SUMMARY
Antimicrobial stewardship is a bundle of integrated interventions employed to optimize the use of antimicrobials in health care settings. While infectious-disease-trained physicians, with clinical pharmacists, are considered the main leaders of antimicrobial stewardship programs, clinical microbiologists can play a key role in these programs. This review is intended to provide a comprehensive discussion of the different components of antimicrobial stewardship in which microbiology laboratories and clinical microbiologists can make significant contributions, including cumulative antimicrobial susceptibility reports, enhanced culture and susceptibility reports, guidance in the preanalytic phase, rapid diagnostic test availability, provider education, and alert and surveillance systems. In reviewing this material, we emphasize how the rapid, and especially the recent, evolution of clinical microbiology has reinforced the importance of clinical microbiologists' collaboration with antimicrobial stewardship programs.
KEYWORDS: antimicrobials, antimicrobial stewardship, microbiologist, optimal use, role, stewardship, rapid tests
INTRODUCTION
Clinical microbiology is a relatively new science. Van Leeuwenhoek, considered “the father of microbiology,” wrote his first letters on microscopy studies in the 17th century (1), but the work by Pasteur and Koch (2), among others, that led to clinical advances in the prevention and management of infectious diseases (ID) and associated improvements of the human condition (3), was not performed until the late 19th century. Once incurable and lethal infections have since become readily diagnosed and easily treatable, contributing to today's lofty expectations of modern medicine in which unsuccessful treatment of infections is considered a major failure.
Threats to these expectations loom, however. The emergence of antimicrobial resistance, including readily transmissible genetic elements in major human bacterial pathogens that confer resistance to most or all available antimicrobials, has foreshadowed the possible return of serious untreatable infection (4). Much of this is attributable to suboptimal—usually excessive—use of antimicrobials in and out of hospital settings, which is estimated to occur in 30 to 50% of all prescriptions (5). Suboptimal antimicrobial usage often stems from inappropriate interpretation or use of microbiological test results: lack of a microbiologically confirmed diagnosis, laboratory test errors, failure to submit appropriate specimens for culture, misuse of microbiology resources, and a general overreliance on empirical antimicrobial therapy with attendant disregard of microbiological results. A comprehensive understanding of these issues and a “modern” approach to their solution, though assembled as early as 1955 (6), has been an elusive operational goal.
Microbiology laboratories and the physicians or scientists who lead them must avoid a potentially paradoxical role in this dynamic. Their reports provide the primary basis for determining the incidence of antimicrobial-resistant infections on which longitudinal assessments of the problem's severity depend and for determining the prevalence of resistance among clinical isolates of common bacterial species that crucially informs empirical antimicrobial therapy strategies. Yet, microbiology input into the design and execution of antimicrobial stewardship interventions is often minimal or absent. Despite recommendations for including clinical microbiologists in hospital antimicrobial stewardship teams in prominent guidelines (7, 8), few if any of the interventions recommended require laboratory input; i.e., the guidelines are often “pharmacy centric.”
This article will review the multiple avenues by which clinical microbiology laboratories can contribute to antimicrobial stewardship efforts and offer a roadmap for clinical microbiologists to seize additional opportunities. It is intended not only for clinical microbiologists but for all health care professionals who want to improve laboratory collaboration in antimicrobial stewardship activities. We recognize that the substantial and growing administrative and managerial responsibilities of clinical microbiologists may hinder their fuller participation in stewardship and other clinical activities but argue here that the rapid pace of recent technological change and the attendant needs for implementation and interpretive guidance described below have produced a greater demand for clinical microbiologists' expertise than at any time in recent memory. Clinical microbiologists must collaborate closely with their clinician colleagues if patients are to fully realize the benefits of these advances.
ANTIMICROBIAL STEWARDSHIP AT THE HELM
Antimicrobial stewardship is a key instrument in working to improve the use of microbiologic data in order to help facilitate the appropriate use of antimicrobials and therefore to minimize antimicrobial resistance, as well as other unintended consequences, such as antimicrobial toxicity, adverse drug reactions, and Clostridium difficile diarrhea (6, 7, 9).
Antimicrobial stewardship can be defined as a bundle of interventions to promote and ensure the optimal use of antimicrobial treatment “that results in the best clinical outcome for the treatment or prevention of infection, with minimal toxicity to the patient and minimal impact on subsequent resistance” (10). The bundle and the key role played in each step by microbiology laboratories can be summarized by the “six D's of antimicrobial stewardship” adapted from other sources (Table 1) (11, 12).
TABLE 1.
The six D's of antimicrobial stewardship and associated key roles of microbiology laboratories
| The 6 D's of antimicrobial stewardship | Description | Examples of the key roles of microbiology laboratories |
|---|---|---|
| Diagnosis | Make and document the right diagnosis | Provide guidance to clinicians in obtaining adequate and significant specimens (e.g., prefer tissues and fluids in adequate volume to swabs) |
| Perform rapid testing for pathogens difficult to identify with standard microbiology (e.g., Legionella urine antigen) | ||
| Perform rapid identification testing of critical specimens (e.g., rapid molecular testing of positive blood cultures) | ||
| Perform timely biomarker testing (e.g., PCT) as indicated by institutional or professional organization recommendations | ||
| Promptly send samples to reference laboratories for appropriate tests not performed on site (e.g., Histoplasma urine antigen) | ||
| Advise clinicians about availability of advanced molecular diagnostic (e.g., 16S rRNA) testing for culture-negative critical access tissues (e.g., brain or bone biopsy specimens, cardiac valves) and provide timely access to reference lab testing as clinically appropriate | ||
| Advise clinicians on the performance characteristics of conventional and emerging RDT methods | ||
| Discard inadequate specimens (e.g., a urine specimen that has leaked from its transport container, external drains, etc.) | ||
| Debridement/drainage | Drainage of abscesses and removal of necrotic tissue or foreign material when required | Provide guidance for obtaining adequate and significant specimens (e.g., fluids in adequate volume rather than just swabs) |
| Prioritize cultures of specimens from operating rooms and interventional radiology (e.g., prepare slides and inoculate agar with specimens as soon as specimens arrive in the laboratory) | ||
| Optimize routing and tracing of specimens to the laboratory (e.g., provide logs to trace specimens from the operating room) | ||
| Drug | Use the right drug empirically according to suspected or confirmed diagnosis, risk factors for resistant pathogens, allergy, or major side effects | Participate in creating local guidelines for common infectious syndromes |
| Provide, revise, and publicize annual cumulative susceptibility reports to clinicians (e.g., provide tables with local susceptibility patterns) and work with ID physicians to interpret these data, e.g., to update recommended empirical regimens | ||
| Provide supplementary testing for susceptibility to new drugs when appropriate | ||
| Use cascade reporting (e.g., do not report carbapenem susceptibility when a pathogen is susceptible to narrower-spectrum drugs) | ||
| Repeat testing and promptly send to reference laboratory unusual susceptibility profiles (e.g., S. aureus resistant to vancomycin) | ||
| Contact clinicians directly and promptly in unusual cases and provide guidance for testing and therapy (e.g., when carbapenem resistance is suspected in a critical specimens and confirmation testing is pending) | ||
| Perform surveillance for emerging pathogens and resistance patterns and inform clinicians and public health authorities as appropriate (e.g., reporting to public health and memo to clinicians when multiple multiresistant Acinetobacter spp. are identified at one institution) | ||
| Dose | Use right dose according to diagnosis, site of infection, or renal or hepatic dysfunction | Collaborate with pharmacists and ID physicians to improve reporting of MICs for dosing based on pharmacokinetic targets |
| Duration | Use drugs for an appropriate duration | Perform biomarker testing and develop protocols to optimize their use for informing therapy duration as indicated |
| De-escalation | Reevaluate diagnosis and therapy routinely and de-escalate therapy to narrow-spectrum and/or oral agents when appropriate | Do not report skin contaminants in noncritical specimens and specify when contamination of critical specimens is or is not suspected (e.g., report S. epidermidis and other skin commensals exclusively from clinically significant specimens such as blood or prosthetic joints) |
| Leverage opportunities to append clinical guidance to microbiological reports, e.g., preferred drugs, likelihood of polymicrobial infection by specimen source (e.g., urine vs intra-abdominal wound), diagnostic follow-up (e.g., that repeat blood cultures are usually required in cases of candidemia, links to respiratory virus panel results in sputum culture reports) |
Antimicrobial stewardship programs have been shown to be beneficial in numerous health care settings, from small community health care centers to nursing homes and academic urban hospitals (13, 14). Reported benefits include, but are not limited to (15), reduction of C. difficile infection incidence (16–19), reduction of antimicrobial resistance (20–24), improving antimicrobial dosing in renally impaired patients (25, 26), improving the use of surgical antimicrobial prophylaxis (27–29), improved infection cure rates (30, 31), decreased mortality rates (14), more rapid administration of effective antimicrobial therapy and appropriate de-escalation in critical infections (32–35), and hospital cost savings (36–41).
Guidelines were published in 2007 by the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) to enhance antimicrobial stewardship activities (7) and updated in 2016 (42). In 2014, the Centers for Disease Control and Prevention (CDC) proposed seven core elements for the success of antimicrobial stewardship programs (Table 2) (5, 43).
TABLE 2.
The CDC's seven core elements of antimicrobial stewardship
| Element | Description |
|---|---|
| Leadership commitment | Dedicating necessary human, financial, and IT resources |
| Accountability | Appointing a single leader responsible for program outcomes |
| Drug expertise | Appointing a single pharmacist leader responsible for working to improve antibiotic use |
| Action | Implementing at least one recommended action with the goal of improving antimicrobial use |
| Tracking | Monitoring antibiotic prescribing and resistance patterns |
| Reporting | Regular reporting of information on antibiotic use and resistance to doctors, nurses, and relevant staff |
| Education | Educating clinicians about resistance and optimal prescribing |
In March 2015, the White House published a National Plan to Combat Antibiotic-Resistant Bacteria (44). The five goals of the plan are to slow the emergence of resistant bacteria and prevent the spread of resistant infections; strengthen national One-Health surveillance efforts to combat resistance; advance the development and use of rapid and innovative diagnostic tests for the identification and characterization of resistant bacteria; accelerate basic and applied research and development for new antibiotics, other therapeutics, and vaccines; and improve international collaboration and capacities for antibiotic resistance prevention, surveillance, control, and antibiotic research and development.
The plan aims to implement antimicrobial stewardship programs in every hospital setting in the United States by 2020 and recognizes antimicrobial stewardship interventions as major elements of the fight against antimicrobial resistance. It addresses antimicrobial resistance not only as a public health problem but also as a potential national security threat (45, 46).
The Joint Commission recently established performance criteria for antimicrobial stewardship for hospitals, critical-access hospitals, and nursing care centers that will become effective in 2017 (47). Elsewhere, Accreditation Canada and the Australian National Safety and Quality Health Service have had similar organizational requirements since 2013 and the National Institute for Health and Care Excellence published quality standards in April 2016 (48–50). Most recently, the issue of antimicrobial resistance was the subject of an unprecedented United Nations meeting, only the fourth health care issue to be accorded its own session of the United Nations General Assembly (51).
THE LABORATORY'S SEAT AT THE CAPTAIN′S TABLE
Because ID can affect all organ systems and encompass all medical disciplines, clinical microbiologists must collaborate with a diverse range of health professionals. Clinical microbiologists and ID physicians should naturally collaborate on a day-to-day basis, and this is considered essential to a successful antimicrobial stewardship program (7). Clinical pharmacists, especially those trained in ID, also play a major role in antimicrobial stewardship programs. Their expertise in antimicrobial effectiveness, toxicity, drug interactions, and pharmacodynamics and pharmacokinetics of antibiotics inform multiple stewardship activities, including, but not limited to, development and editing of order set, clinical pathways, and antibiotic usage policies; providing prior authorization of selected antibiotics; tracking of antimicrobial use and resistance; and interventions with feedback (52).
While medical practices combining both ID and medical microbiology are common in many countries, there is extensive variation in the involvement of clinical microbiologists in antimicrobial stewardship programs around the world (53). In Europe, a large observational study in 170 acute-care hospitals in 32 countries evaluated the role of microbiology in antimicrobial stewardship programs. While there was some geographic variation, a majority of microbiology laboratories participated in day-to-day antimicrobial stewardship activities ranging from advice outside business hours (71%) to daily ward rounds (41%) and cascade reporting (67%) (54).
In many countries of the Commonwealth of Nations, clinical microbiologists assume many clinical functions outside the laboratory because many are also trained in ID. For example, clinical microbiologists were present in more than 90% of acute trust antimicrobial stewardship committees in England and Ireland, making microbiology the most represented specialty in recent surveys (55, 56), perhaps reflecting the clinical roles that clinical microbiologists regularly have played in these countries, especially when there were few clinically trained ID physicians.
In 2011, the Australian Commission on Safety and Quality in Healthcare promulgated multiple recommendations for antimicrobial stewardship programs, including some pertaining to the role of microbiology services (9). The commission recommended that clinical microbiologists provide best practices for the rapid diagnosis of common infections, notify clinicians when critical infections are detected, provide regular patient-specific liaisons with clinicians in high-risk units, perform surveillance for resistance, and run standard antimicrobial susceptibility testing with cascade reporting (described below). The commission emphasized that clinical microbiologists should participate in pharmacy and therapeutics and antimicrobial stewardship committees, as their role is essential and integral to antimicrobial stewardship initiatives. A survey that followed these recommendations in the State of Victoria demonstrated large variations in the implementation of the proposed strategies, mainly depending on the type of institutions (57). Another survey in Queensland found that clinical microbiologists were responsible for providing therapy advice and antimicrobial approval in nearly 40% of the institutions surveyed, though half of the facilities did not have access to in-house clinical microbiologists or ID specialists (58).
In the province of Quebec (Canada), a survey of 68 hospitals in 2008 found that clinical microbiologists participated in 89% of antimicrobial stewardship surveillance programs (59). In this province, as in some other countries, most microbiologists are also trained and certified as ID specialists.
In the United States, clinical microbiologists' training backgrounds can vary between academic (Ph.D.) and medical (M.D.) training. In the latter, most will follow a pathology track while some, more rarely, will additionally be trained in internal medicine and ID. However, many microbiology laboratories focus on processing specimens and providing quality results without engaging in antimicrobial stewardship programs, which are usually led by ID physicians and pharmacists (60). Studies performed in California and Florida showed that microbiologists participated in antimicrobial stewardship activities in 26% and 42% of the hospitals surveyed, respectively (61, 62). A nationwide electronic survey, in which only half of the respondents reported having an institutional antimicrobial stewardship program, found similar results (63).
The 2007 IDSA/SHEA guidelines recommended that the core members of antimicrobial stewardship programs should include an ID physician and a clinical pharmacist with ID training. The participation of clinical microbiologists, along with information system specialists, infection control professionals, and hospital epidemiologists, is considered optimal (7). It is mentioned that “the microbiology laboratory plays a critical role in antimicrobial stewardship by providing patient-specific culture and susceptibility data to optimize individual antimicrobial management and by assisting infection control efforts in the surveillance of resistant organisms and in the molecular epidemiologic investigation of outbreaks” (7). Other potential functions of microbiology laboratories in antimicrobial stewardship programs mentioned in the guidelines include generating cumulative antimicrobial susceptibility reports (CASRs) to inform local guidelines and clinical pathways, collaborating with infection control professionals in outbreak investigations, and surveying for bacterial resistance.
In a 2016 guideline update, six “to-do” recommendations are listed for microbiology laboratory collaboration with antimicrobial stewardship teams, all of which were weak recommendations with low-to-moderate levels of evidence (42): use of stratified CASRs; use of selective or cascade reporting in antimicrobial susceptibility reports, i.e., reporting of algorithm-selected antimicrobial susceptibilities according to local resistance, treatment guidelines, and resistance patterns of a specific organism; use of rapid viral testing for respiratory pathogens; use of rapid diagnostic assays for blood cultures; use of procalcitonin (PCT) testing and algorithms for patients in the intensive care unit; and use of non-culture-based fungal markers for patients with hematologic malignancies.
We believe that clinical microbiologists can play a vital role in clinical services in the 21st century and that antimicrobial stewardship can keep them closer to patient care (64). More than 20 years ago, a survey by Thomson illustrated the changing role of some microbiology laboratories, shifting from research, education, and clinical services to management (65). There is also a worldwide trend in laboratory centralization with stated goals of greater standardization for quality and cost savings. It may seem logical, although unfortunate, that clinical microbiologists facing limited resources focus on more managerial types of activities rather than educational activities. The presence of an antimicrobial stewardship team whose members share responsibilities and in which the laboratory is actively engaged can represent the missing link to reach prescribers and perform the education perceived as missing by laboratorians and/or clinicians. The ways in which the laboratory can contribute to education efforts will be detailed further. In general, these activities should promote more fluid communication between clinicians and the laboratory to increase the clinical microbiologist's visibility, knowledge of the players and issues, and contributions to antimicrobial stewardship. Participation of clinical microbiologists in antimicrobial stewardship committees is thus the first and probably most important step in enhancing collaboration between the laboratory and other participants. Getting to know the current issues and objectives of the program is essential to tailoring what the laboratory can offer.
Many current laboratory practices can be considered stewardship activities and warrant recognition and credit as such. Table 3 presents some of the essential, achievable, and aspirational elements that clinical microbiologists can bring to the table of antimicrobial stewardship.
TABLE 3.
Essential, achievable, and aspirational antimicrobial stewardship activities for the microbiology laboratory
| Stewardship activity level | Descriptiona |
|---|---|
| Essential | Provide timely, reliable, and reproducible identification and antimicrobial susceptibility results |
| Actively participate in antimicrobial stewardship committee or work group | |
| Collaborate in educating local health care workers on microbiology issues that impact treatment and microbial resistance | |
| Promptly report unusual patterns of resistance, test supplementary agents, and provide advice on therapy for patients awaiting results | |
| Optimize communication of critical test result values and alert systems | |
| Provide, revise, and publicize annual CASR consistent with CLSI standards | |
| Provide guidance for adequate collection of microbiology specimens | |
| Develop alert systems for specific multidrug-resistant organisms | |
| Use cascade or selective reporting | |
| Collaborate with ID physicians and pharmacists on updating methods for susceptibility testing | |
| Achievable | Provide specific comments, drafted in collaboration with antimicrobial stewardship team, to guide therapy on microbiology reports |
| Participate in establishing protocols on biomarker use | |
| Use rapid diagnostic and antimicrobial susceptibility technologies for targeted critical specimen types | |
| Use rapid-detection platform for respiratory pathogens | |
| Guide optimal use of diagnostic assays for C. difficile | |
| Develop direct communication pathways with prescribers to help interpret RDT results and discrepant results | |
| Provide guidelines for the interpretation of microbiology test results | |
| Collaborate in audit and feedback of antimicrobial therapies for specific pathogens or syndromes where the role of lab test values is critical (e.g., C. difficile, bloodstream infections) | |
| Aspirational | Evaluate feasibility of and, where possible, perform testing for susceptibility to new drugs |
| Broaden use of validated rapid diagnostic and rapid antimicrobial susceptibility testing | |
| Participate in education of patients and local population on antimicrobial resistance | |
| Participate in national and regional surveillance systems | |
| Promote appropriate use of point-of-care microbiological tests, when available |
CLSI, Clinical and Laboratory Standards Institute; CASR, cumulative antimicrobial susceptibility report; RDT, rapid diagnostic test.
LOW-HANGING-FRUIT INTERVENTIONS OR TREASURES IN SHALLOW WATER
The timely availability of accurate and clinically significant microbiology results is critical for optimal antibiotic use and related clinical outcomes (7, 66). For example, a positive blood culture Gram stain read as Gram-negative bacilli but later identified as Listeria monocytogenes could significantly delay the provision of effective therapy, leading to an adverse outcome, even death (67). While microbiology laboratories usually perform surveillance for sentinel events such as the one described, antimicrobial stewardship teams can assist in this effort, and pathways to report and analyze these potential errors should be clearly defined. The World Health Organization (WHO), the United States Food and Drug Administration (FDA), the CDC, and the Clinical and Laboratory Standards Institute (CLSI) together developed a Laboratory Quality Management Systems Handbook based on previous CLSI documents and International Organization for Standardization standard 15189 to pursue the goal of providing reliable, timely, and accurate results (68, 69). Twelve quality essentials are described in this document: organization, personnel, equipment, purchasing and inventory process control, information management, documents and records, occurrence management, assessment, process improvement, customer service, and safety. Thus, we believe that a culture of quality in the microbiology laboratory and within antimicrobial stewardship can be mutually reinforcing.
Cumulative Antimicrobial Susceptibility Report
CASRs, often referred to simply as “antibiograms,” have many uses, including, but not limited to, helping prescribers select effective therapy when culture results are pending, informing and updating local guidelines for empirical treatment of common infection syndromes, updating periprocedural or perioperative prophylaxis recommendations, providing a rationale for antimicrobial formulary selection, surveying local resistance and benchmarking, identifying targets for stewardship interventions and best practices, and providing the context for new drug susceptibility testing results.
The CLSI first published guidelines for the analysis and presentation of cumulative susceptibility test data in 2002 and updated them most recently in 2014 (70). They included 10 recommendations (Table 4). The clinical microbiologist is in an excellent position to understand how these recommendations influence the utility of the reports and to contribute to antimicrobial stewardship programs on the basis of this expert knowledge. Some institutions have also published their CASRs online, and they can be consulted on the web (71–73).
TABLE 4.
CLSI M39-A4 recommendations for CASRsa
| Recommendationb |
|---|
| Analyze and present CASR at least annually |
| Include only final, verified results |
| Include only species with results for ≥30 isolates |
| Include only diagnostic (not surveillance) isolates |
| Eliminate duplicate isolates by including only first species' isolate/patient/period of analysis |
| Include only routinely tested agents |
| Report % S and exclude % I |
| For Streptococcus pneumoniae, report data for both meningitis and nonmeningitis breakpoints |
| For viridans group streptococci, report both % S and % I |
| For S. aureus, report % S for all isolates and MRSA subset |
Adapted from reference 70 with permission of the publisher.
S, susceptible; I, intermediate.
A 2004 national survey showed that among 474 responding laboratories (74% response rate), 95% published a CASR and 60% published a summary report that was distributed to infection control and medical staff and updated annually. Hospitals with on-site susceptibility testing and greater numbers of laboratory personnel were more likely to be compliant with the three survey elements previously (74).
Zapantis et al. (75) analyzed 209 CASRs from 2000 to 2002 and found that 14.3% showed unusual results such as Enterococcus susceptible to cephalosporins or Stenotrophomonas maltophilia susceptible to imipenem. Others have confirmed these observations (76). More recently, Moehring et al. (77) specifically looked at CASR quality in community hospitals and found that adherence to CLSI guidelines was generally poor. Only 8 (25%) microbiology laboratories excluded data for species with fewer than 30 isolates, while 20 (63%) reported data for nonrecommended pathogen-drug combinations. Only three microbiology laboratories (10%) were fully compliant. Both studies highlight that the first step for microbiology laboratories' contribution to antimicrobial stewardship programs must be to provide reliable data. CASRs benefit from multidisciplinary team interpretation and revision before publication to omit errors that might promote antimicrobial misuse.
While CLSI guidelines provide criteria for standardizing and benchmarking CASR data, challenges remain. Smaller microbiology laboratories may have difficulty meeting the recommended threshold of 30 isolates to report data in CASRs. Combining data at the genus level, from a longer period, or from multiple institutions with shared population characteristics might represent a reasonable option. On the other hand, if the number of isolates is sufficient and results suggest significant differences, data can be stratified by service, unit, resistance mechanisms, body sites, or specimens. Combination therapy susceptibilities can be calculated to help prescribers choose the right second agent for clinical situations, e.g., “Gram-negative sepsis,” where potentially antibiotic-resistant pathogens may warrant the broadened coverage afforded by double therapy while susceptibility results for the specific pathogen are pending (70, 78).
Some pathogen-drug combinations that are not usually recommended for testing can still be included in the CASR, especially when specific resistance phenotypes are locally observed (and antibiotics are systematically tested) or to educate prescribers on the usual nonsusceptibilities of certain pathogens. For example, entering R or 0% susceptibility of S. maltophilia to piperacillin-tazobactam should be considered to discourage its use.
Aggregated CASRs, i.e., the combination of strains from multiple institutions in one CASR, have been attempted, using uniform methodology among hospitals with similar patient populations and other characteristics (79). The State of Hawaii published a statewide antibiogram for selected bacteria of public health significance (80). Though obviously useful among small hospitals with insufficient numbers of specimens for reliable reports, aggregation of hospital antibiograms can also uncover or confirm newly emerging resistance phenotypes. For example, unusually high rates of resistance to fluoroquinolones, third-generation cephalosporins, and carbapenems identified from aggregated susceptibilities of Gram-negative pathogens collected from our long-term and acute-care hospital spurred the development and acceptance of our comprehensive antimicrobial stewardship program (81). Such aggregation is facilitated when hospitals already share some services (information technology [IT] systems, administration, infection control services, etc.).
IT systems might present obstacles to obtaining cumulative susceptibility data. Most available clinical microbiology management systems can extract data adequately. However, some require substantial additional work to obtain the same information. The CLSI also recommends an alternate manual data extraction method (70). In both instances, careful review is mandatory to ensure data accuracy. In any event, the participation of clinical microbiologists in antimicrobial stewardship programs is of great value to ensure that laboratory IT systems are chosen with stewardship considerations in mind.
These challenges should not discourage microbiology laboratories from providing CASRs, as their benefits generally exceed the inconvenience. However, when microbiology laboratories use methods different from those provided by the CLSI, the alternative methods should be clearly stated.
With the adoption of clinical decision support systems in many hospitals, it is also more and more common that CASRs are prepared by members of the antimicrobial stewardship team without the input of the microbiologist. While information provided by these systems can be accurate, errors in interpretation and reporting may lead to the delivery of false information to prescribers. Laboratories should ensure that people who prepare CASRs have received adequate training and have access to the most recent CLSI guidelines. The clinical microbiologist's input is also essential before the publication of the report.
Antimicrobial Susceptibility Reporting: beyond the Horizon
The final step of reporting results is crucial in the process of susceptibility testing (82). From the antimicrobial stewardship standpoint, the method by which the microbiology laboratory communicates results and the use of selective reporting and provision of instructions for how to interpret results can have a profound impact on prescribing habits.
Cascade or selective reporting can be used to promote the judicious use of antimicrobials (42, 83, 84). Cascades consist of algorithm-driven reports that provide only a limited number of tested antimicrobial susceptibilities based on formulary availability, local cumulative susceptibilities, and cost for isolates with no or low levels of resistance and reporting of susceptibility to broader-spectrum drugs only when isolates are resistant to drugs in the first “cascade.” Examples include releasing only gentamicin results when an organism is susceptible to all aminoglycosides, providing only susceptibilities to narrow-spectrum urine agents such as nitrofurantoin and trimethoprim-sulfamethoxazole when organisms isolated from midstream urine cultures are susceptible to these agents and releasing other agents such as quinolones or cephalosporins only when resistance to the former is demonstrated and not releasing non-β-lactam susceptibilities for Streptococcus agalactiae screening cultures if no β-lactam allergy is indicated in the patient chart. While some microbiology laboratories prefer to release all of the information to clinicians, the cascade approach is recommended by the IDSA (42). Careful selection of reported susceptibilities and frequent reevaluation are necessary to ensure the continued value and reliability of the cascade and the quality of the reporting. Unreleased susceptibility data should also be readily available upon clinician request. Some studies suggest an association between the antibiotics listed in antimicrobial susceptibility reporting and the use of these antibiotics by prescribers (85). For example, Cunney et al. found that antimicrobials were half as likely to be prescribed when susceptibility results from noncritical cultures not suggestive of infection were suppressed (86). Similarly, McNulty et al. showed that reporting of cephalexin instead of amoxicillin-clavulanate in urine culture reports resulted in dramatic modification of the use of these two agents in the intervention period even when practitioners were not informed of the change (87). Unfortunately, no guidelines on cascade reporting are currently available (88).
The benefits and potential pitfalls of using comments or additional messages to enhance microbiology reports have not been studied extensively, though this approach is widely used on the basis of local experience. Clear and concise messages on patient reports may be useful to guide therapy (84, 89). However, regulatory agencies may require that some information be included and may overload the reports. The CLSI also recommends few therapy-related comments (90). Some examples are listed in Table 5. One important observation is that automated messages are easier to manage and less likely to be forgotten than when such messages must be added manually. In general, automated susceptibility reporting, available in many laboratory systems, reduces the inherent complexity of managing this process (84, 91).
TABLE 5.
Examples of acceptable therapy-related comments added to patient clinical microbiology reports to improve prescribing of antimicrobials
| Category | Examples |
|---|---|
| CLSI-recommended comments (M100-S25)a | Cefazolin results predict results for oral agents cefaclor, cefdinir, cefpodoxime, cefprozil, cefuroxime, cephalexin, and loracarbef when used for therapy of uncomplicated urinary tract infections due to Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis |
| Rifampin should not be used alone for antimicrobial therapy in infections with Staphylococcus or Streptococcus spp. | |
| Use of penicillins or third-generation cephalosporins for pneumococcal meningitis requires therapy with maximum doses | |
| Dose of intravenous penicillin of at least 2 million units every 4 h in adult with normal renal function (12 million U/day) can be used to treat nonmeningeal pneumococcal infections due to strains with penicillin MICs of ≤2 μg/ml; strains with an intermediate MIC of 4 μg/ml may require penicillin doses of 18 to 24 million U/day in adults with normal renal function | |
| Penicillin- or ampicillin-intermediate isolates may require combined therapy with an aminoglycoside for bactericidal action in streptococcal infections | |
| Combination therapy with ampicillin, penicillin, or vancomycin (for susceptible strains) plus an aminoglycoside is usually indicated for serious enterococcal infections such as endocarditis unless high-level resistance to both gentamicin and streptomycin is documented; such combinations are predicted to result in synergistic killing of the Enterococcus | |
| Other potential comments | |
| Resistance mechanism characterizationb | Enterobacter cloacae with AmpC-type β-lactamase profile; cefepime is usually effective for infections caused by this pathogen |
| Diagnosis issues | Positive urine cultures should prompt targeted antimicrobial therapy only if the patient (i) has symptoms of cystitis or pyelonephritis, (ii) is pregnant, or (iii) will soon undergo an invasive urologic procedure; apart from these clinical indications, patients with asymptomatic bacteriuria do not benefit from antibiotic therapy |
| Specialist consultation | ID consultation is strongly recommended in S. aureus bacteremia |
| Duration of therapy | S. aureus bacteremia usually requires a minimum of 14 days of therapy; longer therapy is often needed to treat or prevent complicated infections; expert consultation is advised |
| Culture interpretation | Gram stain and culture of this specimen represent normal skin flora |
| Reference to documentation | Refer to local guidelines for treatment recommendations of respiratory tract infections |
| Suggestions for alternatives | In our institution, clindamycin is the preferred agent used to treat this pathogen in patients with IgE-mediated allergy to penicillin |
| Selective or cascade susceptibility reporting | Only first-line recommended antimicrobials appear in this report; contact the laboratory for additional susceptibility testing if alternate agents are needed, e.g., due to allergy |
| Reference to antimicrobial stewardship program services | Contact the antimicrobial stewardship team to choose the best agent to treat this infection (e.g., for unusual or multidrug-resistant pathogens) |
| Dosing recommendations | Maximum dosing is recommended to treat severe infections caused by this agent; consider expert consultation |
| Probable contamination or colonization | Candida spp. are rarely pathogenic in respiratory tract or urine cultures |
| Nonstandard methods or lack of interpretation criteria | There are no validated susceptibility criteria for this agent; MICs are provided for information only |
| New interpretation criteria | According to recent published standards, clinical breakpoints for this drug have changed; consult with the laboratory for more information |
| Public health reporting | Infection with this agent is a reportable disease that requires clinical information; this infection will be reported to the public health department; you may be contacted if additional clinical information is needed |
| Infection control recommendations | Contact precautions are mandated in patients with MRSA infection or colonization; refer to infection control procedures for more information |
| Other potential comments | Cost of tested antimicrobials |
| Indication of preferred agents according to local guidelines in the report by highlighting or bolding |
Adapted from reference 90 with permission of the publisher.
To provide insight into agents to avoid or to consider on the basis of specific mechanisms of resistance.
Phone calls remain the method of choice for rapid notification of critical results, but other means of communication, such as paging, text messaging, electronic messaging, and alerting, can be used to communicate with health care professionals in other contexts. Though electronic reporting of microbiological data may improve workflow efficiency, it may impact clinical decisions minimally (9, 92). Person-to-person communication optimally provides reliable transfer of information, increases collegiality, and heightens appreciation of the clinical microbiologist's value.
New Drug Testing and Changes in Interpretation Guidelines
Over the last few decades, a limited number of new antimicrobials have been developed (93). However, new regulations were adopted to promote the development and to speed up the availability of new drugs to patients (94). While new antibiotics should be used with care and only when indicated, some clinicians might find their use urgent, especially when the new agents fill a void in the therapeutic arsenal. Microbiology laboratories should stay abreast of new drug development and assess the laboratory's capacity to test the activity of new agents against appropriate pathogens. Information on clinical breakpoints, quality control, and other drug particularities may be limited when new drugs first come to market or when older drugs, e.g., polymyxins, reemerge as therapies of necessity. Materials for testing may sometimes be available only through drug manufacturers with “research use only” status, and testing may be limited to one or two methods. Thus, a laboratory that previously evaluated, experimented, or validated testing for a specific new drug may play a critical role in the process of approval by a pharmacy and therapeutics committee.
The CLSI, European Committee for Antimicrobial Susceptibility Testing, and other authoritative guidelines are updated frequently and are crucial to microbiology laboratories' ability to provide quality results. Selecting the most appropriate breakpoint guidelines can be challenging, as discussed elsewhere (95, 96). However, once reserved for laboratorians, recommendations and criteria provided by these guidelines are now used in day-to-day patient care by physicians and pharmacists. Interdisciplinary collaboration is essential in analyzing and implementing new breakpoint guidelines, especially in the case of the annual update of the performance standards for antimicrobial susceptibility testing (90, 97). One example is the implementation of CLSI cephalosporin breakpoints for Enterobacteriaceae, changed in 2010, that can impact the epidemiology of resistance and consequently the use of carbapenems (89). New breakpoints should therefore be evaluated for implementation in a timely manner. However, delays in the adoption of these breakpoints by regulators like the FDA, and consequently by the manufacturers of automated platforms, may represent significant barriers to implementation (98). Similarly, as suggested by Heil and Johnson in their paper on clinical breakpoint issues (89), changes in methods that impact identification, susceptibility testing, or simply reporting should also be promptly announced to clinicians to avoid errors in interpretation. Clinical microbiologists, in conjunction with ID physicians and pharmacists, are in the best position to rapidly identify such situations and to provide timely insights and recommendations to antimicrobial stewardship programs.
Guidance in the Preanalytic Phase
Being able to make the right diagnosis is usually a prerequisite to providing effective therapy. Recommendations for drug choice, dosing, or duration may be useless if the diagnosis is wrong. Filice et al. assessed the accuracy of diagnosis and appropriateness of therapy from the medical records of 500 randomly selected hospitalized patients who received antimicrobials. While prescribed antimicrobials were appropriate in the majority (62%) of the cases when the diagnosis was considered accurate on the basis of clinical, radiologic, and laboratory findings, anti-infective appropriateness was abysmal (5%) when the diagnosis was incorrect (66).
One way microbiology laboratories can significantly impact diagnostic accuracy and the quality of antimicrobial prescribing is by providing guidance in the preanalytic phase, i.e., guidance for selecting the appropriate test or culture according to the patient's syndrome, obtaining optimal collection of clinical specimens, and interpreting microbiology test results. Because poorly collected specimens may result in the recovery of commensal or colonizing organisms and are often rejected (99, 100), clinicians need instruction in the appropriate timing and technique of specimen collection. Common problems in the preanalytic phase include contamination of blood cultures, urine cultures in asymptomatic patients, and the failure to use specific testing in specific clinical syndromes (e.g., Legionella urinary antigen in community-acquired pneumonia) (9). The American Society for Microbiology and the IDSA produced detailed guidelines for the laboratory diagnosis of ID, and these are a useful tool for both clinicians and laboratorians as part of antimicrobial stewardship programs (99). Collaborating with other laboratories to optimize the pathway of specimens, as well as minimize superfluous cultures, can also be considered. One example is collaboration with the biochemistry laboratory to use algorithmic pathways between urinalysis and urine cultures that have been shown to reduce antibiotic consumption (101, 102).
Nurses must be included among the recipients of guidance on microbiological test selection and specimen collection, as they also perform diagnostic tests or collect culture specimens, sometimes without or before the physician's evaluation of the patient. Thus, the role of nursing in accurate and standardized specimen collection should be emphasized (43, 103, 104).
BIOMARKERS AND RAPID DIAGNOSTIC AND RAPID SUSCEPTIBILITY TESTING: NEW TRADE WINDS MAY HELP YOU REACH YOUR DESTINATION
Biomarkers
The quest to find a highly sensitive and specific and readily available and interpretable ID biomarker has spanned decades—and such a marker is the object of recent highly promoted prize competitions—but the perfect biomarker has yet to be found. Accurate biomarkers could be a boon to antimicrobial stewardship programs by providing more accurate infection diagnosis, suggesting the class of infectious agent (bacterial, fungal, viral, etc.), monitoring clinical responses, and guiding the duration of treatment (105–107).
C-reactive protein was one of the only commercially available biomarkers until a few years ago. It is widely used to monitor the clinical response in bacterial infections, but high intra- and interindividual variability makes it difficult to use for diagnostic purposes (106).
Newer bacterial infection biomarkers, such as PCT, are used more and more frequently in hospital settings. PCT, a prohormone of calcitonin, is secreted by a number of organs in response to bacterial—but not viral—invasion/infection (107). Serum PCT is detectable as soon as 4 h and peaks between 12 and 48 h after infection onset. Most studies have focused on its use for respiratory infections and sepsis (108–110), and data support its use more often as an indicator to stop, rather to start, therapy. A Cochrane review in 2012 of the use of PCT algorithms in acute respiratory infections found that the median exposure to antimicrobials was reduced from 8 to 4 days without any adverse impact on the mortality rate (109). Similar data were found in patients with sepsis in intensive care units (110).
Five commercial PCT assays are currently approved by the FDA. More detailed reviews have been published elsewhere (108–111). Other potential bacterial biomarkers in development include, but are not limited to, amyloid A, interleukin-10, liposaccharide binding protein, and nCD64 (106).
However useful PCT and other biomarkers may be now or in the future, they cannot replace microbiology analysis. It has been suggested that antimicrobial stewardship team recommendations on the interpretation of biomarker results are required for optimal use (112). Other challenges to biomarker use include cost, turnaround time (optimal with point-of-care testing), limited data in special populations such as immunocompromised patients, physician variability in modifying antibiotics based on available results, and interpretation of intermediate results (105, 113). While significant benefits might result from using biomarkers to guide antimicrobial therapy, multidisciplinary input from antimicrobial stewardship programs that include clinical microbiologists seems essential when developing local protocols for biomarker use.
While biomarker testing may not fall under the responsibility of microbiology laboratories in many institutions, clinical microbiologists' involvement is desirable given their close ties to and ability to integrate this testing with the workflow for other relevant analyses, for example, respiratory virus panels.
Rapid Diagnostic Testing (RDT) and Rapid Antimicrobial Susceptibility Testing
The delayed results of traditional bacterial cultures and antimicrobial susceptibility testing, which may take up to several days to obtain, remain one of the major barriers to providing optimal therapy (84). This is especially important for severe infections such as sepsis and septic shock, for which a delay in initiating effective therapy is a strong predictor of death (114, 115). Emerging RDT methods include a large variety of technologies and vary greatly in terms of complexity, price, speed, and the ability to identify single or multiple pathogens.
The key to successful RDT is the twinning of these technologies to an antimicrobial stewardship team that can notify clinicians about test results and guide their use in initiating or modifying antimicrobial therapy, for without this link between clinical microbiologists and antimicrobial stewardship, the rapid results run the risk of floating adrift at sea (116). A meta-analysis by Buehler et al. found that for patients with bloodstream infections, only rapid diagnostic techniques coupled with direct communication led to significant differences in the time to effective or optimal therapy (117). Most published studies have been performed in larger tertiary-care centers with multiple resources and direct communication of results with guidance on management and therapy provided by clinical pharmacists and/or physicians trained in ID (117, 118). As technologies simplify and become available in more diverse settings, clinical microbiologists will need to collaborate closely with antimicrobial stewardship teams to rapidly communicate results and to interpret their meaning. In our experience, implementation of such technologies and protocols is a team effort. In addition to directing the laboratory-specific steps required to implement a new test, clinical microbiologists must collaborate with the rest of the antimicrobial stewardship team to achieve a consensus on the rules of usage and the presentation and interpretation of the results. Clinicians should receive appropriate information and training before microbiology laboratories go live with RDT, especially when multiplex platforms are used as large amounts of information are available at one time. Clinicians' training should include at least information specific to the RDT method and the technology used, chosen indications for testing in the institution and available alternative testing strategy, advantages and limitations, turnaround time, presentation of the report, and guidance for interpretation. Online sessions provided by professional societies may provide instruction on such topics. While there are multiple advantages to having results faster, clinical microbiologists must guide clinicians in finding the optimal balance between accuracy and rapidity in interpreting rapid diagnostic results.
There is also an ongoing search for a better tool to diagnose ID. The Longitude Prize (https://longitudeprize.org/challenge/antibiotics), launched in 2014 by the United Kingdom and affiliated private partners, will reward with £10 million a team able to build a diagnostic tool that can rapidly rule out the need for antibiotic use or help identify an effective antibiotic to treat a patient (64).
The following paragraphs will review some of the most frequently used assays; more extensive review articles that focus on newer technologies have been published elsewhere (116, 117, 119–121).
Bacterial and Fungal Molecular Assays
Molecular assays have been the main focus in the development of rapid diagnostic technologies in recent years. While methods vary, most bacterial assays focus on critical specimens such as blood cultures.
Peptide nucleic acid fluorescent in situ hybridization (PNA FISH) is a simple molecular assay that requires few instruments and therefore can be used in diverse laboratories. Four panels are available for blood cultures: Enterococcus, Gram-negative bacteria, Candida, and Staphylococcus, the latter being the only one with resistance gene (mecA) detection (121). Laud and Knudsen observed a greater proportion (98% versus 89%) of early appropriate therapy when PNA FISH was used to detect Staphylococcus bacteremia (122). Other studies also found that use of this test was associated with shorter lengths of stay and decreased overall costs (119, 123–125).
The two main multiplex molecular PCR assays currently available in clinical practice are Biofire's FilmArray System and Nanosphere's Verigene System (119). The FilmArray System presently offers four panels: respiratory, gastrointestinal, blood cultures, and meningitis/encephalitis. The Verigene system has five panels: respiratory, enteric pathogens, C. difficile toxins, and Gram-positive and Gram-negative bacteria from blood cultures. Both systems are relatively easy to use, with short hands-on time, excellent performance, and results available in 1 to 2.5 h (35, 119, 126).
Multiple studies have demonstrated important benefits when these technologies are combined with antimicrobial stewardship interventions. A large randomized study by Banerjee et al. evaluated the performance and impact of the FilmArray System Blood Culture Identification (BCID) panel in addition to antimicrobial stewardship interventions performed by a clinical pharmacist or an ID physician. Reduced use of broad-spectrum antibiotics and less frequent treatment of blood culture contaminants were observed; de-escalation was significantly more successful with antimicrobial stewardship guidance, and the mortality rates and overall costs were similar in all groups (35). In another study, 152 causative agents of bacteremia were identified by conventional methods over a 1-month period and 115 (80.4%) were also correctly identified by BCID (127).
In a quasiexperimental study, Sango et al. showed a reduction of 23.4 to 31.1 h in the time to appropriate therapy and significant reductions in the length of stay and hospital costs when the Verigene System was used to rapidly identify Enterococcus bacteremia; there was no difference in the mortality rate (118). Similar results were obtained in community settings (128).
One important concern with multiplex assays is that they are less accurate in detecting polymicrobial infections; thus, clinical microbiologists should consider this possibility when single organisms are reported (35, 129). Detected resistance genes are also limited in number and may not always correlate with phenotypic antimicrobial susceptibility. Thus, risk factor assessment for resistance cannot be dismissed. On the other hand, these molecular assays are extremely sensitive and may detect organisms that would not generally be detected or considered clinically significant by the current gold standards of traditional microbiology. Laboratorians have had to deal with similar situations regularly since the beginning of molecular testing (130). A higher detection of skin contaminants in critical specimens may be challenging in many situations, especially with critically ill patients or when supplementary cultures are not possible, for example, pediatric patients or specimens collected during surgery. Detection of colonizing rather than pathogenic strains of C. difficile also occurs frequently with newer PCR-based assays (131, 132). Microbiology laboratories may want to put in place strategies to identify, track, and analyze discrepant results, especially in the implementation phase of new tests. Interpretation of individual results should always be done in the light of a clinical evaluation of the patient and other available results. We recommend that clinical microbiologists contact prescribers or coordinate responses with antimicrobial stewardship teams in these situations, especially when discrepant results are found in critical specimens, to guide the most appropriate therapeutic strategy. When suspicion for infection is low and the patient is stable, a “wait-and-see” strategy may be the best option.
Viral Molecular Assays
Respiratory viral infections, including influenza, are common mimics of bacterial syndromes that can lead to increased bacterial resistance when inappropriately treated with antibiotics (133, 134). Multiple testing platforms with different technologies are available on the market and are reviewed elsewhere (120, 135–137). While they are recommended in the latest IDSA guidelines (42), most of the evidence supporting the use of these assays is from pediatric studies. As with other rapid diagnostic assays, positive viral tests cannot exclude bacterial super- or coinfection and so may not be sufficient to convince prescribers to discontinue antibacterials. In a study in North Carolina, discontinuation of antibacterials within 48 h following respiratory viral testing with or without PCT was observed in only 10 to 20% of the cases studied (138). In another study, more than a third of the patients with a positive influenza PCR test result were continued on antibiotics more than 24 h after the availability of the test result, suggesting that additional diagnostic tools—an evaluation of the host response that could indicate the presence of a bacterial, viral, or combined infection—or interventions may be required to convince clinicians that antibiotic discontinuation is safe for these patients (139). If used, ease of availability, rapid turnaround time, and prompt notification of results are essential for promoting appropriate antiviral therapy and timely discontinuation of antibacterials when not otherwise indicated (140).
MALDI-TOF MS
Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) can accurately identify a large range of pathogens such as bacteria, yeasts, filamentous fungi, and mycobacteria in as little as a few minutes (141–143). Equipment acquisition costs might be quite high, but the cost per individual test can be as low as $0.41 per sample (144). While conceived for use on isolates grown in routine cultures, protocols for testing specimens directly are commercially available and await FDA clearance (143). Rapid identification with MALDI-TOF MS was also shown to reduce the time to appropriate therapy in 11% to 44% of the cases (34, 145) and to increase the Acinetobacter baumannii infection clinical cure rate by 19% (146). Given the fact it can significantly simplify workflow, MALDI-TOF MS is a reasonable consideration for smaller community institutions. A recent study reported average savings of $3,411 in hospital costs along with a reduced time to appropriate therapy when MALDI-TOF was coupled with a pharmacist intervention for bloodstream infections in two community hospitals in Texas (147)—nicely illustrating the results of successful collaboration between clinical microbiology and antimicrobial stewardship.
Old and New Antimicrobial Susceptibility Testing
Currently available rapid susceptibility tests are limited to the detection of a few specific genes associated with resistance or treatment failure (e.g., mecA in Staphylococcus aureus or blaKPC in Enterobacteriaceae). Therefore, MIC determination by standard procedures is still often required. Rapid-result protocols using standard technologies such as disk diffusion and microdilution have been described mostly for critical specimens such as blood cultures (148–151). These direct methods have shown relatively high categorical agreement with standardized methods but usually require additional labor-intensive steps, with repeat standardized susceptibility testing usually recommended when growth is sufficient (148). It is worth noting that more rapid automated antimicrobial susceptibility tests may have pitfalls. For example, the MICs of vancomycin for S. aureus were reported to be over- or underestimated by automated microdilution systems. Death, however, correlated better with MICs determined by disk diffusion and gradient diffusion (91). While controversial, higher vancomycin MICs may trigger prescribers to use alternative drugs such as daptomycin, linezolid, or ceftaroline that can be more costly, more toxic, and even less effective, depending on the clinical syndrome (152). Reflex protocols to confirm MICs coupled with specific comments, cascade reporting, and/or therapeutic algorithms may lead to more appropriate use of vancomycin and daptomycin and demonstrate cost savings (153).
Additional rapid antimicrobial susceptibility technologies are under development, including automated digital microscopy in real time, flow cytometry, laser scatter, and magnetic resonance (154, 155). An early study with automated digital microscopy in real time speculated that the technology may impact therapies 40% of the time (155). Whole-genome sequencing also has shown some promise, e.g., in Mycobacterium tuberculosis (156, 157).
RDT Bottom Line
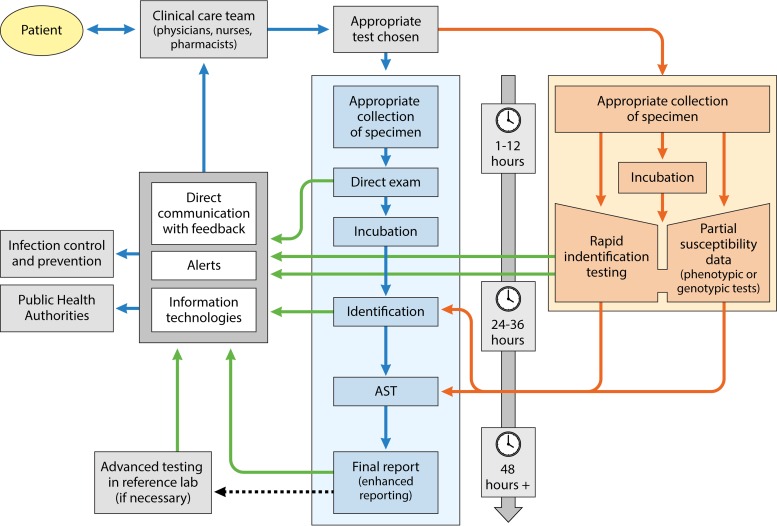
RDT evolves quickly, and many of the RDT methods discussed in this review may be obsolete in as little as a few years. The multiplicity of newer diagnostic approaches, tests, and platforms makes clinical microbiologist collaboration with antimicrobial stewardship programs essential to determine which tests are right for an institution and how best to implement and guide the interpretation of them, given the variation in the tests' performance characteristics and, just as likely, in clinicians' perceptions of them. Figure 1 summarizes the contributions and relationships of RDT in the workflow pathway. For the time being, the limitations of these tests mean that they can supplement but not yet supplant conventional microbiological methods.
FIG 1.
Workflow pathways for conventional microbiology and RDT. Implementation of RDT increases laboratory workflow complexity but can hasten the availability of results. Communication of results is a key factor. Blue arrows represent the conventional microbiology pathway, orange arrows represent the RDT pathway, and green arrows represent opportunities for the laboratory and antimicrobial stewardship teams to improve communication of results. AST, antimicrobial susceptibility testing.
In the end, the complexity and all of the nuances of clinical microbiology testing might make ordering testing by a fixed combination of tests specific for each diagnosis—e.g., a provider orders testing for “community-acquired pneumonia” rather than list all of the specific tests—easier for many providers. This new way of ordering tests could represent a sea change in most hospitals and would require close cooperation of clinical microbiologists, ID physicians, and pharmacy staff and careful monitoring for changing susceptibility patterns.
PROVIDER EDUCATION: HOW TO TIE THE KNOTS
Informational interventions such as provider education have been found to be less effective than coercive interventions in improving antibiotic prescribing in the short term, but those differences become nonsignificant after interventions have been implemented for many months (13). Because the sustainability of the effect of educational interventions is usually low, especially when work forces change over short intervals, as in teaching hospitals, continuous education is considered essential to any antimicrobial stewardship program (7, 42, 158). Clinical microbiologists are experts on a multitude of subjects related to antimicrobial stewardship, such as resistance mechanisms, pathogen interaction with the environment, diagnostic testing, and interpretation of susceptibility reports (159). Their daily decisions as experts in laboratory diagnostics impact clinicians' interpretation of tests and influence patient care. However, the tasks and purposes of clinical microbiologists may not always be fully understood by clinicians (158). Thus, we strongly encourage the participation of clinical microbiologists in designing and delivering antimicrobial stewardship-related teaching, which is ideally multimodal, including rounds and conferences but also staff bulletins and management guidelines (160, 161). To be maximally effective, the clinical microbiologist should visit the ward at least occasionally, in addition to providing educational sessions at physician and staff conferences.
Clinical microbiologists' collaboration with antimicrobial stewardship teams and other clinicians can lead to benefits that are multidirectional. Education sessions provide excellent opportunities to gauge service satisfaction and suggest potential avenues for improving laboratory services while teaching clinical microbiologists about formularies, guidelines, order forms, and other tools in use or under development at their institution, with resultant ideas for collaborating or for adjusting laboratory services to better serve the needs of prescribers. For example, quinolones are no longer recommended as first-line agents for the treatment of uncomplicated urinary tract infections, for which older agents such as nitrofurantoin and fosfomycin are now being used, leading to the need to update urine susceptibility testing (162). Helpful changes in reflex or cascade reporting may also be triggered by feedback gleaned from such interactions.
In addition to publishing the online and/or paper CASR, it also might be beneficial to provide a presentation letter or small conference on a yearly basis to highlight major changes and indicate to prescribers how to use the CASR. The CLSI also suggests supplementary methods for presenting the CASR, such as graphics and tables (70). Different topics can be covered in education sessions. Some of the most relevant from a clinical microbiology perspective are guidance in the preanalytic phase for optimal specimen collection, antimicrobial resistance issues (mechanisms, laboratory testing, therapies, etc.), interpretation of antimicrobial susceptibility reports, antimicrobial resistance surveillance and interpreting CASR annual updates (including infection control and epidemiology), improving clinicians' “microbiologic literacy,” pathogen-specific diagnosis and management (including emerging pathogens), use of new technologies and biomarkers in the institution, updates on testing and interpretation of clinical microbiology testing, and research opportunities and collaborations.
The Cochrane Collaborative performed a meta-analysis of 89 studies on interventions to improve antibiotic practices for inpatients. They found that coercive interventions such as requiring preauthorization of restricted antibiotics or targeting certain antibiotics for specific indications were more rapidly effective than informational interventions such as prescriber education and audit and feedback (13). However, after 6 months, the educational and audit/feedback interventions were as effective as the up-front restrictive or targeted interventions (13).
Because lack of awareness and familiarity is an important factor that influences adherence to medical guidelines, education about the basis of the guidelines is a fairly easy response to this problem (163). However, standard teaching methods focus mainly on increasing the knowledge of participants—without operationalizing that knowledge—and may not always translate to changes in behavior (158).
Antimicrobial stewardship currently receives relatively little attention in medical, nursing, and pharmacy school curricula. Emerging programs are focused mostly on advanced trainees like residents and fellows and practicing clinicians and pharmacists (http://mad-id.org/antimicrobial-stewardship-programs/, http://www.sidp.org/page-1442823). Many of these programs include rudimentary training in clinical microbiology and antimicrobial resistance, but the participation of clinical microbiologists in such programs is limited. Recently published Doctor of Pharmacy student elective curricula include teaching and/or laboratory skill sessions with a clinical microbiologist (164, 165). The success of antimicrobial stewardship in a given institution derives partly from the presence of a culture of antimicrobial stewardship, i.e., the general impression that a better use of antimicrobials is necessary and beneficial for all of the participants of the institution. Clinical microbiologists have a great deal to offer in developing and maintaining such a culture.
Multiple resources in diverse formats now exist to educate providers. In a world where lack of time is the new normal, new ways to reach clinicians that offer more flexibility and interactivity might also help. Many states and hospitals provide online antimicrobial stewardship toolkits with educational material that can be used, for example, the California Department of Public Health (http://www.cdph.ca.gov/programs/hai/Pages/AntimicrobialStewardshipProgramInitiative.aspx) and Nebraska Medicine (Omaha, NE). Massive online open courses (MOOC) and e-learning tools may also be part of the solution (166). One example is the largely publicized MOOC “Antimicrobial Stewardship: Managing Antibiotic Resistance” (http://www.dundee.ac.uk/study/short/antimicrobial-stewardship/) offered by the University of Dundee (Dundee, United Kingdom) and the British Society of Antimicrobial Chemotherapy (Birmingham, United Kingdom). This free 6-week online course provides participants the opportunity to develop skills and carry out interventions that underpin antimicrobial stewardship, learning to promote responsible prescribing and to reduce practice variation, waste, and harm from antibiotic overuse and misuse.
Communication methods have evolved quickly in recent years and are focused on short and efficient messages. Facility with these new tools has the potential to reach a maximum of prescribers and to “trend” some institutional messages. E-mail inboxes fill quickly with information often unread by many. Social media, such as Facebook, Instagram, YouTube, Snapchat, and Twitter, are now commonly used by clinicians, hospitals, health agencies, and organizations to track diseases, raise awareness of the public about health issues, quickly disseminate information, and engage health care professionals (167). The CDC provides guidance and best practices on the use of social media with a dedicated website (http://www.cdc.gov/socialmedia/index.html), and two recent articles reviewed the power and potential of Twitter and Instagram for the practice of microbiology and ID medicine and listed multiple accounts of interest (168, 169).
An important element of antimicrobial stewardship is how to effectively change prescribers' behavior to achieve positive and durable outcomes while respecting their autonomy. The use of clinical guidelines was shown to have limited effects on prescribers' behavior when no other interventions were coupled with them (163). Common barriers include lack of knowledge, insufficient resources, adverse attitudes and beliefs, and lack of time that may be specific to individuals or local practices or widespread among prescribers (158, 163). The potential ability of microbiology laboratories to alter this dynamic favorably is certainly worth exploring.
ALERT AND SURVEILLANCE SYSTEMS: SOUNDING THE ALL-HANDS-ON-DECK ALARM
Surveillance is defined as the ongoing and systematic collection, analysis, and interpretation of health data essential to the planning, implementation, and evaluation of public health practice (170). Surveillance can be passive (with detection via normal laboratory pathways or workflow and alerting on an individual basis) or active (when specific targets are followed by informatics models and processes and acted upon when thresholds are crossed) (170). Most hospital laboratories already participate in some surveillance programs. The Centers for Medicare & Medicaid Services requires reporting of multiple health care-associated infections, such as S. aureus bacteremia or C. difficile infections (171), on which microbiology laboratories must collaborate with infection control teams on a regular basis. Public health departments around the country also require laboratories to report certain pathogens and outbreaks. In both instances, reporting can be complex and automatization is not always seamless (91). While these surveillance systems are important for understanding the regional and national epidemiology of these pathogens and to define national objectives, we will focus on more local approaches for surveillance of resistant organisms in the context of antimicrobial stewardship.
Microbiology laboratories deal with a large volume of information every day. Surveillance and alert systems need to be designed to digest the information and to make it easy to interpret and analyze for antimicrobial stewardship personnel and clinicians (88). On the other hand, if nobody analyzes or acts on the information generated, it is reasonable to question the usefulness of the data. Therefore, microbiology laboratories, in collaboration with other antimicrobial stewardship team members, must choose wisely what information to report, when to report it, and what information it is no longer necessary to report. Relevant microbiology information, appropriate for inclusion in antimicrobial stewardship team alerts, includes positive results (stain[s], detection, culture, etc.) in critical specimens such as normally sterile fluids (blood, cerebrospinal fluid, etc.); identification of specific pathogens that require rapid intervention, such as C. difficile or M. tuberculosis; and specific resistant patterns, such as carbapenem-resistant Enterobacteriaceae or vancomycin-resistant Enterococcus spp. (172, 173).
Critical Specimens
As a patient safety measure, alerts to prescribers concerning positive results obtained with critical specimens are usually handled by protocols in microbiology laboratories. Antimicrobial stewardship interventions performed when these results become available may impact clinical outcomes (115). The value of such alerts has been demonstrated with blood culture results in combination with RDT (35, 127) and for specific pathogens such Candida spp. and S. aureus (174, 175). Pogue et al. evaluated an automated alert system coupled with an antimicrobial stewardship intervention in which pharmacists were alerted in real time when blood cultures turned positive during business hours; they then reviewed charts and provided therapy recommendations. Reviews and recommendations were delayed to the next weekday morning when blood cultures turned positive at night or on weekends. Compared to historical controls where only prescribers were alerted, they found a significantly reduced time to appropriate therapy, length of stay, and infection-related mortality rate in patients with bacteremia (176). Similar results were also found in different settings without automated alert systems (177). Microbiology laboratories can and should participate in developing enhanced alert protocols for high-risk infections that facilitate timely treatment recommendations.
Of note, nurses—because they frequently answer the phones—are often the first professionals to be aware of critical microbiology results in both outpatient and inpatient settings, making them an essential link in the chain for the timely administration of optimal therapy (103, 104). Thus, antimicrobial stewardship teams and clinical microbiologists should ensure that nurses are aware of, and educated about, the meaning of these alerts and their implications.
Resistant Pathogens
Detection of resistance mechanisms in clinical laboratories is controversial. For example, the CLSI does not require screening for extended-spectrum β-lactamases since breakpoints for cephalosporins were lowered in 2010, unless it is required for epidemiological purposes (89, 90). The CLSI lists intrinsic resistance and suggestions for confirmation when uncommon or concerning phenotypes are detected (90). Unusual resistance usually requires investigation and repeat testing to confirm results and exclude clerical, technical, or contamination errors (90). Laboratories should send key resistance isolates for confirmation and alert public health authorities promptly when such results are suspected. It is also necessary to inform clinicians about possible delays in result reporting and advise on alternative therapies while results are pending.
To stop or slow down the emergence of resistance is a goal of antimicrobial stewardship activities. Tracking rates of resistance can also be useful to demonstrate successes of antimicrobial stewardship programs; though antimicrobial consumption metrics are preferred by recent guidelines, resistance trends provide evidence of patient care impact (42). Antimicrobial resistance rates are impacted by multiple factors, including population factors, immunosuppression, infection control measures, use of antibiotics outside the inpatient setting, and others (178). Elligsen et al. (20), in a controlled interrupted time series analysis of audit and feedback for the use of broad-spectrum antibiotics in intensive care units, showed a modest but significant increase in meropenem susceptibility in Gram-negative isolates after the intervention from 78.2 to 83.4% over a 1-year period. To do so, they included the first isolate of each patient in the study period but also included repeated inpatient isolates if patterns of susceptibility to broad-spectrum agents varied, to avoid omitting hospital-acquired strains. Others have focused on specific resistant pathogens, such as vancomycin-resistant Enterococcus spp. and methicillin-resistant S. aureus (MRSA) (179) or specific pathogen-antimicrobial combinations (21). Laxminarayan and Klugman developed a drug resistance index that aggregated resistance to multiple antibiotics into an index similar to stock markets and is intended for much larger populations than hospitals (180). Such disparate efforts and the current absence of standardized methods illustrate the challenges in assessing the impacts of antimicrobial stewardship interventions on antimicrobial resistance (181).
IT systems used in microbiology laboratories are diverse, with multiple systems often in use in a single facility. Some creative IT solutions for advanced clinical decision support systems and reporting algorithms developed more than 20 years ago have shown success (182). Along with electronic health records, multiple platforms are now available to help antimicrobial stewardship teams and were reviewed recently (183). These programs harness extensive information sources (patient information, pharmacy, and microbiology and other laboratories, etc.) to support a large array of tasks from clinical diagnosis to choice of therapy and may support antimicrobial stewardship strategies, such as audit and feedback, formulary authorizations, clinical pathways, and de-escalation protocols. Timely integration of results is thus extremely important to ensure the attainment of their full potential. The popularity of these data mining tools is increasing, especially in larger institutions, but complex and rapid evolution of resistance patterns and clinical standards necessitates frequent evaluation and updating of these systems, whose high costs and resource intensity also impede widespread use (91, 184). Using clinical decision support systems to their full potential may require time to develop meaningful and actionable alerts, and the clinical microbiologist is essential to the successful use of advanced IT applications for antimicrobial stewardship programs. These alerts might be the most useful for critical results such as positive blood cultures or when a mismatch between ongoing therapy and a susceptibility report is detected. This may, however, require more complex programming and interfacing, thus emphasizing the role of IT personnel in antimicrobial stewardship teams.
MICROBIOLOGY AT SEA: SAILING INTO THE SUNSET OR RETURNING TO HARBOR?
Clinical microbiology seems at a crossroads. On the one hand, questions about the cost-effectiveness and clinical utility of traditional microbiological methods and the pressure to cut costs have led to the outsourcing of many hospital microbiology laboratories to off-site commercial or centralized laboratories, thereby increasing the isolation of clinical microbiologists from their clinician colleagues and consigning them to increasingly technical roles (64, 185). On the other, the crisis of antimicrobial resistance and the resultant need to optimize clinical infection management, manifested by the widespread emergence of antimicrobial stewardship programs (186), and the recent proliferation of innovative rapid diagnostic methods—with their attendant uncertainties with respect to instrument selection, performance characteristics, deployment and work flow, costs, interpretive guidance, and rapid technological turnover—could boost the clinical relevance of clinical microbiologists to levels unseen since the time of Koch and Pasteur. As clinicians long immersed in the practice and teaching of ID and antimicrobial stewardship, we are certain that this distancing of microbiology laboratories has deprived bedside medicine of a critical source of nuanced expertise, and we urge clinical microbiologists to seize emerging opportunities to reassert themselves in patient care. In part, this review is intended to offer a roadmap by which this can occur as part of antimicrobial stewardship program development.
Biographies

Philippe Morency-Potvin obtained his medical degree from the Université Laval in Québec City in 2009. He completed his training in medical microbiology and ID at the Université de Montréal, where he graduated in 2014. He is currently in a 2-year fellowship in antimicrobial stewardship at the Rush University Medical Center and the John H. Stroger, Jr. Hospital of Cook County in Chicago under the supervision of Dr. David Schwartz. Upon completion in 2017, he will return to the Centre Hospitalier de l'Université de Montréal, where he will practice as a microbiologist and ID physician and will lead the institution's antimicrobial stewardship team.

David N. Schwartz, since completing his training in 1993, has worked as an attending physician at Cook County Hospital (now the John H. Stroger, Jr. Hospital of Cook County) in Chicago, where he became the chair of the Division of Infectious Diseases in 2008, and he has been on the faculty of nearby Rush Medical College. Dr. Schwartz has chaired the Cook County Hospital Anti-Infective Committee and supervised its antimicrobial stewardship efforts since 1994. He and his colleagues have collaborated on research with the U.S. CDC since 1999, focusing on the design and implementation of interventions to improve antimicrobial use and to refine antimicrobial measurement in hospitals. He has presented research and spoken on these topics at national meetings and has served as a consultant to antimicrobial stewardship collaboratives sponsored by the Illinois Department of Public Health since 2011.

Robert A. Weinstein is the C. Anderson Hedberg Professor of Medicine, Rush Medical Center, the Chairman of Medicine Emeritus, Cook County Hospitals System, and the Founding Chief Operating Officer, Ruth Rothstein CORE Center—all in Chicago. Dr. Weinstein's research interests include healthcare-associated infections, antimicrobial resistance, and infections in intensive care units. Dr. Weinstein is past president of the SHEA and past chair of the CDC Healthcare Infection Control Practices Advisory Committee. Dr. Weinstein currently serves on the CDC Board of Scientific Counselors and on the Presidential Advisory Council Combating Antimicrobial-Resistant Bacteria.
REFERENCES
- 1.Gaynes RP. 2011. Antony van Leeuwenhoek and the birth of microscopy, p 63–77. In Gaynes RP. (ed), Germ theory: medical pioneers in infectious diseases. ASM Press, Washington, DC. [Google Scholar]
- 2.Center for the History of Microbiology/ASM Archives. 2016. Significant events in microbiology 1861-1999. American Society for Microbiology, Washington, DC: https://www.asm.org/index.php/choma2/71-membership/archives/7852-significant-events-in-microbiology-since-1861 Accessed 24 June 2016. [Google Scholar]
- 3.Isenberg HD. 2003. Clinical microbiology: past, present, and future. J Clin Microbiol 41:917–918. doi: 10.1128/JCM.41.3.917-918.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGann P, Snesrud E, Maybank R, Corey B, Ong AC, Clifford R, Hinkle M, Whitman T, Lesho E, Schaecher KE. 2016. Escherichia coli harboring mcr-1 and blaCTX-M on a novel IncF plasmid: first report of mcr-1 in the United States. Antimicrob Agents Chemother 60:4420–4421. doi: 10.1128/AAC.01103-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2014. Core elements of hospital antibiotic stewardship programs. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/getsmart/healthcare/implementation/core-elements.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lepper MH. 1955. Microbial resistance to antibiotics. Ann Intern Med 43:299–315. doi: 10.7326/0003-4819-43-2-299. [DOI] [PubMed] [Google Scholar]
- 7.Dellit TH, Owens RC, McGowan JE, Gerding DN, Weinstein RA, Burke JP, Huskins WC, Paterson DL, Fishman NO, Carpenter CF, Brennan PJ, Billeter M, Hooton TM. 2007. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 8.Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, Pediatric Infectious Diseases Society. 2012. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol 33:322–327. doi: 10.1086/665010. [DOI] [PubMed] [Google Scholar]
- 9.Australian Commission on Safety and Quality in Health Care. 2011. Antimicrobial stewardship in Australian hospitals, January 2011 ed. Australian Commission on Safety and Quality in Health Care, Sydney, NSW, Australia: https://www.safetyandquality.gov.au/our-work/healthcare-associated-infection/antimicrobial-stewardship/book/. [Google Scholar]
- 10.Gerding DN. 2001. The search for good antimicrobial stewardship. Jt Comm J Qual Improv 27:403–404. [DOI] [PubMed] [Google Scholar]
- 11.Joseph J, Rodvold KA. 2008. The role of carbapenems in the treatment of severe nosocomial respiratory tract infections. Expert Opin Pharmacother 9:561–575. doi: 10.1517/14656566.9.4.561. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz DN. 2016. Editorial commentary: antimicrobial stewardship in US hospitals: is the cup half-full yet? Clin Infect Dis 63:450–453. doi: 10.1093/cid/ciw325. [DOI] [PubMed] [Google Scholar]
- 13.Davey P, Brown E, Charani E, Fenelon L, Gould IM, Holmes A, Ramsay CR, Wiffen PJ, Wilcox M. 2013. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 4:CD003543. doi: 10.1002/14651858.CD003543.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Schuts EC, Hulscher MEJL, Mouton JW, Verduin CM, Stuart JWT, Overdiek HWPM, van der Linden PD, Natsch S, Hertogh CMPM, Wolfs TFW, Schouten JA, Kullberg B, Prins JM. 2016. Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis 16:847–856. doi: 10.1016/S1473-3099(16)00065-7. [DOI] [PubMed] [Google Scholar]
- 15.Society for Healthcare Epidemiology of America, Infectious Diseases Society of America. 2014. SHEA/IDSA letter to CMS advancing antimicrobial stewardship as a condition of participation. Infectious Diseases Society of America and Society for Healthcare Epidemiology of America, Arlington, VA: https://www.shea-online.org/images/letters/SHEA_IDSA_ASasCoP.pdf. [Google Scholar]
- 16.Brumley PE, Malani AN, Kabara JJ, Pisani J, Collins CD. 2016. Effect of an antimicrobial stewardship bundle for patients with Clostridium difficile infection. J Antimicrob Chemother 71:836–840. doi: 10.1093/jac/dkv404. [DOI] [PubMed] [Google Scholar]
- 17.Dancer SJ, Kirkpatrick P, Corcoran DS. 2013. Approaching zero: temporal effects of a restrictive antibiotic policy on hospital-acquired Clostridium difficile, extended-spectrum β-lactamase-producing coliforms and methicillin-resistant Staphylococcus aureus. Int J Antimicrob Agents 41:137–142. doi: 10.1016/j.ijantimicag.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Valiquette L, Cossette B, Garant MP, Diab H, Pépin J. 2007. Impact of a reduction in the use of high-risk antibiotics on the course of an epidemic of Clostridium difficile-associated disease caused by the hypervirulent NAP1/027 strain. Clin Infect Dis 45(Suppl 2):S112–S121. doi: 10.1086/519258. [DOI] [PubMed] [Google Scholar]
- 19.Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Signal B. 2013. Clinical and economic outcomes from a community hospital's antimicrobial stewardship program. Am J Infect Control 41:145–148. doi: 10.1016/j.ajic.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Elligsen M, Walker SAN, Pinto R, Simor A, Mubareka S, Rachlis A, Allen V, Daneman N. 2012. Audit and feedback to reduce broad-spectrum antibiotic use among intensive care unit patients: a controlled interrupted time series analysis. Infect Control Hosp Epidemiol 33:354–361. doi: 10.1086/664757. [DOI] [PubMed] [Google Scholar]
- 21.Rahal JJ, Urban C, Horn D, Freeman K, Segal-Maurer S, Maurer J, Mariano N, Marks S, Burns JM, Dominick D, Lim M. 1998. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA 280:1233–1237. doi: 10.1001/jama.280.14.1233. [DOI] [PubMed] [Google Scholar]
- 22.Dortch MJ, Fleming SB, Kauffmann RM, Dossett LA, Talbot TR, May AK. 2011. Infection reduction strategies including antibiotic stewardship protocols in surgical and trauma intensive care units are associated with reduced resistant Gram-negative healthcare-associated infections. Surg Infect (Larchmt) 12:15–25. doi: 10.1089/sur.2009.059. [DOI] [PubMed] [Google Scholar]
- 23.Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. 2000. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit: a proposed solution for indiscriminate antibiotic prescription. Am J Respir Care Med 162:505–511. doi: 10.1164/ajrccm.162.2.9909095. [DOI] [PubMed] [Google Scholar]
- 24.White AC, Atmar RL, Wilson J, Cate TR, Stager CE, Greenberg SB. 1997. Effects of requiring prior authorization for selected antimicrobials: expenditures, susceptibilities, and clinical outcomes. Clin Infect Dis 25:230–239. doi: 10.1086/514545. [DOI] [PubMed] [Google Scholar]
- 25.Dager WE, King JH. 2006. Aminoglycosides in intermittent hemodialysis: pharmacokinetics with individual dosing. Ann Pharmacother 40:9–14. doi: 10.1345/aph.1G064. [DOI] [PubMed] [Google Scholar]
- 26.Bartal C, Danon A, Schlaeffer F, Reisenberg K, Alkan M, Smoliakov R, Sidi A, Almog Y. 2003. Pharmacokinetic dosing of aminoglycosides: a controlled trial. Am J Med 114:194–198. doi: 10.1016/S0002-9343(02)01476-6. [DOI] [PubMed] [Google Scholar]
- 27.Tamayo E, Gualis J, Flórez S, Castrodeza J, Bouza JM, Alvarez FJ. 2008. Comparative study of single-dose and 24-hour multiple-dose antibiotic prophylaxis for cardiac surgery. J Thorac Cardiovasc Surg 136:1522–1527. doi: 10.1016/j.jtcvs.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez L, Jung HS, Goulet JA, Cicalo A, Machado-Aranda DA, Napolitano LM. 2014. Evidence-based protocol for prophylactic antibiotics in open fractures: improved antibiotic stewardship with no increase in infection rates. J Trauma Acute Care Surg 77:400–407. doi: 10.1097/TA.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 29.Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. 2000. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation 101:2916–2921. doi: 10.1161/01.CIR.101.25.2916. [DOI] [PubMed] [Google Scholar]
- 30.Kullar R, Davis SL, Kaye KS, Levine DP, Pogue JM, Rybak MJ. 2013. Implementation of an antimicrobial stewardship pathway with daptomycin for optimal treatment of methicillin-resistant Staphylococcus aureus bacteremia. Pharmacotherapy 33:3–10. doi: 10.1002/phar.1220. [DOI] [PubMed] [Google Scholar]
- 31.Gross R, Morgan AS, Kinky DE, Weiner M, Gibson GA, Fishman NO. 2001. Impact of a hospital-based antimicrobial management program on clinical and economic outcomes. Clin Infect Dis 33:289–295. doi: 10.1086/321880. [DOI] [PubMed] [Google Scholar]
- 32.Nagel JL, Huang AM, Kunapuli A, Gandhi TN, Washer LL, Lassiter J, Patel T, Newton DW. 2014. Impact of antimicrobial stewardship intervention on coagulase-negative Staphylococcus blood cultures in conjunction with rapid diagnostic testing. J Clin Microbiol 52:2849–2854. doi: 10.1128/JCM.00682-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang AM, Newton D, Kunapuli A, Gandhi TN, Washer LL, Isip J, Collins CD, Nagel JL. 2013. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis 57:1237–1245. doi: 10.1093/cid/cit498. [DOI] [PubMed] [Google Scholar]
- 34.Tamma PD, Tan K, Nussenblatt VR, Turnbull AE, Carroll KC, Cosgrove SE. 2013. Can matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) enhance antimicrobial stewardship efforts in the acute care setting? Infect Control Hosp Epidemiol 34:990–995. doi: 10.1086/671731. [DOI] [PubMed] [Google Scholar]
- 35.Banerjee R, Teng CB, Cunningham SA, Ihde SM, Steckelberg JM, Moriarty JP, Shah ND, Mandrekar JN, Patel R. 2015. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis 61:1071–1080. doi: 10.1093/cid/civ447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sick AC, Lehmann CU, Tamma PD, Lee CK, Agwu L. 2013. Sustained savings from a longitudinal cost analysis of an internet-based preapproval antimicrobial stewardship program. Infect Control Hosp Epidemiol 34:573–580. doi: 10.1086/670625. [DOI] [PubMed] [Google Scholar]
- 37.Pate PG, Storey DF, Baum DL. 2012. Implementation of an antimicrobial stewardship program at a 60-bed long-term acute care hospital. Infect Control Hosp Epidemiol 33:405–408. doi: 10.1086/664760. [DOI] [PubMed] [Google Scholar]
- 38.Carling P, Fung T, Killion A, Terrin N, Barza M. 2003. Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect Control Hosp Epidemiol 24:699–706. doi: 10.1086/502278. [DOI] [PubMed] [Google Scholar]
- 39.Standiford HC, Chan S, Tripoli M, Weekes E, Forrest GN. 2012. Antimicrobial stewardship at a large tertiary care academic medical center: cost analysis before, during, and after a 7-year program. Infect Control Hosp Epidemiol 33:338–345. doi: 10.1086/664909. [DOI] [PubMed] [Google Scholar]
- 40.LaRocco A., Jr 2003. Concurrent antibiotic review programs—a role for infectious diseases specialists at small community hospitals. Clin Infect Dis 37:742–743. doi: 10.1086/377286. [DOI] [PubMed] [Google Scholar]
- 41.Day SR, Smith D, Harris K, Cox HL, Mathers AJ. 2015. An infectious diseases physician-led antimicrobial stewardship program at a small community hospital associated with improved susceptibility patterns and cost-savings after the first year. Open Forum Infect Dis 2:ofv064. doi: 10.1093/ofid/ofv064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, Srinivasan A, Dellit TH, Falck-Ytter YT, Fishman NO, Hamilton CW, Jenkins TC, Lipsett PA, Malani PN, May LS, Moran GJ, Neuhauser MM, Newland JG, Ohl CA, Samore MH, Seo SK, Trivedi KK. 2016. Executive summary: implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 62:1197–1202. doi: 10.1093/cid/ciw217. [DOI] [PubMed] [Google Scholar]
- 43.Pollack LA, Srinivasan A. 2014. Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin Infect Dis 59(Suppl 3):S97–S100. doi: 10.1093/cid/ciu542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The White House. 2015. National action plan for combating antibiotic-resistant bacteria. The White House, Washington, DC: https://www.whitehouse.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. [Google Scholar]
- 45.Weiner LM, Fridkin SK, Aponte-Torres Z, Avery L, Coffin N, Dudeck MA, Edwards JR, Jernigan JA, Konnor R, Soe MM, Peterson K, McDonald LC. 2016. Vital signs: preventing antibiotic-resistant infections in hospitals—United States, 2014. MMWR Morb Mortal Wkly Rep 65:235–241. doi: 10.15585/mmwr.mm6509e1. [DOI] [PubMed] [Google Scholar]
- 46.Miller M. 2015. The White House forum on antibiotic stewardship impacts labs across the U.S. MLO Med Lab Obs 47:28. [PubMed] [Google Scholar]
- 47.Joint Commission on Hospital Accreditation. 2016. Approved: new antimicrobial stewardship standard. Jt Comm Perspect 36:1, 3–4, 8. [PubMed] [Google Scholar]
- 48.Accreditation Canada. 2016. Required organizational practices handbooks. Accreditation Canada, Gloucester, ON, Canada: https://accreditation.ca/rop-handbooks Accessed 13 September 2016. [Google Scholar]
- 49.Australian Commission on Safety and Quality in Health Care. 2013. National safety and quality health service standard 3. Australian Commission on Safety and Quality in Health Care, Sydney, NSW, Australia: https://www.safetyandquality.gov.au/our-work/healthcare-associated-infection/antimicrobial-stewardship/ Accessed 13 September 2016. [Google Scholar]
- 50.National Institute for Health and Care Excellence. 2016. Antimicrobial stewardship quality standards (QS121). National Institute for Health and Care Excellence, London, United Kingdom: https://www.nice.org.uk/guidance/qs121 Accessed 13 September 2016. [Google Scholar]
- 51.Stauffer E. 2016. Weekly digest: world leaders meet for UN General Assembly high-level meeting on antimicrobial resistance; alliance to support UN resolution against antimicrobial resistance formed. The Center for Disease Dynamics, Economics & Policy, Washington, DC: http://www.cddep.org/blog/posts/weekly_digest_world_leaders_meet_united_nations_general_assembly_high_level_meeting#sthash.eeZ9mUQb.3Cm4NdHh.dpbs Accessed 26 September 2016. [Google Scholar]
- 52.Kim J, Craft DW, Katzman M. 2015. Building an antimicrobial stewardship program: cooperative roles for pharmacists, infectious diseases specialists, and clinical microbiologists. Lab Med 46:e65–71. doi: 10.1309/LMC0SHRJBY0ONHI9. [DOI] [PubMed] [Google Scholar]
- 53.Howard P, Pulcini C, Levy Hara G, West RM, Gould IM, Harbarth S, Nathwani D, ESCMID Study Group for Antimicrobial Policies (ESGAP); ISC Group on Antimicrobial Stewardship. 2015. An international cross-sectional survey of antimicrobial stewardship programmes in hospitals. J Antimicrob Chemother 70:1245–1255. doi: 10.1093/jac/dku497. [DOI] [PubMed] [Google Scholar]
- 54.MacKenzie FM, Gould IM, Bruce J, Mollison J, Monnet DL, Krcmery V, Cookson B, van der Meer JW. 2007. The role of microbiology and pharmacy departments in the stewardship of antibiotic prescribing in European hospitals. J Hosp Infect 65(Suppl 2):S73–S81. [DOI] [PubMed] [Google Scholar]
- 55.Ashiru-Oredope D, Sharland M, Charani E, McNulty C, Cooke J, ARHAI Antimicrobial Stewardship Group. 2012. Improving the quality of antibiotic prescribing in the NHS by developing a new antimicrobial stewardship programme: start smart—then focus. J Antimicrob Chemother 67(Suppl 1):i51–i63. doi: 10.1093/jac/dks202. [DOI] [PubMed] [Google Scholar]
- 56.Fleming A, Tonna A, O'Connor S, Byrne S, Stewart D. 2014. A cross-sectional survey of the profile and activities of antimicrobial management teams in Irish hospitals. Int J Clin Pharm 36:377–383. doi: 10.1007/s11096-013-9907-4. [DOI] [PubMed] [Google Scholar]
- 57.James RS, McIntosh KA, Luu SB, Cotta MO, Marshall C, Thursky KA, Buising KL. 2013. Antimicrobial stewardship in Victorian hospitals: a statewide survey to identify current gaps. Med J Aust 199:692–695. doi: 10.5694/mja13.10422. [DOI] [PubMed] [Google Scholar]
- 58.Avent ML, Hall L, Davis L, Allen M, Roberts JA, Unwin S, McIntosh KA, Thursky K, Buising K, Paterson DL. 2014. Antimicrobial stewardship activities: a survey of Queensland hospitals. Aust Health Rev 38:557–563. doi: 10.1071/AH13137. [DOI] [PubMed] [Google Scholar]
- 59.Nault V, Beaudoin M, Thirion D, Gosselin M, Cossette B, Valiquette L. 2008. Antimicrobial stewardship in acute care centres: a survey of 68 hospitals in Quebec. Can J Infect Dis Med Microbiol 19:237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trivedi KK, Dumartin C, Gilchrist M, Wade P, Howard P. 2014. Identifying best practices across three countries: hospital antimicrobial stewardship in the United Kingdom, France, and the United States. Clin Infect Dis 59(Suppl 3):S170–S178. doi: 10.1093/cid/ciu538. [DOI] [PubMed] [Google Scholar]
- 61.Trivedi KK, Rosenberg J. 2013. The state of antimicrobial stewardship programs in California. Infect Control Hosp Epidemiol 34:379–384. doi: 10.1086/669876. [DOI] [PubMed] [Google Scholar]
- 62.Abbo L, Lo K, Sinkowitz-Cochran R, Burke A, Hopkins RS, Srinivasan A, Hooton TM. 2013. Antimicrobial stewardship programs in Florida's acute care facilities. Infect Control Hosp Epidemiol 34:634–637. doi: 10.1086/670632. [DOI] [PubMed] [Google Scholar]
- 63.Doron S, Nadkarni L, Price L, Lawrence P, Davidson LE, Evans J, Garber C, Snydman DR. 2013. A nationwide survey of antimicrobial stewardship practices. Clin Ther 35:758–765.e20. doi: 10.1016/j.clinthera.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 64.Dancer SJ, Varon-Lopez C, Moncayo O, Elston A, Humphreys H. 2015. Microbiology service centralization: a step too far. J Hosp Infect 91:292–298. doi: 10.1016/j.jhin.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomson RB., Jr 1995. The changing role of the clinical microbiology laboratory director results of a survey. Diagn Microbiol Infect Dis 23:45–51. doi: 10.1016/0732-8893(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 66.Filice GA, Drekonja DM, Thurn JR, Hamann GM, Masoud BT, Johnson JR. 2015. Diagnostic errors that lead to inappropriate antimicrobial use. Infect Control Hosp Epidemiol 36:949–956. doi: 10.1017/ice.2015.113. [DOI] [PubMed] [Google Scholar]
- 67.Weinstein MP. 2010. Positive blood cultures. Clin Adv Hematol Oncol 8:850–851. [PubMed] [Google Scholar]
- 68.Clinical and Laboratory Standards Institute. 2011. QMS01-A4 quality management system: a model for laboratory services; approved guideline—fourth edition. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 69.World Health Organization. 2011. Laboratory quality management system handbook. World Health Organization, Lyon, France: http://apps.who.int/iris/bitstream/10665/44665/1/9789241548274_eng.pdf. [Google Scholar]
- 70.Clinical and Laboratory Standards Institute. 2014. M39-A4 analysis and presentation of cumulative antimicrobial susceptibility test data; approved guideline—fourth edition. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 71.Sinai Health Systems. 2016. Antibiograms. Mount Sinai Hospital, Toronto, ON, Canada: http://www.mountsinai.on.ca/education/staff-professionals/microbiology/microbiology-laboratory-manual/antibiogram/copy_of_department-of-microbiology Accessed 13 September 2016. [Google Scholar]
- 72.University of Washington. 2016. UW school of medicine antibiograms. University of Washington, Seattle, WA: https://hsl.uw.edu/toolkits/care-provider/uw-school-of-medicine-antibiograms/ Accessed 13 September 2016. [Google Scholar]
- 73.University of Alberta. 2016. Antibiograms. University of Alberta, Edmonton, AB, Canada: http://www.antibiogram.ca/ Accessed 13 September 2016. [Google Scholar]
- 74.Ernst EJ, Diekema DJ, BootsMiller BJ, Vaughn T, Yankey JW, Flach SD, Ward MM, Franciscus CLJ, Acosta E, Pfaller MA, Doebbeling BN. 2004. Are United States hospitals following national guidelines for the analysis and presentation of cumulative antimicrobial susceptibility data? Diagn Microbiol Infect Dis 49:141–145. doi: 10.1016/j.diagmicrobio.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 75.Zapantis A, Lacy MK, Horvat RT, Grauer D, Barnes BJ, O'Neal B, Couldry R. 2005. Nationwide antibiogram analysis using NCCLS M39-A guidelines. J Clin Microbiol 43:2629–2634. doi: 10.1128/JCM.43.6.2629-2634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boehme MS, Somsel PA, Downes FP. 2010. Systematic review of antibiograms: a national laboratory system approach for improving antimicrobial susceptibility testing practices in Michigan. Public Health Rep 125(Suppl 2):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moehring RW, Hazen KC, Hawkins MR, Drew RH, Sexton DJ, Anderson DJ. 2015. Challenges in preparation of cumulative antibiogram reports for community hospitals. J Clin Microbiol 53:2977–2982. doi: 10.1128/JCM.01077-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mizuta M, Linkin DR, Nachamkin I, Fishman NO, Weiner MG, Sheridan A, Lautenbach E. 2006. Identification of optimal combinations for empirical dual antimicrobial therapy of Pseudomonas aeruginosa infection: potential role of a combination antibiogram. Infect Control Hosp Epidemiol 27:413–415. doi: 10.1086/503175. [DOI] [PubMed] [Google Scholar]
- 79.Var SK, Hadi R, Khardori NM. 2015. Evaluation of regional antibiograms to monitor antimicrobial resistance in Hampton Roads, Virginia. Ann Clin Microbiol Antimicrob 14:22. doi: 10.1186/s12941-015-0080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hawaii Antimicrobial Stewardship Collaborative. 2014. 2014 Hawaii statewide antibiogram for selected bacteria of public health significance. Hawaii State Department of Health, Honolulu, HI: http://health.hawaii.gov/docd/files/2015/10/2014_Statewide_Antibiogram_Final_Report.pdf. [Google Scholar]
- 81.Schwartz DN, Abiad H, DeMarais PL, Armeanu E, Trick WE, Wang Y, Weinstein RA. 2007. An educational intervention to improve antimicrobial use in a hospital-based long-term care facility. J Am Geriatr Soc 55:1236–1242. doi: 10.1111/j.1532-5415.2007.01251.x. [DOI] [PubMed] [Google Scholar]
- 82.Turnridge JD, Ferraro MJ, Jorgensen JH. 2011. Susceptibility test methods: general considerations, p 1115–1121. In Versalovic JCK, Jorgensen JH, Funke G, Landry ML, Warnock DW (ed), Manual of clinical microbiology, vol 2 ASM Press, Washington, DC. [Google Scholar]
- 83.Diekema DJ, Lee K, Raney P, Herwaldt LA, Doern GV, Tenover FC. 2004. Accuracy and appropriateness of antimicrobial susceptibility test reporting for bacteria isolated from blood cultures. J Clin Microbiol 42:2258–2260. doi: 10.1128/JCM.42.5.2258-2260.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tenover F, Hindler J. 2010. Reporting of the results, p 89–99. In Courvalin P, Leclerq R, Rice LB (ed), Antibiogram. ASM Press, Washington, DC. [Google Scholar]
- 85.Tan T, McNulty C, Charlett A, Nessa N, Kelly C, Beswick T. 2003. Laboratory antibiotic susceptibility reporting and antibiotic prescribing in general practice. J Antimicrob Chemother 51:379–384. doi: 10.1093/jac/dkg032. [DOI] [PubMed] [Google Scholar]
- 86.Cunney R, Aziz HA, Schubert D, McNamara E, Smyth E. 2000. Interpretative reporting and selective antimicrobial susceptibility release in non-critical microbiology results. J Antimicrob Chemother 45:705–708. doi: 10.1093/jac/45.5.705. [DOI] [PubMed] [Google Scholar]
- 87.McNulty CAM, Lasseter GM, Charlett A, Lovering A, Howell-Jones R, MacGowan A, Thomas M. 2011. Does laboratory antibiotic susceptibility reporting influence primary care prescribing in urinary tract infection and other infections? J Antimicrob Chemother 66:1396–1404. doi: 10.1093/jac/dkr088. [DOI] [PubMed] [Google Scholar]
- 88.Schreckenberger PC, Binnicker MJ. 2011. Optimizing antimicrobial susceptibility test reporting. J Clin Microbiol 49(Suppl):S15–S19. doi: 10.1128/JCM.00712-11. [DOI] [Google Scholar]
- 89.Heil EL, Johnson KJ. 2016. Impact of CLSI breakpoint changes on microbiology laboratories and antimicrobial stewardship programs. J Clin Microbiol 54:840–844. doi: 10.1128/JCM.02424-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clinical and Laboratory Standards Institute. 2015. M100-S25 performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 91.Rhoads DD, Sintchenko V, Rauch CA, Pantanowitz L. 2014. Clinical microbiology informatics. Clin Microbiol Rev 27:1025–1047. doi: 10.1128/CMR.00049-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bruins MJ, Ruijs G, Wolfhagen M, Bloembergen P, Aarts J. 2011. Does electronic clinical microbiology results reporting influence medical decision making: a pre- and post-interview study of medical specialists. BMC Med Inform Decis Mak 11:19. doi: 10.1186/1472-6947-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Talbot GH, Bradley J, Edwards JE, Gilbert D, Scheld M, Bartlett JG, Antimicrobial Availability Task Force of the Infectious Diseases Society of America. 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis 42:657–668. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 94.Food and Drug Administration. 1985. New drug and antibiotic regulations. Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/ScienceResearch/SpecialTopics/RunningClinicalTrials/ucm120020.htm. [Google Scholar]
- 95.Marchese A, Esposito S, Barbieri R, Bassetti M, Debbia E. 2012. Does the adoption of EUCAST susceptibility breakpoints affect the selection of antimicrobials to treat acute community-acquired respiratory tract infections? BMC Infect Dis 12:181. doi: 10.1186/1471-2334-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wolfensberger A, Sax H, Weber R, Zbinden R, Kuster SP, Hombach M. 2013. Change of antibiotic susceptibility testing guidelines from CLSI to EUCAST: influence on cumulative hospital antibiograms. PLoS One 8:e79130. doi: 10.1371/journal.pone.0079130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ginocchio CC. 2002. Role of NCCLS in antimicrobial susceptibility testing and monitoring. Am J Health Syst Pharm 59(8 Suppl 3):S7–S11. [DOI] [PubMed] [Google Scholar]
- 98.Humphries RM, Hindler JA. 2016. Emerging resistance, new antimicrobial agents…but no tests! The challenge of antimicrobial susceptibility testing in the current US regulatory landscape. Clin Infect Dis 63:83–88. doi: 10.1093/cid/ciw201. [DOI] [PubMed] [Google Scholar]
- 99.Baron EJ, Miller JM, Weinstein MP, Richter SS, Gilligan PH, Thomson RB, Bourbeau P, Carroll KC, Kehl SC, Dunne WM, Robinson-Dunn B, Schwartzman JD, Chapin KC, Snyder JW, Forbes BA, Patel R, Rosenblatt JE, Pritt BS. 2013. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin Infect Dis 57:e22–e121. doi: 10.1093/cid/cit278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Johns Hopkins Medicine. 2015. Johns Hopkins medical microbiology specimen collection guidelines—updated 6/2016. Johns Hopkins Medicine, Baltimore, MD: http://www.hopkinsmedicine.org/microbiology/specimen/Specimen_Collection_Guidelines_2016.pdf. [Google Scholar]
- 101.Humphries RM, Bard J. 2016. Point-counterpoint: reflex cultures reduce laboratory workload and improve antimicrobial stewardship in patients suspected of having urinary tract infections. J Clin Microbiol 54:254–258. doi: 10.1128/JCM.03021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Petty LA, Ridgway JP, Pettit NN, Charnot-Katsikas A, Tesic V, Beavis KG, Pisano J. 2015. Effects of the implementation of reflexive urine cultures on antibiotic utilization in hospitalized patients, poster 1497. ID Week 2015, San Diego, CA: http://ofid.oxfordjournals.org/content/2/suppl_1/1497.full. [Google Scholar]
- 103.Olans RN, Olans RD, DeMaria A. 2016. The critical role of the staff nurse in antimicrobial stewardship—unrecognized, but already there. Clin Infect Dis 62:84–89. doi: 10.1093/cid/civ697. [DOI] [PubMed] [Google Scholar]
- 104.Edwards R, Drumright L, Kiernan M, Holmes A. 2011. Covering more territory to fight resistance: considering nurses' role in antimicrobial stewardship. J Infect Prev 12:6–10. doi: 10.1177/1757177410389627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Infectious Diseases Society of America. 2011. An unmet medical need: rapid molecular diagnostics tests for respiratory tract infections. Clin Infect Dis 52(Suppl 4):S384–S395. doi: 10.1093/cid/cir055. [DOI] [PubMed] [Google Scholar]
- 106.Chan T, Gu F. 2011. Early diagnosis of sepsis using serum biomarkers. Expert Rev Mol Diagn 11:487–496. doi: 10.1586/erm.11.26. [DOI] [PubMed] [Google Scholar]
- 107.Gilbert DN. 2010. Use of plasma procalcitonin levels as an adjunct to clinical microbiology. J Clin Microbiol 48:2325–2329. doi: 10.1128/JCM.00655-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. 2013. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis 13:426–435. doi: 10.1016/S1473-3099(12)70323-7. [DOI] [PubMed] [Google Scholar]
- 109.Schuetz P, Müller B, Christ-Crain M, Stolz D, Tamm M, Bouadma L, Luyt CE, Wolff M, Chastre J, Tubach F, Kristoffersen KB, Burkhardt O, Welte T, Schroeder S, Nobre V, Wei L, Bhatnagar N, Bucher HC, Briel M. 2012. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 8:CD007498. doi: 10.1002/14651858.CD007498.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schuetz P, Chiappa V, Briel M, Greenwald JL. 2011. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med 171:1322–1331. doi: 10.1001/archinternmed.2011.318. [DOI] [PubMed] [Google Scholar]
- 111.Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. 2013. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock—a systematic review and meta-analysis. Critical Care 17:R291. doi: 10.1186/cc13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Trienski TL, File TM. 2015. Implementation of a procalcitonin assay requires appropriate stewardship to result in improved antimicrobial use. Infect Dis Clin Pract 23:1–2. doi: 10.1097/IPC.0000000000000232. [DOI] [Google Scholar]
- 113.Albrich WC, Harbarth S. 2015. Pros and cons of using biomarkers versus clinical decisions in start and stop decisions for antibiotics in the critical care setting. Intensive Care Med 41:1739–1751. doi: 10.1007/s00134-015-3978-8. [DOI] [PubMed] [Google Scholar]
- 114.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 115.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, Dodek P, Wood G, Kumar A, Simon D, Peters C, Ahsan M, Chateau D, Cooperative Antimicrobial Therapy of Septic Shock Database Research Group. 2009. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 136:1237–1248. doi: 10.1378/chest.09-0087. [DOI] [PubMed] [Google Scholar]
- 116.Bauer KA, Perez KK, Forrest GN, Goff DA. 2014. Review of rapid diagnostic tests used by antimicrobial stewardship programs. Clin Infect Dis 59(Suppl 3):S134–S145. doi: 10.1093/cid/ciu547. [DOI] [PubMed] [Google Scholar]
- 117.Buehler SS, Madison B, Snyder SR, Derzon JH, Cornish NE, Saubolle MA, Weissfeld AS, Weinstein MP, Liebow EB, Wolk DM. 2016. Effectiveness of practices to increase timeliness of providing targeted therapy for Inpatients with bloodstream infections: a laboratory medicine best practices systematic review and meta-analysis. Clin Microbiol Rev 29:59–103. doi: 10.1128/CMR.00053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sango A, McCarter YS, Johnson D, Ferreira J, Guzman N, Jankowski CA. 2013. Stewardship approach for optimizing antimicrobial therapy through use of a rapid microarray assay on blood cultures positive for Enterococcus species. J Clin Microbiol 51:4008–4011. doi: 10.1128/JCM.01951-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Avdic E, Carroll KC. 2014. The role of the microbiology laboratory in antimicrobial stewardship programs. Infect Dis Clin North Am 28:215–235. doi: 10.1016/j.idc.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 120.Mahony JB, Petrich A, Smieja M. 2011. Molecular diagnosis of respiratory virus infections. Crit Rev Clinical Lab Sci 48:217–249. doi: 10.3109/10408363.2011.640976. [DOI] [PubMed] [Google Scholar]
- 121.Kothari A, Morgan M, Haake DA. 2014. Emerging technologies for rapid identification of bloodstream pathogens. Clin Infect Dis 59:272–278. doi: 10.1093/cid/ciu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Laub RR, Knudsen JD. 2014. Clinical consequences of using PNA-FISH in staphylococcal bacteraemia. Eur J Clin Microbiol Infect Dis 33:599–601. doi: 10.1007/s10096-013-1990-x. [DOI] [PubMed] [Google Scholar]
- 123.Heil EL, Daniels LM, Long DM, Rodino KG, Weber DJ, Miller MB. 2012. Impact of a rapid peptide nucleic acid fluorescence in situ hybridization assay on treatment of Candida infections. Am J Health Syst Pharm 69:1910–1914. doi: 10.2146/ajhp110604. [DOI] [PubMed] [Google Scholar]
- 124.Forrest GN, Roghmann M-CC, Toombs LS, Johnson JK, Weekes E, Lincalis DP, Venezia RA. 2008. Peptide nucleic acid fluorescent in situ hybridization for hospital-acquired enterococcal bacteremia: delivering earlier effective antimicrobial therapy. Antimicrob Agents Chemother 52:3558–3563. doi: 10.1128/AAC.00283-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Forrest GN, Mehta S, Weekes E, Lincalis DP, Johnson JK, Venezia RA. 2006. Impact of rapid in situ hybridization testing on coagulase-negative staphylococci positive blood cultures. J Antimicrob Chemother 58:154–158. doi: 10.1093/jac/dkl146. [DOI] [PubMed] [Google Scholar]
- 126.Ward C, Stocker K, Begum J, Wade P, Ebrahimsa U, Goldenberg SD. 2015. Performance evaluation of the Verigene® (Nanosphere) and FilmArray® (BioFire®) molecular assays for identification of causative organisms in bacterial bloodstream infections. Eur J Clin Microbiol Infect Dis 34:487–496. doi: 10.1007/s10096-014-2252-2. [DOI] [PubMed] [Google Scholar]
- 127.Southern TR, VanSchooneveld TC, Bannister DL, Brown TL, Crismon AS, Buss SN, Iwen PC, Fey PD. 2015. Implementation and performance of the BioFire FilmArray® Blood Culture Identification panel with antimicrobial treatment recommendations for bloodstream infections at a midwestern academic tertiary hospital. Diagn Microbiol Infect Dis 81:96–101. doi: 10.1016/j.diagmicrobio.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 128.Box MJ, Sullivan EL, Ortwine KN, Parmenter MA, Quigley MM, Aguilar-Higgins LM, MacIntosh CL, Goerke KF, Lim RA. 2015. Outcomes of rapid identification for Gram-positive bacteremia in combination with antibiotic stewardship at a community-based hospital system. Pharmacotherapy 35:269–276. doi: 10.1002/phar.1557. [DOI] [PubMed] [Google Scholar]
- 129.Aitken SL, Hemmige VS, Koo HL, Vuong NN, Lasco TM, Garey KW. 2015. Real-world performance of a microarray-based rapid diagnostic for Gram-positive bloodstream infections and potential utility for antimicrobial stewardship. Diagn Microbiol Infect Dis 81:4–8. doi: 10.1016/j.diagmicrobio.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 130.McAdam AJ. 2000. Discrepant analysis: how can we test a test? J Clin Microbiol 38:2027–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Polage CR, Gyorke CE, Kennedy MA, Leslie JL, Chin DL, Wang S, Nguyen HH, Huang B, Tang Y-W, Lee LW, Kim K, Taylor S, Romano PS, Panacek EA, Goodell PB, Solnick JV, Cohen SH. 2015. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med 175:1792–1801. doi: 10.1001/jamainternmed.2015.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Koo HL, Van JN, Zhao M, Ye X, Revell PA, Jiang Z-DD, Grimes CZ, Koo DC, Lasco T, Kozinetz CA, Garey KW, DuPont HL. 2014. Real-time polymerase chain reaction detection of asymptomatic Clostridium difficile colonization and rising C. difficile-associated disease rates. Infect Control Hosp Epidemiol 35:667–673. doi: 10.1086/676433. [DOI] [PubMed] [Google Scholar]
- 133.Crotty MP, Meyers S, Hampton N, Bledsoe S, Ritchie DJ, Buller RS, Storch GA, Kollef MH, Micek ST. 2015. Impact of antibacterials on subsequent resistance and clinical outcomes in adult patients with viral pneumonia: an opportunity for stewardship. Crit Care 19:404. doi: 10.1186/s13054-015-1120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McCulloh RJ, Koster M, Chapin K. 2013. Respiratory viral testing: new frontiers in diagnostics and implications for antimicrobial stewardship. Virulence 4:1–2. doi: 10.4161/viru.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vallières E, Renaud C. 2013. Clinical and economical impact of multiplex respiratory virus assays. Diagn Microbiol Infect Dis 76:255–261. doi: 10.1016/j.diagmicrobio.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Somerville LK, Ratnamohan MV, Dwyer DE, Kok J. 2015. Molecular diagnosis of respiratory viruses. Pathology 47:243–249. doi: 10.1097/PAT.0000000000000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mahony JB. 2008. Detection of respiratory viruses by molecular methods. Clin Microbiol Rev 21:716–747. doi: 10.1128/CMR.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Timbrook T, Maxam M, Bosso J. 2015. Antibiotic discontinuation rates associated with positive respiratory viral panel and low procalcitonin results in proven or suspected respiratory infections. Infect Dis Ther 4:297–306. doi: 10.1007/s40121-015-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ghazi IM, Nicolau DP, Nailor MD, Aslanzadeh J, Ross JW, Kuti JL. 2016. Antibiotic utilization and opportunities for stewardship among hospitalized patients with influenza respiratory tract infection. Infect Control Hosp Epidemiol 37:583–589. doi: 10.1017/ice.2016.17. [DOI] [PubMed] [Google Scholar]
- 140.Oosterheert JJ, van Loon AM, Schuurman R, Hoepelman AI, Hak E, Thijsen S, Nossent G, Schneider MM, Hustinx WM, Bonten MJ. 2005. Impact of rapid detection of viral and atypical bacterial pathogens by real-time polymerase chain reaction for patients with lower respiratory tract infection. Clin Infect Dis 41:1438–1444. doi: 10.1086/497134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Seng P, Abat C, Rolain J, Colson P, Lagier J-C, Gouriet F, Fournier P, Drancourt M, Scola B, Raoult D. 2013. Identification of rare pathogenic bacteria in a clinical microbiology laboratory: impact of matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 51:2182–2194. doi: 10.1128/JCM.00492-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Martiny D, Busson L, Wybo I, Haj ERA, Dediste A, Vandenberg O. 2012. Comparison of the Microflex LT and Vitek MS systems for routine identification of bacteria by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 50:1313–1325. doi: 10.1128/JCM.05971-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Clark AE, Kaleta EJ, Arora A, Wolk DM. 2013. Matrix-assisted laser desorption ionization–time of flight mass spectrometry: a fundamental shift in the routine practice of clinical microbiology. Clin Microbiol Rev 26:547–603. doi: 10.1128/CMR.00072-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gaillot O, Blondiaux N, Loïez C, Wallet F, Lemaître N, Herwegh S, Courcol RJ. 2011. Cost-effectiveness of switch to matrix-assisted laser desorption ionization–time of flight mass spectrometry for routine bacterial identification. J Clin Microbiol 49:4412–4412. doi: 10.1128/JCM.05429-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vlek AL, Bonten MJ, Boel CH. 2012. Direct matrix-assisted laser desorption ionization time-of-flight mass spectrometry improves appropriateness of antibiotic treatment of bacteremia. PLoS One 7:e32589. doi: 10.1371/journal.pone.0032589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wenzler E, Goff DA, Mangino JE, Reed EE, Wehr A, Bauer KA. 2016. Impact of rapid identification of Acinetobacter baumannii via matrix-assisted laser desorption ionization time-of-flight mass spectrometry combined with antimicrobial stewardship in patients with pneumonia and/or bacteremia. Diagn Microbiol Infect Dis 84:63–68. doi: 10.1016/j.diagmicrobio.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 147.Lockwood AM, Perez KK, Musick WL, Ikwuagwu JO, Attia E, Fasoranti OO, Cernoch PL, Olsen RJ, Musser JM. 2016. Integrating rapid diagnostics and antimicrobial stewardship in two community hospitals improved process measures and antibiotic adjustment time. Infect Control Hosp Epidemiol 37:425–432. doi: 10.1017/ice.2015.313. [DOI] [PubMed] [Google Scholar]
- 148.Fay D, Oldfather JE. 1979. Standardization of direct susceptibility test for blood cultures. J Clin Microbiol 9:347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Waites KB, Brookings ES, Moser SA, Zimmer BL. 1998. Direct susceptibility testing with positive BacT/Alert blood cultures by using MicroScan overnight and rapid panels. J Clin Microbiol 36:2052–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ling TK, Liu ZK, Cheng AF. 2003. Evaluation of the VITEK 2 system for rapid direct identification and susceptibility testing of Gram-negative bacilli from positive blood cultures. J Clin Microbiol 41:4705–4707. doi: 10.1128/JCM.41.10.4705-4707.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Trenholme GM, Kaplan RL, Karakusis PH, Stine T, Fuhrer J, Landau W, Levin S. 1989. Clinical impact of rapid identification and susceptibility testing of bacterial blood culture isolates. J Clin Microbiol 27:1342–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kalil AC, Van Schooneveld TC, Fey PD, Rupp ME. 2014. Association between vancomycin minimum inhibitory concentration and mortality among patients with Staphylococcus aureus bloodstream infections: a systematic review and meta-analysis. JAMA 312:1552–1564. doi: 10.1001/jama.2014.6364. [DOI] [PubMed] [Google Scholar]
- 153.Ross JL, Rankin S, Marshik P, Mercier R-CC, Brett M, Walraven CJ. 2015. Antimicrobial stewardship intervention and feedback to infectious disease specialists: a case study in high-dose daptomycin. Antibiotics (Basel) 4:309–320. doi: 10.3390/antibiotics4030309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.van Belkum A, Dunne WM. 2013. Next-generation antimicrobial susceptibility testing. J Clin Microbiol 51:2018–2024. doi: 10.1128/JCM.00313-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Douglas IS, Price CS, Overdier KH, Wolken RF, Metzger SW, Hance KR, Howson DC. 2015. Rapid automated microscopy for microbiological surveillance of ventilator-associated pneumonia. Am J Respir Crit Care Med 191:566–573. doi: 10.1164/rccm.201408-1468OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Köser CU, Bryant JM, Becq J, Török ME, Ellington MJ, Marti-Renom MA, Carmichael AJ, Parkhill J, Smith GP, Peacock SJ. 2013. Whole-genome sequencing for rapid susceptibility testing of M. tuberculosis. N Engl J Med 369:290–292. doi: 10.1056/NEJMc1215305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pak TR, Kasarskis A. 2015. How next-generation sequencing and multiscale data analysis will transform infectious disease management. Clin Infect Dis 61:1695–1702. doi: 10.1093/cid/civ670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ohl CA, Luther VP. 2014. Health care provider education as a tool to enhance antibiotic stewardship practices. Infect Dis Clin North Am 28:177–193. doi: 10.1016/j.idc.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 159.American College of Microbiology. 2008. Clinical microbiology in the 21st century: keeping the pace. American Society for Microbiology, Washington, DC: http://www.asm.org/ccLibraryFiles/FILENAME/000000004806/Clinical_Microbiology_in_the_21st_Century.pdf. [Google Scholar]
- 160.Mack MR, Rohde JM, Jacobsen D, Barron JR, Ko C, Goonewardene M, Rosenberg DJ, Srinivasan A, Flanders SA. 2016. Engaging hospitalists in antimicrobial stewardship: lessons from a multihospital collaborative. J hospital medicine 11:576–580. doi: 10.1002/jhm.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Slain D, Sarwari AR, Petros KO, McKnight RL, Sager RB, Mullett CJ, Wilson A, Thomas JG, Moffett K, Palmer HC, Dedhia HV. 2011. Impact of a multimodal antimicrobial stewardship program on Pseudomonas aeruginosa susceptibility and antimicrobial use in the intensive care unit setting. Crit Care Res Pract 2011:416426. doi: 10.1155/2011/416426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE, Infectious Diseases Society of America, European Society for Microbiology and Infectious Diseases. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–20. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 163.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR. 1999. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA 282:1458–1465. [DOI] [PubMed] [Google Scholar]
- 164.Gauthier TP, Sherman EM, Unger NR. 2015. An elective course on antimicrobial stewardship. Am J Pharm Educ 79:157. doi: 10.5688/ajpe7910157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Falcione BA, Meyer SM. 2014. Development of an antimicrobial stewardship-based infectious diseases elective that incorporates human patient simulation technology. Am J Pharm Educ 78:151. doi: 10.5688/ajpe788151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rocha-Pereira N, Lafferty N, Nathwani D. 2015. Educating healthcare professionals in antimicrobial stewardship: can online-learning solutions help? J Antimicrob Chemother 70:3175–3177. doi: 10.1093/jac/dkv336. [DOI] [PubMed] [Google Scholar]
- 167.Signorini A, Segre A, Polgreen PM. 2011. The use of Twitter to track levels of disease activity and public concern in the U.S. during the influenza A H1N1 pandemic. PLoS One 6:e19467. doi: 10.1371/journal.pone.0019467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Goff DA, Kullar R, Newland JG. 2015. Review of Twitter for infectious diseases clinicians: useful or a waste of time? Clin Infect Dis 60:1533–1540. doi: 10.1093/cid/civ071. [DOI] [PubMed] [Google Scholar]
- 169.Gauthier TP, Spence E. 2015. Instagram and clinical infectious diseases. Clin Infect Dis 61:135–136. doi: 10.1093/cid/civ248. [DOI] [PubMed] [Google Scholar]
- 170.Cantón R. 2005. Role of the microbiology laboratory in infectious disease surveillance, alert and response. Clin Microbiol Infect 11:3–8. doi: 10.1111/j.1469-0691.2005.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Centers for Disease Control and Prevention. 2016. Healthcare-associated infections (HAI) progress report. Centers for Disease Control and Prevention, Atlanta, GA: www.cdc.gov/hai/progress-report. [Google Scholar]
- 172.Schulz L, Osterby K, Fox B. 2013. The use of best practice alerts with the development of an antimicrobial stewardship navigator to promote antibiotic de-escalation in the electronic medical record. Infect Control Hosp Epidemiol 34:1259–1265. doi: 10.1086/673977. [DOI] [PubMed] [Google Scholar]
- 173.Revolinski S. 2015. Implementation of a clinical decision support alert for the management of Clostridium difficile infection. Antibiotics (Basel) 4:667–674. doi: 10.3390/antibiotics4040667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Antworth A, Collins CD, Kunapuli A, Klein K, Carver P, Gandhi T, Washer L, Nagel JL. 2013. Impact of an antimicrobial stewardship program comprehensive care bundle on management of candidemia. Pharmacotherapy 33:137–143. doi: 10.1002/phar.1186. [DOI] [PubMed] [Google Scholar]
- 175.López-Cortés LE, Del Toro MD, Gálvez-Acebal J, Bereciartua-Bastarrica E, Fariñas MCC, Sanz-Franco M, Natera C, Corzo JE, Lomas JMM, Pasquau J, Del Arco A, Martínez MP, Romero A, Muniain MA, de Cueto M, Pascual A, Rodríguez-Baño J, REIPI/SAB group. 2013. Impact of an evidence-based bundle intervention in the quality-of-care management and outcome of Staphylococcus aureus bacteremia. Clin Infect Dis 57:1225–1233. doi: 10.1093/cid/cit499. [DOI] [PubMed] [Google Scholar]
- 176.Pogue JM, Mynatt RP, Marchaim D, Zhao JJ, Barr VO, Moshos J, Sunkara B, Chopra T, Chidurala S, Kaye KS. 2014. Automated alerts coupled with antimicrobial stewardship intervention lead to decreases in length of stay in patients with Gram-negative bacteremia. Infect Control Hosp Epidemiol 35:132–138. doi: 10.1086/674849. [DOI] [PubMed] [Google Scholar]
- 177.Tsukamoto H, Higashi T, Nakamura T, Yano R, Hida Y, Muroi Y, Ikegaya S, Iwasaki H, Masada M. 2014. Clinical effect of a multidisciplinary team approach to the initial treatment of patients with hospital-acquired bloodstream infections at a Japanese university hospital. Am J Infect Control 42:970–975. doi: 10.1016/j.ajic.2014.05.033. [DOI] [PubMed] [Google Scholar]
- 178.Premanandh J, Samara BS, Mazen AN. 2015. Race against antimicrobial resistance requires coordinated action—an overview. Front Microbiol 6:1536. doi: 10.3389/fmicb.2015.01536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kho AN, Doebbeling BN, Cashy JP, Rosenman MB, Dexter PR, Shepherd DC, Lemmon L, Teal E, Khokar S, Overhage JM. 2013. A regional informatics platform for coordinated antibiotic-resistant infection tracking, alerting, and prevention. Clin Infect Dis 57:254–262. doi: 10.1093/cid/cit229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Laxminarayan R, Klugman KP. 2011. Communicating trends in resistance using a drug resistance index. BMJ Open 1:e000135. doi: 10.1136/bmjopen-2011-000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Morris AM. 2014. Antimicrobial stewardship programs: appropriate measures and metrics to study their impact. Curr Treat Options Infect Dis 6:101–112. doi: 10.1007/s40506-014-0015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Evans RS, Pestotnik SL, Classen DC, Clemmer TP, Weaver LK, Orme JF, Lloyd JF, Burke JP. 1998. A computer-assisted management program for antibiotics and other antiinfective agents. N Engl J Med 338:232–238. doi: 10.1056/NEJM199801223380406. [DOI] [PubMed] [Google Scholar]
- 183.Forrest GN, Schooneveld TC, Kullar R, Schulz LT, Duong P, Postelnick M. 2014. Use of electronic health records and clinical decision support systems for antimicrobial stewardship. Clin Infect Dis 59(Suppl 3):S122–S133. doi: 10.1093/cid/ciu565. [DOI] [PubMed] [Google Scholar]
- 184.Parfitt E, Valiquette L, Laupland KB. 2015. When it comes to stewardship, it's time to get with the programmers. Can J Infect Dis Med Microbiol 26:234–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Corbo J, Friedman B, Bijur P, Gallagher EJ. 2004. Limited usefulness of initial blood cultures in community acquired pneumonia. Emerg Med J 21:446–448. [PMC free article] [PubMed] [Google Scholar]
- 186.Pollack LA, van Santen KL, Weiner LM, Dudeck MA, Edwards JR, Srinivasan A. 2016. Antibiotic stewardship programs in U.S. acute care hospitals: findings from the 2014 National Healthcare Safety Network annual hospital survey. Clin Infect Dis 63:443–449. doi: 10.1093/cid/ciw323. [DOI] [PMC free article] [PubMed] [Google Scholar]