Summary
Circulating T and B lymphocytes contribute to the pathogenesis of the neuroinflammatory autoimmune disease, multiple sclerosis (MS). Further progress in the development of MS treatments is dependent upon a greater understanding of the immunological disturbances that underlie the disease. Analyses of circulating immune cells by flow cytometry have revealed MS‐associated alterations in the composition and function of T and B cell subsets, including temporal changes associated with disease activity. Disturbances in circulating immune populations reflect those observed in the central nervous system and include skewing towards proinflammatory CD4+ and CD8+ T cells and B cells, greater proportions of follicular T helper cells and functional defects in the corresponding T and B regulatory subsets. Utilizing the analytical power of modern flow cytometers, researchers are now well positioned to monitor immunological changes associated with disease activity or intervention, describe immunological signatures with predictive value and identify targets for therapeutic drug development. This review discusses the contribution of various T and B lymphocyte subsets to MS pathogenesis, provides current and relevant phenotypical descriptions to assist in experimental design and highlights areas of future research.
Keywords: B cells, flow cytometry, multiple sclerosis, T cells
Introduction
Multiple sclerosis (MS) is a chronic, progressive, neuroinflammatory disease, and the leading cause of neurological disability in young and middle‐aged adults in the developed world 1. While the disease course and symptoms are heterogeneous, the disorder is characterized pathologically by immune‐mediated inflammation, demyelination and axonal damage in the brain and spinal cord [collectively, the central nervous system (CNS)]. Despite decades of research there remains only a small set of reliable markers for diagnosing and monitoring MS. In clinical practice these include magnetic resonance imaging to assess the dissemination of lesions in time and space, and the measurement of immunoglobulin (Ig)G, oligoclonal bands and neurofilament in the cerebrospinal fluid (CSF) 2, 3. However, obtaining these data involves significant expertise and patient burden, and they provide limited information regarding the underlying immunological disturbances on which to base the development of new therapeutic agents.
Peripheral blood (PB), meanwhile, represents an accessible biological sample and provides a ‘window’ into the immunopathogenesis of MS. Immunological characteristics of MS lesions, including infiltration of proinflammatory immune cells and defects in immunoregulation, are reflected in PB immune cells of patients with MS 4, 5, 6, and there is early evidence that certain PB immune disturbances correlate with the severity of disease progression 7. Experience with B cell‐depleting disease‐modifying therapies (DMTs) demonstrate the significant role B cells play in MS pathology 8. However, non‐B cell‐targeted DMTs also display efficacy 9, and an increased risk of MS is associated with polymorphisms in genes related to T helper cells 10. Collectively, these data indicate that the immunological disturbances that underlie MS span a range of immune cell subsets.
Technological advances in flow cytometry have greatly increased the depth of analysis achievable at the single‐cell level, and these developments can be applied to understand more clearly the immunopathology of MS. In this review we provide discussion and phenotypical descriptions of human T and B cell subsets associated with MS pathogenesis, highlighting the importance of multi‐parameter analyses in elucidating subset heterogeneity and identifying pathogenic subsets.
CD4+ T helper cells
Background
Historically, autoimmune diseases such as MS were viewed as interferon (IFN)‐γ, T helper type 1 (Th1)‐mediated conditions, following the Th1/Th2 model first described by Mosmann and colleagues 11, 12. This model was challenged when later studies revealed a protective role of IFN‐γ in the murine model of MS [experimental autoimmune encephalomyelitis (EAE)] 13 and, following the discovery of interleukin (IL)‐17‐producing Th17 cells, the concept of MS as a combined IFN‐γ‐ and IL‐17‐driven condition was developed 13. Substantial evidence implicating Th17 cells, as well as IFN‐γ+IL‐17+ double‐positive Th17.1 cells, in MS pathogenesis has accrued; however, there is now also interest in a third, granulocyte–macrophage colony‐stimulating factor (GM‐CSF)‐producing ‘ThPath’ subset, which may be critical to the disease process 10, 14.
Th17 cells contribute to CNS demyelination via their effects on the protective brain epithelial cells and activation of inflammatory immune cells. IL‐17 impairs the integrity of the blood–brain barrier, permitting entry of circulating immune cells into the CNS, while also stimulating astrocytes and microglia to produce inflammatory mediators 15.
Elevated proportions of PB Th17 cells have been reported in various stages of the disease, including clinically isolated syndrome (CIS, the earliest symptomatic presentation of demyelinating disease) 16, relapsing–remitting MS (RRMS) 6, 17, 18, as well as in the primary progressive (PPMS) and secondary progressive (SPMS) manifestations 7. PB Th17 cells may also be indicative of relapse, as in the active disease phase (the definition of which varies between studies) the percentage of Th17 cells is several‐fold greater than that observed in healthy controls 6, 18.
IFN‐γ+IL‐17+ double‐positive Th17.1 cells emerge from the Th17 population in response to cytokines, including transforming growth factor (TGF)‐β and IL‐23, in the microenvironment 19. Although there is currently little published on Th17.1 cells in MS, two studies reported a significant increase in IFN‐γ+IL‐17+ double‐positive CD4+ T cells in samples from patients with RRMS, the greatest proportions occurring in conjunction with relapse 6, 18. These studies show that, collectively, elevated Th17 cells are detectable across the spectrum of MS phenotypes, and may particularly indicate active disease processes.
Despite evidence for the pathogenic role of IL‐17 in MS and EAE, emerging lines of evidence implicate GM‐CSF‐producing T helper cells in the initiation and maintenance of autoimmune neuroinflammation 10, 14. GM‐CSF promotes the maturation and activation of myeloid cells (monocytes, dendritic cells), which are the predominant immune cells in MS CNS lesions, increasing their antigen presentation and cytokine production 10, 14. In mice, Th17 cells are the predominant source of GM‐CSF, and whereas IL‐17 alone is not mandatory for EAE development, the disease is contingent upon GM‐CSF 14. In humans, GM‐CSF production is associated with IFN‐γ‐producing Th1, rather than Th17, cells, although distinct GM‐CSF single‐positive cells have also been identified 14, 20. Higher proportions of total GM‐CSF+, GM‐CSF+IFN‐γ‐ and GM‐CSF+IFN‐γ+CD4+ T cells in samples from RRMS patients was reported recently by Rasouli and colleagues 20.
Phenotyping
Th cells can be identified through a variety of means, usually involving the use of cell surface markers in combination with lineage‐specific transcription factors (Th1, Th17 and Th17.1 phenotypical markers summarized in Table 1) or intracellular cytokine staining (ICS). The transcription factor T‐bet controls the expression of IFN‐γ 21, and thus serves as a marker of Th1 cells when combined with surface expression of CD3 and CD4, while retinoid‐related orphan receptor gamma t (ROR‐γt) is required for the differentiation of Th17 cells 22. IFN‐γ+IL‐17+ double‐positive Th17.1 cells co‐express T‐bet and ROR‐γt 23. Production of GM‐CSF is enhanced through signal transducer and activator of transcription 5 (STAT‐5) signalling; however, STAT‐5 is non‐specific for GM‐CSF production and also plays a role in regulatory T cell (Treg) development and Th17 differentiation 24.
Table 1.
T helper (Th) subset phenotypical markers
| Th1 | Th17 | Th17.1 | |
|---|---|---|---|
| CD161 | – | + | + |
| CCR6 | – | ++ | + |
| CXCR3 | ++ | – | ++ |
| CCR4 | + | ++ | + |
| T‐bet | + | – | + |
| ROR‐γt | – | + | + |
ROR‐γt = retinoic acid receptor‐related orphan receptor gamma t.
Using ICS, Th1, Th17 and Th17.1 subsets can be identified as CD3+CD4+ T cells with an IFN‐γ+IL‐17‐, IFN‐γ‐IL‐17+ or IFN‐γ+IL‐17+ phenotype, respectively (Fig. 1). The proinflammatory profile of T cells can be elucidated by further staining simultaneously for GM‐CSF to determine the proportion of GM‐CSF single‐positive cells, as well as cells co‐expressing GM‐CSF, IFN‐γ and/or IL‐17. Reports suggest that Th1, Th17 and Th17.1 cells obtained following cell culture are predominantly of a memory (CD45RO+) phenotype 6, whereas CD4+GM‐CSF+ T cells are approximately equally naive (CD45RA+) and memory cells 20. For the purposes of interstudy comparison, it is important to consider the plasticity of T cells in response to cytokines, and that cytokine production is influenced by the method of T cell activation 19, 25.
Figure 1.
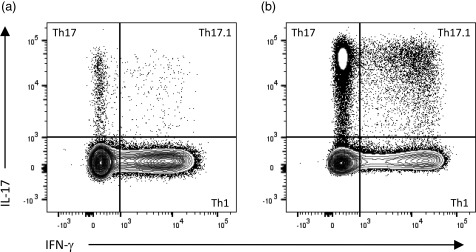
Intracellular cytokine staining to identify T helper type 1 (Th1), Th17 and Th17.1 cells. In comparison to a healthy control sample (a), the proportion of Th17 and Th17.1 cells can be increased substantially in samples from patients with clinically isolated syndrome (CIS), particularly in the setting of active disease (b). Plots represent CD3+CD4+ T cells gated on live, single‐cell peripheral blood mononuclear cells (PBMC), following a 4‐h stimulation with phorbol myristate acetate (PMA)/ionomycin in the presence of brefeldin A (BD leucocyte activation cocktail with GolgiPlug).
Production of IL‐17 is limited to cells that express CD161 26. In addition to CD3, CD4 and CD161, Th17 cells express high levels of the chemokine receptors CCR6 and CCR4, but not the Th1‐identifying CXC chemokine receptor CXCR3 27. The ligand of CCR6, CCL20, is expressed constitutively by epithelial cells of the choroid plexus, and CCR6 expression is essential for Th17 cell migration into the CNS 28. Th17 cells can thus be described as CD3+CD4+CD161+CCR6hiCXCR3–CCR4hi T cells.
In accordance with their mixed Th1/Th17 profile, Th17.1 cells have a CD3+CD4+CD161+CCR6loCXCR3+CCR4lo phenotype. The expression of CXCR3 by these cells may contribute to their pathogenic potential, as its ligand, C‐X‐C motif chemokine 10 (CXCL10), is increased significantly in the CSF of patients with MS 29.
GM‐CSF single‐positive cells have been distinguished from GM‐CSF+ cells co‐expressing IFN‐γ or IL‐17 by their CCR10+CCR4+CCR6–CXCR3– phenotype 14. However, due to conflicting data 20, ICS represents the most reliable method for identifying GM‐CSF+ Th cells at the current time.
An additional marker, CD146 (melanoma cell adhesion molecule), has been associated with greater IL‐17 and GM‐CSF mRNA expression in CD4+CD161+CCR6+ cells, and is found on ∼78% of IFN‐γ+IL‐17+ double‐positive cells 30. Although not expressed exclusively on Th17 or GM‐CSF+ cells, CD146 may be a significant indicator of pathogenic potential due to the preferential migration of CD146+ lymphocytes across the blood–brain barrier 30. CD4+CD146+ cells were found in significantly increased proportions in the PB of patients with CIS, RRMS, SPMS and PPMS, the highest levels being detected during active disease 30.
While Th17 cells are studied regularly in MS, there remains scope to refine our understanding of subset heterogeneity and the relative temporal changes with disease activity and progression. Such knowledge may assist in identifying new drug targets, or guide treatment with existing therapies known to alter the Th17 population 31.
CD4+ regulatory T cells (Treg)
Background
Treg are crucial players in the maintenance of immune tolerance due to their ability to regulate the number and function of autoreactive T cells. A key component in the pathogenesis of MS is a disturbed balance between regulatory and effector compartments of the immune system, resulting in T cell‐driven autoreactive inflammation.
Murine models support a role of Treg in preventing neuroinflammatory demyelinating disease. Depletion or inactivation of Treg increases the susceptibility of mice to the development of EAE 32, whereas adoptive transfer of Treg in Treg‐deficient animals can prevent EAE development 33. The frequency of PB Treg in patients with MS is not significantly different to healthy controls 4, 34; however, Treg from MS patients are less competent at suppressing CD4+ T cell proliferation 4, 34, 35. Accordingly, any examination of Treg in the context of MS should incorporate markers of suppressive function.
Phenotyping
The ability to phenotype Treg accurately was enhanced by the discovery of forkhead box protein 3 (FoxP3), the transcription factor required for the development of regulatory CD4+CD25+ T cells in the thymus 36. As FoxP3 expression correlates inversely with cell surface CD127 expression 37, 38, Treg may be phenotyped as CD4+CD25+FoxP3+ T cells, CD4+CD25+CD127lo/–FoxP3+ T cells, or to circumvent cell permeabilization and conduct functional analyses, as CD4+CD25+CD127lo/– T cells.
In MS, FoxP3 expression appears to change according to the disease stage and activity. It is lowest in RRMS, but is recovered in the later SPMS phase 39, 40. Few data are available regarding Treg in CIS, but the frequency of Treg and levels of FoxP3 mRNA are similar to those reported for RRMS 41, 42. FoxP3 expression and Treg function is restored in response to a number of DMTs, including glucocorticoids, IFN‐β and glatiramer acetate 43. Due to its essential role in Treg function, consistent findings of reduced expression in CIS and RRMS and modulation by DMTs, FoxP3 should be considered an essential marker in MS research.
A subset of Treg that express CD39 is of relevance in MS due to the ability to suppress Th17 cells effectively 44. Deficits in the number and function of CD4+CD25+CD127loFoxP3+CD39+ Treg have been reported in patients with RRMS 44, 45, but not SPMS 44. As CD39+ Treg are predominantly CD45RO+ memory cells 45, this finding matches an earlier report that functionally suppressive CD45RO+ Treg are reduced in early RRMS, but return to normal by the SPMS stage 35.
Another intracellular marker of interest is the Ikaros zinc finger transcription factor, Helios, which is thought to be a marker of activated Treg with increased suppressive function 46, 47. To our knowledge, the only examination of Helios in patients with MS was conducted without comparison to a healthy control group 48. However, treatments that expand the Helios+ Treg pool have shown clinical benefit in EAE studies 48, 49, supporting further investigation of Helios in MS.
CD4+ follicular T helper and regulatory cells
Background
Also found within the CD4+ T cell pool are follicular T helper (Tfh) and follicular T regulatory (Tfr) cells. Tfh cells are involved in the formation of germinal centres (GC), and the activation, expansion and differentiation of B cells into antibody‐producing cells 50, 51. Tfr cells, meanwhile, regulate GC responses and the elimination of autoreactive B cells 52.
It is proposed that Tf cells are recruited to sites of CNS inflammation in response to the chemokine receptor ligand, CXCL13 53, whereupon they contribute to the development of ectopic lymphoid follicle‐like structures (ELF) and antibody‐mediated demyelination 50. ELF in the CNS have been associated with a younger age of MS onset, more pronounced demyelination and more rapid progression to physical disability 54. Potentially reflecting sustained immune activity, the number and proportion of PB Tfh cells are elevated in samples from RRMS and SPMS patients, being most pronounced in active disease 7, 55. Although certain DMTs reduce the frequency of circulating Tfh cells, the levels remain above those in healthy controls 55.
The propensity towards CNS inflammation may be exacerbated by a disturbance in the number and function of Tfr cells 52. In comparison to healthy controls, a significantly decreased frequency of Tfr cells, in conjunction with an increased Tfh : Tfr ratio, was found in RRMS patients in one study 52. Tf cells are currently under‐investigated in MS, although the existing evidence encourages further research.
Phenotyping
Peripheral Tfh cells are heterogeneous, and display differing expression of surface markers depending on their activation status and Th‐like phenotype 51. Characterized primarily as CD4+ T cells expressing CXCR5, the receptor for CXCL13 (Fig. 2a), Tfh cells bearing a memory phenotype (CD45RO+/CD45RA–) may be categorized further as IFN‐γ‐producing Tfh1 cells (CXCR3+CCR6–), IL‐4‐producing Tfh2 cells (CXCR3–CCR6–) or IL‐17‐producing Tfh17 cells (CXCR3–CCR6+) (Fig. 2b) 51.
Figure 2.
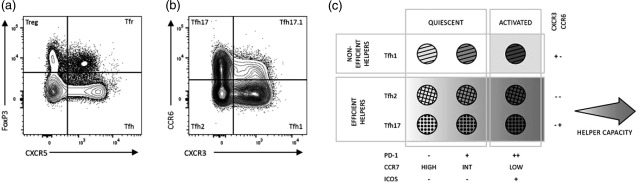
Peripheral blood follicular T cells. These cells are distinguished from conventional CD4+ T cells by the expression of CXCR5 and helper subsets categorized based on chemokine receptor, programmed death 1 (PD)‐1 and inducible T cell co‐stimulator (ICOS) expression. (a) Examining forkhead box protein 3 (FoxP3) and CXCR5 expression on live, single‐cell, CD3+CD4+ T cells reveals four distinct subsets (clockwise from top left quadrant): CCXR5–FoxP3+ regulatory T cells (Treg); CXCR5+FoxP3+ follicular T helper (Tfr); CXCR5+FoxP3– Tfh; and CXCR5–FoxP3– conventional T cells. The absence of CXCR5 fluorochrome‐conjugated antibody in Treg analysis would result in Tfr being analysed simultaneously with conventional Treg. Simultaneously, FoxP3 fluorochrome‐conjugated antibodies (or other regulatory markers) are required to distinguish between Tfh and follicular regulatory T (Tfr) cells. (b) CXCR5+FoxP3–CD45RA– Tfh cells can be categorized as Tfh17 (CCR6+CXCR3–), Tfh17.1 (CCR6+CXCR3+), Tfh1 (CCR6–CXCR3+) and Tfh2 (CCR6–CXCR3–). CCR4 and CD161 expression may be used to refine Tfh subsets further (refer to Table 1). (c) While helper capacity of Tfh1 cells is restricted to the activated ICOS+PD‐1++CCR7lo subset, all Tfh2 and Tfh17 cells are capable of helping B cells with varying capacity. The intensity of the background greyscale reflects the capacity to provide help to B cells. This figure has been adapted from reference 51 with permission from the authors.
Inducible T cell co‐stimulator (ICOS) and programmed cell death protein 1 (PD‐1) are up‐regulated in active PB Tfh cells, differentiating them from their quiescent counterparts (Fig. 2c) 51. The majority of quiescent PB Tfh cells are ICOS–PD‐1–, although a proportion express low levels of PD‐1. Tfh subsets have distinct B cell helper capacities, the most efficient helpers being ICOS–PD‐1+ and ICOS+PD‐12+ Tfh2 and Tfh17 51. A disturbance in the balance of Tfh subsets, specifically lower proportions of non‐efficient Tfh1 cells, has been noted in the PB of RRMS, SPMS and PPMS patients 7.
Regulatory Tf cells retain the activated CD4+CXCR5+PD‐1+ profile and Th‐like subsets, but are differentiated by their expression of regulatory markers such as CD25, FoxP3 and Helios 52. One study has found that Tfr cells, as a percentage of CD4+CD25+CD127– Treg, are reduced in patients with MS, and skewed compositionally towards the Th17‐like subset 52. This study reported a lower percentage of Tfr in samples from MS patients, without a significant difference in Tfh populations 52. The recognition of Tfr cells has implications for Tfh and Treg analysis. An inability to discriminate between CXCR5+ follicular T cells and conventional CD4+ populations would result in misclassification of cells, as can be visualized in Fig. 2a. Thus, the inclusion of regulatory and follicular markers in future Tf and Treg analyses, respectively, is advised.
CD8+ cytotoxic and Treg
Background
While CD4+ T cells have largely been at the centre of T cell research in MS, CD8+ T cells may also be involved in the immunopathogenesis of the disease. Patients with MS have lower frequencies of circulating CD8+ T cells in at least some studies, providing a hypothesized link between Epstein–Barr virus (EBV) reactivation and MS development 56. As EBV infection is normally kept under tight control by CD8+ cytotoxic T cells (Tc) 57, the observed reduction in the frequency of CD8+ T cells potentially impairs defence against EBV to allow development of MS.
In addition to their role in viral defence, CD8+ T cells are implicated in the autoreactivity and immune activation associated with MS, as well as exhibiting impaired regulatory function. CNS autoantigen‐targeted CD8+ T cells can injure neuronal cells directly, and enhance CNS inflammation through cytokine production 58. This includes production of IL‐17 by Tc17 and mucosal‐associated invariant T (MAIT) cells which have been identified in CNS lesions 30, 59. CD8+ T cells can also damage CNS cells through the production of cytotoxic mediators such as perforin (Tc) and granzyme B (MAIT cells) 60.
Although reports on the frequency of CD8+ Treg in MS vary, significant reductions in the frequency and suppressive function of PB and CSF CD8+ Treg have been noted during periods of relapse 61, 62, 63. These disturbances may contribute directly to MS onset or relapse, as CD8+ Treg can inhibit Tfh cells 64, expression of co‐stimulatory molecules on antigen‐presenting cells and proliferation of antigen‐specific CD4+ T cells 63.
Phenotyping
Further to their CD3+CD8+ phenotype, CD8+ T cells can be divided into naive (CD45RA+CCR7+), central memory (CD45RA–CCR7+), effector memory (EM) (CD45RA–CCR7–) and effector memory re‐expressing RA (EMRA) (CD45RA+CCR7–) subsets. In one study the EM and EMRA subsets in particular were reduced as a proportion of PB mononuclear cells (PBMC), but not of CD8+ T cells, in MS patients 56. The consistency of this finding throughout the disease course (CIS, RRMS and SPMS), without alteration with disease severity or duration, suggests that this disturbance reflects a primary defect, rather than being secondary to the disease process.
A number of reports suggest the involvement of CD161‐expressing CD8+ T cells in MS 65, 66, 67. These cells fit closely the description of MAIT cells, which are predominantly CD3+CD4–CD8+ or CD3+CD4–CD8– T cells expressing high levels of CD161, and identified more specifically by the Vα7.2 T cell receptor chain 68. The associations found between MAIT cells and MS, however, have been inconsistent 65, 66, 67. In contradictory studies, Annibali and colleagues 65 found a significantly higher percentage of PB MAIT cells (as a percentage of CD8+ T cells) in samples from patients with RRMS, while two other groups observed that RRMS was associated with lower levels of these cells in circulation (as a percentage of CD8+CD45RA– memory cells 67 or αβT cells 66). The percentage of MAIT cells correlated inversely with disease activity, being lowest during relapse 66. The CCR6+ and EM phenotype of MAIT cells (Fig. 3) is consistent with migration to the CNS, as well with the reduction in CD8+ EM cells observed by Pender and colleagues 56.
Figure 3.
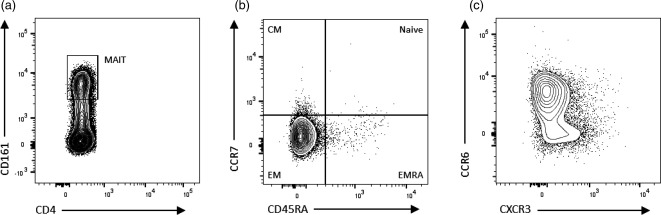
Mucosal‐associated invariant T (MAIT) cells possess a phenotype consistent with central nervous system (CNS) infiltration and proinflammatory cytokine production. (a) CD3+CD4– MAIT cells (predominantly CD8+, not shown) express high levels of CD161, and are predominantly effector memory (EM) (CCR7–CD45RA–) (b) and CCR6+ cells (c). This phenotype is consistent with migration to inflamed tissue, the capacity to cross the blood–brain barrier (BBB) and exert effector function, including interleukin (IL)‐17 production.
CD146, which is associated with IL‐17 production within the CD4+ T cell population 30, is also present on a proportion of CD8+IL‐17+ T cells, and found at increased frequencies on CD8+ T cells in other autoimmune diseases 69. However, we did not identify any research reporting on the frequency of such cells in the circulation of patients with MS. Although both CD161 and CD146 are markers of IL‐17 production in CD4+ T cells, only relatively minor percentages of CD8+CD146+ T cells (∼8·4%) 69 and MAIT cells (∼0·2% 65 to ∼1·5% 66) are IL‐17+. Thus, for the purposes of examining IL‐17 production specifically in CD8+ T cells, ICS represents the most reliable method.
Studies utilizing ICS to examine PB Tc17 (CD3+CD8+IL‐17+) 70, 71 and Tc17 co‐expressing IFN‐γ (CD3+CD8+IL‐17+IFN‐γ+) 70 have found that the frequency of these cells is significantly greater in RRMS patients in comparison to healthy controls, but is stable between relapse and remission. The presence of Tc17 cells in the PB may provide information on disease progression, as it has been reported that Tc17 are not increased in the circulation of patients with CIS or early MS, despite being elevated in the CNS 72.
Regulatory function has been described in CD8+ T cells with the CD8+CD25+CD28– and CD8+FoxP3+ phenotype 61, 62. During relapse, the number of CD8+CD25+CD28– Treg 62, the percentage of CD8+FoxP3+ Treg and the median fluorescence intensity (MFI) of FoxP3 were reduced significantly, while in remission these parameters were not statistically different to healthy controls 61.
B cells
Although often viewed as a T cell‐mediated disease, there is substantial evidence of B cell involvement in MS. Mechanisms by which B cells contribute to MS pathogenesis include the production of proinflammatory cytokines and autoantibodies to myelin proteins, and activation of autoreactive T cells 8. Strongly supporting a pathogenic role of B cells in MS is the efficacy of anti‐CD20 monoclonal antibody therapies (e.g. rituximab and ocrelizumab) in reducing the number of relapses and new gadolinium‐enhancing lesions 73. The specificity of these DMTs for CD20‐expressing pre‐ and mature‐B cells excludes a direct effect on antibody production, and they may act instead by reducing B cell cytokine production and antigen presentation 73, 74. While certain B cell subsets contribute to neuroinflammation, regulatory B cells (Breg) inhibit Th1 and Th17 cell differentiation and induce CD4+ Treg 75, 76, but are reduced numerically and functionally in the PB of patients with MS 77, 78, 79.
A large proportion of B cell research in demyelinating disease examines the CNS, CSF or lymphoid tissues, and is not necessarily generalizable to PB. Here we discuss findings relating to PB, and how changes in circulating B cell populations reflect disease activity in the CNS.
Phenotyping B cells by cell surface markers
Circulating B cells exist in several different stages of maturation and activation. Identified primarily by the pan‐B cell marker CD19, the core B cell subsets are defined by variable expression of CD20, IgD, CD27, CD24, CD38 and CD138 (Table 2). The expression of chemokine receptors and T cell co‐stimulatory molecules reveals further information regarding the propensity for CNS migration and T cell engagement.
Table 2.
Phenotypical description of core B cell subsets
| Transitional | CD19+CD20+IgD+CD27–CD24hiCD38hi |
| Naive | CD19+CD20+IgD+CD27–CD24loCD38lo |
| Double‐negative memory | CD19+CD20+IgD–CD27– |
| Non‐switched memory | CD19+CD20+IgD+CD27+ |
| Switched memory | CD19+CD20+IgD–CD27+ |
| Plasmablasts | CD19+CD20–IgD–CD27hiCD38hiCD138– |
| Plasma cells | CD19+CD20–IgD–CD27hiCD38hiCD138+ |
Ig = immunoglobulin.
Untreated remitting RRMS patients have similar proportions of total B cells and B cell subsets to healthy controls 80, 81, 82, although a higher proportion of CD27+ memory B cells has also been reported 83. Some studies have observed a proportional reduction in CD27+ memory subsets, together with a reciprocal expansion of CD27– B cells, in the PB of patients with CIS and RRMS during active phases of disease 80, 82. This is attributed to the migration of B cells towards chemokine‐producing active lesions. CSF levels of CXCL13, the receptor for which (CXCR5) is expressed by a majority of B cells, are elevated in active relapse and correlate with the accumulation of CD27+ B cells in the CSF 80. Furthermore, the proportion of PB CD27+ B cells expressing CCR5 decreases significantly during relapse 82, possibly in response to the production of CCR5 ligands in active MS lesions 29.
Aside from an increased migratory potential, there is some evidence that the B cell pool in RRMS patients comprises an elevated proportion of CD80+ and CD86+ cells 82, 84. Although increased PB CD80/86+ B cells are not found in all studies, the high proportion of CD80+ B cells in the CSF of CIS and MS patients supports a role for B cells in the generation of CNS‐antigen‐specific T cells 85.
A critical limitation of the studies reporting on chemokine receptor and co‐stimulatory molecule expression is the small number of parameters assessed simultaneously, prohibiting subset categorization and patterns of co‐expression. Current generation multi‐colour flow cytometry is better equipped to contextualize chemokine receptor and co‐stimulatory molecule expression, and revisiting these markers in future projects is required. Underscoring this recommendation is evidence that different CD80/86‐expressing B cell subsets have proinflammatory 78 and regulatory characteristics 86.
In the absence of a unique transcription factor, descriptions of Breg are made by examining the profile of B cells exhibiting suppressor function 76. Such descriptions include transitional B cells 87, CD1d‐expressing naive and memory cells 86, 88, as well as B cells expressing CD25, FoxP3 89 or CD5 76. However, analysis of these Breg in samples from MS patients has yielded conflicting results. Transitional Breg, reported to be numerically and functionally deficient in other autoimmune diseases 75, 87, were reduced in number in CIS and RRMS patients in one study 90, whereas a second found no difference between patients and healthy controls 91. Similarly, the percentage of CD5+ B cells has been associated both inversely 92 and positively 93 with disease progression, while relapse has been associated with both a decrease in the percentage of CD25+ B cells and an increase in FoxP3+ B cells 89. These conflicting results may relate to Breg not being a specific lineage, but rather a phenotype induced by inflammation 76. Thus, different Breg may emerge in different disease settings. Furthermore, the studies mentioned are limited by a lack of in‐depth phenotyping. Expression patterns of Breg are highly complex, and attempts to simplify phenotyping to a few markers may result in misclassification 94. Only through ascertaining their function can Breg be identified confidently 94, and as such ICS may be preferable.
Phenotyping B cells by intracellular cytokine staining
Expression of the regulatory cytokine IL‐10 identifies suppressive B cells. When analysed in conjunction with inflammatory cytokines the crucial balance between pro‐ and anti‐inflammatory B cells can be ascertained. Although both memory and naïve B cells are capable of producing IL‐10, the choice of stimuli is important, as their responses are ligand‐dependent 95.
CD27+ B cells are stimulated to produce IL‐10 in response to Toll‐like receptor (TLR)4‐ and TLR‐9 ligation, whereas CD40 ligation stimulates CD27– B cells specifically 95. Cell culture experiments utilizing CD40 ligation with or without the addition of a TLR‐9 ligand find consistently that B cells from patients with MS produce significantly less IL‐10, but greater amounts of lymphotoxin and IL‐6 than healthy controls, indicating regulatory impairments in naive and memory populations 96, 97. Conflicting results from ICS experiments have been reported, but are probably attributable to the method of stimulation.
In one study, there were significantly fewer IL10+ B cells following stimulation of PBMC with TLR‐9 ligand and phorbol 12‐myristate 13‐acetate (PMA)/ionomycin in samples from patients with MS than in healthy controls 77. In contrast, Ireland and colleagues reported no difference in the number of IL‐10+ B cells or intracellular levels of IL‐10 between patients and controls after stimulating with TLR‐9 ligand and B cell receptor (BCR) 97. However, whereas PMA/ionomycin stimulates B cells to mature into IL‐10+ cells, BCR inhibits the production of IL‐10 that is induced otherwise by TLR‐9 ligation 98. Ireland and colleagues 97 detected significantly greater levels of intracellular IL‐6 in patient samples, providing further support of an imbalanced regulatory : inflammatory B cell profile in MS. Besides IL‐10, Breg that suppress inflammation via the production of IL‐35 or transforming growth factor β have also been described, although their relevance to MS has not been determined 95.
Published ICS analyses suffer the same shortfall as other B cell research in MS; namely, limited subset classification or analysis of chemokine receptor and co‐stimulatory molecule expression. As B cell compartmental shifts are associated with disease activity, an understanding of differential functional capacity would be of benefit.
Summary
Our growing understanding of the pathogenesis of MS has revealed a large set of immunological disturbances involving complex interactions between different subsets of the immune system. Superseding the Th1‐mediated model of MS is one characterized by elevated proportions of a range of inflammatory T and B lymphocytes, coupled with impairments in regulatory populations (summarized in Table 3). Importantly, these disturbances can be observed not only in the CNS, but also through examination of PB immune cells.
Table 3.
Reported changes in peripheral blood (PB) immune cell subsets in different stages of multiple sclerosis (MS) in comparison to healthy controls
| CD4+ Th cells | CD4+ Treg | Tf cells | CD8+ T cells | B cells | |
|---|---|---|---|---|---|
| CIS |
↑ Th17 (% CD4) 16
↑ (↑↑ in active disease) CD146+ (% CD4) 30 |
No significant differences reported in the cited literature |
↔ Tfh (% CD4) ↓ Tfr (% Treg) 52 |
↓ EM/RA (% PBMC) 56
↔ Tc17 (% CD8) 72 ↔ FoxP3+ (% CD8) 61 |
↓ CD27+ (% B cells) in active disease 80 |
| RRMS |
↑ Th17 (% CD4) 17
↔ Th17 (% memory CD4) 6, 18 ↑ CD146+ (% CD4) 30 ↑ GM‐CSF+ (% CD4) 20 |
↓ CD39+ (% Treg) 44
↓ CD39+ (% PBMC) 45 ↓ memory Treg (% CD4) 35 ↓ FoxP3 MFI 39, 40 |
↔ Tfh (% CD4) 52
↑ Tfh (number) 55 ↑ ICOS+ (% Tfh) 7 ↓ Tfh1 (% Tfh) 7 ↓ Tfr (% Treg) 52 ↑ Tfr17 (% Treg) 52 |
↓ EM/RA (% PBMC) 56
↑ Tc17/IFN‐γ+ Tc17 (% CD8) 70 ↑ MAIT (% CD8) 65 ↓ MAIT (% memory CD8 67 and αβT cells 66) ↑ GM‐CSF+ (% CD8) 20 |
↔ B cells (% PBMC) 80
↔ core subsets (% B cells) 80, 81 ↓ IL‐10+ (% B cells) 77 |
| RRMS ‐ active |
↑↑ Th17 and Th17.1 (% memory CD4) 6, 18
↑↑ CD146+ (% CD4) 30 |
↓ CD39+ (% Treg) 44
↓ memory Treg (% CD4) 35 ↓ FoxP3 MFI 39, 40 |
↑↑ number of Tfh and ICOS+ Tfh 55 |
↓ EM/RA (% PBMC) 56
↑ Tc17 70, 71/IFN‐γ+ Tc17 (% CD8) 70 ↓ FoxP3+ (% CD8) and FoxP3 MFI 61 ↓↓ MAIT (% αβT cells) 66 |
↓ CD27+ (% B cells) 80, 82
↓ CCR5+ (% total, CD27+ and CD27– B cells) 82 ↓ IL‐10+ (% B cells) 77 |
| SPMS and PPMS |
↑ Th17 (% CD4) 7
↑ CD146+ (% CD4) 30 |
No significant differences reported in the cited literature |
↑ Tfh17 (% Tfh) 7
↓ Tfr (% Treg) 52 |
↓ EM/RA (% PBMC) 56 | No significant differences reported in the cited literature |
CIS = clinically isolated syndrome; RRMS = relapsing–remitting multiple sclerosis (MS); SPMS = secondary progressive MS; PPMS = primary progressive MS; Treg = regulatory T cell; Tfh = follicular T helper; EM/RA = effector memory re‐expressing RA T cell; ICOS = inducible T cell co‐stimulator; MAIT = mucosal‐associated invariant T; PBMC = peripheral blood mononuclear cells; GM‐CSF = granulocyte–macrophage colony‐stimulatory factor; FoxP3 = forkhead box protein 3; MFI = mean fluorescence intensity; IFN = interferon; Th = T helper.
Beyond providing an overview of MS‐relevant T and B cell subsets, the aim of this review is to bring attention to the need for multi‐parameter analysis capable of examining patterns of co‐expression in identifying novel pathological markers or profiles. Such analyses will facilitate more efficient monitoring of disease activity and the effects of DMTs, as well as aid the development of new, or ‘personalized’, therapeutic interventions. To this end we have noted a number of areas requiring clarification in future research, including the expression of functionally relevant markers within lymphocyte subsets, and characteristics of disease progression. Such questions would be best addressed through thorough, multi‐subset, longitudinal analysis from the earliest presentation of demyelinating disease.
Disclosure
None declared.
Acknowledgements
A. P. J. is a recipient of a Multiple Sclerosis Society of Western Australia Postdoctoral Research Fellowship. R. M. L. is a recipient of a National Health and Medical Research Council Senior Research Fellowship. Our project is funded by a National Health and Medical Research Council Project Grant (ID 1067209).
References
- 1. Koch‐Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol 2010; 9:520–32. [DOI] [PubMed] [Google Scholar]
- 2. Polman CH, Reingold SC, Banwell B et al Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salzer J. The only certain measure of the effectiveness of multiple sclerosis therapy is cerebrospinal neurofilament level – YES. Mult Scler 2015; 21:1239–40. [DOI] [PubMed] [Google Scholar]
- 4. Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KHG. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol 2010; 162:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frisullo G, Nociti V, Iorio R et al Regulatory T cells fail to suppress CD4+T‐bet+ T cells in relapsing multiple sclerosis patients. Immunology 2009; 127:418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Durelli L, Conti L, Clerico M et al T‐helper 17 cells expand in multiple sclerosis and are inhibited by interferon‐beta. Ann Neurol 2009; 65:499–509. [DOI] [PubMed] [Google Scholar]
- 7. Romme Christensen J, Börnsen L, Ratzer R et al Systemic inflammation in progressive multiple sclerosis involves follicular T‐helper, Th17‐ and activated B‐cells and correlates with progression. PLOS ONE 2013; 8:e57820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Claes N, Fraussen J, Stinissen P, Hupperts R, Somers V. B cells are multifunctional players in multiple sclerosis pathogenesis: insights from therapeutic interventions. Front Immunol 2015; 6:642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martin R, Sospedra M, Rosito M, Engelhardt B. Current multiple sclerosis treatments have improved our understanding of MS autoimmune pathogenesis. Eur J Immunol 2016; 46:2078–90. [DOI] [PubMed] [Google Scholar]
- 10. Hartmann FJ, Khademi M, Aram J et al Multiple sclerosis‐associated IL2RA polymorphism controls GM‐CSF production in human TH cells. Nat Commun 2014; 5:5056. [DOI] [PubMed] [Google Scholar]
- 11. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 1986; 136:2348–57. [PubMed] [Google Scholar]
- 12. Stevens TL, Bossie A, Sanders VM et al Regulation of antibody isotype secretion by subsets of antigen‐specific helper T cells. Nature 1988; 334:255–8. [DOI] [PubMed] [Google Scholar]
- 13. Rostami A, Ciric B. Role of Th17 cells in the pathogenesis of CNS demyelination. J Neurol Sci 2013; 333:76–87.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Noster R, Riedel R, Mashreghi MF et al IL‐17 and GM‐CSF expression are antagonistically regulated by human T helper cells. Sci Transl Med 2014; 6:241ra80. [DOI] [PubMed] [Google Scholar]
- 15. Waisman A, Hauptmann J, Regen T. The role of IL‐17 in CNS diseases. Acta Neuropathol 2015; 129:625–37. [DOI] [PubMed] [Google Scholar]
- 16. Zhang X, Tao Y, Chopra M et al IL‐11 induces Th17 cell responses in patients with early relapsing‐remitting multiple sclerosis. J Immunol 2015; 194:5139–49. [DOI] [PubMed] [Google Scholar]
- 17. Teniente‐Serra A, Grau‐López L, Mansilla MJ et al Multiparametric flow cytometric analysis of whole blood reveals changes in minor lymphocyte subpopulations of multiple sclerosis patients. Autoimmunity 2016; 49:219–28. [DOI] [PubMed] [Google Scholar]
- 18. Kebir H, Ifergan I, Alvarez JI et al Preferential recruitment of interferon‐gamma‐expressing TH17 cells in multiple sclerosis. Ann Neurol 2009; 66:390–402. [DOI] [PubMed] [Google Scholar]
- 19. Lee YK, Turner H, Maynard CL et al Late developmental plasticity in the T helper 17 lineage. Immunity 2009; 30:92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rasouli J, Ciric B, Imitola J et al Expression of GM‐CSF in T cells is increased in multiple sclerosis and suppressed by IFN‐β therapy. J Immunol 2015; 194:5085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T‐bet, directs Th1 lineage commitment. Cell 2000; 100:655–69. [DOI] [PubMed] [Google Scholar]
- 22. Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)‐17 cells requires transforming growth factor‐beta and induction of the nuclear receptor RORgammat. Nat Immunol 2008; 9:641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brucklacher‐Waldert V, Stuerner K, Kolster M, Wolthausen J, Tolosa E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain 2009; 132:3329–41. [DOI] [PubMed] [Google Scholar]
- 24. Sheng W, Yang F, Zhou Y et al STAT5 programs a distinct subset of GM‐CSF‐producing T helper cells that is essential for autoimmune neuroinflammation. Cell Res 2014; 24:1387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olsen I, Sollid LM. Pitfalls in determining the cytokine profile of human T cells. J Immunol Methods 2013; 390:106–12. [DOI] [PubMed] [Google Scholar]
- 26. Maggi L, Santarlasci V, Capone M et al CD161 is a marker of all human IL‐17‐producing T‐cell subsets and is induced by RORC. Eur J Immunol 2010; 40:2174–81.] [DOI] [PubMed] [Google Scholar]
- 27. Ramesh R, Kozhaya L, McKevitt K et al Pro‐inflammatory human Th17 cells selectively express P‐glycoprotein and are refractory to glucocorticoids. J Exp Med 2014; 211:89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reboldi A, Coisne C, Baumjohann D et al C‐C chemokine receptor 6‐regulated entry of TH‐17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 2009; 10:514–23. [DOI] [PubMed] [Google Scholar]
- 29. Martínez‐Cáceres EM, Espejo C, Brieva L et al Expression of chemokine receptors in the different clinical forms of multiple sclerosis. Mult Scler 2002; 8:390–5. [DOI] [PubMed] [Google Scholar]
- 30. Larochelle C, Cayrol R, Kebir H et al Melanoma cell adhesion molecule identifies encephalitogenic T lymphocytes and promotes their recruitment to the central nervous system. Brain 2012; 135:2906–24. [DOI] [PubMed] [Google Scholar]
- 31. Dos Passos GR, Sato DK, Becker J, Fujihara K. Th17 cells pathways in multiple sclerosis and neuromyelitis optica spectrum disorders: pathophysiological and therapeutic implications. Mediators Inflamm 2016; 2016:5314541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol 2005; 175:3025–32. [DOI] [PubMed] [Google Scholar]
- 33. Hori S, Haury M, Coutinho A, Demengeot J. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti‐myelin basic protein T cell receptor transgenic mice. Proc Natl Acad Sci USA 2002; 99:8213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haas J, Hug A, Viehöver A et al Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol 2005; 35:3343–52. [DOI] [PubMed] [Google Scholar]
- 35. Venken K, Hellings N, Broekmans T, Hensen K, Rummens JL, Stinissen P. Natural naive CD4+CD25+CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. J Immunol 2008; 180:6411–20. [DOI] [PubMed] [Google Scholar]
- 36. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4:330–6. [DOI] [PubMed] [Google Scholar]
- 37. Liu W, Putnam AL, Xu‐Yu Z et al CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 2006; 203:1701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seddiki N, Santner‐Nanan B, Martinson J et al Expression of interleukin (IL)‐2 and IL‐7 receptors discriminates between human regulatory and activated T cells. J Exp Med 2006; 203:1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Venken K, Hellings N, Hensen K et al Secondary progressive in contrast to relapsing‐remitting multiple sclerosis patients show a normal CD4+CD25+ regulatory T‐cell function and FOXP3 expression. J Neurosci Res 2006; 83:1432–46. [DOI] [PubMed] [Google Scholar]
- 40. Venken K, Hellings N, Thewissen M et al Compromised CD4+ CD25high regulatory T‐cell function in patients with relapsing–remitting multiple sclerosis is correlated with a reduced frequency of FOXP3‐positive cells and reduced FOXP3 expression at the single‐cell level. Immunology 2008; 123:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Basdeo SA, Kelly S, O'Connell K, Tubridy N, McGuigan C, Fletcher JM. Increased expression of Tbet in CD4+ T cells from clinically isolated syndrome patients at high risk of conversion to clinically definite MS. SpringerPlus 2016; 5:779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Feger U, Luther C, Poeschel S, Melms A, Tolosa E, Wiendl H. Increased frequency of CD4+ CD25+ regulatory T cells in the cerebrospinal fluid but not in the blood of multiple sclerosis patients. Clin Exp Immunol 2007; 147:412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Venken K, Hellings N, Liblau R, Stinissen P. Disturbed regulatory T cell homeostasis in multiple sclerosis. Trends Mol Med 2010; 16:58–68. [DOI] [PubMed] [Google Scholar]
- 44. Fletcher JM, Lonergan R, Costelloe L et al CD39+Foxp3+ regulatory T cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol 2009; 183:7602–10. [DOI] [PubMed] [Google Scholar]
- 45. Borsellino G, Kleinewietfeld M, Di Mitri D et al Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 2007; 110:1225–32. [DOI] [PubMed] [Google Scholar]
- 46. Elkord E, Abd Al Samid M, Chaudhary B. Helios, and not FoxP3, is the marker of activated Tregs expressing GARP/LAP. Oncotarget 2015; 6:20026–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zabransky DJ, Nirschl CJ, Durham NM et al Phenotypic and functional properties of Helios+ regulatory T cells. PLOS ONE 2013; 7:e34547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Breuer J, Schwab N, Schneider‐Hohendorf T et al Ultraviolet B light attenuates the systemic immune response in central nervous system autoimmunity. Ann Neurol 2014; 75:739–58. [DOI] [PubMed] [Google Scholar]
- 49. Nashold FE, Nelson CD, Brown LM, Hayes CE. One calcitriol dose transiently increases Helios+ FoxP3+ T cells and ameliorates autoimmune demyelinating disease. J Neuroimmunol 2013; 263:64–74. [DOI] [PubMed] [Google Scholar]
- 50. Ma CS, Deenick EK. Human T follicular helper (Tfh) cells and disease. Immunol Cell Biol 2014; 92:64–71. [DOI] [PubMed] [Google Scholar]
- 51. Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol 2014; 35:436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dhaeze T, Peelen E, Hombrouck A et al Circulating follicular regulatory T cells are defective in multiple sclerosis. J Immunol 2015; 195:832–40. [DOI] [PubMed] [Google Scholar]
- 53. Sellebjerg F, Börnsen L, Khademi M et al Increased cerebrospinal fluid concentrations of the chemokine CXCL13 in active MS. Neurology 2009; 73:2003–10. [DOI] [PubMed] [Google Scholar]
- 54. Magliozzi R, Howell O, Vora A et al Meningeal B‐cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007; 130:1089–104. [DOI] [PubMed] [Google Scholar]
- 55. Fan X, Jin T, Zhao S et al Circulating CCR7+ICOS+ memory T follicular helper cells in patients with multiple sclerosis. PLOS ONE 2015; 10:e0134523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pender MP, Csurhes PA, Pfluger CMM, Burrows SR. Deficiency of CD8+ effector memory T cells is an early and persistent feature of multiple sclerosis. Mult Scler 2014; 20:1825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Khanna R, Burrows SR. Role of cytotoxic T lymphocytes in Epstein‐Barr virus‐associated diseases. Annu Rev Microbiol 2000; 54:19–48. [DOI] [PubMed] [Google Scholar]
- 58. Huseby ES, Huseby PG, Shah S, Smith R, Stadinski BD. Pathogenic CD8 T cells in multiple sclerosis and its experimental models. Front Immunol 2012; 3:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tzartos JS, Friese MA, Craner MJ et al Interleukin‐17 production in central nervous system‐infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 2008; 172:146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Denic A, Wootla B, Rodriguez M. CD8+ T cells in multiple sclerosis. Expert Opin Ther Targets 2013; 17:1053–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Frisullo G, Nociti V, Iorio R et al CD8+Foxp3+ T cells in peripheral blood of relapsing‐remitting multiple sclerosis patients. Hum Immunol 2010; 71:437–41. [DOI] [PubMed] [Google Scholar]
- 62. Aristimuño C, Navarro J, de Andrés C et al Expansion of regulatory CD8+ T‐lymphocytes and fall of activated CD8+ T‐lymphocytes after i.v. methyl‐prednisolone for multiple sclerosis relapse. J Neuroimmunol 2008; 204:131–5. [DOI] [PubMed] [Google Scholar]
- 63. Correale J, Villa A. Role of CD8+ CD25+ Foxp3+ regulatory T cells in multiple sclerosis. Ann Neurol 2010; 67:625–38. [DOI] [PubMed] [Google Scholar]
- 64. Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T‐helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature 2010; 467:328–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Annibali V, Ristori G, Angelini DF et al CD161(high)CD8+T cells bear pathogenetic potential in multiple sclerosis. Brain 2011; 134:542–54. [DOI] [PubMed] [Google Scholar]
- 66. Miyazaki Y, Miyazaki Y, Chiba A, Lantz O, Yamamura T. Mucosal‐associated invariant T cells regulate Th1 response in multiple sclerosis. Int Immunol 2011; 23:529–35. [DOI] [PubMed] [Google Scholar]
- 67. Willing A, Leach OA, Ufer F et al CD8+ MAIT cells infiltrate into the CNS and alterations in their blood frequencies correlate with IL‐18 serum levels in multiple sclerosis. Eur J Immunol 2014; 44:3119–28. [DOI] [PubMed] [Google Scholar]
- 68. Howson LJ, Salio M, Cerundolo V. MR1‐restricted mucosal‐associated invariant t cells and their activation during infectious diseases. Front Immunol 2015; 6:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dagur PK, Biancotto A, Stansky E, Sen HN, Nussenblatt RB, McCoy JP. Secretion of interleukin‐17 by CD8+ T cells expressing CD146 (MCAM). Clin Immunol 2014; 152:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Peelen E, Thewissen M, Knippenberg S et al Fraction of IL‐10+ and IL‐17+ CD8 T cells is increased in MS patients in remission and during a relapse, but is not influenced by immune modulators. J Neuroimmunol 2013; 258:77–84. [DOI] [PubMed] [Google Scholar]
- 71. Wang HH, Dai YQ, Qiu W et al Interleukin‐17‐secreting T cells in neuromyelitis optica and multiple sclerosis during relapse. J Clin Invest 2011; 18:1313–7. [DOI] [PubMed] [Google Scholar]
- 72. Huber M, Heink S, Pagenstecher A et al IL‐17A secretion by CD8+ T cells supports Th17‐mediated autoimmune encephalomyelitis. J Clin Invest 2013; 123:247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sorensen PS, Blinkenberg M. The potential role for ocrelizumab in the treatment of multiple sclerosis: current evidence and future prospects. Ther Adv Neurol Disord 2016; 9:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bar‐Or A, Calabresi PA, Arnold D et al Rituximab in relapsing‐remitting multiple sclerosis: a 72‐week, open‐label, phase I trial. Ann Neurol 2008; 63:395–400. [DOI] [PubMed] [Google Scholar]
- 75. Flores‐Borja F, Bosma A, Ng D et al CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med 2013; 5:173ra23. [DOI] [PubMed] [Google Scholar]
- 76. Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity 2015; 42:607–12. [DOI] [PubMed] [Google Scholar]
- 77. Knippenberg S, Peelen E, Smolders J et al Reduction in IL‐10 producing B cells (Breg) in multiple sclerosis is accompanied by a reduced naïve/memory Breg ratio during a relapse but not in remission. J Neuroimmunol 2011; 239:80–6. [DOI] [PubMed] [Google Scholar]
- 78. Li R, Rezk A, Miyazaki Y et al Proinflammatory GM‐CSF‐producing B cells in multiple sclerosis and B cell depletion therapy. Sci Transl Med 2015; 7:310ra166. [DOI] [PubMed] [Google Scholar]
- 79. Duddy M, Niino M, Adatia F et al Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol 2007; 178:6092–9. [DOI] [PubMed] [Google Scholar]
- 80. Haas J, Bekeredjian‐Ding I, Milkova M et al B cells undergo unique compartmentalized redistribution in multiple sclerosis. J Autoimmun 2011; 37:289–99. [DOI] [PubMed] [Google Scholar]
- 81. Harp CT, Ireland S, Davis LS et al Memory B cells from a subset of treatment‐naïve relapsing‐remitting multiple sclerosis patients elicit CD4+ T‐cell proliferation and IFN‐γ production in response to myelin basic protein and myelin oligodendrocyte glycoprotein. Eur J Immunol 2010; 40:2942–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Niino M, Hirotani M, Miyazaki Y, Sasaki H. Memory and naïve B‐cell subsets in patients with multiple sclerosis. Neurosci Lett 2009; 464:74–8. [DOI] [PubMed] [Google Scholar]
- 83. Dooley J, Pauwels I, Franckaert D et al Immunologic profiles of multiple sclerosis treatments reveal shared early B cell alterations. Neurol Neuroimmunol Neuroinflamm 2016; 3:e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Genç K, Dona DL, Reder AT. Increased CD80(+) B cells in active multiple sclerosis and reversal by interferon beta‐1b therapy. J Clin Invest 1997; 99:2664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sellebjerg F, Jensen J, Ryder LP. Costimulatory CD80 (B7‐1) and CD86 (B7‐2) on cerebrospinal fluid cells in multiple sclerosis. J Neuroimmunol 1998; 84:179–87. [DOI] [PubMed] [Google Scholar]
- 86. Kessel A, Haj T, Peri R et al Human CD19(+)CD25(high) B regulatory cells suppress proliferation of CD4(+) T cells and enhance Foxp3 and CTLA‐4 expression in T‐regulatory cells. Autoimmun Rev 2012; 11:670–7. [DOI] [PubMed] [Google Scholar]
- 87. Blair PA, Noreña LY, Flores‐Borja F et al CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 2010; 32:129–40. [DOI] [PubMed] [Google Scholar]
- 88. Correale J, Farez M, Razzitte G. Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann Neurol 2008; 64:187–99. [DOI] [PubMed] [Google Scholar]
- 89. de Andrés C, Tejera‐Alhambra M, Alonso B et al New regulatory CD19(+)CD25(+) B‐cell subset in clinically isolated syndrome and multiple sclerosis relapse. Changes after glucocorticoids. J Neuroimmunol 2014; 270:37–44. [DOI] [PubMed] [Google Scholar]
- 90. Lee‐Chang C, Top I, Zéphir H et al Primed status of transitional B cells associated with their presence in the cerebrospinal fluid in early phases of multiple sclerosis. Clin Immunol 2011; 139:12–20. [DOI] [PubMed] [Google Scholar]
- 91. Michel L, Chesneau M, Manceau P et al Unaltered regulatory B‐cell frequency and function in patients with multiple sclerosis. Clin Immunol 2014; 155:198–208. [DOI] [PubMed] [Google Scholar]
- 92. Niino M, Fukazawa T, Minami N et al CD5‐positive B cell subsets in secondary progressive multiple sclerosis. Neurosci Lett 2012; 523:56–61. [DOI] [PubMed] [Google Scholar]
- 93. Villar LM, Espiño M, Roldán E et al Increased peripheral blood CD5+ B cells predict earlier conversion to MS in high‐risk clinically isolated syndromes. Mult Scler 2011; 17:690–4. [DOI] [PubMed] [Google Scholar]
- 94. Mauri C, Bosma A. Immune regulatory B cells. Annu Rev Immunol 2012; 30:221–41. [DOI] [PubMed] [Google Scholar]
- 95. Li R, Rezk A, Healy LM et al Cytokine‐defined B cell responses as therapeutic targets in multiple sclerosis. Front Immunol 2015; 6:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bar‐Or A, Fawaz L, Fan B et al Abnormal B‐cell cytokine responses a trigger of T‐cell‐mediated disease in MS? Ann Neurol 2010; 67:452–61. [DOI] [PubMed] [Google Scholar]
- 97. Ireland SJ, Guzman AA, O'Brien DE et al The effect of glatiramer acetate therapy on functional properties of B cells from patients with relapsing‐remitting multiple sclerosis. JAMA Neurol 2014; 71:1421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kalampokis I, Yoshizaki A, Tedder TF. IL‐10‐producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther 2013; 15:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]


