh et al., 2008). In porous media, nZVI can typically reach transport distances between 1 and 5 meters, which is significant in the context of groundwater remediation (Baalousha, 2009; Bennett et al., 2010; Busch et al., 2015; Kocur et al., 2014; Su et al., 2013; Johnson et al., 2013). Although at the scale of the groundwater ecosystem, this appears to be a limited distance, high concentrations of nZVI created at injection points can significantly change the local environmental conditions (e.g., ORP, pH, O2 concentration), likely affecting both microbial community structure and functions within the nZVI reactive zone. In addition, dissolved species released by nZVI, such as Fe2+ will have the capacity to travel further (Shi et al., 2015), potentially expanding the zone of influence of nZVI. In comparison to groundwater systems, higher organic content, lower porosity and water flow usually prevailing in soil, limits nZVI transport capabilities, resulting in shorter nZVI travel distances (Dong and Lo, 2014).
5-2 Entrance points and potential routes to other environments
Pollutant remediation by nZVI is referenced in soils, groundwater, wastewater, and organic wastes (Fu et al., 2014; Mueller et al., 2012; Qu et al., 2013; Tosco et al., 2014; Tuomi et al., 2008). Although applications of nZVI beyond surface and subsurface remediation are extremely limited, organic waste stabilization, odor abatement, and biogas production are also referenced (Carpenter et al., 2015; Su et al., 2013). In this section all applications of nZVI are considered in order to understand nZVI life cycle, and evaluate its potential routes from entrance points to untargeted environments (Mitrano et al., 2015; Nowack, 2009; Som et al., 2010). However, because the detection of nanoparticles in complex environmental media has proven challenging (Hassellöv et al., 2008), data of transport of nZVI in situ are still lacking. Therefore, in this section, the routes taken by nZVI from application sites to other environmental compartments will be hypothesized according to general considerations.
In both groundwater and soil remediation applications, nZVI is deliberately and directly introduced at concentrations typically on the order of 10 g/l (Phenrat et al., 2009; Saleh et al., 2008), although concentrations as low as 0.75 – 1.5 g/l (Elliott and Zhang, 2001), and as high as 50 g/l (Tuomi et al., 2008) have been used. The groundwater remediation process consists of the direct injection of highly concentrated nZVI slurry through drilled boreholes in order to create subsurface reactive zones. In soil, however, treatments involve either subsurface injections or surface amendments. Detailed about field scale experiments can be found elsewhere (Bardos et al., 2015). Given the relatively low transport of nZVI in soil and groundwater, nZVI and oxide end products likely remain close to injection points. However, under particular environmental conditions, transfer of nZVI and oxidation products from groundwater or soil to adjacent surface waters could potentially occur. In this case, nZVI and end products will undergo dilution, aggregation, sedimentation, and further oxidation, likely resulting in the significant decrease of nZVI toxicity (Praetorius et al., 2012; Sun et al., 2014).
Compared to soil and groundwater, water and organic waste treatment plants, although the use of nZVI-based strategies in these systems is extremely limited in practice, can only be considered as a transient compartment for nZVI. Indeed, in organic waste treatment plants, nZVI and end products are expected to follow the treated waste disposal routes. However, the numerous uncertainties concerning the type of industrial plant, treated material, and treatment conditions make it difficult to assess the transfer of nZVI from these systems to other environmental compartments. In wastewater treatment plants, nZVI and end products could partition either to treated effluent or sludge. Given the high tendency of nZVI to adhere to other particles, nZVI is expected to accumulate, for the most part, in treated sludge, which can later be burnt in waste incineration plants, stored in landfills, or amended in agricultural soils as biosolids. The discharge of a small portion of nZVI in treated effluent cannot be excluded, as evidenced for TiO2 nanoparticles (Kiser et al., 2009). Soluble iron ions, however, are expected to be released in the treated effluent and ultimately in surface water. In organic waste treatment plants, nZVI and end products routes into the environment can also be assumed to follow organic wastes disposal routes (i.e., similar to wastewater treated sludge).
Finally, the behaviour of nZVI in landfill is not well documented. To date, almost no data exist on the fate and state of nanoparticles in waste incineration processes, and leaching from landfills. From landfill, the transfer to soil and surface water of Ag, TiO2, Zn, CNT and fullerene nanoparticles has been modelled, and predicted to be negligible for the most part (Sun et al., 2014). Depending on the landfill types (e.g., inert materials, stable materials, or reactive materials), nZVI and end products could undergo cement consolidation, acid washing, grinding and could also be in contact with various organic and inorganic materials (Mueller et al., 2013). Based on these considerations, the stabilization and/or destruction (dissolution) of nZVI in landfill is most likely, and the same behaviour is expected in waste incineration plant.
6- Conclusion and perspectives
The implication of nZVI-based strategies for environmental applications must be thoroughly evaluated before the technology can be further deployed in situ. Because microorganisms are ubiquitous and essential for the functioning of ecosystems, studying the impact of nZVI on microbial communities is of primary importance in evaluating the ecological consequences that could result from the more extensive use of nZVI. Toxicity studies showed that nZVI could exhibit strong to severe toxic effects towards certain microbial species, and although nZVI toxicity is likely to be mitigated by the physico-chemical conditions encountered in situ, substantial shifts in taxonomic composition were reported. From a functional perspective, the introduction of nZVI into the environment will likely disturb the equilibrium between the functional microbial groups carrying out important biogeochemical processes. In addition, based on current and potential future in situ applications of nZVI treatments, untargeted ecosystems might be affected by nZVI. Based on most of the studies conducted to date, whether the focus is directed towards the mechanisms involved in nZVI toxicity at the cellular level, or towards the impact of nZVI at the microbial community level, the environmental conditions encountered in situ definitely have an important role in how nZVI affects microbial communities and ecosystem functions. Therefore, future studies should consider bringing greater emphasis on the effect of environmentally relevant factors on nZVI toxicity in order to provide a better understanding of the ecological impacts related to the use of nZVI.
Highlights.
nZVI toxicity towards microbes is highly modulated by environmental conditions.
Cell membrane disruption and oxidative stress contribute to nZVI toxicity.
Microbes have developed adaptative response to nZVI toxicity.
nZVI disturb microbial community taxonomic and functional composition.
Acknowledgments
Funding for this work was gratefully provided by the NIEHS-supported Duke University Superfund Research Center (NIEHS grant P42-ES010356). The authors would like to thank Erich Pinzón Fuchs and Naresh Kumar for their comments and critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adeleye AS, Keller AA, Miller RJ, Lenihan HS. Persistence of commercial nanoscaled zero-valent iron (nZVI) and by-products. J Nanoparticle Res. 2013;15 doi: 10.1007/s11051-013-1418-7. [DOI] [Google Scholar]
- An Y, Li T, Jin Z, Dong M, Li Q, Wang S. Decreasing ammonium generation using hydrogenotrophic bacteria in the process of nitrate reduction by nanoscale zero-valent iron. Sci Total Environ. 2009;407:5465–5470. doi: 10.1016/j.scitotenv.2009.06.046. [DOI] [PubMed] [Google Scholar]
- An Y, Li T, Jin Z, Dong M, Xia H, Wang X. Effect of bimetallic and polymer-coated Fe nanoparticles on biological denitrification. Bioresour Technol. 2010;101:9825–9828. doi: 10.1016/j.biortech.2010.07.110. [DOI] [PubMed] [Google Scholar]
- Auffan M, Achouak W, Rose J, Roncato MA, Chanéac C, Waite DT, Masion A, Woicik JC, Wiesner MR, Bottero J-Y. Relation between the Redox State of Iron-Based Nanoparticles and Their Cytotoxicity toward Escherichia coli. Environ Sci Technol. 2008;42:6730–6735. doi: 10.1021/es800086f. [DOI] [PubMed] [Google Scholar]
- Auffan M, Rose J, Wiesner MR, Bottero J-Y. Chemical stability of metallic nanoparticles: A parameter controlling their potential cellular toxicity in vitro. Environ Pollut. 2009;157:1127–1133. doi: 10.1016/j.envpol.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Azam Ameer, et al. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. International journal of nanomedicine. 2012;7:6003. doi: 10.2147/IJN.S35347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baalousha M. Aggregation and disaggregation of iron oxide nanoparticles: Influence of particle concentration, pH and natural organic matter. Sci Total Environ. 2009;407:2093–2101. doi: 10.1016/j.scitotenv.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Barnes RJ, Riba O, Gardner MN, Singer AC, Jackman SA, Thompson IP. Inhibition of biological TCE and sulphate reduction in the presence of iron nanoparticles. Chemosphere. 2010;80:554–562. doi: 10.1016/j.chemosphere.2010.04.033. [DOI] [PubMed] [Google Scholar]
- Barton LE, Auffan M, Olivi L, Bottero J-Y, Wiesner MR. Heteroaggregation, transformation and fate of CeO2 nanoparticles in wastewater treatment. Environ Pollut. 2015;203:122–129. doi: 10.1016/j.envpol.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Battesti A, Majdalani N, Gottesman S. The RpoS-Mediated General Stress Response in Escherichia coli *. Annu Rev Microbiol. 2011;65:189–213. doi: 10.1146/annurev-micro-090110-102946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P, He F, Zhao D, Aiken B, Feldman L. In situ testing of metallic iron nanoparticle mobility and reactivity in a shallow granular aquifer. J Contam Hydrol. 2010;116:35–46. doi: 10.1016/j.jconhyd.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Binh ND, Imsapsangworn C, Kim Oanh NT, Parkpian P, Karstensen K, Giao PH, DeLaune RD. Sequential anaerobic–aerobic biodegradation of 2,3,7,8-TCDD contaminated soil in the presence of CMC-coated nZVI and surfactant. Environ Technol. 2015:1–11. doi: 10.1080/09593330.2015.1070918. [DOI] [PubMed] [Google Scholar]
- Busch J, Meißner T, Potthoff A, Bleyl S, Georgi A, Mackenzie K, Trabitzsch R, Werban U, Oswald SE. A field investigation on transport of carbon-supported nanoscale zero-valent iron (nZVI) in groundwater. J Contam Hydrol. 2015;181:59–68. doi: 10.1016/j.jconhyd.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Carpenter AW, Laughton SN, Wiesner MR. Enhanced Biogas Production from Nanoscale Zero Valent Iron-Amended Anaerobic Bioreactors. Environ Eng Sci. 2015;32:647–655. doi: 10.1089/ees.2014.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaithawiwat K, Vangnai A, McEvoy JM, Pruess B, Krajangpan S, Khan E. Impact of nanoscale zero valent iron on bacteria is growth phase dependent. Chemosphere. 2016;144:352–359. doi: 10.1016/j.chemosphere.2015.09.025. [DOI] [PubMed] [Google Scholar]
- Chang YC, Huang SC, Chen KF. Evaluation of the effects of nanoscale zero-valent iron (nZVI) dispersants on intrinsic biodegradation of trichloroethylene (TCE) Water Sci Technol. 2014;69:2357. doi: 10.2166/wst.2014.169. [DOI] [PubMed] [Google Scholar]
- Chen J, Xiu Z, Lowry GV, Alvarez PJJ. Effect of natural organic matter on toxicity and reactivity of nano-scale zero-valent iron. Water Res. 2011;45:1995–2001. doi: 10.1016/j.watres.2010.11.036. [DOI] [PubMed] [Google Scholar]
- Chen Q, Gao M, Li J, Shen F, Wu Y, Xu Z, Yao M. Inactivation and Magnetic Separation of Bacteria from Liquid Suspensions Using Electrosprayed and Nonelectrosprayed nZVI Particles: Observations and Mechanisms. Environ Sci Technol. 2012;46:2360–2367. doi: 10.1021/es204024n. [DOI] [PubMed] [Google Scholar]
- Chowdhury AIA, Krol MM, Kocur CM, Boparai HK, Weber KP, Sleep BE, O’Carroll DM. nZVI injection into variably saturated soils: Field and modeling study. J Contam Hydrol. 2015;183:16–28. doi: 10.1016/j.jconhyd.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Crane RA, Scott TB. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J Hazard Mater. 2012;211(212):112–125. doi: 10.1016/j.jhazmat.2011.11.073. [DOI] [PubMed] [Google Scholar]
- Diao M, Yao M. Use of zero-valent iron nanoparticles in inactivating microbes. Water Res. 2009;43:5243–5251. doi: 10.1016/j.watres.2009.08.051. [DOI] [PubMed] [Google Scholar]
- Dong H, Lo IMC. Transport of Surface-Modified Nano Zero-Valent Iron (SM-NZVI) in Saturated Porous Media: Effects of Surface Stabilizer Type, Subsurface Geochemistry, and Contaminant Loading. Water Air Soil Pollut. 2014:225. doi: 10.1007/s11270-014-2107-6. [DOI] [Google Scholar]
- Dong H, Lo IMC. Influence of calcium ions on the colloidal stability of surface-modified nano zero-valent iron in the absence or presence of humic acid. Water Res. 2013;47:2489–2496. doi: 10.1016/j.watres.2013.02.022. [DOI] [PubMed] [Google Scholar]
- Drever J. The geochemistry of natural waters. New Jersey: prentice Hall; 1988. [Google Scholar]
- Elliott DW, Zhang W. Field Assessment of Nanoscale Bimetallic Particles for Groundwater Treatment. Environ Sci Technol. 2001;35:4922–4926. doi: 10.1021/es0108584. [DOI] [PubMed] [Google Scholar]
- Fajardo C, Ortíz LT, Rodríguez-Membibre ML, Nande M, Lobo MC, Martin M. Assessing the impact of zero-valent iron (ZVI) nanotechnology on soil microbial structure and functionality: A molecular approach. Chemosphere. 2012;86:802–808. doi: 10.1016/j.chemosphere.2011.11.041. [DOI] [PubMed] [Google Scholar]
- Fajardo C, Saccà ML, Martinez-Gomariz M, Costa G, Nande M, Martin M. Transcriptional and proteomic stress responses of a soil bacterium Bacillus cereus to nanosized zero-valent iron (nZVI) particles. Chemosphere. 2013;93:1077–1083. doi: 10.1016/j.chemosphere.2013.05.082. [DOI] [PubMed] [Google Scholar]
- Fajardo C, Gil-Díaz M, Costa G, Alonso J, Guerrero AM, Nande M, Lobo MC, Martín M. Residual impact of aged nZVI on heavy metal-polluted soils. Sci Total Environ. 2015;535:79–84. doi: 10.1016/j.scitotenv.2015.03.067. [DOI] [PubMed] [Google Scholar]
- Fu F, Dionysiou DD, Liu H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J Hazard Mater. 2014;267:194–205. doi: 10.1016/j.jhazmat.2013.12.062. [DOI] [PubMed] [Google Scholar]
- Gottschalk F, Nowack B. The release of engineered nanomaterials to the environment. J Environ Monit. 2011;13:1145–1155. doi: 10.1039/C0em00547a. [DOI] [PubMed] [Google Scholar]
- Gottschalk F, Scholz RW, Nowack B. Probabilistic material flow modeling for assessing the environmental exposure to compounds: Methodology and an application to engineered nano-TiO2 particles. Env Model Softw. 2010;25:320–332. doi: 10.1016/j.envsoft.2009.08.011. [DOI] [Google Scholar]
- Grieger KD, Fjordbøge A, Hartmann NB, Eriksson E, Bjerg PL, Baun A. Environmental benefits and risks of zero-valent iron nanoparticles (nZVI) for in situ remediation: Risk mitigation or trade-off? J Contam Hydrol. 2010;118:165–183. doi: 10.1016/j.jconhyd.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Greenlee LF, Torrey JD, Amaro RL, Shaw JM. Kinetics of Zero Valent Iron Nanoparticle Oxidation in Oxygenated Water. Environ Sci Technol. 2012;46:12913–12920. doi: 10.1021/es303037k. [DOI] [PubMed] [Google Scholar]
- Hassellöv M, Readman JW, Ranville JF, Tiede K. Nanoparticle analysis and characterization methodologies in environmental risk assessment of engineered nanoparticles. Ecotoxicology. 2008;17:344–361. doi: 10.1007/s10646-008-0225-x. [DOI] [PubMed] [Google Scholar]
- Hu Y, Hao X, Zhao D, Fu K. Enhancing the CH4 yield of anaerobic digestion via endogenous CO2 fixation by exogenous H2. Chemosphere. 2015;140:34–39. doi: 10.1016/j.chemosphere.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Hwang Y, Kim D-G, Shin H-S. Effects of synthesis conditions on the characteristics and reactivity of nano scale zero valent iron. Applied Catalysis B: Environmental. 2011;105:144–150. doi: 10.1016/j.apcatb.2011.04.005. [DOI] [Google Scholar]
- Illés E, Tombácz E. The effect of humic acid adsorption on pH-dependent surface charging and aggregation of magnetite nanoparticles. J Colloid Interface Sci. 2006;295:115–123. doi: 10.1016/j.jcis.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Jiang C, Liu Y, Chen Z, Megharaj M, Naidu R. Impact of iron-based nanoparticles on microbial denitrification by Paracoccus sp. strain YF1. Aquat Toxicol. 2013;142(143):329–335. doi: 10.1016/j.aquatox.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Jiang C, Xu X, Megharaj M, Naidu R, Chen Z. Inhibition or promotion of biodegradation of nitrate by Paracoccus sp. in the presence of nanoscale zero-valent iron. Sci Total Environ. 2015;530(531):241–246. doi: 10.1016/j.scitotenv.2015.05.044. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Nurmi JT, O’Brien Johnson GS, Fan D, O’Brien Johnson RL, Shi Z, Salter-Blanc AJ, Tratnyek PG, Lowry GV. Field-Scale Transport and Transformation of Carboxymethylcellulose-Stabilized Nano Zero-Valent Iron. Environ Sci Technol. 2013;47:1573–1580. doi: 10.1021/es304564q. [DOI] [PubMed] [Google Scholar]
- Jung B, O’Carroll D, Sleep B. The influence of humic acid and clay content on the transport of polymer-coated iron nanoparticles through sand. Sci Total Environ. 2014;496:155–164. doi: 10.1016/j.scitotenv.2014.06.075. [DOI] [PubMed] [Google Scholar]
- Kidarsa TA, Shaffer BT, Goebel NC, Roberts DP, Buyer JS, Johnson A, Kobayashi DY, Zabriskie TM, Paulsen I, Loper JE. Genes expressed by the biological control bacterium Pseudomonas protegens Pf-5 on seed surfaces under the control of the global regulators GacA and RpoS: GacA and RpoS regulation of Pf-5 genes on seeds. Environ Microbiol. 2013;15:716–735. doi: 10.1111/1462-2920.12066. [DOI] [PubMed] [Google Scholar]
- Kim H-J, Phenrat T, Tilton RD, Lowry GV. Fe 0 Nanoparticles Remain Mobile in Porous Media after Aging Due to Slow Desorption of Polymeric Surface Modifiers. Environ Sci Technol. 2009;43:3824–3830. doi: 10.1021/es802978s. [DOI] [PubMed] [Google Scholar]
- Kim H-S, Kim T, Ahn J-Y, Hwang K-Y, Park J-Y, Lim T-T, Hwang I. Aging characteristics and reactivity of two types of nanoscale zero-valent iron particles (FeBH and FeH2) in nitrate reduction. Chem Eng J. 2012;197:16–23. doi: 10.1016/j.cej.2012.05.018. [DOI] [Google Scholar]
- Kim JY, Park H-J, Lee C, Nelson KL, Sedlak DL, Yoon J. Inactivation of Escherichia coli by Nanoparticulate Zerovalent Iron and Ferrous Ion. Appl Environ Microbiol. 2010;76:7668–7670. doi: 10.1128/AEM.01009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-M, Murugesan K, Chang Y-Y, Kim E-J, Chang Y-S. Degradation of polybrominated diphenyl ethers by a sequential treatment with nanoscale zero valent iron and aerobic biodegradation. J Chem Technol Biotechnol. 2012;87:216–224. doi: 10.1002/jctb.2699. [DOI] [Google Scholar]
- Kirschling TL, Gregory KB, Minkley EG, Jr, Lowry GV, Tilton RD. Impact of Nanoscale Zero Valent Iron on Geochemistry and Microbial Populations in Trichloroethylene Contaminated Aquifer Materials. Environ Sci Technol. 2010;44:3474–3480. doi: 10.1021/es903744f. [DOI] [PubMed] [Google Scholar]
- Kiser MA, Westerhoff P, Benn T, Wang Y, Pérez-Rivera J, Hristovski K. Titanium Nanomaterial Removal and Release from Wastewater Treatment Plants. Environ Sci Technol. 2009;43:6757–6763. doi: 10.1021/es901102n. [DOI] [PubMed] [Google Scholar]
- Kocur CM, Chowdhury AI, Sakulchaicharoen N, Boparai HK, Weber KP, Sharma P, Krol MM, Austrins L, Peace C, Sleep BE, O’Carroll DM. Characterization of nZVI Mobility in a Field Scale Test. Environ Sci Technol. 2014;48:2862–2869. doi: 10.1021/es4044209. [DOI] [PubMed] [Google Scholar]
- Kocur CMD, Lomheim L, Boparai HK, Chowdhury AIA, Weber KP, Austrins LM, Edwards EA, Sleep BE, O’Carroll DM. Contributions of Abiotic and Biotic Dechlorination Following Carboxymethyl Cellulose Stabilized Nanoscale Zero Valent Iron Injection. Environ Sci Technol. 2015;49:8648–8656. doi: 10.1021/acs.est.5b00719. [DOI] [PubMed] [Google Scholar]
- Kocur CM, O’Carroll DM, Sleep BE. Impact of nZVI stability on mobility in porous media. J Contam Hydrol. 2013;145:17–25. doi: 10.1016/j.jconhyd.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Kumar N, Auffan M, Gattacceca J, Rose J, Olivi L, Borschneck D, Kvapil P, Jublot M, Kaifas D, Malleret L, Doumenq P, Bottero J-Y. Molecular Insights of Oxidation Process of Iron Nanoparticles: Spectroscopic, Magnetic, and Microscopic Evidence. Environ Sci Technol. 2014a;48:13888–13894. doi: 10.1021/es503154q. [DOI] [PubMed] [Google Scholar]
- Kumar N, Omoregie EO, Rose J, Masion A, Lloyd JR, Diels L, Bastiaens L. Inhibition of sulfate reducing bacteria in aquifer sediment by iron nanoparticles. Water Res. 2014b;51:64–72. doi: 10.1016/j.watres.2013.09.042. [DOI] [PubMed] [Google Scholar]
- Lee C, Kim JY, Lee WI, Nelson KL, Yoon J, Sedlak DL. Bactericidal Effect of Zero-Valent Iron Nanoparticles on Escherichia coli. Environ Sci Technol. 2008;42:4927–4933. doi: 10.1021/es800408u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TT, Murugesan K, Kim E-J, Chang Y-S. Effects of inorganic nanoparticles on viability and catabolic activities of Agrobacterium sp. PH-08 during biodegradation of dibenzofuran. Biodegradation. 2014;25:655–668. doi: 10.1007/s10532-014-9689-y. [DOI] [PubMed] [Google Scholar]
- Liu A, Liu J, Pan B, Zhang W. Formation of lepidocrocite (γ-FeOOH) from oxidation of nanoscale zero-valent iron (nZVI) in oxygenated water. RSC Adv. 2014;4:57377–57382. doi: 10.1039/C4RA08988J. [DOI] [Google Scholar]
- Li Z, Greden K, Alvarez PJJ, Gregory KB, Lowry GV. Adsorbed Polymer and NOM Limits Adhesion and Toxicity of Nano Scale Zerovalent Iron to E. coli. Environ Sci Technol. 2010;44:3462–3467. doi: 10.1021/es9031198. [DOI] [PubMed] [Google Scholar]
- Lowry GV, Casman EA. Nanomaterial Transport, Transformation, and Fate in the Environment. In: Linkov I, Steevens J, editors. Nanomaterials: Risks and Benefits. Springer Netherlands; Dordrecht: 2009. pp. 125–137. [Google Scholar]
- Martin JE, Herzing AA, Yan W, Li X, Koel BE, Kiely CJ, Zhang W. Determination of the Oxide Layer Thickness in Core–Shell Zerovalent Iron Nanoparticles. Langmuir. 2008;24:4329–4334. doi: 10.1021/la703689k. [DOI] [PubMed] [Google Scholar]
- Ma Y, Metch JW, Vejerano EP, Miller IJ, Leon EC, Marr LC, Vikesland PJ, Pruden A. Microbial community response of nitrifying sequencing batch reactors to silver, zero-valent iron, titanium dioxide and cerium dioxide nanomaterials. Water Res. 2015;68:87–97. doi: 10.1016/j.watres.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Memic A, Azam A, Ahmed, Oves, Khan, Habib Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomedicine. 2012:6003. doi: 10.2147/IJN.S35347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrano DM, Motellier S, Clavaguera S, Nowack B. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ Int. 2015;77:132–147. doi: 10.1016/j.envint.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Mueller NC, Braun J, Bruns J, Černik M, Rissing P, Rickerby D, Nowack B. Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe. Environ Sci Pollut Res. 2012;19:550–558. doi: 10.1007/s11356-011-0576-3. [DOI] [PubMed] [Google Scholar]
- Mueller NC, Buha J, Wang J, Ulrich A, Nowack B. Modeling the flows of engineered nanomaterials during waste handling. Environ Sci Process Impacts. 2013;15:251–259. doi: 10.1039/c2em30761h. [DOI] [PubMed] [Google Scholar]
- Mueller NC, Nowack B. Exposure Modeling of Engineered Nanoparticles in the Environment. Environ Sci Technol. 2008;42:4447–4453. doi: 10.1021/es7029637. [DOI] [PubMed] [Google Scholar]
- Nowack B. Is anything out there? Nano Today. 2009;4:11–12. doi: 10.1016/j.nantod.2008.10.001. [DOI] [Google Scholar]
- Nurmi JT, Tratnyek PG, Sarathy V, Baer DR, Amonette JE, Pecher K, Wang C, Linehan JC, Matson DW, Penn RL, Driessen MD. Characterization and Properties of Metallic Iron Nanoparticles: Spectroscopy, Electrochemistry, and Kinetics. Environ Sci Technol. 2005;39:1221–1230. doi: 10.1021/es049190u. [DOI] [PubMed] [Google Scholar]
- Otero-González L, García-Saucedo C, Field JA, Sierra-Álvarez R. Toxicity of TiO2, ZrO2, Fe0, Fe2O3, and Mn2O3 nanoparticles to the yeast, Saccharomyces cerevisiae. Chemosphere. 2013;93:1201–1206. doi: 10.1016/j.chemosphere.2013.06.075. [DOI] [PubMed] [Google Scholar]
- Paul Bardos, Jones S, Bardos A, Bartke S, Bone B, Daly P, Elliot D, Garcia de la Calle R, Gens A, Gillett A, Harries N, Limasset E, Lowry G, McCaffrey C, Merly C, Nathanail J, Nathanial P, Oughton D, Tomkiv Y. Promoting Nanoremediation Using Nanoscale Zerovalent Iron (nZVI): Risk-Benefit and Markets Appraisal, Initial Exploitation Strategy and Consultation. 2015 doi: 10.13140/RG.2.1.1921.6800. [DOI] [Google Scholar]
- Pawlett M, Ritz K, Dorey RA, Rocks S, Ramsden J, Harris JA. The impact of zero-valent iron nanoparticles upon soil microbial communities is context dependent. Environ Sci Pollut Res. 2013;20:1041–1049. doi: 10.1007/s11356-012-1196-2. [DOI] [PubMed] [Google Scholar]
- Petosa AR, Jaisi DP, Quevedo IR, Elimelech M, Tufenkji N. Aggregation and Deposition of Engineered Nanomaterials in Aquatic Environments: Role of Physicochemical Interactions. Environ Sci Technol. 2010;44:6532–6549. doi: 10.1021/es100598h. [DOI] [PubMed] [Google Scholar]
- Phenrat T, Liu Y, Tilton RD, Lowry GV. Adsorbed Polyelectrolyte Coatings Decrease Fe 0 Nanoparticle Reactivity with TCE in Water: Conceptual Model and Mechanisms. Environ Sci Technol. 2009;43:1507–1514. doi: 10.1021/es802187d. [DOI] [PubMed] [Google Scholar]
- Phenrat T, Saleh N, Sirk K, Kim H-J, Tilton RD, Lowry GV. Stabilization of aqueous nanoscale zerovalent iron dispersions by anionic polyelectrolytes: adsorbed anionic polyelectrolyte layer properties and their effect on aggregation and sedimentation. J Nanoparticle Res. 2008;10:795–814. doi: 10.1007/s11051-007-9315-6. [DOI] [Google Scholar]
- Phenrat T, Saleh N, Sirk K, Tilton RD, Lowry GV. Aggregation and Sedimentation of Aqueous Nanoscale Zerovalent Iron Dispersions. Environ Sci Technol. 2007;41:284–290. doi: 10.1021/es061349a. [DOI] [PubMed] [Google Scholar]
- Praetorius A, Scheringer M, Hungerbühler K. Development of Environmental Fate Models for Engineered Nanoparticles—A Case Study of TiO2 Nanoparticles in the Rhine River. Environ Sci Technol. 2012;46:6705–6713. doi: 10.1021/es204530n. [DOI] [PubMed] [Google Scholar]
- Ren L-F, Ni S-Q, Liu C, Liang S, Zhang B, Kong Q, Guo N. Effect of zero-valent iron on the start-up performance of anaerobic ammonium oxidation (anammox) process. Environ Sci Pollut Res. 2015;22:2925–2934. doi: 10.1007/s11356-014-3553-9. [DOI] [PubMed] [Google Scholar]
- Saccà ML, Fajardo C, Costa G, Lobo C, Nande M, Martin M. Integrating classical and molecular approaches to evaluate the impact of nanosized zero-valent iron nZVI on soil organisms. Chemosphere. 2014a;104:184–189. doi: 10.1016/j.chemosphere.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Saccà ML, Fajardo C, Martinez-Gomariz M, Costa G, Nande M, Martin M. Molecular Stress Responses to Nano-Sized Zero-Valent Iron (nZVI) Particles in the Soil Bacterium Pseudomonas stutzeri. PLoS ONE. 2014b;9:e89677. doi: 10.1371/journal.pone.0089677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccà ML, Fajardo C, Nande M, Martín M. Effects of Nano Zero-Valent Iron on Klebsiella oxytoca and Stress Response. Microb Ecol. 2013;66:806–812. doi: 10.1007/s00248-013-0269-1. [DOI] [PubMed] [Google Scholar]
- Saleh N, Kim H-J, Phenrat T, Matyjaszewski K, Tilton RD, Lowry GV. Ionic Strength and Composition Affect the Mobility of Surface-Modified Fe 0 Nanoparticles in Water-Saturated Sand Columns. Environ Sci Technol. 2008;42:3349–3355. doi: 10.1021/es071936b. [DOI] [PubMed] [Google Scholar]
- Saleh N, Sirk K, Liu Y, Phenrat T, Dufour B, Matyjaszewski K, Tilton RD, Lowry GV. Surface Modifications Enhance Nanoiron Transport and NAPL Targeting in Saturated Porous Media. Environ Eng Sci. 2007;24:45–57. doi: 10.1089/ees.2007.24.45. [DOI] [Google Scholar]
- Ševců A, El-Temsah S, Joner EJ, Černík M. Oxidative Stress Induced in Microorganisms by Zero-valent Iron Nanoparticles. Microbes Environ. 2011;26:271–281. doi: 10.1264/jsme2.ME11126. [DOI] [PubMed] [Google Scholar]
- Shah V, Dobiášová P, Baldrian P, Nerud F, Kumar A, Seal S. Influence of iron and copper nanoparticle powder on the production of lignocellulose degrading enzymes in the fungus Trametes versicolor. J Hazard Mater. 2010;178:1141–1145. doi: 10.1016/j.jhazmat.2010.01.141. [DOI] [PubMed] [Google Scholar]
- Shin K-H, Cha DK. Microbial reduction of nitrate in the presence of nanoscale zero-valent iron. Chemosphere. 2008;72:257–262. doi: 10.1016/j.chemosphere.2008.01.043. [DOI] [PubMed] [Google Scholar]
- Shi Z, Fan D, Johnson RL, Tratnyek PG, Nurmi JT, Wu Y, Williams KH. Methods for characterizing the fate and effects of nano zerovalent iron during groundwater remediation. J Contam Hydrol. 2015;181:17–35. doi: 10.1016/j.jconhyd.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Signorini L, Pasquini L, Savini L, Carboni R, Boscherini F, Bonetti E, Giglia A, Pedio M, Mahne N, Nannarone S. Size-dependent oxidation in iron/iron oxide core-shell nanoparticles. Phys Rev B. 2003;68 doi: 10.1103/PhysRevB.68.195423. [DOI] [Google Scholar]
- Sirk KM, Saleh NB, Phenrat T, Kim HJ, Dufour B, Ok J, Golas PL, Matyjaszewski K, Lowry GV, Tilton RD. Effect of Adsorbed Polyelectrolytes on Nanoscale Zero Valent Iron Particle Attachment to Soil Surface Models. Environ Sci Technol. 2009;43:3803–3808. doi: 10.1021/es803589t. [DOI] [PubMed] [Google Scholar]
- Som C, Berges M, Chaudhry Q, Dusinska M, Fernandes TF, Olsen SI, Nowack B. The importance of life cycle concepts for the development of safe nanoproducts. Toxicology, Potential Hazard of Nanoparticles: From Properties to Biological & Environmental Effects. 2010;269:160–169. doi: 10.1016/j.tox.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Song H, Carraway ER, Kim YH. Synthesis of nano-sized iron for reductive dechlorination. 2. Effects of Synthesis Conditions on Iron Reactivities. Environ Eng Res. 2005;10:174–180. doi: 10.4491/eer.2005.10.4.174. [DOI] [Google Scholar]
- Stumm W, Morgan J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters 1996 [Google Scholar]
- Su C, Puls RW, Krug TA, Watling MT, O’Hara SK, Quinn JW, Ruiz NE. Travel distance and transformation of injected emulsified zerovalent iron nanoparticles in the subsurface during two and half years. Water Res. 2013;47:4095–4106. doi: 10.1016/j.watres.2012.12.042. [DOI] [PubMed] [Google Scholar]
- Su L, Shi X, Guo G, Zhao A, Zhao Y. Stabilization of sewage sludge in the presence of nanoscale zero-valent iron (nZVI): abatement of odor and improvement of biogas production. J Mater Cycles Waste Manag. 2013;15:461–468. doi: 10.1007/s10163-013-0150-9. [DOI] [Google Scholar]
- Sun TY, Gottschalk F, Hungerbuhler K, Nowack B. Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ Pollut. 2014;185:69–76. doi: 10.1016/j.envpol.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Sun Y-P, Li X-Q, Zhang W-X, Wang HP. A method for the preparation of stable dispersion of zero-valent iron nanoparticles. Colloids Surf Physicochem Eng Asp. 2007;308:60–66. doi: 10.1016/j.colsurfa.2007.05.029. [DOI] [Google Scholar]
- Tilston EL, Collins CD, Mitchell GR, Princivalle J, Shaw LJ. Nanoscale zerovalent iron alters soil bacterial community structure and inhibits chloroaromatic biodegradation potential in Aroclor 1242-contaminated soil. Environ Pollut. 2013;173:38–46. doi: 10.1016/j.envpol.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Tosco T, Petrangeli Papini M, Cruz Viggi C, Sethi R. Nanoscale zerovalent iron particles for groundwater remediation: a review. J Clean Prod. 2014;77:10–21. doi: 10.1016/j.jclepro.2013.12.026. [DOI] [Google Scholar]
- Tratnyek PG, Johnson RL. Nanotechnologies for environmental cleanup. Nano Today. 2006;1:44–48. doi: 10.1016/S1748-0132(06)70048-2. [DOI] [Google Scholar]
- Tuomi P, Hains S, Takala J, Hyodynmaa M, Manni-Rantanen L. Use of nZVI for ground water remediation 2008 [Google Scholar]
- Wei-xian Z. Nanoscale Iron Particles for Environmental Remediation: An Overview. J Nanoparticle Res. 2003;5:323–332. doi: 10.1023/A:1025520116015. [DOI] [Google Scholar]
- Wingender J, Neu TR, Flemming H-C, editors. Microbial extracellular polymeric substances: characterization, structure and function. Springer; Berlin: 1999. [Google Scholar]
- Wu D, Shen Y, Ding A, Mahmood Q, Liu S, Tu Q. Effects of nanoscale zero-valent iron particles on biological nitrogen and phosphorus removal and microorganisms in activated sludge. J Hazard Mater. 2013;262:649–655. doi: 10.1016/j.jhazmat.2013.09.038. [DOI] [PubMed] [Google Scholar]
- Xiu Z, Gregory KB, Lowry GV, Alvarez PJJ. Effect of Bare and Coated Nanoscale Zerovalent Iron on tceA and vcrA Gene Expression in Dehalococcoides spp. Environ Sci Technol. 2010a;44:7647–7651. doi: 10.1021/es101786y. [DOI] [PubMed] [Google Scholar]
- Xiu Z, Jin Z, Li T, Mahendra S, Lowry GV, Alvarez PJJ. Effects of nano-scale zero-valent iron particles on a mixed culture dechlorinating trichloroethylene. Bioresour Technol. 2010b;101:1141–1146. doi: 10.1016/j.biortech.2009.09.057. [DOI] [PubMed] [Google Scholar]
- Yang Y, Guo J, Hu Z. Impact of nano zero valent iron (NZVI) on methanogenic activity and population dynamics in anaerobic digestion. Water Res. 2013;47:6790–6800. doi: 10.1016/j.watres.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Yin K, Lo IMC, Dong H, Rao P, Mak MSH. Lab-scale simulation of the fate and transport of nano zero-valent iron in subsurface environments: Aggregation, sedimentation, and contaminant desorption. J Hazard Mater. 2012;227(228):118–125. doi: 10.1016/j.jhazmat.2012.05.019. [DOI] [PubMed] [Google Scholar]
- Zhou L, Thanh TL, Gong J, Kim J-H, Kim E-J, Chang Y-S. Carboxymethyl cellulose coating decreases toxicity and oxidizing capacity of nanoscale zerovalent iron. Chemosphere. 2014;104:155–161. doi: 10.1016/j.chemosphere.2013.10.085. [DOI] [PubMed] [Google Scholar]


