SUMMARY
Acinetobacter is a complex genus, and historically, there has been confusion about the existence of multiple species. The species commonly cause nosocomial infections, predominantly aspiration pneumonia and catheter-associated bacteremia, but can also cause soft tissue and urinary tract infections. Community-acquired infections by Acinetobacter spp. are increasingly reported. Transmission of Acinetobacter and subsequent disease is facilitated by the organism's environmental tenacity, resistance to desiccation, and evasion of host immunity. The virulence properties demonstrated by Acinetobacter spp. primarily stem from evasion of rapid clearance by the innate immune system, effectively enabling high bacterial density that triggers lipopolysaccharide (LPS)–Toll-like receptor 4 (TLR4)-mediated sepsis. Capsular polysaccharide is a critical virulence factor that enables immune evasion, while LPS triggers septic shock. However, the primary driver of clinical outcome is antibiotic resistance. Administration of initially effective therapy is key to improving survival, reducing 30-day mortality threefold. Regrettably, due to the high frequency of this organism having an extreme drug resistance (XDR) phenotype, early initiation of effective therapy is a major clinical challenge. Given its high rate of antibiotic resistance and abysmal outcomes (up to 70% mortality rate from infections caused by XDR strains in some case series), new preventative and therapeutic options for Acinetobacter spp. are desperately needed.
KEYWORDS: Acinetobacter, Acinetobacter baumannii, Acinetobacter calcoaceticus
INTRODUCTION
It is uncertain when the first isolation of organisms in the Acinetobacter genus occurred (1, 2). Gram-negative coccobacilli that were likely Acinetobacter were isolated as early as 1914 and repeatedly through the 1940s but were previously referred to as Mima polymorphia (now Acinetobacter lwoffii), Herellea vaginicola (now Acinetobacter baumannii or A. calcoaceticus), Bacterium anitratum, B5W, and Moraxella lwoffii (1, 2). Until quite recently, distinguishing A. baumannii from A. calcoaceticus was difficult. Thus, literature from decades past likely reflects a mixture of the two species.
The genus Acinetobacter is highly diverse, comprised of oxidase-positive and -negative, nonpigmented, Gram-negative coccobacilli. Although there are more than 50 species within the diverse Acinetobacter genus (3), the majority are nonpathogenic environmental organisms. The most common species to cause infections is A. baumannii, followed by A. calcoaceticus and A. lwoffii (4). Additional species, including A. haemolyticus, A. johnsonii, A. junii, A. nosocomialis, A. pittii, A. schindleri, and A. ursingii, have occasionally been reported as pathogens (5–11). A. seifertii is an emerging pathogen in Asia; it is genetically closely related to and may be misidentified as A. baumannii (12–14). Multivariate analysis of clinical data and studies of animal models (discussed further below) have demonstrated that A. baumannii is the most virulent of all the species (15).
In general, Acinetobacter spp. are found in wet environments, including moist soil/mud, wetlands, ponds, water treatment plants, fish farms, wastewater, and even seawater (3). These environmental strains often harbor antibiotic resistance mechanisms, including carbapenemases and extended-spectrum β-lactamases (ESBLs) (3), and may thus serve as important environmental reservoirs for resistance elements that transform into clinically relevant strains. Some medically relevant species, such as A. calcoaceticus, A. lwoffii, A. nosocomialis, and A. pittii, have been found on vegetables, meat, dairy products, and human skin (16). Such strains have harbored extensive antibiotic resistance repertoires. Furthermore, A. baumannii strains harboring extensive antibiotic resistance have contaminated commercial food, including meat, vegetables, and various types of livestock, suggesting multiple environmental routes of transmission into human populations (3, 17–19). However, non-baumannii Acinetobacter spp. have predominated in surveillance studies of skin colonization, particularly among healthy individuals, whereas A. baumannii has rarely been identified as a colonizer of skin among healthy patients (3, 20–23). Strains of A. baumannii-A. calcoaceticus complex did colonize 17% of healthy military personnel studied in Texas; however, the colonizing strains were distinct from those found in infected soldiers returning from Iraq and Afghanistan, and hence were unlikely the source of infection (23). Thus, healthy humans seem to rarely harbor more-pathogenic species and strains.
Infections caused by Acinetobacter spp. emerged in earnest during the 1960s and 1970s in parallel with increasing utilization of complex intensive care (1, 2). Acinetobacter was initially considered a commensal opportunist—a low-virulence pathogen of minimal significance. In subsequent decades, however, the increasing ubiquity and intensity of mechanical ventilation, central venous and urinary catheterization, and antibacterial therapy has caused a surge in the frequency and severity of Acinetobacter infections (24–27).
Today, Acinetobacter infections have spread rapidly through hospitals across the globe. The highest density of infections occurs in intensive care units (ICUs). U.S. National Healthcare Safety Network (NHSN) 2009-2010 surveillance data found that Acinetobacter spp. caused 1.8% of all health care-associated infections (27). Based on surveillance studies from hospital networks, the frequency is similar in ICUs across Europe and Latin America (28–32). However, in China, Thailand, Taiwan, Vietnam, and some countries in South America, Acinetobacter causes a much higher proportion of nosocomial infections and may be the predominant nosocomial pathogen. It is also becoming a predominant nosocomial pathogen in India (33–38). In Asian and certain Latin American countries, Acinetobacter is one of the three most common causes of bacteremia and nosocomial pneumonia (39–43). There are an estimated 45,000 (range, 41,400 to 83,000) cases of Acinetobacter infections per year in the United States and 1 million (range, 600,000 to 1,400,000) cases globally per year (44).
TRANSMISSION
Acinetobacter spp. are often transmitted to patients via persistence on environmental surfaces and transient colonization of the hands of health care workers (45, 46). However, nosocomial spread by aerosolized bacteria from infected or colonized patients has been reported. For example, in one well-publicized case, a health care worker developed fulminant pneumonia after inhalation of A. baumannii aerosolized during endotracheal suctioning of a ventilated patient (47). Another study revealed that nearly a quarter of air samples collected from patient rooms were contaminated with carbapenem-resistant A. baumannii (CRAB). These rooms had all housed patients infected with CRAB (46, 48). The air ducts were not colonized, indicating that the patients themselves were the source of the airborne bacteria (46, 48). However, Rock et al. found evidence of air contamination by A. baumannii in only one of a dozen patient rooms evaluated, so the frequency of air contamination by A. baumannii is variable (49). They surmised that the reduced rate of air contamination might have been due to use of frequent air exchanges combined with the fact that their patients were mechanically ventilated (and hence had closed airway circuits) (49). Nevertheless, the unnerving notion of the spread of organisms by settling on patients from contaminated air suggests that episodic cleaning of environmental surfaces may not be able to prevent dissemination unless there are also efforts to disinfect the air in patients' rooms. This concept of airborne spread represents a particular challenge and may require a shift in approach from an infection control standpoint. Specifically, early control of patient respiratory secretions, patient cohorting, and models aimed at reducing environmental dissemination may be equally important as surface disinfection. Novel technologies to enable air decontamination, such as misting, UV light, or vapor technologies may also have roles to play, although clinical data are lacking thus far.
While it is commonly asserted that Acinetobacter spp. cause infections primarily in immunocompromised patients, the predominant predispositions to infection are colonization pressure, selection by exposure to broad-spectrum antibiotics, and disruption of anatomical barriers (e.g., placement of catheters or endotracheal tubes and traumatic or surgical injury to skin and integument). Patients with suppression or depletion of leukocytes constitute the minority of those infected with A. baumannii (25, 50–52). Clinically, Acinetobacter infections are associated with mechanical ventilation, intravenous and urinary catheterization, surgery, invasive procedures, and prolonged broad-spectrum antimicrobials, especially in patients who suffer from burns, have trauma, or are in ICUs (25, 26, 39, 50, 52, 53). Thus, while Acinetobacter is largely an opportunistic pathogen, the “opportunities” that usually result in clinical infection are defects in anatomical host defenses and alteration of normal host flora by exposure to broad-spectrum antibiotics.
Acinetobacter is intrinsically resistant to desiccation, which contributes to its persistence in environments and transmission in health care settings. In addition, community-acquired pneumonia and bacteremia can occur, particularly in hot and humid tropical climates (25, 45). Cases appear to display a seasonal predilection. The National Nosocomial Infections Surveillance (NNIS) System found that between 1987 and 1996 the rate of Acinetobacter infections in the United States increased by 54% between July and October compared to November through June (45). Humidifiers and water baths have often been implicated as environmental reservoirs, and a high level of humidity has been postulated to facilitate growth of the bacteria (45).
PATHOGENESIS
Models of Infection
Various in vivo infection models have been used to study the pathogenesis of A. baumannii. Healthy mice inoculated in the lung or intravenously are generally resistant to lethal infection caused by many strains of Acinetobacter except at very high inocula (i.e., >109 CFU), suggesting dubious relevance to human pathogenesis (54–58). To circumvent the intrinsic resistance of many mouse strains to A. baumannii infection, artificial models have been used, such as infecting mice intraperitoneally (a clinically irrelevant route of entry) or mixing the inoculum with porcine mucin as a foreign body that inhibits the host's immune system from rapidly clearing the organism (59, 60). In addition, mice are often made neutropenic prior to infection even though neutropenia is not a common risk factor for infections caused by A. baumannii, and the vast majority of patients infected with A. baumannii have neither deficiency in leukocyte numbers nor overt defects in leukocyte function (25, 50–53, 61–68). Results from such models must be interpreted circumspectly given the unclear applicability to clinical disease.
In contrast, examination showed that A/J and C3HeB/FeJ strains of mice are intrinsically susceptible to lethal intravenous and lung infections by some clinical isolates of A. baumannii at inocula commensurate to (or lower than) those required for other commonly recognized virulent pathogens, such as Pseudomonas aeruginosa and Staphylococcus aureus (54, 69–72). A/J mice had delayed neutrophil recruitment to the lungs due to diminished CXC chemokine responses to the bacteria, possibly explaining their susceptibility to pulmonary infection (54). Such an explanation for why C3HeB/FeJ mice are more susceptible than other mouse strains to A. baumannii infection has yet to be revealed.
Rats are also susceptible to lethal pneumonia caused by A. baumannii without being immunocompromised. Russo et al. found that inoculating A. baumannii into the lungs of rats resulted in clinically comparable pneumonia, confirmed by histopathology, inflammatory response, physiological injury, and death (73). They also described a skin and soft tissue infection model in rats in which virulence differences among bacterial strains were detected, including greater virulence for clinical isolates than for environmental isolates (73, 74). Thompson et al. describe a wound infection model of Acinetobacter in mice with surgically induced, full-thickness skin incision (68). These investigators used a virulent clinical isolate, A. baumannii AB5075, but like other BALB/c models, they had to pretreat mice with cyclophosphamide to create an immunocompromised state the bacteria could exploit to enable a persistent infection. McConnell et al. have summarized models of meningitis, endocarditis, and osteomyelitis caused by A. baumannii (60).
The wax moth larva of Galleria has also been used as a model of Acinetobacter infection. For example, Peleg et al. found that A. baumannii was more lethal in the Galleria model than non-baumannii Acinetobacter species, including A. baylyi and A. lwoffii, and that antibiotic therapy improved survival of the infected larvae (75). Gebhardt et al. also found that A. baumannii, even a strain considered to be avirulent (ATCC 17978), was more virulent than A. baylyi, in Galleria (76). Wand et al. reported that strain to strain differences in biofilm formation did not correlate with virulence (77). However, if the strains were induced to form biofilms and the biofilm was then disrupted, the sessile bacteria harvested from the biofilms were more virulent in Galleria than the same strain taken from planktonic growth (77). Interestingly, the growth phase of the organism at the time of infection may affect a strain's virulence.
Caenorhabditis elegans has also been used as an occasional invertebrate model of Acinetobacter infection (78, 79). In at least one study, however, virulence outcomes in C. elegans did not correlate with outcomes in mice, so caution is warranted in interpreting translatability of results in this model (80). Finally, a recent study described a model of Acinetobacter virulence in zebrafish larvae (81). In this model, mutant strains that had been shown to have attenuated virulence in mice also had attenuated virulence, and host defenses depended on neutrophil and macrophage uptake, suggesting commonalities between this invertebrate model and mice. Again, caution may be warranted in that the A. baumannii strain background studied was ATCC 17978, a lab-adapted strain which was highly virulent in zebrafish but is essentially avirulent in healthy mice with a normal immune system, suggesting differences between the zebrafish and murine models. The authors also found that bacterial phenylalanine production was critical to triggering neutrophil chemoattraction to the site of infection (81). The phenylalanine catabolic pathway degrades phenylalanine, and a key step in that pathway is the paaA gene. Wild-type strains with intact phenylalanine catabolism produced less phenylalanine, resulting in less neutrophil attraction to the site of infection, whereas a strain with a disrupted paaA gene produced more phenylalanine, resulting in more neutrophil chemoattraction. Thus, phenylalanine degradation may be an important virulence factor of the bacteria, which ameliorates neutrophil chemoattraction and therefore protects the bacteria against early innate immune clearance, at least in zebrafish.
In the aggregate, animal models have been important to identify potential virulence factors driving the outcome of the interactions between the host and Acinetobacter spp. Indeed, in vitro assays, including adherence to human cells (e.g., epithelial cells and/or pneumocytes), cell invasion, and biofilm formation have often lacked correlation with in vivo virulence of Acinetobacter when studied head to head (77, 80, 82, 83). Similarly, a recent survey of A. baumannii and A. pittii clinical isolates found no evidence that the strains mediated adherence, invasion, or damage to lung epithelial cells in in vitro assays despite the fact that they had caused infection and disease in patients (84). These results underscore two points. (i) Discrepancy exists between clinical phenotype and in vitro assays. (ii) Caution must be taken in using such in vitro assays to describe or define virulence factors.
Thus, confirmation of virulence traits in vivo, and in particular in models relevant to human infection, is of great importance to advancing the field. Furthermore, confirmation of physiological injury is important in in vivo models when defining virulence, as 10- to 100-fold differences of bacterial density in the first 24 to 48 h of infection have not translated into survival differences in mice (85).
Acinetobacter Virulence Factors
Multiple studies indicate that A. baumannii intrinsically has more human virulence potential than other Acinetobacter spp., including A. calcoaceticus, A. lwoffii, A. junii, A. baylyi, and A. haemolyticus. For example, A. baumannii grew better at 37°C and was better able to resist macrophage uptake than these other species in one study (86). As mentioned, A. baumannii strains were also more lethal to the wax moth larva of Galleria than strains of A. baylyi and A. lwoffii (75, 76). In another study, a strain of A. junii was nonlethal in neutropenic mice, whereas several A. baumannii strains were lethal (85). Chusri et al. compared clinical outcomes in patients infected with A. nosocomialis, A. pittii, and A. baumannii and then compared the clinical isolates in an animal model (15). By multivariate analysis, infection with a non-baumannii Acinetobacter species resulted in a nearly a 9-fold reduction in mortality compared to A. baumannii. Furthermore, the clinical strains of non-baumannii Acinetobacter species were substantially less lethal during infection in wax moth larvae of Galleria. Similarly, in a case-control study, patients infected with A. ursingii had much lower 28-day mortality than those infected with A. baumannii (6% versus 37%) even though multidrug resistance and inadequate initial therapy were as likely to occur in patients infected with either species (10).
New advances in genetics and molecular biology have facilitated our understanding of basic Acinetobacter physiology and virulence factors (87, 88). Many have made transposon mutant libraries in an effort to better understand Acinetobacter baumannii virulence phenotypes. These libraries have utilized transposon insertion sequencing (TnSeq) in an attempt to identify potential virulence determinants (76, 89–91). When combined, the current mutant collections and whole-genome sequencing provide an invaluable resource for both virulence and antibiotic susceptibility screenings (57, 74, 89, 92).
Paradoxically, despite the etymology of Acinetobacter—from a-kineto, Greek for “nonmotile”—bacteria of this genus are decidedly motile; in fact, motility is one of the genus' putative virulence factors (60, 93). Furthermore, as mentioned, Acinetobacter is resistant to disinfection and desiccation. Ethanol enhanced the growth of A. baumannii in culture media and its salt tolerance, allowing it to grow despite salt concentrations that were inhibitory without alcohol (94). Ethanol exposure also led to marked changes in the organism's proteome and to enhanced virulence in Galleria (95). The bacterial enzyme RecA mediates bacterial DNA repair and resistance to desiccation and prevented A. baumannii killing inside macrophages and contributed to lethality in mice (96).
Under dry conditions, A. baumannii undergoes morphological changes, including thicker cell walls (97, 98), likely contributing to its impressive persistence on environmental surfaces. In outbreak investigations, A. baumannii remained viable in hospital units after months—even years—on a solid surface, underscoring the challenge to eliminating environmental transmission of the organism once it has colonized nosocomial surfaces (97, 98). Subsequent experimental models also highlight a propensity for epidemic isolates to persist in dry conditions (99).
Numerous other potential virulence factors have been suggested, including formation of biofilm, adherence mechanisms, iron acquisition characteristics, activities of polysaccharide membrane and outer membrane protein phospholipases, alteration in penicillin-binding proteins, and outer membrane vesicles (OMVs) (Table 1). Of particular mention is outer membrane protein A (OmpA) which has been suggested to have a variety of functions, including adhesion to host epithelial cells, biofilm function, and complement resistance (100). In a recent lethal model of A. baumannii pneumonia, transposon-mediated disruption of OmpA reduced mortality in small numbers of mice, suggesting a virulence function of the OmpA (100). Additionally, overexpression of chromosomal efflux systems has received considerable attention. The overproduction of these systems confers increased multidrug resistance to antimicrobial agents (101–103).
TABLE 1.
Summary of putative virulence factors for Acinetobacter spp.
| Model type and virulence factor(s) or gene(s), process, or organellea | Model | Outcome(s) | Reference |
|---|---|---|---|
| In vitro only | |||
| OmpA | Cell cytotoxicity | OmpA was administered to eukaryotic cells and induced cell death (note that endotoxin levels on the protein not reported) | 321 |
| OmpA | Complement lysis of OmpA mutant of A. baumannii 19606 vs wild type | OmpA mutant resisted alternative pathway complement lysis in vitro | 322 |
| OmpA | Knockout of OmpA in A. nosocomialis ATCC 17903 | The knockout had reduced biofilm formation and adherence to lung epithelial cells, with no difference in cytotoxicity | 323 |
| CpaA | Blood coagulation | Purified CpaA protease reduced coagulation of human plasma | 324 |
| BfmS | Various assays comparing BfmS mutant on ATCC 17978 background to the wild type | BfmS mutant had diminished biofilm formation, reduced adherence to cells, and sensitization to serum killing | 325 |
| Porins (CarO and OprD-like) | Growth rate, cytotoxicity of a clinical isolate vs ATCC 19606 strain (nonisogenic pair) | A clinical pan-drug-resistant isolate with reduced CarO and OprD-like expression grew more slowly and was less cytotoxic in a cellular assay | 326 |
| CFTR inhibitory factor (CiF) | Gene expression and function | Gene homologous to CiF from Pseudomonas aeuruginosa is found in A. nosocomialis and A. baumannii | 327 |
| Biofilm gene (LH92_11085) | Characterization of gene expression and biofilm formation in A. baumannii MAR002 | MAR002 overexpresses biofilm and has 25-fold increased expression of LH92_11085 | 328 |
| Oxidative resistance (KatG and KatE) | Mutants of A. baumannii and A. nosocomialis tested in vitro | Mutants had increased susceptibility to oxidative killing and neutrophil killing | 329 |
| Adherence, invasion, and cytotoxicity | 5 clinical isolates of A. baumannii and 6 clinical isolates of A. pittii tested in adherence, invasion, and cytotoxicity of lung epithelial cells | Adherence, invasion, and cytotoxicity not detected despite testing strains that had caused clinical disease | 84 |
| In vivo–invertebrate models | |||
| NfuA (iron acquisition scaffold protein) | NfuA knockout in ATCC 19606 strain vs wild-type strain | Knockout strain more sensitive to oxidative stress and modestly less lethal in Galleria | 312 |
| EntA (enterobactin precursor synthetic gene) | EntA knockout in ATCC 19606 vs wild-type strain | Knockout strain modestly less lethal in Galleria | 311 |
| Superoxide dismutase (SOD) | SOD knockout in ATCC 17978 vs wild-type strain | Knockout strain more sensitive to oxidative stress and less lethal in Galleria | 330 |
| TonB (energetics of nutrient uptake) | TonB mutant of ATCC 19606 vs wild-type strain | Variable impact on lethality in Galleria but impacted adherence to epithelial cells | 82 |
| OXA-40 gene (carbapenemase) | Clinical isolates with or without the OXA-40 gene | OXA-40-containing isolates appeared to kill Galleria more slowly | 331 |
| AbuO (outer membrane protein) | AbuO knockout of A. baumannii AYE (origin unclear) with infection in C. elegans | Knockout displayed increased susceptibility to antibiotics and disinfectant and modestly reduced lethality in C. elegans | 332 |
| SecA (iron acquisition) | Transposon mutant disruption of SecA in A. baumannii 19606 | Mutant displayed modest reduction in lethality in Galleria | 315 |
| pmrB (colistin resistance due to altered LPS charge) | Clinical isolate with spontaneous pmrB mutation | Mutant displayed no reduction in strain fitness, growth, or lethality in Galleria | 333 |
| lpxACD, pmrB (colistin resistance due to loss of LPS synthesis genes [lpx] or altered LPS charge [pmr]) | Clinical strains serially passaged on colistin | lpxACD mutants had growth defects and loss of virulence, whereas pmrB mutants had no change in growth or virulence in Galleria | 334 |
| Phospholipase D | Disruption of 3 phospholipase D genes in ATCC 19606 | Reduced virulence in Galleria | 335 |
| Type VI secretion system (T6SS) | T6SS was compared in ATCC 17978, a nonclinical isolate (DSM30011), and 3 clinical isolates | Only the nonclinical isolate expressed a highly functional T6SS, which played a role in colonization in Galleria | 336 |
| lpxD (colistin resistance due to loss of LPS synthesis) | Clinical isolate of A. noscomialis serially passaged in subtherapeutic colistin | lpxD mutant had modestly attenuated virulence in C. elegans | 337 |
| SurA1 (surface antigen protein) | Knockout of SurA1 from A. baumannii CCGGD201101 (an isolate from diseased chicks) | Knockout had decreased growth rate, increased killing in serum, and decreased virulence in Galleria | 338 |
| AdeRS (Acinetobacter drug efflux pump regulator) | Deletion of AdeRS from A. baumannii AYE or S1 | Knockouts had decreased biofilm formation; S1 but not AYE knockout had decreased virulence in Galleria | 103 |
| gacA and gacS (regulator genes), abaI (quorum sensing), paaA (phenylalanine catabolism) | ATCC 17978 and knockouts infected via blood in zebrafish embryos | Knockout strains had attenuated virulence in the zebrafish model, and the paaA knockout produced more phenylalanine, which triggered more neutrophil attraction to the site of infection | 81 |
| Multiple genes regarding stress response, osmotic stress, capsule, and LPS genes | Comparison of Acinetobacter strains in Galleria, including transposon disruptants | Galleria distinguished known avirulent (ATCC 17978) and virulent (5075) strains of A. baumannii, with the former causing some lethality and the latter 100% fatal. A. baylii ADP1 was less virulent than A. baumannii ATCC 17978. A variety of genes disrupted by transposon insertion in A. baumannii 5075 modulated mortality in Galleria. | 76 |
| In vivo–nonlethal vertebrate models | |||
| Serum/complement resistance | Clinical isolates in Long-Evans rat soft tissue infection (subcutaneous) | Sensitivity to complement correlated with rapidity of soft tissue clearance in vivo | 73 |
| Phospholipase D | C57BL/6 intranasal lung infection (>3 × 108 inoculum) with transposon mutant clinical CSF isolate 98-37-09 vs wild type | Disruption resulted in serum sensitivity and no difference in lung bacterial density, but the mutant strain had lower bacterial blood density following pneumonia | 57 |
| Heme consumption | Nonlethal intranasal infection with A. baumannii LAC-4 clinical isolate, treatment with an inhibitor of heme acquisition vs placebo | Mice infected with LAC-4 and treated with heme acquisition inhibitor had modestly reduced lung bacterial density and bacteremia | 313 |
| PTK and EpsA (capsular polysaccharide regulators) | Long-Evans rat soft tissue infection with knockouts on A. baumannii 307-0294 clinical isolate background vs wild type | Disruption resulted in diminished growth in human ascitic fluid, human serum, and rat soft tissue | 74 |
| OmpA, LpsB, GacA | Transposon mutant library of A. baumannii ATCC 17978 infected intranasally into C57BL/6 mice | CFU differences at 24 h detected for strains with mutations of various genes, including lpsB (LPS biosynthesis), ompA, and gacA | 89 |
| Outer membrane vesicles (OMVs) | Outer membrane vesicles purified from A. nosocomialis ATCC 17903 administered to cells in vitro and BALB/c mice intratracheally | OMVs were toxic to eukaryotic cells and triggered inflammatory cytokine production in mouse lungs | 339 |
| LipA (lipase) | Tail vein infection of DBA mice made neutropenic with cyclophosphamide and infected with LipA knockout in A. baumannii ATCC 17978 vs wild type | LipA knockout demonstrated reduced competition fitness during nonlethal infection in mice | 340 |
| gspD (type 2 secretion system [T2SS]) | Intranasal inoculation of C57BL/6 mice with A. nosocomialis M2 with knockout of gspD vs wild type | Modest differences in survival in Galleria and modest differences in bacterial densities during nonlethal infection in mice | 341 |
| AdeABC and AdeIJK (efflux pump regulators) | Intranasal and intraperitoneal infection of C57BL/6 mice with clinical isolate A. baumannii BM4587 or its isogenic Ade mutants | Increase in bacterial burden during intraperitoneal infection with the adeABC mutant, decreased with adeIJK mutant, no change after lung infection (intranasal) | 101 |
| Zur (zinc uptake regulator) | Intranasal infection of C57BL/6 mice with A. baumannii ATCC 17978 or a Zur knockout strain | No difference in lung bacterial burden, but liver burden lower for the Zur knockout strain | 319 |
| ZigA (zinc chaperone) | Intranasal infection of C57BL/6 mice with A. baumannii ATCC 17978 or a ZigA knockout strain | No difference in lung bacterial burden, but liver burden lower for the ZigA knockout strain | 320 |
| FeoB (ferrous iron transport), DDC (cell wall cross-linking), PntB (pyridine metabolism), FepA (enterobactin receptor) | Intravenous infection in CBA/J mice with a transposon mutant library of A. baumannii ATCC 17978 treated with cyclophosphamide to make them neutropenic, using competitive growth by spleen bacterial density, or in human serum, as read-outs | Defects in these genes altered competitive growth/relative bacterial density in the spleens or serum | 90 |
| In vivo–lethal vertebrate models | |||
| Inoculum mixed with porcine mucin | |||
| pmrB | Intraperitoneal infection in C57BL/6 mice with ≥108 organisms of pmrB mutant in A. baumannii ATCC 19606 vs wild type | pmrB mutant had lower bacterial density and less mortality | 59 |
| pmrB | Intraperitoneal infection in C57BL/6 mice with ≥107.9 organisms of a clinical spontaneous pmrB mutant of A. baumannii CR17 (cerebrospinal fluid strain) vs its pretreatment parent strain | pmrB mutant had reduced in vivo fitness in competition with wild type and lower mortality at low, but not high (i.e., >105) inocula | 342 |
| pmrA (altered LPS charge) | Intratracheal lung infection in Sprague-Dawley rats with a spontaneous mutant of A. baumannii respiratory clinical isolate vs its pretreatment isogenic strain | pmrA mutant had reduced lethality | 343 |
| Ciprofloxacin resistance (mutation not described) | Intraperitoneal infection in C57BL/6 mice with 106, 107, or 108 A. baumannii clinical strain serially passaged in subtherapeutic ciprofloxacin vs its parent | Ciprofloxacin-resistant strain induced lower mortality | 344 |
| Acinetobactin (iron siderophore) | Galleria as well as intraperitoneal infection in C57BL/6 mice with 106 or 105 A. baumannii acinetobactin knockouts in ATCC 19606 vs wild type | Knockout strain induced lower mortality in Galleria and in mice | 316 |
| PgILAb (O glycosylation) | Galleria and intraperitoneal infection with porcine mucin in BALB/c mice, using a PgIL knockout in ATCC 17978 vs wild type | Mutant less lethal in Galleria, and less competitive than wild type during growth in mice | 345 |
| pglC (capsule) | Intraperitoneal infection in BALB/c mice infected with pglC knockout in ATCC 17978 vs wild type | Capsule-deficient mutant strain was avirulent compared to wild type | 105 |
| Omp33 | Intraperitoneal infection in C57BL/6 mice with 106, 107, or 108 Omp33 knockout in A. baumannii 17978 vs wild type | Knockout strain displayed growth defect in vitro, and reduced lethality in mice | 171 |
| MapA (Omp33-36) | Intraperitoneal infection in C57BL/6 mice with Omp33-36 knockout in A. baumannii 17978 vs wild type | Knockout displayed a 12-h delay in death (but all mice died) | 346 |
| gacS (sensor kinase) and paaE (phenylacetic acid [PAA] catabolic pathway) | Intraperitoneal infection in BALB/c mice infected with knockouts in ATCC 17978 vs wild type | gacS and paaE mutant strains had attenuated mortality in mice | 347 |
| Infections in wild-type mice without porcine mucin | |||
| RecA (DNA damage repair) | Intraperitoneal infection in CD1 mice with 2 × 108 RecA knockout of A. baumannii ATCC 17978 vs wild type | RecA mutant was more sensitive to oxidative damage, macrophage killing, and heat exposure in vitro and caused mildly reduced lethality compared to wild-type strain (7% vs 20%) | 96 |
| pmrB, lpxA, lpxA, lpxC, lpxD (LPS genes) | Intraperitoneal infection in BALB/c mice with knockouts in A. baumannii 19606 vs wild type | lpx mutants had reduced in vitro growth while pmrB mutant did not; lpx mutants had attenuated virulence in both C. elegans and in mice, but pmrB mutant had attenuated virulence only in C. elegans and not in mice | 80 |
| OmpA | In vitro studies followed by tracheal aspiration pneumonia using Ab5075 strain in wild-type C57BL/6 mice | Transposon-disrupted OmpA strain was nonlethal in 5 mice, whereas 3 of 4 mice infected with wild-type died (note the small numbers of mice) | 100 |
| Capsule | Intraperitoneal infection of C57BL/6 mice with A. baumannii ATCC 17978 strains which were induced to overproduce capsule or strains with mutations in capsule production | Strains expressing enhanced capsule were resistant to serum/complement, and more lethal in mice | 107 |
| UspA (universal stress protein A) | Intranasal and intraperitoneal infection of C57BL/6 mice with UspA knockout in A. baumannii ATCC 17978 vs wild type | Modest difference in lung CFU during nonlethal infection and no significant difference in survival during intraperitoneal lethal infection | 348 |
CFTR, cystic fibrosis transmembrane conductance regulator; PTK, protein tyrosine kinase; DDC, d-Ala-d-Ala-carboxypeptidase.
However, much of the in vivo pathogenesis work published thus far has used avirulent, lab-adapted strains (chiefly ATCC 17978) and/or nonlethal murine models to measure modest changes in bacterial density over time. Table 1 summarizes results of a systematic PubMed search of Acinetobacter virulence research, using title-word “Acinetobacter” and title-word/abstract “virulence.” We have separated studies by whether the assays were strictly in vitro, included invertebrate animal outcomes, included nonlethal vertebrate animal outcomes, or included lethal vertebrate animal outcomes, either conducted or not with artificial introduction of porcine mucin to prevent innate immune clearance of the bacteria (Table 1). It is imperative to consider the results of these pathogenesis studies in the context of the model systems and endpoints used, given their potential limitations.
It is unclear how to interpret virulence factors characterized solely in vitro or in in vivo models in which the animals do not die or suffer physiological injury or in models using routes of infection that rarely occur clinically. Furthermore, as mentioned, in lethal in vivo models, porcine mucin is often used as a foreign body to protect the bacteria from rapid innate immune clearance (which is not physiologically relevant) or render mice immunocompromised by making them neutropenic to achieve the same effect (Table 1). Such models likely mask host-microbe interactions that are of primary importance in determining host outcome. Still, they may unmask secondary virulence functions that become relevant in the context of patients whose immune systems are highly dysfunctional.
In the aggregate, repeated themes become discernible when evaluating virulence studies of A. baumannii (Table 1). Capsule and its negative surface charge may well be the primary virulence function of the pathogen, as it is the primary defense the bacteria have against complement-mediated destruction and opsonization, as well as phagocytic uptake (74, 104–107). Hypervirulent strains have been reported that resist innate immune uptake, which may have unusual or mutated capsular phenotypes (70, 105, 108–110). In one compelling study, the investigators used the lack of virulence of the lab-adapted A. baumannii strain ATCC 17978 to demonstrate a remarkable gain of function of virulence by treating the strain with small molecules that triggered overexpression of capsule (107). Recent elucidation of the O-glycation systems in A. baumannii has begun to clarify capsular chemical structures that protect against innate defenses; A. baumannii glycation favors short-chain, branched, negatively charged amino-containing surface sugars which both protect against host immunity and may serve as a target for future immune interventions to help clear the pathogen (111–116).
Multiple studies have also shown that lipopolysaccharide (LPS) (endotoxin) has a major impact on the organisms' virulence (discussed more below). However, it is not clear that the pmrB mutation, which disrupts phosphoethanolamine addition to the LPS core and thereby affects the negative charge of LPS, actually disrupts virulence. The study results are mixed, likely resulting from yet uncharacterized virulence factor differences underlying the diverse pmrB mutant strains (Table 1). In contrast, defects in the lpxABCD cluster (responsible for LPS biosynthesis) clearly affect the ability of the organism to grow, thereby diminishing virulence (Table 1).
Also of importance to A. baumannii virulence is iron acquisition by multiple routes (Table 1). This is intuitive: without iron, the organism cannot grow. Phospholipases have been suggested in several studies to be important for virulence functions, but the in vivo data are not definitive in vertebrate models.
Immunological Defense Mechanisms
The immune defenses operative against Acinetobacter infection are slowly being elucidated (Table 2). In one of the earliest investigations, van Faassen et al. reported that intranasal pneumonia in mice resulted in rapid neutrophil recruitment to the lung, and as the infection was cleared by the innate immune system, neutrophil recruitment ceased and inflammation rapidly resolved (117). Antibody-mediated depletion of neutrophils increased lung bacterial burden and allowed for extrapulmonary dissemination (117). Nevertheless, the infection remained nonlethal; although inflammation resulted, it remained relatively mild. The nonlethal nature of the model may be the result of using a lab-adapted A. baumannii strain, ATCC 17961, in intrinsically resistant C57BL/6 and BALB/c mice.
TABLE 2.
Summary of host defense elements determining outcomes of Acinetobacter infection
| Model type and host defense factor(s) or process(es) | Model | Outcome(s) | Reference |
|---|---|---|---|
| In vitro models | |||
| Cytokines and pattern recognition receptors | LPS from A. baumannii clinical isolates exposed to human macrophages | High levels of IL-8 and TNF produced but only if TLR4 is functional, not through TLR2—however, whole killed A. baumannii induces cytokines via both TLR4 and TLR2 | 123 |
| Cytokines | LPS from A. baumannii clinical isolates exposed to mouse splenocytes | High levels of TNF released, mitogen stimulation occurred | 122 |
| In vivo–nonlethal models | |||
| Neutrophils | Intranasal infection with A. baumannii ATCC 17961 in C57BL/6 or BALB/c mice with or without neutrophil depletion | Depletion of neutrophils substantially increased bacterial burden in the short term and enabled extrapulmonary dissemination, but inflammation remained mild, and the infection was cleared within several days with no deaths in any group | 117 |
| Neutrophils and macrophages | Intranasal infection with A. baumannii ATCC 17978 in Fus1 knockout or wild-type mice | The knockout mice displayed enhanced NF-κB activation, higher IL-17A production, lower IL-10 production, more rapid neutrophil and macrophage chemotaxis to the lung, and lower bacterial burden | 121 |
| Macrophages | Intranasal infection with A. baumannii ATCC 17961 of C57BL/6 mice treated with liposomal clodronate to deplete macrophages or with placebo | In healthy mice, macrophages are the predominant cells present in the lung through the first 4 h and rapidly take up bacteria. Depletion of macrophages results in substantial increase in bacterial density, but not mortality, as inflammatory cytokine levels are lower (despite higher bacterial density) in depleted mice. | 120 |
| Zinc and manganese sequestration | Mice with calprotectin disrupted or wild-type mice infected intranasally with ATCC 17978 | Mice with calprotectin disrupted had higher bacterial burden in the lung | 317 |
| Pattern recognition receptors and host defense | Intranasal infection with a clinical isolate of A. baumannii RUH 2037 in C57BL/6 mice or congenic CD14, TLR4, or TLR2 knockouts | CD14 and TLR4 knockout mice had higher bacterial density at 4 h and had lower neutrophil influx. At 24 h only, the TLR4 knockout mice had higher bacterial density, but they had cytokine levels similar to those of wild-type mice, and they did not die of infection. TLR2 knockout mice had earlier cellular influx, but their cytokine levels and bacterial density were similar to those of the wild-type mice. | 124 |
| Pattern recognition receptors and host defense | Intranasal infection with A. baumannii ATCC 17978 in C57BL/6 mice or isogenic TLR9 knockout mice | TLR9 knockout mice had higher bacterial burden, attenuated cytokine production, and more severe lung pathology, with no mouse deaths | 129 |
| In vivo–lethal models | |||
| Iron sequestration | Treatment with transferrin or placebo in C3H/FeJ mice lethally infected intravenously with hypervirulent A. baumannii HUMC1 | Mice treated with transferrin had lower blood and tissue bacterial burden and marked improvement in survival | 72 |
| Neutrophils | Intraperitoneal infection with clinical isolates of A. baumannii in C57BL/6 and C3HeB/Fe mice | Antibody depletion of neutrophils but not abrogation of IL-17A or KC (keratinocyte-derived chemokine) increased lethality and bacterial burden of infection | 118 |
| Neutrophils | Intranasal infection with A. baumannii ATCC 17961 in A/J or C57BL/6 mice | A/J mice experienced delayed neutrophil influx into the lungs, resulting in early rapid microbial replication, and later severe inflammation and death, whereas C57BL/6 mice cleared the infection | 54 |
| Superoxide production | Intranasal infection of C57BL/6 mice or congenic gp91phox−/− mice or nitric oxide synthase-deficient mice with A. baumannii ATCC 17961 | Gp91phox−/− mice had normal neutrophil and macrophage recruitment to the lung by 4 h, but 1,000-fold-higher bacterial density, which resulted in marked increase in inflammatory cell influx into the lung by 24 h and death by 48 h. The nitric oxide-deficient mice had relatively normal phenotype and did not die. | 119 |
| Avoidance of innate effector uptake | C3HeB/Fe mice were infected i.v. via the tail vein with >40 clinical isolates of A. baumannii, and then the experiments were repeated with selected strains depleted of combinations of complement, neutrophils, or macrophages in a fatal model of infection | Clinical strains clustered into one of 3 groups: hypervirulent (HUMC1 and LAC-4), virulent (almost all others, including 5075), and avirulent (ATCC 17978 and R2). The differences in virulence (and hence fate of the animal) were definable within 1 h postinfection depending on ability to persist in the blood. Depletion of any of the three components increased bacterial density of the avirulent strain but nonlethally. Double depletion increased bacterial density further but nonlethally, and triple depletion converted the avirulent strain into a hypervirulent strain inducing rapid lethality, while depletion of any individual component had marginal impact on the hypervirulent strain. | 70 |
| LPS-TLR4 governance of outcome | C3HeB/FeJ and C3H/HeJ (TLR4 mutant) mice treated with LpxC inhibitor (blocks LPS production) and C57BL/6 or congenic TLR4 knockout mice, infected i.v. via the tail vein with hypervirulent A. baumannii HUMC1 or avirulent ATCC 17978 | TLR4 mutant and knockout mice had dramatically lower inflammatory cytokine levels, sepsis biomarkers, and 100% survival compared to 100% fatality in the wild-type mice, despite having similar bacterial densities (dissociation of bacterial density from outcome). LpxC inhibition also blocked cytokine levels and protected mice from lethal infection and modestly lowered CFU likely by enhancing macrophage uptake of the bacteria. | 69 |
| Morphine | C57BL/6 and C3HeB/Fe mice with intraperitoneal infection of A. baumannii clinical isolates, treated with morphine or not treated with morphine (control) | Morphine treatment resulted in fatal subcutaneous infection, whereas no control mice died and naltrexone (opiate antagonist) reversed the effect. Morphine did not affect bacterial growth in vitro but increased bacterial density and inflammatory cytokine output in vivo and suppressed phagocyte recruitment to the site of infection. | 130 |
Similarly, in the intraperitoneal infection model (absent porcine mucin), pretreatment of mice with antineutrophil antibodies converted the same nonlethal inoculum of clinical A. baumannii isolates into rapidly lethal infections (118). Interestingly, abrogation of interleukin 17A (IL-17A) or keratinocyte-derived chemokine (KC), both predominant governors of neutrophil chemotaxis, did not alter the outcome. Thus, neutrophils, but not necessarily traditional chemokines that summon neutrophils, are important in early host defense against A. baumannii. Since peritoneal infection by Acinetobacter is highly artificial and rarely encountered in human patients, caution is warranted in interpreting these results. Furthermore, findings in the bloodstream model of infection in another study did not indicate that specific elimination of neutrophils converted nonlethal infection to lethal infection (below).
The role of oxidative killing of A. baumannii in early, innate host defense was delineated by Qiu et al. (119). They reported that gp91phox−/− mice (superoxide-deficient model for chronic granulomatous disease) were hypersusceptible to A. baumannii lung infection compared to congenic wild-type control mice. By 4 h postinfection, the knockout mice had normal levels of neutrophil or macrophage influx, but they were not able to clear the bacteria. The knockout mice developed 1,000-fold-higher bacterial burden such that by 24 h postinfection, they had also developed markedly worse inflammatory influx in the lungs compared to wild-type mice. The knockout mice died by 48 h, while the congenic C57BL/6 wild-type mice, or mice with disrupted nitric oxide synthase, cleared the infection. These results indicated that superoxide production is important in clearance of A. baumannii; this study constitutes one of the earliest to suggest that the inflammatory response to the organism is one of the primary mechanisms by which host death occurs.
Qiu et al. also reported that wild-type A/J mice were more susceptible to pneumonia caused by A. baumannii than C57BL/6 mice were (54). The cause of the increased susceptibility was delayed recruitment of neutrophils which allowed early and rapid microbial replication in the lung, resulting in severe inflammation at later time points. Beyond neutrophils, macrophages are also critical at early stages. The same investigators found that macrophages predominated in bronchoalveolar lavage samples at the time of infection and 2 h postinfection in mice (120). By 4 h postinfection, macrophages were still the predominant cell type present (75%, with neutrophils comprising 25% of cells) and had taken up substantial numbers of A. baumannii in the lung. When mice were depleted of macrophages with liposomal clodronate, bacterial densities rose sharply. However, the infection remained nonlethal and inflammatory cytokine levels were actually lower in the mice with depleted macrophages. These results once again underscore that bacterial density is only one component of determining whether the host survives and demonstrate that the host inflammatory response to infection seems critical to determining outcomes of A. baumannii infection. Even if bacterial density is higher, when inflammatory cytokine output is lower, the host may survive with minimal damage.
The role of early neutrophil and macrophage recruitment in protecting against lung infection caused by A. baumannii is given further credence by a study of mice deficient in the mitochondrial protein Fus1. Fus1-disrupted mice had enhanced neutrophil and macrophage recruitment to the lungs at early time points, likely due to more rapid activation of nuclear factor kappa beta (NF-κΒ) and resulting enhanced IL-17A and decreased IL-10 production (121). Due to enhanced phagocytic recruitment, the knockout mice had lower bacterial burden in the lungs.
LPS from A. baumannii induces inflammatory cytokines, such as IL-8 and tumor necrosis factor (TNF), from mouse splenocytes and human macrophages at levels equivalent to those stimulated by LPS from Escherichia coli (122, 123). The LPS activates cytokine production in a manner requiring TLR4, whereas whole A. baumannii is able to activate cytokines via both TLR4 and, for unclear reasons, TLR2 (123).
In vivo, TLR4-deficient mice exhibited minimal inflammation in response to LPS harvested from A. baumannii that was intranasally administered (124). Furthermore, TLR4-deficient mice had substantially lower and delayed cytokine and chemokine induction in lungs after intranasal infection with A. baumannii, resulting in slower neutrophil recruitment and slower bacterial clearance. Despite having higher bacterial density at 24 h postinfection, the mice had lower inflammatory cytokine levels, and they ultimately cleared the infection. In contrast, TLR2-deficient mice had accelerated inflammation and bacterial lung clearance. A follow-up study used an alternative method (distant turpentine injection) to suppress lung cytokine and chemokine responses during intranasally inoculated pneumonia in mice (125). Turpentine suppressed early cytokine expression and neutrophil recruitment, resulting in higher bacterial density. Yet the treated mice had significantly less alveolar filling consistent with pneumonia and hence less host damage. Once again these data underscore that host outcome is driven by cytokine levels and inflammation as much as or more than bacterial density, and that dampening the inflammatory cytokine response can blunt host damage despite higher bacterial burden.
Nevertheless, since mice with disrupted TLR4 had higher bacterial density, it has been assumed that activation of TLR4 by A. baumannii LPS was critical for host defense. We reiterate that these in vivo models were nonlethal and the outcome measured was slower clearance of bacteria. Thus, the role of TLR4 in host defense against lethal infection was not fully elucidated by these studies. However, these results informed more recent efforts to better delineate the balance between bacterial density and inflammation as well as the factors governing that balance.
More-Recent In Vivo Virulence Assessments
In neutropenic mice with pneumonia, differences in A. baumannii strain virulence were noted using a mortality endpoint (85, 126). To begin describing virulence factors that drive the host's physiological response in noncompromised mice, more than 40 unique clinical isolates of A. baumannii were evaluated in the intravenous infection model of immunocompetent C3HeB/FeJ mice (70). A. baumannii strains were readily separated into virulent and less virulent phenotypes based on survival and sepsis outcomes in the mice (70). In vitro growth variance did not correlate with in vivo virulence differences. However, bacterial blood densities of each strain at 1 h postinfection predicted lethality and correlated with virulence (70). Thus, clinical outcome could be predicted within 1 h after infection, indicated by the bacterial density achieved in blood, even though severe sepsis had not yet developed.
Hypervirulent A. baumannii strains maintained high blood bacterial densities (>107 CFU/ml) at 1 h postinfection, which persisted for 24 h (70). In contrast, the virulent strains achieved 1,000-fold-lower bacterial densities at 1 h postinfection but underwent minimal clearance over the subsequent 23 h (70). The avirulent strain achieved a bacterial density at 1 h postinfection similar to the virulent strains, but underwent an additional 100-fold decrease by 24 h postinfection (70).
The avirulent strain was very susceptible to complement-mediated killing, whereas virulent and hypervirulent strains were considerably less susceptible (70). Macrophage uptake and killing of A. baumannii correlated inversely with strain virulence (70). Furthermore, mice depleted of either macrophages, complement, or neutrophils had modest but significant (10- to-100-fold) increases in blood bacterial density when infected with the avirulent strain (ATCC 17978) (70). Double depletion of any two effectors compounded the increase in blood bacterial density, but the remaining effector was able to maintain a bacterial blood density below the lethal threshold (70). Triple depletion of all three effectors, however, synergistically increased blood bacterial density to levels equivalent to those achieved by the hypervirulent strain in healthy mice; the result was 100% mortality within 24 h postinfection with the avirulent strain (70). These results indicated that lethal infection was not triggered by a specific exotoxin or virulence factor elaborated by A. baumannii ATCC 17978; rather, ATCC 17978 was cleared effectively by all three arms of the innate immune effectors, and all three had to be depleted to enable the organism to achieve sufficient bacterial density in blood to trigger the lethal sepsis response with its LPS.
One of the hypervirulent strains was also tested; A. baumannii HUMC1 is a lung and blood clinical isolate (simultaneously isolated from a patient with bacteremic ventilator-associated pneumonia) that is extremely drug resistant (XDR)—clinically resistant to all antibiotics except colistin (127). In contrast to ATCC 17978, HUMC1 was already relatively resistant to innate effector clearance at baseline, so disruption of individual effectors only marginally impacted its bacterial density (70). Triple depletion, however, further exacerbated bacterial density and severity of infection, indicating that the murine host was simultaneously relying on marginally effective efforts from complement, neutrophils, and macrophages to attempt to control the organism's replication in blood. Only by working in concert were all three arms able to mediate bacterial clearance and growth inhibition in vivo.
Similarly, in studies of a different hypervirulent strain, A. baumannii LAC-4, Harris et al. established lung infection by intranasal inoculation of healthy, wild-type C57BL/6 or BALB/c mice (128). They achieved rapidly lethal infection from severe alveolar pneumonia with secondary bacteremic spread and very potent inflammatory responses in the lungs. Other results with LAC-4 compared to other strains found that LAC-4 and HUMC1 were similarly virulent; both were far more virulent than any other strain they tested (70). Both strains are capable of substantial evasion of initial innate immune clearance by complement and phagocytosis—more so than other strains tested.
Histopathological studies of mice were of further interest. When healthy mice were lethally infected intravenously with A. baumannii HUMC1, no tissue abscesses were revealed (69). Rather, the coccobacilli displayed a surprising inability to extravasate into the parenchyma, instead remaining trapped in the vasculature (69). Thus, Acinetobacter adherence to or penetration through tissues does not appear to occur to a significant degree during systemic infection.
In contrast, interactions between bacterial LPS and host TLR4 clearly drove survival in the model. Contrary to results with nonlethal infection models, TLR4 was antiprotective during lethal infection and mice were protected via disruption of LPS in the bacteria or TLR4 in the host (69). Surprisingly, bacterial burden did not differ between wild-type and TLR4-disrupted mice infected with A. baumannii HUMC1 despite marked, statistically significant differences in sepsis biomarkers and survival (69). Thus, outcomes of infection were driven at least as much by the host response to infection as bacterial density.
Inhibition of LPS production in the A. baumannii HUMC1 bacteria by growth in the presence of an LpxC inhibitor did not slow growth of the bacteria but did abrogate its virulence in mice (69). Treatment of infected mice with the LpxC inhibitor markedly decreased inflammatory cytokines, sepsis response, and death from HUMC1 infection. Bacterial density was also modestly reduced, likely because bacteria exposed to the LpxC inhibitor were easier to phagocytose and clear from blood (69).
TLR9, which recognizes CpG DNA motifs found in bacterial and not eukaryotic cells, also may play a role in host defense against A. baumannii. In nonlethal models of pneumonia or peritoneally administered disseminated infection, TLR9-disrupted mice had increased bacterial burden and attenuated inflammatory responses (129). However, the model was nonlethal, likely due to the use of the lab-adapted A. baumannii ATCC 17978 strain. This study once again demonstrated a dissociation between higher bacterial burden and inflammatory cytokines, where no deaths occurred despite higher bacterial burden likely because inflammation was attenuated.
Interestingly, the critical importance of TLR4 in governing host outcome from A. baumannii may in part account for the proclivity of soldiers injured in battle in Iraq and Afghanistan, who may be treated with morphine, to suffer from A. baumannii infections or at least more severe disease manifestations of infection. Breslow et al. found that morphine treatment resulted in a marked enhancement of death in mice from intraperitoneal infection by clinical isolates of A. baumannii (130). Naltrexone, an opiate antagonist, reversed the effect, demonstrating the specificity of the opiate effect. Morphine-treated mice had markedly increased inflammatory cytokines and bacterial density but suppressed phagocytic recruitment. Morphine has been demonstrated to have the potential to blunt adaptive tolerance to endotoxin, resulting in more persistent septic shock in morphine-treated mice in response to LPS than in control-treated mice (131). Thus, morphine may exacerbate the underlying pathogenesis of A. baumannii, and possibly other Gram-negative, infections. This further highlights the finding that while environmental factors may represent an opportunity for initial exposure, the innate immune system and a variety of host factors play a crucial role in determination of the extent and manifestations of pathogenicity.
Integrated Summary of Current Understanding of A. baumannii Pathogenesis
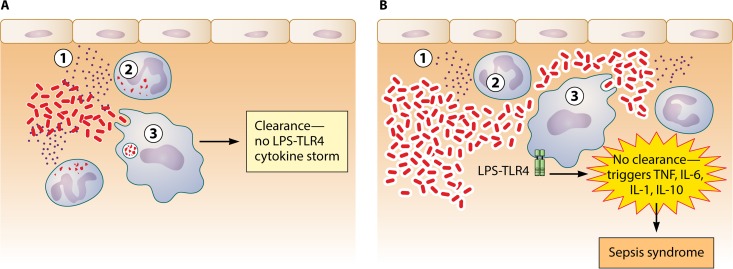
From these studies, an integrative overview of Acinetobacter species virulence is beginning to coalesce (Fig. 1). A. baumannii virulence appears to be driven initially by its ability to evade complement and phagocytosis, likely through its capsular composition and abundance. A large infectious inoculum and depletion or reduction of host innate effectors are also ways that the balance can be tipped in favor of microbial escape. If the organisms are able to evade innate immune clearance, the second virulence phase is initiated by LPS triggering of TLR4-mediated sepsis.
FIG 1.
Host fate during Acinetobacter infection is determined in two stages. (A) Early clearance of the microbe by the three primary innate effectors, complement (circled 1), neutrophils (circled 2), and macrophages (circled 3), results in prevention of a sustained LPS-TLR4 activation and subsequent cytokine storm. (B) If the organism can resist initial innate effector clearance and replicate, it triggers sustained LPS activation of TLR4, resulting in cytokine storm and sepsis syndrome. One mechanism by which the organism may be able to evade clearance is by expression of an altered capsule that resists complement and phagocytic uptake (denoted by thicker shell around the bacteria).
This integrated overview also underscores why clinical outcomes of Acinetobacter infections are much worse when ineffective therapy is administered initially. Early effective therapy helps the host rapidly clear the bacteria, avoiding subsequent host damage from the sepsis response, whereas early administration of ineffective therapy enables the bacteria to persist at higher blood or tissue bacterial densities, triggering host damage.
Antibiotic Resistance and Virulence
As discussed in depth below, the most important determinant in clinical outcome of Acinetobacter infections is antibiotic resistance. While antibiotic resistance may not be a traditional virulence factor, it is by far the biggest driver of clinical outcome by precluding the clinician's ability to kill the infecting strain. Traditional thinking has been that antibiotic resistance causes a metabolic cost to the bacterium and hence is an “anti-virulence” factor. Indeed, in a recent study, high-level antibiotic resistance was shown to exert a decrease in virulence for one A. baumannii strain in mice (132). However, the attenuated virulence was modest. Mutations leading to resistance delayed lethal infection in mice by several days, yet almost all the mice infected with the resistant strain still died in the end. In another recent study, introducing a multidrug-resistant phenotype by altering the Acinetobacter drug efflux (Ade) systems either had minimal impact on virulence or, in one strain, enhanced virulence as assessed by competitive fitness and bacterial burden in nonlethal mouse models (101). Thus, the role of resistance in affecting intrinsic virulence is complex.
Perhaps more clinically important, the therapeutic power of antibiotics is by far the most influential variable in outcome of infections. Resistance to our therapeutic armamentarium does not intrinsically exacerbate the virulence of the infecting strain. However, by eliminating the efficacy of antibiotics, antibiotic resistance precludes our ability to speed clearance of the organism and, in accordance with the model of pathogenesis described above, thereby affects clinical outcomes in patients. Therefore, a limitation of our current models of virulence and infection are that they are not performed in the presence of antibiotics and do not account for antibiotic treatment when assessing an organism's virulence potential. To the extent that studies of virulence expect to correlate with clinical outcomes, new models of understanding virulence and its interactions with antibiotic resistance may be warranted.
ANTIBIOTIC RESISTANCE DRIVES OUTCOMES
The primary challenge of treating Acinetobacter infections centers upon overcoming antibiotic resistance. As early as 1977, a case series found that ventilator-associated pneumonia caused by Acinetobacter initially treated with effective antibiotics had a 14% mortality rate compared to an 82% mortality rate among patients infected with strains resistant to standard β-lactam therapy who received ineffective initial therapy (1). Unfortunately, Acinetobacter is one of the most resistant organisms encountered in clinical medicine, making initiation of effective empirical therapy challenging.
The general resistance of Acinetobacter to antibiotics stems in part from the very small number and size of porins in its outer membrane. The reduced outer membrane porin content confers upon Acinetobacter a low permeability to antibiotics, indeed far lower than for other Gram-negative organisms (133). As a matter of scale, the coefficient of permeability (rate of diffusion from outside to inside the bacteria) for cephalosporins is two- to sevenfold larger in P. aeruginosa than in Acinetobacter (133). Furthermore, the rate of carbapenem diffusion into liposomes containing purified outer membrane derived from A. calcoaceticus was 1 to 3% compared to Escherichia coli outer membrane (134). In addition, Acinetobacter possesses constitutive low-level expression of one or more active efflux systems (e.g., AdeABC and AdeIJK) (133). This interplay between low permeability to antimicrobials and constitutive efflux allows for an inherent resistance to a broad array of antibiotics resulting in limited therapeutic options.
In addition, Acinetobacter possesses a massive resistance island within its genome, which is comprised of 45 resistance genes (135, 136). Moreover, it has the capacity to rapidly acquire additional genetic entities for resistance from other bacterial species (135, 136) and the ability to develop resistance to antibiotics in the middle of a course of therapy (137).
A critical problem is the rise of carbapenem resistance among Acinetobacter. Acinetobacter isolates have shown a complex interaction of multiple mechanisms of resistance to carbapenems, with the production of naturally occurring oxacillinases (OXA) and the absence of PBP2 being most commonly observed; for some isolates, an additional downregulation of porin expression and subsequent reduction in carbapenem entry has been observed (138). The predominant oxacillinases (OXA-23, OXA-24 or -40, OXA-51, OXA-58, and OXA-143) are responsible for the majority of phenotypic resistance to carbapenems detected in the United States, Latin America, Europe, Asia, and in many parts of the world (31, 139–150). OXA-23 is a plasmid- or transposon-encoded β-lactamase, while OXA-51 is a chromosome-based enzyme and is intrinsic to Acinetobacter. OXA-24/40 can be chromosomal or plasmid based, and OXA-58 is plasmid encoded. These class D β-lactamases are not very robust carbapenemases, but the presence of an insertion sequence (IS) element, such as ISAbaI and ISAba9, increases expression of the carbapenemase significantly, resulting in clinical carbapenem resistance (140, 148, 151–155).
Acinetobacter also possesses class B β-lactamases (metallo-β-lactamases [MBLs]) (156). The increase in the number of MBLs in A. baumannii is an ominous development in the global emergence of resistance in this pathogen. The MBLs described—IMP, VIM, SIM, and NDM—are all found in Acinetobacter (156, 157). The presence of NDM, a widespread MBL, in Acinetobacter deserves special mention. Acinetobacter may play a very important role in spreading blaNDM genes from its natural reservoir to Enterobacteriaceae. In Acinetobacter, the blaNDM-type genes are found to be located on either the plasmid or chromosome. Among NDM-producing A. baumannii, the blaNDM gene is usually reported to be found between two copies of the ISAba125 element, forming a composite transposon named Tn125 (158–160). Identification of a truncated form of this composite transposon in Enterobacteriaceae, while reported in its entire form in A. baumannii, strongly suggests that Acinetobacter spp. were the source of those blaNDM genes before their transmission to Enterobacteriaceae (158, 160). Klebsiella pneumoniae carbapenemases (KPCs) and Guiana extended-spectrum (GES) β-lactamases are rarely reported in Acinetobacter (161–165).
In addition to the aforementioned enzymes, porins contribute to carbapenem resistance, the major ones being CarO and OprD (166–171). These porins constitute channels for influx of carbapenems and can contribute to resistance whereby a reduction in carO transcription results in downregulation of the CarO porin system and thus a decrease in carbapenem entry.
One of the remarkable features of antibiotic resistance in Acinetobacter is the flexibility and ease of its transmission. Transposon-mediated passage of resistance mechanisms are well described, including those for AmpC cephalosporinases, OXA carbapenemases, KPC serine carbapenemases, and NDM or VIM metallo-carbapenemases, and aminoglycosides (101, 135, 136, 147, 148, 150, 155, 158, 159, 172–178). Indeed, transposon-mediated resistance to classically described antibiotic classes, including β-lactams, tetracyclines, aminoglycosides, and sulfonamides, had occurred in a global lineage of A. baumannii by the late 1970s; new resistance was subsequently acquired in transposon lineages in the 1980s as new antibiotics became available (136). Transposons can transmit chromosomal resistance mechanisms to plasmids and vice versa and can readily insert in preexisting resistance elements, increasing their expression and hence resulting phenotypic resistance. Furthermore, transposon transfer of resistance can lead to accumulations of large copy numbers of resistance genes or transposons. For example, a patient treated with tobramycin experienced a remarkable rise in resistance concurrent with the isolated strain (172). The MIC increased from 0.5 to 16 μg/ml in only 4 days of therapy and was due to amplification of copy numbers of the Tn6020 transposon containing the aphA1 gene mediating resistance to tobramycin. In only 4 days, the number of the transposons in the strain increased to 65 copies, leading to high-level antibiotic resistance. These data underscore the fluidity and flexibility of A. baumannii to become antibiotic resistant in the middle of a course of antibiotic therapy and the important role that transposons play in this emergence of resistance.
Without a doubt, a key determinant in patient survivability from Acinetobacter infections is the early initiation of effective antimicrobial therapy. Unfortunately, initiation of effective therapy is a particular problem for Acinetobacter infections given the frequency of resistance. Consequently, ineffective antimicrobial treatment is more common for Acinetobacter than most other pathogens, resulting in a dramatic increase in mortality (24, 26, 179, 180).
Acinetobacter is one of several Gram-negative species that routinely demonstrates an XDR phenotype, defined as resistance to all available systemic antibiotics except for those that are known to be less effective or more toxic compared to first-line agents used to treat susceptible pathogens (127). The hallmark of the XDR phenotype is carbapenem resistance. CRAB strains tend to be XDR—that is, resistant to all other antibiotics, with the exception of polymyxins, tigecycline, and sometimes aminoglycosides (50, 52, 53). NHSN and Eurofins surveillance data highlight that carbapenem resistance occurs in more than 50% of A. baumannii ICU isolates in the United States, by far the highest rate of resistance for any pathogen surveyed (27, 181, 182). Resistance rates are even higher in eastern and southern Europe, Latin America, and many Asian countries. Overall, the proportion of global XDR strains of A. baumannii has increased from <4% in 2000 to >60%, while the proportion of XDR strains of A. baumannii in some regional nosocomial settings has more recently approached 90% (27, 31, 35, 42, 43, 183–190).
XDR A. baumannii infections are generally only treatable with relatively ineffective second-line agents, such as tigecycline or polymyxins (discussed further below). Such infections cause longer hospitalization, increased costs, and greater mortality than infections caused by carbapenem-susceptible strains (44, 183). Indeed, due to their resistance to first-line agents, XDR A. baumannii bloodstream infections result in >50 to 60% mortality (44, 191). Furthermore, by multivariate analysis, A. baumannii was one of only two organisms strongly linked to increased mortality, out of 19 microorganisms evaluated (46). The odds ratio for death caused by A. baumannii was 1.53—the highest of all Gram-negative species (192). Similarly, a study of multiple ICUs in a Brazilian hospital found that infection caused by Acinetobacter was more commonly treated with initially ineffective therapy (88% versus 51% of the time) and nearly doubled the mortality rate of infection compared to infection caused by other pathogens encountered (hazard ratio of death of 1.9 by multivariate analysis) (193).
The excess mortality caused by XDR Acinetobacter is underscored by a study in Taiwan, where the most common cause of ICU bloodstream infections is A. baumannii (153). The overall mortality of 33%, while alarming, was even more pronounced when examining strains that were carbapenem resistant or XDR. Nearly a third of patients were infected with such strains. Despite having similar comorbidities to other patients, those infected with XDR strains had a significantly elevated mortality rate of 70% versus the mortality rate of 25% caused by susceptible strains (153). Administering initially effective therapy was the key to improving outcomes for infections caused by all strains (both XDR and non-XDR); 30-day mortality rates were 60% without initially effective therapy compared to 20% with effective therapy (153). For infections caused by XDR/carbapenem-resistant strains, treatment with tigecycline or colistin within 48 h still markedly reduced the mortality rates from >88% to <38% (153). Thus, ineffective initial therapy was likely the primary driver of outcome differences, rather than any other virulence differences between carbapenem-susceptible and -resistant strains. Similarly, in a recent study of patients infected with A. baumannii in U.S. ICUs, receipt of initially ineffective therapy doubled mortality (180).
CLINICAL MANIFESTATIONS
The two most common clinical manifestations of A. baumannii are nosocomial pneumonia and bacteremia. Nosocomial pneumonia occurs as a result of aspiration. In particular, the presence of an endotracheal tube creates an ideal nidus for the environmental transmission of Acinetobacter, which avidly adheres to plastic and can establish biofilms on the tube (194, 195). Aspiration of droplets of Acinetobacter directly into the alveoli of mechanically ventilated patients circumvent natural host barriers, allowing for establishment of infection in tissue. Similarly, A. baumannii bloodstream infections typically occur in the presence of a central venous catheter or secondarily due to extensive pneumonia, facilitating dissemination. Other well-described manifestations of A. baumannii include urinary tract infections (typically associated with urinary catheters or percutaneous nephrostomy tubes), wound infections or osteomyelitis (typically postsurgical or trauma related), endocarditis, and meningitis (typically postsurgical or in the presence of a ventriculostomy) (26, 27, 196–198).
Community-acquired infections predominantly occur in warm, humid, tropical environments, especially in parts of Australia, Oceania, and Asia, including China, Taiwan, and Thailand (199, 200). A prevailing finding has been the presence of comorbidities in such patients, including diabetes, kidney disease, cancer, or chronic obstructive pulmonary disease, and particularly with regard to pneumonia, heavy smoking, and excessive alcohol consumption (26, 199). These community onset infections present with acute pneumonia and, in rare occurrences, with meningitis, cellulitis, or primary bacteremia (26). As with nosocomial cases, inappropriate initial antimicrobials were strongly associated with increased mortality for community onset infections (201).
Another scenario in which Acinetobacter has been a major cause of infection is among wound, soft tissue, and invasive (blood and bone) infections in soldiers in Afghanistan and Iraq, particularly after traumatic injury (197, 202–208). Similar infections have occurred in trauma victims after natural disasters, such as floods and earthquakes, or bystanders in areas of active military conflicts (209–215).
Recent reports of necrotizing fasciitis caused by A. baumannii or A. calcoaceticus are especially alarminģ reflecting a deadly convergence of virulent infections and antibiotic resistance. These cases have typically been reported for immunocompromised patients, mostly in patients with HIV, hepatic cirrhosis, solid-organ transplant, or diabetes mellitus (216–221). They have been either community- or hospital-onset cases and have not necessarily been the result of recognized, antecedent trauma. Acinetobacter has even caused septic shock due to necrotizing fasciitis in a house cat (222).
The latter case emphasizes that household pets can carry and become infected by Acinetobacter. Two recent studies from Réunion Island found that 5 to 10% of household pets (dogs and cats) seen in veterinary clinics on the island carried A. baumannii (223, 224). Most of the pets were seen for routine outpatient clinic visits and were not hospitalized. Although Acinetobacter is an established cause of infection in veterinary hospitals (225), carriage among healthy pets has not been well described outside this unique island setting and merits further investigation in the future.
CURRENT TREATMENT OPTIONS
Because of its propensity to develop resistance to antibiotics, current treatment strategies for Acinetobacter remain extremely limited. β-Lactam antibiotics are the preferred antibacterial choices for susceptible A. baumannii infections, and susceptible pathogens respond briskly to therapy. Due to rising resistance, carbapenems have become an increasingly critical therapeutic option for these infections. For carbapenems (as all β-lactams), the best predictor of efficacy is the time serum carbapenem concentrations remain above the MIC. Extended infusion of carbapenems can maximize time above MIC, thereby optimizing outcomes, particularly for resistant pathogens (226–228). As such, leveraging this property can result in a significant therapeutic effect in strains with low- to intermediate-range MICs.
Risk analysis by Monte Carlo simulation for Acinetobacter spp. predicted that a dose of 1 g meropenem administered every 8 h (administered as a 3-h infusion) would provide optimal bactericidal rates (227). Similarly, a meta-analysis of retrospective clinical studies (not specific for Acinetobacter infections) found that extended carbapenem infusion times resulted in lower mortality rates than rapid infusion, without any evidence of increased rates of resistance emergence (229). However, the pharmacokinetic cost of prolonging the infusion is a decline in the peak level of the drug. At carbapenem MICs of >16 μg/ml, it is likely that peak levels will never exceed the MIC, and modeling indicates lower ability to achieve the time above MIC necessary to optimize bacterial killing with extended infusion (227). Hence, extended infusion should be strongly considered for isolates with MICs of 4 to 16 μg/ml, but intermittent dosing to achieve peak levels above the MIC may be preferred for isolates with higher MICs.
Unfortunately, as mentioned, carbapenem resistance rates for A. baumannii have been rising dramatically both in the United States and globally. For carbapenem-resistant strains, the antibiotic armamentarium is quite limited. There is no consensus on optimal antimicrobial treatments for such strains.
One option to treat XDR infections is tigecycline. While tigecycline often has a low MIC (<2 μg/ml) for A. baumannii strains, the drug's serum concentrations are also low, and in clinical trials, outcomes in patients with ventilator-associated pneumonia and bacteremia have been clearly inferior to alternative agents (230–232). In particular, retrospective clinical data validate the susceptibility breakpoint of tigecycline at 2 μg/ml. Specifically, infections caused by Acinetobacter strains with tigecycline MICs of ≥2 μg/ml result in significantly increased mortality when treated with tigecycline (137, 233–235). Even if the MIC is ≤2 μg/ml, Acinetobacter bacteremia may have inferior outcomes when treated with tigecycline, including worse survival, failure to clear bacteremia, and development of breakthrough bacteremia (236). A recent systemic review and meta-analysis (not specific to Acinetobacter infections) found that tigecycline therapy resulted in higher in-hospital mortality, inferior microbial eradication, and a trend toward longer hospital stays (237).
As an alternative to tigecycline, interest has emerged in the use of minocycline to treat Acinetobacter infections (238–240). Minocycline may retain antimicrobial activity even against strains resistant to other tetracyclines (including tigecycline)—although cross-resistance has been seen. A recent, large, international survey of more than 1,000 global Acinetobacter strains (including XDR strains) found that minocycline was the most active tetracycline in vitro, with more than 70% of strains susceptible per the CLSI breakpoint of MIC of ≤4 μg/ml (241). It should be emphasized that the breakpoint is not well validated based on clinical outcome data and that its value is the same as the mean, peak blood level of minocycline when administered as a 200-mg intravenous (i.v.) dose (239). Thus, caution may be warranted when using minocycline to treat a strain with an MIC of 4 μg/ml, particularly as monotherapy and in the setting of bacteremia. Only limited clinical data are available describing outcomes of Acinetobacter infections treated with minocycline. Goff and colleagues described perhaps the largest case series, in which 55 patients were treated with minocycline, but only 3 received monotherapy (240). More than 70% of patients had a favorable clinical response; 25% died. It is difficult to interpret this efficacy rate absent a control group.
As an alternative to tetracyclines, a unique feature of Acinetobacter infections is the potential therapeutic role of sulbactam. Sulbactam is used to inhibit β-lactamase enzymes for most pathogens, but it has direct antimicrobial activity against Acinetobacter. Sulbactam is a class A β-lactamase inhibitor similar in structure to β-lactams, but it has an intrinsic affinity for the A. baumannii penicillin-binding proteins (242, 243). A major disadvantage of sulbactam is that it is only available in combination with ampicillin within the United States. A more concerning feature is that there have been increasing rates of sulbactam resistance encountered (243). Two major studies (one in Spain and another in Taiwan) identified the rate of sensitivity of Acinetobacter to sulbactam at only 47% and 30%, respectively (243). However, high-level sulbactam resistance may, at least in animal models, convey a significant fitness cost to the organism (242).
Additional potential options for definitive therapy if β-lactams cannot be used include fluoroquinolones and aminoglycosides, although neither should be considered preferred options for empirical therapy because rates of resistance to both classes are high. Resistance to fluoroquinolones occurs via chromosomal mutations of the parC and gyrA genes encoding bacterial topoisomerase IV subunits (151, 155, 244, 245). Reduced permeability of the bacterial outer membrane to quinolones and active efflux mechanisms also contribute to the organism's resistance (244). Among the aminoglycosides, amikacin and tobramycin offer the greatest reliability in susceptibility of Acinetobacter isolates (246). Aminoglycoside-modifying enzymes are widespread and are particularly prevalent in integrons within multidrug-resistant Acinetobacter strains (247). Surveillance data from 2009 found a 59% rate of sensitivity to tobramycin; it is unsurprising that the rate has decreased further since then (248). Prolonged use of aminoglycosides is often avoided due to concerns of drug toxicity. However, a retrospective cohort study found comparable toxicity and microbiologic clearance with tobramycin compared to colistin (246). Therefore, when susceptibility permits, aminoglycosides can be a potential treatment option.
Given the limitations of most of the non-β-lactam antimicrobial options, presently, polymyxins are often the last treatment option for XDR Acinetobacter. Unfortunately, polymyxins suffer from high rates of nephrotoxicity and neurotoxicity. They possess no therapeutic window: doses that are effective are also toxic. Although it remains uncommon, resistance to polymyxins has been reported and is increasing (182, 249).
Clinical data are now emerging that evaluate MIC breakpoints for colistin resistance. In one recent series, patients treated with colistin whose infecting isolate of Acinetobacter had a colistin MIC of 1 to 2 μg/ml had twice the risk of 14-day mortality as patients infected with isolates having lower MICs (250).
A potential limitation to polymyxin therapy is its relatively poor epithelial lining fluid penetration in the lung (251). Imberti et al. found that in critically ill patients, colistin was not detected in bronchoalveolar lavage specimens taken 2 h after intravenous infusion of the drug (251). In contrast, nebulizing polymyxins has the potential to achieve very high concentrations in the lungs while minimizing systemic exposure and toxicity. Numerous investigators have described favorable outcomes of patients with nonbacteremic Acinetobacter pneumonia who were treated with nebulized polymyxins (252–261). In one case-control study, use of inhaled colistin increased microbial eradication rate from the airways in ventilated patients compared to systemic therapy (257). In another study, patients with ventilator-associated pneumonia had superior cure rates, with similar mortality, when inhaled colistin was added to intravenous therapy (258). In contrast, Demirdal et al. found no difference in microbiological eradication, clinical outcome, recurrence, or mortality among 80 patients with Acinetobacter pneumonia who received systemic colistin alone versus 43 patients who received systemic colistin combined with inhaled therapy (262). Fortunately, addition of inhaled colistin did not increase the rate of acute renal injury. Thus, at this point, the data are mixed regarding whether adding inhaled colistin to systemic therapy improves clinical cure. Importantly, several studies have reported favorable outcomes of patients with ventilator-associated pneumonia caused by highly resistant Gram-negative bacteria, including XDR A. baumannii, treated with monotherapy inhalational colistin without adjunctive systemic therapy (255, 259, 261, 263). However, these results should be viewed cautiously, as cases were nonrandomized leading to a potential selection bias.
While it may be true that inhalational therapy minimizes systemic toxicity, colistin can be toxic to lung tissue and induce bronchospasm, and nephrotoxicity can occur with accumulated dosing. Furthermore, resistance has emerged during therapy, although with variable frequency depending on the case series (255, 261, 263).
Indeed, rising rates of resistance to the last-line agents, tigecycline and polymyxins, among A. baumannii are of substantial concern. These strains tend to be pan-resistant, with no available antibiotic therapies left. Tigecycline resistance now exceeds 50% in some regions, and polymyxin resistance rates as high as 20% have been seen in Greece, and are increasingly reported in other regions as well (189, 249, 264–266). These pan-resistant isolates underscore the critical need to identify alternative therapeutic strategies.
COMBINATION ANTIMICROBIAL THERAPY
Given the limitations of monotherapy for XDR strains and the emergence of pan-resistant strains, combination therapy has been advanced as a potential option to improve treatment outcomes and possibly to prevent new emergence of resistance. Experts have long debated whether the use of combination regimens of antibiotics could prevent the emergence of resistance among typical bacterial pathogens, as is clearly the case for Mycobacterium tuberculosis (tuberculosis [TB]) (267–269). However, there are few data to suggest that combination regimens can reduce the emergence of resistance in vivo, particularly for Gram-negative bacterial infections (268, 270, 271). For Acinetobacter specifically, systematic reviews have not found that combination therapy is more effective at preventing emergence of resistance and could not draw general conclusions for therapeutic outcome based on heterogeneity of the data (272, 273). A recent case series from Greece of XDR A. baumannii infections also found no clinical advantage of colistin-based combination regimens compared to colistin alone (189).
With respect to emergence of resistance, it is important not to draw conceptual parallels regarding the use of combination therapy to treat Acinetobacter or TB. M. tuberculosis has no environmental reservoir, spends the majority of its life cycle during infection in a nonreplicating persister state, and is generally treated with drugs with minimal antimicrobial activity against typical bacterial pathogens (e.g., isoniazid, ethambutol, and pyrazinamide; rifampin being the exception). Thus, use of multiple drugs to treat TB in patients results in minimal selection of resistance to other pathogens among the patient's normal flora, and there are no TB bacilli in the environment to have resistance selection occur. For Acinetobacter, the situation is very different. The antimicrobial agents used to treat Acinetobacter do select for resistance, often on mobile genetic elements, among normal flora and among environmental reservoirs of organisms. Even if administration of more than one antibiotic to a patient were to slow the emergence of resistance at the site of infection in that patient, selection of resistance to each drug administered would occur in the patient's normal flora and in the environment. Conceptually, in the long run, this creates the risk of accelerated emergence of resistance, i.e., to each of the multiple drugs used in a combination regimen rather than a single drug used as monotherapy. At the current time, it is not known if there are advantages to combination regimens to prevent the emergence of resistance among Acinetobacter (272, 273) or how rapidly combination regimens would result in selection of resistance to each drug separately among normal flora and in the environment.
However, aside from preventing resistance, combination regimens may be useful to improve clinical outcomes or microbial eradication in patients infected with XDR strains. In vitro models suggest that rifampin is bactericidal for Acinetobacter isolates (59). Despite promising small studies, in a larger randomized, multicenter trial, the mortality was unchanged between patients with XDR A. baumannii infections treated with colistin and rifampin versus colistin alone (274). However, there was superior microbial eradication by culture confirmation in patients randomized to the combination arm. Thus, combination therapy with rifampin may be of benefit in situations where there is an infected foreign body or where the infection is in a sequestered site (e.g., central nervous system [CNS], bone) into which most antibiotics penetrate poorly and would have difficulty sterilizing tissue.
Other combination regimens have been examined and although randomized human studies have not been conducted, in vitro and animal models suggest increased efficacy of sulbactam in combination with cefepime, meropenem, imipenem, amikacin, or rifampin (243). Alternative combinations include colistin-tigecycline and colistin-carbapenem therapy, each without clear evidence of benefit (233). One point is clear: tigecycline combination therapy should not be used to treat isolates with a tigecycline MIC of ≥2 μg/ml. Patients infected with such strains displayed excess mortality when tigecycline was used, even as part of a combination regimen (137, 233).
Among all the combination regimens, the one that may be most rational is colistin-carbapenem, which has been found to be synergistic in multiple in vitro studies (275–279). This synergy may be observed in particular for isolates with intermediate resistance to the carbapenems (e.g., if the carbapenem MIC is 4 to 16 μg/ml). Peak levels of carbapenems exceed these MICs at standard dosing, and in vitro synergy has been described between colistin and carbapenems in this range (276).
Given the complexity and limitations of the data, there remains considerable debate regarding the utility of combination treatment for XDR infections. A retrospective review evaluating monotherapy for Acinetobacter found comparably low efficacy with either tigecycline or colistin, achieving unfavorable clinical success rates of 47% and 48%, respectively (61). However, combination therapy, chiefly with carbapenem or sulbactam, showed a trend toward lower mortality and improved clinical and microbiological success rates (61). Prospective, randomized studies of combination therapy are critically needed to determine whether combination regimens are superior to monotherapy and, if so, what is the preferred combination of agents.
While there are intriguing suggestions that combining colistin with glycopeptides may result in superior outcomes (280), a recent comparative clinical study found no difference in survival or clinical cure, despite a marked decrease in nephrotoxicity among patients treated with the combination versus colistin alone (55% versus 28% [P = 0.04]) (281). Conversely, a retrospective review of 68 patients treated for more than 5 days with colistin plus glycopeptide therapy found no difference in nephrotoxicity and a higher survival rate than colistin monotherapy (282). Given the mixed results—particularly the negative finding in the prospective study—these data await confirmation in a randomized controlled trial.
In summary, absent clear evidence of benefit with combination therapy, monotherapy is preferred for β-lactam-susceptible strains. For XDR strains, however, one rational approach is to consider combining colistin and carbapenem therapy, particularly if the carbapenem MICs are 4 to 16 μg/ml and preferably utilizing extended infusion administration if the MICs are 4 to 8 μg/ml. This approach attempts to supplement the therapeutic effect of the last-line polymyxin therapy with a pharmacokinetically optimized carbapenem. Rifampin may be useful to add for infections of the CNS, bone, or prosthetic materials.
Finally, in cases of nonbacteremic XDR Acinetobacter pneumonia, it is reasonable to consider addition of inhaled colistin, minimizing toxicity and maximizing levels delivered to the lung, as an adjunct to systemic therapy with carbapenem or other agents. The use of nebulized colistin as monotherapy may be a reasonable alternative in nonbacteremic patients with pneumonia to avoid systemic nephrotoxicity. However, the potential for nephrotoxicity is not completely eliminated by this approach, bronchospasm can occur, and the potential for resistance selection has not been fully elucidated. Further study is needed to determine whether other combination regimens are useful, preferably in the form of randomized controlled trials.
FUTURE TREATMENT OPTIONS
New Antibiotics
Several companies are working at the preclinical stage on small-molecule antibiotics that have specific activity focused on Acinetobacter. Entasis Therapeutics has a novel β-lactamase inhibitor combined with sulbactam (283). Spero Therapeutics has a polymyxin-based potentiating peptide that is combined with other antibiotics for Gram-negative bacteria, including Acinetobacter (284). Melinta is developing a novel, ribosomal protein synthesis inhibitor with broad Gram-negative activity, including against A. baumannii (285). Tetraphase Pharmaceuticals is developing eravacycline, a novel fluorotetracycline which has expanded activity against Acinetobacter. Eravacycline has completed phase III clinical trials for complicated intra-abdominal infections (cIAI) and complicated urinary tract infections (cUTI); more trials will likely be required based on unexpectedly low cure rates for cUTI (286).
Antimicrobial Peptides
Novel therapies remain under investigation and offer exciting potential for bypassing the permeability, efflux, and enzymatic resistance mechanisms that make Acinetobacter a tremendously adaptable pathogen. For example, antimicrobial peptides are a common host defense mechanism, and most commonly, they function to disrupt bacterial membranes. The failures of antimicrobial peptides in clinical development are countless, and some key opinion leaders have begun to doubt whether antimicrobial peptides will be viable therapeutic options for systemic infections (in contrast to topical applications) without substantial further scientific advancements (287). Nevertheless, such peptides can have broad-spectrum activity against highly resistant Gram-negative bacteria, including Acinetobacter. For example, cecropin A-melittin hybrid proteins have shown in vitro bactericidal activity against resistant A. baumannii, but a short half-life has limited their utility thus far (288). Peptides derived from frog and toad skin have been shown to have strong bactericidal activity. Highlighting this potential, in preclinical models of blood and wound infections treatment with A3-APO (a proline-rich antibacterial peptide) improved survival and decreased bacterial density more than either imipenem or colistin (288). Before peptide therapies can be reliably used systemically, they must overcome problematic pharmacology, toxicity, and inactivation in the context of biological matrices such as serum, surfactant, and other biological fluids and tissues.
Phototherapy
Phototherapy utilizes the combination of oxygen, infrared light, and a photosensitizer (a nontoxic, photoreactive dye) to generate reactive oxygen species that can damage DNA and disrupt cellular membranes. This modality is limited to topical use and carries the potential for local tissue injury from reactive oxygen species. Although few studies have attempted to examine the roles of photosensitizer agents in Acinetobacter infection, tetrapyrrole porphyrins and phenothiazinium salts have the most support. Studies suggest that phototherapy can exert a bactericidal effect via disruption of lipopolysaccharide with tetrapyrrole choline applied topically, effectively reducing bacterial density 1,000-fold in a mouse model (288).
Bacteriophages
At long last, given the marked rise in resistance to antibiotics and failure of the antibiotic pipeline, there has been renewed interest over the last 2 decades in bacteriophages capable of lysing bacteria. While interest in bacteriophages predates the antibiotic era, by the late 1920s to early 1930s, there were already concerns about the quality of data supporting efficacy in patients and concerns about the potential for biological matrices to neutralize bacteriophage effects (289). While it remains unclear if phages will demonstrate efficacy for systemic infections (as opposed to topical or gastrointestinal), recent years have witnessed a substantial renewed interest in their potential (290).
With respect to Acinetobacter, AB1 and AB2 were identified in 2010 as the first two phages specific for A. baumannii; since that time, numerous other phages have been identified with lytic activity of Acinetobacter (288, 291). Several rodent studies have suggested that strain-specific phages can improve outcomes from intranasally inoculated lung infection, intraperitoneal sepsis, or wound infections (288, 292–294). Lysin peptides from phages have also been studied as effective treatments for A. baumannii infections in mice (295, 296).
The limitations of phage therapy remain substantial. For example, phage therapy requires very precise matching of phage to bacterial strain type; Acinetobacter is an exceedingly diverse genus. In one study, phage AB1 induced lysis in only one of five multidrug-resistant isolates; likewise, phage AB2 was capable of infecting only 25 of 125 (20%) of clinical isolates examined (288). Subsequent studies of phage Abp53 and phage ZZI were able to lyse only 27% and 13% of multidrug-resistant isolates, respectively (288). More recently identified phage strains have shown greater efficacy, but even phage AP22 had activity in only 89 of 130 isolates (68%) and remains one of the most broadly acting phages identified thus far (288).
A cocktail of multiple phages may be able to overcome the limitations of each individual phage. Limited studies of a multiphage cocktail resulted in growth inhibition, but again, with an effect highly dependent on the Acinetobacter strain (297). Regeimbal et al. describe the efficacy of a five-member phage cocktail in reducing the bioburden in a neutropenic murine full-thickness wound (298). Interestingly, this cocktail included four phages that did not directly kill bacteria but rather acted in a combinational manner to delay bacterial growth and targeted encapsulated strains, shifting the population to a predominantly unencapsulated state. The caveat remains that mice were neutropenic, blunting a potential concurrent host immune response that could neutralize the phages, the phage cocktail was individualized to the inoculated Acinetobacter strain, and the cocktail was administered by both intraperitoneal and topical administration. This raises questions about the large-scale applicability of producing personalized phage cocktails and their potential efficacy in a systemic infection model. However, it does raise unique options for phage therapeutics as a means to target specific virulence factors, in this case bacterial capsule, to increase sensitization for eradication by antimicrobial, host immune, or other combinational modalities. Beyond bactericidal activity, phages also hold the potential for decolonization and for disruption of biofilm formation; phage AB7-IBBI and AB7-ABB2 were capable of disrupting 75% of preformed biofilms in one study (288).
As promising as studies have been, phage therapy is far from clinical use. Additional concerns with phage therapy include the potential for secondary effects on human flora, emergence of phage resistance akin to antibiotic resistance, and the potential for production of host inflammatory responses following phage administration. Likewise, the long-term and reproducible viability of phage therapies may be a concern due to rapid clearance by human macrophages and the induction of antiphage antibodies. Clarification of the potential for phage therapy to treat Acinetobacter infections awaits additional study.
Active and Passive Vaccination
Another interest is the development of vaccines targeting Acinetobacter for preventative or therapeutic purposes (299). A major challenge of developing a vaccine targeting Acinetobacter is the diversity of the genus overall and of the A. baumannii species in particular. Nearly 40 serotypes have been identified in A. baumannii, and the prevalence of each serotype is largely unknown. A monoclonal antibody targeting the K1 capsular polysaccharide recognized only 13% of Acinetobacter strains (113). Capsule is an attractive target for an antibody-based vaccine, but such a vaccine would have to be multivalent given the number of different capsular types that might need to be included.
With respect to protein targets, the one that has garnered the most attention is outer membrane protein A (OmpA). OmpA is highly conserved among clinical A. baumannii isolates and has several extracellular loops that might be amenable to vaccination (55). Vaccination with recombinant OmpA did indeed engender protection against bacteremic sepsis in a manner that appeared to be dependent on antibody (55). Higher doses of vaccine were found to result in superior humoral immune responses due to type 2 cytokine polarization (300). Another promising target that has been reported to have protective activity as a vaccine in mice is highly conserved nuclease, NucAb (301). The vaccine markedly reduced bacterial burden, although its improvement of survival was less impressive. Other outer membrane proteins, such as OmpW, have also been reported to be effective vaccines (302, 303). However, a challenge of any active vaccine for Acinetobacter is to define a patient population that is of sufficiently high short-term risk of infection to make efficacy definable in a reasonably sized clinical trial, while still allowing sufficient time for the at-risk patient to generate protective immunity to an active vaccine before infection begins.
In contrast, in passive immunization, antibodies could be administered and result in immediate protection, without having to wait for a lymphocyte response to a vaccine. Indeed, passive transfer of polyclonal anti-OmpA, anti-OmpW, or anti-NucAb immune serum from vaccinated mice transferred protection to recipient mice, validating the approach (300, 301, 303). In other experiments, antibodies against the outer membrane transporters and polysaccharides were able to facilitate opsonization and subsequent phagocytosis of A. baumannii leading to decreased tissue loads (304). The challenge for passive immunization will be to identify a monoclonal antibody or mixture of several monoclonal antibodies that are capable of covering 90 to 95% of clinical isolates of this highly diverse genus or even the A. baumannii species.
Trace Metal Sequestration
Sequestration of host iron and other trace metals is another novel strategy that has potential to ameliorate the severity of Acinetobacter infections (305–308). Multiple studies conducted by prominent laboratories have demonstrated that iron or zinc limitation can suppress Acinetobacter growth and that the organisms have multiple iron and zinc acquisition mechanisms, including catechol production, production of very high-affinity siderophores (e.g., acinetobactin) and companion siderophore uptake receptor and energetic mechanisms, and heme utilization (82, 121, 308–320). Small-molecule iron chelators have in vitro activity at killing Acinetobacter during log growth (318). Thus, combatting iron or zinc uptake in the bacteria is a novel, potentially impactful therapeutic approach.
Transferrin is a predominant iron-sequestering agent in the blood of mammals (306). Despite the much higher affinity for iron of bacterial siderophores compared to transferrin (e.g., affinities of ≥10−30 M versus 10−24 M), sufficient quantities of transferrin can overwhelm and outcompete the siderophores produced by bacteria; the bacteria are thus unable to acquire sufficient iron to maintain viability. Specifically, exposure of Acinetobacter (and other pathogens) to transferrin in vitro resulted in depletion of intracellular iron and zinc levels in the bacteria, with resulting cell membrane depolarization due to inability to maintain cellular energetics absent sufficient iron, and static growth inhibition by time-kill curves (72). Furthermore, i.v. transferrin therapy of mice infected intravenously with XDR, hypervirulent A. baumannii HUMC1 resulted in marked improvement in survival (72).
Another metal-sequestering agent demonstrated to have activity against Acinetobacter is calprotectin (317). Calprotectin is a neutrophil-derived host defense molecule that sequesters zinc and manganese and inhibited A. baumannii growth in vitro (317). Mice with disrupted calprotectin, which diminishes their ability to mediate zinc and manganese sequestration, had higher bacterial burden during A. baumannii pneumonia (317). These data underscore the potential for novel iron-sequestering approaches to serve as viable therapeutic, or perhaps even prophylactic, approaches in patients in the future.
Cytokine and Pattern Recognition Receptor Modulation
Finally, there is great potential for modulation of host inflammation based on LPS-TLR4 interactions to improve outcomes from Acinetobacter infections. We found that disruption of TLR4 resulted in profound resistance of mice to otherwise lethal infection caused by hypervirulent A. baumannii HUMC1 (69). Furthermore, as mentioned previously, small-molecule inhibition of LpxC in A. baumannii, while not altering growth in vitro, completely abrogated its virulence in mice (69). Thus, developing small-molecule or biological approaches that abrogate the LPS-TLR4 interaction during Acinetobacter infection should result in great therapeutic benefit.
Given the relatively barren antimicrobial pipeline for new agents targeting Acinetobacter infections, combined with the dramatic rise in XDR and pan-resistant strains, new therapeutic agents are critically needed for these infections. Novel biological and pathogen-specific approaches are promising to improve outcomes, slow the spread of resistance, and ameliorate the pressure of continuously developing new antibiotics to treat Acinetobacter infections. Given the diversity of approaches under investigation and the proof of concept for many of them at the preclinical stage, it is likely that one or more such approaches will be available clinically in the coming 10 to 15 years.
CONCLUSIONS
After a century of studying this tenacious pathogen, Acinetobacter remains an ever elusive foe, and a tremendous challenge for physicians. The prompt administration of effective therapy remains critically important yet is very difficult to achieve with rising rates of resistance and a dry pipeline targeting this organism. When this pathogen is susceptible, β-lactam antibiotics are the preferred treatment. Whether combination regimens are beneficial is not yet known; however, for XDR strains, and particularly those with carbapenem MICs of 4 to 16 μg/ml, combination carbapenem-polymyxin therapy is a rational approach. For isolated pulmonary disease caused by XDR strains in the absence of bacteremia, inhaled colistin is a reasonable choice to deliver high levels of drug while minimizing systemic exposure.
Ultimately, given our limited therapeutic options, addressing Acinetobacter infections must combine a multidisciplinary approach, including vigilant infection control practices, antimicrobial stewardship, and the combined efforts of multiple health care providers. Additional research on new therapeutics holds promise to improve outcomes in the future. In the meantime, we must learn how to optimize the efficacy of our current antimicrobials, potentially with combinational regimens and extended infusion, to combat the crisis of Acinetobacter infection.
ACKNOWLEDGMENTS
This paper was supported by NIAID R01s AI081719 and AI117211, R42 AI106375, and R21 AI127954, and a grant from Eisai, Inc. (B.S.), and Veterans Affairs Merit Review 1I01BX001974, Geriatric Research Education and Clinical Center VISN, and NIAID R01s AI063517 and R01AI100560 to R.A.B., and NIAID UM1AI104681 to both B.S. and R.A.B.
In the last 12 months, B.S. has received consulting fees from Cempra, The Medicines Company, MedImmune/AstraZeneca, PTC Therapeutics, Entasis, Tetraphase, Merck, and Genentech, DSMB fees from Dipexium, and owned equity in Motif, BioAIM, and Synthetic Biologics, and R.A.B. has been a grant investigator for and received grants from AstraZeneca, Merck, Melinta, Steris, NIH, and Veterans Affairs Merit Review. The other authors have no other conflicts of interest.
Biographies

Darren Wong received his M.D. from the Drexel College of Medicine and completed his internship and residency in internal medicine at Hahnemann University Hospital and Drexel University. He completed an infectious disease fellowship at Montefiore Medical Center of the Albert Einstein College of Medicine in New York. Dr. Wong subsequently joined the Division of Infectious Diseases at the University of Southern California as an assistant professor of clinical medicine. In addition to extensive activity in both medical education and clinical care, Dr. Wong has additional responsibilities in both antimicrobial stewardship, infection control, and the integration of both programs to optimize clinical care. He has a particular interest in the resistance mechanisms and management of multidrug- resistant bacterial infections and in Clostridium difficile disease.

Travis Nielsen is currently a Ph.D. graduate student in medical biology working under the mentorship of Dr. Brad Spellberg in the Keck School of Medicine at the University of Southern California. He began his scientific career as a graduate student in molecular biophysics at Vanderbilt University before subsequently moving to Dr. Spellberg's lab and changing his emphasis to translational immunology and immunotherapy. His thesis focuses on identifying monoclonal antibodies with therapeutic potential for XDR Acinetobacter baumannii and determining their mechanisms of protection. Mr. Nielsen was recently awarded the prestigious, merit-based Graduate School Fellowship from the Keck School of Medicine.

Robert A. Bonomo received his M.D. from the Case Western Reserve University School of Medicine. He trained in internal medicine and infectious diseases at the University Hospitals of Cleveland and received a Certificate of Added Qualification in Geriatrics. Dr. Bonomo served as section chief of the infectious disease division at the Cleveland Veterans Affairs Medical Center before becoming Director of the Veterans Integrated Service Network 10 Geriatric Research, Education and Clinical Center (GRECC). He also serves as Chief of Medicine. He is a Professor of Medicine at the Case Western Reserve University School of Medicine and also holds appointments in the Departments of Pharmacology and Molecular Biology, Biochemistry, and Microbiology. Dr. Bonomo's research focuses on microbial resistance to antibiotics, especially β-lactams, and the discovery of novel therapeutics against multidrug-resistant infections.

Paul Pantapalangkoor earned a bachelor of science in biochemistry (2004) and master of science in biochemistry and molecular biology (2005) from the University of California, Riverside. He previously worked at Amgen Inc. and has worked for Dr. Brad Spellberg for more than five years. He is currently completing a master of science in biomedical sciences at Rosalind Franklin University of Medicine and Science and pursuing medical school.

Brian Luna is currently an Instructor of Research Medicine at the University of Southern California (USC) Keck School of Medicine in the Departments of Medicine and Molecular Microbiology and Immunology. He earned his Ph.D. from the Cellular and Molecular Medicine Program at the Johns Hopkins University School of Medicine. He did his graduate training under Dr. William Bishai at the Center for Tuberculosis Research. His thesis focused on the study of host-pathogen interactions and the development of cavitary lesions in the host. After earning his Ph.D., he went on to do a postdoctoral fellowship with Dr. Elliot Hui at the University of California Irvine in the Department of Biomedical Engineering where he worked on developing microfluidic devices for molecular and microbiological applications. In his current role at USC, he studies antibiotic tolerance mechanisms of bacterial pathogens, including both antibiotic resistance and antibiotic persister cell formation.

Brad Spellberg M.D. F.I.D.S.A. F.A.C.P. is Chief Medical Officer at the Los Angeles County-University of Southern California (LAC+USC) Medical Center. He is also a Professor of Clinical Medicine and Associate Dean for Clinical Affairs at the Keck School of Medicine at USC. Dr. Spellberg has extensive administrative, patient care, and teaching activities. His NIH-funded research interests are diverse, ranging from basic immunology and vaccinology, to pure clinical and outcome research, to process improvement work related to delivery of care, focusing on safety net hospitals. A primary focus of his laboratory is pathogenesis and immunology of, and immunotherapy for, highly resistant Gram-negative bacilli, with a particular emphasis on Acinetobacter spp. Dr. Spellberg has worked for many years on national policy efforts related to antibiotic resistance, has written numerous societal position papers on the topic, testified before Congress, and written an award-winning book, Rising Plague, to inform the public about it.
REFERENCES
- 1.Glew RH, Moellering RC Jr, Kunz LJ. 1977. Infections with Acinetobacter calcoaceticus (Herellea vaginicola): clinical and laboratory studies. Medicine (Baltimore) 56:79–97. doi: 10.1097/00005792-197703000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Daly AK, Postic B, Kass EH. 1962. Infections due to organisms of the genus Herellea. B5W and B anitratum. Arch Intern Med 110:580–591. doi: 10.1001/archinte.1962.03620230026006. [DOI] [PubMed] [Google Scholar]
- 3.Al Atrouni A, Joly-Guillou ML, Hamze M, Kempf M. 2016. Reservoirs of non-baumannii Acinetobacter species. Front Microbiol 7:49. doi: 10.3389/fmicb.2016.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dijkshoorn L, van der Toorn J. 1992. Acinetobacter species: which do we mean? Clin Infect Dis 15:748–749. doi: 10.1093/clind/15.4.748. [DOI] [PubMed] [Google Scholar]
- 5.Chang WN, Lu CH, Huang CR, Chuang YC. 2000. Community-acquired Acinetobacter meningitis in adults. Infection 28:395–397. doi: 10.1007/s150100070013. [DOI] [PubMed] [Google Scholar]
- 6.Visca P, Seifert H, Towner KJ. 2011. Acinetobacter infection–an emerging threat to human health. IUBMB Life 63:1048–1054. doi: 10.1002/iub.534. [DOI] [PubMed] [Google Scholar]
- 7.Dortet L, Legrand P, Soussy CJ, Cattoir V. 2006. Bacterial identification, clinical significance, and antimicrobial susceptibilities of Acinetobacter ursingii and Acinetobacter schindleri, two frequently misidentified opportunistic pathogens. J Clin Microbiol 44:4471–4478. doi: 10.1128/JCM.01535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loubinoux J, Mihaila-Amrouche L, Le Fleche A, Pigne E, Huchon G, Grimont PA, Bouvet A. 2003. Bacteremia caused by Acinetobacter ursingii. J Clin Microbiol 41:1337–1338. doi: 10.1128/JCM.41.3.1337-1338.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endo S, Sasano M, Yano H, Inomata S, Ishibashi N, Aoyagi T, Hatta M, Gu Y, Yamada M, Tokuda K, Kitagawa M, Kunishima H, Hirakata Y, Kaku M. 2012. IMP-1-producing carbapenem-resistant Acinetobacter ursingii from Japan. J Antimicrob Chemother 67:2533–2534. doi: 10.1093/jac/dks249. [DOI] [PubMed] [Google Scholar]
- 10.Chiu CH, Lee YT, Wang YC, Yin T, Kuo SC, Yang YS, Chen TL, Lin JC, Wang FD, Fung CP. 2015. A retrospective study of the incidence, clinical characteristics, identification, and antimicrobial susceptibility of bacteremic isolates of Acinetobacter ursingii. BMC Infect Dis 15:400. doi: 10.1186/s12879-015-1145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salzer HJ, Rolling T, Schmiedel S, Klupp EM, Lange C, Seifert H. 2016. Severe community-acquired bloodstream infection with Acinetobacter ursingii in person who injects drugs. Emerg Infect Dis 22:134–137. doi: 10.3201/eid2201.151298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nemec A, Krizova L, Maixnerova M, Sedo O, Brisse S, Higgins PG. 2015. Acinetobacter seifertii sp. nov., a member of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex isolated from human clinical specimens. Int J Syst Evol Microbiol 65:934–942. doi: 10.1099/ijs.0.000043. [DOI] [PubMed] [Google Scholar]
- 13.Kishii K, Kikuchi K, Tomida J, Kawamura Y, Yoshida A, Okuzumi K, Moriya K. 2016. The first cases of human bacteremia caused by Acinetobacter seifertii in Japan. J Infect Chemother 22:342–345. doi: 10.1016/j.jiac.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Wang J, Fu Y, Ruan Z, Yu Y. 2016. Acinetobacter seifertii isolated from China: genomic sequence and molecular epidemiology analyses. Medicine (Baltimore) 95:e2937. doi: 10.1097/MD.0000000000002937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chusri S, Chongsuvivatwong V, Rivera JI, Silpapojakul K, Singkhamanan K, McNeil E, Doi Y. 2014. Clinical outcomes of hospital-acquired infection with Acinetobacter nosocomialis and Acinetobacter pittii. Antimicrob Agents Chemother 58:4172–4179. doi: 10.1128/AAC.02992-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rafei R, Hamze M, Pailhories H, Eveillard M, Marsollier L, Joly-Guillou ML, Dabboussi F, Kempf M. 2015. Extrahuman epidemiology of Acinetobacter baumannii in Lebanon. Appl Environ Microbiol 81:2359–2367. doi: 10.1128/AEM.03824-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang WJ, Lu Z, Schwarz S, Zhang RM, Wang XM, Si W, Yu S, Chen L, Liu S. 2013. Complete sequence of the bla(NDM-1)-carrying plasmid pNDM-AB from Acinetobacter baumannii of food animal origin. J Antimicrob Chemother 68:1681–1682. doi: 10.1093/jac/dkt066. [DOI] [PubMed] [Google Scholar]
- 18.Lupo A, Vogt D, Seiffert SN, Endimiani A, Perreten V. 2014. Antibiotic resistance and phylogenetic characterization of Acinetobacter baumannii strains isolated from commercial raw meat in Switzerland. J Food Prot 77:1976–1981. doi: 10.4315/0362-028X.JFP-14-073. [DOI] [PubMed] [Google Scholar]
- 19.Berlau J, Aucken HM, Houang E, Pitt TL. 1999. Isolation of Acinetobacter spp. including A. baumannii from vegetables: implications for hospital-acquired infections. J Hosp Infect 42:201–204. doi: 10.1053/jhin.1999.0602. [DOI] [PubMed] [Google Scholar]
- 20.Seifert H, Dijkshoorn L, Gerner-Smidt P, Pelzer N, Tjernberg I, Vaneechoutte M. 1997. Distribution of Acinetobacter species on human skin: comparison of phenotypic and genotypic identification methods. J Clin Microbiol 35:2819–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patil JR, Chopade BA. 2001. Distribution and in vitro antimicrobial susceptibility of Acinetobacter species on the skin of healthy humans. Natl Med J India 14:204–208. [PubMed] [Google Scholar]
- 22.Berlau J, Aucken H, Malnick H, Pitt T. 1999. Distribution of Acinetobacter species on skin of healthy humans. Eur J Clin Microbiol Infect Dis 18:179–183. doi: 10.1007/s100960050254. [DOI] [PubMed] [Google Scholar]
- 23.Griffith ME, Ceremuga JM, Ellis MW, Guymon CH, Hospenthal DR, Murray CK. 2006. Acinetobacter skin colonization of US Army soldiers. Infect Control Hosp Epidemiol 27:659–661. doi: 10.1086/506596. [DOI] [PubMed] [Google Scholar]
- 24.Spellberg B, Bonomo RA. 2014. The deadly impact of extreme drug resistance in Acinetobacter baumannii. Crit Care Med 42:1289–1291. doi: 10.1097/CCM.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maragakis LL, Perl TM. 2008. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis 46:1254–1263. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 26.Joly-Guillou ML. 2005. Clinical impact and pathogenicity of Acinetobacter. Clin Microbiol Infect 11:868–873. doi: 10.1111/j.1469-0691.2005.01227.x. [DOI] [PubMed] [Google Scholar]
- 27.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S, National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities. 2013. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol 34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Bano J, Cisneros JM, Fernandez-Cuenca F, Ribera A, Vila J, Pascual A, Martinez-Martinez L, Bou G, Pachon J, Grupo de Estudio de Infeccion Hospitalaria (GEIH). 2004. Clinical features and epidemiology of Acinetobacter baumannii colonization and infection in Spanish hospitals. Infect Control Hosp Epidemiol 25:819–824. doi: 10.1086/502302. [DOI] [PubMed] [Google Scholar]
- 29.Agodi A, Auxilia F, Barchitta M, Brusaferro S, D'Alessandro D, Montagna MT, Orsi GB, Pasquarella C, Torregrossa V, Suetens C, Mura I, GISIO. 2010. Building a benchmark through active surveillance of intensive care unit-acquired infections: the Italian network SPIN-UTI. J Hosp Infect 74:258–265. doi: 10.1016/j.jhin.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Martins AF, Kuchenbecker RS, Pilger KO, Pagano M, Barth AL, CMCIES-PMPA/SMS Task Force. 2012. High endemic levels of multidrug-resistant Acinetobacter baumannii among hospitals in southern Brazil. Am J Infect Control 40:108–112. doi: 10.1016/j.ajic.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Labarca JA, Salles MJ, Seas C, Guzman-Blanco M. 2016. Carbapenem resistance in Pseudomonas aeruginosa and Acinetobacter baumannii in the nosocomial setting in Latin America. Crit Rev Microbiol 42:276–292. doi: 10.3109/1040841X.2014.940494. [DOI] [PubMed] [Google Scholar]
- 32.Villar M, Cano ME, Gato E, Garnacho-Montero J, Miguel Cisneros J, Ruiz de Alegria C, Fernandez-Cuenca F, Martinez-Martinez L, Vila J, Pascual A, Tomas M, Bou G, Rodriguez-Bano J, GEIH/GEMARA/REIPI-Ab20101 Group. 2014. Epidemiologic and clinical impact of Acinetobacter baumannii colonization and infection: a reappraisal. Medicine (Baltimore) 93:202–210. doi: 10.1097/MD.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta V, Datta P, Chander J. 2006. Prevalence of metallo-beta lactamase (MBL) producing Pseudomonas spp. and Acinetobacter spp. in a tertiary care hospital in India. J Infect 52:311–314. doi: 10.1016/j.jinf.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Mathai AS, Oberoi A, Madhavan S, Kaur P. 2012. Acinetobacter infections in a tertiary level intensive care unit in northern India: epidemiology, clinical profiles and outcomes. J Infect Public Health 5:145–152. doi: 10.1016/j.jiph.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Bali NK, Fomda BA, Bashir H, Zahoor D, Lone S, Koul PA. 2013. Emergence of carbapenem-resistant Acinetobacter in a temperate north Indian State. Br J Biomed Sci 70:156–160. doi: 10.1080/09674845.2013.11669950. [DOI] [PubMed] [Google Scholar]
- 36.Shah S, Singhal T, Naik R. 2015. A 4-year prospective study to determine the incidence and microbial etiology of surgical site infections at a private tertiary care hospital in Mumbai, India. Am J Infect Control 43:59–62. doi: 10.1016/j.ajic.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Golia S, Sangeetha KT, Vasudha CL. 2013. Microbial profile of early and late onset ventilator associated pneumonia in the intensive care unit of a tertiary care hospital in Bangalore, India. J Clin Diagn Res 7:2462–2466. doi: 10.7860/JCDR/2013/6344.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ginawi I, Saleem M, Sigh M, Vaish AK, Ahmad I, Srivastava VK, Abdullah AF. 2014. Hospital acquired infections among patients admitted in the medical and surgical wards of a non-teaching secondary care hospital in northern India. J Clin Diagn Res 8:81–83. doi: 10.7860/JCDR/2014/6673.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung DR, Song JH, Kim SH, Thamlikitkul V, Huang SG, Wang H, So TM, Yasin RM, Hsueh PR, Carlos CC, Hsu LY, Buntaran L, Lalitha MK, Kim MJ, Choi JY, Kim SI, Ko KS, Kang CI, Peck KR. 2011. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med 184:1409–1417. doi: 10.1164/rccm.201102-0349OC. [DOI] [PubMed] [Google Scholar]
- 40.Kuo SC, Chang SC, Wang HY, Lai JF, Chen PC, Shiau YR, Huang IW, Lauderdale TL, TSAR Hospitals. 2012. Emergence of extensively drug-resistant Acinetobacter baumannii complex over 10 years: nationwide data from the Taiwan Surveillance of Antimicrobial Resistance (TSAR) program. BMC Infect Dis 12:200. doi: 10.1186/1471-2334-12-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dejsirilert S, Tiengrim S, Sawanpanyalert P, Aswapokee N, Malathum K. 2009. Antimicrobial resistance of Acinetobacter baumannii: six years of National Antimicrobial Resistance Surveillance Thailand (NARST) surveillance. J Med Assoc Thai 92(Suppl 4):S34–S45. [PubMed] [Google Scholar]
- 42.Madani N, Rosenthal VD, Dendane T, Abidi K, Zeggwagh AA, Abouqal R. 2009. Health-care associated infections rates, length of stay, and bacterial resistance in an intensive care unit of Morocco: findings of the International Nosocomial Infection Control Consortium (INICC). Int Arch Med 2:29. doi: 10.1186/1755-7682-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luna CM, Rodriguez-Noriega E, Bavestrello L, Guzman-Blanco M. 2014. Gram-negative infections in adult intensive care units of Latin America and the Caribbean. Crit Care Res Pract 2014:480463. doi: 10.1155/2014/480463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spellberg B, Rex JH. 2013. The value of single-pathogen antibacterial agents. Nat Rev Drug Discov 12:963. doi: 10.1038/nrd3957-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonald LC, Banerjee SN, Jarvis WR. 1999. Seasonal variation of Acinetobacter infections: 1987-1996. Nosocomial Infections Surveillance System. Clin Infect Dis 29:1133–1137. [DOI] [PubMed] [Google Scholar]
- 46.Spellberg B, Bonomo RA. 2013. “Airborne assault”: a new dimension in Acinetobacter baumannii transmission. Crit Care Med 41:2042–2044. doi: 10.1097/CCM.0b013e31829136c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whitman TJ, Qasba SS, Timpone JG, Babel BS, Kasper MR, English JF, Sanders JW, Hujer KM, Hujer AM, Endimiani A, Eshoo MW, Bonomo RA. 2008. Occupational transmission of Acinetobacter baumannii from a United States serviceman wounded in Iraq to a health care worker. Clin Infect Dis 47:439–443. doi: 10.1086/589247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munoz-Price LS, Fajardo-Aquino Y, Arheart KL, Cleary T, Depascale D, Pizano L, Namias N, Rivera JI, O'Hara JA, Doi Y. 2013. Aerosolization of Acinetobacter baumannii in a trauma ICU. Crit Care Med 41:1915–1918. doi: 10.1097/CCM.0b013e31828a39c0. [DOI] [PubMed] [Google Scholar]
- 49.Rock C, Harris AD, Johnson JK, Bischoff WE, Thom KA. 2015. Infrequent air contamination with Acinetobacter baumannii of air surrounding known colonized or infected patients. Infect Control Hosp Epidemiol 36:830–832. doi: 10.1017/ice.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freire MP, de Oliveira Garcia D, Garcia CP, Campagnari Bueno MF, Camargo CH, Magri AS, Francisco GR, Reghini R, Vieira MF, Ibrahim KY, Rossi F, Hajar L, Levin AS, Hoff PM, Pierrotti LC, Abdala E. 2016. Bloodstream infection caused by extensively drug-resistant Acinetobacter baumannii in cancer patients: high mortality associated with delayed treatment rather than with the degree of neutropenia. Clin Microbiol Infect 22:352–358. doi: 10.1016/j.cmi.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 51.Guan X, He L, Hu B, Hu J, Huang X, Lai G, Li Y, Liu Y, Ni Y, Qiu H, Shao Z, Shi Y, Wang M, Wang R, Wu D, Xie C, Xu Y, Yang F, Yu K, Yu Y, Zhang J, Zhuo C, Chinese XDR Consensus Working Group. 2016. Laboratory diagnosis, clinical management and infection control of the infections caused by extensively drug resistant Gram-negative bacilli: a Chinese consensus statement. Clin Microbiol Infect 22(Suppl 1):S15–S25. doi: 10.1016/j.cmi.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Chopra T, Marchaim D, Johnson PC, Awali RA, Doshi H, Chalana I, Davis N, Zhao JJ, Pogue JM, Parmar S, Kaye KS. 2014. Risk factors and outcomes for patients with bloodstream infection due to Acinetobacter baumannii-calcoaceticus complex. Antimicrob Agents Chemother 58:4630–4635. doi: 10.1128/AAC.02441-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chopra T, Marchaim D, Awali RA, Krishna A, Johnson P, Tansek R, Chaudary K, Lephart P, Slim J, Hothi J, Ahmed H, Pogue J, Zhao JJ, Kaye KS. 2013. Epidemiology of bloodstream infection due to Acinetobacter baumannii and the impact of drug resistance to both carbapenems and ampicillin-sulbactam on clinical outcomes. Antimicrob Agents Chemother 57:6270–6275. doi: 10.1128/AAC.01520-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qiu H, KuoLee R, Harris G, Chen W. 2009. High susceptibility to respiratory Acinetobacter baumannii infection in A/J mice is associated with a delay in early pulmonary recruitment of neutrophils. Microbes Infect 11:946–955. doi: 10.1016/j.micinf.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Luo G, Lin L, Ibrahim AS, Baquir B, Pantapalangkoor P, Bonomo RA, Doi Y, Adams MD, Russo TA, Spellberg B. 2012. Active and passive immunization protects against lethal, extreme drug resistant-Acinetobacter baumannii infection. PLoS One 7:e29446. doi: 10.1371/journal.pone.0029446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo G, Spellberg B, Gebremariam T, Bolaris M, Lee H, Fu Y, French SW, Ibrahim AS. 2012. Diabetic murine models for Acinetobacter baumannii infection. J Antimicrob Chemother 67:1439–1445. doi: 10.1093/jac/dks050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacobs AC, Hood I, Boyd KL, Olson PD, Morrison JM, Carson S, Sayood K, Iwen PC, Skaar EP, Dunman PM. 2010. Inactivation of phospholipase D diminishes Acinetobacter baumannii pathogenesis. Infect Immun 78:1952–1962. doi: 10.1128/IAI.00889-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joly-Guillou ML, Wolff M, Pocidalo JJ, Walker F, Carbon C. 1997. Use of a new mouse model of Acinetobacter baumannii pneumonia to evaluate the postantibiotic effect of imipenem. Antimicrob Agents Chemother 41:345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lopez-Rojas R, Dominguez-Herrera J, McConnell MJ, Docobo-Perez F, Smani Y, Fernandez-Reyes M, Rivas L, Pachon J. 2011. Impaired virulence and in vivo fitness of colistin-resistant Acinetobacter baumannii. J Infect Dis 203:545–548. doi: 10.1093/infdis/jiq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McConnell MJ, Actis L, Pachon J. 2013. Acinetobacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol Rev 37:130–155. doi: 10.1111/j.1574-6976.2012.00344.x. [DOI] [PubMed] [Google Scholar]
- 61.Kim WY, Moon JY, Huh JW, Choi SH, Lim CM, Koh Y, Chong YP, Hong SB. 2016. Comparable efficacy of tigecycline versus colistin therapy for multidrug-resistant and extensively drug-resistant Acinetobacter baumannii pneumonia in critically ill patients. PLoS One 11:e0150642. doi: 10.1371/journal.pone.0150642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ozvatan T, Akalin H, Sinirtas M, Ocakoglu G, Yilmaz E, Heper Y, Kelebek N, Iscimen R, Kahveci F. 2016. Nosocomial Acinetobacter pneumonia: treatment and prognostic factors in 356 cases. Respirology 21:363–369. doi: 10.1111/resp.12698. [DOI] [PubMed] [Google Scholar]
- 63.Henig O, Weber G, Hoshen MB, Paul M, German L, Neuberger A, Gluzman I, Berlin A, Shapira C, Balicer RD. 2015. Risk factors for and impact of carbapenem-resistant Acinetobacter baumannii colonization and infection: matched case-control study. Eur J Clin Microbiol Infect Dis 34:2063–2068. doi: 10.1007/s10096-015-2452-4. [DOI] [PubMed] [Google Scholar]
- 64.Nutman A, Glick R, Temkin E, Hoshen M, Edgar R, Braun T, Carmeli Y. 2014. A case-control study to identify predictors of 14-day mortality following carbapenem-resistant Acinetobacter baumannii bacteraemia. Clin Microbiol Infect 20:O1028–O1034. doi: 10.1111/1469-0691.12716. [DOI] [PubMed] [Google Scholar]
- 65.Ng TM, Teng CB, Lye DC, Apisarnthanarak A. 2014. A multicenter case-case control study for risk factors and outcomes of extensively drug-resistant Acinetobacter baumannii bacteremia. Infect Control Hosp Epidemiol 35:49–55. doi: 10.1086/674387. [DOI] [PubMed] [Google Scholar]
- 66.Zheng YL, Wan YF, Zhou LY, Ye ML, Liu S, Xu CQ, He YQ, Chen JH. 2013. Risk factors and mortality of patients with nosocomial carbapenem-resistant Acinetobacter baumannii pneumonia. Am J Infect Control 41:e59–e63. doi: 10.1016/j.ajic.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 67.Arvaniti K, Lathyris D, Ruimy R, Haidich AB, Koulourida V, Nikolaidis P, Matamis D, Miyakis S. 2012. The importance of colonization pressure in multiresistant Acinetobacter baumannii acquisition in a Greek intensive care unit. Crit Care 16:R102. doi: 10.1186/cc11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thompson MG, Black CC, Pavlicek RL, Honnold CL, Wise MC, Alamneh YA, Moon JK, Kessler JL, Si Y, Williams R, Yildirim S, Kirkup BC Jr, Green RK, Hall ER, Palys TJ, Zurawski DV. 2014. Validation of a novel murine wound model of Acinetobacter baumannii infection. Antimicrob Agents Chemother 58:1332–1342. doi: 10.1128/AAC.01944-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin L, Tan B, Pantapalangkoor P, Ho T, Baquir B, Tomaras A, Montgomery JI, Reilly U, Barbacci EG, Hujer K, Bonomo RA, Fernandez L, Hancock RE, Adams MD, French SW, Buslon VS, Spellberg B. 2012. Inhibition of LpxC protects mice from resistant Acinetobacter baumannii by modulating inflammation and enhancing phagocytosis. mBio 3:e00312-12. doi: 10.1128/mBio.00312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bruhn KW, Pantapalangkoor P, Nielsen T, Tan B, Junus J, Hujer KM, Wright MS, Bonomo RA, Adams MD, Chen W, Spellberg B. 2015. Host fate is rapidly determined by innate effector-microbial interactions during Acinetobacter baumannii bacteremia. J Infect Dis 211:1296–1305. doi: 10.1093/infdis/jiu593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nielsen TB, Bruhn KW, Pantapalangkoor P, Junus JL, Spellberg B. 2015. Cryopreservation of virulent Acinetobacter baumannii to reduce variability of in vivo studies. BMC Microbiol 15:252. doi: 10.1186/s12866-015-0580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin L, Pantapalangkoor P, Tan B, Bruhn KW, Ho T, Nielsen T, Skaar EP, Zhang Y, Bai R, Wang A, Doherty TM, Spellberg B. 2014. Transferrin iron starvation therapy for lethal bacterial and fungal infections. J Infect Dis 210:254–264. doi: 10.1093/infdis/jiu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Russo TA, Beanan JM, Olson R, MacDonald U, Luke NR, Gill SR, Campagnari AA. 2008. Rat pneumonia and soft-tissue infection models for the study of Acinetobacter baumannii biology. Infect Immun 76:3577–3586. doi: 10.1128/IAI.00269-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Russo TA, Luke NR, Beanan JM, Olson R, Sauberan SL, MacDonald U, Schultz LW, Umland TC, Campagnari AA. 2010. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect Immun 78:3993–4000. doi: 10.1128/IAI.00366-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peleg AY, Jara S, Monga D, Eliopoulos GM, Moellering RC Jr, Mylonakis E. 2009. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob Agents Chemother 53:2605–2609. doi: 10.1128/AAC.01533-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gebhardt MJ, Gallagher LA, Jacobson RK, Usacheva EA, Peterson LR, Zurawski DV, Shuman HA. 2015. Joint transcriptional control of virulence and resistance to antibiotic and environmental stress in Acinetobacter baumannii. mBio 6:e01660-15. doi: 10.1128/mBio.01660-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wand ME, Bock LJ, Turton JF, Nugent PG, Sutton JM. 2012. Acinetobacter baumannii virulence is enhanced in Galleria mellonella following biofilm adaptation. J Med Microbiol 61:470–477. doi: 10.1099/jmm.0.037523-0. [DOI] [PubMed] [Google Scholar]
- 78.Vallejo JA, Beceiro A, Rumbo-Feal S, Rodriguez-Palero MJ, Russo TA, Bou G. 9 July 2015. Optimisation of the Caenorhabditis elegans model for studying the pathogenesis of opportunistic Acinetobacter baumannii. Int J Antimicrob Agents doi: 10.1016/j.ijantimicag.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 79.Eijkelkamp BA, Stroeher UH, Hassan KA, Elbourne LD, Paulsen IT, Brown MH. 2013. H-NS plays a role in expression of Acinetobacter baumannii virulence features. Infect Immun 81:2574–2583. doi: 10.1128/IAI.00065-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beceiro A, Moreno A, Fernandez N, Vallejo JA, Aranda J, Adler B, Harper M, Boyce JD, Bou G. 2014. Biological cost of different mechanisms of colistin resistance and their impact on virulence in Acinetobacter baumannii. Antimicrob Agents Chemother 58:518–526. doi: 10.1128/AAC.01597-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bhuiyan MS, Ellett F, Murray GL, Kostoulias X, Cerqueira GM, Schulze KE, Mahamad Maifiah MH, Li J, Creek DJ, Lieschke GJ, Peleg AY. 2016. Acinetobacter baumannii phenylacetic acid metabolism influences infection outcome through a direct effect on neutrophil chemotaxis. Proc Natl Acad Sci U S A 113:9599–9604. doi: 10.1073/pnas.1523116113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zimbler DL, Arivett BA, Beckett AC, Menke SM, Actis LA. 2013. Functional features of TonB energy transduction systems of Acinetobacter baumannii. Infect Immun 81:3382–3394. doi: 10.1128/IAI.00540-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giannouli M, Antunes LC, Marchetti V, Triassi M, Visca P, Zarrilli R. 2013. Virulence-related traits of epidemic Acinetobacter baumannii strains belonging to the international clonal lineages I-III and to the emerging genotypes ST25 and ST78. BMC Infect Dis 13:282. doi: 10.1186/1471-2334-13-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lazaro-Diez M, Navascues-Lejarza T, Remuzgo-Martinez S, Navas J, Icardo JM, Acosta F, Martinez-Martinez L, Ramos-Vivas J. 2016. Acinetobacter baumannii and A. pittii clinical isolates lack adherence and cytotoxicity to lung epithelial cells in vitro. Microbes Infect 18:559–564. doi: 10.1016/j.micinf.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 85.de Breij A, Eveillard M, Dijkshoorn L, van den Broek PJ, Nibbering PH, Joly-Guillou ML. 2012. Differences in Acinetobacter baumannii strains and host innate immune response determine morbidity and mortality in experimental pneumonia. PLoS One 7:e30673. doi: 10.1371/journal.pone.0030673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tayabali AF, Nguyen KC, Shwed PS, Crosthwait J, Coleman G, Seligy VL. 2012. Comparison of the virulence potential of Acinetobacter strains from clinical and environmental sources. PLoS One 7:e37024. doi: 10.1371/journal.pone.0037024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jacobs AC, Thompson MG, Gebhardt M, Corey BW, Yildirim S, Shuman HA, Zurawski DV. 2014. Genetic manipulation of Acinetobacter baumannii. Curr Protoc Microbiol 35:6G.2.1–6G.2-11. doi: 10.1002/9780471729259.mc06g02s35. [DOI] [PubMed] [Google Scholar]
- 88.Biswas I. 2015. Genetic tools for manipulating Acinetobacter baumannii genome: an overview. J Med Microbiol 64:657–669. doi: 10.1099/jmm.0.000081. [DOI] [PubMed] [Google Scholar]
- 89.Wang N, Ozer EA, Mandel MJ, Hauser AR. 2014. Genome-wide identification of Acinetobacter baumannii genes necessary for persistence in the lung. mBio 5:e01163-14. doi: 10.1128/mBio.01163-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Subashchandrabose S, Smith S, DeOrnellas V, Crepin S, Kole M, Zahdeh C, Mobley HL. 2016. Acinetobacter baumannii genes required for bacterial survival during bloodstream infection. mSphere 1:e00013-15. doi: 10.1128/mSphere.00013-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gallagher LA, Ramage E, Weiss EJ, Radey M, Hayden HS, Held KG, Huse HK, Zurawski DV, Brittnacher MJ, Manoil C. 2015. Resources for genetic and genomic analysis of emerging pathogen Acinetobacter baumannii. J Bacteriol 197:2027–2035. doi: 10.1128/JB.00131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Knight D, Dimitrova DD, Rudin SD, Bonomo RA, Rather PN. 2016. Mutations decreasing intrinsic beta-lactam resistance are linked to cell division in the nosocomial pathogen Acinetobacter baumannii. Antimicrob Agents Chemother 60:3751–3758. doi: 10.1128/AAC.00361-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clemmer KM, Bonomo RA, Rather PN. 2011. Genetic analysis of surface motility in Acinetobacter baumannii. Microbiology 157:2534–2544. doi: 10.1099/mic.0.049791-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smith MG, Des Etages SG, Snyder M. 2004. Microbial synergy via an ethanol-triggered pathway. Mol Cell Biol 24:3874–3884. doi: 10.1128/MCB.24.9.3874-3884.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nwugo CC, Arivett BA, Zimbler DL, Gaddy JA, Richards AM, Actis LA. 2012. Effect of ethanol on differential protein production and expression of potential virulence functions in the opportunistic pathogen Acinetobacter baumannii. PLoS One 7:e51936. doi: 10.1371/journal.pone.0051936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aranda J, Bardina C, Beceiro A, Rumbo S, Cabral MP, Barbe J, Bou G. 2011. Acinetobacter baumannii RecA protein in repair of DNA damage, antimicrobial resistance, general stress response, and virulence. J Bacteriol 193:3740–3747. doi: 10.1128/JB.00389-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fournier PE, Richet H. 2006. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis 42:692–699. doi: 10.1086/500202. [DOI] [PubMed] [Google Scholar]
- 98.Houang ET, Sormunen RT, Lai L, Chan CY, Leong AS. 1998. Effect of desiccation on the ultrastructural appearances of Acinetobacter baumannii and Acinetobacter lwoffii. J Clin Pathol 51:786–788. doi: 10.1136/jcp.51.10.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wendt C, Dietze B, Dietz E, Ruden H. 1997. Survival of Acinetobacter baumannii on dry surfaces. J Clin Microbiol 35:1394–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schweppe DK, Harding C, Chavez JD, Wu X, Ramage E, Singh PK, Manoil C, Bruce JE. 2015. Host-microbe protein interactions during bacterial infection. Chem Biol 22:1521–1530. doi: 10.1016/j.chembiol.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yoon EJ, Balloy V, Fiette L, Chignard M, Courvalin P, Grillot-Courvalin C. 2016. Contribution of the Ade resistance-nodulation-cell division-type efflux pumps to fitness and pathogenesis of Acinetobacter baumannii. mBio 7:e00697-16. doi: 10.1128/mBio.00697-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bonomo RA, Szabo D. 2006. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin Infect Dis 43(Suppl 2):S49–S56. doi: 10.1086/504477. [DOI] [PubMed] [Google Scholar]
- 103.Richmond GE, Evans LP, Anderson MJ, Wand ME, Bonney LC, Ivens A, Chua KL, Webber MA, Sutton JM, Peterson ML, Piddock LJ. 2016. The Acinetobacter baumannii two-component system AdeRS regulates genes required for multidrug efflux, biofilm formation, and virulence in a strain-specific manner. mBio 7:e00430-16. doi: 10.1128/mBio.00430-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luke NR, Sauberan SL, Russo TA, Beanan JM, Olson R, Loehfelm TW, Cox AD, St Michael F, Vinogradov EV, Campagnari AA. 2010. Identification and characterization of a glycosyltransferase involved in Acinetobacter baumannii lipopolysaccharide core biosynthesis. Infect Immun 78:2017–2023. doi: 10.1128/IAI.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lees-Miller R, Iwashkiw J, Scott NE, Seper A, Vinogradov E, Schild S, Feldman M. 2013. A common pathway for O-linked protein-glycosylation and synthesis of capsule in Acinetobacter baumannii. Mol Microbiol 89:816–830. doi: 10.1111/mmi.12300. [DOI] [PubMed] [Google Scholar]
- 106.Weber BS, Harding CM, Feldman MF. 2015. Pathogenic Acinetobacter: from the cell surface to infinity and beyond. J Bacteriol 198:880–887. doi: 10.1128/JB.00906-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Geisinger E, Isberg RR. 2015. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog 11:e1004691. doi: 10.1371/journal.ppat.1004691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jones CL, Clancy M, Honnold C, Singh S, Snesrud E, Onmus-Leone F, McGann P, Ong A, Kwak Y, Waterman P, Zurawski DV, Clifford RJ, Lesho E. 2015. A fatal outbreak of an emerging clone of extensively drug-resistant Acinetobacter baumannii with enhanced virulence. Clin Infect Dis 61:145–154. doi: 10.1093/cid/civ225. [DOI] [PubMed] [Google Scholar]
- 109.Ou HY, Kuang SN, He X, Molgora BM, Ewing PJ, Deng Z, Osby M, Chen W, Xu HH. 2015. Complete genome sequence of hypervirulent and outbreak-associated Acinetobacter baumannii strain LAC-4: epidemiology, resistance genetic determinants and potential virulence factors. Sci Rep 5:8643. doi: 10.1038/srep08643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vinogradov E, MacLean L, Xu HH, Chen W. 2014. The structure of the polysaccharide isolated from Acinetobacter baumannii strain LAC-4. Carbohydr Res 390:42–45. doi: 10.1016/j.carres.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 111.Scott NE, Kinsella RL, Edwards AV, Larsen MR, Dutta S, Saba J, Foster LJ, Feldman MF. 2014. Diversity within the O-linked protein glycosylation systems of Acinetobacter species. Mol Cell Proteomics 13:2354–2370. doi: 10.1074/mcp.M114.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kinsella RL, Scott NE, Feldman MF. 2015. Clinical implications of glycoproteomics for Acinetobacter baumannii. Expert Rev Proteomics 12:1–3. doi: 10.1586/14789450.2015.987756. [DOI] [PubMed] [Google Scholar]
- 113.Russo TA, Beanan JM, Olson R, MacDonald U, Cox AD, St Michael F, Vinogradov EV, Spellberg B, Luke-Marshall NR, Campagnari AA. 2013. The K1 capsular polysaccharide from Acinetobacter baumannii is a potential therapeutic target via passive immunization. Infect Immun 81:915–922. doi: 10.1128/IAI.01184-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shashkov AS, Kenyon JJ, Senchenkova SN, Shneider MM, Popova AV, Arbatsky NP, Miroshnikov KA, Volozhantsev NV, Hall RM, Knirel YA. 2016. Acinetobacter baumannii K27 and K44 capsular polysaccharides have the same K unit but different structures due to the presence of distinct wzy genes in otherwise closely related K gene clusters. Glycobiology 26:501–508. doi: 10.1093/glycob/cwv168. [DOI] [PubMed] [Google Scholar]
- 115.Kenyon JJ, Shneider MM, Senchenkova SN, Shashkov AS, Siniagina MN, Malanin SY, Popova AV, Miroshnikov KA, Hall RM, Knirel YA. 2016. The K19 capsular polysaccharide of Acinetobacter baumannii is produced via a Wzy polymerase encoded in a small genomic island rather than the KL19 capsule gene cluster. Microbiology 162:1479–1489. doi: 10.1099/mic.0.000313. [DOI] [PubMed] [Google Scholar]
- 116.Kenyon JJ, Speciale I, Hall RM, De Castro C. 2016. Structure of repeating unit of the capsular polysaccharide from Acinetobacter baumannii D78 and assignment of the K4 gene cluster. Carbohydr Res 434:12–17. doi: 10.1016/j.carres.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 117.van Faassen H, KuoLee R, Harris G, Zhao X, Conlan JW, Chen W. 2007. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infect Immun 75:5597–5608. doi: 10.1128/IAI.00762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Breslow JM, Meissler JJ Jr, Hartzell RR, Spence PB, Truant A, Gaughan J, Eisenstein TK. 2011. Innate immune responses to systemic Acinetobacter baumannii infection in mice: neutrophils, but not IL-17, mediate host resistance. Infect Immun 79:3317–3327. doi: 10.1128/IAI.00069-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Qiu H, Kuolee R, Harris G, Chen W. 2009. Role of NADPH phagocyte oxidase in host defense against acute respiratory Acinetobacter baumannii infection in mice. Infect Immun 77:1015–1021. doi: 10.1128/IAI.01029-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qiu H, Kuolee R, Harris G, Van Rooijen N, Patel GB, Chen W. 2012. Role of macrophages in early host resistance to respiratory Acinetobacter baumannii infection. PLoS One 7:e40019. doi: 10.1371/journal.pone.0040019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hood MI, Uzhachenko R, Boyd K, Skaar EP, Ivanova AV. 2013. Loss of mitochondrial protein Fus1 augments host resistance to Acinetobacter baumannii infection. Infect Immun 81:4461–4469. doi: 10.1128/IAI.00771-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Garcia A, Salgado F, Solar H, Gonzalez CL, Zemelman R, Onate A. 1999. Some immunological properties of lipopolysaccharide from Acinetobacter baumannii. J Med Microbiol 48:479–483. doi: 10.1099/00222615-48-5-479. [DOI] [PubMed] [Google Scholar]
- 123.Erridge C, Moncayo-Nieto OL, Morgan R, Young M, Poxton IR. 2007. Acinetobacter baumannii lipopolysaccharides are potent stimulators of human monocyte activation via Toll-like receptor 4 signalling. J Med Microbiol 56:165–171. doi: 10.1099/jmm.0.46823-0. [DOI] [PubMed] [Google Scholar]
- 124.Knapp S, Wieland CW, Florquin S, Pantophlet R, Dijkshoorn L, Tshimbalanga N, Akira S, van der Poll T. 2006. Differential roles of CD14 and Toll-like receptors 4 and 2 in murine Acinetobacter pneumonia. Am J Respir Crit Care Med 173:122–129. doi: 10.1164/rccm.200505-730OC. [DOI] [PubMed] [Google Scholar]
- 125.Renckens R, Roelofs JJ, Knapp S, de Vos AF, Florquin S, van der Poll T. 2006. The acute-phase response and serum amyloid A inhibit the inflammatory response to Acinetobacter baumannii pneumonia. J Infect Dis 193:187–195. doi: 10.1086/498876. [DOI] [PubMed] [Google Scholar]
- 126.Jacobs AC, Thompson MG, Black CC, Kessler JL, Clark LP, McQueary CN, Gancz HY, Corey BW, Moon JK, Si Y, Owen MT, Hallock JD, Kwak YI, Summers A, Li CZ, Rasko DA, Penwell WF, Honnold CL, Wise MC, Waterman PE, Lesho EP, Stewart RL, Actis LA, Palys TJ, Craft DW, Zurawski DV. 2014. AB5075, a highly virulent isolate of Acinetobacter baumannii, as a model strain for the evaluation of pathogenesis and antimicrobial treatments. mBio 5:e01076-14. doi: 10.1128/mBio.01076-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Infectious Diseases Society of America. 2012. White paper: recommendations on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens. Clin Infect Dis 55:1031–1046. doi: 10.1093/cid/cis688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Harris G, Kuo Lee R, Lam CK, Kanzaki G, Patel GB, Xu HH, Chen W. 2013. A mouse model of Acinetobacter baumannii-associated pneumonia using a clinically isolated hypervirulent strain. Antimicrob Agents Chemother 57:3601–3613. doi: 10.1128/AAC.00944-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Noto MJ, Boyd KL, Burns WJ, Varga MG, Peek RM Jr, Skaar EP. 2015. Toll-like receptor 9 contributes to defense against Acinetobacter baumannii infection. Infect Immun 83:4134–4141. doi: 10.1128/IAI.00410-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Breslow JM, Monroy MA, Daly JM, Meissler JJ, Gaughan J, Adler MW, Eisenstein TK. 2011. Morphine, but not trauma, sensitizes to systemic Acinetobacter baumannii infection. J Neuroimmune Pharmacol 6:551–565. doi: 10.1007/s11481-011-9303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Banerjee S, Meng J, Das S, Krishnan A, Haworth J, Charboneau R, Zeng Y, Ramakrishnan S, Roy S. 2013. Morphine induced exacerbation of sepsis is mediated by tempering endotoxin tolerance through modulation of miR-146a. Sci Rep 3:1977. doi: 10.1038/srep01977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Roux D, Danilchanka O, Guillard T, Cattoir V, Aschard H, Fu Y, Angoulvant F, Messika J, Ricard JD, Mekalanos JJ, Lory S, Pier GB, Skurnik D. 2015. Fitness cost of antibiotic susceptibility during bacterial infection. Sci Transl Med 7:297ra114. doi: 10.1126/scitranslmed.aab1621. [DOI] [PubMed] [Google Scholar]
- 133.Vila J, Marti S, Sanchez-Cespedes J. 2007. Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii. J Antimicrob Chemother 59:1210–1215. doi: 10.1093/jac/dkl509. [DOI] [PubMed] [Google Scholar]
- 134.Sato K, Nakae T. 1991. Outer membrane permeability of Acinetobacter calcoaceticus and its implication in antibiotic resistance. J Antimicrob Chemother 28:35–45. doi: 10.1093/jac/28.1.35. [DOI] [PubMed] [Google Scholar]
- 135.Adams MD, Goglin K, Molyneaux N, Hujer KM, Lavender H, Jamison JJ, MacDonald IJ, Martin KM, Russo T, Campagnari AA, Hujer AM, Bonomo RA, Gill SR. 2008. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J Bacteriol 190:8053–8064. doi: 10.1128/JB.00834-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Blackwell GA, Hamidian M, Hall RM. 2016. IncM plasmid R1215 is the source of chromosomally located regions containing multiple antibiotic resistance genes in the globally disseminated Acinetobacter baumannii GC1 and GC2 clones. mSphere 1:e00117-16. doi: 10.1128/mSphere.00117-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cheng A, Chuang YC, Sun HY, Sheng WH, Yang CJ, Liao CH, Hsueh PR, Yang JL, Shen NJ, Wang JT, Hung CC, Chen YC, Chang SC. 2015. Excess mortality associated with colistin-tigecycline compared with colistin-carbapenem combination therapy for extensively drug-resistant Acinetobacter baumannii bacteremia: a multicenter prospective observational study. Crit Care Med 43:1194–1204. doi: 10.1097/CCM.0000000000000933. [DOI] [PubMed] [Google Scholar]
- 138.Fernandez-Cuenca F, Martinez-Martinez L, Conejo MC, Ayala JA, Perea EJ, Pascual A. 2003. Relationship between beta-lactamase production, outer membrane protein and penicillin-binding protein profiles on the activity of carbapenems against clinical isolates of Acinetobacter baumannii. J Antimicrob Chemother 51:565–574. doi: 10.1093/jac/dkg097. [DOI] [PubMed] [Google Scholar]
- 139.D'Arezzo S, Capone A, Petrosillo N, Visca P, Ballardini M, Bartolini S, Bordi E, Di Stefano A, Galie M, Minniti R, Meledandri M, Pacciani L, Parisi G, Prignano G, Santini C, Valmarin M, Venditti M, Ziantoni S. 2009. Epidemic multidrug-resistant Acinetobacter baumannii related to European clonal types I and II in Rome (Italy). Clin Microbiol Infect 15:347–357. doi: 10.1111/j.1469-0691.2009.02668.x. [DOI] [PubMed] [Google Scholar]
- 140.Higgins PG, Dammhayn C, Hackel M, Seifert H. 2010. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother 65:233–238. doi: 10.1093/jac/dkp428. [DOI] [PubMed] [Google Scholar]
- 141.Adams-Haduch JM, Onuoha EO, Bogdanovich T, Tian GB, Marschall J, Urban CM, Spellberg BJ, Rhee D, Halstead DC, Pasculle AW, Doi Y. 2011. Molecular epidemiology of carbapenem-nonsusceptible Acinetobacter baumannii in the United States. J Clin Microbiol 49:3849–3854. doi: 10.1128/JCM.00619-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ben RJ, Yang MC, Hsueh JC, Shiang JC, Chien ST. 2011. Molecular characterisation of multiple drug-resistant Acinetobacter baumannii isolates in southern Taiwan. Int J Antimicrob Agents 38:403–408. doi: 10.1016/j.ijantimicag.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 143.Wu W, He Y, Lu J, Lu Y, Wu J, Liu Y. 2015. Transition of blaOXA-58-like to blaOXA-23-like in Acinetobacter baumannii clinical isolates in southern China: an 8-year study. PLoS One 10:e0137174. doi: 10.1371/journal.pone.0137174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kamolvit W, Sidjabat HE, Paterson DL. 2015. Molecular epidemiology and mechanisms of carbapenem resistance of Acinetobacter spp. in Asia and Oceania. Microb Drug Resist 21:424–434. doi: 10.1089/mdr.2014.0234. [DOI] [PubMed] [Google Scholar]
- 145.Principe L, Piazza A, Giani T, Bracco S, Caltagirone MS, Arena F, Nucleo E, Tammaro F, Rossolini GM, Pagani L, Luzzaro F, AMCLI-CRAb Survey Participants. 2014. Epidemic diffusion of OXA-23-producing Acinetobacter baumannii isolates in Italy: results of the first cross-sectional countrywide survey. J Clin Microbiol 52:3004–3010. doi: 10.1128/JCM.00291-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ji S, Chen Y, Ruan Z, Fu Y, Ji J, Fu Y, Wang H, Yu Y. 2014. Prevalence of carbapenem-hydrolyzing class D beta-lactamase genes in Acinetobacter spp. isolates in China. Eur J Clin Microbiol Infect Dis 33:989–997. doi: 10.1007/s10096-013-2037-z. [DOI] [PubMed] [Google Scholar]
- 147.Zarrilli R, Pournaras S, Giannouli M, Tsakris A. 2013. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int J Antimicrob Agents 41:11–19. doi: 10.1016/j.ijantimicag.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 148.Nigro SJ, Hall RM. 2016. Structure and context of Acinetobacter transposons carrying the oxa23 carbapenemase gene. J Antimicrob Chemother 71:1135–1147. doi: 10.1093/jac/dkv440. [DOI] [PubMed] [Google Scholar]
- 149.Nigro S, Hall RM. 2015. Distribution of the blaOXA-23-containing transposons Tn2006 and Tn2008 in Australian carbapenem-resistant Acinetobacter baumannii isolates. J Antimicrob Chemother 70:2409–2411. doi: 10.1093/jac/dkv102. [DOI] [PubMed] [Google Scholar]
- 150.Liu LL, Ji SJ, Ruan Z, Fu Y, Fu YQ, Wang YF, Yu YS. 2015. Dissemination of blaOXA-23 in Acinetobacter spp. in China: main roles of conjugative plasmid pAZJ221 and transposon Tn2009. Antimicrob Agents Chemother 59:1998–2005. doi: 10.1128/AAC.04574-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Warner WA, Kuang SN, Hernandez R, Chong MC, Ewing PJ, Fleischer J, Meng J, Chu S, Terashita D, English L, Chen W, Xu HH. 2016. Molecular characterization and antimicrobial susceptibility of Acinetobacter baumannii isolates obtained from two hospital outbreaks in Los Angeles County, California, USA. BMC Infect Dis 16:194. doi: 10.1186/s12879-016-1526-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Agodi A, Voulgari E, Barchitta M, Quattrocchi A, Bellocchi P, Poulou A, Santangelo C, Castiglione G, Giaquinta L, Romeo MA, Vrioni G, Tsakris A. 2014. Spread of a carbapenem- and colistin-resistant Acinetobacter baumannii ST2 clonal strain causing outbreaks in two Sicilian hospitals. J Hosp Infect 86:260–266. doi: 10.1016/j.jhin.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 153.Lee HY, Chen CL, Wu SR, Huang CW, Chiu CH. 2014. Risk factors and outcome analysis of Acinetobacter baumannii complex bacteremia in critical patients. Crit Care Med 42:1081–1088. doi: 10.1097/CCM.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 154.Song JY, Cheong HJ, Choi WS, Heo JY, Noh JY, Kim WJ. 2011. Clinical and microbiological characterization of carbapenem-resistant Acinetobacter baumannii bloodstream infections. J Med Microbiol 60:605–611. doi: 10.1099/jmm.0.029439-0. [DOI] [PubMed] [Google Scholar]
- 155.Adams-Haduch JM, Paterson DL, Sidjabat HE, Pasculle AW, Potoski BA, Muto CA, Harrison LH, Doi Y. 2008. Genetic basis of multidrug resistance in Acinetobacter baumannii clinical isolates at a tertiary medical center in Pennsylvania. Antimicrob Agents Chemother 52:3837–3843. doi: 10.1128/AAC.00570-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. 2007. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dortet L, Poirel L, Errera C, Nordmann P. 2014. CarbAcineto NP test for rapid detection of carbapenemase-producing Acinetobacter spp. J Clin Microbiol 52:2359–2364. doi: 10.1128/JCM.00594-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bonnin RA, Poirel L, Naas T, Pirs M, Seme K, Schrenzel J, Nordmann P. 2012. Dissemination of New Delhi metallo-beta-lactamase-1-producing Acinetobacter baumannii in Europe. Clin Microbiol Infect 18:E362–E365. doi: 10.1111/j.1469-0691.2012.03928.x. [DOI] [PubMed] [Google Scholar]
- 159.Krahn T, Wibberg D, Maus I, Winkler A, Bontron S, Sczyrba A, Nordmann P, Puhler A, Poirel L, Schluter A. 2016. Intraspecies transfer of the chromosomal Acinetobacter baumannii blaNDM-1 carbapenemase gene. Antimicrob Agents Chemother 60:3032–3040. doi: 10.1128/AAC.00124-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bonnin RA, Poirel L, Nordmann P. 2014. New Delhi metallo-beta-lactamase-producing Acinetobacter baumannii: a novel paradigm for spreading antibiotic resistance genes. Future Microbiol 9:33–41. doi: 10.2217/fmb.13.69. [DOI] [PubMed] [Google Scholar]
- 161.Bonnin RA, Nordmann P, Potron A, Lecuyer H, Zahar JR, Poirel L. 2011. Carbapenem-hydrolyzing GES-type extended-spectrum beta-lactamase in Acinetobacter baumannii. Antimicrob Agents Chemother 55:349–354. doi: 10.1128/AAC.00773-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Moubareck C, Bremont S, Conroy MC, Courvalin P, Lambert T. 2009. GES-11, a novel integron-associated GES variant in Acinetobacter baumannii. Antimicrob Agents Chemother 53:3579–3581. doi: 10.1128/AAC.00072-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Castanheira M, Costello SE, Woosley LN, Deshpande LM, Davies TA, Jones RN. 2014. Evaluation of clonality and carbapenem resistance mechanisms among Acinetobacter baumannii-Acinetobacter calcoaceticus complex and Enterobacteriaceae isolates collected in European and Mediterranean countries and detection of two novel beta-lactamases, GES-22 and VIM-35. Antimicrob Agents Chemother 58:7358–7366. doi: 10.1128/AAC.03930-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Robledo IE, Aquino EE, Sante MI, Santana JL, Otero DM, Leon CF, Vazquez GJ. 2010. Detection of KPC in Acinetobacter spp. in Puerto Rico. Antimicrob Agents Chemother 54:1354–1357. doi: 10.1128/AAC.00899-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Robledo IE, Aquino EE, Vazquez GJ. 2011. Detection of the KPC gene in Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii during a PCR-based nosocomial surveillance study in Puerto Rico. Antimicrob Agents Chemother 55:2968–2970. doi: 10.1128/AAC.01633-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mussi MA, Limansky AS, Viale AM. 2005. Acquisition of resistance to carbapenems in multidrug-resistant clinical strains of Acinetobacter baumannii: natural insertional inactivation of a gene encoding a member of a novel family of beta-barrel outer membrane proteins. Antimicrob Agents Chemother 49:1432–1440. doi: 10.1128/AAC.49.4.1432-1440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Siroy A, Molle V, Lemaitre-Guillier C, Vallenet D, Pestel-Caron M, Cozzone AJ, Jouenne T, De E. 2005. Channel formation by CarO, the carbapenem resistance-associated outer membrane protein of Acinetobacter baumannii. Antimicrob Agents Chemother 49:4876–4883. doi: 10.1128/AAC.49.12.4876-4883.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dupont M, Pages JM, Lafitte D, Siroy A, Bollet C. 2005. Identification of an OprD homologue in Acinetobacter baumannii. J Proteome Res 4:2386–2390. doi: 10.1021/pr050143q. [DOI] [PubMed] [Google Scholar]
- 169.Catel-Ferreira M, Coadou G, Molle V, Mugnier P, Nordmann P, Siroy A, Jouenne T, De E. 2011. Structure-function relationships of CarO, the carbapenem resistance-associated outer membrane protein of Acinetobacter baumannii. J Antimicrob Chemother 66:2053–2056. doi: 10.1093/jac/dkr267. [DOI] [PubMed] [Google Scholar]
- 170.Fonseca EL, Scheidegger E, Freitas FS, Cipriano R, Vicente AC. 2013. Carbapenem-resistant Acinetobacter baumannii from Brazil: role of carO alleles expression and blaOXA-23 gene. BMC Microbiol 13:245. doi: 10.1186/1471-2180-13-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Smani Y, Dominguez-Herrera J, Pachon J. 2013. Association of the outer membrane protein Omp33 with fitness and virulence of Acinetobacter baumannii. J Infect Dis 208:1561–1570. doi: 10.1093/infdis/jit386. [DOI] [PubMed] [Google Scholar]
- 172.McGann P, Courvalin P, Snesrud E, Clifford RJ, Yoon EJ, Onmus-Leone F, Ong AC, Kwak YI, Grillot-Courvalin C, Lesho E, Waterman PE. 2014. Amplification of aminoglycoside resistance gene aphA1 in Acinetobacter baumannii results in tobramycin therapy failure. mBio 5:e00915. doi: 10.1128/mBio.00915-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Martinez T, Martinez I, Vazquez GJ, Aquino EE, Robledo IE. 2016. Genetic environment of the KPC gene in Acinetobacter baumannii ST2 clone from Puerto Rico and genomic insights into its drug resistance. J Med Microbiol 65:784–792. doi: 10.1099/jmm.0.000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mosqueda N, Espinal P, Cosgaya C, Viota S, Plasensia V, Alvarez-Lerma F, Montero M, Gomez J, Horcajada JP, Vila J, Roca I. 2013. Globally expanding carbapenemase finally appears in Spain: nosocomial outbreak of Acinetobacter baumannii producing plasmid-encoded OXA-23 in Barcelona, Spain. Antimicrob Agents Chemother 57:5155–5157. doi: 10.1128/AAC.01486-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hamidian M, Hall RM. 2014. pACICU2 is a conjugative plasmid of Acinetobacter carrying the aminoglycoside resistance transposon TnaphA6. J Antimicrob Chemother 69:1146–1148. doi: 10.1093/jac/dkt488. [DOI] [PubMed] [Google Scholar]
- 176.Hamidian M, Hall RM. 2014. Tn6168, a transposon carrying an ISAba1-activated ampC gene and conferring cephalosporin resistance in Acinetobacter baumannii. J Antimicrob Chemother 69:77–80. doi: 10.1093/jac/dkt312. [DOI] [PubMed] [Google Scholar]
- 177.Hamidian M, Holt KE, Pickard D, Dougan G, Hall RM. 2014. A GC1 Acinetobacter baumannii isolate carrying AbaR3 and the aminoglycoside resistance transposon TnaphA6 in a conjugative plasmid. J Antimicrob Chemother 69:955–958. doi: 10.1093/jac/dkt454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Guerrero-Lozano I, Fernandez-Cuenca F, Galan-Sanchez F, Egea P, Rodriguez-Iglesias M, Pascual A. 2015. Description of the OXA-23 beta-lactamase gene located within Tn2007 in a clinical isolate of Acinetobacter baumannii from Spain. Microb Drug Resist 21:215–217. doi: 10.1089/mdr.2014.0155. [DOI] [PubMed] [Google Scholar]
- 179.Blot S, Vandewoude K, Colardyn F. 2003. Nosocomial bacteremia involving Acinetobacter baumannii in critically ill patients: a matched cohort study. Intensive Care Med 29:471–475. doi: 10.1007/s00134-003-1648-8. [DOI] [PubMed] [Google Scholar]
- 180.Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF. 2016. Multidrug resistance, inappropriate empiric therapy, and hospital mortality in Acinetobacter baumannii pneumonia and sepsis. Crit Care 20:221. doi: 10.1186/s13054-016-1392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shlaes DM, Moellering RC Jr. 2002. The United States Food and Drug Administration and the end of antibiotics. Clin Infect Dis 34:420–422. doi: 10.1086/334577. [DOI] [PubMed] [Google Scholar]
- 182.Zilberberg MD, Kollef MH, Shorr AF. 2016. Secular trends in Acinetobacter baumannii resistance in respiratory and blood stream specimens in the United States, 2003 to 2012: a survey study. J Hosp Med 11:21–26. doi: 10.1002/jhm.2477. [DOI] [PubMed] [Google Scholar]
- 183.Lautenbach E, Synnestvedt M, Weiner MG, Bilker WB, Vo L, Schein J, Kim M. 2009. Epidemiology and impact of imipenem resistance in Acinetobacter baumannii. Infect Control Hosp Epidemiol 30:1186–1192. doi: 10.1086/648450. [DOI] [PubMed] [Google Scholar]
- 184.Agodi A, Barchitta M, Quattrocchi A, Maugeri A, Aldisio E, Marchese AE, Mattaliano AR, Tsakris A. 2015. Antibiotic trends of Klebsiella pneumoniae and Acinetobacter baumannii resistance indicators in an intensive care unit of Southern Italy, 2008-2013. Antimicrob Resist Infect Control 4:43. doi: 10.1186/s13756-015-0087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gales AC, Castanheira M, Jones RN, Sader HS. 2012. Antimicrobial resistance among Gram-negative bacilli isolated from Latin America: results from SENTRY Antimicrobial Surveillance Program (Latin America, 2008-2010). Diagn Microbiol Infect Dis 73:354–360. doi: 10.1016/j.diagmicrobio.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 186.Taneja N, Singh G, Singh M, Sharma M. 2011. Emergence of tigecycline & colistin resistant Acinetobacter baumanii in patients with complicated urinary tract infections in north India. Indian J Med Res 133:681–684. [PMC free article] [PubMed] [Google Scholar]
- 187.Perween N, Sehgal S, Prakash SK. 2014. Geographical patterns in antimicrobial resistance of Acinetobacter in clinical isolates. J Clin Diagn Res 8:DC10–DC12. doi: 10.7860/JCDR/2014/8590.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gandra S, Mojica N, Klein EY, Ashok A, Nerurkar V, Kumari M, Ramesh U, Dey S, Vadwai V, Das BR, Laxminarayan R. 2016. Trends in antibiotic resistance among major bacterial pathogens isolated from blood cultures tested at a large private laboratory network in India, 2008-2014. Int J Infect Dis 50:75–82. doi: 10.1016/j.ijid.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tsioutis C, Kritsotakis EI, Karageorgos SA, Stratakou S, Psarologakis C, Kokkini S, Gikas A. 12 August 2016. Clinical epidemiology, treatment and prognostic factors of extensively drug-resistant Acinetobacter baumannii ventilator-associated pneumonia in critically ill patients. Int J Antimicrob Agents doi: 10.1016/j.ijantimicag.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 190.Guo H, Qin J, Xiang J. 17 August 2016. Surveillance for and susceptibility of Acinetobacter baumannii in a large hospital and burn center in Shanghai, China, 2007-2013. Am J Infect Control doi: 10.1016/j.ajic.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 191.Esterly JS, Griffith M, Qi C, Malczynski M, Postelnick MJ, Scheetz MH. 2011. Impact of carbapenem resistance and receipt of active antimicrobial therapy on clinical outcomes of Acinetobacter baumannii bloodstream infections. Antimicrob Agents Chemother 55:4844–4849. doi: 10.1128/AAC.01728-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K. 2009. International study of the prevalence and outcomes of infection in intensive care units. JAMA 302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 193.Leao AC, Menezes PR, Oliveira MS, Levin AS. 2016. Acinetobacter spp. are associated with a higher mortality in intensive care patients with bacteremia: a survival analysis. BMC Infect Dis 16:386. doi: 10.1186/s12879-016-1695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Raad II, Mohamed JA, Reitzel RA, Jiang Y, Dvorak TL, Ghannoum MA, Hachem RY, Chaftari AM. 2011. The prevention of biofilm colonization by multidrug-resistant pathogens that cause ventilator-associated pneumonia with antimicrobial-coated endotracheal tubes. Biomaterials 32:2689–2694. doi: 10.1016/j.biomaterials.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 195.Gil-Perotin S, Ramirez P, Marti V, Sahuquillo JM, Gonzalez E, Calleja I, Menendez R, Bonastre J. 2012. Implications of endotracheal tube biofilm in ventilator-associated pneumonia response: a state of concept. Crit Care 16:R93. doi: 10.1186/cc11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Davis KA, Moran KA, McAllister CK, Gray PJ. 2005. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg Infect Dis 11:1218–1224. doi: 10.3201/1108.050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yun HC, Branstetter JG, Murray CK. 2008. Osteomyelitis in military personnel wounded in Iraq and Afghanistan. J Trauma 64:S163–S168. doi: 10.1097/TA.0b013e318160868c. [DOI] [PubMed] [Google Scholar]
- 198.Carvalho VC, Oliveira PR, Dal-Paz K, Paula AP, Felix CDS, Lima AL. 2012. Gram-negative osteomyelitis: clinical and microbiological profile. Braz J Infect Dis 16:63–67. doi: 10.1016/S1413-8670(12)70276-3. [DOI] [PubMed] [Google Scholar]
- 199.Falagas ME, Karveli EA, Kelesidis I, Kelesidis T. 2007. Community-acquired Acinetobacter infections. Eur J Clin Microbiol Infect Dis 26:857–868. doi: 10.1007/s10096-007-0365-6. [DOI] [PubMed] [Google Scholar]
- 200.Peng C, Zong Z, Fan H. 2012. Acinetobacter baumannii isolates associated with community-acquired pneumonia in West China. Clin Microbiol Infect 18:E491–E493. doi: 10.1111/1469-0691.12017. [DOI] [PubMed] [Google Scholar]
- 201.Anstey NM, Currie BJ, Hassell M, Palmer D, Dwyer B, Seifert H. 2002. Community-acquired bacteremic Acinetobacter pneumonia in tropical Australia is caused by diverse strains of Acinetobacter baumannii, with carriage in the throat in at-risk groups. J Clin Microbiol 40:685–686. doi: 10.1128/JCM.40.2.685-686.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.O'Shea MK. 2012. Acinetobacter in modern warfare. Int J Antimicrob Agents 39:363–375. doi: 10.1016/j.ijantimicag.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 203.Murray CK, Roop SA, Hospenthal DR, Dooley DP, Wenner K, Hammock J, Taufen N, Gourdine E. 2006. Bacteriology of war wounds at the time of injury. Mil Med 171:826–829. doi: 10.7205/MILMED.171.9.826. [DOI] [PubMed] [Google Scholar]
- 204.Murray CK, Wilkins K, Molter NC, Yun HC, Dubick MA, Spott MA, Jenkins D, Eastridge B, Holcomb JB, Blackbourne LH, Hospenthal DR. 2009. Infections in combat casualties during Operations Iraqi and Enduring Freedom. J Trauma 66:S138–S144. doi: 10.1097/TA.0b013e31819d894c. [DOI] [PubMed] [Google Scholar]
- 205.Murray CK, Yun HC, Griffith ME, Thompson B, Crouch HK, Monson LS, Aldous WK, Mende K, Hospenthal DR. 2009. Recovery of multidrug-resistant bacteria from combat personnel evacuated from Iraq and Afghanistan at a single military treatment facility. Mil Med 174:598–604. doi: 10.7205/MILMED-D-03-8008. [DOI] [PubMed] [Google Scholar]
- 206.Petersen K, Riddle MS, Danko JR, Blazes DL, Hayden R, Tasker SA, Dunne JR. 2007. Trauma-related infections in battlefield casualties from Iraq. Ann Surg 245:803–811. doi: 10.1097/01.sla.0000251707.32332.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Scott P, Deye G, Srinivasan A, Murray C, Moran K, Hulten E, Fishbain J, Craft D, Riddell S, Lindler L, Mancuso J, Milstrey E, Bautista CT, Patel J, Ewell A, Hamilton T, Gaddy C, Tenney M, Christopher G, Petersen K, Endy T, Petruccelli B. 2007. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis 44:1577–1584. doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 208.Centers for Disease Control and Prevention. 2004. Acinetobacter baumannii infections among patients at military medical facilities treating injured U.S. service members, 2002-2004. MMWR Morb Mortal Wkly Rep 53:1063–1066. [PubMed] [Google Scholar]
- 209.Oncul O, Keskin O, Acar HV, Kucukardali Y, Evrenkaya R, Atasoyu EM, Top C, Nalbant S, Ozkan S, Emekdas G, Cavuslu S, Us MH, Pahsa A, Gokben M. 2002. Hospital-acquired infections following the 1999 Marmara earthquake. J Hosp Infect 51:47–51. doi: 10.1053/jhin.2002.1205. [DOI] [PubMed] [Google Scholar]
- 210.Maegele M, Gregor S, Steinhausen E, Bouillon B, Heiss MM, Perbix W, Wappler F, Rixen D, Geisen J, Berger-Schreck B, Schwarz R. 2005. The long-distance tertiary air transfer and care of tsunami victims: injury pattern and microbiological and psychological aspects. Crit Care Med 33:1136–1140. doi: 10.1097/01.CCM.0000163269.42524.50. [DOI] [PubMed] [Google Scholar]
- 211.Tao C, Kang M, Chen Z, Xie Y, Fan H, Qin L, Ma Y. 2009. Microbiologic study of the pathogens isolated from wound culture among Wenchuan earthquake survivors. Diagn Microbiol Infect Dis 63:268–270. doi: 10.1016/j.diagmicrobio.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 212.Dallo SF, Weitao T. 2010. Insights into Acinetobacter war-wound infections, biofilms, and control. Adv Skin Wound Care 23:169–174. doi: 10.1097/01.ASW.0000363527.08501.a3. [DOI] [PubMed] [Google Scholar]
- 213.Wang Y, Hao P, Lu B, Yu H, Huang W, Hou H, Dai K. 2010. Causes of infection after earthquake, China, 2008. Emerg Infect Dis 16:974–975. doi: 10.3201/eid1606.091523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhang B, Liu Z, Lin Z, Zhang X, Fu W. 2012. Microbiologic characteristics of pathogenic bacteria from hospitalized trauma patients who survived Wenchuan earthquake. Eur J Clin Microbiol Infect Dis 31:2529–2535. doi: 10.1007/s10096-012-1591-0. [DOI] [PubMed] [Google Scholar]
- 215.Apisarnthanarak A, Khawcharoenporn T, Mundy LM. 2013. Patterns of nosocomial infections, multidrug-resistant microorganisms, and mold detection after extensive black-water flooding: a survey from central Thailand. Infect Control Hosp Epidemiol 34:861–863. doi: 10.1086/671277. [DOI] [PubMed] [Google Scholar]
- 216.Nonaka Y, Nagae M, Omae T, Yamamoto S, Horitani R, Maeda D, Yoshinaga T. 2014. Community-acquired necrotizing fasciitis caused by Acinetobacter calcoaceticus: a case report and literature review. J Infect Chemother 20:330–335. doi: 10.1016/j.jiac.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 217.Sinha N, Niazi M, Lvovsky D. 2014. A fatal case of multidrug resistant Acinetobacter necrotizing fasciitis: the changing scary face of nosocomial infection. Case Rep Infect Dis 2014:705279. doi: 10.1155/2014/705279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Corradino B, Toia F, di Lorenzo S, Cordova A, Moschella F. 2010. A difficult case of necrotizing fasciitis caused by Acinetobacter baumannii. Int J Low Extrem Wounds 9:152–154. doi: 10.1177/1534734610389598. [DOI] [PubMed] [Google Scholar]
- 219.Clemente WT, Sanches MD, Coutinho RL, de Oliveira Junior AR, Lauria MW, Lima CX, de Castro Romanelli RM. 2012. Multidrug-resistant Acinetobacter baumannii causing necrotizing fasciitis in a pancreas-kidney transplant recipient: a case report. Transplantation 94:e37–e38. doi: 10.1097/TP.0b013e318265083b. [DOI] [PubMed] [Google Scholar]
- 220.Sullivan DR, Shields J, Netzer G. 2010. Fatal case of multi-drug resistant Acinetobacter baumannii necrotizing fasciitis. Am Surg 76:651–653. [PubMed] [Google Scholar]
- 221.Charnot-Katsikas A, Dorafshar AH, Aycock JK, David MZ, Weber SG, Frank KM. 2009. Two cases of necrotizing fasciitis due to Acinetobacter baumannii. J Clin Microbiol 47:258–263. doi: 10.1128/JCM.01250-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Brachelente C, Wiener D, Malik Y, Huessy D. 2007. A case of necrotizing fasciitis with septic shock in a cat caused by Acinetobacter baumannii. Vet Dermatol 18:432–438. doi: 10.1111/j.1365-3164.2007.00624.x. [DOI] [PubMed] [Google Scholar]
- 223.Pailhories H, Belmonte O, Kempf M, Lemarie C, Cuziat J, Quinqueneau C, Ramont C, Joly-Guillou ML, Eveillard M. 2015. Diversity of Acinetobacter baumannii strains isolated in humans, companion animals, and the environment in Reunion Island: an exploratory study. Int J Infect Dis 37:64–69. doi: 10.1016/j.ijid.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 224.Belmonte O, Pailhories H, Kempf M, Gaultier MP, Lemarie C, Ramont C, Joly-Guillou ML, Eveillard M. 2014. High prevalence of closely-related Acinetobacter baumannii in pets according to a multicentre study in veterinary clinics, Reunion Island. Vet Microbiol 170:446–450. doi: 10.1016/j.vetmic.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 225.Zordan S, Prenger-Berninghoff E, Weiss R, van der Reijden T, van den Broek P, Baljer G, Dijkshoorn L. 2011. Multidrug-resistant Acinetobacter baumannii in veterinary clinics, Germany. Emerg Infect Dis 17:1751–1754. doi: 10.3201/eid1709.101931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mattoes HM, Kuti JL, Drusano GL, Nicolau DP. 2004. Optimizing antimicrobial pharmacodynamics: dosage strategies for meropenem. Clin Ther 26:1187–1198. doi: 10.1016/S0149-2918(04)80001-8. [DOI] [PubMed] [Google Scholar]
- 227.Lomaestro BM, Drusano GL. 2005. Pharmacodynamic evaluation of extending the administration time of meropenem using a Monte Carlo simulation. Antimicrob Agents Chemother 49:461–463. doi: 10.1128/AAC.49.1.461-463.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lee LS, Kinzig-Schippers M, Nafziger AN, Ma L, Sorgel F, Jones RN, Drusano GL, Bertino JS Jr. 2010. Comparison of 30-min and 3-h infusion regimens for imipenem/cilastatin and for meropenem evaluated by Monte Carlo simulation. Diagn Microbiol Infect Dis 68:251–258. doi: 10.1016/j.diagmicrobio.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 229.Falagas ME, Tansarli GS, Ikawa K, Vardakas KZ. 2013. Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: a systematic review and meta-analysis. Clin Infect Dis 56:272–282. doi: 10.1093/cid/cis857. [DOI] [PubMed] [Google Scholar]
- 230.Prasad P, Sun J, Danner RL, Natanson C. 2012. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 54:1699–1709. doi: 10.1093/cid/cis270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gardiner D, Dukart G, Cooper A, Babinchak T. 2010. Safety and efficacy of intravenous tigecycline in subjects with secondary bacteremia: pooled results from 8 phase III clinical trials. Clin Infect Dis 50:229–238. doi: 10.1086/648720. [DOI] [PubMed] [Google Scholar]
- 232.Freire AT, Melnyk V, Kim MJ, Datsenko O, Dzyublik O, Glumcher F, Chuang YC, Maroko RT, Dukart G, Cooper CA, Korth-Bradley JM, Dartois N, Gandjini H, 311 Study Group. 2010. Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn Microbiol Infect Dis 68:140–151. doi: 10.1016/j.diagmicrobio.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 233.Spellberg B, Bonomo RA. 2015. Combination therapy for extreme drug-resistant Acinetobacter baumannii: ready for prime time? Crit Care Med 43:1332–1334. doi: 10.1097/CCM.0000000000001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chuang YC, Cheng CY, Sheng WH, Sun HY, Wang JT, Chen YC, Chang SC. 2014. Effectiveness of tigecycline-based versus colistin-based therapy for treatment of pneumonia caused by multidrug-resistant Acinetobacter baumannii in a critical setting: a matched cohort analysis. BMC Infect Dis 14:102. doi: 10.1186/1471-2334-14-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lee YT, Tsao SM, Hsueh PR. 2013. Clinical outcomes of tigecycline alone or in combination with other antimicrobial agents for the treatment of patients with healthcare-associated multidrug-resistant Acinetobacter baumannii infections. Eur J Clin Microbiol Infect Dis 32:1211–1220. doi: 10.1007/s10096-013-1870-4. [DOI] [PubMed] [Google Scholar]
- 236.Gordon NC, Wareham DW. 2009. A review of clinical and microbiological outcomes following treatment of infections involving multidrug-resistant Acinetobacter baumannii with tigecycline. J Antimicrob Chemother 63:775–780. doi: 10.1093/jac/dkn555. [DOI] [PubMed] [Google Scholar]
- 237.Ni W, Han Y, Zhao J, Wei C, Cui J, Wang R, Liu Y. 2016. Tigecycline treatment experience against multidrug-resistant Acinetobacter baumannii infections: a systematic review and meta-analysis. Int J Antimicrob Agents 47:107–116. doi: 10.1016/j.ijantimicag.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 238.Goff DA, Kaye KS. 2014. Minocycline: an old drug for a new bug: multidrug-resistant Acinetobacter baumannii. Clin Infect Dis 59(Suppl 6):S365–S366. doi: 10.1093/cid/ciu531. [DOI] [PubMed] [Google Scholar]
- 239.Ritchie DJ, Garavaglia-Wilson A. 2014. A review of intravenous minocycline for treatment of multidrug-resistant Acinetobacter infections. Clin Infect Dis 59(Suppl 6):S374–S380. doi: 10.1093/cid/ciu613. [DOI] [PubMed] [Google Scholar]
- 240.Goff DA, Bauer KA, Mangino JE. 2014. Bad bugs need old drugs: a stewardship program's evaluation of minocycline for multidrug-resistant Acinetobacter baumannii infections. Clin Infect Dis 59(Suppl 6):S381–S387. doi: 10.1093/cid/ciu593. [DOI] [PubMed] [Google Scholar]
- 241.Flamm RK, Castanheira M, Streit JM, Jones RN. 2016. Minocycline activity tested against Acinetobacter baumannii complex, Stenotrophomonas maltophilia, and Burkholderia cepacia species complex isolates from a global surveillance program (2013). Diagn Microbiol Infect Dis 85:352–355. doi: 10.1016/j.diagmicrobio.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 242.Penwell WF, Shapiro AB, Giacobbe RA, Gu RF, Gao N, Thresher J, McLaughlin RE, Huband MD, DeJonge BL, Ehmann DE, Miller AA. 2015. Molecular mechanisms of sulbactam antibacterial activity and resistance determinants in Acinetobacter baumannii. Antimicrob Agents Chemother 59:1680–1689. doi: 10.1128/AAC.04808-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Neonakis IK, Spandidos DA, Petinaki E. 2011. Confronting multidrug-resistant Acinetobacter baumannii: a review. Int J Antimicrob Agents 37:102–109. doi: 10.1016/j.ijantimicag.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 244.Vila J, Ruiz J, Goni P, Jimenez de Anta T. 1997. Quinolone-resistance mutations in the topoisomerase IV parC gene of Acinetobacter baumannii. J Antimicrob Chemother 39:757–762. doi: 10.1093/jac/39.6.757. [DOI] [PubMed] [Google Scholar]
- 245.Hujer KM, Hujer AM, Endimiani A, Thomson JM, Adams MD, Goglin K, Rather PN, Pennella TT, Massire C, Eshoo MW, Sampath R, Blyn LB, Ecker DJ, Bonomo RA. 2009. Rapid determination of quinolone resistance in Acinetobacter spp. J Clin Microbiol 47:1436–1442. doi: 10.1128/JCM.02380-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Fishbain J, Peleg AY. 2010. Treatment of Acinetobacter infections. Clin Infect Dis 51:79–84. doi: 10.1086/653120. [DOI] [PubMed] [Google Scholar]
- 247.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Rhomberg PR, Jones RN. 2009. Summary trends for the Meropenem Yearly Susceptibility Test Information Collection Program: a 10-year experience in the United States (1999-2008). Diagn Microbiol Infect Dis 65:414–426. doi: 10.1016/j.diagmicrobio.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 249.Qureshi ZA, Hittle LE, O'Hara JA, Rivera JI, Syed A, Shields RK, Pasculle AW, Ernst RK, Doi Y. 2015. Colistin-resistant Acinetobacter baumannii: beyond carbapenem resistance. Clin Infect Dis 60:1295–1303. doi: 10.1093/cid/civ048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Choi IS, Lee YJ, Wi YM, Kwan BS, Jung KH, Hong WP, Kim JM. 2016. Predictors of mortality in patients with extensively drug-resistant Acinetobacter baumannii pneumonia receiving colistin therapy. Int J Antimicrob Agents 48:175–180. doi: 10.1016/j.ijantimicag.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 251.Imberti R, Cusato M, Villani P, Carnevale L, Iotti GA, Langer M, Regazzi M. 2010. Steady-state pharmacokinetics and BAL concentration of colistin in critically ill patients after IV colistin methanesulfonate administration. Chest 138:1333–1339. doi: 10.1378/chest.10-0463. [DOI] [PubMed] [Google Scholar]
- 252.Linden PK, Paterson DL. 2006. Parenteral and inhaled colistin for treatment of ventilator-associated pneumonia. Clin Infect Dis 43(Suppl 2):S89–S94. [DOI] [PubMed] [Google Scholar]
- 253.Lin CC, Liu TC, Kuo CF, Liu CP, Lee CM. 2010. Aerosolized colistin for the treatment of multidrug-resistant Acinetobacter baumannii pneumonia: experience in a tertiary care hospital in northern Taiwan. J Microbiol Immunol Infect 43:323–331. doi: 10.1016/S1684-1182(10)60050-3. [DOI] [PubMed] [Google Scholar]
- 254.Rattanaumpawan P, Lorsutthitham J, Ungprasert P, Angkasekwinai N, Thamlikitkul V. 2010. Randomized controlled trial of nebulized colistimethate sodium as adjunctive therapy of ventilator-associated pneumonia caused by Gram-negative bacteria. J Antimicrob Chemother 65:2645–2649. doi: 10.1093/jac/dkq360. [DOI] [PubMed] [Google Scholar]
- 255.Lu Q, Luo R, Bodin L, Yang J, Zahr N, Aubry A, Golmard JL, Rouby JJ, Nebulized Antibiotics Study Group. 2012. Efficacy of high-dose nebulized colistin in ventilator-associated pneumonia caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Anesthesiology 117:1335–1347. doi: 10.1097/ALN.0b013e31827515de. [DOI] [PubMed] [Google Scholar]
- 256.Kwa AL, Loh C, Low JG, Kurup A, Tam VH. 2005. Nebulized colistin in the treatment of pneumonia due to multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Clin Infect Dis 41:754–757. doi: 10.1086/432583. [DOI] [PubMed] [Google Scholar]
- 257.Kuo SC, Lee YT, Yang SP, Chen CP, Chen TL, Hsieh SL, Siu LK, Fung CP. 2012. Eradication of multidrug-resistant Acinetobacter baumannii from the respiratory tract with inhaled colistin methanesulfonate: a matched case-control study. Clin Microbiol Infect 18:870–876. doi: 10.1111/j.1469-0691.2011.03682.x. [DOI] [PubMed] [Google Scholar]
- 258.Korbila IP, Michalopoulos A, Rafailidis PI, Nikita D, Samonis G, Falagas ME. 2010. Inhaled colistin as adjunctive therapy to intravenous colistin for the treatment of microbiologically documented ventilator-associated pneumonia: a comparative cohort study. Clin Microbiol Infect 16:1230–1236. doi: 10.1111/j.1469-0691.2009.03040.x. [DOI] [PubMed] [Google Scholar]
- 259.Falagas ME, Siempos II, Rafailidis PI, Korbila IP, Ioannidou E, Michalopoulos A. 2009. Inhaled colistin as monotherapy for multidrug-resistant gram (-) nosocomial pneumonia: a case series. Respir Med 103:707–713. doi: 10.1016/j.rmed.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 260.Arnold HM, Sawyer AM, Kollef MH. 2012. Use of adjunctive aerosolized antimicrobial therapy in the treatment of Pseudomonas aeruginosa and Acinetobacter baumannii ventilator-associated pneumonia. Respir Care 57:1226–1233. doi: 10.4187/respcare.01556. [DOI] [PubMed] [Google Scholar]
- 261.Choi HK, Kim YK, Kim HY, Uh Y. 2014. Inhaled colistin for treatment of pneumonia due to colistin-only-susceptible Acinetobacter baumannii. Yonsei Med J 55:118–125. doi: 10.3349/ymj.2014.55.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Demirdal T, Sari US, Nemli SA. 2016. Is inhaled colistin beneficial in ventilator associated pneumonia or nosocomial pneumonia caused by Acinetobacter baumannii? Ann Clin Microbiol Antimicrob 15:11. doi: 10.1186/s12941-016-0123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kang CH, Tsai CM, Wu TH, Wu HY, Chung MY, Chen CC, Huang YC, Liu SF, Liao DL, Niu CK, Lee CH, Yu HR. 2014. Colistin inhalation monotherapy for ventilator-associated pneumonia of Acinetobacter baumannii in prematurity. Pediatr Pulmonol 49:381–388. doi: 10.1002/ppul.22750. [DOI] [PubMed] [Google Scholar]
- 264.Oikonomou O, Sarrou S, Papagiannitsis CC, Georgiadou S, Mantzarlis K, Zakynthinos E, Dalekos GN, Petinaki E. 2015. Rapid dissemination of colistin and carbapenem resistant Acinetobacter baumannii in Central Greece: mechanisms of resistance, molecular identification and epidemiological data. BMC Infect Dis 15:559. doi: 10.1186/s12879-015-1297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Pogue JM, Cohen DA, Marchaim D. 2015. Polymyxin-resistant Acinetobacter baumannii: urgent action needed. Clin Infect Dis 60:1304–1307. doi: 10.1093/cid/civ044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Cai Y, Chai D, Wang R, Liang B, Bai N. 2012. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother 67:1607–1615. doi: 10.1093/jac/dks084. [DOI] [PubMed] [Google Scholar]
- 267.Craig WA, Salamone FR. 1988. Do antibiotic combinations prevent the emergence of resistant organisms? Infect Control Hosp Epidemiol 9:417–419. doi: 10.2307/30144309. [DOI] [PubMed] [Google Scholar]
- 268.Tamma PD, Cosgrove SE, Maragakis LL. 2012. Combination therapy for treatment of infections with Gram-negative bacteria. Clin Microbiol Rev 25:450–470. doi: 10.1128/CMR.05041-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Fischbach MA. 2011. Combination therapies for combating antimicrobial resistance. Curr Opin Microbiol 14:519–523. doi: 10.1016/j.mib.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Bliziotis IA, Samonis G, Vardakas KZ, Chrysanthopoulou S, Falagas ME. 2005. Effect of aminoglycoside and beta-lactam combination therapy versus beta-lactam monotherapy on the emergence of antimicrobial resistance: a meta-analysis of randomized, controlled trials. Clin Infect Dis 41:149–158. doi: 10.1086/430912. [DOI] [PubMed] [Google Scholar]
- 271.Paul M, Lador A, Grozinsky-Glasberg S, Leibovici L. 2014. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev 2014:CD003344. doi: 10.1002/14651858.CD003344.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Poulikakos P, Tansarli GS, Falagas ME. 2014. Combination antibiotic treatment versus monotherapy for multidrug-resistant, extensively drug-resistant, and pandrug-resistant Acinetobacter infections: a systematic review. Eur J Clin Microbiol Infect Dis 33:1675–1685. doi: 10.1007/s10096-014-2124-9. [DOI] [PubMed] [Google Scholar]
- 273.Tuon FF, Rocha JL, Merlini AB. 2015. Combined therapy for multi-drug-resistant Acinetobacter baumannii infection–is there evidence outside the laboratory? J Med Microbiol 64:951–959. doi: 10.1099/jmm.0.000144. [DOI] [PubMed] [Google Scholar]
- 274.Durante-Mangoni E, Signoriello G, Andini R, Mattei A, De Cristoforo M, Murino P, Bassetti M, Malacarne P, Petrosillo N, Galdieri N, Mocavero P, Corcione A, Viscoli C, Zarrilli R, Gallo C, Utili R. 2013. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis 57:349–358. doi: 10.1093/cid/cit253. [DOI] [PubMed] [Google Scholar]
- 275.Zusman O, Avni T, Leibovici L, Adler A, Friberg L, Stergiopoulou T, Carmeli Y, Paul M. 2013. In vitro synergy of polymyxins and carbapenems: systematic review and meta analysis. Antimicrob Agents Chemother 57:5104–5111. doi: 10.1128/AAC.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Oleksiuk LM, Nguyen MH, Press EG, Updike CL, O'Hara JA, Doi Y, Clancy CJ, Shields RK. 2014. In vitro responses of Acinetobacter baumannii to two- and three-drug combinations following exposure to colistin and doripenem. Antimicrob Agents Chemother 58:1195–1199. doi: 10.1128/AAC.01779-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Batirel A, Balkan II, Karabay O, Agalar C, Akalin S, Alici O, Alp E, Altay FA, Altin N, Arslan F, Aslan T, Bekiroglu N, Cesur S, Celik AD, Dogan M, Durdu B, Duygu F, Engin A, Engin DO, Gonen I, Guclu E, Guven T, Hatipoglu CA, Hosoglu S, Karahocagil MK, Kilic AU, Ormen B, Ozdemir D, Ozer S, Oztoprak N, Sezak N, Turhan V, Turker N, Yilmaz H. 2014. Comparison of colistin-carbapenem, colistin-sulbactam, and colistin plus other antibacterial agents for the treatment of extremely drug-resistant Acinetobacter baumannii bloodstream infections. Eur J Clin Microbiol Infect Dis 33:1311–1322. doi: 10.1007/s10096-014-2070-6. [DOI] [PubMed] [Google Scholar]
- 278.Dinc G, Demiraslan H, Elmali F, Ahmed SS, Alp E, Doganay M. 2015. Antimicrobial efficacy of doripenem and its combinations with sulbactam, amikacin, colistin, tigecycline in experimental sepsis of carbapenem-resistant Acinetobacter baumannii. New Microbiol 38:67–73. [PubMed] [Google Scholar]
- 279.Le Minh V, Thi Khanh Nhu N, Vinh Phat V, Thompson C, Huong Lan NP, Thieu Nga TV, Thanh Tam PT, Tuyen HT, Hoang Nhu TD, Van Hao N, Thi Loan H, Minh Yen L, Parry CM, Trung Nghia HD, Campbell JI, Hien TT, Thwaites L, Thwaites G, Van Vinh Chau N, Baker S. 2015. In vitro activity of colistin in antimicrobial combination against carbapenem-resistant Acinetobacter baumannii isolated from patients with ventilator-associated pneumonia in Vietnam. J Med Microbiol 64:1162–1169. doi: 10.1099/jmm.0.000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Claeys KC, Fiorvento AD, Rybak MJ. 2014. A review of novel combinations of colistin and lipopeptide or glycopeptide antibiotics for the treatment of multidrug-resistant Acinetobacter baumannii. Infect Dis Ther 3:69–81. doi: 10.1007/s40121-014-0051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Garnacho-Montero J, Amaya-Villar R, Gutierrez-Pizarraya A, Espejo-Gutierrez de Tena E, Artero-Gonzalez ML, Corcia-Palomo Y, Bautista-Paloma J. 2013. Clinical efficacy and safety of the combination of colistin plus vancomycin for the treatment of severe infections caused by carbapenem-resistant Acinetobacter baumannii. Chemotherapy 59:225–231. doi: 10.1159/000356004. [DOI] [PubMed] [Google Scholar]
- 282.Petrosillo N, Giannella M, Antonelli M, Antonini M, Barsic B, Belancic L, Inkaya AC, De Pascale G, Grilli E, Tumbarello M, Akova M. 2014. Clinical experience of colistin-glycopeptide combination in critically ill patients infected with Gram-negative bacteria. Antimicrob Agents Chemother 58:851–858. doi: 10.1128/AAC.00871-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Entasis herapeutics. 2016. Entasis Therapeutics: Pipeline. Entasis Therapeutics, Waltham, MA. [Google Scholar]
- 284.Spero Therapeutics. 2016. Spero Therapeutics unveils data on lead potentiator candidate for the treatment of multidrug resistant Gram-negative infections at ASM Microbe 2016. Spero Therapeutics, Cambridge, MA. [Google Scholar]
- 285.Flamm RK, Rhomberg PR, Jones RN, Farrell DJ. 2015. In vitro activity of RX-P873 against Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter baumannii. Antimicrob Agents Chemother 59:2280–2285. doi: 10.1128/AAC.04840-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Tetraphase Pharmaceuticals. 2016. Tetraphase Pharmaceuticals provides update on eravacycline regulatory and development status. Tetraphase Pharmaceuticals, Watertown, MA. [Google Scholar]
- 287.Czaplewski L, Bax R, Clokie M, Dawson M, Fairhead H, Fischetti VA, Foster S, Gilmore BF, Hancock RE, Harper D, Henderson IR, Hilpert K, Jones BV, Kadioglu A, Knowles D, Olafsdottir S, Payne D, Projan S, Shaunak S, Silverman J, Thomas CM, Trust TJ, Warn P, Rex JH. 2016. Alternatives to antibiotics-a pipeline portfolio review. Lancet Infect Dis 16:239–251. doi: 10.1016/S1473-3099(15)00466-1. [DOI] [PubMed] [Google Scholar]
- 288.Garcia-Quintanilla M, Pulido MR, Lopez-Rojas R, Pachon J, McConnell MJ. 2013. Emerging therapies for multidrug resistant Acinetobacter baumannii. Trends Microbiol 21:157–163. doi: 10.1016/j.tim.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 289.Eaton MD. 1934. Studies on Pneumococcus variation: I. Variants characterized by rapid lysis and absence of normal growth under the routine method of cultivation. J Bacteriol 27:271–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.National Institute of Allergy and Infectious Diseases. 2016. Bacteriophage therapy: an alternative strategy to combat drug resistance. 20 to 21 July 2015. National Institute of Allergy and Infectious Diseases, Rockville, MD. [Google Scholar]
- 291.Defraine V, Schuermans J, Grymonprez B, Govers SK, Aertsen A, Fauvart M, Michiels J, Lavigne R, Briers Y. 2016. Efficacy of artilysin Art-175 against resistant and persistent Acinetobacter baumannii. Antimicrob Agents Chemother 60:3480–3488. doi: 10.1128/AAC.00285-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Jeon J, Ryu CM, Lee JY, Park JH, Yong D, Lee K. 2016. In vivo application of bacteriophage as a potential therapeutic agent to control OXA-66-like carbapenemase-producing Acinetobacter baumannii strains belonging to sequence type 357. Appl Environ Microbiol 82:4200–4208. doi: 10.1128/AEM.00526-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wang Y, Mi Z, Niu W, An X, Yuan X, Liu H, Li P, Liu Y, Feng Y, Huang Y, Zhang X, Zhang Z, Fan H, Peng F, Tong Y, Bai C. 2016. Intranasal treatment with bacteriophage rescues mice from Acinetobacter baumannii-mediated pneumonia. Future Microbiol 11:631–641. doi: 10.2217/fmb.16.11. [DOI] [PubMed] [Google Scholar]
- 294.Shivaswamy VC, Kalasuramath SB, Sadanand CK, Basavaraju AK, Ginnavaram V, Bille S, Ukken SS, Pushparaj UN. 2015. Ability of bacteriophage in resolving wound infection caused by multidrug-resistant Acinetobacter baumannii in uncontrolled diabetic rats. Microb Drug Resist 21:171–177. doi: 10.1089/mdr.2014.0120. [DOI] [PubMed] [Google Scholar]
- 295.Lood R, Winer BY, Pelzek AJ, Diez-Martinez R, Thandar M, Euler CW, Schuch R, Fischetti VA. 2015. Novel phage lysin capable of killing the multidrug-resistant Gram-negative bacterium Acinetobacter baumannii in a mouse bacteremia model. Antimicrob Agents Chemother 59:1983–1991. doi: 10.1128/AAC.04641-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Thandar M, Lood R, Winer BY, Deutsch DR, Euler CW, Fischetti VA. 2016. Novel engineered peptides of a phage lysin as effective antimicrobials against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 60:2671–2679. doi: 10.1128/AAC.02972-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kitti T, Thummeepak R, Leungtongkam U, Kunthalert D, Sitthisak S. 2015. Efficacy of Acinetobacter baumannii bacteriophage cocktail on Acinetobacter baumannii growth. Afr J Microbiol Res 9:2159–2165. [Google Scholar]
- 298.Regeimbal JM, Jacobs AC, Corey BW, Henry MS, Thompson MG, Pavlicek RL, Quinones J, Hannah RM, Ghebremedhin M, Crane NJ, Zurawski DV, Teneza-Mora NC, Biswas B, Hall ER. 2016. Personalized therapeutic cocktail of wild environmental phages rescues mice from Acinetobacter baumannii wound infections. Antimicrob Agents Chemother 60:5806–5816. doi: 10.1128/AAC.02877-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Perez F, Bonomo RA. 2014. Vaccines for Acinetobacter baumannii: thinking “out of the box”. Vaccine 32:2537–2539. doi: 10.1016/j.vaccine.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Lin L, Tan B, Pantapalangkoor P, Ho T, Hujer AM, Taracila MA, Bonomo RA, Spellberg B. 2013. Acinetobacter baumannii rOmpA vaccine dose alters immune polarization and immunodominant epitopes. Vaccine 31:313–318. doi: 10.1016/j.vaccine.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Garg N, Singh R, Shukla G, Capalash N, Sharma P. 2016. Immunoprotective potential of in silico predicted Acinetobacter baumannii outer membrane nuclease, NucAb. Int J Med Microbiol 306:1–9. doi: 10.1016/j.ijmm.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 302.Badmasti F, Ajdary S, Bouzari S, Fooladi AA, Shahcheraghi F, Siadat SD. 2015. Immunological evaluation of OMV(PagL)+Bap(1-487aa) and AbOmpA(8-346aa)+Bap(1-487aa) as vaccine candidates against Acinetobacter baumannii sepsis infection. Mol Immunol 67(2 Part B):552–558. doi: 10.1016/j.molimm.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 303.Huang W, Wang S, Yao Y, Xia Y, Yang X, Long Q, Sun W, Liu C, Li Y, Ma Y. 2015. OmpW is a potential target for eliciting protective immunity against Acinetobacter baumannii infections. Vaccine 33:4479–4485. doi: 10.1016/j.vaccine.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 304.Garcia-Quintanilla M, Pulido MR, Pachon J, McConnell MJ. 2014. Immunization with lipopolysaccharide-deficient whole cells provides protective immunity in an experimental mouse model of Acinetobacter baumannii infection. PLoS One 9:e114410. doi: 10.1371/journal.pone.0114410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Skaar EP. 2010. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog 6:e1000949. doi: 10.1371/journal.ppat.1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Bruhn KW, Spellberg B. 2015. Transferrin-mediated iron sequestration as a novel therapy for bacterial and fungal infections. Curr Opin Microbiol 27:57–61. doi: 10.1016/j.mib.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Kehl-Fie TE, Skaar EP. 2010. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol 14:218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Mortensen BL, Skaar EP. 2013. The contribution of nutrient metal acquisition and metabolism to Acinetobacter baumannii survival within the host. Front Cell Infect Microbiol 3:95. doi: 10.3389/fcimb.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Zimbler DL, Penwell WF, Gaddy JA, Menke SM, Tomaras AP, Connerly PL, Actis LA. 2009. Iron acquisition functions expressed by the human pathogen Acinetobacter baumannii. Biometals 22:23–32. doi: 10.1007/s10534-008-9202-3. [DOI] [PubMed] [Google Scholar]
- 310.Nudel C, Gonzalez R, Castaneda N, Mahler G, Actis LA. 2001. Influence of iron on growth, production of siderophore compounds, membrane proteins, and lipase activity in Acinetobacter calcoaceticus BD 413. Microbiol Res 155:263–269. doi: 10.1016/S0944-5013(01)80003-3. [DOI] [PubMed] [Google Scholar]
- 311.Penwell WF, Arivett BA, Actis LA. 2012. The Acinetobacter baumannii entA gene located outside the acinetobactin cluster is critical for siderophore production, iron acquisition and virulence. PLoS One 7:e36493. doi: 10.1371/journal.pone.0036493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Zimbler DL, Park TM, Arivett BA, Penwell WF, Greer SM, Woodruff TM, Tierney DL, Actis LA. 2012. Stress response and virulence functions of the Acinetobacter baumannii NfuA Fe-S scaffold protein. J Bacteriol 194:2884–2893. doi: 10.1128/JB.00213-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.de Leseleuc L, Harris G, KuoLee R, Xu HH, Chen W. 2014. Serum resistance, gallium nitrate tolerance and extrapulmonary dissemination are linked to heme consumption in a bacteremic strain of Acinetobacter baumannii. Int J Med Microbiol 304:360–369. doi: 10.1016/j.ijmm.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 314.Dorsey CW, Tomaras AP, Connerly PL, Tolmasky ME, Crosa JH, Actis LA. 2004. The siderophore-mediated iron acquisition systems of Acinetobacter baumannii ATCC 19606 and Vibrio anguillarum 775 are structurally and functionally related. Microbiology 150:3657–3667. doi: 10.1099/mic.0.27371-0. [DOI] [PubMed] [Google Scholar]
- 315.Fiester SE, Nwugo CC, Penwell WF, Neary JM, Beckett AC, Arivett BA, Schmidt RE, Geiger SC, Connerly PL, Menke SM, Tomaras AP, Actis LA. 2015. Role of the carboxy terminus of SecA in iron acquisition, protein translocation, and virulence of the bacterial pathogen Acinetobacter baumannii. Infect Immun 83:1354–1365. doi: 10.1128/IAI.02925-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Gaddy JA, Arivett BA, McConnell MJ, Lopez-Rojas R, Pachon J, Actis LA. 2012. Role of acinetobactin-mediated iron acquisition functions in the interaction of Acinetobacter baumannii strain ATCC 19606T with human lung epithelial cells, Galleria mellonella caterpillars, and mice. Infect Immun 80:1015–1024. doi: 10.1128/IAI.06279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Hood MI, Mortensen BL, Moore JL, Zhang Y, Kehl-Fie TE, Sugitani N, Chazin WJ, Caprioli RM, Skaar EP. 2012. Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog 8:e1003068. doi: 10.1371/journal.ppat.1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Thompson MG, Corey BW, Si Y, Craft DW, Zurawski DV. 2012. Antibacterial activities of iron chelators against common nosocomial pathogens. Antimicrob Agents Chemother 56:5419–5421. doi: 10.1128/AAC.01197-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Mortensen BL, Rathi S, Chazin WJ, Skaar EP. 2014. Acinetobacter baumannii response to host-mediated zinc limitation requires the transcriptional regulator Zur. J Bacteriol 196:2616–2626. doi: 10.1128/JB.01650-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Nairn BL, Lonergan ZR, Wang J, Braymer JJ, Zhang Y, Calcutt MW, Lisher JP, Gilston BA, Chazin WJ, de Crecy-Lagard V, Giedroc DP, Skaar EP. 2016. The response of Acinetobacter baumannii to zinc starvation. Cell Host Microbe 19:826–836. doi: 10.1016/j.chom.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Choi CH, Hyun SH, Lee JY, Lee JS, Lee YS, Kim SA, Chae JP, Yoo SM, Lee JC. 2008. Acinetobacter baumannii outer membrane protein A targets the nucleus and induces cytotoxicity. Cell Microbiol 10:309–319. [DOI] [PubMed] [Google Scholar]
- 322.Kim SW, Choi CH, Moon DC, Jin JS, Lee JH, Shin JH, Kim JM, Lee YC, Seol SY, Cho DT, Lee JC. 2009. Serum resistance of Acinetobacter baumannii through the binding of factor H to outer membrane proteins. FEMS Microbiol Lett 301:224–231. doi: 10.1111/j.1574-6968.2009.01820.x. [DOI] [PubMed] [Google Scholar]
- 323.Kim SW, Oh MH, Jun SH, Jeon H, Kim SI, Kim K, Lee YC, Lee JC. 2016. Outer membrane protein A plays a role in pathogenesis of Acinetobacter nosocomialis. Virulence 7:413–426. doi: 10.1080/21505594.2016.1140298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Tilley D, Law R, Warren S, Samis JA, Kumar A. 2014. CpaA a novel protease from Acinetobacter baumannii clinical isolates deregulates blood coagulation. FEMS Microbiol Lett 356:53–61. doi: 10.1111/1574-6968.12496. [DOI] [PubMed] [Google Scholar]
- 325.Liou ML, Soo PC, Ling SR, Kuo HY, Tang CY, Chang KC. 2014. The sensor kinase BfmS mediates virulence in Acinetobacter baumannii. J Microbiol Immunol Infect 47:275–281. doi: 10.1016/j.jmii.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 326.Fernandez-Cuenca F, Smani Y, Gomez-Sanchez MC, Docobo-Perez F, Caballero-Moyano FJ, Dominguez-Herrera J, Pascual A, Pachon J. 2011. Attenuated virulence of a slow-growing pandrug-resistant Acinetobacter baumannii is associated with decreased expression of genes encoding the porins CarO and OprD-like. Int J Antimicrob Agents 38:548–549. doi: 10.1016/j.ijantimicag.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 327.Bahl CD, Hvorecny KL, Bridges AA, Ballok AE, Bomberger JM, Cady KC, O'Toole GA, Madden DR. 2014. Signature motifs identify an Acinetobacter Cif virulence factor with epoxide hydrolase activity. J Biol Chem 289:7460–7469. doi: 10.1074/jbc.M113.518092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Alvarez-Fraga L, Perez A, Rumbo-Feal S, Merino M, Vallejo JA, Ohneck EJ, Edelmann RE, Beceiro A, Vazquez-Ucha JC, Valle J, Actis LA, Bou G, Poza M. 2016. Analysis of the role of the LH92_11085 gene of a biofilm hyper-producing Acinetobacter baumannii strain on biofilm formation and attachment to eukaryotic cells. Virulence 7:443–455. doi: 10.1080/21505594.2016.1145335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Sun D, Crowell SA, Harding CM, De Silva PM, Harrison A, Fernando DM, Mason KM, Santana E, Loewen PC, Kumar A, Liu Y. 2016. KatG and KatE confer Acinetobacter resistance to hydrogen peroxide but sensitize bacteria to killing by phagocytic respiratory burst. Life Sci 148:31–40. doi: 10.1016/j.lfs.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Heindorf M, Kadari M, Heider C, Skiebe E, Wilharm G. 2014. Impact of Acinetobacter baumannii superoxide dismutase on motility, virulence, oxidative stress resistance and susceptibility to antibiotics. PLoS One 9:e101033. doi: 10.1371/journal.pone.0101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Esterly JS, McLaughlin MM, Malczynski M, Qi C, Zembower TR, Scheetz MH. 2014. Pathogenicity of clinical Acinetobacter baumannii isolates in a Galleria mellonella host model according to bla(OXA-40) gene and epidemiological outbreak status. Antimicrob Agents Chemother 58:1240–1242. doi: 10.1128/AAC.02201-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Srinivasan VB, Vaidyanathan V, Rajamohan G. 2015. AbuO, a TolC-like outer membrane protein of Acinetobacter baumannii, is involved in antimicrobial and oxidative stress resistance. Antimicrob Agents Chemother 59:1236–1245. doi: 10.1128/AAC.03626-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Durante-Mangoni E, Del Franco M, Andini R, Bernardo M, Giannouli M, Zarrilli R. 2015. Emergence of colistin resistance without loss of fitness and virulence after prolonged colistin administration in a patient with extensively drug-resistant Acinetobacter baumannii. Diagn Microbiol Infect Dis 82:222–226. doi: 10.1016/j.diagmicrobio.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 334.Wand ME, Bock LJ, Bonney LC, Sutton JM. 2015. Retention of virulence following adaptation to colistin in Acinetobacter baumannii reflects the mechanism of resistance. J Antimicrob Chemother 70:2209–2216. doi: 10.1093/jac/dkv097. [DOI] [PubMed] [Google Scholar]
- 335.Stahl J, Bergmann H, Gottig S, Ebersberger I, Averhoff B. 2015. Acinetobacter baumannii virulence is mediated by the concerted action of three phospholipases D. PLoS One 10:e0138360. doi: 10.1371/journal.pone.0138360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Repizo GD, Gagne S, Foucault-Grunenwald ML, Borges V, Charpentier X, Limansky AS, Gomes JP, Viale AM, Salcedo SP. 2015. Differential role of the T6SS in Acinetobacter baumannii virulence. PLoS One 10:e0138265. doi: 10.1371/journal.pone.0138265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Vila-Farres X, Ferrer-Navarro M, Callarisa AE, Marti S, Espinal P, Gupta S, Rolain JM, Giralt E, Vila J. 2015. Loss of LPS is involved in the virulence and resistance to colistin of colistin-resistant Acinetobacter nosocomialis mutants selected in vitro. J Antimicrob Chemother 70:2981–2986. doi: 10.1093/jac/dkv244. [DOI] [PubMed] [Google Scholar]
- 338.Liu D, Liu ZS, Hu P, Cai L, Fu BQ, Li YS, Lu SY, Liu NN, Ma XL, Chi D, Chang J, Shui YM, Li ZH, Ahmad W, Zhou Y, Ren HL. 2016. Characterization of surface antigen protein 1 (SurA1) from Acinetobacter baumannii and its role in virulence and fitness. Vet Microbiol 186:126–138. doi: 10.1016/j.vetmic.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 339.Nho JS, Jun SH, Oh MH, Park TI, Choi CW, Kim SI, Choi CH, Lee JC. 2015. Acinetobacter nosocomialis secretes outer membrane vesicles that induce epithelial cell death and host inflammatory responses. Microb Pathog 81:39–45. doi: 10.1016/j.micpath.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 340.Johnson TL, Waack U, Smith S, Mobley H, Sandkvist M. 2015. Acinetobacter baumannii is dependent on the type II secretion system and its substrate LipA for lipid utilization and in vivo fitness. J Bacteriol 198:711–719. doi: 10.1128/JB.00622-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Harding CM, Kinsella RL, Palmer LD, Skaar EP, Feldman MF. 2016. Medically relevant Acinetobacter species require a type II secretion system and specific membrane-associated chaperones for the export of multiple substrates and full virulence. PLoS Pathog 12:e1005391. doi: 10.1371/journal.ppat.1005391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Lopez-Rojas R, McConnell MJ, Jimenez-Mejias ME, Dominguez-Herrera J, Fernandez-Cuenca F, Pachon J. 2013. Colistin resistance in a clinical Acinetobacter baumannii strain appearing after colistin treatment: effect on virulence and bacterial fitness. Antimicrob Agents Chemother 57:4587–4589. doi: 10.1128/AAC.00543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Hraiech S, Roch A, Lepidi H, Atieh T, Audoly G, Rolain JM, Raoult D, Brunel JM, Papazian L, Bregeon F. 2013. Impaired virulence and fitness of a colistin-resistant clinical isolate of Acinetobacter baumannii in a rat model of pneumonia. Antimicrob Agents Chemother 57:5120–5121. doi: 10.1128/AAC.00700-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Smani Y, Lopez-Rojas R, Dominguez-Herrera J, Docobo-Perez F, Marti S, Vila J, Pachon J. 2012. In vitro and in vivo reduced fitness and virulence in ciprofloxacin-resistant Acinetobacter baumannii. Clin Microbiol Infect 18:E1–E4. doi: 10.1111/j.1469-0691.2011.03695.x. [DOI] [PubMed] [Google Scholar]
- 345.Iwashkiw JA, Seper A, Weber BS, Scott NE, Vinogradov E, Stratilo C, Reiz B, Cordwell SJ, Whittal R, Schild S, Feldman MF. 2012. Identification of a general O-linked protein glycosylation system in Acinetobacter baumannii and its role in virulence and biofilm formation. PLoS Pathog 8:e1002758. doi: 10.1371/journal.ppat.1002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Rumbo C, Tomas M, Fernandez Moreira E, Soares NC, Carvajal M, Santillana E, Beceiro A, Romero A, Bou G. 2014. The Acinetobacter baumannii Omp33-36 porin is a virulence factor that induces apoptosis and modulates autophagy in human cells. Infect Immun 82:4666–4680. doi: 10.1128/IAI.02034-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Cerqueira GM, Kostoulias X, Khoo C, Aibinu I, Qu Y, Traven A, Peleg AY. 2014. A global virulence regulator in Acinetobacter baumannii and its control of the phenylacetic acid catabolic pathway. J Infect Dis 210:46–55. doi: 10.1093/infdis/jiu024. [DOI] [PubMed] [Google Scholar]
- 348.Elhosseiny NM, Amin MA, Yassin AS, Attia AS. 2015. Acinetobacter baumannii universal stress protein A plays a pivotal role in stress response and is essential for pneumonia and sepsis pathogenesis. Int J Med Microbiol 305:114–123. doi: 10.1016/j.ijmm.2014.11.008. [DOI] [PubMed] [Google Scholar]