Summary
Regulatory T cell (Treg) therapy has been exploited in autoimmune disease, solid organ transplantation and in efforts to prevent or treat graft‐versus‐host disease (GVHD). However, our knowledge on the in‐vivo persistence of transfused Treg is limited. Whether Treg transfusion leads to notable changes in the overall Treg repertoire or whether longevity of Treg in the periphery is restricted to certain clones is unknown. Here we use T cell receptor alpha chain sequencing (TCR‐α‐NGS) to monitor changes in the repertoire of Treg upon polyclonal expansion and after subsequent adoptive transfer. We applied TCR‐α‐NGS to samples from two patients with chronic GVHD who received comparable doses of stem cell donor derived expanded Treg. We found that in‐vitro polyclonal expansion led to notable repertoire changes in vitro and that Treg cell therapy altered the peripheral Treg repertoire considerably towards that of the infused cell product, to different degrees, in each patient. Clonal changes in the peripheral blood were transient and correlated well with the clinical parameters. We suggest that T cell clonotype analyses using TCR sequencing should be considered as a means to monitor longevity and fate of adoptively transferred T cells.
Keywords: cell tracking, next generation sequencing, regulatory T cell therapy, T cell receptor repertoire
Introduction
Regulatory T cells (Treg) have entered the clinic. Cell therapy with freshly isolated or expanded Treg is applied in autoimmunity, transplantation and graft‐versus‐host disease (GVHD) 1, 2, 3, 4, 5, 6, 7, 8, 9, 10. Little is known about the stability and persistence of infused Treg in vivo. Flow cytometry has been used to monitor Treg quantitatively in the periphery post‐transfer. Based on the expression of CD4, CD127, forkhead box protein 3 (FoxP3), and especially the high expression of CD25 on previously expanded Treg, Treg enumerations post‐transfer could be tracked for 2 weeks in the case of third‐party umbilical cord blood origin administered for GVHD prevention 9, and up to 3 months in the case of stem cell donor‐derived Treg for chronic GVHD (cGVHD) treatment 10. Kinetics of transferred Treg in the peripheral blood seem to vary greatly between individuals 1, 6, 10. Recently, Bluestone et al. reported tracking of autologous expanded Treg in type 1 diabetes patients by means of stable isotype labelling of Treg by adding deuterium‐labelled glucose during in‐vitro expansion. Chromatography–mass cytometric analysis of isolated Treg at different time‐points post‐cell therapy revealed 25% of peak labelling at 3 months and the detection of transferred Treg in the periphery for 1 year 1. However, whether longevity is independent of the specificity of Treg or restricted to certain clones is unknown. We aimed to explore the feasibility of T cell receptor (TCR) next‐generation sequencing (NGS) as a tool to measure Treg clonality after expansion and in‐vivo persistence after adoptive transfer, and as a means to track changes in the clonal repertoire of infused allogeneic Treg with time. We chose T cell receptor (TCR)‐α chain sequencing in this feasibility study, as this has been recently developed locally 11. This is, to our knowledge, the first report exploiting Treg TCR‐α‐NGS after adoptive transfer of Treg.
Methods
Patient characteristics and Treg therapy
TCR‐α‐NGS was performed in two patients who received adoptive Treg therapy. Both patients suffered from treatment‐refractory chronic GVHD as defined by National Institute of Health (NIH) criteria 12 after fully matched allogeneic haematopoietic stem cell (HSC) transplantation. The patients received Treg infusions 40·5 months (patient 1) and 28 months (patient 2) after HSC transplantation (40 and 21 months after developing GVHD). Both patients showed full donor chimerism at the time of Treg infusion. Details on original disease, graft characteristics, GVHD manifestation and discontinued GVHD medication are listed in Table 1. Patient 1 showed severe skin chronic GVHD (III°/progressive, maculopapular rash), affected oral cavity (III°/progressive, lichenoid buccal mucosal lesions/ulcerations) and eyes (II°/stable; keratoconjunctivitis sicca). Patient 2 reported severe chronic GVHD affecting the skin (III°/stable, ulcerations, sclerotic features) and the oral cavity (II°/stable). Treg therapy and follow‐up for both patients has been reported previously 10. Briefly, Treg were isolated from a leucapheresis product collected from the original haematopoietic stem cell donor by CD8+ depletion and CD25++ enrichment, expanded for 12 days with two rounds of αCD3αCD28 bead stimulation (Dynabeads Human T‐Activator; Invitrogen, Carlsbad, CA, USA) and high‐dose interleukin (IL)‐2 (Proleukin S; Novartis Pharma, Basel, Switzerland) in the presence of rapamycin. Viability of the final cell product was 98% (patient 1) and 94% (patient 2), as determined by trypan blue staining. Cells were infused at a dosage of 3·7 × 106 Treg cells/kg (patient 1) or 3·8 × 106 Treg cells/kg (patient 2). Adoptive transfer of Treg was conducted within a compassionate use programme. Immunomonitoring after Treg therapy was performed after informed consent within a study protocol approved by the local ethics review committee (protocol no. EK 206082008).
Table 1.
Patient characteristics.
| Type of disease | Conditioning/ GVHD prophylaxis | Stem cell source and characteristics (HLA match) | Donor gender and age | aGVHD | cGVHD organ manifestations at the time of Treg infusion | Discontinued GVHD treatments prior to Treg infusion | GVHD treatment at the time of Treg infusion | |
|---|---|---|---|---|---|---|---|---|
| Patient 1 male 54 years | B‐CLL | Flu, Bu8/CSA, MTX | BM allo unrelated (10/10) | Male, 39 years | Skin II° | Severe skin III° (progressive, maculopapular rash); oral cavity III° (progressive, ulcerations); eyes II° (stable, keratoconjunctivitis sicca) | Tacro, MMF, Alemtu, Rituxi, Dacli, Evero, ECP, MSC | Prednisolone (10 mg/d) |
| Patient 2 female 45 yrs | AML | Flu, 8 Gy TBI/CSA mono | PBSC allo related (10/10) | Female, 51 years | No | Severe skin III° (stable, ulcerations, sclerotic features); oral cavity II° (stable) | Tacro, MMF, ECP, MSC | Prednisolone (7.5 mg/d) Everolimus |
Alemtu = alemtuzumab (3 × 10 mg subcutaneously per week); AML = acute myeloid leukaemia; B‐CLL = B cell chronic lymphocytic leukaemia; BM = bone marrow; Bu = busulfan, Bu8 (8 mg/kg per os total); CSA = cyclosporin; cyclophosphamide (120 mg/kg total); Dacli = daclizumab (1 mg/kg); ECP = extracorporeal photopheresis; Evero = everolimus; Flu = fludarabine (30 mg/m2/day for 5 days); MTX = methotrexate [graft‐versus‐host disease (GVHD) prophylaxis: cumulative dose 45 mg/m2, days 1, 3, 6, 11 after haematopoietic stem cell (HCT); GVHD treatment: 5 mg/m2 per week]; MMF = mycophenolate mofetil; MSC = mesenchymal stromal cells [average dose 1 × 106/kg body weight intravenously (i.v.)]; Rituxi = rituximab (100 mg i.v. per week); Tacro = tacrolimus; TBI = hyperfractionated total body irradiation (dose in Gy); HLA = human leucocyte antigen.
Sample processing
Samples for TCR‐α‐NGS included unexpanded donor Treg (CD4+CD25highCD127low T lymphocytes) and expanded donor Treg, and recipient Treg, CD8+ T lymphocytes and CD4+CD25+CD127+CD45RO+ T lymphocytes obtained immediately prior to infusion and at several time‐points after infusion (Table 3). Cells used for TCR‐α‐NGS were enriched magnetically and subsequently fluorescence activated cell sorter (FACS) purified after staining with CD4‐peridinin chlorophyll (PerCP), clone SK3; CD25‐phycoerythrin (PE), clone M‐A251; CD45RO‐allophycocyanin (APC), clone UCHL1 (all from BD Biosciences, San Jose, CA, USA) and CD127‐eFluor450, clone eBioDDR5 (eBioscience, San Diego, CA, USA). The procedure is outlined schematically in Fig. 3b. Cells were lysed in RLT buffer (RNeasy MiniKit; Qiagen, Hilden, Germany) with 1% β‐mercaptoethanol, snap‐frozen and stored at −80°C.
Table 3.
Sample cell numbers, obtained sequencing reads and clonotypes.
| Cell type/source | Sample/time‐point | Cell number ×106 | Read number ×106 | Clonotype count | |
|---|---|---|---|---|---|
| Patient 1 | Donor Treg | Isolated Treg | 0·040 | 0·25 | 6804 |
| Donor Treg | Expanded Treg | 4·300 | 21·03 | 47 452 | |
| Patient Treg | Preinfusion | 0·004 | 0·08 | 866 | |
| Patient Treg | 24 h post | 0·066 | 0·67 | 19 984 | |
| Patient Treg | 1 week post | 0·081 | 0·93 | 19 134 | |
| Patient Treg | 2 weeks post | 0·116 | 2·06 | 31 703 | |
| Patient Treg | 3 weeks post | 0·078 | 1·93 | 11 154 | |
| Patient Treg | 6.5 weeks post | 0·040 | 0·64 | 11 157 | |
| Patient CD8 | Preinfusion | 0·470 | 5·98 | 7949 | |
| Patient CD8 | 24 h post | 0·510 | 6·77 | 9547 | |
| Patient CD8 | 1 week post | 0·900 | 8·42 | 17 370 | |
| Patient CD8 | 2 weeks post | 0·880 | 10·50 | 7401 | |
| Patient CD8 | 3 weeks post | 1·220 | 21·39 | 11 544 | |
| Patient CD8 | 6.5 weeks post | 1·400 | 20·52 | 15 489 | |
| Patient CD4 | Preinfusion | 0·003 | 0·03 | 762 | |
| Patient CD4 | 24 h post | 0·005 | 0·05 | 1165 | |
| Patient CD4 | 1 week post | 0·008 | 0·07 | 5780 | |
| Patient CD4 | 2 weeks post | 0·007 | 0·12 | 2052 | |
| Patient CD4 | 3 weeks post | 0·010 | 0·23 | 4419 | |
| Patient CD4* | 6.5 weeks post | 0·008 | 0·01 | 2103 | |
| Patient 2 | Donor Treg | Isolated Treg | 0·123 | 1·30 | 6810 |
| Donor Treg | Expanded Treg | 2·560 | 12·80 | 3621 | |
| Patient Treg | Preinfusion | 0·072 | 0·30 | 2957 | |
| Patient Treg | 1 week post | 0·270 | 6·23 | 30 152 | |
| Patient Treg | 5 weeks post | 0·199 | 1·04 | 1535 |
*Sample excluded from analysis for quality reasons. Treg = regulatory T cells.
Figure 3.
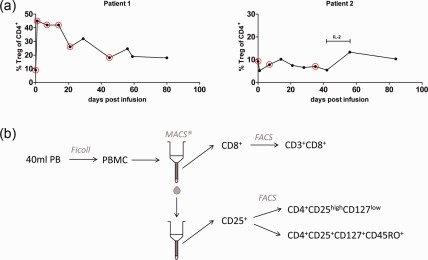
Monitoring of peripheral regulatory T cell (Treg) in patients before and after Treg transfer over time. (a) Percentages of CD4+CD25highCD127lowFOXP3+ T cells (Treg) within the CD4+ compartment were determined by flow cytometry. Circles indicate visits where additional blood samples were drawn for TCR‐α sequencing studies. IL‐2: patient 2 received low‐dose IL‐2 treatment later for the indicated time‐frame. Left graph = patient 1, right graph = patient 2. (b) Layout of the cell isolation process for high‐throughput TCR‐α sequencing. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll density centrifugation from 40 to 50 ml of whole peripheral blood. CD8+ cells were enriched by magnetic activated cell sorting (MACS) and subsequently sorted according to the expression of CD3 and CD8. The CD8– fraction was enriched for CD25 expression and sorted into Treg (CD4+CD25highCD127low) and CD4+ memory T effector cells (CD4+CD25+CD127+CD45RO+).
Library preparation and NGS sequencing for TCR‐α chain
RNA was isolated from the frozen cell pellets using the RNeasy MiniKit (Qiagen). First‐strand cDNA was synthesized utilizing the template switching protocol for TCR‐α, and TCR‐α amplified as described 11. The final product was purified with the QIAquick polymerase chain reaction (PCR) purification kit (Qiagen). Barcoded libraries were pooled and 150 base pairs (bp) reads were generated using the Illumina HiSeq 2500 system. TCR CDR3 region sequences extraction and PCR error correction was carried out as described with MiTCR software (MiLaboratory, Moscow, Russia) 13, 14. Non‐productive TCR sequences were filtered out, resulting in an average of 70% usable reads from the total reads obtained.
Statistical analysis
Analyses were conducted using r (2.15.0 2012‐03‐30; The R Foundation for Statistical Computing, Vienna, Austria) and Konstanz information miner (KNIME) 15. Simpson's Diversity Index was determined as described 16, where an index of 0 is minimal and 1 is maximal diversity. In order to overcome the problem of differing sizes of different biological samples when comparing their Simpson's Diversity, we subsampled the same number of reads from each biological sample according to the smallest sample. This subsampling of reads was repeated 11 times, and the resulting Simpson Diversity indices were averaged. Pearson's correlation was calculated on proportions of clonotypes across time‐points.
Results
Comparison of donor Treg repertoire with preinfusion recipient Treg repertoires
TCR‐α reads within Treg were compared in the SC) recipient and the HSC donor at the time of Treg infusion, 40 months after bone marrow transplant (patient 1) and 28 months after peripheral blood HSC transplant (patient 2) (Fig. 1). There was no overlap in the 100 most frequent TCR‐α clonotypes seen between the recipient and donor Treg in patient 1. In contrast, in patient 2, 12 of the 100 most abundant clonotypes were shared between the recipient and donor. There was no obvious bias of these clonotypes with respect to their TCR‐α variable (TRAV) and TCR‐α joining (TRAJ) genes (data not shown).
Figure 1.
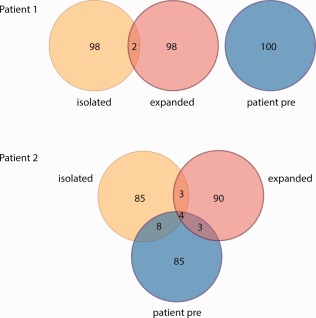
Regulatory T cell (Treg) clonotype overlap between stem cell donor and recipient. High‐throughput T cell receptor (TCR)‐α chain sequencing was used to define distinct Treg clonotypes. Shown are Venn diagrams illustrating overlap in the 100 most frequent Treg clonotypes found in donor‐derived Treg before (yellow), after ex‐vivo expansion (red) and the recipient prior to Treg infusion (blue). Upper diagram = patient 1, lower diagram = patient 2. Numbers illustrate numbers of distinct Treg clonotypes.
TCR‐α diversity is decreased after Treg expansion
We were able to examine the diversity of the TCR‐α repertoire in the pre‐expansion and expanded Treg in patient 2. Few cells were recovered for patient 1. In‐vitro Treg expansion of the Treg for patient 2 was 18‐fold, with a final purity of 91·8% CD4+CD25highCD127lowFoxP3+ cells. We observed a similar gene usage pre‐ and post‐expansion (Fig. 2a). Despite this, there were clear changes in the repertoire as assessed by comparing the frequency of clonotypes pre‐ and post‐expansion (Fig. 2b, left panel). A large number of high‐frequency clones from the isolated cell product were not detected in the expanded Treg sample, whereas others that had frequencies of < 0·001% in the isolated Treg increased 100–1000‐fold in their relative frequency in the expanded preparation. Of 987 clonotypes with read frequencies above 0·01% in the isolated cell product, 416 (42%) were not detectable (read frequencies < 0·0001%) in the expanded cell product. The 100 highest TCR‐α clone frequencies were higher in the expanded Treg preparations than in the isolated Treg (P < 0·0001) (Fig. 2b, right panel). Simpson's diversity indices of the isolated and expanded clonotypes after mathematical subsampling were 0·9996 in the pre‐expanded Treg and 0·9987 in the expanded Treg.
Figure 2.
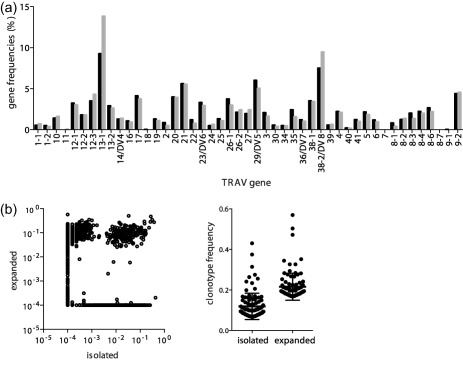
T cell receptor (TCR) gene usage of regulatory T cell (Treg) and clonotype frequencies before and after in‐vitro polyclonal expansion. (a) High‐throughput TCR‐α chain sequencing was used to compare the gene usage of the Treg pool before (black bars) and after in‐vitro expansion (grey bars) for infusion into patient 2. Shown are the frequencies of the individual TCR‐α variable (TRAV) genes. (b) Relative frequencies (in %) of all clones detected pre‐ or post‐expansion were plotted in a correlation scatterplot (left). Clones that were not detected in one of the two samples (zero frequency) were set to 10−04. The relative frequencies (in %) of the 100 most abundant clones are shown additionally in the right graph.
Treg TCR repertoire changes after Treg infusion
We asked whether transferred Treg can be detected in the periphery after infusion and whether the transfer leads to changes in the clonal distribution within the patient's Treg cell pool. For patient 1, a 4.6‐fold increase in the proportion of Treg among the CD4+ T cell pool was observed by flow cytometry 24 h post‐infusion followed by a gradual decline from week 3 post‐infusion (Fig. 3a, left graph). TCR‐α‐NGS sequencing of Treg samples from this patient indicated a rapid change in the peripheral Treg repertoire upon Treg transfer, as shown by the lack of correlation comparing the repertoire pre‐ and 24 h post‐transfusion (Fig. 4a, upper left plot and Fig. 4b, grey bars). Moreover, there was a marked correlation between the repertoire of the expanded Treg cell product and the patient's Treg repertoire at 24 h post‐transfer, suggesting that the increase in Treg was a direct result of the infusion (Fig. 4a, lower left plot and Fig. 4b, black bars). The change in repertoires is also seen in the frequencies of the dominant TCR‐α from the expanded Treg, the preinfusion and the 6·5 weeks post‐infusion repertoires (Fig. 4c, upper row). An increased correlation between the patient's Treg repertoire and the expanded Treg pool compared to patient's repertoire pre‐Treg transfer was observed for the first 3 weeks after infusion in this patient (Fig. 4a,b). At and after 6·5 weeks post‐transfer the repertoire correlation between the expanded cell pool and the patients' cell pool in the periphery decreased markedly. Most of the highest‐frequency TCR‐α in the sample 6·5 weeks post‐infusion was represented in the preinfusion repertoire; few were found only in the expanded repertoire and some were found in both (Fig. 4c, upper panel). We did not detect a change in the CD8+ T cell repertoire upon Treg transfer within the monitored time‐frame (Fig. 4b, lower left bar graph), and only minor changes in the repertoire of peripheral CD4+ T effector memory cells (Fig. 4b, lower right graph). Interestingly, we observed a decrease in activation marker CD69‐positive CD4+ and CD8+ T effector cells during the first 3 weeks post‐Treg transfer by flow cytometry in this patient 10. This was followed by a transient clinical response affecting skin and oral mucosa (grades III–II) from weeks +3 to +8 post‐therapy and a reduction of the corticosteroid dose (Table 2).
Figure 4.
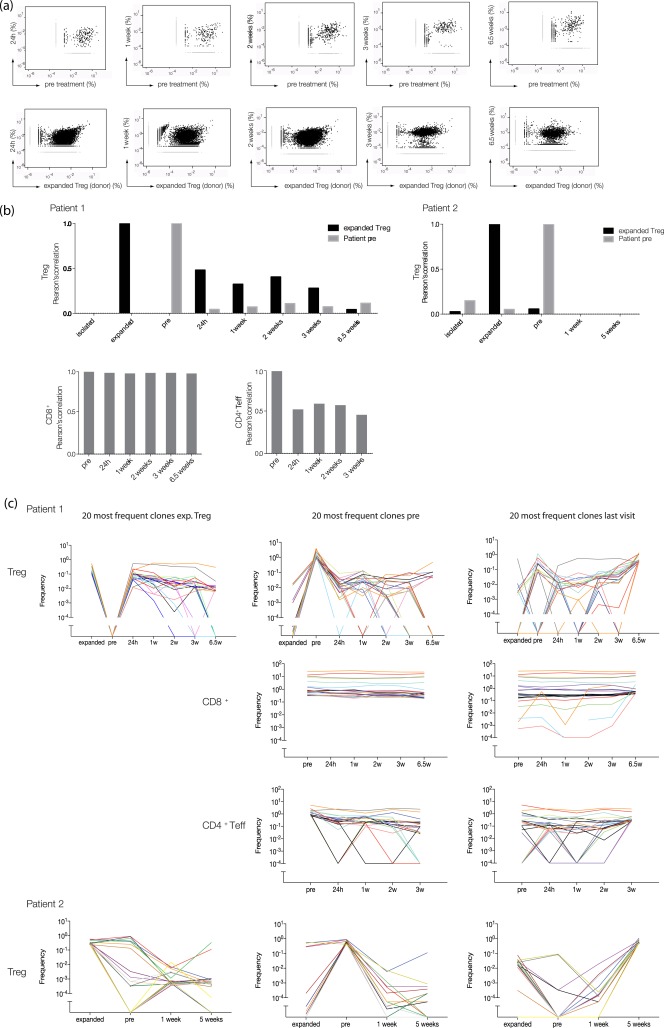
Monitoring of regulatory T cell (Treg) T cell receptor (TCR) repertoire changes after Treg infusion. (a) Comparative frequency analysis of Treg clonotypes using high‐throughput TCR‐α chain sequencing. Scatterplots were generated to compare relative abundance of Treg TCR sequences at different time‐points post‐Treg transfer to their relative frequencies (in %) pretreatment (first row) or to the relative frequencies (in %) of the sequences within the expanded Treg pool (second row). Each dot represents a unique sequence. Sequences that were found only in one of the two compared samples are shown as light grey symbols. (b) Correlation analysis of the Treg repertoires. Shown are Pearson's correlation coefficients as measures of the strength of the association between the TCR repertoire at different time‐points post‐Treg transfer versus the TCR repertoire pre‐Treg therapy (grey bars) or versus the TCR repertoire of the expanded Treg pool (black bars). Bar graph on left = patient 1, bar graph on the right = patient 2. (c) Tracking of the 20 most frequent Treg clones of each sample over time. Every coloured line represents a particular clone. Plots on left: the 20 most abundant Treg clones within the expanded Treg pool followed throughout the indicated timepoints pre‐ and post‐adoptive transfer for patient 1 (first row) and patient 2 (bottom row). Middle column: the 20 most frequent Treg clones pre therapy and their frequencies over time (first row, patient 1; bottom row, patient 2). Second and third rows show the same analysis for CD8+ T cells and CD4+ effector memory T cells [CD4+ T effector (Teff)] isolated from patient 1. Plots on right: the 20 most abundant Treg clones at the last visit post‐Treg transfer and their frequencies before (first row: patient 1, last row: patient 2). The same analysis was conducted for CD8+ (second row) and CD4+ Teff (third row) from patient 1.
Table 2.
Regulatory T cell (Treg) graft characteristics and clinical response.
| Treg purity after CliniMACS isolation (%CD4+CD25hi CD127loFoxP3+) | Duration of Treg culture | Treg fold expansion | Treg purity after expansion (%CD4+CD25hi CD127loFoxP3+) | Total Treg dose (CD4+CD25hi CD127loFoxP3+) (×106) | Treg dose/kg (CD4+CD25hi CD127loFoxP3+) (×106/kg) | Time of Treg infusion (months after HCT) | Clinical response during NGS follow‐up | |
|---|---|---|---|---|---|---|---|---|
| Patient 1 male 54 years | 72·6 | 12 days | ×4 | 84·1 | 4·00 | 3·71 | 40·5 | Partial response week +3 to +8 (skin III°→II°; oral cavity III°→II°) |
| Patient 2 female 45 years | 79·2 | 12 days | ×18 | 91·8 | 4·72 | 3·76 | 28 | Stable disease week +1 to +4; GVHD progression week +5 (skin) |
GVHD = graft‐versus‐host disease; Fox P3 = forkhead box protein 3; NGS = next‐generation sequencing; HCT = haematopoietic stem cell.
Despite infusion of a similar Treg dose and a similar high viability of the cell product, the Treg proportion among CD4+ T cells in the peripheral blood of patient 2 did not increase after infusion as measured by flow cytometry (Fig. 3a, right graph). The Treg TCR‐α repertoires 1 and 5 weeks post‐infusion showed little correlation with the preinfusion or the expanded Treg repertoires (Fig. 4b, right panel). Patient 2 showed no clinical response but stable disease from week +1 to +4 post‐Treg therapy and GVHD progressed after week 5 (skin) (Table 2). Nevertheless, all the most frequent Treg TCR‐α found 5 weeks post‐infusion were seen in the preinfusion or the expanded Treg repertoires (Fig. 4c, bottom panels).
Discussion
Using NGS‐TCR‐α sequencing, we have demonstrated changes in Treg repertoire following Treg cell therapy. The changes were associated with clinical outcome, suggesting that monitoring TCR repertoires following adoptive T cell therapy may be considered as a measure of cell engraftment.
The study was exploratory to assess whether Treg repertoires change after adoptive Treg cell therapy. Despite being small, and thus largely descriptive, it provides insight into the information that can be gained by extensive TCR repertoire analysis of specific cell types, and shows clearly that it can be a useful tool in adoptive T cell therapy. The following observations were of interest. First, follow‐up in two patients demonstrated markedly different outcomes with respect to Treg TCR repertoire changes. One patient (patient 2) had acquired a Treg TCR repertoire 28 months after HSC transplant that overlapped substantially with that of the HSC donor with respect to frequent TCR‐α clonotypes, and showed only minor changes in the peripheral blood Treg TCR repertoire after Treg cell therapy despite a high viability of the cell product at the time of infusion. In contrast, the Treg TCR repertoire in the other patient (patient 1) was very different to that of the bone marrow donor, but changed dramatically to one resembling the repertoire of the infused Treg cells after adoptive Treg cell therapy with a gradual return to a pre‐adoptive Treg cell therapy profile during a period of 1–2 months. The diverse outcomes in the two patients hold promise that monitoring TCR repertoires following adoptive T cell therapy may provide clinically meaningful information. Of note, the CD8+ TCR repertoires did not alter after adoptive Treg cell therapy, showing the specificity of the Treg changes in the patients. The small initial change in the CD4+ T effector repertoire is biased most probably by the small sample size of the pretransfer sample (Table 3). Donor characteristics differed between the two patients. Patient 1 received grafts from a 39‐year‐old unrelated matched donor and patient 2 from her 51‐year‐old sibling. Donor human leucocyte antigen (HLA) match and age have been shown to be associated with the risk of GVHD after allogeneic transplantation. We were also able to assess the Treg repertoire after in‐vitro expansion on one preparation. Several investigators aim currently at prolonged expansion cultures using modified expansion protocols of up to 35 days and to three rounds of restimulation driven by restricted starting material and/or to obtain higher Treg doses 4, 9, 17. A number of studies suggested polyclonality of the expanded cell pool using TCR Vβ repertoire analysis by flow cytometry 9, 18. Bluestone et al. reported recently the gene usage of bead‐expanded Treg by TCR‐β sequencing before and after a 14‐day expansion protocol. Looking at gene usage only, the cell product appeared polyclonal despite an average 500‐fold expansion 1. We had an 18‐fold expansion and were able to confirm polyclonality and a stable gene usage by TCR sequencing. However, we revealed marked changes in the clonal repertoire accompanied by a considerable decrease in diversity after expansion. This contrasts the negligible observed repertoire changes in some reports 19, 20. This finding argues for further investigations by us and other sites aiming at and already reaching far higher numbers of in‐vitro cell doublings before transfusion.
There are at least two limitations of all currently applied approaches to track Treg after adoptive transfer. First, Treg may undergo phenotypical changes including CD25 down‐regulation in vivo, as shown by Singh et al., and thus might not have been isolated by FACS prior to further analysis 21. However, the findings of Bluestone et al., demonstrating that CD4+ T cells other that Treg did not show signs of deuterium labelling after sorting, suggest the plausibility of our approach 1. As infused Treg are CD45RO+, the additional use of this marker for sorting post‐infusion may improve the ability to track cells by TCR sequencing. The use of paired TCR‐α and TCR‐β sequencing using recent techniques 22 is also likely to improve tracking. Secondly, we are currently constrained to limit our analyses to peripheral blood. Treg probably migrate to lymphoid tissue or sites of inflammation where they cannot be detected, and might thus be invisible to us, rather than cleared 23. Their presence in the affected tissue might, at the same time, be of higher relevance for clinical benefit. Based on the similar product viability and dosage, we hypothesize that a retention in lymph nodes or a more rapid sequestration into peripheral tissue might explain the lack of evidence of infused Treg in the peripheral blood of patient 2.
In conclusion, we found that TCR‐α‐NGS Treg is a versatile method to track changes in the Treg repertoire with time. Our results indicate that patients can partially adopt donor Treg specificities after HSC transplantation, and that adoptive Treg cell therapy can lead to transient clonal changes within the circulating peripheral Treg repertoire. The degree of these repertoire changes can differ substantially between individuals. Some of the transferred Treg clones appeared to reside longer in the periphery than others, and overall clonal changes are of transient nature. Thus, we advocate the use of TCR repertoire analyses, together with analyses such as the use of deuterium labelling of cells in patients undergoing adoptive T cell therapies.
Disclosures
The authors declare no commercial, proprietary or financial interests in the products or companies described in this paper.
Author contributions
A. T. planned and supervised Treg isolation and expansion, planned and performed immunomonitoring and FACS sorting and drafted the manuscript; C. W. performed MACS and FACS sorting, M. K. performed data analysis, A. P. performed sequencing data preprocessing, S. T. compiled clinical data, U. O. supervised immunomonitoring for patient 2, A. D. performed sequencing, M. B. initiated and supervised Treg cell therapy and critically read the manuscript, E. B. initiated, planned and supervised the study and contributed to manuscript writing; A. E. planned and supervised the study, performed T cell receptor library preparation, data analysis and contributed to manuscript writing.
Acknowledgements
This work was supported by funding from the DFG‐Center for Regenerative Therapies Dresden, Cluster of Excellence (FZT 111) to M. B. and E. B. The authors would like to thank Anja Maiwald, Anja Zenkel, Dennis Oßmann and Diana Döhler for technical assistance in clinical grade Treg isolation and culture and Sevina Dietz and Denise Walther for technical assistance.
References
- 1. Bluestone JA, Buckner JH, Fitch M et al Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med 2015; 7:315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marek‐Trzonkowska N, Myśliwiec M, Dobyszuk A et al Therapy of type 1 diabetes with CD4(+)CD25(high)CD127‐regulatory T cells prolongs survival of pancreatic islets – results of one year follow‐up. Clin Immunol 2014; 153:23–30. [DOI] [PubMed] [Google Scholar]
- 3. Geissler EK. The ONE Study compares cell therapy products in organ transplantation: introduction to a review series on suppressive monocyte‐derived cells. Transplant Res 2012; 1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Safinia N, Vaikunthanathan Fraser T, Thirkell H et al Successful expansion of functional and stable regulatory T cells for immunotherapy in liver transplantation. Oncotarget 2016; 7:7563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Edinger M, Hoffmann P. Regulatory T cells in stem cell transplantation: strategies and first clinical experiences. Curr Opin Immunol 2011; 23:679–84. [DOI] [PubMed] [Google Scholar]
- 6. Trzonkowski P, Bieniaszewska M, Juścińska J et al First‐in‐man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127– T regulatory cells. Clin Immunol 2009; 133:22–6. [DOI] [PubMed] [Google Scholar]
- 7. Di Ianni M, Falzetti F, Carotti A et al Tregs prevent GVHD and promote immune reconstitution in HLA‐haploidentical transplantation. Blood 2011; 117:3921–8. [DOI] [PubMed] [Google Scholar]
- 8. Martelli MF, Di Ianni M, Ruggeri L et al HLA‐haploidentical transplantation with regulatory and conventional T‐cell adoptive immunotherapy prevents acute leukemia relapse. Blood 2014; 124:638–44. [DOI] [PubMed] [Google Scholar]
- 9. Brunstein CG, Miller JS, McKenna DH et al Umbilical cord blood‐derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood 2016; 127:1044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Theil A, Tuve S, Oelschlägel U et al Adoptive transfer of allogeneic regulatory T cells into patients with chronic graft‐versus‐host disease. Cytotherapy 2015; 17:473–86. [DOI] [PubMed] [Google Scholar]
- 11. Eugster A, Lindner A, Catani M et al High diversity in the TCR repertoire of GAD65 autoantigen‐specific human CD4+ T cells. J Immunol 2015; 194:2531–8. [DOI] [PubMed] [Google Scholar]
- 12. Filipovich AH, Weisdorf D, Pavletic S et al National Institutes of Health consensus development project on criteria for clinical trials in chronic graft‐versus‐host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11:945–56. [DOI] [PubMed] [Google Scholar]
- 13. Mamedov IZ, Britanova OV, Zvyagin IV et al Preparing unbiased T‐cell receptor and antibody cDNA libraries for the deep next generation sequencing profiling. Front Immunol 2013; 4:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bolotin DA, Shugay M, Mamedov IZ et al MiTCR: software for T‐cell receptor sequencing data analysis. Nat Methods 2013; 10:813–4. [DOI] [PubMed] [Google Scholar]
- 15. Berthold MR, Cebron N, Dill F et al. 2008. KNIME: the Konstanz information miner. In: Data Analysis, Machine Learning and Applications: Proceedings of the 31st Annual Conference of the Gesellschaft für Klassifikation e.V., Albert‐Ludwigs‐Universität Freiburg, Preisach C, Burkhardt H, Schmidt‐Thieme L, and Decker R, eds. Berlin: Springer, 2007; 319–326. [Google Scholar]
- 16. Venturi V, Kedzierska K, Turner SJ, Doherty PC, Davenport MP. Methods for comparing the diversity of samples of the T cell receptor repertoire. J Immunol Methods 2007; 321:182–95. [DOI] [PubMed] [Google Scholar]
- 17. Hippen KL, Merkel SC, Schirm DK et al Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med 2011; 3:83ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoffmann P, Eder R, Kunz‐Schughart LA, Andreesen R, Edinger M. Large‐scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood 2004; 104:895–903. [DOI] [PubMed] [Google Scholar]
- 19. Landwehr‐Kenzel S, Issa F, Luu SH et al Novel GMP‐compatible protocol employing an allogeneic B cell bank for clonal expansion of allospecific natural regulatory T cells. Am J Transplant 2014; 14:594–606. [DOI] [PubMed] [Google Scholar]
- 20. Putnam AL, Safinia N, Medvec A et al Clinical grade manufacturing of human alloantigen‐reactive regulatory T cells for use in transplantation. Am J Transplant 2013; 13:3010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh K, Stempora L, Harvey RD et al Superiority of rapamycin over tacrolimus in preserving nonhuman primate Treg half‐life and phenotype after adoptive transfer. Am J Transplant 2014; 14:2691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Turchaninova MA, Britanova OV, Bolotin DA et al Pairing of T‐cell receptor chains via emulsion PCR. Eur J Immunol 2013; 43:2507–15. [DOI] [PubMed] [Google Scholar]
- 23. Imanguli MM, Cowen EW, Rose J et al Comparative analysis of FoxP3(+) regulatory T cells in the target tissues and blood in chronic graft versus host disease. Leukemia 2014; 28:2016–27. [DOI] [PMC free article] [PubMed] [Google Scholar]


