Abstract
Increased fMRI activation in the hippocampus is recognized as a signature characteristic of the amnestic mild cognitive impairment (aMCI) stage of Alzheimer's disease (AD). Previous work has localized this increased activation to the dentate gyrus/CA3 subregion of the hippocampus and showed a correlation with memory impairments in those patients. Increased hippocampal activation has also been reported in carriers of the ApoE-4 allelic variation independently of mild cognitive impairment although these findings were not localized to a hippocampal subregion. To assess the ApoE-4 contribution to increased hippocampal fMRI activation, patients with aMCI genotyped for ApoE-4 status and healthy age-matched control participants completed a high-resolution fMRI scan while performing a memory task designed to tax hippocampal subregion specific functions. Consistent with previous reports, patients with aMCI showed increased hippocampal activation in the left dentate gyrus/CA3 region of the hippocampus as well as memory task errors attributable to this subregion. However, this increased fMRI activation in the hippocampus did not differ between ApoE-4 carriers and ApoE-4 non-carriers and the proportion of memory errors attributable to dentate gyrus/CA3 function did not differ between ApoE-4 carriers and ApoE-4 non-carriers. These results indicate that increased fMRI activation of the hippocampus observed in patients with aMCI is independent of ApoE-4 status and that ApoE-4 does not contribute to the dysfunctional hippocampal activation or the memory errors attributable to this subregion in these patients.
Highlights
-
•
Patients with aMCI show increased fMRI activation in DG/CA3 relative to controls.
-
•
Increased DG/CA3 activation is observed equally in ApoE-4 carriers and non-carriers.
-
•
Hippocampal dysfunction in aMCI is observed independent of ApoE-4 carrier status.
1. Introduction
Amnestic mild cognitive impairment (aMCI) is generally considered an intermediate stage between healthy aging and Alzheimer's disease dementia, defined by memory function that is worse than would be expected for a person's age (Petersen, 2004). Studies using functional magnetic resonance imaging (fMRI) have reported elevated hippocampal activation in the context of impaired memory function in patients with aMCI (Miller et al., 2008, Hämäläinen et al., 2007, Dickerson et al., 2004, Dickerson et al., 2005), which is now considered a characteristic feature of the aMCI phase of Alzheimer's disease (AD) (for a review see Ewers et al., 2011).
Recent high-resolution fMRI studies in patients with aMCI have localized increased hippocampal activation to the dentate gyrus/CA3 (DG/CA3) subregion of the hippocampus. These studies used a three-judgment memory task designed to tax pattern separation, a function ascribed to the granule cells of the dentate gyrus that reduces mnemonic interference by encoding distinctive representations for similar input patterns. In contrast, pattern completion refers to the recovery of a prior representation from partial or degraded input, thought to rely on the extensive recurrent collaterals of the CA3 neurons. Patients with aMCI showed an impairment of memory performance in the context of hippocampal hyperactivation that was consistent with reduced pattern separation and a shift to errors indicative of pattern completion (Yassa et al., 2010a, Bakker et al., 2012, Bakker et al., 2015). Intervention targeting hippocampal hyperactivity with low dose administration of the atypical antiepileptic levetiracetam normalized the fMRI activation and improved task performance in aMCI patients. Longitudinal follow up has shown that hyperactivation of the hippocampus at baseline predicts a trajectory of subsequent cognitive decline in aMCI and progression to dementia (Huijbers et al., 2015, Miller et al., 2008). Moreover, in the clinical phase of aMCI, excess hippocampal activation is significantly correlated with brain atrophy that affects areas vulnerable to neurodegeneration in AD (Putcha et al., 2011), suggesting that hyperactivity plays a role in driving pathophysiology in prodromal AD.
Elevated hippocampal activation observed on fMRI has also been reported in a number of other conditions that confer risk for AD including cognitively normal carriers of the ApoE-4 allelic variation (Bookheimer et al., 2000, Trivedi et al., 2008, Filippini et al., 2009, Dennis et al., 2010). ApoE-4 is the largest known genetic risk factor for sporadic AD (Corder et al., 1993) and increased hippocampal activation has been observed in ApoE-4 carriers decades before any cognitive symptoms would be expected to appear (Bookheimer et al., 2000, Burggren et al., 2002). As ApoE-4 occurs in approximately 15% of the normal population but is present in > 50% of AD patients (Frisoni et al., 1998, Ward et al., 2011), it is possible that the hippocampal hyperactivation observed in MCI is driven by the greater prevalence of ApoE-4 carriers positive status in this population.
In this study, we utilized high-resolution fMRI to directly address this question and assess whether increased hippocampal activation is observed both in aMCI ApoE-4 allele carriers and aMCI ApoE-4 non-carriers when compared to cognitively normal age-matched control participants. Patients with aMCI in this study showed impaired memory function in the context of elevated hippocampal activation characteristic of this condition. Both impaired performance on the three-judgment memory task and hippocampal hyperactivation, localized to the DG/CA3 region, were observed equally in ApoE-4 carriers and ApoE-4 non-carriers with aMCI. Thus elevated hippocampal activation is a clinical characteristic that is phenotypic of aMCI, independent of ApoE geneotype.
2. Methods
2.1. Participants and clinical characterization
Patients with aMCI and cognitively normal control participants were recruited from the Johns Hopkins Alzheimer's disease Research center as well as physician clinics and the community. All participants completed medical, psychiatric and neurological histories, and underwent neuropsychological evaluations including the Mini Mental Status Exam (Folstein et al., 1975), the Buschke Selective Reminding Test (Buschke and Fuld, 1974), the Verbal Paired Associates and the Logical Memory subtests of the Wechsler Memory Scale (Wechsler, 1987), and the Benton Visual Retention Test (Benton, 1974) to examine cognitive performance, as well as the Clinical Dementia Rating Scale (CDR; Morris, 1993) to assess overall functional capacity. Genetic testing was completed for all aMCI patients to assess their Apoliopoprotein E variation. Genetic testing was not completed for control subjects as the primary aim of the study was to assess the contribution of ApoE-4 within the condition of aMCI. All aMCI participants had a global Clinical Dementia Rating scale score of 0.5 with a sum of boxes score not exceeding 2.5 and met criteria for aMCI as proposed by Petersen (2004), which includes a subjective memory complaint corroborated by an informant, impaired memory function on neuropsychological testing (e.g. 1.5 standard deviations below the norm) and no decline in basic activities of daily living. All control participants had a global CDR score of 0. None of the aMCI or control participants met criteria for dementia. Participants were excluded from participation if they acknowledged either current or past major neurological or psychiatric disorders, head trauma with loss of consciousness, recent history of substance abuse or dependency or general contraindications to having an MRI scan (e.g. cardiac pacemaker, aneurysm coils or claustrophobia). The study protocol was approved by the Institutional Review Board of the Johns Hopkins Medical Institutions. All participants provided written informed consent and were paid for their participation in the study.
After excluding participants from analysis due to excessive motion or in-scanner task performance that was inadequate for analysis of the fMRI data, 21 aMCI ApoE-4 carriers and 21 ApoE-4 non-carriers were selected from the study sample such that the aMCI ApoE-4 carrier and aMCI ApoE-4 non-carrier groups, on average, were equivalent for age, education and distribution of sex, functional capacity as measured by the CDR sum of boxes score, as well as cognitive performance on the MMSE, Logical Memory delayed recall, Buschke Selective Reminding Test delayed recall, Verbal Paired Associated delayed recall and the Benton Visual Retention Test. In addition, 35 cognitively normal participants completed the study and were included in the analysis. Data from 32 aMCI patients (16 ApoE-4 carriers and 16 ApoE-4 non-carriers) and 20 control participants described here also contributed to results previously described in Bakker et al., 2012, Bakker et al., 2015.
2.2. fMRI task
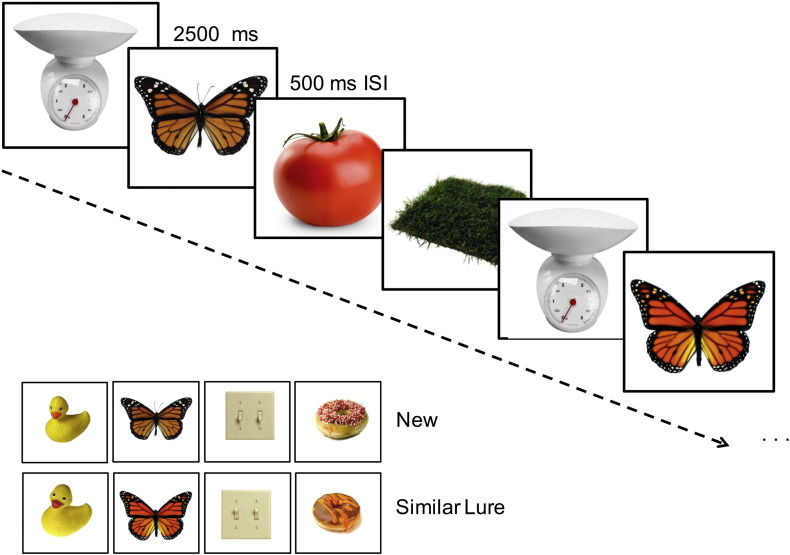
The fMRI task, previously described in detail (Kirwan and Stark, 2007, Bakker et al., 2008, Bakker et al., 2012, Bakker et al., 2015), is a memory task designed to assess pattern separation, a process thought to critically depend on the DG/CA3 subregion of the hippocampus (Marr, 1971). For this task, participants viewed 768 stimuli of common nameable objects. These stimuli included 384 single unrelated pictures of objects used as foils, 96 pairs in which an identical image was repeated, referred to as repeats, and 96 pairs of highly similar but not identical stimuli referred to as lures. Participants completed the task over eight runs with 96 stimuli per run. Stimuli were presented for 2500 ms with a 500 ms inter-stimulus-interval consisting of a blank screen. Trials were presented in a pseudo-random order, with lures or repeated stimuli presented within 30 trials of its pair. The task was presented as a 3-alternative forced choice task, in which participants were asked to judge each stimuli as ‘new’, ‘old’ or ‘similar’ but not identical to a previously seen stimulus (Fig. 1). Data was collected using an Apple Macintosh laptop computer running MATLAB software (The Mathworks, Natick, MA) and a Cedrus RB-610 response box. Participants were given a five-minute practice task prior to the MRI scan to familiarize themselves with the stimuli and the procedures.
Fig. 1.
Stimuli and task design. Participants were shown a series of everyday objects and asked to judge if the item was new (seen for the first time), old (a repeated item) or similar (resembled a previously shown item). The lure items served as the critical trials for assessing performance dependent on the dentate gyrus/CA3.
2.3. fMRI acquisition
Data was acquired on a 3 Tesla Philips scanner equipped with a SENSE parallel imaging head coil (MRI Devices, Inc., Waukesha) and higher order shims to compensate for local field distortions at the F.M. Kirby Research Center for Functional Brain Imaging at the Kennedy Krieger Institute on the Johns Hopkins Medical campus. Functional images were collected using a T2*-weighted echo planar single shot pulse, an echo time of 30 ms and a flip angle of 70, an in-plane resolution of 1.5 × 1.5 mm, a TR of 1.5 s, and an acquisition matrix of 64 × 64. Functional volumes consisted of 19 slices oriented along the principal axis of the hippocampus covering the medial temporal lobe bilaterally. Structural images were acquired using a magnetization prepared rapid gradient echo (MPRAGE) T1-weighted sequence with 231 oblique slices, a 0.65 mm isotropic resolution and a field of view of 240 mm.
2.4. fMRI analysis
Image analysis was performed using the Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996). Functional images were co-registered to correct for slice time acquisition differences and head motion using a three-dimensional registration algorithm creating motion vectors used to remove volumes in which a significant head motion occurred as well as the previous and subsequent TR from further analysis. To avoid confusion, throughout the paper we will refer to items subsequently tested with repetitions as ‘subsequent targets’ and novel items subsequently tested with a similar lure as ‘subsequent lures’. The subsequent items all refer to first presentation trials. During the second presentation, trials will be referred to as targets and lures respectively. Functional runs were concatenated and six vectors were defined to model the different trial types: (1) repeats subsequently called “old”, (2) lures subsequently called “similar”, (3) lures subsequently called “old”, (4) repeats called “old”, (5) lures called “similar” and (6) lures called “old”. Remaining response types (misses, false alarms) were modeled but not included in further analyses. The full set of vectors was used to model each participant's data using a deconvolution approach that was based on a general linear regression treating the single foil presentations that were correctly rejected as a non-zero baseline against all other conditions. The resulting statistical fit coefficient maps represent the difference in activity between each of the trial types and the baseline for a given time point for a given voxel. The sum of fit coefficients over the length of the hemodynamic response (~ 3–12 s after the onset of the trial) was taken as the model's estimate of the response to each trial type. The statistical maps were then smoothed using a Gaussian kernel of 3 mm to account for variations in individual functional anatomy.
2.5. Cross-participant alignment
Alignment procedures used in this study, previously described in detail (Bakker et al., 2012, Bakker et al., 2015), were designed to focus the alignment power to the regions of interest using the segmentation of participant's anatomical image and a custom group template model created from the structural scans collected in this study. Initial affine registration was used to transfer the subject's anatomical and functional images to the Talairach coordinate system (Talairach and Tournoux, 1988). Medial temporal lobe structures and hippocampal subregions were manually segmented using methods previously described in detail (Kirwan and Stark, 2007, Bakker et al., 2008, Bakker et al., 2012, Bakker et al., 2015). Segmented regions include the bilateral entorhinal cortex, perirhinal cortex, parahippocampal cortex and temporopolar cortices as described by Insausti (Insausti et al., 1998). Segmentations of the CA1, DG/CA3, and subiculum regions of the hippocampus followed coronal landmarks described by Duvernoy (2005). Using both the segmentation label-based information for the point-set expectation (PSE) error metric and the grayscale structural image for the pure cross-correlation (PR) error metric, Advanced Normalization Tools (ANTS) (Avants et al., 2008) was used to calculate the 3D vector yield transformation for each subject to the custom group template model by equally weighing the segmentation and T1 grayscale. The 3D vector fields for each individual was then applied to the concatenated fit coefficient maps resulting from the functional analysis.
2.6. Structural MRI analysis
The manual segmentations of the CA1, DG/CA3 and subiculum completed for each individual subject were used as a quantification of the volume of each of the hippocampal subregions.
2.7. Statistical analysis
The primary objective of the study was to examine the effect of the ApoE-4 allelic variation on increased hippocampal activation observed in patients with aMCI. Age, education, neuropsychological and functional assessment scores, and hippocampal subregion volumes between ApoE-4 carrier and non-carrier groups and between aMCI patients and age-matched controls were compared using independent sample t-tests. The distribution of sex between groups was compared using a chi-square test. To facilitate a comparison of the fMRI data between the two ApoE-4 subgroups, an analysis was conducted using a one-way analysis of variance (ANOVA) of trial type including data from only the aMCI patients. To confirm the findings from the initial analysis, an independent confirmatory analysis was conducted based on a one-way ANOVA of trial type in only the control subjects. For both analyses, a voxel threshold of p = 0.05 was used on the overall F-statistic in combination with a spatial extent threshold of 40 voxels to select areas of task-related activation. The resulting areas of activation were then combined with the anatomical segmentations only, in order to include voxels within our areas of interest. This hybrid functional/anatomical analysis resulted in clusters of voxels in each aMCI cohort where activity varied systematically across trial types within each of the anatomical regions of interest. Voxels within each functional/anatomical region of interest were then collapsed for further analysis. Planned post-hoc comparisons using t-tests were used for comparisons between the control and the aMCI groups and for the comparisons between the aMCI ApoE-4 carriers and non-carriers.
3. Results
3.1. Participants
Patients with aMCI did not differ in age, education or proportion of males and females from the participants in the control group but consistent with the diagnosis of aMCI showed significantly impaired performance on the Mini-Mental State Examination (MMSE) (t(75) = 5.57, p < 0.001), Logical Memory delayed recall (t(75) = 8.74, p < 0.001), Buschke Selective Reminding Test delayed recall (t(75) = 11.35, p < 0.001), Verbal Paired Associates delayed recall (t(75) = 7.07, p < 0.001) and the Benton Visual Retention Test (t(75) = 4.91, p < 0.001) when compared to the control participants (Table 1). As a result of matching the ApoE-4 carriers and non-carriers to be equivalent for age, education and distribution of sex, functional capacity as well as cognitive performance ApoE-4 carriers and non-carriers did not differ in performance on the MMSE (t(40) = 1.02, p = 0.31), Logical Memory delayed recall (t(40) = 1.35, p = 0.18), Buschke Selective Reminding Test delayed recall (t(40) = 1.04, p = 0.31), Verbal Paired Associates delayed recall (t(40) = 0.00, p = 1.00, or the Benton Visual Retention Test (t(40) = 0.13, p = 0.90). Results from the complete neuropsychological assessment including non-memory assessments are included in Table 1.
Table 1.
Demographics and clinical characterization of healthy controls and ApoE-4 groups of aMCI participants.
| Controls |
aMCI ε4 − |
aMCI ε4 + |
ε4 p-value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Demographics | |||||||
| Subjects | 35 | 21 | 21 | ||||
| Sex (M/F) | 18/17 | 12/9 | 7/14 | ||||
| Age (years) | 69.03 | 7.73 | 73.14 | 8.39 | 71.71 | 6.18 | 0.53 |
| Education (years) | 16.14 | 2.63 | 16.21 | 2.50 | 14.86 | 2.92 | 0.11 |
| Clinical Dementia Rating | 0.0⁎ | 0.5 | 0.5 | ||||
| Clinical Dementia — sum of boxes | 0.01⁎ | 0.08 | 0.90 | 0.52 | 1.17 | 0.53 | 0.11 |
| General cognition | |||||||
| Clock drawing | 23.60⁎ | 1.14 | 22.24 | 2.96 | 22.24 | 2.70 | 1.00 |
| MMSE | 28.54⁎ | 1.44 | 26.43 | 2.69 | 25.71 | 1.74 | 0.31 |
| Memory | |||||||
| Benton Visual Retention | 6.03⁎ | 1.62 | 4.48 | 1.29 | 4.43 | 1.12 | 0.90 |
| BSRT immediate recall | 49.37⁎ | 7.66 | 34.52 | 9.62 | 31.52 | 5.46 | 0.22 |
| BSRT delayed recall | 8.60⁎ | 2.19 | 3.57 | 2.06 | 2.95 | 1.80 | 0.31 |
| LM immediate recall | 48.06⁎ | 8.83 | 35.19 | 8.99 | 31.86 | 9.04 | 0.24 |
| LM delayed recall | 31.37⁎ | 6.69 | 18.52 | 7.41 | 15.38 | 7.65 | 0.18 |
| R-O CFT immediate copy | 33.37⁎ | 3.09 | 31.07 | 4.79 | 30.21 | 4.54 | 0.55 |
| R-O CFT delayed copy | 15.86⁎ | 7.00 | 8.10 | 5.30 | 7.43 | 5.45 | 0.69 |
| VPA immediate recall | 22.43⁎ | 6.49 | 11.95 | 7.65 | 12.33 | 7.59 | 0.87 |
| VPA delayed recall | 7.26⁎ | 1.17 | 3.90 | 2.45 | 3.90 | 2.79 | 1.00 |
| Working memory | |||||||
| Letter number sequencing | 10.91⁎ | 2.13 | 9.57 | 2.80 | 8.48 | 2.16 | 0.16 |
| Executive functioning | |||||||
| SCWT — word | 94.77⁎ | 13.03 | 90.57 | 15.68 | 86.38 | 13.97 | 0.37 |
| SCWT — color | 61.54⁎ | 11.92 | 55.00 | 12.85 | 56.19 | 11.01 | 0.76 |
| SCWT — color/word | 34.00⁎ | 8.77 | 28.44 | 10.15 | 25.67 | 8.69 | 0.37 |
| Speed of processing | |||||||
| Symbol-digit modalities test | 46.06⁎ | 8.45 | 37.86 | 9.28 | 38.95 | 10.71 | 0.73 |
| Verbal fluency | |||||||
| Verbal fluency (FAS) | 44.29 | 9.49 | 43.86 | 13.21 | 38.14 | 14.39 | 0.19 |
MMSE: Mini Mental Status Exam; BSRT: Buschke Selective Reminding Test; LM: Logical Memory subtest of the Wechsler Memory Scale; R-O CFT: Rey-Osterrieth Complex Figure Test; VPA: Verbal Paired Associates subtest of the Wechsler Memory Scale; SCWT: Stroop Color and Word Test. Listed p-values are based on independent samples t-tests comparing aMCI ApoE-4 carriers and aMCI ApoE-4 non-carriers.
Significant difference between control participants and patients with aMCI collapsed across ApoE-4 groups, p < 0.05.
3.2. Behavioral results
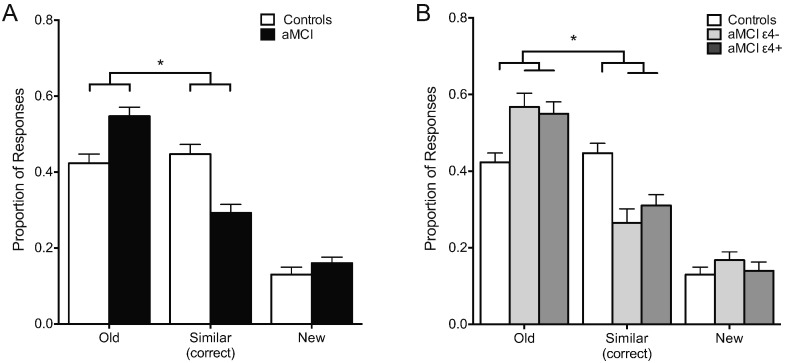
Patients with aMCI showed a significant impairment on the three-judgment memory task relative to age-matched controls assessed by the rates of each response option (old, similar or new) on the critical lure trials. For the lure trials, a between-groups ANOVA showed a significant effect of response type and importantly a significant group by response interaction. A post-hoc analysis of that interaction by a planned contrast showed that aMCI patients incorrectly identified lure items as ‘old’ more often and gave relatively fewer correct responses of ‘similar’ compared to age-matched control participants (Controls vs. aMCI by Old vs. Similar (F(1, 75) = 19.61, p ≤ 0.001) (Fig. 2A). This pattern of results is consistent with reduced pattern separation and a shift towards pattern completion reported in patients with aMCI.
Fig. 2.
Patients with aMCI show impaired task performance independent of ApoE-4 status. A. Patients with aMCI show impaired memory performance by more often incorrectly judging the lure items as “old” instead of “similar” when compared to healthy control subjects. B. aMCI ApoE-4 carriers and aMCI ApoE-4 non-carriers showed equally impaired memory performance with both groups more often incorrectly judging lure items as “old” instead of “similar” when compared to healthy control subjects. Statistics show p-values resulting from a planned post-hoc contrast for the interaction of group as a function of response type (old versus similar). Values are means ± SEM. *p < 0.05.
To assess whether the ApoE-4 allelic variation contributes to impairment on the three-judgment memory task in aMCI, the rates of each response option for the critical lure trials was compared between ApoE4 carrier and non-carrier groups. For the lure trials a between groups ANOVA showed a significant overall effect of response type between ApoE-4 carriers and non-carriers (F(1, 38) = 38.20, p < 0.001). A planned post-hoc comparison show that relative to the aMCI ApoE-4 non-carriers, the ApoE-4 carriers did not differ in the proportion of old and similar responses to lures (ApoE-4 carriers vs. ApoE-4 non-carriers by Old vs. Similar: F(1, 38) = 0.52, p = 0.48) (Fig. 2B). Both ApoE-4 carriers and non-carriers significantly more often incorrectly identified critical lures items as ‘old’ and less often correctly identified those trials as ‘similar’ compared to control subjects.
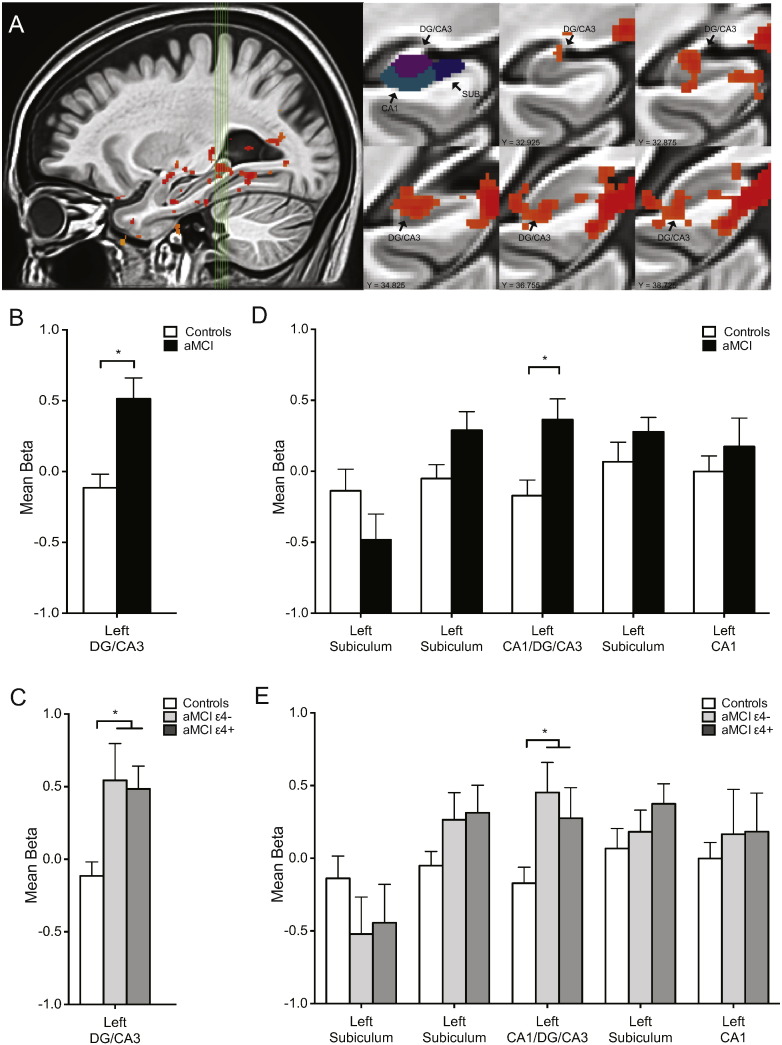
3.3. fMRI results
Functional neuroimaging data was first subjected to a one-way ANOVA of trial type using data from only the aMCI patients to select voxels that showed task related activity. This analysis resulted in a cluster of blood oxygenation level dependent (BOLD) activation localized to the left DG/CA3 subregion of the hippocampus (Fig. 3A). To assess whether increased hippocampal activation was observed in the current study of patients with aMCI, we first compared functional activation during the fMRI memory task performance between the aMCI patients and the age-matched control participants within the cluster of task related activation observed in the left DG/CA3 region. The aMCI patients showed significantly increased BOLD activation during lure trials correctly called similar compared to aged-matched controls (t(75) = 3.42, p < 0.001) (Fig. 3B). Activity in this cluster of increased activation in the left DG/CA3 region showed a positive correlation with the proportion of lures correctly called ‘similar’ (r = − 0.42, p < 0.01) in patients with aMCI. These findings replicate earlier reported findings and supports hippocampal hyperactivation in the context of memory impairment as characteristic of aMCI.
Fig. 3.
Increased hippocampal DG/CA3 activation in the context of impairment memory performance is observed equally in aMCI ApoE-4 carriers and aMCI ApoE-4 non-carriers. A. Sagittal view of the left medial temporal lobe. Green vertical lines identify slices through the hippocampus shown on the right. First coronal slice shows the segmentation of different hippocampal regions of interest including dentate gyrus/CA3 (DG/CA3), CA1 and subiculum (SUB). Coronal slices show statistical maps of the extent of task related activity in the left DG/CA3 from anterior to posterior. B. Patients with aMCI show increased activity in the left DG/CA3 compared to healthy control subjects during critical lure trials. C. Increased activation is observed equally in aMCI ApoE-4 carriers and aMCI non-carriers in the left DG/CA3. D. Patients with aMCI showed increased activation in the left CA1/DG/CA3. No other differences were observed between aMCI patients and healthy control subjects in other clusters of task related activation in the hippocampus. E. Increased activation is observed equally in aMCI ApoE-4 carriers and aMCI non-carriers in the left CA1/DG/CA3. No other differences were observed between aMCI ApoE-4 carriers and aMCI ApoE-4 non-carriers in other clusters of task related activation in the hippocampus. Comparisons are based on independent sample t-tests. Values are means ± SEM. *p < 0.05.
In addition to a cluster of significant BOLD activation in the left DG/CA3 subregion, this analysis also resulted in several other clusters of task related activation in other areas of the hippocampus. Activation during lure trials correctly called similar, did not differ between aMCI patients and age-matched control subjects in these additional areas of activation, which included three separate clusters of activation in the left subiculum separated along the anterior-posterior aspect of the hippocampus (left subiculum 1: t(75) = 1.42, p = 0.16; left subiculum 2: t(75) = 1.06, p = 0.29 and left subiculum 3: t(75) = 1.26, p = 0.21) respectively and a cluster of activation in the left CA1 (t(75) = 0.73, p = 0.47). Finally, a single cluster of activation was observed spanning the CA1/DG/CA3 subregions of the hippocampus showing significantly increased activation in the aMCI patients compared to age-matched controls (t(75) = 2.84, p < 0.01) (Fig. 3D).
To assess the contribution of the ApoE-4 allelic variation to increased hippocampal activation observed in patients with aMCI, we compared functional activation in the aMCI ApoE-4 carriers and aMCI ApoE-4 non-carriers in all observed clusters of activation. When comparing activation between aMCI ApoE-4 carriers and non-carriers during the critical lure trials, no significant differences in activation were observed in the left DG/CA3 (t(40) = 0.20, p = 0.84) and both aMCI ApoE-4 carriers and non-carriers showed significantly increased activation in this subregion of the hippocampus compared to controls (aMCI ApoE-4 carriers vs. Control: t(54) = 3.45, p < 0.01; aMCI ApoE-4 non-carriers vs Control: t(54) = 2.84, p < 0.01) (Fig. 3C). Levels of activation also did not differ between aMCI ApoE-4 carriers and aMCI non-carriers in clusters of activation observed elsewhere in the hippocampus (left subiculum 1: t(40) = 0.21, p = 0.84; left subiculum 2: t(40) = 0.32, p = 0.75; left subiculum 3: t(40) = 0.95, p = 0.35; left CA1: t(40) = 0.04, p = 0.97; left CA1/DG/CA3: t(40) = 0.60, p = 0.55). The increased activation observed in aMCI patients in the left DG/CA3/CA1 was observed equally in aMCI ApoE-4 carriers (aMCI ApoE-4 carriers vs. Control: t(54) = 2.08, p < 0.05) and aMCI ApoE-4 non-carriers (aMCI ApoE-4 non-carriers vs. Control: t(54) = 2.92, p < 0.01) (Fig. 3E).
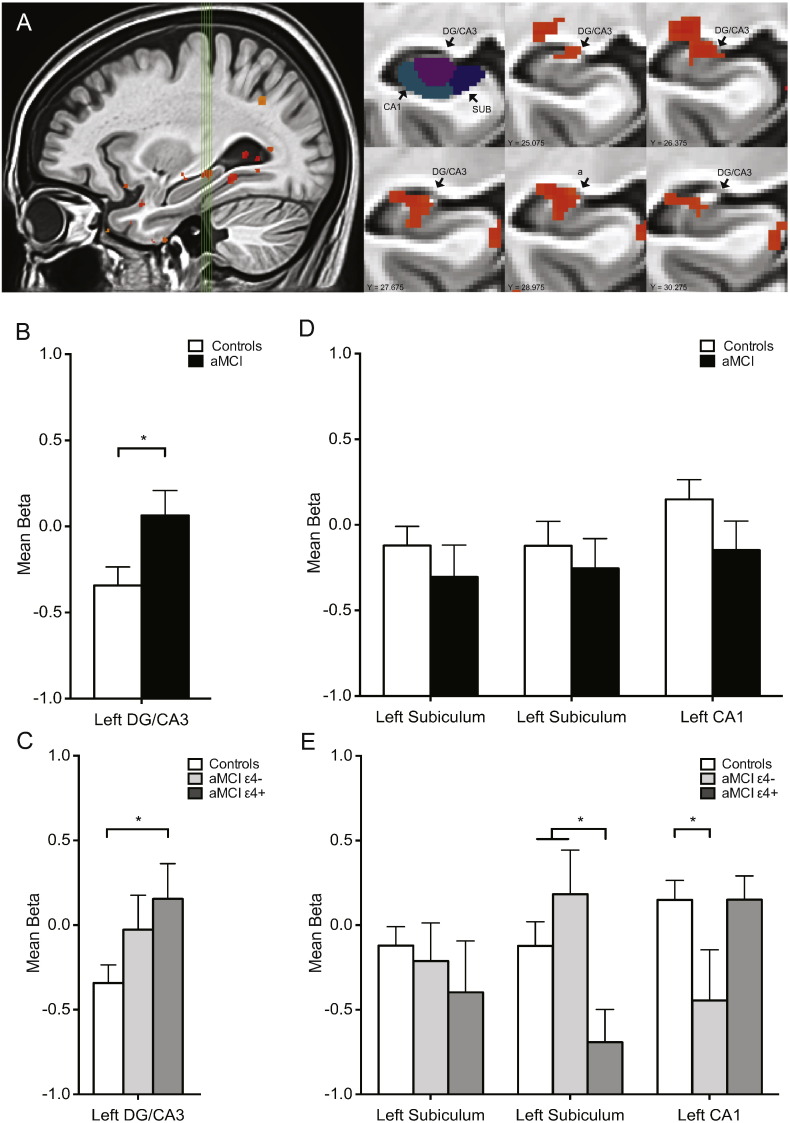
To confirm this finding, a separate analysis was conducted in which voxel selection was based on a one-way ANOVA of trial type only in control subjects. This analysis resulted in a similar cluster of activation in the left DG/CA3 (Fig. 4A). Patients with aMCI again showed increased activation compared to aged-matched controls in the left DG/CA3 (t(75) = 2.12, p < 0.05) (Fig. 4B). Additional clusters of activation in hippocampal subregions from this analysis also did not reveal any differences in activation between aMCI patients and controls in the left anterior subiculum (t(75) = 0.80, p = 0.43), left posterior subiculum t(75) = 0.57, p = 0.57) or left CA1 (t(75) = 1.39; p = 0.17) (Fig. 4D). The effect of ApoE-4 status was confirmed by comparing fMRI activation between the aMCI ApoE-4 carriers and aMCI ApoE-4 non-carriers in these clusters of activation. Activation during the critical lure trials did not differ between ApoE-4 carriers and non-carriers in the left DG/CA3 (t(40) = 0.63, p = 0.54). ApoE-4 carriers showed significantly increased activation in the DG/CA3 subregion of the hippocampus compared to controls (t(54) = 2.35, p = 0.02) but fMRI activation, although increased in aMCI ApoE-4 non-carriers, did not differ from controls in this analysis (t(54) = 1.51, p = 0.14) (Fig. 4C). No differences were observed between aMCI ApoE-4 carriers and non-carriers in the left anterior subiculum (t(40) = 0.49, p = 0.63). In the left posterior subiculum, ApoE-4 carriers showed significantly reduced activation compared to ApoE-4 non-carriers (t(40) = 2.70, p = 0.01) and to age-matched controls (t(54) = 2.40, p = 0.02) but ApoE-4 non-carriers did not differ in activation from age-matched controls (t(54) = 1.12, p = 0.27). In the left CA1, ApoE-4 non-carriers showed significantly reduced activation compared to age-matched controls (t(54) = 2.16, p = 0.04) but their activation did not differ from ApoE-4 carriers (t(54) = 1.80, p = 0.08) and ApoE-4 carriers did not differ in activation from age-matched controls (t(54) = 0.00, p = 0.99) in this subregion of the hippocampus (Fig. 4E).
Fig. 4.
Independent statistical analysis confirms increased hippocampal DG/CA3 activation in the context of impairment memory performance is observed equally in aMCI ApoE-4 carriers and aMCI ApoE-4 non-carriers. A. Sagittal view of the left medial temporal lobe. Green vertical lines identify slices through the hippocampus shown on the right. First coronal slice shows the segmentation of different hippocampal regions of interest including dentate gyrus/CA3 (DG/CA3), CA1 and subiculum (SUB). Coronal slices show statistical maps of the extent of task related activity in the left DG/CA3 from anterior to posterior. B. Patients with aMCI show increased activity in the left DG/CA3 compared to healthy control subjects during critical lure trials. C. Increased activation is observed in aMCI ApoE-4 carriers compared to controls in the left DG/CA3. D. No differences were observed between aMCI patients and healthy control subjects in other clusters of task related activation. E. ApoeE-4 carriers showed increased activation in the left CA1 compared to ApoE-4 non-carriers and reduced activation compared to controls and ApoE-4 non-carriers in the anterior left subiculum. Task related activation in the left DG/CA3/CA1 and posterior left subiculum did not differ between aMCI ApoE-4 carriers and aMCI non-carriers. Comparisons are based on independent sample t-tests. Values are means ± SEM. *p < 0.05.
3.4. Structural MRI results
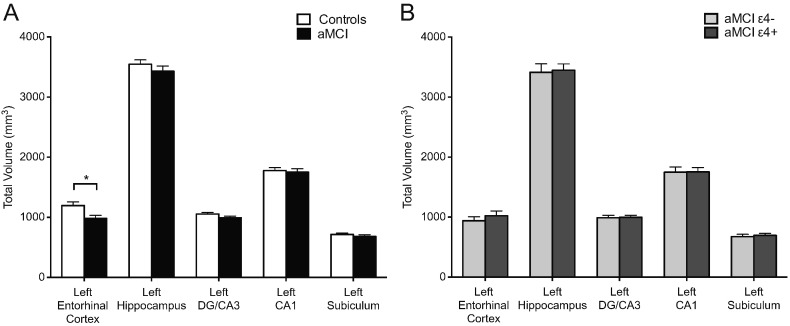
Analysis of the volumetric data comparing aMCI patients and control participants showed significantly reduced volume of the left entorhinal cortex (t(75) = 2.65, p < 0.01) but no differences in overall volume of the left hippocampus or volume of the subregions of the left hippocampus (Fig. 5A). Comparing aMCI ApoE-4 carriers and ApoE-4 non-carriers showed no differences in the volume of the left entorhinal cortex and also showed no differences in volume of the overall hippocampus or hippocampal subregions. ApoE-4 carrier and ApoE-4 non-carrier groups separately did not differ from control participants in the volume of any of the hippocampal subregions (Fig. 5B). The right entorhinal cortex showed a trend towards significantly reduced volume in aMCI patients compared with control participants (control: mean = 1177.82, sd = 298.32; aMCI: mean = 1048.93, sd = 336.84; t(75) = 1.76, p = 0.08) but the right hippocampus (control: mean = 3463.67, sd = 508.20; aMCI: mean = 3433.16, sd = 568.60) or hippocampal subregions did not significantly differ in volume between aMCI patients and controls. No differences were observed between ApoE-4 carriers and ApoE-4 non-carriers in the right entorhinal cortex (ApoE-4 carrier: mean = 1111.26, sd = 413.54; ApoE-4 non-carrier: mean = 986.70, sd = 231.13), right hippocampus (ApoE-4 carrier: mean = 3434.33, sd = 502.92; ApoE-4 non-carrier: mean = 3431.99, sd = 640.19) or any of the hippocampal subregions.
Fig. 5.
Hippocampal subregion volume in patients with aMCI is independent of ApoE-4 status. A. Patients with aMCI showed significantly reduced volume of the left entorhinal cortex but did not show differences in volume of the left hippocampus or in any of the hippocampal subregions compared to control patients. B. Volume of the left entorhinal cortex, left hippocampus or any of the hippocampal subregions did not differ between between ApoE-4 carriers and ApoE-4 non-carriers. Comparisons are based on independent sample t-tests. Values are means ± SEM.
4. Discussion
The current study aimed to assess the role of the ApoE-4 allelic variation in increased hippocampal activation observed in patients with aMCI. Hippocampal hyperactivation observed with fMRI is reliably observed in patients with aMCI relative to age-matched controls and consistently localized to the DG/CA3 subregion of the hippocampus. However, increased hippocampal activation is also observed in ApoE-4 carriers who are cognitively normal and given that over 50% of AD patients are ApoE-4 carriers, it is possible that increased activation in patients with aMCI is driven by the greater prevalence of ApoE-4 in this population.
In the current study, patients with aMCI again showed significantly increased activation of the hippocampus, localized to the DG/CA3 subregion of the hippocampus, which was correlated with impaired memory performance on the scanning task consistent with previous reports (Yassa et al., 2010a, Bakker et al., 2012, Bakker et al., 2015). In two statistically independent analyses, ApoE-4 carriers and ApoE-4 non-carriers showed increased hippocampal activation localized to the DG/CA3 that did not differ between ApoE-4 carriers and ApoE-4 non-carriers. The proportion of memory errors attributable to DG/CA3 function also did not differ between ApoE-4 carrier and ApoE-4 non-carriers with both ApoE-4 groups categorizing critical lure items more frequently as ‘old’ instead of ‘similar’. Volume measures of the hippocampal subregions did not differ between patients with aMCI and age-matched control participants or between ApoE-4 carrier and non-carrier groups confirming that the absence of differences in activation were not due to a difference in hippocampal DG/CA3 volume. These results show that increased fMRI activation of the hippocampus observed in patients with aMCI is independent of ApoE-4 status and that ApoE-4 does not contribute to the dysfunctional hippocampal activation or the memory errors attributable to this subregion in these patients. This finding supports the hypothesis that hippocampal hyperactivity is a clinical feature of aMCI, not driven or enhanced by ApoE-4 status.
In aMCI, increased hippocampal activation may primarily reflect elevation of activity from overactive CA3 neurons and their massive recurrent collaterals. This excessive activation is associated with a computational shift away from pattern separation which facilitates the rapid encoding of novel information and instead retrieves previously encoded representations. This computational shift is reflected in the memory performance of elderly participants who show increased hippocampal activation compared to young adults, making fewer correct responses of ‘similar’ to critical lure items, reflective of pattern separation, and instead calling these items ‘old’, reflecting an overreliance on pattern completion (Yassa et al., 2010a). This condition is further exacerbated in aMCI with additionally increased hippocampal activation relative to age-matched control subjects and a clinically significant shift in the balance between pattern separation and pattern completion (Yassa et al., 2010b, Bakker et al., 2012, Bakker et al., 2015).
Elevated CA3 neural activity has been directly observed in memory-impaired aged rats (Wilson et al., 2005). In this animal model of age-related memory impairment, elevated firing rates of the CA3 neurons specifically are thought to be the result of an age-related loss of inhibitory control and synaptic efficiency of the perforant path projections, giving rise to the memory impairment observed in these animals (Dieguez and Barea-Rodriguez, 2004, Barnes, 1979, Gallagher and Koh, 2011, Wilson et al., 2006). In studies of ApoE-4 knock-in mice and ApoE-4 fragment transgenic mice, a localized reduction of markers for hippocampal inhibitory interneurons and increased excitability of the neurons they innervate was observed, which correlated with the extent of learning and memory deficits in these animals. In this model, toxicity is mediated through a tau-dependent pathway providing a mechanism for the origin of elevated activation in the hippocampus of ApoE-4 carriers, independent of, or in addition to, the mechanisms for age-related impairment described above (Andrews-Zwilling et al., 2010, Palop and Mucke, 2009). The ApoE-4 animal models, in which the hilus and not CA1 was affected, predict that localization particularly to the DG/CA3 region would be observed in ApoE-4 carriers. Increased hippocampal activation in cognitively normal carriers of the ApoE-4 allelic variation has been reported in a number of studies, but the imaging methods used in those studies had insufficient resolution to localize that activation to a specific subregion of the hippocampus (Bookheimer et al., 2000, Burggren et al., 2002). Results from this study robustly localize the observed hippocampal hyperactivity to the DG/CA3 subregion of the hippocampus, consistent with previous observations in aMCI patients and the predictions from animal models of ApoE-4. Furthermore, the analyses in this study resulted in several clusters of activation spanning different subregion locations across the hippocampus. Increased hippocampal activation was only observed in the DG/CA3 subregion. No significant differences in activation between aMCI patients and age-matched controls were observed in any of the other subregions of the hippocampus in either the primary or confirmatory analysis.
Although elevated hippocampal activation was initially thought to provide a compensatory mechanism in patients with aMCI (Dickerson et al., 2005), recent studies have provided evidence of the deleterious effect of hippocampal hyperactivity detected by fMRI. Our previous studies showed that treatment with a low dose of the antiepileptic levetiracetam normalized hippocampal activation and improved memory performance in patients with aMCI, confirming that increased hippocampal activation is not a compensatory mechanism but instead contributes to symptomatic impairment (Bakker et al., 2012, Bakker et al., 2015). Furthermore, increased hippocampal hyperactivity is predictive of future cognitive decline (Miller et al., 2008) and is associated with greater cortical thinning in both MCI patients as well as cognitively normal controls (Putcha et al., 2011). These results suggest that increased hippocampal activation, observed with fMRI, may be an early marker for AD related neuronal dysfunction that contributes to neuronal damage and disease progression, independently of ApoE status if not controlled.
It is important to acknowledge that this study examined only task-activated hippocampal activity. Functional differences in activation in ApoE-4 carriers and non-carriers have been reported in whole brain task-activation (Bondi et al., 2005, Celone et al., 2006, Miller et al., 2008) as well as in resting state fMRI data (Filippini et al., 2009). Increased activation reported outside of the hippocampus could potentially reflect compensatory measures for ApoE-4 carriers compared to non-carriers or purely differences in ApoE-4 driven functional brain organization. A study of cognitively normal ApoE-4 carriers has also observed reduced cortical thickness and longitudinal changes in medial temporal lobe cortical thickness further suggesting that ApoE-4 may contribute to differences in brain organization and age-related brain changes (Putcha et al., 2011). Dissociable activation between ApoE-4 carriers, non-carriers and controls observed here in the left CA1 and subiculum could reflect these differences in brain organization and function.
Together these results show that ApoE-4 does not contribute to increased hippocampal activation in subjects who have progressed to a diagnosis of aMCI and this study provides further evidence for the role of hippocampal hyperactivity as a clinical feature of the aMCI phase of Alzheimer's disease. However, aMCI remains a heterogeneous patient population and biomarker data to support a diagnosis of aMCI due to AD was not available in this study. Further studies examining the role of ApoE-4 and increased hippocampal activation are needed in aMCI patients with biomarker characterization and should also consider both whole brain networks and network changes within the medial temporal lobe as well as further assess the contribution of ApoE-4 to hippocampal hyperactivity and neuronal dysfunction in cognitively normal carriers prior to a diagnosis of aMCI.
Acknowledgements
We would like to thank Dr. Marilyn Albert and Dr. George Rebok for helpful feedback on earlier versions of this manuscript. We would also like to thank the staff of the F.M. Kirby Center for Functional Brain Imaging and Alaina Gold for their assistance with data collection. This work was supported by NIH grants RC2AG036419 and P50AG05146. T.T. is supported by a NIA T32 training grant and a National Defense Science and Engineering Graduate Fellowship (NDSEG) grant awarded by the DOD, Air Force Office of Scientific Research, National Defense Science and Engineering Graduate (NDSEG) Fellowship, 32 CFR 168a. M.G. is the founder of AgeneBio. M.G. and A.B. are inventors on Johns Hopkins University intellectual property with patents pending and licensed to AgeneBio. M.G. consults for the company and owns company stock, which is subject to certain restrictions under University policy. M.G. and A.B.'s role in the current study was in compliance with the conflict of interest policies of the Johns Hopkins School of Medicine.
References
- Andrews-Zwilling Y., Bien-Ly N., Xu Q., Li G., Bernardo A., Yoon S.Y.…Huang Y. Apolipoprotein E4 causes age-and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J. Neurosci. 2010;30(41):13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B.B., Epstein C.L., Grossman M., Gee J.C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A., Albert M.S., Krauss G., Speck C.L., Gallagher M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. NeuroImage. 2015;7:688–698. doi: 10.1016/j.nicl.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A., Kirwan C.B., Miller M., Stark C.E. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319(5870):1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A., Krauss G.L., Albert M.S., Speck C.L., Jones L.R., Stark C.E.…Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74(3):467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C.A. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979;93(1):74. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Benton A.L. Psychological Corporation; 1974. Visual Retention Test. [Google Scholar]
- Bondi M.W., Houston W.S., Eyler L.T., Brown G.G. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64(3):501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S.Y., Strojwas M.H., Cohen M.S., Saunders A.M., Pericak-Vance M.A., Mazziotta J.C., Small G.W. Patterns of brain activation in people at risk for Alzheimer's disease. N. Engl. J. Med. 2000;343(7):450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggren A.C., Small G.W., Sabb F.W., Bookheimer S.Y. Specificity of brain activation patterns in people at genetic risk for Alzheimer disease. Am. J. Geriatr. Psychiatry. 2002;10(1):44–51. [PubMed] [Google Scholar]
- Buschke H., Fuld P.A. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24(11):1019. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- Celone K.A., Calhoun V.D., Dickerson B.C., Atri A., Chua E.F., Miller S.L.…Albert M.S. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J. Neurosci. 2006;26(40):10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder E.H., Saunders A.M., Strittmatter W.J., Schmechel D.E., Gaskell P.C., Small G.…Pericak-Vance M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dennis N.A., Browndyke J.N., Stokes J., Need A., Burke J.R., Welsh-Bohmer K.A., Cabeza R. Temporal lobe functional activity and connectivity in young adult APOE ɛ4 carriers. Alzheimers Dement. 2010;6(4):303–311. doi: 10.1016/j.jalz.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson B.C., Salat D.H., Bates J.F., Atiya M., Killiany R.J., Greve D.N.…Sperling R.A. Medial temporal lobe function and structure in mild cognitive impairment. Ann. Neurol. 2004;56(1):27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson B.C., Salat D.H., Greve D.N., Chua E.F., Rand-Giovannetti E., Rentz D.M.…Albert M.S. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65(3):404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieguez D., Barea-Rodriguez E.J. Aging impairs the late phase of long-term potentiation at the medial perforant path-CA3 synapse in awake rats. Synapse. 2004;52(1):53–61. doi: 10.1002/syn.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy H.M. Springer Science & Business Media; 2005. The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections with MRI. [Google Scholar]
- Ewers M., Sperling R.A., Klunk W.E., Weiner M.W., Hampel H. Neuroimaging markers for the prediction and early diagnosis of Alzheimer's disease dementia. Trends Neurosci. 2011;34(8):430–442. doi: 10.1016/j.tins.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N., MacIntosh B.J., Hough M.G., Goodwin G.M., Frisoni G.B., Smith S.M.…Mackay C.E. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc. Natl. Acad. Sci. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frisoni G.B., Manfredi M., Geroldi C., Binetti G., Zanetti O., Bianchetti A., Trabucchi M. The prevalence of apoE-ε4 in Alzheimer's disease is age dependent. J. Neurol. Neurosurg. Psychiatry. 1998;65(1):103–106. doi: 10.1136/jnnp.65.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M., Koh M.T. Episodic memory on the path to Alzheimer's disease. Curr. Opin. Neurobiol. 2011;21(6):929–934. doi: 10.1016/j.conb.2011.10.021. ( http://doi.org/10.1016/j.conb.2011.10.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen A., Pihlajamäki M., Tanila H., Hänninen T., Niskanen E., Tervo S.…Soininen H. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol. Aging. 2007;28(12):1889–1903. doi: 10.1016/j.neurobiolaging.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Huijbers W., Mormino E.C., Schultz A.P., Wigman S., Ward A.M., Larvie M.…Sperling R.A. Amyloid-β deposition in mild cognitive impairment is associated with increased hippocampal activity, atrophy and clinical progression. Brain. 2015 doi: 10.1093/brain/awv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti, R., Juottonen, K., Soininen, H., Insausti, A. M., Partanen, K., Vainio, P., … & Pitkänen, A., (1998). MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. Am. J. Neuroradiol. 19(4), 659-671. [PMC free article] [PubMed]
- Kirwan C.B., Stark C.E. Overcoming interference: an fMRI investigation of pattern separation in the medial temporal lobe. Learn. Mem. 2007;14(9):625–633. doi: 10.1101/lm.663507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr, D., (1971). Simple memory: a theory for archicortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 262(841), 23–81. http://dx.doi.org/10.1098/rstb.1971.0078 [DOI] [PubMed]
- Miller S.L., Celone K., DePeau K., Diamond E., Dickerson B.C., Rentz D.…Sperling R.A. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc. Natl. Acad. Sci. 2008;105(6):2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993 doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Palop J.J., Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch. Neurol. 2009;66(4):435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Putcha D., Brickhouse M., O'Keefe K., Sullivan C., Rentz D., Marshall G.…Sperling R. Hippocampal hyperactivation associated with cortical thinning in Alzheimer's disease signature regions in non-demented elderly adults. J. Neurosci. 2011;31(48):17680–17688. doi: 10.1523/JNEUROSCI.4740-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach, J., & Tournoux, P. (1988). Co-planar stereotaxic atlas of the human brain. 3-Dimensional proportional system: an approach to cerebral imaging. http://dx.doi.org/10.1017/s0022215100111879.
- Trivedi M.A., Schmitz T.W., Ries M.L., Hess T.M., Fitzgerald M.E., Atwood C.S.…Johnson S.C. fMRI activation during episodic encoding and metacognitive appraisal across the lifespan: risk factors for Alzheimer's disease. Neuropsychologia. 2008;46(6):1667–1678. doi: 10.1016/j.neuropsychologia.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A., Crean S., Mercaldi C.J., Collins J.M., Boyd D., Cook M.N., Arrighi H.M. Prevalence of apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer's disease: a systematic review and meta-analysis. Neuroepidemiology. 2011;38(1):1–17. doi: 10.1159/000334607. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation; 1987. WMS-R: Wechsler Memory Scale-Revised. [Google Scholar]
- Wilson, I.A., Ikonen, S., Gallagher, M., Eichenbaum, H., Tanila, H., (2005). Age-associated alterations of hippocampal place cells are subregion specific. J. Neurosci. 25(29), 6877–6886. http://dx.doi.org/10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed]
- Wilson I.A., Gallagher M., Eichenbaum H., Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29(12):662–670. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa M.A., Muftuler L.T., Stark C.E. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proc. Natl. Acad. Sci. 2010;107(28):12687–12691. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa M.A., Stark S.M., Bakker A., Albert M.S., Gallagher M., Stark C.E. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. NeuroImage. 2010;51(3):1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]