Summary
We showed recently that M3 muscarinic acetylcholine receptor (M3R)‐reactive CD3+ T cells play a pathogenic role in the development of murine autoimmune sialadenitis (MIS), which mimics Sjögren's syndrome (SS). The aim of this study was to determine the effectiveness and mechanism of action of retinoic acid‐related orphan receptor‐gamma t (RORγt) antagonist (A213) in MIS. Splenocytes from M3R knockout (M3R–/–) mice immunized with murine M3R peptide mixture were inoculated into recombination‐activating gene 1 knockout (Rag‐1–/–) mice (M3R–/–→Rag‐1–/–) with MIS. Immunized M3R–/– mice (pretransfer treatment) and M3R–/–→Rag‐1–/– mice (post‐transfer treatment) were treated with A213 every 3 days. Salivary volume, severity of sialadenitis and cytokine production from M3R peptide‐stimulated splenocytes and lymph node cells were examined. Effects of A213 on cytokine production were analysed by enzyme‐linked immunosorbent assay (ELISA) and on T helper type 1 (Th1), Th17 and Th2 differentiation from CD4+ T cells by flow cytometry. Pretransfer A213 treatment maintained salivary volume, improved MIS and reduced interferon (IFN)‐γ and interleukin (IL)‐17 production significantly compared with phosphate‐buffered saline (PBS) (P < 0·05). These suppressive effects involved CD4+ T cells rather than CD11c+ cells. Post‐transfer treatment with A213 increased salivary volume (P < 0·05), suppressed MIS (P < 0·005) and reduced IFN‐γ and IL‐17 production (P < 0·05). In vitro, A213 suppressed IFN‐γ and IL‐17 production from M3R‐stimulated splenocytes and CD4+ T cells of immunized M3R–/– mice (P < 0·05). In contrast with M3R specific responses, A213 suppressed only IL‐17 production from Th17 differentiated CD4+ T cells without any effect on Th1 and Th2 differentiation in vitro. Our findings suggested that RORγt antagonism is potentially suitable treatment strategy for SS‐like sialadenitis through suppression of IL‐17 and IFN‐γ production by M3R‐specific T cells.
Keywords: M3 muscarinic acetylcholine receptor, RORγt antagonist, sialadenitis, Sjögren's syndrome
Introduction
Sjögren's syndrome (SS) is an autoimmune disease in which both autoreactive CD4+ T cells and activated B cells play important pathogenic roles, and is characterized by lymphocytic infiltration into various organs including exocrine glands, such as salivary and lacrimal glands 1, 2. The clinical manifestations of SS are categorized into glandular (dry mouth and dry eyes) and extra‐glandular (other organ disease) involvement 1, 2. Although corticosteroids, immunosuppressants and certain biologicals, such as rituximab, are effective for extra‐glandular manifestations of SS, the usefulness of these drugs against glandular manifestations has not been established 3. At present, palliative therapies, such as eye drops and secretagogues, are used commonly for dryness of SS 3. Therefore, there is a need for new therapies for both the glandular and extra‐glandular manifestations of SS acting that are based on inhibition of specific pathological process of SS.
T helper cells that produce interleukin (IL)‐17 [T helper type 17 (Th17) cells] are implicated in the pathology of several autoimmune diseases, including SS, in addition to multiple sclerosis (MS) and rheumatoid arthritis (RA) 4, 5, 6. IL‐17 is known to enhance inflammatory processes. The discovery of Th17 cells as critical mediators of autoimmune disorders provided a unique opportunity to develop new therapeutic strategies that target inhibition of these cells. The nuclear receptor retinoic acid receptor‐related orphan receptor γt (RORγt) plays an indispensable role in the differentiation of Th17 cells 7, 8, 9, 10, and several synthetic ligands, which bind to and inhibit RORγt (RORγt antagonists), have been developed in recent years 11, 12. RORγt antagonism seems a promising therapeutic strategy against Th17‐mediated autoimmune disorders, acting by the suppression of differentiation and function of Th17 cells 11, 12.
We have established previously a new murine SS model, in which autoreactive CD3+ T cells against M3 muscarinic acetylcholine receptor (M3R) play critical roles in the development of sialadenitis (M3R‐induced sialadenitis; MIS)13. M3R is expressed in exocrine glands, including salivary and lacrimal glands, and plays crucial roles in secretion 14. Recent studies have focused on M3R as a candidate autoantigen in SS 15, 16, 17, 18, 19. Interestingly, both interferon (IFN)‐γ and IL‐17 are required for induction of SS‐like sialadenitis in MIS 20, 21, indicating that M3R‐reactive Th1 and Th17 cells contribute to the pathogenesis of autoimmune sialadenitis. Thus, MIS might be an ideal model to analyse the effectiveness of RORγt antagonists.
Preliminary data indicate that A213, a potent and selective antagonist of RORγt, inhibits Th17 cell differentiation from CD4+ T cells in vitro (Sano et al., personal communication). Moreover, A213 inhibited skin inflammation in the murine psoriasis model successfully by suppressing IL‐17 production and Th17 cells (Sano et al., personal communication). However, to the best of our knowledge, there was neither evidence concerning the treatment effect of A213 on patients with SS nor ongoing studies.
The present study is a critical assessment of RORγt antagonism as a new therapeutic strategy in SS. Specifically, we determined the effectiveness and mechanism(s) of action of A213 in MIS. We also investigated the suppressive effects of A213 on cytokine production from M3R‐reactive T cells. A213 was administered orally in one of two ways: immunized M3R–/– mice (pretransfer treatment) or M3R–/–→Rag‐1–/– mice (post‐transfer treatment) every 3 days. Pretransfer treatment with A213 for MIS had minor inhibitory effects on salivary function and sialadenitis. A213 suppressed both M3R‐reactive Th1‐producing IFN‐γ and Th17‐producing IL‐17 against M3R peptide stimulation between immunized M3R–/– mice treated with A213 and placebo. Post‐transfer treatment with A213 for MIS inhibited significantly the decrease in saliva secretion and sialadenitis through the suppression of both M3R‐reactive Th1 and Th17 cells. The results showed that A213 suppressed immune‐mediated pathology, including Th1 and Th17 cells both in vitro and in vivo, and inhibited salivary dysfunction and sialadenitis successfully in MIS through the suppression of IFN‐γ and IL‐17 production. The findings suggest that inhibition of RORγt is a potentially useful therapeutic strategy for SS through the induction of Th1 and Th17 cell‐based immune responses.
Materials and methods
Mice
C57BL/6J mice (M3R+/+) were purchased from Charles River Laboratory (Yokohama, Japan). Rag1–/– mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). M3R knockout mice (M3R–/–), generated from B6 mice, were kindly provided by Dr Matsui (Fureai Higashitotsuka Hospital, Kanagawa, Japan) 22. All mice were maintained under specific pathogen‐free conditions at the Laboratory Animal Resource Center. All experiments were performed according to the Guide for the Care and Use of Laboratory Animals at University of Tsukuba.
Synthesized peptides encoding M3R extracellular regions
Six types of peptides that encoded murine M3R extracellular domains – N‐terminus 1 (MTLHSNSTTSPLFPNISSSWVHSPSEAGLP, N1), N‐terminus 2 (VHSPSEAGLPLGTVSQLDSYNISGTSGNFS, N2), N‐terminus 3 (NISQTSGNFSSNDTSSDPLGGHTIWQV, N3), first extracellular loop (FTTYIIMNRWALGNLACDLW, 1st), second extracellular loop (QYFVGKRTVPPGECFIQFLSEP, second) and third extracellular loops (VLVNTFCDSCIPKTYWNLGY, third) – were synthesized chemically by a solid‐phase procedure and purified by high‐performance liquid chromatography (AnyGen, Gwangju, Korea).
Induction of M3R‐induced sialadenitis (MIS)
M3R–/– mice (male, aged 8–12 weeks) were immunized intradermally at the base of the tail with an emulsified mixture containing six M3R extracellular peptides (20 μg each), Freund's incomplete adjuvant (IFA) and inactivated Mycobacterium tuberculosis (250 μg). Furthermore, 500 ng of Pertussis toxin was injected intraperitoneally on the day of immunization. The same immunization with intradermal injection of the same emulsified mixture was repeated on day 10 after the first immunization. On day 20, splenocytes were isolated from the immunized M3R–/– mice and suspended in phosphate‐buffered saline (PBS). Then, 1·0 × 107 of these splenocytes were injected intravenously into the recipient adult Rag1–/– mice (male, aged 10–14 weeks) (M3R–/–→Rag‐1–/–). Analysis of Rag1–/– mice was conducted on day 45 after transfer (Fig. 1a).
Figure 1.
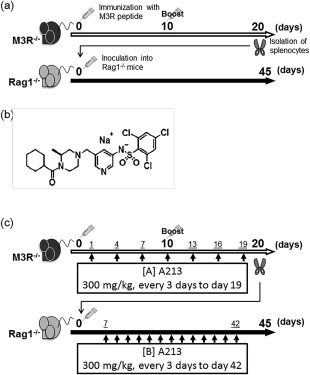
Protocol for induction of muscarinic acetylcholine receptor (M3R)‐induced murine autoimmune sialadenitis (MIS) and treatment with A213 for MIS. (a) M3R–/– mice were immunized with M3R peptide mixture on day 0. On day 10, each mouse was immunized with intradermal injection of the same mixture. On day 20, splenocytes were isolated from immunized M3R–/– mice and inoculated into recombination‐activating gene 1 (Rag‐1)–/– mice. At day 45 after the inoculation, Rag1–/– recipient mice (M3R–/–→Rag‐1–/–) were analysed. (b) Structure of A213 (kindly provided by Daiichi‐Sankyo Company). (c) A213 was dissolved in phosphate‐buffered saline (PBS) and administered orally at 300 mg/kg body weight every 3 days. The administration was started at day 1 after first immunization in immunized M3R–/– mice (protocol A, pretransfer treatment) and at day 7 after inoculation in M3R–/–→Rag‐1–/– mice (protocol B, post‐transfer treatment), and continued until days 19 and 42, respectively.
Treatment protocol with A213
A213 was kindly provided by Daiichi‐Sankyo Company. The chemical structure of A213 is shown in Fig. 1b. The compound was dissolved in PBS at 30 mg/ml, and immunized M3R–/– mice or M3R–/–→Rag‐1–/– mice received 300 mg/kg of A213 (10 μl/g body weight) or vehicle (PBS, 10 μl/g body weight of mice) orally every 3 days (Fig. 1c). Treatment commenced on day 1 after the first immunization (protocol A in Fig. 1c, pretransfer treatment) and on day 7 after intravenous injection of splenocytes into M3R–/–→Rag‐1–/– mice (protocol B in Fig. 1c, post‐transfer treatment), and continued until days 19 and 42, respectively.
Measurement of salivary volume
Mice were first anaesthetized with intraperitoneal injection of pentobarbital (1·0 mg/kg), then injected subcutaneously with pilocarpine (25 mg/kg). We collected saliva from the oral cavity over a period of 15 min using a 200 μl micropipette. The volume of the sample was measured and expressed relative to body weight. Changes in saliva volume were calculated relative to the volume measured at baseline, using the formula [day‐45 saliva volume (ml)/weight (g)]/[day‐0 saliva volume (ml)/weight (g)].
Histopathological analysis
Tissue specimens of salivary glands were embedded in optimal cutting temperature (OCT) compound (Sakura, Torrance, CA, USA) and snap‐frozen. For analysis, 4–5 μm tissue sections were stained with haematoxylin and eosin (H&E) by standard technique. The inflammatory lesions were graded histologically using the focus score (number of focuses per 4 mm2 of each section; one focus was defined as > 50 mononuclear cells accumulation around the salivary gland ducts). Histological evaluation was performed in a blinded manner.
Stimulation of splenocytes and lymph node cell cultures with M3R peptides
At day 45 after splenocyte transfer, splenocytes and cervical lymph nodes (cLN) were isolated from M3R–/–→Rag‐1–/– mice. These cells (2·0 × 105 cells/well) were cultured in RPMI‐1640 medium (Sigma‐Aldrich, St Louis, MO, USA) containing 10% fetal bovine serum (FBS), 100 units/ml of penicillin and 100 μg/ml of streptomycin, with or without mixture of six M3R extracellular peptides (5 μg/ml each) (M3R peptide mixture) in 96‐well round‐bottomed plates (Nunc, Rochester, NY, USA). After 72 h culture, IFN‐γ and IL‐17 concentrations in the culture supernatant were measured using the Duoset enzyme‐linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions.
Criss‐cross co‐cultures using CD4+ T cells and CD11c+ cells under stimulation with M3R peptide
To determine the suppressive effects of A213 on CD4+ T cells and CD11c+ cells at day 20 after the first immunization, the two types of cells were isolated from the spleen of immunized M3R–/– mice treated with A213 or PBS by magnetic activated cell sorting (MACS)‐positive selection using anti‐CD4 microbeads or anti‐CD11c microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). CD11c+ cells were treated with mitomycin C (50 μg/ml) and used as antigen‐presenting cells (APCs). CD4+ T cells and CD11c+ cells obtained from M3R–/– mice treated with A213 or PBS were criss‐cross co‐cultured in RPMI‐1640 medium under stimulation with M3R peptide mixture (5 μg/ml each) at a ratio of 5 : 1 (CD4 T cells: 1·5 × 105 cells/well, CD11c+ cells: 0·3 × 105 cells/well) in 96‐well round‐bottomed plates (Nunc). After 72 h of co‐culture, the amounts of IFN‐γ and IL‐17 were measured in the culture supernatants using the Duoset ELISA kit (R&D Systems).
In‐vitro functional analysis of A213 for M3R‐specific cytokine production
Splenocytes or CD4+ T cells obtained from immunized M3R–/– mice on day 20 after the first immunization were cultured with or without various concentrations of A213 (0·04, 0·2, 1 or 5 μM) under stimulation with M3R peptide mixture (5 μg/ml each). After 72 h of culture, the amounts of IFN‐γ and IL‐17 were measured in the culture supernatants using the Duoset ELISA kit (R&D Systems).
Effects of A213 on the induction of Th1, Th17 and Th2 cell differentiation in vitro
CD4+ cells obtained from the spleens of naive wild‐type (WT) mice were isolated by positive selection using the MACS system with anti‐CD4 mAb (Miltenyi Biotec). The CD4+ T cells were stimulated for 4 days with plate‐bound anti‐CD3 (2 μg/ml) and soluble anti‐CD28 (1 μg/ml) in the presence of recombinant mouse IL‐12 (rmIL‐12) (1 ng/ml; BioLegend, San Diego, CA, USA) and anti‐mouse IL‐4 antibody (10 μg/ml; BioLegend) (induction of Th1 cells) or rmIL‐6 (30 ng/ml; eBioscience, San Diego, CA, USA), recombinant human transforming growth factor (TGF)‐β1 (rhTGF‐β1) (1 ng/ml; R&D Systems), anti‐mouse IFN‐γ antibody (10 μg/ml, BioLegend) and anti‐mouse IL‐4 antibody (10 μg/ml) (induction of Th17 cells) in complete RPMI‐1640 medium containing 10% FBS, 100 units/ml of penicillin and 100 μg/ml of streptomycin. Moreover, the CD4+ T cells were stimulated for 3 days with plate‐bound anti‐CD3 (2 μg/ml) and soluble anti‐CD28 (1 μg/ml) in the presence of rmIL‐4 (50 ng/ml; BioLegend), rhIL‐2 (100 U/ml; Biovision, Milpitas, CA, USA) and anti‐mouse IFN‐γ antibody (10 μg/ml; BioLegend) in complete RPMI‐1640 medium containing 10% FBS, 100 units/ml of penicillin and 100 μg/ml of streptomycin, and then half the medium was changed to that containing rmIL‐4 (50 ng/ml; BioLegend), rhIL‐2 (100 U/ml; Biovision) and anti‐mouse IFN‐γ antibody (10 μg/ml; BioLegend) for 3 more days' culture (induction of Th2 cells). GolgiStop (BD Pharmingen, San Diego, CA, USA), Phorbol myristate acetate (PMA) and ionomycin were added during the last 4 h of each culture. The regulatory T cell (Treg) cell staining kit (eBioscience) was used to stain Th1 and Th17 cells according to the protocol provided by the manufacturer using anti‐RORγt‐APC (eBioscience), anti‐T‐bet‐phycoerythrin (PE) (eBioscience), anti‐IL‐17‐PE‐cyanin 7 (Cy7) (BioLegend) and anti‐IFN‐γ‐fluorescein isothiocyanate (FITC) (BioLegend). Th2 cells were stained with anti‐GATA binding protein 3 (GATA3)‐PE (eBioscience) and anti‐IL‐4‐APC (BioLegend). Dead cells were stained with fixable viability dye eFluor 780 (eBioscience) for the assessment of cell viability. Effects of A213 on cell viability, expression of transcriptional factors and cytokine production of CD4+ cells in each Th1, Th17 and Th2 condition were examined in various concentrations (0, 0·001, 0·003, 0·01, 0·03, 0·1, 0·3, 1, 3 μM). Samples were analysed with BD fluorescence activated cell sorter (FACS)Verse (BD Biosciences), and data were analysed with FlowJo software (Tree Star, Ashland, OR, USA).
Statistical analysis
Data are expressed at mean ± standard error of the mean (s.e.m.) or mean ± standard deviation (s.d.). Differences between groups were examined for statistical significance using Student's t‐test or Kruskal–Wallis test. P‐values less than 0·05 were considered significant.
Results
Pretransfer treatment with A213
Pretransfer A213 treatment of MIS mice maintained salivary secretion at day 45 after transfer, relative to day 0 (Fig. 2a), but there was no significant difference in saliva secretion or body weight between the A213‐ and PBS‐treated groups (Fig. 2a). Mononuclear cell infiltration in salivary glands was observed in both MIS pretransfer treated with A213 and PBS (Fig. 2b). The focus score of sialadenitis in MIS pretransfer treated with A213 tended to be lower than in those treated with PBS, albeit insignificantly (Fig. 2c). M3R‐specific IFN‐γ and IL‐17 production by splenocytes and LN cells from M3R–/–→Rag‐1–/– mice pretransfer treated with A213 were similar to those treated with PBS (Fig. 2d,e). These findings suggest that pretransfer treatment with A213 had no effects on salivary function and sialadenitis in mice with MIS.
Figure 2.
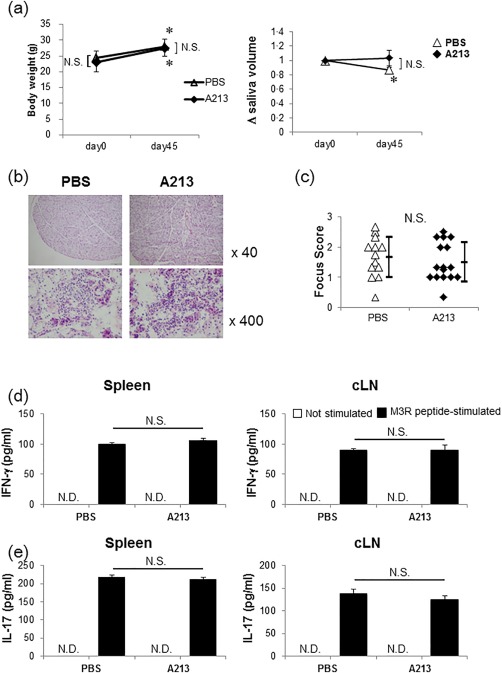
Effects of pretransfer treatment with A213 in murine autoimmune sialadenitis (MIS). (a) Saliva was collected from recombination‐activating gene 1 (Rag‐1)–/– mice after adoptive transfer. The saliva volume was measured on days 0 and 45 after transfer, adjusted for body weight and calculated relative to the volume measured at baseline [A213‐treated mice (n = 11) and phosphate‐buffered saline (PBS)‐treated mice (n = 8)]. Statistical significance was determined using Student's t‐test. *P < 0·05 (day 45 after adoptive transfer versus day 0), not significant (n.s.) between A213‐ and PBS‐treated mice at days 0 and 45. (b) Comparison of haematoxylin and eosin (H&E)‐stained salivary gland sections of muscarinic acetylcholine receptor (M3R)–/–→Rag‐1–/– mice pretransfer treated with A213 and PBS on day 45. Representative images obtained from 13 to 16 mice per group. (c) Histological focus score of inflammatory lesions in salivary glands between M3R–/–→Rag‐1–/– mice pretransfer treated with A213 and PBS on day 45. The focus score was assessed in a blinded manner. Data are mean ± standard deviation (s.d.) of 13–16 mice per group. Statistical significance was determined using Student's t‐test; n.s. between A213‐ and PBS‐treated mice. (d,e) Splenocytes (left panel) and cervical lymph node (cLN) cells (right panel) obtained from M3R–/–→Rag‐1–/– mice on day 45 pretransfer treated with A213 or PBS were cultured with or without M3R peptide mixture. Interferon (IFN)‐γ (d) and interleukin (IL)‐17 (e) levels in culture supernatants were analysed by enzyme‐linked immunosorbent assay (ELISA). Data are mean ± standard error of the mean (s.e.m.) of six mice per group; n.s. (Student's t‐test); not detected (n.d.).
Pretransfer treatment with A213 inhibits cytokine production from M3R‐reactive CD4+ T cells
We compared cytokine production by splenocytes in response to stimulation with M3R peptides between immunized M3R–/– mice treated with A213 and PBS. IL‐17 production from splenocytes stimulated with M3R peptides was significantly lower in immunized M3R–/– mice treated with A213 compared with PBS‐treated mice (P < 0·05) (Fig. 3a, right panel). Similarly, IFN‐γ production against M3R peptides stimulation was also significantly lower in A213‐treated mice than PBS‐treated mice (P < 0·05) (Fig. 3a, left panel).
Figure 3.
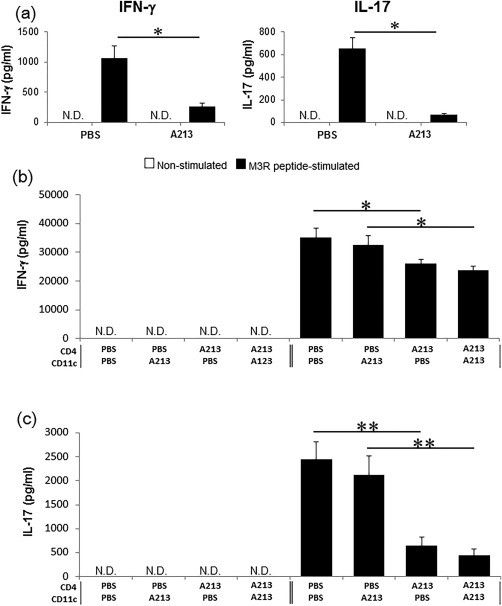
Pretransfer treatment with A213 suppresses production of various cytokines by muscarinic acetylcholine receptor (M3R)‐reactive CD4+ T cells. (a) Comparison of interferon (IFN)‐γ and interleukin (IL)‐17 production from splenocytes treated with M3R peptides obtained from immunized M3R–/– mice treated with A213 and phosphate‐buffered saline (PBS). Data are mean ± standard error of the mean (s.e.m.) of three mice per group (representative data of two independent experiments). *P < 0·05 (Student's t‐test) not detected (n.d.). (b,c) Criss‐cross co‐culture assay using CD4+ T cells and CD11c+ cells (at a ratio of 5 : 1) obtained from immunized M3R–/– mice treated with A213 or PBS under stimulation with M3R peptides. After 72 h of co‐culture, IFN‐γ (b) and IL‐17 (c) levels in the culture supernatants were analysed by enzyme‐linked immunosorbent assay (ELISA). Data are mean ± s.e.m. of three mice per group (representative data of two independent experiments). *P < 0·05; **P < 0·005 (Student's t‐test) not detected (n.d.).
In the next step, we performed criss‐cross co‐culture assays using CD4+ T cells and CD11c+ cells obtained from immunized M3R–/– mice treated with A213 or PBS under stimulation with M3R peptides to clarify whether the suppressive action of A213 on cytokine production was based on CD4+ T cells or CD11c+ cells. M3R‐specific production of both IFN‐γ and IL‐17 from CD4+ T cells was significantly less in A213‐treated M3R–/– mice compared with PBS‐treated mice (P < 0·05), regardless of CD11c+ cell status (Fig. 3b,c). These results suggest that A213 suppresses both M3R‐reactive Th1 and Th17 cells rather than CD11c+ cells.
Post‐transfer treatment with A213
Saliva secretion was examined before and after post‐transfer treatment with A213 (Fig. 1c, protocol B, post‐transfer treatment). The secretion was conserved significantly at day 45 post‐transfer compared with PBS (P < 0·05, Fig. 4a). Infiltration of mononuclear cells was observed in salivary glands in both MIS post‐transfer treated with A213 and PBS (Fig. 4b). The sialadenitis focus score was significantly lower in post‐transfer A213‐treated MIS compared with PBS (P < 0·05) (Fig. 4c). M3R‐specific IFN‐γ and IL‐17 production levels by splenocytes and lymph node cells from post‐transfer A213‐treated M3R–/–→Rag‐1–/– mice were suppressed significantly compared with PBS‐treated mice (P < 0·05, Fig. 4d,e). These findings suggest that post‐transfer treatment of MIS mice with A213 abrogated the decrease in salivary secretion and sialadenitis via suppression of both M3R‐reactive Th1 and Th17 cells.
Figure 4.
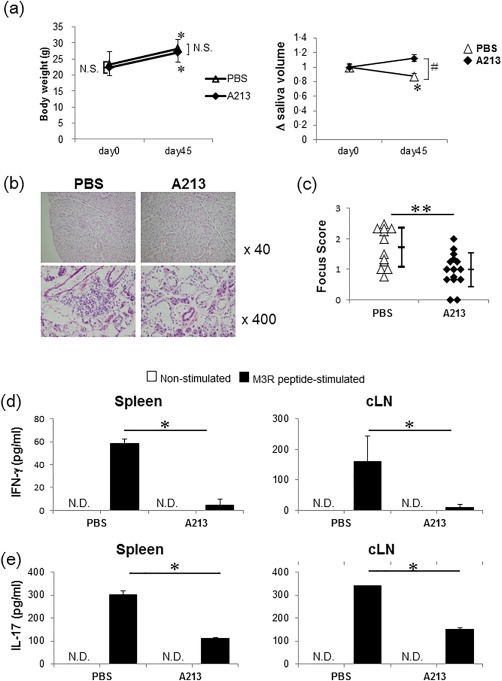
Post‐transfer treatment with A213 for murine autoimmune sialadenitis (MIS). (a) Saliva was collected from recombination‐activating gene 1 (Rag‐1)–/– mice after adoptive transfer. The saliva volume was measured on days 0 and 45, adjusted for body weight, and calculated relative to the volume measured at baseline. [A213‐ and phosphate‐buffered saline (PBS)‐treated mice (each, n=15)]. Statistical significance was determined by Student's t‐test. # P < 0·05 (A213 versus PBS at day 45 after adoptive transfer), *P < 0·05 (day 45 versus day 0 after adoptive transfer). (b) Comparison of haematoxylin and eosin (H&E)‐stained salivary gland sections of muscarinic acetylcholine receptor (M3R)–/–→Rag‐1–/– mice post‐transfer treated with A213 and PBS on day 45. Representative images obtained from 12 to 14 mice per group. (c) Comparison of histological focus score of inflammatory lesions in salivary glands of M3R–/–→Rag‐1–/– mice post‐transfer treated with A213 and PBS on day 45. The focus score was assessed in a blinded manner. Data are mean ± standard deviation (s.d.) of 12–14 mice per group. **P < 0·005 (Student's t‐test). (d,e) Splenocytes (left panel) and cervical lymph node (cLN) cells (right panel) obtained from M3R–/–→Rag‐1–/– mice on day 45 post‐transfer treated with A213 or PBS were cultured with or without M3R peptide mixture. Interferon (IFN)‐γ (d) and interleukin (IL)‐17 (e) levels in culture supernatants were analysed by enzyme‐linked immunosorbent assay (ELISA). Data are mean ± standard error of the mean (s.e.m.) of six mice per group. *P < 0·05 (Student's t‐test) not detected (n.d.).
Effects of A213 on M3R‐specific cytokines production and induction of Th1, Th17 and Th2 cell differentiation in vitro
A213 suppressed M3R‐specific IL‐17 production significantly from splenocytes obtained from immunized M3R–/– mice in vitro (P < 0·05, Fig. 5a, right panel). Interestingly, A213 also suppressed M3R‐specific IFN‐γ production significantly (P < 0·05, Fig. 5a, left panel), similar to its effects in vivo. To test whether the inhibitory effects of A213 on cytokine production and cell differentiation are dose‐dependent, cultured splenocytes and CD4+ T cells from immunized M3R–/– mice under M3R peptide stimulation were treated with different concentrations of A213 (0, 0·04, 0·2, 1 or 5 μM). The results showed that A213 repressed IL‐17 production in a dose‐dependent manner (Fig. 5b,c, right panel), whereas IFN‐γ production under M3R peptides stimulation was decreased significantly only by the highest concentration of A213 (5 μM) (Fig. 5b,c, left panel).
Figure 5.
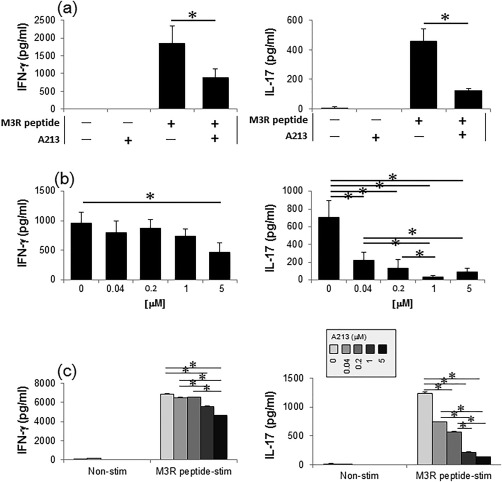
Effects of A213 on production of muscarinic acetylcholine receptor (M3R)‐specific cytokines in vitro. (a,b) Splenocytes from immunized M3R–/– mice obtained on day 20 after the first immunization were cultured without and with different concentrations of A213 [(a) 5 μM, (b) 0·04, 0·2, 1 or 5 μM] under stimulation with M3R peptide mixture (5 μg/ml each). After 72 h of culture, interferon (IFN)‐γ and interferon (IL)‐17 levels in culture supernatants were measured by enzyme‐linked immunosorbent assay (ELISA). Data are mean ± standard error of the mean (s.e.m.) of three mice per group (representative data of two independent experiments). *P < 0·05 [(a) Student's t‐test, (b) Kruskal–Wallis test)]. (c) Effects of A213 at different doses on co‐culture assay using CD4+ T cells and mitomycin C‐treated CD11c+ cells (at a ratio of 5 : 1) obtained from immunized M3R–/– mice and stimulated with M3R peptides. After 72 h of co‐culture, IFN‐γ and IL‐17 levels in culture supernatants were analysed by enzyme‐linked immunosorbent assay (ELISA). Data are mean ± s.e.m. of three mice per group (representative data of two independent experiments). *P < 0·05 (Kruskal–Wallis test).
In addition, CD4+ T cells isolated from naive WT mice were cultured in the presence of IL‐12 and anti‐IL‐4 monoclonal antibodies (mAb) (for induction of Th1 cells), IL‐6, TGF‐β, anti‐IL‐4 mAb and anti‐IFN‐γ mAb (for induction of Th17 cells), IL‐4, IL‐2 and anti‐IFN‐γ mAb (for induction of Th2 cells) with various concentrations of A213 (0, 0·001, 0·003, 0·01, 0·03, 0·1, 0·3, 1, 3 μM). In Th1 and Th2 conditions, cell viability, T‐bet and GATA3 expression levels, and IFN‐γ and IL‐4 production was not affected, even at high concentrations of A213 (Fig. 6a,c). Conversely, IL‐17 expression from Th17‐differentiated CD4+ T cells were clearly suppressed by A213 in a dose‐dependent manner (Fig. 6b, right panel), while cell viability and RORγt expression levels were not affected by A213 at any concentration (Fig. 6b, left and middle panels). These results suggested that A213 suppressed not only M3R‐reactive Th17 cells but also M3R‐reactive Th1 cells at high concentrations, whereas A213 suppressed only IL‐17 production from Th17‐differentiated CD4+ T cells and A213 did not affect Th1 and Th2 differentiation in vitro.
Figure 6.
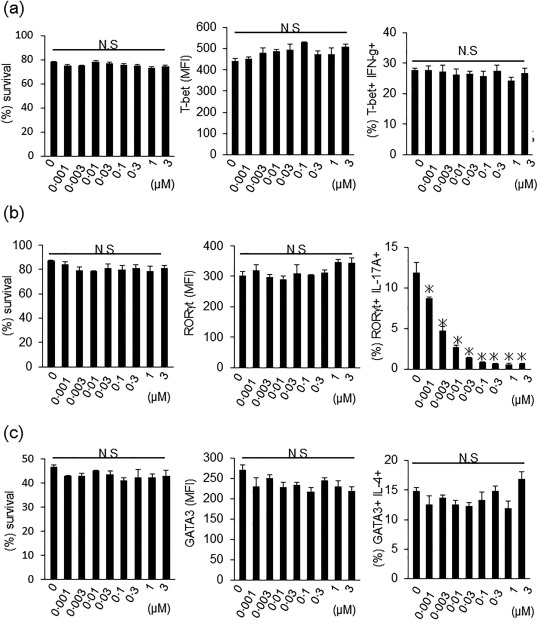
Effects of A213 on the induction of T helper type 1 (Th1), Th17 and Th2 cell differentiation in vitro. (a) CD4+ T cells from naive wild‐type mice were cultured with interleukin (IL)‐12 and anti‐IL‐4 [T helper type 1 (Th1)‐promoting condition]. Four days later, live T cells, expression levels of T‐bet and interferon (IFN)‐γ were analysed by flow cytometry. Data are mean ± standard error of the mean (s.e.m.) of three mice per group (representative data of two independent experiments). *P < 0·05 (Kruskal–Wallis test). (b) CD4+ T cells were cultured with IL‐6, transforming growth factor (TGF)‐β, anti‐IL‐4 and anti‐IFN‐γ (Th17‐promoting condition). Four days later, live T cells, expression levels of retinoic acid‐related orphan receptor‐gamma t (RORγt) and IL‐17 were analysed by flow cytometry. Data are mean ± s.e.m. of three mice per group (representative data of two independent experiments). *P < 0·05 (Kruskal–Wallis test). (c) CD4+ T cells were cultured with IL‐4, IL‐2 and anti‐IFN‐γ (Th2 promoting condition). Six days later, live T cells, expression levels of GATA binding protein 3 (GATA3) and IL‐4 were analysed by flow cytometry. Data are mean ± s.e.m. of three mice per group (representative data of two independent experiments). *P < 0·05 (Kruskal–Wallis test).
Discussion
Due to the known roles of RORγt in autoimmunity, we designed the present study to test whether RORγt targeting is a suitable therapeutic option for the treatment of SS 6. The results showed the efficacy of RORγt‐specific antagonist, A213, in MIS. In a series of studies, Iizuka et al. 20, 21 demonstrated that M3R‐reactive Th17 and Th1 cells contributed to the pathogenesis of autoimmune sialadenitis, highlighting the potential roles of IL‐17 and IFN‐γ in the pathogenesis of SS. Furthermore, IL‐17 and IFN‐γ deficiencies in M3R–/–→Rag‐1–/– mice reduced sialadenitis and improved salivary dysfunction 20, 21. Th17 cells are linked to a number of organ‐specific autoimmune diseases, including psoriasis, and as Sano et al. described the utility of A213 in a murine psoriasis model (personal communication), we felt that this approach was feasible to address specifically the roles of Th17 cells in SS. A213 suppressed immune‐mediated pathology, including Th1 and Th17 cells both in vitro and in vivo, and inhibited salivary dysfunction and sialadenitis in MIS successfully through the suppression of IFN‐γ and IL‐17 production. Our data are consistent with these observations, as we demonstrated that post‐transfer treatment with A213 reduced the severity of sialadenitis. In contrast to post‐transfer treatment with A213, pretransfer treatment with A213 could not suppress sialadenitis in MIS significantly. Both M3R‐reactive Th1 and Th17 cells, which contribute essentially to the induction of MIS in transferred Rag‐1–/– mice, could be generated by immunization of M3R–/– mice with M3R peptide mixtures, as described in our previous reports 13, 20, 21. Thus, we consider that these M3R‐reactive Th1 and Th17 cells could be the target population in this pretransfer treatment with A213. We speculated that suppressed M3R‐reactive Th1 and Th17 cells by pretransfer treatment with A213 could be reactivated after transfer into Rag‐1–/– mice in which M3R antigens existed. In fact, M3R‐specific IFN‐γ and IL‐17 production by splenocytes and LN cells from M3R–/–→Rag‐1–/– mice pretransfer treated with A213 were similar to those treated with PBS.
What is the mechanism of suppressive action of A213 on IFN‐γ expression on M3R‐reactive CD4+ T cells? Although our results did not identify the exact mechanism of low IFN‐γ concentrations, we propose the following three possibilities. First, it is conceivable that A213 inhibits the conversion of Th17 cells into Th1 cells. Th17 cells transform into Th1 cells in the presence of inflammation, including C57BL/6J mice 23. As reported by Lee et al. 24, inoculation of Th17 cells into Rag‐1–/– mice induced severe colitis. A large proportion of transferred cells lost the expression of IL‐17 and became IFN‐γ producers, demonstrating the plasticity of the Th17 lineage in vivo. Consistent with the in‐vivo findings, Th17 cells either retained high expression of IL‐17 or expressed variable levels of IL‐17 and IFN‐γ in vitro 24, 25. IFN‐γ‐ and IL‐17‐producing double‐positive cells have also been identified in primary SS 26. We did not see any substantial inhibition of transformation in vitro using A213. However, this is not surprising, because the in‐vitro conditions rarely mimic the true in‐vivo inflammatory conditions, and as‐yet unknown factors may also be involved in this process (data not shown). Secondly, the reduced IFN‐γ expression on Th1 cells might be RORγt‐dependent, indicating the indirect effects of RORγt on IFN‐γ expression. Considering that RORγt is expressed ubiquitously throughout the body, it is possible that A213 directly affected the development of Th1 and Th17 cells. RORγt is expressed in innate lymphoid cells, and inhibition of RORγt expression could result in decreased cytokine expression in vivo 27, 28, 29, 30. Thirdly, our results revealed that A213 suppressed not only M3R‐reactive Th17 cells but also M3R‐reactive Th1 cells at high concentrations, whereas A213 suppressed only IL‐17 production from Th17‐differentiated CD4+ T cells and A213 did not affect Th1 and Th2 differentiation in vitro. Thus, it is indicated that A213 inhibits both antigen‐specific Th17 and Th1 responses in vivo and especially at high concentrations in vitro, notwithstanding that A213 suppresses IL‐17 production specifically from Th17‐differentiated CD4+ T cells in vitro, without any effect on cell viability, expression levels of transcriptional factors such as RORγt, T‐bet and GATA3 of Th17‐, Th1‐ and Th2‐differentiated CD4+ T cells and IFN‐γ from Th1‐differentiated CD4+ T cells and IL‐4 from Th2‐differentiated CD4+ T cells. Collectively, A213 might represent different modes of action between antigen‐specific processes in vivo and vitro, and cytokine‐dependent T cell differentiation in vitro. Although the above scenarios should be investigated in more detail in future, none of these factors alone seems sufficient to explain the low IFN‐γ level in post‐transfer‐treated mice, because no significant difference was observed in IFN‐γ concentration in pretransfer‐treated mice.
These data suggest that targeting RORγt provided more protection than merely targeting a single cytokine. Support for this hypothesis comes from treatment using corticosteroids 3, 31, 32, 33, 34, 35, 36, conventional immunosuppressants 3, 31, 32, 33, 34, 35, 36 and certain biologicals, such as rituximab 3, 31, 32, 33, 34, 35, 36, 37, 38. These drugs are effective for extra‐glandular manifestations of SS, but their usefulness against glandular manifestations has not been established. In fact, the use of corticosteroids and conventional immunosuppressants is associated with several well‐known adverse effects, such as pneumonia, meningitis, fever, skin itching or rash 3, 31, 32, 33, 34, 35, 36. A213 has a clear treatment target, RORγt, and had no obvious adverse effects, such as weight reduction, in pre‐ or post‐transfer‐treated mice (data not shown). These findings suggest that A213 can be potentially useful in the treatment of SS. Treatment with biologicals also imposes a great burden on patients in relation to the need for intravenous drip and subcutaneous injection 3, 31, 32, 33, 34, 35, 36. A213 was extremely effective when administered orally in post‐transfer‐treated mice, suggesting that it may be more convenient for administration compared with biologicals.
In conclusion, we have demonstrated that regulation of RORγt activity improves MIS outcome, suggesting that small‐molecule inhibitors may prove beneficial for treatment of sialadenitis, as they target many arms of SS pathogenesis.
Disclosure
None.
Acknowledgements
A213 was kindly provided by Daiichi Sankyo. We thank Dr F. G. Issa for the critical reading of the manuscript. This work was supported by a Research Grant from Daiichi Sankyo and the Research Program for Intractable Diseases, Health and Labor Sciences Research Grants from the Ministry of Health, Labor and Welfare, Japan, and the Ministry of Education, Culture, Sports, Science and Technology.
References
- 1. Skopouli FN, Dafni U, Ioannidis JP, Moutsopoulos HM. Clinical evolution, and morbidity and mortality of primary Sjögren's syndrome. Semin Arthritis Rheum 2000; 29:296–304. [DOI] [PubMed] [Google Scholar]
- 2. Malladi AS, Sack KE, Shiboski SC et al Primary Sjögren's syndrome as a systemic disease: a study of participants enrolled in an international Sjögren's syndrome registry. Arthritis Care Res (Hoboken) 2012; 64:911–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramos‐Casals M, Brito‐Zerón P, Sisó‐Almirall A, Bosch X, Tzioufas AG. Topical and systemic medications for the treatment of primary Sjogren's syndrome. Nat Rev Rheumatol 2012; 8:399–411. [DOI] [PubMed] [Google Scholar]
- 4. Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen‐induced arthritis in IL‐17‐deficient mice. J Immunol 2003; 171:6173–7. [DOI] [PubMed] [Google Scholar]
- 5. Jetten AM. Retinoid‐related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal 2009; 7:e003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iizuka M, Tsuboi H, Matsuo N et al A crucial role of RORγt in the development of spontaneous sialadenitis‐like Sjögren's syndrome. J Immunol 2015; 194:56–67. [DOI] [PubMed] [Google Scholar]
- 7. Yang XO, Pappu BP, Nurieva R et al T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity 2008; 28:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ivanov II, McKenzie BS, Zhou L et al The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL‐17+ T helper cells. Cell 2006; 126:1121–33. [DOI] [PubMed] [Google Scholar]
- 9. Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol 2007; 19:409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manel N, Unutmaz D, Littman DR. The differentiation of human TH‐17 cells requires transforming growth factor‐b and induction of the nuclear receptor RORγt. Nat Immunol 2008; 9:641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Solt LA, Banerjee S, Campbell S, Kamenecka TM, Burris TP. ROR inverse agonist suppresses insulitis and prevents hyperglycemia in a mouse model of type 1 diabetes. Endocrinology 2015; 156:869–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang MR, Lyda B, Kamenecka TM, Griffin PR. Pharmacologic repression of retinoic acid receptor‐related orphan nuclear receptor γ is therapeutic in the collagen‐induced arthritis experimental model. Arthritis Rheumatol 2014; 66:579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iizuka M, Wakamatsu E, Tsuboi H et al Pathogenic role of immune response to M3 muscarinic acetylcholine receptor in Sjögren's syndrome‐like sialoadenitis. J Autoimmun 2010; 35:383–9. [DOI] [PubMed] [Google Scholar]
- 14. Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci 2007; 133:3–18. [DOI] [PubMed] [Google Scholar]
- 15. Naito Y, Matsumoto I, Wakamatsu E et al Muscarinic acetylcholine receptor autoantibodies in patients with Sjögren's syndrome. Ann Rheum Dis 2005; 64:510–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bacman S, Berra A, Sterin‐Borda L, Borda E. Muscarinic acetylcholine receptor antibodies as a new marker of dry eye Sjögren's syndrome. Invest Ophthalmol Vis Sci 2001; 42:321–7. [PubMed] [Google Scholar]
- 17. Nakamura Y, Wakamatsu E, Matsumoto I et al High prevalence of autoantibodies to muscarinic‐3 acetylcholine receptor in patients with juvenile onset Sjögren syndrome. Ann Rheum Dis 2008; 67:136–7. [DOI] [PubMed] [Google Scholar]
- 18. Tsuboi H, Matsumoto I, Wakamatsu E et al New epitopes and function of anti‐M3 muscarinic acetylcholine receptor antibodies in patients with Sjögren's syndrome. Clin Exp Immunol 2010; 162:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naito Y, Matsumoto I, Wakamatsu E et al Altered peptide ligands regulate muscarinic acetylcholine receptor reactive T cells of patients with Sjögren's syndrome. Ann Rheum Dis 2006; 65:269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iizuka M, Tsuboi H, Matsuo N et al The crucial roles of IFN‐γ in the development of M3 muscarinic acetylcholine receptor induced Sjögren's syndrome‐like sialadenitis. Mod Rheumatol 2013; 23:614–6. [DOI] [PubMed] [Google Scholar]
- 21. Iizuka M, Tsuboi H, Asashima H et al M3 muscarinic acetylcholine receptor reactive IL‐17 producing T cells promotes development of Sjögren's syndrome like sialadenitis. Mod Rheumatol 2015; 25:158–60. [DOI] [PubMed] [Google Scholar]
- 22. Matsui M, Motomura D, Karasawa H et al Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci U S A 2000; 97:9579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lexberg MH, Taubner A, Albrecht I et al IFN‐g and IL‐12 synergize to convert in vivo generated Th17 into Th1 Th17 cells. Eur J Immunol 2010; 40:3017–27. [DOI] [PubMed] [Google Scholar]
- 24. Lee YK, Turner H, Maynard CL et al Late developmental plasticity in the T helper 17 linage. Immunity 2009; 30:92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lexberg MH, Taubner A, Förster A et al Th memory for interleukin‐17 expression is stable in vivo . Eur J Immunol 2008; 38:2654–64. [DOI] [PubMed] [Google Scholar]
- 26. Sudzius G, Mieliauskaite D, Butrimiene I, Siaurys A, Mackiewicz Z, Dumalakiene I. Activity of T‐helper cells on patients with primary Sjögren's syndrome. In Vivo 2013; 27:263–8. [PubMed] [Google Scholar]
- 27. Maloy KJ, Uhlig HH. ILC1 populations join the border patrol. Immunity 2013; 38:630–2. [DOI] [PubMed] [Google Scholar]
- 28. Klose CS, Flach M, Möhle L et al Differentiation of type 1 ILCs from a common progenitor to all helper‐like innate lymphoid cell lineages. Cell 2014; 157:340–56. [DOI] [PubMed] [Google Scholar]
- 29. Fuchs A, Vermi W, Lee JS et al Intraepithelial type 1 innate lymphoid cells are a unique subset of IL‐12‐ and IL‐15‐responsive IFN‐γ‐producing cells. Immunity 2013; 38:769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Romera‐Hernandez M, Aparicio‐Domingo P, Cupedo T. Damage control: Rorγt+ innate lymphoid cells in tissue regeneration. Curr Opin Immunol 2013; 25:156–60. [DOI] [PubMed] [Google Scholar]
- 31. Tsuboi H, Matsumoto I, Hagiwara S et al Efficacy and safety of abatacept for patients with Sjögren's syndrome associated with rheumatoid arthritis: rheumatoid arthritis with Orencia trial toward Sjögren's syndrome endocrinopathy (ROSE) trial‐an open‐label, one‐year, prospective study –interim analysis of 32 patients for 24 weeks. Mod Rheumatol 2015; 25:187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dass S, Bowman SJ, Vital EM et al Reduction of fatigue in Sjögren syndrome with rituximab: results of a randomized, double‐blind, placebo‐controlled pilot study. Ann Rheum Dis 2008; 67:1541–4. [DOI] [PubMed] [Google Scholar]
- 33. Gottenberg JE, Cinquetti G, Larroche C et al Efficacy of rituximab in systemic manifestations of primary Sjögren's syndrome: results in 78 patients of the AutoImmune and Rituximab registry. Ann Rheum Dis 2013; 72:1026–31. [DOI] [PubMed] [Google Scholar]
- 34. Jiang B, Li T, Guo L, Shen H, Ye S, Chen S. Efficacy and safety of rituximab in systemic lupus erythematosus and Sjogren syndrome patients with refractory thrombocytopenia: a retrospective study of 21 cases. J Clin Rheumatol 2015; 23:244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Devauchelle‐Pensec V, Mariette X, Jousse‐Joulin S et al Treatment of primary Sjögren syndrome with rituximab: a randomized trial. Ann Intern Med 2014; 160:233–42. [DOI] [PubMed] [Google Scholar]
- 36. Faustman DL, Vivino FB, Carsons SE. Treatment of primary Sjögren syndrome with rituximab. Ann Intern Med 2014; 161:376–7. [DOI] [PubMed] [Google Scholar]
- 37. Einfeld DA, Brown JP, Valentine MA, Clark EA, Ledbetter JA. Molecular cloning of the human B cell CD20 receptor predicts a hydrophobic protein with multiple transmembrane domains. EMBO J 1988; 7:711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Valentine MA, Meier KE, Rossie S, Clark EA. Phosphorylation of the CD20 phosphoprotein in resting B lymphocytes. Regulation by protein kinase C. J Biol Chem 1989; 264:11282–7. [PubMed] [Google Scholar]


