Abstract
Introduction
The aim of the present study was to determine the actual state of inter‐day glycemic variability and identify the factors that affect glycemic variability in diabetic outpatients on insulin therapy.
Materials and Methods
The participants were 45 outpatients with diabetes mellitus receiving insulin therapy. The mean plasma glucose (MPG) levels, intra‐day glycemic variability (expressed by standard deviation and mean amplitude of glucose excursion) and inter‐day glycemic variability (expressed by mean of daily differences [MODD] in blood glucose levels) were measured continuously over 7 days with iPro2®. The primary outcome was the relationship between MODD and the life variability index.
Results
MODD values were high in 93.3% of the participants, and significantly higher in patients with lifestyle changes than in those without (higher in patients with high life variability index). MODD values were not associated with age, but significantly higher in women. MODD values correlated significantly with glycated hemoglobin and glycoalbumin levels, and negatively with 1,5‐anhydroglucitol levels. MODD values were significantly higher in type 1 diabetes patients and not associated with duration of disease. MODD values correlated significantly with insulin dose. Multivariate analysis identified the life variability index as a significant determinant of MODD.
Conclusions
iPro2® provided detailed information on glycemic profile in diabetic outpatients receiving insulin therapy. The results suggest that patients with large inter‐day glycemic variability are unlikely to achieve an improvement in their glycated hemoglobin level. Treatment and instructions based on a patient's characteristics, day‐to‐day glycemic variability and lifestyle are important to achieve good glycemic control.
Keywords: Diabetes mellitus, Lifestyle, Mean of daily difference
Introduction
Intensive insulin therapy simulates physiological insulin secretion, and is effective in preventing the development and progression of diabetic complications in type 1 diabetes patients in the Diabetes Control and Complications Trial1 and the Epidemiology of Diabetes Interventions and Complications2, as well as in type 2 diabetes patients in the Kumamoto Study3. Although the number of insulin‐treated patients is increasing, many patients cannot achieve their glycated hemoglobin (HbA1c) targets despite insulin therapy. The results from the Japan Diabetes Clinical Data Management Study Group showed that the mean HbA1c level was 7.6% in type 1 diabetes patients, and among insulin‐treated type 2 diabetes, less than 40% achieved HbA1c level below 7%.
In daily clinical practice, the types and doses of insulin are adjusted based on the results of self‐monitoring of blood glucose. However, daily blood glucose profiles are often unstable, and an intensive insulin adjustment is not provided in many clinical settings. The main causes for failure to provide intensive insulin adjustment are differences in blood glucose profiles associated with the use of various types and classes of antidiabetic agents, and a wide range of age and lifestyle variations among diabetic patients. There are few reports that have objectively shown that these could be the reasons for difficult glycemic control.
Blood glucose profile is useful in planning and assessing treatment for diabetes mellitus. Blood glucose level can be measured continuously using a 24‐h continuous glucose monitoring (CGM) system. CGM can detect glucose variability, which cannot be identified by conventional methods, and provides a true profile of blood glucose in diabetic patients. Among the various CGM systems, iPro2® (Medtronic Inc, Minneapolis, MN, USA) can be used for 7 days to record a complete blood glucose profile in near‐normal life settings.
In the present study, we selected the ‘mean of daily difference’ (MODD) in blood glucose as an indicator of inter‐day glucose variability. MODD represented the mean of the absolute difference between glucose values measured on two successive days, as described previously4. A high MODD value represents a major difference in day‐to‐day glycemic profile. MODD is also used to assess the degree of inter‐day glycemic variability. In the present study, MODD values were evaluated in diabetic outpatients receiving insulin therapy under daily clinical settings using iPro2®, and analyzed the factors that influence MODD values.
Materials and Methods
The present study was a cross‐sectional study that encompassed the recruitment of patients who used iPro2 for 7 days among the diabetic outpatients treated with insulin from July 2012 to July 2013 at Wakamatsu Hospital of the University of Occupational and Environmental Health, Kitakyushu‐shi, Japan. The following exclusion criteria were used for the present study: patients who did not use 24‐h CGM for seven consecutive days; those with infection, ketoacidosis and non‐ketotic hyperosmolar coma; those scheduled for surgery; recent trauma; illness requiring hospitalization; and patients receiving hemo‐ or peritoneal dialysis. The study protocol was approved by the ethics review committee of the University of Occupational and Environmental Health. All patients gave informed consent based on the Helsinki declaration revised in 2000.
Study design
CGM was carried out for 7 days in diabetic outpatients receiving insulin therapy using the CGM system iPro2®. Fasting blood was also collected on the day when iPro2 was removed. The participants also completed a questionnaire on lifestyles during the period of wearing iPro2. The doses and types of glucose‐lowering agents that could influence glycemic control, including insulin, were not changed throughout the period of iPro2 application. The primary outcome was the relationship between MODD and the life variability index. The secondary outcomes were the relationship between MODD and age, and with HbA1c.
Continuous glucose monitoring system
The mean blood glucose level, standard deviation (SD), mean amplitude of glycemic excursions (MAGE), mean postprandial glucose excursion (MPPGE), percentage of time at blood glucose <70 mg/dL, percentage of time at blood glucose ≥180 mg/dL and MODD were measured from the data recorded through CGM using a self‐monitoring blood glucose (SMBG) device. MAGE represents fluctuations in blood glucose levels over a 24‐h period, and was calculated from the daily variations in blood glucose level measured continuously with CGM over a period of 2 days (minimum and maximum SD days)5. MODD was used to assess day‐to‐day glycemic variability, and was calculated based on the absolute difference between the paired CGM values obtained during two successive days (minimum and maximum SD days)4, 6. Previous studies showed that interstitial glucose concentrations measured by the CGM correlate significantly with venous blood glucose levels7. CGM measurements represent glucose concentrations in the interstitial fluid, but since the introduction of the SMBG technique, the measured value is considered to represent blood glucose level.
Measurements of biochemical variables
Fasting venous blood samples were taken in the morning at the start of the study for various laboratory tests. Serum lipids were measured using a Hitachi 7350 autoanalyzer (Hitachi Co., Tokyo, Japan). Low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol and triglycerides were determined by the enzymatic method, and low‐density lipoprotein cholesterol was determined by the direct method. Fasting plasma glucose levels were measured using a standard enzymatic method. HbA1c (%) was measured by high‐performance liquid chromatography using Tosoh HLC‐723 G8 (Tosoh Co., Kyoto, Japan), and expressed as National Glycohemoglobin Standardization Program (NGSP) values by adding 0.4% to the HbA1c value expressed as the conventional Japanese standard substance value8. The 1,5‐anhydroglucitol level was measured by a colorimetric method (Nippon Kayaku, Tokyo, Japan) using a Bio Majesty JCA‐BM 8060 (JEOL, Tokyo, Japan). Glycoalbumin was determined by an enzymatic method using albumin‐specific proteinase, ketoamine oxidase and albumin assay reagent (Lucica GA‐L; Asahi Kasei Pharma Co., Tokyo, Japan).
Measurements of life variability index and life changes
Using a questionnaire, the study participants were asked about the frequency of eating out or buying ready‐to‐eat food, taking snacks between meals, alcohol drinking, exercise level and work schedules. For those items, 0 represented ‘none,’ 1 ‘almost every day,’ 2 ‘3–5 times a week’ and 3 ‘1–2 times a week.’ The total score was defined as the life variability index; a greater life variability index meant larger day‐to‐day lifestyle variability.
In the patient assessment questionnaire, ‘no changes’ represented adoption of the same lifestyle every day over a 1‐week period, whereas ‘changes’ represented adoption of a different lifestyle every day over a period of 1 week.
Patient demographics
Table 1 lists the demographics and characteristics of the participating patients. The participants were 20 men and 25 women, including 13 patients with type 1 diabetes. The mean age was 64.8 ± 11.0 years, body mass index was 23.6 ± 11.0 kg/m2 and duration of diabetes was 21.2 ± 12.1 years. The total insulin dose was 21.2 ± 12.1 U, basal insulin dose was 12.5 ± 9.8 U and bolus insulin dose was 10.3 ± 7.8 U. Intensive insulin therapy was used by 31 patients, mixed preparations were used by three patients, basal insulin alone was used by eight patients, whereas bolus insulin alone was used by three patients. The types of basal insulin preparations used were glargine (n = 36 patients), detemir (n = 2) and degludec (n = 1). The types of bolus insulin used were lispro (n = 25), aspart (n = 7) and glulisine (n = 2). The mixed preparations used were lispro mixture‐25 (n = 2) and aspart 30‐mix (n = 1). The mean HbA1c level was 7.6 ± 1.0% and 14 patients had HbA1c levels less than 7%. With regard to the adverse events, 25 patients developed hypoglycemic episodes (<70 mg/dL), as recorded by the CGM, but all such episodes were mild and asymptomatic.
Table 1.
Patient characteristics
| Males/females (n) | 20/25 |
| Age (years) | 64.8 ± 11.0 |
| Type 1 diabetes/ type 2 diabetes | 13/32 |
| Duration of diabetes (years) | 21.2 ± 12.1 |
| Body mass index (kg/m2) | 23.6 ± 11.0 |
| Systolic blood pressure (mmHg) | 132.5 ± 15.9 |
| Diastolic blood pressure (mmHg) | 69.5 ± 9.9 |
| eGFR (mL/min/1.73 m2) | 64.4 ± 23.9 |
| Triglyceride (mg/dL) | 111.9 ± 73.8 |
| LDL‐C (mg/dL) | 89.8 ± 22.4 |
| HDL‐C (mg/dL) | 65.5 ± 17.9 |
| Fasting plasma glucose (mg/dL) | 176.0 ± 69.1 |
| HbA1c (%) | 7.6 ± 1.0 |
| Glycoalbumin (%) | 21.8 ± 4.8 |
| 1,5‐anhydroglucitol (μg/mL) | 8.2 ± 5.9 |
| Total insulin (unit) | 21.2 ± 12.1 |
| Basal insulin (unit) | 12.5 ± 9.8 |
| Bolus insulin (unit) | 10.3 ± 7.8 |
| Insulin therapy, n (%) | |
| Basal+bolus | 31 (68.8) |
| Basal only | 8 (17.8) |
| Bolus only | 3 (6.7) |
| Mix insulin | 3 (6.7) |
| iPro2 | |
| MPG (mg/dL) | 161.1 ± 37.9 |
| MAGE (mg/dL) | 163.2 ± 66.9 |
| SD (mg/L) | 53.3 ± 22.3 |
| MODD (mg/dL) | 54.2 ± 25.7 |
| Percentage of AUC >180 (%) | 30.2 ± 20.8 |
| Percentage of AOC <70 (%) | 3.6 ± 5.1 |
| Life variability index | 4.2 ± 1.9 |
Data are mean ± standard deviation, n or n (%). AOC, area over the blood concentration‐time curve; AUC, area under the blood concentration‐time curve; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; MAGE, mean amplitude of glycemic excursions.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). Between‐group comparisons were tested by unpaired Mann–Whitney U‐test or χ2‐test. One‐way anova and the Games–Howell test were used to compare the different groups. Factors that could potentially influence MODD were analyzed using Spearman's rank correlation. Multivariate stepwise regression analysis was carried out using MODD as the dependent variable, and several parameters were found to be significantly related to MODD on univariate analysis. A P‐value <0.05 was considered to reflect significant difference. All analyses were carried out using the PASW statistics analysis software v19.0 (SPSS Inc, Chicago, IL, USA).
Results
Severity and extent of MODD
The mean MODD value was 54.2 ± 25.7 mg/dL (range 18.5–120.4). A MODD value <7 mg/dL was defined as ‘normal variability,’ 7–25 mg/dL as ‘stable diabetic’ and >40 mg/dL as ‘unstable diabetic,’ as defined previously8. None of the patients showed a ‘normal variability’ MODD value, whereas just three (6.7%) patients showed ‘stable diabetic’ MODD values, and the remaining 28 (62.2%) patients had ‘unstable diabetic’ MODD values.
Relationship between MODD and various parameters
Table 2 shows the results of correlation analysis for MODD and various parameters.
Table 2.
Correlation between mean of daily differences in blood glucose levels and patient characteristics and glucose metabolism
| r | P‐value | |
|---|---|---|
| Age | – | 0.443 |
| Sex | – | 0.04 |
| Type 1 diabetes/ type 2 diabetes | – | <0.001 |
| Duration of diabetes (years) | 0.122 | 0.425 |
| Body mass index | –0.178 | 0.241 |
| eGFR (mL/min/1.73 m2) | –0.001 | 0.994 |
| HbA1c (%) | 0.5556 | <0.001 |
| 1,5‐anhydroglucitol (μg/mL) | –0.349 | 0.020 |
| Glycoalbumin (%) | 0.386 | 0.010 |
| Insulin total (unit) | 0.462 | 0.001 |
| Bolus (unit) | 0.358 | 0.016 |
| Basal (unit) | 0.483 | 0.001 |
| Bolus/basal | 0.361 | 0.361 |
| Insulin therapy | – | 0.002 |
| Percentage of AOC <70.0 (%) | 0.374 | 0.011 |
| Life variability index | 0.807 | <0.001 |
P‐values by Spearman's rank correlation. Between‐group comparisons were tested by unpaired Mann–Whitney U‐test or χ2‐test. One‐way anova and Games–Howell test were used to compare the four groups. AOC, area over the blood concentration‐time curve; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin.
The MODD values did not correlate significantly with age. The mean MODD value was significantly higher in women than in men (61.0 ± 26.8 vs 45.7 ± 21.9 mg/dL, P = 0.04), and in patients with type 1 diabetes (75.8 ± 26.9 mg/dL) than those with type 2 diabetes (45.4 ± 19.5 mg/dL, P < 0.001).
The MODD values correlated positively with HbA1c levels (r = 0.5556, P < 0.001) and GA levels (r = 0.386, P = 0.01), and negatively with 1,5‐anhydroglucitol levels (r = −0.349, P = 0.02). Patients with poorer glycemic control showed a higher MODD value. The MODD values correlated positively with total insulin dose, bolus insulin dose and basal insulin dose, but not with the ratio of bolus dose to basal dose. The mean MODD value was 61.7 ± 24.9 mg/dL in patients treated with basal and bolus insulin, 43.6 ± 23.7 mg/dL in those with basal insulin alone, 30.1 ± 6.3 mg/dL in those with bolus insulin alone and 28.6 ± 6.7 mg/dL in those with mixed preparations. MODD values correlated significantly with percentage of area over the curve <70.0 mg/dL (r = 0.374, P = 0.011).
Relationship between MODD and lifestyle
The mean MODD value was significantly higher in patients with lifestyle changes than in those without lifestyle changes (67.0 ± 23.0 vs 47.1 ± 24.7 mg/dL, P = 0.002; Figure 1a). Patients with a higher life variability index showed a significantly higher MODD value (P = 0.001; Figure 1b). Multivariate analysis that included MODD value as the dependent variable and sex, type of diabetes mellitus, HbAc1, 1,5‐anhydroglucitol, type of insulin, use of insulin therapy, percentage of area over the curve <70.0 and life variability index as the independent variables identified life variability index (standardized coefficient β = 0.591, P < 0.001), sex (standardized coefficient β = 0.274, P = 0.003), and HbA1c (standardized coefficient β = 0.284, P = 0.008; adjusted multiple R 2 = 0.698) as significant and independent determinants of MODD values (Table 3).
Figure 1.
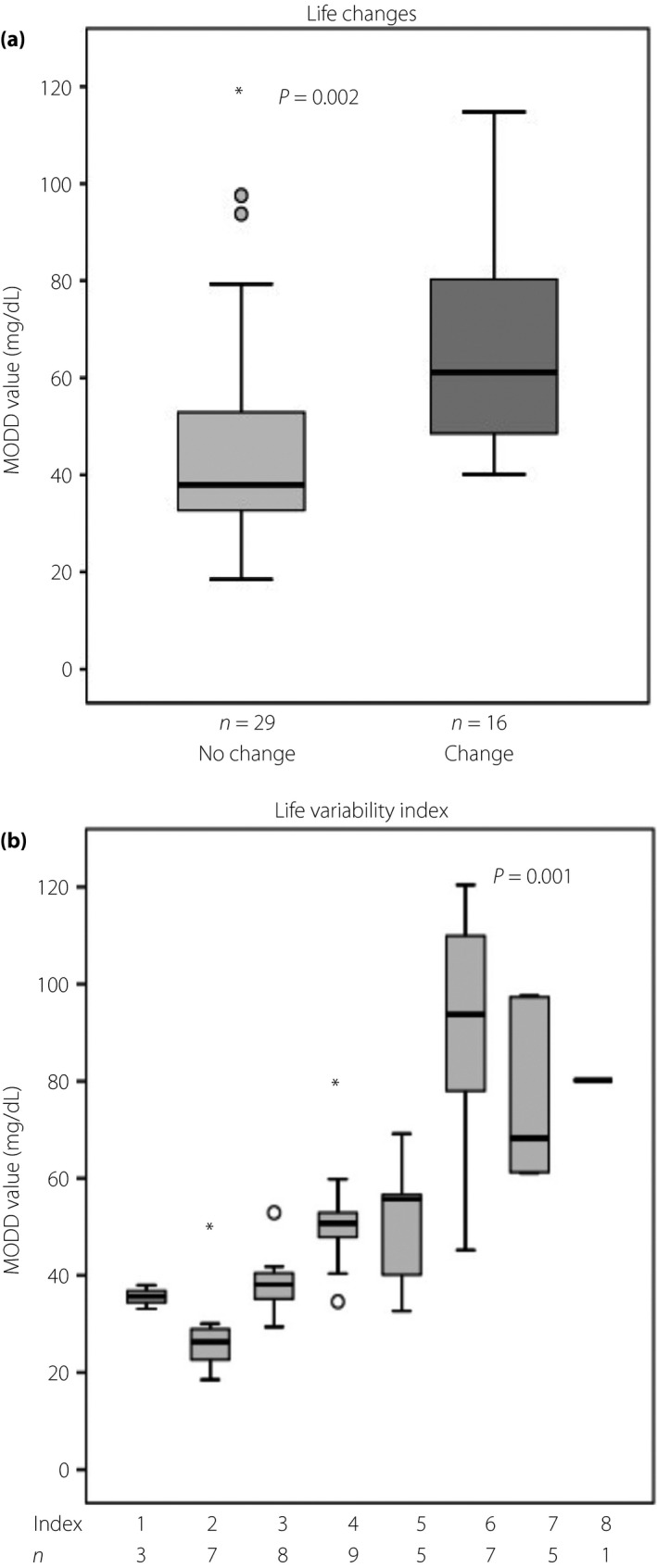
Relationship between mean of daily differences (MODD) in blood glucose levels and life variability. (a) Difference in mean MODD value between patients with lifestyle changes and those without. Between‐group comparisons were tested by unpaired Mann–Whitney U‐test. (b) Mean MODD value according to life variability index. One‐way anova and Games–Howell test were used to compare the groups. In these box‐and‐whisker plots, lines within the boxes represent median values; the upper and lower lines of the boxes represent the 25th and 75th percentiles, respectively; and the upper and lower bars outside the boxes represent the 90th and 10th percentiles, respectively. Asterisk is the extreme values among the outliers. *This represents a case with a value of more than three times the height of the box.
Table 3.
Results of multivariate analysis with mean of daily differences in blood glucose levels as the dependent variable, and sex, type of diabetes, dose of insulin, glycated hemoglobin, insulin therapy, percentage of area over the blood concentration‐time curve <70.0 and life variability index as the independent variables
| Variables | Non‐standardized coefficients | Standardized coefficient r | P‐value | 95% CI | |
|---|---|---|---|---|---|
| Intercept | –57.1154 | 0.004 | –95.203 | –19.026 | |
| Life variability index | 8.061 | 0.591 | <0.001 | 5.262 | 10.860 |
| Sex | 14.009 | 0.274 | 0.003 | 5.143 | 22.874 |
| HbA1c | 7.404 | 0.284 | 0.008 | 2.059 | 12.749 |
| Adjusted multiple R 2 | 0.698 | ||||
CI, confidence interval; HbA1c, glycated hemoglobin.
Discussion
In the present study, the use of iPro2® continuously over a period of 1 week provided actual and detailed glycemic profiles of diabetic outpatients receiving insulin therapy. Approximately 93% of the outpatients showed inter‐day glycemic variability, and 62% showed unstable inter‐day glycemic variability. The inter‐day variability was associated with sex, type of diabetes, insulin dose and regimen. Multivariate analysis identified life variability index as a significant factor that influences MODD.
Zhou et al.9 reported that MODD level was significantly higher in patients with newly diagnosed type 2 diabetes than healthy participants (1.8 ± 0.6 vs 0.8 ± 0.3 mmol/L). The mean MODD value in the present patients was much higher (54.2 ± 25.7 mg/dL) than that reported in the aforementioned study. The high MODD value might reflect the study design; that measurements were made in a real‐life situation where dye was not restricted and no exercise therapy was provided.
Another study reported that glycemic variability influenced HbA1c levels in elderly male patients with type 2 diabetes10. However, in that study, MAGE, rather than MODD, was used for the assessment of glycemic variability. Multivariate analysis in the present study identified HbA1c level as a significant factor that influenced MODD. This finding suggests that minimization of inter‐day glycemic variability is important in improving glycemic control in insulin‐treated patients. Furthermore, the finding could be probably explained by the study design as well; the study patients with high MODD values were not receiving intensive treatment therapy. Physicians sometimes reduce the insulin dose to avoid the chance of hypoglycemia in patients who experienced hypoglycemia. However, physicians often do not increase the insulin dose to avoid hypoglycemia, even in hyperglycemic patients with large glycemic variability. These approaches are often followed in insulin adjustment in actual outpatient clinical settings. In recent years, hypoglycemia has been reported to be a risk factor for cardiovascular events11 and development of dementia12. In the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation study, intensive treatment was associated with frequent episodes of severe hypoglycemia, which increased the risk for micro‐ and macrovasculopathies13. Not only healthcare practitioners, but also patients and families, fear hypoglycemia, which is a serious barrier to intensive treatment14, 15, 16. Although improvement of the HbA1c level is important in the overall management of diabetes mellitus, treatment should be fine‐tuned to avoid the risk of hypoglycemia. It is important to provide intensive insulin treatment that can control blood glucose level, and at the same time minimize intra‐ and inter‐day glycemic variability to avoid hypoglycemia.
The present results showed that lifestyle is significantly associated with MODD. However, lifestyle stabilization is inconsistent with maintaining a high quality of life, which is the main goal of treatment in diabetic patients. In the Diabetes Glycemic Education and Monitoring study carried out in non‐insulin‐treated patients with type 2 diabetes, HbA1c levels at 12 months failed to improve in three groups of patients: (i) patients who did not carry out SMBG; (ii) patients with more intensive SMBG, and (iii) patients with SMBG alone17. The results of that study show that SMBG is not a simple routine method to measure blood glucose level, and it can become useful only when feedback from the results is incorporated into self‐care. Based on the lifestyle and SMBG results, patients should be provided with detailed instructions on diet and exercise therapies, and also regarding decisions about insulin treatment regimens.
Approximately 90% of the insulin‐treated outpatients showed inter‐day glycemic variability. Patients with a high life variability index are likely to have larger inter‐day glycemic variability, resulting in failure to improve HbA1c levels. Treatments and instructions based on patients’ characteristics, day‐to‐day glycemic variability and lifestyle are important for patients receiving insulin therapy.
There were several limitations to the present study. First, the number of participants was relatively small. Second, patients who wore iPro2® might have paid more attention to the disease and have had better glycemic control than those who did not. Third, the life variability index was prepared by our group after failure to recognize in the literature a robust index that comprehensively describes changes in lifestyle after a particular treatment. Although the index was based on various factors, such as eating out, taking snacks, drinking alcohol, doing exercises, job description and working schedules, other lifestyle factors should have been included. Fourth, the data were extracted from CGM, and possible differences between these data and actual blood glucose level cannot be ruled out. Blood glucose levels ≥400 mg/dL or ≤40 mg/dL were not recorded in the present study. Fifth, the present study was carried out in the Outpatients Department in daily clinical settings, and insulin doses were titrated at the discretion of each of the attending physicians without prespecified blood glucose targets. A prospective study needs to be carried out that includes prespecified timing of SMBG, blood glucose targets and titration of insulin doses. Further prospective studies of large sample size are required to determine whether treatment that reduces inter‐day glycemic variability improves HbA1c level.
Disclosure
Dr Okada has received consulting fees and lecture fees from Novo Nordisc Pharma and Eli Lilly Japan.
Acknowledgements
The authors thank Ms N Sakaguchi for her excellent technical assistance. This study was supported in part by Research Grants‐In‐Aid for Scientific Research from the Ministry of Health, Labor and Welfare of Japan; the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Joint Research Association for Japanese Diabetes; and the University of Occupational and Environmental Health, Japan.
J Diabetes Investig 2017; 8: 69–74
Clinical Trial Registry
University Hospital Medical Information Network
UMIN000019727
References
- 1. The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 2. The Diabetes Control and Complications Trial/Epidemiology of Diabetes . Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 2000; 342: 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ohkubo Y, Kishikawa H, Araki E, et al Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study. Diabetes Res Clin Pract 1995; 28: 103–117. [DOI] [PubMed] [Google Scholar]
- 4. Service FJ, Nelson RL. Characteristics of glycemic stability. Diabetes Care 1980; 3: 58–62. [DOI] [PubMed] [Google Scholar]
- 5. Service FJ, Molnar GD, Rosevear JW, et al Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 1970; 19: 644–655. [DOI] [PubMed] [Google Scholar]
- 6. Molnar GD, Taylor WF, Ho MM. Day‐to‐day variation of continuously monitored glycaemia: a further measure of diabetic instability. Diabetologia 1972; 8: 342–348. [DOI] [PubMed] [Google Scholar]
- 7. Boyne MS, Silver DM, Kaplan J, et al Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes 2003; 52: 2790–2794. [DOI] [PubMed] [Google Scholar]
- 8. The Committee of Japan Diabetes Society on the diagnostic criteria of diabetes mellitus . Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010; 1: 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou J, Jia W, Bao Y, et al Glycemic variability and its responses to intensive insulin treatment in newly diagnosed type 2 diabetes. Med Sci Monit 2008; 14: CR552–CR558. [PubMed] [Google Scholar]
- 10. Fang FS, Li ZB, Li CL, et al Influence of glycemic variability on the HbA1c level in elderly male patients with type 2 diabetes. Intern Med 2012; 51: 3109–3113. [DOI] [PubMed] [Google Scholar]
- 11. Desouza VC, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care 2010; 33: 1389–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Whitmer RA, Karter AJ, Yaffe K, et al Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009; 301: 1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zoungas S1, Patel A, Chalmers J, et al Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010; 363: 1410–1418. [DOI] [PubMed] [Google Scholar]
- 14. Frier BM. How hypoglycaemia can affect the life of a person with diabetes. Diabetes Metab Res Rev 2008; 24: 87–92. [DOI] [PubMed] [Google Scholar]
- 15. Guisasola A, Povedano S, Krishnarajah G, et al Hypoglycaemic symptoms, treatment satisfaction, adherence and their associations with glycaemic goal in patients with type 2 diabetes mellitus: findings from the Real‐Life Effectiveness and Care Patterns of Diabetes Management (RECAP‐DM) Study. Diabetes Obes Metab 2008; 10: 25–32. [DOI] [PubMed] [Google Scholar]
- 16. Peyrot M, Bamett AH, Meneghini LF, et al Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med 2012; 29: 682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Farmer A. Impact of self monitoring of blood glucose in the management of patients with non‐insulin treated diabetes: open parallel group randomized trial. Br Med J 2007; 335: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]


