Abstract
Aims/Introduction
Stopping smoking deserves high priority in preventing complications of diabetes; however, only sparse data are available regarding the efficacy of pharmacotherapy in smokers with diabetes. We assessed the efficacy and safety of varenicline in smokers with diabetes who participated in 15 double‐blind, randomized, placebo‐controlled studies.
Materials and Methods
This retrospective pooled analysis included data from smokers of ≥10 cigarettes per day with diabetes. Participants received varenicline 1 mg b.i.d. or placebo for 12 weeks. We examined carbon monoxide‐confirmed continuous abstinence rates (CARs) for weeks 9–12, 9–24 and 9–52, and compared safety in participants with and without diabetes.
Results
Of 6,771 participants, 323 had diabetes (varenicline n = 162; placebo n = 161). Week 9–12 CAR was higher with varenicline than placebo (43.8% vs 24.8%; odds ratio 2.36, 95% CI 1.47–3.79), as was week 9–24 CAR (27.5% vs 14.4%; odds ratio 2.25, 95% CI 1.27–4.00). Week 9–52 CAR was 18.4% for varenicline and 10.1% for placebo (odds ratio 2.00, 95% CI 0.90–4.49). The most commonly‐reported adverse events in participants with diabetes for varenicline vs placebo were: nausea (27.2% vs 8.1%); headache (9.3% vs 9.9%); and insomnia (8.6% vs 5.6%), incidences that were similar in participants without diabetes (29.6% vs 9.7%; 13.4% vs 10.9%; and 11.4% vs 7.1%, respectively). Weight gain in quitters with diabetes (1.7 kg) was similar to that of those without diabetes (2.1 kg).
Conclusions
Varenicline was an effective and well‐tolerated aid for smoking cessation in individuals with diabetes. Safety was comparable with participants without diabetes.
Keywords: Diabetes mellitus, Smoking, Varenicline
Introduction
Cigarette smoking contributes substantially to cardiovascular disease (CVD) and microvascular complications in people with diabetes, and nearly doubles the risk of mortality1. Despite these risks, smoking remains prevalent in individuals with diabetes. In the National Health and Nutrition Examinations Surveys, approximately one‐quarter of people with diabetes or impaired fasting glucose smoked, a similar proportion to the general population2. Furthermore, smoking prevalence among individuals with diabetes or impaired fasting glucose had not changed between 1999 and 20082. Concordant with this observation, excess mortality in USA adults with prediabetes and diabetes has not declined between 1988 and 20063.
Prospective studies show that smoking raises the risk of type 2 diabetes in a dose‐dependent manner, in part by eliciting features of metabolic syndrome and insulin resistance4. In addition, smoking aggravates insulin resistance in patients with diabetes, and is associated with poor glycemic control5, 6. Smoking cessation reduces cardiovascular events7, improves insulin sensitivity8 and carries other clinical benefits, although glycated hemoglobin levels might increase slightly and temporarily after cessation9. Data showing effective interventions for smoking cessation in persons with diabetes are limited10. In addition, large‐scale studies seldom reported results separately for people with diabetes vs other participants11.
Varenicline tartrate is a selective partial agonist at α4β2 nicotinic acetylcholine receptor subtype that has been shown to significantly increase quit rates (compared with placebo and compared with bupropion) in generally healthy smokers12, 13, as well as in smokers with chronic obstructive pulmonary disease (COPD)14, CVD15 or depression16; however, smokers with diabetes have not been studied to date. The present retrospective pooled analysis examined the efficacy and safety of varenicline for smoking cessation in a subgroup of smokers with diabetes that participated in prospectively carried out randomized clinical trials.
Materials and Methods
Studies
The present pooled analysis included all Pfizer‐sponsored phase 2, 3 and 4 clinical trials of varenicline 1 mg b.i.d. completed and published as of 31 October 2014 that were randomized, blinded, parallel‐arm and placebo‐controlled with at least 12 weeks’ treatment duration in smokers aged ≥18 years. In total, 15 studies12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 met these criteria and were included in the present pooled analysis (Table 1)12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26. Trials of varenicline that did not meet these criteria (for example, trials carried out in non‐smoking healthy volunteers or using a flexible dosing regimen) were excluded from the analysis. Of the 15 identified trials, participants with diabetes were found in 12 trials.
Table 1.
Characteristics of the 15 randomized, placebo‐controlled trials of varenicline included in the pooled analysis
| Completed and published varenicline study | ClinicalTrials.gov ID: | Duration of treatment (weeks) | Duration of study (weeks) | Treatment group | Participants with diabetes | Participants without diabetes | Mean age (years) | Mean years smoking |
|---|---|---|---|---|---|---|---|---|
| Dosing strategy study† (Oncken et al.17) | NCT00150254 | 12 | 52 | Varenicline 1 mg b.i.d. Placebo |
2 4 |
251 117 |
43.1 43.0 |
25.0 25.3 |
| Pivotal study† (Gonzales et al.12) | NCT00141206 | 12 | 52 | Varenicline 1 mg b.i.d. Placebo |
3 2 |
346 342 |
42.5 42.6 |
24.3 24.7 |
| Pivotal study† (Jorenby et al.13) | NCT00143364 | 12 | 52 | Varenicline 1 mg b.i.d. Placebo |
0 0 |
343 340 |
44.6 42.3 |
27.2 24.3 |
| Korea and Taiwan study (Tsai et al.18) | NCT00141167 | 12 | 24 | Varenicline 1 mg b.i.d. Placebo |
5 9 |
121 115 |
39.7 40.9 |
20.2 22.1 |
| Japan study† (Nakamura et al.19) | NCT00139750 | 12 | 52 | Varenicline 1 mg b.i.d. Placebo |
0 0 |
156 154 |
39.6 39.8 |
20.7 20.8 |
| China study (Wang et al.20) | NCT00371813 | 12 | 24 | Varenicline 1 mg b.i.d. Placebo |
4 5 |
161 163 |
39.0 38.5 |
20.5 19.6 |
| Smokeless tobacco study (Fagerström et al.21) | NCT00717093 | 12 | 26 | Varenicline 1 mg b.i.d. Placebo |
8 6 |
205 212 |
43.9 43.9 |
20.3‡
21.7‡ |
| CVD study (Rigotti et al.15) | NCT00282984 | 12 | 52 | Varenicline 1 mg b.i.d. Placebo |
58 68 |
295 282 |
57.0 56.0 |
40.0 39.1 |
| COPD study (Tashkin et al.14) | NCT00285012 | 12 | 52 | Varenicline 1 mg b.i.d. Placebo |
13 13 |
235 238 |
57.2 57.1 |
40.4 40.6 |
| Nicotine withdrawal study (Garza et al.22) | NCT00749944 | 12 | 16 | Varenicline 1 mg b.i.d. Placebo |
0 0 |
55 55 |
33.4 33.8 |
16.9 16.8 |
| Africa, Middle East, Latin America study (Bolliger et al.23) | NCT00594204 | 12 | 26 | Varenicline 1 mg b.i.d. Placebo |
24 15 |
366 183 |
43.1 43.9 |
25.0 26.8 |
| Flexible quit date study (Rennard et al.24) | NCT00691483 | 12 | 24 | Varenicline 1 mg b.i.d. Placebo |
14 7 |
472 158 |
43.9 43.2 |
26.0 24.6 |
| Schizophrenia study (Williams et al.25) | NCT00644969 | 12 | 26 | Varenicline 1 mg b.i.d. Placebo |
8 8 |
76 35 |
40.2 43.0 |
23.7 24.9 |
| Depression study (Anthenelli et al.16) | NCT01078298 | 12 | 52 | Varenicline 1 mg b.i.d. Placebo |
11 11 |
245 258 |
45.4 47.1 |
26.0 27.3 |
| Retreatment study (Gonzales et al.26) | NCT01244061 | 12 | 52 | Varenicline 1 mg b.i.d. Placebo |
12 13 |
237 232 |
47.7 47.3 |
30.2 30.0 |
†Study has additional arms not included in this pooled analysis. ‡Number of years of smokeless tobacco use. COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease.
All trials included in the pooled analysis were carried out in accordance with the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice Guidelines, and the institutional review board and/or independent ethics committee at each site approved trial protocols before study start12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26. Written informed consent was obtained from all participants before any procedures were carried out12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26.
Participants
In general, studies included adult smokers aged 18–75 years (some studies had different age ranges, specifically: 18–65 years17; 20–75 years19; 35–75 years15; ≥35 years14, ≥18 years21, 26) who smoked an average of ≥10 cigarettes per day (or, in one study, had been using smokeless tobacco ≥8 times per day21) during the previous year, and who had no period of abstinence greater than 3 months. The diagnosis of diabetes was based on a self‐reported medical history of diabetes or use of antidiabetes medication in the case report form.
Standard exclusion criteria included serious or unstable disease within the 6 months before study entry, diagnosis of or treatment for depression during the previous 12 months (except in one study of smokers with stably treated current or past major depression16); a history of or current psychosis (except in one study of smokers with schizophrenia or schizoaffective disorder25), panic disorder or bipolar disorder; severe COPD (except in one study of smokers with mild to moderate COPD14); clinically significant CVD (except in one study of smokers with stable CVD15), uncontrolled hypertension or systolic blood pressure ≥150 mmHg or diastolic blood pressure ≥95 mmHg at the screening or baseline visit; a history of a cancer (excluding basal cell or squamous cell carcinoma); a history of drug (with the exception of nicotine) or alcohol abuse or dependence; or prior use of nicotine replacement therapy (NRT).
Length of treatment
All studies had a 12‐week randomized treatment period, and the total duration of the studies varied from 16 to 52 weeks (16 weeks22, 24 weeks18, 20, 24, 25, 26 weeks21, 23, 52 weeks12, 13, 14, 15, 16, 17, 19, 26).
On starting treatment, the patients were given ‘Clearing the Air: Quit Smoking Today’27, a smoking cessation self‐help booklet as a guide to the quitting process. At each visit, the participants were provided with brief (up to 10 min), standardized, individual counseling to assist in problem solving and skills training for relapse prevention following recommendations in the Public Health Service Clinical Practice Guideline28.
Outcome measures
Participants in all studies were classified as quitters or non‐quitters based on the primary efficacy end‐point, which was continuous abstinence from smoking for weeks 9–12. A quitter was defined as a participant who self‐reported to be continuously abstinent during the last 4 weeks of treatment (weeks 9–12) and confirmed by a carbon monoxide assessment. Additional efficacy end‐points were continuous abstinence for weeks 9–24 and weeks 9–52.
Adverse event collection and classification
Adverse events (AEs) were collected in patients who received one or more doses of study treatment. All observed or self‐reported AEs were collected in case report forms and followed to resolution or end of study. Descriptions were coded to preferred terms in the Medical Dictionary for Regulatory Activities (MedDRA, version 17.1). Adverse events that resulted in death, hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability or incapacity, or resulted in congenital abnormality or birth defect were classified as serious AEs.
Statistical analysis
Data from all 15 studies were pooled. Continuous abstinence from weeks 9 to 12, the primary efficacy end‐point, was analyzed by generalized linear modeling with treatment as the model term. Each cohort had a separate analysis. These analyses produced estimates (and associated 95% confidence intervals [CIs]) for odds ratio (OR), relative risk and risk difference in the comparison between varenicline and placebo.
The secondary end‐points of continuous abstinence for weeks 9–24 and weeks 9–52 were analyzed in an analogous manner.
Because the 15 studies analyzed had varying designs, end‐point formulation was not perfectly aligned across the studies. Modest departures from true end‐point definition were allowed for the sake of data inclusion. Specifically, a continuous abstinence rate (CAR) 9–26 end‐point from two 26‐week studies21, 23 was used as a surrogate for the CAR 9–24 end‐point within this analysis. There was one 16‐week study22 for which only CAR 9–12 was used.
Results
Participant characteristics
Over the 15 studies, 6,771 smokers were randomized and treated with either varenicline 1 mg b.i.d. or placebo. Of these, 323 participants (74.9% men, 25.1% women) either had a diagnosis of diabetes recorded (type 1 [n = 12]; type 2 [n = 311]) or were receiving antidiabetes medications (n = 41). Of the 327 participants in the diabetes cohort, 276 (85.4%) were receiving diabetes medication, with the majority of patients receiving metformin (67.3%) or sulfonylureas (34.0%). Participant baseline characteristics are shown in Table 2. Compared with participants without diabetes (n = 6,448), participants with diabetes appeared to be older, more likely to be male, had a higher body mass index (BMI), smoked more cigarettes per day, had smoked for longer and had attempted to quit fewer times. In addition, they had higher Fagerström Test for Nicotine Dependence scores, including a shorter time to the first cigarette in the morning. In a subset in which glycated hemoglobin concentrations were measured, as expected, concentrations were higher in the group with diabetes.
Table 2.
Participant characteristics according to diagnosis and treatment
| Participants with diabetes | Participants without diabetes | |||
|---|---|---|---|---|
| Varenicline | Placebo | Varenicline | Placebo | |
| n | 162 | 161 | 3,564 | 2,884 |
| Mean age, years (SD) | 55.0 (9.5) | 56.2 (9.2) | 45.0 (12.2) | 45.1 (12.2) |
| Female, n (%) | 44 (27.2) | 37 (23.0) | 1,357 (38.1) | 1,000 (34.7) |
| Ethnicity, n (%) | ||||
| White | 99 (61.1) | 106 (65.8) | 2,388 (67.0) | 1,940 (67.3) |
| Black | 13 (8.0) | 14 (8.7) | 230 (6.5) | 184 (6.4) |
| Asian | 27 (16.7) | 28 (17.4) | 603 (16.9) | 521 (18.1) |
| Other | 23 (14.2) | 13 (8.1) | 343 (9.6) | 239 (8.3) |
| Mean BMI, kg/m2 (SD) | 29.7 (5.0) | 29.6 (5.0) | 26.4 (4.7) | 26.3 (4.5) |
| Mean years of smoking (SD) | 36.4 (12.8) | 37.3 (12.4) | 26.0 (13.5) | 25.9 (13.6) |
| Mean cigarettes/day (SD) | 23.4 (9.9) | 25.1 (12.9) | 22.3 (9.8) | 22.0 (9.1) |
| Mean previous quit attempts (SD) | 1.9 (3.0) | 2.5 (5.7) | 2.7 (6.0) | 2.7 (5.4) |
| Mean Fagerström score† (SD) | 5.96 (2.18) | 6.02 (2.14) | 5.72 (2.18) | 5.76 (2.11) |
| Time to first cigarette, n (%) | ||||
| Within 5 min | 78 (48.1) | 74 (46.0) | 1,364 (38.3) | 1,071 (37.2) |
| 6–30 min | 58 (84.0) | 58 (82.0) | 1,472 (79.6) | 1,250 (80.6) |
| 31–60 min | 15 (93.2) | 15 (91.3) | 468 (92.8) | 363 (93.2) |
| >60 min | 11 (100) | 14 (100) | 258 (100) | 197 (100) |
| HbA1c, n (%) | (n = 68) | (n = 79) | (n = 480) | (n = 478) |
| <6.5% | 18 (26.5) | 25 (31.6) | 450 (93.8) | 447 (93.5) |
| ≥6.5 to <7.0% | 19 (27.9) | 12 (15.2) | 26 (5.4) | 26 (5.4) |
| ≥7.0% | 31 (45.6) | 42 (53.2) | 4 (0.8) | 5 (1.0) |
†Scores range from 0 to 10, with higher scores indicating greater dependence. BMI, body mass index; SD, standard deviation.
Efficacy
Of the 323 participants with diabetes, 162 received varenicline and 161 received placebo. The CAR during weeks 9–12 of the study was 43.8% in varenicline‐treated participants vs 24.8% in placebo group participants (OR 2.36, 95% CI 1.47–3.79] (Figure 1a). The corresponding CARs for weeks 9–24 and 9–52 were 27.5% vs 14.4% (OR 2.25, 95% CI 1.27–4.00; Figure 1b) and 18.4% vs 10.1% (OR 2.00, 95% CI 0.90–4.49; Figure 1c), respectively. CARs for weeks 9–12, 9–24 and 9–52 in participants without diabetes are shown in Figure 1a–c for comparison.
Figure 1.
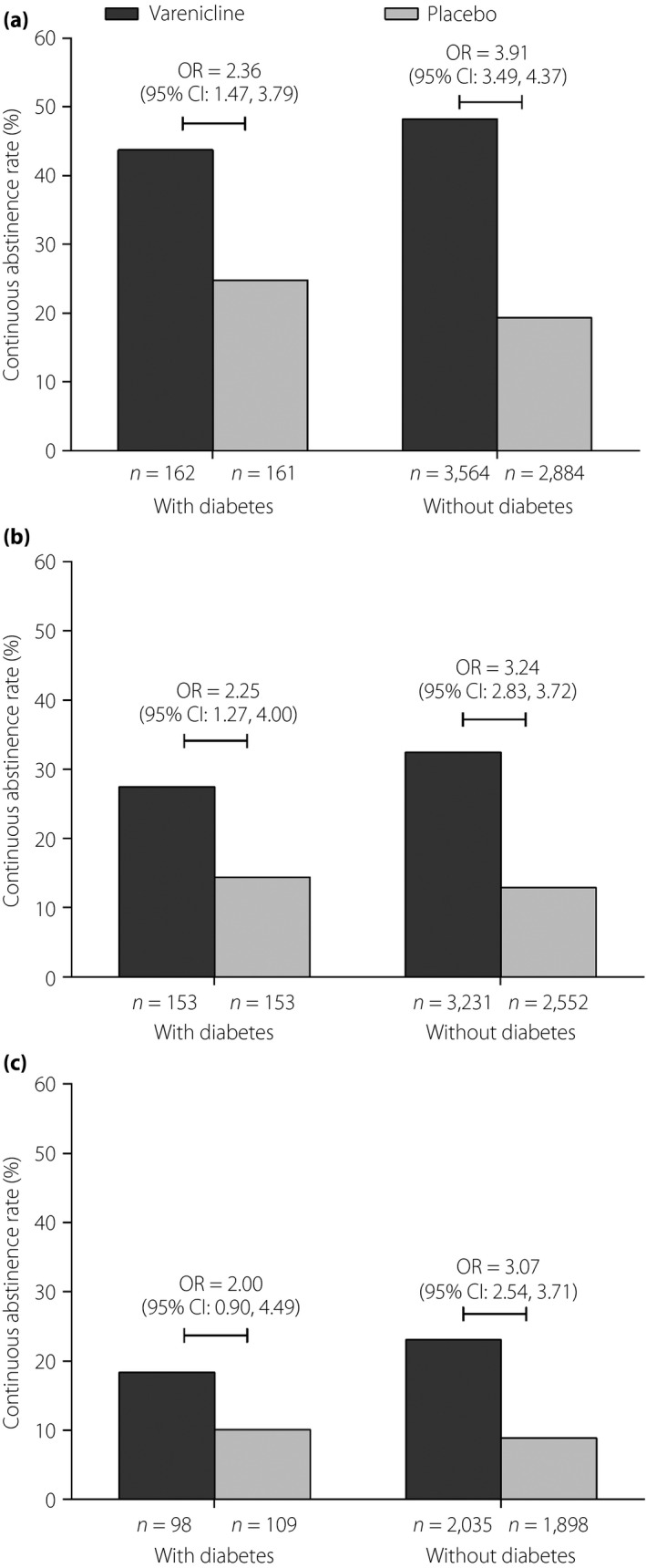
Continuous abstinence rates (CARs) in smokers with and without diabetes from (a) weeks 9–12, (b) weeks 9–24 and (c) weeks 9–52.
AEs
In the cohort with diabetes, 425 AEs occurred in 127 (78.4%) varenicline‐treated participants, and 358 AEs in 114 (70.8%) placebo group participants. Serious AEs occurred in seven (4.3%) and 10 (6.2%) participants in the varenicline and placebo groups, respectively. In the varenicline group, 11 (6.8%) participants permanently discontinued treatment because of an AE, and 19 (11.7%) of participants had their dose reduced or temporarily discontinued because of an AE. The corresponding rates in the placebo group were nine (5.6%) and seven (4.3%).
In the non‐diabetes cohort, a total of 9,105 AEs occurred in 2,759 (77.4%) varenicline‐treated participants, and 5,588 AEs occurred in 1,953 (67.7%) placebo group participants. Serious AEs occurred in 80 (2.2%) and 64 (2.2%) participants in the varenicline and placebo groups, respectively. In the varenicline group, 276 (7.7%) participants permanently discontinued treatment because of an AE, and 275 (7.7%) participants had their dose reduced or temporarily discontinued because of an AE. The corresponding numbers in the placebo group were 174 (6.0%) and 134 (4.6%).
Treatment‐emergent AEs occurring in ≥5% of participants of either treatment group in each cohort are listed in Table 3. The most commonly reported AEs in smokers with diabetes for varenicline vs placebo were nausea (27.2% vs 8.1%), headache (9.3% vs 9.9%), insomnia (8.6% vs 5.6%) and fatigue (8.6% vs 5.0%). In comparison, the most frequent AEs for varenicline vs placebo in smokers without diabetes (n = 6,448) were nausea (29.6% vs 9.7%), headache (13.4% vs 10.9%), insomnia (11.4% vs 7.1%) and abnormal dreams (9.7% vs 3.4%). The incidence of fatigue was higher in participants with diabetes vs participants without diabetes, and insomnia was more commonly reported in participants without diabetes vs participants with diabetes (Table 3).
Table 3.
Treatment‐emergent adverse events occurring in ≥5% participants with and without diabetes in either treatment group
| AE (MedDRA preferred term), n (%) | Participants with diabetes | Participants without diabetes | ||
|---|---|---|---|---|
| Varenicline n = 162 | Placebo n = 161 | Varenicline n = 3,564 | Placebo n = 2,884 | |
| Nausea | 44 (27.2) | 13 (8.1) | 1,056 (29.6) | 280 (9.7) |
| Headache | 15 (9.3) | 16 (9.9) | 478 (13.4) | 315 (10.9) |
| Insomnia | 14 (8.6) | 9 (5.6) | 408 (11.4) | 205 (7.1) |
| Fatigue | 14 (8.6) | 8 (5.0) | 174 (4.9) | 111 (3.8) |
| Vomiting | 12 (7.4) | 4 (2.5) | 168 (4.7) | 53 (1.8) |
| Abnormal dreams | 12 (7.4) | 2 (1.2) | 345 (9.7) | 98 (3.4) |
| Constipation | 12 (7.4) | 5 (3.1) | 218 (6.1) | 86 (3.0) |
| Dizziness | 11 (6.8) | 4 (2.5) | 164 (4.6) | 166 (5.8) |
| Diarrhea | 9 (5.6) | 6 (3.7) | 151 (4.2) | 109 (3.8) |
| Anxiety | 9 (5.6) | 8 (5.0) | 95 (2.7) | 114 (4.0) |
| Nasopharyngitis | 8 (4.9) | 11 (6.8) | 265 (7.4) | 229 (7.9) |
| Upper respiratory tract infection | 7 (4.3) | 8 (5.0) | 210 (5.9) | 211 (7.3) |
| Back pain | 6 (3.7) | 8 (5.0) | 107 (3.0) | 69 (2.4) |
| Influenza | 4 (2.5) | 11 (6.8) | 100 (2.8) | 87 (3.0) |
AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities.
Hypoglycemia occurred in four (2.5%) participants in the diabetes cohort receiving varenicline, and one (0.6%) participant receiving placebo. In comparison, in the non‐diabetes cohort, two (0.1%) participants in the varenicline group and none of the participants in the placebo group reported hypoglycemia.
Hyperglycemia was reported in one (0.6%) participant in the diabetes cohort receiving varenicline, and one (0.6%) participant receiving placebo. In the non‐diabetes cohort, hyperglycemia was reported in five (0.1%) participants in the varenicline group, and one (<0.1%) participant in the placebo group.
Bodyweight and BMI change since baseline at week 12 in quitters vs non‐quitters
Mean bodyweight change from baseline to week 12 in quitters (defined as participants who were continuously abstinent during weeks 9–12) with diabetes was 1.7 kg (standard deviation [SD] 2.6; n = 62) in the varenicline group and 1.7 kg (SD 2.9; n = 29) in the placebo group. Changes from baseline to week 12 in BMI in quitters with diabetes were 0.56 kg/m2 (SD 0.89; n = 62) and 0.54 kg/m2 (SD 0.92; n = 29) in the varenicline and placebo groups, respectively. Quitters without diabetes had mean bodyweight change from baseline to week 12 of 2.1 kg (SD 2.9; n = 1,452) for varenicline and 2.1 kg (SD 2.7; n = 434) for placebo. Mean BMI change from baseline to week 12 in quitters without diabetes were 0.72 kg/m2 (SD 0.99; n = 1,451) and 0.72 kg/m2 (SD 0.91; n = 432) for varenicline and placebo, respectively.
In non‐quitters with diabetes, the mean bodyweight change from baseline to week 12 was 1.3 kg (SD 2.4; n = 70) in the varenicline group and 0.5 kg (SD 3.0; n = 96) in the placebo group. The mean BMI change from baseline to week 12 in non‐quitters with diabetes was 0.44 kg/m2 (SD 0.84; n = 70) for varenicline and 0.18 kg/m2 (SD 0.91; n = 96) for placebo. In non‐quitters without diabetes mean bodyweight change from baseline to week 12 was 1.2 kg (SD 2.6; n = 1,399) for varenicline and 0.6 kg (SD 3.0; n = 1,741) for placebo. The mean BMI change from baseline to week 12 in non‐quitters without diabetes was 0.42 kg/m2 (SD 0.86; n = 1,398) and 0.22 kg/m2 (SD 0.96; n = 1,741) for the varenicline and placebo groups, respectively.
Discussion
Varenicline was effective in promoting smoking cessation in the subgroup of participants with diabetes in data extracted from 15 double‐blind, randomized, placebo‐controlled studies. Quit rates were more than doubled at the end of the 12‐week varenicline vs placebo treatment period, and remained doubled and statistically significantly higher than placebo 24 weeks after the start of treatment (which was 12 weeks after the end of pharmacotherapy). Although the 52‐week quit rate did not achieve statistical significance, this was probably because of the limitations of sample size. Quit rates were numerically doubled at week 52 with varenicline compared with placebo.
There was a priori no specified concern that smokers with diabetes would differ from others in regard to efficacy of varenicline. The rationale of our analysis was based on the need for data on the effects of smoking cessation pharmacotherapy in patients with diabetes. Furthermore, participants with diabetes appeared to be more nicotine dependent than participants without diabetes, as evidenced by their mean Fagerström Test for Nicotine Dependence in Table 1, underscoring the need for more study of efficacious interventions in this group.
The findings show that smokers with diabetes might attain as much benefit as other smokers included in the trials. To date, varenicline has shown efficacy and safety in several clinical populations at high risk for smoking‐associated disease. Quit rates in the present study are comparable with those in smokers with CVD (CAR weeks 9–12 of 47.0% vs 13.9% for varenicline vs placebo, respectively)15 and COPD (CAR weeks 9–12 of 42.3% vs 8.8% for varenicline vs placebo, respectively)14, and appear higher than quit rates in patients with depression (CAR weeks 9–12 of 35.9% vs 15.6% for varenicline vs placebo, respectively)16.
The American Diabetes Association suggested in 2002 that pharmacological supplements be offered as appropriate11. However, we have failed to identify trials of pharmacotherapy vs placebo in populations with diabetes. In one trial, 114 participants in an ongoing type 2 diabetes adult education program were randomized to combined treatment with face‐to‐face motivational interviewing, telephone counseling and free NRT or bupropion. Although there was a trend toward greater cessation at 3 months in those receiving the intervention, this trend was not sustained at 6 months29. Likewise, structured extensive behavior therapy intervention that included NRT was no more successful than physician's advice in promoting cessation; however, the sample size was small30. A study carried out in Spain randomized 280 smokers with diabetes to a nurse‐led intervention vs standard care31. The intervention group showed significantly higher quit rates than standard care after 6 months, but use of NRT in the intervention group was limited. Less than one‐quarter of smokers accepted NRT, and <10% completed the treatment course.
In the current study, motivational and behavioral support was frequent, but not intensive. Evidence regarding the effects of behavioral counseling in smokers with diabetes is limited. Early studies emphasized difficulties of cessation in smokers with diabetes32, and low recruitment and high dropout rates33. The results of quitline studies have been more promising. Tobacco users with diabetes used the quitline in greater proportion than they were represented in the general population, and had quit rates that were comparable with quitline users without diabetes34. More studies are required to understand the best behavioral interventions for smokers with diabetes10.
We found no increase in AEs in participants with diabetes compared with participants without diabetes. Although fatigue was reported more often in participants with diabetes vs participants without diabetes and insomnia was more commonly reported in participants without diabetes vs participants with diabetes, these findings do not have a ready explanation and could be due to chance.
Post‐cessation weight gain is thought to be a major impediment to smoking cessation in individuals with diabetes. In the current study, weight gain was limited to <2 kg (or BMI ≤0.56 kg/m2) in quitters in both the varenicline and placebo groups after 12 weeks, although data were only available for a limited number of participants. Weight gain does not limit the benefits of smoking cessation on reducing CVD7.
These data were obtained from a retrospective pooled analysis of previously collected data and have inherent limitations. Sample size was limited to the number available in the studies. Some of the participants used antidiabetes medications, but did not have a recorded diagnosis of diabetes. We did not have information regarding glycated hemoglobin levels in all participants at baseline, and this information was not collected systematically at the end of the trials.
In conclusion, varenicline appears to be an efficacious and well‐tolerated aid to smoking cessation in smokers with diabetes when added on top of frequent, but non‐intensive, motivational support.
Disclosure
ST has received honoraria for consulting and lectures from manufacturers of smoking cessation medications, including Pfizer Inc., the manufacturer of varenicline. DL is an employee of Pfizer Inc.
Acknowledgments
The authors thank Carla Yunis MD, Theodore Lee MD and Konstantinos Tsilkos MD of Pfizer Inc. for their assistance in the early stages of this analysis and manuscript development. Editorial support in the form of preparing figures and tables, formatting references, collating review comments, and preparing the manuscript for submission was provided by Helen Jones PhD, Alexandra Bound PhD, Michelle Jenvey PhD and Abegale Templar PhD of Engage Scientific, Horsham, UK, and funded by Pfizer Inc. All clinical trials included in the analysis were funded by Pfizer Inc. (ClinicalTrials.gov: NCT00141206, NCT00143364, NCT00285012, NCT00282984, NCT00150254, NCT00141167, NCT00139750, NCT00371813, NCT00594204, NCT00691483, NCT00717093, NCT00749944, NCT00717093, NCT01078298 and NCT01244061).
J Diabetes Investig 2017; 8: 93–100
References
- 1. Nelson KM, Boyko EJ, Koepsell T. All‐cause mortality risk among a national sample of individuals with diabetes. Diabetes Care 2010; 33: 2360–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clair C, Meigs JB, Rigotti NA. Smoking behavior among US adults with diabetes or impaired fasting glucose. Am J Med 2013; 126: 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stokes A, Mehta NK. Mortality and excess risk in US adults with pre‐diabetes and diabetes: a comparison of two nationally representative cohorts, 1988–2006. Popul Health Metr 2013; 11: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Willi C, Bodenmann P, Ghali WA, et al Active smoking and the risk of type 2 diabetes: a systematic review and meta‐analysis. JAMA 2007; 298: 2654–2664. [DOI] [PubMed] [Google Scholar]
- 5. Targher G, Alberiche M, Zenere MB, et al Cigarette smoking and insulin resistance in patients with noninsulin‐dependent diabetes mellitus. J Clin Endocrinol Metab 1997; 82: 3619–3624. [DOI] [PubMed] [Google Scholar]
- 6. Gunton JE, Davies L, Wilmshurst E, et al Cigarette smoking affects glycemic control in diabetes. Diabetes Care 2002; 25: 796–797. [DOI] [PubMed] [Google Scholar]
- 7. Clair C, Rigotti NA, Porneala B, et al Association of smoking cessation and weight change with cardiovascular disease among adults with and without diabetes. JAMA 2013; 309: 1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eliasson B, Attvall S, Taskinen MR, et al Smoking cessation improves insulin sensitivity in healthy middle‐aged men. Eur J Clin Invest 1997; 27: 450–456. [DOI] [PubMed] [Google Scholar]
- 9. Lycett D, Nichols L, Ryan R, et al The association between smoking cessation and glycaemic control in patients with type 2 diabetes: a THIN database cohort study. Lancet Diabetes Endocrinol 2015; 3: 423–430. [DOI] [PubMed] [Google Scholar]
- 10. Nagrebetsky A, Brettell R, Roberts N, et al Smoking cessation in adults with diabetes: a systematic review and meta‐analysis of data from randomised controlled trials. BMJ Open 2014; 4: e004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American Diabetes Association . Smoking and Diabetes. Diabetes Care 2002; 25: S80–S81. [Google Scholar]
- 12. Gonzales D, Rennard SI, Nides M, et al Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained‐release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006; 296: 47–55. [DOI] [PubMed] [Google Scholar]
- 13. Jorenby DE, Hays JT, Rigotti NA, et al Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained‐release bupropion for smoking cessation: a randomized controlled trial. JAMA 2006; 296: 56–63. [DOI] [PubMed] [Google Scholar]
- 14. Tashkin DP, Rennard S, Hays JT, et al Effects of varenicline on smoking cessation in patients with mild to moderate COPD: a randomized controlled trial. Chest 2011; 139: 591–599. [DOI] [PubMed] [Google Scholar]
- 15. Rigotti NA, Pipe AL, Benowitz NL, et al Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation 2010; 121: 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anthenelli RM, Morris C, Ramey TS, et al Effects of varenicline on smoking cessation in adults with stably treated current or past major depression: a randomized trial. Ann Int Med 2013; 159: 390–400. [DOI] [PubMed] [Google Scholar]
- 17. Oncken C, Gonzales D, Nides M, et al Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med 2006; 166: 1571–1577. [DOI] [PubMed] [Google Scholar]
- 18. Tsai ST, Cho HJ, Cheng HS, et al A randomized, placebo‐controlled trial of varenicline, a selective alpha4beta2 nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers. Clin Ther 2007; 29: 1027–1039. [DOI] [PubMed] [Google Scholar]
- 19. Nakamura M, Oshima A, Fujimoto Y, et al Efficacy and tolerability of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, in a 12‐week, randomized, placebo‐controlled, dose‐response study with 40‐week follow‐up for smoking cessation in Japanese smokers. Clin Ther 2007; 29: 1040–1056. [DOI] [PubMed] [Google Scholar]
- 20. Wang C, Xiao D, Chan KP, et al Varenicline for smoking cessation: a placebo‐controlled, randomized study. Respirology 2009; 14: 384–392. [DOI] [PubMed] [Google Scholar]
- 21. Fagerstrom K, Gilljam H, Metcalfe M, et al Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial. BMJ 2010; 341: c6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garza D, Murphy M, Tseng LJ, et al A double‐blind randomized placebo‐controlled pilot study of neuropsychiatric adverse events in abstinent smokers treated with varenicline or placebo. Biol Psychiatry 2011; 69: 1075–1082. [DOI] [PubMed] [Google Scholar]
- 23. Bolliger CT, Issa JS, Posadas‐Valay R, et al Effects of varenicline in adult smokers: a multinational, 24‐week, randomized, double‐blind, placebo‐controlled study. Clin Ther 2011; 33: 465–477. [DOI] [PubMed] [Google Scholar]
- 24. Rennard S, Hughes J, Cinciripini PM, et al A randomized placebo‐controlled trial of varenicline for smoking cessation allowing flexible quit dates. Nicotine Tob Res 2012; 14: 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williams JM, Anthenelli RM, Morris CD, et al A randomized, double‐blind, placebo‐controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry 2012; 73: 654–660. [DOI] [PubMed] [Google Scholar]
- 26. Gonzales D, Hajek P, Pliamm L, et al Retreatment with varenicline for smoking cessation in smokers who have previously taken varenicline: a randomized, placebo‐controlled trial. Clin Pharmacol Ther 2014; 96: 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Cancer Institute . Clearing the Air: Quit Smoking Today. 2003.
- 28. Fiore MC. Treating tobacco use and dependence: an introduction to the US Public Health Service Clinical Practice Guideline. Respir Care 2000; 45: 1196–1199. [PubMed] [Google Scholar]
- 29. Hokanson JM, Anderson RL, Hennrikus DJ, et al Integrated tobacco cessation counseling in a diabetes self‐management training program: a randomized trial of diabetes and reduction of tobacco. Diabetes Educ 2006; 32: 562–570. [DOI] [PubMed] [Google Scholar]
- 30. Sawicki PT, Didjurgeit U, Muhlhauser I, et al Behaviour therapy versus doctor's anti‐smoking advice in diabetic patients. J Intern Med 1993; 234: 407–409. [DOI] [PubMed] [Google Scholar]
- 31. Canga N, De Irala J, Vara E, et al Intervention study for smoking cessation in diabetic patients: a randomized controlled trial in both clinical and primary care settings. Diabetes Care 2000; 23: 1455–1460. [DOI] [PubMed] [Google Scholar]
- 32. Scemama O, Hamo‐Tchatchouang E, Le Faou AL, et al Difficulties of smoking cessation in diabetic inpatients benefiting from a systematic consultation to help them to give up smoking. Diabetes Metab 2006; 32: 435–441. [DOI] [PubMed] [Google Scholar]
- 33. Fowler PM, Hoskins PL, McGill M, et al Anti‐smoking programme for diabetic patients: the agony and the ecstasy. Diabet Med 1989; 6: 698–702. [DOI] [PubMed] [Google Scholar]
- 34. Schauer GL, Bush T, Cerutti B, et al Use and effectiveness of quitlines for smokers with diabetes: cessation and weight outcomes, Washington State Tobacco Quit Line, 2008. Prev Chronic Dis 2013; 10: E105. [DOI] [PMC free article] [PubMed] [Google Scholar]


